JP2013508564A - 銀陰極活性化の改良 - Google Patents
銀陰極活性化の改良 Download PDFInfo
- Publication number
- JP2013508564A JP2013508564A JP2012536945A JP2012536945A JP2013508564A JP 2013508564 A JP2013508564 A JP 2013508564A JP 2012536945 A JP2012536945 A JP 2012536945A JP 2012536945 A JP2012536945 A JP 2012536945A JP 2013508564 A JP2013508564 A JP 2013508564A
- Authority
- JP
- Japan
- Prior art keywords
- cathode
- volts
- acid
- potential
- silver
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/78—Carbon atoms having three bonds to hetero atoms, with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D213/00—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members
- C07D213/02—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members
- C07D213/04—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D213/60—Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom and three or more double bonds between ring members or between ring members and non-ring members having three double bonds between ring members or between ring members and non-ring members having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D213/78—Carbon atoms having three bonds to hetero atoms, with at the most one bond to halogen, e.g. ester or nitrile radicals
- C07D213/79—Acids; Esters
- C07D213/803—Processes of preparation
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B3/00—Electrolytic production of organic compounds
- C25B3/01—Products
- C25B3/11—Halogen containing compounds
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B3/00—Electrolytic production of organic compounds
- C25B3/20—Processes
- C25B3/25—Reduction
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Electrolytic Production Of Non-Metals, Compounds, Apparatuses Therefor (AREA)
- Electrodes For Compound Or Non-Metal Manufacture (AREA)
- Pyridine Compounds (AREA)
Abstract
Description
米国特許第4217185号、第4242183号および第6352635 B2号ならびに米国特許出願公開第2009/0090639号は、対応するより高次のハロゲン化ピリジンおよびピコリン酸誘導体の選択的な電気化学的還元により、特定のハロピリジンおよびハロピコリン酸誘導体を調製することについて記載している。この方法では、銀陰極は、ゼロボルトの初期値から少なくとも+0.3ボルト、好ましくは+0.7ボルトの最終値に電位を増加させることを伴う陽極酸化により活性化される。しかし、不動態化のため、転化が進むにつれて反応速度は通常低下し、バッチを終了するために再陽極酸化により陰極を再活性化することがときには必要である。
Xは、ClまたはBrを表し、
XがClであるとき、YはBrでないことを条件として、Yは、H、F、Cl、Br、またはC1〜C4アルキルを表し、
R1は、ClまたはCO2Hを表し、
R2は、HまたはNH2を表す。)の3−ハロピリジンまたは3−ハロピコリン酸を調製するための改良方法であり、
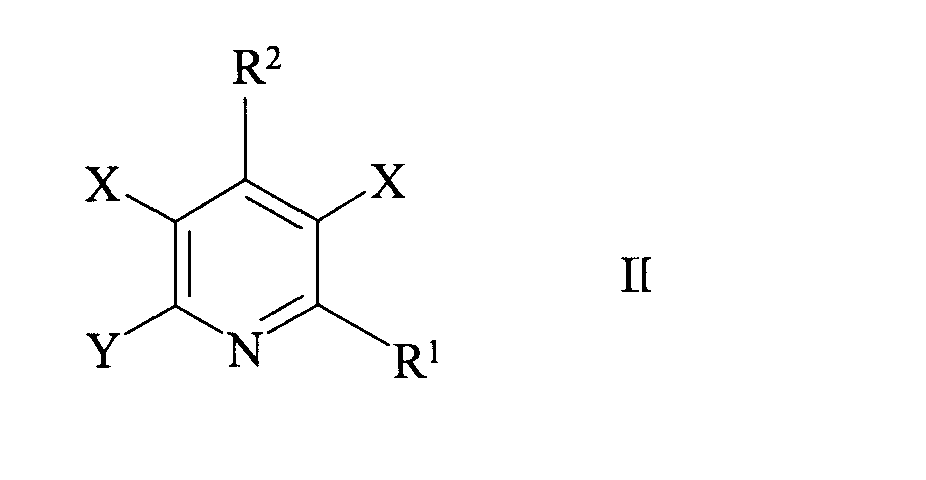
式II:
X、Y、R1、およびR2は、前に定義したとおりであり、ここで、両方のXはClまたはBrのどちらかである。)の3,5−ジハロピリジンまたは3,5−ジハロピコリン酸の溶液中に、Ag/AgCl(3.0M Cl−)参照電極に対する−0.4〜−1.7ボルトの陰極電位にて、直流または交流電流を陽極から銀陰極に流す方法において、
前記改良が、+1.0〜+1.8ボルト、好ましくは+1.2ボルトの最終電位で陰極を活性化することを特徴とする改良方法に関する。
4リットル(L)のフラスコに2420グラム(g)の熱水、250gの50重量%NaOH、30gのNaCl、および300gのピクロラム(95%)を加えた。溶液を30分間(min)撹拌し、1ミクロンのポリプロピレン膜でろ過し、5Lのフィード循環槽に移した。この溶液の重量は3000gであり、9.5重量%の4−アミノ−3,5,6−トリクロロピリジン−2−カルボン酸、2.0〜2.5%の過剰のNaOH、および1.0%のNaClを含有した。比較例と本開示の改良例の両方でこのフィード溶液を使用した。
+0.7ボルトでの陽極酸化を伴う4−アミノ−3,6−ジクロロピリジン−2−カルボン酸の調製(比較)
非分割電気化学セルに500gのピクロラムフィード溶液を加えた。このフィード溶液を毎分4リットル(L/min)の速度および43〜45℃の温度で1つの非分割電気化学セルを通して循環させた。銀メッシュ電極のサイズは1.8cm×15.4cmであった。+0.7ボルト(V)での通常の陽極酸化の後に、セルの極性を反転し、電気分解を開始した。陰極の作用電位は、Ag/AgCl(3.0M Cl−)参照電極に対して−1.35Vに制御した。フィードを再循環させながら、計10mlの50重量%のNaOHを最初の5時間に渡り添加して、NaOH濃度を1.5〜3.0%過剰に維持した。電流を5.0アンペアで開始し、徐々に減らして24時間で0.6アンペアにした。
実施例Aと同じ非分割電気化学セルに500gのピクロラムフィード溶液を加えた。このフィード溶液を4L/minの速度および43〜45℃の温度で1つの非分割電気化学セルを通して循環させた。+1.2ボルト(V)での通常の陽極酸化の後に、セルの極性を反転し、電気分解を開始した。陰極の作用電位は、Ag/AgCl(3.0M Cl−)参照電極に対して−1.35Vに制御した。フィードを再循環させながら、計10mlの50重量%のNaOHを最初の5時間に渡り添加して、NaOH濃度を1.5〜3.0%過剰に維持した。電流を6.5アンペアで開始し、ゆっくり減らして24時間で0.7Vにした。
Claims (3)
- 式I:
Xは、ClまたはBrを表し、
XがClであるとき、YはBrでないことを条件として、Yは、H、F、Cl、Br、またはC1〜C4アルキルを表し、
R1は、ClまたはCO2Hを表し、
R2は、HまたはNH2を表す。)の3−ハロピリジンまたは3−ハロピコリン酸を調製するための改良方法であり、
式II:
X、Y、R1、およびR2は、前に定義したとおりであり、ここで、両方のXはClまたはBrのどちらかである。)の3,5−ジハロピリジンまたは3,5−ジハロピコリン酸の溶液中に、Ag/AgCl(3.0M Cl−)参照電極に対する−0.4〜−1.7ボルトの陰極電位にて、直流または交流電流を陽極から銀陰極に流す方法において、
前記改良が、+1.0〜+1.8ボルトの最終電位で前記陰極を活性化することを特徴とする改良方法。 - 前記銀陰極が、+1.2ボルトの電位での陽極酸化、次いで逆分極により活性化される、請求項1に記載の方法。
- 式Iの前記3−ハロピコリン酸がアミノピラリドであり、式IIの前記3,5−ジハロピコリン酸がピクロラムである、請求項1に記載の方法。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US25518709P | 2009-10-27 | 2009-10-27 | |
| US61/255,187 | 2009-10-27 | ||
| PCT/US2010/054082 WO2011053582A1 (en) | 2009-10-27 | 2010-10-26 | Improved silver cathode activation |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015191549A Division JP6200925B2 (ja) | 2009-10-27 | 2015-09-29 | 銀陰極活性化の改良 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2013508564A true JP2013508564A (ja) | 2013-03-07 |
| JP5818803B2 JP5818803B2 (ja) | 2015-11-18 |
Family
ID=43280201
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012536945A Active JP5818803B2 (ja) | 2009-10-27 | 2010-10-26 | 銀陰極活性化の改良 |
| JP2015191549A Active JP6200925B2 (ja) | 2009-10-27 | 2015-09-29 | 銀陰極活性化の改良 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015191549A Active JP6200925B2 (ja) | 2009-10-27 | 2015-09-29 | 銀陰極活性化の改良 |
Country Status (6)
| Country | Link |
|---|---|
| US (2) | US8685222B2 (ja) |
| EP (1) | EP2494095B1 (ja) |
| JP (2) | JP5818803B2 (ja) |
| KR (2) | KR101847795B1 (ja) |
| CN (1) | CN102597328B (ja) |
| WO (1) | WO2011053582A1 (ja) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101847795B1 (ko) * | 2009-10-27 | 2018-04-10 | 다우 아그로사이언시즈 엘엘씨 | 개선된 은 음극 활성화 |
| CN104621141A (zh) * | 2013-11-15 | 2015-05-20 | 南京华洲药业有限公司 | 一种含氯氨吡啶酸与恶唑酰草胺的混合除草剂 |
| CN104198642B (zh) * | 2014-09-18 | 2015-11-25 | 中华人民共和国南通出入境检验检疫局 | 一种大麦中氯氨吡啶酸农药残留的检测方法 |
| CN105803481B (zh) * | 2016-03-22 | 2018-03-27 | 浙江埃森化学有限公司 | 一种催化电解制备4‑氨基‑3,6‑二氯吡啶‑2‑甲酸的方法 |
| CN110656345B (zh) * | 2019-08-23 | 2021-06-08 | 浙江工业大学 | 一种4-氨基-3,6-二氯吡啶甲酸的电解合成方法 |
| CN113463115A (zh) * | 2021-07-01 | 2021-10-01 | 安徽工业大学 | Amp作为电解液电化学还原二氧化碳的应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS55141585A (en) * | 1979-04-13 | 1980-11-05 | Dow Chemical Co | Active silver electrode |
| JP2003519726A (ja) * | 2000-01-14 | 2003-06-24 | ダウ・アグロサイエンス・エル・エル・シー | ハロゲン化された4−アミノピコリン酸類の選択的な電気化学的還元 |
| WO2008042429A1 (en) * | 2006-10-04 | 2008-04-10 | Dow Agrosciences Llc | Improved electrochemical reduction of halogenated 4-aminopicolinic acids |
| US20090090639A1 (en) * | 2007-10-04 | 2009-04-09 | Chen Wang | Electrochemical reduction of halogenated 4-aminopicolinic acids |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4217185A (en) * | 1979-07-02 | 1980-08-12 | The Dow Chemical Company | Electrolytic production of certain trichloropicolinic acids and/or 3,6-dichloropicolinic acid |
| US4460441A (en) * | 1982-08-31 | 1984-07-17 | The Dow Chemical Company | Expanded metal as more efficient form of silver cathode for electrolytic reduction of polychloropicolinate anions |
| US4755266A (en) | 1986-07-11 | 1988-07-05 | The Dow Chemical Company | Process for silver cathode activation |
| CN101235515B (zh) * | 2008-02-27 | 2010-06-23 | 浙江工业大学 | 一种活性银电极的制备方法 |
| KR101847795B1 (ko) * | 2009-10-27 | 2018-04-10 | 다우 아그로사이언시즈 엘엘씨 | 개선된 은 음극 활성화 |
-
2010
- 2010-10-26 KR KR1020177030125A patent/KR101847795B1/ko active Active
- 2010-10-26 JP JP2012536945A patent/JP5818803B2/ja active Active
- 2010-10-26 KR KR1020127010696A patent/KR101790803B1/ko not_active Expired - Fee Related
- 2010-10-26 CN CN201080048469.2A patent/CN102597328B/zh active Active
- 2010-10-26 EP EP10776000.1A patent/EP2494095B1/en active Active
- 2010-10-26 US US12/912,216 patent/US8685222B2/en active Active
- 2010-10-26 WO PCT/US2010/054082 patent/WO2011053582A1/en not_active Ceased
-
2013
- 2013-05-31 US US13/906,663 patent/US9090977B2/en active Active
-
2015
- 2015-09-29 JP JP2015191549A patent/JP6200925B2/ja active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS55141585A (en) * | 1979-04-13 | 1980-11-05 | Dow Chemical Co | Active silver electrode |
| JP2003519726A (ja) * | 2000-01-14 | 2003-06-24 | ダウ・アグロサイエンス・エル・エル・シー | ハロゲン化された4−アミノピコリン酸類の選択的な電気化学的還元 |
| WO2008042429A1 (en) * | 2006-10-04 | 2008-04-10 | Dow Agrosciences Llc | Improved electrochemical reduction of halogenated 4-aminopicolinic acids |
| US20090090639A1 (en) * | 2007-10-04 | 2009-04-09 | Chen Wang | Electrochemical reduction of halogenated 4-aminopicolinic acids |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5818803B2 (ja) | 2015-11-18 |
| US8685222B2 (en) | 2014-04-01 |
| JP2016035108A (ja) | 2016-03-17 |
| KR101847795B1 (ko) | 2018-04-10 |
| KR20120089692A (ko) | 2012-08-13 |
| JP6200925B2 (ja) | 2017-09-20 |
| WO2011053582A1 (en) | 2011-05-05 |
| EP2494095B1 (en) | 2014-04-23 |
| CN102597328A (zh) | 2012-07-18 |
| US9090977B2 (en) | 2015-07-28 |
| EP2494095A1 (en) | 2012-09-05 |
| KR101790803B1 (ko) | 2017-10-26 |
| CN102597328B (zh) | 2015-09-16 |
| US20110094893A1 (en) | 2011-04-28 |
| US20130256148A1 (en) | 2013-10-03 |
| KR20170121305A (ko) | 2017-11-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN104087968B (zh) | 卤代吡啶甲酸或其盐类化合物的选择性电化学还原方法 | |
| JP4615809B2 (ja) | ハロゲン化された4−アミノピコリン酸類の選択的な電気化学的還元 | |
| JP6200925B2 (ja) | 銀陰極活性化の改良 | |
| JP5198457B2 (ja) | ハロゲン化4−アミノピコリン酸の改善された電気化学的還元 | |
| CN101603179B (zh) | 3,5,6-三氯吡啶甲酸的电解合成方法 | |
| US7666293B2 (en) | Electrochemical reduction of halogenated 4-aminopicolinic acids |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20131017 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20140908 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140916 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20141205 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20141212 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20150114 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150209 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20150901 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20150929 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5818803 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S533 | Written request for registration of change of name |
Free format text: JAPANESE INTERMEDIATE CODE: R313533 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |