KR20140037877A - Cb-183,315 조성물 및 관련 방법 - Google Patents
Cb-183,315 조성물 및 관련 방법 Download PDFInfo
- Publication number
- KR20140037877A KR20140037877A KR1020137033768A KR20137033768A KR20140037877A KR 20140037877 A KR20140037877 A KR 20140037877A KR 1020137033768 A KR1020137033768 A KR 1020137033768A KR 20137033768 A KR20137033768 A KR 20137033768A KR 20140037877 A KR20140037877 A KR 20140037877A
- Authority
- KR
- South Korea
- Prior art keywords
- formulation
- sucrose
- solid
- formulations
- trehalose
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 400
- 238000000034 method Methods 0.000 title claims abstract description 41
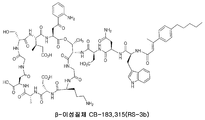
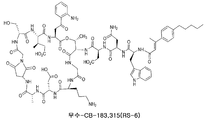
- DYNMYYRPPFVAKR-CWXHRMTKSA-N surotomycin Chemical compound C1=CC(CCCCC)=CC=C1C(\C)=C\C(=O)N[C@H](C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]1C(NCC(=O)N[C@@H](CCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@H](CO)C(=O)N[C@H](C(=O)N[C@@H](CC(=O)C=2C(=CC=CC=2)N)C(=O)O[C@@H]1C)[C@H](C)CC(O)=O)=O)CC1=CNC2=CC=CC=C12 DYNMYYRPPFVAKR-CWXHRMTKSA-N 0.000 claims abstract description 378
- 108010051772 CB-183,315 Proteins 0.000 claims abstract description 376
- 238000009472 formulation Methods 0.000 claims abstract description 346
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims abstract description 140
- 229930006000 Sucrose Natural products 0.000 claims abstract description 140
- 239000005720 sucrose Substances 0.000 claims abstract description 140
- 239000007787 solid Substances 0.000 claims abstract description 101
- 235000000346 sugar Nutrition 0.000 claims abstract description 79
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 claims abstract description 48
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 claims abstract description 48
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 claims abstract description 48
- 150000008163 sugars Chemical class 0.000 claims abstract description 34
- 239000007864 aqueous solution Substances 0.000 claims abstract description 21
- 238000004108 freeze drying Methods 0.000 claims abstract description 16
- 238000001694 spray drying Methods 0.000 claims abstract description 16
- 238000002360 preparation method Methods 0.000 claims abstract description 11
- 239000000243 solution Substances 0.000 claims description 66
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 claims description 42
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 35
- 239000000463 material Substances 0.000 claims description 31
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 30
- 235000019359 magnesium stearate Nutrition 0.000 claims description 21
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 claims description 17
- 229930195725 Mannitol Natural products 0.000 claims description 17
- 229920000168 Microcrystalline cellulose Polymers 0.000 claims description 17
- 239000000594 mannitol Substances 0.000 claims description 17
- 235000010355 mannitol Nutrition 0.000 claims description 17
- 239000008108 microcrystalline cellulose Substances 0.000 claims description 17
- 235000019813 microcrystalline cellulose Nutrition 0.000 claims description 17
- 229940016286 microcrystalline cellulose Drugs 0.000 claims description 17
- 239000007921 spray Substances 0.000 claims description 17
- 229920002785 Croscarmellose sodium Polymers 0.000 claims description 16
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 claims description 16
- 229960001681 croscarmellose sodium Drugs 0.000 claims description 16
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 claims description 16
- 239000008194 pharmaceutical composition Substances 0.000 claims description 14
- 239000000377 silicon dioxide Substances 0.000 claims description 14
- 235000012239 silicon dioxide Nutrition 0.000 claims description 14
- 238000001035 drying Methods 0.000 claims description 9
- 239000002775 capsule Substances 0.000 claims description 7
- 229920002307 Dextran Polymers 0.000 claims description 6
- 239000002671 adjuvant Substances 0.000 claims description 2
- 239000012530 fluid Substances 0.000 claims description 2
- 239000006186 oral dosage form Substances 0.000 claims description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 2
- 239000001913 cellulose Substances 0.000 claims 1
- 229920002678 cellulose Polymers 0.000 claims 1
- 235000010980 cellulose Nutrition 0.000 claims 1
- 238000013329 compounding Methods 0.000 claims 1
- 239000003937 drug carrier Substances 0.000 claims 1
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 claims 1
- 239000000126 substance Substances 0.000 abstract description 27
- 238000004519 manufacturing process Methods 0.000 abstract description 6
- 230000000052 comparative effect Effects 0.000 description 48
- 239000012535 impurity Substances 0.000 description 48
- 239000000843 powder Substances 0.000 description 45
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 30
- 238000003109 Karl Fischer titration Methods 0.000 description 23
- 238000004128 high performance liquid chromatography Methods 0.000 description 20
- 230000015572 biosynthetic process Effects 0.000 description 16
- 238000000576 coating method Methods 0.000 description 11
- 239000008187 granular material Substances 0.000 description 11
- 239000011248 coating agent Substances 0.000 description 10
- 150000001875 compounds Chemical class 0.000 description 10
- 229910052757 nitrogen Inorganic materials 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 238000002156 mixing Methods 0.000 description 6
- 239000002904 solvent Substances 0.000 description 6
- 230000000087 stabilizing effect Effects 0.000 description 6
- 229940085942 formulation r Drugs 0.000 description 5
- 239000008247 solid mixture Substances 0.000 description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 4
- 235000021355 Stearic acid Nutrition 0.000 description 4
- 238000002835 absorbance Methods 0.000 description 4
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 4
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 4
- 239000008117 stearic acid Substances 0.000 description 4
- 241000237858 Gastropoda Species 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 238000005056 compaction Methods 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- MVPICKVDHDWCJQ-UHFFFAOYSA-N ethyl 3-pyrrolidin-1-ylpropanoate Chemical compound CCOC(=O)CCN1CCCC1 MVPICKVDHDWCJQ-UHFFFAOYSA-N 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 239000008213 purified water Substances 0.000 description 3
- 229940045902 sodium stearyl fumarate Drugs 0.000 description 3
- 238000003860 storage Methods 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 230000000844 anti-bacterial effect Effects 0.000 description 2
- 229940075614 colloidal silicon dioxide Drugs 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 239000004570 mortar (masonry) Substances 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 238000005070 sampling Methods 0.000 description 2
- 239000000454 talc Substances 0.000 description 2
- 229910052623 talc Inorganic materials 0.000 description 2
- 241000193163 Clostridioides difficile Species 0.000 description 1
- 241000193403 Clostridium Species 0.000 description 1
- 108010013198 Daptomycin Proteins 0.000 description 1
- 108010028921 Lipopeptides Proteins 0.000 description 1
- 239000005434 MCC/mannitol excipient Substances 0.000 description 1
- 230000010933 acylation Effects 0.000 description 1
- 238000005917 acylation reaction Methods 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 239000000498 cooling water Substances 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- DOAKLVKFURWEDJ-QCMAZARJSA-N daptomycin Chemical compound C([C@H]1C(=O)O[C@H](C)[C@@H](C(NCC(=O)N[C@@H](CCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@H](CO)C(=O)N[C@H](C(=O)N1)[C@H](C)CC(O)=O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)CCCCCCCCC)C(=O)C1=CC=CC=C1N DOAKLVKFURWEDJ-QCMAZARJSA-N 0.000 description 1
- 229960005484 daptomycin Drugs 0.000 description 1
- 230000020176 deacylation Effects 0.000 description 1
- 238000005947 deacylation reaction Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000010511 deprotection reaction Methods 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 239000011361 granulated particle Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 238000005461 lubrication Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 1
- 238000009522 phase III clinical trial Methods 0.000 description 1
- 238000005086 pumping Methods 0.000 description 1
- 238000012958 reprocessing Methods 0.000 description 1
- 238000007873 sieving Methods 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 125000000185 sucrose group Chemical group 0.000 description 1
- -1 sucrose) at pH 2-7 Chemical class 0.000 description 1
- 239000007916 tablet composition Substances 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 239000003643 water by type Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/12—Cyclic peptides, e.g. bacitracins; Polymyxins; Gramicidins S, C; Tyrocidins A, B or C
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
- A61K47/38—Cellulose; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/145—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/146—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with organic macromolecular compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
- A61K9/2018—Sugars, or sugar alcohols, e.g. lactose, mannitol; Derivatives thereof, e.g. polysorbates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/205—Polysaccharides, e.g. alginate, gums; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Inorganic Chemistry (AREA)
- Immunology (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Saccharide Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161490584P | 2011-05-26 | 2011-05-26 | |
| US61/490,584 | 2011-05-26 | ||
| PCT/US2012/039476 WO2012162567A1 (en) | 2011-05-26 | 2012-05-24 | Cb-183,315 compositions and related methods |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20140037877A true KR20140037877A (ko) | 2014-03-27 |
Family
ID=46208839
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020137033768A Withdrawn KR20140037877A (ko) | 2011-05-26 | 2012-05-24 | Cb-183,315 조성물 및 관련 방법 |
Country Status (12)
| Country | Link |
|---|---|
| US (2) | US20130109633A1 (enExample) |
| EP (1) | EP2714012A1 (enExample) |
| JP (1) | JP2014515371A (enExample) |
| KR (1) | KR20140037877A (enExample) |
| CN (1) | CN103687589A (enExample) |
| AR (1) | AR086576A1 (enExample) |
| BR (1) | BR112013030369A2 (enExample) |
| CA (1) | CA2837174A1 (enExample) |
| MX (1) | MX2013013760A (enExample) |
| RU (1) | RU2013157188A (enExample) |
| TW (1) | TW201300124A (enExample) |
| WO (1) | WO2012162567A1 (enExample) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109476704B (zh) * | 2016-04-08 | 2022-08-16 | 港大科桥有限公司 | 抗菌环脂肽 |
| US11667674B2 (en) | 2016-04-08 | 2023-06-06 | Versitech Limited | Antibacterial cyclic lipopeptides |
| US10647746B2 (en) | 2016-04-08 | 2020-05-12 | Versitech Limited | Antibacterial cyclic lipopeptides |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001044271A2 (en) * | 1999-12-15 | 2001-06-21 | Cubist Pharmaceuticals, Inc. | Daptomycin analogs and their use as antibacterial agents |
| CA2413251C (en) * | 2000-06-21 | 2012-06-12 | Cubist Pharmaceuticals, Inc. | Compositions and methods to improve the oral absorption of antimicrobial agents |
| AU2002246688A1 (en) * | 2000-12-18 | 2002-07-30 | Altus Biologics Inc. | Methods for preparing purified daptomycin |
| EP1814588A2 (en) * | 2004-11-12 | 2007-08-08 | Cubist Pharmaceuticals, Inc. | Antiinfective lipopeptides |
| US8158152B2 (en) * | 2005-11-18 | 2012-04-17 | Scidose Llc | Lyophilization process and products obtained thereby |
| RU2512396C2 (ru) * | 2008-12-22 | 2014-04-10 | Кьюбист Фармасьютикалз, Инк. | Новые противобактериальные средства для лечения грамположительных инфекций |
| JP5491119B2 (ja) * | 2009-10-02 | 2014-05-14 | 日東電工株式会社 | 薬物含有微粒子を含む医薬組成物およびその製造方法 |
| CN102060914B (zh) * | 2009-11-13 | 2014-09-17 | 华东理工大学 | 海洋芽孢杆菌b-9987产脂肽类化合物及其制备和应用 |
| BR112012012406B1 (pt) * | 2009-11-23 | 2021-11-16 | Cubist Pharmaceuticals Llc | Composições farmacêuticas sólidas de daptomicina, seu produto farmacêutico e seus métodos de fabricação |
| US20110124551A1 (en) * | 2009-11-23 | 2011-05-26 | Eagle Pharmaceuticals, Inc. | Formulations of daptomycin |
-
2012
- 2012-05-24 RU RU2013157188/15A patent/RU2013157188A/ru not_active Application Discontinuation
- 2012-05-24 MX MX2013013760A patent/MX2013013760A/es unknown
- 2012-05-24 EP EP12725988.5A patent/EP2714012A1/en not_active Withdrawn
- 2012-05-24 BR BR112013030369A patent/BR112013030369A2/pt not_active IP Right Cessation
- 2012-05-24 KR KR1020137033768A patent/KR20140037877A/ko not_active Withdrawn
- 2012-05-24 CA CA2837174A patent/CA2837174A1/en not_active Abandoned
- 2012-05-24 WO PCT/US2012/039476 patent/WO2012162567A1/en not_active Ceased
- 2012-05-24 JP JP2014512126A patent/JP2014515371A/ja active Pending
- 2012-05-24 CN CN201280025551.2A patent/CN103687589A/zh active Pending
- 2012-05-25 US US13/481,009 patent/US20130109633A1/en not_active Abandoned
- 2012-05-25 TW TW101118841A patent/TW201300124A/zh unknown
- 2012-05-28 AR ARP120101868A patent/AR086576A1/es unknown
-
2013
- 2013-10-07 US US14/047,701 patent/US20140135273A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| RU2013157188A (ru) | 2015-07-10 |
| TW201300124A (zh) | 2013-01-01 |
| MX2013013760A (es) | 2014-01-08 |
| BR112013030369A2 (pt) | 2016-12-13 |
| US20140135273A1 (en) | 2014-05-15 |
| US20130109633A1 (en) | 2013-05-02 |
| CA2837174A1 (en) | 2012-11-29 |
| AR086576A1 (es) | 2014-01-08 |
| CN103687589A (zh) | 2014-03-26 |
| EP2714012A1 (en) | 2014-04-09 |
| WO2012162567A1 (en) | 2012-11-29 |
| JP2014515371A (ja) | 2014-06-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102803917B1 (ko) | 루카파립의 고 용량 강도 정제 | |
| EP3606511B1 (en) | Pharmaceutical composition comprising lenvatinib mesylate | |
| HU202100B (en) | Process for producing stabile pharmaceutical compositions containing heterocyclic peptide derivatives as active components | |
| KR101324862B1 (ko) | 클로피도그렐 황산수소염의 구형 입자, 이를 포함하는 약학적 조성물 및 이의 제조방법 | |
| EP2162119A2 (en) | Stable pharmaceutical formulation for a dpp-iv inhibitor | |
| KR20100015764A (ko) | 프레가발린을 포함하는 안정한 약학적 조성물 | |
| KR101977785B1 (ko) | 에제티미브 및 로수바스타틴을 포함하는 경구용 복합제제 및 그 제조방법 | |
| KR20100137572A (ko) | 발데나필 하이드로클로라이드 삼수화물을 포함하는 약물 | |
| EP4025192B1 (en) | Process for producing a tablet comprising glp-1 peptides | |
| EP2197428B1 (en) | Improved pharmaceutical composition containing a pyrrolidone anticonvulsant agent and method for the preparation thereof | |
| KR20140037877A (ko) | Cb-183,315 조성물 및 관련 방법 | |
| KR101197277B1 (ko) | 경구용 결핵의 치료용 또는 예방용 고형 제형 | |
| KR20160002177A (ko) | 오셀타미비어 유리염기를 포함하는 약학 조성물 | |
| EP4436554A1 (en) | Pharmaceutical compositions comprising eltrombopag | |
| KR20120121944A (ko) | 안정성이 개선된 리마프로스트 함유 약제학적 조성물 및 이의 제조방법 | |
| EP2906203B1 (en) | Effervescent cefdinir formulation | |
| KR20080112387A (ko) | 2-아자-비사이클로[3.3.0]-옥탄-3-카르복실산 유도체의 안정한 약학 조성물 | |
| KR101244627B1 (ko) | 올란자핀을 함유하는 확산정 조성물 및 이에 의해 형성된 정제 | |
| JP2022130003A (ja) | 漢方エキス又は植物性生薬エキスを含む固形製剤及びその製造方法、並びに固形製剤の崩壊性を向上させる方法 | |
| KR102102462B1 (ko) | 오셀타미비어 유리염기를 포함하는 약학 조성물 | |
| KR20210073794A (ko) | 생체이용률이 향상된 피오글리타존 함유 약학조성물 | |
| EP4410278A1 (en) | Pharmaceutical composition comprising enavogliflozin | |
| KR100653388B1 (ko) | 안정성이 우수한 ace 저해제를 포함하는 약제학적 조성물 | |
| KR101172539B1 (ko) | 발데나필 하이드로클로라이드 삼수화물을 포함하는 약물 | |
| KR100561025B1 (ko) | 직접 타정법에 의해 제조되며 보관안정성이 증진된 라미프릴 정제 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20131219 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| N231 | Notification of change of applicant | ||
| PN2301 | Change of applicant |
Patent event date: 20160119 Comment text: Notification of Change of Applicant Patent event code: PN23011R01D |
|
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |