JP6193997B2 - 血管壁内へ直接薬剤を送達する針カテーテル - Google Patents
血管壁内へ直接薬剤を送達する針カテーテル Download PDFInfo
- Publication number
- JP6193997B2 JP6193997B2 JP2015527497A JP2015527497A JP6193997B2 JP 6193997 B2 JP6193997 B2 JP 6193997B2 JP 2015527497 A JP2015527497 A JP 2015527497A JP 2015527497 A JP2015527497 A JP 2015527497A JP 6193997 B2 JP6193997 B2 JP 6193997B2
- Authority
- JP
- Japan
- Prior art keywords
- catheter
- positioning
- wire
- lumen
- positioning wire
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000003814 drug Substances 0.000 title description 12
- 229940079593 drug Drugs 0.000 title description 12
- 230000006835 compression Effects 0.000 claims description 7
- 238000007906 compression Methods 0.000 claims description 7
- 238000011282 treatment Methods 0.000 claims description 3
- 238000012276 Endovascular treatment Methods 0.000 claims description 2
- 230000005855 radiation Effects 0.000 claims description 2
- 238000000034 method Methods 0.000 description 18
- 239000003504 photosensitizing agent Substances 0.000 description 17
- 210000002254 renal artery Anatomy 0.000 description 17
- 210000001519 tissue Anatomy 0.000 description 15
- 210000004204 blood vessel Anatomy 0.000 description 14
- 210000002820 sympathetic nervous system Anatomy 0.000 description 13
- 210000005036 nerve Anatomy 0.000 description 12
- 230000006870 function Effects 0.000 description 11
- 210000001367 artery Anatomy 0.000 description 9
- 206010020772 Hypertension Diseases 0.000 description 8
- 230000036772 blood pressure Effects 0.000 description 8
- 230000000694 effects Effects 0.000 description 8
- 239000000126 substance Substances 0.000 description 7
- 210000003734 kidney Anatomy 0.000 description 6
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 5
- 238000001802 infusion Methods 0.000 description 5
- 230000035515 penetration Effects 0.000 description 5
- 230000002889 sympathetic effect Effects 0.000 description 5
- 230000009471 action Effects 0.000 description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- 239000000835 fiber Substances 0.000 description 4
- 230000003287 optical effect Effects 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- 229910052760 oxygen Inorganic materials 0.000 description 4
- 230000002792 vascular Effects 0.000 description 4
- 230000033115 angiogenesis Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 230000002638 denervation Effects 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 230000001965 increasing effect Effects 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 239000011859 microparticle Substances 0.000 description 3
- 238000002428 photodynamic therapy Methods 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- PUUBADHCONCMPA-USOGPTGWSA-N 3-[(21S,22S)-11-ethyl-16-(1-hexoxyethyl)-4-hydroxy-12,17,21,26-tetramethyl-7,23,24,25-tetrazahexacyclo[18.2.1.15,8.110,13.115,18.02,6]hexacosa-1,4,6,8(26),9,11,13(25),14,16,18(24),19-undecaen-22-yl]propanoic acid Chemical compound CCCCCCOC(C)C1=C(C2=NC1=CC3=NC(=CC4=C(C5=C(CC(=C6[C@H]([C@@H](C(=C2)N6)C)CCC(=O)O)C5=N4)O)C)C(=C3C)CC)C PUUBADHCONCMPA-USOGPTGWSA-N 0.000 description 2
- 102000005862 Angiotensin II Human genes 0.000 description 2
- 101800000733 Angiotensin-2 Proteins 0.000 description 2
- 201000001320 Atherosclerosis Diseases 0.000 description 2
- CZGUSIXMZVURDU-JZXHSEFVSA-N Ile(5)-angiotensin II Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC=CC=1)C([O-])=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)[C@@H]([NH3+])CC([O-])=O)C(C)C)C1=CC=C(O)C=C1 CZGUSIXMZVURDU-JZXHSEFVSA-N 0.000 description 2
- 239000000219 Sympatholytic Substances 0.000 description 2
- 206010047139 Vasoconstriction Diseases 0.000 description 2
- 210000003484 anatomy Anatomy 0.000 description 2
- 238000002399 angioplasty Methods 0.000 description 2
- 229950006323 angiotensin ii Drugs 0.000 description 2
- 210000004027 cell Anatomy 0.000 description 2
- 210000000170 cell membrane Anatomy 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 230000005281 excited state Effects 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 238000002594 fluoroscopy Methods 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000002105 nanoparticle Substances 0.000 description 2
- 230000002887 neurotoxic effect Effects 0.000 description 2
- 230000000149 penetrating effect Effects 0.000 description 2
- 150000004032 porphyrins Chemical class 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 208000015658 resistant hypertension Diseases 0.000 description 2
- 208000037803 restenosis Diseases 0.000 description 2
- 230000007480 spreading Effects 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 230000000948 sympatholitic effect Effects 0.000 description 2
- 210000003813 thumb Anatomy 0.000 description 2
- 210000005166 vasculature Anatomy 0.000 description 2
- 230000025033 vasoconstriction Effects 0.000 description 2
- MHIITNFQDPFSES-UHFFFAOYSA-N 25,26,27,28-tetrazahexacyclo[16.6.1.13,6.18,11.113,16.019,24]octacosa-1(25),2,4,6,8(27),9,11,13,15,17,19,21,23-tridecaene Chemical class N1C(C=C2C3=CC=CC=C3C(C=C3NC(=C4)C=C3)=N2)=CC=C1C=C1C=CC4=N1 MHIITNFQDPFSES-UHFFFAOYSA-N 0.000 description 1
- 108091006112 ATPases Proteins 0.000 description 1
- 102000057290 Adenosine Triphosphatases Human genes 0.000 description 1
- 101800000734 Angiotensin-1 Proteins 0.000 description 1
- 102400000344 Angiotensin-1 Human genes 0.000 description 1
- 206010003162 Arterial injury Diseases 0.000 description 1
- 206010051113 Arterial restenosis Diseases 0.000 description 1
- XMWRBQBLMFGWIX-UHFFFAOYSA-N C60 fullerene Chemical compound C12=C3C(C4=C56)=C7C8=C5C5=C9C%10=C6C6=C4C1=C1C4=C6C6=C%10C%10=C9C9=C%11C5=C8C5=C8C7=C3C3=C7C2=C1C1=C2C4=C6C4=C%10C6=C9C9=C%11C5=C5C8=C3C3=C7C1=C1C2=C4C6=C2C9=C5C3=C12 XMWRBQBLMFGWIX-UHFFFAOYSA-N 0.000 description 1
- UJKPHYRXOLRVJJ-MLSVHJFASA-N CC(O)C1=C(C)/C2=C/C3=N/C(=C\C4=C(CCC(O)=O)C(C)=C(N4)/C=C4\N=C(\C=C\1/N\2)C(C)=C4C(C)O)/C(CCC(O)=O)=C3C Chemical class CC(O)C1=C(C)/C2=C/C3=N/C(=C\C4=C(CCC(O)=O)C(C)=C(N4)/C=C4\N=C(\C=C\1/N\2)C(C)=C4C(C)O)/C(CCC(O)=O)=C3C UJKPHYRXOLRVJJ-MLSVHJFASA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 206010020880 Hypertrophy Diseases 0.000 description 1
- 208000031481 Pathologic Constriction Diseases 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 206010048988 Renal artery occlusion Diseases 0.000 description 1
- 206010063897 Renal ischaemia Diseases 0.000 description 1
- 208000001871 Tachycardia Diseases 0.000 description 1
- 208000007536 Thrombosis Diseases 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 229960002478 aldosterone Drugs 0.000 description 1
- 238000002266 amputation Methods 0.000 description 1
- ORWYRWWVDCYOMK-HBZPZAIKSA-N angiotensin I Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C1=CC=C(O)C=C1 ORWYRWWVDCYOMK-HBZPZAIKSA-N 0.000 description 1
- 210000000709 aorta Anatomy 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 206010003119 arrhythmia Diseases 0.000 description 1
- 239000002473 artificial blood Substances 0.000 description 1
- BHPNXACHQYJJJS-UHFFFAOYSA-N bacteriochlorin Chemical compound N1C(C=C2N=C(C=C3NC(=C4)C=C3)CC2)=CC=C1C=C1CCC4=N1 BHPNXACHQYJJJS-UHFFFAOYSA-N 0.000 description 1
- 235000013361 beverage Nutrition 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 229920001222 biopolymer Polymers 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 208000002352 blister Diseases 0.000 description 1
- 206010061592 cardiac fibrillation Diseases 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 230000005779 cell damage Effects 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 230000002526 effect on cardiovascular system Effects 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000000763 evoking effect Effects 0.000 description 1
- 230000003090 exacerbative effect Effects 0.000 description 1
- 229910003472 fullerene Inorganic materials 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 210000002288 golgi apparatus Anatomy 0.000 description 1
- 210000005003 heart tissue Anatomy 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 230000001631 hypertensive effect Effects 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 239000000543 intermediate Substances 0.000 description 1
- 238000013152 interventional procedure Methods 0.000 description 1
- 230000002262 irrigation Effects 0.000 description 1
- 238000003973 irrigation Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 210000003712 lysosome Anatomy 0.000 description 1
- 230000001868 lysosomic effect Effects 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- LKKPNUDVOYAOBB-UHFFFAOYSA-N naphthalocyanine Chemical compound N1C(N=C2C3=CC4=CC=CC=C4C=C3C(N=C3C4=CC5=CC=CC=C5C=C4C(=N4)N3)=N2)=C(C=C2C(C=CC=C2)=C2)C2=C1N=C1C2=CC3=CC=CC=C3C=C2C4=N1 LKKPNUDVOYAOBB-UHFFFAOYSA-N 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 230000008035 nerve activity Effects 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 230000003227 neuromodulating effect Effects 0.000 description 1
- 230000004007 neuromodulation Effects 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 229910001000 nickel titanium Inorganic materials 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 210000001428 peripheral nervous system Anatomy 0.000 description 1
- 230000002186 photoactivation Effects 0.000 description 1
- IEQIEDJGQAUEQZ-UHFFFAOYSA-N phthalocyanine Chemical compound N1C(N=C2C3=CC=CC=C3C(N=C3C4=CC=CC=C4C(=N4)N3)=N2)=C(C=CC=C2)C2=C1N=C1C2=CC=CC=C2C4=N1 IEQIEDJGQAUEQZ-UHFFFAOYSA-N 0.000 description 1
- 230000006461 physiological response Effects 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- -1 polyethylene Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 238000006862 quantum yield reaction Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000011514 reflex Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000008327 renal blood flow Effects 0.000 description 1
- 230000008660 renal denervation Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 210000003935 rough endoplasmic reticulum Anatomy 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000000523 sample Substances 0.000 description 1
- 230000001235 sensitizing effect Effects 0.000 description 1
- 229910001285 shape-memory alloy Inorganic materials 0.000 description 1
- 238000005476 soldering Methods 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 230000036262 stenosis Effects 0.000 description 1
- 208000037804 stenosis Diseases 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 210000000225 synapse Anatomy 0.000 description 1
- 208000037905 systemic hypertension Diseases 0.000 description 1
- 230000006794 tachycardia Effects 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 239000012780 transparent material Substances 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 208000019553 vascular disease Diseases 0.000 description 1
- 230000006442 vascular tone Effects 0.000 description 1
- 230000024883 vasodilation Effects 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/0105—Steering means as part of the catheter or advancing means; Markers for positioning
- A61M25/0133—Tip steering devices
- A61M25/0147—Tip steering devices with movable mechanical means, e.g. pull wires
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/02—Holding devices, e.g. on the body
- A61M25/04—Holding devices, e.g. on the body in the body, e.g. expansible
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0067—Catheters; Hollow probes characterised by the distal end, e.g. tips
- A61M25/0082—Catheter tip comprising a tool
- A61M25/0084—Catheter tip comprising a tool being one or more injection needles
- A61M2025/0089—Single injection needle protruding axially, i.e. along the longitudinal axis of the catheter, from the distal tip
- A61M2025/009—Single injection needle protruding axially, i.e. along the longitudinal axis of the catheter, from the distal tip the needle having a bent tip, i.e. the needle distal tip is angled in relation to the longitudinal axis of the catheter
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M2025/1043—Balloon catheters with special features or adapted for special applications
- A61M2025/1047—Balloon catheters with special features or adapted for special applications having centering means, e.g. balloons having an appropriate shape
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0097—Catheters; Hollow probes characterised by the hub
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Hematology (AREA)
- Pulmonology (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Biophysics (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Mechanical Engineering (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
Description
a.ヘマトポルフィリン誘導体(HPD)
b.ポルフィリン
c.ベンゾポルフィリン誘導体一塩基酸環A(BPD−MA)
d.フタロシアニンおよびナフタロシアニン
e.テキサフィリン
f.カチオン性フラーレン
g.2−[1−ヘキシルオキシエチル]−2−デビニル−ピロフェオホルビド−a(HPPH)
h.クロリンおよびバクテリオクロリン
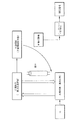
カテーテルの遠位端部を血管内の所望の位置に固定するため位置決めワイヤをカテーテルから離れて湾曲するのが望まれるとき、位置決めワイヤ28a、28b、28c、28dが軸方向に圧縮する負荷を受けて取り付けられる状態を起こすよう構成された位置決め手段もまた有する。位置決め手段は、第1のラック52とピニオン54の組み合わせを有する。第1のピニオンはハンドルの外形から充分露出され、ユーザがピニオンを親指で回転し、それによってラック52を遠位に伸ばすことを可能にする。
Claims (9)
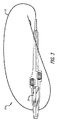
- カテーテル(12)と、ハイポチューブ(20)と、複数の位置決めワイヤ(28)と、及び引張構成要素(100)と、を備える血管内処置用システム(10)であって、
前記カテーテル(12)は、前記ハイポチューブ(20)を受け入れるよう構成された内腔(18)を形成する細長い軸を有し、且つ前記内腔(18)は前記カテーテルの外側の側壁の遠位開口部(24)で終了し、
前記ハイポチューブ(20)は、前記内腔に摺動可能に受け入れられ、前記ハイポチューブ(20)は、鋭くした遠位先端部(22)を有し、且つ前記カテーテル(12)に対して、前記遠位先端部(22)が前記内腔(18)内に完全に収容される引っ込められた状態と、及び前記遠位先端部(20)が前記遠位開口部(24)から突出する進められた状態とを有する様に構成され、
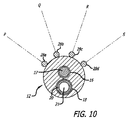
前記複数の位置決めワイヤ(28)は、前記カテーテル(12)に対して外側に伸びており、且つ前記位置決めワイヤ(28)が、前記カテーテル(12)に対して、各位置決めワイヤ(28)が前記カテーテル(12)の外壁に対して畳まれた、血管内送達用の拡張していない状態と、及び各位置決めワイヤ(28)がカテーテル軸から半径方向に離れて伸長する想像上の面に沿って前記カテーテル(12)から離れて曲がり、血管内で前記カテーテル(12)の位置決め用の拡張状態と、を有するよう構成され、
前記想像上の面は、前記カテーテル軸の周囲に180°以下の角度を持つ弧の範囲に全て収容され、
前記引張構成要素(100)は、前記カテーテル(12)の内腔(16)の内部に摺動可能に受け入れられ、各位置決めワイヤ(28)の遠位端(106)が、前記引張構成要素(100)と接続され、それによって、引張構成要素(100)の近位移動が前記位置決めワイヤ(28)を曲げて拡張状態をとり、且つ前記引張構成要素(100)がガイドワイヤ(17)を受け入れるように内腔(102)を形成し、
前記位置決めワイヤ(28)は、拡張状態において、前記弧の中心が遠位開口部(24)の位置の直径方向に反対側であるように、曲げられた前記位置決めワイヤ(28)を前記カテーテル(12)に対して位置付ける、
ことを特徴とする、血管内処置用システム(10)。 - 軸圧縮力を受けて各位置決めワイヤを取り付けるための手段であって、それによって前記各位置決めワイヤが前記拡張状態で前記カテーテルから離れて屈曲する、手段を更に備える、請求項1に記載のシステム。
- 前記軸圧縮力を受けて各位置決めワイヤを取り付けるための手段は、各位置決めワイヤの遠位端に取り付けられる引張構成要素を更に備え、前記引張構成要素は、前記カテーテルに対して近位移動するように構成される、請求項2に記載のシステム。
- 前記ハイポチューブを前記引っ込められた状態から遠位に前記進められた状態に移動する手段をさらに備える、請求項1に記載のシステム。
- 前記ハイポチューブを前記引っ込められた状態から遠位に前記進められた状態に移動する手段が、ラックおよびピニオンを含む、請求項4に記載のシステム。
- 前記複数の位置決めワイヤが合計4本である、請求項1に記載のシステム。
- 前記カテーテルを通って伸長する複数の位置決め内腔を更に備え、前記位置決めワイヤの長さの少なくとも一部に対して前記位置決め内腔を通って前記位置決めワイヤが伸長する、請求項1に記載のシステム。
- 前記位置決めワイヤによって占拠された仮想的な放射平面が前記細長い軸の周囲に120°以下の角度を持つ弧の範囲内で全て収容される、請求項1に記載のシステム。
- 前記位置決めワイヤは前記カテーテルに外側に伸長しており、かつ、外側に伸長する前記ワイヤの長さの中心が、前記カテーテルの軸方向で前記遠位開口部(24)と一致するように構成される、請求項1に記載のシステム。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/586,746 | 2012-08-15 | ||
| US13/586,746 US9033917B2 (en) | 2012-08-15 | 2012-08-15 | Needle catheter for delivery of agents directly into vessel wall |
| PCT/US2013/054188 WO2014028306A1 (en) | 2012-08-15 | 2013-08-08 | Needle catheter for delivery of agents directly into vessel wall |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2015524736A JP2015524736A (ja) | 2015-08-27 |
| JP2015524736A5 JP2015524736A5 (ja) | 2016-09-29 |
| JP6193997B2 true JP6193997B2 (ja) | 2017-09-06 |
Family
ID=49029220
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015527497A Expired - Fee Related JP6193997B2 (ja) | 2012-08-15 | 2013-08-08 | 血管壁内へ直接薬剤を送達する針カテーテル |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US9033917B2 (ja) |
| EP (1) | EP2885041B1 (ja) |
| JP (1) | JP6193997B2 (ja) |
| CN (1) | CN104661699B (ja) |
| WO (1) | WO2014028306A1 (ja) |
Families Citing this family (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20070135875A1 (en) | 2002-04-08 | 2007-06-14 | Ardian, Inc. | Methods and apparatus for thermally-induced renal neuromodulation |
| US20080213331A1 (en) | 2002-04-08 | 2008-09-04 | Ardian, Inc. | Methods and devices for renal nerve blocking |
| US7617005B2 (en) | 2002-04-08 | 2009-11-10 | Ardian, Inc. | Methods and apparatus for thermally-induced renal neuromodulation |
| US9636174B2 (en) | 2002-04-08 | 2017-05-02 | Medtronic Ardian Luxembourg S.A.R.L. | Methods for therapeutic renal neuromodulation |
| US8150519B2 (en) | 2002-04-08 | 2012-04-03 | Ardian, Inc. | Methods and apparatus for bilateral renal neuromodulation |
| US20070129761A1 (en) | 2002-04-08 | 2007-06-07 | Ardian, Inc. | Methods for treating heart arrhythmia |
| US7803168B2 (en) | 2004-12-09 | 2010-09-28 | The Foundry, Llc | Aortic valve repair |
| CN103517731B (zh) | 2011-04-08 | 2016-08-31 | 柯惠有限合伙公司 | 用于去除肾交感神经和离子电渗式药物传递的离子电渗式药物传递系统和方法 |
| JP2015532152A (ja) | 2012-10-11 | 2015-11-09 | ティーブイエー メディカル, インコーポレイテッド | フィステル形成のための装置および方法 |
| CA2905591C (en) | 2013-03-14 | 2023-02-28 | Tva Medical, Inc. | Fistula formulation devices and methods therefor |
| CN105682729B (zh) | 2013-10-25 | 2019-06-18 | 直观外科手术操作公司 | 具有带槽的可操控管的柔性器械 |
| WO2015061692A1 (en) * | 2013-10-25 | 2015-04-30 | Intuitive Surgical Operations, Inc. | Flexible instrument with embedded actuation conduits |
| JP6268673B2 (ja) * | 2014-03-17 | 2018-01-31 | ニプロ株式会社 | 子カテーテル |
| JP6336620B2 (ja) | 2014-05-06 | 2018-06-06 | セント・ジュード・メディカル,カーディオロジー・ディヴィジョン,インコーポレイテッド | 電極支持構造アセンブリ |
| US10118022B2 (en) | 2014-06-05 | 2018-11-06 | St. Jude Medical, Cardiology Division, Inc. | Deflectable catheter shaft section |
| US9844645B2 (en) | 2014-06-17 | 2017-12-19 | St. Jude Medical, Cardiology Division, Inc. | Triple coil catheter support |
| US10898096B2 (en) | 2014-10-27 | 2021-01-26 | St. Jude Medical, Cardiology Division, Inc. | Apparatus and method for connecting elements in medical devices |
| US10603040B1 (en) | 2015-02-09 | 2020-03-31 | Tva Medical, Inc. | Methods for treating hypertension and reducing blood pressure with formation of fistula |
| WO2016176515A1 (en) * | 2015-04-28 | 2016-11-03 | Kassab Ghassan S | Materials and methods of using the same to improve structural integrity of a wall of a mammalian luminal organ |
| US10602983B2 (en) | 2015-05-08 | 2020-03-31 | St. Jude Medical International Holding S.À R.L. | Integrated sensors for medical devices and method of making integrated sensors for medical devices |
| JP6445742B1 (ja) | 2015-10-21 | 2018-12-26 | セント・ジュード・メディカル,カーディオロジー・ディヴィジョン,インコーポレイテッド | 高密度電極マッピングカテーテル |
| BR112018014112A8 (pt) | 2016-01-15 | 2023-02-23 | Tva Medical Inc | Dispositivos e métodos para formação de uma fístula |
| EP3402561B1 (en) | 2016-01-15 | 2024-02-28 | TVA Medical, Inc. | Devices for advancing a wire |
| US10874422B2 (en) | 2016-01-15 | 2020-12-29 | Tva Medical, Inc. | Systems and methods for increasing blood flow |
| WO2017192712A1 (en) | 2016-05-03 | 2017-11-09 | St. Jude Medical, Cardiology Division, Inc. | Irrigated high density electrode catheter |
| US11786705B2 (en) | 2016-10-24 | 2023-10-17 | St. Jude Medical, Cardiology Division, Inc. | Catheter insertion devices |
| US11172858B2 (en) | 2016-10-28 | 2021-11-16 | St. Jude Medical, Cardiology Division, Inc. | Flexible high-density mapping catheter |
| EP3531903B1 (en) | 2017-01-19 | 2021-02-17 | St. Jude Medical, Cardiology Division, Inc. | Sheath visualization |
| CN116369997A (zh) * | 2017-03-27 | 2023-07-04 | 波士顿科学国际有限公司 | 用于影响组织结构移动的系统和方法 |
| US11647935B2 (en) | 2017-07-24 | 2023-05-16 | St. Jude Medical, Cardiology Division, Inc. | Masked ring electrodes |
| EP4115936B1 (en) | 2017-11-28 | 2024-03-06 | St. Jude Medical, Cardiology Division, Inc. | Lumen management catheter |
| US11116561B2 (en) | 2018-01-24 | 2021-09-14 | Medtronic Ardian Luxembourg S.A.R.L. | Devices, agents, and associated methods for selective modulation of renal nerves |
| WO2020039392A2 (en) | 2018-08-23 | 2020-02-27 | St. Jude Medical, Cardiology Division, Inc. | Curved high density electrode mapping catheter |
| WO2020065587A2 (en) | 2018-09-27 | 2020-04-02 | St. Jude Medical, Cardiology Division, Inc. | Uniform mapping balloon |
| US11918762B2 (en) | 2018-10-03 | 2024-03-05 | St. Jude Medical, Cardiology Division, Inc. | Reduced actuation force electrophysiology catheter handle |
| US20210186601A1 (en) * | 2019-12-23 | 2021-06-24 | Ethicon, Inc. | Transesophageal Catheter for Thermal Protection of the Esophagus |
Family Cites Families (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1466889B1 (de) * | 1965-07-28 | 1970-04-23 | Eberhard Dr Regenbogen | Rektoskop zur Endoskopie des als Sigma bezeichneten Bereichs des menschlichen Darmes |
| US4578061A (en) | 1980-10-28 | 1986-03-25 | Lemelson Jerome H | Injection catheter and method |
| US5509900A (en) | 1992-03-02 | 1996-04-23 | Kirkman; Thomas R. | Apparatus and method for retaining a catheter in a blood vessel in a fixed position |
| DE4235506A1 (de) | 1992-10-21 | 1994-04-28 | Bavaria Med Tech | Katheter zur Injektion von Arzneimitteln |
| DE4408108A1 (de) | 1994-03-10 | 1995-09-14 | Bavaria Med Tech | Katheter zur Injektion eines Fluid bzw. eines Arneimittelns |
| US5464395A (en) | 1994-04-05 | 1995-11-07 | Faxon; David P. | Catheter for delivering therapeutic and/or diagnostic agents to the tissue surrounding a bodily passageway |
| US6283951B1 (en) | 1996-10-11 | 2001-09-04 | Transvascular, Inc. | Systems and methods for delivering drugs to selected locations within the body |
| KR19990064209A (ko) | 1995-10-13 | 1999-07-26 | 트랜스바스큘라, 인코포레이티드 | 간질성 경혈관 개입을 위한 장치, 시스템 및 방법 |
| US6302875B1 (en) * | 1996-10-11 | 2001-10-16 | Transvascular, Inc. | Catheters and related devices for forming passageways between blood vessels or other anatomical structures |
| US6375615B1 (en) | 1995-10-13 | 2002-04-23 | Transvascular, Inc. | Tissue penetrating catheters having integral imaging transducers and their methods of use |
| US6726677B1 (en) * | 1995-10-13 | 2004-04-27 | Transvascular, Inc. | Stabilized tissue penetrating catheters |
| GB2327614B (en) | 1997-07-30 | 2002-03-06 | Univ Dundee | A hypodermic needle |
| WO1999065561A1 (en) | 1998-06-19 | 1999-12-23 | Cordis Webster, Inc. | Method and apparatus for transvascular treatment of tachycardia and fibrillation |
| AU736964B2 (en) | 1998-12-09 | 2001-08-09 | Cook Medical Technologies Llc | Hollow, curved, superelastic medical needle |
| US6217554B1 (en) | 1999-02-12 | 2001-04-17 | Pharmaspec Corporation | Methods and apparatus for delivering substances into extravascular tissue |
| US6302870B1 (en) | 1999-04-29 | 2001-10-16 | Precision Vascular Systems, Inc. | Apparatus for injecting fluids into the walls of blood vessels, body cavities, and the like |
| US6213976B1 (en) | 1999-07-22 | 2001-04-10 | Advanced Research And Technology Institute, Inc. | Brachytherapy guide catheter |
| US6629987B1 (en) | 1999-07-30 | 2003-10-07 | C. R. Bard, Inc. | Catheter positioning systems |
| US6458098B1 (en) | 2000-03-17 | 2002-10-01 | Nozomu Kanesaka | Vascular therapy device |
| JP4229621B2 (ja) | 2002-03-05 | 2009-02-25 | 修 加藤 | 薬液注入カテーテル |
| US7141041B2 (en) | 2003-03-19 | 2006-11-28 | Mercator Medsystems, Inc. | Catheters having laterally deployable needles |
| US20060167437A1 (en) | 2003-06-17 | 2006-07-27 | Flowmedica, Inc. | Method and apparatus for intra aortic substance delivery to a branch vessel |
| US7682358B2 (en) * | 2003-10-30 | 2010-03-23 | Medtronic, Inc. | Steerable catheter |
| WO2005049125A1 (en) * | 2003-11-17 | 2005-06-02 | Cook Critical Care Incorporated | Catheter with centering wire |
| CA2609048C (en) * | 2005-05-19 | 2014-01-21 | Spirus Medical Inc. | Rotate-to-advance catheterization system |
| DE102007052513A1 (de) | 2007-10-26 | 2009-04-30 | Karl Storz Gmbh & Co. Kg | Medizinisches Instrument mit seitlich ausspreizbaren Injektionsnadeln |
| JP2012020105A (ja) * | 2010-06-18 | 2012-02-02 | Olympus Corp | カテーテル |
-
2012
- 2012-08-15 US US13/586,746 patent/US9033917B2/en active Active
-
2013
- 2013-08-08 WO PCT/US2013/054188 patent/WO2014028306A1/en active Application Filing
- 2013-08-08 JP JP2015527497A patent/JP6193997B2/ja not_active Expired - Fee Related
- 2013-08-08 EP EP13752760.2A patent/EP2885041B1/en not_active Not-in-force
- 2013-08-08 CN CN201380042759.XA patent/CN104661699B/zh not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| EP2885041A1 (en) | 2015-06-24 |
| CN104661699B (zh) | 2016-06-08 |
| CN104661699A (zh) | 2015-05-27 |
| WO2014028306A1 (en) | 2014-02-20 |
| US9033917B2 (en) | 2015-05-19 |
| US20140052052A1 (en) | 2014-02-20 |
| JP2015524736A (ja) | 2015-08-27 |
| EP2885041B1 (en) | 2015-12-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6193997B2 (ja) | 血管壁内へ直接薬剤を送達する針カテーテル | |
| JP7093744B2 (ja) | 支持構造を備えた脈管周囲組織焼灼カテーテル | |
| JP6932037B2 (ja) | 血管壁注入および血管周囲腎臓除神経のためのカテーテルシステム | |
| US5425703A (en) | Method and apparatus for inducing the permeation of medication into internal tissue | |
| US20020095197A1 (en) | Application of photochemotherapy for the treatment of cardiac arrhythmias | |
| US5429634A (en) | Biogenic implant for drug delivery and method | |
| US20150080709A1 (en) | Implantable Medical Devices, Methods of Use, and Apparatus for Extraction Thereof | |
| US20150343156A1 (en) | Expandable catheter system for fluid injection into and deep to the wall of a blood vessel | |
| EP0814719B1 (en) | Ultrasound therapy device | |
| WO2002009610A1 (en) | Procedures for photodynamic cardiac ablation therapy and devices for those procedures | |
| EP1853189A2 (en) | Photoreactive system and methods for prophylactic treatment of atherosclerosis | |
| CA2339384C (en) | Improved method for targeted topical treatment of disease | |
| AU6086694A (en) | Medicament dispensing stents | |
| Ayaru et al. | Photodynamic therapy for pancreatic and biliary tract carcinoma | |
| JP2015073705A (ja) | 医療用デバイス | |
| US20050130950A1 (en) | Method for improving treatment selectivity and efficacy using intravascular photodynamic therapy | |
| JP2007528754A (ja) | 光ダイナミック療法をするために内腔内に自動調心する光発生装置 | |
| EP1166784B1 (en) | Inhibition of vascular restenosis after angioplasty | |
| Rosenthal | Studies of the laser thermal probe in cardiovascular disease | |
| US20130116496A1 (en) | Apparatus and method for radiation treatment of a desired area in the renal vascular system of a patient | |
| Narciso Jr et al. | SnET2 for the treatment of vascular disease: dose/response study in New Zealand white (NZW) rabbits |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A529 | Written submission of copy of amendment under article 34 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A529 Effective date: 20150317 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160805 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20160805 |
|
| A871 | Explanation of circumstances concerning accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A871 Effective date: 20160805 |
|
| A975 | Report on accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A971005 Effective date: 20160906 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20161025 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170105 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20170214 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170502 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20170711 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20170810 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6193997 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |