KR20140052992A - 뇌혈류에 영향을 미치는 뇌손상 후 결과를 개선시키기 위한 뇌실내 약물 전달 시스템 - Google Patents
뇌혈류에 영향을 미치는 뇌손상 후 결과를 개선시키기 위한 뇌실내 약물 전달 시스템 Download PDFInfo
- Publication number
- KR20140052992A KR20140052992A KR1020137029330A KR20137029330A KR20140052992A KR 20140052992 A KR20140052992 A KR 20140052992A KR 1020137029330 A KR1020137029330 A KR 1020137029330A KR 20137029330 A KR20137029330 A KR 20137029330A KR 20140052992 A KR20140052992 A KR 20140052992A
- Authority
- KR
- South Korea
- Prior art keywords
- therapeutic agent
- calcium channel
- cerebral
- ventricle
- pyrimidine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 208000029028 brain injury Diseases 0.000 title claims abstract description 66
- 230000003727 cerebral blood flow Effects 0.000 title description 9
- 238000012377 drug delivery Methods 0.000 title description 5
- 238000007914 intraventricular administration Methods 0.000 title description 2
- 210000001627 cerebral artery Anatomy 0.000 claims abstract description 244
- 239000000203 mixture Substances 0.000 claims abstract description 218
- 210000002330 subarachnoid space Anatomy 0.000 claims abstract description 184
- 238000013268 sustained release Methods 0.000 claims abstract description 169
- 239000012730 sustained-release form Substances 0.000 claims abstract description 169
- 230000009969 flowable effect Effects 0.000 claims abstract description 86
- 238000000034 method Methods 0.000 claims abstract description 75
- 230000003111 delayed effect Effects 0.000 claims abstract description 53
- 230000006931 brain damage Effects 0.000 claims abstract description 33
- 231100000874 brain damage Toxicity 0.000 claims abstract description 33
- 238000004519 manufacturing process Methods 0.000 claims abstract description 12
- 239000003814 drug Substances 0.000 claims description 232
- 229940124597 therapeutic agent Drugs 0.000 claims description 200
- 206010047163 Vasospasm Diseases 0.000 claims description 186
- 230000001225 therapeutic effect Effects 0.000 claims description 101
- 239000011859 microparticle Substances 0.000 claims description 94
- 238000009472 formulation Methods 0.000 claims description 81
- 239000003937 drug carrier Substances 0.000 claims description 79
- 229940127291 Calcium channel antagonist Drugs 0.000 claims description 69
- 239000000480 calcium channel blocker Substances 0.000 claims description 69
- 238000012384 transportation and delivery Methods 0.000 claims description 63
- 230000000694 effects Effects 0.000 claims description 59
- 241000239290 Araneae Species 0.000 claims description 53
- 108090000623 proteins and genes Proteins 0.000 claims description 42
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 claims description 36
- 229940125400 channel inhibitor Drugs 0.000 claims description 36
- 150000001875 compounds Chemical class 0.000 claims description 33
- 108090000420 L-Type Calcium Channels Proteins 0.000 claims description 32
- 102000004016 L-Type Calcium Channels Human genes 0.000 claims description 32
- 102000004169 proteins and genes Human genes 0.000 claims description 30
- 239000011159 matrix material Substances 0.000 claims description 29
- 208000028867 ischemia Diseases 0.000 claims description 28
- 238000002347 injection Methods 0.000 claims description 25
- 239000007924 injection Substances 0.000 claims description 25
- 230000001054 cortical effect Effects 0.000 claims description 24
- UIAGMCDKSXEBJQ-IBGZPJMESA-N 3-o-(2-methoxyethyl) 5-o-propan-2-yl (4s)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate Chemical group COCCOC(=O)C1=C(C)NC(C)=C(C(=O)OC(C)C)[C@H]1C1=CC=CC([N+]([O-])=O)=C1 UIAGMCDKSXEBJQ-IBGZPJMESA-N 0.000 claims description 21
- 229960000715 nimodipine Drugs 0.000 claims description 21
- 239000000725 suspension Substances 0.000 claims description 21
- YNGDWRXWKFWCJY-UHFFFAOYSA-N 1,4-Dihydropyridine Chemical compound C1C=CNC=C1 YNGDWRXWKFWCJY-UHFFFAOYSA-N 0.000 claims description 20
- 229920002988 biodegradable polymer Polymers 0.000 claims description 20
- 239000004621 biodegradable polymer Substances 0.000 claims description 20
- 206010008118 cerebral infarction Diseases 0.000 claims description 19
- 230000001052 transient effect Effects 0.000 claims description 19
- 210000004055 fourth ventricle Anatomy 0.000 claims description 18
- 108090000583 R-Type Calcium Channels Proteins 0.000 claims description 16
- 102000004059 R-Type Calcium Channels Human genes 0.000 claims description 16
- 102000004129 N-Type Calcium Channels Human genes 0.000 claims description 15
- 108090000699 N-Type Calcium Channels Proteins 0.000 claims description 15
- 210000000211 third ventricle Anatomy 0.000 claims description 15
- 201000006474 Brain Ischemia Diseases 0.000 claims description 14
- 206010008120 Cerebral ischaemia Diseases 0.000 claims description 14
- 239000002308 endothelin receptor antagonist Substances 0.000 claims description 14
- 229920000954 Polyglycolide Polymers 0.000 claims description 12
- 239000000872 buffer Substances 0.000 claims description 12
- 238000009826 distribution Methods 0.000 claims description 12
- RZTAMFZIAATZDJ-HNNXBMFYSA-N 5-o-ethyl 3-o-methyl (4s)-4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate Chemical compound CCOC(=O)C1=C(C)NC(C)=C(C(=O)OC)[C@@H]1C1=CC=CC(Cl)=C1Cl RZTAMFZIAATZDJ-HNNXBMFYSA-N 0.000 claims description 11
- 229920001710 Polyorthoester Polymers 0.000 claims description 11
- 229960003580 felodipine Drugs 0.000 claims description 11
- 239000010419 fine particle Substances 0.000 claims description 11
- 239000000843 powder Substances 0.000 claims description 11
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Polymers OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 claims description 10
- 150000008064 anhydrides Chemical class 0.000 claims description 10
- 239000002745 poly(ortho ester) Substances 0.000 claims description 10
- HMJIYCCIJYRONP-UHFFFAOYSA-N (+-)-Isradipine Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OC(C)C)C1C1=CC=CC2=NON=C12 HMJIYCCIJYRONP-UHFFFAOYSA-N 0.000 claims description 9
- NCUCGYYHUFIYNU-UHFFFAOYSA-N Aranidipine Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OCC(C)=O)C1C1=CC=CC=C1[N+]([O-])=O NCUCGYYHUFIYNU-UHFFFAOYSA-N 0.000 claims description 9
- 229950007556 aranidipine Drugs 0.000 claims description 9
- 229960004427 isradipine Drugs 0.000 claims description 9
- 230000015572 biosynthetic process Effects 0.000 claims description 8
- 210000003140 lateral ventricle Anatomy 0.000 claims description 8
- 230000001419 dependent effect Effects 0.000 claims description 7
- 108010027023 Q-Type Calcium Channels Proteins 0.000 claims description 6
- 239000008194 pharmaceutical composition Substances 0.000 claims description 6
- 230000007480 spreading Effects 0.000 claims description 6
- 238000003892 spreading Methods 0.000 claims description 6
- 206010008089 Cerebral artery occlusion Diseases 0.000 claims description 5
- 206010028980 Neoplasm Diseases 0.000 claims description 5
- 230000002459 sustained effect Effects 0.000 claims description 4
- 230000001839 systemic circulation Effects 0.000 claims description 4
- 238000011287 therapeutic dose Methods 0.000 claims description 4
- 239000003708 ampul Substances 0.000 claims description 2
- 201000011510 cancer Diseases 0.000 claims description 2
- 230000001954 sterilising effect Effects 0.000 claims description 2
- 238000004659 sterilization and disinfection Methods 0.000 claims description 2
- 208000024891 symptom Diseases 0.000 abstract description 35
- 201000008450 Intracranial aneurysm Diseases 0.000 abstract description 6
- 241000124008 Mammalia Species 0.000 abstract description 2
- 230000000144 pharmacologic effect Effects 0.000 description 151
- 208000032851 Subarachnoid Hemorrhage Diseases 0.000 description 144
- 210000004556 brain Anatomy 0.000 description 123
- 230000009467 reduction Effects 0.000 description 110
- 210000001367 artery Anatomy 0.000 description 77
- 239000012528 membrane Substances 0.000 description 65
- 210000004379 membrane Anatomy 0.000 description 65
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 62
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 61
- 108090000765 processed proteins & peptides Proteins 0.000 description 61
- 108091006146 Channels Proteins 0.000 description 54
- 210000004204 blood vessel Anatomy 0.000 description 52
- 206010002329 Aneurysm Diseases 0.000 description 51
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 47
- 230000002490 cerebral effect Effects 0.000 description 47
- 229920002674 hyaluronan Polymers 0.000 description 47
- 229960003160 hyaluronic acid Drugs 0.000 description 47
- 230000001965 increasing effect Effects 0.000 description 44
- 102000003922 Calcium Channels Human genes 0.000 description 42
- 108090000312 Calcium Channels Proteins 0.000 description 42
- 102000005962 receptors Human genes 0.000 description 42
- 108020003175 receptors Proteins 0.000 description 42
- 210000004369 blood Anatomy 0.000 description 39
- 239000008280 blood Substances 0.000 description 39
- 210000004027 cell Anatomy 0.000 description 39
- 208000032843 Hemorrhage Diseases 0.000 description 37
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 36
- 239000002245 particle Substances 0.000 description 35
- 102000004196 processed proteins & peptides Human genes 0.000 description 35
- 201000010099 disease Diseases 0.000 description 34
- 208000034158 bleeding Diseases 0.000 description 32
- 230000000740 bleeding effect Effects 0.000 description 32
- 239000011575 calcium Substances 0.000 description 32
- 208000035475 disorder Diseases 0.000 description 28
- 229920000642 polymer Polymers 0.000 description 28
- 230000025033 vasoconstriction Effects 0.000 description 28
- 206010047139 Vasoconstriction Diseases 0.000 description 26
- 235000018102 proteins Nutrition 0.000 description 26
- 229940079593 drug Drugs 0.000 description 25
- 239000000463 material Substances 0.000 description 25
- -1 4aR Chemical compound 0.000 description 24
- 238000002583 angiography Methods 0.000 description 24
- 239000000126 substance Substances 0.000 description 24
- 210000001519 tissue Anatomy 0.000 description 24
- 229910052791 calcium Inorganic materials 0.000 description 23
- 238000011282 treatment Methods 0.000 description 23
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 22
- 208000001286 intracranial vasospasm Diseases 0.000 description 22
- 239000000556 agonist Substances 0.000 description 21
- 239000003112 inhibitor Substances 0.000 description 21
- 230000002829 reductive effect Effects 0.000 description 21
- 206010057190 Respiratory tract infections Diseases 0.000 description 20
- 210000000278 spinal cord Anatomy 0.000 description 20
- 230000036772 blood pressure Effects 0.000 description 19
- 210000001638 cerebellum Anatomy 0.000 description 19
- 239000003795 chemical substances by application Substances 0.000 description 18
- 239000007787 solid Substances 0.000 description 18
- 101800004490 Endothelin-1 Proteins 0.000 description 17
- 238000002591 computed tomography Methods 0.000 description 17
- 230000007423 decrease Effects 0.000 description 17
- 230000001537 neural effect Effects 0.000 description 17
- 239000012071 phase Substances 0.000 description 16
- 230000008569 process Effects 0.000 description 16
- 239000000779 smoke Substances 0.000 description 16
- 210000003462 vein Anatomy 0.000 description 16
- 206010020772 Hypertension Diseases 0.000 description 15
- 230000027455 binding Effects 0.000 description 15
- 210000002216 heart Anatomy 0.000 description 15
- 210000004720 cerebrum Anatomy 0.000 description 14
- 239000000839 emulsion Substances 0.000 description 14
- 230000028161 membrane depolarization Effects 0.000 description 14
- 210000003205 muscle Anatomy 0.000 description 14
- 210000003388 posterior cerebral artery Anatomy 0.000 description 14
- 230000024883 vasodilation Effects 0.000 description 14
- 206010019196 Head injury Diseases 0.000 description 13
- 230000000747 cardiac effect Effects 0.000 description 13
- 210000004004 carotid artery internal Anatomy 0.000 description 13
- 210000001259 mesencephalon Anatomy 0.000 description 13
- 210000003625 skull Anatomy 0.000 description 13
- 102000038650 voltage-gated calcium channel activity Human genes 0.000 description 13
- 108091023044 voltage-gated calcium channel activity Proteins 0.000 description 13
- 102100033902 Endothelin-1 Human genes 0.000 description 12
- 239000005557 antagonist Substances 0.000 description 12
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 12
- 230000006378 damage Effects 0.000 description 12
- 210000003657 middle cerebral artery Anatomy 0.000 description 12
- 230000002269 spontaneous effect Effects 0.000 description 12
- 230000009885 systemic effect Effects 0.000 description 12
- 230000000472 traumatic effect Effects 0.000 description 12
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 12
- 241001465754 Metazoa Species 0.000 description 11
- 230000036770 blood supply Effects 0.000 description 11
- 239000002872 contrast media Substances 0.000 description 11
- 238000011161 development Methods 0.000 description 11
- 230000018109 developmental process Effects 0.000 description 11
- 230000006870 function Effects 0.000 description 11
- 210000002569 neuron Anatomy 0.000 description 11
- 230000004044 response Effects 0.000 description 11
- 230000002792 vascular Effects 0.000 description 11
- 239000003981 vehicle Substances 0.000 description 11
- 210000002385 vertebral artery Anatomy 0.000 description 11
- 102000002045 Endothelin Human genes 0.000 description 10
- 108050009340 Endothelin Proteins 0.000 description 10
- 102000004310 Ion Channels Human genes 0.000 description 10
- 108090000862 Ion Channels Proteins 0.000 description 10
- 241000237502 Ostreidae Species 0.000 description 10
- 208000006011 Stroke Diseases 0.000 description 10
- 210000002551 anterior cerebral artery Anatomy 0.000 description 10
- 230000017531 blood circulation Effects 0.000 description 10
- 210000000988 bone and bone Anatomy 0.000 description 10
- 230000008602 contraction Effects 0.000 description 10
- 125000000058 cyclopentadienyl group Chemical group C1(=CC=CC1)* 0.000 description 10
- ZSWFCLXCOIISFI-UHFFFAOYSA-N endo-cyclopentadiene Natural products C1C=CC=C1 ZSWFCLXCOIISFI-UHFFFAOYSA-N 0.000 description 10
- ZUBDGKVDJUIMQQ-UBFCDGJISA-N endothelin-1 Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)NC(=O)[C@H]1NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@@H](CC=2C=CC(O)=CC=2)NC(=O)[C@H](C(C)C)NC(=O)[C@H]2CSSC[C@@H](C(N[C@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N2)=O)NC(=O)[C@@H](CO)NC(=O)[C@H](N)CSSC1)C1=CNC=N1 ZUBDGKVDJUIMQQ-UBFCDGJISA-N 0.000 description 10
- 210000003128 head Anatomy 0.000 description 10
- 230000007574 infarction Effects 0.000 description 10
- 230000003834 intracellular effect Effects 0.000 description 10
- 230000007246 mechanism Effects 0.000 description 10
- 210000000653 nervous system Anatomy 0.000 description 10
- 235000020636 oyster Nutrition 0.000 description 10
- 239000000243 solution Substances 0.000 description 10
- 206010033799 Paralysis Diseases 0.000 description 9
- 208000007536 Thrombosis Diseases 0.000 description 9
- 230000004913 activation Effects 0.000 description 9
- 229920001222 biopolymer Polymers 0.000 description 9
- 210000000170 cell membrane Anatomy 0.000 description 9
- 238000003745 diagnosis Methods 0.000 description 9
- 208000003906 hydrocephalus Diseases 0.000 description 9
- 238000002595 magnetic resonance imaging Methods 0.000 description 9
- 230000002265 prevention Effects 0.000 description 9
- 238000004393 prognosis Methods 0.000 description 9
- 239000002904 solvent Substances 0.000 description 9
- 238000001356 surgical procedure Methods 0.000 description 9
- 102000008186 Collagen Human genes 0.000 description 8
- 108010035532 Collagen Proteins 0.000 description 8
- 206010010904 Convulsion Diseases 0.000 description 8
- 241001269524 Dura Species 0.000 description 8
- 206010019233 Headaches Diseases 0.000 description 8
- 206010061216 Infarction Diseases 0.000 description 8
- 230000003872 anastomosis Effects 0.000 description 8
- 210000001841 basilar artery Anatomy 0.000 description 8
- 230000008859 change Effects 0.000 description 8
- 239000011248 coating agent Substances 0.000 description 8
- 229920001436 collagen Polymers 0.000 description 8
- 239000006185 dispersion Substances 0.000 description 8
- 239000012530 fluid Substances 0.000 description 8
- 210000001652 frontal lobe Anatomy 0.000 description 8
- 230000004054 inflammatory process Effects 0.000 description 8
- 210000002464 muscle smooth vascular Anatomy 0.000 description 8
- 230000010412 perfusion Effects 0.000 description 8
- 230000036581 peripheral resistance Effects 0.000 description 8
- 150000003839 salts Chemical class 0.000 description 8
- 239000011734 sodium Substances 0.000 description 8
- 210000003478 temporal lobe Anatomy 0.000 description 8
- 239000003071 vasodilator agent Substances 0.000 description 8
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 7
- 206010061218 Inflammation Diseases 0.000 description 7
- 230000002411 adverse Effects 0.000 description 7
- 210000000576 arachnoid Anatomy 0.000 description 7
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 7
- 238000000576 coating method Methods 0.000 description 7
- 238000010968 computed tomography angiography Methods 0.000 description 7
- 210000000877 corpus callosum Anatomy 0.000 description 7
- 238000009792 diffusion process Methods 0.000 description 7
- 231100000869 headache Toxicity 0.000 description 7
- 239000000017 hydrogel Substances 0.000 description 7
- 208000014674 injury Diseases 0.000 description 7
- 230000003993 interaction Effects 0.000 description 7
- 230000033001 locomotion Effects 0.000 description 7
- 229910052760 oxygen Inorganic materials 0.000 description 7
- 239000001301 oxygen Substances 0.000 description 7
- 239000011148 porous material Substances 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- 230000001105 regulatory effect Effects 0.000 description 7
- 210000002460 smooth muscle Anatomy 0.000 description 7
- 239000005526 vasoconstrictor agent Substances 0.000 description 7
- 229940124549 vasodilator Drugs 0.000 description 7
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- 206010008111 Cerebral haemorrhage Diseases 0.000 description 6
- 206010059109 Cerebral vasoconstriction Diseases 0.000 description 6
- 229920002101 Chitin Polymers 0.000 description 6
- 206010010071 Coma Diseases 0.000 description 6
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 6
- 108030001679 Endothelin-converting enzyme 1 Proteins 0.000 description 6
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 6
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 6
- 208000001953 Hypotension Diseases 0.000 description 6
- ZBBHBTPTTSWHBA-UHFFFAOYSA-N Nicardipine Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OCCN(C)CC=2C=CC=CC=2)C1C1=CC=CC([N+]([O-])=O)=C1 ZBBHBTPTTSWHBA-UHFFFAOYSA-N 0.000 description 6
- 239000002202 Polyethylene glycol Substances 0.000 description 6
- 208000004717 Ruptured Aneurysm Diseases 0.000 description 6
- 230000009471 action Effects 0.000 description 6
- 239000004480 active ingredient Substances 0.000 description 6
- 230000003444 anaesthetic effect Effects 0.000 description 6
- 230000004872 arterial blood pressure Effects 0.000 description 6
- 230000000903 blocking effect Effects 0.000 description 6
- 210000000133 brain stem Anatomy 0.000 description 6
- 239000000969 carrier Substances 0.000 description 6
- 208000026106 cerebrovascular disease Diseases 0.000 description 6
- 230000004087 circulation Effects 0.000 description 6
- 210000001951 dura mater Anatomy 0.000 description 6
- 239000000835 fiber Substances 0.000 description 6
- 210000003016 hypothalamus Anatomy 0.000 description 6
- 239000012729 immediate-release (IR) formulation Substances 0.000 description 6
- 238000001990 intravenous administration Methods 0.000 description 6
- 150000002500 ions Chemical class 0.000 description 6
- 210000005036 nerve Anatomy 0.000 description 6
- 229960001783 nicardipine Drugs 0.000 description 6
- HYIMSNHJOBLJNT-UHFFFAOYSA-N nifedipine Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OC)C1C1=CC=CC=C1[N+]([O-])=O HYIMSNHJOBLJNT-UHFFFAOYSA-N 0.000 description 6
- 210000000869 occipital lobe Anatomy 0.000 description 6
- 239000000546 pharmaceutical excipient Substances 0.000 description 6
- 229920001223 polyethylene glycol Polymers 0.000 description 6
- GPTFURBXHJWNHR-UHFFFAOYSA-N protopine Chemical compound C1=C2C(=O)CC3=CC=C4OCOC4=C3CN(C)CCC2=CC2=C1OCO2 GPTFURBXHJWNHR-UHFFFAOYSA-N 0.000 description 6
- 238000012552 review Methods 0.000 description 6
- 229910052708 sodium Inorganic materials 0.000 description 6
- 230000002861 ventricular Effects 0.000 description 6
- PYWYBTRACMRUQV-UHFFFAOYSA-N 2-(6,7-dimethoxy-3,4-dihydroisoquinolin-1-yl)-2-phenyl-n,n-bis[2-(2,3,4-trimethoxyphenyl)ethyl]acetamide Chemical compound COC1=C(OC)C(OC)=CC=C1CCN(C(=O)C(C=1C2=CC(OC)=C(OC)C=C2CCN=1)C=1C=CC=CC=1)CCC1=CC=C(OC)C(OC)=C1OC PYWYBTRACMRUQV-UHFFFAOYSA-N 0.000 description 5
- 102400000687 Big endothelin-1 Human genes 0.000 description 5
- 101800001398 Big endothelin-1 Proteins 0.000 description 5
- 208000005189 Embolism Diseases 0.000 description 5
- 201000009273 Endometriosis Diseases 0.000 description 5
- 102000048186 Endothelin-converting enzyme 1 Human genes 0.000 description 5
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 5
- 108020004202 Guanylate Kinase Proteins 0.000 description 5
- 108050001421 Transient receptor potential channel, canonical 6 Proteins 0.000 description 5
- 102000014384 Type C Phospholipases Human genes 0.000 description 5
- 108010079194 Type C Phospholipases Proteins 0.000 description 5
- 239000013543 active substance Substances 0.000 description 5
- 230000008485 antagonism Effects 0.000 description 5
- MOTJMGVDPWRKOC-QPVYNBJUSA-N atrasentan Chemical compound C1([C@H]2[C@@H]([C@H](CN2CC(=O)N(CCCC)CCCC)C=2C=C3OCOC3=CC=2)C(O)=O)=CC=C(OC)C=C1 MOTJMGVDPWRKOC-QPVYNBJUSA-N 0.000 description 5
- HZZGDPLAJHVHSP-GKHTVLBPSA-N big endothelin Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O)NC(=O)[C@H]1NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC=2C=CC(O)=CC=2)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]2CSSC[C@@H](C(N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N2)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CSSC1)C1=CN=CN1 HZZGDPLAJHVHSP-GKHTVLBPSA-N 0.000 description 5
- 210000001715 carotid artery Anatomy 0.000 description 5
- 210000003169 central nervous system Anatomy 0.000 description 5
- 210000003710 cerebral cortex Anatomy 0.000 description 5
- 125000004925 dihydropyridyl group Chemical group N1(CC=CC=C1)* 0.000 description 5
- 210000002889 endothelial cell Anatomy 0.000 description 5
- 210000001723 extracellular space Anatomy 0.000 description 5
- 210000003414 extremity Anatomy 0.000 description 5
- 239000010408 film Substances 0.000 description 5
- 239000000499 gel Substances 0.000 description 5
- 239000008187 granular material Substances 0.000 description 5
- 102000006638 guanylate kinase Human genes 0.000 description 5
- 230000000004 hemodynamic effect Effects 0.000 description 5
- 230000036543 hypotension Effects 0.000 description 5
- 230000005764 inhibitory process Effects 0.000 description 5
- 230000000670 limiting effect Effects 0.000 description 5
- 239000003550 marker Substances 0.000 description 5
- 230000001404 mediated effect Effects 0.000 description 5
- 229960001597 nifedipine Drugs 0.000 description 5
- 210000001152 parietal lobe Anatomy 0.000 description 5
- 229940068196 placebo Drugs 0.000 description 5
- 239000000902 placebo Substances 0.000 description 5
- 230000002035 prolonged effect Effects 0.000 description 5
- 230000011514 reflex Effects 0.000 description 5
- 210000000329 smooth muscle myocyte Anatomy 0.000 description 5
- 230000000638 stimulation Effects 0.000 description 5
- 239000000375 suspending agent Substances 0.000 description 5
- 229920001059 synthetic polymer Polymers 0.000 description 5
- 238000002560 therapeutic procedure Methods 0.000 description 5
- DLFVBJFMPXGRIB-UHFFFAOYSA-N Acetamide Chemical compound CC(N)=O DLFVBJFMPXGRIB-UHFFFAOYSA-N 0.000 description 4
- 108091005462 Cation channels Proteins 0.000 description 4
- 206010013935 Dysmenorrhoea Diseases 0.000 description 4
- 102100040611 Endothelin receptor type B Human genes 0.000 description 4
- 102100029109 Endothelin-3 Human genes 0.000 description 4
- 108010072844 Endothelin-3 Proteins 0.000 description 4
- 206010018985 Haemorrhage intracranial Diseases 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 101000637699 Homo sapiens Ryanodine receptor 1 Proteins 0.000 description 4
- 206010022773 Intracranial pressure increased Diseases 0.000 description 4
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 4
- 229920002732 Polyanhydride Polymers 0.000 description 4
- 102000004257 Potassium Channel Human genes 0.000 description 4
- 102100032122 Ryanodine receptor 1 Human genes 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 102000027549 TRPC Human genes 0.000 description 4
- 108060008648 TRPC Proteins 0.000 description 4
- 102000011213 Transient receptor potential channel, canonical 6 Human genes 0.000 description 4
- 206010047700 Vomiting Diseases 0.000 description 4
- 208000027418 Wounds and injury Diseases 0.000 description 4
- 230000009102 absorption Effects 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 229940024606 amino acid Drugs 0.000 description 4
- 210000003484 anatomy Anatomy 0.000 description 4
- 239000008346 aqueous phase Substances 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 230000002146 bilateral effect Effects 0.000 description 4
- 230000008499 blood brain barrier function Effects 0.000 description 4
- 210000001218 blood-brain barrier Anatomy 0.000 description 4
- 230000037396 body weight Effects 0.000 description 4
- 210000001736 capillary Anatomy 0.000 description 4
- ZGOVYTPSWMLYOF-QEADGSHQSA-N chembl1790180 Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)NC(=O)[C@H]1NC(=O)[C@H](CC=2C=CC(O)=CC=2)NC(=O)[C@@H](CC=2C=CC(O)=CC=2)NC(=O)[C@H](C(C)C)NC(=O)[C@H]2CSSC[C@@H](C(N[C@H](CC=3C=CC=CC=3)C(=O)N[C@H](C(=O)N[C@H](CCC=3C=CC(O)=CC=3)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N2)[C@H](C)O)=O)NC(=O)[C@@H]([C@@H](C)O)NC(=O)[C@H](N)CSSC1)C1=CNC=N1 ZGOVYTPSWMLYOF-QEADGSHQSA-N 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 4
- 210000000275 circle of willis Anatomy 0.000 description 4
- 238000013270 controlled release Methods 0.000 description 4
- 230000007812 deficiency Effects 0.000 description 4
- 230000010339 dilation Effects 0.000 description 4
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 4
- 239000002270 dispersing agent Substances 0.000 description 4
- 210000000609 ganglia Anatomy 0.000 description 4
- 150000004676 glycans Chemical class 0.000 description 4
- 229940088597 hormone Drugs 0.000 description 4
- 239000005556 hormone Substances 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 238000003780 insertion Methods 0.000 description 4
- 230000037431 insertion Effects 0.000 description 4
- 208000020658 intracerebral hemorrhage Diseases 0.000 description 4
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 4
- 238000009593 lumbar puncture Methods 0.000 description 4
- 230000014759 maintenance of location Effects 0.000 description 4
- 230000002175 menstrual effect Effects 0.000 description 4
- 210000004165 myocardium Anatomy 0.000 description 4
- 230000000926 neurological effect Effects 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 231100000252 nontoxic Toxicity 0.000 description 4
- 230000003000 nontoxic effect Effects 0.000 description 4
- 210000001636 ophthalmic artery Anatomy 0.000 description 4
- 230000001936 parietal effect Effects 0.000 description 4
- VGEREEWJJVICBM-UHFFFAOYSA-N phloretin Chemical compound C1=CC(O)=CC=C1CCC(=O)C1=C(O)C=C(O)C=C1O VGEREEWJJVICBM-UHFFFAOYSA-N 0.000 description 4
- 239000002953 phosphate buffered saline Substances 0.000 description 4
- 229920002643 polyglutamic acid Polymers 0.000 description 4
- 229920002959 polymer blend Polymers 0.000 description 4
- 229920001282 polysaccharide Polymers 0.000 description 4
- 239000005017 polysaccharide Substances 0.000 description 4
- 108020001213 potassium channel Proteins 0.000 description 4
- 239000003755 preservative agent Substances 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 230000005855 radiation Effects 0.000 description 4
- 230000002441 reversible effect Effects 0.000 description 4
- 230000028327 secretion Effects 0.000 description 4
- 230000001953 sensory effect Effects 0.000 description 4
- 238000000926 separation method Methods 0.000 description 4
- 210000002027 skeletal muscle Anatomy 0.000 description 4
- 210000003491 skin Anatomy 0.000 description 4
- NSUPRLHDCFNOKD-UHFFFAOYSA-N snx 482 Chemical compound N1C(=O)C(CCCNC(N)=N)NC(=O)C(NC(=O)CNC(=O)C(C)NC(=O)C(CCCCN)NC(=O)C(CC(O)=O)NC(=O)C(NC(=O)CN)C(C)C)CSSCC(C(N2CCCC2C(=O)NC(CCCNC(N)=N)C(=O)NC(CC(C)C)C(=O)NCC(=O)N2)=O)NC(=O)C(NC(=O)C(CC(O)=O)NC(=O)C(CC(O)=O)NC(=O)C(CC(N)=O)NC(=O)C(C(C)C)NC(=O)C(CO)NC3=O)CSSCC(C(=O)NC(C)C(=O)NC(CC=4C5=CC=CC=C5NC=4)C(=O)NC(CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(C(C)O)C(=O)NC(CC=4C=CC=CC=4)C(=O)NC(CO)C(=O)NC(CC(O)=O)C(O)=O)NC(=O)C(CC=4C=CC(O)=CC=4)NC(=O)C(CO)NC(=O)C(CC=4C=CC=CC=4)NC(=O)C(CC(C)C)NC(=O)C(CO)NC(=O)C(CC=4N=CNC=4)NC(=O)C2CSSCC3NC(=O)CNC(=O)CNC(=O)C(CC=2C=CC=CC=2)NC(=O)C(CCSC)NC(=O)C1CC1=CC=C(O)C=C1 NSUPRLHDCFNOKD-UHFFFAOYSA-N 0.000 description 4
- 210000004872 soft tissue Anatomy 0.000 description 4
- 230000008673 vomiting Effects 0.000 description 4
- 125000004172 4-methoxyphenyl group Chemical group [H]C1=C([H])C(OC([H])([H])[H])=C([H])C([H])=C1* 0.000 description 3
- 102100026024 Acyl-coenzyme A synthetase ACSM3, mitochondrial Human genes 0.000 description 3
- 206010002383 Angina Pectoris Diseases 0.000 description 3
- 201000001320 Atherosclerosis Diseases 0.000 description 3
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 3
- 206010053942 Cerebral haematoma Diseases 0.000 description 3
- 229920001661 Chitosan Polymers 0.000 description 3
- 241000272095 Dendroaspis angusticeps Species 0.000 description 3
- 206010013887 Dysarthria Diseases 0.000 description 3
- 102000010180 Endothelin receptor Human genes 0.000 description 3
- 108050001739 Endothelin receptor Proteins 0.000 description 3
- 229940118365 Endothelin receptor antagonist Drugs 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- 108010032354 Inositol 1,4,5-Trisphosphate Receptors Proteins 0.000 description 3
- 102000007640 Inositol 1,4,5-Trisphosphate Receptors Human genes 0.000 description 3
- 208000008574 Intracranial Hemorrhages Diseases 0.000 description 3
- 102000016349 Myosin Light Chains Human genes 0.000 description 3
- 108010067385 Myosin Light Chains Proteins 0.000 description 3
- 108700012358 P/Q-type calcium channel Proteins 0.000 description 3
- 102000050761 P/Q-type calcium channel Human genes 0.000 description 3
- 208000002193 Pain Diseases 0.000 description 3
- ZPHBZEQOLSRPAK-UHFFFAOYSA-N Phosphoramidon Natural products C=1NC2=CC=CC=C2C=1CC(C(O)=O)NC(=O)C(CC(C)C)NP(O)(=O)OC1OC(C)C(O)C(O)C1O ZPHBZEQOLSRPAK-UHFFFAOYSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- GTRPODKMSBFDOI-UHFFFAOYSA-N Protopine Natural products CN1Cc2c3OCOc3ccc2C4C1Cc5cc6OCOc6cc5C4=O GTRPODKMSBFDOI-UHFFFAOYSA-N 0.000 description 3
- ZAALQOFZFANFTF-UHFFFAOYSA-N Pseudoprotipine Natural products C1=C2C(=O)CC3=CC=4OCOC=4C=C3CN(C)CCC2=CC2=C1OCO2 ZAALQOFZFANFTF-UHFFFAOYSA-N 0.000 description 3
- 206010037423 Pulmonary oedema Diseases 0.000 description 3
- 108090000030 T-Type Calcium Channels Proteins 0.000 description 3
- 102000003691 T-Type Calcium Channels Human genes 0.000 description 3
- 108010067223 TRPC3 cation channel Proteins 0.000 description 3
- 239000012190 activator Substances 0.000 description 3
- 239000002671 adjuvant Substances 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- 238000002399 angioplasty Methods 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 230000001174 ascending effect Effects 0.000 description 3
- 230000002567 autonomic effect Effects 0.000 description 3
- 230000037424 autonomic function Effects 0.000 description 3
- 229910001424 calcium ion Inorganic materials 0.000 description 3
- 230000003185 calcium uptake Effects 0.000 description 3
- 150000001768 cations Chemical class 0.000 description 3
- 230000003788 cerebral perfusion Effects 0.000 description 3
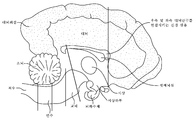
- 210000004289 cerebral ventricle Anatomy 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000013170 computed tomography imaging Methods 0.000 description 3
- 210000002808 connective tissue Anatomy 0.000 description 3
- 229920001577 copolymer Polymers 0.000 description 3
- 210000004351 coronary vessel Anatomy 0.000 description 3
- 230000001186 cumulative effect Effects 0.000 description 3
- 230000034994 death Effects 0.000 description 3
- 231100000517 death Toxicity 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 208000002173 dizziness Diseases 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 230000008030 elimination Effects 0.000 description 3
- 238000003379 elimination reaction Methods 0.000 description 3
- 239000003995 emulsifying agent Substances 0.000 description 3
- 210000003038 endothelium Anatomy 0.000 description 3
- 238000000605 extraction Methods 0.000 description 3
- 210000001508 eye Anatomy 0.000 description 3
- 230000004424 eye movement Effects 0.000 description 3
- 210000001105 femoral artery Anatomy 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 210000002149 gonad Anatomy 0.000 description 3
- 238000003384 imaging method Methods 0.000 description 3
- 230000001771 impaired effect Effects 0.000 description 3
- 230000002779 inactivation Effects 0.000 description 3
- 230000004941 influx Effects 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 238000007917 intracranial administration Methods 0.000 description 3
- 238000007918 intramuscular administration Methods 0.000 description 3
- 230000002427 irreversible effect Effects 0.000 description 3
- 230000000302 ischemic effect Effects 0.000 description 3
- 210000004731 jugular vein Anatomy 0.000 description 3
- 210000000867 larynx Anatomy 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 230000005499 meniscus Effects 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000036407 pain Effects 0.000 description 3
- 230000036961 partial effect Effects 0.000 description 3
- BWSDNRQVTFZQQD-AYVHNPTNSA-N phosphoramidon Chemical compound O([P@@](O)(=O)N[C@H](CC(C)C)C(=O)N[C@H](CC=1[C]2C=CC=CC2=NC=1)C(O)=O)[C@H]1O[C@@H](C)[C@H](O)[C@@H](O)[C@@H]1O BWSDNRQVTFZQQD-AYVHNPTNSA-N 0.000 description 3
- 108010072906 phosphoramidon Proteins 0.000 description 3
- 229910052698 phosphorus Inorganic materials 0.000 description 3
- 230000035790 physiological processes and functions Effects 0.000 description 3
- 229920000728 polyester Polymers 0.000 description 3
- 230000003389 potentiating effect Effects 0.000 description 3
- 208000005333 pulmonary edema Diseases 0.000 description 3
- 229940044551 receptor antagonist Drugs 0.000 description 3
- 239000002464 receptor antagonist Substances 0.000 description 3
- 239000011435 rock Substances 0.000 description 3
- 238000012732 spatial analysis Methods 0.000 description 3
- 210000000701 subdural space Anatomy 0.000 description 3
- 239000001797 sucrose acetate isobutyrate Substances 0.000 description 3
- 235000010983 sucrose acetate isobutyrate Nutrition 0.000 description 3
- UVGUPMLLGBCFEJ-SWTLDUCYSA-N sucrose acetate isobutyrate Chemical compound CC(C)C(=O)O[C@H]1[C@H](OC(=O)C(C)C)[C@@H](COC(=O)C(C)C)O[C@@]1(COC(C)=O)O[C@@H]1[C@H](OC(=O)C(C)C)[C@@H](OC(=O)C(C)C)[C@H](OC(=O)C(C)C)[C@@H](COC(C)=O)O1 UVGUPMLLGBCFEJ-SWTLDUCYSA-N 0.000 description 3
- 210000002820 sympathetic nervous system Anatomy 0.000 description 3
- 208000011580 syndromic disease Diseases 0.000 description 3
- 238000007910 systemic administration Methods 0.000 description 3
- 230000002123 temporal effect Effects 0.000 description 3
- 230000000699 topical effect Effects 0.000 description 3
- 239000003053 toxin Substances 0.000 description 3
- 231100000765 toxin Toxicity 0.000 description 3
- 108700012359 toxins Proteins 0.000 description 3
- 230000008733 trauma Effects 0.000 description 3
- 230000001960 triggered effect Effects 0.000 description 3
- 239000000080 wetting agent Substances 0.000 description 3
- 230000037303 wrinkles Effects 0.000 description 3
- WVTKBKWTSCPRNU-KYJUHHDHSA-N (+)-Tetrandrine Chemical compound C([C@H]1C=2C=C(C(=CC=2CCN1C)OC)O1)C(C=C2)=CC=C2OC(=C2)C(OC)=CC=C2C[C@@H]2N(C)CCC3=CC(OC)=C(OC)C1=C23 WVTKBKWTSCPRNU-KYJUHHDHSA-N 0.000 description 2
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- QHSRPPJQBFQWSC-OJDZSJEKSA-N (2r)-2-[[(2r)-2-[[(2s)-2-(azepane-1-carbonylamino)-4-methylpentanoyl]amino]-3-(1-formylindol-3-yl)propanoyl]amino]-3-(1h-indol-3-yl)propanoic acid Chemical compound N([C@@H](CC(C)C)C(=O)N[C@H](CC=1C2=CC=CC=C2N(C=O)C=1)C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)C(=O)N1CCCCCC1 QHSRPPJQBFQWSC-OJDZSJEKSA-N 0.000 description 2
- LIOKMIQQPDDTNO-UPRLRBBYSA-N (2r)-2-[[(2r)-2-[[(2s)-2-(azepane-1-carbonylamino)-4-methylpentanoyl]amino]-3-(1-methylindol-3-yl)propanoyl]amino]-3-pyridin-2-ylpropanoic acid Chemical compound N([C@@H](CC(C)C)C(=O)N[C@H](CC=1C2=CC=CC=C2N(C)C=1)C(=O)N[C@H](CC=1N=CC=CC=1)C(O)=O)C(=O)N1CCCCCC1 LIOKMIQQPDDTNO-UPRLRBBYSA-N 0.000 description 2
- JVWVTPZZEHLARL-OFEZKSIWSA-N (2r,3r,4s)-4-(1,3-benzodioxol-5-yl)-2-(3-fluoro-4-methoxyphenyl)-1-[2-[pentylsulfonyl(propyl)amino]ethyl]pyrrolidine-3-carboxylic acid Chemical compound C1([C@H]2[C@@H]([C@H](CN2CCN(CCC)S(=O)(=O)CCCCC)C=2C=C3OCOC3=CC=2)C(O)=O)=CC=C(OC)C(F)=C1 JVWVTPZZEHLARL-OFEZKSIWSA-N 0.000 description 2
- UZDORQWMYRRLQV-JHOUSYSJSA-N (2s)-2-[[(2r)-2-[(3,5-dimethylbenzoyl)-methylamino]-3-(4-phenylphenyl)propanoyl]amino]-3-(1h-indol-3-yl)propanoic acid Chemical compound C([C@@H](N(C)C(=O)C=1C=C(C)C=C(C)C=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)C(C=C1)=CC=C1C1=CC=CC=C1 UZDORQWMYRRLQV-JHOUSYSJSA-N 0.000 description 2
- OETKRDJJNWJFOP-FAYOUAAYSA-N (3S)-3-[[(2S)-2-[[(2S)-2-[[(4S,7S,10S,13S,16R)-16-amino-7-benzyl-10-[(4-hydroxyphenyl)methyl]-6,9,12,15-tetraoxo-13-propan-2-yl-1,2-dithia-5,8,11,14-tetrazacycloheptadecane-4-carbonyl]amino]-3-(1H-imidazol-5-yl)propanoyl]amino]-4-methylpentanoyl]amino]-4-[[(2S,3S)-1-[[(2S,3S)-1-[[(1S)-1-carboxy-2-(1H-indol-3-yl)ethyl]amino]-3-methyl-1-oxopentan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-4-oxobutanoic acid Chemical compound CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CSSC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O OETKRDJJNWJFOP-FAYOUAAYSA-N 0.000 description 2
- IUHMIOAKWHUFKU-YINIXLNUSA-N (5s,6r,7r)-5-(1,3-benzodioxol-5-yl)-2-butyl-7-[2-[(2s)-2-carboxypropyl]-4-methoxyphenyl]-6,7-dihydro-5h-cyclopenta[b]pyridine-6-carboxylic acid Chemical compound C1([C@H]2[C@@H]([C@H](C3=CC=C(N=C32)CCCC)C=2C=C3OCOC3=CC=2)C(O)=O)=CC=C(OC)C=C1C[C@H](C)C(O)=O IUHMIOAKWHUFKU-YINIXLNUSA-N 0.000 description 2
- FHGWEHGZBUBQKL-UHFFFAOYSA-N 1,2-benzothiazepine Chemical compound S1N=CC=CC2=CC=CC=C12 FHGWEHGZBUBQKL-UHFFFAOYSA-N 0.000 description 2
- QAMIWBVQNOICHO-XZOQPEGZSA-N 3-[[(2r)-2-[[(2s)-2-(azepane-1-carbonylamino)-4-methylpentanoyl]amino]-3-(1h-indol-3-yl)propanoyl]amino]propanoic acid Chemical compound N([C@@H](CC(C)C)C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCCC(O)=O)C(=O)N1CCCCCC1 QAMIWBVQNOICHO-XZOQPEGZSA-N 0.000 description 2
- 125000004203 4-hydroxyphenyl group Chemical group [H]OC1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- MJRGSRRZKSJHOE-UHFFFAOYSA-N 5-(dimethylamino)-N-(3,4-dimethyl-5-isoxazolyl)-1-naphthalenesulfonamide Chemical compound C1=CC=C2C(N(C)C)=CC=CC2=C1S(=O)(=O)NC=1ON=C(C)C=1C MJRGSRRZKSJHOE-UHFFFAOYSA-N 0.000 description 2
- PZNZOWSLQRURNA-HNRBIFIRSA-N 5-o-[(4s)-5-methoxycarbonyl-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carbonyl] 3-o-methyl (4r)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate Chemical compound C1([C@@H]2C(=C(C)NC(C)=C2C(=O)OC)C(=O)OC(=O)C=2[C@@H](C(=C(C)NC=2C)C(=O)OC)C=2C=C(C=CC=2)[N+]([O-])=O)=CC=CC([N+]([O-])=O)=C1 PZNZOWSLQRURNA-HNRBIFIRSA-N 0.000 description 2
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 2
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 2
- 102000007469 Actins Human genes 0.000 description 2
- 108010085238 Actins Proteins 0.000 description 2
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 2
- VYCMAAOURFJIHD-PJNXIOHISA-N BQ 123 Chemical compound N1C(=O)[C@H](CC(C)C)NC(=O)[C@@H](C(C)C)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](CC(O)=O)NC(=O)[C@H]1CC1=CNC2=CC=CC=C12 VYCMAAOURFJIHD-PJNXIOHISA-N 0.000 description 2
- 108010080719 BQ 485 Proteins 0.000 description 2
- ZBJNAHVLKNFOPP-OJDZSJEKSA-N BQ 485 Chemical compound N([C@@H](CC(C)C)C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)C(=O)N1CCCCCC1 ZBJNAHVLKNFOPP-OJDZSJEKSA-N 0.000 description 2
- KXUDUVUGZWJNHV-QEXRHJMMSA-N BQ 518 Chemical compound C1([C@@H]2C(=O)N[C@H](C(N[C@H](CC=3C4=CC=CC=C4NC=3)C(=O)N[C@H](CC(O)=O)C(=O)N3CCC[C@H]3C(=O)N2)=O)CC(C)C)=CC=CS1 KXUDUVUGZWJNHV-QEXRHJMMSA-N 0.000 description 2
- 108010073982 BQ 610 Proteins 0.000 description 2
- 108010030109 BQ 788 Proteins 0.000 description 2
- BPYKTIZUTYGOLE-IFADSCNNSA-N Bilirubin Chemical compound N1C(=O)C(C)=C(C=C)\C1=C\C1=C(C)C(CCC(O)=O)=C(CC2=C(C(C)=C(\C=C/3C(=C(C=C)C(=O)N\3)C)N2)CCC(O)=O)N1 BPYKTIZUTYGOLE-IFADSCNNSA-N 0.000 description 2
- 108010069674 CP 170687 Proteins 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 101710205660 Calcium-transporting ATPase Proteins 0.000 description 2
- 101710134161 Calcium-transporting ATPase sarcoplasmic/endoplasmic reticulum type Proteins 0.000 description 2
- 102000000584 Calmodulin Human genes 0.000 description 2
- 108010041952 Calmodulin Proteins 0.000 description 2
- 241000282472 Canis lupus familiaris Species 0.000 description 2
- 102000034573 Channels Human genes 0.000 description 2
- 108010034003 Cys(11)-Cys(15)-endothelin-1 (11-21) Proteins 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- 206010012239 Delusion Diseases 0.000 description 2
- 229920002307 Dextran Polymers 0.000 description 2
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 2
- 102000003965 Endothelin-2 Human genes 0.000 description 2
- 108090000387 Endothelin-2 Proteins 0.000 description 2
- 102000003688 G-Protein-Coupled Receptors Human genes 0.000 description 2
- 108090000045 G-Protein-Coupled Receptors Proteins 0.000 description 2
- UGJMXCAKCUNAIE-UHFFFAOYSA-N Gabapentin Chemical compound OC(=O)CC1(CN)CCCCC1 UGJMXCAKCUNAIE-UHFFFAOYSA-N 0.000 description 2
- 229910052688 Gadolinium Inorganic materials 0.000 description 2
- 206010018852 Haematoma Diseases 0.000 description 2
- 208000010496 Heart Arrest Diseases 0.000 description 2
- 206010019280 Heart failures Diseases 0.000 description 2
- 101000910745 Homo sapiens Voltage-dependent calcium channel gamma-3 subunit Proteins 0.000 description 2
- 101000740755 Homo sapiens Voltage-dependent calcium channel subunit alpha-2/delta-1 Proteins 0.000 description 2
- 206010062767 Hypophysitis Diseases 0.000 description 2
- 108010024468 IRL 2500 Proteins 0.000 description 2
- 206010022840 Intraventricular haemorrhage Diseases 0.000 description 2
- 208000032382 Ischaemic stroke Diseases 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- 201000009906 Meningitis Diseases 0.000 description 2
- 208000019695 Migraine disease Diseases 0.000 description 2
- 206010052904 Musculoskeletal stiffness Diseases 0.000 description 2
- 102000003505 Myosin Human genes 0.000 description 2
- 108060008487 Myosin Proteins 0.000 description 2
- 102100035044 Myosin light chain kinase, smooth muscle Human genes 0.000 description 2
- 108010074596 Myosin-Light-Chain Kinase Proteins 0.000 description 2
- HOKKHZGPKSLGJE-GSVOUGTGSA-N N-Methyl-D-aspartic acid Chemical compound CN[C@@H](C(O)=O)CC(O)=O HOKKHZGPKSLGJE-GSVOUGTGSA-N 0.000 description 2
- 206010028813 Nausea Diseases 0.000 description 2
- 208000012902 Nervous system disease Diseases 0.000 description 2
- 206010060860 Neurological symptom Diseases 0.000 description 2
- 101100426589 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) trp-3 gene Proteins 0.000 description 2
- 208000031481 Pathologic Constriction Diseases 0.000 description 2
- 208000006735 Periostitis Diseases 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 108010020346 Polyglutamic Acid Proteins 0.000 description 2
- WWEICYMHWQAHOS-UHFFFAOYSA-N Protopin Natural products CN1CCc2cc3OCOc3cc2C(=O)CCc4cc5OCOc5cc14 WWEICYMHWQAHOS-UHFFFAOYSA-N 0.000 description 2
- 108010053959 RES 701-1 Proteins 0.000 description 2
- FWLPKVQUECFKSW-UHFFFAOYSA-N SKF-96365 hydrochloride Chemical compound Cl.C1=CC(OC)=CC=C1CCCOC(C=1C=CC(OC)=CC=1)CN1C=NC=C1 FWLPKVQUECFKSW-UHFFFAOYSA-N 0.000 description 2
- 108010052164 Sodium Channels Proteins 0.000 description 2
- 102000018674 Sodium Channels Human genes 0.000 description 2
- 108010006431 Sodium-Potassium-Exchanging ATPase Proteins 0.000 description 2
- 208000005392 Spasm Diseases 0.000 description 2
- 108010047918 TAK 044 Proteins 0.000 description 2
- 102000003629 TRPC3 Human genes 0.000 description 2
- 102000003564 TRPC7 Human genes 0.000 description 2
- 101150095096 TRPM2 gene Proteins 0.000 description 2
- 101150037542 Trpc3 gene Proteins 0.000 description 2
- 101150033973 Trpc7 gene Proteins 0.000 description 2
- DRTQHJPVMGBUCF-XVFCMESISA-N Uridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-XVFCMESISA-N 0.000 description 2
- 206010047141 Vasodilatation Diseases 0.000 description 2
- GXBMIBRIOWHPDT-UHFFFAOYSA-N Vasopressin Natural products N1C(=O)C(CC=2C=C(O)C=CC=2)NC(=O)C(N)CSSCC(C(=O)N2C(CCC2)C(=O)NC(CCCN=C(N)N)C(=O)NCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(CCC(N)=O)NC(=O)C1CC1=CC=CC=C1 GXBMIBRIOWHPDT-UHFFFAOYSA-N 0.000 description 2
- 108010004977 Vasopressins Proteins 0.000 description 2
- 102000002852 Vasopressins Human genes 0.000 description 2
- 102100024138 Voltage-dependent calcium channel gamma-3 subunit Human genes 0.000 description 2
- 102100032335 Voltage-dependent calcium channel gamma-8 subunit Human genes 0.000 description 2
- 102100037059 Voltage-dependent calcium channel subunit alpha-2/delta-1 Human genes 0.000 description 2
- MMWCIQZXVOZEGG-HOZKJCLWSA-N [(1S,2R,3S,4S,5R,6S)-2,3,5-trihydroxy-4,6-diphosphonooxycyclohexyl] dihydrogen phosphate Chemical compound O[C@H]1[C@@H](O)[C@H](OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](O)[C@H]1OP(O)(O)=O MMWCIQZXVOZEGG-HOZKJCLWSA-N 0.000 description 2
- OHANKWLYFDFHOJ-RFTFGCRPSA-N [(1r,2r,7s,8ar)-1,8a-dimethyl-6-oxo-7-prop-1-en-2-yl-1,2,3,4,7,8-hexahydronaphthalen-2-yl] (z)-3-methylsulfanylprop-2-enoate Chemical compound O=C1[C@H](C(C)=C)C[C@]2(C)[C@@H](C)[C@H](OC(=O)\C=C/SC)CCC2=C1 OHANKWLYFDFHOJ-RFTFGCRPSA-N 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 125000003668 acetyloxy group Chemical group [H]C([H])([H])C(=O)O[*] 0.000 description 2
- 229960001138 acetylsalicylic acid Drugs 0.000 description 2
- 230000036982 action potential Effects 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 210000004100 adrenal gland Anatomy 0.000 description 2
- UCTWMZQNUQWSLP-UHFFFAOYSA-N adrenaline Chemical compound CNCC(O)C1=CC=C(O)C(O)=C1 UCTWMZQNUQWSLP-UHFFFAOYSA-N 0.000 description 2
- 239000012615 aggregate Substances 0.000 description 2
- 229940072056 alginate Drugs 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 210000003423 ankle Anatomy 0.000 description 2
- KBZOIRJILGZLEJ-LGYYRGKSSA-N argipressin Chemical compound C([C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CSSC[C@@H](C(N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N1)=O)N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(N)=O)C1=CC=CC=C1 KBZOIRJILGZLEJ-LGYYRGKSSA-N 0.000 description 2
- 210000002565 arteriole Anatomy 0.000 description 2
- 210000003403 autonomic nervous system Anatomy 0.000 description 2
- 210000002457 barrier cell Anatomy 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 150000007657 benzothiazepines Chemical class 0.000 description 2
- 239000002876 beta blocker Substances 0.000 description 2
- 229940097320 beta blocking agent Drugs 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 239000002981 blocking agent Substances 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 210000004958 brain cell Anatomy 0.000 description 2
- 210000005013 brain tissue Anatomy 0.000 description 2
- RYYVLZVUVIJVGH-UHFFFAOYSA-N caffeine Chemical compound CN1C(=O)N(C)C(=O)C2=C1N=CN2C RYYVLZVUVIJVGH-UHFFFAOYSA-N 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 210000000269 carotid artery external Anatomy 0.000 description 2
- 150000003943 catecholamines Chemical class 0.000 description 2
- 230000005779 cell damage Effects 0.000 description 2
- 208000037887 cell injury Diseases 0.000 description 2
- 210000004298 cerebral vein Anatomy 0.000 description 2
- 229960004926 chlorobutanol Drugs 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- OROGSEYTTFOCAN-DNJOTXNNSA-N codeine Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)=C[C@H](O)[C@@H]1OC1=C2C3=CC=C1OC OROGSEYTTFOCAN-DNJOTXNNSA-N 0.000 description 2
- 230000019771 cognition Effects 0.000 description 2
- 230000002301 combined effect Effects 0.000 description 2
- 238000007906 compression Methods 0.000 description 2
- 230000006835 compression Effects 0.000 description 2
- 230000021615 conjugation Effects 0.000 description 2
- 230000001595 contractor effect Effects 0.000 description 2
- CVSVTCORWBXHQV-UHFFFAOYSA-N creatine Chemical compound NC(=[NH2+])N(C)CC([O-])=O CVSVTCORWBXHQV-UHFFFAOYSA-N 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 108010031322 cyclo(Trp-Asp-Pro-Val-Leu) Proteins 0.000 description 2
- 108010035609 cyclo(tryptophyl-aspartyl-prolyl-thianylglycyl-leucyl) Proteins 0.000 description 2
- 230000001086 cytosolic effect Effects 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 231100000868 delusion Toxicity 0.000 description 2
- 210000001787 dendrite Anatomy 0.000 description 2
- 238000002059 diagnostic imaging Methods 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- HSUGRBWQSSZJOP-RTWAWAEBSA-N diltiazem Chemical compound C1=CC(OC)=CC=C1[C@H]1[C@@H](OC(C)=O)C(=O)N(CCN(C)C)C2=CC=CC=C2S1 HSUGRBWQSSZJOP-RTWAWAEBSA-N 0.000 description 2
- 229960004166 diltiazem Drugs 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- UWHBIISPHYTOGL-PFSAEEMXSA-L disodium;2-[(2r,5s,8s,11s,14s,17r)-8-(carboxylatomethyl)-17-(1h-indol-3-ylmethyl)-14-(2-methylpropyl)-3,6,9,12,15,18-hexaoxo-5-[2-oxo-2-(4-phenylpiperazin-1-yl)ethyl]-11-thiophen-2-yl-1,4,7,10,13,16-hexazacyclooctadec-2-yl]acetate Chemical compound [Na+].[Na+].C([C@H]1C(=O)N[C@@H](CC([O-])=O)C(=O)N[C@@H](C(=O)N[C@H](C(N[C@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@H](CC([O-])=O)C(=O)N1)=O)CC(C)C)C=1SC=CC=1)C(=O)N(CC1)CCN1C1=CC=CC=C1 UWHBIISPHYTOGL-PFSAEEMXSA-L 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- XIIPOLLCNQAOCP-XQTCEJMISA-N dnc012318 Chemical compound C([C@@H]1C(=O)NCC(=O)N[C@@H](C(N[C@@H](C)C(=O)N2CCC[C@@H]2C(=O)N[C@H](CC(=O)NCC(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CC=2C3=CC=CC=C3NC=2)C(=O)N1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)=O)[C@H](O)C)C1=CNC=N1 XIIPOLLCNQAOCP-XQTCEJMISA-N 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- MLFJHYIHIKEBTQ-IYRKOGFYSA-N endothelin 2 Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)NC(=O)[C@H]1NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC=2C=CC(O)=CC=2)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]2CSSC[C@@H](C(N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC=3C4=CC=CC=C4NC=3)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N2)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CSSC1)C1=CNC=N1 MLFJHYIHIKEBTQ-IYRKOGFYSA-N 0.000 description 2
- 210000002615 epidermis Anatomy 0.000 description 2
- 230000003628 erosive effect Effects 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 2
- 229940093471 ethyl oleate Drugs 0.000 description 2
- 238000013265 extended release Methods 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 210000002950 fibroblast Anatomy 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 210000001061 forehead Anatomy 0.000 description 2
- PGBHMTALBVVCIT-VCIWKGPPSA-N framycetin Chemical compound N[C@@H]1[C@@H](O)[C@H](O)[C@H](CN)O[C@@H]1O[C@H]1[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](N)C[C@@H](N)[C@@H]2O)O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CN)O2)N)O[C@@H]1CO PGBHMTALBVVCIT-VCIWKGPPSA-N 0.000 description 2
- UIWYJDYFSGRHKR-UHFFFAOYSA-N gadolinium atom Chemical compound [Gd] UIWYJDYFSGRHKR-UHFFFAOYSA-N 0.000 description 2
- MEANOSLIBWSCIT-UHFFFAOYSA-K gadolinium trichloride Chemical compound Cl[Gd](Cl)Cl MEANOSLIBWSCIT-UHFFFAOYSA-K 0.000 description 2
- 230000002496 gastric effect Effects 0.000 description 2
- 210000001035 gastrointestinal tract Anatomy 0.000 description 2
- 239000004220 glutamic acid Substances 0.000 description 2
- 235000011187 glycerol Nutrition 0.000 description 2
- LEQAOMBKQFMDFZ-UHFFFAOYSA-N glyoxal Chemical compound O=CC=O LEQAOMBKQFMDFZ-UHFFFAOYSA-N 0.000 description 2
- 238000005469 granulation Methods 0.000 description 2
- 230000003179 granulation Effects 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 208000019622 heart disease Diseases 0.000 description 2
- 230000002008 hemorrhagic effect Effects 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 2
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Substances C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 2
- 238000002513 implantation Methods 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 229940102223 injectable solution Drugs 0.000 description 2
- 210000004692 intercellular junction Anatomy 0.000 description 2
- 210000002425 internal capsule Anatomy 0.000 description 2
- 238000013152 interventional procedure Methods 0.000 description 2
- 201000009941 intracranial hypertension Diseases 0.000 description 2
- 238000007913 intrathecal administration Methods 0.000 description 2
- 230000007794 irritation Effects 0.000 description 2
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 description 2
- 239000004310 lactic acid Substances 0.000 description 2
- 235000014655 lactic acid Nutrition 0.000 description 2
- ICAKDTKJOYSXGC-UHFFFAOYSA-K lanthanum(iii) chloride Chemical compound Cl[La](Cl)Cl ICAKDTKJOYSXGC-UHFFFAOYSA-K 0.000 description 2
- 210000005240 left ventricle Anatomy 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 210000003141 lower extremity Anatomy 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 230000036244 malformation Effects 0.000 description 2
- 230000037323 metabolic rate Effects 0.000 description 2
- VKQFCGNPDRICFG-UHFFFAOYSA-N methyl 2-methylpropyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OCC(C)C)C1C1=CC=CC=C1[N+]([O-])=O VKQFCGNPDRICFG-UHFFFAOYSA-N 0.000 description 2
- ZMJUWFXCWLDAKQ-WZJLIZBTSA-N methyl 4-[[(3r,4s,5s,6r)-4,5-dihydroxy-7-[[4-(hydroxymethyl)phenyl]methyl]-1,1-dioxo-3,6-bis(phenoxymethyl)-1,2,7-thiadiazepan-2-yl]methyl]benzoate Chemical compound C1=CC(C(=O)OC)=CC=C1CN1S(=O)(=O)N(CC=2C=CC(CO)=CC=2)[C@H](COC=2C=CC=CC=2)[C@H](O)[C@@H](O)[C@H]1COC1=CC=CC=C1 ZMJUWFXCWLDAKQ-WZJLIZBTSA-N 0.000 description 2
- 125000000250 methylamino group Chemical group [H]N(*)C([H])([H])[H] 0.000 description 2
- 238000001471 micro-filtration Methods 0.000 description 2
- 239000003094 microcapsule Substances 0.000 description 2
- 206010027599 migraine Diseases 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 210000000663 muscle cell Anatomy 0.000 description 2
- 230000004118 muscle contraction Effects 0.000 description 2
- 230000002107 myocardial effect Effects 0.000 description 2
- 239000002105 nanoparticle Substances 0.000 description 2
- 230000008693 nausea Effects 0.000 description 2
- 229940053050 neomycin sulfate Drugs 0.000 description 2
- 230000007971 neurological deficit Effects 0.000 description 2
- 239000002858 neurotransmitter agent Substances 0.000 description 2
- 210000000440 neutrophil Anatomy 0.000 description 2
- AIKVCUNQWYTVTO-UHFFFAOYSA-N nicardipine hydrochloride Chemical compound Cl.COC(=O)C1=C(C)NC(C)=C(C(=O)OCCN(C)CC=2C=CC=CC=2)C1C1=CC=CC([N+]([O-])=O)=C1 AIKVCUNQWYTVTO-UHFFFAOYSA-N 0.000 description 2
- 229960000227 nisoldipine Drugs 0.000 description 2
- 239000000346 nonvolatile oil Substances 0.000 description 2
- 210000000103 occipital bone Anatomy 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 239000012053 oil suspension Substances 0.000 description 2
- 235000019198 oils Nutrition 0.000 description 2
- 235000008390 olive oil Nutrition 0.000 description 2
- 239000004006 olive oil Substances 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 230000036284 oxygen consumption Effects 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 230000000149 penetrating effect Effects 0.000 description 2
- 210000003460 periosteum Anatomy 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 210000003635 pituitary gland Anatomy 0.000 description 2
- 230000036470 plasma concentration Effects 0.000 description 2
- 229920000515 polycarbonate Polymers 0.000 description 2
- 239000004417 polycarbonate Substances 0.000 description 2
- 229920001184 polypeptide Polymers 0.000 description 2
- 210000000063 presynaptic terminal Anatomy 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 125000002572 propoxy group Chemical group [*]OC([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 2
- 210000001747 pupil Anatomy 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 230000000306 recurrent effect Effects 0.000 description 2
- 230000004648 relaxation of smooth muscle Effects 0.000 description 2
- 210000005241 right ventricle Anatomy 0.000 description 2
- CXMXRPHRNRROMY-UHFFFAOYSA-N sebacic acid Chemical compound OC(=O)CCCCCCCCC(O)=O CXMXRPHRNRROMY-UHFFFAOYSA-N 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 208000018316 severe headache Diseases 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- RSGMPVJSBAIOBP-IJVOEZGUSA-M sodium;(z)-2-(2,1,3-benzothiadiazol-5-yl)-4-(3-fluoro-4-methoxyphenyl)-4-oxo-3-[(3,4,5-trimethoxyphenyl)methyl]but-2-enoate Chemical compound [Na+].C1=C(F)C(OC)=CC=C1C(=O)C(\CC=1C=C(OC)C(OC)=C(OC)C=1)=C(/C([O-])=O)C1=CC2=NSN=C2C=C1 RSGMPVJSBAIOBP-IJVOEZGUSA-M 0.000 description 2
- 238000000638 solvent extraction Methods 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 230000036262 stenosis Effects 0.000 description 2
- 208000037804 stenosis Diseases 0.000 description 2
- 230000002739 subcortical effect Effects 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical compound OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 230000008961 swelling Effects 0.000 description 2
- 238000012385 systemic delivery Methods 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 210000003582 temporal bone Anatomy 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 230000000542 thalamic effect Effects 0.000 description 2
- 230000000451 tissue damage Effects 0.000 description 2
- 231100000827 tissue damage Toxicity 0.000 description 2
- 238000003325 tomography Methods 0.000 description 2
- 238000012549 training Methods 0.000 description 2
- 238000002054 transplantation Methods 0.000 description 2
- 230000032258 transport Effects 0.000 description 2
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 2
- 238000002604 ultrasonography Methods 0.000 description 2
- 208000019553 vascular disease Diseases 0.000 description 2
- 210000003556 vascular endothelial cell Anatomy 0.000 description 2
- 230000000304 vasodilatating effect Effects 0.000 description 2
- 229960003726 vasopressin Drugs 0.000 description 2
- 210000004885 white matter Anatomy 0.000 description 2
- SFLSHLFXELFNJZ-QMMMGPOBSA-N (-)-norepinephrine Chemical compound NC[C@H](O)C1=CC=C(O)C(O)=C1 SFLSHLFXELFNJZ-QMMMGPOBSA-N 0.000 description 1
- AHIFMZUYRNPRJK-MHZLTWQESA-N (1s)-n-(diphenoxyphosphorylmethyl)-2-(4-phenylphenyl)-1-(2h-tetrazol-5-yl)ethanamine Chemical compound C([C@H](NCP(=O)(OC=1C=CC=CC=1)OC=1C=CC=CC=1)C1=NNN=N1)C(C=C1)=CC=C1C1=CC=CC=C1 AHIFMZUYRNPRJK-MHZLTWQESA-N 0.000 description 1
- GTSCNQPMGNAJSH-YHFCJVPQSA-N (2S)-2-[[(2S)-2-[[(4R,7S,10S,16S,19R)-19-[[(3S,6S,9S,12S,15S,21R,26R,29S,32S,35S,38S,41S)-21-[[2-[[(1R,2aR,4S,7S,9aS,10S,12aS,13S,15aS,16S,18aS,19S,22S,25S,28S,31S,34S,37S,40S,43S,46S,52R,57R,60S,63S,66S,69S,72S,75S,81S,84S,87S,90S,93S,96S,99S)-7,63,90-tris(4-aminobutyl)-2a-[[(2S,3S)-2-[[(2S)-2-amino-5-carbamimidamidopentanoyl]amino]-3-methylpentanoyl]amino]-12a-(2-amino-2-oxoethyl)-25-(3-amino-3-oxopropyl)-13-benzyl-16,37,96-tris[(2S)-butan-2-yl]-19,28,46,72-tetrakis(3-carbamimidamidopropyl)-15a,31,43-tris(2-carboxyethyl)-9a,22,60,66-tetrakis[(1R)-1-hydroxyethyl]-40,84-bis(hydroxymethyl)-4,34,99-tris[(4-hydroxyphenyl)methyl]-93-(1H-imidazol-5-ylmethyl)-69,87-dimethyl-81-(2-methylpropyl)-10-(2-methylsulfanylethyl)-1a,2,5,8,8a,11,11a,14,14a,17,17a,20,20a,23,26,29,32,35,38,41,44,47,50,59,62,65,68,71,74,80,83,86,89,92,95,98-hexatriacontaoxo-18a-propan-2-yl-4a,5a,54,55-tetrathia-a,3,6,7a,9,10a,12,13a,15,16a,18,19a,21,24,27,30,33,36,39,42,45,48,51,58,61,64,67,70,73,79,82,85,88,91,94,97-hexatriacontazatricyclo[55.49.14.075,79]icosahectane-52-carbonyl]amino]acetyl]amino]-35-(3-amino-3-oxopropyl)-29-(2-carboxyethyl)-12,32-bis[(1R)-1-hydroxyethyl]-38-[(4-hydroxyphenyl)methyl]-3-(1H-indol-3-ylmethyl)-9-methyl-6-(2-methylsulfanylethyl)-2,5,8,11,14,20,28,31,34,37,40-undecaoxo-23,24-dithia-1,4,7,10,13,19,27,30,33,36,39-undecazatricyclo[39.3.0.015,19]tetratetracontane-26-carbonyl]amino]-16-(4-aminobutyl)-7-(3-carbamimidamidopropyl)-10-(carboxymethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]amino]-4-amino-4-oxobutanoyl]amino]-6-aminohexanoic acid Chemical compound CC[C@H](C)[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@@H]2NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)[C@@H](C)CC)[C@@H](C)O)[C@@H](C)CC)C(=O)NCC(=O)N[C@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@@H]3CCCN3C(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCSC)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H]3CCCN3C2=O)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@H]2CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CCCCN)NC2=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)[C@@H](C)CC)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O GTSCNQPMGNAJSH-YHFCJVPQSA-N 0.000 description 1
- QVTBFVFPWXEIOX-VYAQOJDXSA-N (2r)-2-[[(2s)-2-[3-[[(2r)-2-[[(2r)-2-[[(2s)-2-(azepane-1-carbonylamino)-4-methylpentanoyl]amino]-3-(1h-indol-3-yl)propanoyl]amino]propanoyl]amino]propanoylamino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-phenylpropanoic acid Chemical compound N([C@@H](CC(C)C)C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@H](C)C(=O)NCCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@H](CC=1C=CC=CC=1)C(O)=O)C(=O)N1CCCCCC1 QVTBFVFPWXEIOX-VYAQOJDXSA-N 0.000 description 1
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical class OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 1
- HYNSSBPBTFFZKW-OUPFVLIJSA-N (2s)-2-amino-3-[(2s)-2-[[(2r)-1-[[(2s)-1-[[(2r)-1-(1h-indol-3-yl)-3-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]carbamoyl]-2,5-dihydropyrrol-1-yl]-3-oxopropane-1-sulfonic acid Chemical compound N([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C=O)C(C)C)C(=O)[C@@H]1C=CCN1C(=O)[C@H](N)CS(O)(=O)=O HYNSSBPBTFFZKW-OUPFVLIJSA-N 0.000 description 1
- CRUVAUSVWLATAE-ZDUSSCGKSA-N (2s)-3-dibenzofuran-3-yl-2-(phosphonomethylamino)propanoic acid Chemical compound C1=CC=C2C3=CC=C(C[C@@H](C(=O)O)NCP(O)(O)=O)C=C3OC2=C1 CRUVAUSVWLATAE-ZDUSSCGKSA-N 0.000 description 1
- ZFLWDHHVRRZMEI-CYBMUJFWSA-N (4R)-2,6-dimethyl-5-nitro-4-[2-(trifluoromethyl)phenyl]-1,4-dihydropyridine-3-carboxylic acid methyl ester Chemical compound COC(=O)C1=C(C)NC(C)=C([N+]([O-])=O)[C@@H]1C1=CC=CC=C1C(F)(F)F ZFLWDHHVRRZMEI-CYBMUJFWSA-N 0.000 description 1
- MBHURWYWZFYDQD-HDUXTRFBSA-N (4r)-4-[[(2r)-2-[[(2s)-2-[[(2r,3s)-2-[[(2s)-2-aminopropanoyl]amino]-3-methylpentanoyl]amino]-4-methylpent-4-enoyl]amino]-3-(1h-indol-3-yl)propanoyl]amino]-5-oxopentanoic acid Chemical compound C1=CC=C2C(C[C@@H](NC(=O)[C@H](CC(C)=C)NC(=O)[C@H](NC(=O)[C@H](C)N)[C@@H](C)CC)C(=O)N[C@H](CCC(O)=O)C=O)=CNC2=C1 MBHURWYWZFYDQD-HDUXTRFBSA-N 0.000 description 1
- QGTFISXASKMOQM-MPPIKMIVSA-N (4r)-4-amino-5-[(2s)-2-[[(2r)-1-[[(2s)-1-[[(2r)-1-(1h-indol-3-yl)-3-oxopropan-2-yl]amino]-4-methyl-1-oxopent-4-en-2-yl]amino]-3-methyl-1-oxobutan-2-yl]carbamoyl]pyrrolidin-1-yl]-5-oxopentanoic acid Chemical compound N([C@H](C(C)C)C(=O)N[C@@H](CC(C)=C)C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C=O)C(=O)[C@@H]1CCCN1C(=O)[C@H](N)CCC(O)=O QGTFISXASKMOQM-MPPIKMIVSA-N 0.000 description 1
