JP4767168B2 - ウイルスベクター - Google Patents
ウイルスベクター Download PDFInfo
- Publication number
- JP4767168B2 JP4767168B2 JP2006520905A JP2006520905A JP4767168B2 JP 4767168 B2 JP4767168 B2 JP 4767168B2 JP 2006520905 A JP2006520905 A JP 2006520905A JP 2006520905 A JP2006520905 A JP 2006520905A JP 4767168 B2 JP4767168 B2 JP 4767168B2
- Authority
- JP
- Japan
- Prior art keywords
- virus
- gene
- strain
- cells
- gene encoding
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 241000700605 Viruses Species 0.000 title claims description 127
- 239000013598 vector Substances 0.000 title description 4
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 196
- 102000004169 proteins and genes Human genes 0.000 claims abstract description 49
- 229940002612 prodrug Drugs 0.000 claims abstract description 35
- 239000000651 prodrug Substances 0.000 claims abstract description 35
- 101100508081 Human herpesvirus 1 (strain 17) ICP34.5 gene Proteins 0.000 claims abstract description 29
- 101150027249 RL1 gene Proteins 0.000 claims abstract description 29
- 241001529453 unidentified herpesvirus Species 0.000 claims abstract description 19
- 108090000790 Enzymes Proteins 0.000 claims abstract description 15
- 102000004190 Enzymes Human genes 0.000 claims abstract description 14
- 206010028980 Neoplasm Diseases 0.000 claims description 52
- 241000713813 Gibbon ape leukemia virus Species 0.000 claims description 29
- 241000700588 Human alphaherpesvirus 1 Species 0.000 claims description 26
- 230000004927 fusion Effects 0.000 claims description 20
- 238000000034 method Methods 0.000 claims description 16
- 201000011510 cancer Diseases 0.000 claims description 14
- 108010080611 Cytosine Deaminase Proteins 0.000 claims description 12
- 230000000799 fusogenic effect Effects 0.000 claims description 12
- 230000001225 therapeutic effect Effects 0.000 claims description 9
- 230000000259 anti-tumor effect Effects 0.000 claims description 8
- 102000000311 Cytosine Deaminase Human genes 0.000 claims description 7
- 108090000288 Glycoproteins Proteins 0.000 claims description 7
- 241000701074 Human alphaherpesvirus 2 Species 0.000 claims description 7
- 238000002560 therapeutic procedure Methods 0.000 claims description 6
- 102000003886 Glycoproteins Human genes 0.000 claims description 5
- 239000003085 diluting agent Substances 0.000 claims description 5
- 239000003814 drug Substances 0.000 claims description 4
- 239000003937 drug carrier Substances 0.000 claims description 4
- 239000008194 pharmaceutical composition Substances 0.000 claims description 4
- 241001465754 Metazoa Species 0.000 claims description 3
- 239000004480 active ingredient Substances 0.000 claims description 2
- 238000004113 cell culture Methods 0.000 claims description 2
- 238000011081 inoculation Methods 0.000 claims description 2
- 229940079593 drug Drugs 0.000 claims 3
- 230000002708 enhancing effect Effects 0.000 claims 1
- 230000002601 intratumoral effect Effects 0.000 claims 1
- 230000007910 cell fusion Effects 0.000 abstract description 14
- 230000002519 immonomodulatory effect Effects 0.000 abstract description 7
- 210000004027 cell Anatomy 0.000 description 43
- 210000004881 tumor cell Anatomy 0.000 description 34
- 241000700584 Simplexvirus Species 0.000 description 28
- 230000004957 immunoregulator effect Effects 0.000 description 18
- 101100118916 Gibbon ape leukemia virus env gene Proteins 0.000 description 15
- 230000014509 gene expression Effects 0.000 description 15
- 241000701022 Cytomegalovirus Species 0.000 description 13
- 238000012217 deletion Methods 0.000 description 12
- 230000037430 deletion Effects 0.000 description 12
- 230000000694 effects Effects 0.000 description 12
- 238000012986 modification Methods 0.000 description 12
- 230000004048 modification Effects 0.000 description 12
- 241000714474 Rous sarcoma virus Species 0.000 description 11
- 230000003213 activating effect Effects 0.000 description 11
- 239000013612 plasmid Substances 0.000 description 11
- XRECTZIEBJDKEO-UHFFFAOYSA-N flucytosine Chemical compound NC1=NC(=O)NC=C1F XRECTZIEBJDKEO-UHFFFAOYSA-N 0.000 description 10
- 229960004413 flucytosine Drugs 0.000 description 10
- 230000003612 virological effect Effects 0.000 description 10
- 230000035772 mutation Effects 0.000 description 9
- 230000010076 replication Effects 0.000 description 9
- 239000002773 nucleotide Substances 0.000 description 8
- 125000003729 nucleotide group Chemical group 0.000 description 8
- 241000700159 Rattus Species 0.000 description 7
- 238000001727 in vivo Methods 0.000 description 7
- 241000700589 Herpes simplex virus (type 1 / strain 17) Species 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 102000037865 fusion proteins Human genes 0.000 description 6
- 108020001507 fusion proteins Proteins 0.000 description 6
- 108091008146 restriction endonucleases Proteins 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 5
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 5
- 238000001976 enzyme digestion Methods 0.000 description 5
- 101150046339 fur gene Proteins 0.000 description 5
- 239000005090 green fluorescent protein Substances 0.000 description 5
- 230000028993 immune response Effects 0.000 description 5
- 238000003780 insertion Methods 0.000 description 5
- 230000037431 insertion Effects 0.000 description 5
- 230000001177 retroviral effect Effects 0.000 description 5
- 239000006228 supernatant Substances 0.000 description 5
- 108091000036 uracil phosphoribosyltransferase Proteins 0.000 description 5
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 4
- 101150076998 ICP34.5 gene Proteins 0.000 description 4
- 238000010276 construction Methods 0.000 description 4
- 229960002949 fluorouracil Drugs 0.000 description 4
- 230000006801 homologous recombination Effects 0.000 description 4
- 238000002744 homologous recombination Methods 0.000 description 4
- 238000000338 in vitro Methods 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- 238000003860 storage Methods 0.000 description 4
- 238000006467 substitution reaction Methods 0.000 description 4
- 108091026890 Coding region Proteins 0.000 description 3
- 102100038132 Endogenous retrovirus group K member 6 Pro protein Human genes 0.000 description 3
- 241000700328 Herpes simplex virus (type 1 / strain F) Species 0.000 description 3
- 101100195053 Human herpesvirus 1 (strain 17) RIR1 gene Proteins 0.000 description 3
- 241000700326 Human herpesvirus 1 strain KOS Species 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 230000022534 cell killing Effects 0.000 description 3
- 230000001186 cumulative effect Effects 0.000 description 3
- 239000012634 fragment Substances 0.000 description 3
- 238000001415 gene therapy Methods 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 210000004882 non-tumor cell Anatomy 0.000 description 3
- 239000013605 shuttle vector Substances 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 108010068327 4-hydroxyphenylpyruvate dioxygenase Proteins 0.000 description 2
- 101150096316 5 gene Proteins 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 108010029697 CD40 Ligand Proteins 0.000 description 2
- 102100032937 CD40 ligand Human genes 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- 108020004414 DNA Proteins 0.000 description 2
- 101710121417 Envelope glycoprotein Proteins 0.000 description 2
- 208000032612 Glial tumor Diseases 0.000 description 2
- 206010018338 Glioma Diseases 0.000 description 2
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 2
- 102000004457 Granulocyte-Macrophage Colony-Stimulating Factor Human genes 0.000 description 2
- 108010068250 Herpes Simplex Virus Protein Vmw65 Proteins 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 241000005822 Human endogenous retrovirus W Species 0.000 description 2
- 241000282620 Hylobates sp. Species 0.000 description 2
- 108020004684 Internal Ribosome Entry Sites Proteins 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 241000712079 Measles morbillivirus Species 0.000 description 2
- 101710169105 Minor spike protein Proteins 0.000 description 2
- 101710081079 Minor spike protein H Proteins 0.000 description 2
- 241000713869 Moloney murine leukemia virus Species 0.000 description 2
- 206010060862 Prostate cancer Diseases 0.000 description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 2
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 2
- 102000006601 Thymidine Kinase Human genes 0.000 description 2
- 108020004440 Thymidine kinase Proteins 0.000 description 2
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 2
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 2
- 108700005077 Viral Genes Proteins 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 239000012190 activator Substances 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 208000029742 colonic neoplasm Diseases 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 2
- 230000008014 freezing Effects 0.000 description 2
- 238000007710 freezing Methods 0.000 description 2
- 230000002779 inactivation Effects 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 201000001441 melanoma Diseases 0.000 description 2
- 230000000174 oncolytic effect Effects 0.000 description 2
- 244000309459 oncolytic virus Species 0.000 description 2
- 239000013600 plasmid vector Substances 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 238000007619 statistical method Methods 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 108700020534 tetracycline resistance-encoding transposon repressor Proteins 0.000 description 2
- 108091006106 transcriptional activators Proteins 0.000 description 2
- 108700001624 vesicular stomatitis virus G Proteins 0.000 description 2
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- 102100039358 3-hydroxyacyl-CoA dehydrogenase type-2 Human genes 0.000 description 1
- 102000007469 Actins Human genes 0.000 description 1
- 108010085238 Actins Proteins 0.000 description 1
- 206010003571 Astrocytoma Diseases 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 102100036850 C-C motif chemokine 23 Human genes 0.000 description 1
- 102100032367 C-C motif chemokine 5 Human genes 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 108010055166 Chemokine CCL5 Proteins 0.000 description 1
- 108010012236 Chemokines Proteins 0.000 description 1
- 102000019034 Chemokines Human genes 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 241000724252 Cucumber mosaic virus Species 0.000 description 1
- 108010054576 Deoxyribonuclease EcoRI Proteins 0.000 description 1
- 206010014733 Endometrial cancer Diseases 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- 101001035740 Homo sapiens 3-hydroxyacyl-CoA dehydrogenase type-2 Proteins 0.000 description 1
- 101000713081 Homo sapiens C-C motif chemokine 23 Proteins 0.000 description 1
- 241000725303 Human immunodeficiency virus Species 0.000 description 1
- XQFRJNBWHJMXHO-RRKCRQDMSA-N IDUR Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(I)=C1 XQFRJNBWHJMXHO-RRKCRQDMSA-N 0.000 description 1
- 102000013462 Interleukin-12 Human genes 0.000 description 1
- 108010065805 Interleukin-12 Proteins 0.000 description 1
- 102000015696 Interleukins Human genes 0.000 description 1
- 108010063738 Interleukins Proteins 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- 238000000134 MTT assay Methods 0.000 description 1
- 231100000002 MTT assay Toxicity 0.000 description 1
- 102000009571 Macrophage Inflammatory Proteins Human genes 0.000 description 1
- 108010009474 Macrophage Inflammatory Proteins Proteins 0.000 description 1
- 241000714177 Murine leukemia virus Species 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 101710138657 Neurotoxin Proteins 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 101800001693 R-peptide Proteins 0.000 description 1
- 208000006265 Renal cell carcinoma Diseases 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- GLNADSQYFUSGOU-GPTZEZBUSA-J Trypan blue Chemical compound [Na+].[Na+].[Na+].[Na+].C1=C(S([O-])(=O)=O)C=C2C=C(S([O-])(=O)=O)C(/N=N/C3=CC=C(C=C3C)C=3C=C(C(=CC=3)\N=N\C=3C(=CC4=CC(=CC(N)=C4C=3O)S([O-])(=O)=O)S([O-])(=O)=O)C)=C(O)C2=C1N GLNADSQYFUSGOU-GPTZEZBUSA-J 0.000 description 1
- 101150085955 US11 gene Proteins 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 208000009956 adenocarcinoma Diseases 0.000 description 1
- 125000003275 alpha amino acid group Chemical group 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 230000005975 antitumor immune response Effects 0.000 description 1
- 210000003050 axon Anatomy 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 201000008274 breast adenocarcinoma Diseases 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 208000025997 central nervous system neoplasm Diseases 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 238000000546 chi-square test Methods 0.000 description 1
- 238000012411 cloning technique Methods 0.000 description 1
- 201000010897 colon adenocarcinoma Diseases 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000000139 costimulatory effect Effects 0.000 description 1
- 238000003235 crystal violet staining Methods 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 1
- 230000001461 cytolytic effect Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000001066 destructive effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 230000037433 frameshift Effects 0.000 description 1
- 238000012224 gene deletion Methods 0.000 description 1
- 230000002518 glial effect Effects 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000000415 inactivating effect Effects 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 229940047122 interleukins Drugs 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 101150066555 lacZ gene Proteins 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 239000002581 neurotoxin Substances 0.000 description 1
- 231100000618 neurotoxin Toxicity 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 201000008968 osteosarcoma Diseases 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 239000002953 phosphate buffered saline Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 208000015347 renal cell adenocarcinoma Diseases 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 210000004872 soft tissue Anatomy 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 230000007480 spreading Effects 0.000 description 1
- 238000003892 spreading Methods 0.000 description 1
- 238000000528 statistical test Methods 0.000 description 1
- 108020003113 steroid hormone receptors Proteins 0.000 description 1
- 102000005969 steroid hormone receptors Human genes 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 230000017960 syncytium formation Effects 0.000 description 1
- 238000012353 t test Methods 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 239000003744 tubulin modulator Substances 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 229960005486 vaccine Drugs 0.000 description 1
- 108700026220 vif Genes Proteins 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 230000003245 working effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/76—Viruses; Subviral particles; Bacteriophages
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/76—Viruses; Subviral particles; Bacteriophages
- A61K35/763—Herpes virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/1703—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A61K38/1709—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
- A61K38/191—Tumor necrosis factors [TNF], e.g. lymphotoxin [LT], i.e. TNF-beta
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
- A61K38/193—Colony stimulating factors [CSF]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/45—Transferases (2)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/50—Hydrolases (3) acting on carbon-nitrogen bonds, other than peptide bonds (3.5), e.g. asparaginase
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
- C12N15/869—Herpesviral vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
- C12N15/869—Herpesviral vectors
- C12N15/8695—Herpes simplex virus-based vectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/16011—Herpesviridae
- C12N2710/16611—Simplexvirus, e.g. human herpesvirus 1, 2
- C12N2710/16641—Use of virus, viral particle or viral elements as a vector
- C12N2710/16643—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/10—Plasmid DNA
- C12N2800/108—Plasmid DNA episomal vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2840/00—Vectors comprising a special translation-regulating system
- C12N2840/20—Vectors comprising a special translation-regulating system translation of more than one cistron
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Zoology (AREA)
- Epidemiology (AREA)
- Genetics & Genomics (AREA)
- Immunology (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Organic Chemistry (AREA)
- Biotechnology (AREA)
- Virology (AREA)
- Biomedical Technology (AREA)
- Wood Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Molecular Biology (AREA)
- Physics & Mathematics (AREA)
- Biochemistry (AREA)
- Plant Pathology (AREA)
- Biophysics (AREA)
- Mycology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Cell Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Marine Sciences & Fisheries (AREA)
- Oncology (AREA)
- Hematology (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Description
不活性又は活性が低いプロドラッグを活性型又はより活性の高い形態へと変換することができるプロドラッグ活性化酵素をコードする遺伝子。従って、ウイルスを用いた腫瘍の治療は、プロドラッグの投与を伴う。
- 機能性ICP34.5をコードする遺伝子を欠いたヘルペスウイルスであって、
(i)プロドラッグ変換酵素をコードする遺伝子、
(ii)細胞と細胞との融合を引き起こすことができるタンパク質をコードする遺伝子、及び
(iii)免疫調節タンパク質をコードする遺伝子、
を2以上含む、ヘルペスウイルスを提供する。
(i)プロドラッグ変換酵素をコードする遺伝子、及び
(ii)細胞と細胞との融合を引き起こすことができるタンパク質をコードする遺伝子、
を含む。
- 療法によってヒト又は動物の体を治療する方法に使用するための本発明のヘルペ
スウイルス、
- 癌治療のための薬剤の製造における、本発明のヘルペスウイルスの使用、
- 活性成分として、本発明によるヘルペスウイルス及び製薬上許容可能な担体又は希釈剤を含む、医薬組成物、
- 腫瘍の治療を必要とする個体中において、当該個体に、本発明によるヘルペスウイルスの有効量を投与することによって腫瘍を治療する方法、
を提供する。
本発明のヘルペスウイルスは、効率的に標的腫瘍細胞に感染することができる。ICP34.5をコードする遺伝子は、前記ウイルス中で不活性化している。ICP34.5の変異は、腫瘍退縮活性を選択的とする。ICP34.5遺伝子中の適切な変異については、Chou et al 1990 及び Maclean et al 1991に記載されているが、ICP34.5を非機能性にする任意の変異を使用してよい。このような不活性化が著しく腫瘍退縮効果を低下させる場合、又はこのような欠失がウイルスの腫瘍退縮特性もしくは他の望ましい特性を高める場合、ICP47、ICP6 及び/又はチミジンキナーゼをコードする遺伝子をさらに不活性化してよく、他の遺伝子も同様に不活性化してよい。ICP47をコードする遺伝子が変異している場合、隣接するUS11遺伝子が、HSV複製サイクル中で通常より早い時期に発現するといったように変異してよい。このような変異については、Liu et al 2003に記載されている。本発明のウイルスは、プロドラッグ活性化酵素、細胞と細胞との融合を引き起こすことができるタンパク質及び免疫調節タンパク質を2以上さらにコードする。
好ましくは、非実験室ウイルスは、標準的な統計的試験によって測定した場合、身近な用途に役立つ特定の機能を果たす同等の改変をもつ参照実験室株より高い能力を有する。腫瘍治療のための腫瘍退縮性ウイルスの場合、本発明の非実験室ウイルス株は、好ましくは、同等の改変をもつ参照実験室株と比べて、腫瘍細胞に感染しもしくは腫瘍細胞中で複製し、腫瘍細胞を殺滅し、又は組織中の細胞間で広がるためのより高い能力を有する。さらに好ましくは、該より高い能力は統計的に有意に高い能力である。例えば、本発明によれば、非実験室株は、評価された特性に関して、参照株の1.1倍、1.2倍、1.5倍、2倍、5倍、10倍、20倍、50倍又は100倍までの能力を有する。好ましくは、該参照株はHSV-1株17+、HSV-1(F)及びHSV-1 KOSから選択される。
ウイルスの、腫瘍細胞を殺滅する能力は、目で大体定量することが可能であり、又は、所定の時間及び所定の細胞種に対して経時的に残る生細胞の数を数えることによってさらに正確に定量することができる。例えば、24、48もしくは72時間にわたって比較を行ってよく、任意の既知の腫瘍細胞種を用いてよい。特に、HT29大腸腺癌、LNCaP.FGC前立腺癌、MDA-MB-231乳腺癌、SK-MEL-28悪性黒色腫又は U-87 MG グリア芽星細胞腫細胞を用いることができる。これらの細胞種の任意の1種、又はこれらの細胞種の任意の組合せを使用することが可能であり、他の腫瘍細胞種を用いてもよい。この目的のために、腫瘍細胞種の標準的パネルを構築することが望ましいであろう。所定の時点で残存している生細胞の数を数えるために、トリパンブルー除外細胞(すなわち、生細胞)の数を数えることができる。蛍光活性化細胞分類(FACS)又はMTTアッセイによって定量を実行してもよい。腫瘍細胞殺滅能力は、in vivoで、例えば特定のウイルスによって生じた腫瘍体積の低減を測定することによって測定してもよい。
言及した様々な遺伝子は、当技術分野において周知な幾つかの技法によって機能的に不活性化することができる。例えば、前記遺伝子は、欠失、置換又は挿入によって機能的に不活性化されてよいが、好ましくは欠失による。欠失は、遺伝子の1もしくはそれ以上の部分又は全遺伝子を除去することでよい。例えば、1つのヌクレオチドのみの欠失がなされると、フレームシフトが起こる。しかし、好ましくは例えば、少なくとも25%、さらに好ましくは少なくとも50%の全コーディング配列及び非コーディング配列(或いは、絶対的には、少なくとも10ヌクレオチド、さらに好ましくは少なくとも100ヌクレオチド、最も好ましくは、少なくとも1000ヌクレオチド)といった、より大きい欠失がなされる。全ての遺伝子及び幾つかの隣接配列(フランキング配列)を除去することが特に好ましい。前記遺伝子の、2つ又はそれ以上のコピーがウイルスゲノム中に存在する場合、遺伝子の両方のコピーが機能的に不活性化されていることが好ましい。
本発明のウイルスは、プロドラッグ活性化酵素をコードする異種遺伝子、細胞と細胞との融合を引き起こすことができるタンパク質をコードする異種遺伝子及び免疫調節タンパク質をコードする異種遺伝子を2以上有する。好ましくは、本発明のウイルスは、プロドラッグ活性化酵素をコードする異種遺伝子、並びに融合性タンパク質をコードする異種遺伝子及び免疫調節タンパク質をコードする異種遺伝子の1つ又は両方を含む。前記融合性タンパク質は、免疫調節タンパク質としても機能する。
プロドラッグ変換酵素をコードする遺伝子、細胞と細胞との融合を促進することができるタンパク質をコードする遺伝子及び/又は免疫調節遺伝子並びに制御配列を含む発現カセット及び他の適当な構築物は、当業者に知られた通常のクローニング技法を用いて作製することができる(例えば、Sambrook et al., 1989, Molecular Cloning - A laboratory manual; Cold Spring Harbor Pressを参照)。
ヒト又は動物の体を治療する方法に本発明のウイルスを用いることができる。特に、本発明のウイルスは癌の治療方法に用いることができる。好ましくは、本発明のウイルスは癌の腫瘍退縮治療に用いる。本発明のウイルスは、哺乳類、好ましくはヒトのいかなる固形腫瘍に対する治療上の処置に用いてもよい。例えば、本発明のウイルスを、前立腺癌、乳癌、肺癌、肝癌、腎細胞癌、子宮内膜癌、膀胱癌、大腸癌又は子宮頸部癌、腺癌、黒色腫、リンパ腫、神経膠腫、軟部組織肉腫及び骨肉腫といった肉腫、又は頭頸部癌をもつ被験者に投与してよい。
本発明のウイルスは、治療を必要とする患者、好ましくはヒトの患者に用いてよい。治療を必要とする患者は、癌を患っている個体、好ましくは固形癌を患っている個体である。治療上の処置の目的は、患者の症状の改善である。通常、本発明のウイルスを用いた治療上の処置は、癌の症状を緩和する。本発明による、癌の治療方法は、癌を患っている患者に対する、治療上有効な量の本発明のウイルスの投与を含む。腫瘍を患っている個体に本発明の腫瘍退縮性ウイルスを投与することによって、通常腫瘍細胞が殺滅され、腫瘍の大きさが減少し及び/又は腫瘍から悪性細胞が広がることが妨げられる。
治療上の処置は、ウイルス組成物を標的組織に直接注射することによって実行してよい。該標的組織は、腫瘍、又は該腫瘍に供給を行う血管であってよい。投与されるウイルスの量は、HSVの場合、104から1010 pfuの範囲内であり、好ましくは105から108 pfuの範囲内、さらに好ましくは約106から109 pfuの範囲内である。通常は1から4 ml、例えば2から3 mlの、本質的にウイルス及び製薬上許容可能な適当な担体もしくは希釈剤からなる医薬組成物が、個々の腫瘍への直接注射に用いられる。しかし、幾つかの腫瘍退縮療法の用途については、腫瘍の種類、大きさ及び接種部位によって、10mlまでの大容量を用いてもよい。同様に、1ml未満の少容量を用いてもよい。
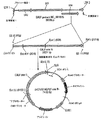
使用したウイルスは、臨床又は「非実験室」HSV1株、JS1を基礎とした。ICP34.5及びICP47をJS1株から完全に欠失した。このウイルスについては、Lui et al 2003に記載されている。GALV env R-(Bateman et al 2000, Galanis et al 2001)及び/又はシトシンデアミナーゼ/ウラシルホスホリボシルトランスフェラーゼ融合遺伝子(Fcy:Fur; Invitrogen)を、その後CMVプロモーター及びRSVプロモーター各々の制御下で、ICP34.5をコードする遺伝子の位置に挿入した。
ステップ1 (図1a): Fcy::Fur 遺伝子を、NcoI及びNheIでpORF Fcy::Fur から切除し、HindIII及びXbaIによる消化の後pRCRSVに挿入した。
GALVのみを発現しているウイルスは、(i) GALV タンパク質の発現を媒介として、in vitroでHT1080、Fadu及びU87MGを含むいくつかのヒト腫瘍細胞株の細胞と細胞との融合を引き起こし、(ii) マウスモデル(Fadu及びHT1080)において、GALV タンパク質を発現していない同等のウイルスと比較して増大した抗腫瘍活性をin vivoで提供する。
ウイルスを含むシトシンデアミナーゼ/ウラシルホスホリボシルトランスフェラーゼ融合遺伝子は、in vitroで5-フルオロシトシンの5-フルオロウラシルへの変換を導き、5-フルオロウラシルが媒介する細胞殺滅が起こることが示された。これは図4に示されており、ここでHT1080細胞は5-フルオロシトシンの存在下又は不在下で3つのウイルスに感染された。その後、これらの細胞からの上清を熱処理して、該上清中に存在するウイルスを不活性化した。次に、これらの上清を用いて新たな細胞を覆った。5-フルオロシトシンが毒性5-フルオロウラシルに変換されていたとすると、これらの新たな細胞はその後死滅させられることとなる。図4から、原HT1080細胞への感染に用いたウイルスがFcy::Fur遺伝子を含んでいた場合、結果として生じた上清で覆った細胞が、Fcy::Fur遺伝子の生物学的活性を示しながら死滅させられたことが確認できる。
図5は、ラットの側腹に移植された縮小腫瘍に対する3つのウイルス(JS1/34.5-/47-/GALV、JS1/34.5/47-/Fcy::Fur 及びJSI/34.5-/47-/GALV/Fcy::Fur)及び「空ベクター」対照(JS1/34.5-/47-)の影響を示す。該ウイルスは、5-フルオロシトシンと併用して投与された。図5から、各々のウイルスが注射された腫瘍の縮小を引き起こしていることが見られる。しかし、GALV env R-又はFcy::Fur単独の送達が、空ベクター対照と比較して改良された腫瘍の縮小を生じさせる一方、GALV env R-及びFcy::Fur両方の併用による送達は、この場合に治癒した全ての腫瘍においてさらに改良された腫瘍縮小効果を生じさせる。従って、プロドラッグ活性化遺伝子及び融合性糖タンパク質の共送達は、腫瘍治療に関して、いずれか一方を単独で用いた場合と比較して改良を生じさせると結論づけることができる。
HSV1株JS1は、仮受託番号01010209の下、2001年1月2日付にてEuropean Collection of Cell Cultures (ECACC)、CAMR、Sailsbury、Wiltshire SP4 0JG、United Kingdomに寄託された。
Chou et al. 1990, Science 250: 1262-1266
Maclean et al. 1991, J. Gen. Virol. 72: 631-639
Gossen M & Bujard H, 1992, PNAS 89: 5547-5551
Gossen M et al. 1995, Science 268: 1766-1769
Thompson et al. 1998, Virus Genes 1(3); 275-286
Meignier et al. 1988, Infect. Dis. 159; 602-614
Liu et al et al 2003, Gene Therapy 10; 292-303
Bateman et al 2000, Cancer Research 60; 1492-1497
Galanis et al 2001, Human Gene Therapy 12; 811-821
Claims (12)
- 機能性ICP34.5をコードする遺伝子を欠いたヘルペスウイルスであって、該ヘルペスウイルスが、
(i)プロドラッグ変換酵素をコードする異種遺伝子、及び
(ii)細胞と細胞との融合を引き起こすことができるタンパク質をコードする異種遺伝子、
を含む、前記ヘルペスウイルス。 - 前記プロドラッグ変換酵素がシトシンデアミナーゼである、請求項1に記載のウイルス。
- 前記細胞と細胞との融合を引き起こすことができるタンパク質がテナガザル白血病ウイルス融合性糖タンパク質である、請求項1又は2に記載のウイルス。
- 前記ウイルスの抗腫瘍治療効果を高めることができる、さらなる異種遺伝子を1以上含む、請求項1〜3のいずれか1項に記載のウイルス。
- ICP47をコードする機能性遺伝子をさらに欠いた、請求項1〜4のいずれか1項に記載のウイルス。
- 単純ヘルペスウイルス1又は2の株である、請求項1〜5のいずれか1項に記載のウイルス。
- 非実験室ウイルス株である、請求項1〜6のいずれか1項に記載のウイルス。
- European collection of cell cultures (ECACC) に受託番号 01010209で寄託されたHSV1 JSIに由来する、請求項1〜7のいずれか1項に記載のウイルス。
- 療法によりヒト又は動物の体を治療する方法に使用する、請求項1〜8のいずれか1項に記載のウイルス。
- 請求項1〜9のいずれか1項に記載のウイルスを含む、癌の治療を目的とした薬剤。
- 前記薬剤が直接腫瘍内接種用である、請求項10に記載の薬剤。
- 有効成分として請求項1〜8のいずれか1項に記載のウイルス及び製薬上許容可能な担体もしくは希釈剤を含む医薬組成物。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0317511.4 | 2003-07-25 | ||
| GBGB0317511.4A GB0317511D0 (en) | 2003-07-25 | 2003-07-25 | Viral vectors |
| PCT/GB2004/003217 WO2005011715A1 (en) | 2003-07-25 | 2004-07-26 | Viral vectors |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2006528484A JP2006528484A (ja) | 2006-12-21 |
| JP2006528484A5 JP2006528484A5 (ja) | 2007-09-06 |
| JP4767168B2 true JP4767168B2 (ja) | 2011-09-07 |
Family
ID=27772737
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2006520905A Expired - Lifetime JP4767168B2 (ja) | 2003-07-25 | 2004-07-26 | ウイルスベクター |
Country Status (18)
| Country | Link |
|---|---|
| US (2) | US7981669B2 (ja) |
| EP (1) | EP1648481B1 (ja) |
| JP (1) | JP4767168B2 (ja) |
| KR (2) | KR20120006582A (ja) |
| CN (1) | CN1829523B (ja) |
| AT (1) | ATE440611T1 (ja) |
| AU (1) | AU2004261037B2 (ja) |
| BR (1) | BRPI0412845A (ja) |
| CA (1) | CA2533338C (ja) |
| DE (1) | DE602004022823D1 (ja) |
| ES (1) | ES2331075T3 (ja) |
| GB (2) | GB0317511D0 (ja) |
| HK (1) | HK1083595A1 (ja) |
| IL (1) | IL173295A0 (ja) |
| MX (1) | MXPA06000876A (ja) |
| PL (1) | PL1648481T3 (ja) |
| WO (1) | WO2005011715A1 (ja) |
| ZA (1) | ZA200600806B (ja) |
Families Citing this family (84)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7063835B2 (en) | 2000-01-21 | 2006-06-20 | Biovex Limited | Virus strains |
| GB0317511D0 (en) | 2003-07-25 | 2003-08-27 | Biovex Ltd | Viral vectors |
| US8133733B2 (en) | 2003-10-24 | 2012-03-13 | Gencia Corporation | Nonviral vectors for delivering polynucleotides to target tissues |
| US20090123468A1 (en) | 2003-10-24 | 2009-05-14 | Gencia Corporation | Transducible polypeptides for modifying metabolism |
| US8507277B2 (en) | 2003-10-24 | 2013-08-13 | Gencia Corporation | Nonviral vectors for delivering polynucleotides |
| US8062891B2 (en) | 2003-10-24 | 2011-11-22 | Gencia Corporation | Nonviral vectors for delivering polynucleotides to plants |
| ES2411962T3 (es) | 2003-10-24 | 2013-07-09 | Gencia Corporation | Métodos y composiciones para suministrar polinucleótidos |
| JP5442452B2 (ja) * | 2007-02-16 | 2014-03-12 | ウイルツ・バイオロジクス・リミテッド | 単純ヘルペスウイルスおよびウイルス複製法 |
| WO2008141151A2 (en) * | 2007-05-09 | 2008-11-20 | Board Of Supervisors Of Louisiana State University And Agricultural And Mechanical College | Synthetic herpes simplex viruses type-1 for treatment of cancers |
| US20130202639A1 (en) * | 2010-03-25 | 2013-08-08 | Konstantin G. Kousoulas | Synthetic Herpes Simplex Viruses for Treatment of Cancers |
| PL2753355T3 (pl) | 2011-09-08 | 2019-04-30 | Benevir Biopharm Inc | Onkolityczny wirus opryszczki pospolitej i jego zastosowania lecznicze |
| US20150250837A1 (en) * | 2012-09-20 | 2015-09-10 | Morningside Technology Ventures Ltd. | Oncolytic virus encoding pd-1 binding agents and uses of the same |
| ES2744837T3 (es) | 2013-10-24 | 2020-02-26 | Amgen Inc | Inyector y procedimiento de ensamblaje |
| JP7051293B2 (ja) | 2013-10-24 | 2022-04-11 | アムジエン・インコーポレーテツド | 温度感知制御を伴う薬剤送達システム |
| WO2015119906A1 (en) | 2014-02-05 | 2015-08-13 | Amgen Inc. | Drug delivery system with electromagnetic field generator |
| BR112016025852B1 (pt) | 2014-05-07 | 2022-11-01 | Amgen Inc | Dispositivo de injeção para aplicação de fármaco |
| KR102416904B1 (ko) | 2014-06-03 | 2022-07-04 | 암겐 인코포레이티드 | 약물 전달 디바이스에 의해 수집된 데이터를 원격으로 프로세싱하기 위한 시스템들 및 방법들 |
| WO2016061220A2 (en) | 2014-10-14 | 2016-04-21 | Amgen Inc. | Drug injection device with visual and audio indicators |
| US11357916B2 (en) | 2014-12-19 | 2022-06-14 | Amgen Inc. | Drug delivery device with live button or user interface field |
| US10799630B2 (en) | 2014-12-19 | 2020-10-13 | Amgen Inc. | Drug delivery device with proximity sensor |
| CA3069716C (en) | 2015-02-17 | 2021-11-09 | Amgen Inc. | Drug delivery device with vacuum assisted securement and/or feedback |
| ES2905870T3 (es) | 2015-02-27 | 2022-04-12 | Amgen Inc | Dispositivo de suministro de fármacos que tiene un mecanismo de protección de aguja con un umbral de resistencia ajustable al movimiento de la protección de aguja |
| US10967015B2 (en) | 2015-06-15 | 2021-04-06 | New York University | Method of treatment using oncolytic viruses |
| WO2017039786A1 (en) | 2015-09-02 | 2017-03-09 | Amgen Inc. | Syringe assembly adapter for a syringe |
| WO2017100501A1 (en) | 2015-12-09 | 2017-06-15 | Amgen Inc. | Auto-injector with signaling cap |
| WO2017120178A1 (en) | 2016-01-06 | 2017-07-13 | Amgen Inc. | Auto-injector with signaling electronics |
| US10612005B2 (en) * | 2016-01-08 | 2020-04-07 | Replimune Limited | Modified oncolytic virus |
| DK3429663T3 (da) | 2016-03-15 | 2020-09-28 | Amgen Inc | Reduktion af sandsynligheden for glasbrud i anordninger til indgivelse af lægemidler |
| WO2017189089A1 (en) | 2016-04-29 | 2017-11-02 | Amgen Inc. | Drug delivery device with messaging label |
| US11389588B2 (en) | 2016-05-02 | 2022-07-19 | Amgen Inc. | Syringe adapter and guide for filling an on-body injector |
| AU2017263558B2 (en) | 2016-05-13 | 2022-12-22 | Amgen Inc. | Vial sleeve assembly |
| WO2017200989A1 (en) | 2016-05-16 | 2017-11-23 | Amgen Inc. | Data encryption in medical devices with limited computational capability |
| EP3465124A1 (en) | 2016-06-03 | 2019-04-10 | Amgen Inc. | Impact testing apparatuses and methods for drug delivery devices |
| US11285266B2 (en) | 2016-07-01 | 2022-03-29 | Amgen Inc. | Drug delivery device having minimized risk of component fracture upon impact events |
| WO2018034784A1 (en) | 2016-08-17 | 2018-02-22 | Amgen Inc. | Drug delivery device with placement detection |
| WO2018081234A1 (en) | 2016-10-25 | 2018-05-03 | Amgen Inc. | On-body injector |
| GB201700350D0 (en) | 2017-01-09 | 2017-02-22 | Replimune Ltd | Altered virus |
| MX2019008432A (es) | 2017-01-17 | 2019-11-18 | Amgen Inc | Dispositivos de inyeccion y metodos relacionados de uso y ensamblaje. |
| AU2018221351B2 (en) | 2017-02-17 | 2023-02-23 | Amgen Inc. | Insertion mechanism for drug delivery device |
| US11752258B2 (en) | 2017-02-17 | 2023-09-12 | Amgen Inc. | Drug delivery device with sterile fluid flowpath and related method of assembly |
| CA3050927A1 (en) | 2017-03-06 | 2018-09-13 | Brian Stonecipher | Drug delivery device with activation prevention feature |
| US11571511B2 (en) | 2017-03-07 | 2023-02-07 | Amgen Inc. | Insertion mechanism and method of inserting a needle of a drug delivery device |
| AU2018230486B2 (en) | 2017-03-09 | 2023-05-11 | Amgen Inc. | Insertion mechanism for drug delivery device |
| HUE063805T2 (hu) | 2017-03-28 | 2024-01-28 | Amgen Inc | Dugattyúrúd és fecskendõ összeállító rendszer és eljárás |
| JP7200134B2 (ja) | 2017-06-08 | 2023-01-06 | アムジエン・インコーポレーテツド | トルク駆動式薬物送達デバイス |
| EP3634539A1 (en) | 2017-06-08 | 2020-04-15 | Amgen Inc. | Syringe assembly for a drug delivery device and method of assembly |
| CN107354136A (zh) * | 2017-06-15 | 2017-11-17 | 杭州睿可特生物科技有限公司 | 重组单纯疱疹病毒及其制备方法和应用 |
| MX2019015472A (es) | 2017-06-22 | 2020-02-19 | Amgen Inc | Reduccion del impacto/choque de la activacion del mecanismo. |
| JP7475860B2 (ja) | 2017-06-23 | 2024-04-30 | アムジエン・インコーポレーテツド | スイッチアセンブリにより起動されるキャップを含む電子薬物送達デバイス |
| JP7408398B2 (ja) | 2017-07-14 | 2024-01-05 | アムジエン・インコーポレーテツド | 二重ねじりばねシステムを有する針挿入後退システム |
| EP3655063A1 (en) | 2017-07-21 | 2020-05-27 | Amgen Inc. | Gas permeable sealing member for drug container and methods of assembly |
| US11617837B2 (en) | 2017-07-25 | 2023-04-04 | Amgen Inc. | Drug delivery device with gear module and related method of assembly |
| MA49676A (fr) | 2017-07-25 | 2020-06-03 | Amgen Inc | Dispositif d'administration de médicament doté d'un système d'accès à un récipient et procédé d'assemblage associé |
| MA49838A (fr) | 2017-08-09 | 2020-06-17 | Amgen Inc | Systèm de administration de médicaments avec pression hydraulique-pneumatique de chambre |
| MA49897A (fr) | 2017-08-18 | 2020-06-24 | Amgen Inc | Injecteur sur-corps avec patch adhésif stérile |
| US11103636B2 (en) | 2017-08-22 | 2021-08-31 | Amgen Inc. | Needle insertion mechanism for drug delivery device |
| ES2939292T3 (es) | 2017-10-04 | 2023-04-20 | Amgen Inc | Adaptador de flujo para dispositivo de administración de fármacos |
| CN111132711B (zh) | 2017-10-06 | 2022-07-01 | 安进公司 | 带有联锁组件的药物递送装置及相关组装方法 |
| US11464903B2 (en) | 2017-10-09 | 2022-10-11 | Amgen Inc. | Drug delivery device with drive assembly and related method of assembly |
| WO2019090079A1 (en) | 2017-11-03 | 2019-05-09 | Amgen Inc. | System and approaches for sterilizing a drug delivery device |
| EP3707075A1 (en) | 2017-11-06 | 2020-09-16 | Amgen Inc. | Fill-finish assemblies and related methods |
| CA3079197A1 (en) | 2017-11-06 | 2019-05-09 | Amgen Inc. | Drug delivery device with placement and flow sensing |
| CN111225696B (zh) | 2017-11-10 | 2023-07-18 | 安进公司 | 用于药物递送装置的柱塞 |
| IL273638B2 (en) | 2017-11-16 | 2024-10-01 | Amgen Inc | Door lock mechanism for drug delivery device |
| US10835685B2 (en) | 2018-05-30 | 2020-11-17 | Amgen Inc. | Thermal spring release mechanism for a drug delivery device |
| US11083840B2 (en) | 2018-06-01 | 2021-08-10 | Amgen Inc. | Modular fluid path assemblies for drug delivery devices |
| MA53379A (fr) | 2018-07-24 | 2021-06-02 | Amgen Inc | Dispositifs d'administration pour l'administration de médicaments |
| WO2020023336A1 (en) | 2018-07-24 | 2020-01-30 | Amgen Inc. | Hybrid drug delivery devices with grip portion |
| WO2020023451A1 (en) | 2018-07-24 | 2020-01-30 | Amgen Inc. | Delivery devices for administering drugs |
| WO2020023220A1 (en) | 2018-07-24 | 2020-01-30 | Amgen Inc. | Hybrid drug delivery devices with tacky skin attachment portion and related method of preparation |
| CA3103105A1 (en) | 2018-07-31 | 2020-02-06 | Amgen Inc. | Fluid path assembly for a drug delivery device |
| US20210346601A1 (en) | 2018-09-24 | 2021-11-11 | Amgen Inc. | Interventional dosing systems and methods |
| EP3856283A1 (en) | 2018-09-28 | 2021-08-04 | Amgen Inc. | Muscle wire escapement activation assembly for a drug delivery device |
| WO2020072577A1 (en) | 2018-10-02 | 2020-04-09 | Amgen Inc. | Injection systems for drug delivery with internal force transmission |
| TWI824026B (zh) | 2018-10-05 | 2023-12-01 | 美商安進公司 | 具有劑量指示器之藥物遞送裝置 |
| SG11202101824VA (en) | 2018-10-15 | 2021-03-30 | Amgen Inc | Platform assembly process for drug delivery device |
| KR20210076935A (ko) | 2018-10-15 | 2021-06-24 | 암젠 인크 | 댐핑 메커니즘을 갖는 약물 전달 장치 |
| CA3113076A1 (en) | 2018-11-01 | 2020-05-07 | Amgen Inc. | Drug delivery devices with partial drug delivery member retraction |
| TWI831847B (zh) | 2018-11-01 | 2024-02-11 | 美商安進公司 | 部分針頭縮回之藥物遞送裝置及其操作方法 |
| WO2020091956A1 (en) | 2018-11-01 | 2020-05-07 | Amgen Inc. | Drug delivery devices with partial drug delivery member retraction |
| WO2020219482A1 (en) | 2019-04-24 | 2020-10-29 | Amgen Inc. | Syringe sterilization verification assemblies and methods |
| EP4017560A2 (en) | 2019-08-23 | 2022-06-29 | Amgen, Inc | Drug delivery device with configurable needle shield engagement components and related methods |
| WO2022170219A1 (en) | 2021-02-05 | 2022-08-11 | Iovance Biotherapeutics, Inc. | Adjuvant therapy for cancer |
| MX2023013640A (es) | 2021-05-21 | 2023-11-30 | Amgen Inc | Metodo de optimizacion de una receta de llenado para un contenedor de medicamento. |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003501445A (ja) * | 1999-06-08 | 2003-01-14 | ユーエイビー リサーチ ファンデーション | 外来遺伝子を発現するヘルペス単純ウイルスおよびそれによる癌の治療方法 |
| JP2003520044A (ja) * | 2000-01-21 | 2003-07-02 | バイオヴェックス リミテッド | ヘルペスウイルス株 |
Family Cites Families (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5328688A (en) * | 1990-09-10 | 1994-07-12 | Arch Development Corporation | Recombinant herpes simplex viruses vaccines and methods |
| GB9102126D0 (en) | 1991-01-31 | 1991-03-13 | Smithkline Beecham Biolog | Novel vaccine |
| US5585096A (en) | 1994-06-23 | 1996-12-17 | Georgetown University | Replication-competent herpes simplex virus mediates destruction of neoplastic cells |
| GB9423663D0 (en) | 1994-11-23 | 1995-01-11 | Cantab Pharma Res | Viral preparations, immunogens, and vaccines |
| NL9500216A (nl) * | 1995-02-06 | 1996-09-02 | Bio Pharma Sciences Bv | Farmaceutische samenstelling voor de behandeling van herpes. |
| IL118942A (en) | 1995-07-27 | 2002-09-12 | American Cyanamid Co | Nonviolent viruses used as killers - tumors and vaccines |
| GB9524973D0 (en) | 1995-12-06 | 1996-02-07 | Lynxvale Ltd | Viral vectors |
| DE69722608T2 (de) | 1996-01-25 | 2004-04-29 | The University Court Of The University Of Glasgow, Glasgow | Hsv-mutant-1716 zur behandlung von mesotheliomen |
| US5824318A (en) * | 1996-07-24 | 1998-10-20 | American Cyanamid Company | Avirulent herpetic viruses useful as tumoricidal agents and vaccines |
| GB9615794D0 (en) | 1996-07-26 | 1996-09-04 | Medical Res Council | Mutant herpes simplex virus strains and uses thereof |
| US5876923A (en) * | 1996-07-26 | 1999-03-02 | Arch Development Corporation | Herpes simplex virus ICP4 as an inhibitor of apoptosis |
| JPH1087503A (ja) * | 1996-09-06 | 1998-04-07 | Nippon Chem Res Kk | 神経系腫瘍細胞のアポトーシス剤 |
| GB9700411D0 (en) | 1997-01-10 | 1997-02-26 | Univ London | Eukaryotic gene expression cassette and uses thereof |
| US20030219409A1 (en) * | 1997-01-10 | 2003-11-27 | Biovex Limited | Eukaryotic gene expression cassette and uses thereof |
| GB9704046D0 (en) | 1997-02-27 | 1997-04-16 | Univ Leeds | Arrestable therapeutic |
| EP0966534B1 (en) * | 1997-03-11 | 2005-02-02 | Mayo Foundation for Medical Education and Research | Compositions and methods for elimination of unwanted cells |
| US6051428A (en) | 1997-03-21 | 2000-04-18 | Sloan-Kettering Institute For Cancer Research | Rapid production of autologous tumor vaccines |
| US5998174A (en) | 1997-05-12 | 1999-12-07 | University Of Pittsburgh Of The Commonwealth System Of Higher Education | Multigene vectors |
| WO1999006583A1 (en) * | 1997-07-31 | 1999-02-11 | University Of Pittsburgh Of The Commonwealth System Of Higher Education | Targeted hsv vectors |
| US20030044384A1 (en) * | 1997-10-09 | 2003-03-06 | Pro-Virus, Inc. | Treatment of neoplasms with viruses |
| GB9801930D0 (en) * | 1998-01-29 | 1998-03-25 | Univ London | Mutant herpes simplex viruses and uses thereof |
| GB9810904D0 (en) | 1998-05-20 | 1998-07-22 | Univ London | Mutant herpes simplex viruses and uses thereof |
| US20040063094A1 (en) * | 1998-05-20 | 2004-04-01 | Biovex Limited | Mutant herpes simplex viruses and uses thereof |
| GB9816781D0 (en) * | 1998-07-31 | 1998-09-30 | Univ London | Herpes virus vectors for dendritic cells |
| GB0009079D0 (en) | 2000-04-12 | 2000-05-31 | Neurovex Ltd | Herpes viruses for immune modulation |
| US6713067B2 (en) * | 1998-07-31 | 2004-03-30 | Biovex Limited | Herpes viruses for immune modulation |
| GB9816856D0 (en) | 1998-08-03 | 1998-09-30 | Univ London | Cell lines for virus growth |
| JP2003535565A (ja) | 1998-12-31 | 2003-12-02 | アーチ ディベロプメント コーポレイション | 新生物疾患の治療に有用な組換え単純ヘルペスウイルス |
| US6428968B1 (en) * | 1999-03-15 | 2002-08-06 | The Trustees Of The University Of Pennsylvania | Combined therapy with a chemotherapeutic agent and an oncolytic virus for killing tumor cells in a subject |
| GB9930418D0 (en) | 1999-12-22 | 2000-02-16 | Neurovex Ltd | Replication incompetent herpes virus vectors |
| GB9930419D0 (en) * | 1999-12-22 | 2000-02-16 | Neurovex Ltd | Replication incompetent herpes virus vectors |
| GB0001476D0 (en) | 2000-01-21 | 2000-03-15 | Neurovex Ltd | Herpes virus strains |
| US7063851B2 (en) * | 2000-04-12 | 2006-06-20 | Biovex Limited | Herpes viruses for immune modulation |
| FI113931B (fi) * | 2002-05-23 | 2004-06-30 | Oplayo Oy | Menetelmä ja järjestelmä HCVQ-vektorikirjaston muodostamiseksi |
| GB0317511D0 (en) | 2003-07-25 | 2003-08-27 | Biovex Ltd | Viral vectors |
-
2003
- 2003-07-25 GB GBGB0317511.4A patent/GB0317511D0/en not_active Ceased
-
2004
- 2004-07-26 ZA ZA200600806A patent/ZA200600806B/xx unknown
- 2004-07-26 CA CA2533338A patent/CA2533338C/en not_active Expired - Lifetime
- 2004-07-26 KR KR1020127000220A patent/KR20120006582A/ko not_active Application Discontinuation
- 2004-07-26 CN CN2004800215652A patent/CN1829523B/zh not_active Expired - Lifetime
- 2004-07-26 PL PL04743547T patent/PL1648481T3/pl unknown
- 2004-07-26 AU AU2004261037A patent/AU2004261037B2/en not_active Expired
- 2004-07-26 JP JP2006520905A patent/JP4767168B2/ja not_active Expired - Lifetime
- 2004-07-26 MX MXPA06000876A patent/MXPA06000876A/es active IP Right Grant
- 2004-07-26 BR BRPI0412845-1A patent/BRPI0412845A/pt not_active Application Discontinuation
- 2004-07-26 EP EP04743547A patent/EP1648481B1/en not_active Expired - Lifetime
- 2004-07-26 WO PCT/GB2004/003217 patent/WO2005011715A1/en active Search and Examination
- 2004-07-26 DE DE602004022823T patent/DE602004022823D1/de not_active Expired - Lifetime
- 2004-07-26 AT AT04743547T patent/ATE440611T1/de not_active IP Right Cessation
- 2004-07-26 GB GB0416628A patent/GB2405406B/en not_active Expired - Lifetime
- 2004-07-26 ES ES04743547T patent/ES2331075T3/es not_active Expired - Lifetime
- 2004-07-26 KR KR1020067001697A patent/KR101177659B1/ko active IP Right Grant
-
2006
- 2006-01-23 IL IL173295A patent/IL173295A0/en active IP Right Grant
- 2006-01-25 US US11/339,804 patent/US7981669B2/en not_active Expired - Lifetime
- 2006-05-15 HK HK06105565.8A patent/HK1083595A1/xx not_active IP Right Cessation
-
2010
- 2010-02-19 US US12/708,692 patent/US8679830B2/en active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003501445A (ja) * | 1999-06-08 | 2003-01-14 | ユーエイビー リサーチ ファンデーション | 外来遺伝子を発現するヘルペス単純ウイルスおよびそれによる癌の治療方法 |
| JP2003520044A (ja) * | 2000-01-21 | 2003-07-02 | バイオヴェックス リミテッド | ヘルペスウイルス株 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN1829523B (zh) | 2010-05-12 |
| AU2004261037B2 (en) | 2009-09-24 |
| EP1648481B1 (en) | 2009-08-26 |
| KR20060039013A (ko) | 2006-05-04 |
| CA2533338A1 (en) | 2005-02-10 |
| CN1829523A (zh) | 2006-09-06 |
| JP2006528484A (ja) | 2006-12-21 |
| GB2405406B (en) | 2008-01-02 |
| AU2004261037A1 (en) | 2005-02-10 |
| KR101177659B1 (ko) | 2012-08-27 |
| BRPI0412845A (pt) | 2006-09-26 |
| US20060188480A1 (en) | 2006-08-24 |
| US7981669B2 (en) | 2011-07-19 |
| PL1648481T3 (pl) | 2010-01-29 |
| WO2005011715A1 (en) | 2005-02-10 |
| KR20120006582A (ko) | 2012-01-18 |
| GB2405406A (en) | 2005-03-02 |
| DE602004022823D1 (de) | 2009-10-08 |
| CA2533338C (en) | 2012-05-08 |
| GB0416628D0 (en) | 2004-08-25 |
| US20100143309A1 (en) | 2010-06-10 |
| ES2331075T3 (es) | 2009-12-21 |
| ATE440611T1 (de) | 2009-09-15 |
| MXPA06000876A (es) | 2006-08-23 |
| GB0317511D0 (en) | 2003-08-27 |
| EP1648481A1 (en) | 2006-04-26 |
| US8679830B2 (en) | 2014-03-25 |
| ZA200600806B (en) | 2007-05-30 |
| IL173295A0 (en) | 2006-06-11 |
| HK1083595A1 (en) | 2006-07-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4767168B2 (ja) | ウイルスベクター | |
| JP6644378B2 (ja) | ウイルス株 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070712 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20070712 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100525 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100824 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20110607 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20110614 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4767168 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140624 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| EXPY | Cancellation because of completion of term |