EP1529006B1 - Spenderflasche für mindestens zwei wirkstofffluide - Google Patents
Spenderflasche für mindestens zwei wirkstofffluide Download PDFInfo
- Publication number
- EP1529006B1 EP1529006B1 EP03792363A EP03792363A EP1529006B1 EP 1529006 B1 EP1529006 B1 EP 1529006B1 EP 03792363 A EP03792363 A EP 03792363A EP 03792363 A EP03792363 A EP 03792363A EP 1529006 B1 EP1529006 B1 EP 1529006B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- dispensing bottle
- active
- container
- weight
- acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D17/00—Detergent materials or soaps characterised by their shape or physical properties
- C11D17/04—Detergent materials or soaps characterised by their shape or physical properties combined with or containing other objects
- C11D17/041—Compositions releasably affixed on a substrate or incorporated into a dispensing means
- C11D17/046—Insoluble free body dispenser
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D1/00—Containers having bodies formed in one piece, e.g. by casting metallic material, by moulding plastics, by blowing vitreous material, by throwing ceramic material, by moulding pulped fibrous material, by deep-drawing operations performed on sheet material
- B65D1/02—Bottles or similar containers with necks or like restricted apertures, designed for pouring contents
- B65D1/04—Multi-cavity bottles
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D1/00—Containers having bodies formed in one piece, e.g. by casting metallic material, by moulding plastics, by blowing vitreous material, by throwing ceramic material, by moulding pulped fibrous material, by deep-drawing operations performed on sheet material
- B65D1/32—Containers adapted to be temporarily deformed by external pressure to expel contents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D81/00—Containers, packaging elements, or packages, for contents presenting particular transport or storage problems, or adapted to be used for non-packaging purposes after removal of contents
- B65D81/32—Containers, packaging elements, or packages, for contents presenting particular transport or storage problems, or adapted to be used for non-packaging purposes after removal of contents for packaging two or more different materials which must be maintained separate prior to use in admixture
- B65D81/3283—Cylindrical or polygonal containers, e.g. bottles, with two or more substantially axially offset, side-by-side compartments for simultaneous dispensing
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D17/00—Detergent materials or soaps characterised by their shape or physical properties
- C11D17/04—Detergent materials or soaps characterised by their shape or physical properties combined with or containing other objects
- C11D17/041—Compositions releasably affixed on a substrate or incorporated into a dispensing means
Definitions
- the invention relates to a dispenser bottle having at least two receptacles for drug fluids which can be stored separately from one another and having the features of the preamble of claim 1.
- drug fluids which are to be stored separately or must. These drug fluids should meet shortly before or during application to the application area, such as a floor, the surface of a toilet bowl, etc., meet. Examples include chlorine-containing bleaching, cleaning, descaling and disinfecting agents (e.g., WO 98/21308 A2). Active fluids of the type in question are also applied, for example, to surfaces in the bathroom or in other hygienically sensitive areas.
- Active fluids are stored in different receptacles, in particular when they are not together stable in storage. But other reasons for a separate storage of active fluids, which are to be applied together, are known, for example, different colors that should communicate different functions of the active fluids, different light sensitivities etc ..
- the dispenser bottle for at least two different non-storage-stable active fluids from which the invention is based has a separate one from the other Chamber, which form the receptacles, having bottle, which is provided at the upper end with immediately adjacent outlets for the active fluids in the two receptacles.
- a receptacle is a first aqueous solution and in the second receptacle, a second aqueous solution.
- concentration of the components in the two aqueous solutions is chosen so that when a certain amount of the first aqueous solution is mixed with a certain amount of the second aqueous solution, the acid bleaching solution desired in this prior art is the result.
- the dispenser bottle of the prior art described above which forms the starting point, has a pump device which can be placed on the outlets of the two receptacles of the dispenser bottle.
- the pumping device the active fluids are brought together and ejected from a discharge nozzle in a common spray. the drug fluids are thus mixed together before they leave the ejection nozzle.
- a similar dispenser bottle in which a cross contamination between the two receptacles can be largely safely avoided, is also known (WO 91/04923 A1, DE 690 16 44 T2).
- pump spray device is provided, but the outlets are simply open and provided with spouts and can be closed again by means of a cap.
- this dispenser bottle is not agreed.
- a dispenser bottle for a drug fluid with a receptacle made of flexible plastic and a discharge nozzle is known (EP 0 911 616 B 1), wherein for optimum application of the active fluid in the toilet bowl, in particular under its inner edge, the ejection nozzle as angled Metering tube is executed.
- a liquid detergent which consists of at least two liquid sub-compositions, the drug fluids are stored separately from each other in a container with at least two chambers (receptacles) and of which at least one imine or Oxaziridine bleach activator and at least one other comprises an alkalizing agent, wherein at least one of the partial compositions contains a peroxygen bleach and each partial composition has a stability leading to the pH.
- the alkalizing agent increases the pH of the final composition so that bleach and bleach activator effectively react with each other.
- European Patent EP 0 807 156 B1 discloses a dispenser with two chambers, the first chamber of which contains an aqueous composition of hydrogen peroxide or an organic peracid having a pH above 2 and below 7 and the second chamber containing an acid component and from which the contents are dispensed together or sequentially onto a surface such that the resulting mixture has a pH of at most 2.
- German Patent Application DE 100 24 251 A1 proposes a bleaching agent which comprises in a first component an aqueous 1 to 40% by weight aqueous imidoperoxycarboxylic acid dispersion and in a second component a substance mixture activating the first component, correspondingly separately Keep the dual-chamber bottle and mix the two components only during use.
- the second component also referred to as a pH-regulating buffer solution in this document, consists of an aqueous solution of sodium bicarbonate and sodium carbonate which has been thickened with the aid of methylcellulose.
- the teaching is now based on the problem to provide a dispenser bottle with at least two receptacles for two active fluids, which is inexpensive to produce and easy to handle by an operator and it allows to apply two active fluids separated from each other, but in an application field clashing.
- the receptacles are preferably designed as compressible containers. By squeezing the receptacle by hand of an operator so the necessary internal pressure is generated in the receptacles to eject the drug fluid from the separately provided ejection nozzles.
- the required pressure can also be generated by gravity if the product delivery is not delivered upwards against gravity, such as in the toilet, but downwards, such as when applying cleaning agents for floor cleaning or entering detergents in the washing machine.
- the active fluids thus mix only after leaving the ejection nozzles in the application field. This results in the application of the two active fluids the desired product to be applied, in particular so the detergent, bleach, etc, which unfolds the desired effect in the application field.
- the claimed dispenser bottle achieved the result described above with a structurally very simple and easy to handle solution, especially waiving a Pumpsprühvorraum.
- the claimed dispenser bottle for use as a mass product is ideally suited, especially for detergents of all kinds, especially for the toilet cleaning.
- the claimed dispenser bottle can be used, for example, for the dosage of textile cleaning agents (detergents in washing machines, etc.), textile pretreatment agents (bleach, etc.), textile aftertreatment agents (fabric softener, etc.), for the dosage of manual and automatic dishwashing detergents and dishwashing aids (rinse aid, limescale removers, etc.), and finally also for the dosing of surface cleaners and surface treatment agents of all kinds.
- active fluids are to be understood as meaning all liquid and other flowable media, from low-viscosity to viscous to gelatinous to pasty substances. It is also possible to apply powdery and particulate active ingredients, such as granules, with the dispenser bottle according to the invention.
- the viscosity of the active substance fluids or fluidity of the active ingredients for the particular application of importance on the other hand, and in a special way, the thixotropy of the active fluids is important (for the explanation of the term thixotropy, the phenomenon that certain active fluids at Liquefy the action of mechanical forces, after the end of the mechanical stress, but if necessary with a considerable time delay again solidify, so have a dependent on the action of mechanical forces viscosity, see R ⁇ MPP LEXIKON chemistry, 10th edition, Georg Thieme Verlag, Stuttgart, 1999, Volume 6, page 4533).
- a particular embodiment consists in the fact that the nozzle channels of the ejection nozzles i.w. are aligned parallel to each other, but each have an asymmetrical to the total flow cross-section arranged cross-sectional constriction.
- the cross-sectional constrictions are arranged on the mutually facing sides of the nozzle channels such that the fluid fluids exiting under pressure have a mutually directed twist.
- the swirl effect is also caused when the openings of the nozzle channels of the Au adopteddüsen are chamfered against each other, ie, the opening planes of the nozzle channels are angled to each other, wherein the longitudinal axis of the nozzle channel inner portion of the wall of the ejection nozzle is longer than the outer axis of the nozzle channel Section of the wall.
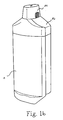
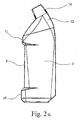
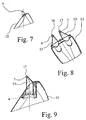
- the invention relates to a dispenser bottle as shown in Fig. 1a and 1b perspective and in Figs. 2a and 2b shown laterally. It can be seen on the left a first receptacle 1 for a first drug fluid and right a second receptacle 2 for a second active fluid.
- a first receptacle 1 for a first drug fluid and right a second receptacle 2 for a second active fluid.
- more than two receptacles 1,2 may be provided, for example, three receptacles for three active fluids or even four receptacles for four active fluids, which are intended to meet in the application area.
- the drug fluids will often be non-shelf stable drug fluids; but this is not a mandatory requirement for the teaching of the invention. Reference may be made to the statements above. Likewise, reference may be made to the above statements with regard to the definition of the term of the active substance fluid in the sense of this patent application and the particular, preferred properties of such active substance fluids.
- the two receptacles 1,2 are either carried out separately and connected to each other, for example by gluing or latching or another connecting element or, as in the illustrated embodiment, integral with each other.
- gluing or latching or another connecting element or, as in the illustrated embodiment, integral with each other.
- a dispenser bottle in which the two receptacles 1, 2 are made in one piece with one another is preferred. This will be explained later.
- Figs. 3 and 4 show the receptacle 1,2 for the first embodiment of the dispenser bottle according to Fig. 1a and 2a separately. It can be seen that the receptacles each have an outlet 3,4 for the respective active substance fluid. The outlets 3, 4 are arranged adjacent to one another such that the two active substance fluids can be applied in a common application field 5, indicated in FIG. 11, of a larger application area.
- the special significance of this external mixing of the active substance fluids from the two receptacles 1, 2 has been pointed out in detail in the general part of the description; reference may be made to this.
- the receptacles were not shown separately, it would be different only that they have no holding area, since the application is carried out by pivoting and the liquid outlet due to gravity.
- the dispenser bottle according to the invention is always explained as if there were only two receptacles 1,2 for two active fluids.
- the receiving containers 1, 2 are each provided with at least one outlet 3.4, in each case at least one, preferably with exactly one ejection nozzle 6, 7 so that the active substance fluids do not intercommunicate with one another until they have left the ejection nozzles 6, 7 be mixed.
- the receptacles 1, 2 are designed as compressible containers, since they are preferably used for a delivery of product against gravity as for a toilet rim dosage.
- the ejection nozzles 6, 7 are preferably inclined with respect to the longitudinal axis of the receptacles 1, 2.
- the ejection nozzles 6, 7 are parallel in the direction of the longitudinal axis of the receptacles 1, 2, since with this dispenser bottle, detergent preferably enters into the dispensing chamber of a washing machine or a dispensing aid for the dispenser
- the receptacles 1, 2 can be designed as compressible containers.
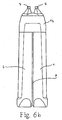
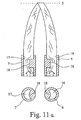
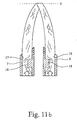
- the ejection nozzles 6, 7 are first recognized in FIGS. 6 a and 6 b, and then in FIG. 8 as well and shown schematically in Fig. 11 a, b.
- the pressure for pushing out the active substance fluids from the receiving containers 1, 2 is applied by the hand of an operator or by gravity after giving more than 90 °.
- the active fluids leave under pressure the ejection nozzles 6, 7, to which they flow from the outlets 3, 4 of the two receptacles 1, 2. Only after leaving the ejection nozzles 6,7 arises, depending on the pressure exerted by the operator, at a certain distance, the meeting of the Flows of the active fluids and their mixing to the applicable product in the application area.
- the receptacle 1.2 consist of a material with a return characteristic and / or have a provision in the original shape supporting shaping.
- the receptacle 1,2 made of a resiliently elastic plastic material.
- a material for the receiving containers 1, 2 can be, for example, a polyolefin, in particular a polypropylene (PP), a polyethylene (PE), a polyvinyl chloride (PVC) or a polyethylene terephthalate (PET), in particular a glycol-modified polyethylene. Terephthalate (PETG), act.
- PP polypropylene
- PE polyethylene
- PVC polyvinyl chloride
- PET polyethylene terephthalate
- PET glycol-modified polyethylene.
- PET polyethylene terephthalate
- Such materials are also suitable for the present application.
- plastic containers with appropriate return characteristic is cost-effective and allows regardless effective dosage of drug fluids in the desired, previously explained manner without premixing.
- Typical volumes of receptacles 1, 2 within the scope of the budget are between 50 ml and 1500 ml, with a preferred range between 300 ml and 500 ml for each of the receptacles 1, 2. Of course, this is application-specific and depends on the drug fluids.
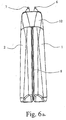
- FIGS. 1a and 1b can be seen in particular in Fig. 4, but also in Fig. 6a and 6b recognize that the receptacle 1,2 as each completed containers and only over at least one, preferably just one between the Receiving containers 1.2 formed connecting web 8 are connected to each other.
- the connecting web 8 is preferably integrally formed on the mutually facing inner sides of the receptacle 1.2, in particular formed, for example, in the blow molding process with the receptacles 1.2 at the same time. It is particularly expedient if the connecting web 8 is arranged approximately centrally and extends iw - possibly with interruptions - over the full length of the receptacle 1.2.
- the connecting web 8 thus forms a stiffening element for the mutually facing walls of the receptacle 1,2, stabilizes them and at the same time leads to the design of a Abutment for the exerted by the hand of the operator pressure forces.
- the receptacle should 1, 2 together have such a cross-section that they can be covered by the hand of an operator in any case for the most part.
- the blow molding process has already been addressed as a convenient method of manufacturing the receptacles 1,2.
- the receptacles 1, 2, which are embodied in one piece with one another have a different translucency and / or a different coloration.
- some drug fluids are photosensitive.
- Other active substance fluids to be administered in conjunction with the respective active substance fluid are less sensitive to light.
- An opaque coloring of the receptacle provided for the more photosensitive active substance fluid eliminates problems here.
- the dispenser bottle shown in the drawings according to Fig. 1a, 2a further characterized by the fact that on the receptacles 1,2 from the hand of an operator to comprehensive holding area 9 by special edge formations 10,11 and / or Surface designs formed and / or identified.
- edge formations 10.11 is specified.
- corrugations, other colorations, etc. are also suitable as surface designs.
- the receptacles 1, 2 in cross-section in the area of the holding area 9 to be encompassed by the hand of an operator have an outer circumference of approximately 18 to approximately 30 cm, preferably approximately 20 to approximately 28 cm, in particular of about 22 to about 26 cm, in particular of about 24 cm.
- Figs. 6a and 6b, Figs. 8, 11a and 11b, Fig. 12 can be explained so far that the design and dimensions of the ejection nozzles 6,7 and the properties of the active fluids are coordinated in that - at average pressure from the hand of an operator or pressure caused by gravitation - the fluid flows come into coverage at a certain distance.
- active substance fluids With regard to the viscosity of the active substance fluids, it is recommended to use active substance fluids with viscosities in the range from 1 to 100,000 mPas, preferably to use about 10,000 mPas, in particular up to about 1,000 mPas. This information is based on the viscosity measured using a Brookfield LVT-II viscometer at 20 rpm. and 20 ° C, spindle 3.
- Figs. 3 and 4 show the receptacle 1,2 with the outlets 3,4.
- the outlets 3,4 are aligned parallel to each other.
- a pre-alignment of the streams of the active fluids can also be provided by already aligning the outlets 3, 4 of the receiving containers 1, 2 slightly inclined towards one another. In terms of manufacturing, however, the illustrated parallel alignment has advantages.
- the ejection nozzle 6, 7 is arranged or formed in a separate, consisting of a dimensionally stable plastic nozzle head 12 and that the nozzle head 12 at the outlet 3, 4 on the receptacle 1, 2 is placed.
- the nozzle head 12 is identified in the figures by reference numeral 12. In the illustrated embodiment, it is true that the nozzle head 12 is latched onto the receptacle 1, 2. Of the. Nozzle head 12 can also be connected to the receptacle 1, 2 in a different way. A snapping is recommended, however, as a particularly simple and convenient manufacturing technique.
- locking connection means for mating locking connection means of the nozzle head 12.
- Such locking connection means are known in corresponding constructions of the prior art. In principle, other connection techniques such as screw connections are applicable.
- the illustrated and preferred embodiments are characterized in particular by the fact that the nozzle heads 12 of the two receptacles 1, 2 are combined in a common nozzle head 12.
- This common nozzle head 12 can be seen in Figs. 7, 8, 9 and 10 and 12, 14.
- the manufacturing technology is very convenient and the connection of the two receptacles 1,2 well adapted.
- nozzle head 12 It is advisable to make the nozzle head 12 of a stiffer plastic material, so that the nozzle head 12 undergoes only slight deformation when the receptacle 1.2 of the dispenser bottle are compressed.
- the nozzle head 12 can be seen in the illustrations given above, as well as in FIGS. 5 and 6.
- the nozzle head 12 can be seen particularly well in section in FIGS. 8, 9, 10. It is shown that the flow of the active substance fluid in the Nozzle head 12 is expedient that the ejection nozzle 6, 7 in the nozzle head 12 asymmetrically, in particular with respect to the center line of the outlet 3, 4 offset in the direction of the further ejection nozzles 7, 6 is arranged. This can be seen in Fig. 8 particularly well.
- the flow of the active substance fluid out of the respective receptacle 1, 2 is brought to the desired distance from the active substance fluid flowing in parallel.
- the nozzle head 12 has an inflow volume 13 tapering from the outlet 3, 4 of the receptacle 1, 2 to the ejection nozzle 6, 7.
- This inflow volume 13 can be understood particularly well in FIGS. 8 and 9.
- the illustrated and preferred embodiment shows a dimensioning such that the lateral center distance of the ejection nozzles 6, 7 is on the outside about 5 mm to about 30 mm, preferably about 15 mm to about 20 mm.
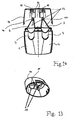
- the ejection nozzle 6, 7 can be closed with a removable closure cap 14, which preferably consists of a dimensionally stable plastic , It is provided that the closure cap 14 a in the ejection nozzle 6; has incoming sealing plug 15. This technique is already proven to avoid cross-contamination (see above WO 91/04923 A1).
- the closure cap 14 laterally adjacent to the in the ejection nozzle 6, 7 entering stopper 15 each have a convex to the longitudinal axis of the cap 14 curved cylinder portion 19 as a positioning aid.
- This cylinder portion 19 is spaced from the sealing plug 15 such that the free ends of the cylinder portion 19 abut on the ejection nozzles 6, 7 in the locking position.
- the lower end of the cylinder portions 19 slide along the inclined surfaces of the ejection nozzles 6, 7, the movement is forced.
- the placement of the closure cap 14 with the cylinder portions 19 as positioning aids and the sealing plug 15 on the ejection nozzles 6,7 is shown schematically in Fig. 14.
- Fig. 1 a, 1 b and 14 show, particularly clearly visible in Fig. 1 a, 1 b and 14, that also applies to the cap 14 that this is summarized for both ejection nozzles 6,7 of the two receptacles 1,2 together.
- This is manufacturing technology appropriate, as well as the nozzle head 12 has been explained as appropriate.
- the cap 14 is made of a similar or the same plastic material as the nozzle head 12th
- the illustrated and so far preferred embodiment shows, however, that the nozzle channels 16,17 of the ejection nozzles 6,7 are aligned parallel to each other. A slight inclination in the context of eg the manufacturing tolerances is of course acceptable.
- the nozzle channels 16, 17 of the ejection nozzles 6, 7 each have a cross-sectional constriction 18 arranged asymmetrically with respect to the overall flow cross-section.
- the cross-sectional constriction 18 in the respective nozzle channel 16, 17 leads to a certain twist being imparted to the streams of the active substance fluids, so that a certain diversion takes place in each case in the exit region of the ejection nozzles 6, 7, so that the streams of the active substance fluids then flow in the application field Distance, which in some way depends on the pressure of the hand of the operator on the receptacles 1,2, impinge mingling.
- a combination of the streams of the active substance fluids is not achieved by aligning the nozzle channels 16, 17, but by influencing the flow.
- FIG. 11a, 11b above shows the functional principle of the cross-sectional constrictions 18, below an example of the arrangement of the cross-sectional constrictions 18 in the adjacent nozzle channels 16,17.
- the cross-sectional constrictions 18 of the nozzle channels 16,17 are executed with angular transitions. In terms of flow, this results in different flow velocities occurring over the flow cross-section of the nozzle channel 16, 17. Distanced from the cross-sectional constriction 18, the active fluid can flow relatively undisturbed, it maintains a high flow velocity with laminar flow.
- the embodiment according to. 11b shows an oblique opening plane of the nozzle channels 16, 17, cf.
- the chamfering of the ends of the nozzle channels also produces the swirl effect due to different flow velocities in the outlet.
- the swirl effect is caused by the fact that the openings of the nozzle channels of the Au conducteddüsen are chamfered against each other.
- the opening planes of the nozzle channels 6, 7 are arranged at an angle to one another, wherein the section of the wall of the ejection nozzle lying inwards to the longitudinal axis of the nozzle channel is longer than the section of the wall lying outside the longitudinal axis of the nozzle channel.
- FIGS. 12a and 12b show further expedient cross-sectional configurations.
- the cross-sectional constrictions 18 it will also be possible to choose different cross-sectional shapes for the cross-sectional constrictions 18 as well as for the nozzle channels 16, 17.
- the length of the cross-sectional constriction 18 of the nozzle channel 16, 17 is only a part of the length of the nozzle channel 16, 17 in total.
- the aspect ratio is about 1: 2 to 1: 4, preferably about 1: 2.5 to 1: 3.
- the total length of the nozzle channel 16, 17 be about 2 mm to about 6 mm, preferably about 3 mm to about 5 mm, in particular approximately 4 mm. Accordingly, the diameter of the nozzle channel 16, 17 is about 1.0 mm to about 4.0 mm, preferably about 1.5 mm to about 3.5 mm, in particular about 2.0 mm to about 2.5 mm ,
- the types of drug fluids, which are applied with the dispenser bottle according to the invention, are directed primarily to the field of application.
- active fluids other than detergents, dishwashing or corrosion inhibitors are used.
- Example 1 specifies different formulations of active substance fluids that can be used in a dispenser bottle according to the invention for toilet cleaning.
- Liquid toilet cleaners are well known in the market. Such products usually contain inorganic or organic acids for the removal of lime and rust deposits, as well as surfactants for cleaning support, abrasives, viscosity regulators, antibacterial additives, dye and perfume for odor removal.
- alkaline WC cleaners are known which are formulated on the basis of sodium hypochlorite, surfactants and the above-mentioned additional components. These products have a good bleaching and disinfecting effect, but are unable to remove calcareous soils.
- acid-free formulations are on the market, which, although having no limescale removal or bleaching action, but by the surfactant support the cleaning performance of organic stains and are easier to perfume due to the lack of acid and bleaching components. However, these cleaners are not very effective in removing stubborn dirt.
- toilet cleaner All traditionally known toilet cleaner has in common that they are offered in monotank plastic bottles with special dosing accessories.
- the formulation of a WC cleaner in a monotank bottle presupposes that the active ingredients used are compatible with one another and also have sufficient storage stability over a longer period of time. This leads to limitations in the formulation of effective cleaners, since the active ingredients used in the rule acid, bleach, perfume oil, abrasives can undergo undesirable reactions, at least for prolonged contact time.
- the dispenser bottle according to the invention enables the expansion of the performance spectrum of toilet cleaners by the use of incompatible or reactive active ingredients.
- acidic peroxide-containing detergents are extremely effective, both in their bleaching and disinfecting action and in the removal of limescale deposits, but have a low storage stability in conventional bottles.
- a cleaning agent which is stable over a considerably longer time is obtained.
- Another example is the combination of an alkaline, hypochlorite-containing bleach in a chamber with an acidic and hence lime-dissolving agent in the second chamber.
- the combination of an acidic agent with a carbonate-containing alkaline phase can be realized only in a dispenser bottle according to the invention. Mixing these two phases (when using the agent) releases carbon dioxide, causing the agent to foam and aid in cleaning performance.
- descaler phase agents described all show good lime release activity, with values according to the IKW standard test of 150 to 350 mg calcium carbonate for the 1: 1 mixture of the two phases.
- the stability of high-quality fragrances and abrasives can be optimally realized in alkaline medium.
- the combination of strongly acidic decalcifier with a fragrance and abrasive phase leads to mechanical and application with the toilet brush for performance and gloss enhancement on the toilet ceramic.
- the perfume is selected so that sufficient stability is ensured in the reduced fragrance / abrasive phase. Reinforcing with the descaling phase (more acidified) provides maximum cleaning efficiency.
- a reactive abrasive component such as calcium carbonate is not possible in a 1-chamber bottle, since a decomposition reaction would occur due to the acid component with strong evolution of gas (carbon dioxide).
- gas carbon dioxide
- the use of a reactive abrasive component is desirable when using the toilet brush, as it is signaled by the gas / foam development for the user on the ceramic surface optically visible effectiveness.
- the reaction achieves an improved spreading behavior and, due to the gas evolution, an optimal distribution of fragrance.
- a color reaction on the ceramic surface when using phenolphthalein as an indicator, for example, pink), which can be achieved only via the dispenser bottle according to the invention.
- suitable indicator dyes with specific pH ranges allows the application in the range neutral-alkaline or neutral-weakly acidic.
- the color change should give the user an indication of the pH change and the with the associated cleaning effect, and on the other hand, the cleaning can be combined with a surprise effect. This is an interesting variant in marketing.
- the combination of hydrogen peroxide and acid, thickened detergent with perfume is not feasible in a 1-chamber bottle, since the product stability is only low due to the effect of the peroxide, which manifests itself for example in a strong decrease in viscosity, but also in a change in the fragrance impression ..
- the combination is desirable for the consumer to use in addition to the effectiveness of the acid and the bleaching and disinfecting effect of hydrogen peroxide; the use of a thickener causes a longer adhesion of the detergent to the surface to be cleaned and thus an increase in the cleaning effect.
- Example 2 gives different formulations of active fluids which can be used in a dispenser bottle according to the invention for manual or automatic dishwashing.
- the dispenser bottle according to the invention not only makes it possible to increase the storage stability of flowable substances or mixtures of substances, in particular of automatic or manual dishwashing detergents, but by using separate receptacles, the stability of the active substances contained in the agents can be improved and at the same time made possible by the separation of chemically incompatible ingredients this approach also the simple and cost-effective packaging of flowable substances in the form of "multi-phase" supply forms.
- polyphase or multiphase for example, the interaction of different active substances in automatic dishwashing detergents can be visualized.
- the volume of the storage container depends, inter alia, on the weight or volume fraction of these active substances in the overall formulation of the automatic dishwashing detergent or the type of preparation of these active substances, for example in the form of the pure substance, as a solution or dispersion.
- all receptacles have the same size, their volume preferably being between 10 and 2000 ml, preferably between 20 and 1500 ml, more preferably between 50 and 1000 ml and in particular between 100 and 800 ml.
- Dispenser bottles according to the invention are suitable for repeated dosing of the flowable automatic dishwasher detergents, accordingly contain at least two, but preferably at least 6, more preferably at least 12, 24 or 36 dosing units.
- the liquids contained in the packings according to the invention can be both water-containing and anhydrous formulations. It is also possible for water-containing and anhydrous formulations to be present separately in a pack.
- aqueous dishwashing detergents have a water content of between 10 and 70% by weight, more preferably between 20 and 60% by weight and in particular between 30 and 50% by weight, based in each case on the total weight of the aqueous machine dishwashing detergent in the context of the present application preferred anhydrous automatic dishwashing detergent has a water content below 6 wt.%, Preferably between 0.5 and 5 wt .-%, particularly preferably between 1 and 4 wt .-%, each based on the total weight of the anhydrous automatic dishwashing detergent, exhibit.
- the liquid matrix of the aforementioned aqueous or anhydrous automatic dishwashing detergents may, of course, also contain other nonaqueous solvents besides the water.
- These non-aqueous solvents are obtained, for example, from the group of monoalcohols, diols, triols or polyols, ethers, esters and / or amides.
- Particular preference is given to non-aqueous solvents which are water-soluble, "water-soluble" solvents in the context of the present application being solvents which are completely water-soluble at room temperature, ie. H. without miscibility, are miscible.
- Nonaqueous solvents which can be used in the dispenser bottles according to the invention are preferably from the group of mono- or polyhydric alcohols, alkanolamines or glycol ethers, provided they are miscible with water in the concentration range indicated.
- the solvents are preferably selected from ethanol, n-propanol or isopropanol, butanols, glycol, propane or butanediol, glycerol, diglycol, propyl- or butyldiglycol, hexylene glycol, ethylene glycol methyl ether, ethylene glycol ethyl ether, ethylene glycol propyl ether, etheylene glycol mono-n-butyl ether, Diethylene glycol methyl ether, diethylene glycol ethyl ether, propylene glycol methyl, ethyl or propyl ether, dipropylene glycol methyl or ethyl ether, methoxy, ethoxy or butoxy
- particularly preferred flowable substances and / or substance mixtures are characterized in that they contain nonaqueous solvents in amounts of from 0.1 to 70% by weight, preferably from 0.5 to 60% by weight, especially preferably from 1 to 50% by weight, very particularly preferably from 2 to 40% by weight and in particular from 2.5 to 30% by weight, based in each case on the solvent-containing flowable substance or the flowable substance mixture, preferred ( s) non-aqueous solvent (s) is / are selected from the group of liquid at room temperature nonionic surfactants, the polyethylene glycols and polypropylene glycols, glycerol, glycerol carbonate, triacetin, ethylene glycol, propylene glycol, propylene carbonate, hexylene glycol, ethanol and n-propanol and / or iso- propanol.
- flowable solids such as, for example, powders, granules or microcompactates, are also considered as flowable substances / substance mixtures in the context of the present application.
- the stated solids may be present in amorphous and / or crystalline and / or partially crystalline form.
- the particle size of these flowable solids is preferably in the range of 10 to 2000 microns, more preferably in the range of 20 to 1000 microns and in particular in the range of 50 to 500 microns.
- flowable solids in which at least 70 wt .-% of the particles, preferably at least 90 wt .-% of the particles have a particle size below 1000 microns, preferably below 800 microns, more preferably below 400 microns have.
- the flowable substances which may preferably contain one or more of the abovementioned non-aqueous solvents
- further active substances preferably from the group of bleaching agents, bleach activators, polymers, builders, surfactants, enzymes, electrolytes, pH adjusters, fragrances, perfume carriers, dyes, Hydrotropes, foam inhibitors, Anti Redepositionsstoff, antimicrobial agents, germicides, fungicides, antioxidants and corrosion inhibitors.
- the dispenser bottle according to the invention is particularly suitable for the separation of incompatible ingredients from detergents.
- a non-exhaustive list of the separation of incompatible ingredients in multi-chambered dual-reservoir bottles is shown in the table below.
- Storage container A Container B bleach bleach bleach enzyme bleach Corrosion inhibitors bleach perfume bleach polymer bleach nonionic surfactant bleach dye bleach Bleach activator, enzyme bleach Bleach activator, corrosion inhibitor bleach Bleach activator, perfume bleach Bleach activator, polymer bleach Bleach activator, nonionic surfactant bleach Bleach activator, dye Bleach, bleach activator enzyme Bleach, bleach activator Corrosion inhibitors Bleach, bleach activator perfume Bleach, bleach activator polymer Bleach, bleach activator nonionic surfactant Bleach, bleach activator dye
- Example 2 specifies different formulations of active substance fluids which can be used in a dispenser bottle according to the invention, also referred to below as a multichamber container, for the manual or automated washing of textiles.
- Example 3 shows that, surprisingly, it has been found that an optimum results from the viewpoint of storage stability and performance of the detergent under conditions of use when using a liquid detergent composition consisting of at least two hydrous subcomponents held separate from each other, a first partial organic peracid contains and a second component composition contains surfactant and enzyme.
- the separation of the partial compositions preferably takes place in that they are present in the dispenser bottle according to the invention as a multi-chamber container, wherein the number of chambers (receptacles) of the container corresponds to the number of partial compositions and only one of the partial compositions is present in each of the chambers.
- Another object of the invention is therefore a combination of a liquid detergent composition defined herein, which consists of at least two, preferably exactly two sub-compositions as drug fluids, and a dispenser bottle, wherein the number of chambers of the container corresponds to the number of sub-compositions and in each one of Chambers each one of the sub-compositions is present.
- the chambers are either carried out separately and connected to each other or made integral with each other.
- Each of the chambers has at least one, preferably exactly one, outlet in the form of an outlet nozzle, from which the component composition can emerge from the respective chamber. This can be done by acting gravity, ie tilting the dispenser bottle so that the partial compositions of the liquid detergent composition flow out.
- the dispenser bottle is compressible so that the outflow of the partial compositions can be accelerated by a pressure on the dispenser bottle exerted, for example, by an operator's hand.
- the outlet of a liquid detergent container is provided with a closure cap, in the case of the present invention, the outlet of each chamber may be provided with its own cap or the cap may be formed so that it closes several, in particular all outlets of the dispenser bottle.
- the dispenser bottle may have grip recesses or handles for ease of handling by the user, the handle may be attached to one or more chambers, or may be part of a chamber, or more chambers each form a handle and they are joined together so that the Dispenser bottle or the multi-chamber container can be grasped by the hand of the user.
- the separate storage in the dispenser bottle or the multi-chamber container causes the sub-compositions of the liquid detergent composition to mix only after leaving the outlets, for example when pouring into a conventional dispensing chamber of a washing machine or into the washing drum of such a washing machine to be introduced metering device, or when spraying the agent on a need to clean textile surface, for example in the context of laundry pre-treatment.
- the chambers of the multi-chamber container each have at least one, preferably exactly one ejection nozzle, and that the nozzle channels Although the ejection nozzles are aligned substantially parallel to each other, but each have an asymmetrical to the total flow cross-section arranged cross-sectional constriction.
- the cross-sectional constrictions are preferably arranged on the mutually facing sides of the nozzle channels in such a way that the sub-assemblies emerging under pressure have a mutually directed twist.
- the application field of the application area can be located, for example, a soiling on a piece of laundry.
- the dispenser bottle may be made of a material having a return characteristic and / or may have a shape supporting the return to the original shape.
- the material for the bottle body or the multi-chamber container is, for example, a polyolefin, in particular polypropylene (PP), polyethylene (PE), polyvinyl chloride (PVC) or polyethylene terephthalate (PET), in particular glycol-modified polyethylene terephthalate (PETG), act.
- PP polypropylene
- PE polyethylene
- PVC polyvinyl chloride
- PET polyethylene terephthalate
- PET glycol-modified polyethylene terephthalate
- the material may also be one or more colors, wherein the individual chambers of the multi-chamber container may have the same color or the same colors or different colors among each other.
- Multi-chamber containers are known, for example, from International Patent Applications WO 02/22467 A1, WO 97/23087 A1, WO 96/12648 A1, WO 95/16023 A1, WO 91/04923, German Patent Application DE 32 20 693 A1 or German Utility Model DE G 93 16 583 U1 known.
- the liquid detergent composition of the invention does not contain a bleach activator.
- the first part composition as the first active fluid consists essentially of water and the organic peracid, which may be dissolved in water, but more preferably at least partially undissolved present in finely divided form.
- the first part composition may also contain organic acid corresponding to the organic peracid and small amounts of conventional stabilizers for bleaching agents, for example the vinyl ether-maleic acid copolymers known from European patent application EP 1 074 607 as dispersants and / or those from European patent EP 0 497 337 known nonionic surfactants and / or complexing agents, which counteract the metal-catalyzed decomposition of peracid.
- the content of organic peracid is preferably 1 wt .-% to 25 wt .-%, in particular 2 wt .-% to 20 wt .-% and particularly preferably 3% to 15 wt .-%, each based on the first part composition.
- the organic peracid may carry aliphatic and / or cyclic, including heterocyclic and / or aromatic, radicals.
- peroxoic acid peroxoacetic acid, peroxopropionic acid, peroxohexanoic acid, peroxobenzoic acid and their substituted derivatives such as m-chloroperoxobenzoic acid, mono- or di-peroxophthalic acids, 1,12-diperoxododecanedioic acid, nonylamidoperoxoadipic acid, 6-hydroxyperoxohexanoic acid, 4-phthalimidoperoxobutanoic acid, 5-phthalimidoperoxopentanoic acid, 6 Phthalimidoperoxohexanoic acid, 7-phthalimidoperoxoheptanoic acid, N, N'-terephthaloyl-di-6-aminoperoxohexanoic acid, and mixtures of these.
- m-chloroperoxobenzoic acid mono- or di-peroxophthalic acids
- 1,12-diperoxododecanedioic acid
- Preferred peracids include 6-phthalimidoperoxohexanoic acid.
- the first part composition preferably has an acidic pH, in particular in the range from pH 1.5 to pH 5 and more preferably from pH 2.5 to pH 4.5, which results from the presence of the organic peracid or more compatible with the system by addition Acids can be adjusted.
- the first part composition contains no hydrogen peroxide. By this is meant to be understood that it contains at most such a small amount of hydrogen peroxide which may eventually result from hydrolysis of the organic peracid.
- the first part composition in one embodiment of the invention may contain anionic acid compatible with the organic peracid in amounts of up to 50% by weight, in particular 10% by weight to 30% by weight, based in each case on the first part composition.
- the second part composition as the second active substance fluid or each of the further optional subcompositions contains, in addition to surfactant, at least one enzyme and is free from oxidative bleaching agents. Mixtures of nonionic and anionic surfactant are particularly preferred, wherein the second subcomposition or any of the further subcomponents may contain a mixture of nonionic and anionic surfactant or at least the second part composition nonionic surfactant and at least one further part composition may contain anionic surfactant. Likewise, enzyme mixtures may be included in the partial compositions, or more enzymes may be distributed to the second and further partial compositions such that each of them contains only one enzyme.
- the second or at least one of the further partial compositions may be alkaline, so that after pouring out of the multi-chamber container, that is, when combining all partial compositions, a preparation results, which has a pH of preferably 4.5 to 10, especially 5 to 9.
- the second part composition contains 8 wt .-% to 70 wt .-%, in particular 20 wt .-% to 55 wt .-% water.
- the surfactants contained in the second subcomposition or the further subcompositions include, in particular, anionic surfactants and nonionic surfactants, although cationic surfactants and amphoteric surfactants may also be suitable.
- anionic surfactants preference is given to using one or more substances from the group of the carboxylic acids, the sulfuric acid half esters and the sulfonic acids, preferably from the group of the fatty acids, the fatty alkyl sulfuric acids and the alkylaryl sulfonic acids.
- the compounds mentioned should have longer-chain hydrocarbon radicals, ie at least 6 carbon atoms in the alkyl or alkenyl radical.
- the C chain distributions of the anionic surfactants are in the range of 6 to 40, preferably 8 to 30 and especially 12 to 22 carbon atoms.
- Carboxylic acids which are used in the form of their alkali metal salts as soaps in detergents and cleaners, are obtained industrially, for the most part, from native fats and oils by hydrolysis. While the alkaline saponification already carried out in the past century led directly to the alkali salts (soaps), today only large amounts of water are used for cleavage, which cleaves the fats into glycerol and the free fatty acids. Examples of industrially applied processes are the autoclave cleavage or continuous high pressure cleavage.
- hexanoic acid (caproic acid), heptanoic acid
- fatty acids such as dodecanoic acid (lauric acid), tetradecanoic acid (myristic acid), hexadecanoic acid (palmitic acid), Octadecanoic acid (stearic acid), eicosanoic acid (arachic acid), docosanoic acid (behenic acid), tetracosanic acid (lignoceric acid), hexacosanoic acid (cerotic acid), triacotanic acid (melissic acid) and the unsaturated Sezies 9c-hexadecenoic acid (palmitoleic acid), 6c-octadecenoic acid (petroselinic acid), 6t-octadecenoic acid (Petroselaidic acid), 9c-octadecenoic acid (oleic acid), 9t-octadecenoic acid (elaidic acid), 9c, 12c-o
- Such mixtures are, for example, coconut oil fatty acid (about 6% by weight C8, 6% by weight C10, 48% by weight C12, 18% by weight C14, 10% by weight C16, 2% by weight C18, 8% by weight C18 ', 1% by weight C18 "), palm kernel oil fatty acid (about 4% by weight C8, 5% by weight C10, 50% by weight C12, 15% by weight C14, 7 Wt% C16, 2 wt% C18, 15 wt% C18 ', 1 wt% C18 "), tallow fatty acid (about 3 wt% C14, 26 wt% C16, 2 wt%) % C16 ', 2 wt.% C17, 17 wt.% C18, 44 wt.% C18', 3 wt.% C18 ", 1 wt.% C18"'
- Sulfuric acid semi-esters of longer-chain alcohols are also anionic surfactants and can be used in the context of the present invention.
- Their alkali metal salts, in particular sodium salts, the so-called fatty alcohol sulfates are commercially available from fatty alcohols, which are reacted with sulfuric acid, chlorosulfonic acid, sulfamic acid or sulfur trioxide to give the corresponding alkyl sulfuric acids and subsequently neutralized.
- the fatty alcohols are thereby obtained from the relevant fatty acids or fatty acid mixtures by high-pressure hydrogenation of fatty acid methyl esters.
- the quantitatively most important industrial process for the production of fatty alkyl sulfuric acids is the sulfation of the alcohols with SO3 / air mixtures in special cascade, falling film or tube bundle reactors.
- alkyl ether sulfuric acids which can be used according to the invention are the alkyl ether sulfuric acids whose salts, the so-called alkyl ether sulfates, are distinguished by a higher water solubility and lower sensitivity to water hardness (solubility of the Ca salts) compared to the alkyl sulfates.
- Alkyl ether sulfuric acids like the alkyl sulfuric acids, are synthesized from fatty alcohols which are reacted with ethylene oxide to give the fatty alcohol ethoxylates in question. Instead of ethylene oxide, propylene oxide can also be used. The subsequent sulfonation with gaseous sulfur trioxide in short-term sulfonation reactors yields over 98% of the relevant alkyl ether sulfuric acids.
- Alkane sulfonic acids and olefin sulfonic acids can also be used in the context of the present invention as anionic surfactants in acid form.
- Alkanesulfonic acids may contain the sulfonic acid group terminally bound (primary alkanesulfonic acids) or along the C chain (secondary alkanesulfonic acids), only the secondary alkanesulfonic acids are of commercial importance. These are prepared by sulfochlorination or sulfoxidation of linear hydrocarbons.
- alkanesulfonic acids Another process for producing alkanesulfonic acids is sulfoxidation, in which n-paraffins are reacted with sulfur dioxide and oxygen under UV light irradiation.
- This radical reaction produces successive alkylsulfonyl radicals, which react further with oxygen to form the alkylsulfonyl radicals.
- the reaction with unreacted paraffin provides an alkyl radical and the alkylpersulfonic acid which decomposes into an alkyl peroxysulfonyl radical and a hydroxyl radical.
- the reaction of the two radicals with unreacted paraffin provides the alkylsulfonic acids or water, which reacts with alkylpersulfonic acid and sulfur dioxide to form sulfuric acid.
- this reaction is usually carried out only up to degrees of conversion of 1% and then terminated.
- Olefinsulfonates are produced industrially by reaction of ⁇ -olefins with sulfur trioxide. Intermediate zwitterions form, which cyclize to form so-called sultones. Under suitable conditions (alkaline or acid hydrolysis), these sultones react to form Hydroxylalkansulfonklaren or alkene sulfonic acids, both of which can also be used as anionic surfactant acids.
- alkyl benzene sulfonates as powerful anionic surfactants have been known since the thirties of our century. At that time, alkylbenzenes were prepared by monochlorination of kogasin fractions and subsequent Friedel-Crafts alkylation, which were sulfonated with oleum and neutralized with sodium hydroxide solution.
- Linear alkylbenzenesulfonates are prepared from linear alkylbenzenes, which in turn are accessible from linear olefins.
- large-scale petroleum fractions are separated with molecular sieves in the n-paraffins of the desired purity and dehydrogenated to the n-olefins, resulting in both ⁇ - and i-olefins.
- the resulting Olefins are then reacted in the presence of acidic catalysts with benzene to the alkylbenzenes, the choice of Friedel-Crafts catalyst has an influence on the isomer distribution of the resulting linear alkylbenzenes:
- the content of the 2-phenyl isomers in the mixture with the 3-, 4-, 5- and other isomers at about 30 wt .-%, however, hydrogen fluoride is used as a catalyst, the content of 2-phenyl isomer can be reduced to about 20 wt .-%.
- C8-16-, preferably C9-13-Alkybenzolsulfonklaren which derive from alkylbenzenes, which have a tetralin content below 5 wt .-%, based on the alkylbenzene.
- alkylbenzenesulphonic acids whose alkylbenzenes have been prepared by the HF process, such that the C8-16-, preferably C9-13-alkylbenzenesulphonic acids used have a content of 2-phenyl-isomer of less than 22% by weight, based on the alkylbenzenesulfonic acid.
- the said anionic surfactants may be used alone or in admixture with each other, mixtures of fatty acids and ether sulfates, in particular in weight ratios of 5: 1 to 1: 5, preferably 2: 1 to 1: 2, being particularly preferred.
- the anionic surfactants described above in their acid form are usually used partially or fully neutralized. Suitable cations for the anionic surfactants are, in addition to the alkali metals (in particular Na and K salts), ammonium and mono-, di- or triethanolammonium ions. Instead of mono-, di- or triethanolamine, the analogous representatives of mono-, di- or trimethanolamine or those of the alkanolamines of higher alcohols can also be quaternized and present as a cation.
- the nonionic surfactants used are preferably alkoxylated, advantageously ethoxylated, in particular primary, alcohols having preferably 8 to 18 carbon atoms and on average 1 to 12 moles of ethylene oxide (EO) per mole of alcohol, in which the alcohol radical can be linear or preferably methyl-branched in the 2-position or linear and methyl-branched radicals in the mixture can contain, as they are usually present in Oxoalkoholresten.
- EO ethylene oxide
- the alcohol radical can be linear or preferably methyl-branched in the 2-position or linear and methyl-branched radicals in the mixture can contain, as they are usually present in Oxoalkoholresten.
- Examples of preferred ethoxylated alcohols include C12-14 alcohols with 3 EO or 4 EO, C9-11 alcohol with 7 EO, C13-15 alcohols with 3 EO, 5 EO, 7 EO or 8 EO, C12-18 alcohols. Alcohols with 3 EO, 5 EO or 7 EO and mixtures of these, such as mixtures of C12-14 alcohol with 3 EO and C12-18 alcohol with 5 EO.
- the degrees of ethoxylation given represent statistical means which, for a particular product, may be an integer or a fractional number.
- Preferred alcohol ethoxylates have a narrow homolog distribution (narrow range ethoxylates, NRE).
- fatty alcohols with more than 12 EO can also be used. Examples include tallow fatty alcohol with 14 EO, 25 EO, 30 EO or 40 EO. Low-foaming nonionic surfactants may also be used which have alternating ethylene oxide and alkylene oxide units. Among these, in turn, surfactants with EO-AO-EO-AO blocks are preferred, wherein in each case one to ten EO or AO groups are bonded to each other before a block of the other groups follows.
- R 1 is a straight-chain or branched, saturated or mono- or polyunsaturated C 6-24-alkyl or alkenyl radical; each group R2 or R3 is independently selected from -CH3; - CH2CH3, -CH2CH2-CH3, CH (CH3) 2 and the indices w, x, y, z independently of one another are integers from 1 to 6.
- R1-OH a straight-chain or branched, saturated or mono- or polyunsaturated C 6-24-alkyl or alkenyl radical
- R2 or R3 is independently selected from -CH3; - CH2CH3, -CH2CH2-CH3, CH (CH3) 2 and the indices w, x, y, z independently of one another are integers from 1 to 6.
- the radical R1 has an even number of carbon atoms and is usually undisplayed, the linear radicals being selected from alcohols of native origin having 12 to 18 carbon atoms, for example from coconut, palm, tallow or oleyl alcohol , are preferred.
- Alcohols which are accessible from synthetic sources are, for example, the Guerbet alcohols or methyl-branched or linear and methyl-branched radicals in the 2-position, as they are usually present in oxo alcohol radicals.
- agents according to the invention are preferred in which R1 in the above formula is an alkyl radical having 6 to 24, preferably 8 to 20, particularly preferably 9 to 15 and in particular 9 to 11 carbon atoms.
- R1 in the above formula is an alkyl radical having 6 to 24, preferably 8 to 20, particularly preferably 9 to 15 and in particular 9 to 11 carbon atoms.
- alkylene oxide unit which may be contained in the nonionic surfactants in an alternating manner with the ethylene oxide unit, in particular butylene oxide is considered in addition to propylene oxide.
- R2 or R3 are independently selected from -CH2CH2-CH3 or CH (CH3) 2, are suitable.
- nonionic surfactants and alkyl glycosides of the general formula RO (G) x can be used in which R is a primary straight-chain or methyl-branched, especially in the 2-position methyl-branched aliphatic radical having 8 to 22, preferably 12 to 18 carbon atoms and G represents a glycose unit having 5 or 6 C atoms, preferably glucose.
- the degree of oligomerization x which indicates the distribution of monoglycosides and oligoglycosides, is any number between 1 and 10; preferably x is 1.2 to 1.4.
- nonionic surfactants used either as the sole nonionic surfactant or in combination with others nonionic surfactants are alkoxylated, preferably ethoxylated or ethoxylated and propoxylated fatty acid alkyl esters, preferably having 1 to 4 carbon atoms in the alkyl chain, in particular fatty acid methyl esters.
- Nonionic surfactants of the amine oxide type for example N-cocoalkyl-N, N-dimethylamine oxide and N-tallowalkyl-N, N-dihydroxyethylamine oxide, and the fatty acid alkanolamides may also be suitable.
- polyhydroxy fatty acid amides of the following formula in which RCO is an aliphatic acyl radical having 6 to 22 carbon atoms, R 1 is hydrogen, an alkyl or hydroxyalkyl radical having 1 to 4 carbon atoms and [Z] is a linear or branched polyhydroxyalkyl radical having 3 to 10 carbon atoms and 3 to 10 hydroxyl groups.
- the polyhydroxy fatty acid amides are known substances which can usually be obtained by reductive amination of a reducing sugar with ammonia, an alkylamine or an alkanolamine and subsequent acylation with a fatty acid, a fatty acid alkyl ester or a fatty acid chloride.
- the group of polyhydroxy fatty acid amides also includes compounds of the formula R is a linear or branched alkyl or alkenyl radical having 7 to 12 carbon atoms, R 1 is a linear, branched or cyclic alkyl radical or an aryl radical having 2 to 8 carbon atoms and R 2 is a linear, branched or cyclic alkyl radical or an aryl radical or an oxy-alkyl radical having 1 to 8 carbon atoms, with C1-4-alkyl or phenyl radicals being preferred and [Z] being a linear polyhydroxyalkyl radical whose alkyl chain is substituted by at least two hydroxyl groups, or alkoxylated, preferably ethoxylated or propoxylated derivatives thereof residue.
- [Z] is preferably obtained by reductive amination of a reduced sugar, for example glucose, fructose, maltose, lactose, galactose, mannose or xylose.
- a reduced sugar for example glucose, fructose, maltose, lactose, galactose, mannose or xylose.
- the N-alkoxy- or N-aryloxy-substituted compounds can then be converted into the desired polyhydroxy fatty acid amides by reaction with fatty acid methyl esters in the presence of an alkoxide as catalyst.
- nonionic surfactants are the end-capped poly (oxyalkylated) surfactants of the formula R1O [CH2CH (R3) O] x [CH2] kCH (OH) [CH 2] j OR 2 in which R 1 and R 2 are linear or branched, saturated or unsaturated, aliphatic or aromatic hydrocarbon radicals having 1 to 30 carbon atoms, R 3 is H or a methyl, ethyl, n-propyl, iso-propyl, n-butyl, 2 Butyl or 2-methyl-2-butyl radical, x for values between 1 and 30, k and j are values between 1 and 12, preferably between 1 and 5.
- each R3 in the above formula may be different.
- R1 and R2 are preferably linear or branched, saturated or unsaturated, aliphatic or aromatic hydrocarbon radicals having 6 to 22 carbon atoms, with radicals having 8 to 18 carbon atoms being particularly preferred.
- R 3 H, -CH 3 or -CH 2 CH 3 are particularly preferred.
- Particularly preferred values for x are in the range from 1 to 20, in particular from 6 to 15.
- nonionic surfactants mixtures of alkoxylated fatty alcohols and alkyl glycosides are preferred. In them, the weight ratio of which is preferably 10: 1 to 1: 2, especially 10: 1 to 2: 1.
- the weight ratio of anionic surfactant to nonionic surfactant is between 10: 1 and 1:10, preferably between 7.5: 1 and 1: 5 and in particular between 5: 1 and 1: 2. It is preferred if surfactant is used in amounts of from 5% by weight to 80% by weight, preferably from 7.5% by weight to 70% by weight, particularly preferably from 10% by weight to 60% by weight. and in particular from 12.5% by weight to 50% by weight.
- the amounts and ratios indicated in one embodiment of the invention relate to the individual (second or further) partial compositions and, in a further embodiment, to the entire composition according to the invention.
- Proteases, amylase, lipase, hemicellulase and / or cellulase belong in particular to the enzymes contained in the second partial composition or the further partial compositions.
- These enzymes are basically of natural origin; Starting from the natural molecules are available for use in detergents and cleaners improved variants are available, which are preferably used accordingly.
- agents according to the invention preferably contain enzymes in total amounts of from 1 ⁇ 10 -6 to 5 percent by weight, based on active protein.
- the protein concentration can be determined by known methods, for example, the BCA method (bicinchoninic acid, 2,2'-biquinolyl-4,4'-dicarboxylic acid) or the biuret method (AG Gornall, CS Bardawill and MM David, J. Biol. Chem. 177 (1948), pp. 751-766).
- the first part composition is free of enzymes.
- the second partial composition contains protease, amylase and cellulase. In this case, further partial compositions (ie, except the first one) may be completely absent.
- subtilisin type those of the subtilisin type are preferable.
- subtilisins BPN 'and Carlsberg the protease PB92, the subtilisins 147 and 309, the alkaline protease from Bacillus lentus, subtilisin DY and the enzymes thermitase, proteinase K and the subtilases, but not the subtilisins in the narrower sense Proteases TW3 and TW7.
- Subtilisin Carlsberg is available in a further developed form under the trade name Alcalase® from Novozymes A / S, Bagsv ⁇ rd, Denmark.
- the subtilisins 147 and 309 are sold under the trade names Esperase® or Savinase® by the company Novozymes.
- the protease from Bacillus lentus DSM 5483 (known from international patent application WO 91/02792) is derived from the variants listed under the name BLAP®, which are described in particular in International Patent Applications WO 92/21760, WO 95/23221 and in the German Patent Applications Patent applications DE 101 21 463 and DE 101 53 792 are described.
- Other usable Proteases from different Bacillus sp. and B. gibsonii are known from the German patent applications DE 101 62 727, DE 101 63 883, DE 101 63 884 and DE 101 62 728.
- proteases are, for example, those under the trade names Durazym®, Relase®, E-verlase®., Nafizym®, Natalase®, Kannase® and Ovozymes® from Novozymes, which are sold under the trade names Purafect®, Purafect® OxP and Properase® , from Genencor, sold under the trade name Protosol® by Advanced Biochemicals Ltd., Thane, India, under the trade name Wuxi® by Wuxi Snyder Bioproducts Ltd., China, under the trade names Proleather® and Protease P® from Amano Pharmaceuticals Ltd., Nagoya, Japan, and the enzyme available under the name Proteinase K-16 from Kao Corp., Tokyo, Japan.
- amylases which can be used according to the invention are the ⁇ -amylases from Bacillus licheniformis, B. amyloliquefaciens or B. stearothermophilus and also their further developments improved for use in detergents and cleaners.
- the B. licheniformis enzyme is available from Novozymes under the name Termamyl® and from Genencor under the name Purastar®ST. Further development products of this ⁇ -amylase are available from Novozymes under the trade names Duramyl® and Termamyl®ultra, from Genencor under the name Purastar®OxAm, and from Daiwa Seiko Inc., Tokyo, Japan, as Keistase®. B.
- amyloliquefaciens ⁇ -amylase is sold by Novozymes under the name BAN®, and variants derived from the B. stearothermophilus ⁇ -amylase under the names BSG® and Novamyl®, also from Novozymes. Furthermore, they are in the international Patent application WO 02/10356 discloses ⁇ -amylase from Bacillus sp. A 7-7 (DSM 12368) and the cyclodextrin glucanotransferase (CGTase) from B.
- DSM 12368 Bacillus sp. A 7-7
- CTTase cyclodextrin glucanotransferase
- agaradherens (DSM 9948) described in International Patent Application PCT / EP01 / 13278; also those belonging to the sequence space of ⁇ -amylases, which is defined in the German patent application DE 101 31 441 A1.
- fusion products of said molecules can be used, for example those known from German patent application DE 101 38 753.
- the further developments of the ⁇ -amylase from Aspergillus niger and A. oryzae available under the trade names Fungamyl® from Novozymes are suitable.
- Another commercial product is, for example, the amylase LT®.
- compositions according to the invention may contain lipases and / or cutinases.
- lipases include, for example, the lipases originally obtainable from Humicola lanuginosa (Thermomyces lanuginosus) or further developed, in particular those with the amino acid exchange D96L. They are sold for example by the company Novozymes under the trade names Lipolase®, Lipolase®Ultra, LipoPrime®, Lipozyme® and Lipex®.
- the cutinases can be used, which were originally isolated from Fusarium solani pisi and Humicola insolens.
- Lipases which are likewise useful are sold by Amano under the names Lipase CE®, Lipase P®, Lipase B® or Lipase CES®, Lipase AKG®, Bacillis sp. Lipase®, Lipase AP®, Lipase M-AP® and Lipase AML®.
- Lipases or cutinases can be used, the initial enzymes were originally isolated from Pseudomonas mendocina and Fusarium solanii.
- compositions of the invention may contain cellulases, depending on the purpose as pure enzymes, as enzyme preparations or in the form of mixtures in which the individual.
- components complement each other in terms of their various performance aspects. These performance aspects include, in particular, contributions to the primary washing performance, the secondary washing performance of the composition (anti-redeposition effect or graying inhibition) and softening (fabric effect), up to the exercise of a "stone washed" effect.
- Cellulase preparation or its further developments are offered by Novozymes under the trade name Celluzyme®.
- Endolase® and Carezyme® which are likewise available from Novozymes, are based on the 50 kD EG or the 43 kD EG from H. insolens DSM 1800. Further commercial products of this company are Cellusoft® and Renozyme®. Likewise, the cellulases disclosed in international patent application WO 97/14804 can be used; for example, the 20 kD EG from Melanocarpus disclosed therein, available from AB Enzymes, Finland, under the trade names Ecostone® and Biotouch®. Further commercial products of AB Enzymes are Econase® and Ecopulp®. Other suitable cellulases from Bacillus sp.
- CBS 670.93 and CBS 669.93 are disclosed in the international patent application WO 96/34092, wherein those derived from Bacillus sp. CBS 670.93 from the company Genencor under the trade name Puradax® is available.
- Other commercial products of Genencor are "Genencor detergent cellulase L" and IndiAge®Neutra.
- compositions according to the invention may contain further enzymes which are included under the term.
- Suitable mannanases are available, for example, under the names Gamanase® and Pektinex AR® from Novozymes, under the name, Rohapec® B1L from AB Enzymes and under the name Pyrolase® from Diversa Corp., San Diego, CA, USA ,
- a suitable ⁇ -glucanase from a B. alcalophilus is disclosed, for example, in International Patent Application WO 99/06573. From. B. subtilis obtained ⁇ -glucanase is available under the name Cereflo® from Novozymes.
- the enzymes used in agents of the invention are either originally from microorganisms, such as the genera Bacillus, Streptomyces, Humicola, or Pseudomonas, and / or are produced by biotechnological methods known per se by suitable microorganisms, such as transgenic expression hosts of the genera Bacillus or filamentous fungi.
- compositions according to the invention may contain enzyme stabilizers for this purpose.
- enzyme stabilizers are reversible protease inhibitors.
- benzamidine hydrochloride, borax, boric acids, boronic acids or their salts or esters are used, including, in particular, derivatives with aromatic groups, for example according to the international patent application WO 95/12655 ortho-substituted, according to International Patent Application WO 92/19707 meta-substituted and according to US Pat. No.
- Further enzyme stabilizers are amino alcohols such as mono-, di-, triethanol- and -propanolamine and mixtures thereof, aliphatic carboxylic acids up to C12, for example from European patent application EP 0 378 261 or international patent application WO 97/05227 known, such as succinic acid, other dicarboxylic acids or salts of said acids.
- German patent application DE 196 50 537 discloses end-group-capped fatty acid amide alkoxylates for this purpose.
- Certain organic acids used as builders are capable, as disclosed in international patent application WO 97/18287, of additionally stabilizing a contained enzyme.
- Lower aliphatic alcohols such as ethanol or propanol, but especially polyols such as glycerol, ethylene glycol, propylene glycol or sorbitol are other useful enzyme stabilizers.
- polyols such as glycerol, ethylene glycol, propylene glycol or sorbitol
- di-glycerol phosphate protects against denaturation by physical influences.
- calcium salts are frequently used, such as, for example, calcium acetate or the calcium formate disclosed for this purpose in European patent EP 0 028 865, and magnesium salts, for example according to European patent application EP 0 378 262. Reducing reducing agents and antioxidants, as disclosed, inter alia, in European Patent Application EP 0 780 466, the stability of enzymes to oxidative degradation.
- Sulfur-containing reducing agents are known, for example, from European patents EP 0 080 748 and EP 0 080 223.
- Other examples include sodium sulfite (according to European patent application EP 0 533 239) and reducing sugars (according to European patent application EP 0 656 058).
- peptide-aldehyde stabilizers is increased according to international patent application WO 98/13462 by combination with boric acid and / or boric acid derivatives and polyols and according to international patent application WO 98/13459 by the additional use of divalent cations, such as calcium Ions further strengthened.
- the second partial composition or the further partial compositions may contain all ingredients customary in liquid detergents which do not unduly interact negatively with the abovementioned. These include, for example, builder materials, complexing agents for heavy metals, nonaqueous water-miscible solvents, thickeners, grayness inhibitors, foam regulators, dye transfer inhibitors, antimicrobial agents, optical brighteners, dyes and fragrances. If desired, such further ingredients may also be included in the first part composition, provided that they do not unreasonably affect the storage stability of the peracid component.
- Builder materials which may be present in the compositions according to the invention are in particular silicates, aluminum silicates (in particular zeolites), carbonates, salts of organic di- and polycarboxylic acids and mixtures of these substances.
- Suitable crystalline, layered sodium silicates have the general formula NaMSixO2x + 1.
- y H2O where M is sodium or hydrogen, x is a number from 1.9 to 4 and y is a number from 0 to 20 and preferred values for x are 2, 3 or 4.
- Such crystalline sheet silicates are described, for example, in European Patent Application EP 0 164 514.
- Preferred crystalline layered silicates of the formula given are those in which M is sodium and x assumes the values 2 or 3.
- both ⁇ - and ⁇ -sodium disilicates Na 2 Si 2 O 5 .yH 2 O are preferred, whereby ⁇ -sodium disilicate can be obtained, for example, by the process described in international patent application WO 91/08171.
- amorphous sodium silicates with one module.
- the dissolution delay compared with conventional amorphous sodium silicates may have been caused in various ways, for example by surface treatment, compounding, compaction / densification or by overdrying.
- the term “amorphous” also "X-ray amorphous" Understood.
- silicates do not yield sharp X-ray reflections typical of crystalline substances in X-ray diffraction experiments, but at most one or more maxima of the scattered X-rays which have a width of several degrees of diffraction angle.
- Such so-called X-ray-amorphous silicates which likewise have a dissolution delay compared with the conventional water glasses, are described, for example, in German patent application DE 44 00 024.
- the optionally used finely crystalline, synthetic and bound water-containing zeolite is preferably zeolite A and / or P.
- zeolite P zeolite MAP® commercial product from Crosfield
- zeolite X and mixtures of A, X and / or P are particularly preferred.
- commercially available and preferably usable in the context of the present invention is, for example, a cocrystal of zeolite X and zeolite A (about 80% by weight of zeolite X) ), by CONDEA Augusta SpA. under the brand name VEGOBOND AX® and by the formula nNa2O. (1-n) K2O. Al2O3. (2 - 2.5) SiO 2.
- the zeolite can be used as spray-dried powder or else as undried, still moist, stabilized suspension of its preparation Use come.
- the zeolite may contain minor additions of nonionic surfactants as stabilizers, for example 1 to 3 wt .-%, based on zeolite, of ethoxylated C12-C18 fatty alcohols having 2 to 5 ethylene oxide groups, C12 C14-fatty alcohols with 4 to 5 ethylene oxide groups or ethoxylated isotridecanols.
- Suitable zeolites have an average particle size of less than 10 ⁇ m (volume distribution, measuring method, for example by means of a Coulter Counter) and preferably contain from 18 to 22% by weight, in particular from 20 to 22% by weight, of bound water.
- phosphates as builders are possible, unless such use should not be avoided for environmental reasons.
- Useful organic builder substances are, for example, the polycarboxylic acids which can be used in the form of their sodium salts, polycarboxylic acids meaning those carboxylic acids which carry more than one acid function. These are, for example, citric acid, adipic acid, succinic acid, glutaric acid, malic acid, tartaric acid, maleic acid, fumaric acid, sugar acids, aminocarboxylic acids, nitrilotriacetic acid (NTA), if such use is not objectionable for ecological reasons, and mixtures of these.
- Preferred salts are the salts of polycarboxylic acids such as citric acid, adipic acid, succinic acid, glutaric acid, tartaric acid, sugar acids and mixtures thereof.
- the acids themselves can also be used.
- the acids typically also have the property of an acidifying component and thus also serve to set a lower one and milder pH of detergents or cleaners.
- citric acid, succinic acid, glutaric acid, adipic acid, gluconic acid and any desired mixtures of these can be mentioned here.
- Further suitable builders are polymeric polycarboxylates, for example the alkali metal salts of polyacrylic acid or polymethacrylic acid, for example those having a relative molecular mass of from 500 to 70,000 g / mol.
- the molecular weights stated for polymeric polycarboxylates are weight-average molecular weights M w of the particular acid form, which can in principle be determined by means of gel permeation chromatography (GPC), a UV detector being used. The measurement is carried out against an external polyacrylic acid standard, which provides realistic molecular weight values due to its structural relationship with the investigated polymers. These data deviate significantly from the molecular weight data in which polystyrene sulfonic acids are used as standard, the molecular weights measured against polystyrenesulfonic acids generally being significantly higher.
- Suitable polymers are, in particular, polyacrylates which preferably have a molecular weight of 2,000 to 20,000 g / mol.
- the short-chain polyacrylates which have molar masses of from 2000 to 10000 g / mol, and particularly preferably from 3000 to 5000 g / mol, may again be preferred from this group.
- copolymeric polycarboxylates in particular those of acrylic acid with methacrylic acid and of acrylic acid or methacrylic acid with maleic acid. Copolymers of acrylic acid with maleic acid which contain 50 to 90% by weight of acrylic acid and 50 to 10% by weight of maleic acid have proven to be particularly suitable.
- the polymers may also contain allyl sulfonic acids, such as allyloxybenzenesulfonic acid and methallylsulfonic acid known from European Patent EP 0 727 448 B1, as monomer.
- biodegradable polymers of more than two different monomer units for example those which according to German Patent Application DE 43 00 772 A1 as monomers salts of acrylic acid and maleic acid and vinyl alcohol or vinyl alcohol derivatives or according to German Patent DE 42 21 381 contain as monomers, salts of acrylic acid and 2-alkylallylsulfonic acid and sugar derivatives.
- Further preferred copolymers are those which are described in the German patent applications DE-A-43 03 320 and DE-A-44 17 734 and preferably have as monomers acrolein and acrylic acid / acrylic acid salts or acrolein and vinyl acetate.
- polymeric aminodicarboxylic acids, their salts or their precursors are also particularly preferred.
- polyaspartic acids or their salts and derivatives of which German Patent Application DE 195 40 086 A1 discloses that, in addition to cobuilder properties, they also have a bleach-stabilizing effect.
- Further suitable builder substances are polyacetals which can be obtained by reacting dialdehydes with polyol carboxylic acids which have 5 to 7 C atoms and at least 3 hydroxyl groups, for example as described in European patent application EP 0 280 223.
- Preferred polyacetals are obtained from dialdehydes such as glyoxal, glutaraldehyde, terephthalaldehyde and mixtures thereof and from polyol carboxylic acids such as gluconic acid and / or glucoheptonic acid.
- dextrins for example oligomers or polymers of carbohydrates, which can be obtained by partial hydrolysis of starches.
- the hydrolysis can be carried out by customary, for example acidic or enzyme-catalyzed processes.
- it is hydrolysis products having average molecular weights in the range of 400 to 500,000 g / mol.
- DE dextrose equivalent
- oxidized derivatives of such dextrins are their reaction products with oxidizing agents which are capable of oxidizing at least one alcohol function of the saccharide ring to the carboxylic acid function.
- Oxydisuccinates and other derivatives of disuccinates are also other suitable builder materials.
- ethylenediamine-N, N'-disuccinate (EDDS) the synthesis of which is described, for example, in US Pat. No. 3,158,615, is preferably used in the form of its sodium or magnesium salts.
- glycerol disuccinates and glycerol trisuccinates are also preferred in this connection.
- organic builders are, for example, acetylated hydroxycarboxylic acids or salts thereof, which may optionally also be present in lactone form and which contain at least 4 carbon atoms and at least one hydroxyl group and a maximum of two acid groups.
- Such builders are described, for example, in International Patent Application WO 95/20029.
- Builder substances, and among these, in particular water-soluble materials, are preferably present in agents according to the invention in amounts of from 1% by weight to 20% by weight, in particular from 1% by weight to 8% by weight, the first part composition preferably being free of builder materials.
- the complexing agents for heavy metals optionally contained in the compositions include phosphoric acid, aminocarboxylic acids and optionally functionally modified phosphonic acids, for example hydroxy- or aminoalkanephosphonic acids.
- useful aminocarboxylic acids include nitrilotriacetic acid, methylglycinediacetic acid and diethylenetriaminepentaacetic acid.
- phosphonic acids are 1-hydroxyethane-1,1-diphosphonic acid (HEDP) or the disodium salt or tetrasodium salt of this acid, 2-phosphonobutane-1,2,4-tricarboxylic acid or the trisodium salt of this acid, ethylenediamine tetramethylenephosphonic acid (EDTMP).
- the N-oxides corresponding to the nitrogen-containing compounds mentioned can also be used.
- the useful complexing agents include ethylenediamine-N, N'-disuccinic acid (EDDS).
- EDDS ethylenediamine-N, N'-disuccinic acid
- the complexing agents mentioned in their acid form can be used as such or in the form of their alkali metal salts, in particular the Sodium salts are used. Preference is given to the use of mixtures of aminocarboxylic acids with phosphonic acids.
- Complexing agents for heavy metals are preferably contained in agents according to the invention in amounts of from 0.05% by weight to 1% by weight, it being possible for them to be present in the first part composition and / or in the second or the further part compositions.
- Non-aqueous solvents which can be used in the compositions according to the invention originate, for example, from the group of monohydric alcohols, alkanolamines or glycol ethers, provided they are miscible with water in the concentration range intended for use.
- the solvents are preferably selected from ethanol, n- or i-propanol, butanols, ethylene glycol methyl ether, ethylene glycol ethyl ether, ethylene glycol propyl ether, ethylene glycol mono-n-butyl ether, diethylene glycol methyl ether, diethylene glycol ethyl ether, propylene glycol methyl, ethyl or propyl ether, dipropylene glycol monomethyl ether.
- nonaqueous solvents can be used in the liquid detergents according to the invention in amounts of up to 40% by weight, preferably from 0.5 to 20% by weight and in particular from 1% by weight to 10% by weight Solvents, the amounts of those which also act as enzyme stabilizers, are included.
- Suitable foam inhibitors which can be used in the compositions according to the invention are, for example, soaps, paraffins or silicone oils.
- silicone oils are used.
- Suitable anti-redeposition agents which are also referred to as soil repellents, are, for example, nonionic cellulose ethers such as methylcellulose and methylhydroxypropylcellulose with a proportion of methoxy groups of 15 to 30% by weight and of hydroxypropyl groups of 1 to 15% by weight, based in each case on the nonionic cellulose ether , as well as the known from the prior art polymers of phthalic acid and / or terephthalic acid or of their derivatives, in particular polymers of ethylene terephthalates and / or polyethylene glycol terephthalates or anionic and / or nonionic modified derivatives thereof. Especially preferred of these are the sulfonated derivatives of the phthalic and terephthalic acid polymers.
- Optical brighteners can be added to the compositions according to the invention in order to eliminate graying and yellowing of the treated textiles. These fabrics are absorbed by the fiber and cause a bleaching and fake bleaching effect by transforming invisible ultraviolet radiation into visible longer wavelength light, emitting ultraviolet light absorbed from the sunlight as faint bluish fluorescence, and pure yellow with the yellowed or yellowed wash White results.
- Suitable compounds are derived, for example, from the substance classes of 4,4'-diamino-2,2'-stilbenedisulfonic acids (flavonic acids), 4,4'-distyrylbiphenyls, methylumbelliferones, coumarins, dihydroquinolinones, 1,3-diarylpyrazolines, naphthalimides, benzoxazole , Benzisoxazole and benzimidazole systems as well as heterocyclic substituted pyrene derivatives.
- the optical brighteners are usually used in amounts between 0.05 and 0.3 wt .-%, based on the finished composition.
- Grayness inhibitors have the task of keeping the dirt detached from the fiber suspended in the liquor and thus preventing the dirt from being rebuilt.
- water-soluble colloids are usually of organic nature, for example, glue, gelatin, salts of E-thersulfonkla the starch or cellulose or salts of acidic sulfuric acid esters of cellulose or starch.
- water-soluble polyamides containing acidic groups are suitable for this purpose.
- soluble starch preparations and other than the above-mentioned starch products can be used, e.g. degraded starch, aldehyde levels, etc. Also polyvinylpyrrolidone is useful.
- cellulose ethers such as carboxymethylcellulose (Na salt), methylcellulose, hydroxyalkylcellulose and mixed ethers such as methylhydroxyethylcellulose, methylhydroxypropylcellulose, methylcarboxymethylcellulose and mixtures thereof in amounts of from 0.1 to 5% by weight, based on the compositions
- compositions according to the invention may contain synthetic crease inhibitors which, however, preferably do not contain the first part composition are included.
- synthetic products based on fatty acids, fatty acid esters, fatty acid amides, alkylol esters, -alkylolamides or fatty alcohols which are usually reacted with ethylene oxide, or products based on lecithin or modified phosphoric acid ester.
- the compositions of the invention may contain antimicrobial agents.
- antimicrobial agents Here one differentiates ever according to antimicrobial spectrum and mechanism of action between bacteriostats and bactericides, fungistatics and fungicides, etc.
- Important substances from these groups are, for example, benzalkonium chlorides, alkylaryl sulfonates, halophenols and phenol mercuriacetate, wherein the compounds according to the invention can be completely dispensed with.
- Thickening agents which can be used in the partial compositions according to the invention are, for example, those from the class of polyurethanes, polyacrylates which may also be present at least partially crosslinked, polyacrylamides and / or polysaccharides or their derivatives.
- an optionally modified polymer of saccharides such as glucose, galactose, mannose, gulose, altrose, allose, etc.
- a water-soluble xanthan as it is commercially available, for example, under the product names Kelzan®, Rhodopol®, Ketrol® or Rheozan®.
- Xanthan is understood as meaning a polysaccharide which corresponds to that which is produced by the bacterial strain Xanthomonas campestris from aqueous solutions of glucose or starch (J. Biochem., Micobiol., Technol.Engineer.Vol.III (1961), pp. 51 to 63). , It consists essentially of glucose, mannose, glucuronic acid and their acetylation products and also contains minor amounts of chemically bound pyruvic acid.
- water-soluble polysaccharide derivatives as can be obtained, for example, by alkoxylation with, for example, ethylene oxide, propylene oxide and / or butylene oxide, by alkylation with, for example, methyl halides and / or dimethyl sulfate, by acylation with carboxylic acid halides or by saponifying deacetylation from the corresponding polysaccharides, is possible.
- Thickening agents are present in the compositions according to the invention in amounts of preferably from 0.05% by weight to 2.5% by weight, in particular from 0.1% by weight to 2% by weight, the proportion of which is not the same in all partial compositions must be big.
- the individual partial compositions are preferably used in equal proportions. This can be achieved in a simple manner by adjusting the viscosity of the partial compositions and / or the type of outflow openings of the chambers of the multi-chamber container, in particular the adaptation of the diameter of the outflow openings, so that the user of the agent by a simple pouring or expressing the multi-chamber container easily useful amount, for example, the necessary for a wash in a washing machine amount of liquid detergent receives.
- surfactant- and enzyme-containing partial compositions T1 and T2 were prepared. These were each filled into a chamber of a polyethylene chamber composed of two chambers of equal size (each volume 750 ml), and the second chamber of the bottle was filled with the same amount of a 5% by weight aqueous phthalimidoperoxohexanoic acid preparation P (Eureco® L, manufacturer Ausimont). filled. Table: surfactant- and enzyme-containing.
Landscapes
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Mechanical Engineering (AREA)
- Organic Chemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Ceramic Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Containers And Packaging Bodies Having A Special Means To Remove Contents (AREA)
- Cleaning By Liquid Or Steam (AREA)
- Containers Having Bodies Formed In One Piece (AREA)
- Detergent Compositions (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SI200330577T SI1529006T1 (sl) | 2002-08-16 | 2003-08-18 | Razdeljevalna steklenica za najmanj dve tekoci snovi |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE2002138431 DE10238431A1 (de) | 2002-08-16 | 2002-08-16 | Spenderflasche für mindestens zwei Wirkstofffluide |
| DE10238431 | 2002-08-16 | ||
| DE10257387A DE10257387A1 (de) | 2002-12-06 | 2002-12-06 | Mehrkomponenten-Flüssigwaschmittel |
| DE10257387 | 2002-12-06 | ||
| PCT/EP2003/009135 WO2004018319A1 (de) | 2002-08-16 | 2003-08-18 | Spenderflasche für mindestens zwei wirkstofffluide |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1529006A1 EP1529006A1 (de) | 2005-05-11 |
| EP1529006B1 true EP1529006B1 (de) | 2006-10-04 |
Family
ID=31947617
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP03792363A Expired - Lifetime EP1529006B1 (de) | 2002-08-16 | 2003-08-18 | Spenderflasche für mindestens zwei wirkstofffluide |
Country Status (7)
| Country | Link |
|---|---|
| EP (1) | EP1529006B1 (ja) |
| JP (1) | JP4147244B2 (ja) |
| AT (1) | ATE341498T1 (ja) |
| AU (1) | AU2003264066A1 (ja) |
| DE (1) | DE50305283D1 (ja) |
| ES (1) | ES2274311T3 (ja) |
| WO (1) | WO2004018319A1 (ja) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2088186B1 (de) | 2003-12-13 | 2013-07-31 | Henkel AG & Co. KGaA | Mehrkomponenten-Thin-To-Thick-System |
| ES2313288T3 (es) | 2004-02-13 | 2009-03-01 | HENKEL AG & CO. KGAA | Botella dispensadora para por lo menos dos fluidos activos. |
| JP4855140B2 (ja) * | 2006-05-18 | 2012-01-18 | 花王株式会社 | 液体漂白洗浄剤組成物 |
| JP5424741B2 (ja) * | 2009-06-25 | 2014-02-26 | 株式会社ユーキケミカル | 二液混合装置、及び、殺菌液生成装置 |
| WO2011106539A1 (en) | 2010-02-24 | 2011-09-01 | Colgate-Palmolive Company | Dispenser cap with selectable reservoirs |
| KR101725085B1 (ko) * | 2012-01-31 | 2017-04-10 | 심상봉 | 이 액 분리 수용형 염색용 튜브용기와 그 제조방법 |
| US20130216631A1 (en) | 2012-02-17 | 2013-08-22 | The Clorox Company | Targeted performance of hypohalite compositions thereof |
| DE102019124860B4 (de) * | 2019-09-16 | 2024-01-25 | MKN Maschinenfabrik Kurt Neubauer GmbH & Co. KG | Verfahren zum Entkalken einer Hochdruckreinigungseinrichtung eines Druckgargeräts sowie dazu eingerichtetes Druckgargerät |
| WO2021130928A1 (ja) * | 2019-12-25 | 2021-07-01 | 車工房株式会社 | クリーニング剤およびクリーニング方法 |
| CN113233169B (zh) * | 2021-05-20 | 2023-03-31 | 江西牛牛乳业有限责任公司 | 双腔瓶杀菌保护装置及其杀菌工艺 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE6901644U (de) | 1968-12-30 | 1969-06-04 | Ingrid Hoffmann | Klein - trampolin |
| GB8922542D0 (en) | 1989-10-06 | 1989-11-22 | Melland Tristan Guy | Multi-compartment bottle |
| US5062550A (en) * | 1990-05-24 | 1991-11-05 | Singh Bharat H | Selective flow dispensing container |
| US5223245A (en) * | 1990-09-11 | 1993-06-29 | Beecham Inc. | Color change mouthrinse |
| US5102016A (en) * | 1990-12-03 | 1992-04-07 | Ball Lee R | Apparatus for dispensing materials in touching association and methods of use thereof |
| US5289950A (en) * | 1992-09-30 | 1994-03-01 | Chesebrough-Pond's Usa Co., Division Of Conopco, Inc. | Multiple chamber dispensing package with closure system |
| GB9300366D0 (en) * | 1993-01-09 | 1993-03-03 | Solvay Interox Ltd | Compositions and uses thereof |
| US5398846A (en) | 1993-08-20 | 1995-03-21 | S. C. Johnson & Son, Inc. | Assembly for simultaneous dispensing of multiple fluids |
| GB2297976A (en) * | 1995-02-01 | 1996-08-21 | Reckitt & Colmann Prod Ltd | Improvements in or relating to a bleaching process |
| US5911909A (en) | 1996-11-12 | 1999-06-15 | S. C. Johnson & Son, Inc. | Acidic bleaching solution, method of preparation and a bleaching system for forming the same |
| ATE204988T1 (de) | 1997-10-27 | 2001-09-15 | Alpla Werke | Flasche, insbesondere zur reinigung von wc- schüsseln |
| US6283316B1 (en) * | 1998-04-27 | 2001-09-04 | Adam Sherman | Orifice reducer for multi-compartment container |
| FR2782840B1 (fr) | 1998-08-25 | 2003-09-05 | Commissariat Energie Atomique | Circuit electronique et procede de realisation d'un circuit electronique integre comprenant au moins un composant electronique de puissance dans une plaque de substrat |
| US6341716B1 (en) * | 1999-11-19 | 2002-01-29 | Owens-Illinois Closure Inc. | Dual-outlet dispensing closure |
| US6247617B1 (en) * | 1999-12-13 | 2001-06-19 | Richard Allen Clyde | Single use container for dispensing separately housed sterile compositions |
| DE10024251A1 (de) | 2000-05-17 | 2001-11-22 | Rudolf Weber | Bleichendes flüssiges Fleckentfernungsmittel und desinfiszierendes Putzmittel mit temperaturunabhängiger Bleichaktivierung |
| ES2231547T3 (es) * | 2000-09-15 | 2005-05-16 | THE PROCTER & GAMBLE COMPANY | Recipiente multicompartimiento y dispositivo dispensador. |
-
2003
- 2003-08-18 AU AU2003264066A patent/AU2003264066A1/en not_active Abandoned
- 2003-08-18 DE DE50305283T patent/DE50305283D1/de not_active Expired - Lifetime
- 2003-08-18 EP EP03792363A patent/EP1529006B1/de not_active Expired - Lifetime
- 2003-08-18 WO PCT/EP2003/009135 patent/WO2004018319A1/de active IP Right Grant
- 2003-08-18 AT AT03792363T patent/ATE341498T1/de active
- 2003-08-18 ES ES03792363T patent/ES2274311T3/es not_active Expired - Lifetime
- 2003-08-18 JP JP2005501206A patent/JP4147244B2/ja not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| DE50305283D1 (de) | 2006-11-16 |
| ES2274311T3 (es) | 2007-05-16 |
| ATE341498T1 (de) | 2006-10-15 |
| EP1529006A1 (de) | 2005-05-11 |
| JP4147244B2 (ja) | 2008-09-10 |
| WO2004018319A1 (de) | 2004-03-04 |
| JP2005535537A (ja) | 2005-11-24 |
| AU2003264066A1 (en) | 2004-03-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1716056B1 (de) | Spenderflasche für flüssigwaschmittel, die aus mindestens zwei teilzusammensetzungen bestehen | |
| US7448556B2 (en) | Dispenser bottle for at least two active fluids | |
| US20050227896A1 (en) | Multicomponent liquid detergent | |
| EP1529006B1 (de) | Spenderflasche für mindestens zwei wirkstofffluide | |
| EP2190337A1 (de) | Verfahren zum maschinellen reinigen von geschirr | |
| DE102009000879A1 (de) | Reinigungsmittel | |
| DE102009002095A1 (de) | Reinigungsmittel | |
| EP2414499A1 (de) | Reinigungsmittel | |
| EP3472290B1 (de) | Konzentrierte isotrope flüssigwaschmittel enthaltend polymere | |
| EP3656839B1 (de) | Reinigungsmittel zur automatischen dosierung | |
| EP4008764A1 (de) | Verbesserte reinigung durch hydrogencarbonat im maschinellen geschirrspülmittel | |
| WO2011157630A1 (de) | Maschinelles reinigungsverfahren | |
| EP3914686B1 (de) | Reinigerformulierungen zum automatischen geschirrspülen mit bleichmittel | |
| DE102018220191A1 (de) | Reinigungsmittel mit Klarspüler für automatische Dosiereinheit | |
| DE10310679B3 (de) | Coating schmelzbarer Substanzen und Substanzgemische | |
| DE102017209213A1 (de) | Konzentrierte isotrope Flüssigwaschmittel enthaltend Polymere | |
| WO2008061817A1 (de) | VERSCHLUß MIT ÜBERKOPFSTANDVORRICHTUNG | |
| EP2879973B1 (de) | Kombinationsprodukt mit einer hohen viskosität | |
| DE102018220188A1 (de) | Transparente Formulierungen zum automatischen Geschirrspülen | |
| DE102018220187A1 (de) | Niedrigviskose Reinigerformulierungen zum automatischen Geschirrspülen | |
| WO2017129310A1 (de) | C8-10-alkylamidoalkylbetain als antiknitterwirkstoff | |
| DE10350930A1 (de) | Verpackungsverfahren mit Tragplatte |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20041214 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL LT LV MK |
|
| DAX | Request for extension of the european patent (deleted) | ||
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20061004 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20061004 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20061004 Ref country code: IE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20061004 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: GERMAN |
|
| REF | Corresponds to: |
Ref document number: 50305283 Country of ref document: DE Date of ref document: 20061116 Kind code of ref document: P |
|
| REG | Reference to a national code |
Ref country code: RO Ref legal event code: EPE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20070104 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20070104 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20070104 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20070110 |
|
| REG | Reference to a national code |
Ref country code: HU Ref legal event code: AG4A Ref document number: E001052 Country of ref document: HU |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20070316 |
|
| NLV1 | Nl: lapsed or annulled due to failure to fulfill the requirements of art. 29p and 29m of the patents act | ||
| ET | Fr: translation filed | ||
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2274311 Country of ref document: ES Kind code of ref document: T3 Ref country code: IE Ref legal event code: FD4D |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20070705 |
|
| BERE | Be: lapsed |
Owner name: HENKEL K.G.A.A. Effective date: 20070831 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070831 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070831 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20070105 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070831 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20061004 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070831 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070818 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20061004 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20061004 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: RO Payment date: 20150724 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SI Payment date: 20150727 Year of fee payment: 13 Ref country code: AT Payment date: 20150820 Year of fee payment: 13 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 14 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 341498 Country of ref document: AT Kind code of ref document: T Effective date: 20160818 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160818 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160818 Ref country code: SI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20160819 |
|
| REG | Reference to a national code |
Ref country code: SI Ref legal event code: KO00 Effective date: 20170418 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 15 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20180920 Year of fee payment: 16 Ref country code: ES Payment date: 20180921 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CZ Payment date: 20180816 Year of fee payment: 16 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190819 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190818 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190818 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20210107 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190819 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20220823 Year of fee payment: 20 Ref country code: DE Payment date: 20220620 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20220823 Year of fee payment: 20 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230530 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 50305283 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20230817 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20230817 |