JP2011529084A - アミロイドβペプチド類似体、これらのオリゴマー、前記類似体またはオリゴマーを調製するためのプロセスおよび前記類似体またはオリゴマーを含む組成物ならびにこれらの使用 - Google Patents
アミロイドβペプチド類似体、これらのオリゴマー、前記類似体またはオリゴマーを調製するためのプロセスおよび前記類似体またはオリゴマーを含む組成物ならびにこれらの使用 Download PDFInfo
- Publication number
- JP2011529084A JP2011529084A JP2011520233A JP2011520233A JP2011529084A JP 2011529084 A JP2011529084 A JP 2011529084A JP 2011520233 A JP2011520233 A JP 2011520233A JP 2011520233 A JP2011520233 A JP 2011520233A JP 2011529084 A JP2011529084 A JP 2011529084A
- Authority
- JP
- Japan
- Prior art keywords
- amino acid
- sequence
- acid residue
- amyloid
- peptide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 108010090849 Amyloid beta-Peptides Proteins 0.000 title claims abstract description 355
- 102000013455 Amyloid beta-Peptides Human genes 0.000 title claims abstract description 355
- 238000000034 method Methods 0.000 title claims abstract description 145
- 239000000203 mixture Substances 0.000 title claims abstract description 53
- 230000008569 process Effects 0.000 title abstract description 15
- 125000000539 amino acid group Chemical group 0.000 claims abstract description 460
- 108090000765 processed proteins & peptides Proteins 0.000 claims abstract description 173
- 239000003795 chemical substances by application Substances 0.000 claims abstract description 82
- 206010002022 amyloidosis Diseases 0.000 claims abstract description 63
- 239000000816 peptidomimetic Substances 0.000 claims abstract description 54
- 230000003053 immunization Effects 0.000 claims abstract description 28
- 238000002649 immunization Methods 0.000 claims abstract description 23
- 235000001014 amino acid Nutrition 0.000 claims description 149
- 229940024606 amino acid Drugs 0.000 claims description 134
- 150000001413 amino acids Chemical group 0.000 claims description 123
- 229930182817 methionine Chemical group 0.000 claims description 107
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 claims description 104
- 230000027455 binding Effects 0.000 claims description 103
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical group CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 claims description 102
- 239000004474 valine Substances 0.000 claims description 101
- 229960004295 valine Drugs 0.000 claims description 101
- 235000004279 alanine Nutrition 0.000 claims description 94
- 229960003767 alanine Drugs 0.000 claims description 94
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical group NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 90
- 229960000310 isoleucine Drugs 0.000 claims description 88
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Chemical group CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 claims description 87
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Chemical group CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 claims description 84
- 229960003136 leucine Drugs 0.000 claims description 84
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical group C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 claims description 79
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical group CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 claims description 78
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical group CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 claims description 73
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Chemical group OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 claims description 73
- 229960001153 serine Drugs 0.000 claims description 73
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical group CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 claims description 72
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical group OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 claims description 71
- 102000036639 antigens Human genes 0.000 claims description 61
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical group OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 60
- 239000000427 antigen Substances 0.000 claims description 60
- 108091007433 antigens Proteins 0.000 claims description 60
- 239000004471 Glycine Chemical group 0.000 claims description 59
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Chemical group OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 claims description 52
- 229960005190 phenylalanine Drugs 0.000 claims description 52
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims description 49
- -1 X 33 Chemical compound 0.000 claims description 41
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical group OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 claims description 34
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 claims description 32
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Chemical group OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 claims description 31
- 229960004441 tyrosine Drugs 0.000 claims description 31
- 239000004472 Lysine Substances 0.000 claims description 30
- 235000018977 lysine Nutrition 0.000 claims description 29
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 28
- 208000024827 Alzheimer disease Diseases 0.000 claims description 22
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 claims description 20
- 239000008194 pharmaceutical composition Substances 0.000 claims description 19
- 238000002360 preparation method Methods 0.000 claims description 19
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical group OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 claims description 18
- 125000000741 isoleucyl group Chemical group [H]N([H])C(C(C([H])([H])[H])C([H])([H])C([H])([H])[H])C(=O)O* 0.000 claims description 18
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 claims description 17
- 239000004475 Arginine Chemical group 0.000 claims description 16
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Chemical group OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 claims description 16
- 235000013922 glutamic acid Nutrition 0.000 claims description 16
- 239000004220 glutamic acid Substances 0.000 claims description 16
- 238000004519 manufacturing process Methods 0.000 claims description 16
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical group NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 claims description 15
- 235000003704 aspartic acid Nutrition 0.000 claims description 15
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Chemical group OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 claims description 14
- 229960002885 histidine Drugs 0.000 claims description 14
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 claims description 14
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Chemical group OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 claims description 13
- 235000009582 asparagine Nutrition 0.000 claims description 13
- 229960001230 asparagine Drugs 0.000 claims description 13
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 claims description 13
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical group OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 claims description 12
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 claims description 12
- 125000000487 histidyl group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C([H])=N1 0.000 claims description 12
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 12
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical group C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 claims description 9
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 claims description 9
- 201000010374 Down Syndrome Diseases 0.000 claims description 8
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical group OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 claims description 8
- 239000003937 drug carrier Substances 0.000 claims description 7
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 claims description 7
- 125000000404 glutamine group Chemical group N[C@@H](CCC(N)=O)C(=O)* 0.000 claims description 7
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Chemical group CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 claims description 6
- 239000004473 Threonine Chemical group 0.000 claims description 6
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 claims description 5
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 claims description 4
- 229960005486 vaccine Drugs 0.000 claims description 3
- 125000003275 alpha amino acid group Chemical group 0.000 abstract description 156
- 210000004027 cell Anatomy 0.000 description 86
- 229960004452 methionine Drugs 0.000 description 78
- 108090000623 proteins and genes Proteins 0.000 description 77
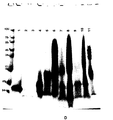
- 108090001109 Thermolysin Proteins 0.000 description 60
- 238000003776 cleavage reaction Methods 0.000 description 53
- 230000007017 scission Effects 0.000 description 53
- 230000015572 biosynthetic process Effects 0.000 description 47
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical group NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 38
- 102000004196 processed proteins & peptides Human genes 0.000 description 35
- 238000000338 in vitro Methods 0.000 description 34
- 102000004169 proteins and genes Human genes 0.000 description 34
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 32
- 235000018102 proteins Nutrition 0.000 description 32
- 230000000890 antigenic effect Effects 0.000 description 29
- 238000002965 ELISA Methods 0.000 description 28
- 230000002255 enzymatic effect Effects 0.000 description 28
- 239000000523 sample Substances 0.000 description 28
- 108020004414 DNA Proteins 0.000 description 26
- 108060003951 Immunoglobulin Proteins 0.000 description 25
- 239000012634 fragment Substances 0.000 description 25
- 102000018358 immunoglobulin Human genes 0.000 description 25
- 241000283973 Oryctolagus cuniculus Species 0.000 description 23
- 125000003277 amino group Chemical group 0.000 description 23
- 150000001875 compounds Chemical class 0.000 description 22
- 230000004927 fusion Effects 0.000 description 22
- 238000001727 in vivo Methods 0.000 description 22
- 108091023037 Aptamer Proteins 0.000 description 21
- 239000004971 Cross linker Substances 0.000 description 21
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 21
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 21
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 20
- 239000013604 expression vector Substances 0.000 description 20
- 230000028993 immune response Effects 0.000 description 20
- 230000004044 response Effects 0.000 description 20
- 239000000178 monomer Substances 0.000 description 19
- 241000699666 Mus <mouse, genus> Species 0.000 description 18
- 125000004429 atom Chemical group 0.000 description 18
- 230000000875 corresponding effect Effects 0.000 description 18
- 238000009472 formulation Methods 0.000 description 18
- 239000011347 resin Substances 0.000 description 18
- 229920005989 resin Polymers 0.000 description 18
- 238000004132 cross linking Methods 0.000 description 17
- 238000012216 screening Methods 0.000 description 17
- 239000000243 solution Substances 0.000 description 16
- 239000000126 substance Substances 0.000 description 16
- 241000699670 Mus sp. Species 0.000 description 15
- 230000014509 gene expression Effects 0.000 description 15
- 238000003259 recombinant expression Methods 0.000 description 15
- 241001465754 Metazoa Species 0.000 description 14
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 14
- 235000018417 cysteine Nutrition 0.000 description 14
- 210000004698 lymphocyte Anatomy 0.000 description 14
- 238000001819 mass spectrum Methods 0.000 description 14
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical group OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 13
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 13
- 150000007523 nucleic acids Chemical class 0.000 description 13
- 238000003786 synthesis reaction Methods 0.000 description 13
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 12
- 238000010586 diagram Methods 0.000 description 12
- 230000029087 digestion Effects 0.000 description 12
- 230000001225 therapeutic effect Effects 0.000 description 12
- 238000006243 chemical reaction Methods 0.000 description 11
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 11
- 239000011780 sodium chloride Substances 0.000 description 11
- 125000003396 thiol group Chemical group [H]S* 0.000 description 11
- 239000013598 vector Substances 0.000 description 11
- 238000004458 analytical method Methods 0.000 description 10
- 239000003550 marker Substances 0.000 description 10
- 230000009467 reduction Effects 0.000 description 10
- 108091005804 Peptidases Proteins 0.000 description 9
- 102000035195 Peptidases Human genes 0.000 description 9
- 206010035226 Plasma cell myeloma Diseases 0.000 description 9
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 9
- 201000000050 myeloid neoplasm Diseases 0.000 description 9
- 239000007790 solid phase Substances 0.000 description 9
- 238000006467 substitution reaction Methods 0.000 description 9
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide Chemical compound CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 8
- 239000004365 Protease Substances 0.000 description 8
- 150000001408 amides Chemical class 0.000 description 8
- 150000001412 amines Chemical class 0.000 description 8
- 239000000872 buffer Substances 0.000 description 8
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 8
- 238000005516 engineering process Methods 0.000 description 8
- 210000004602 germ cell Anatomy 0.000 description 8
- 230000002209 hydrophobic effect Effects 0.000 description 8
- 108020004707 nucleic acids Proteins 0.000 description 8
- 102000039446 nucleic acids Human genes 0.000 description 8
- 230000002441 reversible effect Effects 0.000 description 8
- 239000004094 surface-active agent Substances 0.000 description 8
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 7
- 230000002378 acidificating effect Effects 0.000 description 7
- 238000007792 addition Methods 0.000 description 7
- 125000003118 aryl group Chemical group 0.000 description 7
- 238000003556 assay Methods 0.000 description 7
- 238000006664 bond formation reaction Methods 0.000 description 7
- 239000003153 chemical reaction reagent Substances 0.000 description 7
- 239000003431 cross linking reagent Substances 0.000 description 7
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 7
- 201000010099 disease Diseases 0.000 description 7
- 229960002743 glutamine Drugs 0.000 description 7
- 239000001963 growth medium Substances 0.000 description 7
- 230000035772 mutation Effects 0.000 description 7
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 6
- 125000001433 C-terminal amino-acid group Chemical group 0.000 description 6
- 241001494479 Pecora Species 0.000 description 6
- 108010076504 Protein Sorting Signals Proteins 0.000 description 6
- 241000700159 Rattus Species 0.000 description 6
- 206010044688 Trisomy 21 Diseases 0.000 description 6
- 238000013459 approach Methods 0.000 description 6
- 238000001514 detection method Methods 0.000 description 6
- 239000003814 drug Substances 0.000 description 6
- 239000001257 hydrogen Substances 0.000 description 6
- 229910052739 hydrogen Inorganic materials 0.000 description 6
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 6
- 230000003993 interaction Effects 0.000 description 6
- 125000004430 oxygen atom Chemical group O* 0.000 description 6
- 229920001184 polypeptide Polymers 0.000 description 6
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 6
- 241000894007 species Species 0.000 description 6
- LLXVXPPXELIDGQ-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 3-(2,5-dioxopyrrol-1-yl)benzoate Chemical compound C=1C=CC(N2C(C=CC2=O)=O)=CC=1C(=O)ON1C(=O)CCC1=O LLXVXPPXELIDGQ-UHFFFAOYSA-N 0.000 description 5
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 5
- 241000283707 Capra Species 0.000 description 5
- 101150074155 DHFR gene Proteins 0.000 description 5
- 102000004190 Enzymes Human genes 0.000 description 5
- 108090000790 Enzymes Proteins 0.000 description 5
- 241000287828 Gallus gallus Species 0.000 description 5
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 5
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 5
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 5
- 239000002253 acid Substances 0.000 description 5
- 230000009824 affinity maturation Effects 0.000 description 5
- 229960003965 antiepileptics Drugs 0.000 description 5
- UCMIRNVEIXFBKS-UHFFFAOYSA-N beta-alanine Chemical compound NCCC(O)=O UCMIRNVEIXFBKS-UHFFFAOYSA-N 0.000 description 5
- 229940098773 bovine serum albumin Drugs 0.000 description 5
- 235000013330 chicken meat Nutrition 0.000 description 5
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 5
- 238000010494 dissociation reaction Methods 0.000 description 5
- 230000005593 dissociations Effects 0.000 description 5
- 239000003623 enhancer Substances 0.000 description 5
- 229940088598 enzyme Drugs 0.000 description 5
- 230000036541 health Effects 0.000 description 5
- 230000001900 immune effect Effects 0.000 description 5
- 238000001802 infusion Methods 0.000 description 5
- 238000002823 phage display Methods 0.000 description 5
- 230000017854 proteolysis Effects 0.000 description 5
- JJAHTWIKCUJRDK-UHFFFAOYSA-N succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate Chemical compound C1CC(CN2C(C=CC2=O)=O)CCC1C(=O)ON1C(=O)CCC1=O JJAHTWIKCUJRDK-UHFFFAOYSA-N 0.000 description 5
- 241000701022 Cytomegalovirus Species 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- KSPIYJQBLVDRRI-UHFFFAOYSA-N N-methylisoleucine Chemical compound CCC(C)C(NC)C(O)=O KSPIYJQBLVDRRI-UHFFFAOYSA-N 0.000 description 4
- 125000001429 N-terminal alpha-amino-acid group Chemical group 0.000 description 4
- 108091028043 Nucleic acid sequence Proteins 0.000 description 4
- 108020004511 Recombinant DNA Proteins 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 239000012472 biological sample Substances 0.000 description 4
- 210000004899 c-terminal region Anatomy 0.000 description 4
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 4
- 230000021615 conjugation Effects 0.000 description 4
- 238000005859 coupling reaction Methods 0.000 description 4
- 235000014113 dietary fatty acids Nutrition 0.000 description 4
- 239000002552 dosage form Substances 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- 229930195729 fatty acid Natural products 0.000 description 4
- 239000000194 fatty acid Substances 0.000 description 4
- 150000004665 fatty acids Chemical class 0.000 description 4
- 125000000524 functional group Chemical group 0.000 description 4
- 210000004408 hybridoma Anatomy 0.000 description 4
- 238000003018 immunoassay Methods 0.000 description 4
- 238000003780 insertion Methods 0.000 description 4
- 230000037431 insertion Effects 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 239000012528 membrane Substances 0.000 description 4
- 108020004999 messenger RNA Proteins 0.000 description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 4
- 238000002703 mutagenesis Methods 0.000 description 4
- 231100000350 mutagenesis Toxicity 0.000 description 4
- 239000002773 nucleotide Substances 0.000 description 4
- 125000003729 nucleotide group Chemical group 0.000 description 4
- 235000019419 proteases Nutrition 0.000 description 4
- FSYKKLYZXJSNPZ-UHFFFAOYSA-N sarcosine Chemical compound C[NH2+]CC([O-])=O FSYKKLYZXJSNPZ-UHFFFAOYSA-N 0.000 description 4
- 238000004062 sedimentation Methods 0.000 description 4
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 4
- 150000003573 thiols Chemical group 0.000 description 4
- 230000009261 transgenic effect Effects 0.000 description 4
- 238000013519 translation Methods 0.000 description 4
- DIYPCWKHSODVAP-UHFFFAOYSA-N 1-[3-(2,5-dioxopyrrol-1-yl)benzoyl]oxy-2,5-dioxopyrrolidine-3-sulfonic acid Chemical compound O=C1C(S(=O)(=O)O)CC(=O)N1OC(=O)C1=CC=CC(N2C(C=CC2=O)=O)=C1 DIYPCWKHSODVAP-UHFFFAOYSA-N 0.000 description 3
- CULQNACJHGHAER-UHFFFAOYSA-N 1-[4-[(2-iodoacetyl)amino]benzoyl]oxy-2,5-dioxopyrrolidine-3-sulfonic acid Chemical compound O=C1C(S(=O)(=O)O)CC(=O)N1OC(=O)C1=CC=C(NC(=O)CI)C=C1 CULQNACJHGHAER-UHFFFAOYSA-N 0.000 description 3
- FPIRBHDGWMWJEP-UHFFFAOYSA-N 1-hydroxy-7-azabenzotriazole Chemical compound C1=CN=C2N(O)N=NC2=C1 FPIRBHDGWMWJEP-UHFFFAOYSA-N 0.000 description 3
- SXGMVGOVILIERA-UHFFFAOYSA-N 2,3-diaminobutanoic acid Chemical compound CC(N)C(N)C(O)=O SXGMVGOVILIERA-UHFFFAOYSA-N 0.000 description 3
- OGNSCSPNOLGXSM-UHFFFAOYSA-N 2,4-diaminobutyric acid Chemical compound NCCC(N)C(O)=O OGNSCSPNOLGXSM-UHFFFAOYSA-N 0.000 description 3
- FUOOLUPWFVMBKG-UHFFFAOYSA-N 2-Aminoisobutyric acid Chemical compound CC(C)(N)C(O)=O FUOOLUPWFVMBKG-UHFFFAOYSA-N 0.000 description 3
- YEDUAINPPJYDJZ-UHFFFAOYSA-N 2-hydroxybenzothiazole Chemical compound C1=CC=C2SC(O)=NC2=C1 YEDUAINPPJYDJZ-UHFFFAOYSA-N 0.000 description 3
- 102000007698 Alcohol dehydrogenase Human genes 0.000 description 3
- 108010021809 Alcohol dehydrogenase Proteins 0.000 description 3
- 208000037259 Amyloid Plaque Diseases 0.000 description 3
- 108010039627 Aprotinin Proteins 0.000 description 3
- 101000950981 Bacillus subtilis (strain 168) Catabolic NAD-specific glutamate dehydrogenase RocG Proteins 0.000 description 3
- 241000282832 Camelidae Species 0.000 description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 3
- 102000003846 Carbonic anhydrases Human genes 0.000 description 3
- 108090000209 Carbonic anhydrases Proteins 0.000 description 3
- 206010012289 Dementia Diseases 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- 102000016901 Glutamate dehydrogenase Human genes 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 101000690301 Homo sapiens Aldo-keto reductase family 1 member C4 Proteins 0.000 description 3
- 101001116548 Homo sapiens Protein CBFA2T1 Proteins 0.000 description 3
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 3
- 102000004877 Insulin Human genes 0.000 description 3
- 108090001061 Insulin Proteins 0.000 description 3
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 3
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 3
- 241000829100 Macaca mulatta polyomavirus 1 Species 0.000 description 3
- 102000016943 Muramidase Human genes 0.000 description 3
- 108010014251 Muramidase Proteins 0.000 description 3
- 241000699660 Mus musculus Species 0.000 description 3
- 102000036675 Myoglobin Human genes 0.000 description 3
- 108010062374 Myoglobin Proteins 0.000 description 3
- 102000003505 Myosin Human genes 0.000 description 3
- 108060008487 Myosin Proteins 0.000 description 3
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 3
- 241001460678 Napo <wasp> Species 0.000 description 3
- 102000009097 Phosphorylases Human genes 0.000 description 3
- 108010073135 Phosphorylases Proteins 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 108010022394 Threonine synthase Proteins 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 239000002671 adjuvant Substances 0.000 description 3
- 230000002776 aggregation Effects 0.000 description 3
- 230000003321 amplification Effects 0.000 description 3
- 230000005875 antibody response Effects 0.000 description 3
- 229960004405 aprotinin Drugs 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 239000002299 complementary DNA Substances 0.000 description 3
- 230000008878 coupling Effects 0.000 description 3
- 238000010168 coupling process Methods 0.000 description 3
- 238000012258 culturing Methods 0.000 description 3
- 238000005520 cutting process Methods 0.000 description 3
- 125000004122 cyclic group Chemical group 0.000 description 3
- 238000003745 diagnosis Methods 0.000 description 3
- 102000004419 dihydrofolate reductase Human genes 0.000 description 3
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 108091006047 fluorescent proteins Proteins 0.000 description 3
- 102000034287 fluorescent proteins Human genes 0.000 description 3
- 102000054751 human RUNX1T1 Human genes 0.000 description 3
- NPZTUJOABDZTLV-UHFFFAOYSA-N hydroxybenzotriazole Substances O=C1C=CC=C2NNN=C12 NPZTUJOABDZTLV-UHFFFAOYSA-N 0.000 description 3
- 238000001114 immunoprecipitation Methods 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- ZPNFWUPYTFPOJU-LPYSRVMUSA-N iniprol Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@H]2CSSC[C@H]3C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC=4C=CC=CC=4)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=4C=CC=CC=4)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2N(CCC2)C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N2[C@@H](CCC2)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N2[C@@H](CCC2)C(=O)N3)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H](C(=O)N1)C(C)C)[C@@H](C)O)[C@@H](C)CC)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 ZPNFWUPYTFPOJU-LPYSRVMUSA-N 0.000 description 3
- 229940125396 insulin Drugs 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 239000004325 lysozyme Substances 0.000 description 3
- 229960000274 lysozyme Drugs 0.000 description 3
- 235000010335 lysozyme Nutrition 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 229960000485 methotrexate Drugs 0.000 description 3
- 238000010369 molecular cloning Methods 0.000 description 3
- 238000003199 nucleic acid amplification method Methods 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- 238000010647 peptide synthesis reaction Methods 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 239000002953 phosphate buffered saline Substances 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 238000006798 ring closing metathesis reaction Methods 0.000 description 3
- VUFNRPJNRFOTGK-UHFFFAOYSA-M sodium;1-[4-[(2,5-dioxopyrrol-1-yl)methyl]cyclohexanecarbonyl]oxy-2,5-dioxopyrrolidine-3-sulfonate Chemical compound [Na+].O=C1C(S(=O)(=O)[O-])CC(=O)N1OC(=O)C1CCC(CN2C(C=CC2=O)=O)CC1 VUFNRPJNRFOTGK-UHFFFAOYSA-M 0.000 description 3
- 210000001082 somatic cell Anatomy 0.000 description 3
- 238000010561 standard procedure Methods 0.000 description 3
- 125000001424 substituent group Chemical group 0.000 description 3
- 238000001920 surface-enhanced laser desorption--ionisation mass spectrometry Methods 0.000 description 3
- FPGGTKZVZWFYPV-UHFFFAOYSA-M tetrabutylammonium fluoride Chemical compound [F-].CCCC[N+](CCCC)(CCCC)CCCC FPGGTKZVZWFYPV-UHFFFAOYSA-M 0.000 description 3
- 229940124597 therapeutic agent Drugs 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 239000003053 toxin Substances 0.000 description 3
