JP6281802B2 - 固液混合物処理のための方法及び装置 - Google Patents
固液混合物処理のための方法及び装置 Download PDFInfo
- Publication number
- JP6281802B2 JP6281802B2 JP2011537002A JP2011537002A JP6281802B2 JP 6281802 B2 JP6281802 B2 JP 6281802B2 JP 2011537002 A JP2011537002 A JP 2011537002A JP 2011537002 A JP2011537002 A JP 2011537002A JP 6281802 B2 JP6281802 B2 JP 6281802B2
- Authority
- JP
- Japan
- Prior art keywords
- reactor
- solid
- reaction
- liquid mixture
- carbon
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000000034 method Methods 0.000 title claims description 360
- 239000007788 liquid Substances 0.000 title claims description 185
- 239000000203 mixture Substances 0.000 title claims description 174
- 238000012545 processing Methods 0.000 title claims description 38
- 230000008569 process Effects 0.000 claims description 233
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 197
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 177
- 229910052799 carbon Inorganic materials 0.000 claims description 164
- 238000006243 chemical reaction Methods 0.000 claims description 145
- 239000000463 material Substances 0.000 claims description 135
- 239000000446 fuel Substances 0.000 claims description 113
- 239000000047 product Substances 0.000 claims description 82
- 239000007795 chemical reaction product Substances 0.000 claims description 77
- 239000007787 solid Substances 0.000 claims description 62
- 238000004519 manufacturing process Methods 0.000 claims description 48
- 239000003864 humus Substances 0.000 claims description 47
- 239000011541 reaction mixture Substances 0.000 claims description 41
- 238000002156 mixing Methods 0.000 claims description 40
- 230000002829 reductive effect Effects 0.000 claims description 27
- 239000012528 membrane Substances 0.000 claims description 26
- 108010005094 Advanced Glycation End Products Proteins 0.000 claims description 23
- 239000007858 starting material Substances 0.000 claims description 22
- 238000010438 heat treatment Methods 0.000 claims description 18
- 238000012546 transfer Methods 0.000 claims description 15
- 238000006073 displacement reaction Methods 0.000 claims description 11
- 239000003337 fertilizer Substances 0.000 claims description 7
- 239000012467 final product Substances 0.000 claims description 7
- 238000011533 pre-incubation Methods 0.000 claims description 7
- 238000005086 pumping Methods 0.000 claims description 6
- 239000011248 coating agent Substances 0.000 claims description 5
- 238000000576 coating method Methods 0.000 claims description 5
- 239000002028 Biomass Substances 0.000 description 104
- 241000196324 Embryophyta Species 0.000 description 47
- 239000003054 catalyst Substances 0.000 description 42
- 239000000126 substance Substances 0.000 description 41
- 239000002609 medium Substances 0.000 description 35
- 238000000926 separation method Methods 0.000 description 33
- 230000007062 hydrolysis Effects 0.000 description 32
- 238000006460 hydrolysis reaction Methods 0.000 description 32
- 239000007789 gas Substances 0.000 description 29
- HBMJWWWQQXIZIP-UHFFFAOYSA-N silicon carbide Chemical compound [Si+]#[C-] HBMJWWWQQXIZIP-UHFFFAOYSA-N 0.000 description 29
- 229910010271 silicon carbide Inorganic materials 0.000 description 27
- 239000003245 coal Substances 0.000 description 25
- 238000001035 drying Methods 0.000 description 25
- 230000035484 reaction time Effects 0.000 description 24
- 239000000725 suspension Substances 0.000 description 23
- 238000001914 filtration Methods 0.000 description 21
- 239000002245 particle Substances 0.000 description 21
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 20
- 239000006185 dispersion Substances 0.000 description 20
- 238000003860 storage Methods 0.000 description 20
- 238000005496 tempering Methods 0.000 description 20
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 18
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 18
- 229920002472 Starch Polymers 0.000 description 18
- 239000002803 fossil fuel Substances 0.000 description 17
- 239000007791 liquid phase Substances 0.000 description 17
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 16
- 239000013067 intermediate product Substances 0.000 description 16
- 229910052751 metal Inorganic materials 0.000 description 16
- 239000002184 metal Substances 0.000 description 16
- 235000019698 starch Nutrition 0.000 description 16
- 239000008107 starch Substances 0.000 description 16
- 229910052717 sulfur Inorganic materials 0.000 description 16
- 239000011593 sulfur Substances 0.000 description 16
- 239000002956 ash Substances 0.000 description 15
- 229910010293 ceramic material Inorganic materials 0.000 description 15
- 238000011534 incubation Methods 0.000 description 15
- 230000032258 transport Effects 0.000 description 15
- 230000002378 acidificating effect Effects 0.000 description 13
- 238000009835 boiling Methods 0.000 description 12
- 239000002994 raw material Substances 0.000 description 12
- -1 silicon (silicon) compound Chemical class 0.000 description 12
- 239000007790 solid phase Substances 0.000 description 11
- 238000003756 stirring Methods 0.000 description 11
- 230000015572 biosynthetic process Effects 0.000 description 10
- 239000001569 carbon dioxide Substances 0.000 description 10
- 229910002092 carbon dioxide Inorganic materials 0.000 description 10
- 238000003763 carbonization Methods 0.000 description 10
- 238000002485 combustion reaction Methods 0.000 description 10
- 239000010439 graphite Substances 0.000 description 10
- 229910002804 graphite Inorganic materials 0.000 description 10
- 229910052500 inorganic mineral Inorganic materials 0.000 description 10
- 239000011707 mineral Substances 0.000 description 10
- 239000000376 reactant Substances 0.000 description 10
- 150000001875 compounds Chemical class 0.000 description 9
- 230000007423 decrease Effects 0.000 description 9
- 238000001704 evaporation Methods 0.000 description 9
- 239000000835 fiber Substances 0.000 description 9
- 230000008961 swelling Effects 0.000 description 9
- 238000011144 upstream manufacturing Methods 0.000 description 9
- 239000003610 charcoal Substances 0.000 description 8
- 238000004140 cleaning Methods 0.000 description 8
- 230000008020 evaporation Effects 0.000 description 8
- 239000000499 gel Substances 0.000 description 8
- 238000000227 grinding Methods 0.000 description 8
- 239000000543 intermediate Substances 0.000 description 8
- 239000003077 lignite Substances 0.000 description 8
- 150000002739 metals Chemical class 0.000 description 8
- 239000000377 silicon dioxide Substances 0.000 description 8
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 7
- 229910000831 Steel Inorganic materials 0.000 description 7
- 239000000460 chlorine Substances 0.000 description 7
- 229910052801 chlorine Inorganic materials 0.000 description 7
- 238000001816 cooling Methods 0.000 description 7
- 239000012212 insulator Substances 0.000 description 7
- 229910052757 nitrogen Inorganic materials 0.000 description 7
- 235000012239 silicon dioxide Nutrition 0.000 description 7
- 239000002689 soil Substances 0.000 description 7
- 238000003980 solgel method Methods 0.000 description 7
- 239000010959 steel Substances 0.000 description 7
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 6
- 150000001722 carbon compounds Chemical class 0.000 description 6
- 235000013339 cereals Nutrition 0.000 description 6
- 238000010586 diagram Methods 0.000 description 6
- 239000000428 dust Substances 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- 239000003921 oil Substances 0.000 description 6
- 229910052760 oxygen Inorganic materials 0.000 description 6
- 239000001301 oxygen Substances 0.000 description 6
- 239000003415 peat Substances 0.000 description 6
- 239000011148 porous material Substances 0.000 description 6
- 238000010298 pulverizing process Methods 0.000 description 6
- 238000006722 reduction reaction Methods 0.000 description 6
- 238000007873 sieving Methods 0.000 description 6
- 239000002699 waste material Substances 0.000 description 6
- 239000002351 wastewater Substances 0.000 description 6
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 5
- 238000000137 annealing Methods 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 229910052791 calcium Inorganic materials 0.000 description 5
- 239000011575 calcium Substances 0.000 description 5
- 235000014633 carbohydrates Nutrition 0.000 description 5
- 150000001720 carbohydrates Chemical class 0.000 description 5
- 229920002678 cellulose Polymers 0.000 description 5
- 239000001913 cellulose Substances 0.000 description 5
- 238000010924 continuous production Methods 0.000 description 5
- 238000013461 design Methods 0.000 description 5
- 239000000284 extract Substances 0.000 description 5
- 239000008187 granular material Substances 0.000 description 5
- 229910010272 inorganic material Inorganic materials 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 239000008188 pellet Substances 0.000 description 5
- 238000001556 precipitation Methods 0.000 description 5
- 238000011084 recovery Methods 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 238000001223 reverse osmosis Methods 0.000 description 5
- 239000002023 wood Substances 0.000 description 5
- 229920000049 Carbon (fiber) Polymers 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 4
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 4
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 4
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 4
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 4
- 239000008346 aqueous phase Substances 0.000 description 4
- 239000004917 carbon fiber Substances 0.000 description 4
- 239000002131 composite material Substances 0.000 description 4
- 238000009826 distribution Methods 0.000 description 4
- 238000005485 electric heating Methods 0.000 description 4
- 230000005484 gravity Effects 0.000 description 4
- 229910001385 heavy metal Inorganic materials 0.000 description 4
- 239000001257 hydrogen Substances 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 239000011147 inorganic material Substances 0.000 description 4
- 229920005610 lignin Polymers 0.000 description 4
- 230000007246 mechanism Effects 0.000 description 4
- 229910052750 molybdenum Inorganic materials 0.000 description 4
- 239000011733 molybdenum Substances 0.000 description 4
- 229910017604 nitric acid Inorganic materials 0.000 description 4
- 239000011574 phosphorus Substances 0.000 description 4
- 229910052698 phosphorus Inorganic materials 0.000 description 4
- 238000006116 polymerization reaction Methods 0.000 description 4
- 239000002244 precipitate Substances 0.000 description 4
- 239000003380 propellant Substances 0.000 description 4
- 150000003839 salts Chemical class 0.000 description 4
- 229910052710 silicon Inorganic materials 0.000 description 4
- 239000010703 silicon Substances 0.000 description 4
- 229910001220 stainless steel Inorganic materials 0.000 description 4
- 238000000108 ultra-filtration Methods 0.000 description 4
- 241000219310 Beta vulgaris subsp. vulgaris Species 0.000 description 3
- 244000025254 Cannabis sativa Species 0.000 description 3
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- 235000021536 Sugar beet Nutrition 0.000 description 3
- 238000009825 accumulation Methods 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 210000004027 cell Anatomy 0.000 description 3
- 239000000919 ceramic Substances 0.000 description 3
- 239000011651 chromium Substances 0.000 description 3
- 229910052804 chromium Inorganic materials 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000007906 compression Methods 0.000 description 3
- 230000006835 compression Effects 0.000 description 3
- 239000012141 concentrate Substances 0.000 description 3
- 239000000470 constituent Substances 0.000 description 3
- 230000008878 coupling Effects 0.000 description 3
- 238000010168 coupling process Methods 0.000 description 3
- 238000005859 coupling reaction Methods 0.000 description 3
- 238000000354 decomposition reaction Methods 0.000 description 3
- 238000007791 dehumidification Methods 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 238000000855 fermentation Methods 0.000 description 3
- 230000004151 fermentation Effects 0.000 description 3
- 235000013305 food Nutrition 0.000 description 3
- 229910052909 inorganic silicate Inorganic materials 0.000 description 3
- 230000007774 longterm Effects 0.000 description 3
- 150000002894 organic compounds Chemical class 0.000 description 3
- 239000011368 organic material Substances 0.000 description 3
- 239000010815 organic waste Substances 0.000 description 3
- 239000000123 paper Substances 0.000 description 3
- 239000011236 particulate material Substances 0.000 description 3
- 239000013618 particulate matter Substances 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 3
- 239000004810 polytetrafluoroethylene Substances 0.000 description 3
- 235000019353 potassium silicate Nutrition 0.000 description 3
- 230000001681 protective effect Effects 0.000 description 3
- 108090000623 proteins and genes Proteins 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 238000004062 sedimentation Methods 0.000 description 3
- NTHWMYGWWRZVTN-UHFFFAOYSA-N sodium silicate Chemical compound [Na+].[Na+].[O-][Si]([O-])=O NTHWMYGWWRZVTN-UHFFFAOYSA-N 0.000 description 3
- 239000010902 straw Substances 0.000 description 3
- 108010088751 Albumins Proteins 0.000 description 2
- 102000009027 Albumins Human genes 0.000 description 2
- 229910000570 Cupronickel Inorganic materials 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 2
- 239000004472 Lysine Substances 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- 229910002651 NO3 Inorganic materials 0.000 description 2
- 229910000990 Ni alloy Inorganic materials 0.000 description 2
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- 240000000111 Saccharum officinarum Species 0.000 description 2
- 235000007201 Saccharum officinarum Nutrition 0.000 description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 2
- 240000008042 Zea mays Species 0.000 description 2
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 2
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 2
- HMDDXIMCDZRSNE-UHFFFAOYSA-N [C].[Si] Chemical compound [C].[Si] HMDDXIMCDZRSNE-UHFFFAOYSA-N 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 229910052783 alkali metal Inorganic materials 0.000 description 2
- 150000001340 alkali metals Chemical class 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 150000001413 amino acids Chemical class 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- 230000003750 conditioning effect Effects 0.000 description 2
- 238000011437 continuous method Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- YOCUPQPZWBBYIX-UHFFFAOYSA-N copper nickel Chemical compound [Ni].[Cu] YOCUPQPZWBBYIX-UHFFFAOYSA-N 0.000 description 2
- 235000005822 corn Nutrition 0.000 description 2
- 210000003298 dental enamel Anatomy 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- IJKVHSBPTUYDLN-UHFFFAOYSA-N dihydroxy(oxo)silane Chemical compound O[Si](O)=O IJKVHSBPTUYDLN-UHFFFAOYSA-N 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000011049 filling Methods 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 239000010794 food waste Substances 0.000 description 2
- 238000001879 gelation Methods 0.000 description 2
- 239000005431 greenhouse gas Substances 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- RBTARNINKXHZNM-UHFFFAOYSA-K iron trichloride Chemical compound Cl[Fe](Cl)Cl RBTARNINKXHZNM-UHFFFAOYSA-K 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 2
- 238000001471 micro-filtration Methods 0.000 description 2
- 244000005700 microbiome Species 0.000 description 2
- 238000005065 mining Methods 0.000 description 2
- 238000001728 nano-filtration Methods 0.000 description 2
- 229910001000 nickel titanium Inorganic materials 0.000 description 2
- MWUXSHHQAYIFBG-UHFFFAOYSA-N nitrogen oxide Inorganic materials O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 2
- 238000013386 optimize process Methods 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 239000003002 pH adjusting agent Substances 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- JTJMJGYZQZDUJJ-UHFFFAOYSA-N phencyclidine Chemical class C1CCCCN1C1(C=2C=CC=CC=2)CCCCC1 JTJMJGYZQZDUJJ-UHFFFAOYSA-N 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 229920001592 potato starch Polymers 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000011085 pressure filtration Methods 0.000 description 2
- 230000009257 reactivity Effects 0.000 description 2
- 230000008707 rearrangement Effects 0.000 description 2
- 230000002940 repellent Effects 0.000 description 2
- 239000005871 repellent Substances 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 239000004460 silage Substances 0.000 description 2
- LIVNPJMFVYWSIS-UHFFFAOYSA-N silicon monoxide Chemical compound [Si-]#[O+] LIVNPJMFVYWSIS-UHFFFAOYSA-N 0.000 description 2
- 239000010802 sludge Substances 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 230000003068 static effect Effects 0.000 description 2
- 238000000859 sublimation Methods 0.000 description 2
- 230000008022 sublimation Effects 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- XTQHKBHJIVJGKJ-UHFFFAOYSA-N sulfur monoxide Chemical class S=O XTQHKBHJIVJGKJ-UHFFFAOYSA-N 0.000 description 2
- 229910052815 sulfur oxide Inorganic materials 0.000 description 2
- 239000010936 titanium Substances 0.000 description 2
- 235000013311 vegetables Nutrition 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- 229940100445 wheat starch Drugs 0.000 description 2
- 229920000945 Amylopectin Polymers 0.000 description 1
- 229920000856 Amylose Polymers 0.000 description 1
- 235000016068 Berberis vulgaris Nutrition 0.000 description 1
- 241000335053 Beta vulgaris Species 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 235000008733 Citrus aurantifolia Nutrition 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 241000218691 Cupressaceae Species 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 244000068988 Glycine max Species 0.000 description 1
- 235000010469 Glycine max Nutrition 0.000 description 1
- 229910021577 Iron(II) chloride Inorganic materials 0.000 description 1
- 229910021578 Iron(III) chloride Inorganic materials 0.000 description 1
- GXCLVBGFBYZDAG-UHFFFAOYSA-N N-[2-(1H-indol-3-yl)ethyl]-N-methylprop-2-en-1-amine Chemical compound CN(CCC1=CNC2=C1C=CC=C2)CC=C GXCLVBGFBYZDAG-UHFFFAOYSA-N 0.000 description 1
- 206010033307 Overweight Diseases 0.000 description 1
- 241000209504 Poaceae Species 0.000 description 1
