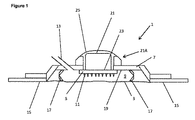
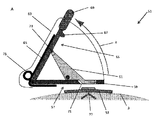
JP6537531B2 - 体液抽出装置、アプリケータ装置及び関連する方法 - Google Patents
体液抽出装置、アプリケータ装置及び関連する方法 Download PDFInfo
- Publication number
- JP6537531B2 JP6537531B2 JP2016565577A JP2016565577A JP6537531B2 JP 6537531 B2 JP6537531 B2 JP 6537531B2 JP 2016565577 A JP2016565577 A JP 2016565577A JP 2016565577 A JP2016565577 A JP 2016565577A JP 6537531 B2 JP6537531 B2 JP 6537531B2
- Authority
- JP
- Japan
- Prior art keywords
- microneedle array
- microneedles
- hammer
- microneedle
- array
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 210000001124 body fluid Anatomy 0.000 title claims description 30
- 239000010839 body fluid Substances 0.000 title claims description 28
- 238000000034 method Methods 0.000 title description 39
- 238000000605 extraction Methods 0.000 title description 13
- 238000002360 preparation method Methods 0.000 claims description 36
- 230000000295 complement effect Effects 0.000 claims description 10
- 210000003491 skin Anatomy 0.000 description 72
- 210000003722 extracellular fluid Anatomy 0.000 description 38
- 239000000463 material Substances 0.000 description 32
- 239000012530 fluid Substances 0.000 description 27
- 230000035515 penetration Effects 0.000 description 17
- 206010019280 Heart failures Diseases 0.000 description 15
- 239000010410 layer Substances 0.000 description 14
- 206010007559 Cardiac failure congestive Diseases 0.000 description 11
- 206010030113 Oedema Diseases 0.000 description 11
- 206010016803 Fluid overload Diseases 0.000 description 10
- 238000011282 treatment Methods 0.000 description 10
- 206010025282 Lymphoedema Diseases 0.000 description 9
- 238000003780 insertion Methods 0.000 description 9
- 230000037431 insertion Effects 0.000 description 9
- 208000002502 lymphedema Diseases 0.000 description 9
- 238000007789 sealing Methods 0.000 description 9
- 238000000502 dialysis Methods 0.000 description 8
- 210000004379 membrane Anatomy 0.000 description 8
- 239000012528 membrane Substances 0.000 description 8
- 239000007787 solid Substances 0.000 description 8
- 239000000853 adhesive Substances 0.000 description 7
- 230000001070 adhesive effect Effects 0.000 description 7
- 239000006260 foam Substances 0.000 description 7
- 210000001519 tissue Anatomy 0.000 description 7
- 208000001647 Renal Insufficiency Diseases 0.000 description 6
- 210000003734 kidney Anatomy 0.000 description 6
- 201000006370 kidney failure Diseases 0.000 description 6
- 206010051055 Deep vein thrombosis Diseases 0.000 description 5
- 206010047249 Venous thrombosis Diseases 0.000 description 5
- 208000014674 injury Diseases 0.000 description 5
- 230000000149 penetrating effect Effects 0.000 description 5
- 238000003860 storage Methods 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- 206010028980 Neoplasm Diseases 0.000 description 4
- 238000003491 array Methods 0.000 description 4
- 201000011510 cancer Diseases 0.000 description 4
- 230000001684 chronic effect Effects 0.000 description 4
- 239000002934 diuretic Substances 0.000 description 4
- 229940030606 diuretics Drugs 0.000 description 4
- 239000000835 fiber Substances 0.000 description 4
- 238000001631 haemodialysis Methods 0.000 description 4
- 230000036541 health Effects 0.000 description 4
- 230000000322 hemodialysis Effects 0.000 description 4
- 230000003116 impacting effect Effects 0.000 description 4
- 238000012959 renal replacement therapy Methods 0.000 description 4
- 230000008733 trauma Effects 0.000 description 4
- 206010015150 Erythema Diseases 0.000 description 3
- 239000004820 Pressure-sensitive adhesive Substances 0.000 description 3
- 206010042674 Swelling Diseases 0.000 description 3
- 238000009825 accumulation Methods 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 230000000875 corresponding effect Effects 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 230000003907 kidney function Effects 0.000 description 3
- 210000001165 lymph node Anatomy 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 239000002207 metabolite Substances 0.000 description 3
- 239000011148 porous material Substances 0.000 description 3
- 238000003825 pressing Methods 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 210000000434 stratum corneum Anatomy 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 230000008961 swelling Effects 0.000 description 3
- 238000002054 transplantation Methods 0.000 description 3
- 230000002485 urinary effect Effects 0.000 description 3
- 208000037157 Azotemia Diseases 0.000 description 2
- 235000017166 Bambusa arundinacea Nutrition 0.000 description 2
- 235000017491 Bambusa tulda Nutrition 0.000 description 2
- 241001330002 Bambuseae Species 0.000 description 2
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 2
- 208000032912 Local swelling Diseases 0.000 description 2
- 206010030124 Oedema peripheral Diseases 0.000 description 2
- 235000015334 Phyllostachys viridis Nutrition 0.000 description 2
- 206010040880 Skin irritation Diseases 0.000 description 2
- 208000027418 Wounds and injury Diseases 0.000 description 2
- 239000012790 adhesive layer Substances 0.000 description 2
- 230000002421 anti-septic effect Effects 0.000 description 2
- 239000011425 bamboo Substances 0.000 description 2
- 230000017531 blood circulation Effects 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 231100000321 erythema Toxicity 0.000 description 2
- 210000003414 extremity Anatomy 0.000 description 2
- 239000000017 hydrogel Substances 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 229960004194 lidocaine Drugs 0.000 description 2
- 210000003141 lower extremity Anatomy 0.000 description 2
- 210000004324 lymphatic system Anatomy 0.000 description 2
- 230000037368 penetrate the skin Effects 0.000 description 2
- 239000012466 permeate Substances 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 239000004926 polymethyl methacrylate Substances 0.000 description 2
- 229920002635 polyurethane Polymers 0.000 description 2
- 239000004814 polyurethane Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 231100000475 skin irritation Toxicity 0.000 description 2
- 230000036556 skin irritation Effects 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 230000002269 spontaneous effect Effects 0.000 description 2
- 230000001954 sterilising effect Effects 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 230000001960 triggered effect Effects 0.000 description 2
- 238000000108 ultra-filtration Methods 0.000 description 2
- 208000009852 uremia Diseases 0.000 description 2
- 206010003226 Arteriovenous fistula Diseases 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 206010007882 Cellulitis Diseases 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- 206010011878 Deafness Diseases 0.000 description 1
- 208000000059 Dyspnea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- 238000000729 Fisher's exact test Methods 0.000 description 1
- 206010016807 Fluid retention Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000002720 Malnutrition Diseases 0.000 description 1
- 208000034906 Medical device complication Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 208000015695 Primary lymphedema Diseases 0.000 description 1
- 206010040047 Sepsis Diseases 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 208000007536 Thrombosis Diseases 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 238000007630 basic procedure Methods 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 230000036996 cardiovascular health Effects 0.000 description 1
- 208000020832 chronic kidney disease Diseases 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 231100000895 deafness Toxicity 0.000 description 1
- 230000000249 desinfective effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 230000002526 effect on cardiovascular system Effects 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000013013 elastic material Substances 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 208000028208 end stage renal disease Diseases 0.000 description 1
- 201000000523 end stage renal failure Diseases 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000010304 firing Methods 0.000 description 1
- 238000011010 flushing procedure Methods 0.000 description 1
- 235000019138 food restriction Nutrition 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 208000016354 hearing loss disease Diseases 0.000 description 1
- 230000004217 heart function Effects 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000010874 in vitro model Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 235000014659 low sodium diet Nutrition 0.000 description 1
- 210000002751 lymph Anatomy 0.000 description 1
- 210000004880 lymph fluid Anatomy 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000001071 malnutrition Effects 0.000 description 1
- 235000000824 malnutrition Nutrition 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 230000027939 micturition Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 208000015380 nutritional deficiency disease Diseases 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 210000003200 peritoneal cavity Anatomy 0.000 description 1
- 210000004303 peritoneum Anatomy 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000037390 scarring Effects 0.000 description 1
- 208000013220 shortness of breath Diseases 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 235000015424 sodium Nutrition 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 239000002441 uremic toxin Substances 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 210000001835 viscera Anatomy 0.000 description 1
- 230000002747 voluntary effect Effects 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150977—Arrays of piercing elements for simultaneous piercing
- A61B5/150984—Microneedles or microblades
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150015—Source of blood
- A61B5/150022—Source of blood for capillary blood or interstitial fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150053—Details for enhanced collection of blood or interstitial fluid at the sample site, e.g. by applying compression, heat, vibration, ultrasound, suction or vacuum to tissue; for reduction of pain or discomfort; Skin piercing elements, e.g. blades, needles, lancets or canulas, with adjustable piercing speed
- A61B5/150061—Means for enhancing collection
- A61B5/150099—Means for enhancing collection by negative pressure, other than vacuum extraction into a syringe by pulling on the piston rod or into pre-evacuated tubes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/151—Devices specially adapted for taking samples of capillary blood, e.g. by lancets, needles or blades
- A61B5/15101—Details
- A61B5/15103—Piercing procedure
- A61B5/15107—Piercing being assisted by a triggering mechanism
- A61B5/15113—Manually triggered, i.e. the triggering requires a deliberate action by the user such as pressing a drive button
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/151—Devices specially adapted for taking samples of capillary blood, e.g. by lancets, needles or blades
- A61B5/15101—Details
- A61B5/15115—Driving means for propelling the piercing element to pierce the skin, e.g. comprising mechanisms based on shape memory alloys, magnetism, solenoids, piezoelectric effect, biased elements, resilient elements, vacuum or compressed fluids
- A61B5/15117—Driving means for propelling the piercing element to pierce the skin, e.g. comprising mechanisms based on shape memory alloys, magnetism, solenoids, piezoelectric effect, biased elements, resilient elements, vacuum or compressed fluids comprising biased elements, resilient elements or a spring, e.g. a helical spring, leaf spring, or elastic strap
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/71—Suction drainage systems
- A61M1/76—Handpieces
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/84—Drainage tubes; Aspiration tips
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B10/00—Instruments for taking body samples for diagnostic purposes; Other methods or instruments for diagnosis, e.g. for vaccination diagnosis, sex determination or ovulation-period determination; Throat striking implements
- A61B10/0045—Devices for taking samples of body liquids
- A61B2010/008—Interstitial fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue
- A61B5/14507—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue specially adapted for measuring characteristics of body fluids other than blood
- A61B5/1451—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue specially adapted for measuring characteristics of body fluids other than blood for interstitial fluid
- A61B5/14514—Measuring characteristics of blood in vivo, e.g. gas concentration or pH-value ; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid or cerebral tissue specially adapted for measuring characteristics of body fluids other than blood for interstitial fluid using means for aiding extraction of interstitial fluid, e.g. microneedles or suction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150374—Details of piercing elements or protective means for preventing accidental injuries by such piercing elements
- A61B5/150381—Design of piercing elements
- A61B5/150412—Pointed piercing elements, e.g. needles, lancets for piercing the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Surgery (AREA)
- Pathology (AREA)
- Molecular Biology (AREA)
- Medical Informatics (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Vascular Medicine (AREA)
- Anesthesiology (AREA)
- Dermatology (AREA)
- Pain & Pain Management (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- External Artificial Organs (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GBGB1401133.2A GB201401133D0 (en) | 2014-01-23 | 2014-01-23 | Fluid extraction device, applicator device and associated methods |
| GB1401133.2 | 2014-01-23 | ||
| PCT/GB2015/050164 WO2015110833A1 (en) | 2014-01-23 | 2015-01-23 | Fluid extraction device, applicator device and associated methods |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2017509449A JP2017509449A (ja) | 2017-04-06 |
| JP2017509449A5 JP2017509449A5 (enExample) | 2018-03-08 |
| JP6537531B2 true JP6537531B2 (ja) | 2019-07-03 |
Family
ID=50287444
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016565577A Expired - Fee Related JP6537531B2 (ja) | 2014-01-23 | 2015-01-23 | 体液抽出装置、アプリケータ装置及び関連する方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US10441691B2 (enExample) |
| EP (1) | EP3096687B1 (enExample) |
| JP (1) | JP6537531B2 (enExample) |
| GB (1) | GB201401133D0 (enExample) |
| WO (1) | WO2015110833A1 (enExample) |
Families Citing this family (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2912965T3 (es) | 2015-09-09 | 2022-05-30 | Drawbridge Health Inc | Dispositivos para la recopilación, estabilización y conservación de muestras |
| CN105266852B (zh) * | 2015-11-24 | 2017-12-01 | 贾爱娟 | 一种医疗检验用装置 |
| EP3395397B1 (en) * | 2015-12-21 | 2022-10-12 | Medrx Co., Ltd. | Microneedle patch applicator housing |
| CN210383905U (zh) | 2017-01-10 | 2020-04-24 | 集联健康有限公司 | 一种用于从受试者收集流体样品的装置以及运输套筒 |
| WO2019126735A1 (en) | 2017-12-21 | 2019-06-27 | Georgia Tech Research Corporation | Methods and systems for improved collection of interstitial fluid |
| JPWO2019188907A1 (ja) * | 2018-03-29 | 2021-04-08 | 富士フイルム株式会社 | 採血用ランセット及び血液検査キット |
| JP2021101749A (ja) * | 2018-03-29 | 2021-07-15 | テルモ株式会社 | 医療器具 |
| US20210353267A1 (en) * | 2018-10-19 | 2021-11-18 | Kenota Inc. | Interstitial fluid extraction |
| WO2020146045A1 (en) * | 2019-01-11 | 2020-07-16 | University Of Cincinnati | Continuous extraction and sensing of interstitial fluid |
| DE102019200557A1 (de) * | 2019-01-17 | 2020-07-23 | Lts Lohmann Therapie-Systeme Ag | Applikator |
| JP7074714B2 (ja) * | 2019-04-17 | 2022-05-24 | 富士フイルム株式会社 | 収容容器、マイクロニードルユニット、収容容器群、及びマイクロニードルユニットの製造方法 |
| US20210153848A1 (en) * | 2019-11-21 | 2021-05-27 | Imcomet B.V. | Skin cancer treatment |
| US12053284B2 (en) * | 2021-11-08 | 2024-08-06 | Satio, Inc. | Dermal patch for collecting a physiological sample |
| US11510602B1 (en) * | 2021-11-08 | 2022-11-29 | Satio, Inc. | Dermal patch for collecting a physiological sample |
| US12023156B2 (en) | 2021-10-13 | 2024-07-02 | Satio, Inc. | Dermal patch for collecting a physiological sample |
| US11877848B2 (en) * | 2021-11-08 | 2024-01-23 | Satio, Inc. | Dermal patch for collecting a physiological sample |
| US12214346B2 (en) | 2021-10-13 | 2025-02-04 | Satio, Inc. | Dermal patch with a diagnostic test strip |
| US11964121B2 (en) | 2021-10-13 | 2024-04-23 | Satio, Inc. | Mono dose dermal patch for pharmaceutical delivery |
| US12178979B2 (en) | 2021-10-13 | 2024-12-31 | Satio, Inc. | Dermal patch for delivering a pharmaceutical |
| US12048543B2 (en) * | 2021-11-08 | 2024-07-30 | Satio, Inc. | Dermal patch for collecting a physiological sample with removable vial |
| US11452474B1 (en) | 2021-04-14 | 2022-09-27 | Satio, Inc. | Dual lever dermal patch system |
| SE546125C2 (en) * | 2022-10-13 | 2024-05-28 | Ascilion Ab | A device for sampling a bodily fluid from a test subject |
| CN115644867A (zh) * | 2022-12-02 | 2023-01-31 | 苏州纳通生物纳米技术有限公司 | 采样装置、采样仪和采样方法 |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2239053T3 (es) * | 1999-12-16 | 2005-09-16 | Alza Corporation | Dispositivo para incrementar el flujo transdermico de sustancias de muestreo. |
| AT409253B (de) | 2000-07-20 | 2002-07-25 | Innova Patent Gmbh | Anlage zur beförderung von personen und verfahren zum betrieb einer derartigen anlage |
| ATE485331T1 (de) | 2003-01-06 | 2010-11-15 | Nektar Therapeutics | Thiolselektive wasserlösliche polymerderivate |
| WO2005044333A2 (en) | 2003-10-31 | 2005-05-19 | Alza Corporation | Self-actuating applicator for microprojection array |
| WO2005123173A1 (en) * | 2004-06-10 | 2005-12-29 | 3M Innovative Properties Company | Patch application device and kit |
| GB0802447D0 (en) | 2008-02-09 | 2008-03-19 | Univ Manchester | Fluid extraction device, associated materials and methods |
| US9295417B2 (en) | 2011-04-29 | 2016-03-29 | Seventh Sense Biosystems, Inc. | Systems and methods for collecting fluid from a subject |
| US10010706B2 (en) * | 2009-07-31 | 2018-07-03 | 3M Innovative Properties Company | Hollow microneedle arrays |
| US20110172645A1 (en) * | 2010-01-08 | 2011-07-14 | Ratio, Inc. | Wearable drug delivery device including integrated pumping and activation elements |
| SG184128A1 (en) | 2010-03-17 | 2012-10-30 | Nanomed Devices Inc | A built-in non-verbal instructional device integratable to applicators |
| ES2565805T3 (es) | 2010-11-09 | 2016-04-07 | Seventh Sense Biosystems, Inc. | Sistemas e interfaces para el muestreo de sangre |
| KR102237667B1 (ko) * | 2011-04-29 | 2021-04-12 | 세븐쓰 센스 바이오시스템즈, 인크. | 유체들의 전달 및/또는 수용 |
-
2014
- 2014-01-23 GB GBGB1401133.2A patent/GB201401133D0/en not_active Ceased
-
2015
- 2015-01-23 WO PCT/GB2015/050164 patent/WO2015110833A1/en not_active Ceased
- 2015-01-23 JP JP2016565577A patent/JP6537531B2/ja not_active Expired - Fee Related
- 2015-01-23 EP EP15701415.0A patent/EP3096687B1/en active Active
- 2015-01-23 US US15/113,249 patent/US10441691B2/en not_active Expired - Fee Related
-
2019
- 2019-09-30 US US16/587,857 patent/US20200101205A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| US10441691B2 (en) | 2019-10-15 |
| GB201401133D0 (en) | 2014-03-12 |
| EP3096687A1 (en) | 2016-11-30 |
| WO2015110833A1 (en) | 2015-07-30 |
| EP3096687B1 (en) | 2021-06-09 |
| US20200101205A1 (en) | 2020-04-02 |
| US20170021067A1 (en) | 2017-01-26 |
| JP2017509449A (ja) | 2017-04-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6537531B2 (ja) | 体液抽出装置、アプリケータ装置及び関連する方法 | |
| US8366649B2 (en) | Manually operated disposable single-needle circuit for extracorporeal treatment of blood | |
| ES2612779T3 (es) | Sistema de parches transdérmicos de administración de fármacos, método de fabricación del mismo y método de uso del mismo | |
| US20150202418A1 (en) | Microneedle-based devices and methods for the removal of fluid from a body | |
| US8585682B2 (en) | Fluid extraction or filtration device, associated materials and methods | |
| US20100100119A1 (en) | Mechanical vein lifter | |
| US20110202014A1 (en) | Adapter device for application of small amounts of fat graft material by use of syringes | |
| JP2012513826A (ja) | マニホールド構造体を用いた減圧創傷治療システムおよび方法 | |
| TWI566791B (zh) | 引流淋巴液至靜脈之裝置 | |
| WO2013067110A1 (en) | Method and device for injecting a fluid into an artificial venous structure | |
| RU2449753C2 (ru) | Прокалывающее кожу устройство для лечения акне | |
| CN112791267B (zh) | 一种输液港 | |
| CN221950437U (zh) | 一种冲击波透皮给药水凝胶微针装置 | |
| JP2020531194A (ja) | 足底筋膜炎の治療及び脂肪移植に用いる方法及び装置 | |
| US20130218104A1 (en) | Devices and methods for delivery of agents to biological tissue | |
| RU2850704C1 (ru) | Имплантируемый инфузионный порт | |
| CN215018680U (zh) | 一种介入治疗用穿刺针 | |
| CN219462148U (zh) | 一种用于固定蝶翼针的皮肤保护垫 | |
| CN101268960A (zh) | 处理痤疮的皮肤刺穿装置 | |
| CN208823733U (zh) | 一种胸腔引流管固定装置 | |
| AU2014202446A1 (en) | Fluid extraction methods | |
| CN119454192A (zh) | 一种麻醉穿刺针导向结构 | |
| CN201684234U (zh) | 皮下组织注入装置 | |
| EP2968771A1 (en) | Subcutaneous hydration system, method, and device | |
| Bell et al. | Could hydrocephalus shunts have a role in the treatment of lymphoedema? |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180123 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20180123 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20181030 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20181031 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190129 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20190507 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20190604 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6537531 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| LAPS | Cancellation because of no payment of annual fees |