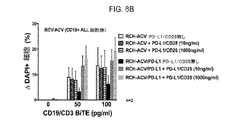
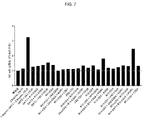
JP2019532017A - がんを治療するための異なるエピトープ結合を示す複数の二重特異性結合ドメイン構築物 - Google Patents
がんを治療するための異なるエピトープ結合を示す複数の二重特異性結合ドメイン構築物 Download PDFInfo
- Publication number
- JP2019532017A JP2019532017A JP2019501628A JP2019501628A JP2019532017A JP 2019532017 A JP2019532017 A JP 2019532017A JP 2019501628 A JP2019501628 A JP 2019501628A JP 2019501628 A JP2019501628 A JP 2019501628A JP 2019532017 A JP2019532017 A JP 2019532017A
- Authority
- JP
- Japan
- Prior art keywords
- seq
- group
- bdc
- epitopes
- cancer antigen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 230000027455 binding Effects 0.000 title claims abstract description 295
- 206010028980 Neoplasm Diseases 0.000 title claims abstract description 289
- 201000011510 cancer Diseases 0.000 title claims abstract description 258
- 230000001747 exhibiting effect Effects 0.000 title 1
- 239000000427 antigen Substances 0.000 claims abstract description 180
- 102000036639 antigens Human genes 0.000 claims abstract description 180
- 108091007433 antigens Proteins 0.000 claims abstract description 180
- 230000005931 immune cell recruitment Effects 0.000 claims abstract description 55
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 130
- 210000004027 cell Anatomy 0.000 claims description 116
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 claims description 108
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 claims description 108
- 101001103039 Homo sapiens Inactive tyrosine-protein kinase transmembrane receptor ROR1 Proteins 0.000 claims description 89
- 101001103036 Homo sapiens Nuclear receptor ROR-alpha Proteins 0.000 claims description 89
- 102100039615 Inactive tyrosine-protein kinase transmembrane receptor ROR1 Human genes 0.000 claims description 89
- 101000934338 Homo sapiens Myeloid cell surface antigen CD33 Proteins 0.000 claims description 76
- 102100025243 Myeloid cell surface antigen CD33 Human genes 0.000 claims description 76
- 108090000623 proteins and genes Proteins 0.000 claims description 65
- 238000000034 method Methods 0.000 claims description 56
- 239000000203 mixture Substances 0.000 claims description 56
- 102000004169 proteins and genes Human genes 0.000 claims description 56
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 claims description 51
- 102100024952 Protein CBFA2T1 Human genes 0.000 claims description 51
- 108010074708 B7-H1 Antigen Proteins 0.000 claims description 49
- 238000011282 treatment Methods 0.000 claims description 47
- 210000002865 immune cell Anatomy 0.000 claims description 46
- 230000008685 targeting Effects 0.000 claims description 33
- -1 MUC-CD Proteins 0.000 claims description 26
- 230000003213 activating effect Effects 0.000 claims description 26
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 claims description 24
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 claims description 24
- 101000998120 Homo sapiens Interleukin-3 receptor subunit alpha Proteins 0.000 claims description 22
- 101000851370 Homo sapiens Tumor necrosis factor receptor superfamily member 9 Proteins 0.000 claims description 22
- 102100033493 Interleukin-3 receptor subunit alpha Human genes 0.000 claims description 22
- 102100036856 Tumor necrosis factor receptor superfamily member 9 Human genes 0.000 claims description 22
- 102100034458 Hepatitis A virus cellular receptor 2 Human genes 0.000 claims description 20
- 102100040678 Programmed cell death protein 1 Human genes 0.000 claims description 20
- 101710089372 Programmed cell death protein 1 Proteins 0.000 claims description 20
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 claims description 18
- 230000001225 therapeutic effect Effects 0.000 claims description 18
- 108010021064 CTLA-4 Antigen Proteins 0.000 claims description 17
- 229940045513 CTLA4 antagonist Drugs 0.000 claims description 17
- 101000666896 Homo sapiens V-type immunoglobulin domain-containing suppressor of T-cell activation Proteins 0.000 claims description 16
- 102100038282 V-type immunoglobulin domain-containing suppressor of T-cell activation Human genes 0.000 claims description 16
- IJJVMEJXYNJXOJ-UHFFFAOYSA-N fluquinconazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1N1C(=O)C2=CC(F)=CC=C2N=C1N1C=NC=N1 IJJVMEJXYNJXOJ-UHFFFAOYSA-N 0.000 claims description 16
- 230000028993 immune response Effects 0.000 claims description 16
- 230000003252 repetitive effect Effects 0.000 claims description 16
- 210000002540 macrophage Anatomy 0.000 claims description 14
- 238000012544 monitoring process Methods 0.000 claims description 14
- 230000001629 suppression Effects 0.000 claims description 14
- 102100037589 OX-2 membrane glycoprotein Human genes 0.000 claims description 12
- 101001098352 Homo sapiens OX-2 membrane glycoprotein Proteins 0.000 claims description 11
- 239000012190 activator Substances 0.000 claims description 10
- 210000000822 natural killer cell Anatomy 0.000 claims description 10
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 claims description 8
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 claims description 8
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 claims description 8
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 claims description 8
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 claims description 8
- 101150013553 CD40 gene Proteins 0.000 claims description 7
- 102100041003 Glutamate carboxypeptidase 2 Human genes 0.000 claims description 7
- 101000892862 Homo sapiens Glutamate carboxypeptidase 2 Proteins 0.000 claims description 7
- 102000003735 Mesothelin Human genes 0.000 claims description 7
- 108090000015 Mesothelin Proteins 0.000 claims description 7
- 102100022748 Wilms tumor protein Human genes 0.000 claims description 7
- 229920001481 poly(stearyl methacrylate) Polymers 0.000 claims description 7
- 230000004936 stimulating effect Effects 0.000 claims description 6
- 101000621309 Homo sapiens Wilms tumor protein Proteins 0.000 claims description 5
- 206010062016 Immunosuppression Diseases 0.000 claims description 5
- 230000001506 immunosuppresive effect Effects 0.000 claims description 5
- 102100038080 B-cell receptor CD22 Human genes 0.000 claims description 4
- 101710083479 Hepatitis A virus cellular receptor 2 homolog Proteins 0.000 claims description 4
- 101000884305 Homo sapiens B-cell receptor CD22 Proteins 0.000 claims description 4
- 101001109503 Homo sapiens NKG2-C type II integral membrane protein Proteins 0.000 claims description 4
- 101001136592 Homo sapiens Prostate stem cell antigen Proteins 0.000 claims description 4
- 108010064548 Lymphocyte Function-Associated Antigen-1 Proteins 0.000 claims description 4
- 102100022683 NKG2-C type II integral membrane protein Human genes 0.000 claims description 4
- 102100036735 Prostate stem cell antigen Human genes 0.000 claims description 4
- 101001051488 Takifugu rubripes Neural cell adhesion molecule L1 Proteins 0.000 claims description 4
- 102000006815 folate receptor Human genes 0.000 claims description 4
- 108020005243 folate receptor Proteins 0.000 claims description 4
- 229960004641 rituximab Drugs 0.000 claims description 4
- 102100029822 B- and T-lymphocyte attenuator Human genes 0.000 claims description 3
- 102100027207 CD27 antigen Human genes 0.000 claims description 3
- 102100038078 CD276 antigen Human genes 0.000 claims description 3
- 102100035793 CD83 antigen Human genes 0.000 claims description 3
- 101000864344 Homo sapiens B- and T-lymphocyte attenuator Proteins 0.000 claims description 3
- 101000914511 Homo sapiens CD27 antigen Proteins 0.000 claims description 3
- 101000946856 Homo sapiens CD83 antigen Proteins 0.000 claims description 3
- 101001137987 Homo sapiens Lymphocyte activation gene 3 protein Proteins 0.000 claims description 3
- 101000914496 Homo sapiens T-cell antigen CD7 Proteins 0.000 claims description 3
- 101000851376 Homo sapiens Tumor necrosis factor receptor superfamily member 8 Proteins 0.000 claims description 3
- 102000017578 LAG3 Human genes 0.000 claims description 3
- 102100027208 T-cell antigen CD7 Human genes 0.000 claims description 3
- 102100025237 T-cell surface antigen CD2 Human genes 0.000 claims description 3
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 claims description 3
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 claims description 3
- 102100036857 Tumor necrosis factor receptor superfamily member 8 Human genes 0.000 claims description 3
- 229960002450 ofatumumab Drugs 0.000 claims description 3
- 230000000638 stimulation Effects 0.000 claims description 3
- BGFTWECWAICPDG-UHFFFAOYSA-N 2-[bis(4-chlorophenyl)methyl]-4-n-[3-[bis(4-chlorophenyl)methyl]-4-(dimethylamino)phenyl]-1-n,1-n-dimethylbenzene-1,4-diamine Chemical compound C1=C(C(C=2C=CC(Cl)=CC=2)C=2C=CC(Cl)=CC=2)C(N(C)C)=CC=C1NC(C=1)=CC=C(N(C)C)C=1C(C=1C=CC(Cl)=CC=1)C1=CC=C(Cl)C=C1 BGFTWECWAICPDG-UHFFFAOYSA-N 0.000 claims description 2
- 108010008014 B-Cell Maturation Antigen Proteins 0.000 claims description 2
- 102000006942 B-Cell Maturation Antigen Human genes 0.000 claims description 2
- 108700012439 CA9 Proteins 0.000 claims description 2
- 102100024423 Carbonic anhydrase 9 Human genes 0.000 claims description 2
- 101000914324 Homo sapiens Carcinoembryonic antigen-related cell adhesion molecule 5 Proteins 0.000 claims description 2
- 101000914321 Homo sapiens Carcinoembryonic antigen-related cell adhesion molecule 7 Proteins 0.000 claims description 2
- 101000617725 Homo sapiens Pregnancy-specific beta-1-glycoprotein 2 Proteins 0.000 claims description 2
- 101000610551 Homo sapiens Prominin-1 Proteins 0.000 claims description 2
- 101000884271 Homo sapiens Signal transducer CD24 Proteins 0.000 claims description 2
- 102100022019 Pregnancy-specific beta-1-glycoprotein 2 Human genes 0.000 claims description 2
- 102100040120 Prominin-1 Human genes 0.000 claims description 2
- 102100038081 Signal transducer CD24 Human genes 0.000 claims description 2
- 108700020467 WT1 Proteins 0.000 claims description 2
- 101150084041 WT1 gene Proteins 0.000 claims description 2
- 229940022353 herceptin Drugs 0.000 claims description 2
- 229940126530 T cell activator Drugs 0.000 claims 2
- 229940126547 T-cell immunoglobulin mucin-3 Drugs 0.000 claims 2
- 102100023990 60S ribosomal protein L17 Human genes 0.000 claims 1
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 claims 1
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 claims 1
- 101000934346 Homo sapiens T-cell surface antigen CD2 Proteins 0.000 claims 1
- 102100025390 Integrin beta-2 Human genes 0.000 claims 1
- 102000040856 WT1 Human genes 0.000 claims 1
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 67
- 235000018102 proteins Nutrition 0.000 description 53
- 230000006044 T cell activation Effects 0.000 description 38
- 150000001413 amino acids Chemical group 0.000 description 37
- 235000001014 amino acid Nutrition 0.000 description 33
- 229940024606 amino acid Drugs 0.000 description 23
- 230000014509 gene expression Effects 0.000 description 22
- 230000000694 effects Effects 0.000 description 20
- 238000006467 substitution reaction Methods 0.000 description 19
- 108010007707 Hepatitis A Virus Cellular Receptor 2 Proteins 0.000 description 16
- 230000004913 activation Effects 0.000 description 16
- 231100000135 cytotoxicity Toxicity 0.000 description 15
- 230000003013 cytotoxicity Effects 0.000 description 15
- 150000003839 salts Chemical class 0.000 description 15
- 229920001223 polyethylene glycol Polymers 0.000 description 14
- 102100020862 Lymphocyte activation gene 3 protein Human genes 0.000 description 13
- 101710092458 Lymphocyte activation gene 3 protein Proteins 0.000 description 13
- 108091008874 T cell receptors Proteins 0.000 description 13
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 13
- 238000013459 approach Methods 0.000 description 13
- 230000006690 co-activation Effects 0.000 description 13
- 239000003814 drug Substances 0.000 description 13
- 210000004881 tumor cell Anatomy 0.000 description 13
- 239000002202 Polyethylene glycol Substances 0.000 description 12
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical group [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 12
- 239000000872 buffer Substances 0.000 description 12
- 108020004414 DNA Proteins 0.000 description 11
- 239000012634 fragment Substances 0.000 description 11
- 238000004519 manufacturing process Methods 0.000 description 11
- 230000008093 supporting effect Effects 0.000 description 11
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 10
- 102000005962 receptors Human genes 0.000 description 10
- 108020003175 receptors Proteins 0.000 description 10
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 9
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 9
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 9
- 230000008901 benefit Effects 0.000 description 9
- 229940079593 drug Drugs 0.000 description 9
- 238000001727 in vivo Methods 0.000 description 9
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 8
- 238000011161 development Methods 0.000 description 8
- 230000018109 developmental process Effects 0.000 description 8
- 230000006870 function Effects 0.000 description 8
- 230000002401 inhibitory effect Effects 0.000 description 8
- 239000003446 ligand Substances 0.000 description 8
- 150000007523 nucleic acids Chemical class 0.000 description 8
- 230000002829 reductive effect Effects 0.000 description 8
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 7
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 7
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 7
- 230000008859 change Effects 0.000 description 7
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 7
- 235000014304 histidine Nutrition 0.000 description 7
- 229960002885 histidine Drugs 0.000 description 7
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 7
- 238000002347 injection Methods 0.000 description 7
- 239000007924 injection Substances 0.000 description 7
- 230000001404 mediated effect Effects 0.000 description 7
- 230000011664 signaling Effects 0.000 description 7
- 238000001890 transfection Methods 0.000 description 7
- 101000831567 Homo sapiens Toll-like receptor 2 Proteins 0.000 description 6
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 6
- 102100024333 Toll-like receptor 2 Human genes 0.000 description 6
- 230000000139 costimulatory effect Effects 0.000 description 6
- 230000001461 cytolytic effect Effects 0.000 description 6
- 230000001086 cytosolic effect Effects 0.000 description 6
- 238000012217 deletion Methods 0.000 description 6
- 230000037430 deletion Effects 0.000 description 6
- 230000001419 dependent effect Effects 0.000 description 6
- 201000010099 disease Diseases 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 238000009472 formulation Methods 0.000 description 6
- 230000002163 immunogen Effects 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- 239000003381 stabilizer Substances 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 102000004127 Cytokines Human genes 0.000 description 5
- 108090000695 Cytokines Proteins 0.000 description 5
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 5
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 5
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 5
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 5
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 5
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 5
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 5
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 5
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 5
- 241000699666 Mus <mouse, genus> Species 0.000 description 5
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 5
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 210000004899 c-terminal region Anatomy 0.000 description 5
- 239000000833 heterodimer Substances 0.000 description 5
- 238000003780 insertion Methods 0.000 description 5
- 230000037431 insertion Effects 0.000 description 5
- 230000000670 limiting effect Effects 0.000 description 5
- 230000006320 pegylation Effects 0.000 description 5
- 229920000642 polymer Polymers 0.000 description 5
- 108090000765 processed proteins & peptides Proteins 0.000 description 5
- 238000011160 research Methods 0.000 description 5
- 230000004044 response Effects 0.000 description 5
- 238000002560 therapeutic procedure Methods 0.000 description 5
- 101100314454 Caenorhabditis elegans tra-1 gene Proteins 0.000 description 4
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 4
- 102100031351 Galectin-9 Human genes 0.000 description 4
- 101710121810 Galectin-9 Proteins 0.000 description 4
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 4
- 101000716102 Homo sapiens T-cell surface glycoprotein CD4 Proteins 0.000 description 4
- 108060003951 Immunoglobulin Proteins 0.000 description 4
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 4
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 4
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 4
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 4
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 4
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 4
- 229930195725 Mannitol Natural products 0.000 description 4
- 206010027476 Metastases Diseases 0.000 description 4
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 4
- 230000005867 T cell response Effects 0.000 description 4
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 description 4
- 239000000443 aerosol Substances 0.000 description 4
- 125000001931 aliphatic group Chemical group 0.000 description 4
- 230000001093 anti-cancer Effects 0.000 description 4
- 229960003008 blinatumomab Drugs 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 239000003937 drug carrier Substances 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 238000000684 flow cytometry Methods 0.000 description 4
- 230000004927 fusion Effects 0.000 description 4
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 4
- 102000018358 immunoglobulin Human genes 0.000 description 4
- 238000001802 infusion Methods 0.000 description 4
- 238000001361 intraarterial administration Methods 0.000 description 4
- 238000007918 intramuscular administration Methods 0.000 description 4
- 238000007912 intraperitoneal administration Methods 0.000 description 4
- 238000007913 intrathecal administration Methods 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 239000008101 lactose Substances 0.000 description 4
- 210000000265 leukocyte Anatomy 0.000 description 4
- 239000000594 mannitol Substances 0.000 description 4
- 235000010355 mannitol Nutrition 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 4
- 230000035772 mutation Effects 0.000 description 4
- 239000000546 pharmaceutical excipient Substances 0.000 description 4
- 229920002451 polyvinyl alcohol Polymers 0.000 description 4
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 4
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 4
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 4
- 238000011321 prophylaxis Methods 0.000 description 4
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- 235000000346 sugar Nutrition 0.000 description 4
- 150000005846 sugar alcohols Chemical class 0.000 description 4
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 4
- 239000004094 surface-active agent Substances 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 238000013268 sustained release Methods 0.000 description 4
- 239000012730 sustained-release form Substances 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- 206010000830 Acute leukaemia Diseases 0.000 description 3
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 3
- 239000004475 Arginine Substances 0.000 description 3
- 102000004082 Calreticulin Human genes 0.000 description 3
- 108090000549 Calreticulin Proteins 0.000 description 3
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 3
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 description 3
- 229920002261 Corn starch Polymers 0.000 description 3
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 3
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- 239000004471 Glycine Substances 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 3
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 3
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 3
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 3
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 3
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 3
- 102000003960 Ligases Human genes 0.000 description 3
- 108090000364 Ligases Proteins 0.000 description 3
- 239000004472 Lysine Substances 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 241000699670 Mus sp. Species 0.000 description 3
- 108091008043 NK cell inhibitory receptors Proteins 0.000 description 3
- 108091028043 Nucleic acid sequence Proteins 0.000 description 3
- 206010057249 Phagocytosis Diseases 0.000 description 3
- 229920002472 Starch Polymers 0.000 description 3
- 229930006000 Sucrose Natural products 0.000 description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 3
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 3
- 108091008035 T cell costimulatory receptors Proteins 0.000 description 3
- 102100033117 Toll-like receptor 9 Human genes 0.000 description 3
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 3
- 230000002159 abnormal effect Effects 0.000 description 3
- 230000002411 adverse Effects 0.000 description 3
- 229940124691 antibody therapeutics Drugs 0.000 description 3
- 102000025171 antigen binding proteins Human genes 0.000 description 3
- 108091000831 antigen binding proteins Proteins 0.000 description 3
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 3
- 229960003121 arginine Drugs 0.000 description 3
- 125000003118 aryl group Chemical group 0.000 description 3
- 230000005812 autoimmune toxicity Effects 0.000 description 3
- 231100001152 autoimmune toxicity Toxicity 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 230000000903 blocking effect Effects 0.000 description 3
- 239000004067 bulking agent Substances 0.000 description 3
- 229920002678 cellulose Polymers 0.000 description 3
- 239000001913 cellulose Substances 0.000 description 3
- 239000002738 chelating agent Substances 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 239000008120 corn starch Substances 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 229940014259 gelatin Drugs 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 229960002449 glycine Drugs 0.000 description 3
- 229940093915 gynecological organic acid Drugs 0.000 description 3
- 210000002443 helper t lymphocyte Anatomy 0.000 description 3
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 3
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 3
- 230000005934 immune activation Effects 0.000 description 3
- 230000005847 immunogenicity Effects 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 238000000099 in vitro assay Methods 0.000 description 3
- 230000015788 innate immune response Effects 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 230000002601 intratumoral effect Effects 0.000 description 3
- 229960003646 lysine Drugs 0.000 description 3
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 235000005985 organic acids Nutrition 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 230000008782 phagocytosis Effects 0.000 description 3
- 102000054765 polymorphisms of proteins Human genes 0.000 description 3
- 229920000136 polysorbate Polymers 0.000 description 3
- 229950008882 polysorbate Drugs 0.000 description 3
- 239000002243 precursor Substances 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 239000000651 prodrug Substances 0.000 description 3
- 229940002612 prodrug Drugs 0.000 description 3
- 238000001542 size-exclusion chromatography Methods 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 235000010356 sorbitol Nutrition 0.000 description 3
- 239000000600 sorbitol Substances 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 239000005720 sucrose Substances 0.000 description 3
- 150000008163 sugars Chemical class 0.000 description 3
- 239000011593 sulfur Substances 0.000 description 3
- 229910052717 sulfur Inorganic materials 0.000 description 3
- 230000002195 synergetic effect Effects 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 230000000699 topical effect Effects 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 238000013518 transcription Methods 0.000 description 3
- 230000035897 transcription Effects 0.000 description 3
- 230000014616 translation Effects 0.000 description 3
- 238000011269 treatment regimen Methods 0.000 description 3
- 239000013598 vector Substances 0.000 description 3
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 2
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- RKDVKSZUMVYZHH-UHFFFAOYSA-N 1,4-dioxane-2,5-dione Chemical compound O=C1COC(=O)CO1 RKDVKSZUMVYZHH-UHFFFAOYSA-N 0.000 description 2
- 108700028369 Alleles Proteins 0.000 description 2
- 108010032595 Antibody Binding Sites Proteins 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- 102100040839 C-type lectin domain family 6 member A Human genes 0.000 description 2
- 101710125370 C-type lectin domain family 6 member A Proteins 0.000 description 2
- 102100040840 C-type lectin domain family 7 member A Human genes 0.000 description 2
- 102000000844 Cell Surface Receptors Human genes 0.000 description 2
- 108010001857 Cell Surface Receptors Proteins 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 108091026890 Coding region Proteins 0.000 description 2
- 206010009944 Colon cancer Diseases 0.000 description 2
- 102000000529 Costimulatory and Inhibitory T-Cell Receptors Human genes 0.000 description 2
- 108010041504 Costimulatory and Inhibitory T-Cell Receptors Proteins 0.000 description 2
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 description 2
- 206010014733 Endometrial cancer Diseases 0.000 description 2
- 206010014759 Endometrial neoplasm Diseases 0.000 description 2
- 241000287828 Gallus gallus Species 0.000 description 2
- 102000018713 Histocompatibility Antigens Class II Human genes 0.000 description 2
- 101001046686 Homo sapiens Integrin alpha-M Proteins 0.000 description 2
- 101001027081 Homo sapiens Killer cell immunoglobulin-like receptor 2DL1 Proteins 0.000 description 2
- 101000945371 Homo sapiens Killer cell immunoglobulin-like receptor 2DL2 Proteins 0.000 description 2
- 101000945331 Homo sapiens Killer cell immunoglobulin-like receptor 2DL4 Proteins 0.000 description 2
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 2
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 description 2
- 101000934372 Homo sapiens Macrosialin Proteins 0.000 description 2
- 101001109501 Homo sapiens NKG2-D type II integral membrane protein Proteins 0.000 description 2
- 101000589305 Homo sapiens Natural cytotoxicity triggering receptor 2 Proteins 0.000 description 2
- 101000763579 Homo sapiens Toll-like receptor 1 Proteins 0.000 description 2
- 101000669447 Homo sapiens Toll-like receptor 4 Proteins 0.000 description 2
- 101000669460 Homo sapiens Toll-like receptor 5 Proteins 0.000 description 2
- 101000669406 Homo sapiens Toll-like receptor 6 Proteins 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 102100022338 Integrin alpha-M Human genes 0.000 description 2
- 102100037363 Killer cell immunoglobulin-like receptor 2DL1 Human genes 0.000 description 2
- 102100033599 Killer cell immunoglobulin-like receptor 2DL2 Human genes 0.000 description 2
- 102100033633 Killer cell immunoglobulin-like receptor 2DL4 Human genes 0.000 description 2
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 2
- 125000000998 L-alanino group Chemical group [H]N([*])[C@](C([H])([H])[H])([H])C(=O)O[H] 0.000 description 2
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 2
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 description 2
- 102100025136 Macrosialin Human genes 0.000 description 2
- 206010027406 Mesothelioma Diseases 0.000 description 2
- 230000006051 NK cell activation Effects 0.000 description 2
- 102100022680 NKG2-D type II integral membrane protein Human genes 0.000 description 2
- 102100032851 Natural cytotoxicity triggering receptor 2 Human genes 0.000 description 2
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 2
- 206010033128 Ovarian cancer Diseases 0.000 description 2
- 206010061535 Ovarian neoplasm Diseases 0.000 description 2
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 229920002873 Polyethylenimine Polymers 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 102100025831 Scavenger receptor cysteine-rich type 1 protein M130 Human genes 0.000 description 2
- 208000003837 Second Primary Neoplasms Diseases 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 230000020385 T cell costimulation Effects 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 2
- 239000004473 Threonine Substances 0.000 description 2
- 108010060818 Toll-Like Receptor 9 Proteins 0.000 description 2
- 102000002689 Toll-like receptor Human genes 0.000 description 2
- 108020000411 Toll-like receptor Proteins 0.000 description 2
- 102100027010 Toll-like receptor 1 Human genes 0.000 description 2
- 102100039360 Toll-like receptor 4 Human genes 0.000 description 2
- 102100039357 Toll-like receptor 5 Human genes 0.000 description 2
- 102100039387 Toll-like receptor 6 Human genes 0.000 description 2
- 229920001615 Tragacanth Polymers 0.000 description 2
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 238000001042 affinity chromatography Methods 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 235000004279 alanine Nutrition 0.000 description 2
- 229960003767 alanine Drugs 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 239000000783 alginic acid Substances 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 229960001126 alginic acid Drugs 0.000 description 2
- 150000004781 alginic acids Chemical class 0.000 description 2
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 230000036436 anti-hiv Effects 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- 239000002246 antineoplastic agent Substances 0.000 description 2
- 229940041181 antineoplastic drug Drugs 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 235000006708 antioxidants Nutrition 0.000 description 2
- 230000006907 apoptotic process Effects 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 235000009582 asparagine Nutrition 0.000 description 2
- 229960001230 asparagine Drugs 0.000 description 2
- 239000000305 astragalus gummifer gum Substances 0.000 description 2
- 210000003719 b-lymphocyte Anatomy 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- 229960000074 biopharmaceutical Drugs 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N biotin Natural products N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 201000003232 brachydactyly type C Diseases 0.000 description 2
- 239000001506 calcium phosphate Substances 0.000 description 2
- 230000005880 cancer cell killing Effects 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- YCIMNLLNPGFGHC-UHFFFAOYSA-N catechol Chemical compound OC1=CC=CC=C1O YCIMNLLNPGFGHC-UHFFFAOYSA-N 0.000 description 2
- 230000010261 cell growth Effects 0.000 description 2
- 238000002659 cell therapy Methods 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 239000003638 chemical reducing agent Substances 0.000 description 2
- 235000013330 chicken meat Nutrition 0.000 description 2
- 208000011654 childhood malignant neoplasm Diseases 0.000 description 2
- 201000010276 collecting duct carcinoma Diseases 0.000 description 2
- 230000004154 complement system Effects 0.000 description 2
- 239000006184 cosolvent Substances 0.000 description 2
- 206010052015 cytokine release syndrome Diseases 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 108010025838 dectin 1 Proteins 0.000 description 2
- 239000003405 delayed action preparation Substances 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 238000010494 dissociation reaction Methods 0.000 description 2
- 230000005593 dissociations Effects 0.000 description 2
- MTZQAGJQAFMTAQ-UHFFFAOYSA-N ethyl benzoate Chemical compound CCOC(=O)C1=CC=CC=C1 MTZQAGJQAFMTAQ-UHFFFAOYSA-N 0.000 description 2
- 210000003527 eukaryotic cell Anatomy 0.000 description 2
- 230000037406 food intake Effects 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 238000001415 gene therapy Methods 0.000 description 2
- 235000013922 glutamic acid Nutrition 0.000 description 2
- 239000004220 glutamic acid Substances 0.000 description 2
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 2
- 235000004554 glutamine Nutrition 0.000 description 2
- 229960002743 glutamine Drugs 0.000 description 2
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 2
- 235000011187 glycerol Nutrition 0.000 description 2
- 201000005787 hematologic cancer Diseases 0.000 description 2
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 238000003364 immunohistochemistry Methods 0.000 description 2
- 230000006054 immunological memory Effects 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 229910052738 indium Inorganic materials 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 108091008042 inhibitory receptors Proteins 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 239000007951 isotonicity adjuster Substances 0.000 description 2
- 229960003136 leucine Drugs 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 229920001427 mPEG Polymers 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 2
- 229930182817 methionine Natural products 0.000 description 2
- 235000006109 methionine Nutrition 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 2
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 2
- 229960002216 methylparaben Drugs 0.000 description 2
- 239000011859 microparticle Substances 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 238000010369 molecular cloning Methods 0.000 description 2
- 210000001616 monocyte Anatomy 0.000 description 2
- PJUIMOJAAPLTRJ-UHFFFAOYSA-N monothioglycerol Chemical compound OCC(O)CS PJUIMOJAAPLTRJ-UHFFFAOYSA-N 0.000 description 2
- DAZSWUUAFHBCGE-KRWDZBQOSA-N n-[(2s)-3-methyl-1-oxo-1-pyrrolidin-1-ylbutan-2-yl]-3-phenylpropanamide Chemical compound N([C@@H](C(C)C)C(=O)N1CCCC1)C(=O)CCC1=CC=CC=C1 DAZSWUUAFHBCGE-KRWDZBQOSA-N 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- 108091027963 non-coding RNA Proteins 0.000 description 2
- 102000042567 non-coding RNA Human genes 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 208000008443 pancreatic carcinoma Diseases 0.000 description 2
- 244000052769 pathogen Species 0.000 description 2
- AQIXEPGDORPWBJ-UHFFFAOYSA-N pentan-3-ol Chemical compound CCC(O)CC AQIXEPGDORPWBJ-UHFFFAOYSA-N 0.000 description 2
- 230000010412 perfusion Effects 0.000 description 2
- 238000002823 phage display Methods 0.000 description 2
- 229920001983 poloxamer Polymers 0.000 description 2
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 description 2
- 238000003752 polymerase chain reaction Methods 0.000 description 2
- 229920001592 potato starch Polymers 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 125000001500 prolyl group Chemical group [H]N1C([H])(C(=O)[*])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 2
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 2
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 2
- 229960003415 propylparaben Drugs 0.000 description 2
- 238000001742 protein purification Methods 0.000 description 2
- 210000003289 regulatory T cell Anatomy 0.000 description 2
- GHMLBKRAJCXXBS-UHFFFAOYSA-N resorcinol Chemical compound OC1=CC=CC(O)=C1 GHMLBKRAJCXXBS-UHFFFAOYSA-N 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 210000003296 saliva Anatomy 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 230000019491 signal transduction Effects 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000001797 sucrose acetate isobutyrate Substances 0.000 description 2
- 235000010983 sucrose acetate isobutyrate Nutrition 0.000 description 2
- UVGUPMLLGBCFEJ-SWTLDUCYSA-N sucrose acetate isobutyrate Chemical compound CC(C)C(=O)O[C@H]1[C@H](OC(=O)C(C)C)[C@@H](COC(=O)C(C)C)O[C@@]1(COC(C)=O)O[C@@H]1[C@H](OC(=O)C(C)C)[C@@H](OC(=O)C(C)C)[C@H](OC(=O)C(C)C)[C@@H](COC(C)=O)O1 UVGUPMLLGBCFEJ-SWTLDUCYSA-N 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- CWERGRDVMFNCDR-UHFFFAOYSA-N thioglycolic acid Chemical compound OC(=O)CS CWERGRDVMFNCDR-UHFFFAOYSA-N 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- 229940074410 trehalose Drugs 0.000 description 2
- URAYPUMNDPQOKB-UHFFFAOYSA-N triacetin Chemical compound CC(=O)OCC(OC(C)=O)COC(C)=O URAYPUMNDPQOKB-UHFFFAOYSA-N 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 239000000811 xylitol Substances 0.000 description 2
- 235000010447 xylitol Nutrition 0.000 description 2
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 2
- 229960002675 xylitol Drugs 0.000 description 2
- ZJIFDEVVTPEXDL-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) hydrogen carbonate Chemical compound OC(=O)ON1C(=O)CCC1=O ZJIFDEVVTPEXDL-UHFFFAOYSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- AGBQKNBQESQNJD-SSDOTTSWSA-N (R)-lipoic acid Chemical compound OC(=O)CCCC[C@@H]1CCSS1 AGBQKNBQESQNJD-SSDOTTSWSA-N 0.000 description 1
- DDMOUSALMHHKOS-UHFFFAOYSA-N 1,2-dichloro-1,1,2,2-tetrafluoroethane Chemical compound FC(F)(Cl)C(F)(F)Cl DDMOUSALMHHKOS-UHFFFAOYSA-N 0.000 description 1
- HNSDLXPSAYFUHK-UHFFFAOYSA-N 1,4-bis(2-ethylhexyl) sulfosuccinate Chemical compound CCCCC(CC)COC(=O)CC(S(O)(=O)=O)C(=O)OCC(CC)CCCC HNSDLXPSAYFUHK-UHFFFAOYSA-N 0.000 description 1
- VOJUXHHACRXLTD-UHFFFAOYSA-N 1,4-dihydroxy-2-naphthoic acid Chemical compound C1=CC=CC2=C(O)C(C(=O)O)=CC(O)=C21 VOJUXHHACRXLTD-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- HTCSFFGLRQDZDE-UHFFFAOYSA-N 2-azaniumyl-2-phenylpropanoate Chemical compound OC(=O)C(N)(C)C1=CC=CC=C1 HTCSFFGLRQDZDE-UHFFFAOYSA-N 0.000 description 1
- HZLCGUXUOFWCCN-UHFFFAOYSA-N 2-hydroxynonadecane-1,2,3-tricarboxylic acid Chemical compound CCCCCCCCCCCCCCCCC(C(O)=O)C(O)(C(O)=O)CC(O)=O HZLCGUXUOFWCCN-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical compound C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 description 1
- QXZGLTYKKZKGLN-UHFFFAOYSA-N 4-(2,5-dioxopyrrolidin-1-yl)oxy-4-oxobutanoic acid Chemical compound OC(=O)CCC(=O)ON1C(=O)CCC1=O QXZGLTYKKZKGLN-UHFFFAOYSA-N 0.000 description 1
- 244000215068 Acacia senegal Species 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 241000251468 Actinopterygii Species 0.000 description 1
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 1
- 206010067484 Adverse reaction Diseases 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 244000303258 Annona diversifolia Species 0.000 description 1
- 235000002198 Annona diversifolia Nutrition 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 108091008875 B cell receptors Proteins 0.000 description 1
- 102100027205 B-cell antigen receptor complex-associated protein alpha chain Human genes 0.000 description 1
- 101710095183 B-cell antigen receptor complex-associated protein alpha chain Proteins 0.000 description 1
- 102100027203 B-cell antigen receptor complex-associated protein beta chain Human genes 0.000 description 1
- 101710166261 B-cell antigen receptor complex-associated protein beta chain Proteins 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 206010005949 Bone cancer Diseases 0.000 description 1
- 208000018084 Bone neoplasm Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 102100021992 CD209 antigen Human genes 0.000 description 1
- 102100038077 CD226 antigen Human genes 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 206010058019 Cancer Pain Diseases 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 108010077544 Chromatin Proteins 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 108700010070 Codon Usage Proteins 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 102000000989 Complement System Proteins Human genes 0.000 description 1
- 108010069112 Complement System Proteins Proteins 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 241000938605 Crocodylia Species 0.000 description 1
- HEBKCHPVOIAQTA-QWWZWVQMSA-N D-arabinitol Chemical compound OC[C@@H](O)C(O)[C@H](O)CO HEBKCHPVOIAQTA-QWWZWVQMSA-N 0.000 description 1
- RGHNJXZEOKUKBD-SQOUGZDYSA-M D-gluconate Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C([O-])=O RGHNJXZEOKUKBD-SQOUGZDYSA-M 0.000 description 1
- WQZGKKKJIJFFOK-QTVWNMPRSA-N D-mannopyranose Chemical compound OC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-QTVWNMPRSA-N 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 239000004338 Dichlorodifluoromethane Substances 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 101150029707 ERBB2 gene Proteins 0.000 description 1
- 102100025137 Early activation antigen CD69 Human genes 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 239000004386 Erythritol Substances 0.000 description 1
- UNXHWFMMPAWVPI-UHFFFAOYSA-N Erythritol Natural products OCC(O)C(O)CO UNXHWFMMPAWVPI-UHFFFAOYSA-N 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- 108010008165 Etanercept Proteins 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 206010015548 Euthanasia Diseases 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- 102000016359 Fibronectins Human genes 0.000 description 1
- 108090000331 Firefly luciferases Proteins 0.000 description 1
- 241000192125 Firmicutes Species 0.000 description 1
- 108010040721 Flagellin Proteins 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 108010024636 Glutathione Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 239000012981 Hank's balanced salt solution Substances 0.000 description 1
- SQUHHTBVTRBESD-UHFFFAOYSA-N Hexa-Ac-myo-Inositol Natural products CC(=O)OC1C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C1OC(C)=O SQUHHTBVTRBESD-UHFFFAOYSA-N 0.000 description 1
- 102100026122 High affinity immunoglobulin gamma Fc receptor I Human genes 0.000 description 1
- 108010093488 His-His-His-His-His-His Proteins 0.000 description 1
- 108010027412 Histocompatibility Antigens Class II Proteins 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000884298 Homo sapiens CD226 antigen Proteins 0.000 description 1
- 101100166600 Homo sapiens CD28 gene Proteins 0.000 description 1
- 101000934374 Homo sapiens Early activation antigen CD69 Proteins 0.000 description 1
- 101000913074 Homo sapiens High affinity immunoglobulin gamma Fc receptor I Proteins 0.000 description 1
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 1
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 1
- 101000945340 Homo sapiens Killer cell immunoglobulin-like receptor 2DS1 Proteins 0.000 description 1
- 101000945339 Homo sapiens Killer cell immunoglobulin-like receptor 2DS2 Proteins 0.000 description 1
- 101000945351 Homo sapiens Killer cell immunoglobulin-like receptor 3DL1 Proteins 0.000 description 1
- 101000945492 Homo sapiens Killer cell immunoglobulin-like receptor 3DS1 Proteins 0.000 description 1
- 101000971538 Homo sapiens Killer cell lectin-like receptor subfamily F member 1 Proteins 0.000 description 1
- 101000971533 Homo sapiens Killer cell lectin-like receptor subfamily G member 1 Proteins 0.000 description 1
- 101001133056 Homo sapiens Mucin-1 Proteins 0.000 description 1
- 101000979342 Homo sapiens Nuclear factor NF-kappa-B p105 subunit Proteins 0.000 description 1
- 101000638251 Homo sapiens Tumor necrosis factor ligand superfamily member 9 Proteins 0.000 description 1
- 238000012450 HuMAb Mouse Methods 0.000 description 1
- 101001057750 Human cytomegalovirus (strain AD169) Uncharacterized protein IRL2 Proteins 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102100022297 Integrin alpha-X Human genes 0.000 description 1
- 102100035678 Interferon gamma receptor 1 Human genes 0.000 description 1
- 102100027268 Interferon-stimulated gene 20 kDa protein Human genes 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 208000005016 Intestinal Neoplasms Diseases 0.000 description 1
- 101150069255 KLRC1 gene Proteins 0.000 description 1
- 101150074862 KLRC3 gene Proteins 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- 102100033631 Killer cell immunoglobulin-like receptor 2DS1 Human genes 0.000 description 1
- 102100033630 Killer cell immunoglobulin-like receptor 2DS2 Human genes 0.000 description 1
- 102100033627 Killer cell immunoglobulin-like receptor 3DL1 Human genes 0.000 description 1
- 102100034833 Killer cell immunoglobulin-like receptor 3DS1 Human genes 0.000 description 1
- 102100021458 Killer cell lectin-like receptor subfamily F member 1 Human genes 0.000 description 1
- 102100021457 Killer cell lectin-like receptor subfamily G member 1 Human genes 0.000 description 1
- 238000012449 Kunming mouse Methods 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- 239000004395 L-leucine Substances 0.000 description 1
- 235000019454 L-leucine Nutrition 0.000 description 1
- 125000000174 L-prolyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(*)=O 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- 125000000510 L-tryptophano group Chemical group [H]C1=C([H])C([H])=C2N([H])C([H])=C(C([H])([H])[C@@]([H])(C(O[H])=O)N([H])[*])C2=C1[H] 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 206010023825 Laryngeal cancer Diseases 0.000 description 1
- 241000713666 Lentivirus Species 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- 108091054437 MHC class I family Proteins 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 108091054438 MHC class II family Proteins 0.000 description 1
- 101100404845 Macaca mulatta NKG2A gene Proteins 0.000 description 1
- 102100025354 Macrophage mannose receptor 1 Human genes 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 208000032271 Malignant tumor of penis Diseases 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 102000012750 Membrane Glycoproteins Human genes 0.000 description 1
- 108010090054 Membrane Glycoproteins Proteins 0.000 description 1
- 244000246386 Mentha pulegium Species 0.000 description 1
- 235000016257 Mentha pulegium Nutrition 0.000 description 1
- 235000004357 Mentha x piperita Nutrition 0.000 description 1
- 208000003445 Mouth Neoplasms Diseases 0.000 description 1
- 102100034256 Mucin-1 Human genes 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- HSHXDCVZWHOWCS-UHFFFAOYSA-N N'-hexadecylthiophene-2-carbohydrazide Chemical compound CCCCCCCCCCCCCCCCNNC(=O)c1cccs1 HSHXDCVZWHOWCS-UHFFFAOYSA-N 0.000 description 1
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 1
- CBQJSKKFNMDLON-JTQLQIEISA-N N-acetyl-L-phenylalanine Chemical group CC(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 CBQJSKKFNMDLON-JTQLQIEISA-N 0.000 description 1
- 230000004988 N-glycosylation Effects 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- 108091008877 NK cell receptors Proteins 0.000 description 1
- 102100022682 NKG2-A/NKG2-B type II integral membrane protein Human genes 0.000 description 1
- 102100022701 NKG2-E type II integral membrane protein Human genes 0.000 description 1
- 208000001894 Nasopharyngeal Neoplasms Diseases 0.000 description 1
- 206010061306 Nasopharyngeal cancer Diseases 0.000 description 1
- 108010004217 Natural Cytotoxicity Triggering Receptor 1 Proteins 0.000 description 1
- 108010004222 Natural Cytotoxicity Triggering Receptor 3 Proteins 0.000 description 1
- 102100032870 Natural cytotoxicity triggering receptor 1 Human genes 0.000 description 1
- 102100032852 Natural cytotoxicity triggering receptor 3 Human genes 0.000 description 1
- 102100021462 Natural killer cells antigen CD94 Human genes 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 102100023050 Nuclear factor NF-kappa-B p105 subunit Human genes 0.000 description 1
- 101710187081 OX-2 membrane glycoprotein Proteins 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 1
- 208000002471 Penile Neoplasms Diseases 0.000 description 1
- 206010034299 Penile cancer Diseases 0.000 description 1
- 108010067902 Peptide Library Proteins 0.000 description 1
- 208000009565 Pharyngeal Neoplasms Diseases 0.000 description 1
- 206010034811 Pharyngeal cancer Diseases 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 206010036790 Productive cough Diseases 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- MUPFEKGTMRGPLJ-RMMQSMQOSA-N Raffinose Natural products O(C[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O[C@@]2(CO)[C@H](O)[C@@H](O)[C@@H](CO)O2)O1)[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 MUPFEKGTMRGPLJ-RMMQSMQOSA-N 0.000 description 1
- 208000015634 Rectal Neoplasms Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- JVWLUVNSQYXYBE-UHFFFAOYSA-N Ribitol Natural products OCC(C)C(O)C(O)CO JVWLUVNSQYXYBE-UHFFFAOYSA-N 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 201000010208 Seminoma Diseases 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 108010029180 Sialic Acid Binding Ig-like Lectin 3 Proteins 0.000 description 1
- 102000001555 Sialic Acid Binding Ig-like Lectin 3 Human genes 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- UQZIYBXSHAGNOE-USOSMYMVSA-N Stachyose Natural products O(C[C@H]1[C@@H](O)[C@H](O)[C@H](O)[C@@H](O[C@@]2(CO)[C@H](O)[C@@H](O)[C@@H](CO)O2)O1)[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@H](CO[C@@H]2[C@@H](O)[C@@H](O)[C@@H](O)[C@H](CO)O2)O1 UQZIYBXSHAGNOE-USOSMYMVSA-N 0.000 description 1
- 108091081024 Start codon Proteins 0.000 description 1
- 208000005718 Stomach Neoplasms Diseases 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 230000006052 T cell proliferation Effects 0.000 description 1
- 206010043276 Teratoma Diseases 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- 238000012338 Therapeutic targeting Methods 0.000 description 1
- 108091036066 Three prime untranslated region Proteins 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 102100032101 Tumor necrosis factor ligand superfamily member 9 Human genes 0.000 description 1
- 101710165474 Tumor necrosis factor receptor superfamily member 5 Proteins 0.000 description 1
- MUPFEKGTMRGPLJ-UHFFFAOYSA-N UNPD196149 Natural products OC1C(O)C(CO)OC1(CO)OC1C(O)C(O)C(O)C(COC2C(C(O)C(O)C(CO)O2)O)O1 MUPFEKGTMRGPLJ-UHFFFAOYSA-N 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 208000002495 Uterine Neoplasms Diseases 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 229960003697 abatacept Drugs 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 239000008351 acetate buffer Substances 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 230000033289 adaptive immune response Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 201000005188 adrenal gland cancer Diseases 0.000 description 1
- 208000024447 adrenal gland neoplasm Diseases 0.000 description 1
- 230000006838 adverse reaction Effects 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 229940040563 agaric acid Drugs 0.000 description 1
- 125000003172 aldehyde group Chemical group 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- AGBQKNBQESQNJD-UHFFFAOYSA-N alpha-Lipoic acid Natural products OC(=O)CCCCC1CCSS1 AGBQKNBQESQNJD-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 230000033115 angiogenesis Effects 0.000 description 1
- 230000001745 anti-biotin effect Effects 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 238000002617 apheresis Methods 0.000 description 1
- 238000003782 apoptosis assay Methods 0.000 description 1
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 229960001716 benzalkonium Drugs 0.000 description 1
- JUHORIMYRDESRB-UHFFFAOYSA-N benzathine Chemical compound C=1C=CC=CC=1CNCCNCC1=CC=CC=C1 JUHORIMYRDESRB-UHFFFAOYSA-N 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 238000007413 biotinylation Methods 0.000 description 1
- 230000006287 biotinylation Effects 0.000 description 1
- HUTDDBSSHVOYJR-UHFFFAOYSA-H bis[(2-oxo-1,3,2$l^{5},4$l^{2}-dioxaphosphaplumbetan-2-yl)oxy]lead Chemical compound [Pb+2].[Pb+2].[Pb+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O HUTDDBSSHVOYJR-UHFFFAOYSA-H 0.000 description 1
- 238000009534 blood test Methods 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 238000004422 calculation algorithm Methods 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 230000009702 cancer cell proliferation Effects 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 229960004424 carbon dioxide Drugs 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 238000012754 cardiac puncture Methods 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 229920006317 cationic polymer Polymers 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 210000002421 cell wall Anatomy 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 239000007958 cherry flavor Substances 0.000 description 1
- VDANGULDQQJODZ-UHFFFAOYSA-N chloroprocaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1Cl VDANGULDQQJODZ-UHFFFAOYSA-N 0.000 description 1
- 229960002023 chloroprocaine Drugs 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- 210000003483 chromatin Anatomy 0.000 description 1
- 238000011097 chromatography purification Methods 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 238000003501 co-culture Methods 0.000 description 1
- 238000011278 co-treatment Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 238000010835 comparative analysis Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 239000008139 complexing agent Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 239000003636 conditioned culture medium Substances 0.000 description 1
- 229940099112 cornstarch Drugs 0.000 description 1
- 108091008034 costimulatory receptors Proteins 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 150000003999 cyclitols Chemical class 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- 230000016396 cytokine production Effects 0.000 description 1
- 210000005220 cytoplasmic tail Anatomy 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 238000012350 deep sequencing Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 239000000412 dendrimer Substances 0.000 description 1
- 229920000736 dendritic polymer Polymers 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 235000019404 dichlorodifluoromethane Nutrition 0.000 description 1
- 229940042935 dichlorodifluoromethane Drugs 0.000 description 1
- 229940087091 dichlorotetrafluoroethane Drugs 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 229940043237 diethanolamine Drugs 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 238000000375 direct analysis in real time Methods 0.000 description 1
- 150000002016 disaccharides Chemical class 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 229960000878 docusate sodium Drugs 0.000 description 1
- 231100000276 dose-dependent cytotoxicity Toxicity 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 239000008298 dragée Substances 0.000 description 1
- 229940112141 dry powder inhaler Drugs 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 238000012063 dual-affinity re-targeting Methods 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 229940073621 enbrel Drugs 0.000 description 1
- 210000002472 endoplasmic reticulum Anatomy 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 230000007515 enzymatic degradation Effects 0.000 description 1
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 1
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- 235000019414 erythritol Nutrition 0.000 description 1
- UNXHWFMMPAWVPI-ZXZARUISSA-N erythritol Chemical compound OC[C@H](O)[C@H](O)CO UNXHWFMMPAWVPI-ZXZARUISSA-N 0.000 description 1
- 229940009714 erythritol Drugs 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 239000013613 expression plasmid Substances 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 239000003889 eye drop Substances 0.000 description 1
- 229940012356 eye drops Drugs 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- FBPFZTCFMRRESA-GUCUJZIJSA-N galactitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-GUCUJZIJSA-N 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 206010017758 gastric cancer Diseases 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 230000030279 gene silencing Effects 0.000 description 1
- 229940050410 gluconate Drugs 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 229960003180 glutathione Drugs 0.000 description 1
- 239000001087 glyceryl triacetate Substances 0.000 description 1
- 235000013773 glyceryl triacetate Nutrition 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 239000003979 granulating agent Substances 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 239000000710 homodimer Substances 0.000 description 1
- 235000001050 hortel pimenta Nutrition 0.000 description 1
- 210000004408 hybridoma Anatomy 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 229940071870 hydroiodic acid Drugs 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000008088 immune pathway Effects 0.000 description 1
- 230000008073 immune recognition Effects 0.000 description 1
- 230000037189 immune system physiology Effects 0.000 description 1
- 238000003119 immunoblot Methods 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 229960000367 inositol Drugs 0.000 description 1
- CDAISMWEOUEBRE-GPIVLXJGSA-N inositol Chemical compound O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O CDAISMWEOUEBRE-GPIVLXJGSA-N 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 238000012482 interaction analysis Methods 0.000 description 1
- 201000002313 intestinal cancer Diseases 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- JJTUDXZGHPGLLC-UHFFFAOYSA-N lactide Chemical compound CC1OC(=O)C(C)OC1=O JJTUDXZGHPGLLC-UHFFFAOYSA-N 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 206010023841 laryngeal neoplasm Diseases 0.000 description 1
- 201000004962 larynx cancer Diseases 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- XMGQYMWWDOXHJM-UHFFFAOYSA-N limonene Chemical compound CC(=C)C1CCC(C)=CC1 XMGQYMWWDOXHJM-UHFFFAOYSA-N 0.000 description 1
- 208000012987 lip and oral cavity carcinoma Diseases 0.000 description 1
- 150000002632 lipids Chemical group 0.000 description 1
- 235000019136 lipoic acid Nutrition 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 238000011528 liquid biopsy Methods 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 201000010453 lymph node cancer Diseases 0.000 description 1
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- RLSSMJSEOOYNOY-UHFFFAOYSA-N m-cresol Chemical compound CC1=CC=CC(O)=C1 RLSSMJSEOOYNOY-UHFFFAOYSA-N 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 238000007898 magnetic cell sorting Methods 0.000 description 1
- 125000005439 maleimidyl group Chemical group C1(C=CC(N1*)=O)=O 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 229960001855 mannitol Drugs 0.000 description 1
- 125000000311 mannosyl group Chemical group C1([C@@H](O)[C@@H](O)[C@H](O)[C@H](O1)CO)* 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 229940100630 metacresol Drugs 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 229960004452 methionine Drugs 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- OSWPMRLSEDHDFF-UHFFFAOYSA-N methyl salicylate Chemical compound COC(=O)C1=CC=CC=C1O OSWPMRLSEDHDFF-UHFFFAOYSA-N 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 230000002297 mitogenic effect Effects 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 102000035118 modified proteins Human genes 0.000 description 1
- 108091005573 modified proteins Proteins 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 210000005087 mononuclear cell Anatomy 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 210000005170 neoplastic cell Anatomy 0.000 description 1
- 230000000802 nitrating effect Effects 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 229960003301 nivolumab Drugs 0.000 description 1
- 230000036963 noncompetitive effect Effects 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- 230000005868 ontogenesis Effects 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 239000007968 orange flavor Substances 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 239000008194 pharmaceutical composition Substances 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 229960000502 poloxamer Drugs 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 229940065514 poly(lactide) Drugs 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 229920001610 polycaprolactone Polymers 0.000 description 1
- 108091033319 polynucleotide Chemical group 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 239000002157 polynucleotide Chemical group 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 229940116317 potato starch Drugs 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 230000005522 programmed cell death Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 230000004845 protein aggregation Effects 0.000 description 1
- 230000004853 protein function Effects 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 210000001938 protoplast Anatomy 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 230000001698 pyrogenic effect Effects 0.000 description 1
- MUPFEKGTMRGPLJ-ZQSKZDJDSA-N raffinose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O2)O)O1 MUPFEKGTMRGPLJ-ZQSKZDJDSA-N 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- HEBKCHPVOIAQTA-ZXFHETKHSA-N ribitol Chemical compound OC[C@H](O)[C@H](O)[C@H](O)CO HEBKCHPVOIAQTA-ZXFHETKHSA-N 0.000 description 1
- 229940100486 rice starch Drugs 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 239000012266 salt solution Substances 0.000 description 1
- 238000003345 scintillation counting Methods 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- CDAISMWEOUEBRE-UHFFFAOYSA-N scyllo-inosotol Natural products OC1C(O)C(O)C(O)C(O)C1O CDAISMWEOUEBRE-UHFFFAOYSA-N 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 238000002864 sequence alignment Methods 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 235000019345 sodium thiosulphate Nutrition 0.000 description 1
- 210000004872 soft tissue Anatomy 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 210000003802 sputum Anatomy 0.000 description 1
- 208000024794 sputum Diseases 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- UQZIYBXSHAGNOE-XNSRJBNMSA-N stachyose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO[C@@H]3[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O3)O)O2)O)O1 UQZIYBXSHAGNOE-XNSRJBNMSA-N 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- SFVFIFLLYFPGHH-UHFFFAOYSA-M stearalkonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CC1=CC=CC=C1 SFVFIFLLYFPGHH-UHFFFAOYSA-M 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 201000011549 stomach cancer Diseases 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 239000008362 succinate buffer Substances 0.000 description 1
- 229960004793 sucrose Drugs 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229940095064 tartrate Drugs 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 229960002663 thioctic acid Drugs 0.000 description 1
- 229940035024 thioglycerol Drugs 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000003151 transfection method Methods 0.000 description 1
- 238000003146 transient transfection Methods 0.000 description 1
- 230000014621 translational initiation Effects 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 229960000575 trastuzumab Drugs 0.000 description 1
- 229960002622 triacetin Drugs 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 description 1
- 229940029284 trichlorofluoromethane Drugs 0.000 description 1
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical class CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 1
- 150000004043 trisaccharides Chemical class 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 229940045136 urea Drugs 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 206010046766 uterine cancer Diseases 0.000 description 1
- 206010046885 vaginal cancer Diseases 0.000 description 1
- 208000013139 vaginal neoplasm Diseases 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 201000011531 vascular cancer Diseases 0.000 description 1
- 206010055031 vascular neoplasm Diseases 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 229940100445 wheat starch Drugs 0.000 description 1
- 239000009637 wintergreen oil Substances 0.000 description 1
- 238000002424 x-ray crystallography Methods 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2896—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against molecules with a "CD"-designation, not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2809—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against the T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2815—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD8
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2827—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against B7 molecules, e.g. CD80, CD86
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2866—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against receptors for cytokines, lymphokines, interferons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/626—Diabody or triabody
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Veterinary Medicine (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662362397P | 2016-07-14 | 2016-07-14 | |
| US62/362,397 | 2016-07-14 | ||
| US201762480230P | 2017-03-31 | 2017-03-31 | |
| US62/480,230 | 2017-03-31 | ||
| PCT/US2017/042264 WO2018014001A1 (en) | 2016-07-14 | 2017-07-14 | Multiple bi-specific binding domain constructs with different epitope binding to treat cancer |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2019532017A true JP2019532017A (ja) | 2019-11-07 |
| JP2019532017A5 JP2019532017A5 (cg-RX-API-DMAC7.html) | 2020-08-20 |
Family
ID=60952209
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019501628A Withdrawn JP2019532017A (ja) | 2016-07-14 | 2017-07-14 | がんを治療するための異なるエピトープ結合を示す複数の二重特異性結合ドメイン構築物 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20190233534A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP3484516A4 (cg-RX-API-DMAC7.html) |
| JP (1) | JP2019532017A (cg-RX-API-DMAC7.html) |
| AU (1) | AU2017297603A1 (cg-RX-API-DMAC7.html) |
| CA (1) | CA3030841A1 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2018014001A1 (cg-RX-API-DMAC7.html) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023519932A (ja) * | 2020-03-31 | 2023-05-15 | フレッド ハッチンソン キャンサー センター | 抗cd33抗体及びその使用 |
| JP2023520399A (ja) * | 2020-03-31 | 2023-05-17 | フレッド ハッチンソン キャンサー センター | ヒト抗cd33抗体及びその使用 |
| JP2023548443A (ja) * | 2020-10-30 | 2023-11-16 | ジャナックス セラピューティクス,インク. | Cd28およびpd-l1を標的とする多特異性抗体ならびにその使用方法 |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12116406B2 (en) | 2017-05-26 | 2024-10-15 | Fred Hutchinson Cancer Center | Anti-CD33 antibodies and uses thereof |
| MX419677B (es) * | 2018-06-21 | 2025-01-14 | Regeneron Pharma | Anticuerpos anti-psma x anti-cd28 biespecíficos y usos de estos |
| KR20210108981A (ko) | 2018-12-21 | 2021-09-03 | 에프. 호프만-라 로슈 아게 | 종양-표적화된 효현성 cd28 항원 결합 분자 |
| CN109485734B (zh) * | 2018-12-30 | 2020-05-12 | 广州百暨基因科技有限公司 | 一种靶向bcma和cd19的双特异性嵌合抗原受体及其应用 |
| WO2020150513A1 (en) * | 2019-01-17 | 2020-07-23 | Fred Hutchinson Cancer Research Center | Methods to enhance the selectivity and effectiveness of cancer treatments |
| US11613576B2 (en) | 2019-04-09 | 2023-03-28 | Sanofi | Trispecific binding proteins, methods, and uses thereof |
| JP7664175B2 (ja) * | 2019-04-09 | 2025-04-17 | サノフイ | 三重特異性結合性タンパク質、方法、およびその使用 |
| JOP20210298A1 (ar) | 2019-05-14 | 2023-01-30 | Provention Bio Inc | طرق وتركيبات للوقاية من مرض السكري من النوع الأول |
| KR102878895B1 (ko) | 2019-05-21 | 2025-10-31 | 노파르티스 아게 | Cd19 결합 분자 및 이의 용도 |
| EP3990022A4 (en) * | 2019-06-26 | 2023-06-28 | Memorial Sloan Kettering Cancer Center | Anti-cd33 antibodies for treating cancer |
| JP2022539038A (ja) * | 2019-06-27 | 2022-09-07 | ヴェルソー セラピューティクス, インコーポレイテッド | 骨髄細胞炎症性表現型を調節するための抗lrrc25組成物及び方法、ならびにその使用 |
| CA3173210A1 (en) * | 2020-03-31 | 2021-10-07 | Cameron J. Turtle | Chimeric antigen receptors targeting cd33 |
| KR20230092863A (ko) | 2020-06-11 | 2023-06-26 | 프로벤션 바이오, 인코포레이티드 | 제1형 당뇨병을 예방하기 위한 방법 및 조성물 |
| US11919958B2 (en) | 2020-08-19 | 2024-03-05 | Xencor, Inc. | Anti-CD28 compositions |
| KR20230166075A (ko) * | 2021-02-02 | 2023-12-06 | 누맙 세러퓨틱스 아게 | Ror1 및 cd3에 대한 특이성을 가지는 다중 특이적 항체 |
| IL314993A (en) | 2022-02-23 | 2024-10-01 | Xencor Inc | Anti-cd28 x anti-psma antibodies |
Family Cites Families (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3920358A1 (de) | 1989-06-22 | 1991-01-17 | Behringwerke Ag | Bispezifische und oligospezifische, mono- und oligovalente antikoerperkonstrukte, ihre herstellung und verwendung |
| GB8928874D0 (en) | 1989-12-21 | 1990-02-28 | Celltech Ltd | Humanised antibodies |
| US5571894A (en) | 1991-02-05 | 1996-11-05 | Ciba-Geigy Corporation | Recombinant antibodies specific for a growth factor receptor |
| SG89295A1 (en) | 1991-03-15 | 2002-06-18 | Amgen Inc | Pegylation of polypeptides |
| GB9114948D0 (en) | 1991-07-11 | 1991-08-28 | Pfizer Ltd | Process for preparing sertraline intermediates |
| FI941572L (fi) | 1991-10-07 | 1994-05-27 | Oncologix Inc | Anti-erbB-2-monoklonaalisten vasta-aineiden yhdistelmä ja käyttömenetelmä |
| DE69333807T2 (de) | 1992-02-06 | 2006-02-02 | Chiron Corp., Emeryville | Marker für krebs und biosynthetisches bindeprotein dafür |
| US5869046A (en) | 1995-04-14 | 1999-02-09 | Genentech, Inc. | Altered polypeptides with increased half-life |
| JP2002540764A (ja) | 1999-02-12 | 2002-12-03 | ジェネティックス・インスチチュート・インコーポレーテッド | B7分子と反応するヒト化免疫グロブリンおよびそれを用いた治療方法 |
| WO2001014424A2 (en) | 1999-08-24 | 2001-03-01 | Medarex, Inc. | Human ctla-4 antibodies and their uses |
| JP2003520828A (ja) | 2000-01-27 | 2003-07-08 | ジェネティクス インスティテュート,エルエルシー | Ctla4(cd152)に対する抗体、これを含む結合体、およびその使用 |
| EP1354600A1 (en) | 2002-04-19 | 2003-10-22 | Affimed Therapeutics AG | Antibody combination useful for tumor therapy |
| AU2004253770C1 (en) | 2003-07-02 | 2010-04-15 | Innate Pharma | PAN-KIR2DL NK-receptor antibodies and their use in diagnostik and therapy |
| US7288638B2 (en) | 2003-10-10 | 2007-10-30 | Bristol-Myers Squibb Company | Fully human antibodies against human 4-1BB |
| ATE475669T1 (de) | 2004-06-29 | 2010-08-15 | Immunocore Ltd | Einen modifizierten t-zellen-rezeptor exprimierende zellen |
| CA2594356C (en) | 2005-01-05 | 2018-07-17 | F-Star Biotechnologische Forschungs- Und Entwicklungsges.M.B.H. | Synthetic immunoglobulin domains with binding properties engineered in regions of the molecule different from the complementarity determining regions |
| EP1896582A4 (en) | 2005-05-09 | 2009-04-08 | Ono Pharmaceutical Co | HUMAN MONOCLONAL ANTIBODIES AGAINST PROGRAMMED CELL DEATH 1 (PD-1) AND METHOD FOR CREATING TREATMENT WITH ANTI-PD-1 ANTIBODIES ALONE OR IN COMBINATION WITH OTHER IMMUNOTHERAPEUTICS |
| WO2008103849A2 (en) | 2007-02-21 | 2008-08-28 | The Regents Of The University Of California | Methods and compounds for lymphoma cell detection and isolation |
| EP1829895A1 (en) | 2006-03-03 | 2007-09-05 | f-star Biotechnologische Forschungs- und Entwicklungsges.m.b.H. | Bispecific molecule binding TLR9 and CD32 and comprising a T cell epitope for treatment of allergies |
| NZ577085A (en) | 2006-11-15 | 2012-06-29 | Medarex Inc | Human monoclonal antibodies to btla and methods of use |
| WO2008076868A2 (en) | 2006-12-18 | 2008-06-26 | Abbott Laboratories | Methods and compositions related to modulation of receptor tyrosine kinase orphan receptor-1 (ror-1) |
| US8163279B2 (en) | 2007-04-13 | 2012-04-24 | Stemline Therapeutics, Inc. | IL3Rα antibody conjugates and uses thereof |
| US20090162359A1 (en) | 2007-12-21 | 2009-06-25 | Christian Klein | Bivalent, bispecific antibodies |
| US8242247B2 (en) | 2007-12-21 | 2012-08-14 | Hoffmann-La Roche Inc. | Bivalent, bispecific antibodies |
| US8227577B2 (en) | 2007-12-21 | 2012-07-24 | Hoffman-La Roche Inc. | Bivalent, bispecific antibodies |
| US9266967B2 (en) | 2007-12-21 | 2016-02-23 | Hoffmann-La Roche, Inc. | Bivalent, bispecific antibodies |
| EP2316491B1 (en) | 2008-07-18 | 2016-10-12 | National University Corporation Nagoya University | Cell proliferation inhibitor |
| US8552154B2 (en) | 2008-09-26 | 2013-10-08 | Emory University | Anti-PD-L1 antibodies and uses therefor |
| WO2010065939A1 (en) | 2008-12-05 | 2010-06-10 | Indiana University Research & Technology Corporation | Combination therapy to enhace nk cell mediated cytotoxicty |
| TW202442680A (zh) | 2008-12-09 | 2024-11-01 | 美商建南德克公司 | 抗pd-l1抗體及其於增進t細胞功能之用途 |
| CN102369215B (zh) | 2009-04-02 | 2015-01-21 | 罗切格利卡特公司 | 包含全长抗体和单链Fab片段的多特异性抗体 |
| ES2537100T3 (es) | 2009-04-07 | 2015-06-02 | Roche Glycart Ag | Anticuerpos biespecíficos trivalentes |
| WO2010124188A1 (en) | 2009-04-23 | 2010-10-28 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Anti-human ror1 antibodies |
| BRPI1007602A2 (pt) | 2009-05-27 | 2016-02-16 | Hoffmann La Roche | "anticorpo tri ou tetraespecífico, método para preparação de um anticorpo triespecífico ou tetraespecífico, célula hospedeira, composição, composição farmacêutica e método para o tratamento de um paciente com necessidade de terapia" |
| US9676845B2 (en) | 2009-06-16 | 2017-06-13 | Hoffmann-La Roche, Inc. | Bispecific antigen binding proteins |
| US8703132B2 (en) | 2009-06-18 | 2014-04-22 | Hoffmann-La Roche, Inc. | Bispecific, tetravalent antigen binding proteins |
| US20120282177A1 (en) | 2009-11-02 | 2012-11-08 | Christian Rohlff | ROR1 as Therapeutic and Diagnostic Target |
| CN102712695B (zh) | 2009-12-18 | 2015-01-21 | 堪瑟拉公司 | 能够诱导细胞死亡的ror1生物抑制剂 |
| US20110165161A1 (en) * | 2009-12-23 | 2011-07-07 | Shih-Yao Lin | Anti-epcam antibodies that induce apoptosis of cancer cells and methods using same |
| JP2013519682A (ja) | 2010-02-11 | 2013-05-30 | アレクシオン ファーマシューティカルズ, インコーポレイテッド | 抗cd200抗体を使用する治療方法 |
| WO2011159847A2 (en) | 2010-06-15 | 2011-12-22 | The Regents Of The University Of California | Receptor tyrosine kinase-like orphan receptor 1 (ror1) single chain fv antibody fragment conjugates and methods of use thereof |
| WO2012045085A1 (en) | 2010-10-01 | 2012-04-05 | Oxford Biotherapeutics Ltd. | Anti-rori antibodies |
| LT3018145T (lt) * | 2010-10-27 | 2018-05-10 | Amgen Research (Munich) Gmbh | Priemonės ir būdai dlbcl gydymui |
| EP2646469B1 (en) * | 2010-12-01 | 2017-11-01 | The United States of America, as represented by The Secretary, Department of Health and Human Services | Chimeric rabbit/human ror1 antibodies |
| GB201020995D0 (en) | 2010-12-10 | 2011-01-26 | Bioinvent Int Ab | Biological materials and uses thereof |
| PT2663579T (pt) | 2011-01-14 | 2017-07-28 | Univ California | Terapêutica de anticorpos contra a proteína r0r-1 e métodos para sua utilização |
| US20140242081A1 (en) * | 2011-07-18 | 2014-08-28 | Micromet Ag | Dosing regimens for treatment of cea-expressing cancers |
| UY34887A (es) | 2012-07-02 | 2013-12-31 | Bristol Myers Squibb Company Una Corporacion Del Estado De Delaware | Optimización de anticuerpos que se fijan al gen de activación de linfocitos 3 (lag-3) y sus usos |
| SMT202100005T1 (it) * | 2012-08-20 | 2021-05-07 | Hutchinson Fred Cancer Res | Metodo e composizioni per immunoterapia cellulare |
| US20140302037A1 (en) | 2013-03-15 | 2014-10-09 | Amgen Inc. | BISPECIFIC-Fc MOLECULES |
| US20140308285A1 (en) | 2013-03-15 | 2014-10-16 | Amgen Inc. | Heterodimeric bispecific antibodies |
| EP2789630A1 (en) * | 2013-04-09 | 2014-10-15 | EngMab AG | Bispecific antibodies against CD3e and ROR1 |
| IL301714A (en) | 2013-12-24 | 2023-05-01 | Janssen Pharmaceutica Nv | Antibodies and anti-VISTA fragments |
| JOP20200096A1 (ar) | 2014-01-31 | 2017-06-16 | Children’S Medical Center Corp | جزيئات جسم مضاد لـ tim-3 واستخداماتها |
| RU2718692C2 (ru) * | 2014-05-29 | 2020-04-13 | Мэкроудженикс, Инк. | Триспецифичные связывающие молекулы, которые специфически связывают антигены множества злокачественных опухолей, и способы их применения |
| RS62332B1 (sr) * | 2014-11-26 | 2021-10-29 | Xencor Inc | Heterodimerna antitela koja vezuju cd3 i cd20 |
-
2017
- 2017-07-14 US US16/317,782 patent/US20190233534A1/en not_active Abandoned
- 2017-07-14 AU AU2017297603A patent/AU2017297603A1/en not_active Abandoned
- 2017-07-14 WO PCT/US2017/042264 patent/WO2018014001A1/en not_active Ceased
- 2017-07-14 CA CA3030841A patent/CA3030841A1/en not_active Abandoned
- 2017-07-14 JP JP2019501628A patent/JP2019532017A/ja not_active Withdrawn
- 2017-07-14 EP EP17828588.8A patent/EP3484516A4/en active Pending
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023519932A (ja) * | 2020-03-31 | 2023-05-15 | フレッド ハッチンソン キャンサー センター | 抗cd33抗体及びその使用 |
| JP2023520399A (ja) * | 2020-03-31 | 2023-05-17 | フレッド ハッチンソン キャンサー センター | ヒト抗cd33抗体及びその使用 |
| JP2023548443A (ja) * | 2020-10-30 | 2023-11-16 | ジャナックス セラピューティクス,インク. | Cd28およびpd-l1を標的とする多特異性抗体ならびにその使用方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3484516A1 (en) | 2019-05-22 |
| WO2018014001A1 (en) | 2018-01-18 |
| AU2017297603A1 (en) | 2019-02-14 |
| CA3030841A1 (en) | 2018-01-18 |
| EP3484516A4 (en) | 2020-03-18 |
| US20190233534A1 (en) | 2019-08-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2019532017A (ja) | がんを治療するための異なるエピトープ結合を示す複数の二重特異性結合ドメイン構築物 | |
| JP2023518049A (ja) | 新規な抗原結合ドメインおよびそれを組み込んだ合成抗原受容体 | |
| AU2019301070A1 (en) | Fusion constructs and methods of using thereof | |
| IL262321B1 (en) | Compositions and methods for selective protein expression | |
| JP2021524269A (ja) | メソテリン及びcd137結合分子 | |
| US20250064934A1 (en) | Genetically engineered cells having anti-cd19 / anti-cd22 chimeric antigen receptors, and uses thereof | |
| IL297916A (en) | Compositions and methods for tcr reprogramming using cd70 specific fusion proteins | |
| TW202504914A (zh) | 嵌合抗原受體及其用途 | |
| WO2022063291A1 (en) | Fibronectin extra domain b (edb) -specific car-t for cancer | |
| WO2025106626A1 (en) | Genetically engineered cells expressing c-x-c chemokine receptor type 4, and uses thereof | |
| US20250090582A1 (en) | Genetically engineered cells having anti-cd133 / anti-egfr chimeric antigen receptors, and uses thereof | |
| CN114685659B (zh) | Cd22特异性人源化抗体及利用其的嵌合抗原受体 | |
| US20230265185A1 (en) | Anti-cd22 single domain antibodies and therapeutic constructs | |
| AU2021305234A1 (en) | Fusion constructs and methods of using thereof | |
| US20230381317A1 (en) | Methods for controlled activation and/or expansion of genetically engineered cells using polyethylene glycol (peg) receptors | |
| WO2025059589A1 (en) | Immune cell engaging molecules | |
| AU2023375625A1 (en) | Genetically engineered cells having anti-nectin4 chimeric antigen receptors, and uses thereof | |
| US20230212286A1 (en) | Antibodies specific for cd22 and uses thereof | |
| WO2025207798A1 (en) | Genetically engineered cells having anti-cd123 chimeric antigen receptors, and uses thereof | |
| WO2025207795A1 (en) | Genetically engineered cells having anti-cd33 / anti-cd123 chimeric antigen receptors, and uses thereof | |
| WO2025245169A1 (en) | Immunotherapy cells equipped with a collagen-targeting payload | |
| WO2020150513A1 (en) | Methods to enhance the selectivity and effectiveness of cancer treatments | |
| HK40056378B (zh) | 人源化抗claudin18.2嵌合抗原受体及其用途 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200709 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20200709 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20210816 |