- ZWTDXYUDJYDHJR-UHFFFAOYSA-N (E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl)-2-propen-1-one Natural products OC1=CC(O)=CC=C1C=CC(=O)C1=CC=C(O)C=C1O ZWTDXYUDJYDHJR-UHFFFAOYSA-N 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- UCTWMZQNUQWSLP-VIFPVBQESA-N (R)-adrenaline Chemical compound CNC[C@H](O)C1=CC=C(O)C(O)=C1 UCTWMZQNUQWSLP-VIFPVBQESA-N 0.000 description 1
- 229930182837 (R)-adrenaline Natural products 0.000 description 1
- PVHUJELLJLJGLN-INIZCTEOSA-N (S)-nitrendipine Chemical compound CCOC(=O)C1=C(C)NC(C)=C(C(=O)OC)[C@@H]1C1=CC=CC([N+]([O-])=O)=C1 PVHUJELLJLJGLN-INIZCTEOSA-N 0.000 description 1
- MGRVRXRGTBOSHW-UHFFFAOYSA-N (aminomethyl)phosphonic acid Chemical compound NCP(O)(O)=O MGRVRXRGTBOSHW-UHFFFAOYSA-N 0.000 description 1
- PSYYHKIRDZMRAC-UHFFFAOYSA-N 1$l^{4},2-benzothiazepine 1-oxide Chemical compound O=S1N=CC=CC2=CC=CC=C12 PSYYHKIRDZMRAC-UHFFFAOYSA-N 0.000 description 1
- ZAIDIVBQUMFXEC-UHFFFAOYSA-N 1,1-dichloroprop-1-ene Chemical compound CC=C(Cl)Cl ZAIDIVBQUMFXEC-UHFFFAOYSA-N 0.000 description 1
- NCUVPPXIIPVXMO-UHFFFAOYSA-N 1,2-benzothiazepine;calcium Chemical compound [Ca].S1N=CC=CC2=CC=CC=C12 NCUVPPXIIPVXMO-UHFFFAOYSA-N 0.000 description 1
- NRZGDXCEAUBUED-UHFFFAOYSA-N 1-(2-methoxyethyl)-2,6-dimethyl-4-(3-nitrophenyl)-3-propan-2-yl-4h-pyridine Chemical compound CC(C)C1=C(C)N(CCOC)C(C)=CC1C1=CC=CC([N+]([O-])=O)=C1 NRZGDXCEAUBUED-UHFFFAOYSA-N 0.000 description 1
- KBPZBOSJIZEELA-UHFFFAOYSA-N 1-[2-[4-methoxy-3-[3-(4-methoxyphenyl)propoxy]phenyl]ethyl]imidazole;hydrochloride Chemical compound Cl.C1=CC(OC)=CC=C1CCCOC1=CC(CCN2C=NC=C2)=CC=C1OC KBPZBOSJIZEELA-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- SGTNSNPWRIOYBX-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-{[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino}-2-(propan-2-yl)pentanenitrile Chemical compound C1=C(OC)C(OC)=CC=C1CCN(C)CCCC(C#N)(C(C)C)C1=CC=C(OC)C(OC)=C1 SGTNSNPWRIOYBX-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 125000003006 2-dimethylaminoethyl group Chemical group [H]C([H])([H])N(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- UCBSDZVRTRBILM-UHFFFAOYSA-N 2-ethoxycarbonylpyridine-3-carboxylic acid hydrochloride Chemical compound CCOC(=O)C1=C(C=CC=N1)C(=O)O.Cl UCBSDZVRTRBILM-UHFFFAOYSA-N 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- XTFPDGZNWTZCMF-DHZHZOJOSA-N 3-o-methyl 5-o-[(e)-3-phenylprop-2-enyl] 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OC\C=C\C=2C=CC=CC=2)C1C1=CC=CC([N+]([O-])=O)=C1 XTFPDGZNWTZCMF-DHZHZOJOSA-N 0.000 description 1
- 125000001255 4-fluorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C([H])=C1F 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 241000238899 Agelenopsis Species 0.000 description 1
- 241000238898 Agelenopsis aperta Species 0.000 description 1
- 101000830639 Agelenopsis aperta Omega-agatoxin-Aa3a Proteins 0.000 description 1
- 208000000044 Amnesia Diseases 0.000 description 1
- 208000031091 Amnestic disease Diseases 0.000 description 1
- 102400000345 Angiotensin-2 Human genes 0.000 description 1
- 101800000733 Angiotensin-2 Proteins 0.000 description 1
- 206010002660 Anoxia Diseases 0.000 description 1
- 241000976983 Anoxia Species 0.000 description 1
- 108090000084 Antiporters Proteins 0.000 description 1
- 102000003669 Antiporters Human genes 0.000 description 1
- 208000019901 Anxiety disease Diseases 0.000 description 1
- 206010003162 Arterial injury Diseases 0.000 description 1
- 206010003225 Arteriospasm coronary Diseases 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- 206010003591 Ataxia Diseases 0.000 description 1
- 206010003658 Atrial Fibrillation Diseases 0.000 description 1
- 208000008035 Back Pain Diseases 0.000 description 1
- 208000019838 Blood disease Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 101800004538 Bradykinin Proteins 0.000 description 1
- 102400000967 Bradykinin Human genes 0.000 description 1
- 206010048962 Brain oedema Diseases 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- GIGDXJSUXRHGNA-UHFFFAOYSA-N CC1C(=C(NC(=C1C(=O)OC(C)C)C)C)C(=O)OC Chemical compound CC1C(=C(NC(=C1C(=O)OC(C)C)C)C)C(=O)OC GIGDXJSUXRHGNA-UHFFFAOYSA-N 0.000 description 1
- KJNNBRVUQZDOET-AREMUKBSSA-N CCCC1=NC(C)=CC=C1O[C@H](C=1C=C2OCOC2=CC=1)C(=O)NS(=O)(=O)C1=CC=C(C(C)C)C=C1 Chemical compound CCCC1=NC(C)=CC=C1O[C@H](C=1C=C2OCOC2=CC=1)C(=O)NS(=O)(=O)C1=CC=C(C(C)C)C=C1 KJNNBRVUQZDOET-AREMUKBSSA-N 0.000 description 1
- 206010007558 Cardiac failure chronic Diseases 0.000 description 1
- 206010007559 Cardiac failure congestive Diseases 0.000 description 1
- 208000031229 Cardiomyopathies Diseases 0.000 description 1
- 208000021479 Cardiovascular injury Diseases 0.000 description 1
- 108010076119 Caseins Proteins 0.000 description 1
- 101001026137 Cavia porcellus Glutathione S-transferase A Proteins 0.000 description 1
- 206010008138 Cerebral venous thrombosis Diseases 0.000 description 1
- 206010008190 Cerebrovascular accident Diseases 0.000 description 1
- KJEBULYHNRNJTE-DHZHZOJOSA-N Cinalong Chemical compound COCCOC(=O)C1=C(C)NC(C)=C(C(=O)OC\C=C\C=2C=CC=CC=2)C1C1=CC=CC([N+]([O-])=O)=C1 KJEBULYHNRNJTE-DHZHZOJOSA-N 0.000 description 1
- 208000028698 Cognitive impairment Diseases 0.000 description 1
- 206010010356 Congenital anomaly Diseases 0.000 description 1
- 208000003890 Coronary Vasospasm Diseases 0.000 description 1
- 206010011224 Cough Diseases 0.000 description 1
- 206010061908 Cranial nerve paralysis Diseases 0.000 description 1
- 102000004420 Creatine Kinase Human genes 0.000 description 1
- 108010042126 Creatine kinase Proteins 0.000 description 1
- 241000238424 Crustacea Species 0.000 description 1
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 1
- 229930105110 Cyclosporin A Natural products 0.000 description 1
- 108010036949 Cyclosporine Proteins 0.000 description 1
- 206010067276 Cytotoxic oedema Diseases 0.000 description 1
- CKLJMWTZIZZHCS-UWTATZPHSA-N D-aspartic acid Chemical compound OC(=O)[C@H](N)CC(O)=O CKLJMWTZIZZHCS-UWTATZPHSA-N 0.000 description 1
- 241000238557 Decapoda Species 0.000 description 1
- 206010051055 Deep vein thrombosis Diseases 0.000 description 1
- 241000272093 Dendroaspis Species 0.000 description 1
- 241000272023 Dendroaspis polylepis Species 0.000 description 1
- 241000272019 Dendroaspis polylepis polylepis Species 0.000 description 1
- 101000723297 Dendroaspis polylepis polylepis Calciseptin Proteins 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- 208000005171 Dysmenorrhea Diseases 0.000 description 1
- 102000016942 Elastin Human genes 0.000 description 1
- 108010014258 Elastin Proteins 0.000 description 1
- 206010014418 Electrolyte imbalance Diseases 0.000 description 1
- 102100029112 Endothelin-converting enzyme 1 Human genes 0.000 description 1
- 238000012276 Endovascular treatment Methods 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 108010022355 Fibroins Proteins 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 108091006027 G proteins Proteins 0.000 description 1
- 102000030782 GTP binding Human genes 0.000 description 1
- 108091000058 GTP-Binding Proteins 0.000 description 1
- 101001026109 Gallus gallus Glutathione S-transferase Proteins 0.000 description 1
- 208000003098 Ganglion Cysts Diseases 0.000 description 1
- 241000206672 Gelidium Species 0.000 description 1
- 208000010412 Glaucoma Diseases 0.000 description 1
- 102000018899 Glutamate Receptors Human genes 0.000 description 1
- 108010027915 Glutamate Receptors Proteins 0.000 description 1
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 1
- 108010068370 Glutens Proteins 0.000 description 1
- 206010018691 Granuloma Diseases 0.000 description 1
- 108010051696 Growth Hormone Proteins 0.000 description 1
- QXZGBUJJYSLZLT-UHFFFAOYSA-N H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH Natural products NC(N)=NCCCC(N)C(=O)N1CCCC1C(=O)N1C(C(=O)NCC(=O)NC(CC=2C=CC=CC=2)C(=O)NC(CO)C(=O)N2C(CCC2)C(=O)NC(CC=2C=CC=CC=2)C(=O)NC(CCCN=C(N)N)C(O)=O)CCC1 QXZGBUJJYSLZLT-UHFFFAOYSA-N 0.000 description 1
- 206010019468 Hemiplegia Diseases 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- 208000016988 Hemorrhagic Stroke Diseases 0.000 description 1
- 206010019909 Hernia Diseases 0.000 description 1
- 101000932850 Homo sapiens Voltage-dependent L-type calcium channel subunit beta-1 Proteins 0.000 description 1
- 101000983956 Homo sapiens Voltage-dependent L-type calcium channel subunit beta-2 Proteins 0.000 description 1
- 101000983936 Homo sapiens Voltage-dependent L-type calcium channel subunit beta-3 Proteins 0.000 description 1
- 101000983947 Homo sapiens Voltage-dependent L-type calcium channel subunit beta-4 Proteins 0.000 description 1
- 101000910759 Homo sapiens Voltage-dependent calcium channel gamma-1 subunit Proteins 0.000 description 1
- 101000910758 Homo sapiens Voltage-dependent calcium channel gamma-2 subunit Proteins 0.000 description 1
- 101000910748 Homo sapiens Voltage-dependent calcium channel gamma-4 subunit Proteins 0.000 description 1
- 101000868383 Homo sapiens Voltage-dependent calcium channel gamma-5 subunit Proteins 0.000 description 1
- 101000868391 Homo sapiens Voltage-dependent calcium channel gamma-6 subunit Proteins 0.000 description 1
- 101000868398 Homo sapiens Voltage-dependent calcium channel gamma-7 subunit Proteins 0.000 description 1
- 101000868545 Homo sapiens Voltage-dependent calcium channel gamma-8 subunit Proteins 0.000 description 1
- WOBHKFSMXKNTIM-UHFFFAOYSA-N Hydroxyethyl methacrylate Chemical compound CC(=C)C(=O)OCCO WOBHKFSMXKNTIM-UHFFFAOYSA-N 0.000 description 1
- 206010020880 Hypertrophy Diseases 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- 241000245954 Hysterocrates gigas Species 0.000 description 1
- CZGUSIXMZVURDU-JZXHSEFVSA-N Ile(5)-angiotensin II Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC=CC=1)C([O-])=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@@H]([NH3+])CC([O-])=O)C(C)C)C1=CC=C(O)C=C1 CZGUSIXMZVURDU-JZXHSEFVSA-N 0.000 description 1
- 108090000723 Insulin-Like Growth Factor I Proteins 0.000 description 1
- 102000004218 Insulin-Like Growth Factor I Human genes 0.000 description 1
- LPHGQDQBBGAPDZ-UHFFFAOYSA-N Isocaffeine Natural products CN1C(=O)N(C)C(=O)C2=C1N(C)C=N2 LPHGQDQBBGAPDZ-UHFFFAOYSA-N 0.000 description 1
- 108010044467 Isoenzymes Proteins 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- 241001484259 Lacuna Species 0.000 description 1
- 208000004852 Lung Injury Diseases 0.000 description 1
- 108090000362 Lymphotoxin-beta Proteins 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 208000010495 Meningocele Diseases 0.000 description 1
- 101710170181 Metalloproteinase inhibitor Proteins 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 1
- DUGOZIWVEXMGBE-UHFFFAOYSA-N Methylphenidate Chemical compound C=1C=CC=CC=1C(C(=O)OC)C1CCCCN1 DUGOZIWVEXMGBE-UHFFFAOYSA-N 0.000 description 1
- HBNPJJILLOYFJU-VMPREFPWSA-N Mibefradil Chemical compound C1CC2=CC(F)=CC=C2[C@H](C(C)C)[C@@]1(OC(=O)COC)CCN(C)CCCC1=NC2=CC=CC=C2N1 HBNPJJILLOYFJU-VMPREFPWSA-N 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 229920001410 Microfiber Polymers 0.000 description 1
- 208000019022 Mood disease Diseases 0.000 description 1
- 102000011131 Myosin-Light-Chain Phosphatase Human genes 0.000 description 1
- 108010037801 Myosin-Light-Chain Phosphatase Proteins 0.000 description 1
- OVRNDRQMDRJTHS-FMDGEEDCSA-N N-acetyl-beta-D-glucosamine Chemical group CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-FMDGEEDCSA-N 0.000 description 1
- YQHMWTPYORBCMF-UHFFFAOYSA-N Naringenin chalcone Natural products C1=CC(O)=CC=C1C=CC(=O)C1=C(O)C=C(O)C=C1O YQHMWTPYORBCMF-UHFFFAOYSA-N 0.000 description 1
- 206010028851 Necrosis Diseases 0.000 description 1
- 208000025966 Neurological disease Diseases 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 102400000050 Oxytocin Human genes 0.000 description 1
- 101800000989 Oxytocin Proteins 0.000 description 1
- XNOPRXBHLZRZKH-UHFFFAOYSA-N Oxytocin Natural products N1C(=O)C(N)CSSCC(C(=O)N2C(CCC2)C(=O)NC(CC(C)C)C(=O)NCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(CCC(N)=O)NC(=O)C(C(C)CC)NC(=O)C1CC1=CC=C(O)C=C1 XNOPRXBHLZRZKH-UHFFFAOYSA-N 0.000 description 1
- 108010075750 P-Type Calcium Channels Proteins 0.000 description 1
- 108010009124 PD 142893 Proteins 0.000 description 1
- 108010007647 PD 145065 Proteins 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 208000037273 Pathologic Processes Diseases 0.000 description 1
- 206010035664 Pneumonia Diseases 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 229920001305 Poly(isodecyl(meth)acrylate) Polymers 0.000 description 1
- 229920002319 Poly(methyl acrylate) Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 208000018238 Primary Headache disease Diseases 0.000 description 1
- 102100024819 Prolactin Human genes 0.000 description 1
- 108010057464 Prolactin Proteins 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 102000002298 Purinergic P2Y Receptors Human genes 0.000 description 1
- 108010000818 Purinergic P2Y Receptors Proteins 0.000 description 1
- 102000001424 Ryanodine receptors Human genes 0.000 description 1
- VWBBKDOYFZXORP-UHFFFAOYSA-N S-Petasin Natural products CC1C(CCC2=CC(=O)C(CC12C)C(=C)C)OC(=O)C=C/CS VWBBKDOYFZXORP-UHFFFAOYSA-N 0.000 description 1
- 102000000395 SH3 domains Human genes 0.000 description 1
- 108050008861 SH3 domains Proteins 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 206010070834 Sensitisation Diseases 0.000 description 1
- 208000032140 Sleepiness Diseases 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 102100038803 Somatotropin Human genes 0.000 description 1
- 206010041349 Somnolence Diseases 0.000 description 1
- 208000007718 Stable Angina Diseases 0.000 description 1
- 229920002472 Starch Chemical class 0.000 description 1
- 208000002847 Surgical Wound Diseases 0.000 description 1
- 102000003673 Symporters Human genes 0.000 description 1
- 108090000088 Symporters Proteins 0.000 description 1
- 208000005400 Synovial Cyst Diseases 0.000 description 1
- 208000008253 Systolic Heart Failure Diseases 0.000 description 1
- 206010042957 Systolic hypertension Diseases 0.000 description 1
- IBUAONLWVYWMIS-UHFFFAOYSA-N TMC-66 Natural products O=C1C2=C(O)C=CC=C2C(=O)C2=C1C=C1CCC(C=C3CC4(N(C(=O)C3=C3O)C(CO4)C(O)=O)C)=C3C1=C2O IBUAONLWVYWMIS-UHFFFAOYSA-N 0.000 description 1
- 102000011040 TRPV Cation Channels Human genes 0.000 description 1
- 108010062740 TRPV Cation Channels Proteins 0.000 description 1
- 108010078577 TTA 386 Proteins 0.000 description 1
- 208000001871 Tachycardia Diseases 0.000 description 1
- 241000239292 Theraphosidae Species 0.000 description 1
- 102000011923 Thyrotropin Human genes 0.000 description 1
- 108010061174 Thyrotropin Proteins 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 108010037150 Transient Receptor Potential Channels Proteins 0.000 description 1
- 102000011753 Transient Receptor Potential Channels Human genes 0.000 description 1
- 101710090554 Transient receptor potential protein Proteins 0.000 description 1
- 206010069363 Traumatic lung injury Diseases 0.000 description 1
- 102000013534 Troponin C Human genes 0.000 description 1
- 208000003443 Unconsciousness Diseases 0.000 description 1
- 208000009443 Vascular Malformations Diseases 0.000 description 1
- 206010047050 Vascular anomaly Diseases 0.000 description 1
- 206010053648 Vascular occlusion Diseases 0.000 description 1
- 206010047115 Vasculitis Diseases 0.000 description 1
- 206010047249 Venous thrombosis Diseases 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 229930003316 Vitamin D Natural products 0.000 description 1
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 description 1
- 208000034698 Vitreous haemorrhage Diseases 0.000 description 1
- 102100025568 Voltage-dependent L-type calcium channel subunit beta-1 Human genes 0.000 description 1
- 102100025807 Voltage-dependent L-type calcium channel subunit beta-2 Human genes 0.000 description 1
- 102100025838 Voltage-dependent L-type calcium channel subunit beta-3 Human genes 0.000 description 1
- 102100025836 Voltage-dependent L-type calcium channel subunit beta-4 Human genes 0.000 description 1
- 102100024142 Voltage-dependent calcium channel gamma-1 subunit Human genes 0.000 description 1
