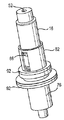
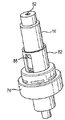
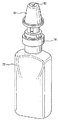
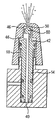
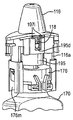
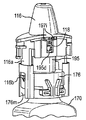
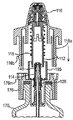
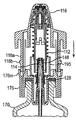
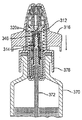
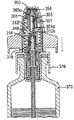
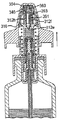
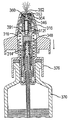
JP5336357B2 - 流体ディスペンサ - Google Patents
流体ディスペンサ Download PDFInfo
- Publication number
- JP5336357B2 JP5336357B2 JP2009512590A JP2009512590A JP5336357B2 JP 5336357 B2 JP5336357 B2 JP 5336357B2 JP 2009512590 A JP2009512590 A JP 2009512590A JP 2009512590 A JP2009512590 A JP 2009512590A JP 5336357 B2 JP5336357 B2 JP 5336357B2
- Authority
- JP
- Japan
- Prior art keywords
- fluid
- fluid dispenser
- chamber
- piston member
- sealing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000012530 fluid Substances 0.000 title claims abstract description 570
- 238000007789 sealing Methods 0.000 claims abstract description 321
- 210000002445 nipple Anatomy 0.000 claims description 40
- 230000007246 mechanism Effects 0.000 claims description 24
- 238000003825 pressing Methods 0.000 claims description 2
- 239000007788 liquid Substances 0.000 description 55
- 238000009826 distribution Methods 0.000 description 38
- 238000000034 method Methods 0.000 description 33
- 238000003780 insertion Methods 0.000 description 25
- 230000037431 insertion Effects 0.000 description 25
- 238000002360 preparation method Methods 0.000 description 19
- 238000004891 communication Methods 0.000 description 18
- 239000000463 material Substances 0.000 description 18
- -1 polypropylene Polymers 0.000 description 18
- 239000004743 Polypropylene Substances 0.000 description 14
- 229920001155 polypropylene Polymers 0.000 description 14
- 229920003023 plastic Polymers 0.000 description 13
- 239000004033 plastic Substances 0.000 description 13
- 239000002585 base Substances 0.000 description 12
- 239000011324 bead Substances 0.000 description 12
- 238000002347 injection Methods 0.000 description 11
- 239000007924 injection Substances 0.000 description 11
- 239000002253 acid Substances 0.000 description 9
- 239000003814 drug Substances 0.000 description 9
- 230000002093 peripheral effect Effects 0.000 description 9
- 150000001875 compounds Chemical class 0.000 description 7
- 229940079593 drug Drugs 0.000 description 7
- 229920001684 low density polyethylene Polymers 0.000 description 7
- 239000004702 low-density polyethylene Substances 0.000 description 7
- 238000007906 compression Methods 0.000 description 6
- 230000006835 compression Effects 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 125000006850 spacer group Chemical group 0.000 description 5
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- 238000010304 firing Methods 0.000 description 4
- 239000003862 glucocorticoid Substances 0.000 description 4
- 208000027866 inflammatory disease Diseases 0.000 description 4
- 239000011148 porous material Substances 0.000 description 4
- 239000007921 spray Substances 0.000 description 4
- 210000002105 tongue Anatomy 0.000 description 4
- 230000003110 anti-inflammatory effect Effects 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 238000005086 pumping Methods 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- KWGRBVOPPLSCSI-WPRPVWTQSA-N (-)-ephedrine Chemical compound CN[C@@H](C)[C@H](O)C1=CC=CC=C1 KWGRBVOPPLSCSI-WPRPVWTQSA-N 0.000 description 2
- LRFVTYWOQMYALW-UHFFFAOYSA-N 9H-xanthine Chemical compound O=C1NC(=O)NC2=C1NC=N2 LRFVTYWOQMYALW-UHFFFAOYSA-N 0.000 description 2
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 2
- 229940123932 Phosphodiesterase 4 inhibitor Drugs 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 229960005305 adenosine Drugs 0.000 description 2
- UCTWMZQNUQWSLP-UHFFFAOYSA-N adrenaline Chemical compound CNCC(O)C1=CC=C(O)C(O)=C1 UCTWMZQNUQWSLP-UHFFFAOYSA-N 0.000 description 2
- 239000000556 agonist Substances 0.000 description 2
- 239000003570 air Substances 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- OROGSEYTTFOCAN-DNJOTXNNSA-N codeine Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)=C[C@H](O)[C@@H]1OC1=C2C3=CC=C1OC OROGSEYTTFOCAN-DNJOTXNNSA-N 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 239000000356 contaminant Substances 0.000 description 2
- 239000013583 drug formulation Substances 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 229920001971 elastomer Polymers 0.000 description 2
- 229920001903 high density polyethylene Polymers 0.000 description 2
- 239000004700 high-density polyethylene Substances 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 239000003199 leukotriene receptor blocking agent Substances 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- BQJCRHHNABKAKU-KBQPJGBKSA-N morphine Chemical compound O([C@H]1[C@H](C=C[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O BQJCRHHNABKAKU-KBQPJGBKSA-N 0.000 description 2
- 210000003928 nasal cavity Anatomy 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- 239000002587 phosphodiesterase IV inhibitor Substances 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 230000002335 preservative effect Effects 0.000 description 2
- 230000000284 resting effect Effects 0.000 description 2
- 206010039083 rhinitis Diseases 0.000 description 2
- 230000000630 rising effect Effects 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 159000000000 sodium salts Chemical class 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- ZFXYFBGIUFBOJW-UHFFFAOYSA-N theophylline Chemical compound O=C1N(C)C(=O)N(C)C2=C1NC=N2 ZFXYFBGIUFBOJW-UHFFFAOYSA-N 0.000 description 2
- 229920002725 thermoplastic elastomer Polymers 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- JWZZKOKVBUJMES-UHFFFAOYSA-N (+-)-Isoprenaline Chemical compound CC(C)NCC(O)C1=CC=C(O)C(O)=C1 JWZZKOKVBUJMES-UHFFFAOYSA-N 0.000 description 1
