JP4332353B2 - 15−ケト−プロスタグランジン類を含む薬物誘発性便秘処置用組成物 - Google Patents
15−ケト−プロスタグランジン類を含む薬物誘発性便秘処置用組成物 Download PDFInfo
- Publication number
- JP4332353B2 JP4332353B2 JP2002586947A JP2002586947A JP4332353B2 JP 4332353 B2 JP4332353 B2 JP 4332353B2 JP 2002586947 A JP2002586947 A JP 2002586947A JP 2002586947 A JP2002586947 A JP 2002586947A JP 4332353 B2 JP4332353 B2 JP 4332353B2
- Authority
- JP
- Japan
- Prior art keywords
- group
- keto
- compound
- constipation
- drug
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- PQZMNQPMCZVXDZ-UTCJRWHESA-N C(C1CC1)/C=C\C1CCCC1 Chemical compound C(C1CC1)/C=C\C1CCCC1 PQZMNQPMCZVXDZ-UTCJRWHESA-N 0.000 description 1
- CBTWSONLCCYHGW-UHFFFAOYSA-N CC(CCC1)C1C1=CC1 Chemical compound CC(CCC1)C1C1=CC1 CBTWSONLCCYHGW-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/557—Eicosanoids, e.g. leukotrienes or prostaglandins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/485—Morphinan derivatives, e.g. morphine, codeine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/557—Eicosanoids, e.g. leukotrienes or prostaglandins
- A61K31/5575—Eicosanoids, e.g. leukotrienes or prostaglandins having a cyclopentane, e.g. prostaglandin E2, prostaglandin F2-alpha
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/10—Laxatives
Landscapes
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Emergency Medicine (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Description
下付1:13,14−不飽和−15−OH
下付2:5,6−および13,14−ジ不飽和−15−OH
下付3:5,6−、13,14−および17,18−トリ不飽和−15−OH。
Aは、−CH2OH、−COCH2OH、−COOHまたはそれらの官能性誘導体;
Bは、−CH2−CH2−、−CH=CH−または−C≡C−;
R1は、非置換またはハロゲン、アルキル、ヒドロキシ、オキソ、アリールまたは複素環で置換された、二価の飽和または不飽和の低〜中級の脂肪族炭化水素残基であり、脂肪族炭化水素における少なくとも1個の炭素原子は任意に酸素、窒素または硫黄で置換されていてもよい;
Raは、非置換またはハロゲン、オキソ、ヒドロキシ、低級アルコキシ、低級アルカノイルオキシ、シクロ(低級)アルキル、シクロ(低級)アルキルオキシ、アリール、アリールオキシ、複素環または複素環オキシで置換された、飽和または不飽和の低〜中級脂肪族炭化水素残基;シクロ(低級)アルキル基;シクロ(低級)アルキルオキシ基;アリール基;アリールオキシ基;複素環基;複素環オキシ基]。
Aは、−CH2OH、−COCH2OH、−COOHまたはそれらの官能性誘導体;
Bは、−CH2−CH2−、−CH=CH−または−C≡C−;
X1およびX2は、水素、低級アルキルまたはハロゲン;
R1は、非置換またはハロゲン、アルキル、ヒドロキシ、オキソ、アリールまたは複素環で置換された、二価の飽和または不飽和の低〜中級の脂肪族炭化水素残基であり、脂肪族炭化水素における少なくとも1個の炭素原子は任意に酸素、窒素または硫黄で置換されていてもよい;
R2は、単結合または低級アルキレン;そして、
R3は、低級アルキル、低級アルコキシ、シクロ(低級)アルキル、シクロ(低級)アルキルオキシ、アリール、アリールオキシ、複素環または複素環オキシ]。
−CH2−CH2−CH2−CH2−CH2−CH2−、
−CH2−CH=CH−CH2−CH2−CH2−、
−CH2−CH2−CH2−CH2−CH=CH−、
−CH2−C≡C−CH2−CH2−CH2−、
−CH2−CH2−CH2−CH2−CH(CH3)−CH2−、
−CH2−CH2−CH2−CH2−O−CH2−、
−CH2−CH=CH−CH2−O−CH2−、
−CH2−C≡C−CH2−O−CH2−、
−CH2−CH2−CH2−CH2−CH2−CH2−CH2−、
−CH2−CH=CH−CH2−CH2−CH2−CH2−、
−CH2−CH2−CH2−CH2−CH2−CH=CH−、
−CH2−C≡C−CH2−CH2−CH2−CH2−、
−CH2−CH2−CH2−CH2−CH2−CH(CH3)−CH2−、
−CH2−CH2−CH2−CH2−CH2−CH2−CH2−CH2−、
−CH2−CH=CH−CH2−CH2−CH2−CH2−CH2−、
−CH2−CH2−CH2−CH2−CH2−CH2−CH=CH−、
−CH2−C≡C−CH2−CH2−CH2−CH2−CH2−、
−CH2−CH2−CH2−CH2−CH2−CH2−CH(CH3)−CH2−、など。
X1’およびX2’は水素、低級アルキル、またはハロゲン;
Yは
R1は非置換またはハロゲン、アルキル、ヒドロキシ、オキソ、アリールまたは複素環で置換された、二価の飽和または不飽和の低〜中級の脂肪族炭化水素残基であり、脂肪族炭化水素における少なくとも1個の炭素原子は任意に酸素、窒素または硫黄で置換されていてもよい;
Ra’は、非置換またはハロゲン、オキソ、ヒドロキシ、低級アルコキシ、低級アルカノイルオキシ、シクロ(低級)アルキル、シクロ(低級)アルキルオキシ、アリール、アリールオキシ、複素環または複素環オキシで置換された、飽和または不飽和の低〜中級脂肪族炭化水素残基;シクロ(低級)アルキル基;シクロ(低級)アルキルオキシ基;アリール基;アリールオキシ基;複素環基;複素環オキシ基;
R3’は水素、低級アルキル、シクロ(低級)アルキル、アリールまたは複素環基]。
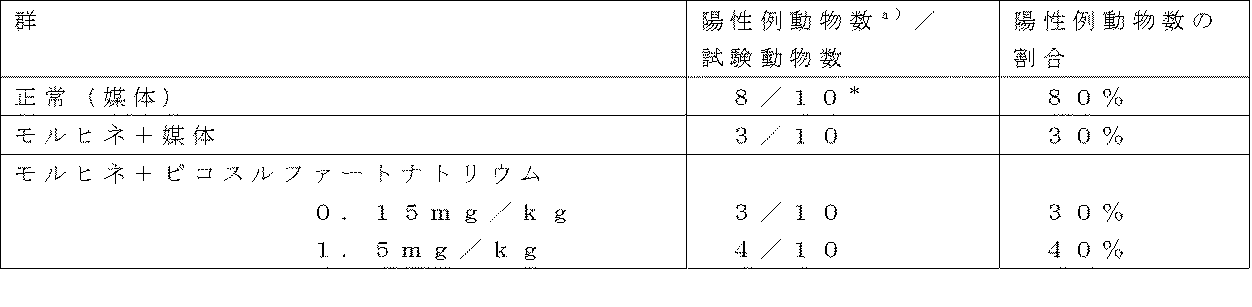
糞食を避けるためワイヤーグリッド底面を持つ飼育ケージ内で一夜絶食したICR系雄性マウスを1群につき15匹用いた。動物に塩酸モルヒネ(武田薬品工業株式会社、大阪、日本)5mg/kgを腹腔内投与した後、直ちに黒鉛マーカー(Pilot INK-30-Bと10%tragacanth mucilageの2:1混合液)0.1mLと、媒体(0.01%ポリソルベート80と0.5%エタノール含有生理食塩水)5mL/kgあるいは被験物質(13,14−ジヒドロ−15−ケト−16,16−ジフルオロ−PGE1)を媒体5mL/kg中、1、10及び100μg/kgの投与用量で経口投与した。正常対照群には、塩酸モルヒネを投与せず、黒鉛マーカーと媒体のみを同用量経口投与した。黒鉛マーカー投与150分後に動物を頚椎脱臼後、開腹し、盲腸内の黒鉛マーカーの存在を調べた。盲腸内に黒鉛マーカーが存在する場合を陽性(陽性例)とした。
モルヒネ誘発性便秘に対する拮抗作用
モルヒネ投与を受けている患者の便秘の治療に臨床で用いられている下剤(センノシド及びピコスルファートナトリウム)のモルヒネ誘発便秘に対する効果を確認した。
糞食を避けるためワイヤーグリッド底面を持つ飼育ケージ内で一夜絶食したICR系雄性マウスの尾をクレンメで挟み、攻撃、噛み付きあるいは鳴き声をあげるまでの反応時間を測定した。反応時間が2秒以内を示す動物18匹を被験動物として用いた。動物に塩酸モルヒネ(武田薬品工業株式会社、大阪、日本)5mg/kgを腹腔内投与後、直ぐに媒体(0.01%ポリソルベート80と0.5%エタノール含有生理食塩水)あるいは媒体に溶解した被験物質(13,14−ジヒドロ−15−ケト−16,16−ジフルオロ−PGE1)1、10または100μg/kgを5mL/kgの投与用量で経口投与した。正常対照群には、塩酸モルヒネを投与せず、媒体のみを同用量経口投与した。
糞食を避けるためワイヤーグリッド底面を持つ飼育ケージ内で一夜絶食したICR系雄性マウスを1群につき10匹用いた。塩酸イミプラミン(和光純薬株式会社、大阪、日本)60mg/kgを腹腔内投与後、直ぐに0.1mLの炭素マーカー(5%アラビアガム中10%炭素粉懸濁液)および媒体(0.01%ポリソルベート80と0.5%エタノール含有生理食塩水)あるいは被験物質(13,14−ジヒドロ−15−ケト−16,16−ジフルオロ−PGE1)を5mL/kgの投与用量で経口投与した。正常対照群には、イミプラミンを投与せず、炭素マーカーおよび媒体のみを同用量経口投与した。炭素マーカー投与の150分後に、動物を頚椎脱臼後、開腹し、盲腸内の炭素マーカーの存在を調べた。盲腸内に炭素マーカーが存在する場合を陽性(陽性例)とした。
イミプラミン(三環系抗うつ剤)誘発性便秘に対する拮抗作用
便秘の処置用に患者に臨床的に用いられている下剤(センノシド)の、イミプラミン誘発性便秘に対する効果を調べた。センノシドの調製と用量レベルは実施例2に記載と同じとした。実験方法は実施例4に記載の方法と同じとした。
Claims (8)
- 一般式(II):
Aは、−COOHまたはその塩、エステルもしくはアミド;
Bは、−CH2−CH2−;
X1およびX2は、水素、低級アルキルまたはハロゲンであって、X1およびX2の少なくとも一方はハロゲンである;
R1は、非置換の二価の飽和または不飽和の炭素数1〜14の直鎖または分枝鎖を有する脂肪族炭化水素(ただし、側鎖は炭素数1〜3のものである);
R2は、単結合または低級アルキレン;そして、
R3は、低級アルキル]
で示される13,14−ジヒドロ−15−ケト−16−モノ―またはジ―ハロゲン−プロスタグランジンE化合物を有効成分として含む、オピオイド化合物または抗コリン作用薬による薬物誘発性便秘処置用組成物。 - 13,14−ジヒドロ−15−ケト−16−モノ―またはジ―ハロゲン−プロスタグランジンE化合物が13,14−ジヒドロ−15−ケト−16−モノ−またはジ−フルオロ−プロスタグランジンE化合物である、請求項1記載の組成物。
- 13,14−ジヒドロ−15−ケト−16−モノ―またはジ―ハロゲン−プロスタグランジンE化合物が13,14−ジヒドロ−15−ケト−16,16−ジ−フルオロ−プロスタグランジンE化合物である、請求項1記載の組成物。
- 13,14−ジヒドロ−15−ケト−16−モノ―またはジ―ハロゲン−プロスタグランジンE化合物が13,14−ジヒドロ−15−ケト−16,16−ジフルオロ−プロスタグランジンE1である請求項1記載の組成物。
- 便秘を誘発する薬物がオピオイド化合物である請求項1〜4のいずれかに記載の組成物。
- オピオイド化合物がモルヒネ化合物あるいはコデイン化合物である請求項5記載の組成物。
- 便秘を誘発する薬物が抗コリン作用薬である、請求項1〜4のいずれかに記載の組成物。
- 抗コリン作用薬が三環系抗うつ剤である請求項7記載の組成物。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US28772001P | 2001-05-02 | 2001-05-02 | |
| PCT/JP2002/004223 WO2002089812A1 (en) | 2001-05-02 | 2002-04-26 | Composition for treating drug-induced constipation with 15-keto-prostaglandins |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2004527567A JP2004527567A (ja) | 2004-09-09 |
| JP2004527567A5 JP2004527567A5 (ja) | 2005-11-17 |
| JP4332353B2 true JP4332353B2 (ja) | 2009-09-16 |
Family
ID=23104042
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002586947A Expired - Fee Related JP4332353B2 (ja) | 2001-05-02 | 2002-04-26 | 15−ケト−プロスタグランジン類を含む薬物誘発性便秘処置用組成物 |
Country Status (19)
| Country | Link |
|---|---|
| US (1) | US6982283B2 (ja) |
| EP (1) | EP1392318B1 (ja) |
| JP (1) | JP4332353B2 (ja) |
| KR (1) | KR100886598B1 (ja) |
| CN (1) | CN1522147B (ja) |
| AR (2) | AR035237A1 (ja) |
| AT (1) | ATE355067T1 (ja) |
| AU (1) | AU2002251554B2 (ja) |
| BR (1) | BR0209327A (ja) |
| CA (1) | CA2444103C (ja) |
| DE (1) | DE60218451T2 (ja) |
| DK (1) | DK1392318T3 (ja) |
| ES (1) | ES2282408T3 (ja) |
| MX (1) | MXPA03010019A (ja) |
| NO (1) | NO335143B1 (ja) |
| NZ (1) | NZ529187A (ja) |
| PT (1) | PT1392318E (ja) |
| TW (1) | TWI302100B (ja) |
| WO (1) | WO2002089812A1 (ja) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006513232A (ja) * | 2002-12-27 | 2006-04-20 | スキャンポ・アーゲー | 腹部不快感の処置のためのプロスタグランジン誘導体 |
Families Citing this family (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2458471C (en) | 2001-08-31 | 2012-07-31 | Sucampo Ag | Prostaglandin analogs as chloride channel opener |
| TWI331920B (en) | 2001-11-14 | 2010-10-21 | Sucampo Ag | Unit dosage form for relieving or treating constipation in human patients |
| TWI263505B (en) | 2001-11-19 | 2006-10-11 | Sucampo Ag | Pharmaceutical composition comprising a C1C-2 channel opener |
| US8114911B2 (en) * | 2002-10-23 | 2012-02-14 | Sucampo Ag | Prostaglandin compounds for the treatment of obesity |
| TWI387454B (zh) * | 2004-09-02 | 2013-03-01 | 蘇坎波公司 | 治療胃腸道疾病之方法及組成物 |
| US8524731B2 (en) | 2005-03-07 | 2013-09-03 | The University Of Chicago | Use of opioid antagonists to attenuate endothelial cell proliferation and migration |
| CN101171010B (zh) * | 2005-03-07 | 2014-09-17 | 芝加哥大学 | 阿片样物质拮抗剂用于减少内皮细胞增殖和迁移的用途 |
| US8518962B2 (en) | 2005-03-07 | 2013-08-27 | The University Of Chicago | Use of opioid antagonists |
| US9662325B2 (en) | 2005-03-07 | 2017-05-30 | The University Of Chicago | Use of opioid antagonists to attenuate endothelial cell proliferation and migration |
| KR20140069182A (ko) * | 2005-04-12 | 2014-06-09 | 수캄포 아게 | 위장 장애 치료를 위한 프로스타글란딘 화합물과 양성자 펌프 억제제의 병용 |
| US20090030072A1 (en) * | 2007-07-03 | 2009-01-29 | Sucampo Ag | Pharmaceutical combination of opioid and prostaglandin compound |
| US20090082442A1 (en) * | 2007-09-26 | 2009-03-26 | Protia, Llc | Deuterium-enriched lubiprostone |
| US9044510B2 (en) * | 2007-11-01 | 2015-06-02 | Washington University | Compositions and methods for treating pruritus |
| US9084815B2 (en) * | 2009-09-16 | 2015-07-21 | Sucampo Ag | Method for treating damage induced by an anti-tumor agent, treating mucositis and treating tumor |
| US8957024B2 (en) | 2011-07-27 | 2015-02-17 | Washington University | Composition and methods for reducing opioid-induced pruritus |
| KR20140052389A (ko) | 2012-10-24 | 2014-05-07 | 주식회사 아리바이오 | 해양 심층수 또는 염지하수로부터 제조된 고경도의 미네랄 워터를 포함하는 변비 예방, 개선 또는 치료용 조성물 |
| US20140116916A1 (en) | 2012-10-31 | 2014-05-01 | 2294719 Ontario Limited | Therapy for Constipation |
| WO2014159679A1 (en) | 2013-03-12 | 2014-10-02 | The United States Of America, As Represented By The Secretary, Department Of Health & Human Services | Methods for using lubiprostone to absorb fluid from the subretinal space |
| CN105777601A (zh) * | 2014-12-26 | 2016-07-20 | 中国人民解放军第二军医大学 | 一种前列地尔衍生物及其药物制剂 |
| CN109475544A (zh) * | 2016-03-29 | 2019-03-15 | 科罗纳里康赛普茨有限责任公司 | 用于治疗便秘的制剂 |
| JP6957610B2 (ja) * | 2016-10-06 | 2021-11-02 | スキャンポ・アーゲーSucampo AG | 医薬品用途のための多層ビーズ |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1581886A (en) * | 1977-05-26 | 1980-12-31 | May & Baker Ltd | Prostanol derivatives |
| US5166174A (en) | 1987-01-28 | 1992-11-24 | K.K. Ueno Seiyaku Oyo Kenkyujo | Prostaglandins E and anti-ulcers containing same |
| CA1322749C (en) | 1987-01-28 | 1993-10-05 | Ryuzo Ueno | Prostaglandins of the d series, and tranquilizers and soporifics containing the same |
| US5221763A (en) | 1987-04-30 | 1993-06-22 | R-Tech Ueno, Ltd. | Prostaglandins of the F series |
| ES2051862T3 (es) * | 1987-10-02 | 1994-07-01 | Ueno Seiyaku Oyo Kenkyujo Kk | Un metodo para producir un medicamento que tiene un efecto catartico. |
| US5317032A (en) | 1987-10-02 | 1994-05-31 | Kabushiki Kaisha Ueno Seiyaku Oyo Kenkyujo | Prostaglandin cathartic |
| CA2027814C (en) * | 1989-10-20 | 1996-07-30 | Ryuji Ueno | Treatment of hepatobiliary disease with 15-keto-prostaglandin compounds |
| TW249226B (ja) | 1990-04-04 | 1995-06-11 | Aderk Ueno Kk | |
| CA2041417C (en) * | 1990-05-01 | 2002-05-21 | Ryuji Ueno | Treatment of pancreatic disease with 15-keto-prostaglandin compounds |
| CA2150287C (en) | 1994-06-03 | 2004-08-10 | Ryuji Ueno | Agent for treating hepato-biliary diseases |
| WO1997047595A1 (en) | 1996-06-10 | 1997-12-18 | R-Tech Ueno, Ltd. | Endothelin antagonist |
| US6414016B1 (en) * | 2000-09-05 | 2002-07-02 | Sucampo, A.G. | Anti-constipation composition |
| ATE387204T1 (de) * | 2001-05-18 | 2008-03-15 | Sucampo Ag | Zusammensetzung mit induzierendem kathartischen effekt |
-
2002
- 2002-04-25 TW TW091108513A patent/TWI302100B/zh not_active IP Right Cessation
- 2002-04-26 ES ES02720623T patent/ES2282408T3/es not_active Expired - Lifetime
- 2002-04-26 NZ NZ529187A patent/NZ529187A/en not_active IP Right Cessation
- 2002-04-26 AT AT02720623T patent/ATE355067T1/de active
- 2002-04-26 CA CA2444103A patent/CA2444103C/en not_active Expired - Lifetime
- 2002-04-26 EP EP02720623A patent/EP1392318B1/en not_active Expired - Lifetime
- 2002-04-26 CN CN028133897A patent/CN1522147B/zh not_active Expired - Lifetime
- 2002-04-26 PT PT02720623T patent/PT1392318E/pt unknown
- 2002-04-26 DE DE60218451T patent/DE60218451T2/de not_active Expired - Lifetime
- 2002-04-26 AU AU2002251554A patent/AU2002251554B2/en not_active Expired
- 2002-04-26 WO PCT/JP2002/004223 patent/WO2002089812A1/en not_active Ceased
- 2002-04-26 MX MXPA03010019A patent/MXPA03010019A/es active IP Right Grant
- 2002-04-26 DK DK02720623T patent/DK1392318T3/da active
- 2002-04-26 JP JP2002586947A patent/JP4332353B2/ja not_active Expired - Fee Related
- 2002-04-26 BR BR0209327-8A patent/BR0209327A/pt not_active Application Discontinuation
- 2002-04-26 KR KR1020037014221A patent/KR100886598B1/ko not_active Expired - Lifetime
- 2002-04-29 AR ARP020101560A patent/AR035237A1/es not_active Application Discontinuation
- 2002-05-01 US US10/135,397 patent/US6982283B2/en not_active Expired - Lifetime
-
2003
- 2003-10-31 NO NO20034864A patent/NO335143B1/no not_active IP Right Cessation
-
2015
- 2015-06-01 AR ARP150101727A patent/AR100696A2/es not_active Application Discontinuation
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006513232A (ja) * | 2002-12-27 | 2006-04-20 | スキャンポ・アーゲー | 腹部不快感の処置のためのプロスタグランジン誘導体 |
| JP2012031202A (ja) * | 2002-12-27 | 2012-02-16 | Sucampo Ag | 腹部不快感の処置のためのプロスタグランジン誘導体 |
| JP4889219B2 (ja) * | 2002-12-27 | 2012-03-07 | スキャンポ・アーゲー | 腹部不快感の処置のためのプロスタグランジン誘導体 |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2444103C (en) | 2010-06-08 |
| US20030073746A1 (en) | 2003-04-17 |
| NO20034864D0 (no) | 2003-10-31 |
| NO335143B1 (no) | 2014-09-29 |
| DE60218451T2 (de) | 2007-11-08 |
| US6982283B2 (en) | 2006-01-03 |
| BR0209327A (pt) | 2004-07-20 |
| DE60218451D1 (de) | 2007-04-12 |
| AR035237A1 (es) | 2004-05-05 |
| CN1522147A (zh) | 2004-08-18 |
| AR100696A2 (es) | 2016-10-26 |
| TWI302100B (en) | 2008-10-21 |
| NZ529187A (en) | 2005-10-28 |
| WO2002089812A1 (en) | 2002-11-14 |
| AU2002251554B2 (en) | 2007-05-24 |
| KR100886598B1 (ko) | 2009-03-05 |
| EP1392318A1 (en) | 2004-03-03 |
| MXPA03010019A (es) | 2004-02-12 |
| ATE355067T1 (de) | 2006-03-15 |
| NO20034864L (no) | 2003-12-23 |
| DK1392318T3 (da) | 2007-06-25 |
| CA2444103A1 (en) | 2002-11-14 |
| JP2004527567A (ja) | 2004-09-09 |
| KR20040008168A (ko) | 2004-01-28 |
| EP1392318B1 (en) | 2007-02-28 |
| ES2282408T3 (es) | 2007-10-16 |
| CN1522147B (zh) | 2010-05-12 |
| PT1392318E (pt) | 2007-05-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4332353B2 (ja) | 15−ケト−プロスタグランジン類を含む薬物誘発性便秘処置用組成物 | |
| US20100298424A1 (en) | Method for treating abdominal discomfort | |
| AU2002251554A1 (en) | Composition for Treating Drug-Induced Constipation | |
| JP5427029B2 (ja) | 消化管の重炭酸分泌を促進するための方法および組成物 | |
| JP5294559B2 (ja) | クロライドチャンネルオープナーとしてプロスタグランジンアナログを含む腸溶性組成物 | |
| JP4705782B2 (ja) | 肥満の処置のためのプロスタグランジン化合物 | |
| EP1791545A1 (en) | Prostaglandin derivatives for treating gastrointestinal disorder |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20040401 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20040401 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20071218 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080318 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20080422 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080716 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20080909 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20081208 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090203 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090501 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20090602 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20090622 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4332353 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120626 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130626 Year of fee payment: 4 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130626 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130626 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130626 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130626 Year of fee payment: 4 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130626 Year of fee payment: 4 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees | ||
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| S533 | Written request for registration of change of name |
Free format text: JAPANESE INTERMEDIATE CODE: R313533 |
|
| S201 | Request for registration of exclusive licence |
Free format text: JAPANESE INTERMEDIATE CODE: R314201 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R314531 |
|
| S533 | Written request for registration of change of name |
Free format text: JAPANESE INTERMEDIATE CODE: R314533 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |