EP3230487B1 - Niederdruck-carbonitrierungsverfahren - Google Patents
Niederdruck-carbonitrierungsverfahren Download PDFInfo
- Publication number
- EP3230487B1 EP3230487B1 EP15817994.5A EP15817994A EP3230487B1 EP 3230487 B1 EP3230487 B1 EP 3230487B1 EP 15817994 A EP15817994 A EP 15817994A EP 3230487 B1 EP3230487 B1 EP 3230487B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- steps
- pressure
- value
- chamber
- during
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000000034 method Methods 0.000 title claims description 48
- 238000005256 carbonitriding Methods 0.000 title claims description 35
- 238000005121 nitriding Methods 0.000 claims description 63
- 238000005255 carburizing Methods 0.000 claims description 27
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 claims description 14
- 230000007935 neutral effect Effects 0.000 claims description 10
- 229910000831 Steel Inorganic materials 0.000 claims description 8
- 239000010959 steel Substances 0.000 claims description 8
- 229910021529 ammonia Inorganic materials 0.000 claims description 7
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 claims description 6
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 claims description 4
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 claims description 4
- 239000001294 propane Substances 0.000 claims description 3
- 239000007789 gas Substances 0.000 description 67
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 49
- 238000009792 diffusion process Methods 0.000 description 37
- 229910052757 nitrogen Inorganic materials 0.000 description 23
- 229910052799 carbon Inorganic materials 0.000 description 21
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 20
- 238000002347 injection Methods 0.000 description 14
- 239000007924 injection Substances 0.000 description 14
- 239000001257 hydrogen Substances 0.000 description 8
- 229910052739 hydrogen Inorganic materials 0.000 description 8
- 238000010791 quenching Methods 0.000 description 8
- 230000000171 quenching effect Effects 0.000 description 8
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 7
- 238000010438 heat treatment Methods 0.000 description 5
- 230000001174 ascending effect Effects 0.000 description 4
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 238000000605 extraction Methods 0.000 description 3
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- 238000004880 explosion Methods 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 150000002430 hydrocarbons Chemical class 0.000 description 2
- 238000004590 computer program Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 239000008246 gaseous mixture Substances 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C8/00—Solid state diffusion of only non-metal elements into metallic material surfaces; Chemical surface treatment of metallic material by reaction of the surface with a reactive gas, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals
- C23C8/06—Solid state diffusion of only non-metal elements into metallic material surfaces; Chemical surface treatment of metallic material by reaction of the surface with a reactive gas, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using gases
- C23C8/34—Solid state diffusion of only non-metal elements into metallic material surfaces; Chemical surface treatment of metallic material by reaction of the surface with a reactive gas, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using gases more than one element being applied in more than one step
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D1/00—General methods or devices for heat treatment, e.g. annealing, hardening, quenching or tempering
- C21D1/06—Surface hardening
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D1/00—General methods or devices for heat treatment, e.g. annealing, hardening, quenching or tempering
- C21D1/74—Methods of treatment in inert gas, controlled atmosphere, vacuum or pulverulent material
- C21D1/76—Adjusting the composition of the atmosphere
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F27—FURNACES; KILNS; OVENS; RETORTS
- F27B—FURNACES, KILNS, OVENS, OR RETORTS IN GENERAL; OPEN SINTERING OR LIKE APPARATUS
- F27B17/00—Furnaces of a kind not covered by any preceding group
- F27B17/0016—Chamber type furnaces
- F27B17/0083—Chamber type furnaces with means for circulating the atmosphere
Definitions
- the present invention relates to processes for treating steel parts, and more particularly to carbonitriding processes, that is to say introducing carbon and nitrogen at the surface of steel parts to improve its performance. hardness and fatigue resistance.
- a first category of carbonitriding processes corresponds to so-called high-pressure carbonitriding processes insofar as the enclosure containing the workpieces is maintained at a pressure generally close to atmospheric pressure for the duration of the treatment.
- Such a process consists, for example, in maintaining the parts at a temperature plateau, for example at approximately 880 ° C., while supplying the enclosure with a gaseous mixture consisting of methanol and ammonia.
- the carbonitriding step is followed by a quenching step, for example an oil quenching, and optionally a hardening step of the treated parts.
- a second category of carbonitriding processes corresponds to so-called low pressure or reduced pressure carbonitriding processes, insofar as the enclosure containing the workpieces is maintained at a pressure generally less than a few hundred pascals (a few millibars).
- An object of an embodiment is to overcome all or part of the disadvantages of low pressure carbonitriding processes and low pressure carbonitriding furnaces previously described.
- Another object of an embodiment is to obtain, precisely and reproducibly, the desired carbon and nitrogen concentration profiles in the treated parts.
- Another object of an embodiment is that the implementation of the carbonitriding process is compatible with the treatment of steel parts in an industrial context.
- an embodiment provides a pressure-based carbonitriding process of a steel part disposed in an enclosure, comprising first stages and second stages, a cementation gas being injected into the enclosure only during the first stages and a nitriding gas being injected into the chamber only during the second stages, at least one of the second stages being situated between two of the first stages, the pressure in the chamber during at least a portion of the said stages; two first steps being maintained at a first value and the pressure in the chamber during at least a portion of said second step located between said first two steps being at a second value strictly greater than the first value.
- the first value is between 0.1 hPa and 20 hPa, preferably between 0.1 hPa and 10 hPa.
- the second value is between 10 hPa and 250 hPa, preferably between 30 hPa and 150 hPa.
- the carburizing gas is propane or acetylene.
- the nitriding gas is ammonia.
- the method further comprises third steps, each third step being located between two of the first steps, between two of the second steps or between one of the first steps and one of the second steps, a neutral gas being injected into the chamber during each third step.
- the method further comprises first, second and third successive phases, the first phase comprising only first steps alternating with third steps, the second phase comprising the successive repetition of a cycle successively comprising a second step, a third step, a first step and a second step, and the third step comprising only second alternate steps with third steps.
- At least one of the third steps directly precedes one of the second steps and the pressure is increased from the first value to the second value during said first step before the beginning of said third step.
- At least one of the third steps directly precedes one of the second steps and the pressure is maintained at the first value until the end of said first step and is increased from the first value to the second value after the beginning of said third step.
- the part is maintained at a temperature step.
- the temperature plateau is between 800 ° C and 1050 ° C.
- the temperature plateau is greater than 900 ° C.
- One embodiment also provides a carbonitriding furnace for receiving a steel part, comprising gas introduction and gas extraction circuits, and a control module adapted to control the gas introduction and gas introduction circuits. extraction of gas to introduce, during the first steps and second steps, a carburizing gas into the chamber only during the first stages and a nitriding gas into the chamber only during the second stages, at least one second steps being located between two first steps, and adapted to maintain the pressure in the chamber during at least a portion of the first two steps at a first value and the pressure in the chamber during at least a portion of said second step located between the first two steps to a second value strictly greater than the first value.
- the furnace further comprises a heating element and the control module is adapted to control the heating element to maintain the room at a temperature step.
- alternating steps A and B means a succession of steps A and B in which each step B, with the exception of the last step of the succession, is located between two steps A and each step A , with the exception of the initial stage of succession, is located between two stages B.
- an alternation of carbon enrichment steps is performed in an enclosure, containing steel parts to be treated maintained at a substantially constant temperature, at least during part of the carbonitriding process.
- cementation steps also known as cementation steps, during which a cementation gas is injected into the chamber maintained at a first reduced pressure
- nitrogen enrichment stages also called nitriding steps, during which a nitriding gas is injected in the chamber maintained at a second pressure greater than the first pressure.
- a diffusion step during which the injection of the carburizing gas and the injection of the nitriding gas into the chamber are interrupted.
- a diffusion step during which the injection of the carburizing gas and the injection of the nitriding gas into the chamber are interrupted.
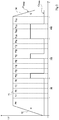
- the figure 1 schematically represents an embodiment of a low-pressure carbonitriding furnace 10.
- the furnace 10 comprises a sealed wall 12 delimiting an internal enclosure 14 in which a charge to be treated 16 is arranged, generally a large number of parts disposed on a suitable support.
- a vacuum at a pressure of a few hectopascals (a few millibars) to a few hundred hectopascals (a few hundred millibars) can be maintained in the chamber 14 through an extraction pipe 18 is connected to a vacuum pump 20.
- An injector 22 makes it possible to introduce gases distributed in the chamber 14.
- gas inlets 22, 24, 26, 28 respectively controlled by valves 30, 32, 34, 36.
- a heating element 38 is disposed in the enclosure 14.
- a control module 40 is connected to the valves 30, 32, 34, 36 and to the vacuum pump 20, and possibly to the heating element 38.
- the control module 40 is adapted to control the closing and opening of each valve 30, 32, 34, 36.
- a pressure sensor 42 and a temperature sensor 44 may be provided in the enclosure 14 and connected to the control module 40. From the signal supplied by the temperature sensor 44, the control module 40 is adapted to control the heating element 38 to maintain the temperature in the enclosure 14 at a value substantially constant. From the signal provided by the pressure sensor 42, the control module 40 is adapted to control the suction power of the vacuum pump 20 to maintain the pressure in the chamber 14 to a set value.
- the control module 40 may comprise a microprocessor or a microcontroller.
- the control module 40 may, in whole or in part, correspond to a dedicated circuit or include a processor adapted to execute instructions of a computer program stored in a memory.
- the figure 2 represents a curve C Temp of temperature evolution and a curve C Pres of evolution of the pressure in the chamber 14 of the carbonitriding furnace 10 of the figure 1 during a carbonitriding cycle according to a carbonitriding process embodiment.
- the method comprises an initial step H corresponding to an increase of the temperature in the chamber 14 containing the charge 16 to a temperature plateau 52 which, in the present example, can correspond to a temperature of between approximately 800 ° C. and about 1050 ° C, preferably between about 880 ° C and about 960 ° C, for example of the order of 930 ° C.
- Step H is followed by a step PH equalizing the temperature of the parts constituting the load 16 at the temperature step 52.
- the steps H and PH can be carried out in the presence of a neutral gas, to which a reducing gas is optionally added.
- the neutral gas is, for example, nitrogen (N 2 ).
- the reducing gas for example hydrogen (H 2 )
- H 2 hydrogen
- the reducing gas may be added in a proportion ranging from 1% to 5% by volume of the neutral gas.
- Step PH is followed by a succession of three phases PI, PII and PIII.
- the phases PI, PII and PIII are carried out by maintaining the temperature in the chamber 14 at the temperature plateau 52.
- a quenching step Q of the load 10 for example a gas quenching, closes the carbonitriding cycle by a decrease in temperature. of the temperature.
- the PI phase may not be present.
- phase PIII may not be present.
- the PI phase comprises an alternation of carbon enrichment stages C I , during which a carburising gas is injected into the chamber 14, and carbon diffusion stages D I during which the carburising gas is not more injected into the enclosure 14.
- the PI phase comprises at least successively a carburizing step, a diffusion step, a carburizing step and a diffusion step.
- the PI phase comprises an alternation of two carburizing steps C I and two diffusion stages D I.
- the carburizing gas is, for example, propane (C 3 H 8 ) or acetylene (C 2 H 2 ). It can also be any other hydrocarbon (C X H Y ) likely to dissociate at the enclosure temperatures to cementer the surface of the parts to be treated.
- Phase PII comprises an alternation of enrichment stages in nitrogen N II , during which a nitriding gas is injected into chamber 14, and enrichment stages in carbon C II during which the gas of cementation is injected into the chamber 14.
- the carburizing gas is not injected into the chamber 14 and, during the carburizing C II stages, the nitriding gas is not injected in the chamber 14.
- a nitriding step N II is followed directly by a cementation step C II .
- a cementation step C II with the exception of the last C II cementation step of the PII phase, is followed directly by a nitriding step N II .
- a diffusion step D II may be provided between each nitriding step N II and the subsequent cementation step C II .
- a diffusion step D II may be provided between each cementation step C II and the next nitriding step N II .
- the PII phase comprises at least successively a nitriding step, a diffusion step, a cementation step and a diffusion step.
- the PII phase comprises two successive cycles each comprising a nitriding step N II , a diffusion step D II , a cementation step C II and a diffusion step D II .
- the nitriding gas is, for example, ammonia (NH 3 ).
- the phase PIII comprises an alternation of enrichment stages in nitrogen N III , during which the nitriding gas is injected into the chamber 14, and carbon diffusion stages D III during which the nitriding gas is more preferably injected into the chamber 14.
- the PIII phase comprises at least successively a nitriding step, a diffusion step, a nitriding step and a diffusion step.
- phase PIII comprises an alternation of two nitriding stages C III and two diffusion stages D III .
- a hydrocarbon (C X H Y ), at the inlet 24 of the nitrogen valve 32, can be fed to the inlet 22 of the valve 30 at the inlet 36 of the valve 34 of the hydrogen and on the inlet 28 of the valve 36 of ammonia.
- the pressure is maintained at a set point in the chamber 14 by the vacuum pump 20 controlled by the control module 40.
- the pressure in the enclosure is, at least on some of these steps, maintained substantially constant at a first value.
- the first pressure value is between 0.1 hPa and 20 hPa, preferably between 0.1 hPa and 10 hPa.
- the pressure in the chamber 14 is kept substantially constant at the first value during at least a portion of each cementation step C I of the first phase P1.
- the pressure in the chamber 14 is kept substantially constant at the first value during at least a portion of each cementation step C II of the second phase PII.
- the pressure in the chamber is maintained, at least over a portion of this step, substantially constant at a second value, strictly greater than the first value. value.
- the second pressure value is between 10 hPa and 250 hPa, preferably between 30 hPa and 150 hPa.
- the pressure in the chamber 14 is kept substantially constant at the second value during each nitriding step N III of the third phase PIII.
- the pressure in the chamber 14 is kept substantially constant at the second value during at least a portion of each nitriding step N II of the third phase PII.
- the carbonitriding process remains a low pressure, or reduced pressure, carbonitriding process insofar as the pressure in the enclosure 14 is less than 500 mbar (500 hPa) during the entire process.
- the pressure in the chamber 14 is, in addition, kept substantially constant at the first value during at least part of each diffusion step D I of the first phase P1, during at least a portion of each diffusion step D II of the second phase PII and / or during at least part of each diffusion step D III of the third phase PIII.
- the pressure in the chamber 14 is furthermore kept substantially constant at the first value during the steps H and PH.
- a neutral gas for example nitrogen (N 2 ), may, in addition, be injected during the H and PH steps and during the carburizing C I , C II , nitriding N II , N III and diffusion stages. D I , D II , D III .
- the neutral gas can be injected only during the diffusion steps D I , D II , D III and not be injected during the cementation steps C I , C II and the nitriding steps N II , N III .
- the passage of the pressure in the chamber 14 from the first value to the second value, which is strictly greater than the first value, can be obtained by temporarily reducing or stopping the suction of the vacuum pump 20.
- increasing the pressure in the chamber 14 from the first value to the second value can be achieved in less than 2 minutes, preferably in less than 1 minute.
- the passage of the pressure in the chamber 14 of the second value to the first value, strictly less than the second value, can be obtained by temporarily increasing the suction of the vacuum pump 20, to reduce the pressure in the enclosure 14, then reducing the suction power of the vacuum pump 20 to a level adapted to maintain the pressure in the chamber 14 to the second value.
- the decrease in the pressure in the chamber 14 from the second value to the first value can be achieved in less than 2 minutes, preferably in less than 1 minute.
- all the gases injected into the enclosure 14 of the furnace 10 or some of them may be In such a variant, for example, during the steps of temperature rise H and of equalization of temperature PH, it is possible to inject directly into the chamber 14 a mixture of nitrogen and hydrogen.
- the Figures 3 to 6 respectively represent curves C 1 , C 2 , C 3 , C 4 of pressure evolution in the chamber 14 and illustrate different configurations of variation of the pressure during the succession of a first diffusion step D1, which may correspond in a step D II or a step D III described above, a nitriding step N, which may correspond to a step N II or a step N III described above, and a second diffusion step D2.
- a first diffusion step D1 which may correspond in a step D II or a step D III described above
- a nitriding step N which may correspond to a step N II or a step N III described above
- nitriding step N nitriding gas is injected into the chamber 14.
- neutral gas is injected into the chamber 14.
- the injection of neutral gas into the chamber 14 can also be carried out during the nitriding step N.
- Each curve C 1 , C 2 , C 3 and C 4 comprises a first pressure bearing LP1 substantially constant at the first value in each diffusion step D1 and D2, a second pressure bearing LP2 substantially constant at the second value in the nitriding step N, an upward phase PUP between the bearing LP1 and the bearing PP2 and a downward phase PDOWN between the LP2 bearing and the LP1 bearing.
- the ascending phase PUP is carried out in the nitriding step N and the downward phase PDOWN is carried out in the diffusion step D2.
- the ascending phase PUP is carried out in the nitriding step N and the downward phase PDOWN is carried out in the nitriding step N.
- the ascending phase PUP is carried out in the diffusion step D1 and the down-phase PDOWN is carried out in the nitriding step N.
- the ascending phase PUP is carried out in the diffusion step D1 and the downward phase PDOWN is carried out in the diffusion step D2.
- the nitriding step N is then advantageously carried out at a substantially constant pressure.
- the figure 7 represents an example of a P C profile of concentration by weight of the carbon element and an example of a P N profile of concentration by weight of the nitrogen element having diffused into a treated part as a function of the depth, measured from the surface of the part during the implementation of a first carbonitriding process in which the pressure in the chamber 14 remains substantially constant at low pressure.
- the figure 8 represents an exemplary profile P C 'of concentration by weight of the carbon element and an example of profile P N ' of concentration by weight of the nitrogen element having diffused into a treated part as a function of the depth, measured from the surface of the part during the implementation of a second carbonitriding process according to the embodiment described above in connection with the figure 2 wherein the pressure is increased during the nitriding steps.
- the carburizing gas was acetylene
- the nitriding gas was ammonia
- the neutral gas was nitrogen.
- the carbonitriding was carried out at a temperature level of 920 ° C.
- the quenching step Q was gas quenching.
- the pressure in the chamber 14 was maintained substantially at 8 mbar (8 hPa) during all the steps H, PH, C I , D I , C II , D II and D III and the pressure in the chamber 14 was maintained substantially at 45 mbar (45 hPa) during stages N II and N III with the exception of the first stage N II which was carried out at the pressure of 8 mbar (8 hPa).
- the inventors have demonstrated that the increase of the pressure during at least certain nitriding steps N II and / or N III makes it possible to obtain an increase in the nitrogen enrichment of the treated parts.
- the nitrogen concentration was 0.1% by weight to 25 ⁇ m, 0.09% by weight to 100 ⁇ m, 0.045% by weight to 200 ⁇ m, and 0.025% by weight to 100 ⁇ m. 300 ⁇ m.
- the nitrogen concentration was 0.4% by weight at 25 ⁇ m, 0.29% by weight at 100 ⁇ m, 0.14% by weight at 200 ⁇ m and 0.06% by weight. at 300 ⁇ m.
- the inventors have demonstrated that the increase of the pressure during at least certain nitriding steps N II and / or N III makes it possible, moreover, to obtain an increase in the carbon enrichment of the treated parts.
- the carbon concentration was 0.725% by weight to 50 ⁇ m, 0.71% by weight to 100 ⁇ m, 0.675% by weight to 200 ⁇ m and 0.6% by weight to 100 ⁇ m. 300 ⁇ m.
- the carbon concentration was 0.8 wt% to 50 ⁇ m, 0.8 wt% to 100 ⁇ m, 0.775 wt% to 200 ⁇ m and 0.68 wt% to 300 wt. .mu.m.
- the nitriding gas can be injected during step H of temperature rise, as soon as the temperature in the chamber 14 exceeds a given temperature, and / or during the equalizing step PH in temperature.
- the nitriding gas is ammonia
- the injection can be performed as soon as the temperature in the enclosure 14 exceeds about 800 ° C.
- the control of the gaseous environment in the chamber 14 can hardly be carried out accurately, which makes it more difficult to obtain accurately and reproducibly, desired nitrogen and carbon concentration profiles of the treated pieces.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Crystallography & Structural Chemistry (AREA)
- Thermal Sciences (AREA)
- Physics & Mathematics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Engineering & Computer Science (AREA)
- Solid-Phase Diffusion Into Metallic Material Surfaces (AREA)
- Furnace Details (AREA)
Claims (12)
- Verfahren zum Niederdruck-Carbonitrieren eines in einer Kammer (14) angeordneten Stahlteils (16), wobei das Verfahren erste Schritte und zweite Schritte aufweist, wobei ein Aufkohlungsgas nur während der ersten Schritte in die Kammer eingelassen wird und ein Nitriergas nur während der zweiten Schritte in die Kammer eingelassen wird, und wobei wenigstens einer der zweiten Schritte zwischen zwei der ersten Schritte stattfindet, dadurch gekennzeichnet, dass der Druck in der Kammer während wenigstens eines Teils der beiden ersten Schritte auf einem ersten Wert gehalten wird und der Druck in der Kammer während wenigstens eines Teils des zweiten Schrittes, der zwischen den beiden ersten Schritten stattfindet, auf einem zweiten Wert liegt, der größer ist als der erste Wert.
- Verfahren nach Anspruch 1, wobei der erste Wert im Bereich von 0,1 hPa bis 20 hPa, vorzugsweise von 0,1 hPa bis 10 hPa liegt.
- Verfahren nach Anspruch 1 oder 2, wobei der zweite Wert im Bereich von 10 hPa bis 250 hPa, vorzugsweise von 30 hPa bis 150 hPa liegt.
- Verfahren nach einem der Ansprüche 1 bis 3, wobei das Aufkohlungsgas Propan oder Acetylen ist.
- Verfahren nach einem der Ansprüche 1 bis 4, wobei das Nitriergas Ammoniak ist.
- Verfahren nach einem der Ansprüche 1 bis 5, das ferner dritte Schritte aufweist, wobei jeder dritte Schritt zwischen zwei der ersten Schritte, zwischen zwei der zweiten Schritte oder zwischen einem der ersten Schritte und einem der zweiten Schritte stattfindet, wobei bei jedem dritten Schritt ein neutrales Gas in die Kammer eingelassen wird.
- Verfahren nach Anspruch 6, das ferner erste, zweite und dritte aufeinanderfolgende Phasen aufweist, und wobei die erste Phase nur erste Schritte im Wechsel mit dritten Schritten aufweist, wobei die zweite Phase die aufeinanderfolgende Wiederholung eines Zyklus aufweist, der aufeinanderfolgend einen zweiten Schritt, einen dritten Schritt, einen ersten Schritt und einen zweiten Schritt aufweist, und wobei die dritte Phase nur zweite Schritte im Wechsel mit dritten Schritten aufweist.
- Verfahren nach Anspruch 6 oder 7, wobei wenigstens einer der dritten Schritte direkt vor einem der zweiten Schritte liegt und wobei der Druck während des ersten Schrittes vor Beginn des dritten Schrittes von dem ersten Wert auf den zweiten Wert erhöht wird.
- Verfahren nach Anspruch 6 oder 7, wobei wenigstens einer der dritten Schritte direkt vor einem der zweiten Schritte liegt und wobei der Druck bis zum Ende des ersten Schrittes auf dem ersten Wert gehalten und nach Beginn des dritten Schrittes vom ersten Wert auf den zweiten Wert erhöht wird.
- Verfahren nach einem der Ansprüche 1 bis 9, wobei das Teil (6) in einer Temperaturhaltestufe gehalten wird.
- Verfahren nach einem der Ansprüche 1 bis 10, wobei die Temperaturhaltestufe im Bereich von 800°C bis 1.050°C liegt.
- Verfahren nach Anspruch 11, wobei die Temperaturhaltestufe höher als 900°C ist.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR1462260A FR3029938B1 (fr) | 2014-12-11 | 2014-12-11 | Procede et four de carbonitruration a basse pression |
| PCT/FR2015/053419 WO2016092219A1 (fr) | 2014-12-11 | 2015-12-10 | Procede et four de carbonitruration a basse pression |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3230487A1 EP3230487A1 (de) | 2017-10-18 |
| EP3230487B1 true EP3230487B1 (de) | 2019-05-08 |
Family
ID=52450486
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP15817994.5A Active EP3230487B1 (de) | 2014-12-11 | 2015-12-10 | Niederdruck-carbonitrierungsverfahren |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US11242594B2 (de) |
| EP (1) | EP3230487B1 (de) |
| JP (1) | JP7177592B2 (de) |
| KR (1) | KR102576343B1 (de) |
| CN (1) | CN107406960B (de) |
| CA (1) | CA2970247C (de) |
| FR (1) | FR3029938B1 (de) |
| MX (1) | MX2017007484A (de) |
| WO (1) | WO2016092219A1 (de) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR3081884B1 (fr) * | 2018-06-05 | 2021-05-21 | Safran Helicopter Engines | Procede de cementation basse pression d'une piece comprenant de l'acier |
| CN112095073B (zh) * | 2020-08-20 | 2022-04-01 | 湖南申亿五金标准件有限公司 | 一种强韧性的qpq处理工艺 |
| AT524143B1 (de) * | 2020-09-10 | 2022-12-15 | Miba Sinter Austria Gmbh | Verfahren zur Härtung eines Sinterbauteils |
| CN111945103A (zh) * | 2020-09-16 | 2020-11-17 | 湖南南方宇航高精传动有限公司 | 一种16Cr3NiWMoVNbE材料低压真空碳氮共渗方法 |
| FR3132720B1 (fr) * | 2022-02-11 | 2024-08-23 | Skf Aerospace France | Procédé de renforcement d’une pièce en acier par carbonitruration |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1462260A (fr) | 1963-12-11 | 1966-04-15 | Velsicol Chemical Corp | Composés herbicides nouveaux, compositions les contenant et procédé pour les préparer |
| JP3867376B2 (ja) * | 1997-12-01 | 2007-01-10 | 日本精工株式会社 | 転動部材の製造方法 |
| DE19909694A1 (de) | 1999-03-05 | 2000-09-14 | Stiftung Inst Fuer Werkstoffte | Verfahren zum Varbonitrieren bei Unterdruckverfahren ohne Plasmaunterstützung |
| DE10118494C2 (de) * | 2001-04-04 | 2003-12-11 | Aichelin Gesmbh Moedling | Verfahren zur Niederdruck-Carbonitrierung von Stahlteilen |
| AU2002221138A1 (en) | 2001-12-13 | 2003-06-23 | Koyo Thermo Systems Co., Ltd. | Vacuum carbo-nitriding method |
| JP4655528B2 (ja) * | 2004-07-12 | 2011-03-23 | 日産自動車株式会社 | 高強度機械構造用部品の製造方法、および高強度機械構造用部品 |
| FR2884523B1 (fr) * | 2005-04-19 | 2008-01-11 | Const Mecaniques Sa Et | Procede et four de carbonitruration a basse pression |
| DE102009058642A1 (de) * | 2009-12-16 | 2011-06-22 | Ipsen International GmbH, 47533 | Verfahren und Einrichtung zur Regelung von Prozessgasen für Wärmebehandlungen von metallischen Werkstoffen/Werkstücken in Industrieöfen |
| DE102010001936A1 (de) * | 2010-02-15 | 2011-08-18 | Robert Bosch GmbH, 70469 | Verfahren zur Carbonitrierung mindestens eines Bauteils in einer Behandlungskammer |
| DE102010028165A1 (de) * | 2010-04-23 | 2011-10-27 | Robert Bosch Gmbh | Verfahren zur Carbonitrierung von metallischen Bauteilen |
| JP2012087384A (ja) * | 2010-10-21 | 2012-05-10 | Ipsen Co Ltd | 工業炉における金属材料/金属ワークピースの熱処理用のプロセスガスを調整する方法および装置 |
-
2014
- 2014-12-11 FR FR1462260A patent/FR3029938B1/fr not_active Expired - Fee Related
-
2015
- 2015-12-10 CN CN201580067754.1A patent/CN107406960B/zh active Active
- 2015-12-10 WO PCT/FR2015/053419 patent/WO2016092219A1/fr active Application Filing
- 2015-12-10 JP JP2017531290A patent/JP7177592B2/ja active Active
- 2015-12-10 EP EP15817994.5A patent/EP3230487B1/de active Active
- 2015-12-10 MX MX2017007484A patent/MX2017007484A/es unknown
- 2015-12-10 CA CA2970247A patent/CA2970247C/fr active Active
- 2015-12-10 US US15/532,270 patent/US11242594B2/en active Active
- 2015-12-10 KR KR1020177016808A patent/KR102576343B1/ko active IP Right Grant
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| JP7177592B2 (ja) | 2022-11-24 |
| CN107406960A (zh) | 2017-11-28 |
| US20170356077A1 (en) | 2017-12-14 |
| EP3230487A1 (de) | 2017-10-18 |
| KR20170093855A (ko) | 2017-08-16 |
| FR3029938B1 (fr) | 2019-04-26 |
| CN107406960B (zh) | 2020-09-22 |
| MX2017007484A (es) | 2017-10-02 |
| US11242594B2 (en) | 2022-02-08 |
| CA2970247A1 (fr) | 2016-06-16 |
| JP2018505301A (ja) | 2018-02-22 |
| FR3029938A1 (fr) | 2016-06-17 |
| WO2016092219A1 (fr) | 2016-06-16 |
| CA2970247C (fr) | 2023-09-12 |
| KR102576343B1 (ko) | 2023-09-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1885904B1 (de) | Niederdruckcarbonitrierverfahren und -vorrichtung | |
| EP3230487B1 (de) | Niederdruck-carbonitrierungsverfahren | |
| EP3218530B1 (de) | Verfahren und anlage zur karbonitrierung einer oder mehrerer stahlteile unter niedrigem druck und bei hoher temperatur | |
| JP5930960B2 (ja) | 浸炭窒化法 | |
| JP5680185B2 (ja) | 金属コンポーネントの浸炭窒化法 | |
| FR2681332A1 (fr) | Procede et dispositif de cementation d'un acier dans une atmosphere a basse pression. | |
| WO2002068707A1 (fr) | Procede de cementation basse pression | |
| KR101245564B1 (ko) | 스테인레스강, 내열강 및 고합금강에 대한 가스질화방법 | |
| WO2013011228A1 (fr) | Procédé de refroidissement de pièces métalliques ayant subi un traitement de nitruration / nitrocarburation en bain de sel fondu, l'installation pour la mise en oeuvre du procédé et les pièces métalliques traitées | |
| FR2678287A1 (fr) | Procede et four de cementation a basse pression. | |
| WO2005038076A1 (fr) | Procede et four de cementation basse pression | |
| FR2999607A1 (fr) | Procede de traitement d'acier comprenant un pretraitement d'affinage du grain | |
| FR3033079A1 (fr) | Procede de passivation d'un substrat et machine pour la mise en oeuvre de ce procede | |
| WO2016124849A1 (fr) | Installation de carbonitruration en série de pièce(s) en acier sous basse pression et haute température | |
| FR3023850A1 (fr) | Procede de nitruration d'une piece en acier inoxydable | |
| FR2874079A1 (fr) | Machine de traitement thermochimique de cementation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20170529 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20180322 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20181120 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: AT Ref legal event code: REF Ref document number: 1130246 Country of ref document: AT Kind code of ref document: T Effective date: 20190515 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602015030057 Country of ref document: DE Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190908 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190808 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190809 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190808 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1130246 Country of ref document: AT Kind code of ref document: T Effective date: 20190508 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602015030057 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 |
|
| 26N | No opposition filed |
Effective date: 20200211 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20191231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20191210 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20191210 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20191210 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20191210 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20191231 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20191231 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20191231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190908 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20151210 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190508 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230522 |
|
| P02 | Opt-out of the competence of the unified patent court (upc) changed |
Effective date: 20230530 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20231124 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20231218 Year of fee payment: 9 Ref country code: IT Payment date: 20231213 Year of fee payment: 9 Ref country code: FR Payment date: 20231228 Year of fee payment: 9 Ref country code: DE Payment date: 20231208 Year of fee payment: 9 |