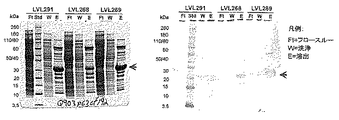
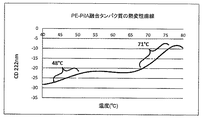
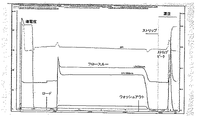
JP6236086B2 - 1種以上の肺炎球菌莢膜糖類コンジュゲートとインフルエンザ菌由来のタンパク質Eおよび/またはPilAを含むタンパク質成分とを含む免疫原性組成物 - Google Patents
1種以上の肺炎球菌莢膜糖類コンジュゲートとインフルエンザ菌由来のタンパク質Eおよび/またはPilAを含むタンパク質成分とを含む免疫原性組成物 Download PDFInfo
- Publication number
- JP6236086B2 JP6236086B2 JP2015537216A JP2015537216A JP6236086B2 JP 6236086 B2 JP6236086 B2 JP 6236086B2 JP 2015537216 A JP2015537216 A JP 2015537216A JP 2015537216 A JP2015537216 A JP 2015537216A JP 6236086 B2 JP6236086 B2 JP 6236086B2
- Authority
- JP
- Japan
- Prior art keywords
- seq
- protein
- pila
- conjugated
- saccharide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/09—Lactobacillales, e.g. aerococcus, enterococcus, lactobacillus, lactococcus, streptococcus
- A61K39/092—Streptococcus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/05—Actinobacteria, e.g. Actinomyces, Streptomyces, Nocardia, Bifidobacterium, Gardnerella, Corynebacterium; Propionibacterium
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/08—Clostridium, e.g. Clostridium tetani
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/099—Bordetella
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/102—Pasteurellales, e.g. Actinobacillus, Pasteurella; Haemophilus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/16—Otologicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/525—Virus
- A61K2039/5252—Virus inactivated (killed)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
- A61K2039/541—Mucosal route
- A61K2039/543—Mucosal route intranasal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/55—Medicinal preparations containing antigens or antibodies characterised by the host/recipient, e.g. newborn with maternal antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55505—Inorganic adjuvants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55544—Bacterial toxins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55566—Emulsions, e.g. Freund's adjuvant, MF59
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/60—Medicinal preparations containing antigens or antibodies characteristics by the carrier linked to the antigen
- A61K2039/6031—Proteins
- A61K2039/6037—Bacterial toxins, e.g. diphteria toxoid [DT], tetanus toxoid [TT]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/70—Multivalent vaccine
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/195—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria
- C07K14/315—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria from Streptococcus (G), e.g. Enterococci
- C07K14/3156—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria from Streptococcus (G), e.g. Enterococci from Streptococcus pneumoniae (Pneumococcus)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2730/00—Reverse transcribing DNA viruses
- C12N2730/00011—Details
- C12N2730/10011—Hepadnaviridae
- C12N2730/10111—Orthohepadnavirus, e.g. hepatitis B virus
- C12N2730/10134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/32011—Picornaviridae
- C12N2770/32611—Poliovirus
- C12N2770/32634—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Immunology (AREA)
- Mycology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Epidemiology (AREA)
- Microbiology (AREA)
- Virology (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Pulmonology (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- AIDS & HIV (AREA)
- Urology & Nephrology (AREA)
- Rheumatology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Pain & Pain Management (AREA)
- Hematology (AREA)
- Ophthalmology & Optometry (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Medicinal Preparation (AREA)
- Gastroenterology & Hepatology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
Applications Claiming Priority (13)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261714956P | 2012-10-17 | 2012-10-17 | |
| US201261714942P | 2012-10-17 | 2012-10-17 | |
| US61/714,956 | 2012-10-17 | ||
| US61/714,942 | 2012-10-17 | ||
| GB1218660.7 | 2012-10-17 | ||
| GBGB1218660.7A GB201218660D0 (en) | 2012-10-17 | 2012-10-17 | Immunogenic composition |
| US13/826,932 | 2013-03-14 | ||
| US13/826,696 | 2013-03-14 | ||
| US13/827,203 | 2013-03-14 | ||
| US13/826,932 US20140193451A1 (en) | 2012-10-17 | 2013-03-14 | Immunogenic composition |
| US13/827,203 US20140105927A1 (en) | 2012-10-17 | 2013-03-14 | Immunogenic composition |
| US13/826,696 US9561268B2 (en) | 2012-10-17 | 2013-03-14 | Immunogenic composition |
| PCT/EP2013/071472 WO2014060383A1 (en) | 2012-10-17 | 2013-10-15 | Immunogenic composition comprising 1 or more streptococcus pneumoniae capsular saccharide conjugates and a protein component comprising protein e and/or pila fromhaemophilus influenzae. |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2015536312A JP2015536312A (ja) | 2015-12-21 |
| JP2015536312A5 JP2015536312A5 (enExample) | 2016-11-10 |
| JP6236086B2 true JP6236086B2 (ja) | 2017-11-22 |
Family
ID=53008966
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015537216A Active JP6236086B2 (ja) | 2012-10-17 | 2013-10-15 | 1種以上の肺炎球菌莢膜糖類コンジュゲートとインフルエンザ菌由来のタンパク質Eおよび/またはPilAを含むタンパク質成分とを含む免疫原性組成物 |
| JP2015537220A Pending JP2015534964A (ja) | 2012-10-17 | 2013-10-15 | 免疫原性組成物 |
| JP2015537218A Expired - Fee Related JP6412500B2 (ja) | 2012-10-17 | 2013-10-15 | 免疫原性組成物 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015537220A Pending JP2015534964A (ja) | 2012-10-17 | 2013-10-15 | 免疫原性組成物 |
| JP2015537218A Expired - Fee Related JP6412500B2 (ja) | 2012-10-17 | 2013-10-15 | 免疫原性組成物 |
Country Status (13)
| Country | Link |
|---|---|
| EP (3) | EP2908855B1 (enExample) |
| JP (3) | JP6236086B2 (enExample) |
| KR (2) | KR20150058571A (enExample) |
| CN (2) | CN104853768B (enExample) |
| AU (2) | AU2013331781A1 (enExample) |
| BR (3) | BR112015008418A2 (enExample) |
| CA (3) | CA2888300A1 (enExample) |
| EA (2) | EA201590490A1 (enExample) |
| ES (1) | ES2640320T3 (enExample) |
| IL (2) | IL238053A0 (enExample) |
| MX (2) | MX365842B (enExample) |
| SG (2) | SG11201502634TA (enExample) |
| WO (3) | WO2014060385A1 (enExample) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB201218660D0 (en) | 2012-10-17 | 2012-11-28 | Glaxosmithkline Biolog Sa | Immunogenic composition |
| US11160855B2 (en) | 2014-01-21 | 2021-11-02 | Pfizer Inc. | Immunogenic compositions comprising conjugated capsular saccharide antigens and uses thereof |
| CN104306965B (zh) * | 2014-10-31 | 2017-05-10 | 康希诺生物股份公司 | 预防肺炎链球菌感染性疾病的免疫原性组合物及制备方法 |
| KR102800419B1 (ko) * | 2015-07-21 | 2025-04-25 | 화이자 인코포레이티드 | 접합된 캡슐형 사카라이드 항원을 포함하는 면역원성 조성물, 그를 포함하는 키트 및 그의 용도 |
| GB201518684D0 (en) * | 2015-10-21 | 2015-12-02 | Glaxosmithkline Biolog Sa | Vaccine |
| GB201603029D0 (en) * | 2016-02-22 | 2016-04-06 | Glaxosmithkline Biolog Sa | Vaccine |
| CN106432512B (zh) * | 2016-09-30 | 2022-03-01 | 康希诺生物股份公司 | 一种增强多糖抗原免疫原性蛋白载体及其制备方法与应用 |
| KR20200111685A (ko) * | 2018-01-19 | 2020-09-29 | 오비아이 파머 인코퍼레이티드 | Crm197 단백질 발현 |
| CN110540597B (zh) * | 2018-12-20 | 2021-04-30 | 湖北工业大学 | 基于流感嗜血杆菌表面蛋白的乳胶微球免疫层析试纸的制备方法 |
| EP4144365A4 (en) * | 2020-05-01 | 2024-06-05 | Sinocelltech Ltd. | METHODS FOR IMPROVING THE IMMUNOGENERICITY OF PROTEIN/PEPTIDE ANTIGEN |
| KR20220142219A (ko) * | 2021-04-14 | 2022-10-21 | 한국생명공학연구원 | 장내 미생물에서 단백질 분비를 유도하는 신호서열 |
| EP4169513A1 (en) | 2021-10-19 | 2023-04-26 | GlaxoSmithKline Biologicals S.A. | Adjuvant composition comprising sting agonists |
| CN117512031B (zh) * | 2023-10-16 | 2024-06-25 | 江苏金迪克生物技术股份有限公司 | 一种肺炎球菌荚膜多糖的纯化方法 |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1162999B1 (en) * | 1999-03-19 | 2006-11-29 | Glaxosmithkline Biologicals S.A. | Vaccine against Streptococcus pneumoniae |
| GB0108364D0 (en) * | 2001-04-03 | 2001-05-23 | Glaxosmithkline Biolog Sa | Vaccine composition |
| CZ20024224A3 (cs) * | 2000-06-29 | 2003-05-14 | Glaxosmithkline Biologicals S. A. | Farmaceutický prostředek |
| GB0022742D0 (en) * | 2000-09-15 | 2000-11-01 | Smithkline Beecham Biolog | Vaccine |
| GB0130215D0 (en) * | 2001-12-18 | 2002-02-06 | Glaxosmithkline Biolog Sa | Vaccine |
| ES2387215T3 (es) * | 2003-12-23 | 2012-09-18 | Nationwide Children's Hospital, Inc. | Pili de tipo IV de la Haemophilus influenzae |
| ATE488526T1 (de) * | 2005-07-08 | 2010-12-15 | Nationwide Childrens Hospital | Chimärer impfstoff für haemophilus influenzae- induzierte infektion |
| NO346497B1 (no) * | 2006-01-17 | 2022-09-05 | Arne Forsgren | Overflateeksponert haemophilus influenzae protein (protein E, pE) |
| US9610339B2 (en) * | 2007-06-26 | 2017-04-04 | Glaxosmithkline Biologicals, S.A. | Vaccine comprising Streptococcus pneumoniae capsular polysaccharide conjugates |
| BRPI0923649A2 (pt) * | 2008-12-24 | 2016-01-19 | Netherlands Vaccine Inst | polipeptídeos de pneumolisina (ply) de streptococcus pneumoniae modificados. |
| ES2670576T3 (es) * | 2009-09-03 | 2018-05-31 | Pfizer Vaccines Llc | Vacuna de PCSK9 |
| US20130183350A1 (en) * | 2009-12-22 | 2013-07-18 | Kevin Harper | Immunogenic compositions |
| CA2819758A1 (en) * | 2010-12-03 | 2012-06-07 | Sanofi Pasteur Limited | Composition for immunization against streptococcus pneumoniae |
| CN103687600B (zh) * | 2011-03-22 | 2016-05-04 | 印度血清研究所私人有限公司 | 一种制备多糖的方法 |
| TW201302779A (zh) * | 2011-04-13 | 2013-01-16 | Glaxosmithkline Biolog Sa | 融合蛋白質及組合疫苗 |
| BR112013029514A2 (pt) * | 2011-05-17 | 2019-09-24 | Glaxosmithkline Biologicals Sa | composição imunogênica, vacina, e, método de tratar ou impedir uma doença |
-
2013
- 2013-10-15 CN CN201380066113.5A patent/CN104853768B/zh not_active Expired - Fee Related
- 2013-10-15 SG SG11201502634TA patent/SG11201502634TA/en unknown
- 2013-10-15 EP EP13779190.1A patent/EP2908855B1/en active Active
- 2013-10-15 CA CA2888300A patent/CA2888300A1/en not_active Abandoned
- 2013-10-15 JP JP2015537216A patent/JP6236086B2/ja active Active
- 2013-10-15 WO PCT/EP2013/071477 patent/WO2014060385A1/en not_active Ceased
- 2013-10-15 CN CN201380066162.9A patent/CN104968366B/zh active Active
- 2013-10-15 BR BR112015008418A patent/BR112015008418A2/pt not_active IP Right Cessation
- 2013-10-15 MX MX2015004949A patent/MX365842B/es active IP Right Grant
- 2013-10-15 KR KR1020157013037A patent/KR20150058571A/ko not_active Withdrawn
- 2013-10-15 EP EP13779191.9A patent/EP2908856B1/en active Active
- 2013-10-15 CA CA2888321A patent/CA2888321A1/en not_active Abandoned
- 2013-10-15 JP JP2015537220A patent/JP2015534964A/ja active Pending
- 2013-10-15 EP EP13776808.1A patent/EP2908854A2/en not_active Ceased
- 2013-10-15 BR BR112015008419-2A patent/BR112015008419B1/pt active IP Right Grant
- 2013-10-15 ES ES13779190.1T patent/ES2640320T3/es active Active
- 2013-10-15 KR KR1020157013039A patent/KR20150072444A/ko not_active Withdrawn
- 2013-10-15 JP JP2015537218A patent/JP6412500B2/ja not_active Expired - Fee Related
- 2013-10-15 SG SG11201502635SA patent/SG11201502635SA/en unknown
- 2013-10-15 MX MX2015005002A patent/MX2015005002A/es unknown
- 2013-10-15 BR BR112015008417A patent/BR112015008417A8/pt not_active Application Discontinuation
- 2013-10-15 CA CA2888310A patent/CA2888310C/en active Active
- 2013-10-15 EA EA201590490A patent/EA201590490A1/ru unknown
- 2013-10-15 WO PCT/EP2013/071472 patent/WO2014060383A1/en not_active Ceased
- 2013-10-15 AU AU2013331781A patent/AU2013331781A1/en not_active Abandoned
- 2013-10-15 AU AU2013333975A patent/AU2013333975A1/en not_active Abandoned
- 2013-10-15 EA EA201590491A patent/EA201590491A1/ru unknown
- 2013-10-15 WO PCT/EP2013/071483 patent/WO2014060389A2/en not_active Ceased
-
2015
- 2015-03-31 IL IL238053A patent/IL238053A0/en unknown
- 2015-04-01 IL IL238079A patent/IL238079A0/en unknown
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6236086B2 (ja) | 1種以上の肺炎球菌莢膜糖類コンジュゲートとインフルエンザ菌由来のタンパク質Eおよび/またはPilAを含むタンパク質成分とを含む免疫原性組成物 | |
| US11198707B2 (en) | Fusion proteins and combination vaccines comprising Haemophilus influenzae Protein E and Pilin A | |
| US9561268B2 (en) | Immunogenic composition | |
| Lu et al. | A bivalent vaccine to protect against Streptococcus pneumoniae and Salmonella typhi | |
| JP2018522978A (ja) | 免疫原性組成物 | |
| EP4511059A1 (en) | Streptococcus suis vaccine composition comprising immunogenic fusion polypeptides | |
| NZ615328B2 (en) | Fusion proteins and combination vaccines comprising haemophilus influenzae protein e and pilin a |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150617 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160916 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20160916 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20170704 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170928 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20171010 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20171027 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6236086 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |