DK152256B - PROCEDURE FOR THE PREPARATION OF A STABLE BLOOD REMOVAL - Google Patents
PROCEDURE FOR THE PREPARATION OF A STABLE BLOOD REMOVAL Download PDFInfo
- Publication number
- DK152256B DK152256B DK307276AA DK307276A DK152256B DK 152256 B DK152256 B DK 152256B DK 307276A A DK307276A A DK 307276AA DK 307276 A DK307276 A DK 307276A DK 152256 B DK152256 B DK 152256B
- Authority
- DK
- Denmark
- Prior art keywords
- emulsion
- plasma
- blood
- tests
- particle size
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0026—Blood substitute; Oxygen transporting formulations; Plasma extender
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/08—Plasma substitutes; Perfusion solutions; Dialytics or haemodialytics; Drugs for electrolytic or acid-base disorders, e.g. hypovolemic shock
Landscapes
- Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Hematology (AREA)
- Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Dermatology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Diabetes (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Description
Opfindelsen angår en fremgangsmåde til fremstilling af en stabil bloderstatning, ved hvilken man homogent blander fysiologisk acceptabelt vand, oxygen-overførende perfluorcarbonforbin-delser og emulgatorer til opnåelse af en grov emulsion, hvorefter denne emulgeres ved, at den injiceres ved en temperatur på op til 55 °C igennem en spalte under et tryk på fra omkring 10 til omkring 50 MPa og derved underkastes forskydningskræfter og blandingsvirkning baseret på en stærk hastighedsgradient, indtil perfluorcarbonforbindelsernes partikelstørrelse i den resulterende emulsion når 0,05 - 0,3 ^um.The invention relates to a process for producing a stable blood substitute in which homogeneously mixed physiologically acceptable water, oxygen-transmitting perfluorocarbon compounds and emulsifiers are obtained to obtain a coarse emulsion, after which it is emulsified by injecting it at a temperature of up to 55 ° C through a gap under a pressure of from about 10 to about 50 MPa, thereby undergoing shear forces and mixing action based on a strong velocity gradient until the particle size of the perfluorocarbon compounds in the resulting emulsion reaches 0.05-0.3 µm.
Det er allerede blevet rapporteret af et antal forskere, at fluor-carbonforbindelse-emulsioner eventuelt kan anvendes som kunstig bloderstatning til pattedyr og som perfusionsvæske til konservering af indre organer, som skal transplanteres, især som en infusionsvæskeerstatning, der er i stand til at transportere oxygen [Leland C. Clark, Jr., F. Becattini, and S. Kaplan: The physiology of syn-thetic biood, Journal of Thoracic Cardiovascular Surgery, 60, 757-773 (1970); R. P. Geyer: Fluorocarbon-polyol artificial biood substitutes, New England Journal of Medicine, 289» 1077-1082 (1973)J ·It has already been reported by a number of researchers that fluorocarbon compound emulsions may optionally be used as artificial blood substitutes for mammals and as perfusion fluid for preservation of internal organs to be transplanted, especially as an infusion fluid replacement capable of carrying oxygen [Leland C. Clark, Jr., F. Becattini, and S. Kaplan: The physiology of syn-thetic biodiversity, Journal of Thoracic Cardiovascular Surgery, 60, 757-773 (1970); R. P. Geyer: Fluorocarbon-polyol artificial biodegraders, New England Journal of Medicine, 289 »1077-1082 (1973) J ·
Disse emulsioner, kan imidlertid ikke antages at være tilfredsstillende nok til praktisk brug med hensyn til deres farmaceutiske stabilitet og sikkerhed for den levende organisme. For at fluor-carbonforbindelse-emulsioner skal være kvalificeret til praktisk brug som kimstig bloderstatning, er det nødvendigt at udvikle et præparat, som er tilstrækkeligt stabilt til at kunne opbevares i lang tid uden ændring i partikelstørrelse.However, these emulsions may not be considered sufficient for practical use in terms of their pharmaceutical stability and safety for the living organism. In order for fluorocarbon compound emulsions to be qualified for practical use as a germ-free blood substitute, it is necessary to develop a composition which is sufficiently stable to be stored for a long time without change in particle size.
I fluorcarbonforbindelse-emulsioner spiller partikelstørrelsen en vigtig rolle for emulsionens toxic.it et og effektivitet [k. Yokoyama, K. Yamanouchi, M. Watanabe, R. Murashima, T. Matsumoto, T. Hamano, H. Ikamoto, T. Suyama, R. Watanabe, and R. Naito: Preparation of perfluorodecalin emulsion, an approach to the red cells substitute, Federation Proceedings, 34, 1478-1483 (May, 1975)]· En emulsion med større partikelstørrelse er mere toxisk og har kortere opholdstid af partiklerne i blodstrømmen. Når fluorcarbonforbindelse-emulsionen er beregnet til brug som kunstig bloderstatning til opretholdelse af livet hos en patient, der lider af massiv blødning, må dens gennemsnitlige partikelstørrelse derfor være 0,3 ^um eller mindre, fortrinsvis 0,2 ^,um eller derunder Cjapansk patentskrift nr. 849 412].In fluorocarbon compound emulsions, particle size plays an important role in the emulsion's toxicity and efficiency [k. Yokoyama, K. Yamanouchi, M. Watanabe, R. Murashima, T. Matsumoto, T. Hamano, H. Ikamoto, T. Suyama, R. Watanabe, and R. Naito: Preparation of perfluorodecalin emulsion, an approach to the red cells substitute, Federation Proceedings, 34, 1478-1483 (May, 1975)] · A larger particle size emulsion is more toxic and has shorter residence time of the particles in the blood stream. Therefore, when the fluorocarbon compound emulsion is intended for use as artificial blood replacement to maintain the life of a patient suffering from massive bleeding, its average particle size must be 0.3 µm or less, preferably 0.2 µm or less, No. 849 412].
Foruden partikelstørrelsen er det nødvendigt, for at fluorcarbon-forbindelse-emulsionen er anvendelig som kunstig bloderstatning, at den intravenøst indgivne fluorcarbonforbindelse efter at være elimineret fra blodstrømmen udskilles fra kroppen så hurtigt som muligt. Yed tidligere undersøgelser af udskillelseshastigheden og toxiciteten af flere slags fluorcarbonforbindelse-emulsioner er det fundet, at perfluorcarbonforbindelser med 9-11 carbonato-mer er anvendelige som materiale i kunstig bloderstatning, idet den bedste forbindelse er perfluordecalin £k.Yokoyama, K. Yamanouchi, and R. Murashima: Excretion of perfluorochemicals after intravenous injection of their emulsion, Chemical Pharmaceutical Bulletin, 23, 1368-1373 (Juni 1975)].In addition to the particle size, it is necessary for the fluorocarbon compound emulsion to be useful as an artificial blood substitute that the intravenously administered fluorocarbon compound, after being eliminated from the blood stream, is excreted from the body as soon as possible. Previous studies on the rate of excretion and toxicity of several types of fluorocarbon compound emulsions have found that perfluorocarbon compounds of 9-11 carbon atoms are useful as artificial blood substitute materials, the best compound being perfluorodecalin. K. Yokoyama, K. Yamanouchi, and R. Murashima: Excretion of perfluorochemicals after intravenous injection of their emulsion, Chemical Pharmaceutical Bulletin, 23, 1368-1373 (June 1975)].
Desuden er det fundet, at der kan fremstilles fine og stabile fluor-carbonforbindelse-emulsioner ud fra disse udvalgte fluorcarbonfor-bindelser med 9-11 carbonatomer ved emulgering af fluorcarbonfor-bindelserne med en blanding af æggeblomme-phospholipider eller soyabønne-phospholipider og en lille mængde fedtsyrer med 8-22 carbonatomer eller salte eller monoglycerider deraf {^japansk patentskrift nr. 954 944 og det tilsvarende DE offentliggørelsesskrift nr. 24 04 564] .Furthermore, it has been found that fine and stable fluorocarbon compound emulsions can be prepared from these selected fluorocarbon compounds of 9-11 carbon atoms by emulsifying the fluorocarbon compounds with a mixture of egg yolk phospholipids or soybean phospholipids and a small amount. fatty acids having 8-22 carbon atoms or salts or monoglycerides thereof {Japanese Patent No. 954,944 and corresponding DE Publication No. 24 04 564].
Sammenlignet med en perfluortributylamin-emulsion stabiliseret med en højmolekylær polyoxyethylen-polyoxypropylen-copolymer (R.P.Compared to a perfluorotributylamine emulsion stabilized with a high molecular weight polyoxyethylene-polyoxypropylene copolymer (R.P.
Geyer, loc.cit.), er den ovennævnte emulsion stabiliseret med både phospholipider og fedtsyrer bedre med hensyn til udskillelseshastighed, men dårligere med hensyn til stabilitet i den cirkulerende blodstrøm efter intravenøs injektion, idet halveringstiden er omkring 2/3 af den førstnævntes.Geyer, loc.cit.), The aforementioned emulsion is stabilized with both phospholipids and fatty acids better in terms of excretion rate, but inferior in stability of circulating blood flow after intravenous injection, with the half-life being about 2/3 of the former.
Endvidere kan en fluorcarbonforbindelse-emulsion, fremstillet med et højmolekylært ikke-ionisk overfladeaktivt middel, såsom perfluortributylamin-emulsion, anvendes som en blanding i ethvert forhold med de kommercielle plasmaforstrækningsmidler, såsom dextran- eller hydroxyethylstivelse- eller modificeret gelatineopløsning, medens den i JP 954 944 og DE 24 04 564 beskrevne perfluordecalin-emulsion ikke kan anvendes i kombination med de nævnte plasmaforstrækningsmidler på grund af dannelse af bundfald, når den blandes med de sidstnævnte. Det ser ud til, at udfældningen skyldes nedbrydningen af emulgerede partikler forårsaget af gensidig indvirkning mellem phospholipiderne, der indeholdes i høj koncentration i emulsionen, og plasmaforstrækningsmidlet, såsom dextran eller hydroxyethyl-stivelse, der er et højmolekylært kolloidt stof.Further, a fluorocarbon compound emulsion prepared with a high molecular weight nonionic surfactant such as perfluorotributylamine emulsion can be used as a mixture in any ratio with the commercial plasma pre-emitting agents such as dextran or hydroxyethyl starch or modified gelatin solution and the perfluorodecaline emulsion described in DE 24 04 564 cannot be used in combination with the aforementioned plasma enhancers due to precipitation formation when mixed with the latter. It appears that the precipitation is due to the degradation of emulsified particles caused by mutual interaction between the high concentration phospholipids contained in the emulsion and the plasma propagating agent such as dextran or hydroxyethyl starch which is a high molecular weight colloidal substance.
Når en fluorcarbonforbindelse-emulsion skal anvendes som infusionvæske eller som kunstig bloderstatning til at redde en patients liv i tilfælde af massiv blødning, bliver kombinationen med et plasmaforstrækningsmiddel vigtig for at frembringe isotonicitet, d.v.s. at udligne de onkotiske tryk af de to kolloidale opløsnin ger, nemlig emulsionen og blodet. Fluorcarbonforbindelse-emulsio-nen leverer oxygen, medens plasmaforstrækningsmidlet gør det muligt at holde det cirkulerende blodvolumen på et passende niveau. Derfor foretrækkes det at anvende et højmolekylært ikke-ionisk overfladeaktivt middel, som er inaktivt over for plasmaforstræknings-midlet, til fremstilling af en fluorcarbonforbindelse-emulsion til anvendelse som kunstigt blod. Selv om disse højmolekylære ikke-iohiske overfladeaktive midler er effektive som emulgeringsmiddel for nogle fluorcarbonforbindelser, såsom perfluortributylamin og andre fluorcarbonforbindelser af amintype, er de ikke egnede for fluorcarbonforbindelser med 9-11 carbonatomer, såsom perfluordeca-lin, som har en.høj udskillelseshastighed.When a fluorocarbon compound emulsion is to be used as an infusion liquid or as an artificial blood substitute to save a patient's life in case of massive bleeding, the combination with a plasma diluent becomes important to produce isotonicity, i.e. to equalize the oncotic pressures of the two colloidal solutions, namely the emulsion and the blood. The fluorocarbon compound emulsion delivers oxygen, while the plasma expanding agent allows the circulating blood volume to be maintained at an appropriate level. Therefore, it is preferred to use a high molecular weight non-ionic surfactant which is inactive against the plasma pre-emitting agent to prepare a fluorocarbon compound emulsion for use as artificial blood. Although these high molecular weight non-ionic surfactants are effective as emulsifiers for some fluorocarbon compounds such as perfluorotributylamine and other amine type fluorocarbons, they are not suitable for fluorocarbon compounds having 9-11 carbon atoms such as perfluorodecline having a high excretion rate.
Under disse omstændigheder har opfinderne gennemført omfattende undersøgelser for'at finde en fremgangsmåde til emulgering af perfluordecalin uden at anvende et phospholipid som hovedemulgeringsmiddel og har som resultat fundet, at når perfluordecalin emulgeres som en blanding med perfluortripropylamin, kan den ønskede emulsion fremstilles ved anvendelse af et ikke-ionisk overfladeaktivt middel som hovedemulgeringsmiddel. Endvidere blev det ved anvendelse af et ikke-ionisk overfladeaktivt middel muligt at tilsætte et plasmaforstrækningsmiddel, som gør emulsionens onkotiske tryk isotonisk. Som resultat heraf nedsættes den hæmo-lytiske virkning, som ellers frembringes af en emulsion, således at dyrets overlevelse bliver mulig, når emulsionen anvendes som kunstig bloderstatning. Det er således udvælgelsen af bestemte perfluorcarbonforbindelser og ikke-ionisk overfladeaktivt middel i forbindelse med et plasmaforstrækningsmiddel, som har gjort . det muligt at fremstille en udmærket fluorcarbonemulsion, der er stabil i den cirkulerende blodstrøm og har lav toxicitet i den levende krop og ønskede egenskaber, så den for første gang i verden har kunnet anvendes som kunstig bloderstatning inden for det kliniske område.In these circumstances, the inventors have conducted extensive studies to find a process for emulsifying perfluorodecaline without using a phospholipid as the main emulsifier and as a result have found that when perfluorodecaline is emulsified as a mixture with perfluorotripropylamine, the desired emulsion can be prepared using a nonionic surfactant as the main emulsifier. Furthermore, using a nonionic surfactant, it was possible to add a plasma pre-emitting agent which renders the emulsion's oncotic pressure isotonic. As a result, the hemolytic effect otherwise produced by an emulsion is reduced so that the animal's survival becomes possible when the emulsion is used as artificial blood substitute. Thus, it has been the selection of particular perfluorocarbon compounds and nonionic surfactant in association with a plasma pre-emitting agent. it is possible to produce an excellent fluorocarbon emulsion that is stable in the circulating bloodstream and has a low toxicity in the living body and desirable properties so that it has been used for the first time in the world as an artificial blood substitute in the clinical field.
Det ejendommelige ved fremgangsmåden ifølge opfindelsen ligger således i udvælgelsen af de optimale komponenter og anvendelsen af disse i det optimale forhold, hvorved der opnås et overraskende fordelagtigt resultat.Thus, the peculiarity of the method according to the invention lies in the selection of the optimum components and their use in the optimum ratio, thereby obtaining a surprisingly advantageous result.
I overensstemmelse hermed er fremgangsmåden ifølge opfindelsen ejendommelig ved det i kravets kendetegnende del anførte.Accordingly, the process of the invention is peculiar to the characterizing part of the claim.
Symbolet "% (vægt/vol.)n betyder mængdeforholdet af et materiale beregnet som vægt (gram) per 100 ml af den resulterende emulsion.The symbol "% (w / v) n means the amount ratio of a material calculated as weight (grams) per 100 ml of the resulting emulsion.
I resten af beskrivelsen betegnes dette mængdeforhold blot somFor the rest of the description, this ratio is simply referred to as
De polyoxyethylen-polyoxypropylen-copolymere, der anvendes som emulgeringsmiddel, har en molekylvægt på 2000-20000. Koncentrationen af dette højmolekylære ikke-ioniske overfladeaktive middel i emulsionen er fra omkring 2,0 til omkring 5,0 %, fortrinsvis 3,0 -3,5 %.The polyoxyethylene-polyoxypropylene copolymers used as emulsifiers have a molecular weight of 2000-20000. The concentration of this high molecular weight nonionic surfactant in the emulsion is from about 2.0 to about 5.0%, preferably 3.0-3.5%.
De phospholipider, der anvendes som emulgeringsmiddel i emulsionen, er sådanne der almindeligt anvendes inden for teknikken, og de, der omfatter æggeblomme-phospholipid eller soyabønne-phos-pholipid, foretrækkes. Den tilstedeværende mængde i emulsionen kan varierer fra omkring 0,1 til 1,0 %, og fortrinsvis omkring 0,4 til omkring 0,6 %.The phospholipids used as the emulsifier in the emulsion are those commonly used in the art and those comprising egg yolk phospholipid or soybean phospholipid are preferred. The amount present in the emulsion may range from about 0.1 to 1.0%, and preferably about 0.4 to about 0.6%.
Den fedtsyreforbindelse, der anvendes som emulgeringsmiddel, er en fedtsyre med 8-22 carbonatomer, et fysiologisk acceptabelt salt deraf, såsom natrium- eller kaliumsaltet, eller et monogly-cerid deraf. Sådanne fedtsyreforbindelser inkluderer f.eks. capryl-syre, caprinsyre, laurinsyre, myristinsyre, palmitinsyre, stearinsyre, behensyre, palmitolsyre, oliesyre, linolsyre og arachidon-syre samt natrium- og kaliumsaltet deraf og monoglycerider deraf. Disse fedtsyreforbindelser kan anvendes alene eller som en blanding af to eller flere slags deraf i så lille en mængde som 0,004-0,1 %, og fortrinsvis fra omkring 0,02 til omkring omkring 0,04 %. Blandt disse fedtsyreforbindelser foretrækkes sådanne med 14-20 carbonatomer og deres fysiologisk acceptable salte, og de mest foretrukne er kaliumpalmitat og kaliumoleat i betragtning af deres gode opløselighed og den lette fremstilling af emulsionen dermed.The fatty acid compound used as an emulsifier is a fatty acid having 8-22 carbon atoms, a physiologically acceptable salt thereof, such as the sodium or potassium salt, or a monoglyceride thereof. Such fatty acid compounds include, e.g. caprylic acid, capric acid, lauric acid, myristic acid, palmitic acid, stearic acid, behenic acid, palmitic acid, oleic acid, linoleic acid and arachidonic acid, and the sodium and potassium salts thereof and monoglycerides thereof. These fatty acid compounds can be used alone or as a mixture of two or more kinds thereof in an amount as low as 0.004-0.1%, and preferably from about 0.02 to about 0.04%. Of these fatty acid compounds, those with 14-20 carbon atoms and their physiologically acceptable salts are preferred, and the most preferred are potassium palmitate and potassium oleate, given their good solubility and ease of preparation of the emulsion.
Fremgangsmåden ifølge opfindelsen gennemføres ved homogent at blande foreskrevne mængder af de førnævnte komponenter i en hvilken som helst rækkefølge i et fysiologisk acceptabelt vandigt medium, såsom destilleret vand, eller en isotonisk opløsning, til opnåelse af en rå emulsion og derpå emulgere den rå emulsion ved at injicere den ved en temperatur på op til 55 °C igennem en spalte under et tryk på fra omkring 10 til omkring 50 MPa, hvorved den underkastes forskydningskræfter og blandingsvirkning baseret på en stærk hastighedsgradient, indtil den førnævnte ønskede partikelstørrelse er opnået.The process of the invention is carried out by homogeneously mixing prescribed amounts of the aforementioned components in any order in a physiologically acceptable aqueous medium, such as distilled water, or an isotonic solution, to obtain a crude emulsion and then emulsifying the crude emulsion by inject it at a temperature of up to 55 ° C through a gap under a pressure of from about 10 to about 50 MPa, subjecting it to shear forces and mixing action based on a strong velocity gradient until the aforementioned desired particle size is achieved.
Den homogene sammenblanding af materialerne udføres ved anvendelse af en konventionel blander, såsom en homoblander eller propel-omrører.The homogeneous mixing of the materials is carried out using a conventional mixer such as a homo mixer or propeller stirrer.
Emulgeringen af den rå emulsion opnås ved hjælp af en højtryksho-mogenisator, som er en højtrykspumpe, der homogeniserer en blanding af to ublandbare væsker ved at injicere den igennem en spalte under et højt tryk med en meget høj hastighed til frembringelse af en forskydning og sammenblanding af væskerne. Den typiske homogenisator på markedet er af Manton-Gaulin-typen (handelsnavn for en homogenisator forhandlet af Manton-Gaulin Manufacturing Co., Inc., U.S.A.), som har en flertrinsventil i kombination af to eller flere ventiler, der hver indeholder en fjeder, hvoraf spalterne dannes.The emulsion of the crude emulsion is obtained by a high pressure homogenizer, which is a high pressure pump which homogenizes a mixture of two immiscible liquids by injecting it through a high pressure slit at a very high speed to produce a shear and mixing. of the fluids. The typical homogenizer on the market is of the Manton-Gaulin type (trade name for a homogenizer sold by Manton-Gaulin Manufacturing Co., Inc., USA), which has a multi-stage valve in combination of two or more valves, each containing a spring, from which the slits are formed.
Blandingen cirkuleres i denne type homogenisator flere gange under et totaltryk på omkring 50 MPa, hvorved der opnås en stabil emulsion. Arbejdstemperaturen holdes i området op til 55 °C, og fortrinsvis ved 25-40 °C.The mixture is circulated in this type of homogenizer several times under a total pressure of about 50 MPa, thus obtaining a stable emulsion. The operating temperature is maintained in the range up to 55 ° C, and preferably at 25-40 ° C.
Den ved fremgangsmåden ifølge opfindelsen fremstillede emulsion har en dispers fase af ultrafine partikler, hvis diameter er mindre end 0,2 ^um eller i hvert fald mindre end 0,3 yum. Endvidere er den stabil, idet den ikke viser nogen vækst i partikelstørrelse, selv når den opvarmes eller opbevares i lang tid. Derfor sikrer emulsionen i høj grad det dyr, hvortil den indgives imod skadelig virkning på grund af agglomerering af emulsionspartiklerne.The emulsion prepared by the process of the invention has a dispersion phase of ultrafine particles whose diameter is less than 0.2 µm or at least less than 0.3 µm. Furthermore, it is stable in that it shows no particle size growth even when heated or stored for a long time. Therefore, the emulsion greatly protects the animal to which it is administered against deleterious effect due to agglomeration of the emulsion particles.
Emulsionen har endvidere en lang tilbageholdelsestid i den cirkulerende blodstrøm, således at den oxygenbærende evne opretholdes i lang tid.Furthermore, the emulsion has a long retention time in the circulating blood stream, so that the oxygen carrying capacity is maintained for a long time.
F.eks. forbliver den ifølge opfindelsen fremstillede emulsion meget længere i dyrets blodstrøm end en fluorcarbonforbindelse-emulsion fremstillet ved anvendelse af phospholipider som emulgeringsmiddel ifølge JP 954 944 og DE 24 04 564. Udskillelsen af den ifølge opfindelsen fremstillede emulsion fra kroppen er meget hurtigere end af en perfluortributylamin-emulsion.Eg. For example, the emulsion prepared according to the invention remains much longer in the animal's blood stream than a fluorocarbon compound emulsion prepared using phospholipids as emulsifier according to JP 954 944 and DE 24 04 564. emulsion.
Den foreliggende emulsion kan anvendes som infusionsvæske, da den er gjort fysiologisk isotonisk med kommercielle plasmaforstrækningsmidler, såsom dextran og hydroxyethylstivelse. Endvidere kan den anvendes som bloderstatning for pattedyr og som per-fusat til konservering af indre organer.The present emulsion can be used as an infusion liquid as it is made physiologically isotonic with commercial plasma extenders such as dextran and hydroxyethyl starch. Furthermore, it can be used as a blood substitute for mammals and as a perfusate for preserving internal organs.
Opfindelsen belyses nærmere ved de følgende eksempler, i hvilke partikelstørrelsen blev målt ved centrifugalsedimenteringsmetoden, som er foreslået af K. Yokoyama, A. Suzuki, I. Utsumi and R. Naito. Chem. Pharm. Buil. 22 (12), 2966-2971 (1974).The invention is further illustrated by the following examples in which the particle size was measured by the centrifugal sedimentation method proposed by K. Yokoyama, A. Suzuki, I. Utsumi and R. Naito. Chem. Pharm. Buil. 22 (12), 2966-2971 (1974).
EKSEMPEL 1 181 destilleret vand opløstes 300 g af en polyoxyethylen-polyoxy-propylen-copolymer (molekylvægt 10800). Til opløsningen sattes 40 g soyabørme-phospholipider, 2 g kaliumo.leat og en blanding bestående af 3 kg perfluordecalin og 300 g perfluortripropylamin.Example 1 181 distilled water was dissolved 300 g of a polyoxyethylene-polyoxy-propylene copolymer (molecular weight 10800). To the solution was added 40 g of soybean phospholipids, 2 g of potassium oleate and a mixture of 3 kg of perfluorodecaline and 300 g of perfluorotripropylamine.
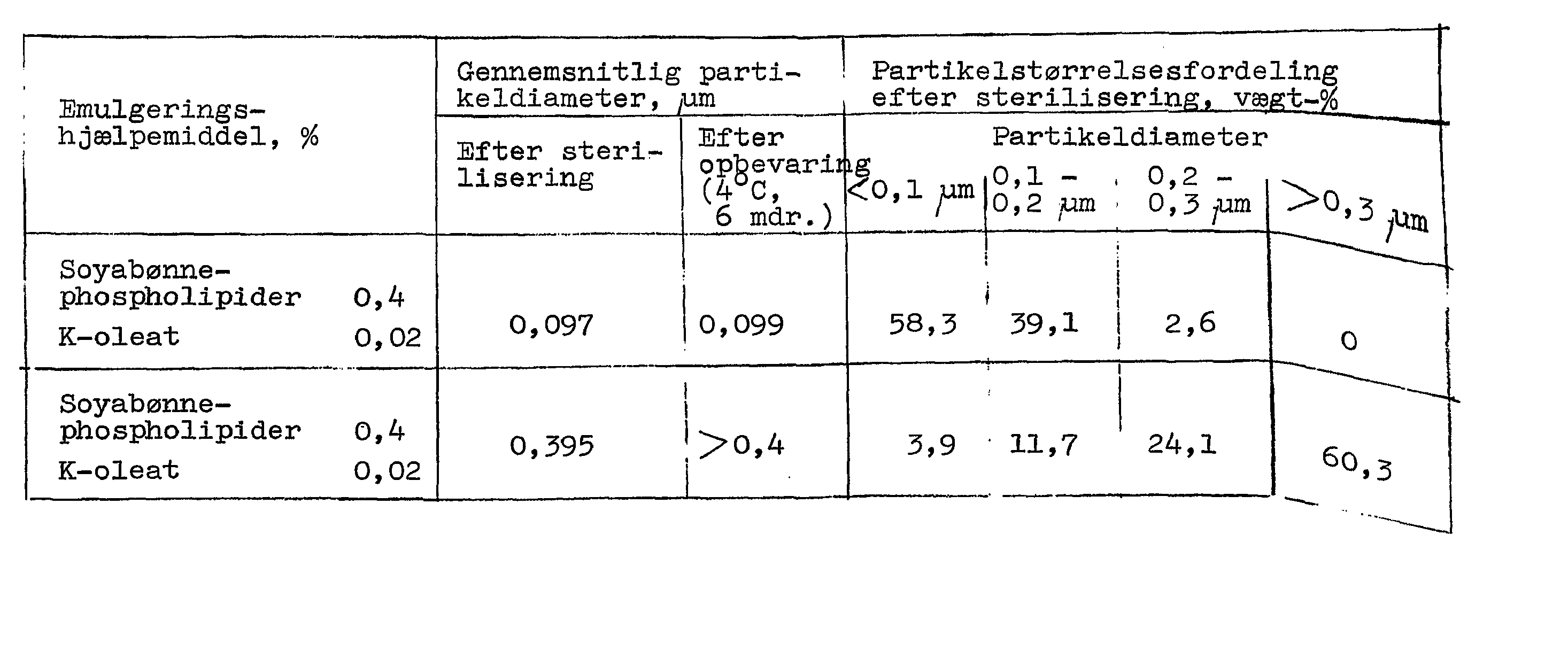
Den resulterende blanding blev omrørt i en blander til dannelse af en grov emulsion. Den resulterende grove emulsion blev fyldt i væsketanken på en jetemulgator (fremstillet af Manton-Gaulin Co.) og emulgeret ved passage 12 gange gange igennem en ventil ved et højt tryk på 20-49 MPa, medens væsketemperaturen holdtes ved 35-5 °C, til frembringelse af emulgering. Den resulterende emulsion indeholdt 30,5 % perfluordecalin og 2,9 % perfluortripropylamin. Den gennemsnitlige partikeldiameter var 0,09 - 0,1 ^um, målt ved centrifugalsedimenteringsmetoden. Emulsionen viste i det væsentlige ingen vækst i partikelstørrelse, når den blev indesluttet i en ampul til injektion og underkastet termisk sterilisering ved 115 °C i 12 minutter i den specielt udformede rotationssterili-sator. I tabel 1 er anført partikelstørrelsesfordelingen af denne emulsion og af en emulsion af perfluordecalin alene, fremstillet uden anvendelse af perfluortripropylamin.The resulting mixture was stirred in a mixer to form a coarse emulsion. The resulting coarse emulsion was charged into the liquid tank of a jet emulsifier (manufactured by Manton-Gaulin Co.) and emulsified by passage 12 times through a valve at a high pressure of 20-49 MPa while maintaining the liquid temperature at 35-5 ° C. to produce emulsification. The resulting emulsion contained 30.5% perfluorodecaline and 2.9% perfluorotripropylamine. The average particle diameter was 0.09 - 0.1 µm, as measured by the centrifugal settling method. The emulsion showed essentially no particle size growth when enclosed in a vial for injection and subjected to thermal sterilization at 115 ° C for 12 minutes in the specially designed rotary sterilizer. Table 1 lists the particle size distribution of this emulsion and of an emulsion of perfluorodecaline alone, prepared without the use of perfluorotripropylamine.
Som det ses af tabel 1, viste den foreliggende emulsion, når den blev opbevaret ved 4 °C i 6 måneder ingen agglomerering, idet mid-delpartikeldiameteren var i det væsentlige uændret.As can be seen from Table 1, the present emulsion when stored at 4 ° C for 6 months showed no agglomeration, the mean particle diameter being essentially unchanged.
Tabel 1. Partikelstørrelsesfordeling af forskellige emulsionerTable 1. Particle size distribution of different emulsions
(Tabellen fortsætter herfra på næste side.(The table continues from here on the next page.
* = polyoxyethylen-polyoxypropylen-copolymer, gennemsnitsmolekylvægt 10 800.* = polyoxyethylene-polyoxypropylene copolymer, average molecular weight 10,800.
Tabel 1 (fortsat)Table 1 (continued)
(fortsat fra side 9 )(continued from page 9)
Til fremstilling af en bloderstatning ved fremgangsmåden ifølge opfindelsen tilsættes emulsionen et plasmaforstrækningsmiddel til at udfylde manglen på onkotisk tryk. Når denne emulsion blev blandet med et plasmaforstrækningsmiddel, blev den reversible udfældning, som kan forårsages af gensidig indvirkning mellem kolloidale opløsninger, ikke iagttaget, hvilket viser, at en af de vanskeligheder, som kunne forventes ved anvendelse af den ifølge opfindelsen fremstillede bloderstatning som infusionsvæske, er elimineret.To prepare a blood substitute by the method of the invention, the emulsion is added to a plasma preening agent to fill the lack of oncotic pressure. When this emulsion was mixed with a plasma pre-emulsifier, the reversible precipitation, which may be caused by mutual interaction between colloidal solutions, was not observed, demonstrating that one of the difficulties to be expected using the blood substitute prepared as an infusion liquid, is eliminated.
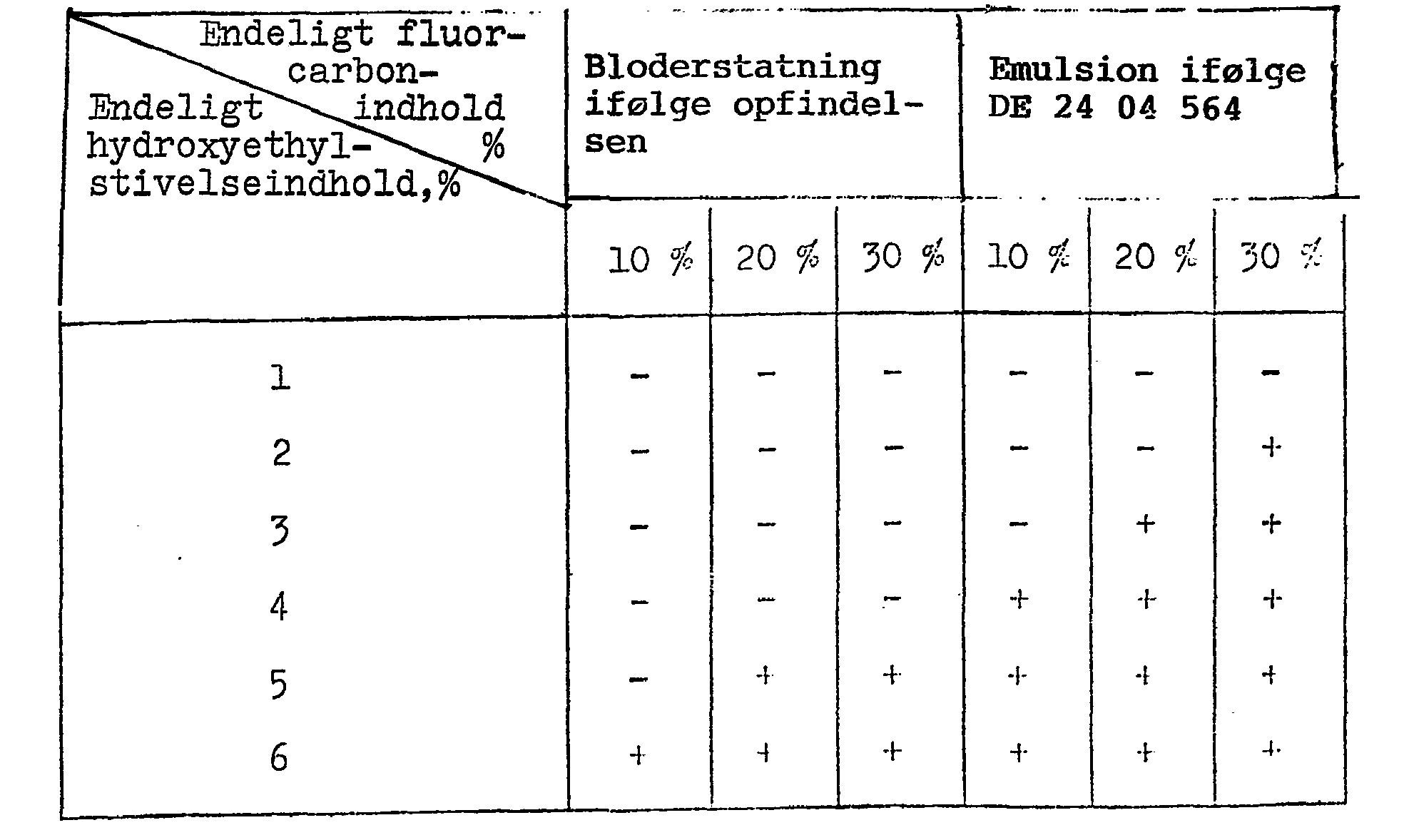
Perfluordecalin/perfluortripropylamin-(10:1)-emulsioner af forskellige koncentrationer fremstillet på samme måde som ovenfor og som reference perfluordecalinemulsioner af forskellige koncentrationer fremstillet ifølge DE offentliggørelsesskrift nr. 24 04 564 [æggeblomme-phospholipid 4%; kaliumoleat 0,02%1 blev hver for sig gjort isotoniske med lactatholdig Ringer's opløsning eller Krebs-Ringer-hydrogencarbonat-opløsning, og derpå blandet med en plasmaerstatning, således at den endelige koncentration af plasmaforstrækningsmidlet blev 1-6%, hvorpå dannelsen af bundfald blev iagttaget visuelt i løbet af 6 timer efter blanding ved stuetemperatur. De anvendte plasmaerstatninger er hydroxyethylstivelse (gennemsnitlig molekylvægt 200 000, 20% i fysiologisk saltopløsning; leveret af Ajinomoto Co., Ltd.) og "Dextran 40" Cgennemsnitlig molekylvægt 40 000, 10% i fysiologisk saltopløsning; leveret af The Green Cross Corp.J.Perfluorodecaline / perfluorotripropylamine (10: 1) emulsions of various concentrations prepared in the same manner as above and by reference perfluorodecalin emulsions of different concentrations prepared according to DE Publication No. 24 04 564 [egg yolk phospholipid 4%; potassium oleate 0.02% 1 was individually made isotonic with lactate-containing Ringer's solution or Krebs-Ringer hydrogen carbonate solution, and then mixed with a plasma substitute so that the final concentration of the plasma excipient was 1-6%, whereupon the formation of precipitate was obtained. visually observed within 6 hours of mixing at room temperature. The plasma replacements used are hydroxyethyl starch (average molecular weight 200,000, 20% in physiological saline; supplied by Ajinomoto Co., Ltd.) and "Dextran 40" average molecular weight 40,000, 10% in physiological saline; provided by The Green Cross Corp.J.
Ved "plasmaerstatning" forstås en opløsning af de højmolekylære forbindelser, "plasmaforstrækningsmidlerne", i et fysiologisk acceptabelt vandigt medium i en sådan koncentration, at de kan anvendes til erstatning af plasma.By "plasma replacement" is meant a solution of the high molecular weight compounds, the "plasma amplifiers", in a physiologically acceptable aqueous medium at such a concentration that they can be used to replace plasma.
Resultaterne er anført i tabel 2 og 3.The results are given in Tables 2 and 3.
Tabel 2Table 2
Tabel 3Table 3
Note: intet bundfald +: dannelse af bundfaldNote: no precipitate +: formation of precipitate
Af de ovenstående resultater fremgår det, at bloderstatningen fremstillet ved fremgangsmåden ifølge opfindelsen påvirkes meget mindre af tilstedeværelsen af et plasmaforstrækningsmiddel end emulsionen ifølge DE offentliggørelsesskrift nr. 24 04 564, hvilket viser, at emulsionen, der anvendes ved fremgangsmåden ifølge opfindelsen kan blandes med "Dextran 40"- og hydroxyethylstivel-seplasmaerstatning i ethvert forhold til at give en bloderstatning med den fysiologisk kolloidale isotonicitet, som opnås ved tilsætning af "Dextran 40" og hydroxyethylstivelse i en endelig koncentration op til henholdsvis 2% og 4%.From the above results, it can be seen that the blood substitute produced by the process of the invention is much less affected by the presence of a plasma pre-emitting agent than the emulsion of DE Publication No. 24 04 564, which shows that the emulsion used in the process of the invention can be mixed with "Dextran". 40 "and hydroxyethyl starch separator replacement in any ratio to provide a blood substitute with the physiological colloidal isotonicity obtained by the addition of" Dextran 40 "and hydroxyethyl starch at a final concentration up to 2% and 4% respectively.
Sammen!iqningsforsøqTogether! Iqningsforsøq
Bloderstatningsemulsionerne blev fremstillet ved proceduren fra eksempel 1 og deres bestanddele var som anført i den efterfølgende tabel 4.The blood replacement emulsions were prepared by the procedure of Example 1 and their constituents were as listed in the following Table 4.
Som udgangsmaterialer anvendtes følgende forbindelser:The following compounds were used as starting materials:
Komponent (A): Perfluordecalin Komponent (B): PerfluortripropylaminComponent (A): Perfluorodecaline Component (B): Perfluorotripropylamine
Emulgeringsmiddel: Polyoxyethylen-polyoxypropylencopolymer ("PLURONIC F-68") i prøverne fremstillet ifølge opfindelsen; phospholipid (æggeblomme) i sammenligningsprøverne .Emulsifier: Polyoxyethylene-polyoxypropylene copolymer ("PLURONIC F-68") in the samples prepared according to the invention; phospholipid (egg yolk) in the comparison samples.
Emulgeringshjælpemiddel: Phospholipid (æggeblomme) og fedtsyre i prøverne fremstillet ifølge opfindelsen; fedtsyre i sammenligningsprøverne.Emulsifier: Phospholipid (egg yolk) and fatty acid in the samples prepared according to the invention; fatty acid in the comparison samples.
Fysiologisk acceptabel bærer: Vandig opløsning indeholdende glycerol (0,8 vægt/vol.%), NaCl (0,6 vægt/vol.%), KCl (0,034 vægt/vol.%), MgCl2 (0,02 vægt-vol.%), CaCl2 (0,028 vægt/vol.%), NaHCO^ (0,21 vægt/vol.%) og glucose (0,18 vægt/vol.%).Physiologically acceptable carrier: Aqueous solution containing glycerol (0.8% w / v), NaCl (0.6% w / v), KCl (0.034% w / v), MgCl2 (0.02% w / v). %), CaCl 2 (0.028 w / v%), NaHCO 3 (0.21 w / v%) and glucose (0.18 w / v%).
Plasmaforstrækningsmiddel: Hydroxyethylstivelse.Plasma prewetting agent: Hydroxyethyl starch.
Sammenligningsforsøgene blev udført med hensyn til følgende emner: 1) Emulsionens partikelstørrelse:The comparison experiments were performed on the following subjects: 1) Particle size of the emulsion:
Emulsionens gennemsnitlige partikelstørrelse blev bestemt efter sterilisering og efter 6 måneders opbevaring ved 4 °C ifølge centrifugalsedimenteringsmetoden, som er beskrevet af K. Yokoyama et al. tchem. Pharm. Buil., 22 (12), 2966-2971 (1974)'].The average particle size of the emulsion was determined after sterilization and after 6 months of storage at 4 ° C according to the centrifugal sedimentation method described by K. Yokoyama et al. Tchem. Pharm. Buil., 22 (12), 2966-2971 (1974) '].
2) Virkning af plasmaforstrækningsmidlet:2) Effect of the plasma pre-emitting agent:
Hydroxyethylstivelse anvendtes som plasmaforstrækningsmiddel i en mængde på 3 vægt/vol.-%, og forekomsten af bundfald blev iagttaget visuelt efter 6 timers opbevaring ved stuetempratur.Hydroxyethyl starch was used as a plasma pre-emulsifier in an amount of 3% w / v, and the occurrence of precipitation was observed visually after 6 hours storage at room temperature.
Hvis der dannes et reversibelt bundfald ved gensidig indvirkning af fluorcarbon(FC)-emulsionen og plasmaforstrækningsmidlet, kan emulsionen ikke anvendes som bloderstatning.If a reversible precipitate is formed by the mutual action of the fluorocarbon (FC) emulsion and the plasma pre-emulsion, the emulsion cannot be used as a blood substitute.
3) Udskiftningstransfusion på rotter:3) Replacement transfusion in rats:
Der gennemførtes sammenligningsforsøg med udskiftningstransfusion under anvendelse af rotter (Wistar-stamme, hanner, vægt 200 -250 g) ved gentagen blødning fra carotidarterien og erstatningstransfusion igennem halevenen skiftevis op til en hæmatokritværdi på 4,0 under 100 % oxygenatmosfære. Derpå bestemtes overlevelsestiden af de udskiftningstransfuserede rotter.Comparative experiments with replacement transfusion were performed using rats (Wistar strain, males, weight 200-250 g) by repeated bleeding from the carotid artery and replacement transfusion through the tail vein alternately up to a hematocrit value of 4.0 under 100% oxygen atmosphere. Then, the survival time of the replacement transfused rats was determined.
4) Hæmolytiske virkninger:4) Hemolytic effects:
Forsøg med FC-emulsionens hæmolytiske virkning i det ekstrakorpo-rale kredsløbssystem blev udført in vivo ved anvendelse af røde blodceller fra kaniner.Experiments with the haemolytic action of FC emulsion in the extracorporeal circulatory system were performed in vivo using rabbit red blood cells.
FC-emulsionen blev blandet med en lactatholdig Ringer"s opløsning, således at den blev i hovedsagen fysiologisk isotonisk, og det resulterende isotoniske emulsionspræparat blev blandet med hepari-niseret kaninblod i et forhold på 1:1 til fremstilling af en prøveopløsning til prøvningen. 8 ml af prøveopløsningen holdtes ved 37 °C i 6 timer, og derpå bestemtes indholdet af frit hæmo- globin ved cyanmethæmoglobinmetoden (Kampen, E.J. and Ziilstram, W.J., Clin. Chim. Acta. 6, 538, 1961). I tilfælde, hvor en lac-tatholdig Ringer's opløsning anvendtes alene som kontrol uden anvendelse af FC-emulsion var det frie hæmoglobinindhold 128 mg.The FC emulsion was mixed with a lactate-containing Ringer's solution so that it became essentially physiologically isotonic, and the resulting isotonic emulsion preparation was mixed with 1: 1 heparinized rabbit blood to prepare a test solution for the test. 8 ml of the sample solution was kept at 37 ° C for 6 hours and then the content of free hemoglobin was determined by the cyanmethemoglobin method (Kampen, EJ and Ziilstram, WJ, Clin. Chim. Acta. 6, 538, 1961). lactated Ringer's solution was used alone as a control without the use of FC emulsion, the free hemoglobin content was 128 mg.
5) Udåndingshastighed af FC-emulsion in vivo.5) Exhalation rate of FC emulsion in vivo.
Udåndingshastigheden af FC-emulsionen in vivo blev bestemt ved den metode, som er beskrevet i Chemical Pharmacological Buil.The rate of exhalation of the FC emulsion in vivo was determined by the method described in Chemical Pharmacological Buil.
26, (3) 956-966, 1978, (K. Yokoyama, "Fate of perfluorochemicals in aminals after intraveneous injection or hemodilution with their emulsions").26, (3) 956-966, 1978, (K. Yokoyama, "Fate of perfluorochemicals in aminals after intravenous injection or hemodilution with their emulsions").
FC-emulsionen blev indgivet intravenøst til rotten med en hastighed på 4 g/kg legemsvægt, og udåndningshastigheden blev vist ved antallet af dage, indtil halvdelen af det indgivne FC var forbrugt ved udånding.The FC emulsion was administered intravenously to the rat at a rate of 4 g / kg body weight and the exhalation rate was shown by the number of days until half of the administered FC was consumed by exhalation.
De opnåede resultater er anført i tabel 4.The results obtained are listed in Table 4.
_________Tabel 4_._______________Table 4 _.______
NOTE: (A) : Perfluordecalin (B) : Perfluortripropylamin F : "PLURONIC F68" L : Phospholipid FA : Fedtsyre HES : HydroxyethylstivelseNOTE: (A): Perfluorodecaline (B): Perfluorotripropylamine F: "PLURONIC F68" L: Phospholipid FA: Fatty Acid HES: Hydroxyethyl Starch
Af de viste resultater kan udledes følgende.From the results shown, the following can be deduced.
I tilfælde af prøve nr. 1 er den resulterende FC-emulsion utilstrækkelig m.h.t. emulgering og har lav stabilitet, således at der ikke kunne opnås en emulsion med en gennemsnitlig partikelstørrelse på under 0,2 yum. Således er den resulterende emulsion helt uegnet som lægemiddel.In case of sample # 1, the resulting FC emulsion is insufficient. emulsion and has low stability so that an emulsion with an average particle size of less than 0.2 µm could not be obtained. Thus, the resulting emulsion is completely unsuitable as a drug.
I tilfælde af prøve nr. 2 var den resulterende emulsion udmærket m.h.t. emulgering og stabilitet, men den dannede bundfald ved blanding med hydroxyethylstivelse, og desuden udviste den en bemærkelsesværdig hæmolytisk virkning, og rotter kunne ikke overleve behandlingen ved udskiftningstransfusion. Således kan denne emulsion heller ikke anvendes som lægemiddel.In case of sample # 2, the resulting emulsion was excellent with respect to emulsification and stability, but it precipitated by mixing with hydroxyethyl starch, and additionally showed a remarkable hemolytic effect and rats could not survive the treatment by replacement transfusion. Thus, this emulsion also cannot be used as a drug.
I tilfælde af prøve nr. 3 har den resulterende emulsion lav stabilitet, men den kan indgives in vivo som lægemiddel, lige efter at den er fremstillet.In the case of sample # 3, the resulting emulsion has low stability, but it can be administered in vivo as a drug just after it is made.
Prøve nr. 4 viste det samme resultat som nr. 2.Sample # 4 showed the same result as # 2.
Prøverne nr. 5, 7 og 9 viste meget udmærkede egenskaber. Derfor kan de anvendes som bloderstatning.Samples Nos. 5, 7 and 9 showed very good properties. Therefore, they can be used as a blood substitute.
Prøverne nr. 6, 8, 10, 12 og 14 viste de samme resultater som nr. 2.Samples Nos. 6, 8, 10, 12 and 14 showed the same results as Nos. 2.
Prøve nr. 11 har lav udåndingshastighed af FC i en levende krop sammenlignet med prøverne nr. 5, 7 og 9, men vil kunne anvendes som lægemiddel under en tilstrækkelig kontrol.Sample # 11 has a low rate of exhalation of FC in a living body compared to samples # 5, 7, and 9, but could be used as a drug under adequate control.
Prøve nr. 13 har meget lav udåndingshastighed, således at den kan forårsage uforudsigelige ulemper i den levende krop. I dette tilfælde døde rotter efter 69 timer, når de blev underkastet udskiftningstransfusion.Sample # 13 has a very low exhalation rate so that it can cause unpredictable disadvantages in the living body. In this case, rats died after 69 hours when subjected to replacement transfusion.
Som det kan ses af disse sammenligningsforsøg viser bloderstat- ningen fremstillet ved fremgangsmåden ifølge opfindelsen (prøverne nr. 3, 5, 7, 9 og 11) væsentlig virkning med hensyn til partiklernes stabilitet, den samtidige anvendelse af plasmaforstrækningsmiddel, udskiftningstransfusion og sikkerhed i kredsløbssystemer uden for legemet sammenlignet med FC-emulsionerne ifølge de tidligere nævnte patentskrifter (prøve nr. 1 er sammensat ifølge DE fremlæggelsesskrift nr. 21 44 094, og prøverne nr. 2, 4, 6, 8, 10, 12 og 14 er sammensat ifølge DE offentliggørelsesskrift nr.As can be seen from these comparative experiments, the blood substitute produced by the method of the invention (samples Nos. 3, 5, 7, 9 and 11) shows significant effect on the stability of the particles, the concomitant use of plasma diluent, replacement transfusion, and safety in circulatory systems without for the body as compared to the FC emulsions of the aforementioned patents (Sample # 1 is composed according to DE Publication No. 21 44 094 and samples Nos. 2, 4, 6, 8, 10, 12 and 14 are composed according to DE disclosure no.
24 04 564). Den ovennævnte forskel i virkning mellem den ifølge opfindelsen fremstillede bloderstatning og de kendte FC-emulsioner skyldes forskellen i FC-emulsionernes bestanddele, som medførte et så væsentligt fremskridt som, at FC-emulsionens toxicitet næsten forsvandt, og det for første gang var muligt at anvende en sådan FC-emulsion til klinisk behandling.24 04 564). The above difference in effect between the blood substitute prepared according to the invention and the known FC emulsions is due to the difference in the constituents of the FC emulsions which led to such a significant advance as the toxicity of the FC emulsion almost disappeared and it was possible for the first time to use such a FC emulsion for clinical treatment.
Klinisk afprøvning På basis af de tidligere rapporterede prøvningsresultater og omfattende yderligere undersøgelser med hensyn til toxicitet, farmakologi, farmakodynamik og effektivitet hos dyr er den ifølge opfindelsen fremstillede bloderstatning, som er kendt under navnet "Fluosol-DA", blevet påvist at være sikker og effektiv som kunstig bloderstatning. Undersøgelserne er nu nået ind i det kliniske stadium.Clinical Testing Based on the previously reported test results and extensive additional studies on toxicity, pharmacology, pharmacodynamics and efficacy in animals, the blood substitute manufactured according to the invention known as "Fluosol-DA" has been shown to be safe and effective such as artificial blood replacement. The studies have now reached the clinical stage.
Sammensætningen i vægt/vol.?i af "Fluosol-DA (20 %)" er som følger:The composition in w / v of "Fluosol-DA (20%)" is as follows:
Perfluordecalin 14,0Perfluorodecaline 14.0
Per fluortripropylamin 6,0 "Pluronic F-68" 2,7 Æggeblommephospholipider 0,4Per fluorotripropylamine 6.0 "Pluronic F-68" 2.7 Egg yolk phospholipids 0.4
Kaliumoleat 0,04Potassium oleate 0.04
Glycerol 0,8Glycerol 0.8
NaCl 0,600 KC1 0,034NaCl 0.600 KCl 0.034
MgCl2 0,020MgCl2 0.020
CaCl2 0,028CaCl2 0.028
NaHC03 0,210NaHCO3 0.210
Glucose 0,180Glucose 0.180
Hydroxyethylstivelse 3,0 1 marts 1979 er fase I undersøgelsen af "Fluosol-DA" hos mennesker blevet gennemført i Japan af professor Mitsuno og Dr. Ohyanagi ved Kobe University Medical School. 10 normale voksne mandlige frivillige, herunder 7 læger, blev infuseret med fra 20 til 500 ml "Fluosol-DA", idet de tre individer, som modtog 500 ml, forinden blev tappet for 200 ml blod.Hydroxyethyl starch 3.0 March 1, 1979, the Phase I study of "Fluosol-DA" in humans has been conducted in Japan by Professor Mitsuno and Dr. Ohyanagi at Kobe University Medical School. Ten normal adult male volunteers, including 7 physicians, were infused with 20 to 500 ml of "Fluosol-DA", the three individuals receiving 500 ml being previously tapped for 200 ml of blood.
Blodprøver blev taget før infusionen og 3, 24 og 48 timer og 1, 2 og 4 uger efter infusionen. De individer, som modtog enten 200 ml eller 500 ml, fik yderligere taget prøver 8 og 14 uger efter infusionen. På prøverne undersøgtes følgende parametre: HæmatologiBlood samples were taken before the infusion and at 3, 24 and 48 hours and 1, 2 and 4 weeks after the infusion. The subjects who received either 200 ml or 500 ml were further sampled 8 and 14 weeks after the infusion. The following parameters were examined in the samples: Hematology
Fuldstændig blodtælling Reticulocyttælling B1odp1adetæl1ing Tælling og differentialtælling af hvide blodceller Serumprotein-elektrophoreseComplete blood count Reticulocyte count B1odp1ad counting Count and differential count of white blood cells Serum protein electrophoresis
KemiChemistry
Thymolturbiditetsprøvning AmylaseThymol Turbidity Test Amylase
Bilirubin, total og direkte Lactatdehydrogenase (LDH)Bilirubin, Total and Direct Lactate Dehydrogenase (LDH)
Cobaltreaktion CholinesteraseCobalt reaction Cholinesterase
Serum-glutamatoxaloacetattransami- nase (SGOT) Blod-urin-nitrogen (BUN)Serum glutamate oxaloacetate transaminase (SGOT) Blood urine nitrogen (BUN)
Serum-glutamatpyruvattransaminase (SGPT) Total cholesterolSerum glutamate pyruvate transaminase (SGPT) Total cholesterol
Alkalisk phosphatase CreatininAlkaline phosphatase Creatinine
Total proteinTotal protein
Desuden udførtes et thromboelastogram, og komplementaktiviteten blev bestemt på blodprøverne taget 3 og 24 timer efter infusionen.In addition, a thromboelastogram was performed and the complement activity was determined on the blood samples taken 3 and 24 hours after the infusion.
Der blev ikke iagttaget nogen ændringer i blodtryk, pulshastighed, åndedrætshastighed, legemstemperatur eller ECE. Ingen andre symptomer blev iagttaget. Ingen skadelige reaktioner eller klager blev rapporteret af nogen af de 10 individer. Under hele undersø-No changes in blood pressure, heart rate, respiratory rate, body temperature or ECE were observed. No other symptoms were observed. No adverse reactions or complaints were reported by any of the 10 individuals. Throughout the investigation
Claims (1)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US65496476A | 1976-02-03 | 1976-02-03 | |
| US65496476 | 1976-02-03 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| DK307276A DK307276A (en) | 1977-08-04 |
| DK152256B true DK152256B (en) | 1988-02-15 |
| DK152256C DK152256C (en) | 1988-08-29 |
Family
ID=24626936
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| DK307276A DK152256C (en) | 1976-02-03 | 1976-07-07 | PROCEDURE FOR THE PREPARATION OF A STABLE BLOOD REMOVAL |
Country Status (22)
| Country | Link |
|---|---|
| JP (1) | JPS5835485B2 (en) |
| AR (1) | AR218864A1 (en) |
| AT (1) | AT355214B (en) |
| BE (1) | BE850992A (en) |
| CA (1) | CA1072446A (en) |
| CH (1) | CH633674A5 (en) |
| DD (1) | DD132231A5 (en) |
| DE (1) | DE2630586C2 (en) |
| DK (1) | DK152256C (en) |
| ES (1) | ES449635A1 (en) |
| FI (1) | FI58071C (en) |
| FR (1) | FR2361867A1 (en) |
| GB (1) | GB1549038A (en) |
| IL (1) | IL51325A (en) |
| LU (1) | LU76698A1 (en) |
| MX (1) | MX4136E (en) |
| NL (1) | NL171533C (en) |
| NO (1) | NO145370C (en) |
| NZ (1) | NZ183096A (en) |
| SE (1) | SE442706B (en) |
| SU (1) | SU797546A3 (en) |
| ZA (1) | ZA77229B (en) |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4186253A (en) * | 1978-10-10 | 1980-01-29 | The Green Cross Corporation | Perfusate for preserving organ to be transplanted and preserving method |
| JPS55100312A (en) * | 1979-01-25 | 1980-07-31 | Toshiro Wada | Contrast medium for blood vessel |
| EP0077114B1 (en) * | 1981-09-08 | 1987-12-02 | Green Cross Corporation | Perfluorochemicals, process for preparing the same and their use as blood substitutes |
| US4423077A (en) * | 1982-07-27 | 1983-12-27 | The University Of Pennsylvania | Perfluorochemical emulsion artificial blood |
| JPS5946218A (en) * | 1982-09-09 | 1984-03-15 | Green Cross Corp:The | Fluorocarbon emulsion preparation |
| JPS59175421A (en) * | 1983-03-24 | 1984-10-04 | Tetsuzou Agishi | Hemocathartic agent |
| WO1993002653A1 (en) * | 1991-08-08 | 1993-02-18 | Segel Leigh D | Fluorocarbon blood substitute |
| US5658962A (en) | 1994-05-20 | 1997-08-19 | Minnesota Mining And Manufacturing Company | Omega-hydrofluoroalkyl ethers, precursor carboxylic acids and derivatives thereof, and their preparation and application |
| US5502094A (en) * | 1994-05-20 | 1996-03-26 | Minnesota Mining And Manufacturing Company | Physiologically acceptable emulsions containing perfluorocarbon ether hydrides and methods for use |
| WO1997025978A1 (en) * | 1996-01-15 | 1997-07-24 | BELOYARTSEV, Arkady Felixovich | Method of obtaining perfluorocarbon emulsions for medical purposes |
| CN1068778C (en) * | 1998-05-15 | 2001-07-25 | 赵超英 | Novel drug composition for treating and curing and its preparing method |
| US7357937B2 (en) | 2002-09-24 | 2008-04-15 | Therox, Inc. | Perfluorocarbon emulsions with non-fluorinated surfactants |
| RU2259819C1 (en) | 2004-03-01 | 2005-09-10 | Кузнецова Ирина Николаевна | Emulsion of perfluoroorganic compounds of medicinal indication and method for its obtaining |
| EA200801973A1 (en) | 2006-03-14 | 2009-02-27 | Сергей Иванович Воробьев | PERFLUOROLEVAL GAS TRANSFER EMULSION FOR MEDICAL AND BIOLOGICAL PURPOSES, METHOD OF ITS RECEIVING AND MEANS FOR TREATMENT (OPTIONS) |
| US8063020B2 (en) * | 2007-12-22 | 2011-11-22 | Simpkins Cuthbert O | Resuscitation fluid |
| GB2467353B (en) | 2009-01-30 | 2011-07-20 | Steven Skill | Apparatus for treatment of fluid streams and method of conducting the same |
| ES2541226B2 (en) * | 2014-01-15 | 2016-02-12 | MONDRAGÓN GOI ESKOLA POLITEKNIKOA J. Mª. ARIZMENDIARRIETA, S.C | Blood phantom |
| RU2745290C2 (en) * | 2019-04-12 | 2021-03-23 | Ирина Николаевна Кузнецова | Emulsion of perfluorocarbon compounds for biomedical purposes and a method for its production |
| WO2024046999A1 (en) * | 2022-08-31 | 2024-03-07 | Johann Wolfgang Goethe-Universität Frankfurt am Main | Lecithin-modified nanoscale oxygen carriers (lenox) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2404564A1 (en) * | 1973-10-05 | 1975-04-17 | Green Cross Corp | Aqueous EMULSION OF SATURATED ALIPHATIC PERFLUORCARBON COMPOUNDS, PROCESS FOR THEIR PRODUCTION AND USE AS BLOOD SUBSTITUTE AND PERFUSION LIQUID |
| DE2144094B2 (en) * | 1970-09-05 | 1980-02-14 | Tanabe Seiyaku Co. Ltd. | Process for the preparation of an oxygen transportable, injectable fluorocarbon particulate emulsion |
-
1976
- 1976-07-01 JP JP51078127A patent/JPS5835485B2/en not_active Expired
- 1976-07-02 GB GB27739/76A patent/GB1549038A/en not_active Expired
- 1976-07-06 SE SE7607727A patent/SE442706B/en not_active IP Right Cessation
- 1976-07-07 CH CH869676A patent/CH633674A5/en not_active IP Right Cessation
- 1976-07-07 ES ES449635A patent/ES449635A1/en not_active Expired
- 1976-07-07 DE DE2630586A patent/DE2630586C2/en not_active Expired
- 1976-07-07 AT AT496676A patent/AT355214B/en not_active IP Right Cessation
- 1976-07-07 DK DK307276A patent/DK152256C/en not_active IP Right Cessation
- 1976-07-07 NL NLAANVRAGE7607514,A patent/NL171533C/en not_active IP Right Cessation
- 1976-07-08 FR FR7620907A patent/FR2361867A1/en active Granted
- 1976-07-08 CA CA256,571A patent/CA1072446A/en not_active Expired
-
1977
- 1977-01-17 NZ NZ183096A patent/NZ183096A/en unknown
- 1977-01-17 ZA ZA770229A patent/ZA77229B/en unknown
- 1977-01-21 MX MX775377U patent/MX4136E/en unknown
- 1977-01-25 IL IL51325A patent/IL51325A/en unknown
- 1977-01-31 AR AR266381A patent/AR218864A1/en active
- 1977-02-01 NO NO770324A patent/NO145370C/en unknown
- 1977-02-01 FI FI770354A patent/FI58071C/en not_active IP Right Cessation
- 1977-02-02 SU SU772447657A patent/SU797546A3/en active
- 1977-02-02 BE BE174577A patent/BE850992A/en not_active IP Right Cessation
- 1977-02-03 LU LU76698A patent/LU76698A1/xx unknown
- 1977-02-03 DD DD7700197227A patent/DD132231A5/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2144094B2 (en) * | 1970-09-05 | 1980-02-14 | Tanabe Seiyaku Co. Ltd. | Process for the preparation of an oxygen transportable, injectable fluorocarbon particulate emulsion |
| DE2404564A1 (en) * | 1973-10-05 | 1975-04-17 | Green Cross Corp | Aqueous EMULSION OF SATURATED ALIPHATIC PERFLUORCARBON COMPOUNDS, PROCESS FOR THEIR PRODUCTION AND USE AS BLOOD SUBSTITUTE AND PERFUSION LIQUID |
| DK137433B (en) * | 1973-10-05 | 1978-03-06 | Green Cross Corp | Process for preparing a stable emulsion of an oxygen-transferring saturated perfluorocarbon compound. |
Also Published As
| Publication number | Publication date |
|---|---|
| ZA77229B (en) | 1977-11-30 |
| FI770354A (en) | 1977-08-04 |
| CA1072446A (en) | 1980-02-26 |
| SU797546A3 (en) | 1981-01-15 |
| SE442706B (en) | 1986-01-27 |
| FI58071B (en) | 1980-08-29 |
| NL171533B (en) | 1982-11-16 |
| FR2361867B1 (en) | 1978-12-15 |
| FI58071C (en) | 1980-12-10 |
| IL51325A0 (en) | 1977-03-31 |
| ATA496676A (en) | 1979-07-15 |
| LU76698A1 (en) | 1977-06-28 |
| FR2361867A1 (en) | 1978-03-17 |
| AU1550576A (en) | 1977-07-28 |
| DE2630586A1 (en) | 1977-08-11 |
| MX4136E (en) | 1982-01-06 |
| ES449635A1 (en) | 1977-11-16 |
| DK152256C (en) | 1988-08-29 |
| NO145370B (en) | 1981-11-30 |
| NL171533C (en) | 1983-04-18 |
| BE850992A (en) | 1977-05-31 |
| SE7607727L (en) | 1977-08-04 |
| NL7607514A (en) | 1977-08-05 |
| DE2630586C2 (en) | 1984-06-07 |
| NO770324L (en) | 1977-08-04 |
| GB1549038A (en) | 1979-08-01 |
| DD132231A5 (en) | 1978-09-13 |
| CH633674A5 (en) | 1982-12-31 |
| AR218864A1 (en) | 1980-07-15 |
| DK307276A (en) | 1977-08-04 |
| NO145370C (en) | 1982-03-10 |
| JPS5835485B2 (en) | 1983-08-03 |
| JPS5296722A (en) | 1977-08-13 |
| AT355214B (en) | 1980-02-25 |
| NZ183096A (en) | 1979-04-26 |
| IL51325A (en) | 1980-05-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4252827A (en) | Oxygen-transferable fluorocarbon emulsion | |
| DK152256B (en) | PROCEDURE FOR THE PREPARATION OF A STABLE BLOOD REMOVAL | |
| AU629832B2 (en) | Improved emulsions of highly fluorinated organic compounds | |
| US4423077A (en) | Perfluorochemical emulsion artificial blood | |
| EP0063149B1 (en) | Aqueous fluorocarbide emulsions indefinitely stable at a given temperature, process for obtaining them and applications | |
| US4397870A (en) | Process for prolonging retention of emulsion particles in the bloodstream | |
| EP2749268B1 (en) | Resuscitation Fluid | |
| IE59175B1 (en) | Stable emulsions of highly fluorinated organic compounds | |
| US4343797A (en) | Synthetic whole blood and a method of making the same | |
| US4439424A (en) | Synthetic whole blood | |
| CN101448485B (en) | Optimized fluorocarbon emulsions for blood substitutes and other therapeutic uses | |
| Vorob’ev | First-and second-generation perfluorocarbon emulsions | |
| JPS5946218A (en) | Fluorocarbon emulsion preparation | |
| RU2745290C2 (en) | Emulsion of perfluorocarbon compounds for biomedical purposes and a method for its production | |
| RU2199311C2 (en) | Composition of perfluorocarbon blood substitute based on emulsion of perfluoroorganic compounds for medical-biological aims | |
| US4874742A (en) | Synthetic whole blood and a process for preparing the same | |
| RU2070033C1 (en) | Method of preparing perfluorocarbon emulsion for medicinal aims | |
| KR810000695B1 (en) | Process for preparing flurocarbon emulsions capable of carrying oxygen | |
| JPS59130813A (en) | Adminiculum for chemotherapy of cancer | |
| AU6031599A (en) | Improved artificial blood fluids | |
| GEYER | Surfactants and perfluorochemical emulsions for use in blood replacement preparations |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PBP | Patent lapsed |