JP2013538857A - ジアミド化合物の結晶性シュウ酸塩 - Google Patents
ジアミド化合物の結晶性シュウ酸塩 Download PDFInfo
- Publication number
- JP2013538857A JP2013538857A JP2013531861A JP2013531861A JP2013538857A JP 2013538857 A JP2013538857 A JP 2013538857A JP 2013531861 A JP2013531861 A JP 2013531861A JP 2013531861 A JP2013531861 A JP 2013531861A JP 2013538857 A JP2013538857 A JP 2013538857A
- Authority
- JP
- Japan
- Prior art keywords
- crystalline oxalate
- crystalline
- hydroxy
- oxalate
- salt
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 title claims abstract description 90
- -1 diamide compound Chemical class 0.000 title claims description 24
- 150000003891 oxalate salts Chemical class 0.000 claims abstract description 43
- 238000000034 method Methods 0.000 claims abstract description 42
- 125000004482 piperidin-4-yl group Chemical group N1CCC(CC1)* 0.000 claims abstract description 32
- QMOVGVNKXUTCQU-UHFFFAOYSA-N (2-phenylphenyl)carbamic acid Chemical compound OC(=O)NC1=CC=CC=C1C1=CC=CC=C1 QMOVGVNKXUTCQU-UHFFFAOYSA-N 0.000 claims abstract description 31
- 125000000031 ethylamino group Chemical group [H]C([H])([H])C([H])([H])N([H])[*] 0.000 claims abstract description 30
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 claims abstract description 25
- 208000019693 Lung disease Diseases 0.000 claims abstract description 19
- 150000003839 salts Chemical class 0.000 claims abstract description 16
- 238000004519 manufacturing process Methods 0.000 claims abstract description 4
- 239000003814 drug Substances 0.000 claims description 46
- 239000008194 pharmaceutical composition Substances 0.000 claims description 34
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 25
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 claims description 20
- 239000012453 solvate Substances 0.000 claims description 19
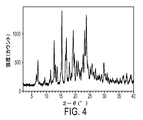
- 238000000634 powder X-ray diffraction Methods 0.000 claims description 15
- 239000002294 steroidal antiinflammatory agent Substances 0.000 claims description 14
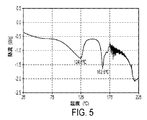
- 238000000113 differential scanning calorimetry Methods 0.000 claims description 13
- 239000000843 powder Substances 0.000 claims description 13
- 238000011282 treatment Methods 0.000 claims description 9
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 claims description 8
- 239000003937 drug carrier Substances 0.000 claims description 8
- 241000124008 Mammalia Species 0.000 claims description 7
- 238000002844 melting Methods 0.000 claims description 7
- 230000008018 melting Effects 0.000 claims description 7
- 208000006673 asthma Diseases 0.000 claims description 6
- 230000007883 bronchodilation Effects 0.000 claims description 6
- 150000004677 hydrates Chemical class 0.000 claims description 3
- 238000002560 therapeutic procedure Methods 0.000 claims description 3
- 239000000203 mixture Substances 0.000 abstract description 60
- ZXZKYYHTWHJHFT-UHFFFAOYSA-N quinoline-2,8-diol Chemical compound C1=CC(=O)NC2=C1C=CC=C2O ZXZKYYHTWHJHFT-UHFFFAOYSA-N 0.000 abstract description 13
- 235000006408 oxalic acid Nutrition 0.000 abstract description 9
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 69
- 150000001875 compounds Chemical class 0.000 description 44
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 43
- 229940124597 therapeutic agent Drugs 0.000 description 43
- 239000000243 solution Substances 0.000 description 34
- 238000002360 preparation method Methods 0.000 description 25
- 210000004027 cell Anatomy 0.000 description 22
- 239000007787 solid Substances 0.000 description 22
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 21
- 238000003556 assay Methods 0.000 description 18
- 239000011541 reaction mixture Substances 0.000 description 18
- 239000012528 membrane Substances 0.000 description 17
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 15
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 13
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 13
- 229940071648 metered dose inhaler Drugs 0.000 description 13
- ZDYUUBIMAGBMPY-UHFFFAOYSA-N oxalic acid;hydrate Chemical compound O.OC(=O)C(O)=O ZDYUUBIMAGBMPY-UHFFFAOYSA-N 0.000 description 13
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 12
- 238000012360 testing method Methods 0.000 description 12
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 11
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 11
- 229940112141 dry powder inhaler Drugs 0.000 description 11
- 239000002244 precipitate Substances 0.000 description 11
- 239000002904 solvent Substances 0.000 description 11
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 10
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 10
- 201000010099 disease Diseases 0.000 description 10
- 238000002411 thermogravimetry Methods 0.000 description 10
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 9
- 239000013078 crystal Substances 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- NZWOPGCLSHLLPA-UHFFFAOYSA-N methacholine Chemical compound C[N+](C)(C)CC(C)OC(C)=O NZWOPGCLSHLLPA-UHFFFAOYSA-N 0.000 description 9
- 229960002329 methacholine Drugs 0.000 description 9
- 239000006199 nebulizer Substances 0.000 description 9
- 239000002002 slurry Substances 0.000 description 9
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 8
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 8
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 8
- 241000700159 Rattus Species 0.000 description 8
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 8
- 238000001914 filtration Methods 0.000 description 8
- BRZYSWJRSDMWLG-CAXSIQPQSA-N geneticin Chemical compound O1C[C@@](O)(C)[C@H](NC)[C@@H](O)[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](C(C)O)O2)N)[C@@H](N)C[C@H]1N BRZYSWJRSDMWLG-CAXSIQPQSA-N 0.000 description 8
- 239000008101 lactose Substances 0.000 description 8
- 239000002245 particle Substances 0.000 description 8
- 238000001144 powder X-ray diffraction data Methods 0.000 description 8
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 7
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 7
- 230000014759 maintenance of location Effects 0.000 description 7
- 239000008188 pellet Substances 0.000 description 7
- 239000000546 pharmaceutical excipient Substances 0.000 description 7
- 239000003380 propellant Substances 0.000 description 7
- 102000014415 Muscarinic acetylcholine receptor Human genes 0.000 description 6
- 108050003473 Muscarinic acetylcholine receptor Proteins 0.000 description 6
- GXCLVBGFBYZDAG-UHFFFAOYSA-N N-[2-(1H-indol-3-yl)ethyl]-N-methylprop-2-en-1-amine Chemical compound CN(CCC1=CNC2=C1C=CC=C2)CC=C GXCLVBGFBYZDAG-UHFFFAOYSA-N 0.000 description 6
- DKGAVHZHDRPRBM-UHFFFAOYSA-N Tert-Butanol Chemical compound CC(C)(C)O DKGAVHZHDRPRBM-UHFFFAOYSA-N 0.000 description 6
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 6
- 238000004458 analytical method Methods 0.000 description 6
- 150000005828 hydrofluoroalkanes Chemical group 0.000 description 6
- 239000010410 layer Substances 0.000 description 6
- 102000005962 receptors Human genes 0.000 description 6
- 108020003175 receptors Proteins 0.000 description 6
- 229920006395 saturated elastomer Polymers 0.000 description 6
- 239000000725 suspension Substances 0.000 description 6
- 108060003345 Adrenergic Receptor Proteins 0.000 description 5
- 102000017910 Adrenergic receptor Human genes 0.000 description 5
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 5
- 101001033280 Homo sapiens Cytokine receptor common subunit beta Proteins 0.000 description 5
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 5
- 239000000048 adrenergic agonist Substances 0.000 description 5
- 229940126157 adrenergic receptor agonist Drugs 0.000 description 5
- 239000000872 buffer Substances 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 238000005516 engineering process Methods 0.000 description 5
- 235000019439 ethyl acetate Nutrition 0.000 description 5
- 239000000706 filtrate Substances 0.000 description 5
- 238000004128 high performance liquid chromatography Methods 0.000 description 5
- 102000055647 human CSF2RB Human genes 0.000 description 5
- 229910052757 nitrogen Inorganic materials 0.000 description 5
- GEVPUGOOGXGPIO-UHFFFAOYSA-N oxalic acid;dihydrate Chemical compound O.O.OC(=O)C(O)=O GEVPUGOOGXGPIO-UHFFFAOYSA-N 0.000 description 5
- ROCHZUNCIZLTRQ-UHFFFAOYSA-N oxalic acid;propan-2-ol Chemical compound CC(C)O.OC(=O)C(O)=O ROCHZUNCIZLTRQ-UHFFFAOYSA-N 0.000 description 5
- 239000001103 potassium chloride Substances 0.000 description 5
- 235000011164 potassium chloride Nutrition 0.000 description 5
- 229910052938 sodium sulfate Inorganic materials 0.000 description 5
- 235000011152 sodium sulphate Nutrition 0.000 description 5
- 239000007858 starting material Substances 0.000 description 5
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 4
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 4
- 229940121948 Muscarinic receptor antagonist Drugs 0.000 description 4
- 238000005481 NMR spectroscopy Methods 0.000 description 4
- 239000002253 acid Substances 0.000 description 4
- 239000000908 ammonium hydroxide Substances 0.000 description 4
- 238000009739 binding Methods 0.000 description 4
- 239000002775 capsule Substances 0.000 description 4
- 239000002552 dosage form Substances 0.000 description 4
- 235000019441 ethanol Nutrition 0.000 description 4
- 239000011521 glass Substances 0.000 description 4
- IXCSERBJSXMMFS-UHFFFAOYSA-N hydrogen chloride Substances Cl.Cl IXCSERBJSXMMFS-UHFFFAOYSA-N 0.000 description 4
- 229910000041 hydrogen chloride Inorganic materials 0.000 description 4
- 230000005764 inhibitory process Effects 0.000 description 4
- 239000012139 lysis buffer Substances 0.000 description 4
- 239000012044 organic layer Substances 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 239000000741 silica gel Substances 0.000 description 4
- 229910002027 silica gel Inorganic materials 0.000 description 4
- 235000017557 sodium bicarbonate Nutrition 0.000 description 4
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 4
- 238000001179 sorption measurement Methods 0.000 description 4
- 238000003756 stirring Methods 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 238000005303 weighing Methods 0.000 description 4
- LVGUZGTVOIAKKC-UHFFFAOYSA-N 1,1,1,2-tetrafluoroethane Chemical compound FCC(F)(F)F LVGUZGTVOIAKKC-UHFFFAOYSA-N 0.000 description 3
- OISVCGZHLKNMSJ-UHFFFAOYSA-N 2,6-dimethylpyridine Chemical compound CC1=CC=CC(C)=N1 OISVCGZHLKNMSJ-UHFFFAOYSA-N 0.000 description 3
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- AKYUMGLGSXQSCR-FAIXQHPJSA-N [1-[3-[[5-[4-[[[(2r)-2-hydroxy-2-(8-hydroxy-2-oxo-1h-quinolin-5-yl)ethyl]amino]methyl]anilino]-5-oxopentyl]-methylamino]-3-oxopropyl]piperidin-4-yl] n-(2-phenylphenyl)carbamate Chemical compound C=1C=C(CNC[C@H](O)C=2C=3C=CC(=O)NC=3C(O)=CC=2)C=CC=1NC(=O)CCCCN(C)C(=O)CCN(CC1)CCC1OC(=O)NC1=CC=CC=C1C1=CC=CC=C1 AKYUMGLGSXQSCR-FAIXQHPJSA-N 0.000 description 3
- 239000000443 aerosol Substances 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- MMXKVMNBHPAILY-UHFFFAOYSA-N ethyl laurate Chemical compound CCCCCCCCCCCC(=O)OCC MMXKVMNBHPAILY-UHFFFAOYSA-N 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 238000010438 heat treatment Methods 0.000 description 3
- 238000007912 intraperitoneal administration Methods 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- 235000019198 oils Nutrition 0.000 description 3
- 239000002953 phosphate buffered saline Substances 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 108090000623 proteins and genes Proteins 0.000 description 3
- 239000002287 radioligand Substances 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- YFMFNYKEUDLDTL-UHFFFAOYSA-N 1,1,1,2,3,3,3-heptafluoropropane Chemical compound FC(F)(F)C(F)C(F)(F)F YFMFNYKEUDLDTL-UHFFFAOYSA-N 0.000 description 2
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 2
- RIXYVXYKLYQCSM-UHFFFAOYSA-N 2-(4-nitrophenyl)-1,3-dioxolane Chemical compound C1=CC([N+](=O)[O-])=CC=C1C1OCCO1 RIXYVXYKLYQCSM-UHFFFAOYSA-N 0.000 description 2
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 2
- CMVZMERLBCRJAH-UHFFFAOYSA-N 5-(methylamino)pentanoic acid Chemical compound CNCCCCC(O)=O CMVZMERLBCRJAH-UHFFFAOYSA-N 0.000 description 2
- HQTMZDNFUYOAMZ-UHFFFAOYSA-N 5-[[2-oxo-4-[4-[(2-phenylphenyl)carbamoyloxy]piperidin-1-yl]butyl]amino]pentanoic acid Chemical compound C1CN(CCC(=O)CNCCCCC(=O)O)CCC1OC(=O)NC1=CC=CC=C1C1=CC=CC=C1 HQTMZDNFUYOAMZ-UHFFFAOYSA-N 0.000 description 2
- 241000700198 Cavia Species 0.000 description 2
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 2
- 206010014561 Emphysema Diseases 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- 239000007995 HEPES buffer Substances 0.000 description 2
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 2
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 2
- 102100032341 PCNA-interacting partner Human genes 0.000 description 2
- 101710196737 PCNA-interacting partner Proteins 0.000 description 2
- OFBQJSOFQDEBGM-UHFFFAOYSA-N Pentane Chemical compound CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 2
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- GGXPEVAVWPDHCQ-UHFFFAOYSA-N [1-[3-[[5-[4-(1,3-dioxolan-2-yl)anilino]-5-oxopentyl]-methylamino]-3-oxopropyl]piperidin-4-yl] n-(2-phenylphenyl)carbamate Chemical compound C1CC(OC(=O)NC=2C(=CC=CC=2)C=2C=CC=CC=2)CCN1CCC(=O)N(C)CCCCC(=O)NC(C=C1)=CC=C1C1OCCO1 GGXPEVAVWPDHCQ-UHFFFAOYSA-N 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 239000012131 assay buffer Substances 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 206010006451 bronchitis Diseases 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- NEHMKBQYUWJMIP-UHFFFAOYSA-N chloromethane Chemical compound ClC NEHMKBQYUWJMIP-UHFFFAOYSA-N 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 239000002178 crystalline material Substances 0.000 description 2
- 238000002425 crystallisation Methods 0.000 description 2
- 230000008025 crystallization Effects 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 150000004683 dihydrates Chemical class 0.000 description 2
- 239000013024 dilution buffer Substances 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- 230000001747 exhibiting effect Effects 0.000 description 2
- 239000012065 filter cake Substances 0.000 description 2
- 238000003818 flash chromatography Methods 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 235000011187 glycerol Nutrition 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- IKGLACJFEHSFNN-UHFFFAOYSA-N hydron;triethylazanium;trifluoride Chemical compound F.F.F.CCN(CC)CC IKGLACJFEHSFNN-UHFFFAOYSA-N 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 239000000543 intermediate Substances 0.000 description 2
- 235000010445 lecithin Nutrition 0.000 description 2
- 239000000787 lecithin Substances 0.000 description 2
- 229940067606 lecithin Drugs 0.000 description 2
- 238000012417 linear regression Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- KKNMRJYPMNVNLR-UHFFFAOYSA-N methyl 5-(methylamino)pentanoate;hydrochloride Chemical compound Cl.CNCCCCC(=O)OC KKNMRJYPMNVNLR-UHFFFAOYSA-N 0.000 description 2
- HNBDRPTVWVGKBR-UHFFFAOYSA-N methyl pentanoate Chemical compound CCCCC(=O)OC HNBDRPTVWVGKBR-UHFFFAOYSA-N 0.000 description 2
- 239000003595 mist Substances 0.000 description 2
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 2
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- 239000006069 physical mixture Substances 0.000 description 2
- 239000006187 pill Substances 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 230000002685 pulmonary effect Effects 0.000 description 2
- MIXMJCQRHVAJIO-TZHJZOAOSA-N qk4dys664x Chemical compound O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O MIXMJCQRHVAJIO-TZHJZOAOSA-N 0.000 description 2
- 238000003653 radioligand binding assay Methods 0.000 description 2
- 230000000241 respiratory effect Effects 0.000 description 2
- 210000002345 respiratory system Anatomy 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 208000017520 skin disease Diseases 0.000 description 2
- 238000002336 sorption--desorption measurement Methods 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 238000002076 thermal analysis method Methods 0.000 description 2
- IDELNEDBPWKHGK-UHFFFAOYSA-N thiobutabarbital Chemical compound CCC(C)C1(CC)C(=O)NC(=S)NC1=O IDELNEDBPWKHGK-UHFFFAOYSA-N 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- RDJLHRNSBYDPRB-UHFFFAOYSA-N (2-phenylphenyl)carbamic acid;hydrochloride Chemical compound Cl.OC(=O)NC1=CC=CC=C1C1=CC=CC=C1 RDJLHRNSBYDPRB-UHFFFAOYSA-N 0.000 description 1
- MSEZLHAVPJYYIQ-VMXHOPILSA-N (8s,9s,10r,13s,14s)-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CCC[C@@]1(C)CC2 MSEZLHAVPJYYIQ-VMXHOPILSA-N 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- FPIRBHDGWMWJEP-UHFFFAOYSA-N 1-hydroxy-7-azabenzotriazole Chemical compound C1=CN=C2N(O)N=NC2=C1 FPIRBHDGWMWJEP-UHFFFAOYSA-N 0.000 description 1
- GGYVTHJIUNGKFZ-UHFFFAOYSA-N 1-methylpiperidin-2-one Chemical compound CN1CCCCC1=O GGYVTHJIUNGKFZ-UHFFFAOYSA-N 0.000 description 1
- ABEXEQSGABRUHS-UHFFFAOYSA-N 16-methylheptadecyl 16-methylheptadecanoate Chemical compound CC(C)CCCCCCCCCCCCCCCOC(=O)CCCCCCCCCCCCCCC(C)C ABEXEQSGABRUHS-UHFFFAOYSA-N 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- XWKFPIODWVPXLX-UHFFFAOYSA-N 2-methyl-5-methylpyridine Natural products CC1=CC=C(C)N=C1 XWKFPIODWVPXLX-UHFFFAOYSA-N 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 1
- XRRRDJZFAVCWEY-UHFFFAOYSA-N 3-[4-[(2-phenylphenyl)carbamoyloxy]piperidin-1-yl]propanoic acid Chemical compound C1CN(CCC(=O)O)CCC1OC(=O)NC1=CC=CC=C1C1=CC=CC=C1 XRRRDJZFAVCWEY-UHFFFAOYSA-N 0.000 description 1
- AGTSXXZWXWHAKH-UHFFFAOYSA-N 4-(1,3-dioxolan-2-yl)aniline Chemical compound C1=CC(N)=CC=C1C1OCCO1 AGTSXXZWXWHAKH-UHFFFAOYSA-N 0.000 description 1
- BXRFQSNOROATLV-UHFFFAOYSA-N 4-nitrobenzaldehyde Chemical compound [O-][N+](=O)C1=CC=C(C=O)C=C1 BXRFQSNOROATLV-UHFFFAOYSA-N 0.000 description 1
- FJNCXZZQNBKEJT-UHFFFAOYSA-N 8beta-hydroxymarrubiin Natural products O1C(=O)C2(C)CCCC3(C)C2C1CC(C)(O)C3(O)CCC=1C=COC=1 FJNCXZZQNBKEJT-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- 229930000680 A04AD01 - Scopolamine Natural products 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 208000000884 Airway Obstruction Diseases 0.000 description 1
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- KUVIULQEHSCUHY-XYWKZLDCSA-N Beclometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O KUVIULQEHSCUHY-XYWKZLDCSA-N 0.000 description 1
- 206010006482 Bronchospasm Diseases 0.000 description 1
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 1
- 210000003771 C cell Anatomy 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- QGBIFMJAQARMNQ-QISPFCDLSA-N C1([C@@H](F)C2)=CC(=O)CC[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H]3O[C@@H](CCC)O[C@@]3(SC)[C@@]2(C)C[C@@H]1O Chemical compound C1([C@@H](F)C2)=CC(=O)CC[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H]3O[C@@H](CCC)O[C@@]3(SC)[C@@]2(C)C[C@@H]1O QGBIFMJAQARMNQ-QISPFCDLSA-N 0.000 description 1
- 206010007559 Cardiac failure congestive Diseases 0.000 description 1
- LUKZNWIVRBCLON-GXOBDPJESA-N Ciclesonide Chemical compound C1([C@H]2O[C@@]3([C@H](O2)C[C@@H]2[C@@]3(C[C@H](O)[C@@H]3[C@@]4(C)C=CC(=O)C=C4CC[C@H]32)C)C(=O)COC(=O)C(C)C)CCCCC1 LUKZNWIVRBCLON-GXOBDPJESA-N 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- QTOWOWRJXDRIRS-UHFFFAOYSA-N Cl.CNCCCCC(=O)OC.COC(CCCCNCC(CCN1CCC(CC1)OC(NC1=C(C=CC=C1)C1=CC=CC=C1)=O)=O)=O Chemical compound Cl.CNCCCCC(=O)OC.COC(CCCCNCC(CCN1CCC(CC1)OC(NC1=C(C=CC=C1)C1=CC=CC=C1)=O)=O)=O QTOWOWRJXDRIRS-UHFFFAOYSA-N 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 241000699802 Cricetulus griseus Species 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 239000012591 Dulbecco’s Phosphate Buffered Saline Substances 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 206010019280 Heart failures Diseases 0.000 description 1
- STECJAGHUSJQJN-GAUPFVANSA-N Hyoscine Natural products C1([C@H](CO)C(=O)OC2C[C@@H]3N([C@H](C2)[C@@H]2[C@H]3O2)C)=CC=CC=C1 STECJAGHUSJQJN-GAUPFVANSA-N 0.000 description 1
- 241000764238 Isis Species 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 1
- 206010028289 Muscle atrophy Diseases 0.000 description 1
- STECJAGHUSJQJN-UHFFFAOYSA-N N-Methyl-scopolamin Natural products C1C(C2C3O2)N(C)C3CC1OC(=O)C(CO)C1=CC=CC=C1 STECJAGHUSJQJN-UHFFFAOYSA-N 0.000 description 1
- GRTSKMXVKQOBGT-UHFFFAOYSA-N N1CCC(CC1)OC(NC1=C(C=CC=C1)C1=CC=CC=C1)=O.C1(=C(C=CC=C1)NC(=O)OC1CCN(CC1)CCC(=O)O)C1=CC=CC=C1 Chemical compound N1CCC(CC1)OC(NC1=C(C=CC=C1)C1=CC=CC=C1)=O.C1(=C(C=CC=C1)NC(=O)OC1CCN(CC1)CCC(=O)O)C1=CC=CC=C1 GRTSKMXVKQOBGT-UHFFFAOYSA-N 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 208000008469 Peptic Ulcer Diseases 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 208000005107 Premature Birth Diseases 0.000 description 1
- 206010036590 Premature baby Diseases 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- 235000019485 Safflower oil Nutrition 0.000 description 1
- 239000004147 Sorbitan trioleate Substances 0.000 description 1
- PRXRUNOAOLTIEF-ADSICKODSA-N Sorbitan trioleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@@H](OC(=O)CCCCCCC\C=C/CCCCCCCC)[C@H]1OC[C@H](O)[C@H]1OC(=O)CCCCCCC\C=C/CCCCCCCC PRXRUNOAOLTIEF-ADSICKODSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 208000025865 Ulcer Diseases 0.000 description 1
- CDTDFZAEQJJCLM-UHFFFAOYSA-N [1-[3-[[5-(4-formylanilino)-5-oxopentyl]-methylamino]-3-oxopropyl]piperidin-4-yl] n-(2-phenylphenyl)carbamate Chemical compound C1CC(OC(=O)NC=2C(=CC=CC=2)C=2C=CC=CC=2)CCN1CCC(=O)N(C)CCCCC(=O)NC1=CC=C(C=O)C=C1 CDTDFZAEQJJCLM-UHFFFAOYSA-N 0.000 description 1
- IQJOOAQPMQDECI-UHFFFAOYSA-N acetic acid;1h-quinolin-2-one Chemical compound CC(O)=O.C1=CC=C2NC(=O)C=CC2=C1 IQJOOAQPMQDECI-UHFFFAOYSA-N 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- OIPILFWXSMYKGL-UHFFFAOYSA-N acetylcholine Chemical compound CC(=O)OCC[N+](C)(C)C OIPILFWXSMYKGL-UHFFFAOYSA-N 0.000 description 1
- 229960004373 acetylcholine Drugs 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- YAJCHEVQCOHZDC-QMMNLEPNSA-N actrapid Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CSSC[C@H]2C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@@H](C(N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3C=CC(O)=CC=3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3N=CNC=3)NC(=O)[C@H](CO)NC(=O)CNC1=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@H](C)O)C(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O)=O)CSSC[C@@H](C(N2)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@H](C)CC)[C@H](C)CC)[C@H](C)O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C(N)=O)C1=CNC=N1 YAJCHEVQCOHZDC-QMMNLEPNSA-N 0.000 description 1
- YKIOKAURTKXMSB-UHFFFAOYSA-N adams's catalyst Chemical compound O=[Pt]=O YKIOKAURTKXMSB-UHFFFAOYSA-N 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 102000030621 adenylate cyclase Human genes 0.000 description 1
- 108060000200 adenylate cyclase Proteins 0.000 description 1
- 238000005273 aeration Methods 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 230000001078 anti-cholinergic effect Effects 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 230000002155 anti-virotic effect Effects 0.000 description 1
- 229940125715 antihistaminic agent Drugs 0.000 description 1
- 239000000739 antihistaminic agent Substances 0.000 description 1
- 229960005475 antiinfective agent Drugs 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 229950000210 beclometasone dipropionate Drugs 0.000 description 1
- NBMKJKDGKREAPL-DVTGEIKXSA-N beclomethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O NBMKJKDGKREAPL-DVTGEIKXSA-N 0.000 description 1
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 1
- 239000012964 benzotriazole Substances 0.000 description 1
- 102000015005 beta-adrenergic receptor activity proteins Human genes 0.000 description 1
- 108040006818 beta-adrenergic receptor activity proteins Proteins 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 239000004044 bronchoconstricting agent Substances 0.000 description 1
- 230000007885 bronchoconstriction Effects 0.000 description 1
- 230000003435 bronchoconstrictive effect Effects 0.000 description 1
- 229940124630 bronchodilator Drugs 0.000 description 1
- 239000000168 bronchodilator agent Substances 0.000 description 1
- 230000000178 bronchoprotective effect Effects 0.000 description 1
- 229960004436 budesonide Drugs 0.000 description 1
- 238000013262 cAMP assay Methods 0.000 description 1
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 239000013553 cell monolayer Substances 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- 229960003728 ciclesonide Drugs 0.000 description 1
- 239000000701 coagulant Substances 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 239000012050 conventional carrier Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 239000006184 cosolvent Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- 238000013480 data collection Methods 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 238000003795 desorption Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 150000001470 diamides Chemical class 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000000921 elemental analysis Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 230000009969 flowable effect Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 229960000676 flunisolide Drugs 0.000 description 1
- 229960001469 fluticasone furoate Drugs 0.000 description 1
- XTULMSXFIHGYFS-VLSRWLAYSA-N fluticasone furoate Chemical compound O([C@]1([C@@]2(C)C[C@H](O)[C@]3(F)[C@@]4(C)C=CC(=O)C=C4[C@@H](F)C[C@H]3[C@@H]2C[C@H]1C)C(=O)SCF)C(=O)C1=CC=CO1 XTULMSXFIHGYFS-VLSRWLAYSA-N 0.000 description 1
- 229960000289 fluticasone propionate Drugs 0.000 description 1
- WMWTYOKRWGGJOA-CENSZEJFSA-N fluticasone propionate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(OC(=O)CC)[C@@]2(C)C[C@@H]1O WMWTYOKRWGGJOA-CENSZEJFSA-N 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 230000005714 functional activity Effects 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 229960005150 glycerol Drugs 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 229960001340 histamine Drugs 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 238000005417 image-selected in vivo spectroscopy Methods 0.000 description 1
- 238000000099 in vitro assay Methods 0.000 description 1
- 238000005462 in vivo assay Methods 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000012739 integrated shape imaging system Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 210000004731 jugular vein Anatomy 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 1
- 239000000347 magnesium hydroxide Substances 0.000 description 1
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 229940050176 methyl chloride Drugs 0.000 description 1
- 229960004584 methylprednisolone Drugs 0.000 description 1
- 125000002816 methylsulfanyl group Chemical group [H]C([H])([H])S[*] 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 229960002744 mometasone furoate Drugs 0.000 description 1
- WOFMFGQZHJDGCX-ZULDAHANSA-N mometasone furoate Chemical compound O([C@]1([C@@]2(C)C[C@H](O)[C@]3(Cl)[C@@]4(C)C=CC(=O)C=C4CC[C@H]3[C@@H]2C[C@H]1C)C(=O)CCl)C(=O)C1=CC=CO1 WOFMFGQZHJDGCX-ZULDAHANSA-N 0.000 description 1
- 229910021421 monocrystalline silicon Inorganic materials 0.000 description 1
- 239000003149 muscarinic antagonist Substances 0.000 description 1
- 230000003551 muscarinic effect Effects 0.000 description 1
- 201000000585 muscular atrophy Diseases 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 230000000414 obstructive effect Effects 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 229960002969 oleic acid Drugs 0.000 description 1
- 235000021313 oleic acid Nutrition 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 239000008055 phosphate buffer solution Substances 0.000 description 1
- 239000002570 phosphodiesterase III inhibitor Substances 0.000 description 1
- 239000002587 phosphodiesterase IV inhibitor Substances 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 229960005205 prednisolone Drugs 0.000 description 1
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 239000012268 protein inhibitor Substances 0.000 description 1
- 229940121649 protein inhibitor Drugs 0.000 description 1
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 1
- 230000001185 psoriatic effect Effects 0.000 description 1
- 208000005069 pulmonary fibrosis Diseases 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000003127 radioimmunoassay Methods 0.000 description 1
- IXTCZMJQGGONPY-XJAYAHQCSA-N rofleponide Chemical compound C1([C@@H](F)C2)=CC(=O)CC[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H]3O[C@@H](CCC)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O IXTCZMJQGGONPY-XJAYAHQCSA-N 0.000 description 1
- 229950004432 rofleponide Drugs 0.000 description 1
- 235000005713 safflower oil Nutrition 0.000 description 1
- 239000003813 safflower oil Substances 0.000 description 1
- STECJAGHUSJQJN-FWXGHANASA-N scopolamine Chemical compound C1([C@@H](CO)C(=O)O[C@H]2C[C@@H]3N([C@H](C2)[C@@H]2[C@H]3O2)C)=CC=CC=C1 STECJAGHUSJQJN-FWXGHANASA-N 0.000 description 1
- 229960002646 scopolamine Drugs 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 230000001568 sexual effect Effects 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000012321 sodium triacetoxyborohydride Substances 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 235000019337 sorbitan trioleate Nutrition 0.000 description 1
- 229960000391 sorbitan trioleate Drugs 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 238000013222 sprague-dawley male rat Methods 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 239000012258 stirred mixture Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 238000000967 suction filtration Methods 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 229940037128 systemic glucocorticoids Drugs 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- CZDYPVPMEAXLPK-UHFFFAOYSA-N tetramethylsilane Chemical compound C[Si](C)(C)C CZDYPVPMEAXLPK-UHFFFAOYSA-N 0.000 description 1
- 229940026152 thiobutabarbital Drugs 0.000 description 1
- 210000003437 trachea Anatomy 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 229960002117 triamcinolone acetonide Drugs 0.000 description 1
- YNDXUCZADRHECN-JNQJZLCISA-N triamcinolone acetonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O YNDXUCZADRHECN-JNQJZLCISA-N 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 230000036269 ulceration Effects 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 238000009423 ventilation Methods 0.000 description 1
- 239000011534 wash buffer Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/444—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a six-membered ring with nitrogen as a ring heteroatom, e.g. amrinone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4709—Non-condensed quinolines and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/08—Bronchodilators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pulmonology (AREA)
- Epidemiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US38814810P | 2010-09-30 | 2010-09-30 | |
| US61/388,148 | 2010-09-30 | ||
| PCT/US2011/053997 WO2012044825A1 (en) | 2010-09-30 | 2011-09-29 | Crystalline oxalate salts of a diamide compound |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015111163A Division JP2015155470A (ja) | 2010-09-30 | 2015-06-01 | ジアミド化合物の結晶性シュウ酸塩 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2013538857A true JP2013538857A (ja) | 2013-10-17 |
| JP2013538857A5 JP2013538857A5 (enExample) | 2014-09-04 |
Family
ID=44903347
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013531861A Ceased JP2013538857A (ja) | 2010-09-30 | 2011-09-29 | ジアミド化合物の結晶性シュウ酸塩 |
| JP2015111163A Pending JP2015155470A (ja) | 2010-09-30 | 2015-06-01 | ジアミド化合物の結晶性シュウ酸塩 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015111163A Pending JP2015155470A (ja) | 2010-09-30 | 2015-06-01 | ジアミド化合物の結晶性シュウ酸塩 |
Country Status (19)
| Country | Link |
|---|---|
| US (1) | US8697724B2 (enExample) |
| EP (1) | EP2621917A1 (enExample) |
| JP (2) | JP2013538857A (enExample) |
| KR (1) | KR20130139919A (enExample) |
| CN (1) | CN103140485B (enExample) |
| AR (1) | AR083115A1 (enExample) |
| AU (1) | AU2011308770B2 (enExample) |
| BR (1) | BR112013007062A2 (enExample) |
| CA (1) | CA2810896A1 (enExample) |
| CL (1) | CL2013000870A1 (enExample) |
| CO (1) | CO6700846A2 (enExample) |
| EA (1) | EA022018B1 (enExample) |
| IL (1) | IL224985A (enExample) |
| MX (1) | MX2013003558A (enExample) |
| NZ (1) | NZ608721A (enExample) |
| PH (1) | PH12013500567A1 (enExample) |
| SG (1) | SG188390A1 (enExample) |
| TW (1) | TW201217338A (enExample) |
| WO (1) | WO2012044825A1 (enExample) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ITRM20110083U1 (it) | 2010-05-13 | 2011-11-14 | De La Cruz Jose Antonio Freire | Piastra per la costruzione di carrelli per aeroplani |
| EP2386555A1 (en) | 2010-05-13 | 2011-11-16 | Almirall, S.A. | New cyclohexylamine derivatives having beta2 adrenergic agonist and m3 muscarinic antagonist activities |
| EP2592078A1 (en) | 2011-11-11 | 2013-05-15 | Almirall, S.A. | New cyclohexylamine derivatives having beta2 adrenergic agonist and M3 muscarinic antagonist activities |
| BR112015013628A2 (pt) | 2012-12-18 | 2017-07-11 | Almirall Sa | derivados de carbamato de ciclo-hexila e quinuclidinila tendo atividades agonista adrenérgica de beta2 e antagonista muscarínica de m3 |
| TWI643853B (zh) | 2013-02-27 | 2018-12-11 | 阿爾米雷爾有限公司 | 同時具有β2腎上腺素受體促效劑和M3毒蕈鹼受體拮抗劑活性之2-氨基-1-羥乙基-8-羥基喹啉-2(1H)-酮衍生物之鹽類 |
| TWI641373B (zh) | 2013-07-25 | 2018-11-21 | 阿爾米雷爾有限公司 | 具有蕈毒鹼受體拮抗劑和β2腎上腺素受體促效劑二者之活性的2-胺基-1-羥乙基-8-羥基喹啉-2(1H)-酮衍生物之鹽 |
| TW201517906A (zh) | 2013-07-25 | 2015-05-16 | Almirall Sa | 含有maba化合物和皮質類固醇之組合 |
| CN104030933A (zh) * | 2014-05-16 | 2014-09-10 | 烟台恒迪克能源科技有限公司 | 一种β-异烷醇基氨基戊酸环己胺的合成方法 |
| TW201617343A (zh) | 2014-09-26 | 2016-05-16 | 阿爾米雷爾有限公司 | 具有β2腎上腺素促效劑及M3蕈毒拮抗劑活性之新穎雙環衍生物 |
| CN117924216A (zh) * | 2024-01-12 | 2024-04-26 | 王叔和生物医药(武汉)有限公司 | 一种1-哌啶丙酸的合成方法 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006522134A (ja) * | 2003-04-01 | 2006-09-28 | セラヴァンス インコーポレーテッド | β2アドレナリン作用性レセプターアゴニスト活性およびムスカリン性レセプターアンタゴニスト活性を有するジアリールメチル化合物および関連化合物 |
| JP2008510015A (ja) * | 2004-08-16 | 2008-04-03 | セラヴァンス, インコーポレーテッド | β2アドレナリン作用性レセプターアゴニスト活性およびムスカリン性レセプターアンタゴニスト活性を有する化合物 |
| JP2009526018A (ja) * | 2006-02-10 | 2009-07-16 | グラクソ グループ リミテッド | ビフェニル−2−イルカルバミン酸1−[2−(2−クロロ−4−{[(r)−2−ヒドロキシ−2−(8−ヒドロキシ−2−オキソ−1,2−ジヒドロキノリン−5−イル)エチルアミノメチル]}−5−メトキシフェニルカルバモイル)エチル]ピペリジン−4−イルエステルのコハク酸塩および肺疾患治療におけるその使用 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6759398B2 (en) | 2000-08-05 | 2004-07-06 | Smithkline Beecham Corporation | Anti-inflammatory androstane derivative |
| CO5310534A1 (es) | 2000-08-05 | 2003-08-29 | Glaxo Group Ltd | Nuevos derivados de androstano anti-inflamatorios |
| US6750210B2 (en) | 2000-08-05 | 2004-06-15 | Smithkline Beecham Corporation | Formulation containing novel anti-inflammatory androstane derivative |
| US6858596B2 (en) | 2000-08-05 | 2005-02-22 | Smithkline Beecham Corporation | Formulation containing anti-inflammatory androstane derivative |
| UA77656C2 (en) | 2001-04-07 | 2007-01-15 | Glaxo Group Ltd | S-fluoromethyl ester of 6-alpha, 9-alpha-difluoro-17-alpha-[(2-furanylcarbonyl)oxy]-11-beta-hydroxy-16- alpha-methyl-3-oxoandrosta-1,4-dien-17-beta-carbothioacid as anti-inflammatory agent |
| PE20040950A1 (es) * | 2003-02-14 | 2005-01-01 | Theravance Inc | DERIVADOS DE BIFENILO COMO AGONISTAS DE LOS RECEPTORES ADRENERGICOS ß2 Y COMO ANTAGONISTAS DE LOS RECEPTORES MUSCARINICOS |
| WO2005080375A1 (en) | 2004-02-13 | 2005-09-01 | Theravance, Inc. | Crystalline form of a biphenyl compound |
| TWI374883B (en) | 2004-08-16 | 2012-10-21 | Theravance Inc | Crystalline form of a biphenyl compound |
| TW200811104A (en) | 2006-04-25 | 2008-03-01 | Theravance Inc | Crystalline forms of a dimethylphenyl compound |
| CN103936716B (zh) * | 2009-04-23 | 2016-09-07 | 施万呼吸有限责任公司 | 具蕈毒碱受体拮抗剂和β2肾上腺素受体激动剂活性的二酰胺化合物 |
-
2011
- 2011-09-26 AR ARP110103518A patent/AR083115A1/es unknown
- 2011-09-29 SG SG2013016043A patent/SG188390A1/en unknown
- 2011-09-29 CA CA2810896A patent/CA2810896A1/en not_active Abandoned
- 2011-09-29 PH PH1/2013/500567A patent/PH12013500567A1/en unknown
- 2011-09-29 WO PCT/US2011/053997 patent/WO2012044825A1/en not_active Ceased
- 2011-09-29 JP JP2013531861A patent/JP2013538857A/ja not_active Ceased
- 2011-09-29 EP EP11771325.5A patent/EP2621917A1/en not_active Withdrawn
- 2011-09-29 NZ NZ60872111A patent/NZ608721A/en not_active IP Right Cessation
- 2011-09-29 US US13/248,840 patent/US8697724B2/en not_active Expired - Fee Related
- 2011-09-29 CN CN201180046585.5A patent/CN103140485B/zh not_active Expired - Fee Related
- 2011-09-29 BR BR112013007062A patent/BR112013007062A2/pt not_active IP Right Cessation
- 2011-09-29 MX MX2013003558A patent/MX2013003558A/es not_active Application Discontinuation
- 2011-09-29 AU AU2011308770A patent/AU2011308770B2/en not_active Ceased
- 2011-09-29 EA EA201390481A patent/EA022018B1/ru not_active IP Right Cessation
- 2011-09-29 KR KR1020137009556A patent/KR20130139919A/ko not_active Withdrawn
- 2011-09-30 TW TW100135675A patent/TW201217338A/zh unknown
-
2013
- 2013-02-28 IL IL224985A patent/IL224985A/en not_active IP Right Cessation
- 2013-03-28 CL CL2013000870A patent/CL2013000870A1/es unknown
- 2013-04-02 CO CO13061812A patent/CO6700846A2/es active IP Right Grant
-
2015
- 2015-06-01 JP JP2015111163A patent/JP2015155470A/ja active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006522134A (ja) * | 2003-04-01 | 2006-09-28 | セラヴァンス インコーポレーテッド | β2アドレナリン作用性レセプターアゴニスト活性およびムスカリン性レセプターアンタゴニスト活性を有するジアリールメチル化合物および関連化合物 |
| JP2008510015A (ja) * | 2004-08-16 | 2008-04-03 | セラヴァンス, インコーポレーテッド | β2アドレナリン作用性レセプターアゴニスト活性およびムスカリン性レセプターアンタゴニスト活性を有する化合物 |
| JP2009526018A (ja) * | 2006-02-10 | 2009-07-16 | グラクソ グループ リミテッド | ビフェニル−2−イルカルバミン酸1−[2−(2−クロロ−4−{[(r)−2−ヒドロキシ−2−(8−ヒドロキシ−2−オキソ−1,2−ジヒドロキノリン−5−イル)エチルアミノメチル]}−5−メトキシフェニルカルバモイル)エチル]ピペリジン−4−イルエステルのコハク酸塩および肺疾患治療におけるその使用 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2621917A1 (en) | 2013-08-07 |
| IL224985A (en) | 2015-08-31 |
| EA201390481A1 (ru) | 2013-08-30 |
| MX2013003558A (es) | 2013-05-01 |
| AR083115A1 (es) | 2013-01-30 |
| JP2015155470A (ja) | 2015-08-27 |
| CA2810896A1 (en) | 2012-04-05 |
| NZ608721A (en) | 2015-03-27 |
| US20120083478A1 (en) | 2012-04-05 |
| AU2011308770A1 (en) | 2013-03-28 |
| BR112013007062A2 (pt) | 2016-06-14 |
| EA022018B1 (ru) | 2015-10-30 |
| CN103140485B (zh) | 2014-10-22 |
| CL2013000870A1 (es) | 2013-07-12 |
| SG188390A1 (en) | 2013-04-30 |
| CN103140485A (zh) | 2013-06-05 |
| CO6700846A2 (es) | 2013-06-28 |
| WO2012044825A1 (en) | 2012-04-05 |
| KR20130139919A (ko) | 2013-12-23 |
| AU2011308770B2 (en) | 2015-03-05 |
| PH12013500567A1 (en) | 2013-05-06 |
| TW201217338A (en) | 2012-05-01 |
| US8697724B2 (en) | 2014-04-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8697724B2 (en) | Crystalline oxalate salts of a diamide compound | |
| TWI570115B (zh) | 具蕈毒鹼受體拮抗劑及β腎上腺素受體激動劑活性之二醯胺化合物 | |
| US7960551B2 (en) | Compound | |
| JP2009535340A (ja) | ジメチルフェニル化合物の結晶性形態 | |
| BRPI0514413B1 (pt) | Sal cristalino de composto de bifenil, sua composição farmacêutica, seus processos de preparação, processo de purificação, usos do referido sal e medicamento. | |
| HK1242699B (en) | Diamide compounds having muscarinic receptor antagonist and beta 2 adrenergic receptor agonist activity | |
| MX2008010205A (es) | Sal de acido succinico de 1-[2-(2-cloro-4-[{[r]-2-hidroxi-2-(8-hidroxi-2-oxo-1, 2-dihidroquinolin-5-il)etilamino]metil}-5-metoxifenilcarbamoil)etil]piperidin-4-il ester de acido bifenil-2-ilcarbamico y su uso en el tratamiento de trastornos pulmonar | |
| HK1119703A (en) | Succinic acid salt of biphenyl-2-ylcarbamic acid 1-[2-(2-chloro-4-{[(r)-2-hydroxy-2-(8-hydroxy-2-oxo-1, 2-dihydroquinolin-5-yl) ethylaminoimethyl]}-5-methoxyphenylcarbamoyl)ethyl]piperidin-4-yl ester and its use for the treatment of pulmonary disorders | |
| HK1119703B (en) | Succinic acid salt of biphenyl-2-ylcarbamic acid 1-[2-(2-chloro-4-{[(r)-2-hydroxy-2-(8-hydroxy-2-oxo-1, 2-dihydroquinolin-5-yl) ethylaminoimethyl]}-5-methoxyphenylcarbamoyl)ethyl]piperidin-4-yl ester and its use for the treatment of pulmonary disorders | |
| HK1184146B (en) | Diamide compounds having muscarinic receptor antagonist and beta 2 adrenergic receptor agonist activity | |
| HK1165799B (en) | Diamide compounds having muscarinic receptor antagonist and beta2 adrenergic receptor agonist activity |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140716 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20140716 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20140725 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20150325 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150601 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20151203 |
|
| A045 | Written measure of dismissal of application [lapsed due to lack of payment] |
Free format text: JAPANESE INTERMEDIATE CODE: A045 Effective date: 20160421 |