- 102100024141 Voltage-dependent calcium channel gamma-2 subunit Human genes 0.000 description 1
- 102100024143 Voltage-dependent calcium channel gamma-4 subunit Human genes 0.000 description 1
- 102100032867 Voltage-dependent calcium channel gamma-5 subunit Human genes 0.000 description 1
- 102100032869 Voltage-dependent calcium channel gamma-7 subunit Human genes 0.000 description 1
- UUMKQZVEZSXWBY-HNNXBMFYSA-N [[(1s)-2-(4-phenylphenyl)-1-(2h-tetrazol-5-yl)ethyl]amino]methylphosphonic acid Chemical compound C([C@H](NCP(O)(=O)O)C1=NNN=N1)C(C=C1)=CC=C1C1=CC=CC=C1 UUMKQZVEZSXWBY-HNNXBMFYSA-N 0.000 description 1
- 208000002223 abdominal aortic aneurysm Diseases 0.000 description 1
- 230000003187 abdominal effect Effects 0.000 description 1
- 230000007488 abnormal function Effects 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 206010000269 abscess Diseases 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000003929 acidic solution Substances 0.000 description 1
- 230000009056 active transport Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 238000005273 aeration Methods 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 229920003232 aliphatic polyester Polymers 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 108090000861 alpha Adrenergic Receptors Proteins 0.000 description 1
- 102000004305 alpha Adrenergic Receptors Human genes 0.000 description 1
- VREFGVBLTWBCJP-UHFFFAOYSA-N alprazolam Chemical compound C12=CC(Cl)=CC=C2N2C(C)=NN=C2CN=C1C1=CC=CC=C1 VREFGVBLTWBCJP-UHFFFAOYSA-N 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 229960000528 amlodipine Drugs 0.000 description 1
- HTIQEAQVCYTUBX-UHFFFAOYSA-N amlodipine Chemical compound CCOC(=O)C1=C(COCCN)NC(C)=C(C(=O)OC)C1C1=CC=CC=C1Cl HTIQEAQVCYTUBX-UHFFFAOYSA-N 0.000 description 1
- 230000006986 amnesia Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229950006323 angiotensin ii Drugs 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 239000003945 anionic surfactant Substances 0.000 description 1
- 230000007953 anoxia Effects 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 230000001567 anti-fibrinolytic effect Effects 0.000 description 1
- 239000001961 anticonvulsive agent Substances 0.000 description 1
- 239000000504 antifibrinolytic agent Substances 0.000 description 1
- 229940082620 antifibrinolytics Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 229940125715 antihistaminic agent Drugs 0.000 description 1
- 239000000739 antihistaminic agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 210000000436 anus Anatomy 0.000 description 1
- 230000036506 anxiety Effects 0.000 description 1
- 210000000709 aorta Anatomy 0.000 description 1
- 208000007474 aortic aneurysm Diseases 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 206010003119 arrhythmia Diseases 0.000 description 1
- 230000008321 arterial blood flow Effects 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000000712 assembly Effects 0.000 description 1
- 238000000429 assembly Methods 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 229950010993 atrasentan Drugs 0.000 description 1
- 230000009910 autonomic response Effects 0.000 description 1
- 238000011888 autopsy Methods 0.000 description 1
- 230000001042 autoregulative effect Effects 0.000 description 1
- 210000003050 axon Anatomy 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 230000037365 barrier function of the epidermis Effects 0.000 description 1
- 210000004227 basal ganglia Anatomy 0.000 description 1
- 210000000721 basilar membrane Anatomy 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 238000010009 beating Methods 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- 235000012216 bentonite Nutrition 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- UIEATEWHFDRYRU-UHFFFAOYSA-N bepridil Chemical group C1CCCN1C(COCC(C)C)CN(C=1C=CC=CC=1)CC1=CC=CC=C1 UIEATEWHFDRYRU-UHFFFAOYSA-N 0.000 description 1
- 229960003665 bepridil Drugs 0.000 description 1
- DRTQHJPVMGBUCF-PSQAKQOGSA-N beta-L-uridine Natural products O[C@H]1[C@@H](O)[C@H](CO)O[C@@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-PSQAKQOGSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 210000002449 bone cell Anatomy 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- 210000003888 boundary cell Anatomy 0.000 description 1
- 210000002302 brachial artery Anatomy 0.000 description 1
- QXZGBUJJYSLZLT-FDISYFBBSA-N bradykinin Chemical compound NC(=N)NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(=O)NCC(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CO)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)CCC1 QXZGBUJJYSLZLT-FDISYFBBSA-N 0.000 description 1
- 208000006752 brain edema Diseases 0.000 description 1
- 210000005252 bulbus oculi Anatomy 0.000 description 1
- 229960001948 caffeine Drugs 0.000 description 1
- VJEONQKOZGKCAK-UHFFFAOYSA-N caffeine Natural products CN1C(=O)N(C)C(=O)C2=C1C=CN2C VJEONQKOZGKCAK-UHFFFAOYSA-N 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 230000010221 calcium permeability Effects 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 230000008061 calcium-channel-blocking effect Effects 0.000 description 1
- 244000309466 calf Species 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 230000003065 cardioinhibitory effect Effects 0.000 description 1
- 210000001168 carotid artery common Anatomy 0.000 description 1
- 239000012876 carrier material Substances 0.000 description 1
- 239000005018 casein Substances 0.000 description 1
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 1
- 235000021240 caseins Nutrition 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 239000003093 cationic surfactant Substances 0.000 description 1
- 210000000711 cavernous sinus Anatomy 0.000 description 1
- 210000005056 cell body Anatomy 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 229920002678 cellulose Chemical class 0.000 description 1
- 239000001913 cellulose Chemical class 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 210000000782 cerebellar granule cell Anatomy 0.000 description 1
- 238000002585 cerebral angiography Methods 0.000 description 1
- 238000001311 chemical methods and process Methods 0.000 description 1
- 230000003399 chemotactic effect Effects 0.000 description 1
- 208000020832 chronic kidney disease Diseases 0.000 description 1
- 208000022831 chronic renal failure syndrome Diseases 0.000 description 1
- 229960001265 ciclosporin Drugs 0.000 description 1
- 229960003020 cilnidipine Drugs 0.000 description 1
- 210000003703 cisterna magna Anatomy 0.000 description 1
- 210000000078 claw Anatomy 0.000 description 1
- 229950007733 clazosentan Drugs 0.000 description 1
- 238000003759 clinical diagnosis Methods 0.000 description 1
- 230000004186 co-expression Effects 0.000 description 1
- 229960004126 codeine Drugs 0.000 description 1
- 208000010877 cognitive disease Diseases 0.000 description 1
- 238000001246 colloidal dispersion Methods 0.000 description 1
- 108091036078 conserved sequence Proteins 0.000 description 1
- 229940039231 contrast media Drugs 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000011443 conventional therapy Methods 0.000 description 1
- 201000011634 coronary artery vasospasm Diseases 0.000 description 1
- 210000005257 cortical tissue Anatomy 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 210000003792 cranial nerve Anatomy 0.000 description 1
- 201000005937 cranial nerve palsy Diseases 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 229960003624 creatine Drugs 0.000 description 1
- 239000006046 creatine Substances 0.000 description 1
- 238000011461 current therapy Methods 0.000 description 1
- 108010017327 cyclo(glutamyl-alanyl-isoleucyl-leucyl-tryptophyl) Proteins 0.000 description 1
- 108010012014 cyclo(glutamyl-prolyl-valyl-leucyl-tryptophyl) Proteins 0.000 description 1
- 108010055679 cyclo(sulfoalanyl-prolyl-valyl-leucyl-tryptophyl) Proteins 0.000 description 1
- 229930182912 cyclosporin Natural products 0.000 description 1
- 208000031513 cyst Diseases 0.000 description 1
- KWGRBVOPPLSCSI-UHFFFAOYSA-N d-ephedrine Natural products CNC(C)C(O)C1=CC=CC=C1 KWGRBVOPPLSCSI-UHFFFAOYSA-N 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- 239000000850 decongestant Substances 0.000 description 1
- 229940124581 decongestants Drugs 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 239000007857 degradation product Substances 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 230000030609 dephosphorylation Effects 0.000 description 1
- 238000006209 dephosphorylation reaction Methods 0.000 description 1
- 210000004207 dermis Anatomy 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000001784 detoxification Methods 0.000 description 1
- SGTNSNPWRIOYBX-HHHXNRCGSA-N dexverapamil Chemical compound C1=C(OC)C(OC)=CC=C1CCN(C)CCC[C@@](C#N)(C(C)C)C1=CC=C(OC)C(OC)=C1 SGTNSNPWRIOYBX-HHHXNRCGSA-N 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 150000001982 diacylglycerols Chemical class 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 229940127292 dihydropyridine calcium channel blocker Drugs 0.000 description 1
- 239000002866 dihydropyridine calcium channel blocker Substances 0.000 description 1
- CHWPMFMUQATVNK-ARYYTZDLSA-N dihydrosporogen AO-1 Natural products O[C@H]1[C@]2(C(C)=C)O[C@@H]2[C@]2(C)[C@@H](C)[C@H](O)CCC2=C1 CHWPMFMUQATVNK-ARYYTZDLSA-N 0.000 description 1
- UGMCXQCYOVCMTB-UHFFFAOYSA-K dihydroxy(stearato)aluminium Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[Al](O)O UGMCXQCYOVCMTB-UHFFFAOYSA-K 0.000 description 1
- 125000002147 dimethylamino group Chemical group [H]C([H])([H])N(*)C([H])([H])[H] 0.000 description 1
- 238000002845 discoloration Methods 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 239000002934 diuretic Substances 0.000 description 1
- 230000001882 diuretic effect Effects 0.000 description 1
- 238000011833 dog model Methods 0.000 description 1
- 230000001091 dromotropic effect Effects 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 239000013583 drug formulation Substances 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 210000003027 ear inner Anatomy 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 210000004177 elastic tissue Anatomy 0.000 description 1
- 229920002549 elastin Polymers 0.000 description 1
- 230000005672 electromagnetic field Effects 0.000 description 1
- 230000010102 embolization Effects 0.000 description 1
- 230000002996 emotional effect Effects 0.000 description 1
- 238000004945 emulsification Methods 0.000 description 1
- 210000003890 endocrine cell Anatomy 0.000 description 1
- 230000007368 endocrine function Effects 0.000 description 1
- 230000003826 endocrine responses Effects 0.000 description 1
- 108091007231 endothelial receptors Proteins 0.000 description 1
- 210000003989 endothelium vascular Anatomy 0.000 description 1
- 229960005139 epinephrine Drugs 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- KAQKFAOMNZTLHT-VVUHWYTRSA-N epoprostenol Chemical compound O1C(=CCCCC(O)=O)C[C@@H]2[C@@H](/C=C/[C@@H](O)CCCCC)[C@H](O)C[C@@H]21 KAQKFAOMNZTLHT-VVUHWYTRSA-N 0.000 description 1
- 229960001123 epoprostenol Drugs 0.000 description 1
- 229930013356 epothilone Natural products 0.000 description 1
- HESCAJZNRMSMJG-KKQRBIROSA-N epothilone A Chemical class C/C([C@@H]1C[C@@H]2O[C@@H]2CCC[C@@H]([C@@H]([C@@H](C)C(=O)C(C)(C)[C@@H](O)CC(=O)O1)O)C)=C\C1=CSC(C)=N1 HESCAJZNRMSMJG-KKQRBIROSA-N 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 230000002964 excitative effect Effects 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 210000003722 extracellular fluid Anatomy 0.000 description 1
- 210000002744 extracellular matrix Anatomy 0.000 description 1
- 210000000744 eyelid Anatomy 0.000 description 1
- 230000004438 eyesight Effects 0.000 description 1
- 230000001815 facial effect Effects 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 239000010685 fatty oil Substances 0.000 description 1
- RZTAMFZIAATZDJ-UHFFFAOYSA-N felodipine Chemical compound CCOC(=O)C1=C(C)NC(C)=C(C(=O)OC)C1C1=CC=CC(Cl)=C1Cl RZTAMFZIAATZDJ-UHFFFAOYSA-N 0.000 description 1
- 238000000855 fermentation Methods 0.000 description 1
- 230000004151 fermentation Effects 0.000 description 1
- 239000003527 fibrinolytic agent Substances 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 230000004907 flux Effects 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 210000002683 foot Anatomy 0.000 description 1
- 210000002532 foramen magnum Anatomy 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 229960002870 gabapentin Drugs 0.000 description 1
- 229960000457 gallopamil Drugs 0.000 description 1
- 230000014509 gene expression Effects 0.000 description 1
- 210000004907 gland Anatomy 0.000 description 1
- 230000004313 glare Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 210000001905 globus pallidus Anatomy 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- 235000021312 gluten Nutrition 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 229940015043 glyoxal Drugs 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 239000000122 growth hormone Substances 0.000 description 1
- 230000003862 health status Effects 0.000 description 1
- 238000005534 hematocrit Methods 0.000 description 1
- 208000014951 hematologic disease Diseases 0.000 description 1
- 208000018706 hematopoietic system disease Diseases 0.000 description 1
- 208000014617 hemorrhoid Diseases 0.000 description 1
- 210000002989 hepatic vein Anatomy 0.000 description 1
- 230000000971 hippocampal effect Effects 0.000 description 1
- 210000001320 hippocampus Anatomy 0.000 description 1
- 230000013632 homeostatic process Effects 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- OROGSEYTTFOCAN-UHFFFAOYSA-N hydrocodone Natural products C1C(N(CCC234)C)C2C=CC(O)C3OC2=C4C1=CC=C2OC OROGSEYTTFOCAN-UHFFFAOYSA-N 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 230000002102 hyperpolarization Effects 0.000 description 1
- 206010020745 hyperreflexia Diseases 0.000 description 1
- 239000000960 hypophysis hormone Substances 0.000 description 1
- 230000002267 hypothalamic effect Effects 0.000 description 1
- 230000002989 hypothyroidism Effects 0.000 description 1
- 208000003532 hypothyroidism Diseases 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 229940102213 injectable suspension Drugs 0.000 description 1
- 229910001410 inorganic ion Inorganic materials 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 210000004020 intracellular membrane Anatomy 0.000 description 1
- 230000002601 intratumoral effect Effects 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 230000009545 invasion Effects 0.000 description 1
- 229940125425 inverse agonist Drugs 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 230000019948 ion homeostasis Effects 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 210000001847 jaw Anatomy 0.000 description 1
- 210000001503 joint Anatomy 0.000 description 1
- 208000017169 kidney disease Diseases 0.000 description 1
- 229960004340 lacidipine Drugs 0.000 description 1
- GKQPCPXONLDCMU-CCEZHUSRSA-N lacidipine Chemical compound CCOC(=O)C1=C(C)NC(C)=C(C(=O)OCC)C1C1=CC=CC=C1\C=C\C(=O)OC(C)(C)C GKQPCPXONLDCMU-CCEZHUSRSA-N 0.000 description 1
- 238000003475 lamination Methods 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 229960004294 lercanidipine Drugs 0.000 description 1
- ZDXUKAKRHYTAKV-UHFFFAOYSA-N lercanidipine Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OC(C)(C)CN(C)CCC(C=2C=CC=CC=2)C=2C=CC=CC=2)C1C1=CC=CC([N+]([O-])=O)=C1 ZDXUKAKRHYTAKV-UHFFFAOYSA-N 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 230000002197 limbic effect Effects 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 238000011866 long-term treatment Methods 0.000 description 1
- 208000012866 low blood pressure Diseases 0.000 description 1
- 231100000053 low toxicity Toxicity 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 231100000515 lung injury Toxicity 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 229960003963 manidipine Drugs 0.000 description 1
- ANEBWFXPVPTEET-UHFFFAOYSA-N manidipine Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OCCN2CCN(CC2)C(C=2C=CC=CC=2)C=2C=CC=CC=2)C1C1=CC=CC([N+]([O-])=O)=C1 ANEBWFXPVPTEET-UHFFFAOYSA-N 0.000 description 1
- 210000002793 maxillary artery Anatomy 0.000 description 1
- 230000008384 membrane barrier Effects 0.000 description 1
- 230000005055 memory storage Effects 0.000 description 1
- 210000002418 meninge Anatomy 0.000 description 1
- 210000002441 meningeal artery Anatomy 0.000 description 1
- 230000009245 menopause Effects 0.000 description 1
- 230000006996 mental state Effects 0.000 description 1
- 210000005033 mesothelial cell Anatomy 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229940126170 metalloproteinase inhibitor Drugs 0.000 description 1
- 239000003475 metalloproteinase inhibitor Substances 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 229960001344 methylphenidate Drugs 0.000 description 1
- 229960004438 mibefradil Drugs 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 239000003658 microfiber Substances 0.000 description 1
- 210000003632 microfilament Anatomy 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 210000004925 microvascular endothelial cell Anatomy 0.000 description 1
- 230000027829 mitochondrial depolarization Effects 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 230000004660 morphological change Effects 0.000 description 1
- 230000007659 motor function Effects 0.000 description 1
- 230000003387 muscular Effects 0.000 description 1
- 208000002089 myocardial stunning Diseases 0.000 description 1
- 210000000107 myocyte Anatomy 0.000 description 1
- 210000001087 myotubule Anatomy 0.000 description 1
- LFWCJABOXHSRGC-UHFFFAOYSA-N n-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2-[2-(2h-tetrazol-5-yl)pyridin-4-yl]pyrimidin-4-yl]-5-methylpyridine-2-sulfonamide Chemical compound COC1=CC=CC=C1OC(C(=NC(=N1)C=2C=C(N=CC=2)C2=NNN=N2)OCCO)=C1NS(=O)(=O)C1=CC=C(C)C=N1 LFWCJABOXHSRGC-UHFFFAOYSA-N 0.000 description 1
- 239000005445 natural material Substances 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 210000001577 neostriatum Anatomy 0.000 description 1
- 210000001640 nerve ending Anatomy 0.000 description 1
- 210000004126 nerve fiber Anatomy 0.000 description 1
- 208000004296 neuralgia Diseases 0.000 description 1
- 230000001272 neurogenic effect Effects 0.000 description 1
- 210000004498 neuroglial cell Anatomy 0.000 description 1
- 208000021722 neuropathic pain Diseases 0.000 description 1
- 230000003957 neurotransmitter release Effects 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- PVNIIMVLHYAWGP-UHFFFAOYSA-N nicotinic acid Natural products OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 1
- UIAGMCDKSXEBJQ-UHFFFAOYSA-N nimodipine Chemical compound COCCOC(=O)C1=C(C)NC(C)=C(C(=O)OC(C)C)C1C1=CC=CC([N+]([O-])=O)=C1 UIAGMCDKSXEBJQ-UHFFFAOYSA-N 0.000 description 1
- PVHUJELLJLJGLN-UHFFFAOYSA-N nitrendipine Chemical compound CCOC(=O)C1=C(C)NC(C)=C(C(=O)OC)C1C1=CC=CC([N+]([O-])=O)=C1 PVHUJELLJLJGLN-UHFFFAOYSA-N 0.000 description 1
- 229960005425 nitrendipine Drugs 0.000 description 1
- 229960002748 norepinephrine Drugs 0.000 description 1
- SFLSHLFXELFNJZ-UHFFFAOYSA-N norepinephrine Natural products NCC(O)C1=CC=C(O)C(O)=C1 SFLSHLFXELFNJZ-UHFFFAOYSA-N 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 231100000862 numbness Toxicity 0.000 description 1
- 230000035764 nutrition Effects 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 230000000414 obstructive effect Effects 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 210000002589 oculomotor nerve Anatomy 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- FDQZTPPHJRQRQQ-NZPQQUJLSA-N omega-conotoxin GVIA Chemical compound C([C@H]1C(=O)N[C@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CSSC[C@H]2C(=O)N[C@@H]3C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(N[C@@H](CC(N)=O)C(=O)N4C[C@H](O)C[C@H]4C(=O)N1)=O)CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CSSC3)C(=O)N[C@@H](CO)C(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(N)=O)C(=O)N2)=O)[C@H](O)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(N)=O)[C@@H](C)O)C1=CC=C(O)C=C1 FDQZTPPHJRQRQQ-NZPQQUJLSA-N 0.000 description 1
- 230000002450 orbitofrontal effect Effects 0.000 description 1
- 210000004789 organ system Anatomy 0.000 description 1
- 150000002895 organic esters Chemical class 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 230000001151 other effect Effects 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 229940094443 oxytocics prostaglandins Drugs 0.000 description 1
- XNOPRXBHLZRZKH-DSZYJQQASA-N oxytocin Chemical compound C([C@H]1C(=O)N[C@H](C(N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CSSC[C@H](N)C(=O)N1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 XNOPRXBHLZRZKH-DSZYJQQASA-N 0.000 description 1
- 229960001723 oxytocin Drugs 0.000 description 1
- 210000003254 palate Anatomy 0.000 description 1
- 210000002741 palatine tonsil Anatomy 0.000 description 1
- 208000021090 palsy Diseases 0.000 description 1
- 239000004031 partial agonist Substances 0.000 description 1
- 230000009054 pathological process Effects 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000007310 pathophysiology Effects 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 210000004197 pelvis Anatomy 0.000 description 1
- 230000008447 perception Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 150000002978 peroxides Chemical class 0.000 description 1
- 230000002688 persistence Effects 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- PHEDXBVPIONUQT-RGYGYFBISA-N phorbol 13-acetate 12-myristate Chemical compound C([C@]1(O)C(=O)C(C)=C[C@H]1[C@@]1(O)[C@H](C)[C@H]2OC(=O)CCCCCCCCCCCCC)C(CO)=C[C@H]1[C@H]1[C@]2(OC(C)=O)C1(C)C PHEDXBVPIONUQT-RGYGYFBISA-N 0.000 description 1
- 239000002644 phorbol ester Substances 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 230000006461 physiological response Effects 0.000 description 1
- 230000001817 pituitary effect Effects 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 231100000614 poison Toxicity 0.000 description 1
- 229920001483 poly(ethyl methacrylate) polymer Polymers 0.000 description 1
- 229920000212 poly(isobutyl acrylate) Polymers 0.000 description 1
- 229920000205 poly(isobutyl methacrylate) Polymers 0.000 description 1
- 229920000196 poly(lauryl methacrylate) Polymers 0.000 description 1
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 1
- 229920000184 poly(octadecyl acrylate) Polymers 0.000 description 1
- 229920002627 poly(phosphazenes) Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 229920001230 polyarylate Polymers 0.000 description 1
- 239000004632 polycaprolactone Substances 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 229920000129 polyhexylmethacrylate Polymers 0.000 description 1
- 229920000197 polyisopropyl acrylate Polymers 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 238000010837 poor prognosis Methods 0.000 description 1
- 210000003240 portal vein Anatomy 0.000 description 1
- 208000028173 post-traumatic stress disease Diseases 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229950004891 pranidipine Drugs 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 229960001233 pregabalin Drugs 0.000 description 1
- AYXYPKUFHZROOJ-ZETCQYMHSA-N pregabalin Chemical compound CC(C)C[C@H](CN)CC(O)=O AYXYPKUFHZROOJ-ZETCQYMHSA-N 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 108010072272 proendothelin 1 Proteins 0.000 description 1
- 229940097325 prolactin Drugs 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 230000001902 propagating effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- KRIOVPPHQSLHCZ-UHFFFAOYSA-N propiophenone Chemical compound CCC(=O)C1=CC=CC=C1 KRIOVPPHQSLHCZ-UHFFFAOYSA-N 0.000 description 1
- 150000003180 prostaglandins Chemical class 0.000 description 1
- 108020001580 protein domains Proteins 0.000 description 1
- KWGRBVOPPLSCSI-WCBMZHEXSA-N pseudoephedrine Chemical compound CN[C@@H](C)[C@@H](O)C1=CC=CC=C1 KWGRBVOPPLSCSI-WCBMZHEXSA-N 0.000 description 1
- 229960003908 pseudoephedrine Drugs 0.000 description 1
- 208000002815 pulmonary hypertension Diseases 0.000 description 1
- 230000008704 pulmonary vasodilation Effects 0.000 description 1
- 210000003742 purkinje fiber Anatomy 0.000 description 1
- 210000002637 putamen Anatomy 0.000 description 1
- 210000002763 pyramidal cell Anatomy 0.000 description 1
- 230000009103 reabsorption Effects 0.000 description 1
- 239000000018 receptor agonist Substances 0.000 description 1
- 229940044601 receptor agonist Drugs 0.000 description 1
- 230000036454 renin-angiotensin system Effects 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 239000013557 residual solvent Substances 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 230000036387 respiratory rate Effects 0.000 description 1
- 230000004043 responsiveness Effects 0.000 description 1
- 230000036390 resting membrane potential Effects 0.000 description 1
- 210000003994 retinal ganglion cell Anatomy 0.000 description 1
- 210000001202 rhombencephalon Anatomy 0.000 description 1
- XMVJITFPVVRMHC-UHFFFAOYSA-N roxarsone Chemical group OC1=CC=C([As](O)(O)=O)C=C1[N+]([O-])=O XMVJITFPVVRMHC-UHFFFAOYSA-N 0.000 description 1
- 108091052345 ryanodine receptor (TC 1.A.3.1) family Proteins 0.000 description 1
- 210000001908 sarcoplasmic reticulum Anatomy 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 229940125723 sedative agent Drugs 0.000 description 1
- 239000000932 sedative agent Substances 0.000 description 1
- 210000002483 sella turcica Anatomy 0.000 description 1
- 230000035807 sensation Effects 0.000 description 1
- 230000037152 sensory function Effects 0.000 description 1
- 230000009209 sensory transmission Effects 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 230000008054 signal transmission Effects 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 230000037321 sleepiness Effects 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 230000000391 smoking effect Effects 0.000 description 1
- 150000003385 sodium Chemical class 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000008247 solid mixture Substances 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000002798 spectrophotometry method Methods 0.000 description 1
- 208000027765 speech disease Diseases 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000008107 starch Chemical class 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 210000003270 subclavian artery Anatomy 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 239000001384 succinic acid Substances 0.000 description 1
- 230000002483 superagonistic effect Effects 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000007460 surgical drainage Methods 0.000 description 1
- 238000013269 sustained drug release Methods 0.000 description 1
- 230000009182 swimming Effects 0.000 description 1
- 230000002889 sympathetic effect Effects 0.000 description 1
- 210000000225 synapse Anatomy 0.000 description 1
- 230000005062 synaptic transmission Effects 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 210000004876 tela submucosa Anatomy 0.000 description 1
- 210000001103 thalamus Anatomy 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- 210000000115 thoracic cavity Anatomy 0.000 description 1
- 210000001578 tight junction Anatomy 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 238000012876 topography Methods 0.000 description 1
- 230000008791 toxic response Effects 0.000 description 1
- 239000003440 toxic substance Substances 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000024033 toxin binding Effects 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 102000042565 transient receptor (TC 1.A.4) family Human genes 0.000 description 1
- 108091053409 transient receptor (TC 1.A.4) family Proteins 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 102000027257 transmembrane receptors Human genes 0.000 description 1
- 108091008578 transmembrane receptors Proteins 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- YFHICDDUDORKJB-UHFFFAOYSA-N trimethylene carbonate Chemical compound O=C1OCCCO1 YFHICDDUDORKJB-UHFFFAOYSA-N 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 238000009827 uniform distribution Methods 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- DRTQHJPVMGBUCF-UHFFFAOYSA-N uracil arabinoside Natural products OC1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-UHFFFAOYSA-N 0.000 description 1
- 229940045145 uridine Drugs 0.000 description 1
- 230000007556 vascular defect Effects 0.000 description 1
- 208000021331 vascular occlusion disease Diseases 0.000 description 1
- 210000004509 vascular smooth muscle cell Anatomy 0.000 description 1
- 230000025102 vascular smooth muscle contraction Effects 0.000 description 1
- 230000006442 vascular tone Effects 0.000 description 1
- 239000002550 vasoactive agent Substances 0.000 description 1
- 230000002455 vasospastic effect Effects 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 239000002435 venom Substances 0.000 description 1
- 231100000611 venom Toxicity 0.000 description 1
- 210000001048 venom Anatomy 0.000 description 1
- 210000005111 ventral hippocampus Anatomy 0.000 description 1
- 210000000264 venule Anatomy 0.000 description 1
- 229960001722 verapamil Drugs 0.000 description 1
- 230000007371 visceral function Effects 0.000 description 1
- 238000011179 visual inspection Methods 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 235000019166 vitamin D Nutrition 0.000 description 1
- 239000011710 vitamin D Substances 0.000 description 1
- 229940046008 vitamin d Drugs 0.000 description 1
- 150000003722 vitamin derivatives Chemical class 0.000 description 1
- 210000004127 vitreous body Anatomy 0.000 description 1
- 230000007279 water homeostasis Effects 0.000 description 1
- 108091058553 ω-conotoxin GVIA Proteins 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4418—Non condensed pyridines; Hydrogenated derivatives thereof having a carbocyclic group directly attached to the heterocyclic ring, e.g. cyproheptadine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4422—1,4-Dihydropyridines, e.g. nifedipine, nicardipine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/4439—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. omeprazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/4523—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems
- A61K31/4545—Non condensed piperidines, e.g. piperocaine containing further heterocyclic ring systems containing a six-membered ring with nitrogen as a ring hetero atom, e.g. pipamperone, anabasine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/34—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyesters, polyamino acids, polysiloxanes, polyphosphazines, copolymers of polyalkylene glycol or poloxamers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0085—Brain, e.g. brain implants; Spinal cord
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1641—Organic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyethylene glycol, poloxamers
- A61K9/1647—Polyesters, e.g. poly(lactide-co-glycolide)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M31/00—Devices for introducing or retaining media, e.g. remedies, in cavities of the body
- A61M31/002—Devices for releasing a drug at a continuous and controlled rate for a prolonged period of time
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/178—Syringes
- A61M5/31—Details
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/02—Antithrombotic agents; Anticoagulants; Platelet aggregation inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Anesthesiology (AREA)
- Inorganic Chemistry (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Vascular Medicine (AREA)
- Dermatology (AREA)
- Psychology (AREA)
- Dispersion Chemistry (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Cardiology (AREA)
- Diabetes (AREA)
- Urology & Nephrology (AREA)
- Biophysics (AREA)
- Pulmonology (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161471779P | 2011-04-05 | 2011-04-05 | |
| US61/471,779 | 2011-04-05 | ||
| PCT/US2012/032317 WO2012138854A1 (en) | 2011-04-05 | 2012-04-05 | Intraventricular drug delivery system for improving outcome after a brain injury affecting cerebral blood flow |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20140052992A true KR20140052992A (ko) | 2014-05-07 |
Family
ID=46969546
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020137029330A Ceased KR20140052992A (ko) | 2011-04-05 | 2012-04-05 | 뇌혈류에 영향을 미치는 뇌손상 후 결과를 개선시키기 위한 뇌실내 약물 전달 시스템 |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US9364432B2 (enExample) |
| EP (1) | EP2694133B1 (enExample) |
| JP (2) | JP6336899B2 (enExample) |
| KR (1) | KR20140052992A (enExample) |
| CN (1) | CN103517726B (enExample) |
| AU (1) | AU2012240131B2 (enExample) |
| CA (1) | CA2832501A1 (enExample) |
| GB (1) | GB2513675A (enExample) |
| HK (1) | HK1198933A1 (enExample) |
| IL (1) | IL228539B (enExample) |
| RU (2) | RU2606169C2 (enExample) |
| SG (2) | SG10201602665UA (enExample) |
| WO (1) | WO2012138854A1 (enExample) |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10092524B2 (en) * | 2008-06-11 | 2018-10-09 | Edge Therapeutics, Inc. | Compositions and their use to treat complications of aneurysmal subarachnoid hemorrhage |
| SG10201602665UA (en) * | 2011-04-05 | 2016-05-30 | Edge Therapeutics | Intraventricular Drug Delivery System For Improving Outcome After A Brain Injury Affecting Cerebral Blood Flow |
| US9399019B2 (en) | 2012-05-09 | 2016-07-26 | Evonik Corporation | Polymorph compositions, methods of making, and uses thereof |
| EP3682912B1 (en) * | 2012-08-17 | 2025-11-26 | AMSilk GmbH | Use of self-assembling polypeptides as tissue adhesives |
| CN103211812A (zh) * | 2013-04-02 | 2013-07-24 | 苏州大学附属第一医院 | Skf96365在治疗蛛网膜下腔出血后发生的脑血管痉挛的应用 |
| BR112019005433B1 (pt) | 2016-09-20 | 2023-04-04 | Bracco Diagnostics Inc | Sistemas e técnicas para gerar, infundir e controlar distribuição de radioisótopo |
| US10646227B2 (en) | 2016-12-29 | 2020-05-12 | Cerepeutics, LLC | Systems and methods for acute treatment to limit intracerebral hemorrhage growth |
| WO2019018306A1 (en) * | 2017-07-17 | 2019-01-24 | Edge Therapeutics, Inc. | ADVANCED MICROPARTICULAR FORMULATIONS CONTAINING FORM 2 OF MICRONIZED POLYMORPHIC NIMODIPINE |
| US20190060109A1 (en) * | 2017-08-31 | 2019-02-28 | Gregory Todd Johnson | Method of Preventing Traumatic Brain Injury (TBI) |
| EP3776598B1 (en) | 2018-03-28 | 2022-05-04 | Bracco Diagnostics Inc. | Systems and techniques for calibrating radioisotope delivery systems with a gamma detector |
| CN112106148B (zh) | 2018-03-28 | 2024-10-29 | 布拉科诊断公司 | 放射性同位素发生器寿命的早期检测 |
| US12016774B2 (en) | 2020-05-08 | 2024-06-25 | Medtronic Vascular, Inc. | Delivery system for prosthetic valve device having controlled release of inflow and outflow ends |
| RU2763254C1 (ru) * | 2020-08-31 | 2021-12-28 | Федеральное государственное бюджетное образовательное учреждение высшего образования "Волгоградский государственный медицинский университет" Министерства здравоохранения Российской Федерации ФГБОУ ВО ВолгГМУ МЗ РФ | Способ лечения церебрального вазоспазма при аневризматическом субарахноидальном кровоизлиянии |
| US11992642B2 (en) | 2020-10-30 | 2024-05-28 | Medtronic, Inc. | Implantable medical device for delivery of pharmacological agents to the deep brain structures |
| US11745003B2 (en) | 2020-10-30 | 2023-09-05 | Medtronic, Inc. | Implantable access port with one-directional filter |
| US11931545B2 (en) | 2020-10-30 | 2024-03-19 | Medtronic, Inc. | Drug infusion port |
| US12226609B2 (en) | 2020-12-16 | 2025-02-18 | Medtronic, Inc. | Method to detect inadvertent delivery of drug to a subcutaneous pocket |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040105888A1 (en) * | 2002-11-26 | 2004-06-03 | Daniel Pratt | Buoyant polymer particles for delivery of therapeutic agents to the central nervous system |
| US20080287879A1 (en) * | 2007-05-16 | 2008-11-20 | Harkins Jr Thomas John | system and method for treating erectile dysfunction |
| JP2009067771A (ja) * | 2008-05-27 | 2009-04-02 | Pacira Pharmaceuticals Inc | 神経学的疾患の治療方法 |
| JP2010529205A (ja) * | 2007-06-11 | 2010-08-26 | アール ロッチ マクドナルド | 脳血管れん縮の予防用薬物送達システム |
Family Cites Families (58)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4757128A (en) | 1986-08-01 | 1988-07-12 | Massachusetts Institute Of Technology | High molecular weight polyanhydride and preparation thereof |
| ATE133087T1 (de) | 1989-05-04 | 1996-02-15 | Southern Res Inst | Einkapselungsverfahren |
| US5650489A (en) | 1990-07-02 | 1997-07-22 | The Arizona Board Of Regents | Random bio-oligomer library, a method of synthesis thereof, and a method of use thereof |
| IT1243390B (it) | 1990-11-22 | 1994-06-10 | Vectorpharma Int | Composizioni farmaceutiche in forma di particelle atte al rilascio controllato di sostanze farmacologicamente attive e procedimento per la loro preparazione. |
| US5399665A (en) | 1992-11-05 | 1995-03-21 | Massachusetts Institute Of Technology | Biodegradable polymers for cell transplantation |
| EP0740791A4 (en) | 1994-01-05 | 2000-04-05 | Arqule Inc | SYSTEMATIC MODULAR PRODUCTION OF AMINIMID AND OXAZOLONE-BASED MOLECULES WITH SELECTED PROPERTIES |
| US5712171A (en) | 1995-01-20 | 1998-01-27 | Arqule, Inc. | Method of generating a plurality of chemical compounds in a spatially arranged array |
| US5747058A (en) | 1995-06-07 | 1998-05-05 | Southern Biosystems, Inc. | High viscosity liquid controlled delivery system |
| US5968542A (en) | 1995-06-07 | 1999-10-19 | Southern Biosystems, Inc. | High viscosity liquid controlled delivery system as a device |
| AT404429B (de) | 1995-06-09 | 1998-11-25 | Immuno Ag | Anti-plasma-antikörper-präparation |
| JP2909418B2 (ja) | 1995-09-18 | 1999-06-23 | 株式会社資生堂 | 薬物の遅延放出型マイクロスフイア |
| EP0932399B1 (en) | 1996-03-12 | 2006-01-04 | PG-TXL Company, L.P. | Water soluble paclitaxel prodrugs |
| WO1998027963A2 (en) * | 1996-12-20 | 1998-07-02 | Alza Corporation | Gel composition and methods |
| US6123956A (en) * | 1997-07-10 | 2000-09-26 | Keith Baker | Methods for universally distributing therapeutic agents to the brain |
| WO1999018949A1 (en) * | 1997-10-15 | 1999-04-22 | Thomas Jefferson University | Nitric oxide donor compositions, methods, apparatus, and kits for preventing or alleviating vasoconstriction or vasospasm in a mammal |
| JP2000070366A (ja) * | 1998-09-02 | 2000-03-07 | Arute:Kk | 容器兼用注射器及び容器兼用注射器への凍結乾燥製剤の封入方法 |
| CA2396016A1 (en) * | 1999-12-29 | 2001-07-05 | Andy Bernhardt | System for reconstituting pastes and methods of using same |
| EP1250166B1 (en) | 2000-01-25 | 2010-03-17 | Edwards Lifesciences Corporation | Delivery systems for treatment of restenosis and anastomotic intimal hyperplasia |
| AU2002335658A1 (en) * | 2001-08-31 | 2003-03-18 | Neuron Therapeutics, Inc. | Treatment of neurologic hemorrhage |
| US20040235801A1 (en) | 2001-10-25 | 2004-11-25 | Jean-Pierre Julien | Therapy for stroke |
| EP1459727B1 (en) | 2001-11-22 | 2010-06-09 | Japan Science and Technology Agency | Nonhuman model animal unresponsive to immunopotentiating synthetic compound |
| JP2005524657A (ja) | 2002-02-27 | 2005-08-18 | ファーメイン, エルティーディー. | 治療剤及び他の物質を送達するための組成物、及び、前記組成物を作製し使用する方法 |
| TW200307011A (en) * | 2002-04-18 | 2003-12-01 | Chugai Pharmaceutical Co Ltd | Hyaluronic acid modifier |
| CN1665925A (zh) | 2002-04-30 | 2005-09-07 | 马克西根控股公司 | 凝血因子Ⅶ或Ⅶa多肽变体 |
| CN1671400A (zh) | 2002-07-03 | 2005-09-21 | 派瑞克科学公司 | 透明质酸组合物以及使用方法 |
| DK1553927T3 (da) | 2002-09-11 | 2011-01-31 | Elan Pharma Int Ltd | Gel-stabiliserede, nanopartikulære aktivstof-sammensætninger |
| JP4092994B2 (ja) * | 2002-09-13 | 2008-05-28 | ニプロ株式会社 | プレフィルドシリンジキット |
| US20070207211A1 (en) | 2003-04-10 | 2007-09-06 | Pr Pharmaceuticals, Inc. | Emulsion-based microparticles and methods for the production thereof |
| JP2007515392A (ja) | 2003-04-10 | 2007-06-14 | ピーアール ファーマシューティカルズ,インコーポレイテッド | エマルジョンベースの微粒子の製造のための方法 |
| CA2533592C (en) | 2003-07-23 | 2015-11-10 | Pr Pharmaceuticals, Inc. | Controlled release compositions |
| US9149440B2 (en) * | 2003-09-02 | 2015-10-06 | University Of South Florida | Nanoparticles for drug-delivery |
| WO2006002365A2 (en) * | 2004-06-24 | 2006-01-05 | Angiotech International Ag | Microparticles with high loadings of a bioactive agent |
| US20060205733A1 (en) | 2004-08-26 | 2006-09-14 | Encysive Pharmaceuticals | Endothelin a receptor antagonists in combination with phosphodiesterase 5 inhibitors and uses thereof |
| US8034762B2 (en) * | 2004-09-02 | 2011-10-11 | Cognosci, Inc. | Treatment of subarachnoid hemorrhage with Apo E analogs |
| WO2006084005A2 (en) | 2005-02-02 | 2006-08-10 | University Of Vermont And State Agricultural College | Emergence of a r-type ca2+channel(cav2.3) contributes to cerebral artery constriction following subarachnoid hemorrhage |
| EP2500031A3 (en) | 2005-02-24 | 2012-12-26 | Joslin Diabetes Center, Inc. | Compositions and Methods for Treating Vascular Permeability |
| US20060217340A1 (en) | 2005-03-23 | 2006-09-28 | University Of Vermont And State Agricultural College | Methods and products for treating hypertension by modulation of TRPC3 channel activity |
| EP1865924B1 (en) * | 2005-03-31 | 2015-12-09 | Lidds Ab | Method for treating prostate diseases based on local delivery of active substances |
| EP1893230A2 (en) | 2005-04-26 | 2008-03-05 | Maxygen Holdings Ltd. | Use of modified factor vii for treating bleeding |
| EP1883465B1 (fr) * | 2005-05-24 | 2010-01-20 | Utéron Pharma S.A. | Dispositif de melange a double chambre pour substances pharmaceutiques visqueuses et procede |
| WO2006133045A1 (en) * | 2005-06-03 | 2006-12-14 | Elan Pharma International, Limited | Nanoparticulate benidipine compositions |
| JP2009501188A (ja) | 2005-07-15 | 2009-01-15 | ノボ ノルディスク ヘルス ケア アーゲー | 抗血小板療法で治療される患者における脳内出血(ICH)後の出血増大及び/又は浮腫形成を予防又は軽減するための第VIIa因子又は第VIIa因子等価物の使用 |
| JP2009516751A (ja) * | 2005-11-21 | 2009-04-23 | ザ・ボード・オブ・トラスティーズ・オブ・ザ・ユニバーシティー・オブ・アラバマ・フォー・アンド・オン・ビハーフ・オブ・ザ・ユニバーシティー・オブ・アラバマ | 神経保護のための小分子化合物を使用する方法 |
| US10092524B2 (en) | 2008-06-11 | 2018-10-09 | Edge Therapeutics, Inc. | Compositions and their use to treat complications of aneurysmal subarachnoid hemorrhage |
| JP5502751B2 (ja) | 2007-12-20 | 2014-05-28 | エボニック コーポレイション | 低残留溶媒濃度を有する微粒子を調製するためのプロセス |
| US20100008968A1 (en) | 2008-06-26 | 2010-01-14 | Lampe John W | Method for treating cardiovascular diseases using rho kinase inhibitor compounds |
| EP2331082B1 (en) | 2008-09-11 | 2021-01-06 | Evonik Corporation | Solvent extraction microencapsulation with tunable extraction rates |
| EP2334288B1 (en) | 2008-09-18 | 2021-05-19 | Evonik Corporation | Microencapsulation process with solvent and salt |
| US20100216948A1 (en) | 2009-01-23 | 2010-08-26 | Tipton Arthur J | Polymer mixtures comprising polymers having different non-repeating units and methods for making and using same |
| US20130059008A1 (en) | 2009-01-23 | 2013-03-07 | Jeffrey L. Atkinson | Drying methods for tuning microparticle properties |
| WO2010085609A2 (en) | 2009-01-23 | 2010-07-29 | Surmodics Pharmaceuticals, Inc. | Controlled release systems from polymer blends |
| US20100291027A1 (en) | 2009-05-14 | 2010-11-18 | Jason Campbell | Hyaluronic acid (ha) injection vehicle |
| US20110033463A1 (en) * | 2009-08-06 | 2011-02-10 | Medtronic, Inc. | Apheresis, administration of agent, or combination thereof |
| WO2011087689A2 (en) | 2009-12-22 | 2011-07-21 | Surmodics Pharmaceuticals,Inc. | Emulsion-based process for preparing microparticles and workhead assembly for use with same |
| GB2490084A (en) | 2010-02-22 | 2012-10-17 | Edge Therapeutics Inc | Methods and compositions to treat hemorrhagic conditions of the brain |
| US9504643B2 (en) | 2010-03-29 | 2016-11-29 | Evonik Corporation | Compositions and methods for improved retention of a pharmaceutical composition at a local administration site |
| JP5900805B2 (ja) * | 2011-01-27 | 2016-04-06 | エルジー・ケム・リミテッド | 間隙充填用スウェリングテープ |
| SG10201602665UA (en) * | 2011-04-05 | 2016-05-30 | Edge Therapeutics | Intraventricular Drug Delivery System For Improving Outcome After A Brain Injury Affecting Cerebral Blood Flow |
-
2012
- 2012-04-05 SG SG10201602665UA patent/SG10201602665UA/en unknown
- 2012-04-05 KR KR1020137029330A patent/KR20140052992A/ko not_active Ceased
- 2012-04-05 JP JP2014503978A patent/JP6336899B2/ja not_active Expired - Fee Related
- 2012-04-05 US US13/440,276 patent/US9364432B2/en not_active Expired - Fee Related
- 2012-04-05 GB GB1317446.1A patent/GB2513675A/en not_active Withdrawn
- 2012-04-05 RU RU2013148938A patent/RU2606169C2/ru not_active IP Right Cessation
- 2012-04-05 HK HK14112548.6A patent/HK1198933A1/xx unknown
- 2012-04-05 AU AU2012240131A patent/AU2012240131B2/en not_active Ceased
- 2012-04-05 EP EP12767399.4A patent/EP2694133B1/en not_active Not-in-force
- 2012-04-05 SG SG2013072848A patent/SG193991A1/en unknown
- 2012-04-05 CN CN201280022344.1A patent/CN103517726B/zh not_active Expired - Fee Related
- 2012-04-05 WO PCT/US2012/032317 patent/WO2012138854A1/en not_active Ceased
- 2012-04-05 CA CA2832501A patent/CA2832501A1/en not_active Abandoned
-
2013
- 2013-09-29 IL IL228539A patent/IL228539B/en not_active IP Right Cessation
-
2016
- 2016-03-14 US US15/069,824 patent/US20160193198A1/en not_active Abandoned
- 2016-10-20 JP JP2016205897A patent/JP2017036320A/ja not_active Withdrawn
- 2016-11-16 RU RU2016144847A patent/RU2016144847A/ru not_active Application Discontinuation
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040105888A1 (en) * | 2002-11-26 | 2004-06-03 | Daniel Pratt | Buoyant polymer particles for delivery of therapeutic agents to the central nervous system |
| US20080287879A1 (en) * | 2007-05-16 | 2008-11-20 | Harkins Jr Thomas John | system and method for treating erectile dysfunction |
| JP2010529205A (ja) * | 2007-06-11 | 2010-08-26 | アール ロッチ マクドナルド | 脳血管れん縮の予防用薬物送達システム |
| JP2009067771A (ja) * | 2008-05-27 | 2009-04-02 | Pacira Pharmaceuticals Inc | 神経学的疾患の治療方法 |
Non-Patent Citations (1)
| Title |
|---|
| 일본 공표특허공보 특표2010-529205호(2010.08.26.) 1부. * |
Also Published As
| Publication number | Publication date |
|---|---|
| RU2016144847A (ru) | 2018-05-17 |
| CN103517726B (zh) | 2016-08-17 |
| NZ616458A (en) | 2016-01-29 |
| SG193991A1 (en) | 2013-11-29 |
| HK1198933A1 (en) | 2015-06-19 |
| RU2016144847A3 (enExample) | 2018-05-17 |
| AU2012240131B2 (en) | 2017-07-20 |
| GB2513675A (en) | 2014-11-05 |
| CA2832501A1 (en) | 2012-10-11 |
| AU2012240131A1 (en) | 2013-10-17 |
| JP2014515744A (ja) | 2014-07-03 |
| JP2017036320A (ja) | 2017-02-16 |
| EP2694133A1 (en) | 2014-02-12 |
| NZ714032A (en) | 2017-03-31 |
| US9364432B2 (en) | 2016-06-14 |
| RU2606169C2 (ru) | 2017-01-10 |
| US20120245561A1 (en) | 2012-09-27 |
| RU2013148938A (ru) | 2015-05-10 |
| EP2694133A4 (en) | 2014-11-19 |
| IL228539B (en) | 2018-10-31 |
| CN103517726A (zh) | 2014-01-15 |
| NZ727856A (en) | 2018-06-29 |
| WO2012138854A1 (en) | 2012-10-11 |
| IL228539A0 (en) | 2013-12-31 |
| SG10201602665UA (en) | 2016-05-30 |
| JP6336899B2 (ja) | 2018-06-06 |
| US20160193198A1 (en) | 2016-07-07 |
| EP2694133B1 (en) | 2018-05-23 |
| GB201317446D0 (en) | 2013-11-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20140052992A (ko) | 뇌혈류에 영향을 미치는 뇌손상 후 결과를 개선시키기 위한 뇌실내 약물 전달 시스템 | |
| EP2164497B1 (en) | A drug delivery system for the prevention of cerebral vasospasm | |
| JP2016512527A (ja) | 動脈瘤性くも膜下出血の合併症を治療するための組成物およびそれらの使用 | |
| JP6146814B2 (ja) | クモ膜下出血のヒトの予後を改善する組成物及び方法 | |
| CA2826890A1 (en) | Compositions and methods for improving prognosis of a human with subarachnoid hemorrhage | |
| HK1230079A1 (en) | Compositions and methods for improving prognosis of a human with subarachnoid hemorrhage | |
| NZ613970B2 (en) | Compositions and methods for improving prognosis of a human with subarachnoid hemorrhage | |
| NZ616458B2 (en) | Intraventricular drug delivery system for improving outcome after a brain injury affecting cerebral blood flow | |
| NZ727856B2 (en) | Intraventricular drug delivery system for improving outcome after a brain injury affecting cerebral blood flow | |
| NZ714032B2 (en) | Intraventricular drug delivery system for improving outcome after a brain injury affecting cerebral blood flow |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20131105 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20170405 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20180412 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20180813 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20180412 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |