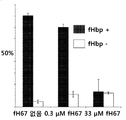
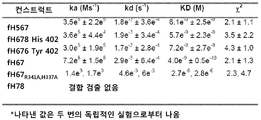
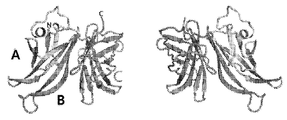
KR20110092282A - 돌연변이 인자 h 결합 단백질을 포함하는 백신 조성물 - Google Patents
돌연변이 인자 h 결합 단백질을 포함하는 백신 조성물 Download PDFInfo
- Publication number
- KR20110092282A KR20110092282A KR1020117011885A KR20117011885A KR20110092282A KR 20110092282 A KR20110092282 A KR 20110092282A KR 1020117011885 A KR1020117011885 A KR 1020117011885A KR 20117011885 A KR20117011885 A KR 20117011885A KR 20110092282 A KR20110092282 A KR 20110092282A
- Authority
- KR
- South Korea
- Prior art keywords
- factor
- composition
- binding protein
- binding
- immune response
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 101710186862 Factor H binding protein Proteins 0.000 title claims abstract description 143
- 239000000203 mixture Substances 0.000 title claims abstract description 70
- 229960005486 vaccine Drugs 0.000 title claims description 22
- 230000028993 immune response Effects 0.000 claims abstract description 40
- 230000002163 immunogen Effects 0.000 claims abstract description 11
- 102000016550 Complement Factor H Human genes 0.000 claims description 61
- 108010053085 Complement Factor H Proteins 0.000 claims description 61
- 241000588650 Neisseria meningitidis Species 0.000 claims description 54
- 108090000623 proteins and genes Proteins 0.000 claims description 45
- 102000004169 proteins and genes Human genes 0.000 claims description 44
- 150000001413 amino acids Chemical class 0.000 claims description 26
- 230000001681 protective effect Effects 0.000 claims description 14
- 238000000034 method Methods 0.000 claims description 13
- 208000015181 infectious disease Diseases 0.000 claims description 11
- 241000588653 Neisseria Species 0.000 claims description 9
- 230000003053 immunization Effects 0.000 claims description 9
- 238000002649 immunization Methods 0.000 claims description 9
- 201000009906 Meningitis Diseases 0.000 claims description 8
- 230000000069 prophylactic effect Effects 0.000 claims description 8
- 230000002829 reductive effect Effects 0.000 claims description 8
- 239000003814 drug Substances 0.000 claims description 7
- 239000008194 pharmaceutical composition Substances 0.000 claims description 7
- 230000001225 therapeutic effect Effects 0.000 claims description 6
- 206010040070 Septic Shock Diseases 0.000 claims description 5
- 230000000694 effects Effects 0.000 claims description 5
- 238000004519 manufacturing process Methods 0.000 claims description 5
- 230000036303 septic shock Effects 0.000 claims description 5
- 206010040047 Sepsis Diseases 0.000 claims description 3
- 125000000539 amino acid group Chemical group 0.000 claims description 3
- 230000002401 inhibitory effect Effects 0.000 claims description 3
- 238000011081 inoculation Methods 0.000 claims description 3
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 3
- 229940021993 prophylactic vaccine Drugs 0.000 claims description 3
- 229940021747 therapeutic vaccine Drugs 0.000 claims description 3
- 241000588652 Neisseria gonorrhoeae Species 0.000 claims description 2
- 239000003937 drug carrier Substances 0.000 claims description 2
- 230000001939 inductive effect Effects 0.000 claims description 2
- 125000003275 alpha amino acid group Chemical group 0.000 claims 1
- 235000018102 proteins Nutrition 0.000 description 39
- 230000003993 interaction Effects 0.000 description 24
- 235000001014 amino acid Nutrition 0.000 description 21
- 229940024606 amino acid Drugs 0.000 description 19
- 230000035772 mutation Effects 0.000 description 18
- 102100032406 Cytosolic carboxypeptidase 6 Human genes 0.000 description 12
- 101000868785 Homo sapiens Cytosolic carboxypeptidase 6 Proteins 0.000 description 12
- 210000002966 serum Anatomy 0.000 description 11
- 239000000427 antigen Substances 0.000 description 10
- 102000036639 antigens Human genes 0.000 description 10
- 108091007433 antigens Proteins 0.000 description 10
- 241000894006 Bacteria Species 0.000 description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 8
- 230000000295 complement effect Effects 0.000 description 8
- 239000013078 crystal Substances 0.000 description 8
- 101710185445 Cytochrome c peroxidase, mitochondrial Proteins 0.000 description 7
- 230000000844 anti-bacterial effect Effects 0.000 description 7
- 201000010099 disease Diseases 0.000 description 7
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 7
- 229920002451 polyvinyl alcohol Polymers 0.000 description 7
- 239000002671 adjuvant Substances 0.000 description 6
- 238000010790 dilution Methods 0.000 description 6
- 239000012895 dilution Substances 0.000 description 6
- 239000012528 membrane Substances 0.000 description 6
- 230000004044 response Effects 0.000 description 6
- 230000008859 change Effects 0.000 description 5
- 230000014509 gene expression Effects 0.000 description 5
- 238000003780 insertion Methods 0.000 description 5
- 230000037431 insertion Effects 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- 238000006467 substitution reaction Methods 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 4
- 241000699670 Mus sp. Species 0.000 description 4
- 239000012491 analyte Substances 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 238000010494 dissociation reaction Methods 0.000 description 4
- 230000005593 dissociations Effects 0.000 description 4
- 230000001965 increasing effect Effects 0.000 description 4
- 244000052769 pathogen Species 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 230000002441 reversible effect Effects 0.000 description 4
- 150000003839 salts Chemical class 0.000 description 4
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 4
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 230000004075 alteration Effects 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- 239000001257 hydrogen Substances 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 230000001404 mediated effect Effects 0.000 description 3
- 230000010076 replication Effects 0.000 description 3
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- 238000001262 western blot Methods 0.000 description 3
- DKJPOZOEBONHFS-ZLUOBGJFSA-N Ala-Ala-Asp Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC(O)=O DKJPOZOEBONHFS-ZLUOBGJFSA-N 0.000 description 2
- MEFILNJXAVSUTO-JXUBOQSCSA-N Ala-Leu-Thr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MEFILNJXAVSUTO-JXUBOQSCSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 102000008300 Mutant Proteins Human genes 0.000 description 2
- 108010021466 Mutant Proteins Proteins 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 235000004279 alanine Nutrition 0.000 description 2
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 230000006472 autoimmune response Effects 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 210000004027 cell Anatomy 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 230000024203 complement activation Effects 0.000 description 2
- 230000009089 cytolysis Effects 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 229910003460 diamond Inorganic materials 0.000 description 2
- 239000010432 diamond Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- 230000005847 immunogenicity Effects 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 208000037909 invasive meningococcal disease Diseases 0.000 description 2
- 208000037941 meningococcal disease Diseases 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 235000013336 milk Nutrition 0.000 description 2
- 239000008267 milk Substances 0.000 description 2
- 210000004080 milk Anatomy 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 239000012452 mother liquor Substances 0.000 description 2
- 238000004806 packaging method and process Methods 0.000 description 2
- 230000001717 pathogenic effect Effects 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 238000001542 size-exclusion chromatography Methods 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 239000011593 sulfur Substances 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 238000011282 treatment Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 125000003287 1H-imidazol-4-ylmethyl group Chemical group [H]N1C([H])=NC(C([H])([H])[*])=C1[H] 0.000 description 1
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- BUANFPRKJKJSRR-ACZMJKKPSA-N Ala-Ala-Gln Chemical compound C[C@H]([NH3+])C(=O)N[C@@H](C)C(=O)N[C@H](C([O-])=O)CCC(N)=O BUANFPRKJKJSRR-ACZMJKKPSA-N 0.000 description 1
- FJVAQLJNTSUQPY-CIUDSAMLSA-N Ala-Ala-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCCN FJVAQLJNTSUQPY-CIUDSAMLSA-N 0.000 description 1
- OILNWMNBLIHXQK-ZLUOBGJFSA-N Ala-Cys-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(O)=O OILNWMNBLIHXQK-ZLUOBGJFSA-N 0.000 description 1
- HMRWQTHUDVXMGH-GUBZILKMSA-N Ala-Glu-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCCN HMRWQTHUDVXMGH-GUBZILKMSA-N 0.000 description 1
- KYDYGANDJHFBCW-DRZSPHRISA-N Ala-Phe-Gln Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N KYDYGANDJHFBCW-DRZSPHRISA-N 0.000 description 1
- DHBKYZYFEXXUAK-ONGXEEELSA-N Ala-Phe-Gly Chemical compound OC(=O)CNC(=O)[C@@H](NC(=O)[C@@H](N)C)CC1=CC=CC=C1 DHBKYZYFEXXUAK-ONGXEEELSA-N 0.000 description 1
- ADSGHMXEAZJJNF-DCAQKATOSA-N Ala-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N ADSGHMXEAZJJNF-DCAQKATOSA-N 0.000 description 1
- CREYEAPXISDKSB-FQPOAREZSA-N Ala-Thr-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O CREYEAPXISDKSB-FQPOAREZSA-N 0.000 description 1
- JCAISGGAOQXEHJ-ZPFDUUQYSA-N Arg-Gln-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCCN=C(N)N)N JCAISGGAOQXEHJ-ZPFDUUQYSA-N 0.000 description 1
- KRQSPVKUISQQFS-FJXKBIBVSA-N Arg-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCN=C(N)N KRQSPVKUISQQFS-FJXKBIBVSA-N 0.000 description 1
- SLNCSSWAIDUUGF-LSJOCFKGSA-N Arg-His-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](C)C(O)=O SLNCSSWAIDUUGF-LSJOCFKGSA-N 0.000 description 1
- SSZGOKWBHLOCHK-DCAQKATOSA-N Arg-Lys-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCN=C(N)N SSZGOKWBHLOCHK-DCAQKATOSA-N 0.000 description 1
- KSUALAGYYLQSHJ-RCWTZXSCSA-N Arg-Met-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KSUALAGYYLQSHJ-RCWTZXSCSA-N 0.000 description 1
- JOTRDIXZHNQYGP-DCAQKATOSA-N Arg-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCCN=C(N)N)N JOTRDIXZHNQYGP-DCAQKATOSA-N 0.000 description 1
- ASQKVGRCKOFKIU-KZVJFYERSA-N Arg-Thr-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O ASQKVGRCKOFKIU-KZVJFYERSA-N 0.000 description 1
- QISZHYWZHJRDAO-CIUDSAMLSA-N Asn-Asp-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC(=O)N)N QISZHYWZHJRDAO-CIUDSAMLSA-N 0.000 description 1
- FMNBYVSGRCXWEK-FOHZUACHSA-N Asn-Thr-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O FMNBYVSGRCXWEK-FOHZUACHSA-N 0.000 description 1
- NAPNAGZWHQHZLG-ZLUOBGJFSA-N Asp-Asp-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CC(=O)O)N NAPNAGZWHQHZLG-ZLUOBGJFSA-N 0.000 description 1
- PZXPWHFYZXTFBI-YUMQZZPRSA-N Asp-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC(O)=O PZXPWHFYZXTFBI-YUMQZZPRSA-N 0.000 description 1
- ODNWIBOCFGMRTP-SRVKXCTJSA-N Asp-His-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(O)=O)CC1=CN=CN1 ODNWIBOCFGMRTP-SRVKXCTJSA-N 0.000 description 1
- LBOVBQONZJRWPV-YUMQZZPRSA-N Asp-Lys-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O LBOVBQONZJRWPV-YUMQZZPRSA-N 0.000 description 1
- QSFHZPQUAAQHAQ-CIUDSAMLSA-N Asp-Ser-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O QSFHZPQUAAQHAQ-CIUDSAMLSA-N 0.000 description 1
- 108010077805 Bacterial Proteins Proteins 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 102000000989 Complement System Proteins Human genes 0.000 description 1
- 108010069112 Complement System Proteins Proteins 0.000 description 1
- 102000003712 Complement factor B Human genes 0.000 description 1
- 108090000056 Complement factor B Proteins 0.000 description 1
- 102100025698 Cytosolic carboxypeptidase 4 Human genes 0.000 description 1
- 102100025717 Cytosolic carboxypeptidase-like protein 5 Human genes 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 241001198387 Escherichia coli BL21(DE3) Species 0.000 description 1
- WLODHVXYKYHLJD-ACZMJKKPSA-N Gln-Asp-Ser Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CO)C(=O)O)N WLODHVXYKYHLJD-ACZMJKKPSA-N 0.000 description 1
- XKBASPWPBXNVLQ-WDSKDSINSA-N Gln-Gly-Asn Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O XKBASPWPBXNVLQ-WDSKDSINSA-N 0.000 description 1
- SFAFZYYMAWOCIC-KKUMJFAQSA-N Gln-Phe-Arg Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N SFAFZYYMAWOCIC-KKUMJFAQSA-N 0.000 description 1
- LPIKVBWNNVFHCQ-GUBZILKMSA-N Gln-Ser-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O LPIKVBWNNVFHCQ-GUBZILKMSA-N 0.000 description 1
- OTQSTOXRUBVWAP-NRPADANISA-N Gln-Ser-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O OTQSTOXRUBVWAP-NRPADANISA-N 0.000 description 1
- UMIRPYLZFKOEOH-YVNDNENWSA-N Glu-Gln-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O UMIRPYLZFKOEOH-YVNDNENWSA-N 0.000 description 1
- DVLZZEPUNFEUBW-AVGNSLFASA-N Glu-His-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCC(=O)O)N DVLZZEPUNFEUBW-AVGNSLFASA-N 0.000 description 1
- WVTIBGWZUMJBFY-GUBZILKMSA-N Glu-His-Ser Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CO)C(O)=O WVTIBGWZUMJBFY-GUBZILKMSA-N 0.000 description 1
- WDTAKCUOIKHCTB-NKIYYHGXSA-N Glu-His-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCC(=O)O)N)O WDTAKCUOIKHCTB-NKIYYHGXSA-N 0.000 description 1
- VMKCPNBBPGGQBJ-GUBZILKMSA-N Glu-Leu-Asn Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)O)N VMKCPNBBPGGQBJ-GUBZILKMSA-N 0.000 description 1
- ILWHFUZZCFYSKT-AVGNSLFASA-N Glu-Lys-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O ILWHFUZZCFYSKT-AVGNSLFASA-N 0.000 description 1
- DCBSZJJHOTXMHY-DCAQKATOSA-N Glu-Pro-Pro Chemical compound OC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 DCBSZJJHOTXMHY-DCAQKATOSA-N 0.000 description 1
- RFTVTKBHDXCEEX-WDSKDSINSA-N Glu-Ser-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)NCC(O)=O RFTVTKBHDXCEEX-WDSKDSINSA-N 0.000 description 1
- KIEICAOUSNYOLM-NRPADANISA-N Glu-Val-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O KIEICAOUSNYOLM-NRPADANISA-N 0.000 description 1
- YPHPEHMXOYTEQG-LAEOZQHASA-N Glu-Val-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCC(O)=O YPHPEHMXOYTEQG-LAEOZQHASA-N 0.000 description 1
- MFVQGXGQRIXBPK-WDSKDSINSA-N Gly-Ala-Glu Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O MFVQGXGQRIXBPK-WDSKDSINSA-N 0.000 description 1
- GGEJHJIXRBTJPD-BYPYZUCNSA-N Gly-Asn-Gly Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O GGEJHJIXRBTJPD-BYPYZUCNSA-N 0.000 description 1
- LXXANCRPFBSSKS-IUCAKERBSA-N Gly-Gln-Leu Chemical compound [H]NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O LXXANCRPFBSSKS-IUCAKERBSA-N 0.000 description 1
- HQRHFUYMGCHHJS-LURJTMIESA-N Gly-Gly-Arg Chemical compound NCC(=O)NCC(=O)N[C@H](C(O)=O)CCCN=C(N)N HQRHFUYMGCHHJS-LURJTMIESA-N 0.000 description 1
- QITBQGJOXQYMOA-ZETCQYMHSA-N Gly-Gly-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)CNC(=O)CN QITBQGJOXQYMOA-ZETCQYMHSA-N 0.000 description 1
- OLPPXYMMIARYAL-QMMMGPOBSA-N Gly-Gly-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)CNC(=O)CN OLPPXYMMIARYAL-QMMMGPOBSA-N 0.000 description 1
- PTIIBFKSLCYQBO-NHCYSSNCSA-N Gly-Lys-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)CN PTIIBFKSLCYQBO-NHCYSSNCSA-N 0.000 description 1
- PCPOYRCAHPJXII-UWVGGRQHSA-N Gly-Lys-Met Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCSC)C(O)=O PCPOYRCAHPJXII-UWVGGRQHSA-N 0.000 description 1
- IRJWAYCXIYUHQE-WHFBIAKZSA-N Gly-Ser-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)CN IRJWAYCXIYUHQE-WHFBIAKZSA-N 0.000 description 1
- FKYQEVBRZSFAMJ-QWRGUYRKSA-N Gly-Ser-Tyr Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 FKYQEVBRZSFAMJ-QWRGUYRKSA-N 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- FPNWKONEZAVQJF-GUBZILKMSA-N His-Asn-Gln Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N FPNWKONEZAVQJF-GUBZILKMSA-N 0.000 description 1
- BXOLYFJYQQRQDJ-MXAVVETBSA-N His-Leu-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1=CN=CN1)N BXOLYFJYQQRQDJ-MXAVVETBSA-N 0.000 description 1
- 101000932585 Homo sapiens Cytosolic carboxypeptidase-like protein 5 Proteins 0.000 description 1
- AQCUAZTZSPQJFF-ZKWXMUAHSA-N Ile-Ala-Gly Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C)C(=O)NCC(O)=O AQCUAZTZSPQJFF-ZKWXMUAHSA-N 0.000 description 1
- LVQDUPQUJZWKSU-PYJNHQTQSA-N Ile-Arg-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N LVQDUPQUJZWKSU-PYJNHQTQSA-N 0.000 description 1
- RPZFUIQVAPZLRH-GHCJXIJMSA-N Ile-Asp-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](C)C(=O)O)N RPZFUIQVAPZLRH-GHCJXIJMSA-N 0.000 description 1
- NZOCIWKZUVUNDW-ZKWXMUAHSA-N Ile-Gly-Ala Chemical compound CC[C@H](C)[C@H](N)C(=O)NCC(=O)N[C@@H](C)C(O)=O NZOCIWKZUVUNDW-ZKWXMUAHSA-N 0.000 description 1
- KFVUBLZRFSVDGO-BYULHYEWSA-N Ile-Gly-Asp Chemical compound CC[C@H](C)[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(O)=O KFVUBLZRFSVDGO-BYULHYEWSA-N 0.000 description 1
- NYEYYMLUABXDMC-NHCYSSNCSA-N Ile-Gly-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)O)N NYEYYMLUABXDMC-NHCYSSNCSA-N 0.000 description 1
- GLYJPWIRLBAIJH-FQUUOJAGSA-N Ile-Lys-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)O)N GLYJPWIRLBAIJH-FQUUOJAGSA-N 0.000 description 1
- GLYJPWIRLBAIJH-UHFFFAOYSA-N Ile-Lys-Pro Natural products CCC(C)C(N)C(=O)NC(CCCCN)C(=O)N1CCCC1C(O)=O GLYJPWIRLBAIJH-UHFFFAOYSA-N 0.000 description 1
- ZLFNNVATRMCAKN-ZKWXMUAHSA-N Ile-Ser-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)NCC(=O)O)N ZLFNNVATRMCAKN-ZKWXMUAHSA-N 0.000 description 1
- KBDIBHQICWDGDL-PPCPHDFISA-N Ile-Thr-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)O)N KBDIBHQICWDGDL-PPCPHDFISA-N 0.000 description 1
- 108010065920 Insulin Lispro Proteins 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ZRLUISBDKUWAIZ-CIUDSAMLSA-N Leu-Ala-Asp Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC(O)=O ZRLUISBDKUWAIZ-CIUDSAMLSA-N 0.000 description 1
- CCQLQKZTXZBXTN-NHCYSSNCSA-N Leu-Gly-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(O)=O CCQLQKZTXZBXTN-NHCYSSNCSA-N 0.000 description 1
- KUIDCYNIEJBZBU-AJNGGQMLSA-N Leu-Ile-Leu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O KUIDCYNIEJBZBU-AJNGGQMLSA-N 0.000 description 1
- VULJUQZPSOASBZ-SRVKXCTJSA-N Leu-Pro-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O VULJUQZPSOASBZ-SRVKXCTJSA-N 0.000 description 1
- MVHXGBZUJLWZOH-BJDJZHNGSA-N Leu-Ser-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O MVHXGBZUJLWZOH-BJDJZHNGSA-N 0.000 description 1
- XOWMDXHFSBCAKQ-SRVKXCTJSA-N Leu-Ser-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C XOWMDXHFSBCAKQ-SRVKXCTJSA-N 0.000 description 1
- HGLKOTPFWOMPOB-MEYUZBJRSA-N Leu-Thr-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HGLKOTPFWOMPOB-MEYUZBJRSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- JCFYLFOCALSNLQ-GUBZILKMSA-N Lys-Ala-Gln Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(O)=O JCFYLFOCALSNLQ-GUBZILKMSA-N 0.000 description 1
- GGAPIOORBXHMNY-ULQDDVLXSA-N Lys-Arg-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCCN)N)O GGAPIOORBXHMNY-ULQDDVLXSA-N 0.000 description 1
- RBEATVHTWHTHTJ-KKUMJFAQSA-N Lys-Leu-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O RBEATVHTWHTHTJ-KKUMJFAQSA-N 0.000 description 1
- WZVSHTFTCYOFPL-GARJFASQSA-N Lys-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CCCCN)N)C(=O)O WZVSHTFTCYOFPL-GARJFASQSA-N 0.000 description 1
- YFQSSOAGMZGXFT-MEYUZBJRSA-N Lys-Thr-Tyr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O YFQSSOAGMZGXFT-MEYUZBJRSA-N 0.000 description 1
- 206010027202 Meningitis bacterial Diseases 0.000 description 1
- 208000034762 Meningococcal Infections Diseases 0.000 description 1
- NHXXGBXJTLRGJI-GUBZILKMSA-N Met-Pro-Ser Chemical compound [H]N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O NHXXGBXJTLRGJI-GUBZILKMSA-N 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- FSVCELGFZIQNCK-UHFFFAOYSA-N N,N-bis(2-hydroxyethyl)glycine Chemical compound OCCN(CCO)CC(O)=O FSVCELGFZIQNCK-UHFFFAOYSA-N 0.000 description 1
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 1
- 241000897304 Neisseria meningitidis H44/76 Species 0.000 description 1
- 102000005608 Neuronal Cell Adhesion Molecules Human genes 0.000 description 1
- 108010059604 Neuronal Cell Adhesion Molecules Proteins 0.000 description 1
- 101100342977 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) leu-1 gene Proteins 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 102000003992 Peroxidases Human genes 0.000 description 1
- BJEYSVHMGIJORT-NHCYSSNCSA-N Phe-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=CC=C1 BJEYSVHMGIJORT-NHCYSSNCSA-N 0.000 description 1
- UHRNIXJAGGLKHP-DLOVCJGASA-N Phe-Ala-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O UHRNIXJAGGLKHP-DLOVCJGASA-N 0.000 description 1
- DJPXNKUDJKGQEE-BZSNNMDCSA-N Phe-Asp-Phe Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O DJPXNKUDJKGQEE-BZSNNMDCSA-N 0.000 description 1
- AEEQKUDWJGOFQI-SRVKXCTJSA-N Phe-Cys-Cys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)O)N AEEQKUDWJGOFQI-SRVKXCTJSA-N 0.000 description 1
- MFQXSDWKUXTOPZ-DZKIICNBSA-N Phe-Gln-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC1=CC=CC=C1)N MFQXSDWKUXTOPZ-DZKIICNBSA-N 0.000 description 1
- RFEXGCASCQGGHZ-STQMWFEESA-N Phe-Gly-Arg Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O RFEXGCASCQGGHZ-STQMWFEESA-N 0.000 description 1
- NAXPHWZXEXNDIW-JTQLQIEISA-N Phe-Gly-Gly Chemical compound OC(=O)CNC(=O)CNC(=O)[C@@H](N)CC1=CC=CC=C1 NAXPHWZXEXNDIW-JTQLQIEISA-N 0.000 description 1
- 241000404883 Pisa Species 0.000 description 1
- ZAUHSLVPDLNTRZ-QXEWZRGKSA-N Pro-Val-Asn Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O ZAUHSLVPDLNTRZ-QXEWZRGKSA-N 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 1
- CAOYHZOWXFFAIR-CIUDSAMLSA-N Ser-His-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CO)C(O)=O CAOYHZOWXFFAIR-CIUDSAMLSA-N 0.000 description 1
- UGTZYIPOBYXWRW-SRVKXCTJSA-N Ser-Phe-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(O)=O)C(O)=O UGTZYIPOBYXWRW-SRVKXCTJSA-N 0.000 description 1
- YEDSOSIKVUMIJE-DCAQKATOSA-N Ser-Val-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O YEDSOSIKVUMIJE-DCAQKATOSA-N 0.000 description 1
- 235000002597 Solanum melongena Nutrition 0.000 description 1
- 244000061458 Solanum melongena Species 0.000 description 1
- 239000012505 Superdex™ Substances 0.000 description 1
- ASJDFGOPDCVXTG-KATARQTJSA-N Thr-Cys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O ASJDFGOPDCVXTG-KATARQTJSA-N 0.000 description 1
- CRZNCABIJLRFKZ-IUKAMOBKSA-N Thr-Ile-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N CRZNCABIJLRFKZ-IUKAMOBKSA-N 0.000 description 1
- RRRRCRYTLZVCEN-HJGDQZAQSA-N Thr-Leu-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O RRRRCRYTLZVCEN-HJGDQZAQSA-N 0.000 description 1
- NDZYTIMDOZMECO-SHGPDSBTSA-N Thr-Thr-Ala Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O NDZYTIMDOZMECO-SHGPDSBTSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- MBFJIHUHHCJBSN-AVGNSLFASA-N Tyr-Asn-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O MBFJIHUHHCJBSN-AVGNSLFASA-N 0.000 description 1
- BYAKMYBZADCNMN-JYJNAYRXSA-N Tyr-Lys-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O BYAKMYBZADCNMN-JYJNAYRXSA-N 0.000 description 1
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 1
- RUCNAYOMFXRIKJ-DCAQKATOSA-N Val-Ala-Lys Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCCN RUCNAYOMFXRIKJ-DCAQKATOSA-N 0.000 description 1
- UDNYEPLJTRDMEJ-RCOVLWMOSA-N Val-Asn-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)NCC(=O)O)N UDNYEPLJTRDMEJ-RCOVLWMOSA-N 0.000 description 1
- XIFAHCUNWWKUDE-DCAQKATOSA-N Val-Cys-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)O)N XIFAHCUNWWKUDE-DCAQKATOSA-N 0.000 description 1
- XPKCFQZDQGVJCX-RHYQMDGZSA-N Val-Lys-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C(C)C)N)O XPKCFQZDQGVJCX-RHYQMDGZSA-N 0.000 description 1
- AJNUKMZFHXUBMK-GUBZILKMSA-N Val-Ser-Arg Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N AJNUKMZFHXUBMK-GUBZILKMSA-N 0.000 description 1
- GTACFKZDQFTVAI-STECZYCISA-N Val-Tyr-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)CC1=CC=C(O)C=C1 GTACFKZDQFTVAI-STECZYCISA-N 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 238000001261 affinity purification Methods 0.000 description 1
- 206010064930 age-related macular degeneration Diseases 0.000 description 1
- 108010076324 alanyl-glycyl-glycine Proteins 0.000 description 1
- 108010024078 alanyl-glycyl-serine Proteins 0.000 description 1
- 108010005233 alanylglutamic acid Proteins 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 230000002547 anomalous effect Effects 0.000 description 1
- 230000002421 anti-septic effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000005875 antibody response Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 1
- 108010047857 aspartylglycine Proteins 0.000 description 1
- 108010092854 aspartyllysine Proteins 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 201000009904 bacterial meningitis Diseases 0.000 description 1
- 238000002869 basic local alignment search tool Methods 0.000 description 1
- 239000007998 bicine buffer Substances 0.000 description 1
- 230000008033 biological extinction Effects 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 230000001332 colony forming effect Effects 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000011437 continuous method Methods 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 238000013480 data collection Methods 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 239000000032 diagnostic agent Substances 0.000 description 1
- 229940039227 diagnostic agent Drugs 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 230000009881 electrostatic interaction Effects 0.000 description 1
- 230000013020 embryo development Effects 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000000684 flow cytometry Methods 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 108010006664 gamma-glutamyl-glycyl-glycine Proteins 0.000 description 1
- 102000034356 gene-regulatory proteins Human genes 0.000 description 1
- 108091006104 gene-regulatory proteins Proteins 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 108010019832 glycyl-asparaginyl-glycine Proteins 0.000 description 1
- 108010001064 glycyl-glycyl-glycyl-glycine Proteins 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 235000014304 histidine Nutrition 0.000 description 1
- 150000002411 histidines Chemical class 0.000 description 1
- 125000001165 hydrophobic group Chemical group 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 210000003000 inclusion body Anatomy 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 230000015788 innate immune response Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- BPHPUYQFMNQIOC-NXRLNHOXSA-N isopropyl beta-D-thiogalactopyranoside Chemical compound CC(C)S[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O BPHPUYQFMNQIOC-NXRLNHOXSA-N 0.000 description 1
- 238000000111 isothermal titration calorimetry Methods 0.000 description 1
- 125000001909 leucine group Chemical class [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 108010034529 leucyl-lysine Proteins 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 108010003700 lysyl aspartic acid Proteins 0.000 description 1
- 108010064235 lysylglycine Proteins 0.000 description 1
- 108010054155 lysyllysine Proteins 0.000 description 1
- 208000002780 macular degeneration Diseases 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 210000003097 mucus Anatomy 0.000 description 1
- 210000001989 nasopharynx Anatomy 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 238000010647 peptide synthesis reaction Methods 0.000 description 1
- 108040007629 peroxidase activity proteins Proteins 0.000 description 1
- 108010070409 phenylalanyl-glycyl-glycine Proteins 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229940093429 polyethylene glycol 6000 Drugs 0.000 description 1
- 229920002704 polyhistidine Polymers 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 208000019880 recessive mitochondrial ataxia syndrome Diseases 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 239000000523 sample Substances 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- WEPNHBQBLCNOBB-FZJVNAOYSA-N sucrose octasulfate Chemical compound OS(=O)(=O)O[C@@H]1[C@H](OS(O)(=O)=O)[C@H](COS(=O)(=O)O)O[C@]1(COS(O)(=O)=O)O[C@@H]1[C@H](OS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@@H](OS(O)(=O)=O)[C@@H](COS(O)(=O)=O)O1 WEPNHBQBLCNOBB-FZJVNAOYSA-N 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 230000001502 supplementing effect Effects 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 238000011285 therapeutic regimen Methods 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/095—Neisseria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Immunology (AREA)
- Epidemiology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Virology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GBGB0819633.9A GB0819633D0 (en) | 2008-10-25 | 2008-10-25 | Composition |
| GB0819633.9 | 2008-10-25 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167035443A Division KR20170000390A (ko) | 2008-10-25 | 2009-10-26 | 돌연변이 인자 h 결합 단백질을 포함하는 백신 조성물 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20110092282A true KR20110092282A (ko) | 2011-08-17 |
Family
ID=40133849
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167035443A Ceased KR20170000390A (ko) | 2008-10-25 | 2009-10-26 | 돌연변이 인자 h 결합 단백질을 포함하는 백신 조성물 |
| KR1020117011885A Ceased KR20110092282A (ko) | 2008-10-25 | 2009-10-26 | 돌연변이 인자 h 결합 단백질을 포함하는 백신 조성물 |
| KR1020197002691A Ceased KR20190011836A (ko) | 2008-10-25 | 2009-10-26 | 돌연변이 인자 h 결합 단백질을 포함하는 백신 조성물 |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167035443A Ceased KR20170000390A (ko) | 2008-10-25 | 2009-10-26 | 돌연변이 인자 h 결합 단백질을 포함하는 백신 조성물 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197002691A Ceased KR20190011836A (ko) | 2008-10-25 | 2009-10-26 | 돌연변이 인자 h 결합 단백질을 포함하는 백신 조성물 |
Country Status (23)
| Country | Link |
|---|---|
| US (1) | US20120052092A1 (enExample) |
| EP (2) | EP3501534B1 (enExample) |
| JP (2) | JP5676458B2 (enExample) |
| KR (3) | KR20170000390A (enExample) |
| CN (2) | CN102245202A (enExample) |
| AU (1) | AU2009306114B2 (enExample) |
| BR (1) | BRPI0919926A2 (enExample) |
| CA (1) | CA2741619A1 (enExample) |
| CO (1) | CO6382140A2 (enExample) |
| DK (1) | DK3501534T5 (enExample) |
| ES (1) | ES2970878T3 (enExample) |
| FI (1) | FI3501534T3 (enExample) |
| GB (1) | GB0819633D0 (enExample) |
| HR (1) | HRP20240226T1 (enExample) |
| HU (1) | HUE065348T2 (enExample) |
| LT (1) | LT3501534T (enExample) |
| MX (1) | MX2011004322A (enExample) |
| NZ (2) | NZ592444A (enExample) |
| PL (1) | PL3501534T3 (enExample) |
| PT (1) | PT3501534T (enExample) |
| RU (1) | RU2559528C2 (enExample) |
| SI (1) | SI3501534T1 (enExample) |
| WO (1) | WO2010046715A1 (enExample) |
Families Citing this family (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DK1093517T3 (da) | 1998-05-01 | 2008-06-23 | Novartis Vaccines & Diagnostic | Neisseria-meningitidis-antigener og præparater |
| NZ515935A (en) | 1999-05-19 | 2004-01-30 | Chiron S | Combination Neisserial compositions and vaccines for the prevention of infections due to Neisseria bacteria such as meningococcal meningitis |
| EP2275553B1 (en) | 1999-10-29 | 2015-05-13 | Novartis Vaccines and Diagnostics S.r.l. | Neisserial antigenic peptides |
| JP4763210B2 (ja) | 2000-02-28 | 2011-08-31 | ノバルティス ヴァクシンズ アンド ダイアグノスティクス エスアールエル | ナイセリアのタンパク質の異種発現 |
| GB0118249D0 (en) | 2001-07-26 | 2001-09-19 | Chiron Spa | Histidine vaccines |
| GB0121591D0 (en) | 2001-09-06 | 2001-10-24 | Chiron Spa | Hybrid and tandem expression of neisserial proteins |
| MXPA05003863A (es) | 2002-10-11 | 2005-09-08 | Chiron Srl | Vacunas polipeptidicas para amplia proteccion contra lineas hipervirulentas de meningococos. |
| GB0227346D0 (en) | 2002-11-22 | 2002-12-31 | Chiron Spa | 741 |
| GB0408977D0 (en) | 2004-04-22 | 2004-05-26 | Chiron Srl | Immunising against meningococcal serogroup Y using proteins |
| GB0524066D0 (en) | 2005-11-25 | 2006-01-04 | Chiron Srl | 741 ii |
| NZ587382A (en) | 2008-02-21 | 2012-01-12 | Novartis Ag | Meningococcal fhbp polypeptides |
| EP2265640B1 (en) | 2008-03-10 | 2015-11-04 | Children's Hospital & Research Center at Oakland | Chimeric factor h binding proteins (fhbp) containing a heterologous b domain and methods of use |
| NZ595234A (en) | 2009-03-24 | 2013-12-20 | Novartis Ag | Adjuvanting meningococcal factor h binding protein |
| WO2010127172A2 (en) | 2009-04-30 | 2010-11-04 | Children's Hospital & Research Center At Oakland | Chimeric factor h binding proteins (fhbp) and methods of use |
| WO2011051893A1 (en) | 2009-10-27 | 2011-05-05 | Novartis Ag | Modified meningococcal fhbp polypeptides |
| WO2011110634A1 (en) * | 2010-03-10 | 2011-09-15 | Glaxosmithkline Biologicals S.A. | Vaccine composition |
| CN110845585A (zh) * | 2010-03-30 | 2020-02-28 | 奥克兰儿童医院及研究中心 | 改性的h因子结合蛋白(fhbp)及其使用方法 |
| EP2585106A1 (en) | 2010-06-25 | 2013-05-01 | Novartis AG | Combinations of meningococcal factor h binding proteins |
| EP2809785B1 (en) | 2012-02-02 | 2017-11-01 | GlaxoSmithKline Biologicals SA | Promoters for increased protein expression in meningococcus |
| WO2013186753A1 (en) | 2012-06-14 | 2013-12-19 | Novartis Ag | Vaccines for serogroup x meningococcus |
| CA2875391A1 (en) | 2012-07-27 | 2014-01-30 | Institut National De La Sante Et De La Recherche Medicale | Cd147 as receptor for pilus-mediated adhesion of meningococci to vascular endothelia |
| WO2014140562A1 (en) * | 2013-03-14 | 2014-09-18 | Isis Innovation Limited | Immunogenic composition to neisseria |
| EP3027206A4 (en) | 2013-08-02 | 2017-03-08 | Children's Hospital & Research Center Oakland | Non-naturally occurring factor h binding proteins (fhbp) and methods of use thereof |
| SI3110442T1 (sl) | 2014-02-28 | 2021-01-29 | Glaxosmithkline Biologicals S.A. | Modificirani meningokokni fHbp polipeptidi |
| EP3172224B1 (en) | 2014-07-23 | 2022-06-29 | Children's Hospital & Research Center at Oakland | Factor h binding protein variants and methods of use thereof |
| KR102709811B1 (ko) * | 2014-08-20 | 2024-09-24 | 스티흐팅 산퀸 불드포르지닝 | 인자 h 강화 항체 및 그의 용도 |
| GB201614687D0 (en) * | 2016-08-31 | 2016-10-12 | Univ Oxford Innovation Ltd | fHbp scaffold |
| EP3607967A1 (en) | 2018-08-09 | 2020-02-12 | GlaxoSmithKline Biologicals S.A. | Modified meningococcal fhbp polypeptides |
| WO2020124159A1 (en) | 2018-12-21 | 2020-06-25 | Griffith University | Compositions, methods and uses for eliciting an immune response |
| WO2021007365A1 (en) * | 2019-07-08 | 2021-01-14 | Crapaud Bio, Inc. | Methods of making and using lipooligosaccharide compositions and vaccines |
| BR112022000739A2 (pt) | 2019-07-17 | 2022-04-12 | Gemini Therapeutics Sub Inc | Anticorpos de potencialização de fator h e usos dos mesmos |
| MX2023009728A (es) | 2021-02-19 | 2023-08-30 | Sanofi Pasteur Inc | Vacuna recombinante meningococica b. |
| TW202423477A (zh) | 2022-08-03 | 2024-06-16 | 美商賽諾菲巴斯德公司 | 針對腦膜炎奈瑟氏菌b的含佐劑免疫原性組成物 |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4786592A (en) * | 1986-06-18 | 1988-11-22 | Scripps Clinic And Research Foundation | Neisseria gonorrhoeae lectin useful as a vaccine and diagnostic marker and means for producing this lectin |
| US5476784A (en) * | 1993-04-06 | 1995-12-19 | Rice; Peter A. | Gonococcal anti-idiotypic antibodies and methods and compositions using them |
| GB0220194D0 (en) * | 2002-08-30 | 2002-10-09 | Chiron Spa | Improved vesicles |
| US20070253964A1 (en) * | 2003-04-16 | 2007-11-01 | Zlotnick Gary W | Novel Immunogenic Compositions for the Prevention and Treatment of Meningococcal Disease |
| EP1855715B1 (en) * | 2005-01-27 | 2013-03-13 | Children's Hospital & Research Center at Oakland | Gna1870-based vesicle vaccines for broad spectrum protection against diseases caused by neisseria meningitidis |
| GB0524066D0 (en) * | 2005-11-25 | 2006-01-04 | Chiron Srl | 741 ii |
| US8039007B2 (en) * | 2006-06-29 | 2011-10-18 | J. Craig Venter Institute, Inc. | Polypeptides from Neisseria meningitidis |
| EP3017828A1 (en) * | 2009-08-27 | 2016-05-11 | GlaxoSmithKline Biologicals SA | Hybrid polypeptides including meningococcal fhbp sequences |
| CN110845585A (zh) * | 2010-03-30 | 2020-02-28 | 奥克兰儿童医院及研究中心 | 改性的h因子结合蛋白(fhbp)及其使用方法 |
-
2008
- 2008-10-25 GB GBGB0819633.9A patent/GB0819633D0/en not_active Ceased
-
2009
- 2009-10-26 FI FIEP18209661.0T patent/FI3501534T3/fi active
- 2009-10-26 CN CN2009801480003A patent/CN102245202A/zh active Pending
- 2009-10-26 US US13/125,957 patent/US20120052092A1/en not_active Abandoned
- 2009-10-26 HU HUE18209661A patent/HUE065348T2/hu unknown
- 2009-10-26 CN CN201510119007.XA patent/CN105412920A/zh active Pending
- 2009-10-26 PL PL18209661.0T patent/PL3501534T3/pl unknown
- 2009-10-26 KR KR1020167035443A patent/KR20170000390A/ko not_active Ceased
- 2009-10-26 KR KR1020117011885A patent/KR20110092282A/ko not_active Ceased
- 2009-10-26 EP EP18209661.0A patent/EP3501534B1/en active Active
- 2009-10-26 SI SI200932190T patent/SI3501534T1/sl unknown
- 2009-10-26 NZ NZ592444A patent/NZ592444A/xx unknown
- 2009-10-26 ES ES18209661T patent/ES2970878T3/es active Active
- 2009-10-26 RU RU2011120706/10A patent/RU2559528C2/ru not_active IP Right Cessation
- 2009-10-26 WO PCT/GB2009/051439 patent/WO2010046715A1/en not_active Ceased
- 2009-10-26 BR BRPI0919926A patent/BRPI0919926A2/pt not_active Application Discontinuation
- 2009-10-26 AU AU2009306114A patent/AU2009306114B2/en not_active Ceased
- 2009-10-26 JP JP2011532725A patent/JP5676458B2/ja not_active Expired - Fee Related
- 2009-10-26 HR HRP20240226TT patent/HRP20240226T1/hr unknown
- 2009-10-26 NZ NZ601538A patent/NZ601538A/en not_active IP Right Cessation
- 2009-10-26 LT LTEP18209661.0T patent/LT3501534T/lt unknown
- 2009-10-26 CA CA2741619A patent/CA2741619A1/en not_active Abandoned
- 2009-10-26 DK DK18209661.0T patent/DK3501534T5/da active
- 2009-10-26 MX MX2011004322A patent/MX2011004322A/es active IP Right Grant
- 2009-10-26 PT PT182096610T patent/PT3501534T/pt unknown
- 2009-10-26 EP EP09753188.3A patent/EP2355845B1/en not_active Not-in-force
- 2009-10-26 KR KR1020197002691A patent/KR20190011836A/ko not_active Ceased
-
2011
- 2011-05-05 CO CO11055224A patent/CO6382140A2/es not_active Application Discontinuation
-
2014
- 2014-12-25 JP JP2014261910A patent/JP6076326B2/ja not_active Expired - Fee Related
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20110092282A (ko) | 돌연변이 인자 h 결합 단백질을 포함하는 백신 조성물 | |
| CN102834410B (zh) | 改性的h因子结合蛋白(fhbp)及其使用方法 | |
| KR100615109B1 (ko) | 모렉셀라 카타르할리스의 uspa1, uspa2 항원 | |
| EP2265640A2 (en) | Chimeric factor h binding proteins (fhbp) containing a heterologous b domain and methods of use | |
| AU2017319218B2 (en) | Modified factor H binding protein | |
| AU2013304832B2 (en) | Neisseria meningitidis fHbp variant and its use for vaccination | |
| KR100509712B1 (ko) | 나이세리아 락토페린 결합 단백질 | |
| AU2017245320B2 (en) | Vaccine compositions comprising a mutated factor h binding protein | |
| US20160030544A1 (en) | Immunogenic composition to neisseria | |
| HK40072982A (en) | Factor h binding proteins (fhbp) with altered properties and methods of use thereof | |
| HK1254940A1 (en) | Factor h binding proteins (fhbp) with altered properties and methods of use thereof | |
| HK1254940B (en) | Factor h binding proteins (fhbp) with altered properties and methods of use thereof | |
| OA19655A (en) | Modified factor H binding protein. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20110525 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20140917 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20160121 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20161124 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20160121 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20161219 |
|
| J201 | Request for trial against refusal decision | ||
| PJ0201 | Trial against decision of rejection |
Patent event date: 20161223 Comment text: Request for Trial against Decision on Refusal Patent event code: PJ02012R01D Patent event date: 20161124 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Appeal kind category: Appeal against decision to decline refusal Appeal identifier: 2016101007205 Request date: 20161223 |
|
| J301 | Trial decision |
Free format text: TRIAL NUMBER: 2016101007205; TRIAL DECISION FOR APPEAL AGAINST DECISION TO DECLINE REFUSAL REQUESTED 20161223 Effective date: 20180330 |
|
| PJ1301 | Trial decision |
Patent event code: PJ13011S01D Patent event date: 20180330 Comment text: Trial Decision on Objection to Decision on Refusal Appeal kind category: Appeal against decision to decline refusal Request date: 20161223 Decision date: 20180330 Appeal identifier: 2016101007205 |