- XWTYSIMOBUGWOL-UHFFFAOYSA-N (+-)-Terbutaline Chemical compound CC(C)(C)NCC(O)C1=CC(O)=CC(O)=C1 XWTYSIMOBUGWOL-UHFFFAOYSA-N 0.000 description 1
- AKNNEGZIBPJZJG-MSOLQXFVSA-N (-)-noscapine Chemical compound CN1CCC2=CC=3OCOC=3C(OC)=C2[C@@H]1[C@@H]1C2=CC=C(OC)C(OC)=C2C(=O)O1 AKNNEGZIBPJZJG-MSOLQXFVSA-N 0.000 description 1
- RZMCXMNNXGCFQG-DQEYMECFSA-N (2s)-3-[4-(4-carbamoylpiperidine-1-carbonyl)oxyphenyl]-2-[[(2s)-4-methyl-2-[[2-(2-methylphenoxy)acetyl]amino]pentanoyl]amino]propanoic acid Chemical compound N([C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC(OC(=O)N2CCC(CC2)C(N)=O)=CC=1)C(O)=O)C(=O)COC1=CC=CC=C1C RZMCXMNNXGCFQG-DQEYMECFSA-N 0.000 description 1
- FUFLCEKSBBHCMO-UHFFFAOYSA-N 11-dehydrocorticosterone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 FUFLCEKSBBHCMO-UHFFFAOYSA-N 0.000 description 1
- YREYLAVBNPACJM-UHFFFAOYSA-N 2-(tert-butylamino)-1-(2-chlorophenyl)ethanol Chemical compound CC(C)(C)NCC(O)C1=CC=CC=C1Cl YREYLAVBNPACJM-UHFFFAOYSA-N 0.000 description 1
- SMNDYUVBFMFKNZ-UHFFFAOYSA-N 2-furoic acid Chemical compound OC(=O)C1=CC=CO1 SMNDYUVBFMFKNZ-UHFFFAOYSA-N 0.000 description 1
- 125000005975 2-phenylethyloxy group Chemical group 0.000 description 1
- PVXFCOPBOYHONF-UHFFFAOYSA-N 3-ethyl-1,3-benzothiazol-2-one Chemical compound C1=CC=C2SC(=O)N(CC)C2=C1 PVXFCOPBOYHONF-UHFFFAOYSA-N 0.000 description 1
- YEJRWHAVMIAJKC-UHFFFAOYSA-N 4-Butyrolactone Chemical compound O=C1CCCO1 YEJRWHAVMIAJKC-UHFFFAOYSA-N 0.000 description 1
- LSLYOANBFKQKPT-DIFFPNOSSA-N 5-[(1r)-1-hydroxy-2-[[(2r)-1-(4-hydroxyphenyl)propan-2-yl]amino]ethyl]benzene-1,3-diol Chemical compound C([C@@H](C)NC[C@H](O)C=1C=C(O)C=C(O)C=1)C1=CC=C(O)C=C1 LSLYOANBFKQKPT-DIFFPNOSSA-N 0.000 description 1
- JFHROPTYMMSOLG-UHFFFAOYSA-N 6-[3-(dimethylcarbamoyl)phenyl]sulfonyl-4-(3-methoxyanilino)-8-methylquinoline-3-carboxamide Chemical compound COC1=CC=CC(NC=2C3=CC(=CC(C)=C3N=CC=2C(N)=O)S(=O)(=O)C=2C=C(C=CC=2)C(=O)N(C)C)=C1 JFHROPTYMMSOLG-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 206010002383 Angina Pectoris Diseases 0.000 description 1
- 229930003347 Atropine Natural products 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 1
- 108010059108 CD18 Antigens Proteins 0.000 description 1
- 229930186147 Cephalosporin Natural products 0.000 description 1
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 1
- LUKZNWIVRBCLON-GXOBDPJESA-N Ciclesonide Chemical compound C1([C@H]2O[C@@]3([C@H](O2)C[C@@H]2[C@@]3(C[C@H](O)[C@@H]3[C@@]4(C)C=CC(=O)C=C4CC[C@H]32)C)C(=O)COC(=O)C(C)C)CCCCC1 LUKZNWIVRBCLON-GXOBDPJESA-N 0.000 description 1
- MFYSYFVPBJMHGN-ZPOLXVRWSA-N Cortisone Chemical compound O=C1CC[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 MFYSYFVPBJMHGN-ZPOLXVRWSA-N 0.000 description 1
- MFYSYFVPBJMHGN-UHFFFAOYSA-N Cortisone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)(O)C(=O)CO)C4C3CCC2=C1 MFYSYFVPBJMHGN-UHFFFAOYSA-N 0.000 description 1
- 201000004624 Dermatitis Diseases 0.000 description 1
- IJVCSMSMFSCRME-KBQPJGBKSA-N Dihydromorphine Chemical compound O([C@H]1[C@H](CC[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O IJVCSMSMFSCRME-KBQPJGBKSA-N 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 1
- 102400000321 Glucagon Human genes 0.000 description 1
- 108060003199 Glucagon Proteins 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- RKUNBYITZUJHSG-UHFFFAOYSA-N Hyosciamin-hydrochlorid Natural products CN1C(C2)CCC1CC2OC(=O)C(CO)C1=CC=CC=C1 RKUNBYITZUJHSG-UHFFFAOYSA-N 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 108010041012 Integrin alpha4 Proteins 0.000 description 1
- HUYWAWARQUIQLE-UHFFFAOYSA-N Isoetharine Chemical compound CC(C)NC(CC)C(O)C1=CC=C(O)C(O)=C1 HUYWAWARQUIQLE-UHFFFAOYSA-N 0.000 description 1
- ZCVMWBYGMWKGHF-UHFFFAOYSA-N Ketotifene Chemical compound C1CN(C)CCC1=C1C2=CC=CC=C2CC(=O)C2=C1C=CS2 ZCVMWBYGMWKGHF-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- UCHDWCPVSPXUMX-TZIWLTJVSA-N Montelukast Chemical compound CC(C)(O)C1=CC=CC=C1CC[C@H](C=1C=C(\C=C\C=2N=C3C=C(Cl)C=CC3=CC=2)C=CC=1)SCC1(CC(O)=O)CC1 UCHDWCPVSPXUMX-TZIWLTJVSA-N 0.000 description 1
- 229940121948 Muscarinic receptor antagonist Drugs 0.000 description 1
- 102100029438 Nitric oxide synthase, inducible Human genes 0.000 description 1
- 101710089543 Nitric oxide synthase, inducible Proteins 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 206010039094 Rhinitis perennial Diseases 0.000 description 1
- 208000036284 Rhinitis seasonal Diseases 0.000 description 1
- GIIZNNXWQWCKIB-UHFFFAOYSA-N Serevent Chemical compound C1=C(O)C(CO)=CC(C(O)CNCCCCCCOCCCCC=2C=CC=CC=2)=C1 GIIZNNXWQWCKIB-UHFFFAOYSA-N 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- 102000001400 Tryptase Human genes 0.000 description 1
- 108060005989 Tryptase Proteins 0.000 description 1
- YEEZWCHGZNKEEK-UHFFFAOYSA-N Zafirlukast Chemical compound COC1=CC(C(=O)NS(=O)(=O)C=2C(=CC=CC=2)C)=CC=C1CC(C1=C2)=CN(C)C1=CC=C2NC(=O)OC1CCCC1 YEEZWCHGZNKEEK-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- NDAUXUAQIAJITI-UHFFFAOYSA-N albuterol Chemical compound CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 NDAUXUAQIAJITI-UHFFFAOYSA-N 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 125000005907 alkyl ester group Chemical group 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- AKNNEGZIBPJZJG-UHFFFAOYSA-N alpha-noscapine Natural products CN1CCC2=CC=3OCOC=3C(OC)=C2C1C1C2=CC=C(OC)C(OC)=C2C(=O)O1 AKNNEGZIBPJZJG-UHFFFAOYSA-N 0.000 description 1
- 239000012080 ambient air Substances 0.000 description 1
- XSDQTOBWRPYKKA-UHFFFAOYSA-N amiloride Chemical compound NC(=N)NC(=O)C1=NC(Cl)=C(N)N=C1N XSDQTOBWRPYKKA-UHFFFAOYSA-N 0.000 description 1
- 229960002576 amiloride Drugs 0.000 description 1
- 229960003556 aminophylline Drugs 0.000 description 1
- FQPFAHBPWDRTLU-UHFFFAOYSA-N aminophylline Chemical compound NCCN.O=C1N(C)C(=O)N(C)C2=C1NC=N2.O=C1N(C)C(=O)N(C)C2=C1NC=N2 FQPFAHBPWDRTLU-UHFFFAOYSA-N 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 230000000954 anitussive effect Effects 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 230000002924 anti-infective effect Effects 0.000 description 1
- 229940124599 anti-inflammatory drug Drugs 0.000 description 1
- 239000000043 antiallergic agent Substances 0.000 description 1
- 239000000739 antihistaminic agent Substances 0.000 description 1
- 229940125715 antihistaminic agent Drugs 0.000 description 1
- 229960005475 antiinfective agent Drugs 0.000 description 1
- 239000003434 antitussive agent Substances 0.000 description 1
- 229940124584 antitussives Drugs 0.000 description 1
- 229960000396 atropine Drugs 0.000 description 1
- RKUNBYITZUJHSG-SPUOUPEWSA-N atropine Chemical compound O([C@H]1C[C@H]2CC[C@@H](C1)N2C)C(=O)C(CO)C1=CC=CC=C1 RKUNBYITZUJHSG-SPUOUPEWSA-N 0.000 description 1
- NBMKJKDGKREAPL-DVTGEIKXSA-N beclomethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O NBMKJKDGKREAPL-DVTGEIKXSA-N 0.000 description 1
- 229940092705 beclomethasone Drugs 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 229940124630 bronchodilator Drugs 0.000 description 1
- 239000000168 bronchodilator agent Substances 0.000 description 1
- 229960004436 budesonide Drugs 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 229940124587 cephalosporin Drugs 0.000 description 1
- 150000001780 cephalosporins Chemical class 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 239000000812 cholinergic antagonist Substances 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 229960003728 ciclesonide Drugs 0.000 description 1
- 229960004126 codeine Drugs 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 239000007799 cork Substances 0.000 description 1
- 229960004544 cortisone Drugs 0.000 description 1
- 229960000265 cromoglicic acid Drugs 0.000 description 1
- KWGRBVOPPLSCSI-UHFFFAOYSA-N d-ephedrine Natural products CNC(C)C(O)C1=CC=CC=C1 KWGRBVOPPLSCSI-UHFFFAOYSA-N 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 229960004166 diltiazem Drugs 0.000 description 1
- HSUGRBWQSSZJOP-RTWAWAEBSA-N diltiazem Chemical compound C1=CC(OC)=CC=C1[C@H]1[C@@H](OC(C)=O)C(=O)N(CCN(C)C)C2=CC=CC=C2S1 HSUGRBWQSSZJOP-RTWAWAEBSA-N 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- VLARUOGDXDTHEH-UHFFFAOYSA-L disodium cromoglycate Chemical compound [Na+].[Na+].O1C(C([O-])=O)=CC(=O)C2=C1C=CC=C2OCC(O)COC1=CC=CC2=C1C(=O)C=C(C([O-])=O)O2 VLARUOGDXDTHEH-UHFFFAOYSA-L 0.000 description 1
- 239000002934 diuretic Substances 0.000 description 1
- 230000001882 diuretic effect Effects 0.000 description 1
- DLNKOYKMWOXYQA-UHFFFAOYSA-N dl-pseudophenylpropanolamine Natural products CC(N)C(O)C1=CC=CC=C1 DLNKOYKMWOXYQA-UHFFFAOYSA-N 0.000 description 1
- 239000003602 elastase inhibitor Substances 0.000 description 1
- 239000013013 elastic material Substances 0.000 description 1
- 229960002179 ephedrine Drugs 0.000 description 1
- OFKDAAIKGIBASY-VFGNJEKYSA-N ergotamine Chemical compound C([C@H]1C(=O)N2CCC[C@H]2[C@]2(O)O[C@@](C(N21)=O)(C)NC(=O)[C@H]1CN([C@H]2C(C3=CC=CC4=NC=C([C]34)C2)=C1)C)C1=CC=CC=C1 OFKDAAIKGIBASY-VFGNJEKYSA-N 0.000 description 1
- 229960004943 ergotamine Drugs 0.000 description 1
- XCGSFFUVFURLIX-UHFFFAOYSA-N ergotaminine Natural products C1=C(C=2C=CC=C3NC=C(C=23)C2)C2N(C)CC1C(=O)NC(C(N12)=O)(C)OC1(O)C1CCCN1C(=O)C2CC1=CC=CC=C1 XCGSFFUVFURLIX-UHFFFAOYSA-N 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 229960001022 fenoterol Drugs 0.000 description 1
- PJMPHNIQZUBGLI-UHFFFAOYSA-N fentanyl Chemical compound C=1C=CC=CC=1N(C(=O)CC)C(CC1)CCN1CCC1=CC=CC=C1 PJMPHNIQZUBGLI-UHFFFAOYSA-N 0.000 description 1
- 229960002428 fentanyl Drugs 0.000 description 1
- 229960000676 flunisolide Drugs 0.000 description 1
- 229960002714 fluticasone Drugs 0.000 description 1
- MGNNYOODZCAHBA-GQKYHHCASA-N fluticasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(O)[C@@]2(C)C[C@@H]1O MGNNYOODZCAHBA-GQKYHHCASA-N 0.000 description 1
- 229960000289 fluticasone propionate Drugs 0.000 description 1
- WMWTYOKRWGGJOA-CENSZEJFSA-N fluticasone propionate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(OC(=O)CC)[C@@]2(C)C[C@@H]1O WMWTYOKRWGGJOA-CENSZEJFSA-N 0.000 description 1
- 229960002848 formoterol Drugs 0.000 description 1
- BPZSYCZIITTYBL-UHFFFAOYSA-N formoterol Chemical compound C1=CC(OC)=CC=C1CC(C)NCC(O)C1=CC=C(O)C(NC=O)=C1 BPZSYCZIITTYBL-UHFFFAOYSA-N 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- MASNOZXLGMXCHN-ZLPAWPGGSA-N glucagon Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 MASNOZXLGMXCHN-ZLPAWPGGSA-N 0.000 description 1
- 229960004666 glucagon Drugs 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- OROGSEYTTFOCAN-UHFFFAOYSA-N hydrocodone Natural products C1C(N(CCC234)C)C2C=CC(O)C3OC2=C4C1=CC=C2OC OROGSEYTTFOCAN-UHFFFAOYSA-N 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- OEXHQOGQTVQTAT-JRNQLAHRSA-N ipratropium Chemical compound O([C@H]1C[C@H]2CC[C@@H](C1)[N@@+]2(C)C(C)C)C(=O)C(CO)C1=CC=CC=C1 OEXHQOGQTVQTAT-JRNQLAHRSA-N 0.000 description 1
- 229960001888 ipratropium Drugs 0.000 description 1
- 229960001268 isoetarine Drugs 0.000 description 1
- 229960001317 isoprenaline Drugs 0.000 description 1
- 229960004958 ketotifen Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- LMOINURANNBYCM-UHFFFAOYSA-N metaproterenol Chemical compound CC(C)NCC(O)C1=CC(O)=CC(O)=C1 LMOINURANNBYCM-UHFFFAOYSA-N 0.000 description 1
- GRVDJDISBSALJP-UHFFFAOYSA-N methyloxidanyl Chemical group [O]C GRVDJDISBSALJP-UHFFFAOYSA-N 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 229960001664 mometasone Drugs 0.000 description 1
- QLIIKPVHVRXHRI-CXSFZGCWSA-N mometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CCl)(O)[C@@]1(C)C[C@@H]2O QLIIKPVHVRXHRI-CXSFZGCWSA-N 0.000 description 1
- 229960005127 montelukast Drugs 0.000 description 1
- 229960005181 morphine Drugs 0.000 description 1
- PLPRGLOFPNJOTN-UHFFFAOYSA-N narcotine Natural products COc1ccc2C(OC(=O)c2c1OC)C3Cc4c(CN3C)cc5OCOc5c4OC PLPRGLOFPNJOTN-UHFFFAOYSA-N 0.000 description 1
- 229940097496 nasal spray Drugs 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 229960004398 nedocromil Drugs 0.000 description 1
- RQTOOFIXOKYGAN-UHFFFAOYSA-N nedocromil Chemical compound CCN1C(C(O)=O)=CC(=O)C2=C1C(CCC)=C1OC(C(O)=O)=CC(=O)C1=C2 RQTOOFIXOKYGAN-UHFFFAOYSA-N 0.000 description 1
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 1
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 1
- 229960004708 noscapine Drugs 0.000 description 1
- 210000001331 nose Anatomy 0.000 description 1
- 229960002657 orciprenaline Drugs 0.000 description 1
- 229960000797 oxitropium Drugs 0.000 description 1
- NVOYVOBDTVTBDX-PMEUIYRNSA-N oxitropium Chemical compound CC[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)C1=CC=CC=C1 NVOYVOBDTVTBDX-PMEUIYRNSA-N 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- XDRYMKDFEDOLFX-UHFFFAOYSA-N pentamidine Chemical compound C1=CC(C(=N)N)=CC=C1OCCCCCOC1=CC=C(C(N)=N)C=C1 XDRYMKDFEDOLFX-UHFFFAOYSA-N 0.000 description 1
- 229960004448 pentamidine Drugs 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229960001802 phenylephrine Drugs 0.000 description 1
- SONNWYBIRXJNDC-VIFPVBQESA-N phenylephrine Chemical compound CNC[C@H](O)C1=CC=CC(O)=C1 SONNWYBIRXJNDC-VIFPVBQESA-N 0.000 description 1
- DLNKOYKMWOXYQA-APPZFPTMSA-N phenylpropanolamine Chemical compound C[C@@H](N)[C@H](O)C1=CC=CC=C1 DLNKOYKMWOXYQA-APPZFPTMSA-N 0.000 description 1
- 229960000395 phenylpropanolamine Drugs 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical compound [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 description 1
- 229960004583 pranlukast Drugs 0.000 description 1
- UAJUXJSXCLUTNU-UHFFFAOYSA-N pranlukast Chemical compound C=1C=C(OCCCCC=2C=CC=CC=2)C=CC=1C(=O)NC(C=1)=CC=C(C(C=2)=O)C=1OC=2C=1N=NNN=1 UAJUXJSXCLUTNU-UHFFFAOYSA-N 0.000 description 1
- 229960005205 prednisolone Drugs 0.000 description 1
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- MIXMJCQRHVAJIO-TZHJZOAOSA-N qk4dys664x Chemical compound O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O MIXMJCQRHVAJIO-TZHJZOAOSA-N 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 229960002720 reproterol Drugs 0.000 description 1
- WVLAAKXASPCBGT-UHFFFAOYSA-N reproterol Chemical compound C1=2C(=O)N(C)C(=O)N(C)C=2N=CN1CCCNCC(O)C1=CC(O)=CC(O)=C1 WVLAAKXASPCBGT-UHFFFAOYSA-N 0.000 description 1
- 230000000452 restraining effect Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 229950004432 rofleponide Drugs 0.000 description 1
- IXTCZMJQGGONPY-XJAYAHQCSA-N rofleponide Chemical compound C1([C@@H](F)C2)=CC(=O)CC[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H]3O[C@@H](CCC)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O IXTCZMJQGGONPY-XJAYAHQCSA-N 0.000 description 1
- MNDBXUUTURYVHR-UHFFFAOYSA-N roflumilast Chemical compound FC(F)OC1=CC=C(C(=O)NC=2C(=CN=CC=2Cl)Cl)C=C1OCC1CC1 MNDBXUUTURYVHR-UHFFFAOYSA-N 0.000 description 1
- 229960002586 roflumilast Drugs 0.000 description 1
- 229960002052 salbutamol Drugs 0.000 description 1
- 229960004017 salmeterol Drugs 0.000 description 1
- 230000001932 seasonal effect Effects 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 229940124530 sulfonamide Drugs 0.000 description 1
- 150000003456 sulfonamides Chemical class 0.000 description 1
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 1
- 229960000195 terbutaline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 229960000278 theophylline Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- LERNTVKEWCAPOY-DZZGSBJMSA-N tiotropium Chemical compound O([C@H]1C[C@@H]2[N+]([C@H](C1)[C@@H]1[C@H]2O1)(C)C)C(=O)C(O)(C=1SC=CC=1)C1=CC=CS1 LERNTVKEWCAPOY-DZZGSBJMSA-N 0.000 description 1
- 229940110309 tiotropium Drugs 0.000 description 1
- 229960005294 triamcinolone Drugs 0.000 description 1
- GFNANZIMVAIWHM-OBYCQNJPSA-N triamcinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 GFNANZIMVAIWHM-OBYCQNJPSA-N 0.000 description 1
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 1
- 239000002750 tryptase inhibitor Substances 0.000 description 1
- 229960000859 tulobuterol Drugs 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 229940075420 xanthine Drugs 0.000 description 1
- 229950000339 xinafoate Drugs 0.000 description 1
- 229960004764 zafirlukast Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/08—Inhaling devices inserted into the nose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M11/00—Sprayers or atomisers specially adapted for therapeutic purposes
- A61M11/02—Sprayers or atomisers specially adapted for therapeutic purposes operated by air or other gas pressure applied to the liquid or other product to be sprayed or atomised
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M11/00—Sprayers or atomisers specially adapted for therapeutic purposes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M11/00—Sprayers or atomisers specially adapted for therapeutic purposes
- A61M11/006—Sprayers or atomisers specially adapted for therapeutic purposes operated by applying mechanical pressure to the liquid to be sprayed or atomised
- A61M11/007—Syringe-type or piston-type sprayers or atomisers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B1/00—Nozzles, spray heads or other outlets, with or without auxiliary devices such as valves, heating means
- B05B1/34—Nozzles, spray heads or other outlets, with or without auxiliary devices such as valves, heating means designed to influence the nature of flow of the liquid or other fluent material, e.g. to produce swirl
- B05B1/3405—Nozzles, spray heads or other outlets, with or without auxiliary devices such as valves, heating means designed to influence the nature of flow of the liquid or other fluent material, e.g. to produce swirl to produce swirl
- B05B1/341—Nozzles, spray heads or other outlets, with or without auxiliary devices such as valves, heating means designed to influence the nature of flow of the liquid or other fluent material, e.g. to produce swirl to produce swirl before discharging the liquid or other fluent material, e.g. in a swirl chamber upstream the spray outlet
- B05B1/3421—Nozzles, spray heads or other outlets, with or without auxiliary devices such as valves, heating means designed to influence the nature of flow of the liquid or other fluent material, e.g. to produce swirl to produce swirl before discharging the liquid or other fluent material, e.g. in a swirl chamber upstream the spray outlet with channels emerging substantially tangentially in the swirl chamber
- B05B1/3431—Nozzles, spray heads or other outlets, with or without auxiliary devices such as valves, heating means designed to influence the nature of flow of the liquid or other fluent material, e.g. to produce swirl to produce swirl before discharging the liquid or other fluent material, e.g. in a swirl chamber upstream the spray outlet with channels emerging substantially tangentially in the swirl chamber the channels being formed at the interface of cooperating elements, e.g. by means of grooves
- B05B1/3436—Nozzles, spray heads or other outlets, with or without auxiliary devices such as valves, heating means designed to influence the nature of flow of the liquid or other fluent material, e.g. to produce swirl to produce swirl before discharging the liquid or other fluent material, e.g. in a swirl chamber upstream the spray outlet with channels emerging substantially tangentially in the swirl chamber the channels being formed at the interface of cooperating elements, e.g. by means of grooves the interface being a plane perpendicular to the outlet axis
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B11/00—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use
- B05B11/01—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use characterised by the means producing the flow
- B05B11/02—Membranes or pistons acting on the contents inside the container, e.g. follower pistons
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B11/00—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use
- B05B11/01—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use characterised by the means producing the flow
- B05B11/10—Pump arrangements for transferring the contents from the container to a pump chamber by a sucking effect and forcing the contents out through the dispensing nozzle
- B05B11/1001—Piston pumps
- B05B11/1004—Piston pumps comprising a movable cylinder and a stationary piston
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B11/00—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use
- B05B11/01—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use characterised by the means producing the flow
- B05B11/10—Pump arrangements for transferring the contents from the container to a pump chamber by a sucking effect and forcing the contents out through the dispensing nozzle
- B05B11/1001—Piston pumps
- B05B11/1016—Piston pumps the outlet valve having a valve seat located downstream a movable valve element controlled by a pressure actuated controlling element
- B05B11/1018—Piston pumps the outlet valve having a valve seat located downstream a movable valve element controlled by a pressure actuated controlling element and the controlling element cooperating with means for opening or closing the inlet valve
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B11/00—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use
- B05B11/01—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use characterised by the means producing the flow
- B05B11/10—Pump arrangements for transferring the contents from the container to a pump chamber by a sucking effect and forcing the contents out through the dispensing nozzle
- B05B11/1042—Components or details
- B05B11/1066—Pump inlet valves
- B05B11/107—Gate valves; Sliding valves
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B11/00—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use
- B05B11/01—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use characterised by the means producing the flow
- B05B11/10—Pump arrangements for transferring the contents from the container to a pump chamber by a sucking effect and forcing the contents out through the dispensing nozzle
- B05B11/1094—Pump arrangements for transferring the contents from the container to a pump chamber by a sucking effect and forcing the contents out through the dispensing nozzle having inlet or outlet valves not being actuated by pressure or having no inlet or outlet valve
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2206/00—Characteristics of a physical parameter; associated device therefor
- A61M2206/10—Flow characteristics
- A61M2206/16—Rotating swirling helical flow, e.g. by tangential inflows
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B11/00—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use
- B05B11/01—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use characterised by the means producing the flow
- B05B11/10—Pump arrangements for transferring the contents from the container to a pump chamber by a sucking effect and forcing the contents out through the dispensing nozzle
- B05B11/1001—Piston pumps
- B05B11/1016—Piston pumps the outlet valve having a valve seat located downstream a movable valve element controlled by a pressure actuated controlling element
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05B—SPRAYING APPARATUS; ATOMISING APPARATUS; NOZZLES
- B05B11/00—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use
- B05B11/01—Single-unit hand-held apparatus in which flow of contents is produced by the muscular force of the operator at the moment of use characterised by the means producing the flow
- B05B11/10—Pump arrangements for transferring the contents from the container to a pump chamber by a sucking effect and forcing the contents out through the dispensing nozzle
- B05B11/1042—Components or details
- B05B11/1073—Springs
- B05B11/1074—Springs located outside pump chambers
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Anesthesiology (AREA)
- Otolaryngology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pulmonology (AREA)
- Mechanical Engineering (AREA)
- Containers And Packaging Bodies Having A Special Means To Remove Contents (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Reciprocating Pumps (AREA)
- Coating Apparatus (AREA)
- Devices For Dispensing Beverages (AREA)
- Closures For Containers (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0610666.0 | 2006-05-30 | ||
| GBGB0610666.0A GB0610666D0 (en) | 2006-05-30 | 2006-05-30 | Fluid dispenser |
| PCT/EP2007/055273 WO2007138084A2 (en) | 2006-05-30 | 2007-05-30 | Fluid dispenser |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009538790A JP2009538790A (ja) | 2009-11-12 |
| JP2009538790A5 JP2009538790A5 (enExample) | 2010-06-17 |
| JP5336357B2 true JP5336357B2 (ja) | 2013-11-06 |
Family
ID=36687944
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009512590A Expired - Fee Related JP5336357B2 (ja) | 2006-05-30 | 2007-05-30 | 流体ディスペンサ |
Country Status (18)
| Country | Link |
|---|---|
| US (1) | US20090236445A1 (enExample) |
| EP (1) | EP2029287B1 (enExample) |
| JP (1) | JP5336357B2 (enExample) |
| KR (1) | KR101410891B1 (enExample) |
| CN (2) | CN101454085B (enExample) |
| AU (1) | AU2007267133B2 (enExample) |
| BR (1) | BRPI0712801A2 (enExample) |
| CA (1) | CA2653405A1 (enExample) |
| GB (1) | GB0610666D0 (enExample) |
| IL (1) | IL195164A (enExample) |
| MX (1) | MX2008014845A (enExample) |
| MY (1) | MY150442A (enExample) |
| NO (1) | NO20084730L (enExample) |
| NZ (1) | NZ572724A (enExample) |
| RU (1) | RU2435651C2 (enExample) |
| SG (1) | SG174743A1 (enExample) |
| WO (1) | WO2007138084A2 (enExample) |
| ZA (1) | ZA200809582B (enExample) |
Families Citing this family (67)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0610666D0 (en) * | 2006-05-30 | 2006-07-05 | Glaxo Group Ltd | Fluid dispenser |
| TWI474870B (zh) * | 2007-05-30 | 2015-03-01 | Glaxo Group Ltd | 流體分配器 |
| AU2008331300B2 (en) | 2007-11-29 | 2014-01-09 | Glaxo Group Limited | A dispensing device |
| ES2609294T5 (es) * | 2008-06-10 | 2021-12-01 | Meadwestvaco Calmar Gmbh | Cabeza de descarga de fluido |
| DE102008027600A1 (de) * | 2008-06-10 | 2009-12-24 | Meadwestvaco Calmar Gmbh | Fluidaustragkopf |
| DE102008027598A1 (de) * | 2008-06-10 | 2009-12-24 | Meadwestvaco Calmar Gmbh | Fluidaustragkopf |
| UA103195C2 (uk) | 2008-08-11 | 2013-09-25 | Глаксосмитклайн Ллк | Похідні пурину для застосування у лікуванні алергій, запальних та інфекційних захворювань |
| JP2011530562A (ja) | 2008-08-11 | 2011-12-22 | グラクソスミスクライン エルエルシー | アレルギー性、炎症性及び感染性疾患治療用のプリン誘導体 |
| CN104530048B (zh) | 2008-08-11 | 2016-09-14 | 葛兰素史密丝克莱恩有限责任公司 | 腺嘌呤衍生物 |
| WO2010094643A1 (en) | 2009-02-17 | 2010-08-26 | Glaxo Group Limited | Quinoline derivatives and their uses for rhinitis and urticaria |
| NZ575252A (en) * | 2009-02-27 | 2011-06-30 | Forlong & Maisey Ltd T A Instr Supplies | Fluid dispenser, typically drench gun, with sleeve to actuate lever to maintain closing of valve despite pressure surges |
| DE102009037164B3 (de) * | 2009-08-03 | 2010-12-09 | Ing. Erich Pfeiffer Gmbh | Austragvorrichtung für flüssige Medien |
| US9180261B2 (en) | 2010-01-12 | 2015-11-10 | Dance Biopharm Inc. | Preservative free insulin formulations and systems and methods for aerosolizing |
| US10842951B2 (en) | 2010-01-12 | 2020-11-24 | Aerami Therapeutics, Inc. | Liquid insulin formulations and methods relating thereto |
| JP2013519644A (ja) | 2010-02-10 | 2013-05-30 | グラクソスミスクライン エルエルシー | プリン誘導体およびそれらの薬学的使用 |
| CA2786973C (en) | 2010-02-10 | 2018-04-10 | Robert Hermann Gibbon | 6-amino-2-{[(1s)-1-methylbutyl] oxy}-9-[5-(1-piperidinyl)-7,9-dihydro-8h-purin-8-one maleate |
| US8662360B2 (en) * | 2010-11-12 | 2014-03-04 | Yuyao Tirrit Co., Ltd. | Liquid distributor and container provided with the liquid distributor |
| FR2975021B1 (fr) * | 2011-05-11 | 2014-03-07 | Jacques Gerbron | Pompe de dosage et de distribution d'un produit liquide ou visqueux |
| EP2734186B1 (en) | 2011-07-22 | 2018-09-12 | GlaxoSmithKline LLC | Composition |
| PL220720B1 (pl) * | 2012-02-08 | 2015-12-31 | Copernicus Spółka Z Ograniczoną Odpowiedzialnością | Urządzenie wstrzykujące z mechanizmem resetu dawki |
| CA2880576A1 (en) | 2012-08-24 | 2014-02-27 | Glaxosmithkline Llc | Pyrazolopyrimidine compounds |
| EP2892476A1 (en) * | 2012-09-07 | 2015-07-15 | Glaxo Group Limited | Liquid droplet dispenser |
| NZ707319A (en) | 2012-11-20 | 2019-09-27 | Glaxosmithkline Llc | 5h-pyrrolo[3,2-d]pyrimidin-4-amine compounds and their use in the treatment of allergic diseases and other inflammatory conditions |
| CN104058180B (zh) * | 2013-03-18 | 2017-03-01 | F·霍尔泽有限责任公司 | 药剂分配器 |
| JP5803045B2 (ja) * | 2013-03-18 | 2015-11-04 | ヤン、ギョンオック | 薬剤ディスペンサ |
| JP2016531245A (ja) * | 2013-06-24 | 2016-10-06 | エスピーエックス フロウ インコーポレイテッド | 圧力バランス型の流体圧装置及び方法 |
| US9968954B2 (en) | 2014-01-20 | 2018-05-15 | Graco Minnesota Inc. | Sprayer with integrated valve seats |
| CN106029668B (zh) | 2014-02-20 | 2018-02-23 | 葛兰素史克知识产权第二有限公司 | 吡咯并[3,2]嘧啶衍生物作为人类干扰素诱导剂 |
| CN203819764U (zh) * | 2014-04-30 | 2014-09-10 | 东莞怡信磁碟有限公司 | 一种便携式乳膏瓶 |
| DE102014221393A1 (de) * | 2014-10-21 | 2016-04-21 | F. Holzer Gmbh | Pumpkopf für eine Dosiervorrichtung, Dosiervorrichtung sowie Verwendungsmöglichkeiten |
| MX2017006302A (es) | 2014-11-13 | 2018-02-16 | Glaxosmithkline Biologicals Sa | Derivados de adenina que son utiles en el tratamiento de enfermedades alergicas u otras afecciones inflamatorias. |
| EP3189800B1 (de) * | 2014-12-29 | 2019-04-03 | Erbe Elektromedizin GmbH | Computerlesbarer speicher mit instruktionen zur implementierung eines steuerverfahrens zum betreiben einer versorgungseinrichtung |
| HK1245116A1 (zh) | 2015-02-25 | 2018-08-24 | Dance Biopharm Inc. | 液体胰岛素制剂及与其相关的方法 |
| CN108704201A (zh) * | 2015-05-16 | 2018-10-26 | 苏州汉方医药有限公司 | 由手动悬浮微颗粒发生器和复方黄芪组成的药盒 |
| MA42628A (fr) | 2015-08-13 | 2018-06-20 | Red Meters LLC | Appareil et procédés pour déterminer la gravité et la densité de solides dans un milieu liquide |
| GB201516243D0 (en) | 2015-09-14 | 2015-10-28 | Glaxosmithkline Ip Dev Ltd | Novel compounds |
| CR20180286A (es) | 2015-12-03 | 2018-07-16 | Glaxosmithkline Ip Dev Ltd | Dinucleotidos de purina cíclicos como moduladores de sting |
| CN108697866B (zh) | 2016-01-25 | 2021-08-20 | 勃林格殷格翰国际有限公司 | 雾化器 |
| RU2018141798A (ru) * | 2016-04-27 | 2020-05-27 | Инновэйшнкооперэйтив3Д, Ллс | Дозатор для регулируемой подачи текучих сред из гибких упаковок с текучими продуктами |
| US11371866B2 (en) | 2017-05-17 | 2022-06-28 | Red Meters LLC | Methods for designing a flow conduit and apparatus that measures deflection at multiple points to determine flow rate |
| GB2553031B (en) | 2017-06-27 | 2021-12-29 | Kohler Mira Ltd | Additive dispenser |
| WO2019014734A1 (pt) * | 2017-07-20 | 2019-01-24 | Guarany Indústria E Comércio Ltda. | Dosador de líquidos, acoplável a pulverizadores manuais com sistema de bomba de pistão |
| CN107377307A (zh) * | 2017-08-25 | 2017-11-24 | 江苏瑞合硕电子科技有限公司 | 流体计量装置 |
| WO2019076995A1 (en) * | 2017-10-18 | 2019-04-25 | Softhale Nv | SEALING STRUCTURE FOR INHALATION DEVICE |
| CN109745603B (zh) * | 2017-11-06 | 2021-05-28 | 微邦科技股份有限公司 | 流体输送装置 |
| US10391269B2 (en) * | 2017-11-29 | 2019-08-27 | Meshil A. M. O. H. Al-Jarba | Nasal sprayer with multiple applicators |
| CN111432868A (zh) * | 2017-12-05 | 2020-07-17 | 马奎特紧急护理公司 | 刺穿组件和呼吸导管套件 |
| TW202005623A (zh) | 2018-04-06 | 2020-02-01 | 美商蒂克利爾公司 | 用於輸送治療劑之系統及方法 |
| WO2019229042A1 (en) * | 2018-05-30 | 2019-12-05 | Softhale Nv | Inhalation device with a pumping unit |
| FR3083997B1 (fr) * | 2018-07-19 | 2023-03-17 | Albea Services | Pompe de distribution d'un produit fluide, tete de distribution equipee d'une telle pompe et flacon associe |
| JP7422279B2 (ja) * | 2018-11-09 | 2024-01-26 | インヴォックス ベルジアム エヌヴイ | 吸入装置のためのリザーバ |
| CN109487693B (zh) * | 2018-11-22 | 2020-10-27 | 徐雨锋 | 一种道路桥梁打点装置 |
| CN109625637B (zh) * | 2019-02-01 | 2024-04-12 | 天舟医疗(苏州)有限公司 | 一种给料装置 |
| KR20210153633A (ko) | 2019-03-28 | 2021-12-17 | 티어클리어 코포레이션 | 안과용 제형의 흐름 제어를 위한 장치 및 방법 |
| US11666931B2 (en) | 2019-05-14 | 2023-06-06 | Kohler Co. | Inline shower device |
| US12031306B2 (en) | 2019-05-14 | 2024-07-09 | Kohler Co. | Inline dispensing device |
| EP3987161B1 (en) * | 2019-08-08 | 2025-05-07 | Cummins, Inc. | Passive piston cooling nozzle control with low speed hot running protection |
| US20220273893A1 (en) * | 2019-08-30 | 2022-09-01 | Noble International, Llc | Nasal spray medicament training device |
| CN115768597A (zh) * | 2020-03-26 | 2023-03-07 | 海别得公司 | 自由调速止回阀 |
| USD1090783S1 (en) | 2020-04-24 | 2025-08-26 | Kohler Co. | Shower system |
| WO2022034036A1 (de) * | 2020-08-10 | 2022-02-17 | Hatchmore Labs GmbH | Nasenapplikator |
| CN116723847A (zh) * | 2020-09-16 | 2023-09-08 | 萨诺蒂泽研究开发公司 | 双腔室喷雾装置 |
| CN112893305B (zh) * | 2021-01-19 | 2022-02-18 | 青岛大学附属医院 | 一种医疗检验科用器皿的清洗消毒设备 |
| USD1104211S1 (en) | 2021-07-01 | 2025-12-02 | Kohler Co. | Shower system |
| USD1104210S1 (en) | 2021-07-01 | 2025-12-02 | Kohler Co. | Shower device |
| CN114247024B (zh) * | 2021-11-16 | 2024-03-19 | 河北谊安奥美医疗设备有限公司 | 一种多孔蒸发装置 |
| FR3130165B1 (fr) * | 2021-12-13 | 2023-11-17 | Aptar France Sas | Dispositif de distribution de produit fluide et son procédé d'amorçage |
Family Cites Families (46)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1579806A (en) * | 1922-02-04 | 1926-04-06 | Technicolor Motion Picture | Registration of complemental images in cinematography |
| US2253738A (en) * | 1939-02-18 | 1941-08-26 | Totschnig Karl | Closure for tubes |
| FR1597387A (enExample) * | 1968-03-29 | 1970-06-22 | ||
| US3696977A (en) * | 1971-04-21 | 1972-10-10 | Johnson & Son Inc S C | Stretch elastomer valve |
| JPS5824183B2 (ja) * | 1974-05-17 | 1983-05-19 | コンドウ ヒロシ | チクアツフンムソウチ |
| US3913803A (en) * | 1974-12-20 | 1975-10-21 | Robert H Laauwe | Aerosol valve actuator with front end discharge governor |
| FR2325346A1 (fr) * | 1975-09-26 | 1977-04-22 | Broilliard Bernard | Perfectionnements a un distributeur doseur pour des produits liquides ou pateux |
| US4132359A (en) * | 1976-04-09 | 1979-01-02 | Yoshino Kogyosho Co., Ltd. | Manually operative atomizer |
| CH641248A5 (en) * | 1977-05-02 | 1984-02-15 | Leeds & Micallef | Manually actuated piston pump for delivering contents from a container, e.g. packaging container, into the open air |
| US4249681A (en) * | 1979-06-11 | 1981-02-10 | The Dow Chemical Company | Leak-proof sprayer |
| DE3445562A1 (de) * | 1984-12-14 | 1986-06-19 | Ing. Erich Pfeiffer GmbH & Co KG, 7760 Radolfzell | Schubkolben-pumpe fuer wirkstoff-spender |
| JPH0344294Y2 (enExample) * | 1985-05-31 | 1991-09-18 | ||
| EP0378935B1 (fr) * | 1988-12-20 | 1992-09-23 | Societe Technique De Pulverisation (S.T.E.P.) | Dispositif pour distribuer un liquide ou un lait par goutte de petit volume et ensemble de distribution associée |
| JP2509203Y2 (ja) * | 1990-03-05 | 1996-08-28 | 伸晃化学株式会社 | 薬液噴霧ノズル |
| FR2671329B1 (fr) * | 1991-01-07 | 1993-03-19 | Valois | Embout-poussoir a jets multiples et a fermeture. |
| US5154325A (en) * | 1991-01-09 | 1992-10-13 | Ryder International Corporation | Solution delivery nozzle and system with antimicrobial features |
| FR2674747B1 (fr) * | 1991-04-05 | 1993-07-30 | Step Soc Tech Pulverisation | Dispositif distributeur de gouttes de petit volume, notamment pour soins ophtalmologiques. |
| FR2708314B1 (fr) * | 1993-07-28 | 1995-09-29 | Conceptair Anstalt | Perfectionnements aux pompes doseuses. |
| US5370313A (en) * | 1994-01-10 | 1994-12-06 | Beard; Walter C. | Sterile liquid dispenser |
| FR2716873B1 (fr) * | 1994-03-03 | 1996-04-19 | Frank Clanet | Dispositif de distribution à fermeture étanche du contenu d'un récipient pressurisé ou d'un récipient à pompe. |
| EP0688608A1 (en) * | 1994-03-25 | 1995-12-27 | GUALA S.p.A. | An atomizer device for manually operated pumps |
| US5464120A (en) * | 1994-05-27 | 1995-11-07 | Flurry International, Inc. | Method and apparatus for frozen dessert dispensing |
| US5842616A (en) * | 1996-04-24 | 1998-12-01 | Ter S.R.L. | Atomized liquid dispenser applicable to manually operated pumps |
| DE19622124A1 (de) * | 1996-06-01 | 1997-12-04 | Alfred Von Schuckmann | Gerät zum Aufbringen von Flüssigkeiten |
