EP0302575A1 - Ein zur mehrmaligen Verwendung bestimmter Kopfteil eines Behältnisses - Google Patents
Ein zur mehrmaligen Verwendung bestimmter Kopfteil eines Behältnisses Download PDFInfo
- Publication number
- EP0302575A1 EP0302575A1 EP88201691A EP88201691A EP0302575A1 EP 0302575 A1 EP0302575 A1 EP 0302575A1 EP 88201691 A EP88201691 A EP 88201691A EP 88201691 A EP88201691 A EP 88201691A EP 0302575 A1 EP0302575 A1 EP 0302575A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- head
- container
- head according
- contents
- passage
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 230000000813 microbial effect Effects 0.000 claims abstract description 11
- 230000004888 barrier function Effects 0.000 claims abstract description 7
- 230000009545 invasion Effects 0.000 claims abstract description 5
- 238000000926 separation method Methods 0.000 claims abstract description 5
- 230000002045 lasting effect Effects 0.000 claims abstract description 3
- 239000002537 cosmetic Substances 0.000 claims description 3
- 239000004599 antimicrobial Substances 0.000 abstract description 14
- 238000010348 incorporation Methods 0.000 abstract description 3
- 230000003190 augmentative effect Effects 0.000 abstract 1
- 238000012258 culturing Methods 0.000 description 15
- BELBBZDIHDAJOR-UHFFFAOYSA-N Phenolsulfonephthalein Chemical compound C1=CC(O)=CC=C1C1(C=2C=CC(O)=CC=2)C2=CC=CC=C2S(=O)(=O)O1 BELBBZDIHDAJOR-UHFFFAOYSA-N 0.000 description 10
- 208000015181 infectious disease Diseases 0.000 description 10
- 229960003531 phenolsulfonphthalein Drugs 0.000 description 10
- 208000034309 Bacterial disease carrier Diseases 0.000 description 9
- 238000011109 contamination Methods 0.000 description 9
- 238000002845 discoloration Methods 0.000 description 9
- 239000006071 cream Substances 0.000 description 7
- 244000005700 microbiome Species 0.000 description 7
- 241000588915 Klebsiella aerogenes Species 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 241000191967 Staphylococcus aureus Species 0.000 description 6
- 230000000845 anti-microbial effect Effects 0.000 description 6
- 230000001580 bacterial effect Effects 0.000 description 6
- 229940092559 enterobacter aerogenes Drugs 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 239000003242 anti bacterial agent Substances 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 208000012868 Overgrowth Diseases 0.000 description 4
- 239000000645 desinfectant Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 229920001817 Agar Polymers 0.000 description 3
- 241000894006 Bacteria Species 0.000 description 3
- 241000588914 Enterobacter Species 0.000 description 3
- 239000008272 agar Substances 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000013401 experimental design Methods 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 239000004033 plastic Substances 0.000 description 3
- 241000228245 Aspergillus niger Species 0.000 description 2
- 241000222122 Candida albicans Species 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- 206010070834 Sensitisation Diseases 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 229940095731 candida albicans Drugs 0.000 description 2
- 230000002939 deleterious effect Effects 0.000 description 2
- 239000013013 elastic material Substances 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000009630 liquid culture Methods 0.000 description 2
- 230000004899 motility Effects 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 229920002379 silicone rubber Polymers 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- 241000589517 Pseudomonas aeruginosa Species 0.000 description 1
- 208000035415 Reinfection Diseases 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 241000191940 Staphylococcus Species 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 230000008952 bacterial invasion Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229940082500 cetostearyl alcohol Drugs 0.000 description 1
- 230000001332 colony forming effect Effects 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 231100001261 hazardous Toxicity 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- -1 iodine, halogen compounds Chemical class 0.000 description 1
- 229940057995 liquid paraffin Drugs 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 230000000050 nutritive effect Effects 0.000 description 1
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 235000019271 petrolatum Nutrition 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 239000004945 silicone rubber Substances 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- OULAJFUGPPVRBK-UHFFFAOYSA-N tetratriacontyl alcohol Natural products CCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCO OULAJFUGPPVRBK-UHFFFAOYSA-N 0.000 description 1
- 239000003871 white petrolatum Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D47/00—Closures with filling and discharging, or with discharging, devices
- B65D47/04—Closures with discharging devices other than pumps
- B65D47/20—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge
- B65D47/2018—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge comprising a valve or like element which is opened or closed by deformation of the container or closure
- B65D47/2031—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge comprising a valve or like element which is opened or closed by deformation of the container or closure the element being formed by a slit, narrow opening or constrictable spout, the size of the outlet passage being able to be varied by increasing or decreasing the pressure
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D47/00—Closures with filling and discharging, or with discharging, devices
- B65D47/04—Closures with discharging devices other than pumps
- B65D47/20—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge
- B65D47/2018—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge comprising a valve or like element which is opened or closed by deformation of the container or closure
- B65D47/205—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge comprising a valve or like element which is opened or closed by deformation of the container or closure the valve being formed by a tubular flexible sleeve surrounding a rod-like element provided with at least one radial passageway which is normally closed by the sleeve
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D47/00—Closures with filling and discharging, or with discharging, devices
- B65D47/04—Closures with discharging devices other than pumps
- B65D47/20—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge
- B65D47/2018—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge comprising a valve or like element which is opened or closed by deformation of the container or closure
- B65D47/2056—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge comprising a valve or like element which is opened or closed by deformation of the container or closure lift valve type
- B65D47/2062—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge comprising a valve or like element which is opened or closed by deformation of the container or closure lift valve type in which the deformation raises or lowers the valve stem
- B65D47/2075—Closures with discharging devices other than pumps comprising hand-operated members for controlling discharge comprising a valve or like element which is opened or closed by deformation of the container or closure lift valve type in which the deformation raises or lowers the valve stem in which the stem is raised by the pressure of the contents and thereby opening the valve
Definitions
- This invention relates to novel heads for different types of containers, whose contents are intended to be discharged as separate aliquots.
- Another option is to prevent contamination by measures taken at the outlet of the container.
- DE-OS-1,586,758 discloses a tube with a closing device to prevent drying out of the tube contents in which point closure is effected by contact between the lips of a tubular member attached to the tube itself.
- GB 417,793 is directed to an opening and closing device which prevents the oxidation of the contents in a tube, and comprises a number of displaceable members held together by a ring which separate to form an aperture when the tube is squeezed.
- GB 326,683 discloses a valve-type closing device for a tube where the opening through which the tube contents are extruded is very short.
- the passage length is no less than 3 mm and is preferably no less than 5 mm, and its width when closed is a maximum of 1 mm, preferably of 0.1 mm.
- the head according to the invention is not to be removed, but stays in its place while the container is being used.
- the head may be fitted with a cap for cover and protection, e.g. against desiccation, which cap must be removed before each use.
- the containers may be of any type known in the art, in particular those in which the volume is reduced and no air is introduced as the contents are extruded.
- Such containers are those provided with a plunger and collapsible tubes, in combination with which the heads of the invention are particularly suitable.
- the contents of the containers for which the heads of the invention are intended may be any of those materials which are susceptible to deterioration by microbial infection, such as pharmaceuticals, cosmetics or foodstuffs.
- the contents should pass readily through the narrow opening or openings of the head, therefore they will be semisolid or liquid.
- the heads may contain certain anti-microbial agents. Also, when a removable cap is used to cover the head, this cap may contain an anti-microbial agent.
- An anti-bacterial agent incorporated in the head or cap according to the present invention has to meet quite different requirements as compared with an anti-bacterial agent which is incorporated in the contents.
- the anti-bacterial agent When the anti-bacterial agent is incorporated in the head or cap, its anti-bacterial effect has to be built up very quickly (due to the frequent transit of fresh contents) in a very small volume of contents, which is consequently unlikely to affect the user in any undesired way.
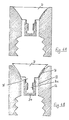
- the embodiment illustrated in Figures 4A, 4B and 4C comprises a housing (11) which may be screwed onto the top of a container (2), and which is provided with a plurality of, in this case, four, openings (13) which extend into the body of the housing.
- the inner surface of the housing extends cylindrically from its upper end for between a quarter and a third of its total length and then tapers radially inwards to meet the top of the container.
- the frusto-conical cavity described by the tapering inner surface is provided with a plug (15) which is made from an elastic material and which comprises an upper stem (15 a ) that is attached inside the central cavity of the housing (16) ending flush with the end surface of the housing.
- the plug stem (15 a ) When the container is squeezed the plug stem (15 a ) is compressed such that its shoulders end flush with the lower surface of the central cavity (17). A narrow elongate channel (18) is thereby formed between the tapering plug tip (15 b ) and the tapering inner surface of the housing through which the container contents are extruded.
- the channel (17) has a length of at least 5 mm, preferably 10 mm and the separation between its walls is no more than 0.1 mm when the passage is closed.
- the embodiment illustrated in Figures 5A and 5B comprises a housing (22) formed from a rigid material, the inner surface of which tapers inwards for about a quarter of its total length and then extends cylindrically to meet the top of the container to which it is attached.
- the housing comprises a central part (24) which is separated from the cylindrical inner surface of the housing by an annular channel (23) and into which the stem (21 a ) of a frusto-conical plug (21) is secured.
- the plug is made from an elastic material and, when the container is not in use, it is in contact with the tapering inner surface of the housing.
- the plug is joined to the stem by a neck section (25) and when the container is squeezed, the pressure caused by the contents in the channel (23) causes elongation of the neck which in turn allows the plug (21) to lift and thereby form an extended elongate channel between the housing and the plug through which the container contents are extruded.
- the channel has a length of at least 5 mm, preferably 10 mm and the separation between its walls is no more than 0.1 mm wide when the passage is closed.
- the invention includes heads as hereinbefore described, provided with anti-microbial agents, as hereinbefore described.
- Cream II is useful for indicating bacterial mutiplication by changing its colour at the place of multiplication to yellow due to acidic metabolism products. Preliminary experiments have ascertained, that the addition of phenol red and sodium hydroxide to cream II does not affect the growth and motility of Staphylococcus or Enterobacter as compared to cream I.
- Example 2 Substantially the same experimental design was employed as in Example 1, the difference being that here infection of every head was done only once, followed by an incubation period at 30°C of 7 or 28 days.
- Example 2 Substantially the same experimental design was employed as in Example 1, the difference being that here Staphylococcus aureus (chosen because it is a common pathogen) was used as the test bacterium. For infecting the free ends of the elastic tubes, about 5x105 CFU were used each time.
- Example 2 The same heads and dummy heads as in Example 1 were employed, and also the same microorganism.
- the heads or dummy heads were attached to disposable plastic 5 ml injection syringes, and the combination was filled with the sterile broth.
- Control for bacterial colonisation within the syringe was performed visually (in the liquid broth, multiplication of bacteria is readily evident by the development of turbidity) and by culturing samples.
- Example 4 Substantially the same experimental design was employed as in Example 4, the difference being that here Staphylococcus aureus was used as the test bacterium for infecting (105 CFU/ml) the broth in the vessel. (The bacterial contents of this organism did not drop below 108 CFU/ml for the duration of the experiment).
- the head according to the first described embodiment ( Figure 1) was used for testing against ingrowth of six different micro-organisms.
- the heads were mounted on the open ends of 30 ml collapsible tubes, filled with the sterile nutritive cream I of Example 1.
- Infection of the tested combination was performed by inserting a liquid culture of Enterobacter aerogenes, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Candida albicans or Aspergillus niger into the free end of the head. Subsequently the combinations were incubated at 30°C for 24 hours.
- Table 2 Bacterial colonisation after single infection of the free end of dummy or complete third-model heads with Enterobacter aerogenes. Heads tested Incubation period (days) Bacterial colonisation indicated by Number of heads tested Number of heads with colonisation Dummy 7 phenolred discoloration 3 3 " " culturing samples 3 3 " 28 phenolred discoloration 3 3 " “ culturing samples 3 3 Complete 7 phenolred discoloration 10 0 " " culturing samples 10 0 " 28 phenolred discoloration 10 0 “ “ culturing samples 10 0 Table 3 Bacterial ingrowth after repeatedly infecting the free end of dummy or complete third-model heads with staphylococcus aureus.
- Heads Bacterial colonisation indicated Number of heads tested Number of heads with colonisation Total percentage heads with colinisation First day Second day Third day Sixth day Seventh day Dummy visually 5 5 5 5 5 5 100 " by culturing samples 5 5 100 complete visually 5 0 0 0 0 0 0 " by culturing samples 5 0 0 Table 5 Bacterial colonitation after placing the free end of dummy or complete third model heads in broth infected with Staphylococcus aureus.
- Heads Bacterial colonisation indicated number of heads tested Number of heads with colonisation Total percentage heads with colonisations First day Second day Third day Seventh day Eight day Dummy visually 5 3 5 5 5 5 5 92 " by culturing samples 5 5 100 complete visually 5 0 0 0 0 0 0 " by culturing samples 5 0 0 Table 6 Microbial colonisation after infection of free end of first model heads.
- test - microorganism starting infection (CFU) number of combinations infected Number of microbiologically positives after 24 hours incubation at 30 ⁇ C before the clip behind the clip Enterobacter aerogenes 2.2x107 2 2 0 Escherichia coli 1.3x106 1 1 0 1.0x103 1 1 0 Pseudomonas aeroginosa 1.4x105 1 1 0 2.2x102 1 1 0 Staphylococcus aureus 2.4x105 1 1 0 1.7x103 1 1 0 Candida albicans 4.8x103 1 1 0 2.0x102 1 1 0 Aspergillus niger 1x104 1 1 0 1x103 1 1 0
Landscapes
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP87201488 | 1987-08-05 | ||
| EP87201488 | 1987-08-05 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP0302575A1 true EP0302575A1 (de) | 1989-02-08 |
Family
ID=8197651
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP88201691A Withdrawn EP0302575A1 (de) | 1987-08-05 | 1988-08-04 | Ein zur mehrmaligen Verwendung bestimmter Kopfteil eines Behältnisses |
Country Status (1)
| Country | Link |
|---|---|
| EP (1) | EP0302575A1 (de) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2738555A1 (fr) * | 1995-09-13 | 1997-03-14 | Sofab | Perfectionnements apportes a un dispositif de distribution pour produit ophtalmologique |
| FR2804846A1 (fr) * | 2000-02-16 | 2001-08-17 | Oreal | Dispositif de conditionnement et d'application comportant une structure poreuse incorporant un agent biocide |
| WO2003029085A3 (en) * | 2001-10-03 | 2004-01-22 | Hunter Group Inc | Collapsible dispensing system |
| EP1391392A3 (de) * | 2002-08-20 | 2004-04-07 | Masuda Masatoshi | Ventilmechanismus für Flüssigkeitstube |
| US7077296B2 (en) | 1991-12-06 | 2006-07-18 | Aptargroup, Inc. | Dispensing valve |
| US7186045B2 (en) | 2000-02-16 | 2007-03-06 | L'oreal S.A. | Device and method for applying a cosmetic product |
| EP1842787A3 (de) * | 2006-04-05 | 2008-10-08 | Coltène/Whaledent GmbH + Co. KG | Spender für viskose Medien mit Spenderbehälter, Ausdrückmechanismus und Spenderkanüle |
| WO2011044531A1 (en) * | 2009-10-09 | 2011-04-14 | Py Daniel C | Device with co-molded closure, one-way valve and variable-volume storage chamber, and related method |
| EP1948522A4 (de) * | 2005-11-03 | 2011-10-19 | Reseal Internat Ltd Partnership | Kontinuierlich abdichtende einwegeventilanordnung und fluidabgabesystem sowie formulierungen zur verwendung darin |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR834140A (fr) * | 1937-02-24 | 1938-11-14 | Embout amovible à obturation automatique | |
| DE834524C (de) * | 1950-08-02 | 1952-03-20 | Bela Seregi | Als Lippenventil ausgebildeter Tubenverschluss |
| FR1092480A (fr) * | 1953-10-26 | 1955-04-21 | Bouchon automatique pour récipients genre tubes à produits pâteux | |
| US2753091A (en) * | 1953-01-09 | 1956-07-03 | Albert M Herzig | Closure for collapsible tubes |
-
1988
- 1988-08-04 EP EP88201691A patent/EP0302575A1/de not_active Withdrawn
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR834140A (fr) * | 1937-02-24 | 1938-11-14 | Embout amovible à obturation automatique | |
| DE834524C (de) * | 1950-08-02 | 1952-03-20 | Bela Seregi | Als Lippenventil ausgebildeter Tubenverschluss |
| US2753091A (en) * | 1953-01-09 | 1956-07-03 | Albert M Herzig | Closure for collapsible tubes |
| FR1092480A (fr) * | 1953-10-26 | 1955-04-21 | Bouchon automatique pour récipients genre tubes à produits pâteux |
Cited By (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7077296B2 (en) | 1991-12-06 | 2006-07-18 | Aptargroup, Inc. | Dispensing valve |
| FR2738555A1 (fr) * | 1995-09-13 | 1997-03-14 | Sofab | Perfectionnements apportes a un dispositif de distribution pour produit ophtalmologique |
| WO1997010160A1 (fr) * | 1995-09-13 | 1997-03-20 | Sofab | Dispositif de distribution pour produit ophtalmologique ou similaire |
| FR2804846A1 (fr) * | 2000-02-16 | 2001-08-17 | Oreal | Dispositif de conditionnement et d'application comportant une structure poreuse incorporant un agent biocide |
| EP1136056A1 (de) * | 2000-02-16 | 2001-09-26 | L'oreal | Vorrichtung zum Aufbewahren und Auftragen mit einer porösen Anordnung welcher ein Biozid enthalt |
| JP2001315860A (ja) * | 2000-02-16 | 2001-11-13 | L'oreal Sa | 滅菌剤を取り入れた多孔構造体を具備する収容且つ供給装置 |
| US7186045B2 (en) | 2000-02-16 | 2007-03-06 | L'oreal S.A. | Device and method for applying a cosmetic product |
| WO2003029085A3 (en) * | 2001-10-03 | 2004-01-22 | Hunter Group Inc | Collapsible dispensing system |
| GB2396684A (en) * | 2001-10-03 | 2004-06-30 | Hunter Group Inc | Collapsible dispensing system |
| GB2396684B (en) * | 2001-10-03 | 2006-02-22 | Hunter Group Inc | One-way valve assembly for dispensing flowable materials |
| US7140517B2 (en) | 2002-08-20 | 2006-11-28 | Masatoshi Masuda | Valve mechanism for tube shaped fluid container |
| EP1391392A3 (de) * | 2002-08-20 | 2004-04-07 | Masuda Masatoshi | Ventilmechanismus für Flüssigkeitstube |
| CN100391805C (zh) * | 2002-08-20 | 2008-06-04 | 增田胜利 | 用于管式流体容器的阀机构 |
| EP1948522A4 (de) * | 2005-11-03 | 2011-10-19 | Reseal Internat Ltd Partnership | Kontinuierlich abdichtende einwegeventilanordnung und fluidabgabesystem sowie formulierungen zur verwendung darin |
| EP1842787A3 (de) * | 2006-04-05 | 2008-10-08 | Coltène/Whaledent GmbH + Co. KG | Spender für viskose Medien mit Spenderbehälter, Ausdrückmechanismus und Spenderkanüle |
| WO2011044531A1 (en) * | 2009-10-09 | 2011-04-14 | Py Daniel C | Device with co-molded closure, one-way valve and variable-volume storage chamber, and related method |
| CN102666300A (zh) * | 2009-10-09 | 2012-09-12 | 丹尼尔·皮 | 具有共模制的封闭件、单向阀和可变容积储存室的设备以及相关方法 |
| CN102666300B (zh) * | 2009-10-09 | 2014-12-17 | 丹尼尔·皮 | 单向阀、包括该单向阀的设备及制造该设备的方法 |
| US8998034B2 (en) | 2009-10-09 | 2015-04-07 | Dr. Py Institute Llc | Device with co-molded closure, one-way valve and variable-volume storage chamber, and related method |
| US10131474B2 (en) | 2009-10-09 | 2018-11-20 | Dr. Py Institute Llc | Apparatus and method for sealing with a liquid sealant |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0302575A1 (de) | Ein zur mehrmaligen Verwendung bestimmter Kopfteil eines Behältnisses | |
| DE69815834T2 (de) | Mit einer die eintretende luft filternden vorrichtung versehene abgabeflasche für eine flüssigkeit, eine creme oder ein gel | |
| ES2859788T3 (es) | Mezcla antimicrobiana de aldehídos, ácidos orgánicos y ésteres de ácidos orgánicos | |
| DE69213248T2 (de) | Abgabevorrichtung für konservierungsmittelfreie sterile flüssigkeit | |
| EP0631770B1 (de) | Neue Verwendung von polymeren Membranen zum Ausgeben von pharmazeutischen Lösungen, die quarternäre, als Konserviermittel dienende Ammoniumverbindungen enthalten und entsprechender Dosierbehälter | |
| EP2338332A2 (de) | Hohles Glukan- oder Zellenwand-Partikel, das eine Terpenkomponente verkapselt | |
| HK1048462A1 (en) | A device for dispensing cells of a probiotic micro organism into liquid and method of making the same | |
| US20100147899A1 (en) | Multidose dispenser for sterile liquid preparations | |
| US11203480B2 (en) | Instant mixing container and product | |
| JP2000509319A (ja) | 液体製品噴霧用抗菌装置 | |
| RU2002120999A (ru) | Предварительно наполненная пипетка одноразового применения | |
| EP2048949B1 (de) | Konservierungsmittel auf basis von carbonsäureanhydriden | |
| US20150031762A1 (en) | Antimicrobial Formulations with Pelargonic Acid | |
| CN102209672A (zh) | 用于保存和释放包含在具有可破裂壁的存储器中的产品的设备 | |
| AU2004207603B2 (en) | One-way valve device | |
| KR20170052323A (ko) | 모린을 포함하는 항생제 내성 황색포도상구균 억제용 항균 조성물 | |
| KR19980032938A (ko) | 매체용 배출장치 | |
| Jabbar et al. | The Effectiveness of Begonia Multangula Blume Leaf Ethanol Extract as Polymicrobial Antibiofilm on Catheters | |
| EP2138171A1 (de) | Veterinärmedizinische Zusammensetzung enthaltend Terpinen-4-ol zur Behandlung und Vorbeugung von Mastitis | |
| EP2394931B9 (de) | Konservierungsmittelfreie Behälter und Verschluss für sterilisierte Produkte oder Produkte mit niedriger Bakterienzahl | |
| Yang et al. | Antimicrobial effects of various red ginger (Zingiber officinale) extract concentrations on Escherichia coli bacteria | |
| Aiemsaard et al. | The effect of some essential oils against subclinical mastitis bacteria isolated from dairy goats. CMUJ | |
| KR100615892B1 (ko) | 쑥추출물을 함유하는 천연 방부제 | |
| EP4081176B1 (de) | Stabiles laktaseprodukt | |
| Heny et al. | In vitro ihbition of Pseudomonas fluorescens bacteria by using curry tree (Murraya koenigii) crude extract |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE ES FR GB GR IT LI LU NL SE |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 19891011 |