JP4896831B2 - 5−ht1a受容体サブタイプ作動薬 - Google Patents
5−ht1a受容体サブタイプ作動薬 Download PDFInfo
- Publication number
- JP4896831B2 JP4896831B2 JP2007179275A JP2007179275A JP4896831B2 JP 4896831 B2 JP4896831 B2 JP 4896831B2 JP 2007179275 A JP2007179275 A JP 2007179275A JP 2007179275 A JP2007179275 A JP 2007179275A JP 4896831 B2 JP4896831 B2 JP 4896831B2
- Authority
- JP
- Japan
- Prior art keywords
- disease
- disorder
- pharmaceutical composition
- schizophrenia
- parkinson
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/08—Drugs for disorders of the alimentary tract or the digestive system for nausea, cinetosis or vertigo; Antiemetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/10—Drugs for genital or sexual disorders; Contraceptives for impotence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/02—Drugs for disorders of the nervous system for peripheral neuropathies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/06—Antimigraine agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/18—Antipsychotics, i.e. neuroleptics; Drugs for mania or schizophrenia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/20—Hypnotics; Sedatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
- A61P25/32—Alcohol-abuse
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P39/00—General protective or antinoxious agents
- A61P39/02—Antidotes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Psychiatry (AREA)
- Epidemiology (AREA)
- Addiction (AREA)
- Endocrinology (AREA)
- Pain & Pain Management (AREA)
- Reproductive Health (AREA)
- Hospice & Palliative Care (AREA)
- Psychology (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Child & Adolescent Psychology (AREA)
- Otolaryngology (AREA)
- Toxicology (AREA)
- Anesthesiology (AREA)
- Gynecology & Obstetrics (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Quinoline Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
Description
本発明は、5−HT1A受容体サブタイプに関連した中枢神経系の障害に罹患した患者を治療するための医薬組成物に関する。有効成分は、カルボスチリル誘導体又はその塩を含む。
米国特許第5,006,528号、欧州特許第367,141号及び特開平7−304740(1995)は、本発明におけるカルボスチリル誘導体として同じ化学構造式を包含しており、それらの薬理学的性質は、精神分裂病に対する治療に有益な薬物である。
本発明の目的は、5−HT1A受容体サブタイプに関連した中枢神経系の障害に罹患した患者を治療する方法を提供することである。
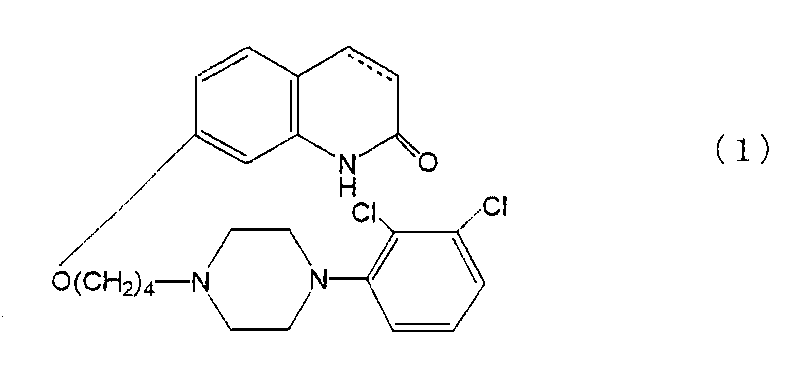
本発明に従って使用される5−HT1A受容体サブタイプ作動薬化合物としては、以下の式(1):
(カルボスチリル骨格の3位及び4位の間の炭素−炭素結合は、単結合又は二重結合である);
で示されるカルボスチリル誘導体が使用される。
1.材料と方法
1.1.試験化合物
7−{4−[4−(2,3−ジクロロフェニル)−1−ピペラジニル]ブトキシ}−3,4−ジヒドロカルボスチリル(アリピプラゾール)が、試験化合物として使用された。
セロトニン(5−HT)及びWAY−100635(N−[2−[4−(2−メトキシフェニル)−1−ピペラジニル]エチル]−N−(2−ピリジニル)−シクロヘキサンカルボキサミド、5−HT1A受容体拮抗薬、RBI(Natick,MA)製)が、参照化合物として使用された。
ジメチルスルホキシド(DMSO)(Sigma Chemicals Co.(St.Louis,MO)製)を溶剤として使用した。
試験化合物を、100%ジメチルスルホキシド(DMSO)に溶解し、100μMの貯蔵溶液を得た(試験化合物を含有するすべての試験管中のDMSOの最終濃度は、1%、v/vであった)。すべてのその他の参照化合物は、DMSOでなく二回蒸留した水を用いて、同じ方法で調製された。
試験化合物及び参照化合物を、10種類の異なった濃度(0.01、0.1、1、5、10、50、100、1000、10000、及び50000nM)で、3回、h5−HT1ACHO細胞膜に対する基礎的[35S]GTPγSの結合への効果を試験した。反応は、GDP(1μM)、[35S]GTPγS(0.1nM)及びh5−HT1ACHO細胞膜(10μgタンパク質/反応;NEN Life Science Products,Boston,MA;カタログ番号CRM035、ロット番号501−60024、GenBank No.X13556)を含有するバッファ(25mM TrisHCl、50mM NaCl,5mM MgCl2、0.1mM EGTA、pH=7.4)792μlと混合した試験薬物/参照薬物、8μlを含有する5mlガラス試験管で行った。反応は、60分間、室温で進行させ、Brandelハーベスター及び4×3ml氷冷バッファ洗浄を使用して、WhatmanGF/B濾紙を通す急速濾過によって終了させた。濾紙に結合した35S放射能を、液体シンチレーション計測(1272Clinigamma,LKB/Wallach)を使用して測定した。
試験化合物を、10種類の異なった濃度(0.01、0.1、1、10、50、100、500、1000、5000及び10000nM)で3回、CHO細胞膜のh5−HT1A受容体(15〜20μgタンパク質;NEN Life Science Products,カタログ番号CRM035、ロット番号501−60024)に結合する[3H]8−OH−DPAT(1nM;NEN Life Sciences;カタログ番号NET929、ロット番号3406035、比活性=124.9Ci/ミリモル)の置換を定量した。膜(396μl)を、[3H]8−OH−DPAT(396μl)、試験化合物又は溶剤(8μl)及びバッファA(50mM Tris.HCl、10mM MgSO4、0.5mM EDTA、0.1%(w/v) アスコルビン酸、pH=7.4)を含有する5mlガラス試験管中でインキュベートした。全てのアッセイは、60分間、室温で行われ、Brandelハーベスター及び4×1mlバッファBで氷冷洗浄を使用して、WhatmanGF/B濾紙(バッファBで前もって浸漬;50mM Tris.HCl)を通す急速濾過によって終了させた。非特異的結合は、10μM(+)8−OH−DPATの存在下で求めた。
セロトニン(5−HT)は、組換えCHO細胞膜で、h5−HT1A受容体に結合する基礎的[35S]GTPγSの増加を促進する、完全5−HT1A受容体作動体である。試験化合物を10種類の濃度で試験して、それらの基礎的[35S]GTPγSの結合への効果を10μM5−HTによって得られた効果と比較して定量した。相対活性(EC50、95%信頼区間)及び固有作動作用(10μM5−HTに対するEmaxの%)を、完全濃度−効果データのコンピュータ化した非線形回帰分析によって、各化合物につき計算した。h5−HT1A受容体における試験化合物の結合親和性は、この受容体を発現するCHO細胞膜に結合する[3H]8−OH−DPATを妨げる能力によって定量した。競合結合データの非線形回帰分析を使用して、[3H]8−OH−DPATによって特異的に結合されたh5−HT1A部位の半分を占拠する試験化合物の濃度である、阻害定数(IC50、95%信頼区間)を計算した。試験化合物に対するh5−HT1A受容体の親和性(Ki、95%信頼区間)は、式、Ki=(IC50)/(1+([[3H]8−OH−DPAT]/Kd)、ここでh5−HT1Aにおける[3H]8−OH−DPATのKd=0.69nM(NEN Life Sciences)、によって計算した。h5−HT1A受容体における薬物結合親和性、力価及び固有の効果の全ての推定値は、Windows(登録商標)用のGraphPad Prism ver.3.00(GrapPad Software,San Diego,CA)を使用して計算した。
試験化合物及び5−HTは、基礎的[35S]GTPγS結合以上に濃度依存的に増加をもたらした。1%DMSOのみでの試験では、基礎的又は薬物誘発[35S]GTPγS結合には効果がなかった。
Claims (7)
- 自閉症;ダウン症候群;注意欠陥多動障害(ADHD);アルツハイマー病又はパーキンソン病から選択される神経変性疾患;強迫性障害(OCD);睡眠障害;性的機能不全;アルコール及び薬物耽溺;嘔吐;乗物酔い;肥満;片頭痛;アルツハイマー病又はパーキンソン病から選択される神経変性疾患に起因する認知障害からなる群から選ばれた5−HT1A受容体サブタイプに関連した中枢神経系の障害を治療するための医薬組成物であって、式(1):
(カルボスチリル骨格の3位及び4位の間の炭素−炭素結合は、単結合又は二重結合である);
のカルボスチリル化合物、及び医薬として許容されるその塩又は溶媒和物の治療有効量を含む医薬組成物。 - 障害が、自閉症、ダウン症候群又は注意欠陥多動障害(ADHD)である、請求項1記載の医薬組成物。
- 障害がアルツハイマー病又はパーキンソン病から選択される神経変性疾患である、請求項1記載の医薬組成物。
- 障害が、強迫性障害(OCD)、睡眠障害、性的機能不全、アルコール及び薬物耽溺、嘔吐、乗物酔い、肥満又は片頭痛である、請求項1記載の医薬組成物。
- 障害が、性的機能不全;アルコール及び薬物耽溺;アルツハイマー病又はパーキンソン病から選択される神経変性疾患に起因する認知障害;アルツハイマー病又はパーキンソン病から選択される神経変性疾患;自閉症;注意欠陥多動障害(ADHD)である、請求項1記載の医薬組成物。
- 障害が、アルツハイマー病又はパーキンソン病から選択される神経変性疾患に起因する認知障害である、請求項1記載の医薬組成物。
- カルボスチリル化合物が、7−{4−[4−(2,3−ジクロロフェニル)−1−ピペラジニル]ブトキシ}−3,4−ジヒドロカルボスチリルである、請求項1乃至6のいずれか一項に記載の医薬組成物。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US77021001A | 2001-01-29 | 2001-01-29 | |
| US09/770,210 | 2001-01-29 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002560616A Division JP4178032B2 (ja) | 2001-01-29 | 2002-01-29 | 5−ht1a受容体サブタイプ作動薬 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011133033A Division JP2011184460A (ja) | 2001-01-29 | 2011-06-15 | 5−ht1a受容体サブタイプ作動薬 |
| JP2011133032A Division JP5683010B2 (ja) | 2001-01-29 | 2011-06-15 | 5−ht1a受容体サブタイプ作動薬 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2007297405A JP2007297405A (ja) | 2007-11-15 |
| JP4896831B2 true JP4896831B2 (ja) | 2012-03-14 |
Family
ID=25087808
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002560616A Expired - Lifetime JP4178032B2 (ja) | 2001-01-29 | 2002-01-29 | 5−ht1a受容体サブタイプ作動薬 |
| JP2007179275A Expired - Lifetime JP4896831B2 (ja) | 2001-01-29 | 2007-07-09 | 5−ht1a受容体サブタイプ作動薬 |
| JP2011133032A Expired - Lifetime JP5683010B2 (ja) | 2001-01-29 | 2011-06-15 | 5−ht1a受容体サブタイプ作動薬 |
| JP2011133033A Pending JP2011184460A (ja) | 2001-01-29 | 2011-06-15 | 5−ht1a受容体サブタイプ作動薬 |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002560616A Expired - Lifetime JP4178032B2 (ja) | 2001-01-29 | 2002-01-29 | 5−ht1a受容体サブタイプ作動薬 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011133032A Expired - Lifetime JP5683010B2 (ja) | 2001-01-29 | 2011-06-15 | 5−ht1a受容体サブタイプ作動薬 |
| JP2011133033A Pending JP2011184460A (ja) | 2001-01-29 | 2011-06-15 | 5−ht1a受容体サブタイプ作動薬 |
Country Status (19)
| Country | Link |
|---|---|
| EP (3) | EP1355639B1 (ja) |
| JP (4) | JP4178032B2 (ja) |
| KR (5) | KR100713607B1 (ja) |
| CN (3) | CN1879624A (ja) |
| AR (4) | AR032641A1 (ja) |
| AT (3) | ATE322894T1 (ja) |
| AU (4) | AU2002226752C1 (ja) |
| BR (1) | BR0206237A (ja) |
| CA (2) | CA2429496C (ja) |
| CY (3) | CY1105631T1 (ja) |
| DE (3) | DE60239711D1 (ja) |
| DK (3) | DK1712225T3 (ja) |
| ES (3) | ES2363366T3 (ja) |
| MX (2) | MX344556B (ja) |
| MY (1) | MY129355A (ja) |
| PH (1) | PH12014500937A1 (ja) |
| PT (3) | PT1712225E (ja) |
| TW (2) | TWI302832B (ja) |
| WO (1) | WO2002060423A2 (ja) |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AR032641A1 (es) * | 2001-01-29 | 2003-11-19 | Otsuka Pharma Co Ltd | Agonista de subtipo de receptor 5-ht 1a. |
| US7053092B2 (en) | 2001-01-29 | 2006-05-30 | Otsuka Pharmaceutical Co., Ltd. | 5-HT1a receptor subtype agonist |
| US8703772B2 (en) | 2001-09-25 | 2014-04-22 | Otsuka Pharmaceutical Co., Ltd. | Low hygroscopic aripiprazole drug substance and processes for the preparation thereof |
| AR033485A1 (es) | 2001-09-25 | 2003-12-26 | Otsuka Pharma Co Ltd | Sustancia medicinal de aripiprazol de baja higroscopicidad y proceso para la preparacion de la misma |
| DE10148233A1 (de) * | 2001-09-28 | 2003-04-10 | Boehringer Ingelheim Pharma | Verbindungen zur Reduzierung übermäßiger Nahrungsaufnahme |
| WO2004010932A2 (en) * | 2002-07-30 | 2004-02-05 | Peter Migaly | Combination therapy for depression, prevention of suicide, and varous medical and psychiatric conditions |
| KR100842694B1 (ko) | 2002-12-27 | 2008-07-01 | 오쓰까 세이야꾸 가부시키가이샤 | 기분 장애 치료용 카르보스티릴 유도체 및 세로토닌 재흡수억제제 |
| AR042806A1 (es) * | 2002-12-27 | 2005-07-06 | Otsuka Pharma Co Ltd | Combinacion de derivados de carboestirilo e inhibidores de la reabsorcion de serotonina para el tratamiento de trastornos del animo |
| US9125939B2 (en) | 2003-05-23 | 2015-09-08 | Otsuka Pharmaceutical Co., Ltd. | Carbostyril derivatives and mood stabilizers for treating mood disorders |
| US7160888B2 (en) * | 2003-08-22 | 2007-01-09 | Warner Lambert Company Llc | [1,8]naphthyridin-2-ones and related compounds for the treatment of schizophrenia |
| FR2865650B1 (fr) * | 2004-01-30 | 2008-06-13 | Biocortech | Utilisation du 14,15 dihydro 20,21-dinoreburnamenin14-ol pour traiter et/ou prevenir les depressions majeures et les desordres du cycle veille-sommeil |
| WO2006090273A2 (en) * | 2005-02-22 | 2006-08-31 | Warner-Lambert Company Llc | [1,8]naphthyridin-2-ones and related compounds with keto or hydroxyl linkers for the treatment of schizophrenia |
| EP1912650B8 (en) * | 2005-08-03 | 2017-10-18 | Sprout Pharmaceuticals, Inc. | Use of flibanserin in the treatment of obesity |
| TW200848041A (en) | 2007-03-30 | 2008-12-16 | Otsuka Pharma Co Ltd | A medicament for treating schizophrenia comprising cilostazol |
| GB2456183A (en) | 2008-01-04 | 2009-07-08 | Gw Pharma Ltd | Anti-psychotic composition comprising cannabinoids and anti-psychotic medicament |
| JP2009286740A (ja) * | 2008-05-30 | 2009-12-10 | Otsuka Pharmaceut Co Ltd | アリピプラゾールを含有する逆耐性抑制剤 |
| EP2338873A1 (en) | 2009-12-22 | 2011-06-29 | Gmeiner, Peter | New aminotetraline derivatives |
| TW201343201A (zh) | 2012-03-06 | 2013-11-01 | Otsuka Pharma Co Ltd | 持續釋放型口服固體製劑 |
| JOP20200109A1 (ar) | 2012-04-23 | 2017-06-16 | Otsuka Pharma Co Ltd | مستحضر قابل للحقن |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS54130587A (en) * | 1978-03-30 | 1979-10-09 | Otsuka Pharmaceut Co Ltd | Carbostyryl derivative |
| JPS5646812A (en) * | 1979-09-27 | 1981-04-28 | Otsuka Pharmaceut Co Ltd | Central nervous system depressant |
| US4764416A (en) | 1986-07-01 | 1988-08-16 | Mitsubishi Denki Kabushiki Kaisha | Electric element circuit using oxidation-reduction substances |
| JP2608788B2 (ja) * | 1988-10-31 | 1997-05-14 | 大塚製薬 株式会社 | 精神分裂病治療剤 |
| US5006528A (en) * | 1988-10-31 | 1991-04-09 | Otsuka Pharmaceutical Co., Ltd. | Carbostyril derivatives |
| WO1992020655A1 (en) * | 1991-05-20 | 1992-11-26 | The Upjohn Company | Carboxamido-(1,2n)-carbocyclic-2-aminotetralin derivatives |
| US5532240A (en) * | 1991-12-26 | 1996-07-02 | Yoshitomi Pharmaceutical Industries, Ltd. | Condensed thiophene compound and pharmaceutical use thereof |
| WO1994009765A1 (en) * | 1992-10-23 | 1994-05-11 | New York University | Functional interactions between glial s-100b and central nervous system serotonergic neurons |
| DK148292D0 (da) * | 1992-12-09 | 1992-12-09 | Lundbeck & Co As H | Forbindelser |
| JP2959615B2 (ja) * | 1993-06-24 | 1999-10-06 | 吉富製薬株式会社 | 縮合型チオフェン化合物およびその医薬用途 |
| JPH09291034A (ja) * | 1996-02-27 | 1997-11-11 | Yoshitomi Pharmaceut Ind Ltd | 縮合ピリジン化合物およびその医薬としての用途 |
| US5688950A (en) * | 1996-04-23 | 1997-11-18 | Neurogen Corporation | Tricyclic aminoalkylcarboxamides; novel dopamine D3 receptor subtype specific ligands |
| DK0904273T3 (da) * | 1996-05-07 | 2003-07-07 | Pfizer | Mesylat-trihydratsalt af 5-(2-(4-(1,2-benzisothiazol-3-yl)-1-piperazinyl)ethyl-6-chlor-1,3-dihydro-2(1H)-indol-2-on(=ziprasidon), dets fremstilling og dets anvendelse som dipamin D2 antagonist |
| JP4012994B2 (ja) * | 1996-05-08 | 2007-11-28 | 大塚製薬株式会社 | 抗不安薬 |
| CN1234023A (zh) * | 1996-08-27 | 1999-11-03 | 美国家用产品公司 | 作为多巴胺d2激动剂和5ht1a的配位体的4-氨基乙氧基吲哚 |
| WO1999038864A1 (en) * | 1998-02-03 | 1999-08-05 | American Home Products Corporation | Oxazole derivatives as serotonin-1a receptor agonists |
| AU3555099A (en) * | 1998-04-13 | 1999-11-01 | American Home Products Corporation | 4-amino-(ethylamino)-oxindole dopamine autoreceptor agonists |
| JPH11335286A (ja) * | 1998-05-25 | 1999-12-07 | Mitsui Chem Inc | ドーパミン拮抗薬の効果増強剤 |
| AR032641A1 (es) * | 2001-01-29 | 2003-11-19 | Otsuka Pharma Co Ltd | Agonista de subtipo de receptor 5-ht 1a. |
| WO2002102297A2 (en) * | 2001-06-19 | 2002-12-27 | Mueller Norbert | Use of cox-2 inhibitors for the treatment of schizophrenia, delusional disorders, affective disorders, autism or tic disorders |
| AR033485A1 (es) * | 2001-09-25 | 2003-12-26 | Otsuka Pharma Co Ltd | Sustancia medicinal de aripiprazol de baja higroscopicidad y proceso para la preparacion de la misma |
-
2002
- 2002-01-15 AR ARP020100118A patent/AR032641A1/es not_active Application Discontinuation
- 2002-01-25 TW TW091101289A patent/TWI302832B/zh not_active IP Right Cessation
- 2002-01-25 TW TW093127321A patent/TWI331919B/zh not_active IP Right Cessation
- 2002-01-26 MY MYPI20020297A patent/MY129355A/en unknown
- 2002-01-29 EP EP02716434A patent/EP1355639B1/en not_active Expired - Lifetime
- 2002-01-29 KR KR1020067014046A patent/KR100713607B1/ko not_active Expired - Lifetime
- 2002-01-29 DE DE60239711T patent/DE60239711D1/de not_active Expired - Lifetime
- 2002-01-29 CN CNA2006100943881A patent/CN1879624A/zh active Pending
- 2002-01-29 CN CNB028035518A patent/CN1239154C/zh not_active Expired - Lifetime
- 2002-01-29 PT PT06015782T patent/PT1712225E/pt unknown
- 2002-01-29 KR KR1020077010561A patent/KR100825705B1/ko not_active Expired - Lifetime
- 2002-01-29 CA CA2429496A patent/CA2429496C/en not_active Expired - Lifetime
- 2002-01-29 BR BR0206237-2A patent/BR0206237A/pt not_active Application Discontinuation
- 2002-01-29 WO PCT/JP2002/000626 patent/WO2002060423A2/en not_active Ceased
- 2002-01-29 DE DE60210581T patent/DE60210581T2/de not_active Expired - Lifetime
- 2002-01-29 PT PT05023971T patent/PT1621198E/pt unknown
- 2002-01-29 AT AT02716434T patent/ATE322894T1/de active
- 2002-01-29 ES ES06015782T patent/ES2363366T3/es not_active Expired - Lifetime
- 2002-01-29 CA CA2700314A patent/CA2700314C/en not_active Expired - Lifetime
- 2002-01-29 KR KR1020057019896A patent/KR100653591B1/ko not_active Expired - Lifetime
- 2002-01-29 MX MX2011010975A patent/MX344556B/es unknown
- 2002-01-29 DK DK06015782.3T patent/DK1712225T3/da active
- 2002-01-29 DE DE60220325T patent/DE60220325T2/de not_active Expired - Lifetime
- 2002-01-29 EP EP05023971A patent/EP1621198B1/en not_active Revoked
- 2002-01-29 PT PT02716434T patent/PT1355639E/pt unknown
- 2002-01-29 AU AU2002226752A patent/AU2002226752C1/en not_active Expired
- 2002-01-29 ES ES05023971T patent/ES2286755T3/es not_active Expired - Lifetime
- 2002-01-29 JP JP2002560616A patent/JP4178032B2/ja not_active Expired - Lifetime
- 2002-01-29 KR KR1020067005164A patent/KR100763288B1/ko not_active Ceased
- 2002-01-29 CN CNB2005100228288A patent/CN100450485C/zh not_active Expired - Lifetime
- 2002-01-29 KR KR1020037008565A patent/KR100601073B1/ko not_active Expired - Lifetime
- 2002-01-29 EP EP06015782A patent/EP1712225B1/en not_active Revoked
- 2002-01-29 MX MXPA03006603A patent/MXPA03006603A/es active IP Right Grant
- 2002-01-29 ES ES02716434T patent/ES2261652T3/es not_active Expired - Lifetime
- 2002-01-29 DK DK05023971T patent/DK1621198T3/da active
- 2002-01-29 AT AT06015782T patent/ATE504293T1/de active
- 2002-01-29 AT AT05023971T patent/ATE362763T1/de active
- 2002-01-29 DK DK02716434T patent/DK1355639T3/da active
-
2005
- 2005-04-27 AU AU2005201772A patent/AU2005201772C1/en not_active Expired
-
2006
- 2006-06-28 CY CY20061100884T patent/CY1105631T1/el unknown
-
2007
- 2007-04-17 AU AU2007201701A patent/AU2007201701B2/en not_active Expired
- 2007-07-09 JP JP2007179275A patent/JP4896831B2/ja not_active Expired - Lifetime
- 2007-08-20 CY CY20071101093T patent/CY1108031T1/el unknown
-
2009
- 2009-10-29 AU AU2009233591A patent/AU2009233591B2/en not_active Expired
-
2010
- 2010-12-28 AR ARP100104973A patent/AR079761A2/es not_active Application Discontinuation
-
2011
- 2011-04-08 AR ARP110101189A patent/AR080849A2/es not_active Application Discontinuation
- 2011-04-18 CY CY20111100391T patent/CY1111392T1/el unknown
- 2011-06-15 JP JP2011133032A patent/JP5683010B2/ja not_active Expired - Lifetime
- 2011-06-15 JP JP2011133033A patent/JP2011184460A/ja active Pending
-
2014
- 2014-04-28 PH PH12014500937A patent/PH12014500937A1/en unknown
-
2015
- 2015-03-13 AR ARP150100766A patent/AR099754A2/es not_active Application Discontinuation
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4896831B2 (ja) | 5−ht1a受容体サブタイプ作動薬 | |
| US9006248B2 (en) | 5-HT1A receptor subtype agonist | |
| AU2002226752A1 (en) | Substituted carbostyril derivatives as 5-HT1A receptor subtype agonists | |
| AU2013203248A1 (en) | Substituted carbostyril derivatives as 5-HT 1A receptor subtype agonists | |
| HK1091403B (en) | 5-ht 1a receptor subtype agonist | |
| HK1095541A (en) | 5-ht 1a receptor subtype agonist | |
| HK1061805B (en) | 5-ht 1a receptor subtype agonist |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20101105 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20101227 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20101227 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110422 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110615 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20110615 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20111216 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20111221 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4896831 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150106 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |