JP6085845B2 - 改良型放射線不透過性マーカ方式の血管内デリバリシステム - Google Patents
改良型放射線不透過性マーカ方式の血管内デリバリシステム Download PDFInfo
- Publication number
- JP6085845B2 JP6085845B2 JP2015517290A JP2015517290A JP6085845B2 JP 6085845 B2 JP6085845 B2 JP 6085845B2 JP 2015517290 A JP2015517290 A JP 2015517290A JP 2015517290 A JP2015517290 A JP 2015517290A JP 6085845 B2 JP6085845 B2 JP 6085845B2
- Authority
- JP
- Japan
- Prior art keywords
- delivery system
- prosthesis
- marker
- markers
- holder
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000003550 marker Substances 0.000 title claims description 115
- 239000000463 material Substances 0.000 claims description 73
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 28
- 229910052697 platinum Inorganic materials 0.000 claims description 14
- 229910052741 iridium Inorganic materials 0.000 claims description 13
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 claims description 13
- 238000000034 method Methods 0.000 description 63
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 24
- -1 polyethylene Polymers 0.000 description 24
- 239000012530 fluid Substances 0.000 description 20
- 230000003447 ipsilateral effect Effects 0.000 description 18
- 239000004810 polytetrafluoroethylene Substances 0.000 description 18
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 18
- 208000007474 aortic aneurysm Diseases 0.000 description 17
- 210000004204 blood vessel Anatomy 0.000 description 14
- 238000002513 implantation Methods 0.000 description 13
- 238000002594 fluoroscopy Methods 0.000 description 12
- 210000005166 vasculature Anatomy 0.000 description 10
- 150000004985 diamines Chemical class 0.000 description 9
- 210000003090 iliac artery Anatomy 0.000 description 9
- 229920001577 copolymer Polymers 0.000 description 8
- 238000011282 treatment Methods 0.000 description 8
- 208000002223 abdominal aortic aneurysm Diseases 0.000 description 7
- 229920000642 polymer Polymers 0.000 description 7
- 230000035945 sensitivity Effects 0.000 description 7
- 206010002329 Aneurysm Diseases 0.000 description 6
- 239000004743 Polypropylene Substances 0.000 description 6
- 210000000709 aorta Anatomy 0.000 description 6
- 230000008859 change Effects 0.000 description 6
- 238000004891 communication Methods 0.000 description 6
- 238000003384 imaging method Methods 0.000 description 6
- 230000036961 partial effect Effects 0.000 description 6
- 230000008439 repair process Effects 0.000 description 6
- 238000000576 coating method Methods 0.000 description 5
- GYZLOYUZLJXAJU-UHFFFAOYSA-N diglycidyl ether Chemical compound C1OC1COCC1CO1 GYZLOYUZLJXAJU-UHFFFAOYSA-N 0.000 description 5
- 239000000017 hydrogel Substances 0.000 description 5
- 230000035699 permeability Effects 0.000 description 5
- 229920000728 polyester Polymers 0.000 description 5
- 229920000139 polyethylene terephthalate Polymers 0.000 description 5
- 239000005020 polyethylene terephthalate Substances 0.000 description 5
- 229920001155 polypropylene Polymers 0.000 description 5
- 238000001356 surgical procedure Methods 0.000 description 5
- 230000002792 vascular Effects 0.000 description 5
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 4
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 4
- 239000004952 Polyamide Substances 0.000 description 4
- 201000008982 Thoracic Aortic Aneurysm Diseases 0.000 description 4
- 230000004323 axial length Effects 0.000 description 4
- 239000011248 coating agent Substances 0.000 description 4
- 208000003457 familial thoracic 1 aortic aneurysm Diseases 0.000 description 4
- 229920001903 high density polyethylene Polymers 0.000 description 4
- 239000004700 high-density polyethylene Substances 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 229920002647 polyamide Polymers 0.000 description 4
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 4
- 229910052721 tungsten Inorganic materials 0.000 description 4
- 239000010937 tungsten Substances 0.000 description 4
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 239000004677 Nylon Substances 0.000 description 3
- 239000004698 Polyethylene Substances 0.000 description 3
- 239000004721 Polyphenylene oxide Substances 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 229920000249 biocompatible polymer Polymers 0.000 description 3
- 210000001105 femoral artery Anatomy 0.000 description 3
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 3
- 229910052737 gold Inorganic materials 0.000 description 3
- 239000010931 gold Substances 0.000 description 3
- 229910001000 nickel titanium Inorganic materials 0.000 description 3
- 229920001778 nylon Polymers 0.000 description 3
- 229920000570 polyether Polymers 0.000 description 3
- 229920000573 polyethylene Polymers 0.000 description 3
- NLKNQRATVPKPDG-UHFFFAOYSA-M potassium iodide Chemical compound [K+].[I-] NLKNQRATVPKPDG-UHFFFAOYSA-M 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- FVAUCKIRQBBSSJ-UHFFFAOYSA-M sodium iodide Chemical compound [Na+].[I-] FVAUCKIRQBBSSJ-UHFFFAOYSA-M 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 229910052715 tantalum Inorganic materials 0.000 description 3
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- FJKROLUGYXJWQN-UHFFFAOYSA-N 4-hydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 2
- 238000012276 Endovascular treatment Methods 0.000 description 2
- 239000004812 Fluorinated ethylene propylene Substances 0.000 description 2
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 2
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 2
- 229920002614 Polyether block amide Polymers 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 239000004642 Polyimide Substances 0.000 description 2
- 239000004793 Polystyrene Substances 0.000 description 2
- 229920002125 Sokalan® Polymers 0.000 description 2
- KKEYFWRCBNTPAC-UHFFFAOYSA-N Terephthalic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-N 0.000 description 2
- 210000003484 anatomy Anatomy 0.000 description 2
- 229910052788 barium Inorganic materials 0.000 description 2
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 2
- TZCXTZWJZNENPQ-UHFFFAOYSA-L barium sulfate Chemical compound [Ba+2].[O-]S([O-])(=O)=O TZCXTZWJZNENPQ-UHFFFAOYSA-L 0.000 description 2
- 239000000560 biocompatible material Substances 0.000 description 2
- 229910052797 bismuth Inorganic materials 0.000 description 2
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 229920001971 elastomer Polymers 0.000 description 2
- 239000000806 elastomer Substances 0.000 description 2
- HQQADJVZYDDRJT-UHFFFAOYSA-N ethene;prop-1-ene Chemical group C=C.CC=C HQQADJVZYDDRJT-UHFFFAOYSA-N 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 150000002334 glycols Chemical class 0.000 description 2
- 229920001519 homopolymer Polymers 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 230000000877 morphologic effect Effects 0.000 description 2
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 2
- 229910052763 palladium Inorganic materials 0.000 description 2
- 229920009441 perflouroethylene propylene Polymers 0.000 description 2
- 229920002401 polyacrylamide Polymers 0.000 description 2
- 239000004417 polycarbonate Substances 0.000 description 2
- 229920000515 polycarbonate Polymers 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920001721 polyimide Polymers 0.000 description 2
- 229920000098 polyolefin Polymers 0.000 description 2
- 229920001451 polypropylene glycol Polymers 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- 229920002635 polyurethane Polymers 0.000 description 2
- 239000004814 polyurethane Substances 0.000 description 2
- 229920002689 polyvinyl acetate Polymers 0.000 description 2
- 239000011118 polyvinyl acetate Substances 0.000 description 2
- 229920000915 polyvinyl chloride Polymers 0.000 description 2
- 239000004800 polyvinyl chloride Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 210000002254 renal artery Anatomy 0.000 description 2
- 239000004753 textile Substances 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- 238000012800 visualization Methods 0.000 description 2
- 230000003313 weakening effect Effects 0.000 description 2
- HQUZVILJINRCDT-UHFFFAOYSA-N 1,7-diamino-4-(2-hydroxyethoxymethyl)heptan-4-ol Chemical compound NCCCC(O)(CCCN)COCCO HQUZVILJINRCDT-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- UXMYUFHUUYBDLL-UHFFFAOYSA-N 2,2-dimethyl-3-(oxiran-2-ylmethoxy)propan-1-ol Chemical compound OCC(C)(C)COCC1CO1 UXMYUFHUUYBDLL-UHFFFAOYSA-N 0.000 description 1
- WTYYGFLRBWMFRY-UHFFFAOYSA-N 2-[6-(oxiran-2-ylmethoxy)hexoxymethyl]oxirane Chemical compound C1OC1COCCCCCCOCC1CO1 WTYYGFLRBWMFRY-UHFFFAOYSA-N 0.000 description 1
- FSYPIGPPWAJCJG-UHFFFAOYSA-N 2-[[4-(oxiran-2-ylmethoxy)phenoxy]methyl]oxirane Chemical compound C1OC1COC(C=C1)=CC=C1OCC1CO1 FSYPIGPPWAJCJG-UHFFFAOYSA-N 0.000 description 1
- NYEZZYQZRQDLEH-UHFFFAOYSA-N 2-ethyl-4,5-dihydro-1,3-oxazole Chemical class CCC1=NCCO1 NYEZZYQZRQDLEH-UHFFFAOYSA-N 0.000 description 1
- UUODQIKUTGWMPT-UHFFFAOYSA-N 2-fluoro-5-(trifluoromethyl)pyridine Chemical compound FC1=CC=C(C(F)(F)F)C=N1 UUODQIKUTGWMPT-UHFFFAOYSA-N 0.000 description 1
- OMIGHNLMNHATMP-UHFFFAOYSA-N 2-hydroxyethyl prop-2-enoate Chemical compound OCCOC(=O)C=C OMIGHNLMNHATMP-UHFFFAOYSA-N 0.000 description 1
- HUHDYASLFWQVOL-WZTVWXICSA-N 3-[[2-[[3-[acetyl(methyl)amino]-2,4,6-triiodo-5-(methylcarbamoyl)benzoyl]amino]acetyl]amino]-5-(2-hydroxyethylcarbamoyl)-2,4,6-triiodobenzoic acid;(2r,3r,4r,5s)-6-(methylamino)hexane-1,2,3,4,5-pentol Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.CNC(=O)C1=C(I)C(N(C)C(C)=O)=C(I)C(C(=O)NCC(=O)NC=2C(=C(C(=O)NCCO)C(I)=C(C(O)=O)C=2I)I)=C1I HUHDYASLFWQVOL-WZTVWXICSA-N 0.000 description 1
- MECNWXGGNCJFQJ-UHFFFAOYSA-N 3-piperidin-1-ylpropane-1,2-diol Chemical group OCC(O)CN1CCCCC1 MECNWXGGNCJFQJ-UHFFFAOYSA-N 0.000 description 1
- 229940090248 4-hydroxybenzoic acid Drugs 0.000 description 1
- SQDAZGGFXASXDW-UHFFFAOYSA-N 5-bromo-2-(trifluoromethoxy)pyridine Chemical compound FC(F)(F)OC1=CC=C(Br)C=N1 SQDAZGGFXASXDW-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 1