- 231100000765 toxin Toxicity 0.000 description 3
- 108700012359 toxins Proteins 0.000 description 3
- 238000013518 transcription Methods 0.000 description 3
- 230000035897 transcription Effects 0.000 description 3
- 238000011830 transgenic mouse model Methods 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- GKSPIZSKQWTXQG-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-[1-(pyridin-2-yldisulfanyl)ethyl]benzoate Chemical compound C=1C=C(C(=O)ON2C(CCC2=O)=O)C=CC=1C(C)SSC1=CC=CC=N1 GKSPIZSKQWTXQG-UHFFFAOYSA-N 0.000 description 2
- BVAUMRCGVHUWOZ-ZETCQYMHSA-N (2s)-2-(cyclohexylazaniumyl)propanoate Chemical compound OC(=O)[C@H](C)NC1CCCCC1 BVAUMRCGVHUWOZ-ZETCQYMHSA-N 0.000 description 2
- MRTPISKDZDHEQI-YFKPBYRVSA-N (2s)-2-(tert-butylamino)propanoic acid Chemical compound OC(=O)[C@H](C)NC(C)(C)C MRTPISKDZDHEQI-YFKPBYRVSA-N 0.000 description 2
- NPDBDJFLKKQMCM-SCSAIBSYSA-N (2s)-2-amino-3,3-dimethylbutanoic acid Chemical compound CC(C)(C)[C@H](N)C(O)=O NPDBDJFLKKQMCM-SCSAIBSYSA-N 0.000 description 2
- 125000006727 (C1-C6) alkenyl group Chemical group 0.000 description 2
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 2
- 125000006728 (C1-C6) alkynyl group Chemical group 0.000 description 2
- 125000003088 (fluoren-9-ylmethoxy)carbonyl group Chemical group 0.000 description 2
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- AASYSXRGODIQGY-UHFFFAOYSA-N 1-[1-(2,5-dioxopyrrol-1-yl)hexyl]pyrrole-2,5-dione Chemical compound O=C1C=CC(=O)N1C(CCCCC)N1C(=O)C=CC1=O AASYSXRGODIQGY-UHFFFAOYSA-N 0.000 description 2
- JFLSOKIMYBSASW-UHFFFAOYSA-N 1-chloro-2-[chloro(diphenyl)methyl]benzene Chemical compound ClC1=CC=CC=C1C(Cl)(C=1C=CC=CC=1)C1=CC=CC=C1 JFLSOKIMYBSASW-UHFFFAOYSA-N 0.000 description 2
- GVJXGCIPWAVXJP-UHFFFAOYSA-N 2,5-dioxo-1-oxoniopyrrolidine-3-sulfonate Chemical class ON1C(=O)CC(S(O)(=O)=O)C1=O GVJXGCIPWAVXJP-UHFFFAOYSA-N 0.000 description 2
- BLSAPDZWVFWUTL-UHFFFAOYSA-N 2,5-dioxopyrrolidine-3-sulfonic acid Chemical class OS(=O)(=O)C1CC(=O)NC1=O BLSAPDZWVFWUTL-UHFFFAOYSA-N 0.000 description 2
- 150000003923 2,5-pyrrolediones Chemical class 0.000 description 2
- QQZOUYFHWKTGEY-UHFFFAOYSA-N 4-azido-n-[2-[2-[(4-azido-2-hydroxybenzoyl)amino]ethyldisulfanyl]ethyl]-2-hydroxybenzamide Chemical compound OC1=CC(N=[N+]=[N-])=CC=C1C(=O)NCCSSCCNC(=O)C1=CC=C(N=[N+]=[N-])C=C1O QQZOUYFHWKTGEY-UHFFFAOYSA-N 0.000 description 2
- JJMDCOVWQOJGCB-UHFFFAOYSA-N 5-aminopentanoic acid Chemical compound [NH3+]CCCCC([O-])=O JJMDCOVWQOJGCB-UHFFFAOYSA-N 0.000 description 2
- 108700004914 Ac-Nal(1)-Cpa(2)-Pal(3,6)-Arg(5)-Ala(10)- LHRH Proteins 0.000 description 2
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 2
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 241000282836 Camelus dromedarius Species 0.000 description 2
- 208000028698 Cognitive impairment Diseases 0.000 description 2
- 206010010099 Combined immunodeficiency Diseases 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- 150000008574 D-amino acids Chemical class 0.000 description 2
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 2
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 2
- RHGKLRLOHDJJDR-BYPYZUCNSA-N L-citrulline Chemical compound NC(=O)NCCC[C@H]([NH3+])C([O-])=O RHGKLRLOHDJJDR-BYPYZUCNSA-N 0.000 description 2
- QEFRNWWLZKMPFJ-ZXPFJRLXSA-N L-methionine (R)-S-oxide Chemical compound C[S@@](=O)CC[C@H]([NH3+])C([O-])=O QEFRNWWLZKMPFJ-ZXPFJRLXSA-N 0.000 description 2
- QEFRNWWLZKMPFJ-UHFFFAOYSA-N L-methionine sulphoxide Natural products CS(=O)CCC(N)C(O)=O QEFRNWWLZKMPFJ-UHFFFAOYSA-N 0.000 description 2
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 2
- 108060001084 Luciferase Proteins 0.000 description 2
- 239000005089 Luciferase Substances 0.000 description 2
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 229930195725 Mannitol Natural products 0.000 description 2
- VEYYWZRYIYDQJM-ZETCQYMHSA-N N(2)-acetyl-L-lysine Chemical compound CC(=O)N[C@H](C([O-])=O)CCCC[NH3+] VEYYWZRYIYDQJM-ZETCQYMHSA-N 0.000 description 2
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 2
- AKCRVYNORCOYQT-YFKPBYRVSA-N N-methyl-L-valine Chemical compound CN[C@@H](C(C)C)C(O)=O AKCRVYNORCOYQT-YFKPBYRVSA-N 0.000 description 2
- 102220606690 Neurotrimin_D23N_mutation Human genes 0.000 description 2
- 239000000020 Nitrocellulose Substances 0.000 description 2
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 2
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 2
- 108010033276 Peptide Fragments Proteins 0.000 description 2
- 102000007079 Peptide Fragments Human genes 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 241000276498 Pollachius virens Species 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 2
- 238000011579 SCID mouse model Methods 0.000 description 2
- 108010077895 Sarcosine Proteins 0.000 description 2
- 102220538768 Superoxide dismutase [Cu-Zn]_E22G_mutation Human genes 0.000 description 2
- 210000001744 T-lymphocyte Anatomy 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- 108700019146 Transgenes Proteins 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- HAXFWIACAGNFHA-UHFFFAOYSA-N aldrithiol Chemical compound C=1C=CC=NC=1SSC1=CC=CC=N1 HAXFWIACAGNFHA-UHFFFAOYSA-N 0.000 description 2
- 125000002877 alkyl aryl group Chemical group 0.000 description 2
- 108010064397 amyloid beta-protein (1-40) Proteins 0.000 description 2
- 108010064539 amyloid beta-protein (1-42) Proteins 0.000 description 2
- 230000003941 amyloidogenesis Effects 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 239000001506 calcium phosphate Substances 0.000 description 2
- 229910000389 calcium phosphate Inorganic materials 0.000 description 2
- 235000011010 calcium phosphates Nutrition 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- 230000002490 cerebral effect Effects 0.000 description 2
- 238000010367 cloning Methods 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 208000010877 cognitive disease Diseases 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 239000002577 cryoprotective agent Substances 0.000 description 2
- 239000012228 culture supernatant Substances 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 230000002950 deficient Effects 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 238000010511 deprotection reaction Methods 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000002405 diagnostic procedure Methods 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 239000002612 dispersion medium Substances 0.000 description 2
- 210000003527 eukaryotic cell Anatomy 0.000 description 2
- BTCSSZJGUNDROE-UHFFFAOYSA-N gamma-aminobutyric acid Chemical compound NCCCC(O)=O BTCSSZJGUNDROE-UHFFFAOYSA-N 0.000 description 2
- 229910052736 halogen Inorganic materials 0.000 description 2
- 150000002367 halogens Chemical class 0.000 description 2
- 230000002949 hemolytic effect Effects 0.000 description 2
- 125000001072 heteroaryl group Chemical group 0.000 description 2
- 230000000971 hippocampal effect Effects 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 229940127121 immunoconjugate Drugs 0.000 description 2
- 230000002163 immunogen Effects 0.000 description 2
- 230000016784 immunoglobulin production Effects 0.000 description 2
- 229940072221 immunoglobulins Drugs 0.000 description 2
- 230000002779 inactivation Effects 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- 229940102223 injectable solution Drugs 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- PGLTVOMIXTUURA-UHFFFAOYSA-N iodoacetamide Chemical compound NC(=O)CI PGLTVOMIXTUURA-UHFFFAOYSA-N 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 239000007951 isotonicity adjuster Substances 0.000 description 2
- 238000005304 joining Methods 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 239000008297 liquid dosage form Substances 0.000 description 2
- 229920002521 macromolecule Polymers 0.000 description 2
- 210000004962 mammalian cell Anatomy 0.000 description 2
- 239000000594 mannitol Substances 0.000 description 2
- 235000010355 mannitol Nutrition 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 238000000816 matrix-assisted laser desorption--ionisation Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 238000001823 molecular biology technique Methods 0.000 description 2
- 210000002682 neurofibrillary tangle Anatomy 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 229920001220 nitrocellulos Polymers 0.000 description 2
- 229960003104 ornithine Drugs 0.000 description 2
- 230000003204 osmotic effect Effects 0.000 description 2
- 238000004091 panning Methods 0.000 description 2
- 125000002255 pentenyl group Chemical group C(=CCCC)* 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 235000019833 protease Nutrition 0.000 description 2
- 125000006239 protecting group Chemical group 0.000 description 2
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 2
- 230000009257 reactivity Effects 0.000 description 2
- 230000010076 replication Effects 0.000 description 2
- 108091008146 restriction endonucleases Proteins 0.000 description 2
- 238000003757 reverse transcription PCR Methods 0.000 description 2
- 102200131541 rs121912450 Human genes 0.000 description 2
- 102220249000 rs200856000 Human genes 0.000 description 2
- 102200158871 rs33955330 Human genes 0.000 description 2
- 102200151424 rs5198 Human genes 0.000 description 2
- 102220092852 rs754853086 Human genes 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 230000003248 secreting effect Effects 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 238000007086 side reaction Methods 0.000 description 2
- 230000011664 signaling Effects 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 210000004989 spleen cell Anatomy 0.000 description 2
- 230000006641 stabilisation Effects 0.000 description 2
- 238000011105 stabilization Methods 0.000 description 2
- 238000012409 standard PCR amplification Methods 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 238000010254 subcutaneous injection Methods 0.000 description 2
- 239000007929 subcutaneous injection Substances 0.000 description 2
- TYFQFVWCELRYAO-UHFFFAOYSA-N suberic acid Chemical compound OC(=O)CCCCCCC(O)=O TYFQFVWCELRYAO-UHFFFAOYSA-N 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 238000013268 sustained release Methods 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- 230000002103 transcriptional effect Effects 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 2
- 241000701161 unidentified adenovirus Species 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 210000005253 yeast cell Anatomy 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- QYEAAMBIUQLHFQ-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 6-[3-(pyridin-2-yldisulfanyl)propanoylamino]hexanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCCCCNC(=O)CCSSC1=CC=CC=N1 QYEAAMBIUQLHFQ-UHFFFAOYSA-N 0.000 description 1
- IYKLZBIWFXPUCS-VIFPVBQESA-N (2s)-2-(naphthalen-1-ylamino)propanoic acid Chemical compound C1=CC=C2C(N[C@@H](C)C(O)=O)=CC=CC2=C1 IYKLZBIWFXPUCS-VIFPVBQESA-N 0.000 description 1
- BEJKOYIMCGMNRB-GRHHLOCNSA-N (2s)-2-amino-3-(4-hydroxyphenyl)propanoic acid;(2s)-2-amino-3-phenylpropanoic acid Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1.OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 BEJKOYIMCGMNRB-GRHHLOCNSA-N 0.000 description 1
- LJRDOKAZOAKLDU-UDXJMMFXSA-N (2s,3s,4r,5r,6r)-5-amino-2-(aminomethyl)-6-[(2r,3s,4r,5s)-5-[(1r,2r,3s,5r,6s)-3,5-diamino-2-[(2s,3r,4r,5s,6r)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6-hydroxycyclohexyl]oxy-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl]oxyoxane-3,4-diol;sulfuric ac Chemical compound OS(O)(=O)=O.N[C@@H]1[C@@H](O)[C@H](O)[C@H](CN)O[C@@H]1O[C@H]1[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](N)C[C@@H](N)[C@@H]2O)O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)N)O[C@@H]1CO LJRDOKAZOAKLDU-UDXJMMFXSA-N 0.000 description 1
- UKAUYVFTDYCKQA-UHFFFAOYSA-N -2-Amino-4-hydroxybutanoic acid Natural products OC(=O)C(N)CCO UKAUYVFTDYCKQA-UHFFFAOYSA-N 0.000 description 1
- BWKMGYQJPOAASG-UHFFFAOYSA-N 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid Chemical compound C1=CC=C2CNC(C(=O)O)CC2=C1 BWKMGYQJPOAASG-UHFFFAOYSA-N 0.000 description 1
- STGOXLCCKKFIGD-UHFFFAOYSA-N 1-hydroxy-2,5-dioxopyrrolidine-3,3-disulfonic acid Chemical compound ON1C(=O)CC(S(O)(=O)=O)(S(O)(=O)=O)C1=O STGOXLCCKKFIGD-UHFFFAOYSA-N 0.000 description 1
- GZCWLCBFPRFLKL-UHFFFAOYSA-N 1-prop-2-ynoxypropan-2-ol Chemical compound CC(O)COCC#C GZCWLCBFPRFLKL-UHFFFAOYSA-N 0.000 description 1
- ASNTZYQMIUCEBV-UHFFFAOYSA-N 2,5-dioxo-1-[6-[3-(pyridin-2-yldisulfanyl)propanoylamino]hexanoyloxy]pyrrolidine-3-sulfonic acid Chemical compound O=C1C(S(=O)(=O)O)CC(=O)N1OC(=O)CCCCCNC(=O)CCSSC1=CC=CC=N1 ASNTZYQMIUCEBV-UHFFFAOYSA-N 0.000 description 1
- NYCRCTMDYITATC-UHFFFAOYSA-N 2-fluorophenylalanine Chemical compound OC(=O)C(N)CC1=CC=CC=C1F NYCRCTMDYITATC-UHFFFAOYSA-N 0.000 description 1
- DJBRKGZFUXKLKO-UHFFFAOYSA-N 3-(pyridin-2-yldisulfanyl)propanoic acid Chemical compound OC(=O)CCSSC1=CC=CC=N1 DJBRKGZFUXKLKO-UHFFFAOYSA-N 0.000 description 1
- CORXEUSRKLNMDW-UHFFFAOYSA-N 3-[[1-(2,5-dioxopyrrolidin-1-yl)-2h-pyridin-2-yl]disulfanyl]propanoic acid Chemical compound OC(=O)CCSSC1C=CC=CN1N1C(=O)CCC1=O CORXEUSRKLNMDW-UHFFFAOYSA-N 0.000 description 1
- CMUHFUGDYMFHEI-QMMMGPOBSA-N 4-amino-L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N)C=C1 CMUHFUGDYMFHEI-QMMMGPOBSA-N 0.000 description 1
- TVZGACDUOSZQKY-LBPRGKRZSA-N 4-aminofolic acid Chemical compound C1=NC2=NC(N)=NC(N)=C2N=C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 TVZGACDUOSZQKY-LBPRGKRZSA-N 0.000 description 1
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical compound NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 description 1
- KKWOBMDHVBWFRT-UHFFFAOYSA-N 9H-fluoren-9-ylmethyl 2-amino-2-(2,4-dimethoxyphenyl)-2-[4-[2-[[(4-methylphenyl)-phenylmethyl]amino]-2-oxoethoxy]phenyl]acetate Chemical compound COC1=C(C=CC(=C1)OC)C(C1=CC=C(OCC(=O)NC(C2=CC=C(C=C2)C)C2=CC=CC=C2)C=C1)(N)C(=O)OCC1C2=CC=CC=C2C2=CC=CC=C12 KKWOBMDHVBWFRT-UHFFFAOYSA-N 0.000 description 1
- JDDWRLPTKIOUOF-UHFFFAOYSA-N 9h-fluoren-9-ylmethyl n-[[4-[2-[bis(4-methylphenyl)methylamino]-2-oxoethoxy]phenyl]-(2,4-dimethoxyphenyl)methyl]carbamate Chemical compound COC1=CC(OC)=CC=C1C(C=1C=CC(OCC(=O)NC(C=2C=CC(C)=CC=2)C=2C=CC(C)=CC=2)=CC=1)NC(=O)OCC1C2=CC=CC=C2C2=CC=CC=C21 JDDWRLPTKIOUOF-UHFFFAOYSA-N 0.000 description 1
- 102000013563 Acid Phosphatase Human genes 0.000 description 1
- 108010051457 Acid Phosphatase Proteins 0.000 description 1
- 241000242764 Aequorea victoria Species 0.000 description 1
- 241000607620 Aliivibrio fischeri Species 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 241000143060 Americamysis bahia Species 0.000 description 1
- 102000001049 Amyloid Human genes 0.000 description 1
- 108010094108 Amyloid Proteins 0.000 description 1
- 102000002659 Amyloid Precursor Protein Secretases Human genes 0.000 description 1
- 241000024188 Andala Species 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 101100002951 Caenorhabditis elegans asp-17 gene Proteins 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-N Caprylic acid Natural products CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 108010025905 Cystine-Knot Miniproteins Proteins 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-UHFFFAOYSA-N D-alpha-Ala Natural products CC([NH3+])C([O-])=O QNAYBMKLOCPYGJ-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- KDXKERNSBIXSRK-RXMQYKEDSA-N D-lysine Chemical compound NCCCC[C@@H](N)C(O)=O KDXKERNSBIXSRK-RXMQYKEDSA-N 0.000 description 1
- 208000031124 Dementia Alzheimer type Diseases 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- YXHKONLOYHBTNS-UHFFFAOYSA-N Diazomethane Chemical compound C=[N+]=[N-] YXHKONLOYHBTNS-UHFFFAOYSA-N 0.000 description 1
- 241000581356 Dictyosoma Species 0.000 description 1
- 108010092408 Eosinophil Peroxidase Proteins 0.000 description 1
- 102000044708 Eosinophil peroxidases Human genes 0.000 description 1
- YQYJSBFKSSDGFO-UHFFFAOYSA-N Epihygromycin Natural products OC1C(O)C(C(=O)C)OC1OC(C(=C1)O)=CC=C1C=C(C)C(=O)NC1C(O)C(O)C2OCOC2C1O YQYJSBFKSSDGFO-UHFFFAOYSA-N 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- NIGWMJHCCYYCSF-UHFFFAOYSA-N Fenclonine Chemical compound OC(=O)C(N)CC1=CC=C(Cl)C=C1 NIGWMJHCCYYCSF-UHFFFAOYSA-N 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 108010044091 Globulins Proteins 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 1
- 239000007821 HATU Substances 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- 102000009786 Immunoglobulin Constant Regions Human genes 0.000 description 1
- 108010009817 Immunoglobulin Constant Regions Proteins 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- QUOGESRFPZDMMT-UHFFFAOYSA-N L-Homoarginine Natural products OC(=O)C(N)CCCCNC(N)=N QUOGESRFPZDMMT-UHFFFAOYSA-N 0.000 description 1
- 235000019766 L-Lysine Nutrition 0.000 description 1
- FFEARJCKVFRZRR-UHFFFAOYSA-N L-Methionine Natural products CSCCC(N)C(O)=O FFEARJCKVFRZRR-UHFFFAOYSA-N 0.000 description 1
- ZGUNAGUHMKGQNY-ZETCQYMHSA-N L-alpha-phenylglycine zwitterion Chemical compound OC(=O)[C@@H](N)C1=CC=CC=C1 ZGUNAGUHMKGQNY-ZETCQYMHSA-N 0.000 description 1
- QUOGESRFPZDMMT-YFKPBYRVSA-N L-homoarginine Chemical compound OC(=O)[C@@H](N)CCCCNC(N)=N QUOGESRFPZDMMT-YFKPBYRVSA-N 0.000 description 1
- FFFHZYDWPBMWHY-VKHMYHEASA-N L-homocysteine Chemical compound OC(=O)[C@@H](N)CCS FFFHZYDWPBMWHY-VKHMYHEASA-N 0.000 description 1
- JTTHKOPSMAVJFE-VIFPVBQESA-N L-homophenylalanine Chemical compound OC(=O)[C@@H](N)CCC1=CC=CC=C1 JTTHKOPSMAVJFE-VIFPVBQESA-N 0.000 description 1
- UKAUYVFTDYCKQA-VKHMYHEASA-N L-homoserine Chemical compound OC(=O)[C@@H](N)CCO UKAUYVFTDYCKQA-VKHMYHEASA-N 0.000 description 1
- 229930195722 L-methionine Natural products 0.000 description 1
- DGYHPLMPMRKMPD-UHFFFAOYSA-N L-propargyl glycine Natural products OC(=O)C(N)CC#C DGYHPLMPMRKMPD-UHFFFAOYSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 108090001090 Lectins Proteins 0.000 description 1
- 102000004856 Lectins Human genes 0.000 description 1
- 229920000057 Mannan Polymers 0.000 description 1
- 108010049175 N-substituted Glycines Proteins 0.000 description 1
- 125000000729 N-terminal amino-acid group Chemical group 0.000 description 1
- RHGKLRLOHDJJDR-UHFFFAOYSA-N Ndelta-carbamoyl-DL-ornithine Natural products OC(=O)C(N)CCCNC(N)=O RHGKLRLOHDJJDR-UHFFFAOYSA-N 0.000 description 1
- 241001045988 Neogene Species 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 102000007339 Nerve Growth Factor Receptors Human genes 0.000 description 1
- 108010032605 Nerve Growth Factor Receptors Proteins 0.000 description 1
- 108010058846 Ovalbumin Proteins 0.000 description 1
- 238000012408 PCR amplification Methods 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 108010043958 Peptoids Chemical class 0.000 description 1
- 108700020962 Peroxidase Proteins 0.000 description 1
- 102000003992 Peroxidases Human genes 0.000 description 1
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical compound OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 1
- 101000622060 Photinus pyralis Luciferin 4-monooxygenase Proteins 0.000 description 1
- 108010004729 Phycoerythrin Proteins 0.000 description 1
- 229920002732 Polyanhydride Polymers 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 108010007127 Pulmonary Surfactant-Associated Protein D Proteins 0.000 description 1
- 102100027845 Pulmonary surfactant-associated protein D Human genes 0.000 description 1
- 238000001069 Raman spectroscopy Methods 0.000 description 1
- 102000006382 Ribonucleases Human genes 0.000 description 1
- 108010083644 Ribonucleases Proteins 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 108010055044 Tetanus Toxin Proteins 0.000 description 1
- 102100024554 Tetranectin Human genes 0.000 description 1
- 102000009843 Thyroglobulin Human genes 0.000 description 1
- 108010034949 Thyroglobulin Proteins 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 206010054094 Tumour necrosis Diseases 0.000 description 1
- 108090000848 Ubiquitin Proteins 0.000 description 1
- 102000044159 Ubiquitin Human genes 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 239000003070 absorption delaying agent Substances 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- YDPHAWREMXVRAC-UHFFFAOYSA-N acetic acid;2-aminoacetic acid Chemical compound CC([O-])=O.[NH3+]CC(O)=O YDPHAWREMXVRAC-UHFFFAOYSA-N 0.000 description 1
- 239000003929 acidic solution Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- DZBUGLKDJFMEHC-UHFFFAOYSA-O acridine;hydron Chemical compound C1=CC=CC2=CC3=CC=CC=C3[NH+]=C21 DZBUGLKDJFMEHC-UHFFFAOYSA-O 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 238000005054 agglomeration Methods 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 230000029936 alkylation Effects 0.000 description 1
- 238000005804 alkylation reaction Methods 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- 102000038380 alpha-secretases Human genes 0.000 description 1
- 108091007736 alpha-secretases Proteins 0.000 description 1
- 150000001414 amino alcohols Chemical group 0.000 description 1
- 229960003896 aminopterin Drugs 0.000 description 1
- 239000003708 ampul Substances 0.000 description 1
- DZHSAHHDTRWUTF-SIQRNXPUSA-N amyloid-beta polypeptide 42 Chemical compound C([C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O)[C@@H](C)CC)C(C)C)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(C)C)C1=CC=CC=C1 DZHSAHHDTRWUTF-SIQRNXPUSA-N 0.000 description 1
- 238000002583 angiography Methods 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 230000009830 antibody antigen interaction Effects 0.000 description 1
- 210000000628 antibody-producing cell Anatomy 0.000 description 1
- 229930193936 anticarin Natural products 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000006286 aqueous extract Substances 0.000 description 1
- 150000001510 aspartic acids Chemical class 0.000 description 1
- 238000002820 assay format Methods 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- GONOPSZTUGRENK-UHFFFAOYSA-N benzyl(trichloro)silane Chemical compound Cl[Si](Cl)(Cl)CC1=CC=CC=C1 GONOPSZTUGRENK-UHFFFAOYSA-N 0.000 description 1
- WTOFYLAWDLQMBZ-LURJTMIESA-N beta(2-thienyl)alanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CS1 WTOFYLAWDLQMBZ-LURJTMIESA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 102000005936 beta-Galactosidase Human genes 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- 229940000635 beta-alanine Drugs 0.000 description 1
- 108091007737 beta-secretases Proteins 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 229920000249 biocompatible polymer Polymers 0.000 description 1
- 239000013060 biological fluid Substances 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- JCZLABDVDPYLRZ-AWEZNQCLSA-N biphenylalanine Chemical compound C1=CC(C[C@H](N)C(O)=O)=CC=C1C1=CC=CC=C1 JCZLABDVDPYLRZ-AWEZNQCLSA-N 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000002798 bone marrow cell Anatomy 0.000 description 1
- 239000006189 buccal tablet Substances 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- DQXBYHZEEUGOBF-UHFFFAOYSA-N but-3-enoic acid;ethene Chemical compound C=C.OC(=O)CC=C DQXBYHZEEUGOBF-UHFFFAOYSA-N 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 150000001718 carbodiimides Chemical class 0.000 description 1
- 150000003857 carboxamides Chemical group 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 238000000423 cell based assay Methods 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 238000010382 chemical cross-linking Methods 0.000 description 1
- 125000003636 chemical group Chemical group 0.000 description 1
- 238000012993 chemical processing Methods 0.000 description 1
- 229960002173 citrulline Drugs 0.000 description 1
- 235000013477 citrulline Nutrition 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 239000000084 colloidal system Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000008139 complexing agent Substances 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- IQFVPQOLBLOTPF-HKXUKFGYSA-L congo red Chemical compound [Na+].[Na+].C1=CC=CC2=C(N)C(/N=N/C3=CC=C(C=C3)C3=CC=C(C=C3)/N=N/C3=C(C4=CC=CC=C4C(=C3)S([O-])(=O)=O)N)=CC(S([O-])(=O)=O)=C21 IQFVPQOLBLOTPF-HKXUKFGYSA-L 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000002079 cooperative effect Effects 0.000 description 1
- 239000007822 coupling agent Substances 0.000 description 1
- 230000009260 cross reactivity Effects 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 210000001787 dendrite Anatomy 0.000 description 1
- 238000000326 densiometry Methods 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- KZNICNPSHKQLFF-UHFFFAOYSA-N dihydromaleimide Natural products O=C1CCC(=O)N1 KZNICNPSHKQLFF-UHFFFAOYSA-N 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 206010013023 diphtheria Diseases 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000012645 endogenous antigen Substances 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- AEOCXXJPGCBFJA-UHFFFAOYSA-N ethionamide Chemical compound CCC1=CC(C(N)=S)=CC=N1 AEOCXXJPGCBFJA-UHFFFAOYSA-N 0.000 description 1
- 239000005038 ethylene vinyl acetate Substances 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 230000005294 ferromagnetic effect Effects 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 102000034240 fibrous proteins Human genes 0.000 description 1
- 108091005899 fibrous proteins Proteins 0.000 description 1
- 239000005308 flint glass Substances 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 229960003692 gamma aminobutyric acid Drugs 0.000 description 1
- 102000038383 gamma-secretases Human genes 0.000 description 1
- 108091007739 gamma-secretases Proteins 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical group [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- 210000002443 helper t lymphocyte Anatomy 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 239000012145 high-salt buffer Substances 0.000 description 1
- 238000002744 homologous recombination Methods 0.000 description 1
- 230000006801 homologous recombination Effects 0.000 description 1
- 229940040731 human interleukin-12 Drugs 0.000 description 1
- 230000008348 humoral response Effects 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 108010045069 keyhole-limpet hemocyanin Proteins 0.000 description 1
- 238000011813 knockout mouse model Methods 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 150000002605 large molecules Chemical class 0.000 description 1
- 239000004816 latex Substances 0.000 description 1
- 229920000126 latex Polymers 0.000 description 1
- 239000002523 lectin Substances 0.000 description 1
- 231100000636 lethal dose Toxicity 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 230000027928 long-term synaptic potentiation Effects 0.000 description 1
- 210000001165 lymph node Anatomy 0.000 description 1
- 239000008176 lyophilized powder Substances 0.000 description 1
- VWHRYODZTDMVSS-QMMMGPOBSA-N m-fluoro-L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC(F)=C1 VWHRYODZTDMVSS-QMMMGPOBSA-N 0.000 description 1
- 230000005291 magnetic effect Effects 0.000 description 1
- 239000006249 magnetic particle Substances 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- MGJXBDMLVWIYOQ-UHFFFAOYSA-N methylazanide Chemical compound [NH-]C MGJXBDMLVWIYOQ-UHFFFAOYSA-N 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- NKAAEMMYHLFEFN-UHFFFAOYSA-M monosodium tartrate Chemical compound [Na+].OC(=O)C(O)C(O)C([O-])=O NKAAEMMYHLFEFN-UHFFFAOYSA-M 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N n-hexanoic acid Natural products CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 101150091879 neo gene Proteins 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 230000002981 neuropathic effect Effects 0.000 description 1
- 230000009871 nonspecific binding Effects 0.000 description 1
- 229940013982 octagam Drugs 0.000 description 1
- 238000006384 oligomerization reaction Methods 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 239000007935 oral tablet Substances 0.000 description 1
- 229940092253 ovalbumin Drugs 0.000 description 1
- 239000003002 pH adjusting agent Substances 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 229960001639 penicillamine Drugs 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 125000001151 peptidyl group Chemical group 0.000 description 1
- 210000005105 peripheral blood lymphocyte Anatomy 0.000 description 1
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- HXEACLLIILLPRG-UHFFFAOYSA-N pipecolic acid Chemical compound OC(=O)C1CCCCN1 HXEACLLIILLPRG-UHFFFAOYSA-N 0.000 description 1
- 239000011505 plaster Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 239000004633 polyglycolic acid Substances 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 239000002952 polymeric resin Substances 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 229940068977 polysorbate 20 Drugs 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000008057 potassium phosphate buffer Substances 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 230000000770 proinflammatory effect Effects 0.000 description 1
- 210000001236 prokaryotic cell Anatomy 0.000 description 1
- 230000009465 prokaryotic expression Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000013777 protein digestion Effects 0.000 description 1
- 238000002818 protein evolution Methods 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 230000007026 protein scission Effects 0.000 description 1
- 230000004850 protein–protein interaction Effects 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 229950010131 puromycin Drugs 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 239000002516 radical scavenger Substances 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 239000012217 radiopharmaceutical Substances 0.000 description 1
- 229940121896 radiopharmaceutical Drugs 0.000 description 1
- 230000002799 radiopharmaceutical effect Effects 0.000 description 1
- 238000002708 random mutagenesis Methods 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 238000004007 reversed phase HPLC Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000002702 ribosome display Methods 0.000 description 1
- 229940043230 sarcosine Drugs 0.000 description 1
- 210000004116 schwann cell Anatomy 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 238000002922 simulated annealing Methods 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 239000012064 sodium phosphate buffer Substances 0.000 description 1
- 229940074404 sodium succinate Drugs 0.000 description 1
- ZDQYSKICYIVCPN-UHFFFAOYSA-L sodium succinate (anhydrous) Chemical compound [Na+].[Na+].[O-]C(=O)CCC([O-])=O ZDQYSKICYIVCPN-UHFFFAOYSA-L 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 210000004988 splenocyte Anatomy 0.000 description 1
- 238000001694 spray drying Methods 0.000 description 1
- 230000007480 spreading Effects 0.000 description 1
- 238000003892 spreading Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 238000011146 sterile filtration Methods 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 229960002317 succinimide Drugs 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 229920003002 synthetic resin Polymers 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 102000013498 tau Proteins Human genes 0.000 description 1
- 108010026424 tau Proteins Proteins 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 108010013645 tetranectin Proteins 0.000 description 1
- 150000007970 thio esters Chemical class 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 229960002175 thyroglobulin Drugs 0.000 description 1
- 230000008791 toxic response Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- SBUXRMKDJWEXRL-ZWKOTPCHSA-N trans-body Chemical compound O=C([C@@H]1N(C2=O)[C@H](C3=C(C4=CC=CC=C4N3)C1)CC)N2C1=CC=C(F)C=C1 SBUXRMKDJWEXRL-ZWKOTPCHSA-N 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 241001515965 unidentified phage Species 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000002023 wood Substances 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- C07K14/4701—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals not used
- C07K14/4711—Alzheimer's disease; Amyloid plaque core protein
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/46—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans from vertebrates
- G01N2333/47—Assays involving proteins of known structure or function as defined in the subgroups
- G01N2333/4701—Details
- G01N2333/4709—Amyloid plaque core protein
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2500/00—Screening for compounds of potential therapeutic value
- G01N2500/04—Screening involving studying the effect of compounds C directly on molecule A (e.g. C are potential ligands for a receptor A, or potential substrates for an enzyme A)
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/28—Neurological disorders
- G01N2800/2814—Dementia; Cognitive disorders
- G01N2800/2821—Alzheimer
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Toxicology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Immunology (AREA)
- Neurosurgery (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Psychiatry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Hospice & Palliative Care (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US8358908P | 2008-07-25 | 2008-07-25 | |
| US61/083,589 | 2008-07-25 | ||
| PCT/US2009/051721 WO2010011947A2 (en) | 2008-07-25 | 2009-07-24 | AMYLOID ß PEPTIDE ANALOGUES, OLIGOMERS THEREOF, PROCESSES FOR PREPARING AND COMPOSITIONS COMPRISING SAID ANALOGUES OR OLIGOMERS, AND THEIR USES |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2011529084A true JP2011529084A (ja) | 2011-12-01 |
| JP2011529084A5 JP2011529084A5 (cg-RX-API-DMAC7.html) | 2012-05-31 |
Family
ID=41570887
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011520233A Pending JP2011529084A (ja) | 2008-07-25 | 2009-07-24 | アミロイドβペプチド類似体、これらのオリゴマー、前記類似体またはオリゴマーを調製するためのプロセスおよび前記類似体またはオリゴマーを含む組成物ならびにこれらの使用 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20110092445A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP2303920A4 (cg-RX-API-DMAC7.html) |
| JP (1) | JP2011529084A (cg-RX-API-DMAC7.html) |
| CN (1) | CN102203124A (cg-RX-API-DMAC7.html) |
| CA (1) | CA2730804A1 (cg-RX-API-DMAC7.html) |
| MX (1) | MX2011000975A (cg-RX-API-DMAC7.html) |
| WO (1) | WO2010011947A2 (cg-RX-API-DMAC7.html) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016505391A (ja) * | 2012-10-27 | 2016-02-25 | フォルシュングスツェントルム・ユーリッヒ・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツング | 基板上にナノ多孔性層を製造する方法 |
| JP2017532289A (ja) * | 2014-07-07 | 2017-11-02 | アッヴィ・ドイチュラント・ゲー・エム・ベー・ハー・ウント・コー・カー・ゲー | 突然変異タンパク質アミロイドβ(Aβ)アミノ酸配列に基づく免疫原性産物およびその使用 |
| JP2021123551A (ja) * | 2020-02-04 | 2021-08-30 | 国立大学法人京都大学 | アミロイドβ42架橋アナログペプチド |
Families Citing this family (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10303974A1 (de) | 2003-01-31 | 2004-08-05 | Abbott Gmbh & Co. Kg | Amyloid-β(1-42)-Oligomere, Verfahren zu deren Herstellung und deren Verwendung |
| PT1954718E (pt) | 2005-11-30 | 2014-12-16 | Abbvie Inc | Anticorpos anti-globulómeros aβ, suas porções de ligação ao antigénio, correspondentes hibridomas, ácidos nucleicos, vectores, células hospedeiras, métodos de produção dos ditos anticorpos, composições compreendendo os ditos anticorpos, usos dos ditos anticorpos e métodos para uso dos ditos anticorpos |
| PL1976877T5 (pl) | 2005-11-30 | 2017-09-29 | Abbvie Inc | Przeciwciała monoklonalne przeciwko białku amyloidu beta oraz ich zastosowania |
| US8455626B2 (en) | 2006-11-30 | 2013-06-04 | Abbott Laboratories | Aβ conformer selective anti-aβ globulomer monoclonal antibodies |
| US20100311767A1 (en) | 2007-02-27 | 2010-12-09 | Abbott Gmbh & Co. Kg | Method for the treatment of amyloidoses |
| EP2262526B1 (en) * | 2008-04-14 | 2015-12-02 | Alzinova AB | Stable amyloid beta monomers and oligomers |
| AU2009266552B2 (en) * | 2008-07-01 | 2014-01-23 | De Staat Der Nederlanden, Vert. Door De Minister Van Vws | Vaccine against amyloid folding intermediate |
| US8486940B2 (en) | 2009-09-11 | 2013-07-16 | Probiodrug Ag | Inhibitors |
| MX341536B (es) * | 2010-03-03 | 2016-08-24 | The Univ Of British Columbia * | Epitopo y anticuerpos beta amiloide de oligomero especifico. |
| AU2013205000B2 (en) * | 2010-03-03 | 2015-05-28 | The University Of British Columbia | Oligomer-specific amyloid beta epitope and antibodies |
| CN102933601B (zh) | 2010-04-15 | 2016-06-08 | Abbvie公司 | β淀粉样蛋白结合蛋白 |
| US9364449B2 (en) | 2010-06-09 | 2016-06-14 | New York University | Peptoid and synthetic oligomers, pharmaceutical compositions and methods of using same |
| MX358739B (es) | 2010-08-14 | 2018-09-03 | Abbvie Inc Star | Proteinas de union a amiloide beta. |
| US20120089291A1 (en) * | 2010-10-12 | 2012-04-12 | Halder Bibhrajit | Autonomous machine control system |
| US11231426B2 (en) | 2010-11-29 | 2022-01-25 | Inven2 As | Methods and compositions for monitoring phagocytic activity |
| WO2012072611A1 (de) * | 2010-11-29 | 2012-06-07 | Philipps-Universität Marburg | SYNTHETISCHE LIGANDEN FÜR HUMANE ANTI-Aβ-ANTIKÖRPER |
| JP6220344B2 (ja) * | 2011-11-29 | 2017-10-25 | プロクララ バイオサイエンシーズ, インコーポレイテッド | アミロイド結合剤としてのバクテリオファージのp3の使用 |
| US20130164217A1 (en) * | 2011-12-21 | 2013-06-27 | Meso Scale Technologies, Llc | Method of diagnosing, preventing and/or treating dementia & related disorders |
| EP2746402A1 (en) * | 2013-03-12 | 2014-06-25 | Academisch Medisch Centrum | Method of prognosis of Alzheimers disease and substrates for use therein |
| EP2787349A1 (en) | 2013-04-03 | 2014-10-08 | Affiris AG | Method for detecting proteinopathy-specific antibodies in a biological sample |
| EP2787347A1 (en) * | 2013-04-03 | 2014-10-08 | Affiris AG | Method for detecting Aß-specific antibodies in a biological sample |
| KR102331422B1 (ko) | 2014-01-31 | 2021-11-25 | 카그니션 테라퓨틱스, 인코퍼레이티드 | 아이소인돌린 조성물 및 신경퇴행성 질환을 치료하는 방법 |
| JP6675403B2 (ja) * | 2014-09-05 | 2020-04-01 | システム オブ システムズ アナリティクス インコーポレイテッド | アミロイドベータオリゴマーを検出する方法 |
| ES2571055B1 (es) * | 2016-02-15 | 2016-12-28 | Araclon Biotech, S.L. | Conjugado amiloide y usos y procedimientos del mismo |
| WO2018005980A1 (en) * | 2016-07-01 | 2018-01-04 | The Scripps Research Institute | Compositions and diagnostic methods related to transthyretin amyloid diseases |
| US11214540B2 (en) | 2017-05-15 | 2022-01-04 | Cognition Therapeutics, Inc. | Compositions for treating neurodegenerative diseases |
| PL3461819T3 (pl) | 2017-09-29 | 2020-11-30 | Probiodrug Ag | Inhibitory cyklazy glutaminylowej |
| CN108148115A (zh) * | 2018-01-30 | 2018-06-12 | 中国药科大学 | 一种环肽合成新方法及其在药物开发中的应用 |
| US20210262006A1 (en) * | 2018-06-18 | 2021-08-26 | Emory University | Parallel Enzyme Digestion for Protein Biomarker Detection |
| CN109912687A (zh) * | 2019-03-20 | 2019-06-21 | 横琴欣健生物科技研究院有限公司 | 一种治疗老年痴呆症的多肽药物 |
| CN109851660A (zh) * | 2019-03-20 | 2019-06-07 | 横琴欣健生物科技研究院有限公司 | 一种治疗老年痴呆症的多肽及其疫苗 |
| KR102767892B1 (ko) * | 2021-12-30 | 2025-02-14 | 고려대학교 산학협력단 | 신규한 단백질 변이체 및 이를 이용한 신경퇴행성 질환 치료용 조성물 |
| US12037375B2 (en) * | 2021-12-30 | 2024-07-16 | Korea University Research And Business Foundation | Protein variant and composition for treating neurodegenerative disease using the same |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5750349A (en) * | 1993-01-25 | 1998-05-12 | Takeda Chemical Industries Ltd. | Antibodies to β-amyloids or their derivatives and use thereof |
| DE10101430B4 (de) * | 2001-01-13 | 2008-10-02 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Lösliche cyclische Analoga zur Modulation der Amyloidogenese |
| US20020132758A1 (en) * | 2001-01-18 | 2002-09-19 | Shell John W. | Method for identifying compounds to treat medical pathologies associated with molecular crystallization |
| US20070213512A1 (en) * | 2002-10-01 | 2007-09-13 | Krafft Grant A | Amyloid beta peptide analogs and assemblies thereof |
| DE10303974A1 (de) * | 2003-01-31 | 2004-08-05 | Abbott Gmbh & Co. Kg | Amyloid-β(1-42)-Oligomere, Verfahren zu deren Herstellung und deren Verwendung |
| EP1676859A1 (en) * | 2004-12-30 | 2006-07-05 | Pevion Biotech Ltd. | Immunogenic compositions of cyclic peptides derived from the beta-amyloid peptide |
| CN101365717A (zh) * | 2005-03-05 | 2009-02-11 | 艾博特股份有限两合公司 | 筛选方法、纯化非扩散A-β寡聚体的方法、抗所述非扩散A-β寡聚体的选择性抗体以及制备所述抗体的方法 |
| WO2007064917A2 (en) * | 2005-11-30 | 2007-06-07 | Abbott Laboratories | Methods of preparation of recombinant forms of human beta-amyloid protein and uses of these proteins |
| DK2091948T3 (da) * | 2006-11-30 | 2012-07-23 | Probiodrug Ag | Nye inhibitorer af glutaminylcyclase |
-
2009
- 2009-07-24 CA CA2730804A patent/CA2730804A1/en not_active Abandoned
- 2009-07-24 US US12/509,325 patent/US20110092445A1/en not_active Abandoned
- 2009-07-24 EP EP09801088A patent/EP2303920A4/en not_active Withdrawn
- 2009-07-24 MX MX2011000975A patent/MX2011000975A/es not_active Application Discontinuation
- 2009-07-24 WO PCT/US2009/051721 patent/WO2010011947A2/en not_active Ceased
- 2009-07-24 JP JP2011520233A patent/JP2011529084A/ja active Pending
- 2009-07-24 CN CN200980137576XA patent/CN102203124A/zh active Pending
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016505391A (ja) * | 2012-10-27 | 2016-02-25 | フォルシュングスツェントルム・ユーリッヒ・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツング | 基板上にナノ多孔性層を製造する方法 |
| JP2017532289A (ja) * | 2014-07-07 | 2017-11-02 | アッヴィ・ドイチュラント・ゲー・エム・ベー・ハー・ウント・コー・カー・ゲー | 突然変異タンパク質アミロイドβ(Aβ)アミノ酸配列に基づく免疫原性産物およびその使用 |
| JP2021001203A (ja) * | 2014-07-07 | 2021-01-07 | アッヴィ・ドイチュラント・ゲー・エム・ベー・ハー・ウント・コー・カー・ゲー | 突然変異タンパク質アミロイドβ(Aβ)アミノ酸配列に基づく免疫原性産物およびその使用 |
| JP2023036606A (ja) * | 2014-07-07 | 2023-03-14 | アッヴィ・ドイチュラント・ゲー・エム・ベー・ハー・ウント・コー・カー・ゲー | 突然変異タンパク質アミロイドβ(Aβ)アミノ酸配列に基づく免疫原性産物およびその使用 |
| JP2021123551A (ja) * | 2020-02-04 | 2021-08-30 | 国立大学法人京都大学 | アミロイドβ42架橋アナログペプチド |
| JP7357354B2 (ja) | 2020-02-04 | 2023-10-06 | 国立大学法人京都大学 | アミロイドβ42架橋アナログペプチド |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2010011947A2 (en) | 2010-01-28 |
| US20110092445A1 (en) | 2011-04-21 |
| MX2011000975A (es) | 2011-05-25 |
| EP2303920A4 (en) | 2011-11-09 |
| CN102203124A (zh) | 2011-09-28 |
| EP2303920A2 (en) | 2011-04-06 |
| CA2730804A1 (en) | 2010-01-28 |
| WO2010011947A3 (en) | 2010-04-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2011529084A (ja) | アミロイドβペプチド類似体、これらのオリゴマー、前記類似体またはオリゴマーを調製するためのプロセスおよび前記類似体またはオリゴマーを含む組成物ならびにこれらの使用 | |
| CA2514582C (en) | Amyloid-.beta.(1-42) oligomers, derivatives thereof, antibodies for the same, method for production and use thereof | |
| JP2023036606A (ja) | 突然変異タンパク質アミロイドβ(Aβ)アミノ酸配列に基づく免疫原性産物およびその使用 | |
| JP5722770B2 (ja) | アミロイド折りたたみ中間体に対するワクチン | |
| JP4709149B2 (ja) | プリオン特異的ペプチド試薬 | |
| US20060025575A1 (en) | Immunological detection of prions | |
| WO2010010469A2 (en) | Abeta (x-38..43) oligomers, and processes, compositions, and uses thereof | |
| JP2000516572A (ja) | プリオンタンパク質に結合し、イソ型PrP▲上c▼とPrP▲上sc▼を識別することが可能なシャペロン | |
| US11319348B2 (en) | Synthetic beta-amyloid peptides capable of forming stable antigenic oligomers | |
| JP4079775B2 (ja) | β−アミロイドペプチドの可溶性環状類似体 | |
| JP2008543791A (ja) | Psp94診断薬およびアッセイ | |
| AU2003288434B2 (en) | Peptides, antibodies thereto, and their use in the treatment of central nervous system damage | |
| US20060057636A1 (en) | Composite peptide compounds for diagnosis and treatment of diseases caused by prion proteins | |
| US20090099343A1 (en) | Isolation of pathogenic prions | |
| US20090191571A1 (en) | Isolation and Detection of Pathogenic Prions | |
| WO1999046574A2 (en) | Antibodies for assessing protein post-translational modification status and methods of making and using the same | |
| Oboznaya et al. | Production of monoclonal antibodies to the prion protein and their characterization | |
| BR112017000428B1 (pt) | Produto imunogênico com base em sequências de aminoácidos muteína amiloide beta, composição compreendendo o produto e uso do mesmo para tratar ou prevenir amiloidose |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120404 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20120404 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A712 Effective date: 20130701 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130822 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20131203 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20140603 |