| FR2758801B1 (fr) * | 1997-01-27 | 1999-03-26 | Valois | Systeme d'obturation d'un dispositif de distribution de produit fluide |
| DE19729516C2 (de) * | 1997-07-10 | 1999-04-22 | Georg Wiegner | Pumpe zum dosierten Austragen von flüssigen, gelartigen oder viskosen Substanzen |
| FR2773784B1 (fr) * | 1998-01-16 | 2000-03-24 | Valois Sa | Tete de pulverisation pour un distributeur de produit fluide |
| FR2775262B1 (fr) * | 1998-02-25 | 2000-05-12 | Oreal | Tete de distribution pour la distribution d'un produit et ensemble de distribution sous pression equipe de cette tete |
| AU769830B2 (en) * | 1998-10-27 | 2004-02-05 | Garth T. Webb | Vaporizing device for administering sterile medication |
| US6302101B1 (en) * | 1999-12-14 | 2001-10-16 | Daniel Py | System and method for application of medicament into the nasal passage |
| FR2815611B1 (fr) * | 2000-10-23 | 2003-04-11 | Valois Sa | Tete de distribution et distributeur de produit fluide comportant une telle tete de distribution |
| US6516976B2 (en) * | 2000-12-19 | 2003-02-11 | Kimberly-Clark Worldwide, Inc. | Dosing pump for liquid dispensers |
| US6540117B2 (en) * | 2001-03-30 | 2003-04-01 | Kimberly-Clark Worldwide, Inc. | Dosing pump for liquid dispensers |
| FR2828871B1 (fr) * | 2001-08-22 | 2003-12-19 | Oreal | Dispositif de conditionnement et de distribution sous pression d'un produit |
| US6685109B2 (en) * | 2001-09-24 | 2004-02-03 | Daniel Py | System and method for a two piece spray nozzle |
| FR2837179B1 (fr) * | 2002-03-15 | 2004-07-09 | Valois Sa | Dispositif de distribution de produit fluide |
| FR2838783B1 (fr) * | 2002-04-17 | 2004-07-09 | Valois Sa | Pompe de distribution de produit fluide |
| SE0202800D0 (sv) * | 2002-09-23 | 2002-09-23 | Pharmacia Ab | Dispensing apparatus and method for liquid products, particularly medicinal products |
| FR2852934B1 (fr) * | 2003-03-27 | 2005-12-23 | Rexam Dispensing Sys | Distributeur de produit comprenant une pompe a actionnement par poussoir |
| FR2862107B1 (fr) * | 2003-11-07 | 2006-02-10 | Valois Sas | Pompe de distribution de produit fluide. |
| FR2862009B1 (fr) * | 2003-11-07 | 2007-01-05 | Valois Sas | Tete de pulverisation de produit fluide et pompe de distribution comportant une telle tete. |
| KR100525455B1 (ko) * | 2003-11-26 | 2005-11-04 | 쓰리애플즈코스메틱스 주식회사 | 노즐헤드 하강형 진공타입 화장품 용기의 정량 토출구조 |
| GB0402690D0 (en) * | 2004-02-06 | 2004-03-10 | Glaxo Group Ltd | A fluid dispenser |
| FR2876601B1 (fr) * | 2004-10-19 | 2006-12-29 | Claude Jaunay | Procede de securisation, procede de fabrication et embout securise de pulverisation |
| GB0610666D0 (en) * | 2006-05-30 | 2006-07-05 | Glaxo Group Ltd | Fluid dispenser |
| TWI474870B (zh) * | 2007-05-30 | 2015-03-01 | Glaxo Group Ltd | 流體分配器 |
-
2006
- 2006-05-30 GB GBGB0610666.0A patent/GB0610666D0/en not_active Ceased
-
2007
- 2007-05-30 WO PCT/EP2007/055273 patent/WO2007138084A2/en not_active Ceased
- 2007-05-30 CN CN2007800198608A patent/CN101454085B/zh not_active Expired - Fee Related
- 2007-05-30 MX MX2008014845A patent/MX2008014845A/es active IP Right Grant
- 2007-05-30 AU AU2007267133A patent/AU2007267133B2/en not_active Ceased
- 2007-05-30 BR BRPI0712801-0A patent/BRPI0712801A2/pt not_active Application Discontinuation
- 2007-05-30 JP JP2009512590A patent/JP5336357B2/ja not_active Expired - Fee Related
- 2007-05-30 US US12/302,515 patent/US20090236445A1/en not_active Abandoned
- 2007-05-30 SG SG2011061678A patent/SG174743A1/en unknown
- 2007-05-30 RU RU2008145608/05A patent/RU2435651C2/ru not_active IP Right Cessation
- 2007-05-30 NZ NZ572724A patent/NZ572724A/en not_active IP Right Cessation
- 2007-05-30 MY MYPI20084873 patent/MY150442A/en unknown
- 2007-05-30 CN CN2012104728396A patent/CN102974013A/zh active Pending
- 2007-05-30 CA CA002653405A patent/CA2653405A1/en not_active Abandoned
- 2007-05-30 EP EP07729686.1A patent/EP2029287B1/en active Active
- 2007-05-30 KR KR1020087032111A patent/KR101410891B1/ko not_active Expired - Fee Related
-
2008
- 2008-11-06 IL IL195164A patent/IL195164A/en not_active IP Right Cessation
- 2008-11-10 NO NO20084730A patent/NO20084730L/no not_active Application Discontinuation
- 2008-11-10 ZA ZA2008/09582A patent/ZA200809582B/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| CN101454085B (zh) | 2013-01-02 |
| EP2029287B1 (en) | 2017-12-13 |
| ZA200809582B (en) | 2014-01-29 |
| RU2008145608A (ru) | 2010-07-10 |
| JP2009538790A (ja) | 2009-11-12 |
| US20090236445A1 (en) | 2009-09-24 |
| MX2008014845A (es) | 2008-12-05 |
| WO2007138084A3 (en) | 2008-03-20 |
| RU2435651C2 (ru) | 2011-12-10 |
| KR101410891B1 (ko) | 2014-06-23 |
| SG174743A1 (en) | 2011-10-28 |
| KR20090017662A (ko) | 2009-02-18 |
| MY150442A (en) | 2014-01-30 |
| WO2007138084A2 (en) | 2007-12-06 |
| AU2007267133A1 (en) | 2007-12-06 |
| NZ572724A (en) | 2011-10-28 |
| CN102974013A (zh) | 2013-03-20 |
| IL195164A0 (en) | 2009-08-03 |
| BRPI0712801A2 (pt) | 2012-10-23 |
| AU2007267133B2 (en) | 2012-04-05 |
| GB0610666D0 (en) | 2006-07-05 |
| CA2653405A1 (en) | 2007-12-06 |
| CN101454085A (zh) | 2009-06-10 |
| EP2029287A2 (en) | 2009-03-04 |
| IL195164A (en) | 2013-09-30 |
| NO20084730L (no) | 2008-12-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5336357B2 (ja) | 流体ディスペンサ | |
| KR101618351B1 (ko) | 유체 디스펜서 | |
| JP5427185B2 (ja) | 分配デバイス | |
| AU2013263698A1 (en) | Fluid dispenser |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100420 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100420 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20120518 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120529 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120823 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120830 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20121129 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20130709 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20130801 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5336357 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |