JP2009501046A - 肥満及び糖尿病を治療するための胃腸及び膵臓用装置 - Google Patents
肥満及び糖尿病を治療するための胃腸及び膵臓用装置 Download PDFInfo
- Publication number
- JP2009501046A JP2009501046A JP2008521036A JP2008521036A JP2009501046A JP 2009501046 A JP2009501046 A JP 2009501046A JP 2008521036 A JP2008521036 A JP 2008521036A JP 2008521036 A JP2008521036 A JP 2008521036A JP 2009501046 A JP2009501046 A JP 2009501046A
- Authority
- JP
- Japan
- Prior art keywords
- current
- subject
- electrodes
- electrode
- configuring
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 230000002496 gastric effect Effects 0.000 title description 30
- 208000008589 Obesity Diseases 0.000 title description 26
- 235000020824 obesity Nutrition 0.000 title description 25
- 206010012601 diabetes mellitus Diseases 0.000 title description 22
- 238000000034 method Methods 0.000 claims abstract description 129
- 210000001198 duodenum Anatomy 0.000 claims abstract description 89
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 claims abstract description 86
- 210000004369 blood Anatomy 0.000 claims abstract description 62
- 239000008280 blood Substances 0.000 claims abstract description 62
- 102000004877 Insulin Human genes 0.000 claims abstract description 43
- 108090001061 Insulin Proteins 0.000 claims abstract description 43
- 229940125396 insulin Drugs 0.000 claims abstract description 43
- 230000003213 activating effect Effects 0.000 claims abstract description 19
- 230000000638 stimulation Effects 0.000 claims description 60
- 210000000496 pancreas Anatomy 0.000 claims description 58
- 210000001519 tissue Anatomy 0.000 claims description 58
- 230000002183 duodenal effect Effects 0.000 claims description 55
- 210000000105 enteric nervous system Anatomy 0.000 claims description 51
- 210000002784 stomach Anatomy 0.000 claims description 51
- 230000000694 effects Effects 0.000 claims description 48
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 47
- 239000008103 glucose Substances 0.000 claims description 47
- 210000001186 vagus nerve Anatomy 0.000 claims description 38
- 230000004044 response Effects 0.000 claims description 34
- 210000000277 pancreatic duct Anatomy 0.000 claims description 33
- 210000001187 pylorus Anatomy 0.000 claims description 33
- 210000001035 gastrointestinal tract Anatomy 0.000 claims description 28
- 238000011282 treatment Methods 0.000 claims description 27
- 235000005686 eating Nutrition 0.000 claims description 25
- 210000003205 muscle Anatomy 0.000 claims description 19
- 230000008855 peristalsis Effects 0.000 claims description 19
- 239000002775 capsule Substances 0.000 claims description 18
- 239000012530 fluid Substances 0.000 claims description 18
- 230000030136 gastric emptying Effects 0.000 claims description 15
- 230000004936 stimulating effect Effects 0.000 claims description 15
- 230000001965 increasing effect Effects 0.000 claims description 13
- 238000004519 manufacturing process Methods 0.000 claims description 13
- 230000036186 satiety Effects 0.000 claims description 13
- 235000019627 satiety Nutrition 0.000 claims description 13
- 210000002237 B-cell of pancreatic islet Anatomy 0.000 claims description 12
- 230000001079 digestive effect Effects 0.000 claims description 12
- 230000008602 contraction Effects 0.000 claims description 11
- 230000037406 food intake Effects 0.000 claims description 10
- 230000002572 peristaltic effect Effects 0.000 claims description 10
- 238000003780 insertion Methods 0.000 claims description 9
- 230000037431 insertion Effects 0.000 claims description 9
- 210000001819 pancreatic juice Anatomy 0.000 claims description 9
- 210000005036 nerve Anatomy 0.000 claims description 8
- 210000000941 bile Anatomy 0.000 claims description 7
- 210000000214 mouth Anatomy 0.000 claims description 7
- 230000003187 abdominal effect Effects 0.000 claims description 6
- 238000002513 implantation Methods 0.000 claims description 6
- DTHNMHAUYICORS-KTKZVXAJSA-N Glucagon-like peptide 1 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 DTHNMHAUYICORS-KTKZVXAJSA-N 0.000 claims description 5
- 210000000227 basophil cell of anterior lobe of hypophysis Anatomy 0.000 claims description 5
- 230000001404 mediated effect Effects 0.000 claims description 4
- 230000037361 pathway Effects 0.000 claims description 4
- 230000002051 biphasic effect Effects 0.000 claims description 3
- 235000019525 fullness Nutrition 0.000 claims description 3
- 102100025101 GATA-type zinc finger protein 1 Human genes 0.000 claims 1
- 101710198884 GATA-type zinc finger protein 1 Proteins 0.000 claims 1
- 241000700159 Rattus Species 0.000 description 44
- 238000002474 experimental method Methods 0.000 description 28
- 230000005684 electric field Effects 0.000 description 21
- 230000000968 intestinal effect Effects 0.000 description 19
- 238000010521 absorption reaction Methods 0.000 description 14
- 210000004027 cell Anatomy 0.000 description 12
- 238000001514 detection method Methods 0.000 description 12
- 230000028327 secretion Effects 0.000 description 12
- 230000006698 induction Effects 0.000 description 11
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 11
- 102000004190 Enzymes Human genes 0.000 description 10
- 108090000790 Enzymes Proteins 0.000 description 10
- 238000010586 diagram Methods 0.000 description 9
- 235000013305 food Nutrition 0.000 description 8
- 230000001537 neural effect Effects 0.000 description 8
- 230000007423 decrease Effects 0.000 description 7
- 230000002829 reductive effect Effects 0.000 description 7
- 230000001154 acute effect Effects 0.000 description 6
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 6
- 230000003914 insulin secretion Effects 0.000 description 6
- 210000005070 sphincter Anatomy 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 210000001015 abdomen Anatomy 0.000 description 5
- 210000000683 abdominal cavity Anatomy 0.000 description 5
- 235000019789 appetite Nutrition 0.000 description 5
- 230000036528 appetite Effects 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 230000033001 locomotion Effects 0.000 description 5
- 230000011218 segmentation Effects 0.000 description 5
- 230000001515 vagal effect Effects 0.000 description 5
- 101800000224 Glucagon-like peptide 1 Proteins 0.000 description 4
- 206010022489 Insulin Resistance Diseases 0.000 description 4
- 102100040918 Pro-glucagon Human genes 0.000 description 4
- 230000004913 activation Effects 0.000 description 4
- 210000000013 bile duct Anatomy 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 210000001072 colon Anatomy 0.000 description 4
- 238000002350 laparotomy Methods 0.000 description 4
- 235000012054 meals Nutrition 0.000 description 4
- 210000002569 neuron Anatomy 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 230000003820 β-cell dysfunction Effects 0.000 description 4
- 241001631457 Cannula Species 0.000 description 3
- 206010021518 Impaired gastric emptying Diseases 0.000 description 3
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 3
- 108010087230 Sincalide Proteins 0.000 description 3
- 230000001133 acceleration Effects 0.000 description 3
- 230000036982 action potential Effects 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- 230000001174 ascending effect Effects 0.000 description 3
- 230000033228 biological regulation Effects 0.000 description 3
- 235000019577 caloric intake Nutrition 0.000 description 3
- 230000000747 cardiac effect Effects 0.000 description 3
- 238000010609 cell counting kit-8 assay Methods 0.000 description 3
- 239000000306 component Substances 0.000 description 3
- 230000008878 coupling Effects 0.000 description 3
- 238000010168 coupling process Methods 0.000 description 3
- 238000005859 coupling reaction Methods 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 235000014632 disordered eating Nutrition 0.000 description 3
- 210000003238 esophagus Anatomy 0.000 description 3
- 229940088597 hormone Drugs 0.000 description 3
- 239000005556 hormone Substances 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 238000009413 insulation Methods 0.000 description 3
- 230000006362 insulin response pathway Effects 0.000 description 3
- 210000000936 intestine Anatomy 0.000 description 3
- 210000004153 islets of langerhan Anatomy 0.000 description 3
- 229960002725 isoflurane Drugs 0.000 description 3
- 238000007726 management method Methods 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 230000004899 motility Effects 0.000 description 3
- 210000004126 nerve fiber Anatomy 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 210000004798 organs belonging to the digestive system Anatomy 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 108090000623 proteins and genes Proteins 0.000 description 3
- IZTQOLKUZKXIRV-YRVFCXMDSA-N sincalide Chemical compound C([C@@H](C(=O)N[C@@H](CCSC)C(=O)NCC(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(N)=O)NC(=O)[C@@H](N)CC(O)=O)C1=CC=C(OS(O)(=O)=O)C=C1 IZTQOLKUZKXIRV-YRVFCXMDSA-N 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 230000004083 survival effect Effects 0.000 description 3
- 230000001960 triggered effect Effects 0.000 description 3
- 208000030814 Eating disease Diseases 0.000 description 2
- 208000019454 Feeding and Eating disease Diseases 0.000 description 2
- 206010016654 Fibrosis Diseases 0.000 description 2
- 208000002705 Glucose Intolerance Diseases 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 2
- 206010028980 Neoplasm Diseases 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- OIPILFWXSMYKGL-UHFFFAOYSA-N acetylcholine Chemical group CC(=O)OCC[N+](C)(C)C OIPILFWXSMYKGL-UHFFFAOYSA-N 0.000 description 2
- 229960004373 acetylcholine Drugs 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 210000001367 artery Anatomy 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 230000036765 blood level Effects 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 238000010292 electrical insulation Methods 0.000 description 2
- 230000029142 excretion Effects 0.000 description 2
- 239000000835 fiber Substances 0.000 description 2
- 230000004761 fibrosis Effects 0.000 description 2
- 235000012631 food intake Nutrition 0.000 description 2
- 230000002641 glycemic effect Effects 0.000 description 2
- 210000003767 ileocecal valve Anatomy 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 230000008991 intestinal motility Effects 0.000 description 2
- 230000002045 lasting effect Effects 0.000 description 2
- WABPQHHGFIMREM-UHFFFAOYSA-N lead(0) Chemical compound [Pb] WABPQHHGFIMREM-UHFFFAOYSA-N 0.000 description 2
- 229960004194 lidocaine Drugs 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 210000004877 mucosa Anatomy 0.000 description 2
- 210000003249 myenteric plexus Anatomy 0.000 description 2
- 230000003183 myoelectrical effect Effects 0.000 description 2
- 235000015816 nutrient absorption Nutrition 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 230000009996 pancreatic endocrine effect Effects 0.000 description 2
- 210000004923 pancreatic tissue Anatomy 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- 210000004303 peritoneum Anatomy 0.000 description 2
- 230000000291 postprandial effect Effects 0.000 description 2
- 201000009104 prediabetes syndrome Diseases 0.000 description 2
- GCYXWQUSHADNBF-AAEALURTSA-N preproglucagon 78-108 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 GCYXWQUSHADNBF-AAEALURTSA-N 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 210000000813 small intestine Anatomy 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 210000004876 tela submucosa Anatomy 0.000 description 2
- 229930003347 Atropine Natural products 0.000 description 1
- 208000032841 Bulimia Diseases 0.000 description 1
- 206010006550 Bulimia nervosa Diseases 0.000 description 1
- 241000272173 Calidris Species 0.000 description 1
- 108010089335 Cholecystokinin A Receptor Proteins 0.000 description 1
- 102000004859 Cholecystokinin Receptors Human genes 0.000 description 1
- 108090001085 Cholecystokinin Receptors Proteins 0.000 description 1
- 102100034927 Cholecystokinin receptor type A Human genes 0.000 description 1
- 208000032928 Dyslipidaemia Diseases 0.000 description 1
- 241000611421 Elia Species 0.000 description 1
- 208000010235 Food Addiction Diseases 0.000 description 1
- 102000051325 Glucagon Human genes 0.000 description 1
- 108060003199 Glucagon Proteins 0.000 description 1
- VZJFGSRCJCXDSG-UHFFFAOYSA-N Hexamethonium Chemical compound C[N+](C)(C)CCCCCC[N+](C)(C)C VZJFGSRCJCXDSG-UHFFFAOYSA-N 0.000 description 1
- 241000167880 Hirundinidae Species 0.000 description 1
- 101000976075 Homo sapiens Insulin Proteins 0.000 description 1
- RKUNBYITZUJHSG-UHFFFAOYSA-N Hyosciamin-hydrochlorid Natural products CN1C(C2)CCC1CC2OC(=O)C(CO)C1=CC=CC=C1 RKUNBYITZUJHSG-UHFFFAOYSA-N 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- MRAUNPAHJZDYCK-BYPYZUCNSA-N L-nitroarginine Chemical compound OC(=O)[C@@H](N)CCCNC(=N)N[N+]([O-])=O MRAUNPAHJZDYCK-BYPYZUCNSA-N 0.000 description 1
- 208000017170 Lipid metabolism disease Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 208000002693 Multiple Abnormalities Diseases 0.000 description 1
- 206010033307 Overweight Diseases 0.000 description 1
- 208000006809 Pancreatic Fistula Diseases 0.000 description 1
- 102000052651 Pancreatic hormone Human genes 0.000 description 1
- 108700020479 Pancreatic hormone Proteins 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- BELBBZDIHDAJOR-UHFFFAOYSA-N Phenolsulfonephthalein Chemical compound C1=CC(O)=CC=C1C1(C=2C=CC(O)=CC=2)C2=CC=CC=C2S(=O)(=O)O1 BELBBZDIHDAJOR-UHFFFAOYSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- RKUNBYITZUJHSG-SPUOUPEWSA-N atropine Chemical compound O([C@H]1C[C@H]2CC[C@@H](C1)N2C)C(=O)C(CO)C1=CC=CC=C1 RKUNBYITZUJHSG-SPUOUPEWSA-N 0.000 description 1
- 229960000396 atropine Drugs 0.000 description 1
- 210000003403 autonomic nervous system Anatomy 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 238000005842 biochemical reaction Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000012503 blood component Substances 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 230000002060 circadian Effects 0.000 description 1
- 208000012696 congenital leptin deficiency Diseases 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 230000000875 corresponding effect Effects 0.000 description 1
- 210000001100 crypt cell Anatomy 0.000 description 1
- 230000001351 cycling effect Effects 0.000 description 1
- 206010061428 decreased appetite Diseases 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000001687 destabilization Effects 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000000378 dietary effect Effects 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 230000003028 elevating effect Effects 0.000 description 1
- 230000002124 endocrine Effects 0.000 description 1
- 238000001839 endoscopy Methods 0.000 description 1
- 230000002964 excitative effect Effects 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 210000003608 fece Anatomy 0.000 description 1
- 235000011389 fruit/vegetable juice Nutrition 0.000 description 1
- 230000005176 gastrointestinal motility Effects 0.000 description 1
- 210000005095 gastrointestinal system Anatomy 0.000 description 1
- 229960004666 glucagon Drugs 0.000 description 1
- MASNOZXLGMXCHN-ZLPAWPGGSA-N glucagon Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 MASNOZXLGMXCHN-ZLPAWPGGSA-N 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 230000002650 habitual effect Effects 0.000 description 1
- 210000002216 heart Anatomy 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 229950002932 hexamethonium Drugs 0.000 description 1
- 208000021760 high fever Diseases 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 230000030214 innervation Effects 0.000 description 1
- 239000012212 insulator Substances 0.000 description 1
- PBGKTOXHQIOBKM-FHFVDXKLSA-N insulin (human) Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CSSC[C@H]2C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=3C=CC(O)=CC=3)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3C=CC(O)=CC=3)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=3NC=NC=3)NC(=O)[C@H](CO)NC(=O)CNC1=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O)=O)CSSC[C@@H](C(N2)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC=1C=CC=CC=1)C(C)C)C1=CN=CN1 PBGKTOXHQIOBKM-FHFVDXKLSA-N 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 210000001630 jejunum Anatomy 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 238000012317 liver biopsy Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000028161 membrane depolarization Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 230000000116 mitigating effect Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 208000001022 morbid obesity Diseases 0.000 description 1
- 210000000663 muscle cell Anatomy 0.000 description 1
- 230000004118 muscle contraction Effects 0.000 description 1
- 230000003880 negative regulation of appetite Effects 0.000 description 1
- 230000008035 nerve activity Effects 0.000 description 1
- 230000007383 nerve stimulation Effects 0.000 description 1
- 230000007230 neural mechanism Effects 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 239000004025 pancreas hormone Substances 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 229940032957 pancreatic hormone Drugs 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 229960003531 phenolsulfonphthalein Drugs 0.000 description 1
- 230000010287 polarization Effects 0.000 description 1
- 210000003240 portal vein Anatomy 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 210000002460 smooth muscle Anatomy 0.000 description 1
- 210000000329 smooth muscle myocyte Anatomy 0.000 description 1
- IFGCUJZIWBUILZ-UHFFFAOYSA-N sodium 2-[[2-[[hydroxy-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyphosphoryl]amino]-4-methylpentanoyl]amino]-3-(1H-indol-3-yl)propanoic acid Chemical compound [Na+].C=1NC2=CC=CC=C2C=1CC(C(O)=O)NC(=O)C(CC(C)C)NP(O)(=O)OC1OC(C)C(O)C(O)C1O IFGCUJZIWBUILZ-UHFFFAOYSA-N 0.000 description 1
- 210000003594 spinal ganglia Anatomy 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 239000000021 stimulant Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 210000000470 submucous plexus Anatomy 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- 229960004799 tryptophan Drugs 0.000 description 1
- 210000002438 upper gastrointestinal tract Anatomy 0.000 description 1
- 230000009278 visceral effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/36007—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation of urogenital or gastrointestinal organs, e.g. for incontinence control
Landscapes
- Health & Medical Sciences (AREA)
- Gastroenterology & Hepatology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Radiology & Medical Imaging (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Electrotherapy Devices (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US69944205P | 2005-07-13 | 2005-07-13 | |
| US72095105P | 2005-09-26 | 2005-09-26 | |
| US11/279,355 US20070016262A1 (en) | 2005-07-13 | 2006-04-11 | Gi and pancreatic device for treating obesity and diabetes |
| PCT/IL2006/000819 WO2007007339A2 (en) | 2005-07-13 | 2006-07-13 | Gi and pancreatic device for treating obesity and diabetes |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009501046A true JP2009501046A (ja) | 2009-01-15 |
| JP2009501046A5 JP2009501046A5 (cg-RX-API-DMAC7.html) | 2009-09-17 |
Family
ID=37637592
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008521036A Pending JP2009501046A (ja) | 2005-07-13 | 2006-07-13 | 肥満及び糖尿病を治療するための胃腸及び膵臓用装置 |
Country Status (4)
| Country | Link |
|---|---|
| US (3) | US20070016262A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP1907046A4 (cg-RX-API-DMAC7.html) |
| JP (1) | JP2009501046A (cg-RX-API-DMAC7.html) |
| WO (1) | WO2007007339A2 (cg-RX-API-DMAC7.html) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021519632A (ja) * | 2018-03-29 | 2021-08-12 | ネヴロ コーポレイション | 2型糖尿病を含む血糖異常を治療する、および/またはHbA1cレベルを低下させるための治療的調節、ならびに関連するシステムおよび方法 |
Families Citing this family (269)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8825152B2 (en) * | 1996-01-08 | 2014-09-02 | Impulse Dynamics, N.V. | Modulation of intracellular calcium concentration using non-excitatory electrical signals applied to the tissue |
| US7167748B2 (en) | 1996-01-08 | 2007-01-23 | Impulse Dynamics Nv | Electrical muscle controller |
| US9289618B1 (en) | 1996-01-08 | 2016-03-22 | Impulse Dynamics Nv | Electrical muscle controller |
| US8346363B2 (en) * | 1999-03-05 | 2013-01-01 | Metacure Limited | Blood glucose level control |
| US9101765B2 (en) * | 1999-03-05 | 2015-08-11 | Metacure Limited | Non-immediate effects of therapy |
| WO2006073671A1 (en) | 2004-12-09 | 2006-07-13 | Impulse Dynamics Nv | Protein activity modification |
| US8666495B2 (en) * | 1999-03-05 | 2014-03-04 | Metacure Limited | Gastrointestinal methods and apparatus for use in treating disorders and controlling blood sugar |
| US8700161B2 (en) * | 1999-03-05 | 2014-04-15 | Metacure Limited | Blood glucose level control |
| US8019421B2 (en) * | 1999-03-05 | 2011-09-13 | Metacure Limited | Blood glucose level control |
| US20040249421A1 (en) * | 2000-09-13 | 2004-12-09 | Impulse Dynamics Nv | Blood glucose level control |
| US8914114B2 (en) * | 2000-05-23 | 2014-12-16 | The Feinstein Institute For Medical Research | Inhibition of inflammatory cytokine production by cholinergic agonists and vagus nerve stimulation |
| US8845672B2 (en) | 2002-05-09 | 2014-09-30 | Reshape Medical, Inc. | Balloon system and methods for treating obesity |
| US7338433B2 (en) | 2002-08-13 | 2008-03-04 | Allergan, Inc. | Remotely adjustable gastric banding method |
| EP1553878B1 (en) * | 2002-08-28 | 2010-02-24 | Allergan, Inc. | Fatigue-resistant gastric banding device |
| US7901419B2 (en) * | 2002-09-04 | 2011-03-08 | Allergan, Inc. | Telemetrically controlled band for regulating functioning of a body organ or duct, and methods of making, implantation and use |
| US8070743B2 (en) | 2002-11-01 | 2011-12-06 | Valentx, Inc. | Devices and methods for attaching an endolumenal gastrointestinal implant |
| US9060844B2 (en) | 2002-11-01 | 2015-06-23 | Valentx, Inc. | Apparatus and methods for treatment of morbid obesity |
| US20040172084A1 (en) | 2003-02-03 | 2004-09-02 | Knudson Mark B. | Method and apparatus for treatment of gastro-esophageal reflux disease (GERD) |
| US7444183B2 (en) | 2003-02-03 | 2008-10-28 | Enteromedics, Inc. | Intraluminal electrode apparatus and method |
| US7844338B2 (en) | 2003-02-03 | 2010-11-30 | Enteromedics Inc. | High frequency obesity treatment |
| CN1787850B (zh) * | 2003-03-10 | 2015-12-16 | 脉冲动力公司 | 用于传送电信号以修改心脏组织中基因表达的装置与方法 |
| AU2003245593B2 (en) * | 2003-06-20 | 2010-06-03 | Apollo Endosurgery, Inc. | Two-way slit valve |
| US8792985B2 (en) * | 2003-07-21 | 2014-07-29 | Metacure Limited | Gastrointestinal methods and apparatus for use in treating disorders and controlling blood sugar |
| US20090259236A2 (en) | 2003-07-28 | 2009-10-15 | Baronova, Inc. | Gastric retaining devices and methods |
| US8821521B2 (en) * | 2003-07-28 | 2014-09-02 | Baronova, Inc. | Gastro-intestinal device and method for treating addiction |
| US8048169B2 (en) * | 2003-07-28 | 2011-11-01 | Baronova, Inc. | Pyloric valve obstructing devices and methods |
| US9700450B2 (en) * | 2003-07-28 | 2017-07-11 | Baronova, Inc. | Devices and methods for gastrointestinal stimulation |
| US9498366B2 (en) * | 2003-07-28 | 2016-11-22 | Baronova, Inc. | Devices and methods for pyloric anchoring |
| PT2399528E (pt) | 2004-01-23 | 2013-02-26 | Allergan Inc | Banda gástrica regulável de uma só peça, que pode ser fixada de forma amovível |
| JP4718539B2 (ja) * | 2004-03-08 | 2011-07-06 | アラーガン・メディカル・ソシエテ・ア・レスポンサビリテ・リミテ | 管状器官用の閉鎖システム |
| US8352031B2 (en) | 2004-03-10 | 2013-01-08 | Impulse Dynamics Nv | Protein activity modification |
| ATE517652T1 (de) * | 2004-03-18 | 2011-08-15 | Allergan Inc | Vorrichtung zur volumeneinstellung intragastraler ballons |
| US10912712B2 (en) | 2004-03-25 | 2021-02-09 | The Feinstein Institutes For Medical Research | Treatment of bleeding by non-invasive stimulation |
| US20080077186A1 (en) * | 2006-04-18 | 2008-03-27 | Proteus Biomedical, Inc. | High phrenic, low capture threshold pacing devices and methods |
| US20080255647A1 (en) * | 2004-12-22 | 2008-10-16 | Marc Jensen | Implantable Addressable Segmented Electrodes |
| US11207518B2 (en) | 2004-12-27 | 2021-12-28 | The Feinstein Institutes For Medical Research | Treating inflammatory disorders by stimulation of the cholinergic anti-inflammatory pathway |
| EP1833559B1 (en) | 2004-12-27 | 2010-11-24 | The Feinstein Institute for Medical Research | Treating inflammatory disorders by electrical vagus nerve stimulation |
| US9821158B2 (en) | 2005-02-17 | 2017-11-21 | Metacure Limited | Non-immediate effects of therapy |
| WO2006097934A2 (en) * | 2005-03-18 | 2006-09-21 | Metacure Limited | Pancreas lead |
| US8251888B2 (en) * | 2005-04-13 | 2012-08-28 | Mitchell Steven Roslin | Artificial gastric valve |
| EP1898991B1 (en) * | 2005-05-04 | 2016-06-29 | Impulse Dynamics NV | Protein activity modification |
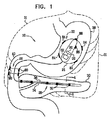
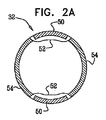
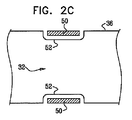
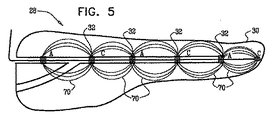
| US20070016262A1 (en) | 2005-07-13 | 2007-01-18 | Betastim, Ltd. | Gi and pancreatic device for treating obesity and diabetes |
| JP2009502302A (ja) | 2005-07-25 | 2009-01-29 | グロス,ヨシ | 血管の電気刺激 |
| US20070100368A1 (en) * | 2005-10-31 | 2007-05-03 | Quijano Rodolfo C | Intragastric space filler |
| US20070100369A1 (en) * | 2005-10-31 | 2007-05-03 | Cragg Andrew H | Intragastric space filler |
| US8204586B2 (en) * | 2005-11-22 | 2012-06-19 | Proteus Biomedical, Inc. | External continuous field tomography |
| US8043206B2 (en) | 2006-01-04 | 2011-10-25 | Allergan, Inc. | Self-regulating gastric band with pressure data processing |
| US7798954B2 (en) | 2006-01-04 | 2010-09-21 | Allergan, Inc. | Hydraulic gastric band with collapsible reservoir |
| US7881797B2 (en) * | 2006-04-25 | 2011-02-01 | Valentx, Inc. | Methods and devices for gastrointestinal stimulation |
| US20090143777A1 (en) * | 2006-05-23 | 2009-06-04 | Andrew Pacey | Apparatus and method for treating tissue such as tumours |
| US20070288033A1 (en) * | 2006-06-09 | 2007-12-13 | Allergan, Inc. | Intragastric balloon retrieval mechanisms |
| US20080097566A1 (en) * | 2006-07-13 | 2008-04-24 | Olivier Colliou | Focused segmented electrode |
| US20080039916A1 (en) * | 2006-08-08 | 2008-02-14 | Olivier Colliou | Distally distributed multi-electrode lead |
| US9326877B2 (en) | 2006-09-29 | 2016-05-03 | Apollo Endosurgery, Inc. | Apparatus and method for intragastric balloon with in situ adjustment means |
| US20080114230A1 (en) * | 2006-11-14 | 2008-05-15 | Bruce Addis | Electrode support |
| US8926648B2 (en) * | 2007-02-13 | 2015-01-06 | Brian Charles Weiner | Multi-method and multi-apparatus for treating obesity |
| WO2008100974A2 (en) * | 2007-02-13 | 2008-08-21 | Sharma Virender K | Method and apparatus for electrical stimulation of the pancreatico-biliary system |
| US8068918B2 (en) | 2007-03-09 | 2011-11-29 | Enteromedics Inc. | Remote monitoring and control of implantable devices |
| WO2008112915A1 (en) * | 2007-03-13 | 2008-09-18 | The Feinstein Institute For Medical Research | Treatment of inflammation by non-invasive stimulation |
| US8226602B2 (en) * | 2007-03-30 | 2012-07-24 | Reshape Medical, Inc. | Intragastric balloon system and therapeutic processes and products |
| US20080255601A1 (en) * | 2007-04-13 | 2008-10-16 | Allergan, Inc. | Apparatus and method for remote deflation of intragastric balloon |
| US7962214B2 (en) * | 2007-04-26 | 2011-06-14 | Cyberonics, Inc. | Non-surgical device and methods for trans-esophageal vagus nerve stimulation |
| US9364666B2 (en) * | 2007-05-07 | 2016-06-14 | Transtimulation Research, Inc. | Method of using a gastrointestinal stimulator device for digestive and eating disorders |
| US20080281365A1 (en) * | 2007-05-09 | 2008-11-13 | Tweden Katherine S | Neural signal duty cycle |
| US20080300645A1 (en) * | 2007-05-31 | 2008-12-04 | Pacesetter, Inc. | Treatment of cardiomyopathy, heart failure and cardiac ischemia using stimulation induced secretion of glp-1 |
| US8532787B2 (en) | 2007-05-31 | 2013-09-10 | Enteromedics Inc. | Implantable therapy system having multiple operating modes |
| US20080300648A1 (en) * | 2007-05-31 | 2008-12-04 | Pacesetter, Inc. | Treatment of cardiomyopathy, heart failure and cardiac ischemia using stimulation induced secretion of glp-1 |
| US7634315B2 (en) * | 2007-05-31 | 2009-12-15 | Pacesetter, Inc. | Techniques to monitor and trend nerve damage and recovery |
| US20080300646A1 (en) * | 2007-05-31 | 2008-12-04 | Pacesetter, Inc. | Treatment of cardiomyopathy, heart failure and cardiac ischemia using stimulation induced secretion of glp-1 |
| US20080300647A1 (en) * | 2007-05-31 | 2008-12-04 | Pacesetter, Inc. | Treatment of cardiomyopathy, heart failure and cardiac ischemia using stimulation induced secretion of glp-1 |
| US20090012544A1 (en) * | 2007-06-08 | 2009-01-08 | Valen Tx, Inc. | Gastrointestinal bypass sleeve as an adjunct to bariatric surgery |
| EP2164558A4 (en) * | 2007-06-08 | 2010-08-04 | Valentx Inc | METHODS AND DEVICES FOR INTRAGASTRIC SUPPORT OF FUNCTIONAL OR PROSTHETIC GASTROINTESTINAL DEVICES |
| WO2008154594A2 (en) * | 2007-06-11 | 2008-12-18 | Valentx, Inc. | Endoscopic delivery devices and methods |
| US8032222B2 (en) | 2007-06-19 | 2011-10-04 | Loushin Michael K H | Device for electrically and mechanically stimulating a compartment in a body |
| US8142469B2 (en) * | 2007-06-25 | 2012-03-27 | Reshape Medical, Inc. | Gastric space filler device, delivery system, and related methods |
| WO2009013749A2 (en) * | 2007-07-24 | 2009-01-29 | Betastim, Ltd. | Duodenal eating sensor |
| US9392445B2 (en) * | 2007-08-17 | 2016-07-12 | Qualcomm Incorporated | Handoff at an ad-hoc mobile service provider |
| US9398453B2 (en) | 2007-08-17 | 2016-07-19 | Qualcomm Incorporated | Ad hoc service provider's ability to provide service for a wireless network |
| US20090047930A1 (en) * | 2007-08-17 | 2009-02-19 | Qualcomm Incorporated | Method for a heterogeneous wireless ad hoc mobile service provider |
| WO2009029614A1 (en) | 2007-08-27 | 2009-03-05 | The Feinstein Institute For Medical Research | Devices and methods for inhibiting granulocyte activation by neural stimulation |
| CA2843571C (en) | 2007-09-07 | 2018-02-06 | Baronova, Inc. | Device for intermittently obstructing a gastric opening and method of use |
| EP2211789B1 (en) * | 2007-10-11 | 2023-06-07 | Implantica Patent Ltd. | Apparatus and method for controlling food flow through a compartmentalized stomach of a patient |
| EA033368B1 (ru) * | 2007-10-11 | 2019-10-31 | Implantica Patent Ltd | Устройство для управления потоком в органе тела |
| DK2211984T3 (da) * | 2007-10-11 | 2022-05-16 | Implantica Patent Ltd | Apparat til behandling af seksuel dysfunktion hos kvinder |
| EP2214601B1 (en) * | 2007-10-11 | 2023-11-29 | Implantica Patent Ltd. | Apparatus for controlling food flow through the stomach of a patient |
| AU2008317024A1 (en) * | 2007-10-23 | 2009-04-30 | Allergan, Inc. | Pressure sensing intragastric balloon |
| WO2009064408A1 (en) * | 2007-11-12 | 2009-05-22 | Dilorenzo Daniel J | Method and apparatus for programming of autonomic neuromodulation for the treatment of obesity |
| EP2231060B1 (en) * | 2007-12-10 | 2015-05-27 | Medtronic Ablation Frontiers LLC | Ablation catheter |
| US20090177223A1 (en) * | 2008-01-03 | 2009-07-09 | Tara Chand Singhal | System and method for management of type 2 diabetes |
| US8538535B2 (en) | 2010-08-05 | 2013-09-17 | Rainbow Medical Ltd. | Enhancing perfusion by contraction |
| WO2009102969A1 (en) * | 2008-02-14 | 2009-08-20 | Enteromedics, Inc. | Treatment of excess weight by neural downregulation in combination with compositions |
| US9662490B2 (en) | 2008-03-31 | 2017-05-30 | The Feinstein Institute For Medical Research | Methods and systems for reducing inflammation by neuromodulation and administration of an anti-inflammatory drug |
| US9211409B2 (en) | 2008-03-31 | 2015-12-15 | The Feinstein Institute For Medical Research | Methods and systems for reducing inflammation by neuromodulation of T-cell activity |
| CN105031811B (zh) * | 2008-04-04 | 2017-09-08 | 安特罗麦迪克斯公司 | 用于葡萄糖调节的方法和系统 |
| US8768469B2 (en) | 2008-08-08 | 2014-07-01 | Enteromedics Inc. | Systems for regulation of blood pressure and heart rate |
| BRPI0917925A2 (pt) * | 2008-08-26 | 2015-11-10 | Centocor Ortho Biotech Inc | estimulação da liberação de hormônio da saciedade |
| EP2362762A1 (en) * | 2008-10-06 | 2011-09-07 | Allergan Medical Sàrl | Mechanical gastric band with cushions |
| WO2010042686A1 (en) | 2008-10-09 | 2010-04-15 | Sharma Virender K | Method and apparatus for stimulating the vascular system |
| US10603489B2 (en) | 2008-10-09 | 2020-03-31 | Virender K. Sharma | Methods and apparatuses for stimulating blood vessels in order to control, treat, and/or prevent a hemorrhage |
| US20100185049A1 (en) * | 2008-10-22 | 2010-07-22 | Allergan, Inc. | Dome and screw valves for remotely adjustable gastric banding systems |
| EP2355893B1 (en) * | 2008-11-18 | 2013-12-25 | Setpoint Medical Corporation | Devices for optimizing electrode placement for anti-inflamatory stimulation |
| US9174031B2 (en) * | 2009-03-13 | 2015-11-03 | Reshape Medical, Inc. | Device and method for deflation and removal of implantable and inflatable devices |
| EP2414025A4 (en) * | 2009-04-03 | 2017-08-02 | ReShape Medical, Inc. | Improved intragastric space fillers and methods of manufacturing including in vitro testing |
| US8788034B2 (en) | 2011-05-09 | 2014-07-22 | Setpoint Medical Corporation | Single-pulse activation of the cholinergic anti-inflammatory pathway to treat chronic inflammation |
| US8996116B2 (en) | 2009-10-30 | 2015-03-31 | Setpoint Medical Corporation | Modulation of the cholinergic anti-inflammatory pathway to treat pain or addiction |
| EP2424583A2 (en) * | 2009-05-01 | 2012-03-07 | Allergan, Inc. | Laparoscopic gastric band with active agents |
| US9211410B2 (en) | 2009-05-01 | 2015-12-15 | Setpoint Medical Corporation | Extremely low duty-cycle activation of the cholinergic anti-inflammatory pathway to treat chronic inflammation |
| US20110184229A1 (en) * | 2009-05-01 | 2011-07-28 | Allergan, Inc. | Laparoscopic gastric band with active agents |
| US20110066175A1 (en) * | 2009-05-07 | 2011-03-17 | Rainbow Medical Ltd. | Gastric anchor |
| US20100286628A1 (en) * | 2009-05-07 | 2010-11-11 | Rainbow Medical Ltd | Gastric anchor |
| US8414559B2 (en) * | 2009-05-07 | 2013-04-09 | Rainbow Medical Ltd. | Gastroretentive duodenal pill |
| US9179367B2 (en) | 2009-05-26 | 2015-11-03 | Qualcomm Incorporated | Maximizing service provider utility in a heterogeneous wireless ad-hoc network |
| US20100305655A1 (en) * | 2009-05-28 | 2010-12-02 | ElectroCore, LLC | Methods and apparatus for treating gastrointestinal disorders using electrical signals |
| AU2010258792B2 (en) | 2009-06-09 | 2015-07-02 | Setpoint Medical Corporation | Nerve cuff with pocket for leadless stimulator |
| EP2456507A4 (en) | 2009-07-22 | 2013-07-03 | Reshape Medical Inc | RETENTION MECHANISMS FOR IMPLANTABLE MEDICAL DEVICES |
| US9604038B2 (en) | 2009-07-23 | 2017-03-28 | Reshape Medical, Inc. | Inflation and deflation mechanisms for inflatable medical devices |
| US9050174B2 (en) | 2009-07-23 | 2015-06-09 | Reshape Medical, Inc. | Deflation and removal of implantable medical devices |
| CN102770151B (zh) * | 2009-08-03 | 2018-07-31 | 因卡伯实验室有限责任公司 | 用于刺激肠道内肠促胰岛素产生的吞咽式囊和方法 |
| US20110137112A1 (en) * | 2009-08-28 | 2011-06-09 | Allergan, Inc. | Gastric band with electric stimulation |
| WO2011031400A2 (en) * | 2009-08-28 | 2011-03-17 | Allergan, Inc. | Gastric band with electric stimulation |
| EP2480279A4 (en) | 2009-09-24 | 2017-11-15 | Reshape Medical, Inc. | Normalization and stabilization of balloon surfaces for deflation |
| AU2010308498B2 (en) | 2009-10-21 | 2016-02-11 | Apollo Endosurgery, Inc. | Bariatric device and method for weight loss |
| WO2014169145A1 (en) | 2013-04-10 | 2014-10-16 | Setpoint Medical Corporation | Closed-loop vagus nerve stimulation |
| US9833621B2 (en) | 2011-09-23 | 2017-12-05 | Setpoint Medical Corporation | Modulation of sirtuins by vagus nerve stimulation |
| WO2011079309A2 (en) | 2009-12-23 | 2011-06-30 | Setpoint Medical Corporation | Neural stimulation devices and systems for treatment of chronic inflammation |
| US8759284B2 (en) | 2009-12-24 | 2014-06-24 | Rani Therapeutics, Llc | Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US20110184258A1 (en) * | 2010-01-28 | 2011-07-28 | Abbott Diabetes Care Inc. | Balloon Catheter Analyte Measurement Sensors and Methods for Using the Same |
| WO2011092710A2 (en) | 2010-02-01 | 2011-08-04 | Metacure Limited | Gastrointestinal electrical therapy |
| WO2011097637A1 (en) | 2010-02-08 | 2011-08-11 | Reshape Medical, Inc. | Materials and methods for improved intragastric balloon devices |
| EP2533845A4 (en) | 2010-02-08 | 2016-04-06 | Reshape Medical Inc | IMPROVED AND REINFORCED EXTRACTION METHOD AND MECHANISMS FOR INTRAGASTRAL DEVICES |
| US8678993B2 (en) * | 2010-02-12 | 2014-03-25 | Apollo Endosurgery, Inc. | Remotely adjustable gastric banding system |
| US20110201874A1 (en) * | 2010-02-12 | 2011-08-18 | Allergan, Inc. | Remotely adjustable gastric banding system |
| US8758221B2 (en) * | 2010-02-24 | 2014-06-24 | Apollo Endosurgery, Inc. | Source reservoir with potential energy for remotely adjustable gastric banding system |
| US8840541B2 (en) | 2010-02-25 | 2014-09-23 | Apollo Endosurgery, Inc. | Pressure sensing gastric banding system |
| US8764624B2 (en) | 2010-02-25 | 2014-07-01 | Apollo Endosurgery, Inc. | Inductively powered remotely adjustable gastric banding system |
| EP2539011A4 (en) | 2010-02-25 | 2014-03-26 | Reshape Medical Inc | Improved and enhanced explant processes and mechanisms for intragastric devices |
| AU2011227357B2 (en) | 2010-03-15 | 2015-04-02 | Boston Scientific Scimed, Inc. | Bariatric device and method for weight loss |
| WO2011127205A1 (en) | 2010-04-06 | 2011-10-13 | Reshape Medical , Inc. | Inflation devices for intragastric devices with improved attachment and detachment and associated systems and methods |
| US20110270024A1 (en) | 2010-04-29 | 2011-11-03 | Allergan, Inc. | Self-adjusting gastric band having various compliant components |
| US9044298B2 (en) | 2010-04-29 | 2015-06-02 | Apollo Endosurgery, Inc. | Self-adjusting gastric band |
| US9028394B2 (en) | 2010-04-29 | 2015-05-12 | Apollo Endosurgery, Inc. | Self-adjusting mechanical gastric band |
| US20110270025A1 (en) | 2010-04-30 | 2011-11-03 | Allergan, Inc. | Remotely powered remotely adjustable gastric band system |
| US9226840B2 (en) | 2010-06-03 | 2016-01-05 | Apollo Endosurgery, Inc. | Magnetically coupled implantable pump system and method |
| US8517915B2 (en) | 2010-06-10 | 2013-08-27 | Allergan, Inc. | Remotely adjustable gastric banding system |
| US8825164B2 (en) | 2010-06-11 | 2014-09-02 | Enteromedics Inc. | Neural modulation devices and methods |
| US10010439B2 (en) | 2010-06-13 | 2018-07-03 | Synerz Medical, Inc. | Intragastric device for treating obesity |
| US8628554B2 (en) | 2010-06-13 | 2014-01-14 | Virender K. Sharma | Intragastric device for treating obesity |
| US9526648B2 (en) | 2010-06-13 | 2016-12-27 | Synerz Medical, Inc. | Intragastric device for treating obesity |
| US10420665B2 (en) | 2010-06-13 | 2019-09-24 | W. L. Gore & Associates, Inc. | Intragastric device for treating obesity |
| US9211207B2 (en) | 2010-08-18 | 2015-12-15 | Apollo Endosurgery, Inc. | Power regulated implant |
| US8698373B2 (en) | 2010-08-18 | 2014-04-15 | Apollo Endosurgery, Inc. | Pare piezo power with energy recovery |
| US20120059216A1 (en) | 2010-09-07 | 2012-03-08 | Allergan, Inc. | Remotely adjustable gastric banding system |
| ES2566498T3 (es) | 2010-10-18 | 2016-04-13 | Apollo Endosurgery, Inc. | Implantes intragástricos con anclajes duodenales |
| US9233016B2 (en) | 2010-10-18 | 2016-01-12 | Apollo Endosurgery, Inc. | Elevating stomach stimulation device |
| ES2565348T3 (es) | 2010-10-18 | 2016-04-04 | Apollo Endosurgery, Inc. | Dispositivos de implante intragástrico reactivos |
| US9463107B2 (en) | 2010-10-18 | 2016-10-11 | Apollo Endosurgery, Inc. | Variable size intragastric implant devices |
| US8870966B2 (en) | 2010-10-18 | 2014-10-28 | Apollo Endosurgery, Inc. | Intragastric balloon for treating obesity |
| US9095405B2 (en) | 2010-10-19 | 2015-08-04 | Apollo Endosurgery, Inc. | Space-filling intragastric implants with fluid flow |
| US9398969B2 (en) | 2010-10-19 | 2016-07-26 | Apollo Endosurgery, Inc. | Upper stomach gastric implants |
| WO2012054522A2 (en) | 2010-10-19 | 2012-04-26 | Allergan, Inc. | Anchored non-piercing duodenal sleeve and delivery systems |
| US8864840B2 (en) | 2010-10-19 | 2014-10-21 | Apollo Endosurgery, Inc. | Intragastric implants with collapsible frames |
| US8920447B2 (en) | 2010-10-19 | 2014-12-30 | Apollo Endosurgery, Inc. | Articulated gastric implant clip |
| US9198790B2 (en) | 2010-10-19 | 2015-12-01 | Apollo Endosurgery, Inc. | Upper stomach gastric implants |
| US9498365B2 (en) | 2010-10-19 | 2016-11-22 | Apollo Endosurgery, Inc. | Intragastric implants with multiple fluid chambers |
| US8961393B2 (en) | 2010-11-15 | 2015-02-24 | Apollo Endosurgery, Inc. | Gastric band devices and drive systems |
| MX2013006546A (es) * | 2010-12-17 | 2013-09-05 | Neural Diabetes Llc | Metodo, sistema y aparato para controlar la funcion de celulas beta pancreaticas para mejorar la homeostasis de glucosa y la produccion de insulina. |
| US9149617B2 (en) | 2010-12-23 | 2015-10-06 | Rani Therapeutics, Llc | Device, system and methods for the oral delivery of therapeutic compounds |
| US9861683B2 (en) | 2010-12-23 | 2018-01-09 | Rani Therapeutics, Llc | Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US8846040B2 (en) | 2010-12-23 | 2014-09-30 | Rani Therapeutics, Llc | Therapeutic agent preparations comprising etanercept for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US9259386B2 (en) | 2010-12-23 | 2016-02-16 | Rani Therapeutics, Llc | Therapeutic preparation comprising somatostatin or somatostatin analogoue for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US8980822B2 (en) | 2010-12-23 | 2015-03-17 | Rani Therapeutics, Llc | Therapeutic agent preparations comprising pramlintide for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US8809271B2 (en) | 2010-12-23 | 2014-08-19 | Rani Therapeutics, Llc | Therapeutic agent preparations comprising liraglutide for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US9629799B2 (en) | 2010-12-23 | 2017-04-25 | Rani Therapeutics, Llc | Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US9284367B2 (en) | 2010-12-23 | 2016-03-15 | Rani Therapeutics, Llc | Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US8734429B2 (en) | 2010-12-23 | 2014-05-27 | Rani Therapeutics, Llc | Device, system and methods for the oral delivery of therapeutic compounds |
| US9283179B2 (en) | 2010-12-23 | 2016-03-15 | Rani Therapeutics, Llc | GnRH preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US10639272B2 (en) | 2010-12-23 | 2020-05-05 | Rani Therapeutics, Llc | Methods for delivering etanercept preparations into a lumen of the intestinal tract using a swallowable drug delivery device |
| US8809269B2 (en) | 2010-12-23 | 2014-08-19 | Rani Therapeutics, Llc | Therapeutic agent preparations comprising insulin for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US9402807B2 (en) | 2010-12-23 | 2016-08-02 | Rani Therapeutics, Llc | Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US8764733B2 (en) | 2010-12-23 | 2014-07-01 | Rani Therapeutics, Llc | Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US8969293B2 (en) | 2010-12-23 | 2015-03-03 | Rani Therapeutics, Llc | Therapeutic agent preparations comprising exenatide for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US9402806B2 (en) | 2010-12-23 | 2016-08-02 | Rani Therapeutics, Llc | Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US9415004B2 (en) | 2010-12-23 | 2016-08-16 | Rani Therapeutics, Llc | Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device |
| US8696616B2 (en) | 2010-12-29 | 2014-04-15 | Ethicon Endo-Surgery, Inc. | Obesity therapy and heart rate variability |
| CN108392263A (zh) | 2011-01-19 | 2018-08-14 | 弗拉克泰尔实验室公司 | 用于组织处理的装置与方法 |
| US8888732B2 (en) | 2011-03-11 | 2014-11-18 | Apollo Endosurgery, Inc. | Intraluminal sleeve with active agents |
| US12172017B2 (en) | 2011-05-09 | 2024-12-24 | Setpoint Medical Corporation | Vagus nerve stimulation to treat neurodegenerative disorders |
| EP2872070B1 (en) | 2011-09-09 | 2018-02-07 | Enopace Biomedical Ltd. | Wireless endovascular stent-based electrodes |
| CN102500057B (zh) * | 2011-09-28 | 2014-02-26 | 上海交通大学 | 多功能植入式胃肠电刺激系统 |
| US10548585B2 (en) | 2011-11-16 | 2020-02-04 | VentureMD Innovations, LLC | Soft tissue attachment |
| US10675014B2 (en) | 2011-11-16 | 2020-06-09 | Crossroads Extremity Systems, Llc | Knotless soft tissue attachment |
| US10470756B2 (en) | 2011-11-16 | 2019-11-12 | VentureMD Innovations, LLC | Suture anchor and method |
| US10136883B2 (en) | 2011-11-16 | 2018-11-27 | VentureMD Innovations, LLC | Method of anchoring a suture |
| US8876694B2 (en) | 2011-12-07 | 2014-11-04 | Apollo Endosurgery, Inc. | Tube connector with a guiding tip |
| JP2013123484A (ja) * | 2011-12-13 | 2013-06-24 | Olympus Corp | 神経刺激装置および神経刺激システム |
| US8961394B2 (en) | 2011-12-20 | 2015-02-24 | Apollo Endosurgery, Inc. | Self-sealing fluid joint for use with a gastric band |
| US8979887B2 (en) | 2012-02-24 | 2015-03-17 | Elwha Llc | Devices, systems, and methods to control stomach volume |
| CA2865567C (en) | 2012-02-27 | 2022-10-11 | Fractyl Laboratories, Inc. | Heat ablation systems, devices and methods for the treatment of tissue |
| US20150111918A1 (en) | 2012-03-08 | 2015-04-23 | Medtronic Ardian Luxembourg S.a.r.l | Immune system neuromodulation and associated systems and methods |
| WO2013134763A2 (en) | 2012-03-09 | 2013-09-12 | Enteromedics Inc. | Safety features for use in medical devices |
| US9572983B2 (en) | 2012-03-26 | 2017-02-21 | Setpoint Medical Corporation | Devices and methods for modulation of bone erosion |
| WO2013143603A1 (en) * | 2012-03-30 | 2013-10-03 | Ethicon Endo-Surgery, Inc. | Devices and methods for the treatment of metabolic disorders |
| WO2013143599A1 (en) * | 2012-03-30 | 2013-10-03 | Ethicon Endo-Surgery, Inc. | Devices and methods for the treatment of metabolic disorders. |
| WO2013143600A1 (en) * | 2012-03-30 | 2013-10-03 | Ethicon Endo-Surgery, Inc. | Devices and methods for the treatment of metabolic disorders. |
| WO2013143609A1 (en) * | 2012-03-30 | 2013-10-03 | Ethicon Endo-Surgery, Inc. | Devices and methods for the treatment of metabolic disorders |
| CA2869904C (en) | 2012-04-19 | 2020-04-21 | Fractyl Laboratories, Inc. | Systems for expanding tissue prior to energy ablation |
| US9681975B2 (en) | 2012-05-31 | 2017-06-20 | Valentx, Inc. | Devices and methods for gastrointestinal bypass |
| US9566181B2 (en) | 2012-05-31 | 2017-02-14 | Valentx, Inc. | Devices and methods for gastrointestinal bypass |
| US20130324906A1 (en) | 2012-05-31 | 2013-12-05 | Valen Tx, Inc. | Devices and methods for gastrointestinal bypass |
| US9456916B2 (en) | 2013-03-12 | 2016-10-04 | Medibotics Llc | Device for selectively reducing absorption of unhealthy food |
| EP2879605A4 (en) | 2012-07-30 | 2016-04-06 | Fractyl Lab Inc | ELECTRICITY CONTROL SYSTEMS, DEVICES AND METHOD FOR TREATMENT OF TISSUE |
| EP2882362B1 (en) | 2012-08-09 | 2024-01-03 | Fractyl Health, Inc. | Ablation systems, devices and methods for the treatment of tissue |
| EP2903626A4 (en) | 2012-10-05 | 2016-10-19 | Fractyl Lab Inc | METHODS, SYSTEMS AND DEVICES FOR CARRYING OUT MULTIPLE TREATMENTS OF A PATIENT |
| EP2934540B1 (en) | 2012-12-24 | 2023-08-02 | Neurogastrx, Inc. | Methods for treating gi tract disorders |
| CN109044528B (zh) | 2013-01-31 | 2021-11-02 | 迪格玛医疗有限公司 | 用于减少受试者的器官中的神经活性的方法和系统 |
| US9067070B2 (en) | 2013-03-12 | 2015-06-30 | Medibotics Llc | Dysgeusia-inducing neurostimulation for modifying consumption of a selected nutrient type |
| US9011365B2 (en) | 2013-03-12 | 2015-04-21 | Medibotics Llc | Adjustable gastrointestinal bifurcation (AGB) for reduced absorption of unhealthy food |
| US9168000B2 (en) | 2013-03-13 | 2015-10-27 | Ethicon Endo-Surgery, Inc. | Meal detection devices and methods |
| US9757264B2 (en) | 2013-03-13 | 2017-09-12 | Valentx, Inc. | Devices and methods for gastrointestinal bypass |
| BR112015023344B1 (pt) | 2013-03-15 | 2022-05-31 | Baronova, Inc | Dispositivo para intermitentemente obstruir uma abertura gástrica; e método de reconfiguração instalação de um dispositivo de oclusão |
| EP3003461B1 (en) | 2013-06-04 | 2019-05-01 | Fractyl Laboratories, Inc. | Systems and devices for reducing the luminal surface area of the gastrointestinal tract |
| US11986235B2 (en) | 2013-09-12 | 2024-05-21 | Fractyl Health, Inc. | Systems, methods and devices for treatment of target tissue |
| US10779965B2 (en) | 2013-11-06 | 2020-09-22 | Enopace Biomedical Ltd. | Posts with compliant junctions |
| JP6603660B2 (ja) | 2013-11-22 | 2019-11-06 | フラクティル ラボラトリーズ インコーポレイテッド | 消化管における治療制約の生成のためのシステム、デバイスおよび方法 |
| US20150188765A1 (en) * | 2013-12-31 | 2015-07-02 | Microsoft Corporation | Multimode gaming server |
| US9486623B2 (en) | 2014-03-05 | 2016-11-08 | Rainbow Medical Ltd. | Electrical stimulation of a pancreas |
| US10959774B2 (en) | 2014-03-24 | 2021-03-30 | Fractyl Laboratories, Inc. | Injectate delivery devices, systems and methods |
| US10537387B2 (en) | 2014-04-17 | 2020-01-21 | Digma Medical Ltd. | Methods and systems for blocking neural activity in an organ of a subject, preferably in the small intestine or the duodenum |
| US9844554B2 (en) | 2014-06-24 | 2017-12-19 | Neurogastrx, Inc. | Prodrugs of metopimazine |
| US9492396B2 (en) | 2014-07-15 | 2016-11-15 | Yossi Gross | Enhanced drug delivery pill |
| US11185367B2 (en) | 2014-07-16 | 2021-11-30 | Fractyl Health, Inc. | Methods and systems for treating diabetes and related diseases and disorders |
| WO2016011269A1 (en) | 2014-07-16 | 2016-01-21 | Fractyl Laboratories, Inc. | Methods and systems for treating diabetes and related diseases and disorders |
| US9844641B2 (en) | 2014-07-16 | 2017-12-19 | Fractyl Laboratories, Inc. | Systems, devices and methods for performing medical procedures in the intestine |
| US11311725B2 (en) | 2014-10-24 | 2022-04-26 | Setpoint Medical Corporation | Systems and methods for stimulating and/or monitoring loci in the brain to treat inflammation and to enhance vagus nerve stimulation |
| JP2017536187A (ja) | 2014-12-03 | 2017-12-07 | メタベンション インコーポレイテッド | 神経または他の組織を調節するためのシステムおよび方法 |
| WO2016126807A1 (en) | 2015-02-03 | 2016-08-11 | Setpoint Medical Corporation | Apparatus and method for reminding, prompting, or alerting a patient with an implanted stimulator |
| US20220062621A1 (en) | 2015-02-24 | 2022-03-03 | Elira, Inc. | Electrical Stimulation-Based Weight Management System |
| US10376145B2 (en) | 2015-02-24 | 2019-08-13 | Elira, Inc. | Systems and methods for enabling a patient to achieve a weight loss objective using an electrical dermal patch |
| US10765863B2 (en) | 2015-02-24 | 2020-09-08 | Elira, Inc. | Systems and methods for using a transcutaneous electrical stimulation device to deliver titrated therapy |
| US9956393B2 (en) | 2015-02-24 | 2018-05-01 | Elira, Inc. | Systems for increasing a delay in the gastric emptying time for a patient using a transcutaneous electro-dermal patch |
| US10335302B2 (en) | 2015-02-24 | 2019-07-02 | Elira, Inc. | Systems and methods for using transcutaneous electrical stimulation to enable dietary interventions |
| CN115227969A (zh) | 2015-02-24 | 2022-10-25 | 伊莱拉股份有限公司 | 使用电极皮肤贴实现食欲调节或改善饮食依从性的方法 |
| US10864367B2 (en) | 2015-02-24 | 2020-12-15 | Elira, Inc. | Methods for using an electrical dermal patch in a manner that reduces adverse patient reactions |
| US11337749B2 (en) * | 2015-10-07 | 2022-05-24 | Mayo Foundation For Medical Education And Research | Electroporation for obesity or diabetes treatment |
| US10596367B2 (en) | 2016-01-13 | 2020-03-24 | Setpoint Medical Corporation | Systems and methods for establishing a nerve block |
| US11471681B2 (en) | 2016-01-20 | 2022-10-18 | Setpoint Medical Corporation | Batteryless implantable microstimulators |
| CN108882885A (zh) | 2016-01-20 | 2018-11-23 | 赛博恩特医疗器械公司 | 迷走神经刺激的控制 |
| US10314501B2 (en) | 2016-01-20 | 2019-06-11 | Setpoint Medical Corporation | Implantable microstimulators and inductive charging systems |
| US10583304B2 (en) | 2016-01-25 | 2020-03-10 | Setpoint Medical Corporation | Implantable neurostimulator having power control and thermal regulation and methods of use |
| US10779980B2 (en) | 2016-04-27 | 2020-09-22 | Synerz Medical, Inc. | Intragastric device for treating obesity |
| EP3496800B1 (en) | 2016-08-14 | 2020-11-04 | Digma Medical Ltd. | Apparatus for nerve ablation in the wall of the gastointestinal tract |
| US10575904B1 (en) | 2016-08-14 | 2020-03-03 | Digma Medical Ltd. | Apparatus and method for selective submucosal ablation |
| WO2018136967A1 (en) | 2017-01-23 | 2018-07-26 | Baronova, Inc. | Gastric obstruction device deployment assembly and methods of delivering and deploying a gastric obstruction device |
| US10537454B2 (en) | 2017-06-16 | 2020-01-21 | Proximate Concepts Llc | Electrophysiologically active transducer intragastric balloon system and method |
| EP3668402B1 (en) | 2017-08-14 | 2024-07-31 | Setpoint Medical Corporation | Vagus nerve stimulation pre-screening test |
| US11420051B2 (en) | 2018-05-17 | 2022-08-23 | Imam Abdulrahman Bin Faisal University | Medical device for treating diabetes |
| US10675248B2 (en) | 2018-08-14 | 2020-06-09 | Alma Therapeutics Ltd. | Expandable pill |
| US11260229B2 (en) | 2018-09-25 | 2022-03-01 | The Feinstein Institutes For Medical Research | Methods and apparatuses for reducing bleeding via coordinated trigeminal and vagal nerve stimulation |
| US20200261730A1 (en) * | 2019-02-14 | 2020-08-20 | Morton M. Mower | Method and apparatus for electrical current therapy or biological tissue and insulin release therefrom |
| CN113613713B (zh) | 2019-03-06 | 2024-07-19 | 浙江迈达佩思医疗科技有限公司 | 将疗法递送至肠道肌肉的装置、系统和方法 |
| WO2020210786A1 (en) | 2019-04-12 | 2020-10-15 | Setpoint Medical Corporation | Vagus nerve stimulation to treat neurodegenerative disorders |
| US12017073B2 (en) | 2019-05-16 | 2024-06-25 | Enteromed Ltd | Devices, systems, and methods for delivering therapy to a sacral nerve |
| EP4048179A1 (en) | 2019-10-21 | 2022-08-31 | Endogenex, Inc. | Devices, systems, and methods for pulsed electric field treatment of the duodenum |
| WO2021146535A1 (en) | 2020-01-15 | 2021-07-22 | Fractyl Laboratories, Inc. | Automated tissue treatment devices, systems, and methods |
| US10836757B1 (en) | 2020-04-02 | 2020-11-17 | Neurogastrx, Inc. | Polymorphic forms of metopimazine |
| KR20230012585A (ko) | 2020-05-21 | 2023-01-26 | 더 파인스타인 인스티튜츠 포 메디칼 리서치 | 미주 신경 자극을 위한 시스템들 및 방법들 |
| US20220152195A1 (en) * | 2020-11-13 | 2022-05-19 | Leonhardt Ventures Llc | Methods of preventing and treating viral infections |
| WO2022245878A1 (en) | 2021-05-17 | 2022-11-24 | Setpoint Medical Corporation | Neurostimulation parameter authentication and expiration system for neurostimulation |
| US11400299B1 (en) | 2021-09-14 | 2022-08-02 | Rainbow Medical Ltd. | Flexible antenna for stimulator |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004112563A2 (en) * | 2003-06-20 | 2004-12-29 | Metacure N.V., | Gastrointestinal methods and apparatus for use in treating disorders |
| WO2005007232A2 (en) * | 2003-07-21 | 2005-01-27 | Metacure N.V. | Gastrointestinal methods and apparatus for use in treating disorders and controlling blood sugar |
Family Cites Families (66)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4416267A (en) | 1981-12-10 | 1983-11-22 | Garren Lloyd R | Method and apparatus for treating obesity |
| US4899747A (en) | 1981-12-10 | 1990-02-13 | Garren Lloyd R | Method and appartus for treating obesity |
| US4723547A (en) | 1985-05-07 | 1988-02-09 | C. R. Bard, Inc. | Anti-obesity balloon placement system |
| GB8517092D0 (en) | 1985-07-05 | 1985-08-14 | Taylor T V | Artificial bezoar |
| US4694827A (en) | 1986-01-14 | 1987-09-22 | Weiner Brian C | Inflatable gastric device for treating obesity and method of using the same |
| US4739758A (en) | 1986-05-19 | 1988-04-26 | Criticare Systems, Inc. | Apparatus for stomach cavity reduction |
| US5188104A (en) | 1991-02-01 | 1993-02-23 | Cyberonics, Inc. | Treatment of eating disorders by nerve stimulation |
| US5263480A (en) * | 1991-02-01 | 1993-11-23 | Cyberonics, Inc. | Treatment of eating disorders by nerve stimulation |
| US5234454A (en) | 1991-08-05 | 1993-08-10 | Akron City Hospital | Percutaneous intragastric balloon catheter and method for controlling body weight therewith |
| IT1260485B (it) | 1992-05-29 | 1996-04-09 | Procedimento e dispositivo per il trattamento dell'obesita' di un paziente | |
| US5541951A (en) | 1994-11-14 | 1996-07-30 | Intelligent Surgical Lasers, Inc. | Device and method for high-power end pumping |
| US6575969B1 (en) | 1995-05-04 | 2003-06-10 | Sherwood Services Ag | Cool-tip radiofrequency thermosurgery electrode system for tumor ablation |
| US5690691A (en) * | 1996-05-08 | 1997-11-25 | The Center For Innovative Technology | Gastro-intestinal pacemaker having phased multi-point stimulation |
| CA2264831C (en) * | 1996-09-05 | 2003-08-12 | The Governors Of The University Of Alberta | Gastro-intestinal electrical pacemaker |
| UA65549C2 (uk) | 1996-11-05 | 2004-04-15 | Елі Ліллі Енд Компані | Спосіб регулювання ожиріння шляхом периферійного введення аналогів та похідних glp-1 (варіанти) та фармацевтична композиція |
| US6404755B1 (en) | 1996-11-07 | 2002-06-11 | Harris Broadband Wireless Access, Inc. | Multi-level information mapping system and method |
| IT1290866B1 (it) | 1996-12-24 | 1998-12-14 | Francesco Garbagnati | Sonda-catetere per il trattamento di tumori di organi parenchimatosi con ipertermia interstiziale indotta da radiofrequenza |
| US5836994A (en) * | 1997-04-30 | 1998-11-17 | Medtronic, Inc. | Method and apparatus for electrical stimulation of the gastrointestinal tract |
| US5868141A (en) | 1997-05-14 | 1999-02-09 | Ellias; Yakub A. | Endoscopic stomach insert for treating obesity and method for use |
| US5987060A (en) | 1997-06-13 | 1999-11-16 | Innova Corporation | System and method of radio communications with an up-down digital signal link |
| US5919216A (en) * | 1997-06-16 | 1999-07-06 | Medtronic, Inc. | System and method for enhancement of glucose production by stimulation of pancreatic beta cells |
| US6093167A (en) | 1997-06-16 | 2000-07-25 | Medtronic, Inc. | System for pancreatic stimulation and glucose measurement |
| US7006871B1 (en) * | 1997-07-16 | 2006-02-28 | Metacure N.V. | Blood glucose level control |
| ATE353689T1 (de) | 1997-07-16 | 2007-03-15 | Metacure Nv | Einrichtung zur steuerung eines glatten muskels |
| US6091992A (en) | 1997-12-15 | 2000-07-18 | Medtronic, Inc. | Method and apparatus for electrical stimulation of the gastrointestinal tract |
| JP2000026434A (ja) * | 1998-06-05 | 2000-01-25 | Zeria Pharmaceut Co Ltd | 新規1,5−ベンゾジアゼピン誘導体 |
| CN1260653A (zh) | 1998-07-24 | 2000-07-19 | 休斯电子公司 | 多模式、多调制点对多点通信 |
| US8700161B2 (en) | 1999-03-05 | 2014-04-15 | Metacure Limited | Blood glucose level control |
| US20040249421A1 (en) | 2000-09-13 | 2004-12-09 | Impulse Dynamics Nv | Blood glucose level control |
| US8019421B2 (en) | 1999-03-05 | 2011-09-13 | Metacure Limited | Blood glucose level control |
| EP1159030B1 (en) | 1999-03-05 | 2007-06-13 | Impulse Dynamics N.V. | Blood glucose level control |
| JP3589086B2 (ja) | 1999-05-17 | 2004-11-17 | 松下電器産業株式会社 | 電源装置 |
| US6853862B1 (en) | 1999-12-03 | 2005-02-08 | Medtronic, Inc. | Gastroelectric stimulation for influencing pancreatic secretions |
| US6600953B2 (en) | 2000-12-11 | 2003-07-29 | Impulse Dynamics N.V. | Acute and chronic electrical signal therapy for obesity |
| MXPA00001922A (es) | 2000-02-24 | 2002-03-08 | De Hayos Garza Andres | Cateter de balon intragastrico percutaneo para tratamiento de la obesidad. |
| FR2805986B1 (fr) | 2000-03-13 | 2002-10-11 | Districlass Madical | Dispositif intra-gastrique a volume variable |
| US6826428B1 (en) | 2000-04-11 | 2004-11-30 | The Board Of Regents Of The University Of Texas System | Gastrointestinal electrical stimulation |
| US6658297B2 (en) | 2000-09-07 | 2003-12-02 | Alfred E. Mann Institute For Biomedical Engineering At The University Of Southern California | Method and apparatus for control of bowel function |
| US6895279B2 (en) | 2000-09-15 | 2005-05-17 | Alfred E. Mann Institute For Biomedical Engineering At The University Of Southern California | Method and apparatus to treat disorders of gastrointestinal peristalsis |
| US7016296B2 (en) | 2000-10-16 | 2006-03-21 | Broadcom Corporation | Adaptive modulation for fixed wireless link in cable transmission system |
| US6579301B1 (en) * | 2000-11-17 | 2003-06-17 | Syntheon, Llc | Intragastric balloon device adapted to be repeatedly varied in volume without external assistance |
| US6832114B1 (en) | 2000-11-21 | 2004-12-14 | Advanced Bionics Corporation | Systems and methods for modulation of pancreatic endocrine secretion and treatment of diabetes |
| US6684105B2 (en) | 2001-08-31 | 2004-01-27 | Biocontrol Medical, Ltd. | Treatment of disorders by unidirectional nerve stimulation |
| US6928320B2 (en) | 2001-05-17 | 2005-08-09 | Medtronic, Inc. | Apparatus for blocking activation of tissue or conduction of action potentials while other tissue is being therapeutically activated |
| RU2211712C2 (ru) | 2001-08-23 | 2003-09-10 | Надточий Александр Иванович | Адаптивный электростимулятор |
| US7047029B1 (en) | 2001-09-10 | 2006-05-16 | The Directv Group, Inc. | Adaptive transmission system |
| ES2279463T3 (es) | 2001-10-12 | 2007-08-16 | Nokia Corporation | Sistema de radiocomunicaciones de microondas de punto a punto. |
| AU2002343193A1 (en) | 2001-11-29 | 2003-06-10 | Impulse Dynamics Nv | Sensing of pancreatic electrical activity |
| FR2834202B1 (fr) | 2001-12-28 | 2004-03-19 | Cie Euro Etude Rech Paroscopie | Ballon intra-gastrique a poches multiples, dispositif chirurgical d'expansion dudit ballon et procede de fabrication correspondant |
| US6733512B2 (en) | 2002-03-07 | 2004-05-11 | Mcghan Jim J. | Self-deflating intragastric balloon |
| US7239912B2 (en) * | 2002-03-22 | 2007-07-03 | Leptos Biomedical, Inc. | Electric modulation of sympathetic nervous system |
| US7069078B2 (en) * | 2002-04-22 | 2006-06-27 | Medtronic, Inc. | Insulin-mediated glucose uptake monitor |
| AU2003263816A1 (en) * | 2002-07-26 | 2004-02-16 | Transneuronix, Inc. | Improved process for electrostimulation treatment of morbid obesity |
| AU2003298801B9 (en) | 2002-12-02 | 2008-07-31 | Gi Dynamics, Inc. | Bariatric sleeve |
| US20040172084A1 (en) * | 2003-02-03 | 2004-09-02 | Knudson Mark B. | Method and apparatus for treatment of gastro-esophageal reflux disease (GERD) |
| US7444183B2 (en) | 2003-02-03 | 2008-10-28 | Enteromedics, Inc. | Intraluminal electrode apparatus and method |
| JP4149838B2 (ja) * | 2003-03-04 | 2008-09-17 | オリンパス株式会社 | カプセル型医療装置 |
| FR2852821B1 (fr) | 2003-03-31 | 2007-06-01 | Cie Euro Etude Rech Paroscopie | Ballon intra-gastrique enduit de parylene, procede de fabrication d'un tel ballon et utilisation de parylene pour revetir un ballon intra-gastrique |
| US7427909B2 (en) | 2003-06-12 | 2008-09-23 | Nec Tokin Corporation | Coil component and fabrication method of the same |
| KR20030068070A (ko) | 2003-06-26 | 2003-08-19 | 이정환 | 비만치료를 위한 내시경적 풍선 삽입장치 및 삽입기술 |
| US20070060971A1 (en) * | 2003-07-21 | 2007-03-15 | Ofer Glasberg | Hepatic device for treatment or glucose detection |
| CA2588727C (en) | 2003-11-28 | 2014-01-14 | University Technologies International Inc. | Gastrointestinal motility control |
| US7194307B2 (en) * | 2003-12-22 | 2007-03-20 | Cardiac Pacemakers, Inc. | Pacing method and device for preserving native conduction system |
| EP1735047A4 (en) | 2004-03-18 | 2011-02-09 | Metacure Nv | GASTROINTESTINAL METHODS AND APPARATUSES FOR USE IN THE TREATMENT OF DISORDERS AND GLYCEMIA CONTROL |
| WO2006018851A2 (en) | 2004-08-18 | 2006-02-23 | Metacure Ltd. | Monitoring, analysis, and regulation of eating habits |
| US20070016262A1 (en) | 2005-07-13 | 2007-01-18 | Betastim, Ltd. | Gi and pancreatic device for treating obesity and diabetes |
-
2006
- 2006-04-11 US US11/279,355 patent/US20070016262A1/en not_active Abandoned
- 2006-07-13 EP EP06766139A patent/EP1907046A4/en not_active Ceased
- 2006-07-13 US US11/995,663 patent/US8095218B2/en not_active Expired - Fee Related
- 2006-07-13 WO PCT/IL2006/000819 patent/WO2007007339A2/en not_active Ceased
- 2006-07-13 JP JP2008521036A patent/JP2009501046A/ja active Pending
-
2011
- 2011-12-15 US US13/326,631 patent/US20120083855A1/en not_active Abandoned
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004112563A2 (en) * | 2003-06-20 | 2004-12-29 | Metacure N.V., | Gastrointestinal methods and apparatus for use in treating disorders |
| WO2005007232A2 (en) * | 2003-07-21 | 2005-01-27 | Metacure N.V. | Gastrointestinal methods and apparatus for use in treating disorders and controlling blood sugar |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021519632A (ja) * | 2018-03-29 | 2021-08-12 | ネヴロ コーポレイション | 2型糖尿病を含む血糖異常を治療する、および/またはHbA1cレベルを低下させるための治療的調節、ならびに関連するシステムおよび方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| US8095218B2 (en) | 2012-01-10 |
| US20090062881A1 (en) | 2009-03-05 |
| EP1907046A4 (en) | 2009-10-21 |
| WO2007007339A2 (en) | 2007-01-18 |
| WO2007007339A3 (en) | 2009-04-30 |
| EP1907046A2 (en) | 2008-04-09 |
| US20070016262A1 (en) | 2007-01-18 |
| US20120083855A1 (en) | 2012-04-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8095218B2 (en) | GI and pancreatic device for treating obesity and diabetes | |
| US20240157126A1 (en) | Modular Stimulation System for the Treatment of Gastrointestinal Disorders | |
| US9821158B2 (en) | Non-immediate effects of therapy | |
| US9101765B2 (en) | Non-immediate effects of therapy | |
| US9037244B2 (en) | Method and apparatus for electrical stimulation of the pancreatico-biliary system | |
| US7738961B2 (en) | Method and apparatus for treatment of the gastrointestinal tract | |
| US6993391B2 (en) | Acute and chronic electrical signal therapy for obesity | |
| EP1874402B1 (en) | Weight loss device | |
| US8543210B2 (en) | Device and implantation system for electrical stimulation of biological systems | |
| CN1856338B (zh) | 用于治疗疾病和控制血糖的胃肠方法和装置 | |
| US20120277619A1 (en) | Detecting food intake based on impedance | |
| CN1838978B (zh) | 用于治疗疾病的胃肠方法和装置 | |
| US20070255336A1 (en) | Gastric constriction device with selectable electrode combinations | |
| US20080086180A1 (en) | Techniques for gall bladder stimulation | |
| US20090234417A1 (en) | Methods And Apparatus For The Treatment Of Metabolic Disorders | |
| US20080183238A1 (en) | Process for electrostimulation treatment of morbid obesity | |
| US20070255335A1 (en) | Controller for gastric constriction device with selectable electrode configurations | |
| JP2008509794A (ja) | 疾患治療の動的な神経刺激 | |
| WO2009114008A1 (en) | Method and apparatus for treatment of the gastrointestinal tract | |
| EP1868679B1 (en) | Non-immediate effects of therapy | |
| WO2005087310A2 (en) | Gastrointestinal methods and apparatus for use in treating disorders and controlling blood sugar | |
| US20110190843A1 (en) | Appendicular and rectal stimulator device for digestive and eating disorders | |
| CN101516441A (zh) | 用于治疗肥胖症和糖尿病的胃肠和胰腺装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090713 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20090713 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090728 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20111115 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20120703 |