- 229920001131 Pulp (paper) Polymers 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 235000011941 Tilia x europaea Nutrition 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 239000002154 agricultural waste Substances 0.000 description 1
- 230000001476 alcoholic effect Effects 0.000 description 1
- 229910052910 alkali metal silicate Inorganic materials 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 239000010868 animal carcass Substances 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 239000010828 animal waste Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 239000011942 biocatalyst Substances 0.000 description 1
- 230000003851 biochemical process Effects 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 238000010170 biological method Methods 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 229920001222 biopolymer Polymers 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 235000008429 bread Nutrition 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 210000002421 cell wall Anatomy 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000001311 chemical methods and process Methods 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 239000002817 coal dust Substances 0.000 description 1
- 238000001246 colloidal dispersion Methods 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 238000009264 composting Methods 0.000 description 1
- 230000001143 conditioned effect Effects 0.000 description 1
- 238000009770 conventional sintering Methods 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 238000009295 crossflow filtration Methods 0.000 description 1
- 230000001351 cycling effect Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000010791 domestic waste Substances 0.000 description 1
- 238000000921 elemental analysis Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 238000005265 energy consumption Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 239000002657 fibrous material Substances 0.000 description 1
- 238000005429 filling process Methods 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 239000010419 fine particle Substances 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000013505 freshwater Substances 0.000 description 1
- 239000002737 fuel gas Substances 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 230000008570 general process Effects 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 239000004519 grease Substances 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 239000002440 industrial waste Substances 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 239000011810 insulating material Substances 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- NMCUIPGRVMDVDB-UHFFFAOYSA-L iron dichloride Chemical compound Cl[Fe]Cl NMCUIPGRVMDVDB-UHFFFAOYSA-L 0.000 description 1
- BAUYGSIQEAFULO-UHFFFAOYSA-L iron(2+) sulfate (anhydrous) Chemical compound [Fe+2].[O-]S([O-])(=O)=O BAUYGSIQEAFULO-UHFFFAOYSA-L 0.000 description 1
- 229910000359 iron(II) sulfate Inorganic materials 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 239000011133 lead Substances 0.000 description 1
- 235000021190 leftovers Nutrition 0.000 description 1
- 239000004571 lime Substances 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 238000007885 magnetic separation Methods 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000010907 mechanical stirring Methods 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 150000002736 metal compounds Chemical class 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 239000002086 nanomaterial Substances 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- 229910000510 noble metal Inorganic materials 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 238000004091 panning Methods 0.000 description 1
- AYEKKSTZQYEZPU-RYUDHWBXSA-N pentosidine Chemical compound OC(=O)[C@@H](N)CCCCN1C=CC=C2N=C(NCCC[C@H](N)C(O)=O)N=C12 AYEKKSTZQYEZPU-RYUDHWBXSA-N 0.000 description 1
- 238000005191 phase separation Methods 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000008635 plant growth Effects 0.000 description 1
- 239000010908 plant waste Substances 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 238000012805 post-processing Methods 0.000 description 1
- 235000012015 potatoes Nutrition 0.000 description 1
- 238000007781 pre-processing Methods 0.000 description 1
- 239000010970 precious metal Substances 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 238000004064 recycling Methods 0.000 description 1
- 239000011819 refractory material Substances 0.000 description 1
- 230000002787 reinforcement Effects 0.000 description 1
- 230000003014 reinforcing effect Effects 0.000 description 1
- 238000012958 reprocessing Methods 0.000 description 1
- 239000004576 sand Substances 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 239000004065 semiconductor Substances 0.000 description 1
- 230000037152 sensory function Effects 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 150000003377 silicon compounds Chemical class 0.000 description 1
- 229910052814 silicon oxide Inorganic materials 0.000 description 1
- 238000005245 sintering Methods 0.000 description 1
- 238000007613 slurry method Methods 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000003892 spreading Methods 0.000 description 1
- 230000007480 spreading Effects 0.000 description 1
- 238000011146 sterile filtration Methods 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 239000011232 storage material Substances 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 230000001502 supplementing effect Effects 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 238000009283 thermal hydrolysis Methods 0.000 description 1
- 238000009997 thermal pre-treatment Methods 0.000 description 1
- 229910052723 transition metal Inorganic materials 0.000 description 1
- 150000003624 transition metals Chemical class 0.000 description 1
- 238000003828 vacuum filtration Methods 0.000 description 1
- 239000002918 waste heat Substances 0.000 description 1
- 238000003466 welding Methods 0.000 description 1
- 238000009279 wet oxidation reaction Methods 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L5/00—Solid fuels
- C10L5/40—Solid fuels essentially based on materials of non-mineral origin
- C10L5/44—Solid fuels essentially based on materials of non-mineral origin on vegetable substances
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/515—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on non-oxide ceramics
- C04B35/56—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on non-oxide ceramics based on carbides or oxycarbides
- C04B35/565—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on non-oxide ceramics based on carbides or oxycarbides based on silicon carbide
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L9/00—Treating solid fuels to improve their combustion
- C10L9/08—Treating solid fuels to improve their combustion by heat treatments, e.g. calcining
- C10L9/086—Hydrothermal carbonization
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/38—Non-oxide ceramic constituents or additives
- C04B2235/3817—Carbides
- C04B2235/3826—Silicon carbides
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/42—Non metallic elements added as constituents or additives, e.g. sulfur, phosphor, selenium or tellurium
- C04B2235/422—Carbon
- C04B2235/425—Graphite
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/60—Aspects relating to the preparation, properties or mechanical treatment of green bodies or pre-forms
- C04B2235/614—Gas infiltration of green bodies or pre-forms
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L2290/00—Fuel preparation or upgrading, processes or apparatus therefore, comprising specific process steps or apparatus units
- C10L2290/06—Heat exchange, direct or indirect
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L2290/00—Fuel preparation or upgrading, processes or apparatus therefore, comprising specific process steps or apparatus units
- C10L2290/10—Recycling of a stream within the process or apparatus to reuse elsewhere therein
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L2290/00—Fuel preparation or upgrading, processes or apparatus therefore, comprising specific process steps or apparatus units
- C10L2290/14—Injection, e.g. in a reactor or a fuel stream during fuel production
- C10L2290/141—Injection, e.g. in a reactor or a fuel stream during fuel production of additive or catalyst
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L2290/00—Fuel preparation or upgrading, processes or apparatus therefore, comprising specific process steps or apparatus units
- C10L2290/14—Injection, e.g. in a reactor or a fuel stream during fuel production
- C10L2290/146—Injection, e.g. in a reactor or a fuel stream during fuel production of water
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L2290/00—Fuel preparation or upgrading, processes or apparatus therefore, comprising specific process steps or apparatus units
- C10L2290/46—Compressors or pumps
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L2290/00—Fuel preparation or upgrading, processes or apparatus therefore, comprising specific process steps or apparatus units
- C10L2290/54—Specific separation steps for separating fractions, components or impurities during preparation or upgrading of a fuel
- C10L2290/548—Membrane- or permeation-treatment for separating fractions, components or impurities during preparation or upgrading of a fuel
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E50/00—Technologies for the production of fuel of non-fossil origin
- Y02E50/10—Biofuels, e.g. bio-diesel
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E50/00—Technologies for the production of fuel of non-fossil origin
- Y02E50/30—Fuel from waste, e.g. synthetic alcohol or diesel
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Ceramic Engineering (AREA)
- Thermal Sciences (AREA)
- Combustion & Propulsion (AREA)
- Physics & Mathematics (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Structural Engineering (AREA)
- Processing Of Solid Wastes (AREA)
- Solid Fuels And Fuel-Associated Substances (AREA)
- Carbon And Carbon Compounds (AREA)
Description
・ メイラード反応又はメイラード様反応の反応生成物と液相、固相の存在。
・ 供給原料によって変化する強力かつ集中的な臭気の形成。臭気の形成は、基本的にメイラード反応生成物によるものである。
・ 他の自然発生と比較して改良された電気伝送度。
・ 高い炭素含有量を持つ炭素化合物。
・ 黒色石炭、石炭のような燃料。
・ 同一の炭素の割合を持つ化石燃料と比較して安定した化合物。
・ 燃焼による低い灰分の発生、低い窒素含量。
・ 硫黄、硝酸、重金属との反応性。つまり、同様に高炭素の割合を持つ化石燃料と比較して、低い自己発火温度。
・ 同様に高炭素の割合を持つ化石燃料と比較し、低いダメージを与える燃料ガスの組成物。
・ 本発明の方法を用いることにより、バイオマスから芝土、黒炭のような燃料が得られる。
・ 燃料値は、プロセスの手段に依存しており、特に反応時間に依存する。燃料値は反応時間な反応器内の滞留時間と共に増加する。
・ 同一の炭素の含有割合では、従来のもの、又は化石燃料よりも成分の揮発性が低い。
・ エネルギー収率は石炭の0.7から0.95にまで上げられる。低炭水化物で、高いエネルギー収率がある、
・ 90から95%:リグニン又は細菌のバイオマス。
・ 燃料は、より反応性が高く、同等の炭素の割合を有する化石燃料よりも低い自己発火温度を持つ。
・ リグナイトや黒石炭などの化石燃料は、本発明の方法で製造される燃料と比較して、同様の発熱量を持っているが、組成や特性に関しては明瞭に異なる。
・ 供給原料や、供給原料の混合物、プロセス手順、触媒混合物、及び、工程水の組成。
・ 供給原料の脂質の多い部分、供給されるエネルギー量。
・ 高い発熱量を持つ燃料。例えば、汚泥の処理中に、発熱量は34から36MJ/kgとなる。
・ 燃料値が30から33MJ/kgで燃料を燃焼した後の灰分は、17から20MJ/kgの燃料値供給原料を燃焼した場合に比較して75%まで減少する。
・ 燃料値が30から33MJ/kgで燃料を燃焼した後の硫黄分は、17から20MJ/kgの燃料値供給原料を燃焼した場合に比較して50%まで減少する。
・ 微細な粒子状物質とガスの排出量は、供給原料に比べて低くなる。
・ 燃焼の結果はプロセス手順、条件に依存する燃料の品質及び燃焼技術に関連する全てのパラメーターにより決定される。
・ 燃料は、比較的均一な特性を持ち、供給原料の組成や触媒だけでなく、プロセス手順を経由して算出し制御できる特性を持つ燃料である。
・ 既に述べたように燃料特性の違いに加えて、黒炭、褐炭、泥炭といったような化石燃料には異なる特性が追加される。
・ 純粋、超純粋の材料も本発明に係る生成物に属する。ろれらは、有利な特性を持っており、その特性は主に供給原料に比べてミネラル性材料の削減をすることができる特性である、純粋な石炭は、主に石炭の可燃部分として理解され、もっとも純粋な石炭は活性炭として理解される。超純粋の石炭のミネラル含有量は0.1重量%未満である。
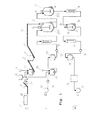
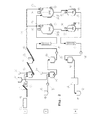
様の符号をつけている。反応器(19,24又は32,33)は連続的な出入の流れができるように,並列に配置されている。連続的な出入の流れは,少なくとも反応時間の10から30%,30から60%,又は60から90%の間続いており,上流に接続された装置を介し,下流に接続された反応器に流れている。熱交換器は,反応混合物の加熱及び冷却のための装置である。
る。液化ポンプはコンベア力,エッセントリックスパイラル,スパイラル変位,又はピストンメンブレンポンプとし設計されている。
はコンパクトグランド炭化ケイ素粉末を高温で異なる結着剤を用いて焼く。この手順の欠点は,特定の高温と長い焼結時間に加えて,結果物に気孔があくことである。
炭化ケイ素含有ガスが改善されることによって,初めて大規模に適用できるようになることによって,調整される。炭素と追加される粒状物質の他の物質の微細な分布により,アニール処理中に形成する酸化ケイ素含有ガスは,すぐに炭素と追加される他の物質と反応することができ,対象物表面近くの拡散プロセスですぐに利用可能である。
所望の最終形状及び/又は最終測定値に応じて,最初のステップで製造され,炭素含有物質の対象物は,炭素を豊富に含む二酸化ケイ素に囲まれており,アニール温度で,保護ガス雰囲気下で少なくともアニールされる。ここでは,二酸化ケイ素粒状材料はケイ素と炭素を含有したガスを放出し,そのガスは,対象物を通過し,そして,炭素含有物質を炭化ケイ素に変換する。
2 導管
3 粉砕装置
4 貯水タンク
5 コンテナ
6 混合槽
7 搬送装置
8 インキュベーション容器
9 コンベア装置
10 コンベア装置
11
12 貯蔵容器
13 搬送装置
14 混合装置
15 コンテナ
16 インキュベーション容器
17 搬送装置
18 熱交換ユニット
19 反応容器
20 攪拌装置
21 供給装置
22 熱交換装置
23 二重壁
24 反応器
25 熱交換ユニット
26 液化容器
27 熱交換器
28 液化ポンプ
29 液化容器
30 固液分離器
31 ストア
32 反応器
33 反応器
34
35
36
37
38
39
40 加水分解反応器
41 供給装置
42 熱交換器
43 二重壁
Claims (18)
- 水と炭素含有物を含む固液混合物からの,燃料,腐植土,メイラード反応生成物又はメイラード反応と類似した反応による生成物の製造方法であって,
前記固液混合物を100〜300℃の温度で,かつ,5〜34バールの圧力で少なくとも1つの反応器(19,24)において処理するために,
a)供給原料の中のポンプ輸送不可能な出発物質を,第1搬送ライン(流れ1)を介して、コンベア装置(9,10)及び供給装置(21又は41)を利用して前記少なくとも1つの反応器(19,24)にポンプを用いずに搬送し、
b)前記供給原料の中のポンプ輸送可能な搬送中に加熱される出発物質を,前記第1搬送ラインに平行な又は前記第1搬送ラインから分派した、第2搬送ライン(流れ2)を介して,搬送装置(17)を利用して前記少なくとも1つの反応器(19,24)にポンプを用いて搬送する方法において、
全体容積の25から97パーセントの固形分を有する出発物質は,前記ポンプ輸送不可能な出発物質として前記第1搬送ラインを介して前記少なくとも1つの反応器に搬送され,全体容積の3から50パーセントの固形分を有する出発物質は,前記ポンプ輸送可能な物質として前記第1搬送ラインに平行な又は前記第1搬送ラインから分派した第2搬送ラインを介して前記少なくとも1つの反応器に搬送され,前記ポンプ輸送不可能な出発物質と前記ポンプ輸送可能な出発物質の処理容積の比率が1:20〜0.1である,ことを特徴とする方法。 - 請求項1に記載の方法であって,
前記ポンプ輸送不可能な出発物質は,前記生成物から抽出された工程水の水蒸気の圧力を5〜34バールにした状態で,前記第1搬送ラインから前記少なくとも1つの反応器へ搬送される,方法。 - 前記ポンプ輸送不可能な出発物質が,工程水の水蒸気の圧力を5〜34バールにした状態で,前記第1搬送ラインから前記少なくとも1つの反応器へ供給される供給装置には,インジェクター,ダブルスクリューエクストルーダー,エキセントリックスパイラルポンプ,ピストンポンプのいずれかが,スパイラルコンプレッサー又はダブルスクリューコンプレッサーの有無に関わらず,備えられている請求項1又は請求項2のいずれか1項に記載の方法。
- 前記ポンプ輸送不可能な出発物質と前記ポンプ輸送可能な出発物質の処理容積の比率が,1:20〜0.1,又は,1:5〜1である請求項1から請求項3のいずれか1項に記載の方法。
- 100〜300℃の温度で,かつ,5〜34バールの圧力で処理される,水と炭素含有物を含む固液混合物からの,燃料,腐植土,メイラード反応生成物又はメイラードと類似した反応の生成物の製造方法において,
前記固液混合物は,加熱のために熱交換器を通り,前記熱交換器のチューブの軸の水平面に対する角度は,10度よりも大きいことを特徴とする請求項1から請求項4のいずれか1項に記載の方法。 - 前記固液混合物は,加熱のために熱交換器を通り,前記固液混合物は,前記熱交換器内のチューブ全長の少なくとも60%の垂直チューブ部分を通って搬送される,請求項5に記載の方法。
- 前記固液混合物の粘度が前記熱交換器を通ることで減少し,最終生成物の粘度が少なくとも出発物質の粘度の4分の3に減少する請求項5又は請求項6に記載の方法。
- 前記固液混合物を搬送するためのポンプ及び/又は逆圧ポンプが,エキセントリックポンプ,スパイラルディスプレイスメントポンプ,ピストンポンプである請求項5から請求項7のいずれか1項に記載の方法。
- 100〜300℃の温度で,かつ,5〜34バールの圧力で処理される,水と炭素含有物を含む固液混合物からの,燃料,腐植土,メイラード反応生成物又はメイラードと類似した反応の生成物の製造方法において,
出発物質は,前方の反応器の反応サイクル時間の10分の6以上の間,熱交換器を介して前記前方の反応器に連続的に供給され,
反応混合物は,バッチ形式で前記前方の反応器から後方の反応器に搬送され,
反応生成物は,前記後方の反応器の反応サイクル時間の10分の6以上の間,前記後方の反応器から連続的に排出されることを特徴とする請求項1から請求項8のいずれか1項に記載の方法。 - 閉じられた反応器において,反応サイクル時間の100分の1の間,逆流混合を防ぐためのバッチが維持され,前記閉じられた反応器は,流れ方向中において,前記少なくとも1つの反応器の後方に配置される請求項9に記載の方法。
- 100〜300℃の温度で,かつ,5〜34バールの圧力で処理される,水と炭素含有物を含む固液混合物からの,燃料,腐植土,メイラード反応生成物又はメイラードと類似した反応の生成物の製造方法において,
膜反応器における反応中に,水は連続的に又は間隔を開けて固液混合物から引き抜かれることを特徴とする請求項1から請求項8のいずれか1項に記載の方法。 - 100〜300℃の温度で,かつ,5〜34バールの圧力で処理される,水と炭素含有物を含む固液混合物からの,燃料,腐植土,メイラード反応生成物又はメイラードと類似した反応の生成物の製造方法において,
濃縮された前記生成物から抽出された工程水は,出発物質,ポンプ輸送可能な固液混合物のプレインキュベーションのため,出発物質の予備加熱のため,ポンプ搬送可能な固液混合物の製造のため,反応器への搬送のため,プラントの反応器において前記出発物質をコーティングし又は混合するため,及び/又は肥料要素として使用するために利用される請求項1から請求項8のいずれか1項に記載の方法。 - 反応器を有し,水と炭素含有物を含む固液混合物を,100〜300℃の温度で,かつ,5〜34バールの圧力で処理する請求項1に記載の方法を実施するための装置であって,
前記装置は、容積比で少なくとも10パーセントの固形物を有する固液混合物を搬送するためのポンプ又は熱交換器を含み,前記熱交換器のチューブの軸の水平面に対する角度は,10度よりも大きいことを特徴とする装置。 - 前記熱交換器が,少なくとも60パーセントの垂直チューブの部分からなる請求項13に記載の装置。
- 前記熱交換器は,10から120バールの圧力に設定されており,又は,前記熱交換器の構成部分は,少なくとも60から120バールの圧力に設定されている,請求項13又は請求項14に記載の装置。
- 反応器を有し,水と炭素含有物を含む固液混合物を,100〜300℃の温度で,かつ,5〜34バールの圧力で処理する装置であって,
a.少なくとも10パーセントの固形物を有する固液混合物を搬送するためのポンプ,及び/又は
b.前記熱交換器のチューブの軸の水平面に対する角度は,10度よりも大きい熱交換器を備えた装置を含む、請求項14又は請求項15に記載の装置。 - 100〜300℃の温度で,かつ,5〜34バールの圧力で処理され,水と炭素含有物を含む固液混合物から,燃料,腐植土,メイラード反応生成物又はメイラードと類似した反応の生成物の製造装置において,
前記製造装置は,前記生成物から抽出された工程水の濃縮のため,及び濃縮された前記工程水を固液混合物に戻すための装置を有することを特徴とする請求項13から請求項15のいずれか1項に記載の装置。 - 100〜300℃の温度で,かつ,5〜34バールの圧力で処理される,水と炭素含有物を含む固液混合物からの,燃料,腐植土,メイラード反応生成物又はメイラードと類似した反応の生成物の請求項1に記載の製造方法により得られた濃縮された前記生成物から抽出された工程水が,プレインキュベーションのため,出発物質の予備加熱のため,ポンプ搬送可能な固液混合物の製造のため,反応器への搬送のため,プラントの反応器において前記出発物質をコーティングし又は混合するため,及び/又は肥料要素として使用するため、に利用される、濃縮水の使用方法。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102008058444.4 | 2008-11-21 | ||
| DE102008058444.4A DE102008058444B4 (de) | 2007-11-21 | 2008-11-21 | Verfahren und Verwendung einer Vorrichtung zur Herstellung von Brennstoffen, Humus oder Suspensionen davon |
| PCT/IB2009/055293 WO2010058377A2 (de) | 2008-11-21 | 2009-11-23 | Verfahren und vorrichtung zur herstellung von werk-oder brennstoffen |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012509383A JP2012509383A (ja) | 2012-04-19 |
| JP6281802B2 true JP6281802B2 (ja) | 2018-02-21 |
Family
ID=42199030
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011537002A Expired - Fee Related JP6281802B2 (ja) | 2008-11-21 | 2009-11-23 | 固液混合物処理のための方法及び装置 |
Country Status (14)
| Country | Link |
|---|---|
| US (1) | US20110226603A1 (ja) |
| EP (2) | EP2467201B1 (ja) |
| JP (1) | JP6281802B2 (ja) |
| CN (1) | CN102325587A (ja) |
| AR (1) | AR074660A1 (ja) |
| BR (1) | BRPI0922500B1 (ja) |
| DE (1) | DE102008058444B4 (ja) |
| DK (1) | DK2467201T3 (ja) |
| ES (1) | ES2751198T3 (ja) |
| HU (1) | HUE046579T2 (ja) |
| LT (1) | LT2467201T (ja) |
| PL (1) | PL2467201T3 (ja) |
| PT (1) | PT2467201T (ja) |
| WO (1) | WO2010058377A2 (ja) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024205153A1 (ko) * | 2023-03-27 | 2024-10-03 | 이화여자대학교 산학협력단 | 연속식 및 유가식 고온 고압의 가공향 반응기 |
Families Citing this family (45)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102008058444B4 (de) * | 2007-11-21 | 2020-03-26 | Antacor Ltd. | Verfahren und Verwendung einer Vorrichtung zur Herstellung von Brennstoffen, Humus oder Suspensionen davon |
| US20110056125A1 (en) * | 2008-04-17 | 2011-03-10 | Csl Carbon Solutions Ltd. | Process for converting biomass to coal-like material using hydrothermal carbonisation |
| EP2366757B1 (en) | 2008-11-17 | 2016-02-17 | Ingelia, S.L. | Pressure and temperature control system for at least one chemical reactor for treating biomass |
| DE102009027007A1 (de) * | 2009-06-17 | 2010-12-23 | Technische Universität Berlin | Verfahren zur Herstellung von mineralischem Biodünger |
| DE102009032370A1 (de) | 2009-07-08 | 2011-01-13 | Sartorius Stedim Biotech Gmbh | Plattenwärmetauscher |
| DE102010013050A1 (de) | 2010-03-27 | 2011-09-29 | Terranova Energy Gmbh | Additiv zur Verbesserung der Hydrothermalen Karbonisierung von Biomasse |
| DE102010006263A1 (de) | 2010-01-28 | 2011-08-18 | TerraNova Energy GmbH, 40231 | Verfahren zur thermischen Verwertung von durch hydrothermale Karbonisierung hergestellte Brennstoffen, insbesondere Kohlestaub |
| DE102010020712A1 (de) | 2010-05-17 | 2011-11-17 | Terranova Energy Gmbh | Verfahren zur Erwärmung und Trocknung der Stoffströme bei der Hydrothermalen Karbonisierung |
| DE102010000576B4 (de) | 2010-02-26 | 2013-06-27 | G+R Technology Group Ag | Anlage und Verfahren zur hydrothermalen Karbonisierung von Biomasse |
| DE102010012613B4 (de) * | 2010-03-24 | 2020-06-10 | Antacor Ltd. | Vorrichtung und Verwendung zur Behandlung von Fest-Flüssig-Gemischen |
| DE102010064715B3 (de) | 2010-03-24 | 2022-04-28 | Antacor Ltd. | Verfahren und Verwendung eines Rohrreaktors zur Behandlung von Fest-Flüssig-Gemischen |
| GB2479924A (en) * | 2010-04-29 | 2011-11-02 | Mortimer Tech Holdings | Torrefaction Process |
| EP2388305A3 (de) | 2010-05-17 | 2012-01-25 | TerraNova Energy GmbH | Thermische Verwertung fester Brennstoffe |
| DE102011012756A1 (de) | 2011-03-01 | 2012-09-06 | Terranova Energy Gmbh | Verfahren zur Reinigung von Abwasser aus der Hydrothermalen Karbonisierung von Biomasse |
| DE202011110246U1 (de) * | 2011-03-04 | 2013-04-25 | Ava-Co2 Schweiz Ag | Vorrichtung zur hydrothermalen Karbonisierung |
| DE102011051269A1 (de) * | 2011-06-22 | 2012-12-27 | DIL Deutsches Institut für Lebensmitteltechnik e.V. | Beschickungsbehälter und Verfahren zur zeitgleichen Hochdruck- und Temperaturbehandlung eines Nahrungsmittels in einem Hochdruckkessel |
| BE1020209A5 (nl) * | 2011-08-30 | 2013-06-04 | Renovius Man | Opwerking van vervuilde biomassa stromen. |
| DE102011055983A1 (de) * | 2011-12-02 | 2013-06-06 | Thomas Reichhart | Verfahren sowie Vorrichtung zur hydrothermalen Karbonisierung von Biomasse |
| DE102011055987A1 (de) * | 2011-12-02 | 2013-06-06 | Thomas Reichhart | Vorrichtung zur hydrothermalen Karbonisierung von Biomasse |
| DE102012002098A1 (de) * | 2012-02-06 | 2013-08-08 | Eurofoam Deutschland Gmbh | Hydrothermale Karbonisierung von Kunststoffmaterial |
| EP2653451A1 (en) * | 2012-04-20 | 2013-10-23 | CS Carbon Solutions | Method and apparatus for the treatment of process water from an organic material conversion process |
| DE102012104309A1 (de) * | 2012-05-18 | 2013-11-21 | L'Air Liquide, Société Anonyme pour l'Etude et l'Exploitation des Procédés Georges Claude | Hydrothermales Carbonisierungsverfahren zur Inkohlung kohlenhydrathaltiger Biomasse |
| JP6114754B2 (ja) | 2012-08-29 | 2017-04-12 | 日本パワーグラファイト株式会社 | 負極炭素材料製造装置及び該装置を用いる負極炭素材料の製造方法 |
| CN102873078A (zh) * | 2012-09-30 | 2013-01-16 | 浙江爱科乐环保有限公司 | 厨余垃圾处理方法 |
| DE102012019659A1 (de) | 2012-10-08 | 2014-04-10 | Terranova Energy Gmbh | Verfahren zur Herstellung von Düngemittel |
| EP2765178A1 (en) * | 2013-02-07 | 2014-08-13 | Arbaflame Technology AS | Method of producing carbon-enriched biomass material |
| FR3008693B1 (fr) | 2013-07-18 | 2019-05-03 | Terranova Energy Gmbh | Procede de carbonisation hydrothermale optimise et installation pour sa mise en oeuvre |
| DE102013013085B3 (de) | 2013-08-07 | 2015-02-05 | Eurofoam Deutschland Gmbh Schaumstoffe | Partikel eines kohleähnlichen Feststoffs, Verwendungen und Herstellungsverfahren |
| CN103480312B (zh) * | 2013-09-18 | 2015-03-11 | 基伊埃工程技术(中国)有限公司 | 一种管式美拉德反应设备 |
| DE102014004056A1 (de) | 2014-03-21 | 2015-09-24 | Terranova Energy Gmbh | Verfahren zur Vermeidung von Teer bei der Entwässerung von Kohleschlamm |
| DE102015002416A1 (de) | 2015-02-26 | 2016-09-01 | Terranova Energy Gmbh | Verfahren zur Abtrennung von Phosphor aus Klärschlamm |
| CN104927046B (zh) * | 2015-06-25 | 2017-06-06 | 安庆市虹泰新材料有限责任公司 | 一种聚酰胺的生产系统及其生产方法 |
| CN105174325B (zh) * | 2015-09-28 | 2017-06-16 | 中广核环保产业有限公司 | 一种利用糠醛渣制备糠醛废水处理剂的方法 |
| DE102015116366A1 (de) | 2015-09-28 | 2017-03-30 | Grenol Ip Gmbh | System zur Aufarbeitung von organischen Reststoffen |
| DE102015016194A1 (de) | 2015-12-15 | 2017-06-22 | Terranova Energy Gmbh | Verfahren zur Faulung und hydrothermalen Karbonisierung von Klärschlamm |
| FR3068708B1 (fr) * | 2017-07-07 | 2020-09-18 | Ifp Energies Now | Procede de traitement de la biomasse par co-broyage avec une charge fossile |
| DE102017123281A1 (de) | 2017-10-06 | 2019-04-11 | Terranova Energy Gmbh | Verfahren zur Herstellung einer geruchsfreien HTC-Kohle und geruchsfreie HTC-Kohle |
| US20190234649A1 (en) * | 2018-01-29 | 2019-08-01 | Brian Gregory Phillips | Tubular-shaped and modular air handling unit (ahu) for heating, ventilating, and air conditioning (hvac) systems |
| CN109401931A (zh) * | 2018-12-08 | 2019-03-01 | 内蒙古弘达生物环保科技有限责任公司 | 一种沼气工程原料的进料及预处理系统 |
| CN112662444B (zh) * | 2019-10-16 | 2022-09-13 | 柳晶(溧阳)环保科技有限公司 | 一种超声分离铸造除尘灰中煤粉的方法 |
| US11254883B2 (en) | 2020-06-30 | 2022-02-22 | Duke Technologies, Llc | Method and system for treating renewable feedstocks |
| US11525096B2 (en) | 2020-06-30 | 2022-12-13 | Duke Technologies, Llc | Method for treating renewable feedstocks |
| US12098334B2 (en) | 2020-06-30 | 2024-09-24 | Duke Technologies, Llc | Method for treating renewable feedstocks |
| CN111955634A (zh) * | 2020-08-25 | 2020-11-20 | 福建拓天生物科技有限公司 | 一种复合薏米红曲粉,及其高效复配制备方法 |
| CN116813173A (zh) * | 2023-07-03 | 2023-09-29 | 中国海洋大学 | 一种海底采矿土资源化利用方法 |
Family Cites Families (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1471949A (en) * | 1974-06-19 | 1977-04-27 | Shell Int Research | Process for the upgrading of coal or the like |
| US4266083A (en) * | 1979-06-08 | 1981-05-05 | The Rust Engineering Company | Biomass liquefaction process |
| FI58346C (fi) * | 1979-12-18 | 1981-01-12 | Tampella Oy Ab | Foerfarande foer kontinuerlig foersockring av cellulosa av vaextmaterial |
| US4513030A (en) * | 1982-06-18 | 1985-04-23 | The United States Of America As Represented By The United States Department Of Energy | Method of producing silicon carbide articles |
| US4700445A (en) * | 1982-07-12 | 1987-10-20 | Rubin Raskin | Method of manufacturing heat transfer panels by inflation |
| US4579562A (en) | 1984-05-16 | 1986-04-01 | Institute Of Gas Technology | Thermochemical beneficiation of low rank coals |
| GB8511587D0 (en) * | 1985-05-08 | 1985-06-12 | Shell Int Research | Producing hydrocarbon-containing liquids |
| DE3928815A1 (de) * | 1988-12-13 | 1990-06-21 | Still Otto Gmbh | Verfahren zur behandlung von biomassen, z. b. bei der biologischen abwasserreinigung anfallenden klaerschlaemmen, guelle, sonstigen mikrobiologischen oder nachwachsenden biomassen |
| DE4403391A1 (de) * | 1994-02-04 | 1995-08-10 | Thyssen Still Otto Gmbh | Verfahren zur Gewinnung von Wasserstoff und Carbonsäuren aus Biomassenhydrolysat |
| DE19723519A1 (de) | 1997-06-05 | 1998-12-10 | Kuesters Eduard Maschf | Kalander |
| DE19723510C1 (de) * | 1997-06-05 | 1999-02-18 | Atz Evus | Verfahren und Vorrichtung zur Behandlung biogener Restmassen |
| JP2000189781A (ja) * | 1998-12-28 | 2000-07-11 | Toshiba Corp | 高圧処理装置、高圧処理装置への供給方法および高圧処理装置の保護方法 |
| EP1184443A1 (en) * | 2000-09-04 | 2002-03-06 | Biofuel B.V. | Process for the production of liquid fuels from biomass |
| JP3901984B2 (ja) * | 2001-10-25 | 2007-04-04 | 日揮株式会社 | バイオマス水スラリー及びその製造方法 |
| JP2005205252A (ja) * | 2004-01-20 | 2005-08-04 | Kobe Steel Ltd | バイオマスを含む高濃度スラリー、および高濃度スラリーの製造方法、並びにバイオマス燃料の製造方法 |
| DE102006055469A1 (de) * | 2006-11-23 | 2008-05-29 | Universität Paderborn | Verfahren zur Herstellung eines Gegenstandes zumindest teilweise mit Siliziumkarbidgefüge aus einem Rohling aus einem kohlenstoffhaltigen Material |
| DE102007056170A1 (de) * | 2006-12-28 | 2008-11-06 | Dominik Peus | Semikontinuierliches Verfahren zur Herstellung von Brennstoff aus Biomasse |
| TR201901643T4 (tr) * | 2007-02-08 | 2019-02-21 | Grenol Ip Gmbh | Biyokütlelerin hidrotermal karbonizasyonu. |
| DE102007012112C5 (de) * | 2007-03-13 | 2016-08-18 | Loritus Gmbh | Vorrichtung und Verfahren zur hydrothermalen Karbonisierung von Biomasse |
| JP2008253861A (ja) * | 2007-03-30 | 2008-10-23 | Bussan Food Science Kk | バイオマス処理用連続式高圧水熱反応装置 |
| DE102007022840A1 (de) * | 2007-05-11 | 2008-12-24 | Suncoal Industries Gmbh | Verfahren zur Kühlung und Vorwärmung einer Anlage zur hydrothermalen Carbonisierung von Biomasse |
| WO2008138637A2 (de) * | 2007-05-11 | 2008-11-20 | Suncoal Industries Gmbh | Verfahren und vorrichtung zur hydrothermalen karbonisierung (htc) von biomasse mit einer htc-anlage |
| DE102008058444B4 (de) * | 2007-11-21 | 2020-03-26 | Antacor Ltd. | Verfahren und Verwendung einer Vorrichtung zur Herstellung von Brennstoffen, Humus oder Suspensionen davon |
| DE102008004732A1 (de) * | 2008-01-16 | 2009-07-23 | Lucia Viviane Sanders | Hydrothermale Karbonisierung von Biomasse |
| DE202008012419U1 (de) * | 2008-09-18 | 2008-11-20 | Agrokraft Gmbh | Vorrichtung zur Behandlung von Biomasse |
-
2008
- 2008-11-21 DE DE102008058444.4A patent/DE102008058444B4/de active Active
-
2009
- 2009-11-23 PL PL09827256T patent/PL2467201T3/pl unknown
- 2009-11-23 WO PCT/IB2009/055293 patent/WO2010058377A2/de active Application Filing
- 2009-11-23 BR BRPI0922500-5A patent/BRPI0922500B1/pt not_active IP Right Cessation
- 2009-11-23 EP EP09827256.0A patent/EP2467201B1/de active Active
- 2009-11-23 PT PT98272560T patent/PT2467201T/pt unknown
- 2009-11-23 EP EP19186330.7A patent/EP3656836A1/de active Pending
- 2009-11-23 LT LT09827256T patent/LT2467201T/lt unknown
- 2009-11-23 CN CN2009801550932A patent/CN102325587A/zh active Pending
- 2009-11-23 HU HUE09827256A patent/HUE046579T2/hu unknown
- 2009-11-23 DK DK09827256T patent/DK2467201T3/da active
- 2009-11-23 US US13/130,578 patent/US20110226603A1/en not_active Abandoned
- 2009-11-23 ES ES09827256T patent/ES2751198T3/es active Active
- 2009-11-23 JP JP2011537002A patent/JP6281802B2/ja not_active Expired - Fee Related
- 2009-11-24 AR ARP090104516A patent/AR074660A1/es active IP Right Grant
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024205153A1 (ko) * | 2023-03-27 | 2024-10-03 | 이화여자대학교 산학협력단 | 연속식 및 유가식 고온 고압의 가공향 반응기 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2012509383A (ja) | 2012-04-19 |
| US20110226603A1 (en) | 2011-09-22 |
| WO2010058377A2 (de) | 2010-05-27 |
| DK2467201T3 (da) | 2019-10-28 |
| BRPI0922500A2 (pt) | 2018-06-05 |
| AR074660A1 (es) | 2011-02-02 |
| DE102008058444B4 (de) | 2020-03-26 |
| ES2751198T3 (es) | 2020-03-30 |
| WO2010058377A3 (de) | 2011-06-23 |
| DE102008058444A1 (de) | 2009-05-28 |
| PL2467201T3 (pl) | 2020-03-31 |
| EP2467201B1 (de) | 2019-07-17 |
| EP2467201A2 (de) | 2012-06-27 |
| BRPI0922500B1 (pt) | 2021-07-27 |
| CN102325587A (zh) | 2012-01-18 |
| EP2467201A4 (de) | 2016-01-20 |
| LT2467201T (lt) | 2019-11-11 |
| EP3656836A1 (de) | 2020-05-27 |
| HUE046579T2 (hu) | 2020-03-30 |
| PT2467201T (pt) | 2019-11-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6281802B2 (ja) | 固液混合物処理のための方法及び装置 | |
| US11124723B2 (en) | Apparatus and system for producing solid fuel from biomass | |
| CN102260506B (zh) | 一种橡胶树加工板材的废弃料综合利用的方法 | |
| CN100549131C (zh) | 生物质分级控温慢速热解工艺及其系统 | |
| TWI427142B (zh) | 用以處理材料與燃料的方法及裝置 | |
| CN101747919B (zh) | 生物质延迟焦化处理的蒸汽除焦方法与装置 | |
| CN101121895A (zh) | 生物油及其用生物质磁稳定流化床反应器中温快速热裂解制生物油的方法 | |
| AU2013201163A1 (en) | Apparatus and method for producing carbon enriched material and fuel from biomass |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD02 | Notification of acceptance of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7422 Effective date: 20120410 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20120411 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20121122 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20140207 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140218 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140513 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140526 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140617 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140624 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140718 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140813 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140818 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20141216 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150416 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20150522 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150515 |
|
| A912 | Re-examination (zenchi) completed and case transferred to appeal board |
Free format text: JAPANESE INTERMEDIATE CODE: A912 Effective date: 20150724 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20161024 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170208 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20170706 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20170810 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20170906 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20171011 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20180112 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6281802 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |