JP4375951B2 - ガイドワイヤ - Google Patents
ガイドワイヤ Download PDFInfo
- Publication number
- JP4375951B2 JP4375951B2 JP2002232166A JP2002232166A JP4375951B2 JP 4375951 B2 JP4375951 B2 JP 4375951B2 JP 2002232166 A JP2002232166 A JP 2002232166A JP 2002232166 A JP2002232166 A JP 2002232166A JP 4375951 B2 JP4375951 B2 JP 4375951B2
- Authority
- JP
- Japan
- Prior art keywords
- wire
- outer diameter
- guide wire
- diameter gradually
- guide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 229910045601 alloy Inorganic materials 0.000 claims description 49
- 239000000956 alloy Substances 0.000 claims description 49
- 239000000463 material Substances 0.000 claims description 45
- 230000003247 decreasing effect Effects 0.000 claims description 41
- 230000007423 decrease Effects 0.000 claims description 21
- 238000003466 welding Methods 0.000 claims description 19
- 239000000470 constituent Substances 0.000 claims description 15
- 239000010935 stainless steel Substances 0.000 claims description 13
- 229910001220 stainless steel Inorganic materials 0.000 claims description 11
- 229910000531 Co alloy Inorganic materials 0.000 claims description 9
- PXHVJJICTQNCMI-UHFFFAOYSA-N nickel Substances [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 25
- 238000005452 bending Methods 0.000 description 14
- 238000000034 method Methods 0.000 description 13
- 210000004204 blood vessel Anatomy 0.000 description 10
- 210000004351 coronary vessel Anatomy 0.000 description 10
- 229910052759 nickel Inorganic materials 0.000 description 10
- KHYBPSFKEHXSLX-UHFFFAOYSA-N iminotitanium Chemical compound [Ti]=N KHYBPSFKEHXSLX-UHFFFAOYSA-N 0.000 description 8
- 229910001000 nickel titanium Inorganic materials 0.000 description 8
- 206010057469 Vascular stenosis Diseases 0.000 description 6
- 230000005540 biological transmission Effects 0.000 description 5
- 239000011248 coating agent Substances 0.000 description 5
- 238000000576 coating method Methods 0.000 description 5
- 239000011162 core material Substances 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 239000007769 metal material Substances 0.000 description 5
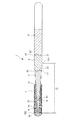
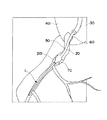
- 238000010586 diagram Methods 0.000 description 4
- 238000005304 joining Methods 0.000 description 4
- 210000002376 aorta thoracic Anatomy 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 230000002093 peripheral effect Effects 0.000 description 3
- -1 polyethylene Polymers 0.000 description 3
- 239000002861 polymer material Substances 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 229910001030 Iron–nickel alloy Inorganic materials 0.000 description 2
- 229910018054 Ni-Cu Inorganic materials 0.000 description 2
- 229910018487 Ni—Cr Inorganic materials 0.000 description 2
- 229910018481 Ni—Cu Inorganic materials 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 2
- 229910000510 noble metal Inorganic materials 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229920002401 polyacrylamide Polymers 0.000 description 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 description 2
- 229910000679 solder Inorganic materials 0.000 description 2
- 239000006104 solid solution Substances 0.000 description 2
- 229910001256 stainless steel alloy Inorganic materials 0.000 description 2
- PKAUISBSZACZJI-UHFFFAOYSA-N CC(=CC(=O)N)C.C(C(=C)C)(=O)O Chemical compound CC(=CC(=O)N)C.C(C(=C)C)(=O)O PKAUISBSZACZJI-UHFFFAOYSA-N 0.000 description 1
- 229910017518 Cu Zn Inorganic materials 0.000 description 1
- 229910017752 Cu-Zn Inorganic materials 0.000 description 1
- 229910017943 Cu—Zn Inorganic materials 0.000 description 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- 229910003310 Ni-Al Inorganic materials 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 208000031481 Pathologic Constriction Diseases 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- 238000002583 angiography Methods 0.000 description 1
- 229910052790 beryllium Inorganic materials 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 238000005219 brazing Methods 0.000 description 1
- 229920003174 cellulose-based polymer Polymers 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- TVZPLCNGKSPOJA-UHFFFAOYSA-N copper zinc Chemical compound [Cu].[Zn] TVZPLCNGKSPOJA-UHFFFAOYSA-N 0.000 description 1
- 238000007887 coronary angioplasty Methods 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 229920000840 ethylene tetrafluoroethylene copolymer Polymers 0.000 description 1
- 210000001105 femoral artery Anatomy 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 238000002594 fluoroscopy Methods 0.000 description 1
- 229910052733 gallium Inorganic materials 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 229920002050 silicone resin Polymers 0.000 description 1
- 230000036262 stenosis Effects 0.000 description 1
- 208000037804 stenosis Diseases 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 229920003002 synthetic resin Polymers 0.000 description 1
- 239000000057 synthetic resin Substances 0.000 description 1
- 229910052718 tin Inorganic materials 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 238000004804 winding Methods 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Images
Landscapes
- Media Introduction/Drainage Providing Device (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002232166A JP4375951B2 (ja) | 2002-08-08 | 2002-08-08 | ガイドワイヤ |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002232166A JP4375951B2 (ja) | 2002-08-08 | 2002-08-08 | ガイドワイヤ |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2004065797A JP2004065797A (ja) | 2004-03-04 |
| JP2004065797A5 JP2004065797A5 (enExample) | 2005-10-27 |
| JP4375951B2 true JP4375951B2 (ja) | 2009-12-02 |
Family
ID=32017664
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002232166A Expired - Lifetime JP4375951B2 (ja) | 2002-08-08 | 2002-08-08 | ガイドワイヤ |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP4375951B2 (enExample) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060047223A1 (en) * | 2004-08-31 | 2006-03-02 | Ryan Grandfield | Apparatus and method for joining stainless steel guide wire portion to nitinol portion, without a hypotube |
| CN101984920B (zh) * | 2004-04-05 | 2012-09-26 | Hi-Lex株式会社 | 骨髓采集用的医疗器具 |
| US7998090B2 (en) | 2004-08-31 | 2011-08-16 | Abbott Cardiovascular Systems Inc. | Guide wire with core having welded wire segments |
| JP5019868B2 (ja) * | 2006-12-26 | 2012-09-05 | テルモ株式会社 | ガイドワイヤ |
| US9061088B2 (en) | 2012-02-02 | 2015-06-23 | Abbott Cardiovascular Systems, Inc. | Guide wire core wire made from a substantially titanium-free alloy for enhanced guide wire steering response |
| US9636485B2 (en) | 2013-01-17 | 2017-05-02 | Abbott Cardiovascular Systems, Inc. | Methods for counteracting rebounding effects during solid state resistance welding of dissimilar materials |
| US11278701B2 (en) | 2016-10-13 | 2022-03-22 | Lake Region Manufacturing, Inc. | Apparatus including multiple joined hypotubes and method of making same |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01135363A (ja) * | 1987-11-20 | 1989-05-29 | Terumo Corp | カテーテル用ガイドワイヤー |
| JP2516444B2 (ja) * | 1990-02-06 | 1996-07-24 | テルモ株式会社 | カテ―テル用ガイドワイヤ― |
| JP2761694B2 (ja) * | 1992-02-05 | 1998-06-04 | 住友金属工業株式会社 | クラッド線材 |
| US7883474B1 (en) * | 1993-05-11 | 2011-02-08 | Target Therapeutics, Inc. | Composite braided guidewire |
| JP3309573B2 (ja) * | 1993-06-30 | 2002-07-29 | 株式会社日立製作所 | 形状記憶合金管継手及び水中管 |
| JPH0768386A (ja) * | 1993-08-31 | 1995-03-14 | Furukawa Electric Co Ltd:The | Ni−Ti系合金の接合方法 |
| EP0852955B1 (en) * | 1993-12-10 | 2001-10-17 | Schneider (Usa) Inc. | Guiding catheter |
| US5596996A (en) * | 1995-03-30 | 1997-01-28 | Medtronic, Inc. | High support nitinol tube guidewire with plastic plug transition |
| JPH1157014A (ja) * | 1997-08-11 | 1999-03-02 | Terumo Corp | ガイドワイヤ |
| JPH10309319A (ja) * | 1997-05-12 | 1998-11-24 | Tokin Corp | カテーテル用ガイドワイヤ |
| US6234981B1 (en) * | 1998-12-30 | 2001-05-22 | Advanced Cardiovascular Systems, Inc. | Vapor deposition coated intracorporeal device |
-
2002
- 2002-08-08 JP JP2002232166A patent/JP4375951B2/ja not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| JP2004065797A (ja) | 2004-03-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4203358B2 (ja) | ガイドワイヤ | |
| JP4138582B2 (ja) | ガイドワイヤ | |
| JP4138583B2 (ja) | ガイドワイヤ | |
| JP4734015B2 (ja) | ガイドワイヤの製造方法 | |
| JP2005270466A (ja) | ガイドワイヤ | |
| JP4375951B2 (ja) | ガイドワイヤ | |
| JP4376048B2 (ja) | ガイドワイヤ | |
| JP4783343B2 (ja) | ガイドワイヤ | |
| JP5073713B2 (ja) | ガイドワイヤ | |
| JP5328835B2 (ja) | ガイドワイヤの製造方法 | |
| JP4405252B2 (ja) | 医療用ワイヤ | |
| JP3962652B2 (ja) | ガイドワイヤ | |
| JP2004065796A (ja) | ガイドワイヤ | |
| JP4455808B2 (ja) | ガイドワイヤ | |
| JP4734029B2 (ja) | ガイドワイヤの製造方法 | |
| JP4447597B2 (ja) | ガイドワイヤ | |
| JP4138467B2 (ja) | ガイドワイヤ | |
| JP4116944B2 (ja) | ガイドワイヤ | |
| JP5296143B2 (ja) | ガイドワイヤ | |
| JP4376073B2 (ja) | ガイドワイヤ | |
| JP2004065794A (ja) | ガイドワイヤ | |
| JP2008110266A (ja) | ガイドワイヤ | |
| JP5019868B2 (ja) | ガイドワイヤ | |
| JP5135452B2 (ja) | ガイドワイヤ | |
| JP4783345B2 (ja) | ガイドワイヤ |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20050728 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050728 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20060920 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20061026 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20061222 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20070320 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070518 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20070524 |
|
| A912 | Re-examination (zenchi) completed and case transferred to appeal board |
Free format text: JAPANESE INTERMEDIATE CODE: A912 Effective date: 20070629 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20090908 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4375951 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120918 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130918 Year of fee payment: 4 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |