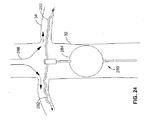
JP2006508776A - 腎臓内カテーテルを介する選択的物質送達のための方法および装置 - Google Patents
腎臓内カテーテルを介する選択的物質送達のための方法および装置 Download PDFInfo
- Publication number
- JP2006508776A JP2006508776A JP2005501060A JP2005501060A JP2006508776A JP 2006508776 A JP2006508776 A JP 2006508776A JP 2005501060 A JP2005501060 A JP 2005501060A JP 2005501060 A JP2005501060 A JP 2005501060A JP 2006508776 A JP2006508776 A JP 2006508776A
- Authority
- JP
- Japan
- Prior art keywords
- renal
- delivery
- distal
- kidney
- proximal
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims description 129
- 239000000126 substance Substances 0.000 title claims description 61
- 210000002254 renal artery Anatomy 0.000 claims abstract description 310
- 210000003734 kidney Anatomy 0.000 claims abstract description 243
- 239000012530 fluid Substances 0.000 claims abstract description 126
- 210000000702 aorta abdominal Anatomy 0.000 claims abstract description 106
- 210000002796 renal vein Anatomy 0.000 claims abstract description 65
- 230000002146 bilateral effect Effects 0.000 claims abstract description 51
- 239000003795 chemical substances by application Substances 0.000 claims abstract description 45
- 238000011282 treatment Methods 0.000 claims abstract description 36
- 239000000463 material Substances 0.000 claims abstract description 24
- 230000003187 abdominal effect Effects 0.000 claims abstract description 23
- 238000002347 injection Methods 0.000 claims description 69
- 239000007924 injection Substances 0.000 claims description 69
- 210000000709 aorta Anatomy 0.000 claims description 63
- 238000001802 infusion Methods 0.000 claims description 52
- 239000000523 sample Substances 0.000 claims description 45
- 230000017531 blood circulation Effects 0.000 claims description 43
- 230000002792 vascular Effects 0.000 claims description 37
- 210000004204 blood vessel Anatomy 0.000 claims description 36
- 210000004369 blood Anatomy 0.000 claims description 25
- 239000008280 blood Substances 0.000 claims description 25
- 239000003550 marker Substances 0.000 claims description 21
- 229940124549 vasodilator Drugs 0.000 claims description 20
- 239000003071 vasodilator agent Substances 0.000 claims description 20
- 210000005227 renal system Anatomy 0.000 claims description 18
- 238000003780 insertion Methods 0.000 claims description 17
- 230000037431 insertion Effects 0.000 claims description 17
- 230000033001 locomotion Effects 0.000 claims description 15
- 230000003287 optical effect Effects 0.000 claims description 15
- 230000000694 effects Effects 0.000 claims description 12
- 238000002594 fluoroscopy Methods 0.000 claims description 11
- XQYZDYMELSJDRZ-UHFFFAOYSA-N papaverine Chemical compound C1=C(OC)C(OC)=CC=C1CC1=NC=CC2=CC(OC)=C(OC)C=C12 XQYZDYMELSJDRZ-UHFFFAOYSA-N 0.000 claims description 11
- VYFYYTLLBUKUHU-UHFFFAOYSA-N dopamine Chemical compound NCCC1=CC=C(O)C(O)=C1 VYFYYTLLBUKUHU-UHFFFAOYSA-N 0.000 claims description 10
- 238000012544 monitoring process Methods 0.000 claims description 10
- 238000004891 communication Methods 0.000 claims description 8
- 238000003384 imaging method Methods 0.000 claims description 8
- 239000002131 composite material Substances 0.000 claims description 7
- 230000036961 partial effect Effects 0.000 claims description 7
- 238000004873 anchoring Methods 0.000 claims description 6
- 238000000429 assembly Methods 0.000 claims description 6
- 230000000712 assembly Effects 0.000 claims description 6
- 238000011084 recovery Methods 0.000 claims description 6
- 238000002560 therapeutic procedure Methods 0.000 claims description 6
- 229930008281 A03AD01 - Papaverine Natural products 0.000 claims description 5
- 239000002934 diuretic Substances 0.000 claims description 5
- 229960003638 dopamine Drugs 0.000 claims description 5
- 229960001789 papaverine Drugs 0.000 claims description 5
- 238000002604 ultrasonography Methods 0.000 claims description 5
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 claims description 4
- 230000001882 diuretic effect Effects 0.000 claims description 4
- 230000007704 transition Effects 0.000 claims description 4
- 229940127291 Calcium channel antagonist Drugs 0.000 claims description 3
- 239000000480 calcium channel blocker Substances 0.000 claims description 3
- ZZUFCTLCJUWOSV-UHFFFAOYSA-N furosemide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC(C(O)=O)=C1NCC1=CC=CO1 ZZUFCTLCJUWOSV-UHFFFAOYSA-N 0.000 claims description 3
- 229960003883 furosemide Drugs 0.000 claims description 3
- 239000003223 protective agent Substances 0.000 claims description 3
- 239000007787 solid Substances 0.000 claims description 3
- 239000005526 vasoconstrictor agent Substances 0.000 claims description 3
- 230000008320 venous blood flow Effects 0.000 claims description 3
- JBMKAUGHUNFTOL-UHFFFAOYSA-N Aldoclor Chemical class C1=C(Cl)C(S(=O)(=O)N)=CC2=C1NC=NS2(=O)=O JBMKAUGHUNFTOL-UHFFFAOYSA-N 0.000 claims description 2
- 206010067171 Regurgitation Diseases 0.000 claims description 2
- 239000000556 agonist Substances 0.000 claims description 2
- 229960001597 nifedipine Drugs 0.000 claims description 2
- HYIMSNHJOBLJNT-UHFFFAOYSA-N nifedipine Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OC)C1C1=CC=CC=C1[N+]([O-])=O HYIMSNHJOBLJNT-UHFFFAOYSA-N 0.000 claims description 2
- 230000000452 restraining effect Effects 0.000 claims description 2
- 230000000717 retained effect Effects 0.000 claims description 2
- 239000003451 thiazide diuretic agent Substances 0.000 claims description 2
- 239000002550 vasoactive agent Substances 0.000 claims description 2
- 230000008602 contraction Effects 0.000 claims 1
- 238000012285 ultrasound imaging Methods 0.000 claims 1
- 239000003814 drug Substances 0.000 abstract description 63
- 229940079593 drug Drugs 0.000 abstract description 56
- 210000001367 artery Anatomy 0.000 description 63
- 230000003014 reinforcing effect Effects 0.000 description 22
- 230000009286 beneficial effect Effects 0.000 description 21
- 230000008901 benefit Effects 0.000 description 17
- 208000033626 Renal failure acute Diseases 0.000 description 14
- 201000011040 acute kidney failure Diseases 0.000 description 14
- 239000000975 dye Substances 0.000 description 14
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 12
- 229920000642 polymer Polymers 0.000 description 12
- 239000011780 sodium chloride Substances 0.000 description 12
- 206010007559 Cardiac failure congestive Diseases 0.000 description 11
- 238000013459 approach Methods 0.000 description 11
- 201000003068 rheumatic fever Diseases 0.000 description 11
- 238000011144 upstream manufacturing Methods 0.000 description 11
- 230000006378 damage Effects 0.000 description 10
- 230000009885 systemic effect Effects 0.000 description 10
- 238000004519 manufacturing process Methods 0.000 description 9
- 238000003745 diagnosis Methods 0.000 description 7
- 238000012377 drug delivery Methods 0.000 description 7
- 230000023597 hemostasis Effects 0.000 description 7
- 230000002787 reinforcement Effects 0.000 description 7
- 229940124597 therapeutic agent Drugs 0.000 description 7
- 229920002614 Polyether block amide Polymers 0.000 description 6
- 238000001647 drug administration Methods 0.000 description 6
- 210000001105 femoral artery Anatomy 0.000 description 6
- 210000000056 organ Anatomy 0.000 description 6
- 230000010412 perfusion Effects 0.000 description 6
- 238000011200 topical administration Methods 0.000 description 6
- 230000000699 topical effect Effects 0.000 description 6
- 230000036772 blood pressure Effects 0.000 description 5
- 208000026817 47,XYY syndrome Diseases 0.000 description 4
- 238000002583 angiography Methods 0.000 description 4
- 230000000747 cardiac effect Effects 0.000 description 4
- 210000004351 coronary vessel Anatomy 0.000 description 4
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 4
- 239000000032 diagnostic agent Substances 0.000 description 4
- 229940039227 diagnostic agent Drugs 0.000 description 4
- 229960002724 fenoldopam Drugs 0.000 description 4
- TVURRHSHRRELCG-UHFFFAOYSA-N fenoldopam Chemical compound C1=CC(O)=CC=C1C1C2=CC(O)=C(O)C(Cl)=C2CCNC1 TVURRHSHRRELCG-UHFFFAOYSA-N 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 230000003907 kidney function Effects 0.000 description 4
- 230000000670 limiting effect Effects 0.000 description 4
- 230000007246 mechanism Effects 0.000 description 4
- 230000002441 reversible effect Effects 0.000 description 4
- 238000007910 systemic administration Methods 0.000 description 4
- 230000024883 vasodilation Effects 0.000 description 4
- 208000009304 Acute Kidney Injury Diseases 0.000 description 3
- 206010019280 Heart failures Diseases 0.000 description 3
- 208000007536 Thrombosis Diseases 0.000 description 3
- 208000027418 Wounds and injury Diseases 0.000 description 3
- 208000012998 acute renal failure Diseases 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 230000004087 circulation Effects 0.000 description 3
- 239000002872 contrast media Substances 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 230000001965 increasing effect Effects 0.000 description 3
- 208000014674 injury Diseases 0.000 description 3
- 208000017169 kidney disease Diseases 0.000 description 3
- 229910001000 nickel titanium Inorganic materials 0.000 description 3
- 230000002265 prevention Effects 0.000 description 3
- 238000011477 surgical intervention Methods 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 241000408529 Libra Species 0.000 description 2
- 208000031481 Pathologic Constriction Diseases 0.000 description 2
- 206010047139 Vasoconstriction Diseases 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 210000003484 anatomy Anatomy 0.000 description 2
- 238000002399 angioplasty Methods 0.000 description 2
- 230000001010 compromised effect Effects 0.000 description 2
- 238000012790 confirmation Methods 0.000 description 2
- 239000000994 contrast dye Substances 0.000 description 2
- 229940109239 creatinine Drugs 0.000 description 2
- 230000006866 deterioration Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 238000002405 diagnostic procedure Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 238000002224 dissection Methods 0.000 description 2
- 230000009977 dual effect Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 210000003191 femoral vein Anatomy 0.000 description 2
- 238000001631 haemodialysis Methods 0.000 description 2
- 230000000322 hemodialysis Effects 0.000 description 2
- 230000002452 interceptive effect Effects 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 230000004807 localization Effects 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 238000002601 radiography Methods 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 230000036262 stenosis Effects 0.000 description 2
- 208000037804 stenosis Diseases 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- ZFXYFBGIUFBOJW-UHFFFAOYSA-N theophylline Chemical compound O=C1N(C)C(=O)N(C)C2=C1NC=N2 ZFXYFBGIUFBOJW-UHFFFAOYSA-N 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 238000004448 titration Methods 0.000 description 2
- 230000025033 vasoconstriction Effects 0.000 description 2
- 230000002861 ventricular Effects 0.000 description 2
- 230000000007 visual effect Effects 0.000 description 2
- 238000012800 visualization Methods 0.000 description 2
- SGTNSNPWRIOYBX-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-{[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino}-2-(propan-2-yl)pentanenitrile Chemical compound C1=C(OC)C(OC)=CC=C1CCN(C)CCCC(C#N)(C(C)C)C1=CC=C(OC)C(OC)=C1 SGTNSNPWRIOYBX-UHFFFAOYSA-N 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- 208000037260 Atherosclerotic Plaque Diseases 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- 208000006017 Cardiac Tamponade Diseases 0.000 description 1
- 108091006146 Channels Proteins 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 206010016803 Fluid overload Diseases 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- PWKSKIMOESPYIA-BYPYZUCNSA-N L-N-acetyl-Cysteine Chemical compound CC(=O)N[C@@H](CS)C(O)=O PWKSKIMOESPYIA-BYPYZUCNSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 208000010378 Pulmonary Embolism Diseases 0.000 description 1
- 206010037423 Pulmonary oedema Diseases 0.000 description 1
- 206010061481 Renal injury Diseases 0.000 description 1
- 206010040047 Sepsis Diseases 0.000 description 1
- 206010040070 Septic Shock Diseases 0.000 description 1
- 102000003990 Urokinase-type plasminogen activator Human genes 0.000 description 1
- 108090000435 Urokinase-type plasminogen activator Proteins 0.000 description 1
- 208000024248 Vascular System injury Diseases 0.000 description 1
- 208000012339 Vascular injury Diseases 0.000 description 1
- HZEWFHLRYVTOIW-UHFFFAOYSA-N [Ti].[Ni] Chemical compound [Ti].[Ni] HZEWFHLRYVTOIW-UHFFFAOYSA-N 0.000 description 1
- 208000002223 abdominal aortic aneurysm Diseases 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- OIPILFWXSMYKGL-UHFFFAOYSA-N acetylcholine Chemical compound CC(=O)OCC[N+](C)(C)C OIPILFWXSMYKGL-UHFFFAOYSA-N 0.000 description 1
- 229960004373 acetylcholine Drugs 0.000 description 1
- -1 acetylcystein Chemical compound 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 239000000464 adrenergic agent Substances 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 230000033115 angiogenesis Effects 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 239000002220 antihypertensive agent Substances 0.000 description 1
- 229940127088 antihypertensive drug Drugs 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 208000007474 aortic aneurysm Diseases 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 230000008081 blood perfusion Effects 0.000 description 1
- 210000000746 body region Anatomy 0.000 description 1
- 210000002302 brachial artery Anatomy 0.000 description 1
- 210000001715 carotid artery Anatomy 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 238000005253 cladding Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000002586 coronary angiography Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 230000003292 diminished effect Effects 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 229940030606 diuretics Drugs 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 210000003414 extremity Anatomy 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 239000003527 fibrinolytic agent Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000002637 fluid replacement therapy Methods 0.000 description 1
- 238000011010 flushing procedure Methods 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 210000004013 groin Anatomy 0.000 description 1
- 230000009931 harmful effect Effects 0.000 description 1
- 230000000004 hemodynamic effect Effects 0.000 description 1
- 230000002439 hemostatic effect Effects 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 230000009610 hypersensitivity Effects 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000009545 invasion Effects 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- 230000000302 ischemic effect Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 210000003141 lower extremity Anatomy 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 238000010297 mechanical methods and process Methods 0.000 description 1
- 210000001363 mesenteric artery superior Anatomy 0.000 description 1
- 230000037125 natural defense Effects 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 1
- 210000004789 organ system Anatomy 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 230000035479 physiological effects, processes and functions Effects 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 208000005333 pulmonary edema Diseases 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 230000008327 renal blood flow Effects 0.000 description 1
- 230000008085 renal dysfunction Effects 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000036303 septic shock Effects 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 230000001839 systemic circulation Effects 0.000 description 1
- 229960000278 theophylline Drugs 0.000 description 1
- 230000002885 thrombogenetic effect Effects 0.000 description 1
- 239000012780 transparent material Substances 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 229960005356 urokinase Drugs 0.000 description 1
- 230000003966 vascular damage Effects 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 229960001722 verapamil Drugs 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/02—Holding devices, e.g. on the body
- A61M25/04—Holding devices, e.g. on the body in the body, e.g. expansible
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0043—Catheters; Hollow probes characterised by structural features
- A61M25/0054—Catheters; Hollow probes characterised by structural features with regions for increasing flexibility
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M29/00—Dilators with or without means for introducing media, e.g. remedies
- A61M29/02—Dilators made of swellable material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M2025/0008—Catheters; Hollow probes having visible markings on its surface, i.e. visible to the naked eye, for any purpose, e.g. insertion depth markers, rotational markers or identification of type
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0021—Catheters; Hollow probes characterised by the form of the tubing
- A61M25/0023—Catheters; Hollow probes characterised by the form of the tubing by the form of the lumen, e.g. cross-section, variable diameter
- A61M25/0026—Multi-lumen catheters with stationary elements
- A61M2025/0034—Multi-lumen catheters with stationary elements characterized by elements which are assembled, connected or fused, e.g. splittable tubes, outer sheaths creating lumina or separate cores
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0021—Catheters; Hollow probes characterised by the form of the tubing
- A61M25/0023—Catheters; Hollow probes characterised by the form of the tubing by the form of the lumen, e.g. cross-section, variable diameter
- A61M25/0026—Multi-lumen catheters with stationary elements
- A61M2025/0037—Multi-lumen catheters with stationary elements characterized by lumina being arranged side-by-side
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/0105—Steering means as part of the catheter or advancing means; Markers for positioning
- A61M25/0133—Tip steering devices
- A61M2025/0161—Tip steering devices wherein the distal tips have two or more deflection regions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M2025/0188—Introducing, guiding, advancing, emplacing or holding catheters having slitted or breakaway lumens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1002—Balloon catheters characterised by balloon shape
- A61M2025/1004—Balloons with folds, e.g. folded or multifolded
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M2025/1043—Balloon catheters with special features or adapted for special applications
- A61M2025/1047—Balloon catheters with special features or adapted for special applications having centering means, e.g. balloons having an appropriate shape
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M2025/1043—Balloon catheters with special features or adapted for special applications
- A61M2025/1052—Balloon catheters with special features or adapted for special applications for temporarily occluding a vessel for isolating a sector
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0021—Catheters; Hollow probes characterised by the form of the tubing
- A61M25/0023—Catheters; Hollow probes characterised by the form of the tubing by the form of the lumen, e.g. cross-section, variable diameter
- A61M25/0026—Multi-lumen catheters with stationary elements
- A61M25/0029—Multi-lumen catheters with stationary elements characterized by features relating to least one lumen located at the middle part of the catheter, e.g. slots, flaps, valves, cuffs, apertures, notches, grooves or rapid exchange ports
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/0105—Steering means as part of the catheter or advancing means; Markers for positioning
- A61M25/0133—Tip steering devices
- A61M25/0147—Tip steering devices with movable mechanical means, e.g. pull wires
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/0105—Steering means as part of the catheter or advancing means; Markers for positioning
- A61M25/0133—Tip steering devices
- A61M25/0152—Tip steering devices with pre-shaped mechanisms, e.g. pre-shaped stylets or pre-shaped outer tubes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M39/02—Access sites
- A61M39/06—Haemostasis valves, i.e. gaskets sealing around a needle, catheter or the like, closing on removal thereof
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Hematology (AREA)
- Animal Behavior & Ethology (AREA)
- Engineering & Computer Science (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Pulmonology (AREA)
- Biophysics (AREA)
- Vascular Medicine (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- External Artificial Organs (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US41247602P | 2002-09-20 | 2002-09-20 | |
| US41234302P | 2002-09-20 | 2002-09-20 | |
| US47634703P | 2003-06-05 | 2003-06-05 | |
| US50260003P | 2003-09-13 | 2003-09-13 | |
| PCT/US2003/029744 WO2004032791A2 (en) | 2002-09-20 | 2003-09-22 | Method and apparatus for selective material delivery via an intra-renal catheter |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2006508776A true JP2006508776A (ja) | 2006-03-16 |
| JP2006508776A5 JP2006508776A5 (enExample) | 2007-01-25 |
Family
ID=32097072
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2005501060A Pending JP2006508776A (ja) | 2002-09-20 | 2003-09-22 | 腎臓内カテーテルを介する選択的物質送達のための方法および装置 |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US7914503B2 (enExample) |
| EP (1) | EP1539291A4 (enExample) |
| JP (1) | JP2006508776A (enExample) |
| AU (1) | AU2003276903A1 (enExample) |
| WO (1) | WO2004032791A2 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007521233A (ja) * | 2003-08-05 | 2007-08-02 | フロウメディカ, インコーポレイテッド | 放射線造影剤誘発性腎症の予防のためのシステムおよび方法 |
| JP2014508563A (ja) * | 2011-01-11 | 2014-04-10 | サイメティス エスアー | 経カテーテル大動脈弁埋込に有用な方法および装置 |
| JP2018064786A (ja) * | 2016-10-20 | 2018-04-26 | 株式会社八光 | 分岐管 |
| JP2020142108A (ja) * | 2013-06-04 | 2020-09-10 | プリスティーン アクセス テクノロジーズ エルティーディーPristine Access Technologies Ltd | デュアルチップ血液透析カテーテル |
Families Citing this family (162)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6749598B1 (en) * | 1999-01-11 | 2004-06-15 | Flowmedica, Inc. | Apparatus and methods for treating congestive heart disease |
| US7147661B2 (en) | 2001-12-20 | 2006-12-12 | Boston Scientific Santa Rosa Corp. | Radially expandable stent |
| US20160193078A1 (en) * | 2002-01-16 | 2016-07-07 | Zoll Circulation, Inc. | Apparatus and Method Of Gastric Cooling Using Balloon Catheter |
| US7620451B2 (en) | 2005-12-29 | 2009-11-17 | Ardian, Inc. | Methods and apparatus for pulsed electric field neuromodulation via an intra-to-extravascular approach |
| US8145317B2 (en) | 2002-04-08 | 2012-03-27 | Ardian, Inc. | Methods for renal neuromodulation |
| US20070129761A1 (en) | 2002-04-08 | 2007-06-07 | Ardian, Inc. | Methods for treating heart arrhythmia |
| US8131371B2 (en) | 2002-04-08 | 2012-03-06 | Ardian, Inc. | Methods and apparatus for monopolar renal neuromodulation |
| US20140018880A1 (en) | 2002-04-08 | 2014-01-16 | Medtronic Ardian Luxembourg S.A.R.L. | Methods for monopolar renal neuromodulation |
| US6978174B2 (en) * | 2002-04-08 | 2005-12-20 | Ardian, Inc. | Methods and devices for renal nerve blocking |
| US8347891B2 (en) | 2002-04-08 | 2013-01-08 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for performing a non-continuous circumferential treatment of a body lumen |
| US9308043B2 (en) | 2002-04-08 | 2016-04-12 | Medtronic Ardian Luxembourg S.A.R.L. | Methods for monopolar renal neuromodulation |
| US20080213331A1 (en) | 2002-04-08 | 2008-09-04 | Ardian, Inc. | Methods and devices for renal nerve blocking |
| US7653438B2 (en) | 2002-04-08 | 2010-01-26 | Ardian, Inc. | Methods and apparatus for renal neuromodulation |
| US7617005B2 (en) | 2002-04-08 | 2009-11-10 | Ardian, Inc. | Methods and apparatus for thermally-induced renal neuromodulation |
| US7162303B2 (en) * | 2002-04-08 | 2007-01-09 | Ardian, Inc. | Renal nerve stimulation method and apparatus for treatment of patients |
| US8774922B2 (en) | 2002-04-08 | 2014-07-08 | Medtronic Ardian Luxembourg S.A.R.L. | Catheter apparatuses having expandable balloons for renal neuromodulation and associated systems and methods |
| US20070135875A1 (en) | 2002-04-08 | 2007-06-14 | Ardian, Inc. | Methods and apparatus for thermally-induced renal neuromodulation |
| US8150519B2 (en) | 2002-04-08 | 2012-04-03 | Ardian, Inc. | Methods and apparatus for bilateral renal neuromodulation |
| US9636174B2 (en) | 2002-04-08 | 2017-05-02 | Medtronic Ardian Luxembourg S.A.R.L. | Methods for therapeutic renal neuromodulation |
| US7853333B2 (en) | 2002-04-08 | 2010-12-14 | Ardian, Inc. | Methods and apparatus for multi-vessel renal neuromodulation |
| US9308044B2 (en) | 2002-04-08 | 2016-04-12 | Medtronic Ardian Luxembourg S.A.R.L. | Methods for therapeutic renal neuromodulation |
| US7756583B2 (en) | 2002-04-08 | 2010-07-13 | Ardian, Inc. | Methods and apparatus for intravascularly-induced neuromodulation |
| US8145316B2 (en) | 2002-04-08 | 2012-03-27 | Ardian, Inc. | Methods and apparatus for renal neuromodulation |
| US20110207758A1 (en) * | 2003-04-08 | 2011-08-25 | Medtronic Vascular, Inc. | Methods for Therapeutic Renal Denervation |
| US20060206150A1 (en) * | 2002-04-08 | 2006-09-14 | Ardian, Inc. | Methods and apparatus for treating acute myocardial infarction |
| US8774913B2 (en) | 2002-04-08 | 2014-07-08 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for intravasculary-induced neuromodulation |
| US7993325B2 (en) | 2002-09-20 | 2011-08-09 | Angio Dynamics, Inc. | Renal infusion systems and methods |
| WO2004030718A2 (en) | 2002-09-20 | 2004-04-15 | Flowmedica, Inc. | Method and apparatus for intra aortic substance delivery to a branch vessel |
| US9956377B2 (en) * | 2002-09-20 | 2018-05-01 | Angiodynamics, Inc. | Method and apparatus for intra-aortic substance delivery to a branch vessel |
| AU2003276903A1 (en) * | 2002-09-20 | 2004-05-04 | Flowmedica, Inc. | Method and apparatus for selective material delivery via an intra-renal catheter |
| DE10261575A1 (de) * | 2002-12-23 | 2004-07-08 | Nova Lung Gmbh | Vorrichtung zur Kanülierung eines Blut führenden Gefäßes und deren Verwendung zur Kanülierung von Blut führenden Gefäßen |
| US20050043585A1 (en) * | 2003-01-03 | 2005-02-24 | Arindam Datta | Reticulated elastomeric matrices, their manufacture and use in implantable devices |
| CA2525792C (en) | 2003-05-15 | 2015-10-13 | Biomerix Corporation | Reticulated elastomeric matrices, their manufacture and use in implantable devices |
| JP2006526464A (ja) * | 2003-06-05 | 2006-11-24 | フローメディカ,インコーポレイテッド | 分枝した身体管腔において両側介入または診断を行うためのシステムおよび方法 |
| EP2260776A1 (en) | 2003-11-21 | 2010-12-15 | Vnus Medical Technologies, Inc. | Apparatus for treating the carotid artery |
| US7763077B2 (en) | 2003-12-24 | 2010-07-27 | Biomerix Corporation | Repair of spinal annular defects and annulo-nucleoplasty regeneration |
| WO2005091910A2 (en) * | 2004-03-04 | 2005-10-06 | Flowmedica, Inc. | Sheath for use in peripheral interventions |
| US20050267520A1 (en) * | 2004-05-12 | 2005-12-01 | Modesitt D B | Access and closure device and method |
| WO2005112980A2 (en) * | 2004-05-14 | 2005-12-01 | Flowmedica, Inc. | Bi-lateral local renal delivery for treating congestive heart failure and for bnp therapy |
| US20070190108A1 (en) * | 2004-05-17 | 2007-08-16 | Arindam Datta | High performance reticulated elastomeric matrix preparation, properties, reinforcement, and use in surgical devices, tissue augmentation and/or tissue repair |
| US7678133B2 (en) * | 2004-07-10 | 2010-03-16 | Arstasis, Inc. | Biological tissue closure device and method |
| US20060069323A1 (en) * | 2004-09-24 | 2006-03-30 | Flowmedica, Inc. | Systems and methods for bi-lateral guidewire cannulation of branched body lumens |
| US20070083239A1 (en) * | 2005-09-23 | 2007-04-12 | Denise Demarais | Methods and apparatus for inducing, monitoring and controlling renal neuromodulation |
| US7937143B2 (en) * | 2004-11-02 | 2011-05-03 | Ardian, Inc. | Methods and apparatus for inducing controlled renal neuromodulation |
| US8409191B2 (en) | 2004-11-04 | 2013-04-02 | Boston Scientific Scimed, Inc. | Preshaped ablation catheter for ablating pulmonary vein ostia within the heart |
| US20060116714A1 (en) * | 2004-11-26 | 2006-06-01 | Ivan Sepetka | Coupling and release devices and methods for their assembly and use |
| US7891085B1 (en) | 2005-01-11 | 2011-02-22 | Boston Scientific Neuromodulation Corporation | Electrode array assembly and method of making same |
| JP5188389B2 (ja) | 2005-05-05 | 2013-04-24 | ボストン サイエンティフィック リミテッド | 肺静脈口を画像として再構築する予成形した位置確認カテーテル及びシステム |
| CN101217916B (zh) * | 2005-05-12 | 2013-04-10 | 阿尔斯塔西斯公司 | 穿刺和闭合装置以及方法 |
| US8608789B2 (en) * | 2005-05-24 | 2013-12-17 | Trireme Medical, Inc. | Delivery system for bifurcation stents |
| US7894906B2 (en) | 2006-06-06 | 2011-02-22 | Cardiac Pacemakers, Inc. | Amelioration of chronic pain by endolymphatic stimulation |
| US8126538B2 (en) | 2006-06-06 | 2012-02-28 | Cardiac Pacemakers, Inc. | Method and apparatus for introducing endolymphatic instrumentation |
| US7734341B2 (en) | 2006-06-06 | 2010-06-08 | Cardiac Pacemakers, Inc. | Method and apparatus for gastrointestinal stimulation via the lymphatic system |
| US20070282376A1 (en) | 2006-06-06 | 2007-12-06 | Shuros Allan C | Method and apparatus for neural stimulation via the lymphatic system |
| US7771401B2 (en) * | 2006-06-08 | 2010-08-10 | Angiodynamics, Inc. | Selective renal cannulation and infusion systems and methods |
| DE102006042639A1 (de) * | 2006-09-01 | 2008-03-20 | Novalung Gmbh | Vorrichtung zur Kanülierung eines Blut-führenden Gefäßes |
| US8905999B2 (en) | 2006-09-01 | 2014-12-09 | Cardiac Pacemakers, Inc. | Method and apparatus for endolymphatic drug delivery |
| US20080154186A1 (en) * | 2006-11-07 | 2008-06-26 | Angiodynamics, Inc. | Multiple lumen catheter with proximal port |
| US8317773B2 (en) * | 2006-11-07 | 2012-11-27 | Angio Dynamics, Inc. | Catheter with open faced sloped end portion |
| WO2008074027A1 (en) * | 2006-12-13 | 2008-06-19 | Biomerix Corporation | Aneurysm occlusion devices |
| WO2008095046A2 (en) * | 2007-01-30 | 2008-08-07 | Loma Vista Medical, Inc., | Biological navigation device |
| US9037244B2 (en) | 2007-02-13 | 2015-05-19 | Virender K. Sharma | Method and apparatus for electrical stimulation of the pancreatico-biliary system |
| US20080221551A1 (en) * | 2007-03-09 | 2008-09-11 | Flowmedica, Inc. | Acute kidney injury treatment systems and methods |
| US20090306625A1 (en) * | 2007-03-09 | 2009-12-10 | Angiodynamics, Inc. | Treatment systems and methods for renal-related diseases |
| KR20150042870A (ko) | 2007-04-10 | 2015-04-21 | 씨. 알. 바드, 인크. | 저 전력 초음파 시스템 |
| FR2916978B1 (fr) * | 2007-06-07 | 2009-07-31 | Chater Pierre El | Drain chirurgical sans fixation cutanee (el chater) |
| US20090198172A1 (en) * | 2008-02-05 | 2009-08-06 | Garrison Michi E | Interventional sheath with retention features |
| US8157760B2 (en) | 2007-07-18 | 2012-04-17 | Silk Road Medical, Inc. | Methods and systems for establishing retrograde carotid arterial blood flow |
| US8858490B2 (en) | 2007-07-18 | 2014-10-14 | Silk Road Medical, Inc. | Systems and methods for treating a carotid artery |
| US20090105799A1 (en) * | 2007-10-23 | 2009-04-23 | Flowmedica, Inc. | Renal assessment systems and methods |
| JP2011509714A (ja) * | 2008-01-11 | 2011-03-31 | ボストン サイエンティフィック サイムド, インコーポレイテッド | 内視鏡係留デバイス |
| JP2011510796A (ja) | 2008-02-05 | 2011-04-07 | シルク・ロード・メディカル・インコーポレイテッド | 介入カテーテルシステム及び方法 |
| EP2268347A4 (en) * | 2008-03-24 | 2011-09-21 | M D Joe Sam Robinson | INTRAVASCULAR BRAIN CATHETER AND METHOD FOR ITS USE |
| WO2009131583A1 (en) * | 2008-04-24 | 2009-10-29 | Angiodynamics, Inc. | Catheter with open faced beveled tip |
| US20090299327A1 (en) * | 2008-06-02 | 2009-12-03 | Lorna Vista Medical, Inc. | Inflatable medical devices |
| CA2731435A1 (en) * | 2008-07-21 | 2010-01-28 | Arstasis, Inc. | Devices, methods, and kits for forming tracts in tissue |
| CA2731493A1 (en) * | 2008-07-21 | 2010-01-28 | Arstasis, Inc. | Devices and methods for forming tracts in tissue |
| WO2010014568A1 (en) * | 2008-07-28 | 2010-02-04 | William Beaumont Hospital | Multiple port introducer for thrombolysis |
| WO2010042686A1 (en) | 2008-10-09 | 2010-04-15 | Sharma Virender K | Method and apparatus for stimulating the vascular system |
| US10603489B2 (en) | 2008-10-09 | 2020-03-31 | Virender K. Sharma | Methods and apparatuses for stimulating blood vessels in order to control, treat, and/or prevent a hemorrhage |
| USD603044S1 (en) | 2008-12-15 | 2009-10-27 | Angiodynamics, Inc. | Catheter tip |
| US8652129B2 (en) | 2008-12-31 | 2014-02-18 | Medtronic Ardian Luxembourg S.A.R.L. | Apparatus, systems, and methods for achieving intravascular, thermally-induced renal neuromodulation |
| WO2010141752A1 (en) | 2009-06-03 | 2010-12-09 | Silk Road Medical, Inc. | System and methods for controlling retrograde carotid arterial blood flow |
| EP2456503B1 (en) | 2009-07-23 | 2017-09-06 | Acist Medical Systems, Inc. | Endoventricular injection catheter system with integrated echocardiographic capabilities |
| CA2921304C (en) | 2009-07-30 | 2018-06-05 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| US20100204708A1 (en) * | 2010-02-23 | 2010-08-12 | Sanjiv Sharma | Carotid guiding catheter (sheath) for carotid percutaneous intervention/stenting with internal fixation device to prevent migration of the Carotid guiding catheter (sheath) |
| US9642991B2 (en) * | 2011-05-03 | 2017-05-09 | Lxs, Llc | Apparatus and methods for accessing the lymphatic system |
| WO2012009486A2 (en) | 2010-07-13 | 2012-01-19 | Loma Vista Medical, Inc. | Inflatable medical devices |
| US8585601B2 (en) | 2010-10-18 | 2013-11-19 | CardioSonic Ltd. | Ultrasound transducer |
| WO2012052920A1 (en) | 2010-10-18 | 2012-04-26 | CardioSonic Ltd. | Therapeutics reservoir |
| WO2012061153A1 (en) | 2010-10-25 | 2012-05-10 | Medtronic Ardian Luxembourg S.A.R.L. | Devices, systems and methods for evaluation and feedback of neuromodulation treatment |
| US10188436B2 (en) | 2010-11-09 | 2019-01-29 | Loma Vista Medical, Inc. | Inflatable medical devices |
| US20130325101A1 (en) * | 2010-12-14 | 2013-12-05 | Transcatheter Technologies Gmbh | Apparatus Comprising an Aligning Device, Set and Method |
| GB201102867D0 (en) * | 2011-02-18 | 2011-04-06 | Univ Leicester | Catheter and method |
| EP2522307B1 (en) * | 2011-05-08 | 2020-09-30 | ITSO Medical AB | Device for delivery of medical devices to a cardiac valve |
| US9526856B2 (en) | 2011-12-15 | 2016-12-27 | The Board Of Trustees Of The Leland Stanford Junior University | Devices and methods for preventing tracheal aspiration |
| CN104271062B (zh) | 2012-03-08 | 2017-07-07 | 美敦力Af卢森堡有限责任公司 | 采用神经调节装置的生物标志物取样和相关系统及方法 |
| AU2013230781B2 (en) | 2012-03-08 | 2015-12-03 | Medtronic Af Luxembourg S.A.R.L. | Ovarian neuromodulation and associated systems and methods |
| US10357304B2 (en) | 2012-04-18 | 2019-07-23 | CardioSonic Ltd. | Tissue treatment |
| US9180242B2 (en) | 2012-05-17 | 2015-11-10 | Tandem Diabetes Care, Inc. | Methods and devices for multiple fluid transfer |
| US20130317481A1 (en) | 2012-05-25 | 2013-11-28 | Arstasis, Inc. | Vascular access configuration |
| US20130317438A1 (en) | 2012-05-25 | 2013-11-28 | Arstasis, Inc. | Vascular access configuration |
| EP2854703A4 (en) * | 2012-05-25 | 2016-01-27 | Arstasis Inc | VASCULAR ACCESS CONFIGURATION |
| US11357447B2 (en) | 2012-05-31 | 2022-06-14 | Sonivie Ltd. | Method and/or apparatus for measuring renal denervation effectiveness |
| CN108742951B (zh) | 2012-06-06 | 2021-05-25 | 洋红医疗有限公司 | 人工肾脏瓣膜 |
| US20140110296A1 (en) | 2012-10-19 | 2014-04-24 | Medtronic Ardian Luxembourg S.A.R.L. | Packaging for Catheter Treatment Devices and Associated Devices, Systems, and Methods |
| US9233225B2 (en) | 2012-11-10 | 2016-01-12 | Curvo Medical, Inc. | Coaxial bi-directional catheter |
| US9549666B2 (en) | 2012-11-10 | 2017-01-24 | Curvo Medical, Inc. | Coaxial micro-endoscope |
| US9180033B2 (en) | 2012-11-20 | 2015-11-10 | Indiana University Research And Technology Corp. | Intravascular shunt for traumatized arteries |
| WO2014081947A1 (en) | 2012-11-21 | 2014-05-30 | Syed Mubin I | System for the intravascular placement of a medical device |
| CN113616920B (zh) | 2013-03-13 | 2024-10-25 | 马真塔医药有限公司 | 血液泵浦装置及制造血液泵浦的方法 |
| US10583231B2 (en) | 2013-03-13 | 2020-03-10 | Magenta Medical Ltd. | Blood pump |
| US9173998B2 (en) | 2013-03-14 | 2015-11-03 | Tandem Diabetes Care, Inc. | System and method for detecting occlusions in an infusion pump |
| US9421329B2 (en) | 2013-03-15 | 2016-08-23 | Tandem Diabetes Care, Inc. | Infusion device occlusion detection system |
| MX2015015137A (es) * | 2013-04-30 | 2016-07-08 | Lake Region Mfg Inc D B A Lake Region Medical | Dipositivo de ablación de bucle inverso. |
| WO2014188430A2 (en) | 2013-05-23 | 2014-11-27 | CardioSonic Ltd. | Devices and methods for renal denervation and assessment thereof |
| WO2015016139A1 (ja) * | 2013-08-01 | 2015-02-05 | テルモ株式会社 | 腎動脈用ガイディングカテーテル及びその使用方法 |
| DE102013216030A1 (de) * | 2013-08-13 | 2015-02-19 | Olympus Winter & Ibe Gmbh | Führungskatheter mit Stabilisierungsmechanismus und Verfahren zum Einführen eines Führungskatheters |
| US9770194B2 (en) | 2013-11-05 | 2017-09-26 | Ciel Medical, Inc. | Devices and methods for airway measurement |
| US9764113B2 (en) | 2013-12-11 | 2017-09-19 | Magenta Medical Ltd | Curved catheter |
| US9980766B1 (en) | 2014-03-28 | 2018-05-29 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and systems for renal neuromodulation |
| US10194980B1 (en) | 2014-03-28 | 2019-02-05 | Medtronic Ardian Luxembourg S.A.R.L. | Methods for catheter-based renal neuromodulation |
| US10194979B1 (en) | 2014-03-28 | 2019-02-05 | Medtronic Ardian Luxembourg S.A.R.L. | Methods for catheter-based renal neuromodulation |
| US10016591B2 (en) | 2014-10-24 | 2018-07-10 | Medtronic, Inc. | Medical electrical lead |
| US9901732B2 (en) | 2014-10-24 | 2018-02-27 | Medtronic, Inc. | Medical electrical lead |
| US10532206B2 (en) | 2014-10-24 | 2020-01-14 | Medtronic, Inc. | Coronary sinus medical electrical lead |
| EP3223712B1 (en) * | 2014-11-26 | 2023-08-30 | Sonivie Ltd. | Devices for pulmonary hypertension treatment |
| CR20170245A (es) * | 2014-12-05 | 2017-09-14 | Edwards Lifesciences Corp | Cateter dirigible con cable de tracción |
| US10434287B2 (en) * | 2015-04-28 | 2019-10-08 | Cook Medical Technologies Llc | Transradial celiac artery catherer |
| WO2016185473A1 (en) | 2015-05-18 | 2016-11-24 | Magenta Medical Ltd. | Blood pump |
| US10492936B2 (en) | 2015-10-30 | 2019-12-03 | Ram Medical Innovations, Llc | Apparatus and method for improved access of procedural catheter in tortuous vessels |
| US11020256B2 (en) * | 2015-10-30 | 2021-06-01 | Ram Medical Innovations, Inc. | Bifurcated “Y” anchor support for coronary interventions |
| US10327929B2 (en) | 2015-10-30 | 2019-06-25 | Ram Medical Innovations, Llc | Apparatus and method for stabilization of procedural catheter in tortuous vessels |
| US10779976B2 (en) | 2015-10-30 | 2020-09-22 | Ram Medical Innovations, Llc | Apparatus and method for stabilization of procedural catheter in tortuous vessels |
| WO2017096362A1 (en) * | 2015-12-04 | 2017-06-08 | Barrish Mark D | Lateral articulation anchors for catheters and other uses |
| US10173031B2 (en) | 2016-06-20 | 2019-01-08 | Mubin I. Syed | Interchangeable flush/selective catheter |
| EP3518825B1 (en) | 2016-09-29 | 2020-05-27 | Magenta Medical Ltd. | Blood vessel tube |
| CN115040776A (zh) | 2016-10-25 | 2022-09-13 | 马真塔医药有限公司 | 心室辅助装置 |
| US11033727B2 (en) | 2016-11-23 | 2021-06-15 | Magenta Medical Ltd. | Blood pumps |
| AU2017379821B2 (en) * | 2016-12-20 | 2020-09-17 | W. L. Gore & Associates, Inc. | Anticannulation web |
| EP3600434A4 (en) | 2017-03-20 | 2021-01-06 | Sonievie Ltd. | Pulmonary hypertension treatment |
| IL258461A (en) * | 2017-04-06 | 2018-06-28 | Continuse Biometrics Ltd | Blood pressure monitoring system |
| AU2018254569B2 (en) * | 2017-04-20 | 2022-05-12 | Endotronix, Inc. | Anchoring system for a catheter delivered device |
| EP3691731B1 (en) * | 2017-10-03 | 2021-12-01 | Sanford Health | Bi-lateral catheter system |
| JP7214731B2 (ja) | 2017-11-30 | 2023-01-30 | シー・アール・バード・インコーポレーテッド | 血管内装置 |
| WO2019108941A1 (en) * | 2017-12-01 | 2019-06-06 | Generations International Asset Management Company Llc D/B/A International Private Bank | Memory catheter |
| CN115177858A (zh) | 2018-01-10 | 2022-10-14 | 马真塔医药有限公司 | 心室辅助装置 |
| US10905808B2 (en) | 2018-01-10 | 2021-02-02 | Magenta Medical Ltd. | Drive cable for use with a blood pump |
| US11007075B2 (en) * | 2018-02-18 | 2021-05-18 | Ram Medical Innovations, Inc. | Vascular access devices and methods for lower limb interventions |
| US11191556B2 (en) | 2018-03-01 | 2021-12-07 | Covidien Lp | Catheter including an expandable member |
| US10893927B2 (en) | 2018-03-29 | 2021-01-19 | Magenta Medical Ltd. | Inferior vena cava blood-flow implant |
| US12350454B2 (en) | 2018-10-04 | 2025-07-08 | The University Of Chicago | LIMA crossover integrated catheter system |
| CN113226436B (zh) * | 2018-12-19 | 2024-12-31 | 波士顿科学国际有限公司 | 循环支撑装置 |
| EP3782666B1 (en) | 2019-01-24 | 2021-08-11 | Magenta Medical Ltd. | Manufacturing an impeller |
| FR3092000B1 (fr) | 2019-01-28 | 2023-02-24 | Hopitaux Paris Assist Publique | Canule d’injection, système d’injection d’un fluide |
| CN114040794B (zh) | 2019-05-23 | 2025-01-24 | 马真塔医药有限公司 | 血液泵 |
| CN112972166A (zh) * | 2019-12-17 | 2021-06-18 | 先健科技(深圳)有限公司 | 医疗器械系统 |
| US12097339B2 (en) * | 2019-12-31 | 2024-09-24 | Biosense Webster (Israel) Ltd. | System and methods of using a catheter with an anchoring mechanism |
| WO2021205346A2 (en) | 2020-04-07 | 2021-10-14 | Magenta Medical Ltd | Ventricular assist device |
| WO2021242471A1 (en) | 2020-05-26 | 2021-12-02 | Penumbra, Inc. | Hemostasis valve |
| US20210402156A1 (en) * | 2020-06-30 | 2021-12-30 | Becton, Dickinson And Company | Catheter tip passive control device and related systems and methods |
| CN116490239A (zh) | 2020-11-09 | 2023-07-25 | 敏捷设备有限公司 | 用于操纵导管的装置 |
Family Cites Families (245)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1696018A (en) * | 1926-07-10 | 1928-12-18 | Schellberg Oscar Boto | Colonic theraphy apparatus |
| US2499045A (en) | 1948-08-16 | 1950-02-28 | Walker Frank Ray | Rectal dilator and medicator |
| US3144868A (en) | 1960-10-21 | 1964-08-18 | Mario E Jascalevich | Drainage and feeding cannulae |
| US3455298A (en) | 1967-04-10 | 1969-07-15 | George L Anstadt | Instrument for direct mechanical cardiac massage |
| US3516408A (en) | 1967-09-21 | 1970-06-23 | Vincent L Montanti | Arterial bypass |
| US3667069A (en) | 1970-03-27 | 1972-06-06 | Univ Minnesota | Jet pump cardiac replacement and assist device and method of at least partially replacing a disabled right heart |
| US3730186A (en) | 1971-03-05 | 1973-05-01 | Univ California | Adjustable implantable artery-constricting device |
| US3791374A (en) | 1971-08-09 | 1974-02-12 | Department Of Health Education | Programmer for segmented balloon pump |
| US3841331A (en) | 1972-11-17 | 1974-10-15 | J Wilder | Suction-pump assembly for drawing body fluids |
| US3995623A (en) | 1974-12-23 | 1976-12-07 | American Hospital Supply Corporation | Multipurpose flow-directed catheter |
| US4169464A (en) * | 1977-12-16 | 1979-10-02 | Cordis Corporation | Catheter for selective catheterization of aortic branches |
| US4248224A (en) * | 1978-08-01 | 1981-02-03 | Jones James W | Double venous cannula |
| CA1153264A (en) | 1979-02-08 | 1983-09-06 | Hidenaga Yoshimura | Medical vascular guide wire and self-guiding type catheter |
| US4493697A (en) | 1979-05-10 | 1985-01-15 | Krause Horst E | Method and apparatus for pumping blood within a vessel |
| US4309994A (en) | 1980-02-25 | 1982-01-12 | Grunwald Ronald P | Cardiovascular cannula |
| US4407271A (en) | 1980-07-28 | 1983-10-04 | Peter Schiff | Apparatus for left heart assist |
| FR2502499B1 (fr) | 1981-03-27 | 1987-01-23 | Farcot Jean Christian | Appareil pour la retroperfusion sanguine, destine notamment au traitement d'infarctus par injection de sang arteriel dans le sinus coronaire |
| IT1155105B (it) | 1982-03-03 | 1987-01-21 | Roberto Parravicini | Dispositivo di impianto per il sostentamento dell attivita del miocardio |
| US4423725A (en) | 1982-03-31 | 1984-01-03 | Baran Ostap E | Multiple surgical cuff |
| US4636195A (en) | 1982-04-02 | 1987-01-13 | Harvey Wolinsky | Method and apparatus for removing arterial constriction |
| US4490374A (en) | 1982-09-30 | 1984-12-25 | Ortho Pharmaceutical Corporation | 5,6-Dialkoxy-3,4-optionally substituted-2(1H)quinazolinones, composition and method of use |
| US4714460A (en) | 1983-07-29 | 1987-12-22 | Reynaldo Calderon | Methods and systems for retrograde perfusion in the body for curing it of the disease or immume deficiency |
| US4546759A (en) | 1983-07-29 | 1985-10-15 | Mladen Solar | Method and apparatus for assisting human heart function |
| US4685446A (en) | 1984-02-21 | 1987-08-11 | Choy Daniel S J | Method for using a ventricular assist device |
| US4705507A (en) | 1984-05-02 | 1987-11-10 | Boyles Paul W | Arterial catheter means |
| US4554284A (en) | 1984-09-12 | 1985-11-19 | Smithkline Beckman Corporation | 7-(2-Aminoethyl)-1,3-benzthia- or oxa-zol-2(3H)-ones |
| US4705502A (en) | 1985-11-06 | 1987-11-10 | The Kendall Company | Suprapubic catheter with dual balloons |
| SE455164B (sv) | 1985-12-05 | 1988-06-27 | Data Promeditech Inc Ab | Pump for biologiska vetskor |
| US5158540A (en) | 1985-12-19 | 1992-10-27 | Leocor, Inc. | Perfusion catheter |
| SE454942B (sv) | 1986-05-22 | 1988-06-13 | Astra Tech Ab | Hjerthjelpanordning for inoperation i brosthalan |
| IT8629545V0 (it) | 1986-06-12 | 1986-06-12 | Fina Ernesto | Set cateteri ureterali coassiali a palloncino per estrazione di calcoli ureterali |
| DE3621350A1 (de) | 1986-06-26 | 1988-01-14 | Bonzel Tassilo | Dilatationskatheter mit einem aufweitbaren ballon |
| US4723939A (en) | 1986-07-31 | 1988-02-09 | The Research Foundation Of State Univ. Of New York | Apparatus and method for multiple organ procurement |
| US4840172A (en) | 1986-09-04 | 1989-06-20 | Augustine Scott D | Device for positioning an endotracheal tube |
| US4990139A (en) | 1986-09-10 | 1991-02-05 | Jang G David | Tandem independently inflatable/deflatable multiple diameter balloon angioplasty catheter systems |
| US4712551A (en) | 1986-10-14 | 1987-12-15 | Rayhanabad Simon B | Vascular shunt |
| US4753221A (en) | 1986-10-22 | 1988-06-28 | Intravascular Surgical Instruments, Inc. | Blood pumping catheter and method of use |
| US5002532A (en) | 1987-01-06 | 1991-03-26 | Advanced Cardiovascular Systems, Inc. | Tandem balloon dilatation catheter |
| US4781716A (en) | 1987-02-13 | 1988-11-01 | Marc Richelsoph | Artificial heart |
| US4925443A (en) | 1987-02-27 | 1990-05-15 | Heilman Marlin S | Biocompatible ventricular assist and arrhythmia control device |
| US4861330A (en) | 1987-03-12 | 1989-08-29 | Gene Voss | Cardiac assist device and method |
| US4902272A (en) | 1987-06-17 | 1990-02-20 | Abiomed Cardiovascular, Inc. | Intra-arterial cardiac support system |
| US4938766A (en) | 1987-08-28 | 1990-07-03 | Jarvik Robert K | Prosthetic compliance devices |
| US4834707A (en) | 1987-09-16 | 1989-05-30 | Evans Phillip H | Venting apparatus and method for cardiovascular pumping application |
| US5073094A (en) | 1987-11-17 | 1991-12-17 | Regents Of The University Of Minnesota | Zero net external displacement implantable pump and driver |
| US4817586A (en) | 1987-11-24 | 1989-04-04 | Nimbus Medical, Inc. | Percutaneous bloom pump with mixed-flow output |
| IL85249A0 (en) | 1988-01-29 | 1988-07-31 | Galram Technology Ind Ltd | Heart assist device |
| US4863461A (en) | 1988-03-21 | 1989-09-05 | Symbion, Inc. | Artificial ventricle |
| US4846831A (en) | 1988-04-27 | 1989-07-11 | Skillin David E | Manual back-up drive for artificial heart |
| US4906229A (en) | 1988-05-03 | 1990-03-06 | Nimbus Medical, Inc. | High-frequency transvalvular axisymmetric blood pump |
| US4909252A (en) | 1988-05-26 | 1990-03-20 | The Regents Of The Univ. Of California | Perfusion balloon catheter |
| US4888011A (en) | 1988-07-07 | 1989-12-19 | Abiomed, Inc. | Artificial heart |
| JPH0255064A (ja) | 1988-08-03 | 1990-02-23 | Toa O | カテーテルを用いた血管内血栓の血栓除去装置 |
| US4964864A (en) | 1988-09-27 | 1990-10-23 | American Biomed, Inc. | Heart assist pump |
| US4919647A (en) | 1988-10-13 | 1990-04-24 | Kensey Nash Corporation | Aortically located blood pumping catheter and method of use |
| US5069662A (en) | 1988-10-21 | 1991-12-03 | Delcath Systems, Inc. | Cancer treatment |
| US5053023A (en) | 1988-10-25 | 1991-10-01 | Vas-Cath Incorporated | Catheter for prolonged access |
| US4994069A (en) * | 1988-11-02 | 1991-02-19 | Target Therapeutics | Vaso-occlusion coil and method |
| US4927412A (en) | 1988-12-08 | 1990-05-22 | Retroperfusion Systems, Inc. | Coronary sinus catheter |
| FR2641692A1 (fr) * | 1989-01-17 | 1990-07-20 | Nippon Zeon Co | Bouchon de fermeture d'une breche pour application medicale et dispositif pour bouchon de fermeture l'utilisant |
| US5044369A (en) | 1989-01-23 | 1991-09-03 | Harvinder Sahota | Bent topless catheters |
| US4976691A (en) | 1989-01-23 | 1990-12-11 | Harvinder Sahota | Topless catheters |
| US5087244A (en) | 1989-01-31 | 1992-02-11 | C. R. Bard, Inc. | Catheter and method for locally applying medication to the wall of a blood vessel or other body lumen |
| US4902291A (en) | 1989-01-31 | 1990-02-20 | University Of Utah Research Foundation | Collapsible artificial ventricle and pumping shell |
| US5328470A (en) | 1989-03-31 | 1994-07-12 | The Regents Of The University Of Michigan | Treatment of diseases by site-specific instillation of cells or site-specific transformation of cells and kits therefor |
| US4973312A (en) | 1989-05-26 | 1990-11-27 | Andrew Daniel E | Method and system for inserting spinal catheters |
| US4927407A (en) | 1989-06-19 | 1990-05-22 | Regents Of The University Of Minnesota | Cardiac assist pump with steady rate supply of fluid lubricant |
| US4995864A (en) | 1989-08-15 | 1991-02-26 | Imed Corporation | Dual chamber pumping apparatus |
| US4950226A (en) | 1989-09-11 | 1990-08-21 | Bruce Barron | Surgical shunt for liver isolation |
| SE465017B (sv) | 1989-11-24 | 1991-07-15 | Lars Knutson | Anordning foer segmentell perfusion/aspiration av grovtarmen |
| GB2239675A (en) | 1989-12-05 | 1991-07-10 | Man Fai Shiu | Pump for pumping liquid |
| US5067960A (en) | 1989-12-06 | 1991-11-26 | Medtronic, Inc. | Muscle fitness detection by colorimetry |
| US5358519A (en) | 1989-12-06 | 1994-10-25 | Medtronic, Inc. | Muscle control and monitoring system |
| US5098442A (en) | 1989-12-06 | 1992-03-24 | Medtronic, Inc. | Muscle contraction control by intramuscular pressure monitoring |
| US5089019A (en) | 1989-12-06 | 1992-02-18 | Medtronic, Inc. | Muscle work output monitor by intramuscular temperature variation measurement |
| US5069680A (en) | 1989-12-06 | 1991-12-03 | Medtronic, Inc. | Muscle stimulator with variable duty cycle |
| US5308319A (en) | 1989-12-28 | 1994-05-03 | Sumitmo Bakelite Company Limited | Cardio assist system and insertion device therefor |
| US5345927A (en) * | 1990-03-02 | 1994-09-13 | Bonutti Peter M | Arthroscopic retractors |
| US5163910A (en) | 1990-04-10 | 1992-11-17 | Mayo Foundation For Medical Education And Research | Intracatheter perfusion pump apparatus and method |
| US5108407A (en) * | 1990-06-08 | 1992-04-28 | Rush-Presbyterian St. Luke's Medical Center | Method and apparatus for placement of an embolic coil |
| US5131905A (en) | 1990-07-16 | 1992-07-21 | Grooters Ronald K | External cardiac assist device |
| CA2022019C (en) | 1990-07-26 | 1992-12-29 | Michael Black | Catheter |
| US5135474A (en) | 1990-08-03 | 1992-08-04 | University Of Medicine And Dentistry Of New Jersey | Hepatic bypass catheter |
| US4976692A (en) | 1990-09-13 | 1990-12-11 | Travenol Laboratories (Israel) Ltd. | Catheter particularly useful for inducing labor and/or for the application of a pharmaceutical substance to the cervix of the uterus |
| US5205810A (en) | 1990-10-15 | 1993-04-27 | Medtronic, Inc. | Muscle powered cardiac assist system |
| WO1992008500A1 (en) | 1990-11-09 | 1992-05-29 | Mcgill University | Cardiac assist method and apparatus |
| US5119804A (en) | 1990-11-19 | 1992-06-09 | Anstadt George L | Heart massage apparatus |
| US5308320A (en) | 1990-12-28 | 1994-05-03 | University Of Pittsburgh Of The Commonwealth System Of Higher Education | Portable and modular cardiopulmonary bypass apparatus and associated aortic balloon catheter and associated method |
| US5108420A (en) * | 1991-02-01 | 1992-04-28 | Temple University | Aperture occlusion device |
| EP0581866B1 (en) | 1991-04-24 | 1998-03-04 | Baxter International Inc. | Exchangeable integrated-wire balloon catheter |
| US5167628A (en) | 1991-05-02 | 1992-12-01 | Boyles Paul W | Aortic balloon catheter assembly for indirect infusion of the coronary arteries |
| US5112301A (en) | 1991-06-19 | 1992-05-12 | Strato Medical Corporation | Bidirectional check valve catheter |
| US5180364A (en) | 1991-07-03 | 1993-01-19 | Robert Ginsburg | Valved self-perfusing catheter guide |
| US5766151A (en) | 1991-07-16 | 1998-06-16 | Heartport, Inc. | Endovascular system for arresting the heart |
| US5282784A (en) | 1991-10-09 | 1994-02-01 | Mentor Corporation | Injection stent system |
| US5226888A (en) | 1991-10-25 | 1993-07-13 | Michelle Arney | Coiled, perfusion balloon catheter |
| US5368566A (en) | 1992-04-29 | 1994-11-29 | Cardiovascular Dynamics, Inc. | Delivery and temporary stent catheter having a reinforced perfusion lumen |
| US5383840A (en) | 1992-07-28 | 1995-01-24 | Vascor, Inc. | Biocompatible ventricular assist and arrhythmia control device including cardiac compression band-stay-pad assembly |
| US5290227A (en) | 1992-08-06 | 1994-03-01 | Pasque Michael K | Method of implanting blood pump in ascending aorta or main pulmonary artery |
| US5332403A (en) | 1992-08-17 | 1994-07-26 | Jack Kolff | LVAD with t-shape and unidirectional valve |
| US5257974A (en) | 1992-08-19 | 1993-11-02 | Scimed Life Systems, Inc. | Performance enhancement adaptor for intravascular balloon catheter |
| US5382259A (en) * | 1992-10-26 | 1995-01-17 | Target Therapeutics, Inc. | Vasoocclusion coil with attached tubular woven or braided fibrous covering |
| US5376114A (en) | 1992-10-30 | 1994-12-27 | Jarvik; Robert | Cannula pumps for temporary cardiac support and methods of their application and use |
| US5536250A (en) | 1994-04-01 | 1996-07-16 | Localmed, Inc. | Perfusion shunt device and method |
| US5336178A (en) | 1992-11-02 | 1994-08-09 | Localmed, Inc. | Intravascular catheter with infusion array |
| US5326374A (en) | 1992-12-01 | 1994-07-05 | Michael N. Ilbawi | Body-implantable device for controlling the size of a fluid passageway |
| US5256141A (en) | 1992-12-22 | 1993-10-26 | Nelson Gencheff | Biological material deployment method and apparatus |
| US5292309A (en) | 1993-01-22 | 1994-03-08 | Schneider (Usa) Inc. | Surgical depth measuring instrument and method |
| US6125852A (en) | 1993-02-22 | 2000-10-03 | Heartport, Inc. | Minimally-invasive devices and methods for treatment of congestive heart failure |
| US5643171A (en) | 1993-05-04 | 1997-07-01 | Neocardia, Llc | Method and apparatus for uniform radiation treatment of vascular lumens |
| US5453084A (en) | 1993-05-19 | 1995-09-26 | Moses; John A. | Vascular graft with internal shunt |
| US5464449A (en) | 1993-07-08 | 1995-11-07 | Thomas J. Fogarty | Internal graft prosthesis and delivery system |
| DE4324637A1 (de) | 1993-07-22 | 1995-03-09 | Schreiber Hans | Verfahren und Bausatz zur Organtransplantation |
| US5370617A (en) | 1993-09-17 | 1994-12-06 | Sahota; Harvinder | Blood perfusion balloon catheter |
| ES2077519B1 (es) | 1993-11-22 | 1996-07-01 | Fernandez De Lomana Euge Anaya | Cateter intraaortico para perfusion y conservacion renal. |
| US5397307A (en) | 1993-12-07 | 1995-03-14 | Schneider (Usa) Inc. | Drug delivery PTCA catheter and method for drug delivery |
| US5417708A (en) * | 1994-03-09 | 1995-05-23 | Cook Incorporated | Intravascular treatment system and percutaneous release mechanism therefor |
| US5613949A (en) | 1994-04-01 | 1997-03-25 | Advanced Cardiovascular Systems, Inc. | Double balloon catheter assembly |
| US5599306A (en) | 1994-04-01 | 1997-02-04 | Localmed, Inc. | Method and apparatus for providing external perfusion lumens on balloon catheters |
| US5935924A (en) | 1994-04-15 | 1999-08-10 | Genentech, Inc. | Treatment of congestive heart failure |
| US5484385A (en) | 1994-04-21 | 1996-01-16 | C. R. Bard, Inc. | Intra-aortic balloon catheter |
| US5762599A (en) | 1994-05-02 | 1998-06-09 | Influence Medical Technologies, Ltd. | Magnetically-coupled implantable medical devices |
| US5478331A (en) | 1994-05-11 | 1995-12-26 | Localmed, Inc. | Multi-function proximal end adapter for catheter |
| US5456694A (en) | 1994-05-13 | 1995-10-10 | Stentco, Inc. | Device for delivering and deploying intraluminal devices |
| US5476453A (en) * | 1994-05-20 | 1995-12-19 | Mehta; Sameer | Catheter for simultaneous right and left coronary cannulization |
| US5509428A (en) | 1994-05-31 | 1996-04-23 | Dunlop; Richard W. | Method and apparatus for the creation of tricuspid regurgitation |
| US5695457A (en) * | 1994-07-28 | 1997-12-09 | Heartport, Inc. | Cardioplegia catheter system |
| US5807895A (en) | 1994-11-29 | 1998-09-15 | Schwarz Pharma, Inc. | Use of prostaglandin E1, E2 or analogs to prevent renal failure induced by medical tests that utilize contrast media agents |
| US5879366A (en) * | 1996-12-20 | 1999-03-09 | W.L. Gore & Associates, Inc. | Self-expanding defect closure device and method of making and using |
| US5613980A (en) | 1994-12-22 | 1997-03-25 | Chauhan; Tusharsindhu C. | Bifurcated catheter system and method |
| US5643215A (en) | 1995-02-24 | 1997-07-01 | The Research Foundation Of State University Of New York | Gas exchange apparatus and method |
| US5609628A (en) | 1995-04-20 | 1997-03-11 | Keranen; Victor J. | Intravascular graft and catheter |
| NO962336L (no) * | 1995-06-06 | 1996-12-09 | Target Therapeutics Inc | Vaso-okklusiv spiral |
| US6280413B1 (en) * | 1995-06-07 | 2001-08-28 | Medtronic Ave, Inc. | Thrombolytic filtration and drug delivery catheter with a self-expanding portion |
| US5713853A (en) | 1995-06-07 | 1998-02-03 | Interventional Innovations Corporation | Methods for treating thrombosis |
| US5913852A (en) | 1995-07-21 | 1999-06-22 | Nemours Foundation | Drain cannula |
| FR2738154B1 (fr) | 1995-09-05 | 1997-12-26 | Pourchez Thierry | Catheter multiconduits, notamment d'hemodialyse |
| DE19535781C2 (de) | 1995-09-26 | 1999-11-11 | Fraunhofer Ges Forschung | Vorrichtung zur aktiven Strömungsunterstützung von Körperflüssigkeiten |
| US5669924A (en) | 1995-10-26 | 1997-09-23 | Shaknovich; Alexander | Y-shuttle stent assembly for bifurcating vessels and method of using the same |
| WO1997016119A1 (en) * | 1995-10-30 | 1997-05-09 | Children's Medical Center Corporation | Self-centering umbrella-type septal closure device |
| US5797876A (en) | 1995-11-27 | 1998-08-25 | Therox, Inc. | High pressure perfusion device |
| JP2750569B2 (ja) | 1995-12-07 | 1998-05-13 | 幸夫 堀口 | 血管内装着型血流調節器及びバイパス用人工血管 |
| US5871537A (en) * | 1996-02-13 | 1999-02-16 | Scimed Life Systems, Inc. | Endovascular apparatus |
| US5853422A (en) * | 1996-03-22 | 1998-12-29 | Scimed Life Systems, Inc. | Apparatus and method for closing a septal defect |
| AR001590A1 (es) * | 1996-04-10 | 1997-11-26 | Jorge Alberto Baccaro | Dispositivo oclusor de comunicaciones vasculares anormales y cartucho aplicador de dicho dispositivo |
| US20010029349A1 (en) | 1996-04-12 | 2001-10-11 | Boris Leschinsky | Method and apparatus for treating aneurysms |
| UA58485C2 (uk) * | 1996-05-03 | 2003-08-15 | Медінол Лтд. | Спосіб виготовлення роздвоєного стента (варіанти) та роздвоєний стент (варіанти) |
| US6251133B1 (en) * | 1996-05-03 | 2001-06-26 | Medinol Ltd. | Bifurcated stent with improved side branch aperture and method of making same |
| US5617878A (en) | 1996-05-31 | 1997-04-08 | Taheri; Syde A. | Stent and method for treatment of aortic occlusive disease |
| CA2211249C (en) | 1996-07-24 | 2007-07-17 | Cordis Corporation | Balloon catheter and methods of use |
| US5702343A (en) | 1996-10-02 | 1997-12-30 | Acorn Medical, Inc. | Cardiac reinforcement device |
| EP0835673A3 (en) | 1996-10-10 | 1998-09-23 | Schneider (Usa) Inc. | Catheter for tissue dilatation and drug delivery |
| US5902336A (en) | 1996-10-15 | 1999-05-11 | Mirimedical, Inc. | Implantable device and method for removing fluids from the blood of a patient method for implanting such a device and method for treating a patient experiencing renal failure |
| AU747403B2 (en) | 1996-10-18 | 2002-05-16 | Cardio Technologies, Inc. | Method and apparatus for assisting a heart |
| US6325826B1 (en) * | 1998-01-14 | 2001-12-04 | Advanced Stent Technologies, Inc. | Extendible stent apparatus |
| US5976178A (en) * | 1996-11-07 | 1999-11-02 | Vascular Science Inc. | Medical grafting methods |
| US5807311A (en) | 1996-11-29 | 1998-09-15 | Palestrant; Aubrey M. | Dialysis catheter having rigid and collapsible lumens and related method |
| US5733329A (en) * | 1996-12-30 | 1998-03-31 | Target Therapeutics, Inc. | Vaso-occlusive coil with conical end |
| US6050936A (en) | 1997-01-02 | 2000-04-18 | Myocor, Inc. | Heart wall tension reduction apparatus |
| US5916213A (en) * | 1997-02-04 | 1999-06-29 | Medtronic, Inc. | Systems and methods for tissue mapping and ablation |
| US5720735A (en) | 1997-02-12 | 1998-02-24 | Dorros; Gerald | Bifurcated endovascular catheter |
| US6096073A (en) * | 1997-02-25 | 2000-08-01 | Scimed Life Systems, Inc. | Method of deploying a stent at a lesion site located at a bifurcation in a parent vessel |
| US6013054A (en) * | 1997-04-28 | 2000-01-11 | Advanced Cardiovascular Systems, Inc. | Multifurcated balloon catheter |
| US6217546B1 (en) | 1997-05-19 | 2001-04-17 | United States Surgical Corporation | Catheter system |
| US6129704A (en) | 1997-06-12 | 2000-10-10 | Schneider (Usa) Inc. | Perfusion balloon catheter having a magnetically driven impeller |
| US5817046A (en) | 1997-07-14 | 1998-10-06 | Delcath Systems, Inc. | Apparatus and method for isolated pelvic perfusion |
| EP1003422B1 (en) * | 1997-08-05 | 2006-06-14 | Boston Scientific Limited | Detachable aneurysm neck bridge |
| US6063070A (en) * | 1997-08-05 | 2000-05-16 | Target Therapeutics, Inc. | Detachable aneurysm neck bridge (II) |
| US5947953A (en) | 1997-08-06 | 1999-09-07 | Hemocleanse, Inc. | Splittable multiple catheter assembly and methods of inserting the same |
| US5968013A (en) | 1997-08-21 | 1999-10-19 | Scimed Life Systems, Inc. | Multi-function dilatation catheter |
| US5984955A (en) | 1997-09-11 | 1999-11-16 | Wisselink; Willem | System and method for endoluminal grafting of bifurcated or branched vessels |
| US6387037B1 (en) | 1997-10-09 | 2002-05-14 | Orqis Medical Corporation | Implantable heart assist system and method of applying same |
| US6889082B2 (en) | 1997-10-09 | 2005-05-03 | Orqis Medical Corporation | Implantable heart assist system and method of applying same |
| US6390969B1 (en) | 1997-10-09 | 2002-05-21 | Orqis Medical Corporation | Implantable heart assist system and method of applying same |
| WO1999022784A1 (en) | 1997-11-03 | 1999-05-14 | Cardio Technologies, Inc. | Method and apparatus for assisting a heart to pump blood |
| EP0951310B1 (en) * | 1997-11-07 | 2005-05-18 | Ave Connaught | Balloon catheter for repairing bifurcated vessels |
| US6036720A (en) * | 1997-12-15 | 2000-03-14 | Target Therapeutics, Inc. | Sheet metal aneurysm neck bridge |
| US6699231B1 (en) | 1997-12-31 | 2004-03-02 | Heartport, Inc. | Methods and apparatus for perfusion of isolated tissue structure |
| AU2022999A (en) | 1997-12-31 | 1999-07-19 | Heartport, Inc. | Methods and apparatus for perfusion of isolated tissue structure |
| US6156016A (en) | 1998-01-06 | 2000-12-05 | Maginot Vascular Systems | Catheter systems and associated methods utilizing removable inner catheter or catheters |
| US6063100A (en) * | 1998-03-10 | 2000-05-16 | Cordis Corporation | Embolic coil deployment system with improved embolic coil |
| US5902229A (en) | 1998-03-30 | 1999-05-11 | Cardio Technologies, Inc. | Drive system for controlling cardiac compression |
| US5928132A (en) | 1998-03-31 | 1999-07-27 | Datascope Investment Corp. | Closed chest intra-aortic balloon based ventricular assist device |
| US6086527A (en) | 1998-04-02 | 2000-07-11 | Scimed Life Systems, Inc. | System for treating congestive heart failure |
| US6261273B1 (en) * | 1998-05-07 | 2001-07-17 | Carlos E. Ruiz | Access system for branched vessels amd methods of use |
| US5935148A (en) * | 1998-06-24 | 1999-08-10 | Target Therapeutics, Inc. | Detachable, varying flexibility, aneurysm neck bridge |
| US6143002A (en) * | 1998-08-04 | 2000-11-07 | Scimed Life Systems, Inc. | System for delivering stents to bifurcation lesions |
| US6117117A (en) * | 1998-08-24 | 2000-09-12 | Advanced Cardiovascular Systems, Inc. | Bifurcated catheter assembly |
| US6280414B1 (en) * | 1998-09-30 | 2001-08-28 | Medtronic Ave, Inc. | Method and apparatus for local delivery of therapeutic agent |
| US6086557A (en) * | 1998-10-01 | 2000-07-11 | Cardiothoracic Systems, Inc. | Bifurcated venous cannula |
| US6077256A (en) | 1998-10-06 | 2000-06-20 | Mann; Michael J. | Delivery of a composition to the lung |
| US7780628B1 (en) | 1999-01-11 | 2010-08-24 | Angiodynamics, Inc. | Apparatus and methods for treating congestive heart disease |
| US7481803B2 (en) | 2000-11-28 | 2009-01-27 | Flowmedica, Inc. | Intra-aortic renal drug delivery catheter |
| US6749598B1 (en) | 1999-01-11 | 2004-06-15 | Flowmedica, Inc. | Apparatus and methods for treating congestive heart disease |
| US6261316B1 (en) | 1999-03-11 | 2001-07-17 | Endologix, Inc. | Single puncture bifurcation graft deployment system |
| JP2000300571A (ja) * | 1999-04-19 | 2000-10-31 | Nissho Corp | 経カテーテル手術用閉鎖栓 |
| US6595959B1 (en) | 1999-04-23 | 2003-07-22 | Alexander A. Stratienko | Cardiovascular sheath/catheter |
| US6884258B2 (en) * | 1999-06-04 | 2005-04-26 | Advanced Stent Technologies, Inc. | Bifurcation lesion stent delivery using multiple guidewires |
| US6960651B2 (en) * | 1999-06-29 | 2005-11-01 | Millennium Pharmaceuticals, Inc. | TANGO 332 polypeptides |
| US6190304B1 (en) | 1999-07-13 | 2001-02-20 | University Of North Texas Health Science Center At Fort Worth | Enhanced intra-aortic balloon assist device |
| US6544279B1 (en) | 2000-08-09 | 2003-04-08 | Incept, Llc | Vascular device for emboli, thrombus and foreign body removal and methods of use |
| WO2001035715A2 (en) * | 1999-11-18 | 2001-05-25 | Petrus Besselink | Method for placing bifurcated stents |
| US6592567B1 (en) | 1999-12-07 | 2003-07-15 | Chf Solutions, Inc. | Kidney perfusion catheter |
| US6547761B2 (en) | 2000-01-07 | 2003-04-15 | Scimed Life Systems, Inc. | Drainage catheter |
| US6514226B1 (en) | 2000-02-10 | 2003-02-04 | Chf Solutions, Inc. | Method and apparatus for treatment of congestive heart failure by improving perfusion of the kidney |
| US6287608B1 (en) | 2000-04-11 | 2001-09-11 | Intellicardia, Inc. | Method and apparatus for treatment of congestive heart failure by improving perfusion of the kidney by infusion of a vasodilator |
| US6533747B1 (en) | 2000-05-23 | 2003-03-18 | Chf Solutions, Inc. | Extracorporeal circuit for peripheral vein fluid removal |
| US6695832B2 (en) | 2000-06-01 | 2004-02-24 | Twincath, Llc | Multilumen catheter and methods for making the catheter |
| WO2001097717A1 (en) | 2000-06-20 | 2001-12-27 | Chf Solutions, Inc. | Implantable flow diversion device |
| WO2001097879A1 (en) | 2000-06-20 | 2001-12-27 | Chf Solutions, Inc. | Apparatus and method for perfusing the kidney with venous blood |
| WO2001097687A1 (en) | 2000-06-20 | 2001-12-27 | Chf Solutions, Inc. | Instrumented stent |
| WO2001097878A1 (en) | 2000-06-20 | 2001-12-27 | Intellicardia, Inc. | Split circulation apparatus and method |
| US6482211B1 (en) * | 2000-07-31 | 2002-11-19 | Advanced Cardiovascular Systems, Inc. | Angulated stent delivery system and method of use |
| US6468241B1 (en) | 2000-10-26 | 2002-10-22 | Chf Solutions, Inc. | Artificial kidney set with electronic key |
| US20020091355A1 (en) | 2000-11-17 | 2002-07-11 | Ascend Medical, Inc. | Compliant delivery catheter |
| JP2005501573A (ja) | 2000-12-01 | 2005-01-20 | ネフロス セラピューティクス, インコーポレイテッド | 血管内薬物送達デバイスおよびその使用 |
| US20020099326A1 (en) | 2001-01-24 | 2002-07-25 | Wilson Jon S. | Multi-lumen catheter with attachable hub |
| US7374560B2 (en) * | 2001-05-01 | 2008-05-20 | St. Jude Medical, Cardiology Division, Inc. | Emboli protection devices and related methods of use |
| US7604612B2 (en) * | 2001-05-01 | 2009-10-20 | St. Jude Medical, Cardiology Division, Inc. | Emboli protection devices and related methods of use |
| US6749628B1 (en) * | 2001-05-17 | 2004-06-15 | Advanced Cardiovascular Systems, Inc. | Stent and catheter assembly and method for treating bifurcations |
| AU2003214945A1 (en) | 2002-02-01 | 2003-09-02 | Robert J. Goldman | Multi-function catheter and use thereof |
| AU2003216517A1 (en) | 2002-03-07 | 2003-09-22 | Cordis Corporation | Iliac bifurcation balloon catheter |
| US7105016B2 (en) * | 2002-04-23 | 2006-09-12 | Medtronic Vascular, Inc. | Integrated mechanical handle with quick slide mechanism |
| US6911039B2 (en) * | 2002-04-23 | 2005-06-28 | Medtronic Vascular, Inc. | Integrated mechanical handle with quick slide mechanism |
| US7655036B2 (en) * | 2002-04-24 | 2010-02-02 | Medtronic Vascular, Inc. | Bifurcated endoluminal prosthetic assembly and method |
| WO2003099108A2 (en) * | 2002-05-28 | 2003-12-04 | The Cleveland Clinic Foundation | Minimally invasive treatment system for aortic aneurysms |
| US7163520B2 (en) | 2002-06-26 | 2007-01-16 | Chf Solutions, Inc. | Method and device for removal of radiocontrast media from blood |
| US6887258B2 (en) * | 2002-06-26 | 2005-05-03 | Advanced Cardiovascular Systems, Inc. | Embolic filtering devices for bifurcated vessels |
| WO2004034767A2 (en) * | 2002-09-20 | 2004-04-29 | Flowmedica, Inc. | Catheter system for renal therapy |
| WO2004030718A2 (en) * | 2002-09-20 | 2004-04-15 | Flowmedica, Inc. | Method and apparatus for intra aortic substance delivery to a branch vessel |
| AU2003278858A1 (en) * | 2002-09-20 | 2004-04-08 | Flowmedica, Inc. | Method and apparatus for selective drug infusion via an intraaortic flow diverter delivery catheter |
| AU2003276903A1 (en) * | 2002-09-20 | 2004-05-04 | Flowmedica, Inc. | Method and apparatus for selective material delivery via an intra-renal catheter |
| US7993325B2 (en) * | 2002-09-20 | 2011-08-09 | Angio Dynamics, Inc. | Renal infusion systems and methods |
| US7393339B2 (en) | 2003-02-21 | 2008-07-01 | C. R. Bard, Inc. | Multi-lumen catheter with separate distal tips |
| EP1599240A4 (en) | 2003-02-24 | 2007-06-06 | Plc Medical Systems Inc | CATHETER METHOD AND SYSTEM APPLICABLE IN CASE OF ACUTE RENAL FAILURE |
| US6945992B2 (en) * | 2003-04-22 | 2005-09-20 | Medtronic Vascular, Inc. | Single-piece crown stent |
| ES2332135T3 (es) | 2003-06-03 | 2010-01-27 | Novartis Ag | Inhibidores p-38 basados en heterociclo de 5 miembros. |
| JP2006526464A (ja) * | 2003-06-05 | 2006-11-24 | フローメディカ,インコーポレイテッド | 分枝した身体管腔において両側介入または診断を行うためのシステムおよび方法 |
| JP2006527629A (ja) | 2003-06-17 | 2006-12-07 | フローメディカ,インコーポレイテッド | 分岐血管に大動脈内物質を供給するための方法および装置 |
| WO2005091910A2 (en) * | 2004-03-04 | 2005-10-06 | Flowmedica, Inc. | Sheath for use in peripheral interventions |
| WO2005112980A2 (en) * | 2004-05-14 | 2005-12-01 | Flowmedica, Inc. | Bi-lateral local renal delivery for treating congestive heart failure and for bnp therapy |
| US7470252B2 (en) | 2004-09-16 | 2008-12-30 | Boston Scientific Scimed, Inc. | Expandable multi-port therapeutic delivery system |
| US20060069323A1 (en) * | 2004-09-24 | 2006-03-30 | Flowmedica, Inc. | Systems and methods for bi-lateral guidewire cannulation of branched body lumens |
| US7491188B2 (en) | 2004-10-12 | 2009-02-17 | Boston Scientific Scimed, Inc. | Reinforced and drug-eluting balloon catheters and methods for making same |
| US20060259066A1 (en) * | 2005-04-28 | 2006-11-16 | Euteneuer Charles L | Bifurcated artery filter system |
-
2003
- 2003-09-22 AU AU2003276903A patent/AU2003276903A1/en not_active Abandoned
- 2003-09-22 WO PCT/US2003/029744 patent/WO2004032791A2/en not_active Ceased
- 2003-09-22 JP JP2005501060A patent/JP2006508776A/ja active Pending
- 2003-09-22 EP EP03808092A patent/EP1539291A4/en not_active Withdrawn
-
2005
- 2005-03-16 US US11/084,738 patent/US7914503B2/en not_active Expired - Fee Related
-
2007
- 2007-06-26 US US11/768,390 patent/US8012121B2/en not_active Expired - Fee Related
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007521233A (ja) * | 2003-08-05 | 2007-08-02 | フロウメディカ, インコーポレイテッド | 放射線造影剤誘発性腎症の予防のためのシステムおよび方法 |
| JP2014508563A (ja) * | 2011-01-11 | 2014-04-10 | サイメティス エスアー | 経カテーテル大動脈弁埋込に有用な方法および装置 |
| JP2020142108A (ja) * | 2013-06-04 | 2020-09-10 | プリスティーン アクセス テクノロジーズ エルティーディーPristine Access Technologies Ltd | デュアルチップ血液透析カテーテル |
| JP7003180B2 (ja) | 2013-06-04 | 2022-01-20 | プリスティーン アクセス テクノロジーズ エルティーディー | デュアルチップ血液透析カテーテル |
| JP2018064786A (ja) * | 2016-10-20 | 2018-04-26 | 株式会社八光 | 分岐管 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2004032791A2 (en) | 2004-04-22 |
| US7914503B2 (en) | 2011-03-29 |
| US20070249997A1 (en) | 2007-10-25 |
| EP1539291A4 (en) | 2010-03-10 |
| WO2004032791A3 (en) | 2004-07-01 |
| US8012121B2 (en) | 2011-09-06 |
| US20060036218A1 (en) | 2006-02-16 |
| AU2003276903A8 (en) | 2004-05-04 |
| EP1539291A2 (en) | 2005-06-15 |
| AU2003276903A1 (en) | 2004-05-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2006508776A (ja) | 腎臓内カテーテルを介する選択的物質送達のための方法および装置 | |
| JP2006508776A5 (enExample) | ||
| US7364566B2 (en) | Method and apparatus for intra-aortic substance delivery to a branch vessel | |
| US6994700B2 (en) | Apparatus and method for inserting an intra-aorta catheter through a delivery sheath | |
| US7766961B2 (en) | Systems and methods for performing bi-lateral interventions or diagnosis in branched body lumens | |
| US20060030814A1 (en) | Method and apparatus for selective drug infusion via an intra-aortic flow diverter delivery catheter | |
| US20060167437A1 (en) | Method and apparatus for intra aortic substance delivery to a branch vessel | |
| JP2009509644A (ja) | 腎臓への注入システムおよび方法 | |
| US9126016B2 (en) | Augmented delivery catheter and method | |
| WO2004107965A2 (en) | Systems and methods for performing bi-lateral interventions or diagnosis in branched body lumens | |
| JP2006513809A5 (enExample) | ||
| US9956377B2 (en) | Method and apparatus for intra-aortic substance delivery to a branch vessel | |
| AU2013308439A1 (en) | Devices and methods for the treatment of vascular disease | |
| WO2015104631A1 (en) | Devices and methods for imaging and treating blood vessels | |
| JP2009511199A (ja) | 可変管腔構成の血管シース | |
| WO2005002660A1 (en) | Method and apparatus for intra aortic substance delivery to a branch vessel | |
| CN119604326A (zh) | 用于局部区域灌注系统的导管 | |
| EP4501387A1 (en) | A dual catheter arrangement and system for reperfusion of an ischemic tissue region via a coronary vessel |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20060731 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20060731 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20061108 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090710 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20091009 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20091019 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20100201 |