KR20080068089A - 항체 의존성 세포 독성 활성이 증강된 항체, 그 생산 및사용 방법 - Google Patents
항체 의존성 세포 독성 활성이 증강된 항체, 그 생산 및사용 방법 Download PDFInfo
- Publication number
- KR20080068089A KR20080068089A KR1020087012185A KR20087012185A KR20080068089A KR 20080068089 A KR20080068089 A KR 20080068089A KR 1020087012185 A KR1020087012185 A KR 1020087012185A KR 20087012185 A KR20087012185 A KR 20087012185A KR 20080068089 A KR20080068089 A KR 20080068089A
- Authority
- KR
- South Korea
- Prior art keywords
- antibody
- antibodies
- composition
- milk
- oligomannose
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/283—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against Fc-receptors, e.g. CD16, CD32, CD64
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/04—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies from milk
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2875—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF/TNF superfamily, e.g. CD70, CD95L, CD153, CD154
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/10—Immunoglobulins specific features characterized by their source of isolation or production
- C07K2317/12—Immunoglobulins specific features characterized by their source of isolation or production isolated from milk
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/40—Immunoglobulins specific features characterized by post-translational modification
- C07K2317/41—Glycosylation, sialylation, or fucosylation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/71—Decreased effector function due to an Fc-modification
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/72—Increased effector function due to an Fc-modification
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/732—Antibody-dependent cellular cytotoxicity [ADCC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/734—Complement-dependent cytotoxicity [CDC]
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Rheumatology (AREA)
- Hematology (AREA)
- Mycology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Pain & Pain Management (AREA)
- Transplantation (AREA)
- Oncology (AREA)
- Microbiology (AREA)
- Physical Education & Sports Medicine (AREA)
- Epidemiology (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US72905405P | 2005-10-21 | 2005-10-21 | |
| US60/729,054 | 2005-10-21 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020137019819A Division KR101507027B1 (ko) | 2005-10-21 | 2006-10-23 | 항체 의존성 세포 독성 활성이 증강된 항체, 그 생산 및 사용 방법 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20080068089A true KR20080068089A (ko) | 2008-07-22 |
Family
ID=37820635
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020087012185A Abandoned KR20080068089A (ko) | 2005-10-21 | 2006-10-23 | 항체 의존성 세포 독성 활성이 증강된 항체, 그 생산 및사용 방법 |
| KR1020137019819A Expired - Fee Related KR101507027B1 (ko) | 2005-10-21 | 2006-10-23 | 항체 의존성 세포 독성 활성이 증강된 항체, 그 생산 및 사용 방법 |
| KR20147030540A Withdrawn KR20140133958A (ko) | 2005-10-21 | 2006-10-23 | 항체 의존성 세포 독성 활성이 증강된 항체, 그 생산 및 사용 방법 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020137019819A Expired - Fee Related KR101507027B1 (ko) | 2005-10-21 | 2006-10-23 | 항체 의존성 세포 독성 활성이 증강된 항체, 그 생산 및 사용 방법 |
| KR20147030540A Withdrawn KR20140133958A (ko) | 2005-10-21 | 2006-10-23 | 항체 의존성 세포 독성 활성이 증강된 항체, 그 생산 및 사용 방법 |
Country Status (13)
| Country | Link |
|---|---|
| US (3) | US20080118501A1 (enExample) |
| EP (3) | EP2388272A1 (enExample) |
| JP (2) | JP5328019B2 (enExample) |
| KR (3) | KR20080068089A (enExample) |
| CN (2) | CN103435695A (enExample) |
| AU (1) | AU2006304886B2 (enExample) |
| CA (1) | CA2626542C (enExample) |
| DK (2) | DK2388273T3 (enExample) |
| ES (2) | ES2417147T3 (enExample) |
| IL (1) | IL218943A0 (enExample) |
| NZ (1) | NZ568433A (enExample) |
| PL (1) | PL1945666T3 (enExample) |
| WO (1) | WO2007048077A2 (enExample) |
Families Citing this family (61)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080019905A9 (en) * | 2005-02-18 | 2008-01-24 | Strome Scott E | Method of using an anti-CD137 antibody as an agent for radioimmunotherapy or radioimmunodetection |
| NZ568433A (en) * | 2005-10-21 | 2012-07-27 | Gtc Biotherapeutics Inc | Milk-derived antibodies with enhanced antibody-dependent cellular cytoxicity activity, methods of their production and use |
| US7846724B2 (en) | 2006-04-11 | 2010-12-07 | Hoffmann-La Roche Inc. | Method for selecting CHO cell for production of glycosylated antibodies |
| WO2008030564A2 (en) * | 2006-09-08 | 2008-03-13 | Verenium Corporation | Aglycosylated antibodies and methods of making and using those antibodies |
| FR2915398B1 (fr) * | 2007-04-25 | 2012-12-28 | Lab Francais Du Fractionnement | "ensemble de moyens pour le traitement d'une pathologie maligne, d'une maladie auto-immune ou d'une maladie infectieuse" |
| EP1995309A1 (en) * | 2007-05-21 | 2008-11-26 | Vivalis | Recombinant protein production in avian EBx® cells |
| EP2725035A1 (en) * | 2007-10-02 | 2014-04-30 | Avaxia Biologics, Inc. | Antibody therapy for use in the digestive tract |
| US8647626B2 (en) | 2007-10-02 | 2014-02-11 | Avaxia Biologics, Incorporated | Compositions comprising TNF-specific antibodies for oral delivery |
| ES2336873B1 (es) | 2007-11-07 | 2011-01-24 | Proyecto De Biomedicina Cima, S.L. | Composicion farmaceutica para el tratamiento de cancer. |
| WO2009114641A1 (en) * | 2008-03-11 | 2009-09-17 | Genentech, Inc. | Antibodies with enhanced adcc function |
| WO2009124109A1 (en) | 2008-04-04 | 2009-10-08 | The Government Of The U.S. A. As Represented By The Secretary Of The Dept. Of Health &Human Services | Human monoclonal antibodies specific for cd22 |
| US20110229460A1 (en) * | 2008-05-01 | 2011-09-22 | Gtc Biotherapeutics, Inc. | anti-cd137 antibody as an agent in the treatment of inflammatory conditions |
| US8080415B2 (en) | 2008-09-26 | 2011-12-20 | Eureka Therapeutics, Inc. | Modified host cells and uses thereof |
| US8475790B2 (en) | 2008-10-06 | 2013-07-02 | Bristol-Myers Squibb Company | Combination of CD137 antibody and CTLA-4 antibody for the treatment of proliferative diseases |
| TWI409079B (zh) * | 2009-08-14 | 2013-09-21 | Roche Glycart Ag | 非典型岩藻醣化cd20抗體與苯達莫斯汀(bendamustine)之組合療法 |
| HUE033758T2 (en) | 2009-10-26 | 2017-12-28 | Hoffmann La Roche | A method for producing glycosylated immunoglobulin |
| FR2962908A1 (fr) | 2010-07-20 | 2012-01-27 | Lfb Biotechnologies | Formulation d'anticorps anti-cd20 |
| PH12013501365A1 (en) | 2010-12-30 | 2013-09-16 | Laboratoire Francais Du Fractionnment Et Des Biotechnologies | Glycols as pathogen inactivating agents |
| FR2976811A1 (fr) | 2011-06-22 | 2012-12-28 | Lfb Biotechnologies | Utilisation d'un anticorps anti-cd20 a haute adcc pour le traitement de la maladie de waldenstrom |
| WO2013013193A1 (en) | 2011-07-20 | 2013-01-24 | Zepteon, Incorporated | Polypeptide separation methods |
| BR112014003110A2 (pt) * | 2011-08-10 | 2018-10-09 | Lab Francais Du Fractionnement | composição, método para produção de uma população altamente galactosilada de anticorpos, células epiteliais de glândula mamária e mamífero não humano tramsgênico |
| US20130302274A1 (en) * | 2011-11-25 | 2013-11-14 | Roche Glycart Ag | Combination therapy |
| US20150017687A1 (en) * | 2012-01-30 | 2015-01-15 | Dr. Reddy's Laboratories Limited | Method for obtaining a glycoprotein composition |
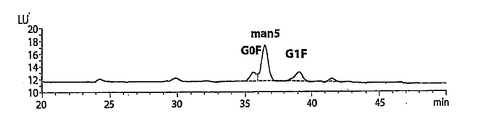
| US10059770B2 (en) | 2012-01-30 | 2018-08-28 | Dr. Reddy's Laboratories Limited | Process of modulating man5 and/or afucosylation content of a glycoprotein composition |
| AR091977A1 (es) | 2012-08-03 | 2015-03-11 | Revo Biolog Inc | El uso de antitrombina en la oxigenacion de membranas extracorporeas |
| DK2925350T3 (da) * | 2012-12-03 | 2019-05-13 | Bristol Myers Squibb Co | Øgning af virksomheden mod cancer af immunomodulatoriske fc- fusionsproteiner |
| US20160185847A1 (en) * | 2012-12-17 | 2016-06-30 | Laboratoire Francais Du Fractionnement Et Des Biotechnologies | Use of monoclonal antibodies for the treatment of inflammation and bacterial infections |
| BR112015019341A2 (pt) | 2013-02-13 | 2017-08-22 | Lab Francais Du Fractionnement | Anticorpo anti-tnf-alfa, composição que compreende o anticorpo, método para produzir uma população de anticorpos, células epiteliais da glândula mamária, mamífero não humano transgênico, e, composição de anticorpo anti-tnf monoclonal |
| MX2015010429A (es) | 2013-02-13 | 2016-05-16 | Lab Francais Du Fractionnement | Cetuximab con glicosilación modificada y sus usos. |
| AR094781A1 (es) * | 2013-02-13 | 2015-08-26 | Laboratoire Français Du Fractionnement Et Des Biotechnologies | Anticuerpos anti-her2 (receptor 2 del factor de crecimiento epidérmico humano) altamente galactosilados y sus usos |
| EP2956003A2 (en) | 2013-02-13 | 2015-12-23 | Laboratoire Français du Fractionnement et des Biotechnologies | Proteins with modified glycosylation and methods of production thereof |
| PL2991683T3 (pl) | 2013-05-02 | 2020-03-31 | Glykos Finland Oy | Koniugaty glikoproteiny lub glikanu z toksycznym ładunkiem |
| JP2016532100A (ja) | 2013-07-05 | 2016-10-13 | ラボラトワール・フランセ・デュ・フラクシオンマン・エ・デ・ビョテクノロジーLaboratoire Francais Du Fractionnement Et Des Biotechnologies | アフィニティークロマトグラフィーマトリックス |
| FR3016633B1 (fr) | 2014-01-17 | 2018-04-13 | Laboratoire Francais Du Fractionnement Et Des Biotechnologies | Immunoglobuline anti-toxine du charbon |
| US10780182B2 (en) | 2014-04-25 | 2020-09-22 | The Trustees Of The University Of Pennsylvania | Methods and compositions for treating metastatic breast cancer and other cancers in the brain |
| FR3025515B1 (fr) | 2014-09-05 | 2016-09-09 | Lab Francais Du Fractionnement | Procede de purification d'un anticorps monoclonal |
| FR3034420A1 (fr) | 2015-03-31 | 2016-10-07 | Lab Francais Du Fractionnement | Anticorps monoclonaux anti-cd303 |
| FR3038517B1 (fr) | 2015-07-06 | 2020-02-28 | Laboratoire Francais Du Fractionnement Et Des Biotechnologies | Utilisation de fragments fc modifies en immunotherapie |
| CN105886656B (zh) * | 2016-06-24 | 2019-11-12 | 河北医科大学第四医院 | Gif基因在食管鳞癌诊治中的应用 |
| CN106199005B (zh) * | 2016-07-26 | 2019-02-01 | 浙江大学 | 采用cd137表达量检测淋巴细胞抗大肠癌活性的方法 |
| CN107987160A (zh) * | 2016-10-26 | 2018-05-04 | 无锡科捷诺生物科技有限责任公司 | 一种无岩藻糖基化的单克隆抗体 |
| IL300095A (en) * | 2016-11-23 | 2023-03-01 | Translational Drug Dev Llc | Benzamide and tumor necrosis factor receptor superfamily agonist compositions and uses thereof |
| FR3060394B1 (fr) | 2016-12-16 | 2019-05-24 | Laboratoire Francais Du Fractionnement Et Des Biotechnologies | Combinaison d'anticorps anti-cd303 et anti-amhrii |
| FR3060395B1 (fr) | 2016-12-16 | 2019-05-24 | Laboratoire Francais Du Fractionnement Et Des Biotechnologies | Combinaison d'anticorps anti-cd303 et anti-her2 |
| CN107083362A (zh) * | 2017-06-19 | 2017-08-22 | 金浩范 | 一种自体nk细胞制备方法及实施该方法的试剂盒 |
| EP3498293A1 (en) | 2017-12-15 | 2019-06-19 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Treatment of monogenic diseases with an anti-cd45rc antibody |
| FR3076294B1 (fr) | 2017-12-29 | 2022-01-28 | Lab Francais Du Fractionnement | Procede de purification d'anticorps a partir de lait brut |
| US11555071B2 (en) | 2018-06-03 | 2023-01-17 | Lamkap Bio Beta Ltd. | Bispecific antibodies against CEACAM5 and CD47 |
| EP3626265A1 (en) | 2018-09-21 | 2020-03-25 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Anti-human cd45rc antibodies and uses thereof |
| TWI839395B (zh) | 2018-10-09 | 2024-04-21 | 瑞士商Numab治療公司 | 靶向cd137的抗體及其使用方法 |
| EP3636320A1 (en) * | 2018-10-09 | 2020-04-15 | Numab Therapeutics AG | Antibodies targeting cd137 and methods of use thereof |
| EP3914616A1 (en) | 2019-01-23 | 2021-12-01 | Encefa | Cd31 competitors and uses thereof |
| EP3831849A1 (en) | 2019-12-02 | 2021-06-09 | LamKap Bio beta AG | Bispecific antibodies against ceacam5 and cd47 |
| EP4055055B1 (en) | 2020-12-18 | 2023-11-22 | LamKap Bio beta AG | Bispecific antibodies against ceacam5 and cd47 |
| CN112724263B (zh) * | 2021-04-02 | 2021-08-03 | 上海偌妥生物科技有限公司 | 改造抗cd20单克隆抗体以提高其药物疗效的方法及其应用 |
| CN113648405B (zh) * | 2021-08-19 | 2023-06-02 | 重庆医科大学 | 一种口服重组幽门螺杆菌蛋白疫苗纳米粒及其制备方法 |
| WO2023041717A1 (en) | 2021-09-16 | 2023-03-23 | Aboleris Pharma | Anti-human cd45rc binding domains and uses thereof |
| MX2024007676A (es) | 2021-12-20 | 2024-08-28 | Vetoquinol Sa | Anticuerpos anti-receptor a de interleucina 31 canina (il-31ra) y sus usos. |
| WO2023242351A1 (en) | 2022-06-16 | 2023-12-21 | Lamkap Bio Beta Ag | Combination therapy of bispecific antibodies against ceacam5 and cd47 and bispecific antibodies against ceacam5 and cd3 |
| WO2024165823A2 (fr) | 2023-02-09 | 2024-08-15 | Commissariat A L'energie Atomique Et Aux Energies Alternatives | Fragment fab mutant pour l'obtention de conjugués mono- ou bi-fonctionnalisés site-spécifiques |
| WO2025114614A1 (en) | 2023-11-30 | 2025-06-05 | Vetoquinol Sa | Anti-canine interleukine-4-receptor alpha (il-4rα) antibodies and the uses thereof |
Family Cites Families (75)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2459619B1 (fr) * | 1979-06-26 | 1983-07-29 | Agronomique Inst Nat Rech | Procede pour l'obtention a partir de lactoserum, d'un produit enrichi en alpha-lactalbumine et applications dudit procede |
| US4257774A (en) * | 1979-07-16 | 1981-03-24 | Meloy Laboratories, Inc. | Intercalation inhibition assay for compounds that interact with DNA or RNA |
| US4361544A (en) * | 1980-03-03 | 1982-11-30 | Goldenberg Milton David | Tumor localization and therapy with labeled antibodies specific to intracellular tumor-associated markers |
| GB8308235D0 (en) * | 1983-03-25 | 1983-05-05 | Celltech Ltd | Polypeptides |
| US4816567A (en) * | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| DE3432718C1 (de) * | 1984-09-06 | 1986-05-22 | Biotest Pharma GmbH, 6000 Frankfurt | Verfahren zur Herstellung einer Loesung von Milch- und/oder Kolostralimmunglobulinen |
| US5322775A (en) * | 1986-06-30 | 1994-06-21 | Pharmaceutical Proteins Ltd. | Peptide production |
| US4946778A (en) * | 1987-09-21 | 1990-08-07 | Genex Corporation | Single polypeptide chain binding molecules |
| US5750172A (en) * | 1987-06-23 | 1998-05-12 | Pharming B.V. | Transgenic non human mammal milk |
| US4873316A (en) * | 1987-06-23 | 1989-10-10 | Biogen, Inc. | Isolation of exogenous recombinant proteins from the milk of transgenic mammals |
| US5202238A (en) * | 1987-10-27 | 1993-04-13 | Oncogen | Production of chimeric antibodies by homologous recombination |
| GB8823869D0 (en) * | 1988-10-12 | 1988-11-16 | Medical Res Council | Production of antibodies |
| US4897465A (en) * | 1988-10-12 | 1990-01-30 | Abbott Laboratories | Enrichment and concentration of proteins by ultrafiltration |
| US20060002904A9 (en) * | 1988-11-07 | 2006-01-05 | Kwon Byoung S | Receptor and related products and methods |
| US6362325B1 (en) * | 1988-11-07 | 2002-03-26 | Advanced Research And Technology Institute, Inc. | Murine 4-1BB gene |
| US6303121B1 (en) * | 1992-07-30 | 2001-10-16 | Advanced Research And Technology | Method of using human receptor protein 4-1BB |
| US6355476B1 (en) * | 1988-11-07 | 2002-03-12 | Advanced Research And Technologyinc | Nucleic acid encoding MIP-1α Lymphokine |
| US5175384A (en) | 1988-12-05 | 1992-12-29 | Genpharm International | Transgenic mice depleted in mature t-cells and methods for making transgenic mice |
| US5633076A (en) * | 1989-12-01 | 1997-05-27 | Pharming Bv | Method of producing a transgenic bovine or transgenic bovine embryo |
| US6150584A (en) | 1990-01-12 | 2000-11-21 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| US5965789A (en) * | 1991-01-11 | 1999-10-12 | American Red Cross | Engineering protein posttranslational modification by PACE/furin in transgenic non-human mammals |
| JP3507486B2 (ja) | 1991-03-15 | 2004-03-15 | アムジエン・インコーポレーテツド | 顆粒球コロニー刺激因子の肺内投与 |
| US5714350A (en) * | 1992-03-09 | 1998-02-03 | Protein Design Labs, Inc. | Increasing antibody affinity by altering glycosylation in the immunoglobulin variable region |
| US7138500B1 (en) * | 1993-05-07 | 2006-11-21 | Immunex Corporation | Antibodies to human 4-1BB |
| US5674704A (en) * | 1993-05-07 | 1997-10-07 | Immunex Corporation | Cytokine designated 4-IBB ligand |
| US7211259B1 (en) * | 1993-05-07 | 2007-05-01 | Immunex Corporation | 4-1BB polypeptides and DNA encoding 4-1BB polypeptides |
| US5824837A (en) * | 1993-07-22 | 1998-10-20 | Merck & Co., Inc. | Expression of human interleukin-1β in a transgenic animal |
| DE69435261D1 (de) * | 1993-09-16 | 2010-02-25 | Univ Indiana Res & Tech Corp | Menschlicher rezeptor h4-1bb |
| US5827690A (en) * | 1993-12-20 | 1998-10-27 | Genzyme Transgenics Corporatiion | Transgenic production of antibodies in milk |
| AU692841B2 (en) * | 1994-03-09 | 1998-06-18 | Abbott Laboratories | Transgenic animals producing oligosaccharides and glycoconjugates |
| CA2186455A1 (en) * | 1994-03-29 | 1995-10-05 | Raymond John Owens | Antibodies against e-selectin |
| US5451569A (en) | 1994-04-19 | 1995-09-19 | Hong Kong University Of Science And Technology R & D Corporation Limited | Pulmonary drug delivery system |
| EP0690452A3 (en) | 1994-06-28 | 1999-01-07 | Advanced Micro Devices, Inc. | Electrically erasable memory and method of erasure |
| BR9510490B1 (pt) * | 1994-12-23 | 2010-10-05 | composição farmacêutica combinada de dois componentes estruturada para uso em um hospedeiro. | |
| JP2911056B2 (ja) * | 1995-04-08 | 1999-06-23 | 株式会社エルジ化学 | ヒト4−1bbに特異的なモノクローナル抗体およびこれを産生する細胞株 |
| US6998467B1 (en) | 1995-04-28 | 2006-02-14 | The Hospital For Sick Children | Antibody specific for presenilin 1 and method of use thereof |
| US5874240A (en) * | 1996-03-15 | 1999-02-23 | Human Genome Sciences, Inc. | Human 4-1BB receptor splicing variant |
| US6268487B1 (en) * | 1996-05-13 | 2001-07-31 | Genzyme Transgenics Corporation | Purification of biologically active peptides from milk |
| ATE230801T1 (de) * | 1996-10-03 | 2003-01-15 | Canon Kk | Verfahren zur detektion von zielnukleinsäure, verfahren zu ihrer quantifizierung und pyrylium- verbindungen zur chemilumineszenz-analyse |
| CA2268340A1 (en) * | 1996-10-11 | 1998-04-23 | Bristol-Myers Squibb Company | Methods and compositions for immunomodulation |
| US5945577A (en) | 1997-01-10 | 1999-08-31 | University Of Massachusetts As Represented By Its Amherst Campus | Cloning using donor nuclei from proliferating somatic cells |
| DE69834490T2 (de) * | 1997-05-29 | 2007-03-01 | Agresearch Ltd. | Verfahren zur herstellung von immunoglobulin a in milch |
| US20030175754A1 (en) | 1998-06-29 | 2003-09-18 | Incyte Genomics, Inc. | RVP-1 variant differentially expressed in crohns disease |
| US20030096976A1 (en) * | 1998-11-17 | 2003-05-22 | Hong Hyo Jeong | Humanized antibodies LB-00503 and LB-00506 specific for human 4-1BB and pharmaceutical compositions comprising said humanized antibodies |
| KR20000034847A (ko) * | 1998-11-17 | 2000-06-26 | 성재갑 | 인간 4-1비비 분자에 대한 인간화 항체 및 이를 포함하는 약학조성물 |
| US7414170B2 (en) * | 1999-11-19 | 2008-08-19 | Kirin Beer Kabushiki Kaisha | Transgenic bovines capable of human antibody production |
| WO2001057088A1 (en) * | 2000-02-03 | 2001-08-09 | Hammarstroem Lennart | RUMINANT MHC CLASS I-LIKE Fc RECEPTORS |
| FR2807767B1 (fr) * | 2000-04-12 | 2005-01-14 | Lab Francais Du Fractionnement | Anticorps monoclonaux anti-d |
| US20040247563A1 (en) * | 2000-11-02 | 2004-12-09 | Lynch David H. | Method of enhancing lymphocyte-mediated immune responses |
| WO2003006058A1 (en) * | 2001-07-12 | 2003-01-23 | Wyeth | Cd25+ differential markers and uses thereof |
| ATE435655T1 (de) * | 2001-10-09 | 2009-07-15 | Mayo Foundation | Verwendung von agonistischen 4-1bb antikörpern zur erhöhung der immunantwort |
| US7053202B2 (en) * | 2001-10-19 | 2006-05-30 | Millennium Pharmaceuticals, Inc. | Immunoglobulin DNA cassette molecules, monobody constructs, methods of production, and methods of use therefor |
| US20040132101A1 (en) * | 2002-09-27 | 2004-07-08 | Xencor | Optimized Fc variants and methods for their generation |
| US20030223989A1 (en) * | 2002-04-18 | 2003-12-04 | Pluenneke John D. | CD137 agonists to treat patients with IgE-mediated conditions |
| CA2492561A1 (en) * | 2002-07-15 | 2004-01-22 | Mayo Foundation For Medical Education And Research | Treatment and prophylaxis with 4-1bb-binding agents |
| SI1523496T1 (sl) * | 2002-07-18 | 2011-11-30 | Merus B V | Rekombinantno proizvajanje zmesi protiteles |
| US6887673B2 (en) * | 2002-07-30 | 2005-05-03 | Bristol-Myers Squibb Company | Humanized antibodies against human 4-1BB |
| WO2004012499A2 (en) * | 2002-08-01 | 2004-02-12 | Gtc Biotherapeutics, Inc. | Method of selecting cells for somatic cell nuclear transfer |
| CA2506629A1 (en) * | 2002-11-27 | 2004-06-17 | Gtc Biotherapeutics, Inc. | Modified antibodies stably produced in milk and methods of producing same |
| US20060121030A1 (en) * | 2002-12-16 | 2006-06-08 | Herbert Schwarz | Use of cd 137 antagonists for the treatment of tumors |
| EP1587540B1 (en) * | 2003-01-09 | 2021-09-15 | MacroGenics, Inc. | IDENTIFICATION AND ENGINEERING OF ANTIBODIES WITH VARIANT Fc REGIONS AND METHODS OF USING SAME |
| US7471635B2 (en) * | 2003-07-16 | 2008-12-30 | Qlogic, Corporation | Method and apparatus for test pattern generation |
| NZ545776A (en) * | 2003-08-22 | 2009-05-31 | Biogen Idec Inc | Improved antibodies having altered effector function and methods for making the same |
| US7288638B2 (en) * | 2003-10-10 | 2007-10-30 | Bristol-Myers Squibb Company | Fully human antibodies against human 4-1BB |
| WO2005095653A2 (en) * | 2004-04-02 | 2005-10-13 | Hematologics, Inc. | Method for collecting purified cells |
| US20080019905A9 (en) * | 2005-02-18 | 2008-01-24 | Strome Scott E | Method of using an anti-CD137 antibody as an agent for radioimmunotherapy or radioimmunodetection |
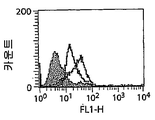
| US20060182744A1 (en) * | 2005-02-15 | 2006-08-17 | Strome Scott E | Anti-CD137 antibody as an agent in the treatment of cancer and glycosylation variants thereof |
| EP1850872A4 (en) * | 2005-02-15 | 2008-10-15 | Gtc Biotherapeutics Inc | METHOD FOR USE OF ANTI-CD-137 ANTIBODY AS RADIOIMMUN THERAPY OR RADIO IMMUNE DETECTION MEANS |
| EP1851244A4 (en) * | 2005-02-15 | 2009-04-15 | Gtc Biotherapeutics Inc | ANTI-CD137 ANTIBODY AS A MEANS FOR THE TREATMENT OF CANCER, AND GLYCOSYLATION VARIANTS THEREOF |
| JP2009507482A (ja) * | 2005-09-09 | 2009-02-26 | グライコフィ, インコーポレイテッド | 主にman7glcnac2、man8glcnac2グリコフォームを含むイムノグロブリン |
| NZ568433A (en) * | 2005-10-21 | 2012-07-27 | Gtc Biotherapeutics Inc | Milk-derived antibodies with enhanced antibody-dependent cellular cytoxicity activity, methods of their production and use |
| DK1945665T3 (da) * | 2005-10-21 | 2012-02-06 | Genzyme Corp | Antistof-baserede terapimidler med forhøjet ADCC-aktivitet |
| US20110229460A1 (en) * | 2008-05-01 | 2011-09-22 | Gtc Biotherapeutics, Inc. | anti-cd137 antibody as an agent in the treatment of inflammatory conditions |
| US10798205B2 (en) | 2016-04-13 | 2020-10-06 | Facebook, Inc. | Cache system for live broadcast streaming |
-
2006
- 2006-10-23 NZ NZ568433A patent/NZ568433A/en not_active IP Right Cessation
- 2006-10-23 AU AU2006304886A patent/AU2006304886B2/en not_active Ceased
- 2006-10-23 CN CN2013101589165A patent/CN103435695A/zh active Pending
- 2006-10-23 WO PCT/US2006/041656 patent/WO2007048077A2/en not_active Ceased
- 2006-10-23 JP JP2008536622A patent/JP5328019B2/ja not_active Expired - Fee Related
- 2006-10-23 EP EP11167181A patent/EP2388272A1/en not_active Ceased
- 2006-10-23 DK DK11167183.0T patent/DK2388273T3/en active
- 2006-10-23 ES ES06844220T patent/ES2417147T3/es active Active
- 2006-10-23 US US11/586,431 patent/US20080118501A1/en not_active Abandoned
- 2006-10-23 PL PL06844220T patent/PL1945666T3/pl unknown
- 2006-10-23 EP EP06844220A patent/EP1945666B1/en active Active
- 2006-10-23 EP EP11167183.0A patent/EP2388273B1/en active Active
- 2006-10-23 KR KR1020087012185A patent/KR20080068089A/ko not_active Abandoned
- 2006-10-23 KR KR1020137019819A patent/KR101507027B1/ko not_active Expired - Fee Related
- 2006-10-23 CN CN200680048006.XA patent/CN101484470B/zh not_active Expired - Fee Related
- 2006-10-23 DK DK06844220.1T patent/DK1945666T3/da active
- 2006-10-23 ES ES11167183.0T patent/ES2642787T3/es active Active
- 2006-10-23 CA CA2626542A patent/CA2626542C/en not_active Expired - Fee Related
- 2006-10-23 KR KR20147030540A patent/KR20140133958A/ko not_active Withdrawn
-
2012
- 2012-03-29 IL IL218943A patent/IL218943A0/en unknown
- 2012-07-27 JP JP2012167078A patent/JP2012250984A/ja active Pending
-
2013
- 2013-10-18 US US14/057,243 patent/US20140046033A1/en not_active Abandoned
-
2020
- 2020-02-21 US US16/798,047 patent/US20200255518A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| EP1945666B1 (en) | 2013-03-27 |
| EP2388272A1 (en) | 2011-11-23 |
| US20200255518A1 (en) | 2020-08-13 |
| DK2388273T3 (en) | 2017-10-09 |
| US20140046033A1 (en) | 2014-02-13 |
| CA2626542A1 (en) | 2007-04-26 |
| NZ568433A (en) | 2012-07-27 |
| US20080118501A1 (en) | 2008-05-22 |
| CA2626542C (en) | 2018-07-03 |
| ES2642787T3 (es) | 2017-11-20 |
| JP2012250984A (ja) | 2012-12-20 |
| EP2388273B1 (en) | 2017-07-05 |
| CN101484470A (zh) | 2009-07-15 |
| AU2006304886A1 (en) | 2007-04-26 |
| IL218943A0 (en) | 2012-05-31 |
| KR101507027B1 (ko) | 2015-03-31 |
| JP2009512694A (ja) | 2009-03-26 |
| EP1945666A2 (en) | 2008-07-23 |
| DK1945666T3 (da) | 2013-07-01 |
| PL1945666T3 (pl) | 2013-09-30 |
| KR20140133958A (ko) | 2014-11-20 |
| AU2006304886B2 (en) | 2012-04-12 |
| EP2388273A1 (en) | 2011-11-23 |
| CN101484470B (zh) | 2014-07-23 |
| WO2007048077A3 (en) | 2007-07-05 |
| JP5328019B2 (ja) | 2013-10-30 |
| WO2007048077A2 (en) | 2007-04-26 |
| CN103435695A (zh) | 2013-12-11 |
| ES2417147T3 (es) | 2013-08-06 |
| KR20130089673A (ko) | 2013-08-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101507027B1 (ko) | 항체 의존성 세포 독성 활성이 증강된 항체, 그 생산 및 사용 방법 | |
| EP2741769B1 (en) | Highly galactosylated antibodies | |
| EP2925350B1 (en) | Enhancing anti-cancer activity of immunomodulatory fc fusion proteins | |
| JP2021506244A (ja) | 抗trem2抗体及び関連する方法 | |
| US20130149301A1 (en) | Anti-cd137 antibody as an agent in the treatment of inflammatory conditions | |
| US20190106502A1 (en) | Antibody fab and fc variants | |
| KR20160003634A (ko) | 고도로 갈락토실화된 항-her2 항체 및 이의 용도 | |
| AU2012201010B2 (en) | Antibodies with enhanced antibody-dependent cellular cytoxicity activity, methods of their production and use | |
| AU2015201981A1 (en) | Antibodies with enhanced antibody-dependent cellular cytoxicity activity, methods of their production and use | |
| AU2012293420B2 (en) | Highly galactosylated antibodies |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20080521 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| AMND | Amendment | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20111021 Comment text: Request for Examination of Application |
|
| N231 | Notification of change of applicant | ||
| PN2301 | Change of applicant |
Patent event date: 20111117 Comment text: Notification of Change of Applicant Patent event code: PN23011R01D |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20130425 Patent event code: PE09021S01D |
|
| A107 | Divisional application of patent | ||
| AMND | Amendment | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20130725 |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20131029 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20130425 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| J201 | Request for trial against refusal decision | ||
| PJ0201 | Trial against decision of rejection |
Patent event date: 20140129 Comment text: Request for Trial against Decision on Refusal Patent event code: PJ02012R01D Patent event date: 20131029 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Appeal kind category: Appeal against decision to decline refusal Decision date: 20160303 Appeal identifier: 2014101000540 Request date: 20140129 |
|
| AMND | Amendment | ||
| PB0901 | Examination by re-examination before a trial |
Comment text: Amendment to Specification, etc. Patent event date: 20140228 Patent event code: PB09011R02I Comment text: Request for Trial against Decision on Refusal Patent event date: 20140129 Patent event code: PB09011R01I Comment text: Amendment to Specification, etc. Patent event date: 20130725 Patent event code: PB09011R02I Comment text: Amendment to Specification, etc. Patent event date: 20111021 Patent event code: PB09011R02I |
|
| N231 | Notification of change of applicant | ||
| PN2301 | Change of applicant |
Patent event date: 20141020 Comment text: Notification of Change of Applicant Patent event code: PN23011R01D |
|
| B601 | Maintenance of original decision after re-examination before a trial | ||
| PB0601 | Maintenance of original decision after re-examination before a trial | ||
| J301 | Trial decision |
Free format text: TRIAL DECISION FOR APPEAL AGAINST DECISION TO DECLINE REFUSAL REQUESTED 20140129 Effective date: 20160303 Free format text: TRIAL NUMBER: 2014101000540; TRIAL DECISION FOR APPEAL AGAINST DECISION TO DECLINE REFUSAL REQUESTED 20140129 Effective date: 20160303 |
|
| PJ1301 | Trial decision |
Patent event code: PJ13011S01D Patent event date: 20160303 Comment text: Trial Decision on Objection to Decision on Refusal Appeal kind category: Appeal against decision to decline refusal Request date: 20140129 Decision date: 20160303 Appeal identifier: 2014101000540 |
|
| PS0901 | Examination by remand of revocation | ||
| S901 | Examination by remand of revocation | ||
| GRNO | Decision to grant (after opposition) | ||
| PS0701 | Decision of registration after remand of revocation |
Patent event date: 20160309 Patent event code: PS07012S01D Comment text: Decision to Grant Registration Patent event date: 20160304 Patent event code: PS07011S01I Comment text: Notice of Trial Decision (Remand of Revocation) |
|
| PC1904 | Unpaid initial registration fee |