JP6846352B2 - キメラ抗原受容体 - Google Patents
キメラ抗原受容体 Download PDFInfo
- Publication number
- JP6846352B2 JP6846352B2 JP2017541919A JP2017541919A JP6846352B2 JP 6846352 B2 JP6846352 B2 JP 6846352B2 JP 2017541919 A JP2017541919 A JP 2017541919A JP 2017541919 A JP2017541919 A JP 2017541919A JP 6846352 B2 JP6846352 B2 JP 6846352B2
- Authority
- JP
- Japan
- Prior art keywords
- cells
- car
- domain
- seq
- receptor
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 title claims description 309
- 210000004027 cell Anatomy 0.000 claims description 171
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 135
- 230000001086 cytosolic effect Effects 0.000 claims description 129
- 239000000427 antigen Substances 0.000 claims description 87
- 102000036639 antigens Human genes 0.000 claims description 87
- 108091007433 antigens Proteins 0.000 claims description 87
- 230000003834 intracellular effect Effects 0.000 claims description 81
- 102000004495 STAT3 Transcription Factor Human genes 0.000 claims description 77
- 108010017324 STAT3 Transcription Factor Proteins 0.000 claims description 76
- 230000004068 intracellular signaling Effects 0.000 claims description 66
- 150000007523 nucleic acids Chemical class 0.000 claims description 65
- 102000039446 nucleic acids Human genes 0.000 claims description 62
- 108020004707 nucleic acids Proteins 0.000 claims description 62
- 230000027455 binding Effects 0.000 claims description 55
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 claims description 54
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 claims description 54
- 102000001712 STAT5 Transcription Factor Human genes 0.000 claims description 48
- 108010029477 STAT5 Transcription Factor Proteins 0.000 claims description 47
- 239000012634 fragment Substances 0.000 claims description 41
- 102000010789 Interleukin-2 Receptors Human genes 0.000 claims description 33
- 108010038453 Interleukin-2 Receptors Proteins 0.000 claims description 32
- 239000013598 vector Substances 0.000 claims description 31
- 102000042838 JAK family Human genes 0.000 claims description 28
- 108091082332 JAK family Proteins 0.000 claims description 28
- 108010076504 Protein Sorting Signals Proteins 0.000 claims description 27
- 125000000539 amino acid group Chemical group 0.000 claims description 27
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 22
- 239000000203 mixture Substances 0.000 claims description 22
- 238000010361 transduction Methods 0.000 claims description 20
- 230000026683 transduction Effects 0.000 claims description 20
- 201000010099 disease Diseases 0.000 claims description 19
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 claims description 16
- 241000124008 Mammalia Species 0.000 claims description 14
- 101000946843 Homo sapiens T-cell surface glycoprotein CD8 alpha chain Proteins 0.000 claims description 13
- 102100034922 T-cell surface glycoprotein CD8 alpha chain Human genes 0.000 claims description 13
- 210000002865 immune cell Anatomy 0.000 claims description 13
- 102100036856 Tumor necrosis factor receptor superfamily member 9 Human genes 0.000 claims description 12
- 238000003776 cleavage reaction Methods 0.000 claims description 10
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 10
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 claims description 9
- 230000007017 scission Effects 0.000 claims description 9
- 238000004519 manufacturing process Methods 0.000 claims description 8
- 238000001890 transfection Methods 0.000 claims description 7
- 230000019491 signal transduction Effects 0.000 claims description 6
- 230000005809 anti-tumor immunity Effects 0.000 claims description 5
- 108091006106 transcriptional activators Proteins 0.000 claims description 5
- 102000040430 polynucleotide Human genes 0.000 claims description 3
- 108091033319 polynucleotide Proteins 0.000 claims description 3
- 239000002157 polynucleotide Substances 0.000 claims description 3
- 230000002463 transducing effect Effects 0.000 claims description 3
- 239000000825 pharmaceutical preparation Substances 0.000 claims 1
- 229940127557 pharmaceutical product Drugs 0.000 claims 1
- 150000001413 amino acids Chemical class 0.000 description 92
- 235000001014 amino acid Nutrition 0.000 description 81
- 102000005962 receptors Human genes 0.000 description 44
- 108020003175 receptors Proteins 0.000 description 44
- 102000015696 Interleukins Human genes 0.000 description 41
- 108010063738 Interleukins Proteins 0.000 description 41
- 238000000034 method Methods 0.000 description 38
- 206010028980 Neoplasm Diseases 0.000 description 34
- 230000011664 signaling Effects 0.000 description 32
- 102000002467 interleukin receptors Human genes 0.000 description 22
- 108010093036 interleukin receptors Proteins 0.000 description 22
- 108090000765 processed proteins & peptides Proteins 0.000 description 22
- 102000004196 processed proteins & peptides Human genes 0.000 description 21
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 19
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 16
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 16
- 229920001184 polypeptide Polymers 0.000 description 16
- 108090000623 proteins and genes Proteins 0.000 description 16
- 101000716102 Homo sapiens T-cell surface glycoprotein CD4 Proteins 0.000 description 15
- 230000000638 stimulation Effects 0.000 description 15
- 201000011510 cancer Diseases 0.000 description 14
- 230000035755 proliferation Effects 0.000 description 14
- 241000282414 Homo sapiens Species 0.000 description 13
- 241000699670 Mus sp. Species 0.000 description 13
- 241000700605 Viruses Species 0.000 description 13
- 230000036961 partial effect Effects 0.000 description 13
- 101001018097 Homo sapiens L-selectin Proteins 0.000 description 12
- 102100033467 L-selectin Human genes 0.000 description 12
- 108091008874 T cell receptors Proteins 0.000 description 12
- 102000004527 Interleukin-21 Receptors Human genes 0.000 description 11
- 108010017411 Interleukin-21 Receptors Proteins 0.000 description 11
- 230000000139 costimulatory effect Effects 0.000 description 11
- 230000004913 activation Effects 0.000 description 10
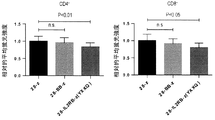
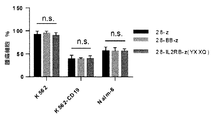
- 238000007427 paired t-test Methods 0.000 description 10
- 230000001177 retroviral effect Effects 0.000 description 10
- 125000006850 spacer group Chemical group 0.000 description 10
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 9
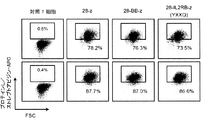
- 238000000684 flow cytometry Methods 0.000 description 9
- 235000018102 proteins Nutrition 0.000 description 9
- 102000004169 proteins and genes Human genes 0.000 description 9
- 230000004083 survival effect Effects 0.000 description 9
- 108010002350 Interleukin-2 Proteins 0.000 description 8
- 102000000588 Interleukin-2 Human genes 0.000 description 8
- 238000002347 injection Methods 0.000 description 8
- 239000007924 injection Substances 0.000 description 8
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 8
- 239000013603 viral vector Substances 0.000 description 8
- VDABVNMGKGUPEY-UHFFFAOYSA-N 6-carboxyfluorescein succinimidyl ester Chemical compound C=1C(O)=CC=C2C=1OC1=CC(O)=CC=C1C2(C1=C2)OC(=O)C1=CC=C2C(=O)ON1C(=O)CCC1=O VDABVNMGKGUPEY-UHFFFAOYSA-N 0.000 description 7
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 7
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 7
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical group OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 7
- 230000000295 complement effect Effects 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 230000006870 function Effects 0.000 description 7
- 230000026731 phosphorylation Effects 0.000 description 7
- 238000006366 phosphorylation reaction Methods 0.000 description 7
- 238000006467 substitution reaction Methods 0.000 description 7
- 241001430294 unidentified retrovirus Species 0.000 description 7
- -1 CD49A Proteins 0.000 description 6
- 108091007741 Chimeric antigen receptor T cells Proteins 0.000 description 6
- 102000004127 Cytokines Human genes 0.000 description 6
- 108090000695 Cytokines Proteins 0.000 description 6
- 108020004414 DNA Proteins 0.000 description 6
- 239000004480 active ingredient Substances 0.000 description 6
- 210000004899 c-terminal region Anatomy 0.000 description 6
- 230000032823 cell division Effects 0.000 description 6
- 210000004408 hybridoma Anatomy 0.000 description 6
- 230000031146 intracellular signal transduction Effects 0.000 description 6
- 102000034285 signal transducing proteins Human genes 0.000 description 6
- 108091006024 signal transducing proteins Proteins 0.000 description 6
- 210000004881 tumor cell Anatomy 0.000 description 6
- 108010075944 Erythropoietin Receptors Proteins 0.000 description 5
- 102100036509 Erythropoietin receptor Human genes 0.000 description 5
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 5
- 101000611023 Homo sapiens Tumor necrosis factor receptor superfamily member 6 Proteins 0.000 description 5
- 102100040403 Tumor necrosis factor receptor superfamily member 6 Human genes 0.000 description 5
- 210000003719 b-lymphocyte Anatomy 0.000 description 5
- 238000012217 deletion Methods 0.000 description 5
- 230000037430 deletion Effects 0.000 description 5
- 208000032839 leukemia Diseases 0.000 description 5
- 238000004806 packaging method and process Methods 0.000 description 5
- 239000002245 particle Substances 0.000 description 5
- 210000000130 stem cell Anatomy 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 238000012546 transfer Methods 0.000 description 5
- 201000008827 tuberculosis Diseases 0.000 description 5
- 241001529453 unidentified herpesvirus Species 0.000 description 5
- 102100038080 B-cell receptor CD22 Human genes 0.000 description 4
- 241000894006 Bacteria Species 0.000 description 4
- 101000884305 Homo sapiens B-cell receptor CD22 Proteins 0.000 description 4
- 101000946860 Homo sapiens T-cell surface glycoprotein CD3 epsilon chain Proteins 0.000 description 4
- 101000934341 Homo sapiens T-cell surface glycoprotein CD5 Proteins 0.000 description 4
- 102000012937 Interleukin-21 Receptor alpha Subunit Human genes 0.000 description 4
- 239000005089 Luciferase Substances 0.000 description 4
- 108091028043 Nucleic acid sequence Proteins 0.000 description 4
- 108010038807 Oligopeptides Proteins 0.000 description 4
- 102000015636 Oligopeptides Human genes 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 241000194017 Streptococcus Species 0.000 description 4
- 102100025237 T-cell surface antigen CD2 Human genes 0.000 description 4
- 102100035794 T-cell surface glycoprotein CD3 epsilon chain Human genes 0.000 description 4
- 102100025244 T-cell surface glycoprotein CD5 Human genes 0.000 description 4
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 4
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 4
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 4
- 230000006907 apoptotic process Effects 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 4
- 230000001472 cytotoxic effect Effects 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 4
- 208000027866 inflammatory disease Diseases 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 239000003446 ligand Substances 0.000 description 4
- 210000003071 memory t lymphocyte Anatomy 0.000 description 4
- 210000000822 natural killer cell Anatomy 0.000 description 4
- 210000004986 primary T-cell Anatomy 0.000 description 4
- 230000003612 virological effect Effects 0.000 description 4
- 208000035473 Communicable disease Diseases 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 108010092372 Granulocyte-Macrophage Colony-Stimulating Factor Receptors Proteins 0.000 description 3
- 102000016355 Granulocyte-Macrophage Colony-Stimulating Factor Receptors Human genes 0.000 description 3
- 102100020948 Growth hormone receptor Human genes 0.000 description 3
- 101001010593 Homo sapiens Interleukin-21 receptor Proteins 0.000 description 3
- 101000997835 Homo sapiens Tyrosine-protein kinase JAK1 Proteins 0.000 description 3
- 241000725303 Human immunodeficiency virus Species 0.000 description 3
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 3
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 3
- 230000004163 JAK-STAT signaling pathway Effects 0.000 description 3
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 3
- 102000043136 MAP kinase family Human genes 0.000 description 3
- 108091054455 MAP kinase family Proteins 0.000 description 3
- 102100028389 Melanoma antigen recognized by T-cells 1 Human genes 0.000 description 3
- 108010052285 Membrane Proteins Proteins 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 102000004140 Oncostatin M Human genes 0.000 description 3
- 108090000630 Oncostatin M Proteins 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- 108020004511 Recombinant DNA Proteins 0.000 description 3
- 108010068542 Somatotropin Receptors Proteins 0.000 description 3
- 108010070774 Thrombopoietin Receptors Proteins 0.000 description 3
- 102100034196 Thrombopoietin receptor Human genes 0.000 description 3
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 3
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- 230000004663 cell proliferation Effects 0.000 description 3
- 238000010367 cloning Methods 0.000 description 3
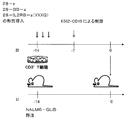
- 238000010586 diagram Methods 0.000 description 3
- 239000000539 dimer Substances 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 3
- 208000006454 hepatitis Diseases 0.000 description 3
- 231100000283 hepatitis Toxicity 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 210000004698 lymphocyte Anatomy 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 3
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 3
- 239000010452 phosphate Substances 0.000 description 3
- 238000003752 polymerase chain reaction Methods 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 230000002441 reversible effect Effects 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 230000008685 targeting Effects 0.000 description 3
- 229940124597 therapeutic agent Drugs 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 102000003390 tumor necrosis factor Human genes 0.000 description 3
- 239000004474 valine Substances 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- LKKMLIBUAXYLOY-UHFFFAOYSA-N 3-Amino-1-methyl-5H-pyrido[4,3-b]indole Chemical compound N1C2=CC=CC=C2C2=C1C=C(N)N=C2C LKKMLIBUAXYLOY-UHFFFAOYSA-N 0.000 description 2
- WEVYNIUIFUYDGI-UHFFFAOYSA-N 3-[6-[4-(trifluoromethoxy)anilino]-4-pyrimidinyl]benzamide Chemical compound NC(=O)C1=CC=CC(C=2N=CN=C(NC=3C=CC(OC(F)(F)F)=CC=3)C=2)=C1 WEVYNIUIFUYDGI-UHFFFAOYSA-N 0.000 description 2
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 2
- LRFVTYWOQMYALW-UHFFFAOYSA-N 9H-xanthine Chemical compound O=C1NC(=O)NC2=C1NC=N2 LRFVTYWOQMYALW-UHFFFAOYSA-N 0.000 description 2
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 2
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 2
- 239000004475 Arginine Substances 0.000 description 2
- 208000023275 Autoimmune disease Diseases 0.000 description 2
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 2
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- 102100035793 CD83 antigen Human genes 0.000 description 2
- 102100025466 Carcinoembryonic antigen-related cell adhesion molecule 3 Human genes 0.000 description 2
- 241000701022 Cytomegalovirus Species 0.000 description 2
- 201000004624 Dermatitis Diseases 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 101001004391 Drosophila melanogaster Protein jim lovell Proteins 0.000 description 2
- 241000709661 Enterovirus Species 0.000 description 2
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 2
- 241000233866 Fungi Species 0.000 description 2
- 229930182566 Gentamicin Natural products 0.000 description 2
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 2
- 102100041003 Glutamate carboxypeptidase 2 Human genes 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101100383038 Homo sapiens CD19 gene Proteins 0.000 description 2
- 101000914337 Homo sapiens Carcinoembryonic antigen-related cell adhesion molecule 3 Proteins 0.000 description 2
- 101000914324 Homo sapiens Carcinoembryonic antigen-related cell adhesion molecule 5 Proteins 0.000 description 2
- 101001042362 Homo sapiens Leukemia inhibitory factor receptor Proteins 0.000 description 2
- 101000578784 Homo sapiens Melanoma antigen recognized by T-cells 1 Proteins 0.000 description 2
- 101000934338 Homo sapiens Myeloid cell surface antigen CD33 Proteins 0.000 description 2
- 101000842302 Homo sapiens Protein-cysteine N-palmitoyltransferase HHAT Proteins 0.000 description 2
- 101000934346 Homo sapiens T-cell surface antigen CD2 Proteins 0.000 description 2
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 2
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 2
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 2
- 102000003814 Interleukin-10 Human genes 0.000 description 2
- 108090000174 Interleukin-10 Proteins 0.000 description 2
- 102000004889 Interleukin-6 Human genes 0.000 description 2
- 108090001005 Interleukin-6 Proteins 0.000 description 2
- 102100037795 Interleukin-6 receptor subunit beta Human genes 0.000 description 2
- 102100031413 L-dopachrome tautomerase Human genes 0.000 description 2
- 101710093778 L-dopachrome tautomerase Proteins 0.000 description 2
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 2
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 2
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 2
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 2
- 102000004058 Leukemia inhibitory factor Human genes 0.000 description 2
- 108090000581 Leukemia inhibitory factor Proteins 0.000 description 2
- 102100021747 Leukemia inhibitory factor receptor Human genes 0.000 description 2
- 241000186779 Listeria monocytogenes Species 0.000 description 2
- 108060001084 Luciferase Proteins 0.000 description 2
- 201000009906 Meningitis Diseases 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 241000186364 Mycobacterium intracellulare Species 0.000 description 2
- 102100025243 Myeloid cell surface antigen CD33 Human genes 0.000 description 2
- 241000588652 Neisseria gonorrhoeae Species 0.000 description 2
- 108091000080 Phosphotransferase Proteins 0.000 description 2
- 206010035226 Plasma cell myeloma Diseases 0.000 description 2
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 2
- 102100030616 Protein-cysteine N-palmitoyltransferase HHAT Human genes 0.000 description 2
- 241000589516 Pseudomonas Species 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 241000607142 Salmonella Species 0.000 description 2
- 108091058545 Secretory proteins Proteins 0.000 description 2
- 101710173694 Short transient receptor potential channel 2 Proteins 0.000 description 2
- 102100024040 Signal transducer and activator of transcription 3 Human genes 0.000 description 2
- 206010041925 Staphylococcal infections Diseases 0.000 description 2
- 241000191967 Staphylococcus aureus Species 0.000 description 2
- 241000193985 Streptococcus agalactiae Species 0.000 description 2
- 241000193998 Streptococcus pneumoniae Species 0.000 description 2
- 241000193996 Streptococcus pyogenes Species 0.000 description 2
- 230000006044 T cell activation Effects 0.000 description 2
- 230000006052 T cell proliferation Effects 0.000 description 2
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 2
- 239000004473 Threonine Substances 0.000 description 2
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 description 2
- 102000040856 WT1 Human genes 0.000 description 2
- 108700020467 WT1 Proteins 0.000 description 2
- 101150084041 WT1 gene Proteins 0.000 description 2
- 238000007792 addition Methods 0.000 description 2
- 230000000259 anti-tumor effect Effects 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 208000010668 atopic eczema Diseases 0.000 description 2
- 230000005784 autoimmunity Effects 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 2
- 125000002091 cationic group Chemical group 0.000 description 2
- 230000006037 cell lysis Effects 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 230000005754 cellular signaling Effects 0.000 description 2
- 239000012707 chemical precursor Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000003501 co-culture Methods 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 102000003675 cytokine receptors Human genes 0.000 description 2
- 108010057085 cytokine receptors Proteins 0.000 description 2
- 229940104302 cytosine Drugs 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 210000004443 dendritic cell Anatomy 0.000 description 2
- 230000004069 differentiation Effects 0.000 description 2
- 239000012636 effector Substances 0.000 description 2
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 2
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 229960002518 gentamicin Drugs 0.000 description 2
- 239000001963 growth medium Substances 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 238000003384 imaging method Methods 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 230000002458 infectious effect Effects 0.000 description 2
- 206010022000 influenza Diseases 0.000 description 2
- 238000001361 intraarterial administration Methods 0.000 description 2
- 108020001756 ligand binding domains Proteins 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 210000002751 lymph Anatomy 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 208000015688 methicillin-resistant staphylococcus aureus infectious disease Diseases 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 201000000050 myeloid neoplasm Diseases 0.000 description 2
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 2
- 239000002773 nucleotide Substances 0.000 description 2
- 125000003729 nucleotide group Chemical group 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 210000005259 peripheral blood Anatomy 0.000 description 2
- 239000011886 peripheral blood Substances 0.000 description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 2
- 102000020233 phosphotransferase Human genes 0.000 description 2
- 239000002504 physiological saline solution Substances 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 230000010076 replication Effects 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 108010056030 retronectin Proteins 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 238000002741 site-directed mutagenesis Methods 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 229940031000 streptococcus pneumoniae Drugs 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 206010052366 systemic mycosis Diseases 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 229940104230 thymidine Drugs 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 230000009258 tissue cross reactivity Effects 0.000 description 2
- 230000004614 tumor growth Effects 0.000 description 2
- 229940035893 uracil Drugs 0.000 description 2
- 230000035899 viability Effects 0.000 description 2
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- WEYNBWVKOYCCQT-UHFFFAOYSA-N 1-(3-chloro-4-methylphenyl)-3-{2-[({5-[(dimethylamino)methyl]-2-furyl}methyl)thio]ethyl}urea Chemical compound O1C(CN(C)C)=CC=C1CSCCNC(=O)NC1=CC=C(C)C(Cl)=C1 WEYNBWVKOYCCQT-UHFFFAOYSA-N 0.000 description 1
- ZVEUWSJUXREOBK-DKWTVANSSA-N 2-aminoacetic acid;(2s)-2-amino-3-hydroxypropanoic acid Chemical group NCC(O)=O.OC[C@H](N)C(O)=O ZVEUWSJUXREOBK-DKWTVANSSA-N 0.000 description 1
- 102100030310 5,6-dihydroxyindole-2-carboxylic acid oxidase Human genes 0.000 description 1
- 101710163881 5,6-dihydroxyindole-2-carboxylic acid oxidase Proteins 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 208000002874 Acne Vulgaris Diseases 0.000 description 1
- 102100021305 Acyl-CoA:lysophosphatidylglycerol acyltransferase 1 Human genes 0.000 description 1
- 229930024421 Adenine Natural products 0.000 description 1
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 description 1
- 101710137115 Adenylyl cyclase-associated protein 1 Proteins 0.000 description 1
- 102100023635 Alpha-fetoprotein Human genes 0.000 description 1
- 102000052587 Anaphase-Promoting Complex-Cyclosome Apc3 Subunit Human genes 0.000 description 1
- 108700004606 Anaphase-Promoting Complex-Cyclosome Apc3 Subunit Proteins 0.000 description 1
- 101100288313 Arabidopsis thaliana KTI4 gene Proteins 0.000 description 1
- 101000719121 Arabidopsis thaliana Protein MEI2-like 1 Proteins 0.000 description 1
- 102100035526 B melanoma antigen 1 Human genes 0.000 description 1
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 1
- 108010064528 Basigin Proteins 0.000 description 1
- 102000015279 Basigin Human genes 0.000 description 1
- 108060000903 Beta-catenin Proteins 0.000 description 1
- 102000015735 Beta-catenin Human genes 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 102100024217 CAMPATH-1 antigen Human genes 0.000 description 1
- 108010065524 CD52 Antigen Proteins 0.000 description 1
- 108010052382 CD83 antigen Proteins 0.000 description 1
- 102100037904 CD9 antigen Human genes 0.000 description 1
- 101150108242 CDC27 gene Proteins 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 102100039510 Cancer/testis antigen 2 Human genes 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 108090000538 Caspase-8 Proteins 0.000 description 1
- 102100026548 Caspase-8 Human genes 0.000 description 1
- ZEOWTGPWHLSLOG-UHFFFAOYSA-N Cc1ccc(cc1-c1ccc2c(n[nH]c2c1)-c1cnn(c1)C1CC1)C(=O)Nc1cccc(c1)C(F)(F)F Chemical compound Cc1ccc(cc1-c1ccc2c(n[nH]c2c1)-c1cnn(c1)C1CC1)C(=O)Nc1cccc(c1)C(F)(F)F ZEOWTGPWHLSLOG-UHFFFAOYSA-N 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 241000193449 Clostridium tetani Species 0.000 description 1
- 241000711573 Coronaviridae Species 0.000 description 1
- 241000709687 Coxsackievirus Species 0.000 description 1
- 108010025464 Cyclin-Dependent Kinase 4 Proteins 0.000 description 1
- 102000013701 Cyclin-Dependent Kinase 4 Human genes 0.000 description 1
- 102100026234 Cytokine receptor common subunit gamma Human genes 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 241000702421 Dependoparvovirus Species 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 101100216227 Dictyostelium discoideum anapc3 gene Proteins 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- 208000010772 Dog disease Diseases 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 101150084967 EPCAM gene Proteins 0.000 description 1
- 241001115402 Ebolavirus Species 0.000 description 1
- 241001466953 Echovirus Species 0.000 description 1
- 241000991587 Enterovirus C Species 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 102100028043 Fibroblast growth factor 3 Human genes 0.000 description 1
- 102100028075 Fibroblast growth factor 6 Human genes 0.000 description 1
- 108090000382 Fibroblast growth factor 6 Proteins 0.000 description 1
- 102100023593 Fibroblast growth factor receptor 1 Human genes 0.000 description 1
- 101710182386 Fibroblast growth factor receptor 1 Proteins 0.000 description 1
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 1
- 108700007698 Genetic Terminator Regions Proteins 0.000 description 1
- BCCRXDTUTZHDEU-VKHMYHEASA-N Gly-Ser Chemical compound NCC(=O)N[C@@H](CO)C(O)=O BCCRXDTUTZHDEU-VKHMYHEASA-N 0.000 description 1
- 102100039622 Granulocyte colony-stimulating factor receptor Human genes 0.000 description 1
- 102100028972 HLA class I histocompatibility antigen, A alpha chain Human genes 0.000 description 1
- 108010075704 HLA-A Antigens Proteins 0.000 description 1
- 241000590002 Helicobacter pylori Species 0.000 description 1
- 208000002250 Hematologic Neoplasms Diseases 0.000 description 1
- 241000711549 Hepacivirus C Species 0.000 description 1
- 241000709721 Hepatovirus A Species 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 102100022132 High affinity immunoglobulin epsilon receptor subunit gamma Human genes 0.000 description 1
- 108091010847 High affinity immunoglobulin epsilon receptor subunit gamma Proteins 0.000 description 1
- 102100026122 High affinity immunoglobulin gamma Fc receptor I Human genes 0.000 description 1
- 101001042227 Homo sapiens Acyl-CoA:lysophosphatidylglycerol acyltransferase 1 Proteins 0.000 description 1
- 101000874316 Homo sapiens B melanoma antigen 1 Proteins 0.000 description 1
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 1
- 101100165850 Homo sapiens CA9 gene Proteins 0.000 description 1
- 101000946856 Homo sapiens CD83 antigen Proteins 0.000 description 1
- 101000738354 Homo sapiens CD9 antigen Proteins 0.000 description 1
- 101000889345 Homo sapiens Cancer/testis antigen 2 Proteins 0.000 description 1
- 101000914321 Homo sapiens Carcinoembryonic antigen-related cell adhesion molecule 7 Proteins 0.000 description 1
- 101001055227 Homo sapiens Cytokine receptor common subunit gamma Proteins 0.000 description 1
- 101000892862 Homo sapiens Glutamate carboxypeptidase 2 Proteins 0.000 description 1
- 101000746364 Homo sapiens Granulocyte colony-stimulating factor receptor Proteins 0.000 description 1
- 101000913074 Homo sapiens High affinity immunoglobulin gamma Fc receptor I Proteins 0.000 description 1
- 101100341519 Homo sapiens ITGAX gene Proteins 0.000 description 1
- 101001078133 Homo sapiens Integrin alpha-2 Proteins 0.000 description 1
- 101000994375 Homo sapiens Integrin alpha-4 Proteins 0.000 description 1
- 101001078143 Homo sapiens Integrin alpha-IIb Proteins 0.000 description 1
- 101001046683 Homo sapiens Integrin alpha-L Proteins 0.000 description 1
- 101001046686 Homo sapiens Integrin alpha-M Proteins 0.000 description 1
- 101001046677 Homo sapiens Integrin alpha-V Proteins 0.000 description 1
- 101000935043 Homo sapiens Integrin beta-1 Proteins 0.000 description 1
- 101000935040 Homo sapiens Integrin beta-2 Proteins 0.000 description 1
- 101001015004 Homo sapiens Integrin beta-3 Proteins 0.000 description 1
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 1
- 101001083151 Homo sapiens Interleukin-10 receptor subunit alpha Proteins 0.000 description 1
- 101001002657 Homo sapiens Interleukin-2 Proteins 0.000 description 1
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 1
- 101000599056 Homo sapiens Interleukin-6 receptor subunit beta Proteins 0.000 description 1
- 101000777628 Homo sapiens Leukocyte antigen CD37 Proteins 0.000 description 1
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 1
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 description 1
- 101001051093 Homo sapiens Low-density lipoprotein receptor Proteins 0.000 description 1
- 101001134060 Homo sapiens Melanocyte-stimulating hormone receptor Proteins 0.000 description 1
- 101001133056 Homo sapiens Mucin-1 Proteins 0.000 description 1
- 101000623901 Homo sapiens Mucin-16 Proteins 0.000 description 1
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 description 1
- 101001024605 Homo sapiens Next to BRCA1 gene 1 protein Proteins 0.000 description 1
- 101000617725 Homo sapiens Pregnancy-specific beta-1-glycoprotein 2 Proteins 0.000 description 1
- 101000874141 Homo sapiens Probable ATP-dependent RNA helicase DDX43 Proteins 0.000 description 1
- 101001109419 Homo sapiens RNA-binding protein NOB1 Proteins 0.000 description 1
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 1
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 1
- 101001094545 Homo sapiens Retrotransposon-like protein 1 Proteins 0.000 description 1
- 101000857677 Homo sapiens Runt-related transcription factor 1 Proteins 0.000 description 1
- 101000826373 Homo sapiens Signal transducer and activator of transcription 3 Proteins 0.000 description 1
- 101000648075 Homo sapiens Trafficking protein particle complex subunit 1 Proteins 0.000 description 1
- 101000801234 Homo sapiens Tumor necrosis factor receptor superfamily member 18 Proteins 0.000 description 1
- 101000851376 Homo sapiens Tumor necrosis factor receptor superfamily member 8 Proteins 0.000 description 1
- 101000955999 Homo sapiens V-set domain-containing T-cell activation inhibitor 1 Proteins 0.000 description 1
- 241000701085 Human alphaherpesvirus 3 Species 0.000 description 1
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- 101710123134 Ice-binding protein Proteins 0.000 description 1
- 101710082837 Ice-structuring protein Proteins 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 108050002021 Integrator complex subunit 2 Proteins 0.000 description 1
- 102100025305 Integrin alpha-2 Human genes 0.000 description 1
- 102100032818 Integrin alpha-4 Human genes 0.000 description 1
- 102100025306 Integrin alpha-IIb Human genes 0.000 description 1
- 102100022339 Integrin alpha-L Human genes 0.000 description 1
- 102100022338 Integrin alpha-M Human genes 0.000 description 1
- 102100022337 Integrin alpha-V Human genes 0.000 description 1
- 102100022297 Integrin alpha-X Human genes 0.000 description 1
- 102100025304 Integrin beta-1 Human genes 0.000 description 1
- 102100025390 Integrin beta-2 Human genes 0.000 description 1
- 102100032999 Integrin beta-3 Human genes 0.000 description 1
- 102100037850 Interferon gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102100027268 Interferon-stimulated gene 20 kDa protein Human genes 0.000 description 1
- 108010017550 Interleukin-10 Receptors Proteins 0.000 description 1
- 102000004551 Interleukin-10 Receptors Human genes 0.000 description 1
- 102100030236 Interleukin-10 receptor subunit alpha Human genes 0.000 description 1
- 108010017521 Interleukin-11 Receptors Proteins 0.000 description 1
- 102000004553 Interleukin-11 Receptors Human genes 0.000 description 1
- 108010017515 Interleukin-12 Receptors Proteins 0.000 description 1
- 102000004560 Interleukin-12 Receptors Human genes 0.000 description 1
- 108010017511 Interleukin-13 Receptors Proteins 0.000 description 1
- 102000004559 Interleukin-13 Receptors Human genes 0.000 description 1
- 102000003812 Interleukin-15 Human genes 0.000 description 1
- 108010017535 Interleukin-15 Receptors Proteins 0.000 description 1
- 102000004556 Interleukin-15 Receptors Human genes 0.000 description 1
- 102100030704 Interleukin-21 Human genes 0.000 description 1
- 108010079728 Interleukin-21 Receptor alpha Subunit Proteins 0.000 description 1
- 102100036672 Interleukin-23 receptor Human genes 0.000 description 1
- 101710195550 Interleukin-23 receptor Proteins 0.000 description 1
- 108010066979 Interleukin-27 Proteins 0.000 description 1
- 108010038452 Interleukin-3 Receptors Proteins 0.000 description 1
- 102000010790 Interleukin-3 Receptors Human genes 0.000 description 1
- 102000004388 Interleukin-4 Human genes 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 108010038486 Interleukin-4 Receptors Proteins 0.000 description 1
- 102000010787 Interleukin-4 Receptors Human genes 0.000 description 1
- 108010002616 Interleukin-5 Proteins 0.000 description 1
- 102100039897 Interleukin-5 Human genes 0.000 description 1
- 108010038484 Interleukin-5 Receptors Proteins 0.000 description 1
- 102000010786 Interleukin-5 Receptors Human genes 0.000 description 1
- 108010038501 Interleukin-6 Receptors Proteins 0.000 description 1
- 102000010781 Interleukin-6 Receptors Human genes 0.000 description 1
- 102100021592 Interleukin-7 Human genes 0.000 description 1
- 108010002586 Interleukin-7 Proteins 0.000 description 1
- 108010038498 Interleukin-7 Receptors Proteins 0.000 description 1
- 102000010782 Interleukin-7 Receptors Human genes 0.000 description 1
- 102000000585 Interleukin-9 Human genes 0.000 description 1
- 108010002335 Interleukin-9 Proteins 0.000 description 1
- 108010038414 Interleukin-9 Receptors Proteins 0.000 description 1
- 102000010682 Interleukin-9 Receptors Human genes 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- 241000589248 Legionella Species 0.000 description 1
- 208000007764 Legionnaires' Disease Diseases 0.000 description 1
- MPSBSKHOWJQHBS-IHRRRGAJSA-N Leu-His-Met Chemical group CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CCSC)C(=O)O)N MPSBSKHOWJQHBS-IHRRRGAJSA-N 0.000 description 1
- 102100031586 Leukocyte antigen CD37 Human genes 0.000 description 1
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 description 1
- 102100024640 Low-density lipoprotein receptor Human genes 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 108010010995 MART-1 Antigen Proteins 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 108091054437 MHC class I family Proteins 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 1
- 241000712079 Measles morbillivirus Species 0.000 description 1
- 208000000172 Medulloblastoma Diseases 0.000 description 1
- 102100022430 Melanocyte protein PMEL Human genes 0.000 description 1
- 102100034216 Melanocyte-stimulating hormone receptor Human genes 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 102100034256 Mucin-1 Human genes 0.000 description 1
- 102100023123 Mucin-16 Human genes 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 241000186359 Mycobacterium Species 0.000 description 1
- 241000186363 Mycobacterium kansasii Species 0.000 description 1
- 241000187479 Mycobacterium tuberculosis Species 0.000 description 1
- 102100031789 Myeloid-derived growth factor Human genes 0.000 description 1
- 102000003505 Myosin Human genes 0.000 description 1
- 108060008487 Myosin Proteins 0.000 description 1
- 241000588653 Neisseria Species 0.000 description 1
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 101710160107 Outer membrane protein A Proteins 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 108060006580 PRAME Proteins 0.000 description 1
- 102000036673 PRAME Human genes 0.000 description 1
- 102100034640 PWWP domain-containing DNA repair factor 3A Human genes 0.000 description 1
- 108050007154 PWWP domain-containing DNA repair factor 3A Proteins 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 229930040373 Paraformaldehyde Natural products 0.000 description 1
- 208000002606 Paramyxoviridae Infections Diseases 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 102100021768 Phosphoserine aminotransferase Human genes 0.000 description 1
- 241000709664 Picornaviridae Species 0.000 description 1
- 241000276498 Pollachius virens Species 0.000 description 1
- 102100022019 Pregnancy-specific beta-1-glycoprotein 2 Human genes 0.000 description 1
- 102100035724 Probable ATP-dependent RNA helicase DDX43 Human genes 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 108010072866 Prostate-Specific Antigen Proteins 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- 241000125945 Protoparvovirus Species 0.000 description 1
- 101800001295 Putative ATP-dependent helicase Proteins 0.000 description 1
- 101800001006 Putative helicase Proteins 0.000 description 1
- 102100022491 RNA-binding protein NOB1 Human genes 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 1
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 1
- 102100023606 Retinoic acid receptor alpha Human genes 0.000 description 1
- 102100025373 Runt-related transcription factor 1 Human genes 0.000 description 1
- 108050003189 SH2B adapter protein 1 Proteins 0.000 description 1
- 108010044012 STAT1 Transcription Factor Proteins 0.000 description 1
- 102000040739 Secretory proteins Human genes 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 101710173693 Short transient receptor potential channel 1 Proteins 0.000 description 1
- 102100029904 Signal transducer and activator of transcription 1-alpha/beta Human genes 0.000 description 1
- 102100035748 Squamous cell carcinoma antigen recognized by T-cells 3 Human genes 0.000 description 1
- 101710185775 Squamous cell carcinoma antigen recognized by T-cells 3 Proteins 0.000 description 1
- 241000295644 Staphylococcaceae Species 0.000 description 1
- 241000191940 Staphylococcus Species 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 101800001271 Surface protein Proteins 0.000 description 1
- 108700019889 TEL-AML1 fusion Proteins 0.000 description 1
- 102100033082 TNF receptor-associated factor 3 Human genes 0.000 description 1
- 101150033985 TPI gene Proteins 0.000 description 1
- 101150032817 TPI1 gene Proteins 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- 102100025256 Trafficking protein particle complex subunit 1 Human genes 0.000 description 1
- 108700009124 Transcription Initiation Site Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000000887 Transcription factor STAT Human genes 0.000 description 1
- 108050007918 Transcription factor STAT Proteins 0.000 description 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 1
- 102100030742 Transforming growth factor beta-1 proprotein Human genes 0.000 description 1
- 108700015934 Triose-phosphate isomerases Proteins 0.000 description 1
- 102100033598 Triosephosphate isomerase Human genes 0.000 description 1
- LVTKHGUGBGNBPL-UHFFFAOYSA-N Trp-P-1 Chemical compound N1C2=CC=CC=C2C2=C1C(C)=C(N)N=C2C LVTKHGUGBGNBPL-UHFFFAOYSA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 102100033728 Tumor necrosis factor receptor superfamily member 18 Human genes 0.000 description 1
- 102100036857 Tumor necrosis factor receptor superfamily member 8 Human genes 0.000 description 1
- 101710107540 Type-2 ice-structuring protein Proteins 0.000 description 1
- WDIJBEWLXLQQKD-ULQDDVLXSA-N Tyr-Arg-His Chemical group C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)N)O WDIJBEWLXLQQKD-ULQDDVLXSA-N 0.000 description 1
- 102100033438 Tyrosine-protein kinase JAK1 Human genes 0.000 description 1
- 102100038929 V-set domain-containing T-cell activation inhibitor 1 Human genes 0.000 description 1
- 241000700618 Vaccinia virus Species 0.000 description 1
- 206010046865 Vaccinia virus infection Diseases 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 206010000496 acne Diseases 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 229960000643 adenine Drugs 0.000 description 1
- 210000001789 adipocyte Anatomy 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 230000000735 allogeneic effect Effects 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 230000001745 anti-biotin effect Effects 0.000 description 1
- 230000000719 anti-leukaemic effect Effects 0.000 description 1
- 230000000692 anti-sense effect Effects 0.000 description 1
- 210000000628 antibody-producing cell Anatomy 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000029918 bioluminescence Effects 0.000 description 1
- 238000005415 bioluminescence Methods 0.000 description 1
- 239000000090 biomarker Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 238000010322 bone marrow transplantation Methods 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 230000020411 cell activation Effects 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 230000007910 cell fusion Effects 0.000 description 1
- 239000002771 cell marker Substances 0.000 description 1
- 230000012292 cell migration Effects 0.000 description 1
- 238000002659 cell therapy Methods 0.000 description 1
- 230000003833 cell viability Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 108700010039 chimeric receptor Proteins 0.000 description 1
- 230000007012 clinical effect Effects 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 230000004940 costimulation Effects 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 230000006806 disease prevention Effects 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 230000005713 exacerbation Effects 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 210000004700 fetal blood Anatomy 0.000 description 1
- 239000012091 fetal bovine serum Substances 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 108091006047 fluorescent proteins Proteins 0.000 description 1
- 102000034287 fluorescent proteins Human genes 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 201000005787 hematologic cancer Diseases 0.000 description 1
- 230000002489 hematologic effect Effects 0.000 description 1
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 1
- 210000003494 hepatocyte Anatomy 0.000 description 1
- 239000000833 heterodimer Substances 0.000 description 1
- 125000000487 histidyl group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C([H])=N1 0.000 description 1
- 102000054189 human CD80 Human genes 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 229910000042 hydrogen bromide Inorganic materials 0.000 description 1
- 125000001165 hydrophobic group Chemical group 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 238000000099 in vitro assay Methods 0.000 description 1
- 230000005917 in vivo anti-tumor Effects 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 210000004969 inflammatory cell Anatomy 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 108010001618 interleukin-20 receptor Proteins 0.000 description 1
- 108010074108 interleukin-21 Proteins 0.000 description 1
- 108010027445 interleukin-22 receptor Proteins 0.000 description 1
- 210000002510 keratinocyte Anatomy 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 201000003265 lymphadenitis Diseases 0.000 description 1
- 230000002101 lytic effect Effects 0.000 description 1
- 108010026228 mRNA guanylyltransferase Proteins 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 210000002901 mesenchymal stem cell Anatomy 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 238000012737 microarray-based gene expression Methods 0.000 description 1
- 239000011325 microbead Substances 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 210000001589 microsome Anatomy 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 210000005087 mononuclear cell Anatomy 0.000 description 1
- 230000004660 morphological change Effects 0.000 description 1
- 229940126619 mouse monoclonal antibody Drugs 0.000 description 1
- 238000012243 multiplex automated genomic engineering Methods 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 150000003833 nucleoside derivatives Chemical class 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 201000008968 osteosarcoma Diseases 0.000 description 1
- 239000006174 pH buffer Substances 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 229920002866 paraformaldehyde Polymers 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 239000002953 phosphate buffered saline Substances 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 210000001778 pluripotent stem cell Anatomy 0.000 description 1
- 229920001481 poly(stearyl methacrylate) Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 210000003289 regulatory T cell Anatomy 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000008672 reprogramming Effects 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 108091008726 retinoic acid receptors α Proteins 0.000 description 1
- 229920002477 rna polymer Polymers 0.000 description 1
- 235000002020 sage Nutrition 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 238000004062 sedimentation Methods 0.000 description 1
- 210000001082 somatic cell Anatomy 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 208000003265 stomatitis Diseases 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 238000012353 t test Methods 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000009261 transgenic effect Effects 0.000 description 1
- 102000035160 transmembrane proteins Human genes 0.000 description 1
- 108091005703 transmembrane proteins Proteins 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 210000003171 tumor-infiltrating lymphocyte Anatomy 0.000 description 1
- 241000701161 unidentified adenovirus Species 0.000 description 1
- 241000712461 unidentified influenza virus Species 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 208000007089 vaccinia Diseases 0.000 description 1
- 108700026220 vif Genes Proteins 0.000 description 1
- 230000009385 viral infection Effects 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 229940075420 xanthine Drugs 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/7051—T-cell receptor (TcR)-CD3 complex
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70517—CD8
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70521—CD28, CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/715—Receptors; Cell surface antigens; Cell surface determinants for cytokines; for lymphokines; for interferons
- C07K14/7155—Receptors; Cell surface antigens; Cell surface determinants for cytokines; for lymphokines; for interferons for interleukins [IL]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/62—DNA sequences coding for fusion proteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Medicinal Chemistry (AREA)
- Zoology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Toxicology (AREA)
- Cell Biology (AREA)
- Gastroenterology & Hepatology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Epidemiology (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562115527P | 2015-02-12 | 2015-02-12 | |
| US62/115,527 | 2015-02-12 | ||
| PCT/CA2016/050126 WO2016127257A1 (en) | 2015-02-12 | 2016-02-11 | Chimeric antigen receptors |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020186004A Division JP2021036883A (ja) | 2015-02-12 | 2020-11-06 | キメラ抗原受容体 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2018509893A JP2018509893A (ja) | 2018-04-12 |
| JP6846352B2 true JP6846352B2 (ja) | 2021-03-24 |
Family
ID=56614021
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2017541919A Active JP6846352B2 (ja) | 2015-02-12 | 2016-02-11 | キメラ抗原受容体 |
| JP2020186004A Pending JP2021036883A (ja) | 2015-02-12 | 2020-11-06 | キメラ抗原受容体 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020186004A Pending JP2021036883A (ja) | 2015-02-12 | 2020-11-06 | キメラ抗原受容体 |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US10336810B2 (enExample) |
| EP (1) | EP3256496B1 (enExample) |
| JP (2) | JP6846352B2 (enExample) |
| KR (1) | KR102607152B1 (enExample) |
| CA (1) | CA2974998C (enExample) |
| ES (1) | ES2857998T3 (enExample) |
| WO (1) | WO2016127257A1 (enExample) |
Families Citing this family (46)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3256496B1 (en) | 2015-02-12 | 2020-12-30 | University Health Network | Chimeric antigen receptors |
| DK3298033T4 (da) | 2015-05-18 | 2023-10-02 | Tcr2 Therapeutics Inc | Sammensætninger og medicinske anvendelser til tcr-reprogrammering under anvendelse af fusionsproteiner |
| GB201518816D0 (en) * | 2015-10-23 | 2015-12-09 | Autolus Ltd | Receptor |
| JP7178906B2 (ja) * | 2016-05-19 | 2022-11-28 | ザ ジェネラル ホスピタル コーポレイション | ナチュラルキラー細胞および制御性T細胞の活性を強化するためのプラットフォームである、その受容体IL-2Rβに繋ぎ止められたインターロイキン-2 |
| JP7109789B2 (ja) | 2016-08-02 | 2022-08-01 | ティーシーアール2 セラピューティクス インク. | 融合タンパク質を使用したtcrの再プログラム化のための組成物及び方法 |
| CN109996811A (zh) | 2016-08-03 | 2019-07-09 | 约翰·F·迪帕西奥 | 用于用嵌合抗原受体治疗t细胞恶性肿瘤的car-t细胞的基因编辑 |
| US20190119636A1 (en) | 2017-10-23 | 2019-04-25 | Poseida Therapeutics, Inc. | Modified stem cell memory t cells, methods of making and methods of using same |
| CA3036745A1 (en) | 2016-10-07 | 2018-04-12 | TCR2 Therapeutics Inc. | Compositions and methods for t-cell receptors reprogramming using fusion proteins |
| CA3044593A1 (en) | 2016-11-22 | 2018-05-31 | TCR2 Therapeutics Inc. | Compositions and methods for tcr reprogramming using fusion proteins |
| JP7206214B2 (ja) * | 2016-12-13 | 2023-01-17 | シアトル チルドレンズ ホスピタル (ディービーエイ シアトル チルドレンズ リサーチ インスティテュート) | インビトロ及びインビボで操作された細胞において発現された化学誘導シグナル伝達複合体の外因性薬物活性化の方法 |
| IL317065A (en) * | 2018-03-02 | 2025-01-01 | Allogene Therapeutics Inc | Chimeric inducible cytokine receptors |
| WO2019175328A1 (en) | 2018-03-14 | 2019-09-19 | Imba - Institut Für Molekulare Biotechnologie Gmbh | Bh4pathwayactivationandusethereoffortreatingcancer |
| SG11202007874XA (en) | 2018-04-27 | 2020-09-29 | Seattle Childrens Hospital Dba Seattle Childrens Res Inst | Rapamycin resistant cells |
| EP3784272A1 (en) * | 2018-04-27 | 2021-03-03 | CRISPR Therapeutics AG | Anti-bcma car-t-cells for plasma cell depletion |
| CN110615843B (zh) * | 2018-06-20 | 2023-05-09 | 上海隆耀生物科技有限公司 | 一种包含第三信号受体的嵌合抗原受体及其应用 |
| GB201814203D0 (en) * | 2018-08-31 | 2018-10-17 | King S College London | Engineered regulatory t cell |
| US20210252069A1 (en) * | 2018-09-05 | 2021-08-19 | Seogkyoung KONG | Chimeric antigen receptor for solid cancer and t cells expressing chimeric antigen receptor |
| WO2020172643A2 (en) * | 2019-02-21 | 2020-08-27 | Luk John M | Artificial immunosurveillance chimeric antigen receptor (ai-car) and cells expressing the same |
| MX2021010443A (es) | 2019-03-01 | 2021-12-10 | Allogene Therapeutics Inc | Receptores de citocinas quimericos constitutivamente activos. |
| AU2020232216B2 (en) | 2019-03-01 | 2025-09-25 | Allogene Therapeutics, Inc. | Chimeric cytokine receptors bearing a PD-1 ectodomain |
| GB201911187D0 (en) * | 2019-08-05 | 2019-09-18 | Autolus Ltd | Receptor |
| GB201912515D0 (en) * | 2019-08-30 | 2019-10-16 | King S College London | Engineered regulatory T cell |
| AU2020342544A1 (en) * | 2019-09-05 | 2022-03-24 | Poseida Therapeutics, Inc. | Allogeneic cell compositions and methods of use |
| CN110717956B (zh) * | 2019-09-30 | 2023-06-20 | 重庆大学 | 一种有限角投影超像素引导的l0范数最优化重建方法 |
| TWI717880B (zh) * | 2019-10-24 | 2021-02-01 | 中國醫藥大學附設醫院 | Hla-g特異性嵌合抗原受體、核酸、hla-g特異性嵌合抗原受體表達質體、表達hla-g特異性嵌合抗原受體細胞、其用途以及用於治療癌症之醫藥組合物 |
| CN115038453A (zh) * | 2020-01-14 | 2022-09-09 | 辛德凯因股份有限公司 | 具有改变的icd stat信号转导的cd122 |
| EP4090383A4 (en) * | 2020-01-14 | 2024-01-24 | Synthekine, Inc. | IL2 ORTHOLOGIST AND APPLICATION METHOD |
| CA3167065A1 (en) | 2020-02-24 | 2021-09-02 | Regina Junhui LIN | Bcma car-t cells with enhanced activities |
| GB2609103A (en) * | 2020-02-25 | 2023-01-25 | Quell Therapeutics Ltd | Chimeric receptors for use in engineered cells |
| US20230121433A1 (en) * | 2020-03-11 | 2023-04-20 | Poseida Therapeutics, Inc. | Chimeric stimulatory receptors and methods of use in t cell activation and differentiation |
| EP4135726A4 (en) * | 2020-04-17 | 2024-09-25 | The Council of the Queensland Institute of Medical Research | IMMUNE CELLS WITH IMPROVED FUNCTION |
| US20230167191A1 (en) * | 2020-04-24 | 2023-06-01 | Sorrento Therapeutics, Inc. | Memory Dimeric Antigen Receptors (mDARs) |
| KR102297396B1 (ko) | 2020-07-29 | 2021-09-06 | (주)티카로스 | 면역시냅스를 안정화시키는 키메라 항원 수용체(car) t 세포 |
| US12012441B2 (en) | 2020-10-26 | 2024-06-18 | Neptune Biosciences Llc | Engineered human IL-21 cytokines and methods for using the same |
| CN116782937A (zh) | 2020-12-10 | 2023-09-19 | 优特力克斯有限公司 | 抗pd-1抗体及其用途 |
| CN114716548B (zh) | 2021-01-05 | 2024-11-05 | (株)爱恩德生物 | 抗-fgfr3抗体及其用途 |
| CN113512125B (zh) * | 2021-04-26 | 2024-01-19 | 北京双赢科创生物科技有限公司 | 一种携带stat结合基序的嵌合抗原受体分子及表达该嵌合抗原受体分子的nk细胞 |
| CN118251410A (zh) | 2021-09-17 | 2024-06-25 | 帕克癌症免疫治疗研究所 | 使用il-9信号传导结构域的开关受体 |
| WO2023158997A2 (en) * | 2022-02-15 | 2023-08-24 | Memorial Sloan-Kettering Cancer Center | Compositions including killer innate-like t cells and uses therof |
| US20250154463A1 (en) * | 2022-02-15 | 2025-05-15 | Memorial Sloan-Kettering Cancer Center | Compositions including cytotoxic innate lymphoid cells and uses thereof |
| AU2023307698A1 (en) * | 2022-07-11 | 2025-01-23 | The Board Of Trustees Of The Leland Stanford Junior University | Tunable cytokine receptor signaling domains |
| KR20240016216A (ko) | 2022-07-26 | 2024-02-06 | (주)에임드바이오 | 항-ror1 항체 및 이의 용도 |
| EP4342907A1 (en) | 2022-09-21 | 2024-03-27 | AvenCell Europe GmbH | Switchable chimeric antigen receptors and their use |
| JP2025532948A (ja) | 2022-09-30 | 2025-10-03 | カリブー・バイオサイエンシーズ・インコーポレイテッド | 抗ror1キメラ抗原受容体(car)、carを発現する細胞、及び関連する方法 |
| EP4633648A1 (en) * | 2022-12-15 | 2025-10-22 | Carisma Therapeutics Inc. | Chimeric antigen receptors including jak/stat binding domains and modified immune cells |
| WO2025096673A1 (en) * | 2023-10-30 | 2025-05-08 | The Board Of Trustees Of The Leland Stanford Junior University | Compositions comprising cells targeting cancer and methods of using the same |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4694778A (en) | 1984-05-04 | 1987-09-22 | Anicon, Inc. | Chemical vapor deposition wafer boat |
| US5741899A (en) * | 1995-02-02 | 1998-04-21 | Cell Genesys, Inc. | Chimeric receptors comprising janus kinase for regulating cellular pro liferation |
| US20130266551A1 (en) | 2003-11-05 | 2013-10-10 | St. Jude Children's Research Hospital, Inc. | Chimeric receptors with 4-1bb stimulatory signaling domain |
| EP1795599A1 (en) | 2005-12-09 | 2007-06-13 | Schuler, Gerold, Prof. Dr. | Methods for generating antigen-specific effector T cells |
| US8119772B2 (en) | 2006-09-29 | 2012-02-21 | California Institute Of Technology | MART-1 T cell receptors |
| CA2751762A1 (en) | 2009-02-09 | 2010-08-12 | Helmholtz Zentrum Muenchen Deutsches Forschungszentrum Fuer Gesundheit U Nd Umwelt (Gmbh) | Repertoire of allo-restricted peptide-specific t cell receptor sequences and use thereof |
| WO2012154858A1 (en) | 2011-05-09 | 2012-11-15 | Whitehead Institute For Biomedical Research | Chaperone interaction assays and uses thereof |
| AU2013204922B2 (en) * | 2012-12-20 | 2015-05-14 | Celgene Corporation | Chimeric antigen receptors |
| EP2997134B1 (en) * | 2013-05-14 | 2020-06-24 | Board of Regents, The University of Texas System | Human application of engineered chimeric antigen receptor (car) t-cells |
| EP3004168A4 (en) | 2013-05-24 | 2017-03-01 | Board of Regents, The University of Texas System | Chimeric antigen receptor-targeting monoclonal antibodies |
| EP3256496B1 (en) | 2015-02-12 | 2020-12-30 | University Health Network | Chimeric antigen receptors |
-
2016
- 2016-02-11 EP EP16748518.4A patent/EP3256496B1/en active Active
- 2016-02-11 US US15/550,645 patent/US10336810B2/en active Active
- 2016-02-11 JP JP2017541919A patent/JP6846352B2/ja active Active
- 2016-02-11 KR KR1020177025120A patent/KR102607152B1/ko active Active
- 2016-02-11 WO PCT/CA2016/050126 patent/WO2016127257A1/en not_active Ceased
- 2016-02-11 ES ES16748518T patent/ES2857998T3/es active Active
- 2016-02-11 CA CA2974998A patent/CA2974998C/en active Active
-
2019
- 2019-05-14 US US16/411,941 patent/US10822392B2/en active Active
-
2020
- 2020-11-06 JP JP2020186004A patent/JP2021036883A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| JP2018509893A (ja) | 2018-04-12 |
| CA2974998C (en) | 2022-04-26 |
| US10822392B2 (en) | 2020-11-03 |
| US20190359685A1 (en) | 2019-11-28 |
| CA2974998A1 (en) | 2016-08-18 |
| EP3256496A1 (en) | 2017-12-20 |
| ES2857998T3 (es) | 2021-09-29 |
| KR102607152B1 (ko) | 2023-11-27 |
| EP3256496B1 (en) | 2020-12-30 |
| EP3256496A4 (en) | 2018-12-12 |
| US10336810B2 (en) | 2019-07-02 |
| JP2021036883A (ja) | 2021-03-11 |
| KR20170116084A (ko) | 2017-10-18 |
| WO2016127257A1 (en) | 2016-08-18 |
| US20180037630A1 (en) | 2018-02-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6846352B2 (ja) | キメラ抗原受容体 | |
| AU2024227744A1 (en) | Methods of making chimeric antigen receptor-expressing cells | |
| US20200370012A1 (en) | Methods of making chimeric antigen receptor-expressing cells | |
| JP2024518011A (ja) | 多様な免疫細胞のための単鎖および多鎖合成抗原受容体 | |
| KR20220124173A (ko) | Lilrb1-기반 키메라 항원 수용체 | |
| CA3032054A1 (en) | Combination therapies of chimeric antigen receptors and pd-1 inhibitors | |
| JP2023002556A (ja) | 固形腫瘍を治療するためのキメラ抗原受容体細胞 | |
| US20210379149A1 (en) | Increasing or Maintaining T-Cell Subpopulations in Adoptive T-Cell Therapy | |
| CN110257338B (zh) | 嵌合细胞因子受体 | |
| CN110511912B (zh) | 免疫细胞的功能调节 | |
| US20230192848A1 (en) | Engineered cell compositions and methods of use thereof | |
| TW202026006A (zh) | 使用融合蛋白進行tcr再程式化之組成物及方法 | |
| CN111655720A (zh) | Nkg2d daric受体 | |
| WO2022232277A1 (en) | COMPOSITIONS AND METHODS FOR TCR REPROGRAMMING USING FUSION PROTEINS AND TGFβR SWITCH | |
| US20240226154A9 (en) | Car-t constructs comprising a novel cd19 binder combined with il18 and methods of using the same | |
| CN117202921A (zh) | 用于多种免疫细胞的单链和多链合成抗原受体 | |
| US11701385B2 (en) | Modulation of cell function for immunotherapy | |
| KR20250046298A (ko) | 자연 살해 세포 기능을 향상시키기 위한 표적 유전자의 유전자 편집 | |
| US20240269182A1 (en) | Modified Chimeric Antigen Receptor and Use thereof | |
| KR20240034205A (ko) | 항EGFRviii 항체, 폴리펩타이드, 상기 폴리펩타이드를 발현하는 세포, 상기 세포를 포함하는 의약 조성물, 상기 세포의 제조 방법 및 상기 폴리펩타이드를 코딩하는 염기서열을 포함하는 폴리뉴클레오티드 또는 벡터 | |
| US12043654B2 (en) | Anti-GCC antibody and CAR thereof for treating digestive system cancer | |
| CN116732068A (zh) | 一种编码嵌合抗原受体的基因及其载体、修饰细胞和应用 | |
| TW202216752A (zh) | 用於過繼細胞療法之提供靶向共刺激之嵌合分子 | |
| JP2022001021A (ja) | Cd26特異的キメラ抗原受容体 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20181121 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20190924 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20191001 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20200106 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200228 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20200707 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20201106 |
|
| C60 | Trial request (containing other claim documents, opposition documents) |
Free format text: JAPANESE INTERMEDIATE CODE: C60 Effective date: 20201106 |
|
| C11 | Written invitation by the commissioner to file amendments |
Free format text: JAPANESE INTERMEDIATE CODE: C11 Effective date: 20201117 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20201207 |
|
| C21 | Notice of transfer of a case for reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C21 Effective date: 20201208 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20210202 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20210301 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6846352 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |