JP4346248B2 - アラゴナイト結晶系炭酸カルシウムの製造方法 - Google Patents
アラゴナイト結晶系炭酸カルシウムの製造方法 Download PDFInfo
- Publication number
- JP4346248B2 JP4346248B2 JP2001030184A JP2001030184A JP4346248B2 JP 4346248 B2 JP4346248 B2 JP 4346248B2 JP 2001030184 A JP2001030184 A JP 2001030184A JP 2001030184 A JP2001030184 A JP 2001030184A JP 4346248 B2 JP4346248 B2 JP 4346248B2
- Authority
- JP
- Japan
- Prior art keywords
- calcium carbonate
- reaction
- aragonite
- reaction tank
- concentration
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 title claims description 131
- 229910000019 calcium carbonate Inorganic materials 0.000 title claims description 45
- 238000004519 manufacturing process Methods 0.000 title claims description 24
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 claims description 99
- 238000006243 chemical reaction Methods 0.000 claims description 62
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims description 57
- 238000000034 method Methods 0.000 claims description 45
- 235000017550 sodium carbonate Nutrition 0.000 claims description 44
- 229910000029 sodium carbonate Inorganic materials 0.000 claims description 44
- 239000013078 crystal Substances 0.000 claims description 40
- ODINCKMPIJJUCX-UHFFFAOYSA-N Calcium oxide Chemical compound [Ca]=O ODINCKMPIJJUCX-UHFFFAOYSA-N 0.000 claims description 35
- 235000008733 Citrus aurantifolia Nutrition 0.000 claims description 27
- 235000011941 Tilia x europaea Nutrition 0.000 claims description 27
- 239000004571 lime Substances 0.000 claims description 27
- 239000008267 milk Substances 0.000 claims description 26
- 235000013336 milk Nutrition 0.000 claims description 26
- 210000004080 milk Anatomy 0.000 claims description 26
- 238000009993 causticizing Methods 0.000 claims description 24
- 239000000292 calcium oxide Substances 0.000 claims description 18
- 235000012255 calcium oxide Nutrition 0.000 claims description 18
- 239000007864 aqueous solution Substances 0.000 claims description 17
- 239000000920 calcium hydroxide Substances 0.000 claims description 13
- 235000011116 calcium hydroxide Nutrition 0.000 claims description 13
- 239000000945 filler Substances 0.000 claims description 12
- 239000000049 pigment Substances 0.000 claims description 12
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 claims description 11
- 229910001861 calcium hydroxide Inorganic materials 0.000 claims description 11
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 claims description 8
- 238000000576 coating method Methods 0.000 claims description 7
- 239000011248 coating agent Substances 0.000 claims description 6
- 238000010924 continuous production Methods 0.000 claims description 3
- 239000000463 material Substances 0.000 claims 1
- 235000010216 calcium carbonate Nutrition 0.000 description 38
- 239000002245 particle Substances 0.000 description 15
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 10
- 239000000047 product Substances 0.000 description 9
- 239000011734 sodium Substances 0.000 description 9
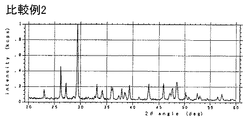
- 230000000052 comparative effect Effects 0.000 description 8
- 238000002360 preparation method Methods 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- 239000007788 liquid Substances 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 229910021532 Calcite Inorganic materials 0.000 description 5
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 5
- 239000001569 carbon dioxide Substances 0.000 description 5
- 229910002092 carbon dioxide Inorganic materials 0.000 description 5
- 239000007795 chemical reaction product Substances 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
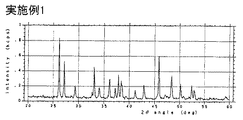
- 238000002441 X-ray diffraction Methods 0.000 description 3
- 239000003513 alkali Substances 0.000 description 3
- 238000011437 continuous method Methods 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
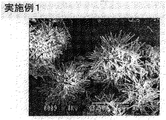
- 238000001878 scanning electron micrograph Methods 0.000 description 3
- 235000019738 Limestone Nutrition 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 239000006227 byproduct Substances 0.000 description 2
- 239000004202 carbamide Substances 0.000 description 2
- 239000000470 constituent Substances 0.000 description 2
- 230000001276 controlling effect Effects 0.000 description 2
- 238000010411 cooking Methods 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 238000007865 diluting Methods 0.000 description 2
- 238000010304 firing Methods 0.000 description 2
- 239000006028 limestone Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 239000011164 primary particle Substances 0.000 description 2
- 239000002002 slurry Substances 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 206010010071 Coma Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000012670 alkaline solution Substances 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 230000002308 calcification Effects 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- BRPQOXSCLDDYGP-UHFFFAOYSA-N calcium oxide Chemical compound [O-2].[Ca+2] BRPQOXSCLDDYGP-UHFFFAOYSA-N 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 238000012824 chemical production Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 229910052570 clay Inorganic materials 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 239000003337 fertilizer Substances 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000004898 kneading Methods 0.000 description 1
- 238000010299 mechanically pulverizing process Methods 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 229940088417 precipitated calcium carbonate Drugs 0.000 description 1
- 238000007639 printing Methods 0.000 description 1
- 230000003134 recirculating effect Effects 0.000 description 1
- 238000007670 refining Methods 0.000 description 1
- 230000001172 regenerating effect Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 239000010802 sludge Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 239000004408 titanium dioxide Substances 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Landscapes
- Compounds Of Alkaline-Earth Elements, Aluminum Or Rare-Earth Metals (AREA)
- Pigments, Carbon Blacks, Or Wood Stains (AREA)
- Paper (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2001030184A JP4346248B2 (ja) | 2001-02-06 | 2001-02-06 | アラゴナイト結晶系炭酸カルシウムの製造方法 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2001030184A JP4346248B2 (ja) | 2001-02-06 | 2001-02-06 | アラゴナイト結晶系炭酸カルシウムの製造方法 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2002234725A JP2002234725A (ja) | 2002-08-23 |
| JP2002234725A5 JP2002234725A5 (enExample) | 2006-06-15 |
| JP4346248B2 true JP4346248B2 (ja) | 2009-10-21 |
Family
ID=18894432
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2001030184A Expired - Fee Related JP4346248B2 (ja) | 2001-02-06 | 2001-02-06 | アラゴナイト結晶系炭酸カルシウムの製造方法 |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP4346248B2 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104695270A (zh) * | 2015-02-14 | 2015-06-10 | 浙江理工大学 | 一种非木材制浆碱回收绿液低温苛化控硅方法 |
| WO2022110127A1 (zh) * | 2020-11-27 | 2022-06-02 | 广西华纳新材料科技有限公司 | 类立方纳米碳酸钙的制备方法 |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1321243C (zh) * | 2004-07-20 | 2007-06-13 | 上海东升新材料有限公司 | 一种绿液清洁苛化回收超细碳酸钙的方法 |
| JP4565074B2 (ja) * | 2004-09-03 | 2010-10-20 | 地方独立行政法人北海道立総合研究機構 | チョーク |
| JP4813075B2 (ja) * | 2005-03-30 | 2011-11-09 | 日本製紙株式会社 | アラゴナイト系針状炭酸カルシウムの製造方法 |
| CN100400719C (zh) * | 2005-11-03 | 2008-07-09 | 福建师范大学 | 一种晶须碳酸钙的制备方法 |
| JP5767556B2 (ja) * | 2011-10-18 | 2015-08-19 | 三菱製紙株式会社 | 炭酸カルシウムの製造方法 |
| CN103708522A (zh) * | 2014-01-03 | 2014-04-09 | 三三环保科技(北京)有限公司 | 利用碱回收绿液进行苛化反应生产高品质碳酸钙的方法 |
-
2001
- 2001-02-06 JP JP2001030184A patent/JP4346248B2/ja not_active Expired - Fee Related
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104695270A (zh) * | 2015-02-14 | 2015-06-10 | 浙江理工大学 | 一种非木材制浆碱回收绿液低温苛化控硅方法 |
| WO2022110127A1 (zh) * | 2020-11-27 | 2022-06-02 | 广西华纳新材料科技有限公司 | 类立方纳米碳酸钙的制备方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2002234725A (ja) | 2002-08-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6623555B1 (en) | Composite precipitated calcium carbonate/silicon compound pigment and method of making same | |
| US7097819B2 (en) | Method for producing calcium carbonate | |
| JP2010524813A (ja) | Pccの製造方法 | |
| JP4346248B2 (ja) | アラゴナイト結晶系炭酸カルシウムの製造方法 | |
| EP0953544B1 (en) | Processes for preparing calcium carbonate | |
| JP3874978B2 (ja) | 炭酸カルシウム及び水酸化ナトリウムの製造方法 | |
| JP2010077009A (ja) | 炭酸カルシウムの製造方法 | |
| JP4375842B2 (ja) | 炭酸カルシウムの製造方法、及び石灰石からの沈降製炭酸カルシウムの白化方法 | |
| US6123855A (en) | Dewatering of calcium carbonate | |
| JP4339528B2 (ja) | 炭酸カルシウムの製造方法 | |
| JP3902718B2 (ja) | アラゴナイト結晶系炭酸カルシウムの製造方法 | |
| JP4191943B2 (ja) | 炭酸カルシウムウィスカーの製造方法 | |
| JP3872611B2 (ja) | 炭酸カルシウムの製造方法 | |
| JP4236818B2 (ja) | 炭酸カルシウムウィスカーの製造方法 | |
| JP4273561B2 (ja) | アラゴナイト系針状炭酸カルシウムの製造方法 | |
| JP4761791B2 (ja) | ウィスカー状炭酸カルシウムの製造方法 | |
| JP2002234725A5 (enExample) | ||
| JPH10226517A (ja) | 炭酸カルシウムの製造方法 | |
| JPH0433730B2 (enExample) | ||
| JP3872610B2 (ja) | 炭酸カルシウムの製造方法 | |
| JPH10292283A (ja) | 炭酸カルシウムの製造方法 | |
| JP3874958B2 (ja) | 炭酸カルシウムの製造方法 | |
| JP4813075B2 (ja) | アラゴナイト系針状炭酸カルシウムの製造方法 | |
| CN120019030A (zh) | 生产沉淀碳酸钙(pcc)的方法、pcc产品和pcc的用途 | |
| JP4663816B2 (ja) | ホタテ貝殻焼成物を用いる苛性化炭酸カルシウムの製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20060428 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20060428 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20081216 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090403 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090601 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20090615 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20090714 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120724 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120724 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150724 Year of fee payment: 6 |
|
| LAPS | Cancellation because of no payment of annual fees |