JP2012529281A - 抗EpCAM抗体 - Google Patents
抗EpCAM抗体 Download PDFInfo
- Publication number
- JP2012529281A JP2012529281A JP2012514538A JP2012514538A JP2012529281A JP 2012529281 A JP2012529281 A JP 2012529281A JP 2012514538 A JP2012514538 A JP 2012514538A JP 2012514538 A JP2012514538 A JP 2012514538A JP 2012529281 A JP2012529281 A JP 2012529281A
- Authority
- JP
- Japan
- Prior art keywords
- antibody
- seq
- sequence
- epcam
- amino acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 108010066687 Epithelial Cell Adhesion Molecule Proteins 0.000 claims abstract description 248
- 238000000034 method Methods 0.000 claims abstract description 103
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 97
- 239000000203 mixture Substances 0.000 claims abstract description 67
- 201000011510 cancer Diseases 0.000 claims abstract description 57
- 229940127121 immunoconjugate Drugs 0.000 claims abstract description 56
- 238000011282 treatment Methods 0.000 claims abstract description 34
- 102000018651 Epithelial Cell Adhesion Molecule Human genes 0.000 claims abstract description 12
- 241000282414 Homo sapiens Species 0.000 claims description 178
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 142
- 230000027455 binding Effects 0.000 claims description 129
- 102100035360 Cerebellar degeneration-related antigen 1 Human genes 0.000 claims description 110
- 239000000427 antigen Substances 0.000 claims description 85
- 150000007523 nucleic acids Chemical class 0.000 claims description 85
- 108091007433 antigens Proteins 0.000 claims description 75
- 102000036639 antigens Human genes 0.000 claims description 75
- 108020004707 nucleic acids Proteins 0.000 claims description 74
- 102000039446 nucleic acids Human genes 0.000 claims description 74
- 239000012634 fragment Substances 0.000 claims description 51
- 239000003814 drug Substances 0.000 claims description 50
- 150000001413 amino acids Chemical class 0.000 claims description 46
- 241001465754 Metazoa Species 0.000 claims description 41
- 238000012360 testing method Methods 0.000 claims description 38
- 239000013604 expression vector Substances 0.000 claims description 32
- 229940124597 therapeutic agent Drugs 0.000 claims description 31
- 108010021625 Immunoglobulin Fragments Proteins 0.000 claims description 30
- 102000008394 Immunoglobulin Fragments Human genes 0.000 claims description 30
- 102000023732 binding proteins Human genes 0.000 claims description 25
- 108091008324 binding proteins Proteins 0.000 claims description 25
- 239000002246 antineoplastic agent Substances 0.000 claims description 23
- 241000282693 Cercopithecidae Species 0.000 claims description 22
- 239000003795 chemical substances by application Substances 0.000 claims description 22
- 239000000032 diagnostic agent Substances 0.000 claims description 20
- 229940039227 diagnostic agent Drugs 0.000 claims description 20
- 206010006187 Breast cancer Diseases 0.000 claims description 19
- 208000026310 Breast neoplasm Diseases 0.000 claims description 19
- 238000006467 substitution reaction Methods 0.000 claims description 19
- 210000001519 tissue Anatomy 0.000 claims description 18
- 238000002560 therapeutic procedure Methods 0.000 claims description 17
- 239000002773 nucleotide Substances 0.000 claims description 15
- 125000003729 nucleotide group Chemical group 0.000 claims description 15
- 229940127089 cytotoxic agent Drugs 0.000 claims description 14
- -1 another antibody Substances 0.000 claims description 13
- 230000001939 inductive effect Effects 0.000 claims description 13
- 238000003745 diagnosis Methods 0.000 claims description 11
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 9
- 230000015572 biosynthetic process Effects 0.000 claims description 9
- 238000004519 manufacturing process Methods 0.000 claims description 9
- 241000700605 Viruses Species 0.000 claims description 8
- 239000000539 dimer Substances 0.000 claims description 8
- 206010017758 gastric cancer Diseases 0.000 claims description 8
- 238000003384 imaging method Methods 0.000 claims description 8
- 201000011549 stomach cancer Diseases 0.000 claims description 8
- 102000004127 Cytokines Human genes 0.000 claims description 7
- 108090000695 Cytokines Proteins 0.000 claims description 7
- 230000006907 apoptotic process Effects 0.000 claims description 7
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 6
- 239000004037 angiogenesis inhibitor Substances 0.000 claims description 6
- 239000002254 cytotoxic agent Substances 0.000 claims description 6
- 108010012236 Chemokines Proteins 0.000 claims description 5
- 102000019034 Chemokines Human genes 0.000 claims description 5
- 206010009944 Colon cancer Diseases 0.000 claims description 5
- 206010060862 Prostate cancer Diseases 0.000 claims description 5
- 239000000362 adenosine triphosphatase inhibitor Substances 0.000 claims description 5
- 239000000701 coagulant Substances 0.000 claims description 5
- 150000003431 steroids Chemical class 0.000 claims description 5
- 238000001959 radiotherapy Methods 0.000 claims description 4
- 229940121819 ATPase inhibitor Drugs 0.000 claims description 3
- 208000022072 Gallbladder Neoplasms Diseases 0.000 claims description 3
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 3
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 3
- 108010003723 Single-Domain Antibodies Proteins 0.000 claims description 3
- 238000012258 culturing Methods 0.000 claims description 3
- 201000010175 gallbladder cancer Diseases 0.000 claims description 3
- 208000020816 lung neoplasm Diseases 0.000 claims description 3
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 3
- 201000002528 pancreatic cancer Diseases 0.000 claims description 3
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 3
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 2
- 206010005003 Bladder cancer Diseases 0.000 claims description 2
- 206010008342 Cervix carcinoma Diseases 0.000 claims description 2
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims description 2
- 208000032612 Glial tumor Diseases 0.000 claims description 2
- 206010018338 Glioma Diseases 0.000 claims description 2
- 208000008839 Kidney Neoplasms Diseases 0.000 claims description 2
- 206010061523 Lip and/or oral cavity cancer Diseases 0.000 claims description 2
- 206010062038 Lip neoplasm Diseases 0.000 claims description 2
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims description 2
- 208000003445 Mouth Neoplasms Diseases 0.000 claims description 2
- 206010033128 Ovarian cancer Diseases 0.000 claims description 2
- 206010038389 Renal cancer Diseases 0.000 claims description 2
- 208000006265 Renal cell carcinoma Diseases 0.000 claims description 2
- 208000000453 Skin Neoplasms Diseases 0.000 claims description 2
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 claims description 2
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 claims description 2
- 210000004369 blood Anatomy 0.000 claims description 2
- 239000008280 blood Substances 0.000 claims description 2
- 201000010881 cervical cancer Diseases 0.000 claims description 2
- 201000010536 head and neck cancer Diseases 0.000 claims description 2
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 2
- 206010073071 hepatocellular carcinoma Diseases 0.000 claims description 2
- 231100000844 hepatocellular carcinoma Toxicity 0.000 claims description 2
- 201000010982 kidney cancer Diseases 0.000 claims description 2
- 201000006721 lip cancer Diseases 0.000 claims description 2
- 201000007270 liver cancer Diseases 0.000 claims description 2
- 208000014018 liver neoplasm Diseases 0.000 claims description 2
- 201000005202 lung cancer Diseases 0.000 claims description 2
- 238000011275 oncology therapy Methods 0.000 claims description 2
- 229910052721 tungsten Inorganic materials 0.000 claims description 2
- 201000005112 urinary bladder cancer Diseases 0.000 claims description 2
- 206010046885 vaginal cancer Diseases 0.000 claims description 2
- 208000013139 vaginal neoplasm Diseases 0.000 claims description 2
- 230000009278 visceral effect Effects 0.000 claims description 2
- 208000000461 Esophageal Neoplasms Diseases 0.000 claims 1
- 206010029098 Neoplasm skin Diseases 0.000 claims 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 claims 1
- 238000000375 direct analysis in real time Methods 0.000 claims 1
- 238000012063 dual-affinity re-targeting Methods 0.000 claims 1
- 201000004101 esophageal cancer Diseases 0.000 claims 1
- 230000002496 gastric effect Effects 0.000 claims 1
- 208000015347 renal cell adenocarcinoma Diseases 0.000 claims 1
- 230000000694 effects Effects 0.000 abstract description 29
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 abstract description 25
- 230000009260 cross reactivity Effects 0.000 abstract description 6
- 230000008901 benefit Effects 0.000 abstract description 5
- 102100031940 Epithelial cell adhesion molecule Human genes 0.000 description 277
- 210000004027 cell Anatomy 0.000 description 145
- 108090000623 proteins and genes Proteins 0.000 description 74
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 73
- 102000004169 proteins and genes Human genes 0.000 description 59
- 235000018102 proteins Nutrition 0.000 description 55
- 235000001014 amino acid Nutrition 0.000 description 50
- 229950009084 adecatumumab Drugs 0.000 description 46
- 229940024606 amino acid Drugs 0.000 description 42
- 101000920667 Homo sapiens Epithelial cell adhesion molecule Proteins 0.000 description 35
- 210000004881 tumor cell Anatomy 0.000 description 31
- 238000009472 formulation Methods 0.000 description 29
- 230000004540 complement-dependent cytotoxicity Effects 0.000 description 28
- 238000000338 in vitro Methods 0.000 description 27
- 230000001225 therapeutic effect Effects 0.000 description 27
- 201000010099 disease Diseases 0.000 description 23
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 23
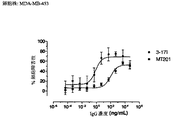
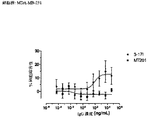
- 238000003556 assay Methods 0.000 description 22
- 230000014509 gene expression Effects 0.000 description 22
- 238000001727 in vivo Methods 0.000 description 20
- 238000002965 ELISA Methods 0.000 description 19
- 239000000523 sample Substances 0.000 description 19
- 239000013598 vector Substances 0.000 description 17
- 239000000651 prodrug Substances 0.000 description 16
- 229940002612 prodrug Drugs 0.000 description 16
- 238000009396 hybridization Methods 0.000 description 15
- 230000000259 anti-tumor effect Effects 0.000 description 13
- 241000282567 Macaca fascicularis Species 0.000 description 12
- 101100501585 Mus musculus Epcam gene Proteins 0.000 description 12
- 230000006870 function Effects 0.000 description 12
- 229940051026 immunotoxin Drugs 0.000 description 11
- 230000002637 immunotoxin Effects 0.000 description 11
- 239000002596 immunotoxin Substances 0.000 description 11
- 231100000608 immunotoxin Toxicity 0.000 description 11
- 230000035772 mutation Effects 0.000 description 11
- 238000003259 recombinant expression Methods 0.000 description 11
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 10
- 241000699666 Mus <mouse, genus> Species 0.000 description 10
- 108091028043 Nucleic acid sequence Proteins 0.000 description 10
- 238000010790 dilution Methods 0.000 description 10
- 239000012895 dilution Substances 0.000 description 10
- 230000001965 increasing effect Effects 0.000 description 10
- 230000002147 killing effect Effects 0.000 description 10
- 239000003550 marker Substances 0.000 description 10
- 230000004048 modification Effects 0.000 description 10
- 238000012986 modification Methods 0.000 description 10
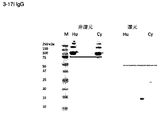
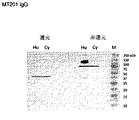
- 238000001262 western blot Methods 0.000 description 10
- 108010039209 Blood Coagulation Factors Proteins 0.000 description 9
- 102000015081 Blood Coagulation Factors Human genes 0.000 description 9
- 239000012980 RPMI-1640 medium Substances 0.000 description 9
- 238000004458 analytical method Methods 0.000 description 9
- 239000003114 blood coagulation factor Substances 0.000 description 9
- 238000000684 flow cytometry Methods 0.000 description 9
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 9
- 239000003053 toxin Substances 0.000 description 9
- 231100000765 toxin Toxicity 0.000 description 9
- 108700012359 toxins Proteins 0.000 description 9
- 241000282412 Homo Species 0.000 description 8
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 8
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 8
- 229940079593 drug Drugs 0.000 description 8
- 230000004927 fusion Effects 0.000 description 8
- 239000001963 growth medium Substances 0.000 description 8
- 230000001976 improved effect Effects 0.000 description 8
- 230000005764 inhibitory process Effects 0.000 description 8
- 241000894007 species Species 0.000 description 8
- 238000002474 experimental method Methods 0.000 description 7
- 210000000987 immune system Anatomy 0.000 description 7
- 239000012528 membrane Substances 0.000 description 7
- 108090000765 processed proteins & peptides Proteins 0.000 description 7
- 239000000047 product Substances 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 239000000126 substance Substances 0.000 description 7
- 108060003951 Immunoglobulin Proteins 0.000 description 6
- 241000699670 Mus sp. Species 0.000 description 6
- 241000283973 Oryctolagus cuniculus Species 0.000 description 6
- 210000004899 c-terminal region Anatomy 0.000 description 6
- 230000000295 complement effect Effects 0.000 description 6
- 230000009089 cytolysis Effects 0.000 description 6
- 231100000599 cytotoxic agent Toxicity 0.000 description 6
- 238000001514 detection method Methods 0.000 description 6
- 102000018358 immunoglobulin Human genes 0.000 description 6
- 238000011534 incubation Methods 0.000 description 6
- 229920001184 polypeptide Polymers 0.000 description 6
- 238000002360 preparation method Methods 0.000 description 6
- 102000004196 processed proteins & peptides Human genes 0.000 description 6
- 238000000159 protein binding assay Methods 0.000 description 6
- 210000002966 serum Anatomy 0.000 description 6
- 241000196324 Embryophyta Species 0.000 description 5
- 241000700159 Rattus Species 0.000 description 5
- 108010039491 Ricin Proteins 0.000 description 5
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 5
- 230000009471 action Effects 0.000 description 5
- 125000000539 amino acid group Chemical group 0.000 description 5
- 239000002585 base Substances 0.000 description 5
- 230000000903 blocking effect Effects 0.000 description 5
- 238000010367 cloning Methods 0.000 description 5
- 238000012875 competitive assay Methods 0.000 description 5
- 230000006378 damage Effects 0.000 description 5
- 239000012636 effector Substances 0.000 description 5
- 102000037865 fusion proteins Human genes 0.000 description 5
- 108020001507 fusion proteins Proteins 0.000 description 5
- 210000004602 germ cell Anatomy 0.000 description 5
- 239000005090 green fluorescent protein Substances 0.000 description 5
- 238000009169 immunotherapy Methods 0.000 description 5
- 239000003446 ligand Substances 0.000 description 5
- 238000002703 mutagenesis Methods 0.000 description 5
- 231100000350 mutagenesis Toxicity 0.000 description 5
- 239000013641 positive control Substances 0.000 description 5
- 238000000746 purification Methods 0.000 description 5
- 230000002829 reductive effect Effects 0.000 description 5
- 238000003786 synthesis reaction Methods 0.000 description 5
- 238000013518 transcription Methods 0.000 description 5
- 230000035897 transcription Effects 0.000 description 5
- 230000009466 transformation Effects 0.000 description 5
- 230000009261 transgenic effect Effects 0.000 description 5
- 238000005406 washing Methods 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- IAKHMKGGTNLKSZ-INIZCTEOSA-N (S)-colchicine Chemical compound C1([C@@H](NC(C)=O)CC2)=CC(=O)C(OC)=CC=C1C1=C2C=C(OC)C(OC)=C1OC IAKHMKGGTNLKSZ-INIZCTEOSA-N 0.000 description 4
- 241000283690 Bos taurus Species 0.000 description 4
- 241000283707 Capra Species 0.000 description 4
- 241000701022 Cytomegalovirus Species 0.000 description 4
- 108010070675 Glutathione transferase Proteins 0.000 description 4
- 102000005720 Glutathione transferase Human genes 0.000 description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- 235000003332 Ilex aquifolium Nutrition 0.000 description 4
- 241000209027 Ilex aquifolium Species 0.000 description 4
- 241000124008 Mammalia Species 0.000 description 4
- 102100038895 Myc proto-oncogene protein Human genes 0.000 description 4
- 101710135898 Myc proto-oncogene protein Proteins 0.000 description 4
- 229930193140 Neomycin Natural products 0.000 description 4
- 229930012538 Paclitaxel Natural products 0.000 description 4
- 241001494479 Pecora Species 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 241000282887 Suidae Species 0.000 description 4
- 210000001744 T-lymphocyte Anatomy 0.000 description 4
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 4
- 101710150448 Transcriptional regulator Myc Proteins 0.000 description 4
- 239000013504 Triton X-100 Substances 0.000 description 4
- 229920004890 Triton X-100 Polymers 0.000 description 4
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 4
- 238000010171 animal model Methods 0.000 description 4
- 230000001093 anti-cancer Effects 0.000 description 4
- 230000001580 bacterial effect Effects 0.000 description 4
- 229960000074 biopharmaceutical Drugs 0.000 description 4
- 230000032823 cell division Effects 0.000 description 4
- 238000005119 centrifugation Methods 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 238000002512 chemotherapy Methods 0.000 description 4
- 208000029742 colonic neoplasm Diseases 0.000 description 4
- 239000000562 conjugate Substances 0.000 description 4
- 238000007796 conventional method Methods 0.000 description 4
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 4
- 230000001419 dependent effect Effects 0.000 description 4
- 230000002538 fungal effect Effects 0.000 description 4
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 4
- 229940072221 immunoglobulins Drugs 0.000 description 4
- 239000003112 inhibitor Substances 0.000 description 4
- 210000004962 mammalian cell Anatomy 0.000 description 4
- 229960004927 neomycin Drugs 0.000 description 4
- 229960001592 paclitaxel Drugs 0.000 description 4
- 239000008194 pharmaceutical composition Substances 0.000 description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 4
- 238000010188 recombinant method Methods 0.000 description 4
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 4
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 4
- 206010069754 Acquired gene mutation Diseases 0.000 description 3
- 102100026189 Beta-galactosidase Human genes 0.000 description 3
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 3
- 108020004705 Codon Proteins 0.000 description 3
- 238000012286 ELISA Assay Methods 0.000 description 3
- 241000588724 Escherichia coli Species 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 241000238631 Hexapoda Species 0.000 description 3
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 3
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 3
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 3
- 206010028851 Necrosis Diseases 0.000 description 3
- 206010033645 Pancreatitis Diseases 0.000 description 3
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 3
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 3
- 230000003213 activating effect Effects 0.000 description 3
- 238000007792 addition Methods 0.000 description 3
- 239000000611 antibody drug conjugate Substances 0.000 description 3
- 229940049595 antibody-drug conjugate Drugs 0.000 description 3
- 108010005774 beta-Galactosidase Proteins 0.000 description 3
- 210000000481 breast Anatomy 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 230000022131 cell cycle Effects 0.000 description 3
- 230000006037 cell lysis Effects 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000012217 deletion Methods 0.000 description 3
- 230000037430 deletion Effects 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 229960004679 doxorubicin Drugs 0.000 description 3
- 229960001776 edrecolomab Drugs 0.000 description 3
- 230000006698 induction Effects 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- 210000005075 mammary gland Anatomy 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 230000001404 mediated effect Effects 0.000 description 3
- 239000000178 monomer Substances 0.000 description 3
- 238000010172 mouse model Methods 0.000 description 3
- 230000017074 necrotic cell death Effects 0.000 description 3
- 230000002018 overexpression Effects 0.000 description 3
- 230000003389 potentiating effect Effects 0.000 description 3
- 210000001236 prokaryotic cell Anatomy 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 230000000392 somatic effect Effects 0.000 description 3
- 230000037439 somatic mutation Effects 0.000 description 3
- 210000002784 stomach Anatomy 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 3
- 230000014616 translation Effects 0.000 description 3
- 238000013519 translation Methods 0.000 description 3
- 229960003048 vinblastine Drugs 0.000 description 3
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 3
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 2
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 2
- OTLLEIBWKHEHGU-UHFFFAOYSA-N 2-[5-[[5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy]-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,5-dihydroxy-4-phosphonooxyhexanedioic acid Chemical compound C1=NC=2C(N)=NC=NC=2N1C(C(C1O)O)OC1COC1C(CO)OC(OC(C(O)C(OP(O)(O)=O)C(O)C(O)=O)C(O)=O)C(O)C1O OTLLEIBWKHEHGU-UHFFFAOYSA-N 0.000 description 2
- NDMPLJNOPCLANR-UHFFFAOYSA-N 3,4-dihydroxy-15-(4-hydroxy-18-methoxycarbonyl-5,18-seco-ibogamin-18-yl)-16-methoxy-1-methyl-6,7-didehydro-aspidospermidine-3-carboxylic acid methyl ester Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 NDMPLJNOPCLANR-UHFFFAOYSA-N 0.000 description 2
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 2
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 2
- LRFVTYWOQMYALW-UHFFFAOYSA-N 9H-xanthine Chemical compound O=C1NC(=O)NC2=C1NC=N2 LRFVTYWOQMYALW-UHFFFAOYSA-N 0.000 description 2
- 229930024421 Adenine Natural products 0.000 description 2
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 description 2
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 2
- 206010055113 Breast cancer metastatic Diseases 0.000 description 2
- 102100023700 C-C motif chemokine 16 Human genes 0.000 description 2
- 102100025250 C-X-C motif chemokine 14 Human genes 0.000 description 2
- 108050007957 Cadherin Proteins 0.000 description 2
- 102000000905 Cadherin Human genes 0.000 description 2
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 2
- 241000282472 Canis lupus familiaris Species 0.000 description 2
- 108010035563 Chloramphenicol O-acetyltransferase Proteins 0.000 description 2
- 102100023804 Coagulation factor VII Human genes 0.000 description 2
- 206010053567 Coagulopathies Diseases 0.000 description 2
- 108091035707 Consensus sequence Proteins 0.000 description 2
- 108020004414 DNA Proteins 0.000 description 2
- 241000702421 Dependoparvovirus Species 0.000 description 2
- 101150084967 EPCAM gene Proteins 0.000 description 2
- 101710147220 Ent-copalyl diphosphate synthase, chloroplastic Proteins 0.000 description 2
- YQYJSBFKSSDGFO-UHFFFAOYSA-N Epihygromycin Natural products OC1C(O)C(C(=O)C)OC1OC(C(=C1)O)=CC=C1C=C(C)C(=O)NC1C(O)C(O)C2OCOC2C1O YQYJSBFKSSDGFO-UHFFFAOYSA-N 0.000 description 2
- 108010023321 Factor VII Proteins 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- 108090000331 Firefly luciferases Proteins 0.000 description 2
- 108700004714 Gelonium multiflorum GEL Proteins 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 101710154606 Hemagglutinin Proteins 0.000 description 2
- 101000978375 Homo sapiens C-C motif chemokine 16 Proteins 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 102000003781 Inhibitor of growth protein 1 Human genes 0.000 description 2
- 108090000191 Inhibitor of growth protein 1 Proteins 0.000 description 2
- 102000006992 Interferon-alpha Human genes 0.000 description 2
- 108010047761 Interferon-alpha Proteins 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 2
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 2
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 2
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- 241000219823 Medicago Species 0.000 description 2
- 241001529936 Murinae Species 0.000 description 2
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 2
- 108091007491 NSP3 Papain-like protease domains Proteins 0.000 description 2
- 108700020796 Oncogene Proteins 0.000 description 2
- 101710093908 Outer capsid protein VP4 Proteins 0.000 description 2
- 101710135467 Outer capsid protein sigma-1 Proteins 0.000 description 2
- 229930182555 Penicillin Natural products 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- 101710176177 Protein A56 Proteins 0.000 description 2
- 101000762949 Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) Exotoxin A Proteins 0.000 description 2
- 241000508269 Psidium Species 0.000 description 2
- 108020004511 Recombinant DNA Proteins 0.000 description 2
- 108020005091 Replication Origin Proteins 0.000 description 2
- 208000007660 Residual Neoplasm Diseases 0.000 description 2
- 108010083644 Ribonucleases Proteins 0.000 description 2
- 102000006382 Ribonucleases Human genes 0.000 description 2
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 2
- 229920002684 Sepharose Polymers 0.000 description 2
- 241000700584 Simplexvirus Species 0.000 description 2
- FKNQFGJONOIPTF-UHFFFAOYSA-N Sodium cation Chemical compound [Na+] FKNQFGJONOIPTF-UHFFFAOYSA-N 0.000 description 2
- 239000012505 Superdex™ Substances 0.000 description 2
- 101150057140 TACSTD1 gene Proteins 0.000 description 2
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 2
- 239000004473 Threonine Substances 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 2
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 2
- 229930003448 Vitamin K Natural products 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 229960000643 adenine Drugs 0.000 description 2
- 229940009456 adriamycin Drugs 0.000 description 2
- 229940100198 alkylating agent Drugs 0.000 description 2
- 239000002168 alkylating agent Substances 0.000 description 2
- 238000009175 antibody therapy Methods 0.000 description 2
- 238000010913 antigen-directed enzyme pro-drug therapy Methods 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 2
- 239000003124 biologic agent Substances 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 230000036760 body temperature Effects 0.000 description 2
- BQRGNLJZBFXNCZ-UHFFFAOYSA-N calcein am Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC(CN(CC(=O)OCOC(C)=O)CC(=O)OCOC(C)=O)=C(OC(C)=O)C=C1OC1=C2C=C(CN(CC(=O)OCOC(C)=O)CC(=O)OCOC(=O)C)C(OC(C)=O)=C1 BQRGNLJZBFXNCZ-UHFFFAOYSA-N 0.000 description 2
- 239000001110 calcium chloride Substances 0.000 description 2
- 235000011148 calcium chloride Nutrition 0.000 description 2
- 229960002713 calcium chloride Drugs 0.000 description 2
- 229910001628 calcium chloride Inorganic materials 0.000 description 2
- 150000001720 carbohydrates Chemical group 0.000 description 2
- 239000006143 cell culture medium Substances 0.000 description 2
- 239000012592 cell culture supplement Substances 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 239000006285 cell suspension Substances 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 239000012707 chemical precursor Substances 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 230000035602 clotting Effects 0.000 description 2
- 229960001338 colchicine Drugs 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000004590 computer program Methods 0.000 description 2
- 230000021615 conjugation Effects 0.000 description 2
- 239000013068 control sample Substances 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 229940104302 cytosine Drugs 0.000 description 2
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 231100000517 death Toxicity 0.000 description 2
- 231100000673 dose–response relationship Toxicity 0.000 description 2
- 230000009977 dual effect Effects 0.000 description 2
- 238000004520 electroporation Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 239000003623 enhancer Substances 0.000 description 2
- YJGVMLPVUAXIQN-UHFFFAOYSA-N epipodophyllotoxin Natural products COC1=C(OC)C(OC)=CC(C2C3=CC=4OCOC=4C=C3C(O)C3C2C(OC3)=O)=C1 YJGVMLPVUAXIQN-UHFFFAOYSA-N 0.000 description 2
- 230000005284 excitation Effects 0.000 description 2
- 230000007717 exclusion Effects 0.000 description 2
- 239000002095 exotoxin Substances 0.000 description 2
- 231100000776 exotoxin Toxicity 0.000 description 2
- 229940012413 factor vii Drugs 0.000 description 2
- 239000012894 fetal calf serum Substances 0.000 description 2
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 238000001415 gene therapy Methods 0.000 description 2
- 230000013595 glycosylation Effects 0.000 description 2
- 238000006206 glycosylation reaction Methods 0.000 description 2
- 239000000185 hemagglutinin Substances 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 239000012216 imaging agent Substances 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 230000002163 immunogen Effects 0.000 description 2
- 238000003364 immunohistochemistry Methods 0.000 description 2
- 238000000099 in vitro assay Methods 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 229960000310 isoleucine Drugs 0.000 description 2
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 2
- 235000014705 isoleucine Nutrition 0.000 description 2
- 238000012417 linear regression Methods 0.000 description 2
- 238000011068 loading method Methods 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 230000010534 mechanism of action Effects 0.000 description 2
- 229960000485 methotrexate Drugs 0.000 description 2
- 238000000520 microinjection Methods 0.000 description 2
- 230000003278 mimic effect Effects 0.000 description 2
- 230000011278 mitosis Effects 0.000 description 2
- 239000013642 negative control Substances 0.000 description 2
- 230000010807 negative regulation of binding Effects 0.000 description 2
- 229940049954 penicillin Drugs 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 2
- SHUZOJHMOBOZST-UHFFFAOYSA-N phylloquinone Natural products CC(C)CCCCC(C)CCC(C)CCCC(=CCC1=C(C)C(=O)c2ccccc2C1=O)C SHUZOJHMOBOZST-UHFFFAOYSA-N 0.000 description 2
- 239000013612 plasmid Substances 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 210000002307 prostate Anatomy 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 230000002285 radioactive effect Effects 0.000 description 2
- 230000003439 radiotherapeutic effect Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 2
- IGLNJRXAVVLDKE-UHFFFAOYSA-N rubidium atom Chemical compound [Rb] IGLNJRXAVVLDKE-UHFFFAOYSA-N 0.000 description 2
- 235000020183 skimmed milk Nutrition 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 2
- 229910001415 sodium ion Inorganic materials 0.000 description 2
- 230000009870 specific binding Effects 0.000 description 2
- 238000001228 spectrum Methods 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- 239000013589 supplement Substances 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 238000007910 systemic administration Methods 0.000 description 2
- 230000008685 targeting Effects 0.000 description 2
- 229940104230 thymidine Drugs 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 239000013638 trimer Substances 0.000 description 2
- 230000004614 tumor growth Effects 0.000 description 2
- 230000005909 tumor killing Effects 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 241000701161 unidentified adenovirus Species 0.000 description 2
- 241001430294 unidentified retrovirus Species 0.000 description 2
- 229940035893 uracil Drugs 0.000 description 2
- 239000004474 valine Substances 0.000 description 2
- 235000014393 valine Nutrition 0.000 description 2
- 229910052720 vanadium Inorganic materials 0.000 description 2
- 229960004528 vincristine Drugs 0.000 description 2
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 2
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 2
- 229960004355 vindesine Drugs 0.000 description 2
- UGGWPQSBPIFKDZ-KOTLKJBCSA-N vindesine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(N)=O)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1N=C1[C]2C=CC=C1 UGGWPQSBPIFKDZ-KOTLKJBCSA-N 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- 239000011712 vitamin K Substances 0.000 description 2
- 235000019168 vitamin K Nutrition 0.000 description 2
- 150000003721 vitamin K derivatives Chemical class 0.000 description 2
- 229940046010 vitamin k Drugs 0.000 description 2
- DQJCDTNMLBYVAY-ZXXIYAEKSA-N (2S,5R,10R,13R)-16-{[(2R,3S,4R,5R)-3-{[(2S,3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-5-(ethylamino)-6-hydroxy-2-(hydroxymethyl)oxan-4-yl]oxy}-5-(4-aminobutyl)-10-carbamoyl-2,13-dimethyl-4,7,12,15-tetraoxo-3,6,11,14-tetraazaheptadecan-1-oic acid Chemical class NCCCC[C@H](C(=O)N[C@@H](C)C(O)=O)NC(=O)CC[C@H](C(N)=O)NC(=O)[C@@H](C)NC(=O)C(C)O[C@@H]1[C@@H](NCC)C(O)O[C@H](CO)[C@H]1O[C@H]1[C@H](NC(C)=O)[C@@H](O)[C@H](O)[C@@H](CO)O1 DQJCDTNMLBYVAY-ZXXIYAEKSA-N 0.000 description 1
- YJGVMLPVUAXIQN-LGWHJFRWSA-N (5s,5ar,8ar,9r)-5-hydroxy-9-(3,4,5-trimethoxyphenyl)-5a,6,8a,9-tetrahydro-5h-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one Chemical compound COC1=C(OC)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O)[C@@H]3[C@@H]2C(OC3)=O)=C1 YJGVMLPVUAXIQN-LGWHJFRWSA-N 0.000 description 1
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 1
- MQLACMBJVPINKE-UHFFFAOYSA-N 10-[(3-hydroxy-4-methoxyphenyl)methylidene]anthracen-9-one Chemical compound C1=C(O)C(OC)=CC=C1C=C1C2=CC=CC=C2C(=O)C2=CC=CC=C21 MQLACMBJVPINKE-UHFFFAOYSA-N 0.000 description 1
- PNDPGZBMCMUPRI-HVTJNCQCSA-N 10043-66-0 Chemical compound [131I][131I] PNDPGZBMCMUPRI-HVTJNCQCSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- WUAPFZMCVAUBPE-NJFSPNSNSA-N 188Re Chemical compound [188Re] WUAPFZMCVAUBPE-NJFSPNSNSA-N 0.000 description 1
- UAIUNKRWKOVEES-UHFFFAOYSA-N 3,3',5,5'-tetramethylbenzidine Chemical compound CC1=C(N)C(C)=CC(C=2C=C(C)C(N)=C(C)C=2)=C1 UAIUNKRWKOVEES-UHFFFAOYSA-N 0.000 description 1
- TVZGACDUOSZQKY-LBPRGKRZSA-N 4-aminofolic acid Chemical compound C1=NC2=NC(N)=NC(N)=C2N=C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 TVZGACDUOSZQKY-LBPRGKRZSA-N 0.000 description 1
- 108010066676 Abrin Proteins 0.000 description 1
- 241000589156 Agrobacterium rhizogenes Species 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 201000004384 Alopecia Diseases 0.000 description 1
- 101800002638 Alpha-amanitin Proteins 0.000 description 1
- 102000009840 Angiopoietins Human genes 0.000 description 1
- 108010009906 Angiopoietins Proteins 0.000 description 1
- 102400000068 Angiostatin Human genes 0.000 description 1
- 108010079709 Angiostatins Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 108010002913 Asialoglycoproteins Proteins 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 241000228212 Aspergillus Species 0.000 description 1
- 101100136076 Aspergillus oryzae (strain ATCC 42149 / RIB 40) pel1 gene Proteins 0.000 description 1
- 101000669426 Aspergillus restrictus Ribonuclease mitogillin Proteins 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 108050001427 Avidin/streptavidin Proteins 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 231100000699 Bacterial toxin Toxicity 0.000 description 1
- 241000255789 Bombyx mori Species 0.000 description 1
- 102100032912 CD44 antigen Human genes 0.000 description 1
- 101100028791 Caenorhabditis elegans pbs-5 gene Proteins 0.000 description 1
- 101100314454 Caenorhabditis elegans tra-1 gene Proteins 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 102400000730 Canstatin Human genes 0.000 description 1
- 101800000626 Canstatin Proteins 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 102000016289 Cell Adhesion Molecules Human genes 0.000 description 1
- 108010067225 Cell Adhesion Molecules Proteins 0.000 description 1
- 102100022641 Coagulation factor IX Human genes 0.000 description 1
- 108091026890 Coding region Proteins 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- 102000002554 Cyclin A Human genes 0.000 description 1
- 108010068192 Cyclin A Proteins 0.000 description 1
- 102000003909 Cyclin E Human genes 0.000 description 1
- 108090000257 Cyclin E Proteins 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- IGXWBGJHJZYPQS-SSDOTTSWSA-N D-Luciferin Chemical compound OC(=O)[C@H]1CSC(C=2SC3=CC=C(O)C=C3N=2)=N1 IGXWBGJHJZYPQS-SSDOTTSWSA-N 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 230000004543 DNA replication Effects 0.000 description 1
- 230000007023 DNA restriction-modification system Effects 0.000 description 1
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 1
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 1
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 1
- CYCGRDQQIOGCKX-UHFFFAOYSA-N Dehydro-luciferin Natural products OC(=O)C1=CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 CYCGRDQQIOGCKX-UHFFFAOYSA-N 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 238000009007 Diagnostic Kit Methods 0.000 description 1
- 108010053187 Diphtheria Toxin Proteins 0.000 description 1
- 102000016607 Diphtheria Toxin Human genes 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- 229910052692 Dysprosium Inorganic materials 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 102000009025 Endorphins Human genes 0.000 description 1
- 108010049140 Endorphins Proteins 0.000 description 1
- 102400001047 Endostatin Human genes 0.000 description 1
- 108010079505 Endostatins Proteins 0.000 description 1
- 241000702371 Enterobacteria phage f1 Species 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 229910052691 Erbium Inorganic materials 0.000 description 1
- 108010029144 Factor IIa Proteins 0.000 description 1
- 108010076282 Factor IX Proteins 0.000 description 1
- 108010048049 Factor IXa Proteins 0.000 description 1
- 108010054265 Factor VIIa Proteins 0.000 description 1
- 108010014173 Factor X Proteins 0.000 description 1
- 108010074860 Factor Xa Proteins 0.000 description 1
- CWYNVVGOOAEACU-UHFFFAOYSA-N Fe2+ Chemical compound [Fe+2] CWYNVVGOOAEACU-UHFFFAOYSA-N 0.000 description 1
- BJGNCJDXODQBOB-UHFFFAOYSA-N Fivefly Luciferin Natural products OC(=O)C1CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 BJGNCJDXODQBOB-UHFFFAOYSA-N 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- 229910052688 Gadolinium Inorganic materials 0.000 description 1
- GYHNNYVSQQEPJS-OIOBTWANSA-N Gallium-67 Chemical compound [67Ga] GYHNNYVSQQEPJS-OIOBTWANSA-N 0.000 description 1
- GYHNNYVSQQEPJS-YPZZEJLDSA-N Gallium-68 Chemical compound [68Ga] GYHNNYVSQQEPJS-YPZZEJLDSA-N 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 1
- 229910052689 Holmium Inorganic materials 0.000 description 1
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 description 1
- 101000589305 Homo sapiens Natural cytotoxicity triggering receptor 2 Proteins 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- 108090000144 Human Proteins Proteins 0.000 description 1
- 102000003839 Human Proteins Human genes 0.000 description 1
- 241000701109 Human adenovirus 2 Species 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 108700001097 Insect Genes Proteins 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102100023915 Insulin Human genes 0.000 description 1
- 102100026720 Interferon beta Human genes 0.000 description 1
- 102100037850 Interferon gamma Human genes 0.000 description 1
- 108090000467 Interferon-beta Proteins 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 108091029795 Intergenic region Proteins 0.000 description 1
- 102000013462 Interleukin-12 Human genes 0.000 description 1
- 108010065805 Interleukin-12 Proteins 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 102000000588 Interleukin-2 Human genes 0.000 description 1
- ZCYVEMRRCGMTRW-AHCXROLUSA-N Iodine-123 Chemical compound [123I] ZCYVEMRRCGMTRW-AHCXROLUSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- 108010007622 LDL Lipoproteins Proteins 0.000 description 1
- 102000007330 LDL Lipoproteins Human genes 0.000 description 1
- 108090001090 Lectins Proteins 0.000 description 1
- 102000004856 Lectins Human genes 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- DDWFXDSYGUXRAY-UHFFFAOYSA-N Luciferin Natural products CCc1c(C)c(CC2NC(=O)C(=C2C=C)C)[nH]c1Cc3[nH]c4C(=C5/NC(CC(=O)O)C(C)C5CC(=O)O)CC(=O)c4c3C DDWFXDSYGUXRAY-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 241000829100 Macaca mulatta polyomavirus 1 Species 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- WAEMQWOKJMHJLA-UHFFFAOYSA-N Manganese(2+) Chemical compound [Mn+2] WAEMQWOKJMHJLA-UHFFFAOYSA-N 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 108090000143 Mouse Proteins Proteins 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 241000282341 Mustela putorius furo Species 0.000 description 1
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 1
- 230000004988 N-glycosylation Effects 0.000 description 1
- 108091061960 Naked DNA Proteins 0.000 description 1
- 241001460678 Napo <wasp> Species 0.000 description 1
- 108010004217 Natural Cytotoxicity Triggering Receptor 1 Proteins 0.000 description 1
- 108010004222 Natural Cytotoxicity Triggering Receptor 3 Proteins 0.000 description 1
- 102100032870 Natural cytotoxicity triggering receptor 1 Human genes 0.000 description 1
- 102100032851 Natural cytotoxicity triggering receptor 2 Human genes 0.000 description 1
- 102100032852 Natural cytotoxicity triggering receptor 3 Human genes 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 101100076448 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) med-8 gene Proteins 0.000 description 1
- VEQPNABPJHWNSG-UHFFFAOYSA-N Nickel(2+) Chemical compound [Ni+2] VEQPNABPJHWNSG-UHFFFAOYSA-N 0.000 description 1
- 230000004989 O-glycosylation Effects 0.000 description 1
- 206010033647 Pancreatitis acute Diseases 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- MHRARRIFWSILEQ-UHFFFAOYSA-N Pederin Natural products COCC(CC1OC2C(NC(=O)C(O)C3(CC(=O)C(C)C(C)O3)OC)OCOC2C(OC)C1(C)C)OC MHRARRIFWSILEQ-UHFFFAOYSA-N 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108010081690 Pertussis Toxin Proteins 0.000 description 1
- 241000235648 Pichia Species 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 229940123573 Protein synthesis inhibitor Drugs 0.000 description 1
- 108010094028 Prothrombin Proteins 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- 108090000040 Russellysin Proteins 0.000 description 1
- RXGJTYFDKOHJHK-UHFFFAOYSA-N S-deoxo-amaninamide Natural products CCC(C)C1NC(=O)CNC(=O)C2Cc3c(SCC(NC(=O)CNC1=O)C(=O)NC(CC(=O)N)C(=O)N4CC(O)CC4C(=O)NC(C(C)C(O)CO)C(=O)N2)[nH]c5ccccc35 RXGJTYFDKOHJHK-UHFFFAOYSA-N 0.000 description 1
- 238000011579 SCID mouse model Methods 0.000 description 1
- 108010005173 SERPIN-B5 Proteins 0.000 description 1
- 241000235343 Saccharomycetales Species 0.000 description 1
- 108010084592 Saporins Proteins 0.000 description 1
- 108090000184 Selectins Proteins 0.000 description 1
- 102000003800 Selectins Human genes 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 102100030333 Serpin B5 Human genes 0.000 description 1
- 241000256248 Spodoptera Species 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- GKLVYJBZJHMRIY-OUBTZVSYSA-N Technetium-99 Chemical compound [99Tc] GKLVYJBZJHMRIY-OUBTZVSYSA-N 0.000 description 1
- 229910052771 Terbium Inorganic materials 0.000 description 1
- 108010000499 Thromboplastin Proteins 0.000 description 1
- 102000002262 Thromboplastin Human genes 0.000 description 1
- 102000002070 Transferrins Human genes 0.000 description 1
- 108010015865 Transferrins Proteins 0.000 description 1
- 108090000704 Tubulin Proteins 0.000 description 1
- 102000004243 Tubulin Human genes 0.000 description 1
- 206010054094 Tumour necrosis Diseases 0.000 description 1
- 244000274883 Urtica dioica Species 0.000 description 1
- 235000009108 Urtica dioica Nutrition 0.000 description 1
- 102000011731 Vacuolar Proton-Translocating ATPases Human genes 0.000 description 1
- 108010037026 Vacuolar Proton-Translocating ATPases Proteins 0.000 description 1
- 229940122803 Vinca alkaloid Drugs 0.000 description 1
- VWQVUPCCIRVNHF-OUBTZVSYSA-N Yttrium-90 Chemical compound [90Y] VWQVUPCCIRVNHF-OUBTZVSYSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 201000003229 acute pancreatitis Diseases 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 238000001261 affinity purification Methods 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 239000004007 alpha amanitin Substances 0.000 description 1
- 108090000183 alpha-2-Antiplasmin Proteins 0.000 description 1
- 102000003801 alpha-2-Antiplasmin Human genes 0.000 description 1
- CIORWBWIBBPXCG-SXZCQOKQSA-N alpha-amanitin Chemical compound O=C1N[C@@H](CC(N)=O)C(=O)N2C[C@H](O)C[C@H]2C(=O)N[C@@H]([C@@H](C)[C@@H](O)CO)C(=O)N[C@@H](C2)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@H]1C[S@@](=O)C1=C2C2=CC=C(O)C=C2N1 CIORWBWIBBPXCG-SXZCQOKQSA-N 0.000 description 1
- CIORWBWIBBPXCG-UHFFFAOYSA-N alpha-amanitin Natural products O=C1NC(CC(N)=O)C(=O)N2CC(O)CC2C(=O)NC(C(C)C(O)CO)C(=O)NC(C2)C(=O)NCC(=O)NC(C(C)CC)C(=O)NCC(=O)NC1CS(=O)C1=C2C2=CC=C(O)C=C2N1 CIORWBWIBBPXCG-UHFFFAOYSA-N 0.000 description 1
- 229960003896 aminopterin Drugs 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- 230000002491 angiogenic effect Effects 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 229940045799 anthracyclines and related substance Drugs 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 229940125644 antibody drug Drugs 0.000 description 1
- 230000005875 antibody response Effects 0.000 description 1
- 238000011394 anticancer treatment Methods 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 229940034982 antineoplastic agent Drugs 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- FIVPIPIDMRVLAY-UHFFFAOYSA-N aspergillin Natural products C1C2=CC=CC(O)C2N2C1(SS1)C(=O)N(C)C1(CO)C2=O FIVPIPIDMRVLAY-UHFFFAOYSA-N 0.000 description 1
- FZCSTZYAHCUGEM-UHFFFAOYSA-N aspergillomarasmine B Natural products OC(=O)CNC(C(O)=O)CNC(C(O)=O)CC(O)=O FZCSTZYAHCUGEM-UHFFFAOYSA-N 0.000 description 1
- 108010044540 auristatin Proteins 0.000 description 1
- OHDRQQURAXLVGJ-HLVWOLMTSA-N azane;(2e)-3-ethyl-2-[(e)-(3-ethyl-6-sulfo-1,3-benzothiazol-2-ylidene)hydrazinylidene]-1,3-benzothiazole-6-sulfonic acid Chemical compound [NH4+].[NH4+].S/1C2=CC(S([O-])(=O)=O)=CC=C2N(CC)C\1=N/N=C1/SC2=CC(S([O-])(=O)=O)=CC=C2N1CC OHDRQQURAXLVGJ-HLVWOLMTSA-N 0.000 description 1
- 239000000688 bacterial toxin Substances 0.000 description 1
- 238000013321 baculovirus-insect cell expression system Methods 0.000 description 1
- 229930192649 bafilomycin Natural products 0.000 description 1
- XDHNQDDQEHDUTM-UHFFFAOYSA-N bafliomycin A1 Natural products COC1C=CC=C(C)CC(C)C(O)C(C)C=C(C)C=C(OC)C(=O)OC1C(C)C(O)C(C)C1(O)OC(C(C)C)C(C)C(O)C1 XDHNQDDQEHDUTM-UHFFFAOYSA-N 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 239000012472 biological sample Substances 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 238000007413 biotinylation Methods 0.000 description 1
- 230000006287 biotinylation Effects 0.000 description 1
- 229910052797 bismuth Inorganic materials 0.000 description 1
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 1
- 230000023555 blood coagulation Effects 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- 239000003710 calcium ionophore Substances 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 230000003185 calcium uptake Effects 0.000 description 1
- 238000004422 calculation algorithm Methods 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 229930195731 calicheamicin Natural products 0.000 description 1
- HXCHCVDVKSCDHU-LULTVBGHSA-N calicheamicin Chemical compound C1[C@H](OC)[C@@H](NCC)CO[C@H]1O[C@H]1[C@H](O[C@@H]2C\3=C(NC(=O)OC)C(=O)C[C@](C/3=C/CSSSC)(O)C#C\C=C/C#C2)O[C@H](C)[C@@H](NO[C@@H]2O[C@H](C)[C@@H](SC(=O)C=3C(=C(OC)C(O[C@H]4[C@@H]([C@H](OC)[C@@H](O)[C@H](C)O4)O)=C(I)C=3C)OC)[C@@H](O)C2)[C@@H]1O HXCHCVDVKSCDHU-LULTVBGHSA-N 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000005779 cell damage Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 208000037887 cell injury Diseases 0.000 description 1
- 230000022534 cell killing Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- BFGKITSFLPAWGI-UHFFFAOYSA-N chromium(3+) Chemical compound [Cr+3] BFGKITSFLPAWGI-UHFFFAOYSA-N 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 238000000975 co-precipitation Methods 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- XLJKHNWPARRRJB-UHFFFAOYSA-N cobalt(2+) Chemical compound [Co+2] XLJKHNWPARRRJB-UHFFFAOYSA-N 0.000 description 1
- AGVAZMGAQJOSFJ-WZHZPDAFSA-M cobalt(2+);[(2r,3s,4r,5s)-5-(5,6-dimethylbenzimidazol-1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl] [(2r)-1-[3-[(1r,2r,3r,4z,7s,9z,12s,13s,14z,17s,18s,19r)-2,13,18-tris(2-amino-2-oxoethyl)-7,12,17-tris(3-amino-3-oxopropyl)-3,5,8,8,13,15,18,19-octamethyl-2 Chemical compound [Co+2].N#[C-].[N-]([C@@H]1[C@H](CC(N)=O)[C@@]2(C)CCC(=O)NC[C@@H](C)OP(O)(=O)O[C@H]3[C@H]([C@H](O[C@@H]3CO)N3C4=CC(C)=C(C)C=C4N=C3)O)\C2=C(C)/C([C@H](C\2(C)C)CCC(N)=O)=N/C/2=C\C([C@H]([C@@]/2(CC(N)=O)C)CCC(N)=O)=N\C\2=C(C)/C2=N[C@]1(C)[C@@](C)(CC(N)=O)[C@@H]2CCC(N)=O AGVAZMGAQJOSFJ-WZHZPDAFSA-M 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 230000006957 competitive inhibition Effects 0.000 description 1
- 239000000306 component Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- RYGMFSIKBFXOCR-AKLPVKDBSA-N copper-67 Chemical compound [67Cu] RYGMFSIKBFXOCR-AKLPVKDBSA-N 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 230000000445 cytocidal effect Effects 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 239000002619 cytotoxin Substances 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 229940124447 delivery agent Drugs 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 206010013023 diphtheria Diseases 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 229960003668 docetaxel Drugs 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- KBQHZAAAGSGFKK-UHFFFAOYSA-N dysprosium atom Chemical compound [Dy] KBQHZAAAGSGFKK-UHFFFAOYSA-N 0.000 description 1
- 235000013601 eggs Nutrition 0.000 description 1
- 108010030074 endodeoxyribonuclease MluI Proteins 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- UYAHIZSMUZPPFV-UHFFFAOYSA-N erbium Chemical compound [Er] UYAHIZSMUZPPFV-UHFFFAOYSA-N 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 229960004222 factor ix Drugs 0.000 description 1
- 229940012414 factor viia Drugs 0.000 description 1
- 239000012091 fetal bovine serum Substances 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 238000002825 functional assay Methods 0.000 description 1
- UIWYJDYFSGRHKR-UHFFFAOYSA-N gadolinium atom Chemical compound [Gd] UIWYJDYFSGRHKR-UHFFFAOYSA-N 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 238000012215 gene cloning Methods 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- FIVPIPIDMRVLAY-RBJBARPLSA-N gliotoxin Chemical compound C1C2=CC=C[C@H](O)[C@H]2N2[C@]1(SS1)C(=O)N(C)[C@@]1(CO)C2=O FIVPIPIDMRVLAY-RBJBARPLSA-N 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- CBMIPXHVOVTTTL-UHFFFAOYSA-N gold(3+) Chemical compound [Au+3] CBMIPXHVOVTTTL-UHFFFAOYSA-N 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 208000024963 hair loss Diseases 0.000 description 1
- 230000003676 hair loss Effects 0.000 description 1
- 239000002874 hemostatic agent Substances 0.000 description 1
- 210000003494 hepatocyte Anatomy 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- 125000000487 histidyl group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C([H])=N1 0.000 description 1
- KJZYNXUDTRRSPN-UHFFFAOYSA-N holmium atom Chemical compound [Ho] KJZYNXUDTRRSPN-UHFFFAOYSA-N 0.000 description 1
- 239000012456 homogeneous solution Substances 0.000 description 1
- 230000021158 homophilic cell adhesion Effects 0.000 description 1
- 230000003100 immobilizing effect Effects 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 238000003119 immunoblot Methods 0.000 description 1
- 230000002055 immunohistochemical effect Effects 0.000 description 1
- 238000001114 immunoprecipitation Methods 0.000 description 1
- 230000001024 immunotherapeutic effect Effects 0.000 description 1
- 238000005462 in vivo assay Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- APFVFJFRJDLVQX-AHCXROLUSA-N indium-111 Chemical compound [111In] APFVFJFRJDLVQX-AHCXROLUSA-N 0.000 description 1
- APFVFJFRJDLVQX-YPZZEJLDSA-N indium-113 Chemical compound [113In] APFVFJFRJDLVQX-YPZZEJLDSA-N 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 102000006495 integrins Human genes 0.000 description 1
- 108010044426 integrins Proteins 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- XMBWDFGMSWQBCA-YPZZEJLDSA-N iodane Chemical compound [125IH] XMBWDFGMSWQBCA-YPZZEJLDSA-N 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- BPHPUYQFMNQIOC-NXRLNHOXSA-N isopropyl beta-D-thiogalactopyranoside Chemical compound CC(C)S[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O BPHPUYQFMNQIOC-NXRLNHOXSA-N 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 238000012933 kinetic analysis Methods 0.000 description 1
- CZMAIROVPAYCMU-UHFFFAOYSA-N lanthanum(3+) Chemical compound [La+3] CZMAIROVPAYCMU-UHFFFAOYSA-N 0.000 description 1
- 239000002523 lectin Substances 0.000 description 1
- 235000005772 leucine Nutrition 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- GZQKNULLWNGMCW-PWQABINMSA-N lipid A (E. coli) Chemical compound O1[C@H](CO)[C@@H](OP(O)(O)=O)[C@H](OC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)[C@@H](NC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)[C@@H]1OC[C@@H]1[C@@H](O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OP(O)(O)=O)O1 GZQKNULLWNGMCW-PWQABINMSA-N 0.000 description 1
- 238000001638 lipofection Methods 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 231100000053 low toxicity Toxicity 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 210000001161 mammalian embryo Anatomy 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 235000013372 meat Nutrition 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- QSHDDOUJBYECFT-AHCXROLUSA-N mercury-197 Chemical compound [197Hg] QSHDDOUJBYECFT-AHCXROLUSA-N 0.000 description 1
- QSHDDOUJBYECFT-NJFSPNSNSA-N mercury-203 Chemical compound [203Hg] QSHDDOUJBYECFT-NJFSPNSNSA-N 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 208000037819 metastatic cancer Diseases 0.000 description 1
- 208000011575 metastatic malignant neoplasm Diseases 0.000 description 1
- 208000010658 metastatic prostate carcinoma Diseases 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- CFCUWKMKBJTWLW-BKHRDMLASA-N mithramycin Chemical compound O([C@@H]1C[C@@H](O[C@H](C)[C@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1C)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](O[C@@H]2O[C@H](C)[C@H](O)[C@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@@](C)(O)C3)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@@H](O)[C@H](O)[C@@H](C)O1 CFCUWKMKBJTWLW-BKHRDMLASA-N 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000002715 modification method Methods 0.000 description 1
- 238000001823 molecular biology technique Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 239000003471 mutagenic agent Substances 0.000 description 1
- 231100000707 mutagenic chemical Toxicity 0.000 description 1
- 239000002636 mycotoxin Substances 0.000 description 1
- OHDXDNUPVVYWOV-UHFFFAOYSA-N n-methyl-1-(2-naphthalen-1-ylsulfanylphenyl)methanamine Chemical compound CNCC1=CC=CC=C1SC1=CC=CC2=CC=CC=C12 OHDXDNUPVVYWOV-UHFFFAOYSA-N 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- QEFYFXOXNSNQGX-UHFFFAOYSA-N neodymium atom Chemical compound [Nd] QEFYFXOXNSNQGX-UHFFFAOYSA-N 0.000 description 1
- 239000003865 nucleic acid synthesis inhibitor Substances 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 150000003833 nucleoside derivatives Chemical class 0.000 description 1
- 238000011580 nude mouse model Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 108010087558 pectate lyase Proteins 0.000 description 1
- ZNEZZONMADKYTB-VRCUBXEUSA-N pederin Chemical compound C1[C@@H](O)C(C)(C)[C@@H](C[C@@H](COC)OC)O[C@@H]1[C@H](OC)NC(=O)[C@@H](O)[C@]1(OC)O[C@H](C)[C@H](C)C(=C)C1 ZNEZZONMADKYTB-VRCUBXEUSA-N 0.000 description 1
- 101150040383 pel2 gene Proteins 0.000 description 1
- 101150050446 pelB gene Proteins 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 238000010647 peptide synthesis reaction Methods 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 238000009520 phase I clinical trial Methods 0.000 description 1
- 238000009521 phase II clinical trial Methods 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 230000003169 placental effect Effects 0.000 description 1
- 239000003123 plant toxin Substances 0.000 description 1
- 239000013600 plasmid vector Substances 0.000 description 1
- 229960003171 plicamycin Drugs 0.000 description 1
- 229960001237 podophyllotoxin Drugs 0.000 description 1
- YJGVMLPVUAXIQN-XVVDYKMHSA-N podophyllotoxin Chemical compound COC1=C(OC)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@H](O)[C@@H]3[C@@H]2C(OC3)=O)=C1 YJGVMLPVUAXIQN-XVVDYKMHSA-N 0.000 description 1
- YVCVYCSAAZQOJI-UHFFFAOYSA-N podophyllotoxin Natural products COC1=C(O)C(OC)=CC(C2C3=CC=4OCOC=4C=C3C(O)C3C2C(OC3)=O)=C1 YVCVYCSAAZQOJI-UHFFFAOYSA-N 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 231100001271 preclinical toxicology Toxicity 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 238000009117 preventive therapy Methods 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 108020001580 protein domains Proteins 0.000 description 1
- 239000000007 protein synthesis inhibitor Substances 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- FFISLTWEISOMFC-UHFFFAOYSA-N pyridin-2-yl propanedithioate Chemical compound CCC(=S)SC1=CC=CC=N1 FFISLTWEISOMFC-UHFFFAOYSA-N 0.000 description 1
- 238000003127 radioimmunoassay Methods 0.000 description 1
- 238000002708 random mutagenesis Methods 0.000 description 1
- 229940051173 recombinant immunotoxin Drugs 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 238000012827 research and development Methods 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- WUAPFZMCVAUBPE-IGMARMGPSA-N rhenium-186 Chemical compound [186Re] WUAPFZMCVAUBPE-IGMARMGPSA-N 0.000 description 1
- 229920002477 rna polymer Polymers 0.000 description 1
- 229910052701 rubidium Inorganic materials 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- DOSGOCSVHPUUIA-UHFFFAOYSA-N samarium(3+) Chemical compound [Sm+3] DOSGOCSVHPUUIA-UHFFFAOYSA-N 0.000 description 1
- 208000011571 secondary malignant neoplasm Diseases 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 201000010088 skin benign neoplasm Diseases 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 238000010532 solid phase synthesis reaction Methods 0.000 description 1
- 210000001082 somatic cell Anatomy 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 235000011149 sulphuric acid Nutrition 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 230000001502 supplementing effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- GZCRRIHWUXGPOV-UHFFFAOYSA-N terbium atom Chemical compound [Tb] GZCRRIHWUXGPOV-UHFFFAOYSA-N 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- DSNBHJFQCNUKMA-SCKDECHMSA-N thromboxane A2 Chemical compound OC(=O)CCC\C=C/C[C@@H]1[C@@H](/C=C/[C@@H](O)CCCCC)O[C@@H]2O[C@H]1C2 DSNBHJFQCNUKMA-SCKDECHMSA-N 0.000 description 1
- 239000003768 thromboxane synthase inhibitor Substances 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 231100000440 toxicity profile Toxicity 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000011830 transgenic mouse model Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 230000014621 translational initiation Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- PXSOHRWMIRDKMP-UHFFFAOYSA-N triaziquone Chemical compound O=C1C(N2CC2)=C(N2CC2)C(=O)C=C1N1CC1 PXSOHRWMIRDKMP-UHFFFAOYSA-N 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 230000005747 tumor angiogenesis Effects 0.000 description 1
- 238000013414 tumor xenograft model Methods 0.000 description 1
- 241000701447 unidentified baculovirus Species 0.000 description 1
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 210000001835 viscera Anatomy 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
- 229940075420 xanthine Drugs 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
- 229960005502 α-amanitin Drugs 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/21—Immunoglobulins specific features characterized by taxonomic origin from primates, e.g. man
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/732—Antibody-dependent cellular cytotoxicity [ADCC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/734—Complement-dependent cytotoxicity [CDC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Cell Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Veterinary Medicine (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Animal Behavior & Ethology (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US18538709P | 2009-06-09 | 2009-06-09 | |
| US61/185,387 | 2009-06-09 | ||
| GB0909904.5 | 2009-06-09 | ||
| GBGB0909904.5A GB0909904D0 (en) | 2009-06-09 | 2009-06-09 | Product |
| PCT/GB2010/050969 WO2010142990A1 (en) | 2009-06-09 | 2010-06-09 | ANTI-EpCAM ANTIBODIES |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012529281A true JP2012529281A (ja) | 2012-11-22 |
| JP2012529281A5 JP2012529281A5 (OSRAM) | 2013-07-25 |
Family
ID=40937119
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012514538A Pending JP2012529281A (ja) | 2009-06-09 | 2010-06-09 | 抗EpCAM抗体 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US8637017B2 (OSRAM) |
| EP (1) | EP2440580A1 (OSRAM) |
| JP (1) | JP2012529281A (OSRAM) |
| CN (1) | CN102549017A (OSRAM) |
| BR (1) | BRPI1011005A2 (OSRAM) |
| EA (1) | EA201171463A1 (OSRAM) |
| GB (1) | GB0909904D0 (OSRAM) |
| WO (1) | WO2010142990A1 (OSRAM) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016510755A (ja) * | 2013-03-06 | 2016-04-11 | メリマック ファーマシューティカルズ インコーポレーティッド | 抗C−METタンデムFc二重特異性抗体 |
| JP2020515262A (ja) * | 2017-03-29 | 2020-05-28 | エフ・ホフマン−ラ・ロシュ・アクチェンゲゼルシャフト | 共刺激tnf受容体のための二重特異性抗原結合分子 |
| JP2022506072A (ja) * | 2018-10-26 | 2022-01-17 | イミュノジェン, インコーポレイテッド | EpCAM抗体、活性化可能抗体、および免疫複合体、ならびにそれらの使用 |
Families Citing this family (48)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2826735C (en) | 2003-04-30 | 2019-06-04 | University Of Zurich | Methods for treating cancer using an immunotoxin |
| US7612181B2 (en) | 2005-08-19 | 2009-11-03 | Abbott Laboratories | Dual variable domain immunoglobulin and uses thereof |
| AU2007312367B2 (en) | 2006-10-12 | 2012-09-06 | Chugai Seiyaku Kabushiki Kaisha | Diagnosis and treatment of cancer using anti-EREG antibody |
| US20120141501A1 (en) | 2009-05-29 | 2012-06-07 | Forerunner Pharma Research Co. Ltd | Pharmaceutical Composition Containing Antagonist of EGF Family Ligand as Component |
| JP6130307B2 (ja) | 2011-03-17 | 2017-05-17 | ザ ユニバーシティ オブ バーミンガム | 再指向性免疫療法 |
| CN107936121B (zh) | 2011-05-16 | 2022-01-14 | 埃泰美德(香港)有限公司 | 多特异性fab融合蛋白及其使用方法 |
| TWI593705B (zh) * | 2011-12-28 | 2017-08-01 | Chugai Pharmaceutical Co Ltd | Humanized anti-epiregulin antibody and cancer therapeutic agent containing the antibody as an active ingredient |
| JP6163502B2 (ja) * | 2012-03-02 | 2017-07-12 | アカデミア シニカAcademia Sinica | 抗上皮細胞接着分子(EpCAM)抗体及びその使用方法 |
| IN2015DN01967A (OSRAM) * | 2012-09-19 | 2015-08-14 | Abbvie Biotherapeutics Inc | |
| CN103387990B (zh) * | 2012-09-24 | 2016-01-06 | 厦门大学 | 上皮细胞粘附分子的核酸适体EpCAM B及其制备方法 |
| US20140248292A1 (en) * | 2013-03-04 | 2014-09-04 | Oslo Universitetssykehus Hf | Compositions and methods for treating cancer |
| CA2909153A1 (en) * | 2013-04-12 | 2014-10-16 | Viventia Bio Inc. | Compositions and methods for detection and treatment of hepatocellular carcinoma |
| CN103275226B (zh) * | 2013-06-09 | 2017-08-29 | 中国科学技术大学 | 特异性抗人上皮细胞粘附分子(EpCAM)的单克隆抗体的制备、鉴定及应用 |
| WO2014208482A1 (ja) * | 2013-06-24 | 2014-12-31 | 中外製薬株式会社 | ヒト化抗Epiregulin抗体を有効成分として含む腺癌以外の非小細胞肺癌の治療剤 |
| DK3052525T3 (da) | 2013-10-02 | 2019-10-07 | Viventia Bio Inc | Anti-epcam-antistoffer og anvendelsesfremgangsmåder |
| JP6824183B2 (ja) | 2015-03-12 | 2021-02-03 | セセン バイオ,インコーポレイテッド | Epcam陽性膀胱癌の処置方法 |
| AU2016228760B2 (en) | 2015-03-12 | 2020-07-16 | Viventia Bio Inc. | Dosing strategies for targeting EPCAM positive bladder cancer |
| EP3277716B1 (en) | 2015-04-03 | 2020-06-24 | XOMA Technology Ltd. | Treatment of cancer using inhibitors of tgf-beta and pd-1 |
| US11098131B2 (en) | 2015-07-31 | 2021-08-24 | Sutro Biopharma, Inc. | Anti-EpCAM antibodies, compositions comprising anti-EpCAM antibodies and methods of making and using anti-EpCAM antibodies |
| US10870701B2 (en) | 2016-03-15 | 2020-12-22 | Generon (Shanghai) Corporation Ltd. | Multispecific fab fusion proteins and use thereof |
| AU2017301826A1 (en) | 2016-07-26 | 2019-03-14 | Tessa Therapeutics Ltd. | Chimeric antigen receptor |
| CN107814846A (zh) * | 2016-11-14 | 2018-03-20 | 杭州华得森生物技术有限公司 | 针对EpCAM和Cytokeratin5的特异性抗体及其制备方法 |
| MX420258B (es) * | 2017-09-07 | 2025-02-10 | Dragonfly Therapeutics Inc | Proteínas de unión a nkg2d, cd16 y nectina 4. |
| US12384847B2 (en) | 2018-02-08 | 2025-08-12 | Dragonfly Therapeutics, Inc. | Cancer therapy involving an anti-PD1 antibody and a multi-specific binding protein that binds NKG2D, CD16, and a tumor-associated antigen |
| US11884733B2 (en) | 2018-02-08 | 2024-01-30 | Dragonfly Therapeutics, Inc. | Antibody variable domains targeting the NKG2D receptor |
| US12215157B2 (en) | 2018-02-20 | 2025-02-04 | Dragonfly Therapeutics, Inc. | Multi-specific binding proteins that bind CD33, NKG2D, and CD16, and methods of use |
| CN108484771A (zh) * | 2018-04-24 | 2018-09-04 | 南京市妇幼保健院 | EpCAM单域抗体G7 |
| US20210261669A1 (en) | 2018-06-20 | 2021-08-26 | Chugai Seiyaku Kabushiki Kaisha | Method for activating immune response of target cell and composition therefor |
| GB201811410D0 (en) | 2018-07-12 | 2018-08-29 | F Star Beta Ltd | OX40 Binding molecules |
| WO2020018964A1 (en) | 2018-07-20 | 2020-01-23 | Fred Hutchinson Cancer Research Center | Compositions and methods for controlled expression of antigen-specific receptors |
| MA53284A (fr) | 2018-08-08 | 2022-01-26 | Dragonfly Therapeutics Inc | Protéines de liaison à nkg2d, à cd16 et à un antigène associé à une tumeur |
| EP3833392A4 (en) | 2018-08-08 | 2022-05-18 | Dragonfly Therapeutics, Inc. | Multi-specific binding proteins that bind bcma, nkg2d and cd16, and methods of use |
| EA202091888A1 (ru) | 2018-08-08 | 2020-10-23 | Драгонфлай Терапьютикс, Инк. | Вариабельные домены антител, нацеленные на рецептор nkg2d |
| EP3847196A4 (en) | 2018-09-07 | 2023-01-04 | ITabMed (HK) Limited | BISPECIFIC ANTIGEN BINDING PROTEINS AND USES THEREOF |
| CN119896730A (zh) | 2018-12-17 | 2025-04-29 | 雷维托普有限公司 | 双免疫细胞衔接物 |
| CN113597432B (zh) * | 2019-04-22 | 2023-05-02 | 江苏恒瑞医药股份有限公司 | 抗EpCAM抗体及其应用 |
| CN110950959B (zh) * | 2020-02-25 | 2020-07-03 | 和铂医药(上海)有限责任公司 | 靶向EpCAM的抗体及其制备和应用 |
| CN118063614A (zh) * | 2020-03-11 | 2024-05-24 | 南京融捷康生物科技有限公司 | 可特异性结合EpCAM的单域抗体及其应用 |
| TW202208428A (zh) | 2020-05-06 | 2022-03-01 | 美商蜻蜓醫療公司 | 結合nkg2d、cd16及clec12a之蛋白質 |
| WO2021231237A2 (en) * | 2020-05-11 | 2021-11-18 | Augmenta Bioworks, Inc. | Antibodies for sars-cov-2 and uses thereof |
| CN112359052B (zh) * | 2020-08-20 | 2023-01-03 | 山东兴瑞生物科技有限公司 | 抗EpCAM嵌合抗原受体的编码基因、制备方法、具有该基因的质粒、免疫细胞及其应用 |
| WO2022150792A1 (en) * | 2021-01-11 | 2022-07-14 | Soteria Biotherapeutics, Inc. | Indinavir based chemical dimerization t cell engager compositions |
| EP4301774A4 (en) | 2021-03-03 | 2025-08-13 | Dragonfly Therapeutics Inc | METHODS OF TREATING CANCER USING MULTI-SPECIFIC BINDING PROTEINS THAT BIND TO NKG2D, CD16, AND A TUMOR-ASSOCIATED ANTIGEN |
| AR126009A1 (es) | 2021-06-02 | 2023-08-30 | Hoffmann La Roche | Moléculas agonistas de unión al antígeno cd28 que se dirigen a epcam |
| CN113563474B (zh) * | 2021-06-11 | 2023-05-02 | 广州医科大学附属第三医院(广州重症孕产妇救治中心、广州柔济医院) | EpCAM-CD16-NKG2D三特异性抗体及其应用 |
| WO2024082269A1 (zh) * | 2022-10-21 | 2024-04-25 | 武汉友芝友生物制药股份有限公司 | 双特异性抗体在免疫细胞治疗方面的应用 |
| WO2024258870A2 (en) | 2023-06-12 | 2024-12-19 | Amgen Inc. | Lymphotoxin beta receptor agonist binding proteins |
| CN119409829B (zh) * | 2024-11-15 | 2025-08-22 | 康元医疗科技(大连)有限公司 | 抗EpCAM纳米抗体及其应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004533248A (ja) * | 2001-05-03 | 2004-11-04 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフトング | 腫瘍特異的組換え抗体およびその使用 |
| US7227002B1 (en) * | 1997-04-14 | 2007-06-05 | Micromet Ag | Human antibodies that bind human 17-A1/EpCAM tumor antigen |
| WO2008122551A2 (en) * | 2007-04-04 | 2008-10-16 | Sigma-Tau Industrie Farmaceutiche Riunite S.P.A. | Anti-epcam antibody and uses thereof |
| JP2009511521A (ja) * | 2005-10-11 | 2009-03-19 | ミクロメット・アクチェンゲゼルシャフト | 交差種特異的(cross−species−specific)抗体を含む組成物および該組成物の使用 |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2437213A1 (fr) | 1978-09-28 | 1980-04-25 | Cm Ind | Produits cytotoxiques formes par liaison covalente de la chaine a de la ricine avec un anticorps et leur procede de preparation |
| US4736866A (en) | 1984-06-22 | 1988-04-12 | President And Fellows Of Harvard College | Transgenic non-human mammals |
| WO1990010457A1 (en) | 1989-03-14 | 1990-09-20 | New York University | Method of treating hiv infections using immunotoxins |
| DE3920358A1 (de) | 1989-06-22 | 1991-01-17 | Behringwerke Ag | Bispezifische und oligospezifische, mono- und oligovalente antikoerperkonstrukte, ihre herstellung und verwendung |
| EP0617706B1 (en) | 1991-11-25 | 2001-10-17 | Enzon, Inc. | Multivalent antigen-binding proteins |
| US5965132A (en) | 1992-03-05 | 1999-10-12 | Board Of Regents, The University Of Texas System | Methods and compositions for targeting the vasculature of solid tumors |
| WO1993017715A1 (en) | 1992-03-05 | 1993-09-16 | Board Of Regents, The University Of Texas System | Diagnostic and/or therapeutic agents, targeted to neovascular endothelial cells |
| US5660827A (en) | 1992-03-05 | 1997-08-26 | Board Of Regents, The University Of Texas System | Antibodies that bind to endoglin |
| US8236561B2 (en) | 1999-04-15 | 2012-08-07 | Crucell Holland B.V. | Efficient production of IgA in recombinant mammalian cells |
| GB9911569D0 (en) | 1999-05-18 | 1999-07-21 | Oxford Biomedica Ltd | Antibodies |
| DE60035434T2 (de) | 1999-12-27 | 2008-05-15 | Crucell Holland B.V. | Auswahl von epitop-bindungsfähigen verbindungen aus bibliotheken |
| DE10143106C1 (de) | 2001-09-03 | 2002-10-10 | Artus Ges Fuer Molekularbiolog | Vermehrung von Ribonukleinsäuren |
| CN1812999A (zh) * | 2003-05-31 | 2006-08-02 | 麦克罗梅特股份公司 | 包含EpCAM特异构建体的药物组合物 |
| US8802442B2 (en) | 2011-11-30 | 2014-08-12 | Eric B. Wheeldon | Apparatus and method for the remote sensing of blood in human feces and urine |
-
2009
- 2009-06-09 GB GBGB0909904.5A patent/GB0909904D0/en not_active Ceased
-
2010
- 2010-06-09 EA EA201171463A patent/EA201171463A1/ru unknown
- 2010-06-09 WO PCT/GB2010/050969 patent/WO2010142990A1/en not_active Ceased
- 2010-06-09 CN CN2010800351667A patent/CN102549017A/zh active Pending
- 2010-06-09 EP EP10724121A patent/EP2440580A1/en not_active Withdrawn
- 2010-06-09 US US12/797,052 patent/US8637017B2/en not_active Expired - Fee Related
- 2010-06-09 JP JP2012514538A patent/JP2012529281A/ja active Pending
- 2010-06-09 BR BRPI1011005A patent/BRPI1011005A2/pt not_active IP Right Cessation
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7227002B1 (en) * | 1997-04-14 | 2007-06-05 | Micromet Ag | Human antibodies that bind human 17-A1/EpCAM tumor antigen |
| JP2004533248A (ja) * | 2001-05-03 | 2004-11-04 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフトング | 腫瘍特異的組換え抗体およびその使用 |
| JP2009511521A (ja) * | 2005-10-11 | 2009-03-19 | ミクロメット・アクチェンゲゼルシャフト | 交差種特異的(cross−species−specific)抗体を含む組成物および該組成物の使用 |
| WO2008122551A2 (en) * | 2007-04-04 | 2008-10-16 | Sigma-Tau Industrie Farmaceutiche Riunite S.P.A. | Anti-epcam antibody and uses thereof |
Non-Patent Citations (3)
| Title |
|---|
| JPN5012014465; LUTTERBUESE PETRA: 'EXCHANGING HUMAN FCgamma1 WITH MURINE FCgamma2A HIGHLY POTENTIATES ANTI-TUMOR ACTIVITY 以下備考' CANCER IMMUNOLOGY IMMUNOTHERAPY V56 N4, 200704, P459-468 * |
| JPN5012014470; RAUM T: 'ANTI-SELF ANTIBODIES SELECTED FROM A HUMAN IGD HEAVY CHAIN REPERTOIRE: A NOVEL APPROACH 以下備考' CANCER IMMUNOLOGY AND IMMUNOTHERAPY V50 N3, 20010501, P141-150, SPRINGER-VERLAG * |
| JPN6014042231; Br. J. Cancer, 2005, 92(2), pp.342-349 * |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016510755A (ja) * | 2013-03-06 | 2016-04-11 | メリマック ファーマシューティカルズ インコーポレーティッド | 抗C−METタンデムFc二重特異性抗体 |
| JP2020515262A (ja) * | 2017-03-29 | 2020-05-28 | エフ・ホフマン−ラ・ロシュ・アクチェンゲゼルシャフト | 共刺激tnf受容体のための二重特異性抗原結合分子 |
| JP7196094B2 (ja) | 2017-03-29 | 2022-12-26 | エフ・ホフマン-ラ・ロシュ・アクチェンゲゼルシャフト | 共刺激tnf受容体のための二重特異性抗原結合分子 |
| US11639394B2 (en) | 2017-03-29 | 2023-05-02 | Hoffmann-La Roche Inc. | Bispecific antigen binding molecule for a costimulatory TNF receptor |
| JP2022506072A (ja) * | 2018-10-26 | 2022-01-17 | イミュノジェン, インコーポレイテッド | EpCAM抗体、活性化可能抗体、および免疫複合体、ならびにそれらの使用 |
| JP7592009B2 (ja) | 2018-10-26 | 2024-11-29 | イミュノジェン, インコーポレイテッド | EpCAM抗体、活性化可能抗体、および免疫複合体、ならびにそれらの使用 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2010142990A1 (en) | 2010-12-16 |
| US20100310463A1 (en) | 2010-12-09 |
| BRPI1011005A2 (pt) | 2018-03-06 |
| EA201171463A1 (ru) | 2012-07-30 |
| US8637017B2 (en) | 2014-01-28 |
| CN102549017A (zh) | 2012-07-04 |
| GB0909904D0 (en) | 2009-07-22 |
| EP2440580A1 (en) | 2012-04-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8637017B2 (en) | Anti-EpCAM antibodies | |
| US8822664B2 (en) | Antibodies | |
| US9353185B2 (en) | Antibodies | |
| AU2011340264B2 (en) | Anti CCR4 antibodies and uses thereof | |
| US20090070890A1 (en) | Product | |
| US10266599B2 (en) | Antibodies which bind to the human CC chemokine receptor 4 and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130606 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20130606 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20141007 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20141222 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20150205 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20150306 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20150609 |