- 206010057453 Aortic dilatation Diseases 0.000 description 1
- GAWIXWVDTYZWAW-UHFFFAOYSA-N C[CH]O Chemical group C[CH]O GAWIXWVDTYZWAW-UHFFFAOYSA-N 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 229920002101 Chitin Polymers 0.000 description 1
- 229920001287 Chondroitin sulfate Polymers 0.000 description 1
- 229910000531 Co alloy Inorganic materials 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 229920004934 Dacron® Polymers 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 244000043261 Hevea brasiliensis Species 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 241001272720 Medialuna californiensis Species 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 1
- 239000004696 Poly ether ether ketone Substances 0.000 description 1
- 229920001744 Polyaldehyde Polymers 0.000 description 1
- 229920002873 Polyethylenimine Polymers 0.000 description 1
- 229920002396 Polyurea Polymers 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 239000004830 Super Glue Substances 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- ZJCCRDAZUWHFQH-UHFFFAOYSA-N Trimethylolpropane Chemical compound CCC(CO)(CO)CO ZJCCRDAZUWHFQH-UHFFFAOYSA-N 0.000 description 1
- HZEWFHLRYVTOIW-UHFFFAOYSA-N [Ti].[Ni] Chemical compound [Ti].[Ni] HZEWFHLRYVTOIW-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 125000003282 alkyl amino group Chemical group 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 210000000702 aorta abdominal Anatomy 0.000 description 1
- 210000002413 aortic body Anatomy 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229940059329 chondroitin sulfate Drugs 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- GPLRAVKSCUXZTP-UHFFFAOYSA-N diglycerol Chemical compound OCC(O)COCC(O)CO GPLRAVKSCUXZTP-UHFFFAOYSA-N 0.000 description 1
- 239000004205 dimethyl polysiloxane Substances 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000013013 elastic material Substances 0.000 description 1
- 229910000701 elgiloys (Co-Cr-Ni Alloy) Inorganic materials 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- FGBJXOREULPLGL-UHFFFAOYSA-N ethyl cyanoacrylate Chemical compound CCOC(=O)C(=C)C#N FGBJXOREULPLGL-UHFFFAOYSA-N 0.000 description 1
- 229920000295 expanded polytetrafluoroethylene Polymers 0.000 description 1
- 210000003414 extremity Anatomy 0.000 description 1
- 238000005429 filling process Methods 0.000 description 1
- 238000011010 flushing procedure Methods 0.000 description 1
- 125000003055 glycidyl group Chemical group C(C1CO1)* 0.000 description 1
- 230000023597 hemostasis Effects 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 210000001621 ilium bone Anatomy 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000012977 invasive surgical procedure Methods 0.000 description 1
- NBQNWMBBSKPBAY-UHFFFAOYSA-N iodixanol Chemical compound IC=1C(C(=O)NCC(O)CO)=C(I)C(C(=O)NCC(O)CO)=C(I)C=1N(C(=O)C)CC(O)CN(C(C)=O)C1=C(I)C(C(=O)NCC(O)CO)=C(I)C(C(=O)NCC(O)CO)=C1I NBQNWMBBSKPBAY-UHFFFAOYSA-N 0.000 description 1
- NTHXOOBQLCIOLC-UHFFFAOYSA-N iohexol Chemical compound OCC(O)CN(C(=O)C)C1=C(I)C(C(=O)NCC(O)CO)=C(I)C(C(=O)NCC(O)CO)=C1I NTHXOOBQLCIOLC-UHFFFAOYSA-N 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- MIKKOBKEXMRYFQ-WZTVWXICSA-N meglumine amidotrizoate Chemical compound C[NH2+]C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.CC(=O)NC1=C(I)C(NC(C)=O)=C(I)C(C([O-])=O)=C1I MIKKOBKEXMRYFQ-WZTVWXICSA-N 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- XJRBAMWJDBPFIM-UHFFFAOYSA-N methyl vinyl ether Chemical compound COC=C XJRBAMWJDBPFIM-UHFFFAOYSA-N 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 125000005487 naphthalate group Chemical group 0.000 description 1
- 229920003052 natural elastomer Polymers 0.000 description 1
- 229920001194 natural rubber Polymers 0.000 description 1
- 229910052758 niobium Inorganic materials 0.000 description 1
- 239000010955 niobium Substances 0.000 description 1
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 description 1
- WXZMFSXDPGVJKK-UHFFFAOYSA-N pentaerythritol Chemical compound OCC(CO)(CO)CO WXZMFSXDPGVJKK-UHFFFAOYSA-N 0.000 description 1
- 229920000233 poly(alkylene oxides) Polymers 0.000 description 1
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 1
- 229920003207 poly(ethylene-2,6-naphthalate) Polymers 0.000 description 1
- 229920002627 poly(phosphazenes) Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 229920002239 polyacrylonitrile Polymers 0.000 description 1
- 229920002530 polyetherether ketone Polymers 0.000 description 1
- 239000011112 polyethylene naphthalate Substances 0.000 description 1
- 229920000223 polyglycerol Polymers 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 229920000193 polymethacrylate Polymers 0.000 description 1
- 229920000379 polypropylene carbonate Polymers 0.000 description 1
- 229920003226 polyurethane urea Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- HNJBEVLQSNELDL-UHFFFAOYSA-N pyrrolidin-2-one Chemical compound O=C1CCCN1 HNJBEVLQSNELDL-UHFFFAOYSA-N 0.000 description 1
- 238000002601 radiography Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 230000002787 reinforcement Effects 0.000 description 1
- 230000000452 restraining effect Effects 0.000 description 1
- 229910001285 shape-memory alloy Inorganic materials 0.000 description 1
- 229920002050 silicone resin Polymers 0.000 description 1
- 229920002379 silicone rubber Polymers 0.000 description 1
- 239000004945 silicone rubber Substances 0.000 description 1
- 238000004513 sizing Methods 0.000 description 1
- 210000003625 skull Anatomy 0.000 description 1
- 235000009518 sodium iodide Nutrition 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229920003048 styrene butadiene rubber Polymers 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 229920002994 synthetic fiber Polymers 0.000 description 1
- 239000012209 synthetic fiber Substances 0.000 description 1
- 229920001897 terpolymer Polymers 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 229920001169 thermoplastic Polymers 0.000 description 1
- 229920002725 thermoplastic elastomer Polymers 0.000 description 1
- 229920005992 thermoplastic resin Polymers 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 230000007556 vascular defect Effects 0.000 description 1
- 208000019553 vascular disease Diseases 0.000 description 1
- NLVXSWCKKBEXTG-UHFFFAOYSA-N vinylsulfonic acid Chemical compound OS(=O)(=O)C=C NLVXSWCKKBEXTG-UHFFFAOYSA-N 0.000 description 1
- 238000007794 visualization technique Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/962—Instruments specially adapted for placement or removal of stents or stent-grafts having an outer sleeve
- A61F2/966—Instruments specially adapted for placement or removal of stents or stent-grafts having an outer sleeve with relative longitudinal movement between outer sleeve and prosthesis, e.g. using a push rod
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/9522—Means for mounting a stent or stent-graft onto or into a placement instrument
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2002/9505—Instruments specially adapted for placement or removal of stents or stent-grafts having retaining means other than an outer sleeve, e.g. male-female connector between stent and instrument
- A61F2002/9511—Instruments specially adapted for placement or removal of stents or stent-grafts having retaining means other than an outer sleeve, e.g. male-female connector between stent and instrument the retaining means being filaments or wires
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/962—Instruments specially adapted for placement or removal of stents or stent-grafts having an outer sleeve
- A61F2/966—Instruments specially adapted for placement or removal of stents or stent-grafts having an outer sleeve with relative longitudinal movement between outer sleeve and prosthesis, e.g. using a push rod
- A61F2002/9665—Instruments specially adapted for placement or removal of stents or stent-grafts having an outer sleeve with relative longitudinal movement between outer sleeve and prosthesis, e.g. using a push rod with additional retaining means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0063—Three-dimensional shapes
- A61F2230/0069—Three-dimensional shapes cylindrical
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0003—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having an inflatable pocket filled with fluid, e.g. liquid or gas
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0058—Additional features; Implant or prostheses properties not otherwise provided for
- A61F2250/0096—Markers and sensors for detecting a position or changes of a position of an implant, e.g. RF sensors, ultrasound markers
- A61F2250/0098—Markers and sensors for detecting a position or changes of a position of an implant, e.g. RF sensors, ultrasound markers radio-opaque, e.g. radio-opaque markers
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
Description
本願は、2012年6月15日に出願された米国特許仮出願第61/660,413号の権益主張出願である。
dgap/dθ=−R(sinθ+cosθ)
であり、他方、本明細書において説明した実施形態において構成される第2のマーカのないシステムの場合、隙間の変化率は次式で表される。
dgap/dθ=−Rsinθ
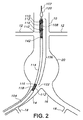
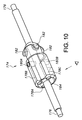
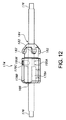
開口したルーメン並びに互いに反対側に位置した近位端及び遠位端を有する細長い外側管状器具を含み、上記近位端と上記遠位端との間には中間部分が設けられ、
上記外側管状器具内に設けられたプロテーゼホルダを含み、上記プロテーゼホルダは、
上記プロテーゼホルダを貫通して延びる軸方向ガイドワイヤを有し、
上記軸方向ガイドワイヤを包囲した本体を有し、上記本体は、上記軸方向ガイドワイヤに平行な方向に整列し且つ各々が上記軸方向ガイドワイヤから等距離間隔を置いて位置した少なくとも2つの全体として円筒形のマーカを有し、
外面を有し、プロテーゼを運搬に先立って上記外面に固定することができる、デリバリシステム。
〔実施態様項2〕 上記プロテーゼホルダは、蛍光性でもなく放射線不透過性でもない材料で作られている、実施態様項1記載のデリバリシステム。
〔実施態様項3〕 上記軸方向ガイドワイヤは、蛍光性であり又は放射線不透過性である材料で作られている、実施態様項1記載のデリバリシステム。
〔実施態様項4〕 上記全体として円筒形のマーカの各々は、蛍光性であり又は放射線不透過性である材料で作られている、実施態様項1記載のデリバリシステム。
〔実施態様項5〕 上記全体として円筒形のマーカの各々は、白金とイリジウムの組み合わせで作られている、実施態様項4記載のデリバリシステム。
〔実施態様項6〕 上記軸方向ガイドワイヤに平行な方向に整列した第3の全体として円筒形のマーカを更に含み、上記全体として円筒形のマーカの各々は、上記軸方向ガイドワイヤから等距離間隔を置いて配置されている、実施態様項1記載のデリバリシステム。
〔実施態様項7〕 上記全体として円筒形のマーカの各々は、上記軸方向ガイドワイヤによって形成された軸線回りに測定して約90°間隔で配置されている、実施態様項6記載のデリバリシステム。
〔実施態様項8〕 上記全体として円筒形のマーカの各々は、蛍光性であり又は放射線不透過性である材料で作られている、実施態様項7記載のデリバリシステム。
〔実施態様項9〕 上記全体として円筒形のマーカの各々は、白金とイリジウムの組み合わせで作られている、実施態様項8記載のデリバリシステム。
〔実施態様項10〕 少なくとも1つの垂直のマーカを更に含み、上記垂直マーカは、上記軸方向ガイドワイヤに対して垂直の角度をなして設けられている、実施態様項1記載のデリバリシステム。
〔実施態様項11〕 上記垂直マーカは、蛍光性であり又は放射線不透過性である材料で作られている、実施態様項10記載のデリバリシステム。
〔実施態様項12〕 上記垂直マーカは、白金とイリジウムの組み合わせで作られている、実施態様項11記載のデリバリシステム。
〔実施態様項13〕 少なくとも1つの垂直のマーカを更に含み、上記垂直マーカは、上記軸方向ガイドワイヤに対して垂直の角度をなして設けられている、実施態様項6記載のデリバリシステム。
〔実施態様項14〕 上記垂直マーカは、蛍光性であり又は放射線不透過性である材料で作られている、実施態様項13記載のデリバリシステム。
〔実施態様項15〕 上記垂直マーカは、白金とイリジウムの組み合わせで作られている、実施態様項14記載のデリバリシステム。
〔実施態様項16〕 上記プロテーゼは、ステント‐グラフトである、実施態様項1記載のデリバリシステム。
〔実施態様項17〕 上記プロテーゼホルダは、上記ステント‐グラフトを上記プロテーゼホルダの上記外面に固定するための複数のアンカを有する、実施態様項16記載のデリバリシステム。
〔実施態様項18〕 上記全体として円筒形のマーカは、上記本体内に圧力嵌めされている、実施態様項1記載のデリバリシステム。
〔実施態様項19〕 上記全体として円筒形のマーカは、上記本体内に成形されている、実施態様項1記載のデリバリシステム。
〔実施態様項20〕 上記全体として円筒形のマーカは、約0.030インチ(0.762mm)の直径を有する、実施態様項1記載のデリバリシステム。
〔実施態様項21〕 上記軸方向ガイドワイヤは、約0.035インチ(0.889mm)の直径を有する、実施態様項1記載のデリバリシステム。
〔実施態様項22〕 上記全体として円筒形のマーカは、上記本体内に圧力嵌めされている、実施態様項6記載のデリバリシステム。
〔実施態様項23〕 上記全体として円筒形のマーカは、上記本体内に成形されている、実施態様項6記載のデリバリシステム。
〔実施態様項24〕 上記全体として円筒形のマーカは、約0.010インチ(0.254mm)〜約0.040インチ(1.016mm)の直径を有する、実施態様項6記載のデリバリシステム。
〔実施態様項25〕 上記軸方向ガイドワイヤは、約0.010インチ(0.254mm)〜約0.050インチ(1.270mm)の直径を有する、実施態様項6記載のデリバリシステム。
〔実施態様項26〕 上記全体として円筒形のマーカは、上記本体内に上記軸方向ガイドワイヤから約0.010インチ(0.254mm)〜約0.015インチ(0.381mm)の距離を置いたところに設けられている、実施態様項1記載のデリバリシステム。
〔実施態様項27〕 上記全体として円筒形のマーカの各々は、上記本体内に上記軸方向ガイドワイヤから約0.010インチ(0.254mm)〜約0.015インチ(0.381mm)の距離を置いたところに設けられている、実施態様項6記載のデリバリシステム。
〔実施態様項28〕 プロテーゼを体内管腔内で運搬する方法であって、
(a)デリバリシステムを用意するステップを含み、上記デリバリシステムは、
(i)開口したルーメン並びに互いに反対側に位置した近位端及び遠位端を有する細長い外側管状器具を含み、上記近位端と上記遠位端との間には中間部分が設けられ、
(ii)上記外側管状器具内に設けられたプロテーゼホルダを含み、上記プロテーゼホルダは、
上記プロテーゼホルダを貫通して延びる軸方向ガイドワイヤを有し、
上記軸方向ガイドワイヤを包囲した本体を有し、上記本体は、上記軸方向ガイドワイヤに平行な方向に整列し且つ各々が上記軸方向ガイドワイヤから等距離間隔を置いて位置した少なくとも2つの全体として円筒形のマーカを有し、
外面を有し、
上記外面に固定されたプロテーゼを有し、
(b)上記デリバリシステムを体内管腔内に挿入して上記プロテーゼホルダを上記ルーメン内の所望の場所に方向付けるステップを含み、
(c)装置を用いて上記全体として円筒形のマーカの配置場所を観察するステップを含み、
(d)上記プロテーゼホルダを上記全体として円筒形のマーカに基づいて回転角度で整列させるステップを含み、
(e)上記プロテーゼを上記体内管腔内でリリースするステップを含む、方法。
〔実施態様項29〕 上記プロテーゼホルダは、蛍光性でもなく放射線不透過性でもない材料で作られている、実施態様項28記載の方法。
〔実施態様項30〕 上記軸方向ガイドワイヤは、蛍光性であり又は放射線不透過性である材料で作られている、実施態様項28記載の方法。
〔実施態様項31〕 上記全体として円筒形のマーカの各々は、蛍光性であり又は放射線不透過性である材料で作られている、実施態様項28記載の方法。
〔実施態様項32〕 上記全体として円筒形のマーカの各々は、白金とイリジウムの組み合わせで作られている、実施態様項31記載の方法。
〔実施態様項33〕 上記デリバリシステムは、上記軸方向ガイドワイヤに平行な方向に整列した第3の全体として円筒形のマーカを更に含み、上記全体として円筒形のマーカの各々は、上記軸方向ガイドワイヤから等距離間隔を置いて配置されている、実施態様項28記載の方法。
〔実施態様項34〕 上記全体として円筒形のマーカの各々は、上記軸方向ガイドワイヤによって形成された軸線回りに測定して約90°間隔で配置されている、実施態様項33記載の方法。
〔実施態様項35〕 上記全体として円筒形のマーカの各々は、蛍光性であり又は放射線不透過性である材料で作られている、実施態様項34記載の方法。
〔実施態様項36〕 上記全体として円筒形のマーカの各々は、白金とイリジウムの組み合わせで作られている、実施態様項35記載の方法。
〔実施態様項37〕 上記デリバリシステムは、少なくとも1つの垂直のマーカを更に含み、上記垂直マーカは、上記軸方向ガイドワイヤに対して垂直の角度をなして設けられている、実施態様項28記載の方法。
〔実施態様項38〕 上記垂直マーカは、蛍光性であり又は放射線不透過性である材料で作られている、実施態様項37記載の方法。
〔実施態様項37〕 上記垂直マーカは、白金とイリジウムの組み合わせで作られている、実施態様項38記載の方法。
〔実施態様項38〕 上記デリバリシステムは、少なくとも1つの垂直のマーカを更に含み、上記垂直マーカは、上記軸方向ガイドワイヤに対して垂直の角度をなして設けられている、実施態様項33記載の方法。
〔実施態様項39〕 上記垂直マーカは、蛍光性であり又は放射線不透過性である材料で作られている、実施態様項38記載の方法。
〔実施態様項40〕 上記垂直マーカは、白金とイリジウムの組み合わせで作られている、実施態様項39記載の方法。
〔実施態様項41〕 上記プロテーゼは、ステント‐グラフトである、実施態様項28記載の方法。
〔実施態様項42〕 上記プロテーゼホルダは、上記ステント‐グラフトを上記プロテーゼホルダの上記外面に固定するための複数のアンカを有する、実施態様項41記載の方法。
〔実施態様項43〕 上記全体として円筒形のマーカは、上記本体内に圧力嵌めされている、実施態様項28記載の方法。
〔実施態様項44〕 上記全体として円筒形のマーカは、上記本体内に成形されている、実施態様項28記載の方法。
〔実施態様項45〕 上記全体として円筒形のマーカは、約0.030インチ(0.762mm)の直径を有する、実施態様項28記載の方法。
〔実施態様項46〕 上記軸方向ガイドワイヤは、約0.035インチ(0.889mm)の直径を有する、実施態様項28記載の方法。
〔実施態様項47〕 上記全体として円筒形のマーカは、上記本体内に圧力嵌めされている、実施態様項33記載の方法。
〔実施態様項48〕 上記全体として円筒形のマーカは、上記本体内に成形されている、実施態様項33記載の方法。
〔実施態様項49〕 上記全体として円筒形のマーカは、約0.030インチ(0.762mm)の直径を有する、実施態様項33記載の方法。
〔実施態様項50〕 上記軸方向ガイドワイヤは、約0.035インチ(0.889mm)の直径を有する、実施態様項33記載の方法。
〔実施態様項51〕 上記全体として円筒形のマーカの各々は、上記軸方向ガイドワイヤから約0.010インチ(0.254mm)〜約0.015インチ(0.381mm)の距離を置いて上記本体内に配置されていることを特徴とする実施態様項28記載の方法。
〔実施態様項52〕 上記全体として円筒形のマーカの各々は、上記軸方向ガイドワイヤから約0.010インチ(0.254mm)〜約0.015インチ(0.381mm)の距離を置いて上記本体内に配置されていることを特徴とする実施態様項33記載の方法。〔実施態様項53〕 上記器具は、X線撮影又は蛍光物質を観察するモニタを有することを特徴とする実施態様項28記載の方法。
〔実施態様項54〕 上記モニタは、上記軸方向ガイドワイヤにより形成される上記軸線に垂直の角度をなして患者の管腔の画像を読み取ることを特徴とする実施態様項53記載の方法。
〔実施態様項55〕 上記プロテーゼホルダを上記円筒形マーカに基づいて回転角度で位置合わせする上記ステップ(d)は、(i)上記モニタを観察するステップと、(ii)上記全体として円筒形のマーカと上記軸方向ガイドワイヤとの間の距離のサイズを測定するステップと、(iii)上記プロテーゼホルダを回転させてついには、上記全体として円筒形のマーカと上記軸方向ガイドワイヤとの間の距離がその最大の状態になるようにするステップとを含むことを特徴とする実施態様項54記載の方法。
Claims (16)
- 血管内デリバリシステムであって、
開口したルーメン並びに互いに反対側に位置した近位端及び遠位端を有する細長い外側シースを含み、前記近位端と前記遠位端との間には中間部分が設けられ、
前記外側シース内に設けられたホルダを含み、このホルダは、前記ホルダを貫通して延びる軸方向ガイドワイヤを有し、
前記ホルダは、前記軸方向ガイドワイヤを包囲した本体を有し、
前記ホルダは、プロテーゼが運搬に先立って固定される外面を有し、
前記本体は、少なくとも3つのほぼ円筒形のマーカを有し、この3つのマーカは、前記軸方向ガイドワイヤに平行な方向に整列し且つ各々が前記軸方向ガイドワイヤから等距離間隔を置いて配置されており、前記3つのマーカのうちの1つは、中間の円筒形のマーカであり、前記3つのマーカのうちの他の2つは、サイドの円筒形のマーカであり、
前記本体は、少なくとも2つの垂直マーカを有し、この垂直マーカは、前記軸方向ガイドワイヤに対して垂直の角度をなして設けられており、
前記ほぼ円筒形のマーカの各々は、前記軸方向ガイドワイヤによって形成された軸線回りに測定して約90°間隔で配置され、
前記ホルダが第1の位置に回転する際には、前記中間の円筒形のマーカ及び前記2つの垂直マーカのみが視認可能であり、前記ホルダが第2の位置に回転する際には、前記2つのサイドマーカのみが視認可能であることを特徴とするデリバリシステム。 - 前記本体は、蛍光性でもなく放射線不透過性でもない材料で作られている請求項1記載のデリバリシステム。
- 前記軸方向ガイドワイヤは、蛍光性であり又は放射線不透過性である材料で作られている請求項1記載のデリバリシステム。
- 前記全体として円筒形のマーカの各々は、蛍光性であり又は放射線不透過性である材料で作られている請求項1記載のデリバリシステム。
- 前記全体として円筒形のマーカの各々は、白金とイリジウムの組み合わせで作られている請求項4記載のデリバリシステム。
- 前記垂直マーカは、蛍光性であり又は放射線不透過性である材料で作られている請求項1記載のデリバリシステム。
- 前記垂直マーカは、白金とイリジウムの組み合わせで作られている請求項6記載のデリバリシステム。
- 前記プロテーゼは、ステント−グラフトである請求項1記載のデリバリシステム。
- 前記本体は、前記ステント−グラフトを前記本体の前記外面に固定するための複数のアンカを有する請求項8記載のデリバリシステム。
- 前記全体として円筒形のマーカは、前記本体内に圧力嵌めされている請求項1記載のデリバリシステム。
- 前記全体として円筒形のマーカは、前記本体内に成形されている請求項1記載のデリバリシステム。
- 前記全体として円筒形のマーカは、約0.030インチ(0.762mm)の直径を有する請求項1記載のデリバリシステム。
- 前記軸方向ガイドワイヤは、約0.035インチ(0.889mm)の直径を有する請求項1記載のデリバリシステム。
- 前記全体として円筒形のマーカは、約0.010インチ(0.254mm)〜約0.040インチ(1.016mm)の直径を有する請求項1記載のデリバリシステム。
- 前記軸方向ガイドワイヤは、約0.010インチ(0.254mm)〜約0.050インチ(1.270mm)の直径を有する請求項1記載のデリバリシステム。
- 前記全体として円筒形のマーカは、前記本体内に前記軸方向ガイドワイヤから約0.010インチ(0.254mm)〜約0.015インチ(0.381mm)の距離を置いたところに設けられている請求項1記載のデリバリシステム。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261660413P | 2012-06-15 | 2012-06-15 | |
| US61/660,413 | 2012-06-15 | ||
| PCT/US2013/043615 WO2013188132A1 (en) | 2012-06-15 | 2013-05-31 | Endovascular delivery system with an improved radiopaque marker scheme |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2015519178A JP2015519178A (ja) | 2015-07-09 |
| JP2015519178A5 JP2015519178A5 (ja) | 2016-07-07 |
| JP6085845B2 true JP6085845B2 (ja) | 2017-03-01 |
Family
ID=48741485
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015517290A Active JP6085845B2 (ja) | 2012-06-15 | 2013-05-31 | 改良型放射線不透過性マーカ方式の血管内デリバリシステム |
Country Status (4)
| Country | Link |
|---|---|
| US (4) | US9233015B2 (ja) |
| EP (1) | EP2861189B1 (ja) |
| JP (1) | JP6085845B2 (ja) |
| WO (1) | WO2013188132A1 (ja) |
Families Citing this family (60)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102007043830A1 (de) | 2007-09-13 | 2009-04-02 | Lozonschi, Lucian, Madison | Herzklappenstent |
| EP3649985B8 (en) | 2009-12-08 | 2021-04-21 | Avalon Medical Ltd. | Device and system for transcatheter mitral valve replacement |
| US9480559B2 (en) | 2011-08-11 | 2016-11-01 | Tendyne Holdings, Inc. | Prosthetic valves and related inventions |
| US9827092B2 (en) | 2011-12-16 | 2017-11-28 | Tendyne Holdings, Inc. | Tethers for prosthetic mitral valve |
| US9233015B2 (en) * | 2012-06-15 | 2016-01-12 | Trivascular, Inc. | Endovascular delivery system with an improved radiopaque marker scheme |
| WO2014022124A1 (en) | 2012-07-28 | 2014-02-06 | Tendyne Holdings, Inc. | Improved multi-component designs for heart valve retrieval device, sealing structures and stent assembly |
| US9675454B2 (en) | 2012-07-30 | 2017-06-13 | Tendyne Holdings, Inc. | Delivery systems and methods for transcatheter prosthetic valves |
| US11406498B2 (en) | 2012-12-20 | 2022-08-09 | Philips Image Guided Therapy Corporation | Implant delivery system and implants |
| EP2943153A1 (en) | 2013-01-10 | 2015-11-18 | TriVascular, Inc. | Gate wire for contralateral leg access |
| US9655754B2 (en) | 2013-01-10 | 2017-05-23 | Trivascular, Inc. | Systems and methods for guidewire crossover for bifurcated prostheses |
| US9486306B2 (en) | 2013-04-02 | 2016-11-08 | Tendyne Holdings, Inc. | Inflatable annular sealing device for prosthetic mitral valve |
| US10463489B2 (en) | 2013-04-02 | 2019-11-05 | Tendyne Holdings, Inc. | Prosthetic heart valve and systems and methods for delivering the same |
| US11224510B2 (en) | 2013-04-02 | 2022-01-18 | Tendyne Holdings, Inc. | Prosthetic heart valve and systems and methods for delivering the same |
| US10478293B2 (en) | 2013-04-04 | 2019-11-19 | Tendyne Holdings, Inc. | Retrieval and repositioning system for prosthetic heart valve |
| US9610159B2 (en) | 2013-05-30 | 2017-04-04 | Tendyne Holdings, Inc. | Structural members for prosthetic mitral valves |
| WO2014210124A1 (en) | 2013-06-25 | 2014-12-31 | Mark Christianson | Thrombus management and structural compliance features for prosthetic heart valves |
| JP6465883B2 (ja) | 2013-08-01 | 2019-02-06 | テンダイン ホールディングス,インコーポレイテッド | 心外膜アンカーデバイス及び方法 |
| WO2015058039A1 (en) | 2013-10-17 | 2015-04-23 | Robert Vidlund | Apparatus and methods for alignment and deployment of intracardiac devices |
| ES2773255T3 (es) | 2013-10-28 | 2020-07-10 | Tendyne Holdings Inc | Válvula cardiaca protésica y sistemas para suministrar la misma |
| US9526611B2 (en) | 2013-10-29 | 2016-12-27 | Tendyne Holdings, Inc. | Apparatus and methods for delivery of transcatheter prosthetic valves |
| EP3409243B1 (en) | 2014-01-09 | 2022-07-20 | TriVascular, Inc. | Endovasular delivery system |
| WO2015120122A2 (en) | 2014-02-05 | 2015-08-13 | Robert Vidlund | Apparatus and methods for transfemoral delivery of prosthetic mitral valve |
| US9986993B2 (en) | 2014-02-11 | 2018-06-05 | Tendyne Holdings, Inc. | Adjustable tether and epicardial pad system for prosthetic heart valve |
| CA2937566C (en) | 2014-03-10 | 2023-09-05 | Tendyne Holdings, Inc. | Devices and methods for positioning and monitoring tether load for prosthetic mitral valve |
| JP6959003B2 (ja) * | 2014-03-10 | 2021-11-02 | トリバスキュラー インコーポレイテッド | 大動脈用途向きのインフレート可能な閉塞ワイヤ・バルーン |
| EP3128909B1 (en) * | 2014-04-11 | 2021-11-10 | Koninklijke Philips N.V. | Implant delivery system |
| ES2731434T3 (es) | 2014-09-23 | 2019-11-15 | Bolton Medical Inc | Dispositivos de reparación vascular |
| JP2017530814A (ja) * | 2014-10-13 | 2017-10-19 | シメティス・ソシエテ・アノニムSymetis Sa | ステント弁のためのカテーテル送達システム |
| EP3226814A1 (en) | 2014-12-04 | 2017-10-11 | TriVascular, Inc. | Internal iliac preservation devices and methods |
| US10149777B2 (en) | 2014-12-18 | 2018-12-11 | Cook Medical Technologies Llc | Orientation marker on pusher for deployment of endoluminal prostheses |
| EP3242630A2 (en) | 2015-01-07 | 2017-11-15 | Tendyne Holdings, Inc. | Prosthetic mitral valves and apparatus and methods for delivery of same |
| EP3884906A1 (en) | 2015-02-05 | 2021-09-29 | Tendyne Holdings, Inc. | Expandable epicardial pads and devices and methods for delivery of same |
| AU2016248314B2 (en) | 2015-04-16 | 2020-05-21 | Tendyne Holdings, Inc. | Apparatus and methods for delivery, repositioning, and retrieval of transcatheter prosthetic valves |
| WO2016183128A1 (en) | 2015-05-11 | 2016-11-17 | Trivascular, Inc. | Stent-graft with improved flexibility |
| WO2017019913A1 (en) * | 2015-07-30 | 2017-02-02 | Trivascular, Inc. | Endoluminal prosthesis deployment devices and methods |
| US10327894B2 (en) | 2015-09-18 | 2019-06-25 | Tendyne Holdings, Inc. | Methods for delivery of prosthetic mitral valves |
| JP2018535754A (ja) | 2015-12-03 | 2018-12-06 | テンダイン ホールディングス,インコーポレイテッド | 人工僧帽弁用のフレーム特徴 |
| JP6795591B2 (ja) | 2015-12-28 | 2020-12-02 | テンダイン ホールディングス,インコーポレイテッド | 人工心臓弁用の心房ポケットクロージャ |
| US10052185B2 (en) | 2016-02-12 | 2018-08-21 | Covidien Lp | Vascular device marker attachment |
| US10265089B2 (en) | 2016-02-12 | 2019-04-23 | Covidien Lp | Vascular device visibility |
| WO2017176730A1 (en) * | 2016-04-05 | 2017-10-12 | Bolton Medical, Inc. | Stent graft with internal tunnels and fenestrations and methods of use |
| US10470877B2 (en) | 2016-05-03 | 2019-11-12 | Tendyne Holdings, Inc. | Apparatus and methods for anterior valve leaflet management |
| WO2017218375A1 (en) | 2016-06-13 | 2017-12-21 | Tendyne Holdings, Inc. | Sequential delivery of two-part prosthetic mitral valve |
| US11090157B2 (en) | 2016-06-30 | 2021-08-17 | Tendyne Holdings, Inc. | Prosthetic heart valves and apparatus and methods for delivery of same |
| EP3484411A1 (en) | 2016-07-12 | 2019-05-22 | Tendyne Holdings, Inc. | Apparatus and methods for trans-septal retrieval of prosthetic heart valves |
| US11638655B2 (en) | 2016-11-02 | 2023-05-02 | Daniel Ezra Walzman | Orientable intracranial occlusion device and method |
| US11045177B2 (en) | 2016-11-02 | 2021-06-29 | Daniel Ezra Walzman | Orientable intracranial occlusion device and method |
| US11154399B2 (en) | 2017-07-13 | 2021-10-26 | Tendyne Holdings, Inc. | Prosthetic heart valves and apparatus and methods for delivery of same |
| EP3675774B1 (en) | 2017-08-28 | 2023-06-21 | Tendyne Holdings, Inc. | Prosthetic heart valves with tether coupling features |
| US10441449B1 (en) | 2018-05-30 | 2019-10-15 | Vesper Medical, Inc. | Rotary handle stent delivery system and method |
| US10449073B1 (en) | 2018-09-18 | 2019-10-22 | Vesper Medical, Inc. | Rotary handle stent delivery system and method |
| WO2020251777A1 (en) | 2019-06-12 | 2020-12-17 | Walzman Daniel Ezra | Orientable intracranial occlusion device and method |
| EP3982876A4 (en) * | 2019-06-12 | 2023-10-25 | Daniel Ezra Walzmann | DEVICE AND METHOD FOR ORIENTABLE INTRACRANIAL OCCLUSION |
| CN113939327A (zh) * | 2019-06-24 | 2022-01-14 | 美敦力公司 | 具有扭矩机构和阀减压组件的导管手柄 |
| EP3831343B1 (en) | 2019-12-05 | 2024-01-31 | Tendyne Holdings, Inc. | Braided anchor for mitral valve |
| US11648114B2 (en) | 2019-12-20 | 2023-05-16 | Tendyne Holdings, Inc. | Distally loaded sheath and loading funnel |
| US11951002B2 (en) | 2020-03-30 | 2024-04-09 | Tendyne Holdings, Inc. | Apparatus and methods for valve and tether fixation |
| US11219541B2 (en) | 2020-05-21 | 2022-01-11 | Vesper Medical, Inc. | Wheel lock for thumbwheel actuated device |
| US11678980B2 (en) | 2020-08-19 | 2023-06-20 | Tendyne Holdings, Inc. | Fully-transseptal apical pad with pulley for tensioning |
| US20230190502A1 (en) * | 2021-12-20 | 2023-06-22 | Medtronic Vascular, Inc. | Delivery system for delivering a cardiovascular device |
Family Cites Families (345)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4041931A (en) | 1976-05-17 | 1977-08-16 | Elliott Donald P | Radiopaque anastomosis marker |
| US4202349A (en) | 1978-04-24 | 1980-05-13 | Jones James W | Radiopaque vessel markers |
| US4616652A (en) | 1983-10-19 | 1986-10-14 | Advanced Cardiovascular Systems, Inc. | Dilatation catheter positioning apparatus |
| US5275622A (en) * | 1983-12-09 | 1994-01-04 | Harrison Medical Technologies, Inc. | Endovascular grafting apparatus, system and method and devices for use therewith |
| US5669936A (en) | 1983-12-09 | 1997-09-23 | Endovascular Technologies, Inc. | Endovascular grafting system and method for use therewith |
| US4917088A (en) | 1985-05-02 | 1990-04-17 | C. R. Bard, Inc. | Balloon dilation probe |
| US5102390A (en) | 1985-05-02 | 1992-04-07 | C. R. Bard, Inc. | Microdilatation probe and system for performing angioplasty in highly stenosed blood vessels |
| US4733665C2 (en) * | 1985-11-07 | 2002-01-29 | Expandable Grafts Partnership | Expandable intraluminal graft and method and apparatus for implanting an expandable intraluminal graft |
| JPS62261371A (ja) * | 1986-05-08 | 1987-11-13 | テルモ株式会社 | カテ−テル |
| US4762128A (en) | 1986-12-09 | 1988-08-09 | Advanced Surgical Intervention, Inc. | Method and apparatus for treating hypertrophy of the prostate gland |
| US4793359A (en) | 1987-04-24 | 1988-12-27 | Gv Medical, Inc. | Centering balloon structure for transluminal angioplasty catheter |
| US4796637A (en) | 1987-06-17 | 1989-01-10 | Victory Engineering Company | Radiopaque marker for stereotaxic catheter |
| US4781681A (en) | 1987-09-15 | 1988-11-01 | Gv Medical, Inc. | Inflatable tip for laser catheterization |
| US4830003A (en) | 1988-06-17 | 1989-05-16 | Wolff Rodney G | Compressive stent and delivery system |
| AU4191989A (en) | 1988-08-24 | 1990-03-23 | Marvin J. Slepian | Biodegradable polymeric endoluminal sealing |
| CA1301007C (en) | 1989-01-30 | 1992-05-19 | Geoffrey S. Martin | Angioplasty catheter with spiral balloon |
| US5007434A (en) | 1989-02-07 | 1991-04-16 | Advanced Cardiovascular Systems, Inc. | Catheter tip attitude controlling guide wire |
| US4928693A (en) | 1989-03-13 | 1990-05-29 | Schneider (Usa), Inc. | Pressure monitor catheter |
| US4976690A (en) | 1989-08-10 | 1990-12-11 | Scimed Life Systems, Inc. | Variable stiffness angioplasty catheter |
| US5476100A (en) | 1994-07-07 | 1995-12-19 | Guided Medical Systems, Inc. | Catheter steerable by directional jets with remotely controlled closures |
| US5318529A (en) | 1989-09-06 | 1994-06-07 | Boston Scientific Corporation | Angioplasty balloon catheter and adaptor |
| US5158084A (en) | 1989-11-22 | 1992-10-27 | Board Of Regents, The University Of Texas System | Modified localization wire for excisional biopsy |
| US7033325B1 (en) | 1989-12-19 | 2006-04-25 | Scimed Life Systems, Inc. | Guidewire with multiple radiopaque marker sections |
| US5209730A (en) | 1989-12-19 | 1993-05-11 | Scimed Life Systems, Inc. | Method for placement of a balloon dilatation catheter across a stenosis and apparatus therefor |
| US5209749A (en) | 1990-05-11 | 1993-05-11 | Applied Urology Inc. | Fluoroscopically alignable cutter assembly and method of using the same |
| US5034005A (en) | 1990-07-09 | 1991-07-23 | Appling William M | Radiopaque marker |
| IT9084979A1 (it) | 1990-07-30 | 1992-01-31 | Imad Sheiban | Catetere per angioplastica coronarica transluminale percutanea con due palloncini alla sua estremita' distale uno di diametro piccolo (1, 5mm. seguito da un altro palloncino di diametro maggiore variabile da 2, 5 a 4 mm il palloncino piccolo ha la fu |
| US5395332A (en) | 1990-08-28 | 1995-03-07 | Scimed Life Systems, Inc. | Intravascualr catheter with distal tip guide wire lumen |
| US5246420A (en) | 1990-11-19 | 1993-09-21 | Danforth Biomedical Incorporated | Highly steerable dilatation balloon catheter system |
| US5174302A (en) | 1990-12-04 | 1992-12-29 | Cordis Corporation | Variable radiopacity guidewire with spaced highly radiopaque regions |
| CA2202800A1 (en) | 1991-04-11 | 1992-10-12 | Alec A. Piplani | Endovascular graft having bifurcation and apparatus and method for deploying the same |
| DE69224636T2 (de) | 1991-04-24 | 1998-11-05 | Advanced Cardiovascular System | Auswechselbarer ballonkatheter mit integriertem führungsdraht |
| US5263928A (en) | 1991-06-14 | 1993-11-23 | Baxter International Inc. | Catheter and endoscope assembly and method of use |
| CA2068584C (en) | 1991-06-18 | 1997-04-22 | Paul H. Burmeister | Intravascular guide wire and method for manufacture thereof |
| US5253653A (en) | 1991-10-31 | 1993-10-19 | Boston Scientific Corp. | Fluoroscopically viewable guidewire for catheters |
| US5318032A (en) | 1992-02-05 | 1994-06-07 | Devices For Vascular Intervention | Guiding catheter having soft tip |
| US5944712A (en) | 1992-03-02 | 1999-08-31 | Medtronic Ave, Inc. | Catheter size designation system |
| US5353808A (en) | 1992-03-04 | 1994-10-11 | Cordis Corporation | Guidewire having distally located marker segment |
| US5203777A (en) | 1992-03-19 | 1993-04-20 | Lee Peter Y | Radiopaque marker system for a tubular device |
| ZA931943B (en) | 1992-03-30 | 1993-11-16 | Pameda Nv | Inflatable shaft catheter |
| US5261878A (en) | 1992-05-19 | 1993-11-16 | The Regents Of The University Of California | Double balloon pediatric ductus arteriosus stent catheter and method of using the same |
| US5707376A (en) | 1992-08-06 | 1998-01-13 | William Cook Europe A/S | Stent introducer and method of use |
| US5562725A (en) | 1992-09-14 | 1996-10-08 | Meadox Medicals Inc. | Radially self-expanding implantable intraluminal device |
| US5413557A (en) | 1993-08-24 | 1995-05-09 | Pameda N.V. | Dilatation catheter with eccentric balloon |
| US5315747A (en) | 1992-10-30 | 1994-05-31 | Pameda N.V. | Method of preparing a balloon dilatation catheter |
| US5336178A (en) | 1992-11-02 | 1994-08-09 | Localmed, Inc. | Intravascular catheter with infusion array |
| US5480423A (en) * | 1993-05-20 | 1996-01-02 | Boston Scientific Corporation | Prosthesis delivery |
| US5318535A (en) | 1993-06-21 | 1994-06-07 | Baxter International Inc. | Low-profile dual-lumen perfusion balloon catheter with axially movable inner guide sheath |
| US5458615A (en) | 1993-07-06 | 1995-10-17 | Advanced Cardiovascular Systems, Inc. | Stent delivery system |
| JPH08507243A (ja) | 1993-07-23 | 1996-08-06 | クック インコーポレイティッド | シート材料から形成されたパターンを有するフレキシブルなステント |
| US6113576A (en) | 1993-08-04 | 2000-09-05 | Lake Region Manufacturing, Inc. | Thrombolysis catheter system with fixed length infusion zone |
| CA2125258C (en) | 1993-08-05 | 1998-12-22 | Dinah B Quiachon | Multicapsule intraluminal grafting system and method |
| US5669880A (en) | 1993-08-24 | 1997-09-23 | Cordis Corporation | Stent delivery system |
| US5429605A (en) | 1994-01-26 | 1995-07-04 | Target Therapeutics, Inc. | Microballoon catheter |
| US5479938A (en) | 1994-02-07 | 1996-01-02 | Cordis Corporation | Lumen diameter reference guidewire |
| US5609627A (en) | 1994-02-09 | 1997-03-11 | Boston Scientific Technology, Inc. | Method for delivering a bifurcated endoluminal prosthesis |
| US6051020A (en) | 1994-02-09 | 2000-04-18 | Boston Scientific Technology, Inc. | Bifurcated endoluminal prosthesis |
| US6165213A (en) | 1994-02-09 | 2000-12-26 | Boston Scientific Technology, Inc. | System and method for assembling an endoluminal prosthesis |
| US5429597A (en) | 1994-03-01 | 1995-07-04 | Boston Scientific Corporation | Kink resistant balloon catheter and method for use |
| US5948489A (en) | 1994-03-03 | 1999-09-07 | Cordis Corporation | Catheter having extruded, flexible, pliable and compliant marker band |
| US5406960A (en) | 1994-04-13 | 1995-04-18 | Cordis Corporation | Guidewire with integral core and marker bands |
| US5824044A (en) | 1994-05-12 | 1998-10-20 | Endovascular Technologies, Inc. | Bifurcated multicapsule intraluminal grafting system |
| US5824041A (en) * | 1994-06-08 | 1998-10-20 | Medtronic, Inc. | Apparatus and methods for placement and repositioning of intraluminal prostheses |
| US5683451A (en) | 1994-06-08 | 1997-11-04 | Cardiovascular Concepts, Inc. | Apparatus and methods for deployment release of intraluminal prostheses |
| US5743874A (en) | 1994-08-29 | 1998-04-28 | Fischell; Robert E. | Integrated catheter for balloon angioplasty and stent delivery |
| US6331188B1 (en) | 1994-08-31 | 2001-12-18 | Gore Enterprise Holdings, Inc. | Exterior supported self-expanding stent-graft |
| US5558652A (en) | 1994-10-06 | 1996-09-24 | B. Braun Medical, Inc. | Introducer with radiopaque marked tip and method of manufacture therefor |
| CA2163708C (en) | 1994-12-07 | 2007-08-07 | Robert E. Fischell | Integrated dual-function catheter system for balloon angioplasty and stent delivery |
| EP0723786A1 (en) | 1995-01-30 | 1996-07-31 | Cardiovascular Concepts, Inc. | Lesion measurement catheter and method |
| US5549552A (en) | 1995-03-02 | 1996-08-27 | Scimed Life Systems, Inc. | Balloon dilation catheter with improved pushability, trackability and crossability |
| US5836892A (en) | 1995-10-30 | 1998-11-17 | Cordis Corporation | Guidewire with radiopaque markers |
| US5782810A (en) | 1995-11-22 | 1998-07-21 | O'donnell; Miles C. | Multipart radiopaque and/or magnetically detectable tube catheter and method of fabrication thereof |
| US5690642A (en) | 1996-01-18 | 1997-11-25 | Cook Incorporated | Rapid exchange stent delivery balloon catheter |
| US5645532A (en) | 1996-03-04 | 1997-07-08 | Sil-Med Corporation | Radiopaque cuff peritoneal dialysis catheter |
| IL117472A0 (en) | 1996-03-13 | 1996-07-23 | Instent Israel Ltd | Radiopaque stent markers |
| US7686846B2 (en) | 1996-06-06 | 2010-03-30 | Devax, Inc. | Bifurcation stent and method of positioning in a body lumen |
| US7238197B2 (en) | 2000-05-30 | 2007-07-03 | Devax, Inc. | Endoprosthesis deployment system for treating vascular bifurcations |
| US6077273A (en) | 1996-08-23 | 2000-06-20 | Scimed Life Systems, Inc. | Catheter support for stent delivery |
| US5676146B1 (en) | 1996-09-13 | 2000-04-18 | Osteotech Inc | Surgical implant containing a resorbable radiopaque marker and method of locating such within a body |
| DE69739342D1 (ja) | 1996-09-23 | 2009-05-14 | Best Vascular Inc | |
| DE69736676T2 (de) | 1996-11-04 | 2007-01-11 | Advanced Stent Technologies, Inc., Pleasanton | Aufweitbarer doppelstent |
| US6692483B2 (en) | 1996-11-04 | 2004-02-17 | Advanced Stent Technologies, Inc. | Catheter with attached flexible side sheath |
| US6599316B2 (en) | 1996-11-04 | 2003-07-29 | Advanced Stent Technologies, Inc. | Extendible stent apparatus |
| US5843090A (en) | 1996-11-05 | 1998-12-01 | Schneider (Usa) Inc. | Stent delivery device |
| US5713913A (en) | 1996-11-12 | 1998-02-03 | Interventional Technologies Inc. | Device and method for transecting a coronary artery |
| US5968052A (en) | 1996-11-27 | 1999-10-19 | Scimed Life Systems Inc. | Pull back stent delivery system with pistol grip retraction handle |
| US5779731A (en) | 1996-12-20 | 1998-07-14 | Cordis Corporation | Balloon catheter having dual markers and method |
| US5759174A (en) | 1997-01-29 | 1998-06-02 | Cathco, Inc. | Angioplasty balloon with an expandable external radiopaque marker band |
| US5792144A (en) | 1997-03-31 | 1998-08-11 | Cathco, Inc. | Stent delivery catheter system |
| US20040215168A1 (en) | 1997-04-30 | 2004-10-28 | Beth Israel Deaconess Medical Center | Kit for transvenously accessing the pericardial space via the right atrium |
| US5741327A (en) | 1997-05-06 | 1998-04-21 | Global Therapeutics, Inc. | Surgical stent featuring radiopaque markers |
| US6004328A (en) | 1997-06-19 | 1999-12-21 | Solar; Ronald J. | Radially expandable intraluminal stent and delivery catheter therefore and method of using the same |
| US5921978A (en) * | 1997-06-20 | 1999-07-13 | Ep Technologies, Inc. | Catheter tip steering plane marker |
| US6174330B1 (en) | 1997-08-01 | 2001-01-16 | Schneider (Usa) Inc | Bioabsorbable marker having radiopaque constituents |
| US6340367B1 (en) | 1997-08-01 | 2002-01-22 | Boston Scientific Scimed, Inc. | Radiopaque markers and methods of using the same |
| US5980531A (en) | 1997-09-11 | 1999-11-09 | Schneider Inc | Stent deployment device with two balloons |
| JP4292710B2 (ja) | 1997-09-24 | 2009-07-08 | エム イー ディ インスチィチュート インク | 半径方向に拡張可能なステント |
| US6371928B1 (en) | 1997-11-07 | 2002-04-16 | Prolifix Medical, Inc. | Guidewire for positioning a catheter against a lumen wall |
| AUPP083597A0 (en) | 1997-12-10 | 1998-01-08 | William A Cook Australia Pty Ltd | Endoluminal aortic stents |
| US6022374A (en) | 1997-12-16 | 2000-02-08 | Cardiovasc, Inc. | Expandable stent having radiopaque marker and method |
| US6240231B1 (en) | 1997-12-22 | 2001-05-29 | Micrus Corporation | Variable stiffness fiber optic shaft |
| US6395019B2 (en) | 1998-02-09 | 2002-05-28 | Trivascular, Inc. | Endovascular graft |
| US6285903B1 (en) | 1998-06-30 | 2001-09-04 | Eclipse Surgical Technologies, Inc. | Intracorporeal device with radiopaque marker |
| US6053913A (en) | 1998-09-10 | 2000-04-25 | Tu; Lily Chen | Rapid exchange stented balloon catheter having ablation capabilities |
| US6368345B1 (en) | 1998-09-30 | 2002-04-09 | Edwards Lifesciences Corporation | Methods and apparatus for intraluminal placement of a bifurcated intraluminal garafat |
| CA2319443C (en) | 1998-12-01 | 2009-09-29 | Cook Biotech, Inc. | Collagenous biomaterials formed with submucosal tissue |
| EP1152711B1 (en) | 1999-01-27 | 2005-07-06 | Boston Scientific Limited | Bifurcation stent delivery system |
| US6361557B1 (en) | 1999-02-05 | 2002-03-26 | Medtronic Ave, Inc. | Staplebutton radiopaque marker |
| US6730116B1 (en) | 1999-04-16 | 2004-05-04 | Medtronic, Inc. | Medical device for intraluminal endovascular stenting |
| US6726712B1 (en) | 1999-05-14 | 2004-04-27 | Boston Scientific Scimed | Prosthesis deployment device with translucent distal end |
| US6375676B1 (en) | 1999-05-17 | 2002-04-23 | Advanced Cardiovascular Systems, Inc. | Self-expanding stent with enhanced delivery precision and stent delivery system |
| US6858034B1 (en) | 1999-05-20 | 2005-02-22 | Scimed Life Systems, Inc. | Stent delivery system for prevention of kinking, and method of loading and using same |
| US7387639B2 (en) | 1999-06-04 | 2008-06-17 | Advanced Stent Technologies, Inc. | Short sleeve stent delivery catheter and methods |
| US6210396B1 (en) | 1999-06-24 | 2001-04-03 | Medtronic, Inc. | Guiding catheter with tungsten loaded band |
| US6613075B1 (en) | 1999-10-27 | 2003-09-02 | Cordis Corporation | Rapid exchange self-expanding stent delivery catheter system |
| US6702802B1 (en) | 1999-11-10 | 2004-03-09 | Endovascular Technologies, Inc. | Catheters with improved transition |
| US7758624B2 (en) | 2000-11-13 | 2010-07-20 | C. R. Bard, Inc. | Implant delivery device |
| US6306162B1 (en) | 1999-12-15 | 2001-10-23 | Advanced Cardiovascular Systems, Inc. | Stent delivery system utilizing novel balloon for obtaining variable post-deployment stent characteristics |
| US6443979B1 (en) | 1999-12-20 | 2002-09-03 | Advanced Cardiovascular Systems, Inc. | Expandable stent delivery sheath and method of use |
| US6540721B1 (en) | 1999-12-29 | 2003-04-01 | Advanced Cardiovascular Systems, Inc. | Balloon catheter with flexible radiopaque polymeric marker |
| US6520934B1 (en) | 1999-12-29 | 2003-02-18 | Advanced Cardiovascular Systems, Inc. | Catheter assemblies with flexible radiopaque marker |
| US6457365B1 (en) | 2000-02-09 | 2002-10-01 | Endosonics Corporation | Method and apparatus for ultrasonic imaging |
| US7056294B2 (en) | 2000-04-13 | 2006-06-06 | Ev3 Sunnyvale, Inc | Method and apparatus for accessing the left atrial appendage |
| US6432130B1 (en) | 2000-04-20 | 2002-08-13 | Scimed Life Systems, Inc. | Fully sheathed balloon expandable stent delivery system |
| US20030114918A1 (en) | 2000-04-28 | 2003-06-19 | Garrison Michi E. | Stent graft assembly and method |
| US6520984B1 (en) | 2000-04-28 | 2003-02-18 | Cardiovasc, Inc. | Stent graft assembly and method |
| US6554848B2 (en) * | 2000-06-02 | 2003-04-29 | Advanced Cardiovascular Systems, Inc. | Marker device for rotationally orienting a stent delivery system prior to deploying a curved self-expanding stent |
| GB0020491D0 (en) | 2000-08-18 | 2000-10-11 | Angiomed Ag | Stent with attached element and method of making such a stent |
| US6945989B1 (en) | 2000-09-18 | 2005-09-20 | Endotex Interventional Systems, Inc. | Apparatus for delivering endoluminal prostheses and methods of making and using them |
| US6428512B1 (en) | 2000-10-10 | 2002-08-06 | Advanced Cardiovascular Systems, Inc. | Guidewire with improved lesion measurement |
| US6786918B1 (en) | 2000-10-17 | 2004-09-07 | Medtronic Vascular, Inc. | Stent delivery system |
| WO2003061502A1 (en) | 2000-10-26 | 2003-07-31 | Scimed Life Systems, Inc. | Stent having radiopaque markers and method of fabricating the same |
| US6761708B1 (en) | 2000-10-31 | 2004-07-13 | Advanced Cardiovascular Systems, Inc. | Radiopaque marker for a catheter and method of making |
| US6623504B2 (en) | 2000-12-08 | 2003-09-23 | Scimed Life Systems, Inc. | Balloon catheter with radiopaque distal tip |
| US7208002B2 (en) | 2001-01-04 | 2007-04-24 | Boston Scientific Scimed, Inc. | Expansion-assisting delivery system for self-expanding stent |
| US6602241B2 (en) | 2001-01-17 | 2003-08-05 | Transvascular, Inc. | Methods and apparatus for acute or chronic delivery of substances or apparatus to extravascular treatment sites |
| US20030097169A1 (en) | 2001-02-26 | 2003-05-22 | Brucker Gregory G. | Bifurcated stent and delivery system |
| US6623518B2 (en) | 2001-02-26 | 2003-09-23 | Ev3 Peripheral, Inc. | Implant delivery system with interlock |
| US6761733B2 (en) | 2001-04-11 | 2004-07-13 | Trivascular, Inc. | Delivery system and method for bifurcated endovascular graft |
| US6733521B2 (en) | 2001-04-11 | 2004-05-11 | Trivascular, Inc. | Delivery system and method for endovascular graft |
| US7422579B2 (en) | 2001-05-01 | 2008-09-09 | St. Jude Medical Cardiology Divison, Inc. | Emboli protection devices and related methods of use |
| US6673106B2 (en) | 2001-06-14 | 2004-01-06 | Cordis Neurovascular, Inc. | Intravascular stent device |
| US6818013B2 (en) | 2001-06-14 | 2004-11-16 | Cordis Corporation | Intravascular stent device |
| JP2004529735A (ja) | 2001-06-18 | 2004-09-30 | イーバ コーポレイション | 補綴植接集合体とその使用方法 |
| US20020198559A1 (en) | 2001-06-26 | 2002-12-26 | Bhavesh Mistry | Radiopaque balloon |
| WO2003003944A2 (en) | 2001-07-06 | 2003-01-16 | Angiomed Gmbh & Co. Medizintechnik Kg | Delivery system having a rapid pusher assembly for self-expanding stent, and stent exchange configuration |
| CA2459234C (en) | 2001-09-04 | 2013-03-26 | Micro Therapeutics, Inc. | Occlusion catheter having compliant balloon for use with complex vasculature |
| US7252679B2 (en) | 2001-09-13 | 2007-08-07 | Cordis Corporation | Stent with angulated struts |
| US6863683B2 (en) | 2001-09-19 | 2005-03-08 | Abbott Laboratoris Vascular Entities Limited | Cold-molding process for loading a stent onto a stent delivery system |
| EP1430925B1 (en) | 2001-09-28 | 2011-05-18 | Kaneka Corporation | Stent delivery catheter |
| GB0123633D0 (en) | 2001-10-02 | 2001-11-21 | Angiomed Ag | Stent delivery system |
| US20050228479A1 (en) | 2001-11-29 | 2005-10-13 | Cook Incorporated | Medical device delivery system |
| US6960188B2 (en) | 2001-11-30 | 2005-11-01 | Abbott Laboratories Vascular Entities Limited | Catheter having enhanced distal pushability |
| US7309350B2 (en) | 2001-12-03 | 2007-12-18 | Xtent, Inc. | Apparatus and methods for deployment of vascular prostheses |
| US20040186551A1 (en) | 2003-01-17 | 2004-09-23 | Xtent, Inc. | Multiple independent nested stent structures and methods for their preparation and deployment |
| US20030176914A1 (en) | 2003-01-21 | 2003-09-18 | Rabkin Dmitry J. | Multi-segment modular stent and methods for manufacturing stents |
| US7147660B2 (en) | 2001-12-20 | 2006-12-12 | Boston Scientific Santa Rosa Corp. | Advanced endovascular graft |
| US7125464B2 (en) | 2001-12-20 | 2006-10-24 | Boston Scientific Santa Rosa Corp. | Method for manufacturing an endovascular graft section |
| US7147661B2 (en) | 2001-12-20 | 2006-12-12 | Boston Scientific Santa Rosa Corp. | Radially expandable stent |
| US6776604B1 (en) | 2001-12-20 | 2004-08-17 | Trivascular, Inc. | Method and apparatus for shape forming endovascular graft material |
| US7090693B1 (en) | 2001-12-20 | 2006-08-15 | Boston Scientific Santa Rosa Corp. | Endovascular graft joint and method for manufacture |
| US20100016943A1 (en) | 2001-12-20 | 2010-01-21 | Trivascular2, Inc. | Method of delivering advanced endovascular graft |
| US20040073283A1 (en) | 2001-12-21 | 2004-04-15 | Ewers Richard C. | Stent delivery system and method |
| EP1467678A1 (en) | 2001-12-21 | 2004-10-20 | Cardiovasc, Inc. | Composite stent with polymeric covering and bioactive coating |
| US20030135256A1 (en) | 2002-01-14 | 2003-07-17 | Gallagher Brendan P. | Stent delivery system |
| US8221482B2 (en) | 2002-01-28 | 2012-07-17 | Orbusneich Medical, Inc. | Flared ostial endoprosthesis and delivery system |
| US7004964B2 (en) | 2002-02-22 | 2006-02-28 | Scimed Life Systems, Inc. | Apparatus and method for deployment of an endoluminal device |
| US6989024B2 (en) | 2002-02-28 | 2006-01-24 | Counter Clockwise, Inc. | Guidewire loaded stent for delivery through a catheter |
| US20030187495A1 (en) | 2002-04-01 | 2003-10-02 | Cully Edward H. | Endoluminal devices, embolic filters, methods of manufacture and use |
| US7691461B1 (en) | 2002-04-01 | 2010-04-06 | Advanced Cardiovascular Systems, Inc. | Hybrid stent and method of making |
| US7083822B2 (en) | 2002-04-26 | 2006-08-01 | Medtronic Vascular, Inc. | Overlapping coated stents |
| US7105031B2 (en) | 2002-04-26 | 2006-09-12 | Medtronic Vascular, Inc. | Balloon-tipped, multi-lumen catheter for endoluminal repair of endoluminal leaks in aortic or aorto-iliac endoluminal grafts |
| US6833003B2 (en) | 2002-06-24 | 2004-12-21 | Cordis Neurovascular | Expandable stent and delivery system |
| US20040006380A1 (en) | 2002-07-05 | 2004-01-08 | Buck Jerrick C. | Stent delivery system |
| US6999809B2 (en) | 2002-07-16 | 2006-02-14 | Edwards Lifesciences Corporation | Central venous catheter having a soft tip and fiber optics |
| US20040015229A1 (en) | 2002-07-22 | 2004-01-22 | Syntheon, Llc | Vascular stent with radiopaque markers |
| US8133236B2 (en) | 2006-11-07 | 2012-03-13 | Flowcardia, Inc. | Ultrasound catheter having protective feature against breakage |
| EP1393771B1 (en) | 2002-08-06 | 2007-10-24 | Abbott Laboratories Vascular Enterprises Limited | Balloon catheter with radioopaque marker |
| US6945995B2 (en) | 2002-08-29 | 2005-09-20 | Boston Scientific Scimed, Inc. | Stent overlap point markers |
| US7001422B2 (en) | 2002-09-23 | 2006-02-21 | Cordis Neurovascular, Inc | Expandable stent and delivery system |
| US6733489B2 (en) | 2002-09-26 | 2004-05-11 | Angiodynamics, Inc. | Vascular orientation marker for determining the orientation of a blood vessel |
| US20040068190A1 (en) | 2002-10-04 | 2004-04-08 | Cespedes Eduardo Ignacio | Imaging catheter with indicia and methods of use |
| US7331986B2 (en) | 2002-10-09 | 2008-02-19 | Boston Scientific Scimed, Inc. | Intraluminal medical device having improved visibility |
| US8936632B2 (en) | 2002-10-10 | 2015-01-20 | Sam Ciamacco, Jr. | Stent delivery and deployment system |
| US7485139B1 (en) | 2002-10-10 | 2009-02-03 | Ciamacco Jr Sam | Stent delivery and deployment system |
| US20040088039A1 (en) | 2002-11-01 | 2004-05-06 | Lee Nathan T. | Method of securing radiopaque markers to an implant |
| US6814746B2 (en) | 2002-11-01 | 2004-11-09 | Ev3 Peripheral, Inc. | Implant delivery system with marker interlock |
| US6970734B2 (en) * | 2002-12-02 | 2005-11-29 | Boston Scientific Scimed, Inc. | Flexible marker bands |
| US20040111143A1 (en) | 2002-12-06 | 2004-06-10 | Fischell Robert E. | Introducer sheath for the ostial placement of a stent |
| US6951554B2 (en) | 2002-12-16 | 2005-10-04 | Intraluminal Therapeutics Inc. | Deflecting catheter |
| WO2004062458A2 (en) | 2003-01-15 | 2004-07-29 | Angiomed Gmbh & C0. Medizintechnik Kg | Trans-luminal surgical device |
| US20040193141A1 (en) | 2003-02-14 | 2004-09-30 | Leopold Eric W. | Intravascular flow modifier and reinforcement device and deployment system for same |
| WO2004075789A2 (en) | 2003-02-26 | 2004-09-10 | Cook Incorporated | PROTHESIS ADAPTED FOR PLACEDd UNDER EXTERNAL IMAGING |
| US7150758B2 (en) | 2003-03-06 | 2006-12-19 | Boston Scientific Santa Rosa Corp. | Kink resistant endovascular graft |
| US7771463B2 (en) | 2003-03-26 | 2010-08-10 | Ton Dai T | Twist-down implant delivery technologies |
| WO2004087006A2 (en) | 2003-03-26 | 2004-10-14 | Cardiomind, Inc. | Implant delivery technologies |
| US20040254627A1 (en) | 2003-04-04 | 2004-12-16 | Thompson Paul J. | Stent with end adapted for flaring |
| DE10317241A1 (de) | 2003-04-10 | 2004-10-28 | Biotronik Meß- und Therapiegeräte GmbH & Co. Ingenieurbüro Berlin | Stent |
| US7473271B2 (en) * | 2003-04-11 | 2009-01-06 | Boston Scientific Scimed, Inc. | Stent delivery system with securement and deployment accuracy |
| US7717953B2 (en) | 2004-10-13 | 2010-05-18 | Tryton Medical, Inc. | Delivery system for placement of prosthesis at luminal OS |
| US20040215314A1 (en) | 2003-04-25 | 2004-10-28 | Kantor John D. | Stent deployment assembly with collars for drug-eluting stent |
| US7625398B2 (en) | 2003-05-06 | 2009-12-01 | Abbott Laboratories | Endoprosthesis having foot extensions |
| US7625401B2 (en) | 2003-05-06 | 2009-12-01 | Abbott Laboratories | Endoprosthesis having foot extensions |
| US7758520B2 (en) | 2003-05-27 | 2010-07-20 | Boston Scientific Scimed, Inc. | Medical device having segmented construction |
| DE10325678A1 (de) | 2003-06-02 | 2004-12-23 | Biotronik Meß- und Therapiegeräte GmbH & Co. Ingenieurbüro Berlin | Verbindungssystem zur Verbindung eines Stents mit einem radioopaken Marker sowie Verfahren zur Herstellung einer Verbindung zwischen einem Stent und zwei oder mehreren radioopaken Markern |
| US20040254637A1 (en) | 2003-06-16 | 2004-12-16 | Endotex Interventional Systems, Inc. | Sleeve stent marker |
| US7105015B2 (en) | 2003-06-17 | 2006-09-12 | Medtronic Vascular, Inc. | Method and system for treating an ostium of a side-branch vessel |
| US8021418B2 (en) | 2003-06-19 | 2011-09-20 | Boston Scientific Scimed, Inc. | Sandwiched radiopaque marker on covered stent |
| US20040267281A1 (en) | 2003-06-25 | 2004-12-30 | Eran Harari | Delivery system for self-expandable diverter |
| US7879024B2 (en) | 2003-06-26 | 2011-02-01 | St. Jude Medical, Atrial Fibrillation Division, Inc. | Splittable cannula having radiopaque marker |
| WO2005011790A1 (en) | 2003-07-31 | 2005-02-10 | Wilson-Cook Medical Inc. | System for introducing multiple medical devices |
| WO2005013855A2 (en) | 2003-08-01 | 2005-02-17 | Cook Urological, Incorporated | Implant delivery device |
| US20050049666A1 (en) | 2003-08-26 | 2005-03-03 | Chien Thomas Yung-Hui | Stent delivery system |
| US7763012B2 (en) | 2003-09-02 | 2010-07-27 | St. Jude Medical, Cardiology Division, Inc. | Devices and methods for crossing a chronic total occlusion |
| US8292943B2 (en) | 2003-09-03 | 2012-10-23 | Bolton Medical, Inc. | Stent graft with longitudinal support member |
| US7235083B1 (en) | 2003-09-10 | 2007-06-26 | Endovascular Technologies, Inc. | Methods and devices for aiding in situ assembly of repair devices |
| US20050060025A1 (en) | 2003-09-12 | 2005-03-17 | Mackiewicz David A. | Radiopaque markers for medical devices |
| US20050255317A1 (en) | 2003-09-22 | 2005-11-17 | Advanced Cardiovascular Systems, Inc. | Polymeric marker with high radiopacity for use in medical devices |
| US20050064223A1 (en) | 2003-09-22 | 2005-03-24 | Bavaro Vincent Peter | Polymeric marker with high radiopacity |
| GB0322511D0 (en) | 2003-09-25 | 2003-10-29 | Angiomed Ag | Lining for bodily lumen |
| US7208008B2 (en) | 2003-10-02 | 2007-04-24 | Medtronic Vascular, Inc. | Balloonless direct stenting device |
| US7175654B2 (en) | 2003-10-16 | 2007-02-13 | Cordis Corporation | Stent design having stent segments which uncouple upon deployment |
| US20080208307A1 (en) | 2003-11-03 | 2008-08-28 | B-Balloon Ltd. | Treatment of Vascular Bifurcations |
| US7655040B2 (en) | 2003-11-12 | 2010-02-02 | Medtronic Vascular, Inc. | Cardiac valve annulus reduction system |
| DE10361942A1 (de) | 2003-12-24 | 2005-07-21 | Restate Patent Ag | Radioopaker Marker für medizinische Implantate |
| US7641647B2 (en) | 2003-12-29 | 2010-01-05 | Boston Scientific Scimed, Inc. | Medical device with modified marker band |
| CA2552649A1 (en) | 2004-01-07 | 2005-07-28 | Boston Scientific Santa Rosa Corporation | Methods, compositions, and devices for embolizing body lumens |
| US7922753B2 (en) | 2004-01-13 | 2011-04-12 | Boston Scientific Scimed, Inc. | Bifurcated stent delivery system |
| US7972350B2 (en) | 2004-01-29 | 2011-07-05 | Boston Scientific Scimed, Inc. | Catheter tip |
| US7243408B2 (en) | 2004-02-09 | 2007-07-17 | Boston Scientific Scimed, Inc. | Process method for attaching radio opaque markers to shape memory stent |
| US7480973B2 (en) | 2004-03-01 | 2009-01-27 | Boston Scientific Scimed, Inc. | Automated marker band nest placement crimper |
| US7753951B2 (en) | 2004-03-04 | 2010-07-13 | Y Med, Inc. | Vessel treatment devices |
| US8431145B2 (en) | 2004-03-19 | 2013-04-30 | Abbott Laboratories | Multiple drug delivery from a balloon and a prosthesis |
| EP1735042B1 (en) | 2004-03-19 | 2011-11-23 | Abbott Laboratories | Multiple drug delivery from a balloon and a prosthesis |
| WO2005094725A1 (en) | 2004-03-31 | 2005-10-13 | Merlin Md Pte Ltd | A method for treating aneurysms |
| US20050246008A1 (en) | 2004-04-30 | 2005-11-03 | Novostent Corporation | Delivery system for vascular prostheses and methods of use |
| US20050278011A1 (en) | 2004-06-10 | 2005-12-15 | Peckham John E | Stent delivery system |
| US20050283226A1 (en) | 2004-06-18 | 2005-12-22 | Scimed Life Systems, Inc. | Medical devices |
| US7303580B2 (en) | 2004-07-26 | 2007-12-04 | Cook Incorporated | Stent delivery system allowing controlled release of a stent |
| US7819841B2 (en) | 2004-08-18 | 2010-10-26 | Medtronic Vascular, Inc. | Vessel isolation device |
| US20060064064A1 (en) | 2004-09-17 | 2006-03-23 | Jang G D | Two-step/dual-diameter balloon angioplasty catheter for bifurcation and side-branch vascular anatomy |
| US20060074477A1 (en) * | 2004-09-29 | 2006-04-06 | Medtronic Vascular, Inc. | Self-expanding stent delivery system |
| US20080195137A1 (en) | 2004-10-26 | 2008-08-14 | Alleyne Cargill H | Devices and Methods for Aneurysm Treatment |
| EP1833417B1 (en) | 2004-11-10 | 2012-02-01 | Boston Scientific Scimed, Inc. | Atraumatic stent with reduced deployment force and method for making the same |
| US9101500B2 (en) | 2005-01-10 | 2015-08-11 | Trireme Medical, Inc. | Stent with self-deployable portion having wings of different lengths |
| EP1835855B1 (en) | 2005-01-11 | 2017-04-05 | Volcano Corporation | Vascular image co-registration |
| US7578838B2 (en) | 2005-01-12 | 2009-08-25 | Cook Incorporated | Delivery system with helical shaft |
| EP1681077A1 (en) | 2005-01-12 | 2006-07-19 | Acrostak Corp. | A positioning device and a procedure for treating the walls of a resection cavity |
| US20080188803A1 (en) | 2005-02-03 | 2008-08-07 | Jang G David | Triple-profile balloon catheter |
| US20060184225A1 (en) * | 2005-02-11 | 2006-08-17 | Medtronic Vascular, Inc. | Force distributing system for delivering a self-expanding stent |
| US20060222596A1 (en) | 2005-04-01 | 2006-10-05 | Trivascular, Inc. | Non-degradable, low swelling, water soluble radiopaque hydrogel polymer |
| US20060233991A1 (en) | 2005-04-13 | 2006-10-19 | Trivascular, Inc. | PTFE layers and methods of manufacturing |
| US7922754B2 (en) | 2005-04-18 | 2011-04-12 | Trireme Medical, Inc. | Apparatus and methods for delivering prostheses to luminal bifurcations |
| CA2608160C (en) | 2005-05-09 | 2013-12-03 | Jurgen Dorn | Implant delivery device |
| WO2006128016A2 (en) | 2005-05-25 | 2006-11-30 | Prescient Medical, Inc. | Methods and systems for treating vulnerable plaques |
| US7320702B2 (en) | 2005-06-08 | 2008-01-22 | Xtent, Inc. | Apparatus and methods for deployment of multiple custom-length prostheses (III) |
| US8951225B2 (en) | 2005-06-10 | 2015-02-10 | Acclarent, Inc. | Catheters with non-removable guide members useable for treatment of sinusitis |
| EP1895938B1 (en) | 2005-06-30 | 2019-02-20 | Abbott Laboratories | Endoprosthesis having foot extensions |
| ATE487445T1 (de) | 2005-07-25 | 2010-11-15 | Invatec Spa | Endoluminale prothese mit bioresorbierbaren abschnitten |
| US20070083252A1 (en) | 2005-09-27 | 2007-04-12 | Mcdonald Michael B | Method for placing a stent through a constricted lumen, and medical device |
| WO2007053791A1 (en) | 2005-11-07 | 2007-05-10 | Med Institute, Inc. | Stent with orientation-dependent properties |
| JP5254805B2 (ja) | 2005-12-23 | 2013-08-07 | シー・アール・バード・インコーポレーテッド | ベント穴が中央に設けられたバルーンカテーテル |
| US20070156230A1 (en) | 2006-01-04 | 2007-07-05 | Dugan Stephen R | Stents with radiopaque markers |
| US20080004689A1 (en) | 2006-01-19 | 2008-01-03 | Linda Jahnke | Systems and Methods for Making Medical Devices |
| US20070208302A1 (en) | 2006-01-26 | 2007-09-06 | Webster Mark W | Deflection control catheters, support catheters and methods of use |
| NZ569760A (en) | 2006-02-17 | 2011-01-28 | Invatec Srl | Endoluminal prosthesis |
| GB0603685D0 (en) | 2006-02-23 | 2006-04-05 | Angiomed Ag | Vascular prosthesis for aneurysms, set of vascular prostheses, method for manufacturing a vascular prosthesis and method for inserting a vascular prosthesis |
| US20070225790A1 (en) | 2006-03-24 | 2007-09-27 | Fischell Robert E | Introducer sheath for the placement of a stent at the ostium of an artery |
| US20070225788A1 (en) | 2006-03-24 | 2007-09-27 | Fischell Robert E | Device and method for placing a stent at the ostium of a blood vessel |
| US8585680B2 (en) | 2006-04-17 | 2013-11-19 | Cook Medical Technologies Llc | Endovascular device tip assembly incorporating a marker device and method for making the same |
| US8690935B2 (en) | 2006-04-28 | 2014-04-08 | DePuy Synthes Products, LLC | Stent delivery system with threaded engagement and method |
| US20070266542A1 (en) | 2006-05-08 | 2007-11-22 | Cook Incorporated | Radiopaque marker for intraluminal medical device |
| US20070270691A1 (en) | 2006-05-19 | 2007-11-22 | Bailey Michael L | Radiopaque compositions, articles and methods of making and using same |
| US8585594B2 (en) | 2006-05-24 | 2013-11-19 | Phoenix Biomedical, Inc. | Methods of assessing inner surfaces of body lumens or organs |
| US7651520B2 (en) | 2006-05-30 | 2010-01-26 | Ostial Solutions, Llc | Means and method for the accurate placement of a stent at the ostium of an artery |
| US20070288082A1 (en) | 2006-06-07 | 2007-12-13 | Abbott Cardiovascular Systems Inc. | Stent and catheter assembly and method for treating bifurcations |
| JP2009542319A (ja) | 2006-06-30 | 2009-12-03 | ボストン サイエンティフィック リミテッド | 円周に沿って可変拡張コラムを備えるステント |
| US8029558B2 (en) | 2006-07-07 | 2011-10-04 | Abbott Cardiovascular Systems, Inc. | Stent and catheter assembly and method for treating bifurcations |
| DE102006033399B4 (de) * | 2006-07-19 | 2009-04-09 | Jotec Gmbh | Markersystem und Einführsystem für ein solches Markersystem |
| ATE419814T1 (de) | 2006-07-24 | 2009-01-15 | Cardiatis Sa | Vorrichtung zum reversiblen einbringen einer endoprothese |
| US8439961B2 (en) | 2006-07-31 | 2013-05-14 | Boston Scientific Scimed, Inc. | Stent retaining mechanisms |
| DE102006038233A1 (de) | 2006-08-07 | 2008-02-14 | Biotronik Vi Patent Ag | Markerkomposit für medizinische Implantate |
| US20080097404A1 (en) | 2006-08-16 | 2008-04-24 | Abbott Laboratories | Catheter having markers to indicate rotational orientation |
| US20080051867A1 (en) | 2006-08-28 | 2008-02-28 | Davila Luis A | Multiple in vivo implant delivery device |
| GB0617074D0 (en) | 2006-08-30 | 2006-10-11 | Angiomed Ag | Arteriovenous fistula |
| US20080077223A1 (en) | 2006-09-21 | 2008-03-27 | Fischell Robert E | Stent delivery system with improved deliverabilty features |
| WO2008070262A2 (en) | 2006-10-06 | 2008-06-12 | The Cleveland Clinic Foundation | Apparatus and method for targeting a body tissue |
| US20070073331A1 (en) | 2006-10-17 | 2007-03-29 | Conor Medsystems, Inc. | Rapid exchange stent delivery catheter |
| US7963960B2 (en) | 2006-11-07 | 2011-06-21 | Medtronic Vascular, Inc. | Cutting radio frequency catheter for creating fenestrations in graft cloth |
| US8470024B2 (en) | 2006-12-19 | 2013-06-25 | Sorin Group Italia S.R.L. | Device for in situ positioning of cardiac valve prosthesis |
| KR101250555B1 (ko) | 2007-02-09 | 2013-04-03 | 파이오락스 메디칼 디바이스, 인코포레이션 | 스텐트 |
| GB0703379D0 (en) | 2007-02-21 | 2007-03-28 | Angiomed Ag | Stent with radiopaque marker |
| EP2129325B1 (en) | 2007-03-12 | 2016-05-18 | Cook Medical Technologies LLC | Stent graft with side arm |
| US7967807B2 (en) | 2007-03-16 | 2011-06-28 | Medtronic Vascular, Inc. | Vascular fluoroscopic marker |
| US20080255654A1 (en) | 2007-03-22 | 2008-10-16 | Bay Street Medical | System for delivering a stent |
| US8486132B2 (en) | 2007-03-22 | 2013-07-16 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US20080243069A1 (en) | 2007-04-02 | 2008-10-02 | Medtronic Vascular, Inc. | Self-Crimping Radiopaque marker |
| US7815608B2 (en) | 2007-04-02 | 2010-10-19 | William Cook Australia Pty. Ltd. | High flex introducer assembly |
| GB0706499D0 (en) | 2007-04-03 | 2007-05-09 | Angiomed Ag | Bendable stent |
| US8052639B2 (en) | 2007-04-10 | 2011-11-08 | Wilson David B | Clampless anastomotic device |
| US20080255447A1 (en) | 2007-04-16 | 2008-10-16 | Henry Bourang | Diagnostic catheter |
| US8197442B2 (en) | 2007-04-27 | 2012-06-12 | Codman & Shurtleff, Inc. | Interventional medical device system having a slotted section and radiopaque marker and method of making the same |
| US8388673B2 (en) | 2008-05-02 | 2013-03-05 | Abbott Cardiovascular Systems Inc. | Polymeric stent |
| US8500786B2 (en) | 2007-05-15 | 2013-08-06 | Abbott Laboratories | Radiopaque markers comprising binary alloys of titanium |
| US20090005858A1 (en) | 2007-06-26 | 2009-01-01 | Eugene Young | Integration of markers into bar arms using a warm-forming process |
| US9144508B2 (en) | 2007-07-19 | 2015-09-29 | Back Bay Medical Inc. | Radially expandable stent |
| JP2010536430A (ja) | 2007-08-15 | 2010-12-02 | ウィルソン−クック・メディカル・インコーポレーテッド | ソフトステント用展開システム |
| GB0717481D0 (en) | 2007-09-07 | 2007-10-17 | Angiomed Ag | Self-expansible stent with radiopaque markers |
| US9393137B2 (en) | 2007-09-24 | 2016-07-19 | Boston Scientific Scimed, Inc. | Method for loading a stent into a delivery system |
| US7691125B2 (en) | 2007-10-04 | 2010-04-06 | Wilson-Cook Medical Inc. | System and method for forming a stent of a desired length at an endoluminal site |
| EP2194921B1 (en) | 2007-10-04 | 2018-08-29 | TriVascular, Inc. | Modular vascular graft for low profile percutaneous delivery |
| US8114144B2 (en) | 2007-10-17 | 2012-02-14 | Abbott Cardiovascular Systems Inc. | Rapid-exchange retractable sheath self-expanding delivery system with incompressible inner member and flexible distal assembly |
| EP3659664A1 (en) | 2007-10-22 | 2020-06-03 | Bridgepoint Medical, Inc. | Devices for crossing chronic total occlusions |
| CA2703665C (en) | 2007-10-25 | 2016-05-10 | Symetis Sa | Stents, valved-stents and methods and systems for delivery thereof |
| US20090131785A1 (en) | 2007-11-15 | 2009-05-21 | University Of Florida Research Foundation, Inc. | Variable occlusional balloon catheter assembly |
| US20090138065A1 (en) | 2007-11-28 | 2009-05-28 | Wilson-Cook Medical Inc. | Double loaded stent delivery system |
| WO2009100129A2 (en) | 2008-02-05 | 2009-08-13 | Chad John Kugler | Crossing occlusions in blood vessels |
| US20090204203A1 (en) | 2008-02-07 | 2009-08-13 | Medtronic Vascular, Inc. | Bioabsorbable Stent Having a Radiopaque Marker |
| EP2242527A4 (en) | 2008-02-19 | 2011-07-13 | Portaero Inc | DEVICES AND METHODS FOR ADMINISTERING A THERAPEUTIC AGENT THROUGH PNEUMOSTOMY |
| EP2257239A2 (en) | 2008-02-28 | 2010-12-08 | Fistulink Ltd. | Apparatus and method for creating arteriovenous fistulas |
| US8100960B2 (en) | 2008-03-20 | 2012-01-24 | Medtronic Vascular, Inc. | Bloused stent-graft and fenestration method |
| CA2720452A1 (en) | 2008-04-02 | 2009-10-08 | Laurimed, Llc | Methods and devices for delivering injections |
| US20090264987A1 (en) | 2008-04-18 | 2009-10-22 | Medtronic Vascular, Inc. | Stent Graft Delivery System and Method of Use |
| US20090264990A1 (en) | 2008-04-21 | 2009-10-22 | Medtronic Vascular, Inc. | Radiopaque Imprinted Ink Marker for Stent Graft |
| AU2009274126A1 (en) | 2008-07-22 | 2010-01-28 | Covidien Lp | Vascular remodeling device |
| US8694078B2 (en) | 2008-09-04 | 2014-04-08 | Freedom Medi-Tech Ventures Llc | Method and device for inserting electrical leads |
| ES2428901T3 (es) | 2008-09-12 | 2013-11-12 | Alexander Grigorievich Viller | Sistema de suministro de doble balón para un estent implantable biselado |
| US8690936B2 (en) | 2008-10-10 | 2014-04-08 | Edwards Lifesciences Corporation | Expandable sheath for introducing an endovascular delivery device into a body |
| EP2349123B1 (en) * | 2008-10-10 | 2015-11-04 | Veryan Medical Limited | A medical device |
| US8257420B2 (en) | 2008-11-14 | 2012-09-04 | Abbott Cardiovascular Systems Inc. | Stent delivery catheter system with high tensile strength |
| US20100137966A1 (en) | 2008-12-01 | 2010-06-03 | Cook Incorporated | System and method for sequentially deploying two or more implantable medical devices |
| CN101779992B (zh) | 2009-01-19 | 2012-08-22 | 加奇生物科技(上海)有限公司 | 可回撤自弹式脑神经支架的输送装置 |
| JP5687216B2 (ja) | 2009-03-13 | 2015-03-18 | ボルトン メディカル インコーポレイテッド | 手術部位で管腔内プロテーゼを展開するためのシステム及び方法 |
| US8882678B2 (en) | 2009-03-13 | 2014-11-11 | Atrium Medical Corporation | Pleural drainage system and method of use |
| US8460168B2 (en) | 2009-03-27 | 2013-06-11 | Circulite, Inc. | Transseptal cannula device, coaxial balloon delivery device, and methods of using the same |
| JP5284165B2 (ja) | 2009-03-31 | 2013-09-11 | テルモ株式会社 | 生体器官病変部改善用器具 |
| US20100274189A1 (en) | 2009-04-22 | 2010-10-28 | Pressure Products Medical Supplies Inc. | Balloon catheter and method of manufacture of the same |
| US20100274276A1 (en) * | 2009-04-22 | 2010-10-28 | Ricky Chow | Aneurysm treatment system, device and method |
| US20110054586A1 (en) | 2009-04-28 | 2011-03-03 | Endologix, Inc. | Apparatus and method of placement of a graft or graft system |
| US8795345B2 (en) | 2009-07-08 | 2014-08-05 | Concentric Medical, Inc. | Vascular and bodily duct treatment devices and methods |
| EP2477617B1 (en) | 2009-09-18 | 2018-01-31 | Bioinspire Technologies Inc. | Free-standing biodegradable patch |
| AU2010308367A1 (en) | 2009-10-19 | 2012-05-17 | Cook Medical Technologies Llc | Balloon-tipped endoscopic system with inverted sleeve |
| US9173705B2 (en) | 2010-05-13 | 2015-11-03 | Ncontact Surgical, Inc. | Subxyphoid epicardial ablation |
| US9125765B2 (en) * | 2010-12-13 | 2015-09-08 | Cook Medical Technologies Llc | Implant deployment restraint device |
| US9233015B2 (en) * | 2012-06-15 | 2016-01-12 | Trivascular, Inc. | Endovascular delivery system with an improved radiopaque marker scheme |
-
2013
- 2013-03-14 US US13/803,050 patent/US9233015B2/en active Active
- 2013-05-31 JP JP2015517290A patent/JP6085845B2/ja active Active
- 2013-05-31 WO PCT/US2013/043615 patent/WO2013188132A1/en active Application Filing
- 2013-05-31 EP EP13733113.8A patent/EP2861189B1/en active Active
-
2015
- 2015-12-16 US US14/970,621 patent/US10034787B2/en active Active
-
2018
- 2018-07-30 US US16/049,560 patent/US11013626B2/en active Active
-
2021
- 2021-05-24 US US17/328,985 patent/US20210275331A1/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| US11013626B2 (en) | 2021-05-25 |
| US20180344491A1 (en) | 2018-12-06 |
| EP2861189A1 (en) | 2015-04-22 |
| US20130338752A1 (en) | 2013-12-19 |
| US20210275331A1 (en) | 2021-09-09 |
| US9233015B2 (en) | 2016-01-12 |
| JP2015519178A (ja) | 2015-07-09 |
| WO2013188132A1 (en) | 2013-12-19 |
| US20160235566A1 (en) | 2016-08-18 |
| EP2861189B1 (en) | 2022-12-28 |
| US10034787B2 (en) | 2018-07-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6085845B2 (ja) | 改良型放射線不透過性マーカ方式の血管内デリバリシステム | |
| US11779479B2 (en) | Bifurcated endovascular prosthesis having tethered contralateral leg | |
| US20210236313A1 (en) | Systems and methods for guidewire crossover for bifurcated prostheses | |
| EP3116439B1 (en) | Inflatable occlusion wire-balloon for aortic applications | |
| US20140194970A1 (en) | Gate wire for contralateral leg access | |
| US9066828B2 (en) | Endovascular delivery system with flexible and torqueable hypotube | |
| JP2010540190A (ja) | 低プロファイル経皮的送達のためのモジュラー式血管グラフト | |
| EP3409243B1 (en) | Endovasular delivery system |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160517 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20160517 |
|
| A871 | Explanation of circumstances concerning accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A871 Effective date: 20160517 |
|
| A975 | Report on accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A971005 Effective date: 20160628 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20160704 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20161003 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20170104 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20170112 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6085845 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |