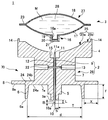
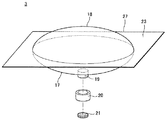
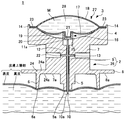
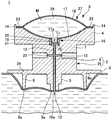
JP2012010971A - 薬剤容器、及び薬剤注射装置 - Google Patents
薬剤容器、及び薬剤注射装置 Download PDFInfo
- Publication number
- JP2012010971A JP2012010971A JP2010150494A JP2010150494A JP2012010971A JP 2012010971 A JP2012010971 A JP 2012010971A JP 2010150494 A JP2010150494 A JP 2010150494A JP 2010150494 A JP2010150494 A JP 2010150494A JP 2012010971 A JP2012010971 A JP 2012010971A
- Authority
- JP
- Japan
- Prior art keywords
- needle
- drug
- container
- medicine
- injection device
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000003814 drug Substances 0.000 title claims abstract description 301
- 229940079593 drug Drugs 0.000 title claims abstract description 172
- 238000002347 injection Methods 0.000 title claims abstract description 110
- 239000007924 injection Substances 0.000 title claims abstract description 110
- 238000005304 joining Methods 0.000 claims abstract description 7
- 238000009434 installation Methods 0.000 claims description 56
- 239000000463 material Substances 0.000 claims description 33
- 238000003780 insertion Methods 0.000 claims description 31
- 230000037431 insertion Effects 0.000 claims description 31
- 239000007788 liquid Substances 0.000 claims description 29
- 230000000149 penetrating effect Effects 0.000 claims description 5
- 238000012423 maintenance Methods 0.000 claims 1
- 238000007789 sealing Methods 0.000 abstract description 4
- 239000000243 solution Substances 0.000 abstract description 4
- 229940090044 injection Drugs 0.000 description 98
- 210000003491 skin Anatomy 0.000 description 87
- 238000003825 pressing Methods 0.000 description 33
- 230000002093 peripheral effect Effects 0.000 description 21
- 239000003795 chemical substances by application Substances 0.000 description 20
- 230000000087 stabilizing effect Effects 0.000 description 20
- 239000000126 substance Substances 0.000 description 19
- 238000000034 method Methods 0.000 description 14
- -1 polyethylene Polymers 0.000 description 8
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- 230000004927 fusion Effects 0.000 description 6
- 239000004698 Polyethylene Substances 0.000 description 5
- 239000004743 Polypropylene Substances 0.000 description 5
- 210000000852 deltoid muscle Anatomy 0.000 description 5
- 238000000465 moulding Methods 0.000 description 5
- 229920000573 polyethylene Polymers 0.000 description 5
- 229920001155 polypropylene Polymers 0.000 description 5
- 229960005486 vaccine Drugs 0.000 description 5
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 4
- 239000000853 adhesive Substances 0.000 description 4
- 230000001070 adhesive effect Effects 0.000 description 4
- 239000000470 constituent Substances 0.000 description 4
- 210000004207 dermis Anatomy 0.000 description 4
- 238000010586 diagram Methods 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 210000002615 epidermis Anatomy 0.000 description 4
- 230000006641 stabilisation Effects 0.000 description 4
- 238000011105 stabilization Methods 0.000 description 4
- 230000004888 barrier function Effects 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- 230000035699 permeability Effects 0.000 description 3
- 229920005989 resin Polymers 0.000 description 3
- 239000011347 resin Substances 0.000 description 3
- 229920002725 thermoplastic elastomer Polymers 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 239000012611 container material Substances 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 229920001971 elastomer Polymers 0.000 description 2
- 239000005038 ethylene vinyl acetate Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 2
- 229920000728 polyester Polymers 0.000 description 2
- 229920000306 polymethylpentene Polymers 0.000 description 2
- 229920000098 polyolefin Polymers 0.000 description 2
- 229940071643 prefilled syringe Drugs 0.000 description 2
- 229920000178 Acrylic resin Polymers 0.000 description 1
- 239000004925 Acrylic resin Substances 0.000 description 1
- 229910000838 Al alloy Inorganic materials 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 244000043261 Hevea brasiliensis Species 0.000 description 1
- JHWNWJKBPDFINM-UHFFFAOYSA-N Laurolactam Chemical compound O=C1CCCCCCCCCCCN1 JHWNWJKBPDFINM-UHFFFAOYSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 229920000299 Nylon 12 Polymers 0.000 description 1
- 229920002292 Nylon 6 Polymers 0.000 description 1
- 229920000305 Nylon 6,10 Polymers 0.000 description 1
- 229920002302 Nylon 6,6 Polymers 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000005062 Polybutadiene Substances 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 229910001069 Ti alloy Inorganic materials 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- XECAHXYUAAWDEL-UHFFFAOYSA-N acrylonitrile butadiene styrene Chemical compound C=CC=C.C=CC#N.C=CC1=CC=CC=C1 XECAHXYUAAWDEL-UHFFFAOYSA-N 0.000 description 1
- 239000004676 acrylonitrile butadiene styrene Substances 0.000 description 1
- 229920000122 acrylonitrile butadiene styrene Polymers 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229940035674 anesthetics Drugs 0.000 description 1
- 229940125644 antibody drug Drugs 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 238000005452 bending Methods 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 238000000071 blow moulding Methods 0.000 description 1
- 238000007664 blowing Methods 0.000 description 1
- 229920005557 bromobutyl Polymers 0.000 description 1
- 229920005549 butyl rubber Polymers 0.000 description 1
- 229920005556 chlorobutyl Polymers 0.000 description 1
- 239000002872 contrast media Substances 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 1
- 238000007599 discharging Methods 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 239000013013 elastic material Substances 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000003631 expected effect Effects 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 239000003193 general anesthetic agent Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 229940093181 glucose injection Drugs 0.000 description 1
- 229940095529 heparin calcium Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 206010022000 influenza Diseases 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- 229920003049 isoprene rubber Polymers 0.000 description 1
- 239000005001 laminate film Substances 0.000 description 1
- 229920001684 low density polyethylene Polymers 0.000 description 1
- 239000004702 low-density polyethylene Substances 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 239000004081 narcotic agent Substances 0.000 description 1
- 229920003052 natural elastomer Polymers 0.000 description 1
- 229920001194 natural rubber Polymers 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 206010033675 panniculitis Diseases 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920002857 polybutadiene Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- PHZLMBHDXVLRIX-UHFFFAOYSA-M potassium lactate Chemical compound [K+].CC(O)C([O-])=O PHZLMBHDXVLRIX-UHFFFAOYSA-M 0.000 description 1
- 239000001521 potassium lactate Substances 0.000 description 1
- 229960001304 potassium lactate Drugs 0.000 description 1
- 235000011085 potassium lactate Nutrition 0.000 description 1
- 238000002310 reflectometry Methods 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 229920002379 silicone rubber Polymers 0.000 description 1
- 239000004945 silicone rubber Substances 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229960002668 sodium chloride Drugs 0.000 description 1
- 235000002639 sodium chloride Nutrition 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 239000000021 stimulant Substances 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 210000004304 subcutaneous tissue Anatomy 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 229920003002 synthetic resin Polymers 0.000 description 1
- 239000000057 synthetic resin Substances 0.000 description 1
- KKEYFWRCBNTPAC-UHFFFAOYSA-L terephthalate(2-) Chemical compound [O-]C(=O)C1=CC=C(C([O-])=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-L 0.000 description 1
- 229920002397 thermoplastic olefin Polymers 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 150000003722 vitamin derivatives Chemical class 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
Images
Landscapes
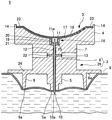
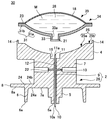
- Infusion, Injection, And Reservoir Apparatuses (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010150494A JP2012010971A (ja) | 2010-06-30 | 2010-06-30 | 薬剤容器、及び薬剤注射装置 |
| PCT/JP2011/064061 WO2012002190A1 (ja) | 2010-06-30 | 2011-06-20 | 薬剤注射装置及び薬剤容器 |
| US13/807,401 US20130110053A1 (en) | 2010-06-30 | 2011-06-20 | Drug injection apparatus and drug container |
| CN201180033074XA CN102971026A (zh) | 2010-06-30 | 2011-06-20 | 药剂注射装置和药剂容器 |
| EP11800655.0A EP2589397A4 (en) | 2010-06-30 | 2011-06-20 | MEDICATION INJECTION APPARATUS AND MEDICINE CONTAINER |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010150494A JP2012010971A (ja) | 2010-06-30 | 2010-06-30 | 薬剤容器、及び薬剤注射装置 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012010971A true JP2012010971A (ja) | 2012-01-19 |
| JP2012010971A5 JP2012010971A5 (cg-RX-API-DMAC7.html) | 2013-08-01 |
Family
ID=45598134
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010150494A Pending JP2012010971A (ja) | 2010-06-30 | 2010-06-30 | 薬剤容器、及び薬剤注射装置 |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP2012010971A (cg-RX-API-DMAC7.html) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101768647B1 (ko) | 2015-10-07 | 2017-08-17 | 최종수 | 멀티 주사 바늘 및 무선 자동주사장치 |
| JP2024103573A (ja) * | 2017-12-11 | 2024-08-01 | デカ・プロダクツ・リミテッド・パートナーシップ | 使い捨て筐体アセンブリと吹込成型されたリザーバとを有する注入ポンプアセンブリ |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001511037A (ja) * | 1997-01-17 | 2001-08-07 | ロシュ ダイアグノスティックス ゲーエムベーハー | 皮下組織注射システム |
| WO2008083209A2 (en) * | 2006-12-29 | 2008-07-10 | Amir Genosar | Hypodermic drug delivery reservoir and apparatus |
| JP2009516572A (ja) * | 2005-11-21 | 2009-04-23 | ベクトン・ディキンソン・アンド・カンパニー | 皮内送出装置 |
| JP2010502364A (ja) * | 2006-09-06 | 2010-01-28 | キャリブラ メディカル インコーポレイテッド | 折り畳可能の空気封じ込めリザーバを備える使い捨て注入装置 |
-
2010
- 2010-06-30 JP JP2010150494A patent/JP2012010971A/ja active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001511037A (ja) * | 1997-01-17 | 2001-08-07 | ロシュ ダイアグノスティックス ゲーエムベーハー | 皮下組織注射システム |
| JP2009516572A (ja) * | 2005-11-21 | 2009-04-23 | ベクトン・ディキンソン・アンド・カンパニー | 皮内送出装置 |
| JP2010502364A (ja) * | 2006-09-06 | 2010-01-28 | キャリブラ メディカル インコーポレイテッド | 折り畳可能の空気封じ込めリザーバを備える使い捨て注入装置 |
| WO2008083209A2 (en) * | 2006-12-29 | 2008-07-10 | Amir Genosar | Hypodermic drug delivery reservoir and apparatus |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101768647B1 (ko) | 2015-10-07 | 2017-08-17 | 최종수 | 멀티 주사 바늘 및 무선 자동주사장치 |
| JP2024103573A (ja) * | 2017-12-11 | 2024-08-01 | デカ・プロダクツ・リミテッド・パートナーシップ | 使い捨て筐体アセンブリと吹込成型されたリザーバとを有する注入ポンプアセンブリ |
| JP2024103575A (ja) * | 2017-12-11 | 2024-08-01 | デカ・プロダクツ・リミテッド・パートナーシップ | 使い捨て筐体アセンブリと吹込成型されたリザーバとを有する注入ポンプアセンブリ |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20130110053A1 (en) | Drug injection apparatus and drug container | |
| JP6284595B2 (ja) | 注射針組立体及び薬剤注射装置 | |
| JP5667571B2 (ja) | 注射針組立体及び薬剤注射装置 | |
| EP2554207A1 (en) | Prefilled syringe | |
| WO2011125560A1 (ja) | プレフィルドシリンジ | |
| WO2011125561A1 (ja) | プレフィルドシリンジ及びプレフィルドシリンジの組立方法 | |
| JP6605585B2 (ja) | 薬剤注射装置 | |
| JP5756793B2 (ja) | 注射針組立体及び薬剤注射装置 | |
| WO2011122221A1 (ja) | 薬剤投与器具及び薬剤注射装置 | |
| JPWO2011122393A1 (ja) | プレフィルドシリンジ | |
| WO2016117164A1 (ja) | 注射針組立体及びそれを備えた皮膚上層部への薬液注入用注射器 | |
| JP2012029723A (ja) | 注射針組立体、及び薬剤注射装置 | |
| WO2011068131A1 (ja) | プレフィルドシリンジ | |
| JP2011115345A (ja) | プレフィルドシリンジ | |
| JP2011212183A (ja) | プレフィルドシリンジ | |
| JP2012010971A (ja) | 薬剤容器、及び薬剤注射装置 | |
| JP2012010970A (ja) | 薬剤注射装置 | |
| JP2011212184A (ja) | プレフィルドシリンジ | |
| JP5432272B2 (ja) | 注射補助具及び薬剤注射装置 | |
| WO2012160852A1 (ja) | 注射針組立体及び薬剤注射装置 | |
| WO2011068130A1 (ja) | プレフィルドシリンジ | |
| JP5270424B2 (ja) | 薬剤注射装置および注射針組立体 | |
| WO2012157318A1 (ja) | 注射針組立体及び薬剤注射装置 | |
| WO2012147415A1 (ja) | 注射針組立体及び薬剤注射装置 | |
| JP2016187431A (ja) | 注射針組立体および薬剤注射装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130617 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20130617 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140422 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20140812 |