JP5116697B2 - 静脈内電流測定バイオセンサのためのヒドロゲル - Google Patents
静脈内電流測定バイオセンサのためのヒドロゲル Download PDFInfo
- Publication number
- JP5116697B2 JP5116697B2 JP2008556412A JP2008556412A JP5116697B2 JP 5116697 B2 JP5116697 B2 JP 5116697B2 JP 2008556412 A JP2008556412 A JP 2008556412A JP 2008556412 A JP2008556412 A JP 2008556412A JP 5116697 B2 JP5116697 B2 JP 5116697B2
- Authority
- JP
- Japan
- Prior art keywords
- chitosan
- genipin
- oxidase
- solution
- electrode
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000000017 hydrogel Substances 0.000 title claims abstract description 67
- 238000001990 intravenous administration Methods 0.000 title claims abstract description 12
- 229920001661 Chitosan Polymers 0.000 claims abstract description 64
- AZKVWQKMDGGDSV-BCMRRPTOSA-N Genipin Chemical compound COC(=O)C1=CO[C@@H](O)[C@@H]2C(CO)=CC[C@H]12 AZKVWQKMDGGDSV-BCMRRPTOSA-N 0.000 claims abstract description 42
- AZKVWQKMDGGDSV-UHFFFAOYSA-N genipin Natural products COC(=O)C1=COC(O)C2C(CO)=CCC12 AZKVWQKMDGGDSV-UHFFFAOYSA-N 0.000 claims abstract description 42
- 239000000243 solution Substances 0.000 claims abstract description 37
- 108010015776 Glucose oxidase Proteins 0.000 claims abstract description 31
- 235000019420 glucose oxidase Nutrition 0.000 claims abstract description 31
- 239000004366 Glucose oxidase Substances 0.000 claims abstract description 30
- 229940116332 glucose oxidase Drugs 0.000 claims abstract description 30
- 238000000034 method Methods 0.000 claims abstract description 30
- 239000011159 matrix material Substances 0.000 claims abstract description 11
- 239000007787 solid Substances 0.000 claims abstract description 9
- 239000000872 buffer Substances 0.000 claims abstract description 8
- 230000003100 immobilizing effect Effects 0.000 claims abstract description 6
- 239000003929 acidic solution Substances 0.000 claims abstract description 3
- 108090000854 Oxidoreductases Proteins 0.000 claims description 20
- 102000004316 Oxidoreductases Human genes 0.000 claims description 20
- 239000000758 substrate Substances 0.000 claims description 19
- 238000005259 measurement Methods 0.000 claims description 6
- 238000002791 soaking Methods 0.000 claims description 5
- 239000012503 blood component Substances 0.000 claims description 4
- 239000003795 chemical substances by application Substances 0.000 abstract description 5
- 238000009792 diffusion process Methods 0.000 abstract description 5
- 239000003431 cross linking reagent Substances 0.000 abstract description 4
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 44
- 239000008103 glucose Substances 0.000 description 44
- 238000012360 testing method Methods 0.000 description 26
- 239000000376 reactant Substances 0.000 description 22
- 239000008280 blood Substances 0.000 description 19
- 210000004369 blood Anatomy 0.000 description 19
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 18
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 17
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 15
- 108090000790 Enzymes Proteins 0.000 description 14
- 102000004190 Enzymes Human genes 0.000 description 14
- 229940088598 enzyme Drugs 0.000 description 14
- 230000004044 response Effects 0.000 description 10
- 238000006243 chemical reaction Methods 0.000 description 9
- 239000012528 membrane Substances 0.000 description 9
- 238000007254 oxidation reaction Methods 0.000 description 9
- 229910052697 platinum Inorganic materials 0.000 description 9
- 238000004132 cross linking Methods 0.000 description 8
- 239000010408 film Substances 0.000 description 8
- 230000006870 function Effects 0.000 description 7
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 6
- 239000000976 ink Substances 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 230000003647 oxidation Effects 0.000 description 6
- 229910052760 oxygen Inorganic materials 0.000 description 6
- 239000001301 oxygen Substances 0.000 description 6
- 239000004971 Cross linker Substances 0.000 description 5
- 239000012491 analyte Substances 0.000 description 5
- 230000008859 change Effects 0.000 description 5
- 239000008363 phosphate buffer Substances 0.000 description 5
- 239000000499 gel Substances 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 239000000523 sample Substances 0.000 description 4
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000007654 immersion Methods 0.000 description 3
- 238000012544 monitoring process Methods 0.000 description 3
- 238000006722 reduction reaction Methods 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 239000010409 thin film Substances 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 239000004642 Polyimide Substances 0.000 description 2
- 229910021607 Silver chloride Inorganic materials 0.000 description 2
- 239000012080 ambient air Substances 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 239000007979 citrate buffer Substances 0.000 description 2
- 239000004020 conductor Substances 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 239000011888 foil Substances 0.000 description 2
- 229910002804 graphite Inorganic materials 0.000 description 2
- 239000010439 graphite Substances 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 231100000252 nontoxic Toxicity 0.000 description 2
- 230000003000 nontoxic effect Effects 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 229920001721 polyimide Polymers 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 229910052709 silver Inorganic materials 0.000 description 2
- 239000004332 silver Substances 0.000 description 2
- HKZLPVFGJNLROG-UHFFFAOYSA-M silver monochloride Chemical compound [Cl-].[Ag+] HKZLPVFGJNLROG-UHFFFAOYSA-M 0.000 description 2
- 125000006850 spacer group Chemical group 0.000 description 2
- 230000032258 transport Effects 0.000 description 2
- 241000228245 Aspergillus niger Species 0.000 description 1
- 229920002101 Chitin Polymers 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 241000238424 Crustacea Species 0.000 description 1
- PHOQVHQSTUBQQK-SQOUGZDYSA-N D-glucono-1,5-lactone Chemical compound OC[C@H]1OC(=O)[C@H](O)[C@@H](O)[C@@H]1O PHOQVHQSTUBQQK-SQOUGZDYSA-N 0.000 description 1
- 239000004593 Epoxy Substances 0.000 description 1
- 240000001972 Gardenia jasminoides Species 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 208000007536 Thrombosis Diseases 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 230000009056 active transport Effects 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000003570 air Substances 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000023555 blood coagulation Effects 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 210000003191 femoral vein Anatomy 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 229940068517 fruit extracts Drugs 0.000 description 1
- 235000012209 glucono delta-lactone Nutrition 0.000 description 1
- 229960003681 gluconolactone Drugs 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 239000007770 graphite material Substances 0.000 description 1
- 241000411851 herbal medicine Species 0.000 description 1
- 230000002218 hypoglycaemic effect Effects 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 230000000873 masking effect Effects 0.000 description 1
- VUZPPFZMUPKLLV-UHFFFAOYSA-N methane;hydrate Chemical compound C.O VUZPPFZMUPKLLV-UHFFFAOYSA-N 0.000 description 1
- 238000004377 microelectronic Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 206010033675 panniculitis Diseases 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 238000000206 photolithography Methods 0.000 description 1
- 150000003057 platinum Chemical class 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000027756 respiratory electron transport chain Effects 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 210000004304 subcutaneous tissue Anatomy 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 238000009827 uniform distribution Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue
- A61B5/1486—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue using enzyme electrodes, e.g. with immobilised oxidase
- A61B5/14865—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue using enzyme electrodes, e.g. with immobilised oxidase invasive, e.g. introduced into the body by a catheter or needle or using implanted sensors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue
- A61B5/14532—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue for measuring glucose, e.g. by tissue impedance measurement
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/001—Enzyme electrodes
- C12Q1/002—Electrode membranes
- C12Q1/003—Functionalisation
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/001—Enzyme electrodes
- C12Q1/005—Enzyme electrodes involving specific analytes or enzymes
- C12Q1/006—Enzyme electrodes involving specific analytes or enzymes for glucose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B2562/00—Details of sensors; Constructional details of sensor housings or probes; Accessories for sensors
- A61B2562/02—Details of sensors specially adapted for in-vivo measurements
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- General Health & Medical Sciences (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Molecular Biology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Immunology (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Analytical Chemistry (AREA)
- Genetics & Genomics (AREA)
- Emergency Medicine (AREA)
- Optics & Photonics (AREA)
- Pathology (AREA)
- Microbiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Medical Informatics (AREA)
- Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Description
特許のための本出願は、2006年2月27日に出願され、そして本出願の譲受人に譲渡され、そして本明細書中に参考として明示して本明細書によって援用される米国仮出願番号第60/777,254号への優先権を主張している。
本発明は、血液化学を測定するための電流測定バイオセンサに関する。特に、本発明は、静脈内電流測定バイオセンサに関する。
電流測定バイオセンサは、血液化学を分析するための医療産業で公知である。酵素電極としてもまた知られる初期のバイオセンサは、ClarkおよびLyonsによって最初に提案され、そしてUpdikeおよびHicksによって履行された。酵素電極は、代表的には、電極の表面で透析膜の後に固定化されるグルコースオキシダーゼのようなオキシダーゼ酵素を含む。血液の存在下で、この膜は、目的の分析物、例えば、グルコースを、上記酸化酵素まで選択的に通過させ、そこで、それは、酸化または還元、例えば、グルコースのグルコノラクトンへの酸化を受ける。電流測定バイオセンサは、反応を持続するに十分な電位が、反応体の存在下で2つの電極間に付与されるとき、電流を生成することによって機能する。例えば、グルコースとグルコースオキシダーゼとの反応では、過酸化水素副産物が、電極への電子移動によって次に酸化され得る。電極中の電流の得られる流れは、目的の分析物の濃度の指標である。
Gargら、「経皮的リアルタイム連続的グルコースセンサでの血糖可動域における改良」、Diabetes Care、2006年1月
本発明は、静脈内使用および連続的分析物モニタリングのために設計された電流測定バイオセンサとの使用のための新規ヒドロゲルを開示する。電極を有するセンサ上でヒドロゲルは、少なくとも1つの反応性酵素を有する層として形成され得る。このヒドロゲル層は、少なくとも1つのさらなる架橋剤と架橋されたキトサンの固定化するマトリックスを含み得、層の全体に反応性酵素を固定化する。1つの実施形態では、この反応性酵素は、グルコースオキシダーゼのようなオキシダーゼを含み得る。別の実施形態では、ジェニピンが架橋剤として用いられる。その他の実施形態では、上記センサは、薄膜センサであり得、そして上記電極は白金電極であり得る。ヒドロゲル自体は、律速層として機能し得、ヒドロゲル層への拡散の速度を選択的に制御する。
本発明は、静脈内電流測定バイオセンサにおける作業電極上の反応性酵素層としての使用のために理想的な酵素を固定化する頑丈な生体適合性ヒドロゲルを開示する。このヒドロゲルは、(i)キトサン、(ii)反応性酵素、および(iii)架橋剤から形成され得る。本明細書に記載されるように、この組み合わせは、反応性酵素を固定化し、そしてそれをヒドロゲル全体に均一に分配する機械的に強い架橋されたマトリックスを提供することが示された。1つの実施形態では、キトサンは、ジェニピンと架橋され得、グルコースオキシダーゼのようなオキシダーゼを固定化する。ヒドロゲル中で架橋することは、形成を強化し、そしてそれが水性溶液中で溶解することを防ぐ。
H2O2 −1 → 2H+ + O2 0 +2e−
に従って、作業電極19の表面上で酸化する。
Ag0 → Ag+ + e−
対電極17は、白金またはグラファイトのような伝導性材料から構築され得る。これらの材料は、厚膜プロセスそしてそれに合うように硬化を用いて基板13への付与のためのインクとして処方され得る。対電極17は、酸化化学から産生される電子の大部分を、血液溶液に伝導して戻す作業領域を提供する。そうでなければ、すべての電流は、参照電極15を通過する可能性があり、そしてその耐用年数を低減し得る。1つの実施形態では、上記対電極17は、作業電極19の表面積より大きい表面積を備えて形成され得る。
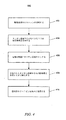
プロトタイプグルコースセンサの集団が、白金電極上に形成されたキトサン−ジェニピンヒドロゲル中にグルコースオキシダーゼを固定化するために方法400を用いて実験室中で製作された。これらグルコースセンサは、種々の機械的および化学的性質について試験された。試験された主な機械的性質は、接着、および浸漬後の接着であった。試験された主な化学的性質は、グルコース応答であった。タイプ7グルコースオキシダーゼをこれら実験のために用いた。以下の開示は、試験センサ製作手順および上記試験センサ集団上で実施された主な試験から得られた結果を提示する。
Claims (11)
- 静脈内電流測定バイオセンサであって:
可撓性基質;
該基質に結合された電極;および
該電極に結合されたヒドロゲル層であって、キトサン、オキシダーゼ酵素、および該キトサンに架橋され、該オキシダーゼ酵素を固定化するマトリックスを形成するジェニピンを含む、ヒドロゲル層、
を含む、静脈内電流測定バイオセンサ。 - 前記ヒドロゲル層が、前記オキシダーゼ酵素と反応するために血液成分を選択的に拡散するための律速層を提供する、請求項1に記載のバイオセンサ。
- 電極上にオキシダーゼを固定化するためのヒドロゲルを形成する方法であって:
キトサンを酸性溶液に溶解する工程;
該キトサン溶液にオキシダーゼを添加する工程;
該キトサン溶液を該電極の表面に付与する工程;
付与された該キトサン溶液をそれが固体膜を形成するまで硬化する工程;および
該固体膜をジェニピン溶液中に浸漬し、該キトサンおよび該ジェニピンを架橋し、それによって該オキシダーゼを固定化する工程、を包含する、方法。 - 前記溶解する工程が、0.1〜2.0重量%のキトサンのキトサン濃度を生成する、請求項3に記載の方法。
- 前記溶解する工程が、0.25重量%キトサンのキトサン濃度を生成する、請求項4に記載の方法。
- 前記オキシダーゼが、グルコースオキシダーゼを含む、請求項3に記載の方法。
- 前記添加する工程が、1/120,000〜1/10,000重量%のオキシダーゼ濃度を生成する、請求項3に記載の方法。
- 前記浸漬する工程が、前記固体膜をジェニピン溶液中に2〜10時間の持続時間の間浸漬することを含む、請求項3に記載の方法。
- 前記ジェニピン溶液が、4〜10のpHを有する緩衝液を含む、請求項3に記載の方法。
- 前記ジェニピン溶液が、1%重量/容量のジェニピンを含む、請求項3に記載の方法。
- 前記架橋されたジェニピンおよびキトサンに、ジェニピンおよびキトサンの第2のより密に架橋された層を付加する工程をさらに包含し、前記オキシダーゼと反応するために血液成分を選択的に拡散するための律速層を提供する、請求項3に記載の方法。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US77725406P | 2006-02-27 | 2006-02-27 | |
| US60/777,254 | 2006-02-27 | ||
| PCT/US2007/004536 WO2007100588A1 (en) | 2006-02-27 | 2007-02-20 | Hydrogel for an intravenous amperometric biosensor |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009528083A JP2009528083A (ja) | 2009-08-06 |
| JP5116697B2 true JP5116697B2 (ja) | 2013-01-09 |
Family
ID=38261532
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008556412A Expired - Fee Related JP5116697B2 (ja) | 2006-02-27 | 2007-02-20 | 静脈内電流測定バイオセンサのためのヒドロゲル |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US20080029390A1 (ja) |
| EP (1) | EP1991111B1 (ja) |
| JP (1) | JP5116697B2 (ja) |
| CN (1) | CN101360449A (ja) |
| AT (1) | ATE504236T1 (ja) |
| CA (1) | CA2630537A1 (ja) |
| DE (1) | DE602007013718D1 (ja) |
| WO (1) | WO2007100588A1 (ja) |
Families Citing this family (54)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7497827B2 (en) | 2004-07-13 | 2009-03-03 | Dexcom, Inc. | Transcutaneous analyte sensor |
| US8886273B2 (en) | 2003-08-01 | 2014-11-11 | Dexcom, Inc. | Analyte sensor |
| US20190357827A1 (en) | 2003-08-01 | 2019-11-28 | Dexcom, Inc. | Analyte sensor |
| US7591801B2 (en) | 2004-02-26 | 2009-09-22 | Dexcom, Inc. | Integrated delivery device for continuous glucose sensor |
| US8626257B2 (en) | 2003-08-01 | 2014-01-07 | Dexcom, Inc. | Analyte sensor |
| US7920906B2 (en) | 2005-03-10 | 2011-04-05 | Dexcom, Inc. | System and methods for processing analyte sensor data for sensor calibration |
| US9247900B2 (en) | 2004-07-13 | 2016-02-02 | Dexcom, Inc. | Analyte sensor |
| US20080197024A1 (en) * | 2003-12-05 | 2008-08-21 | Dexcom, Inc. | Analyte sensor |
| US8364230B2 (en) * | 2006-10-04 | 2013-01-29 | Dexcom, Inc. | Analyte sensor |
| US8425417B2 (en) * | 2003-12-05 | 2013-04-23 | Dexcom, Inc. | Integrated device for continuous in vivo analyte detection and simultaneous control of an infusion device |
| US8774886B2 (en) | 2006-10-04 | 2014-07-08 | Dexcom, Inc. | Analyte sensor |
| US8425416B2 (en) * | 2006-10-04 | 2013-04-23 | Dexcom, Inc. | Analyte sensor |
| US8364231B2 (en) | 2006-10-04 | 2013-01-29 | Dexcom, Inc. | Analyte sensor |
| US8808228B2 (en) | 2004-02-26 | 2014-08-19 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| US8886272B2 (en) * | 2004-07-13 | 2014-11-11 | Dexcom, Inc. | Analyte sensor |
| US20090143658A1 (en) * | 2006-02-27 | 2009-06-04 | Edwards Lifesciences Corporation | Analyte sensor |
| US8447376B2 (en) | 2006-10-04 | 2013-05-21 | Dexcom, Inc. | Analyte sensor |
| US8449464B2 (en) * | 2006-10-04 | 2013-05-28 | Dexcom, Inc. | Analyte sensor |
| US8478377B2 (en) * | 2006-10-04 | 2013-07-02 | Dexcom, Inc. | Analyte sensor |
| US8275438B2 (en) * | 2006-10-04 | 2012-09-25 | Dexcom, Inc. | Analyte sensor |
| US8562528B2 (en) * | 2006-10-04 | 2013-10-22 | Dexcom, Inc. | Analyte sensor |
| US8298142B2 (en) | 2006-10-04 | 2012-10-30 | Dexcom, Inc. | Analyte sensor |
| CA2688184A1 (en) | 2007-06-08 | 2008-12-18 | Dexcom, Inc. | Integrated medicament delivery device for use with continuous analyte sensor |
| EP2227132B1 (en) | 2007-10-09 | 2023-03-08 | DexCom, Inc. | Integrated insulin delivery system with continuous glucose sensor |
| US8000918B2 (en) * | 2007-10-23 | 2011-08-16 | Edwards Lifesciences Corporation | Monitoring and compensating for temperature-related error in an electrochemical sensor |
| KR20100105564A (ko) * | 2007-11-02 | 2010-09-29 | 에드워즈 라이프사이언시스 코포레이션 | 시스템의 수송 또는 주 전력 손실에서 이용되는 백업 전원을 갖는 분석물질 모니터링 시스템 |
| US20090188811A1 (en) * | 2007-11-28 | 2009-07-30 | Edwards Lifesciences Corporation | Preparation and maintenance of sensors |
| US9143569B2 (en) | 2008-02-21 | 2015-09-22 | Dexcom, Inc. | Systems and methods for processing, transmitting and displaying sensor data |
| US8396528B2 (en) | 2008-03-25 | 2013-03-12 | Dexcom, Inc. | Analyte sensor |
| US20100072062A1 (en) * | 2008-05-05 | 2010-03-25 | Edwards Lifesciences Corporation | Membrane For Use With Amperometric Sensors |
| PL235185B1 (pl) | 2008-07-02 | 2020-06-01 | Univ Jagiellonski | Polimer chitozanowy do zastosowania do usuwania heparyny |
| EP2329255A4 (en) * | 2008-08-27 | 2014-04-09 | Edwards Lifesciences Corp | analyte |
| WO2010051421A2 (en) * | 2008-10-31 | 2010-05-06 | Edwards Lifesciences Corporation | Analyte sensor with non-working electrode layer |
| US20100160756A1 (en) * | 2008-12-24 | 2010-06-24 | Edwards Lifesciences Corporation | Membrane Layer for Electrochemical Biosensor and Method of Accommodating Electromagnetic and Radiofrequency Fields |
| JP5727932B2 (ja) * | 2009-06-19 | 2015-06-03 | 日産化学工業株式会社 | 低分子ゲル化剤により化合物が内包されたゲル |
| US20110054284A1 (en) * | 2009-08-28 | 2011-03-03 | Edwards Lifesciences Corporation | Anti-Coagulant Calibrant Infusion Fluid Source |
| US8858883B2 (en) * | 2010-12-02 | 2014-10-14 | University Of Maryland, College Park | Method and system for capture and use of intact vesicles on electrodeposited hydrophobically modified biopolymer films |
| EP3575796B1 (en) | 2011-04-15 | 2020-11-11 | DexCom, Inc. | Advanced analyte sensor calibration and error detection |
| BR112014010978B1 (pt) | 2011-11-07 | 2020-11-03 | Wild Flavors, Inc. | método para preparar corantes vermelhos a partir de um material rico em genipina do fruto de genipa americana |
| US8992751B2 (en) * | 2012-04-09 | 2015-03-31 | Compose Element Limited | Test strips and preparation method thereof |
| CN111044591A (zh) * | 2013-01-11 | 2020-04-21 | 东北大学 | 唾液葡萄糖监测系统 |
| US9655999B2 (en) | 2013-03-12 | 2017-05-23 | Carnegie Mellon University | Coated vaso-occlusive device for treatment of aneurysms |
| DE102014112972A1 (de) * | 2013-09-12 | 2015-03-12 | Endress + Hauser Conducta Gesellschaft für Mess- und Regeltechnik mbH + Co. KG | Messmembran für einen optochemischen oder amperometrischen Sensor |
| GB2538724A (en) * | 2015-05-26 | 2016-11-30 | Imp Innovations Ltd | Methods |
| WO2018088391A1 (ja) * | 2016-11-08 | 2018-05-17 | Jsr株式会社 | 酵素センサ及び酵素センサシステム |
| WO2018186657A1 (ko) * | 2017-04-04 | 2018-10-11 | 주식회사 스칼라팍스트롯 | 바이오 칩 |
| KR102037800B1 (ko) * | 2017-04-04 | 2019-10-30 | 주식회사 스칼라팍스트롯 | 바이오 칩 |
| DK3700416T3 (da) | 2017-10-24 | 2024-09-30 | Dexcom Inc | På forhånd forbundne analytsensorer |
| US11331022B2 (en) | 2017-10-24 | 2022-05-17 | Dexcom, Inc. | Pre-connected analyte sensors |
| WO2019094387A1 (en) * | 2017-11-08 | 2019-05-16 | The Regents Of The University Of Colorado, A Body Corporate | Hydrogel drug delivery systems for the treatment of pediatric growth plate injuries |
| EP3805304A4 (en) * | 2018-06-08 | 2022-03-02 | i-Sens, Inc. | CROSSLINKER WITH GENIPIN FOR USE IN MAKING A SENSOR FILM OR A DIFFUSION CONTROL FILM OF AN ELECTROCHEMICAL SENSOR |
| CN111839532A (zh) * | 2020-07-14 | 2020-10-30 | 天津大学 | 一种基于导电水凝胶的柔性表皮电化学生物传感器 |
| CN112642018B (zh) * | 2020-12-18 | 2023-05-23 | 河南科技大学第一附属医院 | 一种与静脉留置针配合的血糖检测装置及其检测方法 |
| WO2023215131A1 (en) * | 2022-05-03 | 2023-11-09 | Massachusetts Institute Of Technology | Flexible electronics for analyte detection |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5928358Y2 (ja) * | 1978-08-28 | 1984-08-16 | 株式会社クラレ | 医療用のfetセンサ− |
| US4937444A (en) * | 1982-09-29 | 1990-06-26 | Vpl Research, Inc. | Optical flex sensor |
| US4542291A (en) * | 1982-09-29 | 1985-09-17 | Vpl Research Inc. | Optical flex sensor |
| JPH0716409B2 (ja) * | 1986-12-19 | 1995-03-01 | サントリー株式会社 | 酵素を固定化する方法 |
| GB8725936D0 (en) * | 1987-11-05 | 1987-12-09 | Genetics Int Inc | Sensing system |
| US5322063A (en) * | 1991-10-04 | 1994-06-21 | Eli Lilly And Company | Hydrophilic polyurethane membranes for electrochemical glucose sensors |
| US5423883A (en) * | 1993-07-14 | 1995-06-13 | Pacesetter, Inc. | Implantable myocardial stimulation lead with sensors thereon |
| JPH0980010A (ja) * | 1995-09-08 | 1997-03-28 | Daikin Ind Ltd | 使い捨て型酵素電極およびその製造方法 |
| JPH09182738A (ja) * | 1995-12-28 | 1997-07-15 | Fujitsu Ltd | 酸素電極およびその製造方法 |
| EP0929341B1 (en) * | 1996-03-07 | 2005-08-24 | Axon Engineering, Inc. | Polymer-metal foil structure for neural stimulating electrodes |
| JP2910670B2 (ja) * | 1996-04-12 | 1999-06-23 | 日本電気株式会社 | 半導体実装構造 |
| GB9801286D0 (en) * | 1998-01-21 | 1998-03-18 | Univ Cambridge Tech | Sensor |
| US6587705B1 (en) * | 1998-03-13 | 2003-07-01 | Lynn Kim | Biosensor, iontophoretic sampling system, and methods of use thereof |
| JP2004532526A (ja) * | 2001-05-03 | 2004-10-21 | マシモ・コーポレイション | フレックス回路シールド光学センサ及び該フレックス回路シールド光学センサを製造する方法 |
| AU2003215330B2 (en) * | 2002-02-21 | 2008-03-13 | Encelle, Inc. | Immobilized bioactive hydrogel matrices as surface coatings |
| WO2004012676A2 (en) * | 2002-08-02 | 2004-02-12 | Gp Medical | Drug-loaded biological material chemically treated with genipin |
| US6885107B2 (en) * | 2002-08-29 | 2005-04-26 | Micron Technology, Inc. | Flip-chip image sensor packages and methods of fabrication |
| US7883615B2 (en) * | 2003-02-12 | 2011-02-08 | University Of Maryland, College Park | Controlled electrochemical deposition of polysaccharide films and hydrogels, and materials formed therefrom |
| WO2005061127A1 (en) * | 2003-12-11 | 2005-07-07 | University Of Maryland College Park | Biolithographical deposition, and materials and devices formed therefrom |
| JP4593957B2 (ja) * | 2004-03-31 | 2010-12-08 | テルモ株式会社 | 電子光学式検出装置 |
| US7282194B2 (en) * | 2004-10-05 | 2007-10-16 | Gp Medical, Inc. | Nanoparticles for protein drug delivery |
-
2007
- 2007-02-20 AT AT07751306T patent/ATE504236T1/de not_active IP Right Cessation
- 2007-02-20 WO PCT/US2007/004536 patent/WO2007100588A1/en active Application Filing
- 2007-02-20 CA CA002630537A patent/CA2630537A1/en not_active Abandoned
- 2007-02-20 US US11/709,114 patent/US20080029390A1/en not_active Abandoned
- 2007-02-20 CN CNA2007800014680A patent/CN101360449A/zh active Pending
- 2007-02-20 JP JP2008556412A patent/JP5116697B2/ja not_active Expired - Fee Related
- 2007-02-20 EP EP07751306A patent/EP1991111B1/en not_active Not-in-force
- 2007-02-20 DE DE602007013718T patent/DE602007013718D1/de active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CA2630537A1 (en) | 2007-09-07 |
| WO2007100588A1 (en) | 2007-09-07 |
| EP1991111B1 (en) | 2011-04-06 |
| JP2009528083A (ja) | 2009-08-06 |
| DE602007013718D1 (de) | 2011-05-19 |
| CN101360449A (zh) | 2009-02-04 |
| EP1991111A1 (en) | 2008-11-19 |
| ATE504236T1 (de) | 2011-04-15 |
| US20080029390A1 (en) | 2008-02-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5116697B2 (ja) | 静脈内電流測定バイオセンサのためのヒドロゲル | |
| JP5234967B2 (ja) | 静脈内電流測定バイオセンサのための流束制限膜 | |
| EP1841363B1 (en) | Catheter-free implantable needle biosensor | |
| JP5595038B2 (ja) | 分析物センサ装置ならびにその製造方法、組成物及びキット | |
| JP3194434B2 (ja) | 植込み可能なグルコースセンサー | |
| JP5996791B2 (ja) | 折り返しセンサとその製作および使用方法 | |
| Ming Li et al. | Implantable electrochemical sensors for biomedical and clinical applications: progress, problems, and future possibilities. | |
| JP4731554B2 (ja) | 被検物センサならびにその製造方法および使用方法 | |
| CA2888644C (en) | Microarray electrodes useful with analyte sensors and methods for making and using them | |
| JP6088064B2 (ja) | 電圧を印加することによってセンサ機能を最適化する方法およびシステム | |
| CN109312383B (zh) | 用于连续葡萄糖传感器的原位化学堆栈 | |
| US20150122645A1 (en) | Enzyme matrices for biosensors | |
| JP2002506209A (ja) | 電気化学分析物センサ | |
| US11311215B2 (en) | Measurement of device materials using non-Faradaic electrochemical impedance spectroscopy | |
| Vadgama et al. | In vivo biosensors | |
| CN111683596A (zh) | 展现减少的体内炎性反应的可植入聚合物表面 | |
| Wang | A Membrane Biosensor for the Detection of Lactate in Body Fluids | |
| Madden | Development and characterisation of macro-disc and micro-band electrodes for electrochemical sensing applications | |
| CN118777398A (zh) | 与分析物传感器一起使用的分析物转运膜 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100209 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110204 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20120215 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120229 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120528 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120604 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120628 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120705 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120727 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120803 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120828 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20120919 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20121016 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 Ref document number: 5116697 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20151026 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |