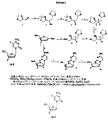
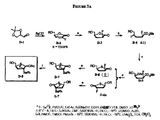
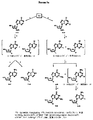
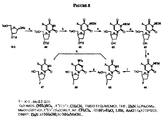
JP2005503358A - β−2’−または3’−ハロヌクレオシド - Google Patents
β−2’−または3’−ハロヌクレオシド Download PDFInfo
- Publication number
- JP2005503358A JP2005503358A JP2003506646A JP2003506646A JP2005503358A JP 2005503358 A JP2005503358 A JP 2005503358A JP 2003506646 A JP2003506646 A JP 2003506646A JP 2003506646 A JP2003506646 A JP 2003506646A JP 2005503358 A JP2005503358 A JP 2005503358A
- Authority
- JP
- Japan
- Prior art keywords
- alkyl
- aryl
- pharmaceutically acceptable
- halonucleoside
- alkoxyalkyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 150000001875 compounds Chemical class 0.000 claims abstract description 341
- 238000000034 method Methods 0.000 claims abstract description 161
- 238000011282 treatment Methods 0.000 claims abstract description 89
- 230000002159 abnormal effect Effects 0.000 claims abstract description 27
- 230000010261 cell growth Effects 0.000 claims abstract description 25
- -1 dimethylaminomethylene Chemical group 0.000 claims description 130
- 150000003839 salts Chemical class 0.000 claims description 89
- 229940002612 prodrug Drugs 0.000 claims description 85
- 239000000651 prodrug Substances 0.000 claims description 85
- 229910052731 fluorine Inorganic materials 0.000 claims description 78
- 125000001153 fluoro group Chemical group F* 0.000 claims description 66
- KDCGOANMDULRCW-UHFFFAOYSA-N Purine Natural products N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 claims description 63
- 239000001226 triphosphate Substances 0.000 claims description 59
- 235000011178 triphosphate Nutrition 0.000 claims description 58
- 125000005140 aralkylsulfonyl group Chemical group 0.000 claims description 56
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical class C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 claims description 55
- 125000004390 alkyl sulfonyl group Chemical group 0.000 claims description 55
- 125000004391 aryl sulfonyl group Chemical group 0.000 claims description 55
- 125000000539 amino acid group Chemical group 0.000 claims description 54
- 239000003814 drug Substances 0.000 claims description 53
- 229910052740 iodine Inorganic materials 0.000 claims description 49
- 229910052736 halogen Inorganic materials 0.000 claims description 47
- 150000002367 halogens Chemical class 0.000 claims description 46
- 239000008194 pharmaceutical composition Substances 0.000 claims description 46
- 229910019142 PO4 Chemical class 0.000 claims description 44
- 239000003937 drug carrier Substances 0.000 claims description 44
- 229910052794 bromium Inorganic materials 0.000 claims description 42
- 229910052801 chlorine Inorganic materials 0.000 claims description 42
- 239000010452 phosphate Chemical class 0.000 claims description 41
- 230000002265 prevention Effects 0.000 claims description 41
- 229910052760 oxygen Inorganic materials 0.000 claims description 40
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical class [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 claims description 38
- 229910052717 sulfur Inorganic materials 0.000 claims description 37
- UNXRWKVEANCORM-UHFFFAOYSA-N triphosphoric acid Chemical compound OP(O)(=O)OP(O)(=O)OP(O)(O)=O UNXRWKVEANCORM-UHFFFAOYSA-N 0.000 claims description 37
- 229940079593 drug Drugs 0.000 claims description 35
- 229910052739 hydrogen Inorganic materials 0.000 claims description 35
- 239000001257 hydrogen Substances 0.000 claims description 33
- 150000003212 purines Chemical class 0.000 claims description 33
- 125000001246 bromo group Chemical group Br* 0.000 claims description 31
- 125000001309 chloro group Chemical group Cl* 0.000 claims description 31
- 125000002346 iodo group Chemical group I* 0.000 claims description 31
- 239000003795 chemical substances by application Substances 0.000 claims description 29
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 claims description 29
- 150000002431 hydrogen Chemical class 0.000 claims description 29
- 208000015181 infectious disease Diseases 0.000 claims description 29
- 125000000217 alkyl group Chemical group 0.000 claims description 27
- 238000004519 manufacturing process Methods 0.000 claims description 26
- 230000001225 therapeutic effect Effects 0.000 claims description 25
- 208000031886 HIV Infections Diseases 0.000 claims description 24
- 239000005450 thionucleoside Substances 0.000 claims description 24
- 125000002252 acyl group Chemical group 0.000 claims description 23
- 125000002264 triphosphate group Chemical class [H]OP(=O)(O[H])OP(=O)(O[H])OP(=O)(O[H])O* 0.000 claims description 22
- 208000037357 HIV infectious disease Diseases 0.000 claims description 21
- 208000033519 human immunodeficiency virus infectious disease Diseases 0.000 claims description 21
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 claims description 20
- 230000002829 reductive effect Effects 0.000 claims description 20
- 230000001028 anti-proliverative effect Effects 0.000 claims description 18
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 16
- 102000014150 Interferons Human genes 0.000 claims description 14
- 108010050904 Interferons Proteins 0.000 claims description 14
- 125000001181 organosilyl group Chemical group [SiH3]* 0.000 claims description 14
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 claims description 13
- 229940079322 interferon Drugs 0.000 claims description 13
- 238000011321 prophylaxis Methods 0.000 claims description 13
- IGFXRKMLLMBKSA-UHFFFAOYSA-N purine Chemical compound N1=C[N]C2=NC=NC2=C1 IGFXRKMLLMBKSA-UHFFFAOYSA-N 0.000 claims description 13
- NQDJXKOVJZTUJA-UHFFFAOYSA-N nevirapine Chemical compound C12=NC=CC=C2C(=O)NC=2C(C)=CC=NC=2N1C1CC1 NQDJXKOVJZTUJA-UHFFFAOYSA-N 0.000 claims description 12
- 125000006239 protecting group Chemical group 0.000 claims description 12
- WREGKURFCTUGRC-POYBYMJQSA-N Zalcitabine Chemical compound O=C1N=C(N)C=CN1[C@@H]1O[C@H](CO)CC1 WREGKURFCTUGRC-POYBYMJQSA-N 0.000 claims description 11
- XPOQHMRABVBWPR-ZDUSSCGKSA-N efavirenz Chemical compound C([C@]1(C2=CC(Cl)=CC=C2NC(=O)O1)C(F)(F)F)#CC1CC1 XPOQHMRABVBWPR-ZDUSSCGKSA-N 0.000 claims description 11
- XQSPYNMVSIKCOC-UHFFFAOYSA-N 4-amino-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one Chemical compound C1=C(F)C(N)=NC(=O)N1C1OC(CO)SC1 XQSPYNMVSIKCOC-UHFFFAOYSA-N 0.000 claims description 10
- 230000003213 activating effect Effects 0.000 claims description 10
- 239000002259 anti human immunodeficiency virus agent Substances 0.000 claims description 10
- 229910052698 phosphorus Inorganic materials 0.000 claims description 10
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 claims description 10
- MLILORUFDVLTSP-UHFFFAOYSA-N emivirine Chemical compound O=C1NC(=O)N(COCC)C(CC=2C=CC=CC=2)=C1C(C)C MLILORUFDVLTSP-UHFFFAOYSA-N 0.000 claims description 9
- BXZVVICBKDXVGW-NKWVEPMBSA-N Didanosine Chemical compound O1[C@H](CO)CC[C@@H]1N1C(NC=NC2=O)=C2N=C1 BXZVVICBKDXVGW-NKWVEPMBSA-N 0.000 claims description 8
- 230000004663 cell proliferation Effects 0.000 claims description 8
- XSSYCIGJYCVRRK-RQJHMYQMSA-N (-)-carbovir Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@@H]1C[C@H](CO)C=C1 XSSYCIGJYCVRRK-RQJHMYQMSA-N 0.000 claims description 7
- HINZVVDZPLARRP-YSVIXOAZSA-N (4r,5s,6s,7r)-1,3-bis[(3-aminophenyl)methyl]-4,7-dibenzyl-5,6-dihydroxy-1,3-diazepan-2-one;methanesulfonic acid Chemical compound CS(O)(=O)=O.CS(O)(=O)=O.NC1=CC=CC(CN2C(N(CC=3C=C(N)C=CC=3)[C@H](CC=3C=CC=CC=3)[C@H](O)[C@@H](O)[C@H]2CC=2C=CC=CC=2)=O)=C1 HINZVVDZPLARRP-YSVIXOAZSA-N 0.000 claims description 7
- RLAHNGKRJJEIJL-RFZPGFLSSA-N [(2r,4r)-4-(2,6-diaminopurin-9-yl)-1,3-dioxolan-2-yl]methanol Chemical compound C12=NC(N)=NC(N)=C2N=CN1[C@H]1CO[C@@H](CO)O1 RLAHNGKRJJEIJL-RFZPGFLSSA-N 0.000 claims description 7
- MKUXAQIIEYXACX-UHFFFAOYSA-N aciclovir Chemical compound N1C(N)=NC(=O)C2=C1N(COCCO)C=N2 MKUXAQIIEYXACX-UHFFFAOYSA-N 0.000 claims description 7
- 229960002989 glutamic acid Drugs 0.000 claims description 7
- 239000001301 oxygen Substances 0.000 claims description 7
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical compound CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 claims description 6
- JNTOCHDNEULJHD-UHFFFAOYSA-N Penciclovir Chemical compound N1C(N)=NC(=O)C2=C1N(CCC(CO)CO)C=N2 JNTOCHDNEULJHD-UHFFFAOYSA-N 0.000 claims description 6
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 6
- 229960004150 aciclovir Drugs 0.000 claims description 6
- 229960004396 famciclovir Drugs 0.000 claims description 6
- GGXKWVWZWMLJEH-UHFFFAOYSA-N famcyclovir Chemical compound N1=C(N)N=C2N(CCC(COC(=O)C)COC(C)=O)C=NC2=C1 GGXKWVWZWMLJEH-UHFFFAOYSA-N 0.000 claims description 6
- 229960000689 nevirapine Drugs 0.000 claims description 6
- 229960001852 saquinavir Drugs 0.000 claims description 6
- QWAXKHKRTORLEM-UGJKXSETSA-N saquinavir Chemical compound C([C@@H]([C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)C=1N=C2C=CC=CC2=CC=1)C1=CC=CC=C1 QWAXKHKRTORLEM-UGJKXSETSA-N 0.000 claims description 6
- 229940054565 sustiva Drugs 0.000 claims description 6
- XNKLLVCARDGLGL-JGVFFNPUSA-N Stavudine Chemical compound O=C1NC(=O)C(C)=CN1[C@H]1C=C[C@@H](CO)O1 XNKLLVCARDGLGL-JGVFFNPUSA-N 0.000 claims description 5
- MCGSCOLBFJQGHM-SCZZXKLOSA-N abacavir Chemical compound C=12N=CN([C@H]3C=C[C@@H](CO)C3)C2=NC(N)=NC=1NC1CC1 MCGSCOLBFJQGHM-SCZZXKLOSA-N 0.000 claims description 5
- 229960004748 abacavir Drugs 0.000 claims description 5
- CBVCZFGXHXORBI-PXQQMZJSSA-N indinavir Chemical compound C([C@H](N(CC1)C[C@@H](O)C[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H]2C3=CC=CC=C3C[C@H]2O)C(=O)NC(C)(C)C)N1CC1=CC=CN=C1 CBVCZFGXHXORBI-PXQQMZJSSA-N 0.000 claims description 5
- 229960001936 indinavir Drugs 0.000 claims description 5
- YWBHROUQJYHSOR-UHFFFAOYSA-N $l^{1}-selanylbenzene Chemical compound [Se]C1=CC=CC=C1 YWBHROUQJYHSOR-UHFFFAOYSA-N 0.000 claims description 4
- 125000005843 halogen group Chemical group 0.000 claims description 4
- 239000011593 sulfur Substances 0.000 claims description 4
- LTYMSROWYAPPGB-UHFFFAOYSA-N diphenyl sulfide Chemical compound C=1C=CC=CC=1SC1=CC=CC=C1 LTYMSROWYAPPGB-UHFFFAOYSA-N 0.000 claims description 3
- 239000012039 electrophile Substances 0.000 claims description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 2
- 150000001413 amino acids Chemical class 0.000 claims description 2
- 150000003013 phosphoric acid derivatives Chemical class 0.000 claims 27
- 229910005965 SO 2 Inorganic materials 0.000 claims 26
- VKKXEIQIGGPMHT-UHFFFAOYSA-N 7h-purine-2,8-diamine Chemical compound NC1=NC=C2NC(N)=NC2=N1 VKKXEIQIGGPMHT-UHFFFAOYSA-N 0.000 claims 4
- WZPBZJONDBGPKJ-VEHQQRBSSA-N aztreonam Chemical compound O=C1N(S([O-])(=O)=O)[C@@H](C)[C@@H]1NC(=O)C(=N/OC(C)(C)C(O)=O)\C1=CSC([NH3+])=N1 WZPBZJONDBGPKJ-VEHQQRBSSA-N 0.000 claims 4
- 229940024606 amino acid Drugs 0.000 claims 1
- 238000002651 drug therapy Methods 0.000 claims 1
- 239000000203 mixture Substances 0.000 abstract description 112
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 121
- 239000002777 nucleoside Substances 0.000 description 121
- 210000004027 cell Anatomy 0.000 description 101
- 238000005481 NMR spectroscopy Methods 0.000 description 95
- 238000003786 synthesis reaction Methods 0.000 description 95
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 89
- 230000015572 biosynthetic process Effects 0.000 description 88
- 239000000243 solution Substances 0.000 description 88
- 230000000694 effects Effects 0.000 description 73
- 238000000921 elemental analysis Methods 0.000 description 60
- 125000003835 nucleoside group Chemical group 0.000 description 60
- 241000700721 Hepatitis B virus Species 0.000 description 57
- 238000001644 13C nuclear magnetic resonance spectroscopy Methods 0.000 description 54
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 46
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 45
- 230000000840 anti-viral effect Effects 0.000 description 44
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 44
- 239000007787 solid Substances 0.000 description 42
- 229910001868 water Inorganic materials 0.000 description 41
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 40
- 238000006243 chemical reaction Methods 0.000 description 40
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 40
- 235000019439 ethyl acetate Nutrition 0.000 description 40
- 206010028980 Neoplasm Diseases 0.000 description 39
- 239000000047 product Substances 0.000 description 39
- 239000011737 fluorine Substances 0.000 description 35
- 241000725303 Human immunodeficiency virus Species 0.000 description 34
- 239000011541 reaction mixture Substances 0.000 description 34
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 33
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 32
- 150000003833 nucleoside derivatives Chemical class 0.000 description 32
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 31
- OKKJLVBELUTLKV-MZCSYVLQSA-N Deuterated methanol Chemical compound [2H]OC([2H])([2H])[2H] OKKJLVBELUTLKV-MZCSYVLQSA-N 0.000 description 30
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 30
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical compound CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 description 28
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical class [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 27
- 230000036436 anti-hiv Effects 0.000 description 27
- 238000003556 assay Methods 0.000 description 27
- 239000003921 oil Substances 0.000 description 27
- 235000019198 oils Nutrition 0.000 description 27
- 239000000741 silica gel Substances 0.000 description 27
- 229910002027 silica gel Inorganic materials 0.000 description 27
- 241000700605 Viruses Species 0.000 description 26
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 26
- CSNNHWWHGAXBCP-UHFFFAOYSA-L magnesium sulphate Substances [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 26
- 108020004414 DNA Proteins 0.000 description 24
- 238000002360 preparation method Methods 0.000 description 24
- 230000003612 virological effect Effects 0.000 description 24
- 201000011510 cancer Diseases 0.000 description 23
- 238000010898 silica gel chromatography Methods 0.000 description 23
- 238000000746 purification Methods 0.000 description 22
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 21
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 21
- 239000003443 antiviral agent Substances 0.000 description 20
- 239000002585 base Substances 0.000 description 20
- 229940104302 cytosine Drugs 0.000 description 19
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 19
- 230000003647 oxidation Effects 0.000 description 19
- 238000007254 oxidation reaction Methods 0.000 description 19
- 230000003389 potentiating effect Effects 0.000 description 19
- 239000002904 solvent Substances 0.000 description 19
- 239000006228 supernatant Substances 0.000 description 19
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 18
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 18
- 239000012043 crude product Substances 0.000 description 18
- 208000002672 hepatitis B Diseases 0.000 description 18
- 239000000523 sample Substances 0.000 description 18
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 18
- 238000012360 testing method Methods 0.000 description 18
- 102100034343 Integrase Human genes 0.000 description 17
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 17
- 238000000354 decomposition reaction Methods 0.000 description 17
- 239000002609 medium Substances 0.000 description 17
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 16
- 239000007864 aqueous solution Substances 0.000 description 16
- 238000003379 elimination reaction Methods 0.000 description 16
- 235000021317 phosphate Nutrition 0.000 description 16
- 229940113082 thymine Drugs 0.000 description 16
- 230000008569 process Effects 0.000 description 15
- FTVLMFQEYACZNP-UHFFFAOYSA-N trimethylsilyl trifluoromethanesulfonate Chemical compound C[Si](C)(C)OS(=O)(=O)C(F)(F)F FTVLMFQEYACZNP-UHFFFAOYSA-N 0.000 description 15
- 230000009385 viral infection Effects 0.000 description 15
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Natural products O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 14
- 239000000706 filtrate Substances 0.000 description 14
- 238000003818 flash chromatography Methods 0.000 description 14
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 14
- 108090000623 proteins and genes Proteins 0.000 description 14
- UHDGCWIWMRVCDJ-UHFFFAOYSA-N 1-beta-D-Xylofuranosyl-NH-Cytosine Natural products O=C1N=C(N)C=CN1C1C(O)C(O)C(CO)O1 UHDGCWIWMRVCDJ-UHFFFAOYSA-N 0.000 description 13
- HBUBKKRHXORPQB-UUOKFMHZSA-N 2-fluoroadenosine Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O HBUBKKRHXORPQB-UUOKFMHZSA-N 0.000 description 13
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 13
- 102000055025 Adenosine deaminases Human genes 0.000 description 13
- 125000003118 aryl group Chemical group 0.000 description 13
- 230000004071 biological effect Effects 0.000 description 13
- 229960002949 fluorouracil Drugs 0.000 description 13
- 239000000126 substance Substances 0.000 description 13
- 239000000758 substrate Substances 0.000 description 13
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 12
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 12
- 102000004190 Enzymes Human genes 0.000 description 12
- 108090000790 Enzymes Proteins 0.000 description 12
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 12
- 241000711549 Hepacivirus C Species 0.000 description 12
- 238000011529 RT qPCR Methods 0.000 description 12
- 208000036142 Viral infection Diseases 0.000 description 12
- 239000003153 chemical reaction reagent Substances 0.000 description 12
- UHDGCWIWMRVCDJ-ZAKLUEHWSA-N cytidine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-ZAKLUEHWSA-N 0.000 description 12
- 229940088598 enzyme Drugs 0.000 description 12
- 238000004992 fast atom bombardment mass spectroscopy Methods 0.000 description 12
- 208000006454 hepatitis Diseases 0.000 description 12
- FFUAGWLWBBFQJT-UHFFFAOYSA-N hexamethyldisilazane Chemical compound C[Si](C)(C)N[Si](C)(C)C FFUAGWLWBBFQJT-UHFFFAOYSA-N 0.000 description 12
- 239000003112 inhibitor Substances 0.000 description 12
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 12
- 229920006395 saturated elastomer Polymers 0.000 description 12
- 101710169336 5'-deoxyadenosine deaminase Proteins 0.000 description 11
- 238000006482 condensation reaction Methods 0.000 description 11
- 238000000338 in vitro Methods 0.000 description 11
- 208000030507 AIDS Diseases 0.000 description 10
- 229930024421 Adenine Natural products 0.000 description 10
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 10
- NYHBQMYGNKIUIF-UUOKFMHZSA-N Guanosine Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O NYHBQMYGNKIUIF-UUOKFMHZSA-N 0.000 description 10
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 10
- 239000002253 acid Substances 0.000 description 10
- 229960000643 adenine Drugs 0.000 description 10
- 239000002246 antineoplastic agent Substances 0.000 description 10
- 230000003013 cytotoxicity Effects 0.000 description 10
- 231100000135 cytotoxicity Toxicity 0.000 description 10
- 238000011156 evaluation Methods 0.000 description 10
- 239000006260 foam Substances 0.000 description 10
- 108020004707 nucleic acids Proteins 0.000 description 10
- 102000039446 nucleic acids Human genes 0.000 description 10
- 150000007523 nucleic acids Chemical class 0.000 description 10
- 230000010076 replication Effects 0.000 description 10
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 description 9
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 9
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 9
- UHDGCWIWMRVCDJ-PSQAKQOGSA-N Cytidine Natural products O=C1N=C(N)C=CN1[C@@H]1[C@@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-PSQAKQOGSA-N 0.000 description 9
- 229960005305 adenosine Drugs 0.000 description 9
- 230000000259 anti-tumor effect Effects 0.000 description 9
- 238000002844 melting Methods 0.000 description 9
- 230000008018 melting Effects 0.000 description 9
- FPGGTKZVZWFYPV-UHFFFAOYSA-M tetrabutylammonium fluoride Chemical compound [F-].CCCC[N+](CCCC)(CCCC)CCCC FPGGTKZVZWFYPV-UHFFFAOYSA-M 0.000 description 9
- 241000710780 Bovine viral diarrhea virus 1 Species 0.000 description 8
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 8
- 108020005196 Mitochondrial DNA Proteins 0.000 description 8
- IWUCXVSUMQZMFG-AFCXAGJDSA-N Ribavirin Chemical compound N1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 IWUCXVSUMQZMFG-AFCXAGJDSA-N 0.000 description 8
- 108020005202 Viral DNA Proteins 0.000 description 8
- 239000012267 brine Substances 0.000 description 8
- 150000002148 esters Chemical class 0.000 description 8
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 8
- 239000000543 intermediate Substances 0.000 description 8
- 150000002576 ketones Chemical class 0.000 description 8
- 125000003729 nucleotide group Chemical group 0.000 description 8
- 239000012074 organic phase Substances 0.000 description 8
- 235000017557 sodium bicarbonate Nutrition 0.000 description 8
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 8
- 238000003756 stirring Methods 0.000 description 8
- 238000005160 1H NMR spectroscopy Methods 0.000 description 7
- ZKBQDFAWXLTYKS-UHFFFAOYSA-N 6-Chloro-1H-purine Chemical compound ClC1=NC=NC2=C1NC=N2 ZKBQDFAWXLTYKS-UHFFFAOYSA-N 0.000 description 7
- 108010085238 Actins Proteins 0.000 description 7
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical class N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 7
- 241000282412 Homo Species 0.000 description 7
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 7
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Natural products O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 7
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 7
- 108020000999 Viral RNA Proteins 0.000 description 7
- 239000006227 byproduct Substances 0.000 description 7
- 238000004587 chromatography analysis Methods 0.000 description 7
- 238000010511 deprotection reaction Methods 0.000 description 7
- XRECTZIEBJDKEO-UHFFFAOYSA-N flucytosine Chemical compound NC1=NC(=O)NC=C1F XRECTZIEBJDKEO-UHFFFAOYSA-N 0.000 description 7
- 229960004413 flucytosine Drugs 0.000 description 7
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 7
- 229940029575 guanosine Drugs 0.000 description 7
- 230000005764 inhibitory process Effects 0.000 description 7
- 230000002438 mitochondrial effect Effects 0.000 description 7
- 230000000269 nucleophilic effect Effects 0.000 description 7
- 239000002773 nucleotide Substances 0.000 description 7
- 125000002467 phosphate group Chemical class [H]OP(=O)(O[H])O[*] 0.000 description 7
- 238000012746 preparative thin layer chromatography Methods 0.000 description 7
- 229960000329 ribavirin Drugs 0.000 description 7
- 235000000346 sugar Nutrition 0.000 description 7
- 238000002560 therapeutic procedure Methods 0.000 description 7
- 210000001519 tissue Anatomy 0.000 description 7
- 229940035893 uracil Drugs 0.000 description 7
- ZTRXFCGYDLPIDD-UHFFFAOYSA-N 4-amino-1-benzoylpyrimidin-2-one Chemical compound O=C1N=C(N)C=CN1C(=O)C1=CC=CC=C1 ZTRXFCGYDLPIDD-UHFFFAOYSA-N 0.000 description 6
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 6
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 6
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 239000012298 atmosphere Substances 0.000 description 6
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 6
- GBBJCSTXCAQSSJ-XQXXSGGOSA-N clevudine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1[C@H](F)[C@@H](O)[C@H](CO)O1 GBBJCSTXCAQSSJ-XQXXSGGOSA-N 0.000 description 6
- 230000000875 corresponding effect Effects 0.000 description 6
- 238000005828 desilylation reaction Methods 0.000 description 6
- 201000010099 disease Diseases 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 239000000284 extract Substances 0.000 description 6
- LLYJISDUHFXOHK-GOCONZMPSA-N ferroptocide Chemical compound C[C@@H]1CC[C@@]23C[C@@H](C(=O)[C@]2([C@@]1([C@@H](C[C@H]([C@@H]3C)C4=CCN5C(=O)N(C(=O)N5C4)C6=CC=CC=C6)OC(=O)CCl)C)O)O LLYJISDUHFXOHK-GOCONZMPSA-N 0.000 description 6
- 231100000283 hepatitis Toxicity 0.000 description 6
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 6
- 239000011630 iodine Substances 0.000 description 6
- 230000002503 metabolic effect Effects 0.000 description 6
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 6
- 239000002212 purine nucleoside Substances 0.000 description 6
- 239000002718 pyrimidine nucleoside Substances 0.000 description 6
- HZCAHMRRMINHDJ-DBRKOABJSA-N ribavirin Natural products O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1N=CN=C1 HZCAHMRRMINHDJ-DBRKOABJSA-N 0.000 description 6
- 239000012047 saturated solution Substances 0.000 description 6
- 239000007858 starting material Substances 0.000 description 6
- 150000003463 sulfur Chemical class 0.000 description 6
- JTEGQNOMFQHVDC-RQJHMYQMSA-N 4-amino-1-[(2s,5r)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one Chemical compound O=C1N=C(N)C=CN1[C@@H]1O[C@H](CO)SC1 JTEGQNOMFQHVDC-RQJHMYQMSA-N 0.000 description 5
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 5
- 102100022900 Actin, cytoplasmic 1 Human genes 0.000 description 5
- MIKUYHXYGGJMLM-GIMIYPNGSA-N Crotonoside Natural products C1=NC2=C(N)NC(=O)N=C2N1[C@H]1O[C@@H](CO)[C@H](O)[C@@H]1O MIKUYHXYGGJMLM-GIMIYPNGSA-N 0.000 description 5
- MNQZXJOMYWMBOU-VKHMYHEASA-N D-glyceraldehyde Chemical compound OC[C@@H](O)C=O MNQZXJOMYWMBOU-VKHMYHEASA-N 0.000 description 5
- NYHBQMYGNKIUIF-UHFFFAOYSA-N D-guanosine Natural products C1=2NC(N)=NC(=O)C=2N=CN1C1OC(CO)C(O)C1O NYHBQMYGNKIUIF-UHFFFAOYSA-N 0.000 description 5
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 5
- UGQMRVRMYYASKQ-KQYNXXCUSA-N Inosine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(O)=C2N=C1 UGQMRVRMYYASKQ-KQYNXXCUSA-N 0.000 description 5
- MNQZXJOMYWMBOU-GSVOUGTGSA-N L-(-)-glyceraldehyde Chemical compound OC[C@H](O)C=O MNQZXJOMYWMBOU-GSVOUGTGSA-N 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 5
- 239000007832 Na2SO4 Substances 0.000 description 5
- 239000004098 Tetracycline Substances 0.000 description 5
- 150000007513 acids Chemical class 0.000 description 5
- 150000003838 adenosines Chemical class 0.000 description 5
- 229940100198 alkylating agent Drugs 0.000 description 5
- 239000002168 alkylating agent Substances 0.000 description 5
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 5
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 5
- 235000011130 ammonium sulphate Nutrition 0.000 description 5
- 230000000340 anti-metabolite Effects 0.000 description 5
- 229940100197 antimetabolite Drugs 0.000 description 5
- 239000002256 antimetabolite Substances 0.000 description 5
- 230000003115 biocidal effect Effects 0.000 description 5
- 229910052799 carbon Inorganic materials 0.000 description 5
- 239000012230 colorless oil Substances 0.000 description 5
- 238000004440 column chromatography Methods 0.000 description 5
- 238000002648 combination therapy Methods 0.000 description 5
- 125000004122 cyclic group Chemical group 0.000 description 5
- CSJLBAMHHLJAAS-UHFFFAOYSA-N diethylaminosulfur trifluoride Chemical compound CCN(CC)S(F)(F)F CSJLBAMHHLJAAS-UHFFFAOYSA-N 0.000 description 5
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 238000011534 incubation Methods 0.000 description 5
- 230000002458 infectious effect Effects 0.000 description 5
- 230000002401 inhibitory effect Effects 0.000 description 5
- 208000032839 leukemia Diseases 0.000 description 5
- 150000002632 lipids Chemical class 0.000 description 5
- YNESATAKKCNGOF-UHFFFAOYSA-N lithium bis(trimethylsilyl)amide Chemical compound [Li+].C[Si](C)(C)[N-][Si](C)(C)C YNESATAKKCNGOF-UHFFFAOYSA-N 0.000 description 5
- 230000004060 metabolic process Effects 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- 230000035772 mutation Effects 0.000 description 5
- 229910052757 nitrogen Inorganic materials 0.000 description 5
- ZJAOAACCNHFJAH-UHFFFAOYSA-N phosphonoformic acid Chemical compound OC(=O)P(O)(O)=O ZJAOAACCNHFJAH-UHFFFAOYSA-N 0.000 description 5
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 238000006722 reduction reaction Methods 0.000 description 5
- 238000010992 reflux Methods 0.000 description 5
- 239000003419 rna directed dna polymerase inhibitor Substances 0.000 description 5
- 150000003333 secondary alcohols Chemical class 0.000 description 5
- 229910052938 sodium sulfate Inorganic materials 0.000 description 5
- 238000011272 standard treatment Methods 0.000 description 5
- 125000001424 substituent group Chemical group 0.000 description 5
- 238000006467 substitution reaction Methods 0.000 description 5
- 229930101283 tetracycline Natural products 0.000 description 5
- 229960002180 tetracycline Drugs 0.000 description 5
- 235000019364 tetracycline Nutrition 0.000 description 5
- 150000003522 tetracyclines Chemical class 0.000 description 5
- 238000004809 thin layer chromatography Methods 0.000 description 5
- 230000001988 toxicity Effects 0.000 description 5
- 231100000419 toxicity Toxicity 0.000 description 5
- RIOQSEWOXXDEQQ-UHFFFAOYSA-N triphenylphosphine Chemical compound C1=CC=CC=C1P(C=1C=CC=CC=1)C1=CC=CC=C1 RIOQSEWOXXDEQQ-UHFFFAOYSA-N 0.000 description 5
- 210000003501 vero cell Anatomy 0.000 description 5
- HBOMLICNUCNMMY-XLPZGREQSA-N zidovudine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](N=[N+]=[N-])C1 HBOMLICNUCNMMY-XLPZGREQSA-N 0.000 description 5
- ZSNNBSPEFVIUDS-SHYZEUOFSA-N 1-[(2r,4s,5s)-4-azido-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione Chemical compound C1[C@H](N=[N+]=[N-])[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 ZSNNBSPEFVIUDS-SHYZEUOFSA-N 0.000 description 4
- XKKCQTLDIPIRQD-JGVFFNPUSA-N 1-[(2r,5s)-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)CC1 XKKCQTLDIPIRQD-JGVFFNPUSA-N 0.000 description 4
- OZAIFHULBGXAKX-UHFFFAOYSA-N 2-(2-cyanopropan-2-yldiazenyl)-2-methylpropanenitrile Chemical compound N#CC(C)(C)N=NC(C)(C)C#N OZAIFHULBGXAKX-UHFFFAOYSA-N 0.000 description 4
- OROGUZVNAFJPHA-UHFFFAOYSA-N 3-hydroxy-2,4-dimethyl-2H-thiophen-5-one Chemical compound CC1SC(=O)C(C)=C1O OROGUZVNAFJPHA-UHFFFAOYSA-N 0.000 description 4
- MOMUJZRKXYLWMH-SHYZEUOFSA-N 4-amino-1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)thiolan-2-yl]pyrimidin-2-one Chemical class O=C1N=C(N)C=CN1[C@@H]1S[C@H](CO)[C@@H](O)C1 MOMUJZRKXYLWMH-SHYZEUOFSA-N 0.000 description 4
- STRZQWQNZQMHQR-UAKXSSHOSA-N 5-fluorocytidine Chemical compound C1=C(F)C(N)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 STRZQWQNZQMHQR-UAKXSSHOSA-N 0.000 description 4
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 4
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 4
- ODZBBRURCPAEIQ-DJLDLDEBSA-N Brivudine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(C=CBr)=C1 ODZBBRURCPAEIQ-DJLDLDEBSA-N 0.000 description 4
- KCBAMQOKOLXLOX-BSZYMOERSA-N CC1=C(SC=N1)C2=CC=C(C=C2)[C@H](C)NC(=O)[C@@H]3C[C@H](CN3C(=O)[C@H](C(C)(C)C)NC(=O)CCCCCCCCCCNCCCONC(=O)C4=C(C(=C(C=C4)F)F)NC5=C(C=C(C=C5)I)F)O Chemical compound CC1=C(SC=N1)C2=CC=C(C=C2)[C@H](C)NC(=O)[C@@H]3C[C@H](CN3C(=O)[C@H](C(C)(C)C)NC(=O)CCCCCCCCCCNCCCONC(=O)C4=C(C(=C(C=C4)F)F)NC5=C(C=C(C=C5)I)F)O KCBAMQOKOLXLOX-BSZYMOERSA-N 0.000 description 4
- 241000713730 Equine infectious anemia virus Species 0.000 description 4
- 229930010555 Inosine Natural products 0.000 description 4
- 239000002841 Lewis acid Substances 0.000 description 4
- 241000699670 Mus sp. Species 0.000 description 4
- 102000043276 Oncogene Human genes 0.000 description 4
- 108700020796 Oncogene Proteins 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 229910021627 Tin(IV) chloride Inorganic materials 0.000 description 4
- 230000009102 absorption Effects 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- WOZSCQDILHKSGG-UHFFFAOYSA-N adefovir depivoxil Chemical compound N1=CN=C2N(CCOCP(=O)(OCOC(=O)C(C)(C)C)OCOC(=O)C(C)(C)C)C=NC2=C1N WOZSCQDILHKSGG-UHFFFAOYSA-N 0.000 description 4
- 230000001093 anti-cancer Effects 0.000 description 4
- 230000003602 anti-herpes Effects 0.000 description 4
- 230000000692 anti-sense effect Effects 0.000 description 4
- 239000000427 antigen Substances 0.000 description 4
- 108091007433 antigens Proteins 0.000 description 4
- 102000036639 antigens Human genes 0.000 description 4
- 125000003710 aryl alkyl group Chemical group 0.000 description 4
- CBHOOMGKXCMKIR-UHFFFAOYSA-N azane;methanol Chemical compound N.OC CBHOOMGKXCMKIR-UHFFFAOYSA-N 0.000 description 4
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 4
- 238000012925 biological evaluation Methods 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 230000015556 catabolic process Effects 0.000 description 4
- 230000003197 catalytic effect Effects 0.000 description 4
- 238000002512 chemotherapy Methods 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 4
- 229940125833 compound 23 Drugs 0.000 description 4
- 239000012228 culture supernatant Substances 0.000 description 4
- 238000006731 degradation reaction Methods 0.000 description 4
- 238000006345 epimerization reaction Methods 0.000 description 4
- 238000001704 evaporation Methods 0.000 description 4
- 230000008020 evaporation Effects 0.000 description 4
- 150000002243 furanoses Chemical class 0.000 description 4
- 229960003786 inosine Drugs 0.000 description 4
- 230000003834 intracellular effect Effects 0.000 description 4
- 150000002500 ions Chemical class 0.000 description 4
- JTEGQNOMFQHVDC-NKWVEPMBSA-N lamivudine Chemical compound O=C1N=C(N)C=CN1[C@H]1O[C@@H](CO)SC1 JTEGQNOMFQHVDC-NKWVEPMBSA-N 0.000 description 4
- 150000007517 lewis acids Chemical class 0.000 description 4
- 239000011777 magnesium Substances 0.000 description 4
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 4
- 239000013641 positive control Substances 0.000 description 4
- 150000003230 pyrimidines Chemical class 0.000 description 4
- 108700022487 rRNA Genes Proteins 0.000 description 4
- 238000002976 reverse transcriptase assay Methods 0.000 description 4
- 238000006884 silylation reaction Methods 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- 230000000707 stereoselective effect Effects 0.000 description 4
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 4
- RAHZWNYVWXNFOC-UHFFFAOYSA-N sulfur dioxide Inorganic materials O=S=O RAHZWNYVWXNFOC-UHFFFAOYSA-N 0.000 description 4
- 239000006188 syrup Substances 0.000 description 4
- 235000020357 syrup Nutrition 0.000 description 4
- 229940104230 thymidine Drugs 0.000 description 4
- HPGGPRDJHPYFRM-UHFFFAOYSA-J tin(iv) chloride Chemical compound Cl[Sn](Cl)(Cl)Cl HPGGPRDJHPYFRM-UHFFFAOYSA-J 0.000 description 4
- XJDNKRIXUMDJCW-UHFFFAOYSA-J titanium tetrachloride Chemical compound Cl[Ti](Cl)(Cl)Cl XJDNKRIXUMDJCW-UHFFFAOYSA-J 0.000 description 4
- 125000002221 trityl group Chemical group [H]C1=C([H])C([H])=C([H])C([H])=C1C([*])(C1=C(C(=C(C(=C1[H])[H])[H])[H])[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 4
- 230000029812 viral genome replication Effects 0.000 description 4
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 4
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 3
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 3
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 3
- JTEGQNOMFQHVDC-UHFFFAOYSA-N 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one Chemical compound O=C1N=C(N)C=CN1C1OC(CO)SC1 JTEGQNOMFQHVDC-UHFFFAOYSA-N 0.000 description 3
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical class OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 3
- 241000283690 Bos taurus Species 0.000 description 3
- IVOMOUWHDPKRLL-KQYNXXCUSA-N Cyclic adenosine monophosphate Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=CN=C2N)=C2N=C1 IVOMOUWHDPKRLL-KQYNXXCUSA-N 0.000 description 3
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 3
- XQSPYNMVSIKCOC-NTSWFWBYSA-N Emtricitabine Chemical compound C1=C(F)C(N)=NC(=O)N1[C@H]1O[C@@H](CO)SC1 XQSPYNMVSIKCOC-NTSWFWBYSA-N 0.000 description 3
- 229940126656 GS-4224 Drugs 0.000 description 3
- 229940126544 HIV-1 protease inhibitor Drugs 0.000 description 3
- 208000005176 Hepatitis C Diseases 0.000 description 3
- XQFRJNBWHJMXHO-RRKCRQDMSA-N IDUR Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(I)=C1 XQFRJNBWHJMXHO-RRKCRQDMSA-N 0.000 description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 3
- 241000701076 Macacine alphaherpesvirus 1 Species 0.000 description 3
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 3
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 3
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical compound OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 3
- 230000018199 S phase Effects 0.000 description 3
- 229910000831 Steel Inorganic materials 0.000 description 3
- IVOMOUWHDPKRLL-UHFFFAOYSA-N UNPD107823 Natural products O1C2COP(O)(=O)OC2C(O)C1N1C(N=CN=C2N)=C2N=C1 IVOMOUWHDPKRLL-UHFFFAOYSA-N 0.000 description 3
- 229960000583 acetic acid Drugs 0.000 description 3
- 230000002378 acidificating effect Effects 0.000 description 3
- 125000003282 alkyl amino group Chemical group 0.000 description 3
- 125000002877 alkyl aryl group Chemical group 0.000 description 3
- 230000003321 amplification Effects 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- 125000001769 aryl amino group Chemical group 0.000 description 3
- 230000006696 biosynthetic metabolic pathway Effects 0.000 description 3
- 210000001772 blood platelet Anatomy 0.000 description 3
- 210000000481 breast Anatomy 0.000 description 3
- 238000004113 cell culture Methods 0.000 description 3
- 230000001413 cellular effect Effects 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 239000003638 chemical reducing agent Substances 0.000 description 3
- IJOOHPMOJXWVHK-UHFFFAOYSA-N chlorotrimethylsilane Chemical compound C[Si](C)(C)Cl IJOOHPMOJXWVHK-UHFFFAOYSA-N 0.000 description 3
- 208000037976 chronic inflammation Diseases 0.000 description 3
- 230000006020 chronic inflammation Effects 0.000 description 3
- 230000000295 complement effect Effects 0.000 description 3
- 229940095074 cyclic amp Drugs 0.000 description 3
- 231100000433 cytotoxic Toxicity 0.000 description 3
- 230000001472 cytotoxic effect Effects 0.000 description 3
- SUYVUBYJARFZHO-RRKCRQDMSA-N dATP Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 SUYVUBYJARFZHO-RRKCRQDMSA-N 0.000 description 3
- SUYVUBYJARFZHO-UHFFFAOYSA-N dATP Natural products C1=NC=2C(N)=NC=NC=2N1C1CC(O)C(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 SUYVUBYJARFZHO-UHFFFAOYSA-N 0.000 description 3
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 230000014509 gene expression Effects 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 238000004128 high performance liquid chromatography Methods 0.000 description 3
- 230000007062 hydrolysis Effects 0.000 description 3
- 238000006460 hydrolysis reaction Methods 0.000 description 3
- 150000002596 lactones Chemical class 0.000 description 3
- 229960001627 lamivudine Drugs 0.000 description 3
- 239000003446 ligand Substances 0.000 description 3
- 229910052749 magnesium Inorganic materials 0.000 description 3
- 238000000302 molecular modelling Methods 0.000 description 3
- 238000010606 normalization Methods 0.000 description 3
- 238000010899 nucleation Methods 0.000 description 3
- 238000003199 nucleic acid amplification method Methods 0.000 description 3
- 230000003287 optical effect Effects 0.000 description 3
- 239000008188 pellet Substances 0.000 description 3
- 210000005259 peripheral blood Anatomy 0.000 description 3
- 239000011886 peripheral blood Substances 0.000 description 3
- 238000001050 pharmacotherapy Methods 0.000 description 3
- LCEFEIBEOBPPSJ-UHFFFAOYSA-N phenyl selenohypobromite Chemical compound Br[Se]C1=CC=CC=C1 LCEFEIBEOBPPSJ-UHFFFAOYSA-N 0.000 description 3
- 230000002062 proliferating effect Effects 0.000 description 3
- 238000011002 quantification Methods 0.000 description 3
- 238000003753 real-time PCR Methods 0.000 description 3
- 230000035945 sensitivity Effects 0.000 description 3
- 238000013207 serial dilution Methods 0.000 description 3
- 210000002966 serum Anatomy 0.000 description 3
- 239000002002 slurry Substances 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- 238000012453 sprague-dawley rat model Methods 0.000 description 3
- 239000010959 steel Substances 0.000 description 3
- 125000000446 sulfanediyl group Chemical group *S* 0.000 description 3
- QAOWNCQODCNURD-UHFFFAOYSA-N sulfuric acid Substances OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 3
- 230000001629 suppression Effects 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 208000011580 syndromic disease Diseases 0.000 description 3
- 238000010189 synthetic method Methods 0.000 description 3
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- DUYAAUVXQSMXQP-UHFFFAOYSA-M thioacetate Chemical compound CC([S-])=O DUYAAUVXQSMXQP-UHFFFAOYSA-M 0.000 description 3
- PNKZBZPLRKCVLI-UHFFFAOYSA-N (2-methylpropan-2-yl)oxybenzene Chemical compound CC(C)(C)OC1=CC=CC=C1 PNKZBZPLRKCVLI-UHFFFAOYSA-N 0.000 description 2
- IVWWFWFVSWOTLP-YVZVNANGSA-N (3'as,4r,7'as)-2,2,2',2'-tetramethylspiro[1,3-dioxolane-4,6'-4,7a-dihydro-3ah-[1,3]dioxolo[4,5-c]pyran]-7'-one Chemical compound C([C@@H]1OC(O[C@@H]1C1=O)(C)C)O[C@]21COC(C)(C)O2 IVWWFWFVSWOTLP-YVZVNANGSA-N 0.000 description 2
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 2
- YNGDWRXWKFWCJY-UHFFFAOYSA-N 1,4-Dihydropyridine Chemical compound C1C=CNC=C1 YNGDWRXWKFWCJY-UHFFFAOYSA-N 0.000 description 2
- HDMHBHNRWDNNCD-UHFFFAOYSA-N 1-[(2-hydroxyethoxy)methyl]-6-(phenylsulfanyl)thymine Chemical compound OCCOCN1C(=O)NC(=O)C(C)=C1SC1=CC=CC=C1 HDMHBHNRWDNNCD-UHFFFAOYSA-N 0.000 description 2
- FLAHATSCJQFZKB-UAKXSSHOSA-N 1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)thiolan-2-yl]-5-fluoropyrimidine-2,4-dione Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)S[C@H]1N1C(=O)NC(=O)C(F)=C1 FLAHATSCJQFZKB-UAKXSSHOSA-N 0.000 description 2
- IPVFGAYTKQKGBM-BYPJNBLXSA-N 1-[(2r,3s,4r,5r)-3-fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidine-2,4-dione Chemical compound F[C@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(I)=C1 IPVFGAYTKQKGBM-BYPJNBLXSA-N 0.000 description 2
- SCMFKRCYKTYWIW-UHFFFAOYSA-N 1-[3-(1,4,8,11-tetrazacyclotetradec-1-yl)propyl]-1,4,8,11-tetrazacyclotetradecane Chemical compound C1CCNCCNCCCNCCN1CCCN1CCCNCCNCCCNCC1 SCMFKRCYKTYWIW-UHFFFAOYSA-N 0.000 description 2
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 2
- BLGJHQMNSBYLEZ-UHFFFAOYSA-N 2-hydroxyethanesulfonamide Chemical compound NS(=O)(=O)CCO BLGJHQMNSBYLEZ-UHFFFAOYSA-N 0.000 description 2
- CLAHOZSYMRNIPY-UHFFFAOYSA-N 2-hydroxyethylurea Chemical compound NC(=O)NCCO CLAHOZSYMRNIPY-UHFFFAOYSA-N 0.000 description 2
- ZOOGRGPOEVQQDX-UUOKFMHZSA-N 3',5'-cyclic GMP Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=C(NC2=O)N)=C2N=C1 ZOOGRGPOEVQQDX-UUOKFMHZSA-N 0.000 description 2
- ZHHOTKZTEUZTHX-SHYZEUOFSA-N 4-amino-1-[(2r,3r,5s)-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@H](O)C[C@@H](CO)O1 ZHHOTKZTEUZTHX-SHYZEUOFSA-N 0.000 description 2
- OOBICGOWICFMIX-POYBYMJQSA-N 4-amino-1-[(2r,5s)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]pyrimidin-2-one Chemical compound O=C1N=C(N)C=CN1[C@H]1C=C[C@@H](CO)O1 OOBICGOWICFMIX-POYBYMJQSA-N 0.000 description 2
- HSBKFSPNDWWPSL-CAHLUQPWSA-N 4-amino-5-fluoro-1-[(2r,5s)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]pyrimidin-2-one Chemical compound C1=C(F)C(N)=NC(=O)N1[C@H]1C=C[C@@H](CO)O1 HSBKFSPNDWWPSL-CAHLUQPWSA-N 0.000 description 2
- FCZOVUJWOBSMSS-UHFFFAOYSA-N 5-[(6-aminopurin-9-yl)methyl]-5-methyl-3-methylideneoxolan-2-one Chemical compound C1=NC2=C(N)N=CN=C2N1CC1(C)CC(=C)C(=O)O1 FCZOVUJWOBSMSS-UHFFFAOYSA-N 0.000 description 2
- NFYXXBIRONUIPP-UHFFFAOYSA-N 5-ethyl-1-(phenylmethoxymethyl)-6-pyridin-2-ylsulfanylpyrimidine-2,4-dione Chemical compound C=1C=CC=CC=1COCN1C(=O)NC(=O)C(CC)=C1SC1=CC=CC=N1 NFYXXBIRONUIPP-UHFFFAOYSA-N 0.000 description 2
- ZPMSFPSRIQLYQI-UHFFFAOYSA-N 6-benzyl-1-(ethoxymethyl)-5-ethylpyrimidine-2,4-dione Chemical compound O=C1NC(=O)N(COCC)C(CC=2C=CC=CC=2)=C1CC ZPMSFPSRIQLYQI-UHFFFAOYSA-N 0.000 description 2
- UNRIYCIDCQDGQE-UHFFFAOYSA-N 6-chloro-2-fluoro-7h-purine Chemical compound FC1=NC(Cl)=C2NC=NC2=N1 UNRIYCIDCQDGQE-UHFFFAOYSA-N 0.000 description 2
- OYXZMSRRJOYLLO-UHFFFAOYSA-N 7alpha-Hydroxycholesterol Natural products OC1C=C2CC(O)CCC2(C)C2C1C1CCC(C(C)CCCC(C)C)C1(C)CC2 OYXZMSRRJOYLLO-UHFFFAOYSA-N 0.000 description 2
- OYXZMSRRJOYLLO-KGZHIOMZSA-N 7beta-hydroxycholesterol Chemical compound C([C@@H]1O)=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 OYXZMSRRJOYLLO-KGZHIOMZSA-N 0.000 description 2
- OZAIFHULBGXAKX-VAWYXSNFSA-N AIBN Substances N#CC(C)(C)\N=N\C(C)(C)C#N OZAIFHULBGXAKX-VAWYXSNFSA-N 0.000 description 2
- 206010001513 AIDS related complex Diseases 0.000 description 2
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 2
- 108700040115 Adenosine deaminases Proteins 0.000 description 2
- 201000004384 Alopecia Diseases 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 2
- 101100002068 Bacillus subtilis (strain 168) araR gene Proteins 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- 101150086475 COXII gene Proteins 0.000 description 2
- 241000282472 Canis lupus familiaris Species 0.000 description 2
- 206010008909 Chronic Hepatitis Diseases 0.000 description 2
- 102000016903 DNA Polymerase gamma Human genes 0.000 description 2
- 108010014080 DNA Polymerase gamma Proteins 0.000 description 2
- 230000006820 DNA synthesis Effects 0.000 description 2
- 102100026816 DNA-dependent metalloprotease SPRTN Human genes 0.000 description 2
- 101710175461 DNA-dependent metalloprotease SPRTN Proteins 0.000 description 2
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 2
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 2
- ZMJOVJSTYLQINE-UHFFFAOYSA-N Dichloroacetylene Chemical compound ClC#CCl ZMJOVJSTYLQINE-UHFFFAOYSA-N 0.000 description 2
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 206010059866 Drug resistance Diseases 0.000 description 2
- 241000283073 Equus caballus Species 0.000 description 2
- 241000713800 Feline immunodeficiency virus Species 0.000 description 2
- 206010016654 Fibrosis Diseases 0.000 description 2
- 241000710831 Flavivirus Species 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- 206010019759 Hepatitis chronic persistent Diseases 0.000 description 2
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 2
- 101900297506 Human immunodeficiency virus type 1 group M subtype B Reverse transcriptase/ribonuclease H Proteins 0.000 description 2
- 102000015696 Interleukins Human genes 0.000 description 2
- 108010063738 Interleukins Proteins 0.000 description 2
- 239000003810 Jones reagent Substances 0.000 description 2
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 2
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 2
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 2
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 2
- LVDRREOUMKACNJ-BKMJKUGQSA-N N-[(2R,3S)-2-(4-chlorophenyl)-1-(1,4-dimethyl-2-oxoquinolin-7-yl)-6-oxopiperidin-3-yl]-2-methylpropane-1-sulfonamide Chemical compound CC(C)CS(=O)(=O)N[C@H]1CCC(=O)N([C@@H]1c1ccc(Cl)cc1)c1ccc2c(C)cc(=O)n(C)c2c1 LVDRREOUMKACNJ-BKMJKUGQSA-N 0.000 description 2
- QOVYHDHLFPKQQG-NDEPHWFRSA-N N[C@@H](CCC(=O)N1CCC(CC1)NC1=C2C=CC=CC2=NC(NCC2=CN(CCCNCCCNC3CCCCC3)N=N2)=N1)C(O)=O Chemical compound N[C@@H](CCC(=O)N1CCC(CC1)NC1=C2C=CC=CC2=NC(NCC2=CN(CCCNCCCNC3CCCCC3)N=N2)=N1)C(O)=O QOVYHDHLFPKQQG-NDEPHWFRSA-N 0.000 description 2
- 206010028813 Nausea Diseases 0.000 description 2
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 2
- 108091093105 Nuclear DNA Proteins 0.000 description 2
- 108091028043 Nucleic acid sequence Proteins 0.000 description 2
- 235000019502 Orange oil Nutrition 0.000 description 2
- 241000282579 Pan Species 0.000 description 2
- 229930182555 Penicillin Natural products 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- 241000710778 Pestivirus Species 0.000 description 2
- 108091000080 Phosphotransferase Proteins 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- 102100036286 Purine nucleoside phosphorylase Human genes 0.000 description 2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- 239000007983 Tris buffer Substances 0.000 description 2
- 229920004890 Triton X-100 Polymers 0.000 description 2
- 239000013504 Triton X-100 Substances 0.000 description 2
- HBHJTJKGFOGKMG-LADGJGSJSA-N [(2r)-3-[[(2s,3s,5r)-3-azido-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-2-tetradecanoyloxypropyl] tetradecanoate Chemical compound C1[C@H](N=[N+]=[N-])[C@@H](COP(O)(=O)OC[C@@H](COC(=O)CCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)O[C@H]1N1C(=O)NC(=O)C(C)=C1 HBHJTJKGFOGKMG-LADGJGSJSA-N 0.000 description 2
- MKSZAUGMIGSRNS-UHFFFAOYSA-N [2-dodecanoyloxy-3-[hydroxy-[hydroxy-[[5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy]phosphoryl]oxyphosphoryl]oxypropyl] dodecanoate Chemical compound O1C(COP(O)(=O)OP(O)(=O)OCC(COC(=O)CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)CCC1N1C(=O)NC(=O)C(C)=C1 MKSZAUGMIGSRNS-UHFFFAOYSA-N 0.000 description 2
- 150000008065 acid anhydrides Chemical class 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 231100000354 acute hepatitis Toxicity 0.000 description 2
- 230000010933 acylation Effects 0.000 description 2
- 238000005917 acylation reaction Methods 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 229910052783 alkali metal Inorganic materials 0.000 description 2
- 150000001340 alkali metals Chemical class 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- 150000001342 alkaline earth metals Chemical class 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 230000029936 alkylation Effects 0.000 description 2
- 238000005804 alkylation reaction Methods 0.000 description 2
- 238000005576 amination reaction Methods 0.000 description 2
- 235000019270 ammonium chloride Nutrition 0.000 description 2
- 150000001450 anions Chemical class 0.000 description 2
- 229940124411 anti-hiv antiviral agent Drugs 0.000 description 2
- 239000004599 antimicrobial Substances 0.000 description 2
- 229940041181 antineoplastic drug Drugs 0.000 description 2
- 101150044616 araC gene Proteins 0.000 description 2
- 125000004104 aryloxy group Chemical group 0.000 description 2
- 239000012911 assay medium Substances 0.000 description 2
- 230000000975 bioactive effect Effects 0.000 description 2
- 235000010290 biphenyl Nutrition 0.000 description 2
- 239000004305 biphenyl Substances 0.000 description 2
- 210000001185 bone marrow Anatomy 0.000 description 2
- 125000002837 carbocyclic group Chemical group 0.000 description 2
- 150000001720 carbohydrates Chemical group 0.000 description 2
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 2
- 230000006652 catabolic pathway Effects 0.000 description 2
- 238000006555 catalytic reaction Methods 0.000 description 2
- KQIADDMXRMTWHZ-UHFFFAOYSA-N chloro-tri(propan-2-yl)silane Chemical compound CC(C)[Si](Cl)(C(C)C)C(C)C KQIADDMXRMTWHZ-UHFFFAOYSA-N 0.000 description 2
- 229960001231 choline Drugs 0.000 description 2
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 2
- ZCDOYSPFYFSLEW-UHFFFAOYSA-N chromate(2-) Chemical compound [O-][Cr]([O-])(=O)=O ZCDOYSPFYFSLEW-UHFFFAOYSA-N 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 2
- 230000007882 cirrhosis Effects 0.000 description 2
- 208000019425 cirrhosis of liver Diseases 0.000 description 2
- 229960004316 cisplatin Drugs 0.000 description 2
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 2
- 238000009833 condensation Methods 0.000 description 2
- 230000005494 condensation Effects 0.000 description 2
- 108091036078 conserved sequence Proteins 0.000 description 2
- 239000003246 corticosteroid Substances 0.000 description 2
- 229960001334 corticosteroids Drugs 0.000 description 2
- 239000010779 crude oil Substances 0.000 description 2
- 238000012866 crystallographic experiment Methods 0.000 description 2
- ZOOGRGPOEVQQDX-UHFFFAOYSA-N cyclic GMP Natural products O1C2COP(O)(=O)OC2C(O)C1N1C=NC2=C1NC(N)=NC2=O ZOOGRGPOEVQQDX-UHFFFAOYSA-N 0.000 description 2
- 229960000684 cytarabine Drugs 0.000 description 2
- 230000009615 deamination Effects 0.000 description 2
- 238000006481 deamination reaction Methods 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 239000005549 deoxyribonucleoside Substances 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- YMWUJEATGCHHMB-DICFDUPASA-N dichloromethane-d2 Chemical compound [2H]C([2H])(Cl)Cl YMWUJEATGCHHMB-DICFDUPASA-N 0.000 description 2
- 238000006251 dihalogenation reaction Methods 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 230000008030 elimination Effects 0.000 description 2
- 125000004185 ester group Chemical group 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 238000003682 fluorination reaction Methods 0.000 description 2
- 229960005102 foscarnet Drugs 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 2
- 239000012362 glacial acetic acid Substances 0.000 description 2
- 230000002140 halogenating effect Effects 0.000 description 2
- 230000026030 halogenation Effects 0.000 description 2
- 238000005658 halogenation reaction Methods 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 125000000623 heterocyclic group Chemical group 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- 229940031575 hydroxyethyl urea Drugs 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000002779 inactivation Effects 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 210000004347 intestinal mucosa Anatomy 0.000 description 2
- 230000009545 invasion Effects 0.000 description 2
- 230000026045 iodination Effects 0.000 description 2
- 238000006192 iodination reaction Methods 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 231100001231 less toxic Toxicity 0.000 description 2
- 229910052744 lithium Inorganic materials 0.000 description 2
- AMXOYNBUYSYVKV-UHFFFAOYSA-M lithium bromide Chemical compound [Li+].[Br-] AMXOYNBUYSYVKV-UHFFFAOYSA-M 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 229910001629 magnesium chloride Inorganic materials 0.000 description 2
- IERSPCAGKAOLSQ-UHFFFAOYSA-M magnesium;2-methanidyl-1,3-dioxolane;bromide Chemical group [Mg+2].[Br-].[CH2-]C1OCCO1 IERSPCAGKAOLSQ-UHFFFAOYSA-M 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- LBSANEJBGMCTBH-UHFFFAOYSA-N manganate Chemical compound [O-][Mn]([O-])(=O)=O LBSANEJBGMCTBH-UHFFFAOYSA-N 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 2
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 2
- 150000004712 monophosphates Chemical class 0.000 description 2
- 210000004165 myocardium Anatomy 0.000 description 2
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 2
- ORTIQSICVORWBJ-UHFFFAOYSA-N n-[4-chloro-3-(3-methylbut-2-enoxy)phenyl]-2-methylthiophene-3-carbothioamide Chemical compound C1=C(Cl)C(OCC=C(C)C)=CC(NC(=S)C2=C(SC=C2)C)=C1 ORTIQSICVORWBJ-UHFFFAOYSA-N 0.000 description 2
- 230000008693 nausea Effects 0.000 description 2
- LBQAJLBSGOBDQF-UHFFFAOYSA-N nitro azanylidynemethanesulfonate Chemical compound [O-][N+](=O)OS(=O)(=O)C#N LBQAJLBSGOBDQF-UHFFFAOYSA-N 0.000 description 2
- 239000012299 nitrogen atmosphere Substances 0.000 description 2
- 239000002726 nonnucleoside reverse transcriptase inhibitor Substances 0.000 description 2
- 108010009099 nucleoside phosphorylase Proteins 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 239000010502 orange oil Substances 0.000 description 2
- 125000002524 organometallic group Chemical group 0.000 description 2
- 230000020477 pH reduction Effects 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 229960001179 penciclovir Drugs 0.000 description 2
- 229940049954 penicillin Drugs 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- YBYRMVIVWMBXKQ-UHFFFAOYSA-N phenylmethanesulfonyl fluoride Chemical compound FS(=O)(=O)CC1=CC=CC=C1 YBYRMVIVWMBXKQ-UHFFFAOYSA-N 0.000 description 2
- UEZVMMHDMIWARA-UHFFFAOYSA-M phosphonate Chemical compound [O-]P(=O)=O UEZVMMHDMIWARA-UHFFFAOYSA-M 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 102000020233 phosphotransferase Human genes 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- YIQPUIGJQJDJOS-UHFFFAOYSA-N plerixafor Chemical compound C=1C=C(CN2CCNCCCNCCNCCC2)C=CC=1CN1CCCNCCNCCCNCC1 YIQPUIGJQJDJOS-UHFFFAOYSA-N 0.000 description 2
- 229920001467 poly(styrenesulfonates) Polymers 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 125000001844 prenyl group Chemical group [H]C([*])([H])C([H])=C(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 235000018102 proteins Nutrition 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 235000011962 puddings Nutrition 0.000 description 2
- 150000003214 pyranose derivatives Chemical class 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 230000002285 radioactive effect Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 229960000311 ritonavir Drugs 0.000 description 2
- NCDNCNXCDXHOMX-XGKFQTDJSA-N ritonavir Chemical compound N([C@@H](C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](CC=1C=CC=CC=1)NC(=O)OCC=1SC=NC=1)CC=1C=CC=CC=1)C(=O)N(C)CC1=CSC(C(C)C)=N1 NCDNCNXCDXHOMX-XGKFQTDJSA-N 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 239000010703 silicon Substances 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000011537 solubilization buffer Substances 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical compound OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 230000032895 transmembrane transport Effects 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- 230000005748 tumor development Effects 0.000 description 2
- 241001430294 unidentified retrovirus Species 0.000 description 2
- 230000002861 ventricular Effects 0.000 description 2
- 239000003039 volatile agent Substances 0.000 description 2
- UAOUIVVJBYDFKD-XKCDOFEDSA-N (1R,9R,10S,11R,12R,15S,18S,21R)-10,11,21-trihydroxy-8,8-dimethyl-14-methylidene-4-(prop-2-enylamino)-20-oxa-5-thia-3-azahexacyclo[9.7.2.112,15.01,9.02,6.012,18]henicosa-2(6),3-dien-13-one Chemical compound C([C@@H]1[C@@H](O)[C@@]23C(C1=C)=O)C[C@H]2[C@]12C(N=C(NCC=C)S4)=C4CC(C)(C)[C@H]1[C@H](O)[C@]3(O)OC2 UAOUIVVJBYDFKD-XKCDOFEDSA-N 0.000 description 1
- AMBQJZQQDVCAQE-RUAOOFDTSA-N (2R,3R,5S)-5-[[tert-butyl(diphenyl)silyl]oxymethyl]-2-(6-chloro-2-fluoropurin-9-yl)-3-fluoro-3-phenylthiolane-2-selenol Chemical compound C(C)(C)(C)[Si](OC[C@@H]1C[C@]([C@](S1)(N1C2=NC(=NC(=C2N=C1)Cl)F)[SeH])(C1=CC=CC=C1)F)(C1=CC=CC=C1)C1=CC=CC=C1 AMBQJZQQDVCAQE-RUAOOFDTSA-N 0.000 description 1
- HBUBKKRHXORPQB-FJFJXFQQSA-N (2R,3S,4S,5R)-2-(6-amino-2-fluoro-9-purinyl)-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@@H]1O HBUBKKRHXORPQB-FJFJXFQQSA-N 0.000 description 1
- SEUZBEQSAMGKOO-MRXNPFEDSA-N (2r)-2-[[tert-butyl(diphenyl)silyl]oxymethyl]-4-fluoro-2h-furan-5-one Chemical compound C=1C=CC=CC=1[Si](C=1C=CC=CC=1)(C(C)(C)C)OC[C@@H]1OC(=O)C(F)=C1 SEUZBEQSAMGKOO-MRXNPFEDSA-N 0.000 description 1
- IUSARDYWEPUTPN-OZBXUNDUSA-N (2r)-n-[(2s,3r)-4-[[(4s)-6-(2,2-dimethylpropyl)spiro[3,4-dihydropyrano[2,3-b]pyridine-2,1'-cyclobutane]-4-yl]amino]-3-hydroxy-1-[3-(1,3-thiazol-2-yl)phenyl]butan-2-yl]-2-methoxypropanamide Chemical compound C([C@H](NC(=O)[C@@H](C)OC)[C@H](O)CN[C@@H]1C2=CC(CC(C)(C)C)=CN=C2OC2(CCC2)C1)C(C=1)=CC=CC=1C1=NC=CS1 IUSARDYWEPUTPN-OZBXUNDUSA-N 0.000 description 1
- SEUZBEQSAMGKOO-INIZCTEOSA-N (2s)-2-[[tert-butyl(diphenyl)silyl]oxymethyl]-4-fluoro-2h-furan-5-one Chemical compound C=1C=CC=CC=1[Si](C=1C=CC=CC=1)(C(C)(C)C)OC[C@H]1OC(=O)C(F)=C1 SEUZBEQSAMGKOO-INIZCTEOSA-N 0.000 description 1
- IYKXRORSPVZSHP-ZELIPEIJSA-N (2s)-n-[(2s,3r)-4-[tert-butylcarbamoyl(3-methylbutyl)amino]-3-hydroxy-1-phenylbutan-2-yl]-3,3-dimethyl-2-[[2-(methylamino)acetyl]amino]butanamide;hydrochloride Chemical compound Cl.CNCC(=O)N[C@@H](C(C)(C)C)C(=O)N[C@H]([C@H](O)CN(CCC(C)C)C(=O)NC(C)(C)C)CC1=CC=CC=C1 IYKXRORSPVZSHP-ZELIPEIJSA-N 0.000 description 1
- QPVWMQXBTCSLCB-BYAJYZPISA-N (2s)-n-[(2s,3s,4r,5s)-3,4-dihydroxy-5-[[(2s)-3-methyl-2-[[methyl(pyridin-2-ylmethyl)carbamoyl]amino]butanoyl]amino]-1,6-diphenylhexan-2-yl]-3-methyl-2-[[methyl(pyridin-2-ylmethyl)carbamoyl]amino]butanamide Chemical compound N([C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)[C@H](O)[C@H](O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)N(C)CC=1N=CC=CC=1)C(C)C)C(=O)N(C)CC1=CC=CC=N1 QPVWMQXBTCSLCB-BYAJYZPISA-N 0.000 description 1
- STBLNCCBQMHSRC-BATDWUPUSA-N (2s)-n-[(3s,4s)-5-acetyl-7-cyano-4-methyl-1-[(2-methylnaphthalen-1-yl)methyl]-2-oxo-3,4-dihydro-1,5-benzodiazepin-3-yl]-2-(methylamino)propanamide Chemical compound O=C1[C@@H](NC(=O)[C@H](C)NC)[C@H](C)N(C(C)=O)C2=CC(C#N)=CC=C2N1CC1=C(C)C=CC2=CC=CC=C12 STBLNCCBQMHSRC-BATDWUPUSA-N 0.000 description 1
- LDSJMFGYNFIFRK-IUCAKERBSA-N (2s,3s)-3-azaniumyl-2-hydroxy-4-phenylbutanoate Chemical compound OC(=O)[C@@H](O)[C@@H](N)CC1=CC=CC=C1 LDSJMFGYNFIFRK-IUCAKERBSA-N 0.000 description 1
- JBZYNPYBIPKTAW-RRKCRQDMSA-N (2s,3s,5r)-2-(hydroxymethyl)-5-(6-oxo-3h-purin-9-yl)oxolane-3-carboxylic acid Chemical compound C1[C@H](C(O)=O)[C@@H](CO)O[C@H]1N1C(NC=NC2=O)=C2N=C1 JBZYNPYBIPKTAW-RRKCRQDMSA-N 0.000 description 1
- WIHAFSSIBZQPSO-VQIMIIECSA-N (3R,5R)-5-[[tert-butyl(diphenyl)silyl]oxymethyl]-3-fluorooxolane-2-thione Chemical compound [Si](C1=CC=CC=C1)(C1=CC=CC=C1)(C(C)(C)C)OC[C@H]1C[C@H](C(=S)O1)F WIHAFSSIBZQPSO-VQIMIIECSA-N 0.000 description 1
- OPGKSOIBRDZMGZ-KRWDZBQOSA-N (3S)-3-[[tert-butyl(diphenyl)silyl]oxymethyl]oxolane-2-thione Chemical compound [Si](C1=CC=CC=C1)(C1=CC=CC=C1)(C(C)(C)C)OC[C@H]1C(=S)OCC1 OPGKSOIBRDZMGZ-KRWDZBQOSA-N 0.000 description 1
- DLRSFCJLDNERBG-RZIURPKCSA-N (3S)-4-[[tert-butyl(diphenyl)silyl]oxymethyl]-3-phenyl-3-selanyloxolane-2-thione Chemical compound [Si](C1=CC=CC=C1)(C1=CC=CC=C1)(C(C)(C)C)OCC1[C@@](C(=S)OC1)(C1=CC=CC=C1)[SeH] DLRSFCJLDNERBG-RZIURPKCSA-N 0.000 description 1
- WIHAFSSIBZQPSO-LPHOPBHVSA-N (3S,5S)-5-[[tert-butyl(diphenyl)silyl]oxymethyl]-3-fluorooxolane-2-thione Chemical compound [Si](C1=CC=CC=C1)(C1=CC=CC=C1)(C(C)(C)C)OC[C@@H]1C[C@@H](C(=S)O1)F WIHAFSSIBZQPSO-LPHOPBHVSA-N 0.000 description 1
- SIGIJBMOORTVPB-YTXCHSKXSA-N (3r,4r,5r)-2-(6-aminopurin-9-yl)-5-(hydroxymethyl)thiolane-3,4-diol Chemical compound C1=NC=2C(N)=NC=NC=2N1C1S[C@H](CO)[C@H](O)[C@H]1O SIGIJBMOORTVPB-YTXCHSKXSA-N 0.000 description 1
- GYUNRABPNCIDMG-VQIMIIECSA-N (3r,5r)-5-[[tert-butyl(diphenyl)silyl]oxymethyl]-3-fluorooxolan-2-one Chemical compound C=1C=CC=CC=1[Si](C=1C=CC=CC=1)(C(C)(C)C)OC[C@H]1C[C@@H](F)C(=O)O1 GYUNRABPNCIDMG-VQIMIIECSA-N 0.000 description 1
- GYUNRABPNCIDMG-LPHOPBHVSA-N (3s,5s)-5-[[tert-butyl(diphenyl)silyl]oxymethyl]-3-fluorooxolan-2-one Chemical compound C=1C=CC=CC=1[Si](C=1C=CC=CC=1)(C(C)(C)C)OC[C@@H]1C[C@H](F)C(=O)O1 GYUNRABPNCIDMG-LPHOPBHVSA-N 0.000 description 1
- ZYZCALPXKGUGJI-DDVDASKDSA-M (e,3r,5s)-7-[3-(4-fluorophenyl)-2-phenyl-5-propan-2-ylimidazol-4-yl]-3,5-dihydroxyhept-6-enoate Chemical compound C=1C=C(F)C=CC=1N1C(\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)=C(C(C)C)N=C1C1=CC=CC=C1 ZYZCALPXKGUGJI-DDVDASKDSA-M 0.000 description 1
- GWKIPRVERALPRD-ZDUSSCGKSA-N (s)-4-isopropoxycarbonyl-6-methoxy-3-methylthiomethyl-3,4-dihydroquinoxalin-2(1h)-thione Chemical compound N1C(=S)[C@H](CSC)N(C(=O)OC(C)C)C2=CC(OC)=CC=C21 GWKIPRVERALPRD-ZDUSSCGKSA-N 0.000 description 1
- SCYULBFZEHDVBN-UHFFFAOYSA-N 1,1-Dichloroethane Chemical compound CC(Cl)Cl SCYULBFZEHDVBN-UHFFFAOYSA-N 0.000 description 1
- FYADHXFMURLYQI-UHFFFAOYSA-N 1,2,4-triazine Chemical compound C1=CN=NC=N1 FYADHXFMURLYQI-UHFFFAOYSA-N 0.000 description 1
- CXWGKAYMVASWDQ-UHFFFAOYSA-N 1,2-dithiane Chemical compound C1CCSSC1 CXWGKAYMVASWDQ-UHFFFAOYSA-N 0.000 description 1
- DJMOXMNDXFFONV-UHFFFAOYSA-N 1,3-dimethyl-7-[2-(n-methylanilino)ethyl]purine-2,6-dione Chemical compound C1=NC=2N(C)C(=O)N(C)C(=O)C=2N1CCN(C)C1=CC=CC=C1 DJMOXMNDXFFONV-UHFFFAOYSA-N 0.000 description 1
- KKHFRAFPESRGGD-UHFFFAOYSA-N 1,3-dimethyl-7-[3-(n-methylanilino)propyl]purine-2,6-dione Chemical compound C1=NC=2N(C)C(=O)N(C)C(=O)C=2N1CCCN(C)C1=CC=CC=C1 KKHFRAFPESRGGD-UHFFFAOYSA-N 0.000 description 1
- SYZIUAAQNFJPJY-UHFFFAOYSA-N 1,4-dihydro-3,1-benzoxazin-2-one Chemical compound C1=CC=C2COC(=O)NC2=C1 SYZIUAAQNFJPJY-UHFFFAOYSA-N 0.000 description 1
- SXSIHVNMXOMEDS-UHFFFAOYSA-N 1-(2-chlorophenyl)-n-methylpropan-2-amine Chemical compound CNC(C)CC1=CC=CC=C1Cl SXSIHVNMXOMEDS-UHFFFAOYSA-N 0.000 description 1
- BIAAQBNMRITRDV-UHFFFAOYSA-N 1-(chloromethoxy)-2-methoxyethane Chemical compound COCCOCCl BIAAQBNMRITRDV-UHFFFAOYSA-N 0.000 description 1
- IGFIJVXZVHIJST-UHFFFAOYSA-N 1-(ethoxymethyl)-5-ethyl-6-phenylselanylpyrimidine-2,4-dione Chemical compound O=C1NC(=O)N(COCC)C([Se]C=2C=CC=CC=2)=C1CC IGFIJVXZVHIJST-UHFFFAOYSA-N 0.000 description 1
- CJUYXNGOWAETEX-UHFFFAOYSA-N 1-(ethoxymethyl)-5-ethyl-6-phenylsulfanylpyrimidine-2,4-dione Chemical compound O=C1NC(=O)N(COCC)C(SC=2C=CC=CC=2)=C1CC CJUYXNGOWAETEX-UHFFFAOYSA-N 0.000 description 1
- KQZLRWGGWXJPOS-NLFPWZOASA-N 1-[(1R)-1-(2,4-dichlorophenyl)ethyl]-6-[(4S,5R)-4-[(2S)-2-(hydroxymethyl)pyrrolidin-1-yl]-5-methylcyclohexen-1-yl]pyrazolo[3,4-b]pyrazine-3-carbonitrile Chemical compound ClC1=C(C=CC(=C1)Cl)[C@@H](C)N1N=C(C=2C1=NC(=CN=2)C1=CC[C@@H]([C@@H](C1)C)N1[C@@H](CCC1)CO)C#N KQZLRWGGWXJPOS-NLFPWZOASA-N 0.000 description 1
- QWZNMUPRNVZTEK-XVKPBYJWSA-N 1-[(2R,5S)-2-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione Chemical compound O=C1NC(=O)C(C)=CN1[C@]1(N=[N+]=[N-])O[C@H](CO)CC1 QWZNMUPRNVZTEK-XVKPBYJWSA-N 0.000 description 1
- IVUDHNYLTWOARV-XLPZGREQSA-N 1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)thiolan-2-yl]-5-methylpyrimidine-2,4-dione Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1S[C@H](CO)[C@@H](O)C1 IVUDHNYLTWOARV-XLPZGREQSA-N 0.000 description 1
- IAYDHFWPBIVIBO-MUWHJKNJSA-N 1-[(2s,5r)-3-fluoro-5-(hydroxymethyl)-2,5-dihydrothiophen-2-yl]-5-methylpyrimidine-2,4-dione Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1C(F)=C[C@H](CO)S1 IAYDHFWPBIVIBO-MUWHJKNJSA-N 0.000 description 1
- JPSWGILVBMOSJN-XRGYYRRGSA-N 1-[(2s,5r)-3-fluoro-5-(hydroxymethyl)-2,5-dihydrothiophen-2-yl]pyrimidine-2,4-dione Chemical compound FC1=C[C@H](CO)S[C@@H]1N1C(=O)NC(=O)C=C1 JPSWGILVBMOSJN-XRGYYRRGSA-N 0.000 description 1
- KZKYYGSZDVZKNJ-XDSPWSPCSA-N 1-[(5r,6r,8r,9r)-4-amino-9-[tert-butyl(dimethyl)silyl]oxy-6-[[tert-butyl(dimethyl)silyl]oxymethyl]-2,2-dioxo-1,7-dioxa-2$l^{6}-thiaspiro[4.4]non-3-en-8-yl]-3,5-dimethylpyrimidine-2,4-dione Chemical compound O=C1N(C)C(=O)C(C)=CN1[C@H]1[C@H](O[Si](C)(C)C(C)(C)C)[C@@]2(C(=CS(=O)(=O)O2)N)[C@@H](CO[Si](C)(C)C(C)(C)C)O1 KZKYYGSZDVZKNJ-XDSPWSPCSA-N 0.000 description 1
- VSNHCAURESNICA-NJFSPNSNSA-N 1-oxidanylurea Chemical compound N[14C](=O)NO VSNHCAURESNICA-NJFSPNSNSA-N 0.000 description 1
- FUFLCEKSBBHCMO-UHFFFAOYSA-N 11-dehydrocorticosterone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 FUFLCEKSBBHCMO-UHFFFAOYSA-N 0.000 description 1
- GCKMFJBGXUYNAG-UHFFFAOYSA-N 17alpha-methyltestosterone Natural products C1CC2=CC(=O)CCC2(C)C2C1C1CCC(C)(O)C1(C)CC2 GCKMFJBGXUYNAG-UHFFFAOYSA-N 0.000 description 1
- ASOMNDIOOKDVDC-UHFFFAOYSA-N 1h-indol-2-yl-[4-[3-(propan-2-ylamino)pyridin-2-yl]piperazin-1-yl]methanone Chemical compound CC(C)NC1=CC=CN=C1N1CCN(C(=O)C=2NC3=CC=CC=C3C=2)CC1 ASOMNDIOOKDVDC-UHFFFAOYSA-N 0.000 description 1
- MGAXHFMCFLLMNG-UHFFFAOYSA-N 1h-pyrimidine-6-thione Chemical compound SC1=CC=NC=N1 MGAXHFMCFLLMNG-UHFFFAOYSA-N 0.000 description 1
- YKBGVTZYEHREMT-KVQBGUIXSA-N 2'-deoxyguanosine Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)O1 YKBGVTZYEHREMT-KVQBGUIXSA-N 0.000 description 1
- YKBGVTZYEHREMT-UHFFFAOYSA-N 2'-deoxyguanosine Natural products C1=2NC(N)=NC(=O)C=2N=CN1C1CC(O)C(CO)O1 YKBGVTZYEHREMT-UHFFFAOYSA-N 0.000 description 1
- BTOTXLJHDSNXMW-POYBYMJQSA-N 2,3-dideoxyuridine Chemical compound O1[C@H](CO)CC[C@@H]1N1C(=O)NC(=O)C=C1 BTOTXLJHDSNXMW-POYBYMJQSA-N 0.000 description 1
- OOYZSQIVHXKAPI-UHFFFAOYSA-N 2,3-dihydroxybutanedioic acid;propanedioic acid Chemical compound OC(=O)CC(O)=O.OC(=O)C(O)C(O)C(O)=O OOYZSQIVHXKAPI-UHFFFAOYSA-N 0.000 description 1
- JYWKEVKEKOTYEX-UHFFFAOYSA-N 2,6-dibromo-4-chloroiminocyclohexa-2,5-dien-1-one Chemical compound ClN=C1C=C(Br)C(=O)C(Br)=C1 JYWKEVKEKOTYEX-UHFFFAOYSA-N 0.000 description 1
- WGFNXGPBPIJYLI-UHFFFAOYSA-N 2,6-difluoro-3-[(3-fluorophenyl)sulfonylamino]-n-(3-methoxy-1h-pyrazolo[3,4-b]pyridin-5-yl)benzamide Chemical compound C1=C2C(OC)=NNC2=NC=C1NC(=O)C(C=1F)=C(F)C=CC=1NS(=O)(=O)C1=CC=CC(F)=C1 WGFNXGPBPIJYLI-UHFFFAOYSA-N 0.000 description 1
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical class NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 1
- OIZHACWNMXZCRJ-UHFFFAOYSA-N 2-[2-(5-ethyl-6-methyl-2-oxo-1h-pyridin-3-yl)ethyl]isoindole-1,3-dione Chemical compound O=C1NC(C)=C(CC)C=C1CCN1C(=O)C2=CC=CC=C2C1=O OIZHACWNMXZCRJ-UHFFFAOYSA-N 0.000 description 1
- VVCMGAUPZIKYTH-VGHSCWAPSA-N 2-acetyloxybenzoic acid;[(2s,3r)-4-(dimethylamino)-3-methyl-1,2-diphenylbutan-2-yl] propanoate;1,3,7-trimethylpurine-2,6-dione Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O.CN1C(=O)N(C)C(=O)C2=C1N=CN2C.C([C@](OC(=O)CC)([C@H](C)CN(C)C)C=1C=CC=CC=1)C1=CC=CC=C1 VVCMGAUPZIKYTH-VGHSCWAPSA-N 0.000 description 1
- NPRYCHLHHVWLQZ-TURQNECASA-N 2-amino-9-[(2R,3S,4S,5R)-4-fluoro-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-7-prop-2-ynylpurin-8-one Chemical compound NC1=NC=C2N(C(N(C2=N1)[C@@H]1O[C@@H]([C@H]([C@H]1O)F)CO)=O)CC#C NPRYCHLHHVWLQZ-TURQNECASA-N 0.000 description 1
- SWNBTENWDRLOJC-MOFOKWOHSA-N 2-amino-9-[(2s,5r)-3-fluoro-5-(hydroxymethyl)-2,5-dihydrothiophen-2-yl]-3h-purin-6-one Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1[C@H]1S[C@@H](CO)C=C1F SWNBTENWDRLOJC-MOFOKWOHSA-N 0.000 description 1
- VBYYJZIWKPTMIE-UHFFFAOYSA-N 2-chloropurin-2-amine Chemical compound NC1(Cl)N=CC2=NC=NC2=N1 VBYYJZIWKPTMIE-UHFFFAOYSA-N 0.000 description 1
- YZHIXLCGPOTQNB-UHFFFAOYSA-N 2-methyl-furan-3-carbothioic acid [4-chloro-3-(3-methyl-but-2-enyloxy)-phenyl]-amide Chemical compound C1=C(Cl)C(OCC=C(C)C)=CC(NC(=S)C2=C(OC=C2)C)=C1 YZHIXLCGPOTQNB-UHFFFAOYSA-N 0.000 description 1
- KPGXRSRHYNQIFN-UHFFFAOYSA-N 2-oxoglutaric acid Chemical compound OC(=O)CCC(=O)C(O)=O KPGXRSRHYNQIFN-UHFFFAOYSA-N 0.000 description 1
- BPSNETAIJADFTO-UHFFFAOYSA-M 2-pyridin-2-ylacetate Chemical compound [O-]C(=O)CC1=CC=CC=N1 BPSNETAIJADFTO-UHFFFAOYSA-M 0.000 description 1
- FTZIQBGFCYJWKA-UHFFFAOYSA-N 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Chemical compound S1C(C)=C(C)N=C1[N+]1=NC(C=2C=CC=CC=2)=NN1C1=CC=CC=C1 FTZIQBGFCYJWKA-UHFFFAOYSA-N 0.000 description 1
- 125000005917 3-methylpentyl group Chemical group 0.000 description 1
- LTDCCBLBAQXNKP-SHYZEUOFSA-N 4-amino-1-[(2r,3r,5s)-3-fluoro-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@H](F)C[C@@H](CO)O1 LTDCCBLBAQXNKP-SHYZEUOFSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-YDKYIBAVSA-N 4-amino-1-[(2r,3s,4r,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-YDKYIBAVSA-N 0.000 description 1
- CKTSBUTUHBMZGZ-ULQXZJNLSA-N 4-amino-1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-tritiopyrimidin-2-one Chemical class O=C1N=C(N)C([3H])=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 CKTSBUTUHBMZGZ-ULQXZJNLSA-N 0.000 description 1
- YCSAJGCTBARBQI-SPGJFGJESA-N 4-amino-1-[(2r,5r)-3,4-dihydroxy-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]pyrimidin-2-one Chemical compound O=C1N=C(N)C=CN1[C@H]1C(O)=C(O)[C@@H](CO)O1 YCSAJGCTBARBQI-SPGJFGJESA-N 0.000 description 1
- CKTSBUTUHBMZGZ-CHKWXVPMSA-N 4-amino-1-[(2s,4r,5s)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one Chemical compound O=C1N=C(N)C=CN1[C@H]1O[C@@H](CO)[C@H](O)C1 CKTSBUTUHBMZGZ-CHKWXVPMSA-N 0.000 description 1
- IAYLNBJBDSGLMT-QPPQHZFASA-N 4-amino-5-fluoro-1-[(2r,4r,5r)-4-fluoro-5-(hydroxymethyl)thiolan-2-yl]pyrimidin-2-one Chemical compound C1=C(F)C(N)=NC(=O)N1[C@@H]1S[C@H](CO)[C@H](F)C1 IAYLNBJBDSGLMT-QPPQHZFASA-N 0.000 description 1
- IAYLNBJBDSGLMT-UBKIQSJTSA-N 4-amino-5-fluoro-1-[(2r,4s,5r)-4-fluoro-5-(hydroxymethyl)thiolan-2-yl]pyrimidin-2-one Chemical compound C1=C(F)C(N)=NC(=O)N1[C@@H]1S[C@H](CO)[C@@H](F)C1 IAYLNBJBDSGLMT-UBKIQSJTSA-N 0.000 description 1
- RJKXXGNCKMQXTH-WDSKDSINSA-N 4-amino-5-fluoro-1-[(2s,4s)-2-(hydroxymethyl)-1,3-dioxolan-4-yl]pyrimidin-2-one Chemical compound C1=C(F)C(N)=NC(=O)N1[C@H]1O[C@@H](CO)OC1 RJKXXGNCKMQXTH-WDSKDSINSA-N 0.000 description 1
- YCEGZCXRAXIXRH-VHWMUFPASA-N 4-amino-5-fluoro-1-[(2s,5r)-3-fluoro-5-(hydroxymethyl)-2,5-dihydrothiophen-2-yl]pyrimidin-2-one Chemical compound C1=C(F)C(N)=NC(=O)N1[C@@H]1C(F)=C[C@H](CO)S1 YCEGZCXRAXIXRH-VHWMUFPASA-N 0.000 description 1
- 229960000549 4-dimethylaminophenol Drugs 0.000 description 1
- CJNLDUJVMLMBRT-UHFFFAOYSA-N 4-fluoro-2h-furan-5-one Chemical compound FC1=CCOC1=O CJNLDUJVMLMBRT-UHFFFAOYSA-N 0.000 description 1
- ZMGMDXCADSRNCX-UHFFFAOYSA-N 5,6-dihydroxy-1,3-diazepan-2-one Chemical compound OC1CNC(=O)NCC1O ZMGMDXCADSRNCX-UHFFFAOYSA-N 0.000 description 1
- JVPRTWJSEPKQGH-FDDDBJFASA-N 5-(2-bromoethoxy)-1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(OCCBr)=C1 JVPRTWJSEPKQGH-FDDDBJFASA-N 0.000 description 1
- NMUSYJAQQFHJEW-UHFFFAOYSA-N 5-Azacytidine Natural products O=C1N=C(N)N=CN1C1C(O)C(O)C(CO)O1 NMUSYJAQQFHJEW-UHFFFAOYSA-N 0.000 description 1
- MHIWAWLDGNOCRU-PIXDULNESA-N 5-[(e)-2-bromoethenyl]-1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)thiolan-2-yl]pyrimidine-2,4-dione Chemical compound C1[C@H](O)[C@@H](CO)S[C@H]1N1C(=O)NC(=O)C(\C=C\Br)=C1 MHIWAWLDGNOCRU-PIXDULNESA-N 0.000 description 1
- YNWUIBSEOAWSHW-HIQWKFPQSA-N 5-[(e)-2-bromoethenyl]-1-[(2s,4s)-2-(hydroxymethyl)-1,3-dioxolan-4-yl]pyrimidine-2,4-dione Chemical compound O1[C@@H](CO)OC[C@H]1N1C(=O)NC(=O)C(\C=C\Br)=C1 YNWUIBSEOAWSHW-HIQWKFPQSA-N 0.000 description 1
- SVXNJCYYMRMXNM-UHFFFAOYSA-N 5-amino-2h-1,2,4-triazin-3-one Chemical compound NC=1C=NNC(=O)N=1 SVXNJCYYMRMXNM-UHFFFAOYSA-N 0.000 description 1
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 1
- NPYPQKXJJZZSAX-UHFFFAOYSA-N 5-benzylpyrimidine Chemical compound C=1N=CN=CC=1CC1=CC=CC=C1 NPYPQKXJJZZSAX-UHFFFAOYSA-N 0.000 description 1
- HXXVIKZQIFTJOQ-UHFFFAOYSA-N 5-ethenylpyrimidine Chemical compound C=CC1=CN=CN=C1 HXXVIKZQIFTJOQ-UHFFFAOYSA-N 0.000 description 1
- SWFJAJRDLUUIOA-IBCQBUCCSA-N 5-ethyl-1-[(2r,3s,4r,5r)-3-fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione Chemical compound O=C1NC(=O)C(CC)=CN1[C@H]1[C@@H](F)[C@H](O)[C@@H](CO)O1 SWFJAJRDLUUIOA-IBCQBUCCSA-N 0.000 description 1
- APMTWPKBXAIALZ-MYKRQKDWSA-N 5-fluoro-1-[(2r,3s,5s)-3-hydroxy-5-(hydroxymethyl)thiolan-2-yl]pyrimidine-2,4-dione Chemical compound S1[C@H](CO)C[C@H](O)[C@@H]1N1C(=O)NC(=O)C(F)=C1 APMTWPKBXAIALZ-MYKRQKDWSA-N 0.000 description 1
- MDLZHMJLMTWRIO-UBKIQSJTSA-N 5-fluoro-1-[(2r,4s,5r)-4-fluoro-5-(hydroxymethyl)thiolan-2-yl]pyrimidine-2,4-dione Chemical compound C1[C@H](F)[C@@H](CO)S[C@H]1N1C(=O)NC(=O)C(F)=C1 MDLZHMJLMTWRIO-UBKIQSJTSA-N 0.000 description 1
- KSPDSMOWMQFPBL-UHFFFAOYSA-N 5-fluoropyrimidine Chemical compound FC1=CN=CN=C1 KSPDSMOWMQFPBL-UHFFFAOYSA-N 0.000 description 1
- KSNXJLQDQOIRIP-UHFFFAOYSA-N 5-iodouracil Chemical compound IC1=CNC(=O)NC1=O KSNXJLQDQOIRIP-UHFFFAOYSA-N 0.000 description 1
- LRSASMSXMSNRBT-UHFFFAOYSA-N 5-methylcytosine Chemical compound CC1=CNC(=O)N=C1N LRSASMSXMSNRBT-UHFFFAOYSA-N 0.000 description 1
- NOYDQGFVFOQSAJ-UHFFFAOYSA-N 5-nitropyrimidine Chemical compound [O-][N+](=O)C1=CN=CN=C1 NOYDQGFVFOQSAJ-UHFFFAOYSA-N 0.000 description 1
- RSIWALKZYXPAGW-NSHDSACASA-N 6-(3-fluorophenyl)-3-methyl-7-[(1s)-1-(7h-purin-6-ylamino)ethyl]-[1,3]thiazolo[3,2-a]pyrimidin-5-one Chemical compound C=1([C@@H](NC=2C=3N=CNC=3N=CN=2)C)N=C2SC=C(C)N2C(=O)C=1C1=CC=CC(F)=C1 RSIWALKZYXPAGW-NSHDSACASA-N 0.000 description 1
- 150000005020 6-aminopurines Chemical class 0.000 description 1
- PVRBGBGMDLPYKG-UHFFFAOYSA-N 6-benzyl-7h-purine Chemical compound N=1C=NC=2N=CNC=2C=1CC1=CC=CC=C1 PVRBGBGMDLPYKG-UHFFFAOYSA-N 0.000 description 1
- RYYIULNRIVUMTQ-UHFFFAOYSA-N 6-chloroguanine Chemical class NC1=NC(Cl)=C2N=CNC2=N1 RYYIULNRIVUMTQ-UHFFFAOYSA-N 0.000 description 1
- VVIAGPKUTFNRDU-UHFFFAOYSA-N 6S-folinic acid Natural products C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-UHFFFAOYSA-N 0.000 description 1
- RURYYXGWPHMEPS-FWOIEVBISA-N 9-[(2s,5r)-3-fluoro-5-(hydroxymethyl)-2,5-dihydrothiophen-2-yl]-3h-purin-6-one Chemical compound FC1=C[C@H](CO)S[C@@H]1N1C(NC=NC2=O)=C2N=C1 RURYYXGWPHMEPS-FWOIEVBISA-N 0.000 description 1
- MSSXOMSJDRHRMC-UHFFFAOYSA-N 9H-purine-2,6-diamine Chemical compound NC1=NC(N)=C2NC=NC2=N1 MSSXOMSJDRHRMC-UHFFFAOYSA-N 0.000 description 1
- 208000004998 Abdominal Pain Diseases 0.000 description 1
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- 241001388119 Anisotremus surinamensis Species 0.000 description 1
- 102000015790 Asparaginase Human genes 0.000 description 1
- 108010024976 Asparaginase Proteins 0.000 description 1
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical class C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 1
- 208000012526 B-cell neoplasm Diseases 0.000 description 1
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- JQUCWIWWWKZNCS-LESHARBVSA-N C(C1=CC=CC=C1)(=O)NC=1SC[C@H]2[C@@](N1)(CO[C@H](C2)C)C=2SC=C(N2)NC(=O)C2=NC=C(C=C2)OC(F)F Chemical compound C(C1=CC=CC=C1)(=O)NC=1SC[C@H]2[C@@](N1)(CO[C@H](C2)C)C=2SC=C(N2)NC(=O)C2=NC=C(C=C2)OC(F)F JQUCWIWWWKZNCS-LESHARBVSA-N 0.000 description 1
- CRWLATSHAHNVOV-FKMOCYPUSA-N C1([C@@H](O)[C@@H](O)[C@@H](O1)CO)C1=NC=C2NC=NC2=N1 Chemical compound C1([C@@H](O)[C@@H](O)[C@@H](O1)CO)C1=NC=C2NC=NC2=N1 CRWLATSHAHNVOV-FKMOCYPUSA-N 0.000 description 1
- ILBLWHXGVVVXNI-IZGCPZNMSA-N CC(C)(C)[Si](C1=CC=CC=C1)(C2=CC=CC=C2)OC[C@H]3C[C@@]([C@@](S3)(N4CN=C(C=C4)C(=O)C5=CC=CC=C5)[SeH])(C6=CC=CC=C6)F Chemical compound CC(C)(C)[Si](C1=CC=CC=C1)(C2=CC=CC=C2)OC[C@H]3C[C@@]([C@@](S3)(N4CN=C(C=C4)C(=O)C5=CC=CC=C5)[SeH])(C6=CC=CC=C6)F ILBLWHXGVVVXNI-IZGCPZNMSA-N 0.000 description 1
- QAGYKUNXZHXKMR-UHFFFAOYSA-N CPD000469186 Natural products CC1=C(O)C=CC=C1C(=O)NC(C(O)CN1C(CC2CCCCC2C1)C(=O)NC(C)(C)C)CSC1=CC=CC=C1 QAGYKUNXZHXKMR-UHFFFAOYSA-N 0.000 description 1
- 101100512078 Caenorhabditis elegans lys-1 gene Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 241001227713 Chiron Species 0.000 description 1
- 241000282552 Chlorocebus aethiops Species 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- PTOAARAWEBMLNO-KVQBGUIXSA-N Cladribine Chemical compound C1=NC=2C(N)=NC(Cl)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)O1 PTOAARAWEBMLNO-KVQBGUIXSA-N 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 102000007644 Colony-Stimulating Factors Human genes 0.000 description 1
- 108010071942 Colony-Stimulating Factors Proteins 0.000 description 1
- 229940126639 Compound 33 Drugs 0.000 description 1
- 208000032170 Congenital Abnormalities Diseases 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- MFYSYFVPBJMHGN-ZPOLXVRWSA-N Cortisone Chemical compound O=C1CC[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 MFYSYFVPBJMHGN-ZPOLXVRWSA-N 0.000 description 1
- MFYSYFVPBJMHGN-UHFFFAOYSA-N Cortisone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)(O)C(=O)CO)C4C3CCC2=C1 MFYSYFVPBJMHGN-UHFFFAOYSA-N 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- 102100026846 Cytidine deaminase Human genes 0.000 description 1
- 125000003317 D-arabinofuranosyl group Chemical group [H]OC([H])([H])[C@@]1([H])OC([H])(*)[C@@]([H])(O[H])[C@]1([H])O[H] 0.000 description 1
- WHUUTDBJXJRKMK-GSVOUGTGSA-N D-glutamic acid Chemical compound OC(=O)[C@H](N)CCC(O)=O WHUUTDBJXJRKMK-GSVOUGTGSA-N 0.000 description 1
- 229930182847 D-glutamic acid Natural products 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 230000008836 DNA modification Effects 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 206010012735 Diarrhoea Diseases 0.000 description 1
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- 231100000036 EC90 Toxicity 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 208000031637 Erythroblastic Acute Leukemia Diseases 0.000 description 1
- 208000036566 Erythroleukaemia Diseases 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 238000005033 Fourier transform infrared spectroscopy Methods 0.000 description 1
- 230000010190 G1 phase Effects 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- UXDDRFCJKNROTO-UHFFFAOYSA-N Glycerol 1,2-diacetate Chemical compound CC(=O)OCC(CO)OC(C)=O UXDDRFCJKNROTO-UHFFFAOYSA-N 0.000 description 1
- 206010019233 Headaches Diseases 0.000 description 1
- 206010019708 Hepatic steatosis Diseases 0.000 description 1
- 206010019799 Hepatitis viral Diseases 0.000 description 1
- 208000028782 Hereditary disease Diseases 0.000 description 1
- 101000756632 Homo sapiens Actin, cytoplasmic 1 Proteins 0.000 description 1
- 101000777693 Homo sapiens Cytidine and dCMP deaminase domain-containing protein 1 Proteins 0.000 description 1
- 101000912053 Homo sapiens Cytidine deaminase Proteins 0.000 description 1
- 101000917383 Homo sapiens Deoxycytidine kinase Proteins 0.000 description 1
- 101000796134 Homo sapiens Thymidine phosphorylase Proteins 0.000 description 1
- 241000700588 Human alphaherpesvirus 1 Species 0.000 description 1
- 241000701074 Human alphaherpesvirus 2 Species 0.000 description 1
- 241001492344 Human immunodeficiency virus 3 Species 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- 206010061598 Immunodeficiency Diseases 0.000 description 1
- 208000029462 Immunodeficiency disease Diseases 0.000 description 1
- 102100020873 Interleukin-2 Human genes 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 206010023126 Jaundice Diseases 0.000 description 1
- 108010000668 KNI 102 Proteins 0.000 description 1
- XCVUOCMQYKSJJR-IGRGDXOOSA-N Kni 102 Chemical compound CC(C)(C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)OCC=1C=CC=CC=1)CC1=CC=CC=C1 XCVUOCMQYKSJJR-IGRGDXOOSA-N 0.000 description 1
- NJBBLOIWMSYVCQ-VZTVMPNDSA-N Kynostatin 272 Chemical compound C([C@H](NC(=O)[C@@H](NC(=O)COC=1C2=CC=NC=C2C=CC=1)CSC)[C@H](O)C(=O)N1[C@@H](CSC1)C(=O)NC(C)(C)C)C1=CC=CC=C1 NJBBLOIWMSYVCQ-VZTVMPNDSA-N 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- HMFHBZSHGGEWLO-OWMBCFKOSA-N L-ribofuranose Chemical compound OC[C@@H]1OC(O)[C@@H](O)[C@H]1O HMFHBZSHGGEWLO-OWMBCFKOSA-N 0.000 description 1
- 108010000817 Leuprolide Proteins 0.000 description 1
- HLFSDGLLUJUHTE-SNVBAGLBSA-N Levamisole Chemical compound C1([C@H]2CN3CCSC3=N2)=CC=CC=C1 HLFSDGLLUJUHTE-SNVBAGLBSA-N 0.000 description 1
- 206010067125 Liver injury Diseases 0.000 description 1
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 208000028018 Lymphocytic leukaemia Diseases 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- 102000007651 Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 108010046938 Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 208000001940 Massive Hepatic Necrosis Diseases 0.000 description 1
- 208000024556 Mendelian disease Diseases 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 1
- GCKMFJBGXUYNAG-HLXURNFRSA-N Methyltestosterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@](C)(O)[C@@]1(C)CC2 GCKMFJBGXUYNAG-HLXURNFRSA-N 0.000 description 1
- 208000001984 Multiple Symmetrical Lipomatosis Diseases 0.000 description 1
- 241000714177 Murine leukemia virus Species 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 208000021642 Muscular disease Diseases 0.000 description 1
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 1
- 201000009623 Myopathy Diseases 0.000 description 1
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 1
- UHUIZSSIHRBLES-FEEQUYSTSA-N N-[1-[(2S,3S,5R)-5-[[tert-butyl(diphenyl)silyl]oxymethyl]-3-fluoro-3-phenyl-2-selanylthiolan-2-yl]-5-fluoro-2-oxopyrimidin-4-yl]benzamide Chemical compound C(C1=CC=CC=C1)(=O)NC1=NC(N(C=C1F)[C@]1([C@](C[C@@H](S1)CO[Si](C1=CC=CC=C1)(C1=CC=CC=C1)C(C)(C)C)(C1=CC=CC=C1)F)[SeH])=O UHUIZSSIHRBLES-FEEQUYSTSA-N 0.000 description 1
- ZWZYBHKNWHAHQR-UHFFFAOYSA-N N-ethyl-N-(triethyl-lambda4-sulfanyl)ethanamine Chemical compound C(C)S(N(CC)CC)(CC)CC ZWZYBHKNWHAHQR-UHFFFAOYSA-N 0.000 description 1
- 206010029155 Nephropathy toxic Diseases 0.000 description 1
- 241000208125 Nicotiana Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- 102000007999 Nuclear Proteins Human genes 0.000 description 1
- 108010089610 Nuclear Proteins Proteins 0.000 description 1
- 229940122313 Nucleoside reverse transcriptase inhibitor Drugs 0.000 description 1
- QUQCFZTWLNPOGU-UHFFFAOYSA-N OC(C(=O)N)(CCC)N Chemical compound OC(C(=O)N)(CCC)N QUQCFZTWLNPOGU-UHFFFAOYSA-N 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 108090000854 Oxidoreductases Proteins 0.000 description 1
- 102000004316 Oxidoreductases Human genes 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 206010033645 Pancreatitis Diseases 0.000 description 1
- 206010034133 Pathogen resistance Diseases 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 208000037581 Persistent Infection Diseases 0.000 description 1
- 108010047620 Phytohemagglutinins Proteins 0.000 description 1
- 208000012641 Pigmentation disease Diseases 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 102000001253 Protein Kinase Human genes 0.000 description 1
- 206010037660 Pyrexia Diseases 0.000 description 1
- 101800001554 RNA-directed RNA polymerase Proteins 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 101150104269 RT gene Proteins 0.000 description 1
- 108700005075 Regulator Genes Proteins 0.000 description 1
- 206010057190 Respiratory tract infections Diseases 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- 101001047650 Rhyparobia maderae Leucokinin-3 Proteins 0.000 description 1
- 102000000505 Ribonucleotide Reductases Human genes 0.000 description 1
- 108010041388 Ribonucleotide Reductases Proteins 0.000 description 1
- NCDNCNXCDXHOMX-UHFFFAOYSA-N Ritonavir Natural products C=1C=CC=CC=1CC(NC(=O)OCC=1SC=NC=1)C(O)CC(CC=1C=CC=CC=1)NC(=O)C(C(C)C)NC(=O)N(C)CC1=CSC(C(C)C)=N1 NCDNCNXCDXHOMX-UHFFFAOYSA-N 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 241000713311 Simian immunodeficiency virus Species 0.000 description 1
- PNUZDKCDAWUEGK-CYZMBNFOSA-N Sitafloxacin Chemical compound C([C@H]1N)N(C=2C(=C3C(C(C(C(O)=O)=CN3[C@H]3[C@H](C3)F)=O)=CC=2F)Cl)CC11CC1 PNUZDKCDAWUEGK-CYZMBNFOSA-N 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- 238000002105 Southern blotting Methods 0.000 description 1
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 1
- RZCIEJXAILMSQK-JXOAFFINSA-N TTP Chemical compound O=C1NC(=O)C(C)=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 RZCIEJXAILMSQK-JXOAFFINSA-N 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 1
- 101710183280 Topoisomerase Proteins 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- DRTQHJPVMGBUCF-XVFCMESISA-N Uridine Natural products O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-XVFCMESISA-N 0.000 description 1
- 208000002495 Uterine Neoplasms Diseases 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 229940122803 Vinca alkaloid Drugs 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- LNNPIUNWZAYXKK-YNODCEANSA-N [(2r)-5-acetyl-3,3-difluoro-5-hydroxyoxolan-2-yl]methyl benzoate Chemical compound O1C(C(=O)C)(O)CC(F)(F)[C@H]1COC(=O)C1=CC=CC=C1 LNNPIUNWZAYXKK-YNODCEANSA-N 0.000 description 1
- GIUYCYHIANZCFB-UUOKFMHZSA-N [(2r,3s,4r,5r)-5-(6-amino-2-fluoropurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O GIUYCYHIANZCFB-UUOKFMHZSA-N 0.000 description 1
- AXODMUZULWFLIS-MOFOKWOHSA-N [(2r,5s)-5-(6-amino-2-fluoropurin-9-yl)-4-fluoro-2,5-dihydrothiophen-2-yl]methanol Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@H]1S[C@@H](CO)C=C1F AXODMUZULWFLIS-MOFOKWOHSA-N 0.000 description 1
- CNXKXOFTMXPMTH-UAKXSSHOSA-N [1-[(2r,3r,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-2,4-dioxopyrimidin-5-yl] hypoiodite Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(OI)=C1 CNXKXOFTMXPMTH-UAKXSSHOSA-N 0.000 description 1
- 238000005263 ab initio calculation Methods 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- IKHGUXGNUITLKF-XPULMUKRSA-N acetaldehyde Chemical compound [14CH]([14CH3])=O IKHGUXGNUITLKF-XPULMUKRSA-N 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 150000008043 acidic salts Chemical class 0.000 description 1
- 238000001994 activation Methods 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 208000021841 acute erythroid leukemia Diseases 0.000 description 1
- 125000002015 acyclic group Chemical group 0.000 description 1
- SUPKOOSCJHTBAH-UHFFFAOYSA-N adefovir Chemical compound NC1=NC=NC2=C1N=CN2CCOCP(O)(O)=O SUPKOOSCJHTBAH-UHFFFAOYSA-N 0.000 description 1
- 229960001997 adefovir Drugs 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 150000001447 alkali salts Chemical class 0.000 description 1
- 125000004183 alkoxy alkyl group Chemical group 0.000 description 1
- 150000008051 alkyl sulfates Chemical class 0.000 description 1
- 230000002152 alkylating effect Effects 0.000 description 1
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 150000003862 amino acid derivatives Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 230000001195 anabolic effect Effects 0.000 description 1
- 239000003098 androgen Substances 0.000 description 1
- 229940030486 androgens Drugs 0.000 description 1
- 208000007502 anemia Diseases 0.000 description 1
- 208000022531 anorexia Diseases 0.000 description 1
- RGHILYZRVFRRNK-UHFFFAOYSA-N anthracene-1,2-dione Chemical class C1=CC=C2C=C(C(C(=O)C=C3)=O)C3=CC2=C1 RGHILYZRVFRRNK-UHFFFAOYSA-N 0.000 description 1
- 229940045799 anthracyclines and related substance Drugs 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000002280 anti-androgenic effect Effects 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 230000001512 anti-cytomegaloviral effect Effects 0.000 description 1
- 229940046836 anti-estrogen Drugs 0.000 description 1
- 230000001833 anti-estrogenic effect Effects 0.000 description 1
- 230000003432 anti-folate effect Effects 0.000 description 1
- 230000000798 anti-retroviral effect Effects 0.000 description 1
- 238000002832 anti-viral assay Methods 0.000 description 1
- 239000000051 antiandrogen Substances 0.000 description 1
- 229940030495 antiandrogen sex hormone and modulator of the genital system Drugs 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 229940127074 antifolate Drugs 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 229940045686 antimetabolites antineoplastic purine analogs Drugs 0.000 description 1
- 229940045719 antineoplastic alkylating agent nitrosoureas Drugs 0.000 description 1
- 230000004596 appetite loss Effects 0.000 description 1
- 239000000010 aprotic solvent Substances 0.000 description 1
- OIRDTQYFTABQOQ-UHFFFAOYSA-N ara-adenosine Natural products Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O OIRDTQYFTABQOQ-UHFFFAOYSA-N 0.000 description 1
- 125000005160 aryl oxy alkyl group Chemical group 0.000 description 1
- 229940072107 ascorbate Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 229960003272 asparaginase Drugs 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-M asparaginate Chemical compound [O-]C(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-M 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 229960002756 azacitidine Drugs 0.000 description 1
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 1
- 150000001540 azides Chemical class 0.000 description 1
- HNYOPLTXPVRDBG-UHFFFAOYSA-N barbituric acid Chemical compound O=C1CC(=O)NC(=O)N1 HNYOPLTXPVRDBG-UHFFFAOYSA-N 0.000 description 1
- 210000002469 basement membrane Anatomy 0.000 description 1
- 150000007514 bases Chemical class 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229940050390 benzoate Drugs 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- PASDCCFISLVPSO-UHFFFAOYSA-N benzoyl chloride Chemical compound ClC(=O)C1=CC=CC=C1 PASDCCFISLVPSO-UHFFFAOYSA-N 0.000 description 1
- AGEZXYOZHKGVCM-UHFFFAOYSA-N benzyl bromide Chemical compound BrCC1=CC=CC=C1 AGEZXYOZHKGVCM-UHFFFAOYSA-N 0.000 description 1
- TZRRVSCDIPHXHN-RRUVMKMCSA-N benzyl n-[(2s)-1-[[(2s,3r,4r,5s)-3,4-dihydroxy-5-[[(2s)-3-methyl-2-(phenylmethoxycarbonylamino)butanoyl]amino]-1,6-diphenylhexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]carbamate Chemical compound N([C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)[C@@H](O)[C@H](O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)OCC=1C=CC=CC=1)C(C)C)C(=O)OCC1=CC=CC=C1 TZRRVSCDIPHXHN-RRUVMKMCSA-N 0.000 description 1
- DRTQHJPVMGBUCF-PSQAKQOGSA-N beta-L-uridine Natural products O[C@H]1[C@@H](O)[C@H](CO)O[C@@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-PSQAKQOGSA-N 0.000 description 1
- 229920001222 biopolymer Polymers 0.000 description 1
- 125000006267 biphenyl group Chemical group 0.000 description 1
- SIPUZPBQZHNSDW-UHFFFAOYSA-N bis(2-methylpropyl)aluminum Chemical compound CC(C)C[Al]CC(C)C SIPUZPBQZHNSDW-UHFFFAOYSA-N 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 230000036765 blood level Effects 0.000 description 1
- 238000009534 blood test Methods 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 244000309464 bull Species 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 150000001669 calcium Chemical class 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 150000001733 carboxylic acid esters Chemical class 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 231100000357 carcinogen Toxicity 0.000 description 1
- 239000003183 carcinogenic agent Substances 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000022131 cell cycle Effects 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 230000003833 cell viability Effects 0.000 description 1
- 108091092356 cellular DNA Proteins 0.000 description 1
- 230000019522 cellular metabolic process Effects 0.000 description 1
- 201000007455 central nervous system cancer Diseases 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000007810 chemical reaction solvent Substances 0.000 description 1
- 239000013626 chemical specie Substances 0.000 description 1
- 229940044683 chemotherapy drug Drugs 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- DDKMFOUTRRODRE-UHFFFAOYSA-N chloromethanone Chemical compound Cl[C]=O DDKMFOUTRRODRE-UHFFFAOYSA-N 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 229940117975 chromium trioxide Drugs 0.000 description 1
- WGLPBDUCMAPZCE-UHFFFAOYSA-N chromium trioxide Inorganic materials O=[Cr](=O)=O WGLPBDUCMAPZCE-UHFFFAOYSA-N 0.000 description 1
- GAMDZJFZMJECOS-UHFFFAOYSA-N chromium(6+);oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[Cr+6] GAMDZJFZMJECOS-UHFFFAOYSA-N 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 238000004737 colorimetric analysis Methods 0.000 description 1
- 229940125877 compound 31 Drugs 0.000 description 1
- 229940125878 compound 36 Drugs 0.000 description 1
- 229940125807 compound 37 Drugs 0.000 description 1
- 238000002809 confirmatory assay Methods 0.000 description 1
- 239000000039 congener Substances 0.000 description 1
- 230000001268 conjugating effect Effects 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 229960004544 cortisone Drugs 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000004210 cyclohexylmethyl group Chemical group [H]C([H])(*)C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 108010023472 cytochrome C oxidase subunit II Proteins 0.000 description 1
- 230000003436 cytoskeletal effect Effects 0.000 description 1
- 238000002784 cytotoxicity assay Methods 0.000 description 1
- 231100000263 cytotoxicity test Toxicity 0.000 description 1
- NHVNXKFIZYSCEB-XLPZGREQSA-N dTTP Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)C1 NHVNXKFIZYSCEB-XLPZGREQSA-N 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 238000013480 data collection Methods 0.000 description 1
- 230000020176 deacylation Effects 0.000 description 1
- 238000005947 deacylation reaction Methods 0.000 description 1
- 230000020335 dealkylation Effects 0.000 description 1
- 238000006900 dealkylation reaction Methods 0.000 description 1
- 206010061428 decreased appetite Diseases 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- WHBIGIKBNXZKFE-UHFFFAOYSA-N delavirdine Chemical compound CC(C)NC1=CC=CN=C1N1CCN(C(=O)C=2NC3=CC=C(NS(C)(=O)=O)C=C3C=2)CC1 WHBIGIKBNXZKFE-UHFFFAOYSA-N 0.000 description 1
- 230000030609 dephosphorylation Effects 0.000 description 1
- 238000006209 dephosphorylation reaction Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- VGONTNSXDCQUGY-UHFFFAOYSA-N desoxyinosine Natural products C1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 VGONTNSXDCQUGY-UHFFFAOYSA-N 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 229960002086 dextran Drugs 0.000 description 1
- 229960000633 dextran sulfate Drugs 0.000 description 1
- LJSQFQKUNVCTIA-UHFFFAOYSA-N diethyl sulfide Chemical compound CCSCC LJSQFQKUNVCTIA-UHFFFAOYSA-N 0.000 description 1
- RGLYKWWBQGJZGM-ISLYRVAYSA-N diethylstilbestrol Chemical compound C=1C=C(O)C=CC=1C(/CC)=C(\CC)C1=CC=C(O)C=C1 RGLYKWWBQGJZGM-ISLYRVAYSA-N 0.000 description 1
- 229960000452 diethylstilbestrol Drugs 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- IJKVHSBPTUYDLN-UHFFFAOYSA-N dihydroxy(oxo)silane Chemical compound O[Si](O)=O IJKVHSBPTUYDLN-UHFFFAOYSA-N 0.000 description 1
- 238000005906 dihydroxylation reaction Methods 0.000 description 1
- 239000012470 diluted sample Substances 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- VAYGXNSJCAHWJZ-UHFFFAOYSA-N dimethyl sulfate Chemical compound COS(=O)(=O)OC VAYGXNSJCAHWJZ-UHFFFAOYSA-N 0.000 description 1
- 239000001177 diphosphate Substances 0.000 description 1
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical class [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 1
- 230000008034 disappearance Effects 0.000 description 1
- 231100000676 disease causative agent Toxicity 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- LOZWAPSEEHRYPG-UHFFFAOYSA-N dithiane Natural products C1CSCCS1 LOZWAPSEEHRYPG-UHFFFAOYSA-N 0.000 description 1
- 150000004252 dithioacetals Chemical class 0.000 description 1
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 229940000406 drug candidate Drugs 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 230000000857 drug effect Effects 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 229960000366 emtricitabine Drugs 0.000 description 1
- QDGZDCVAUDNJFG-FXQIFTODSA-N entecavir (anhydrous) Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1[C@H]1C[C@H](O)[C@@H](CO)C1=C QDGZDCVAUDNJFG-FXQIFTODSA-N 0.000 description 1
- 230000007515 enzymatic degradation Effects 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 239000002532 enzyme inhibitor Substances 0.000 description 1
- 238000006735 epoxidation reaction Methods 0.000 description 1
- 208000009724 equine infectious anemia Diseases 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- 239000000328 estrogen antagonist Substances 0.000 description 1
- 229960004756 ethanol Drugs 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 206010016256 fatigue Diseases 0.000 description 1
- 239000012894 fetal calf serum Substances 0.000 description 1
- 210000003754 fetus Anatomy 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- YLRFCQOZQXIBAB-RBZZARIASA-N fluoxymesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1CC[C@](C)(O)[C@@]1(C)C[C@@H]2O YLRFCQOZQXIBAB-RBZZARIASA-N 0.000 description 1
- 229960001751 fluoxymesterone Drugs 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 238000005187 foaming Methods 0.000 description 1
- 239000004052 folic acid antagonist Substances 0.000 description 1
- VVIAGPKUTFNRDU-ABLWVSNPSA-N folinic acid Chemical compound C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-ABLWVSNPSA-N 0.000 description 1
- 235000008191 folinic acid Nutrition 0.000 description 1
- 239000011672 folinic acid Substances 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- IRSCQMHQWWYFCW-UHFFFAOYSA-N ganciclovir Chemical compound O=C1NC(N)=NC2=C1N=CN2COC(CO)CO IRSCQMHQWWYFCW-UHFFFAOYSA-N 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- 238000012239 gene modification Methods 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 230000005017 genetic modification Effects 0.000 description 1
- 230000007614 genetic variation Effects 0.000 description 1
- 235000013617 genetically modified food Nutrition 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- XLXSAKCOAKORKW-UHFFFAOYSA-N gonadorelin Chemical class C1CCC(C(=O)NCC(N)=O)N1C(=O)C(CCCN=C(N)N)NC(=O)C(CC(C)C)NC(=O)CNC(=O)C(NC(=O)C(CO)NC(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C(CC=1NC=NC=1)NC(=O)C1NC(=O)CC1)CC1=CC=C(O)C=C1 XLXSAKCOAKORKW-UHFFFAOYSA-N 0.000 description 1
- 208000024963 hair loss Diseases 0.000 description 1
- 230000003676 hair loss Effects 0.000 description 1
- 201000009277 hairy cell leukemia Diseases 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- 230000005802 health problem Effects 0.000 description 1
- 231100000234 hepatic damage Toxicity 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 238000000703 high-speed centrifugation Methods 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 102000057462 human TYMP Human genes 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical group 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 230000033444 hydroxylation Effects 0.000 description 1
- 238000005805 hydroxylation reaction Methods 0.000 description 1
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 1
- 239000005457 ice water Substances 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 230000007813 immunodeficiency Effects 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 230000000415 inactivating effect Effects 0.000 description 1
- NUBQKPWHXMGDLP-BDEHJDMKSA-N indinavir sulfate Chemical compound OS(O)(=O)=O.C([C@H](N(CC1)C[C@@H](O)C[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H]2C3=CC=CC=C3C[C@H]2O)C(=O)NC(C)(C)C)N1CC1=CC=CN=C1 NUBQKPWHXMGDLP-BDEHJDMKSA-N 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 206010022000 influenza Diseases 0.000 description 1
- 238000002329 infrared spectrum Methods 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000004491 isohexyl group Chemical group C(CCC(C)C)* 0.000 description 1
- 125000001972 isopentyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- 108010075606 kynostatin 272 Proteins 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 208000006443 lactic acidosis Diseases 0.000 description 1
- 229960001691 leucovorin Drugs 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 201000002364 leukopenia Diseases 0.000 description 1
- 231100001022 leukopenia Toxicity 0.000 description 1
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 1
- 229960004338 leuprorelin Drugs 0.000 description 1
- 229960001614 levamisole Drugs 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 238000012317 liver biopsy Methods 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 230000008818 liver damage Effects 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 229960002247 lomustine Drugs 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 238000011866 long-term treatment Methods 0.000 description 1
- 208000019017 loss of appetite Diseases 0.000 description 1
- 235000021266 loss of appetite Nutrition 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 208000003747 lymphoid leukemia Diseases 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- 206010025482 malaise Diseases 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical class ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- 229960004616 medroxyprogesterone Drugs 0.000 description 1
- PSGAAPLEWMOORI-PEINSRQWSA-N medroxyprogesterone acetate Chemical compound C([C@@]12C)CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2CC[C@]2(C)[C@@](OC(C)=O)(C(C)=O)CC[C@H]21 PSGAAPLEWMOORI-PEINSRQWSA-N 0.000 description 1
- 229960004296 megestrol acetate Drugs 0.000 description 1
- RQZAXGRLVPAYTJ-GQFGMJRRSA-N megestrol acetate Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 RQZAXGRLVPAYTJ-GQFGMJRRSA-N 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229960001810 meprednisone Drugs 0.000 description 1
- PIDANAQULIKBQS-RNUIGHNZSA-N meprednisone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)CC2=O PIDANAQULIKBQS-RNUIGHNZSA-N 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 230000001394 metastastic effect Effects 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- HRDXJKGNWSUIBT-UHFFFAOYSA-N methoxybenzene Chemical group [CH2]OC1=CC=CC=C1 HRDXJKGNWSUIBT-UHFFFAOYSA-N 0.000 description 1
- 125000004184 methoxymethyl group Chemical group [H]C([H])([H])OC([H])([H])* 0.000 description 1
- CYZUCLUOBWBLDS-QFIPXVFZSA-N methyl (2s)-2-acetylsulfanyl-5-[tert-butyl(diphenyl)silyl]oxypentanoate Chemical compound C=1C=CC=CC=1[Si](C(C)(C)C)(OCCC[C@@H](C(=O)OC)SC(C)=O)C1=CC=CC=C1 CYZUCLUOBWBLDS-QFIPXVFZSA-N 0.000 description 1
- 150000004702 methyl esters Chemical class 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- PQIOSYKVBBWRRI-UHFFFAOYSA-N methylphosphonyl difluoride Chemical group CP(F)(F)=O PQIOSYKVBBWRRI-UHFFFAOYSA-N 0.000 description 1
- 229960001566 methyltestosterone Drugs 0.000 description 1
- 125000004092 methylthiomethyl group Chemical group [H]C([H])([H])SC([H])([H])* 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 108091064355 mitochondrial RNA Proteins 0.000 description 1
- 230000007625 mitochondrial abnormality Effects 0.000 description 1
- 208000012268 mitochondrial disease Diseases 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 230000004660 morphological change Effects 0.000 description 1
- 210000004400 mucous membrane Anatomy 0.000 description 1
- 201000011171 multiple symmetric lipomatosis Diseases 0.000 description 1
- VMGAPWLDMVPYIA-HIDZBRGKSA-N n'-amino-n-iminomethanimidamide Chemical compound N\N=C\N=N VMGAPWLDMVPYIA-HIDZBRGKSA-N 0.000 description 1
- RLKHFSNWQCZBDC-UHFFFAOYSA-N n-(benzenesulfonyl)-n-fluorobenzenesulfonamide Chemical compound C=1C=CC=CC=1S(=O)(=O)N(F)S(=O)(=O)C1=CC=CC=C1 RLKHFSNWQCZBDC-UHFFFAOYSA-N 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- MRWXACSTFXYYMV-FDDDBJFASA-N nebularine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC=C2N=C1 MRWXACSTFXYYMV-FDDDBJFASA-N 0.000 description 1
- QAGYKUNXZHXKMR-HKWSIXNMSA-N nelfinavir Chemical compound CC1=C(O)C=CC=C1C(=O)N[C@H]([C@H](O)CN1[C@@H](C[C@@H]2CCCC[C@@H]2C1)C(=O)NC(C)(C)C)CSC1=CC=CC=C1 QAGYKUNXZHXKMR-HKWSIXNMSA-N 0.000 description 1
- 229960000884 nelfinavir Drugs 0.000 description 1
- 125000001971 neopentyl group Chemical group [H]C([*])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 230000001613 neoplastic effect Effects 0.000 description 1
- 230000007694 nephrotoxicity Effects 0.000 description 1
- 231100000417 nephrotoxicity Toxicity 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- 125000004355 nitrogen functional group Chemical group 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 229910000069 nitrogen hydride Inorganic materials 0.000 description 1
- 229940042402 non-nucleoside reverse transcriptase inhibitor Drugs 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000000655 nuclear magnetic resonance spectrum Methods 0.000 description 1
- 239000012038 nucleophile Substances 0.000 description 1
- 229940127073 nucleoside analogue Drugs 0.000 description 1
- KOSYAAIZOGNATQ-UHFFFAOYSA-N o-phenyl chloromethanethioate Chemical compound ClC(=S)OC1=CC=CC=C1 KOSYAAIZOGNATQ-UHFFFAOYSA-N 0.000 description 1
- NRGLDOQPRFMCPR-UHFFFAOYSA-N o-phenyl methanethioate Chemical compound S=COC1=CC=CC=C1 NRGLDOQPRFMCPR-UHFFFAOYSA-N 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 239000012044 organic layer Substances 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 239000007800 oxidant agent Substances 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 125000001151 peptidyl group Chemical group 0.000 description 1
- 208000033808 peripheral neuropathy Diseases 0.000 description 1
- 238000005502 peroxidation Methods 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 238000006363 phenylselenylation reaction Methods 0.000 description 1
- 239000008055 phosphate buffer solution Substances 0.000 description 1
- 150000008298 phosphoramidates Chemical class 0.000 description 1
- 125000004437 phosphorous atom Chemical group 0.000 description 1
- 230000001885 phytohemagglutinin Effects 0.000 description 1
- 230000019612 pigmentation Effects 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 230000037048 polymerization activity Effects 0.000 description 1
- 231100000683 possible toxicity Toxicity 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 125000001501 propionyl group Chemical group O=C([*])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 108060006633 protein kinase Proteins 0.000 description 1
- FVLAYJRLBLHIPV-UHFFFAOYSA-N pyrimidin-5-amine Chemical compound NC1=CN=CN=C1 FVLAYJRLBLHIPV-UHFFFAOYSA-N 0.000 description 1
- HBCQSNAFLVXVAY-UHFFFAOYSA-N pyrimidine-2-thiol Chemical compound SC1=NC=CC=N1 HBCQSNAFLVXVAY-UHFFFAOYSA-N 0.000 description 1
- XVIAPHVAGFEFFN-UHFFFAOYSA-N pyrimidine-5-carbonitrile Chemical compound N#CC1=CN=CN=C1 XVIAPHVAGFEFFN-UHFFFAOYSA-N 0.000 description 1
- WTTIBCHOELPGFK-LBPRGKRZSA-N r82150 Chemical compound C1N(CC=C(C)C)[C@@H](C)CN2C(=S)NC3=CC=CC1=C32 WTTIBCHOELPGFK-LBPRGKRZSA-N 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 238000006476 reductive cyclization reaction Methods 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- BOLDJAUMGUJJKM-LSDHHAIUSA-N renifolin D Natural products CC(=C)[C@@H]1Cc2c(O)c(O)ccc2[C@H]1CC(=O)c3ccc(O)cc3O BOLDJAUMGUJJKM-LSDHHAIUSA-N 0.000 description 1
- 230000003362 replicative effect Effects 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 238000011268 retreatment Methods 0.000 description 1
- 230000001177 retroviral effect Effects 0.000 description 1
- 238000010839 reverse transcription Methods 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 238000006798 ring closing metathesis reaction Methods 0.000 description 1
- 238000007363 ring formation reaction Methods 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000007127 saponification reaction Methods 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000004088 simulation Methods 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- AWUCVROLDVIAJX-GSVOUGTGSA-N sn-glycerol 3-phosphate Chemical compound OC[C@@H](O)COP(O)(O)=O AWUCVROLDVIAJX-GSVOUGTGSA-N 0.000 description 1
- 150000003385 sodium Chemical class 0.000 description 1
- FQENQNTWSFEDLI-UHFFFAOYSA-J sodium diphosphate Chemical compound [Na+].[Na+].[Na+].[Na+].[O-]P([O-])(=O)OP([O-])([O-])=O FQENQNTWSFEDLI-UHFFFAOYSA-J 0.000 description 1
- 239000012312 sodium hydride Substances 0.000 description 1
- 229910000104 sodium hydride Inorganic materials 0.000 description 1
- 229940048086 sodium pyrophosphate Drugs 0.000 description 1
- 238000005063 solubilization Methods 0.000 description 1
- 230000007928 solubilization Effects 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 239000012258 stirred mixture Substances 0.000 description 1
- 208000003265 stomatitis Diseases 0.000 description 1
- 229960001052 streptozocin Drugs 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 238000005556 structure-activity relationship Methods 0.000 description 1
- 229940086735 succinate Drugs 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 239000001384 succinic acid Substances 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 150000003871 sulfonates Chemical class 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- IQFYYKKMVGJFEH-CSMHCCOUSA-N telbivudine Chemical compound O=C1NC(=O)C(C)=CN1[C@H]1O[C@@H](CO)[C@H](O)C1 IQFYYKKMVGJFEH-CSMHCCOUSA-N 0.000 description 1
- VCMJCVGFSROFHV-WZGZYPNHSA-N tenofovir disoproxil fumarate Chemical compound OC(=O)\C=C\C(O)=O.N1=CN=C2N(C[C@@H](C)OCP(=O)(OCOC(=O)OC(C)C)OCOC(=O)OC(C)C)C=NC2=C1N VCMJCVGFSROFHV-WZGZYPNHSA-N 0.000 description 1
- OQHZMGOXOOOFEE-SYQUUIDJSA-N tert-butyl n-[(2s,3r)-3-hydroxy-4-[[(2r,3s)-2-hydroxy-3-[(2-methylpropan-2-yl)oxycarbonylamino]-4-[4-(2-morpholin-4-yl-2-oxoethoxy)phenyl]butyl]amino]-1-phenylbutan-2-yl]carbamate Chemical compound C([C@H](NC(=O)OC(C)(C)C)[C@H](O)CNC[C@@H](O)[C@H](CC=1C=CC(OCC(=O)N2CCOCC2)=CC=1)NC(=O)OC(C)(C)C)C1=CC=CC=C1 OQHZMGOXOOOFEE-SYQUUIDJSA-N 0.000 description 1
- ILMRJRBKQSSXGY-UHFFFAOYSA-N tert-butyl(dimethyl)silicon Chemical group C[Si](C)C(C)(C)C ILMRJRBKQSSXGY-UHFFFAOYSA-N 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- DPKBAXPHAYBPRL-UHFFFAOYSA-M tetrabutylazanium;iodide Chemical compound [I-].CCCC[N+](CCCC)(CCCC)CCCC DPKBAXPHAYBPRL-UHFFFAOYSA-M 0.000 description 1
- 235000019818 tetrasodium diphosphate Nutrition 0.000 description 1
- 239000001577 tetrasodium phosphonato phosphate Substances 0.000 description 1
- 229960001196 thiotepa Drugs 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-M toluene-4-sulfonate Chemical compound CC1=CC=C(S([O-])(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-M 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 238000002723 toxicity assay Methods 0.000 description 1
- 230000002110 toxicologic effect Effects 0.000 description 1
- 231100001265 toxicological assessment Toxicity 0.000 description 1
- 231100000723 toxicological property Toxicity 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 125000004665 trialkylsilyl group Chemical group 0.000 description 1
- DBGVGMSCBYYSLD-UHFFFAOYSA-N tributylstannane Chemical compound CCCC[SnH](CCCC)CCCC DBGVGMSCBYYSLD-UHFFFAOYSA-N 0.000 description 1
- YNJBWRMUSHSURL-UHFFFAOYSA-N trichloroacetic acid Chemical compound OC(=O)C(Cl)(Cl)Cl YNJBWRMUSHSURL-UHFFFAOYSA-N 0.000 description 1
- 125000000026 trimethylsilyl group Chemical group [H]C([H])([H])[Si]([*])(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- JBWKIWSBJXDJDT-UHFFFAOYSA-N triphenylmethyl chloride Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(Cl)C1=CC=CC=C1 JBWKIWSBJXDJDT-UHFFFAOYSA-N 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 102000003390 tumor necrosis factor Human genes 0.000 description 1
- 230000007306 turnover Effects 0.000 description 1
- 238000002211 ultraviolet spectrum Methods 0.000 description 1
- DRTQHJPVMGBUCF-UHFFFAOYSA-N uracil arabinoside Natural products OC1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-UHFFFAOYSA-N 0.000 description 1
- 229940045145 uridine Drugs 0.000 description 1
- 210000003932 urinary bladder Anatomy 0.000 description 1
- 206010046766 uterine cancer Diseases 0.000 description 1
- 229960005486 vaccine Drugs 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 201000001862 viral hepatitis Diseases 0.000 description 1
- 230000008673 vomiting Effects 0.000 description 1
- 238000003260 vortexing Methods 0.000 description 1
- 239000003643 water by type Substances 0.000 description 1
- 230000005428 wave function Effects 0.000 description 1
- 229960000523 zalcitabine Drugs 0.000 description 1
- 229960002555 zidovudine Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
- A61K31/52—Purines, e.g. adenine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
- A61K31/52—Purines, e.g. adenine
- A61K31/522—Purines, e.g. adenine having oxo groups directly attached to the heterocyclic ring, e.g. hypoxanthine, guanine, acyclovir
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
- A61K31/675—Phosphorus compounds having nitrogen as a ring hetero atom, e.g. pyridoxal phosphate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7084—Compounds having two nucleosides or nucleotides, e.g. nicotinamide-adenine dinucleotide, flavine-adenine dinucleotide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H19/00—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof
- C07H19/02—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof sharing nitrogen
- C07H19/04—Heterocyclic radicals containing only nitrogen atoms as ring hetero atom
- C07H19/06—Pyrimidine radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H9/00—Compounds containing a hetero ring sharing at least two hetero atoms with a saccharide radical
- C07H9/02—Compounds containing a hetero ring sharing at least two hetero atoms with a saccharide radical the hetero ring containing only oxygen as ring hetero atoms
- C07H9/04—Cyclic acetals
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Epidemiology (AREA)
- Virology (AREA)
- Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Biochemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Genetics & Genomics (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- AIDS & HIV (AREA)
- Tropical Medicine & Parasitology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Saccharide Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US30035601P | 2001-06-22 | 2001-06-22 | |
| US30538601P | 2001-07-13 | 2001-07-13 | |
| PCT/US2002/020245 WO2003000200A2 (en) | 2001-06-22 | 2002-06-24 | β-2'-OR 3'-HALONUCLEOSIDES |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2005503358A true JP2005503358A (ja) | 2005-02-03 |
| JP2005503358A5 JP2005503358A5 (enExample) | 2009-11-19 |
Family
ID=26971734
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2003506646A Pending JP2005503358A (ja) | 2001-06-22 | 2002-06-24 | β−2’−または3’−ハロヌクレオシド |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US6949522B2 (enExample) |
| EP (1) | EP1478322A4 (enExample) |
| JP (1) | JP2005503358A (enExample) |
| KR (1) | KR20050059975A (enExample) |
| CN (1) | CN100343268C (enExample) |
| AU (1) | AU2002322325A1 (enExample) |
| BR (1) | BR0210594A (enExample) |
| CA (1) | CA2451745A1 (enExample) |
| WO (1) | WO2003000200A2 (enExample) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015125781A1 (ja) * | 2014-02-18 | 2015-08-27 | 富士フイルム株式会社 | チオラン骨格型糖化合物の製造方法およびチオラン骨格型糖化合物 |
| US9815812B2 (en) | 2014-02-19 | 2017-11-14 | Fujifilm Corporation | Thiopyranose compound and method for producing same |
| US9896471B2 (en) | 2012-03-28 | 2018-02-20 | Fujifilm Corporation | Salt of 1-(2-deoxy-2-fluoro-4-thio-β-D-arabinofuranosyl)cytosine |
| US10059734B2 (en) | 2014-10-31 | 2018-08-28 | Fujifilm Corporation | Thionucleoside derivative or salt thereof, and pharmaceutical composition |
| US10093645B2 (en) | 2012-08-13 | 2018-10-09 | Fujifilm Corporation | Synthetic intermediate of 1-(2-deoxy-2-fluoro-4-thio-β-D-arabinofuranosyl)cytosine, synthetic intermediate of thionucleoside, and method for producing the same |
| US11141421B2 (en) | 2018-01-29 | 2021-10-12 | Fujifilm Corporation | Antitumor agent for biliary tract cancer and method for treating biliary tract cancer |
| US11369625B2 (en) | 2016-08-31 | 2022-06-28 | Fujifilm Corporation | Anti-tumor agent, anti-tumor effect enhancer, and anti-tumor kit |
Families Citing this family (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MY164523A (en) | 2000-05-23 | 2017-12-29 | Univ Degli Studi Cagliari | Methods and compositions for treating hepatitis c virus |
| MXPA02011691A (es) | 2000-05-26 | 2004-05-17 | Idenix Cayman Ltd | Metodos y composiciones para el tratamiento de flavivirus y pestivirus. |
| EP1360325A2 (en) * | 2000-10-18 | 2003-11-12 | Pharmasset Limited | Multiplex quantification of nucleic acids in diseased cells |
| US8481712B2 (en) | 2001-01-22 | 2013-07-09 | Merck Sharp & Dohme Corp. | Nucleoside derivatives as inhibitors of RNA-dependent RNA viral polymerase |
| EP1355916B1 (en) | 2001-01-22 | 2007-01-10 | Merck & Co., Inc. | Nucleoside derivatives as inhibitors of rna-dependent rna viral polymerase |
| US7105499B2 (en) | 2001-01-22 | 2006-09-12 | Merck & Co., Inc. | Nucleoside derivatives as inhibitors of RNA-dependent RNA viral polymerase |
| AU2002343604C1 (en) | 2001-10-30 | 2009-09-17 | Conforma Therapeutics Corporation | Purine analogs having HSP90-inhibiting activity |
| MXPA04012802A (es) | 2002-06-28 | 2005-04-19 | Idenix Cayman Ltd | Ester 2'-c-metil-3'-o-l-valina de ribofuranosil-citidina para el tratamiento de infecciones por flaviviridae. |
| US7608600B2 (en) * | 2002-06-28 | 2009-10-27 | Idenix Pharmaceuticals, Inc. | Modified 2′ and 3′-nucleoside prodrugs for treating Flaviviridae infections |
| CN101172993A (zh) | 2002-06-28 | 2008-05-07 | 埃迪尼克斯(开曼)有限公司 | 用于治疗黄病毒感染的2′-c-甲基-3′-o-l-缬氨酸酯核糖呋喃基胞苷 |
| ES2624353T3 (es) | 2002-11-15 | 2017-07-13 | Idenix Pharmaceuticals Llc | 2'-Metil nucleósidos en combinación con interferón y mutación de Flaviviridae |
| RU2005121904A (ru) | 2002-12-12 | 2006-01-20 | Айденикс (Кайман) Лимитед (Ky) | Способ получения 2`-разветвленных нуклеозидов |
| EP1610797A1 (en) * | 2003-03-27 | 2006-01-04 | Boehringer Ingelheim International GmbH | Antiviral combination of nevirapine and a further antiretroviral compound |
| EP2345658A1 (en) | 2003-05-30 | 2011-07-20 | Pharmasset, Inc. | Modified fluorinated nucleoside analogues |
| WO2005028434A2 (en) | 2003-09-18 | 2005-03-31 | Conforma Therapeutics Corporation | Novel heterocyclic compounds as hsp90-inhibitors |
| JP2005289964A (ja) * | 2004-03-12 | 2005-10-20 | Ajinomoto Co Inc | 2−デオキシ−l−リボース化合物の製造方法 |
| GB0505781D0 (en) * | 2005-03-21 | 2005-04-27 | Univ Cardiff | Chemical compounds |
| KR20080004550A (ko) | 2005-03-30 | 2008-01-09 | 콘포마 세러퓨틱스 코포레이션 | Hsp90-억제제로서 알키닐 피롤로피리미딘 및 관련유사체 |
| CA2623522C (en) | 2005-09-26 | 2015-12-08 | Pharmasset, Inc. | Modified 4'-nucleosides as antiviral agents |
| WO2007075876A2 (en) | 2005-12-23 | 2007-07-05 | Idenix Pharmaceuticals, Inc. | Process for preparing a synthetic intermediate for preparation of branched nucleosides |
| CA2717788A1 (en) * | 2007-07-09 | 2009-01-15 | Eastern Virginia Medical School | Substituted nucleoside derivatives with antiviral and antimicrobial properties |
| WO2012142075A1 (en) | 2011-04-13 | 2012-10-18 | Merck Sharp & Dohme Corp. | 2'-azido substituted nucleoside derivatives and methods of use thereof for the treatment of viral diseases |
| JP2014511875A (ja) | 2011-04-13 | 2014-05-19 | メルク・シャープ・アンド・ドーム・コーポレーション | 2’−シアノ置換ヌクレオシド誘導体およびウイルス疾患の治療のためのその使用方法 |
| BR112013026345A2 (pt) | 2011-04-13 | 2019-04-24 | Merck Sharp & Dohe Corp. | composto, composição farmacêutica, uso de um composto, e, método para tratar um paciente infectado com hcv |
| WO2013009735A1 (en) | 2011-07-13 | 2013-01-17 | Merck Sharp & Dohme Corp. | 5'-substituted nucleoside derivatives and methods of use thereof for the treatment of viral diseases |
| EP2731433A4 (en) | 2011-07-13 | 2014-12-31 | Merck Sharp & Dohme | 5'-SUBSTITUTED NUCLEOSIDE ANALOGA AND METHOD FOR THEIR USE FOR THE TREATMENT OF VIRUS DISEASES |
| WO2013123138A2 (en) | 2012-02-14 | 2013-08-22 | University Of Georgia Research Foundation, Inc. | Spiro [2.4]heptanes for treatment of flaviviridae infections |
| CN107427530B (zh) | 2015-03-06 | 2020-09-08 | 阿堤亚制药公司 | 用于HCV治疗的β-D-2’-脱氧-2’α-氟-2’-β-C-取代的-2-改性的-N6-取代的嘌呤核苷酸 |
| LU100724B1 (en) | 2016-07-14 | 2018-07-31 | Atea Pharmaceuticals Inc | Beta-d-2'-deoxy-2'-alpha-fluoro-2'-beta-c-substituted-4'-fluoro-n6-substituted-6-amino-2-substituted purine nucleotides for the treatment of hepatitis c virus infection |
| CN109689672B (zh) | 2016-09-07 | 2023-05-30 | 阿堤亚制药公司 | 用于rna病毒治疗的2’-取代的-n6-取代的嘌呤核苷酸 |
| GEP20227457B (en) | 2017-02-01 | 2023-01-10 | Atea Pharmaceuticals Inc | Nucleotide hemi-sulfate salt for treatment of hepatitis c virus |
| JP2021521118A (ja) | 2018-04-10 | 2021-08-26 | アテア ファーマシューティカルズ, インコーポレイテッド | 肝硬変を伴うhcv感染患者の治療 |
| US10874687B1 (en) | 2020-02-27 | 2020-12-29 | Atea Pharmaceuticals, Inc. | Highly active compounds against COVID-19 |
| CN115667280B (zh) * | 2020-03-25 | 2025-07-18 | 西蒙弗雷泽大学 | 一种合成核苷及其类似物的方法和试剂 |
| KR20240022574A (ko) | 2021-06-17 | 2024-02-20 | 아테아 파마슈티컬즈, 인크. | 유리한 항-hcv 조합 요법 |
Family Cites Families (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4211773A (en) | 1978-10-02 | 1980-07-08 | Sloan Kettering Institute For Cancer Research | 5-Substituted 1-(2'-Deoxy-2'-substituted-β-D-arabinofuranosyl)pyrimidine nucleosides |
| US4625020A (en) | 1983-11-18 | 1986-11-25 | Bristol-Myers Company | Nucleoside process |
| US4666892A (en) | 1984-03-06 | 1987-05-19 | Sloan-Kettering Memorial Cancer Center | Method and composition for hepatitis treatment with pyrimidine nucleoside compounds |
| US4681933A (en) * | 1986-05-01 | 1987-07-21 | University Of Georgia Research Foundation, Inc. | 2',3'-dideoxy-5-substituted uridines and related compounds as antiviral agents |
| US5446029A (en) * | 1986-07-04 | 1995-08-29 | Medivir Ab | Anti-retroviral activity of 2',3'-dideoxy-3'-fluoronucleosides |
| DD279407A1 (de) * | 1986-07-24 | 1990-06-06 | Akad Wissenschaften Ddr | Verfahren zur herstellung eines mittels gegen aids |
| US5215970A (en) | 1987-04-16 | 1993-06-01 | Medivir Ab | Nucleosides and nucleotide analogues, pharmaceutical composition and processes for the preparation of the compounds |
| DE3715666A1 (de) | 1987-05-11 | 1988-11-24 | Kodak Ag | Kassettenentlade- und beladegeraet |
| GB8712115D0 (en) | 1987-05-22 | 1987-06-24 | Hoffmann La Roche | Pyrimidine derivatives |
| US5246924A (en) | 1987-09-03 | 1993-09-21 | Sloan-Kettering Institute For Cancer Research | Method for treating hepatitis B virus infections using 1-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)-5-ethyluracil |
| GB8726136D0 (en) | 1987-11-07 | 1987-12-09 | Wellcome Found | Therapeutic nucleosides |
| US4908440A (en) | 1987-11-12 | 1990-03-13 | Bristol Myers Company | 2',3'-dideoxy-2'-fluoroarabinopyrimidine nucleosides |
| SE8802173D0 (sv) | 1988-06-10 | 1988-06-10 | Astra Ab | Pyrimidine derivatives |
| SE8802687D0 (sv) | 1988-07-20 | 1988-07-20 | Astra Ab | Nucleoside derivatives |
| US5034518A (en) | 1989-05-23 | 1991-07-23 | Southern Research Institute | 2-fluoro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl) adenine nucleosides |
| DD293498A5 (de) | 1989-07-20 | 1991-09-05 | Zi Fuer Molekularbiologie Der Adw,De | Verfahren zur herstellung eines mittels fuer die behandlung oder prophylaxe von hepatits-infektionen bei mensch und tier |
| US5128458A (en) | 1990-04-20 | 1992-07-07 | Southern Research Institute | 2',3'-dideoxy-4'-thioribonucleosides as antiviral agents |
| EP0463470A3 (en) | 1990-06-22 | 1992-08-05 | F. Hoffmann-La Roche Ag | Process for the preparation of a fluorotetrahydrofuranone derivative |
| JPH0477485A (ja) * | 1990-07-19 | 1992-03-11 | Yamasa Shoyu Co Ltd | 2’,3’―ジデヒドロ―2’,3’―ジデオキシウリジン誘導体およびその製造法 |
| US5817799A (en) | 1990-07-23 | 1998-10-06 | The United States Of America As Represented By The Department Of Health And Human Services | 2'-Fluorofuranosyl derivatives and methods for preparing 2'-fluoropyrimidine and 2'-fluoropurine nucleosides |
| IT1246983B (it) | 1990-11-13 | 1994-12-12 | Consiglio Nazionale Ricerche | L-2'-desossiuridine e composizioni farmaceutiche che le contengono. |
| GB9200150D0 (en) | 1992-01-06 | 1992-02-26 | Wellcome Found | Therapeutic nucleosides |
| ATE178335T1 (de) | 1992-01-06 | 1999-04-15 | Wellcome Found | Therapeutische nukleoside der 2',3'-dideoxy-3'- fluor -purin reihe |
| US5426183A (en) | 1992-06-22 | 1995-06-20 | Eli Lilly And Company | Catalytic stereoselective glycosylation process for preparing 2'-deoxy-2',2'-difluoronucleosides and 2'-deoxy-2'-fluoronucleosides |
| AU5691094A (en) | 1992-12-18 | 1994-07-19 | University Of Alberta, The | Dihydropyrimidine nucleosides with antiviral properties |
| US5424416A (en) | 1993-08-25 | 1995-06-13 | Eli Lilly And Company | Process for preparation of 2-deoxy-2,2-difluoro-D-ribofuranosyl-3,5-hydroxy protected-1-alkyl and aryl sulfonates and their use in preparation of 2',2'-difluoro-2'-deoxy nucleosides |
| US5587362A (en) | 1994-01-28 | 1996-12-24 | Univ. Of Ga Research Foundation | L-nucleosides |
| US5703058A (en) * | 1995-01-27 | 1997-12-30 | Emory University | Compositions containing 5-fluoro-2',3'-didehydro-2',3'-dideoxycytidine or a mono-, di-, or triphosphate thereof and a second antiviral agent |
| US6355790B1 (en) * | 1997-06-03 | 2002-03-12 | University Of Rochester | Inhibition of HIV replication using a mutated transfer RNA primer |
| WO1999043691A1 (en) | 1998-02-25 | 1999-09-02 | Emory University | 2'-fluoronucleosides |
| MY164523A (en) | 2000-05-23 | 2017-12-29 | Univ Degli Studi Cagliari | Methods and compositions for treating hepatitis c virus |
| MXPA02011691A (es) | 2000-05-26 | 2004-05-17 | Idenix Cayman Ltd | Metodos y composiciones para el tratamiento de flavivirus y pestivirus. |
-
2002
- 2002-06-24 BR BR0210594-2A patent/BR0210594A/pt not_active IP Right Cessation
- 2002-06-24 WO PCT/US2002/020245 patent/WO2003000200A2/en not_active Ceased
- 2002-06-24 KR KR1020037016775A patent/KR20050059975A/ko not_active Ceased
- 2002-06-24 EP EP02756310A patent/EP1478322A4/en not_active Withdrawn
- 2002-06-24 JP JP2003506646A patent/JP2005503358A/ja active Pending
- 2002-06-24 US US10/179,612 patent/US6949522B2/en not_active Expired - Fee Related
- 2002-06-24 CN CNB028164555A patent/CN100343268C/zh not_active Expired - Fee Related
- 2002-06-24 CA CA002451745A patent/CA2451745A1/en not_active Abandoned
- 2002-06-24 AU AU2002322325A patent/AU2002322325A1/en not_active Abandoned
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9896471B2 (en) | 2012-03-28 | 2018-02-20 | Fujifilm Corporation | Salt of 1-(2-deoxy-2-fluoro-4-thio-β-D-arabinofuranosyl)cytosine |
| US10093645B2 (en) | 2012-08-13 | 2018-10-09 | Fujifilm Corporation | Synthetic intermediate of 1-(2-deoxy-2-fluoro-4-thio-β-D-arabinofuranosyl)cytosine, synthetic intermediate of thionucleoside, and method for producing the same |
| US10570112B2 (en) | 2012-08-13 | 2020-02-25 | Fujifilm Corporation | Synthetic intermediate of 1-(2-deoxy-2-fluoro-4-thio-β-D-arabinofuranosyl)cytosine, synthetic intermediate of thionucleoside, and method for producing the same |
| WO2015125781A1 (ja) * | 2014-02-18 | 2015-08-27 | 富士フイルム株式会社 | チオラン骨格型糖化合物の製造方法およびチオラン骨格型糖化合物 |
| JPWO2015125781A1 (ja) * | 2014-02-18 | 2017-03-30 | 富士フイルム株式会社 | チオラン骨格型糖化合物の製造方法およびチオラン骨格型糖化合物 |
| US9884882B2 (en) | 2014-02-18 | 2018-02-06 | Fujifilm Corporation | Method for producing thiolane skeleton-type glycoconjugate, and thiolane skeleton-type glycoconjugate |
| US9815812B2 (en) | 2014-02-19 | 2017-11-14 | Fujifilm Corporation | Thiopyranose compound and method for producing same |
| US10059734B2 (en) | 2014-10-31 | 2018-08-28 | Fujifilm Corporation | Thionucleoside derivative or salt thereof, and pharmaceutical composition |
| US10385089B2 (en) | 2014-10-31 | 2019-08-20 | Fujifilm Corporation | Thionucleoside derivative or salt thereof, and pharmaceutical composition |
| US11369625B2 (en) | 2016-08-31 | 2022-06-28 | Fujifilm Corporation | Anti-tumor agent, anti-tumor effect enhancer, and anti-tumor kit |
| US11141421B2 (en) | 2018-01-29 | 2021-10-12 | Fujifilm Corporation | Antitumor agent for biliary tract cancer and method for treating biliary tract cancer |
Also Published As
| Publication number | Publication date |
|---|---|
| US6949522B2 (en) | 2005-09-27 |
| HK1076473A1 (en) | 2006-01-20 |
| EP1478322A4 (en) | 2007-08-08 |
| US20050119286A1 (en) | 2005-06-02 |
| WO2003000200A3 (en) | 2004-09-02 |
| AU2002322325A1 (en) | 2003-01-08 |
| CN1599744A (zh) | 2005-03-23 |
| KR20050059975A (ko) | 2005-06-21 |
| EP1478322A2 (en) | 2004-11-24 |
| CN100343268C (zh) | 2007-10-17 |
| CA2451745A1 (en) | 2003-01-03 |
| BR0210594A (pt) | 2005-11-01 |
| WO2003000200A2 (en) | 2003-01-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6949522B2 (en) | β-2′- or 3′-halonucleosides | |
| EP1058686B1 (en) | 2'-fluoronucleosides | |
| US8895531B2 (en) | 2′-fluoronucleoside phosphonates as antiviral agents | |
| JP2005503358A5 (enExample) | ||
| MX2007006961A (es) | Analogos nucleosidos de ciclobutilo 2' y 3'-sustituidos para el tratamiento de infeccciones virales y proliferacion celular anormal. | |
| CN101240001B (zh) | 2'-氟代核苷 | |
| KR100886653B1 (ko) | 2'-플루오로뉴클레오사이드 | |
| AU2007203174B2 (en) | 2'-Fluoronucleosides | |
| EP1754710A2 (en) | 2'-Fluoroncucleosides | |
| HK1076473B (en) | β-2'-OR 3'-HALONUCLEOSIDES |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050624 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090317 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20090610 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20090617 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090917 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20090917 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20091117 |