CN113924311A - 包含修饰的il-2多肽的多肽及其用途 - Google Patents
包含修饰的il-2多肽的多肽及其用途 Download PDFInfo
- Publication number
- CN113924311A CN113924311A CN202080018011.6A CN202080018011A CN113924311A CN 113924311 A CN113924311 A CN 113924311A CN 202080018011 A CN202080018011 A CN 202080018011A CN 113924311 A CN113924311 A CN 113924311A
- Authority
- CN
- China
- Prior art keywords
- leu
- polypeptide
- binding domain
- antigen binding
- lys
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 438
- 102000004196 processed proteins & peptides Human genes 0.000 title claims abstract description 434
- 229920001184 polypeptide Polymers 0.000 title claims abstract description 433
- 108010002350 Interleukin-2 Proteins 0.000 title claims abstract description 39
- 210000001744 T-lymphocyte Anatomy 0.000 claims abstract description 155
- 230000027455 binding Effects 0.000 claims description 326
- 239000000427 antigen Substances 0.000 claims description 318
- 108091007433 antigens Proteins 0.000 claims description 318
- 102000036639 antigens Human genes 0.000 claims description 318
- 210000004027 cell Anatomy 0.000 claims description 141
- 101000716102 Homo sapiens T-cell surface glycoprotein CD4 Proteins 0.000 claims description 113
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 claims description 113
- 238000006467 substitution reaction Methods 0.000 claims description 110
- 238000000034 method Methods 0.000 claims description 105
- 206010028980 Neoplasm Diseases 0.000 claims description 75
- 102100034922 T-cell surface glycoprotein CD8 alpha chain Human genes 0.000 claims description 65
- 241000282414 Homo sapiens Species 0.000 claims description 63
- 150000001413 amino acids Chemical group 0.000 claims description 46
- 201000011510 cancer Diseases 0.000 claims description 43
- 210000000822 natural killer cell Anatomy 0.000 claims description 41
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 claims description 39
- 230000035772 mutation Effects 0.000 claims description 38
- 210000001266 CD8-positive T-lymphocyte Anatomy 0.000 claims description 30
- 230000001965 increasing effect Effects 0.000 claims description 26
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 claims description 25
- 229960002621 pembrolizumab Drugs 0.000 claims description 24
- 239000003814 drug Substances 0.000 claims description 23
- 102000039446 nucleic acids Human genes 0.000 claims description 23
- 108020004707 nucleic acids Proteins 0.000 claims description 23
- 150000007523 nucleic acids Chemical class 0.000 claims description 23
- 108010021064 CTLA-4 Antigen Proteins 0.000 claims description 22
- 229940045513 CTLA4 antagonist Drugs 0.000 claims description 22
- 102220096696 rs62625272 Human genes 0.000 claims description 22
- 230000006052 T cell proliferation Effects 0.000 claims description 21
- 238000002560 therapeutic procedure Methods 0.000 claims description 20
- 102220638990 Beta-enolase_H16A_mutation Human genes 0.000 claims description 18
- -1 F42R Proteins 0.000 claims description 18
- 108010004217 Natural Cytotoxicity Triggering Receptor 1 Proteins 0.000 claims description 18
- 102100032870 Natural cytotoxicity triggering receptor 1 Human genes 0.000 claims description 18
- 102220503468 Superoxide dismutase [Cu-Zn]_D84S_mutation Human genes 0.000 claims description 18
- 239000003795 chemical substances by application Substances 0.000 claims description 18
- 229940124597 therapeutic agent Drugs 0.000 claims description 18
- 239000002246 antineoplastic agent Substances 0.000 claims description 16
- 101100044298 Drosophila melanogaster fand gene Proteins 0.000 claims description 15
- 101150064015 FAS gene Proteins 0.000 claims description 15
- 101100335198 Pneumocystis carinii fol1 gene Proteins 0.000 claims description 15
- 230000004663 cell proliferation Effects 0.000 claims description 15
- 102000017578 LAG3 Human genes 0.000 claims description 14
- 239000000556 agonist Substances 0.000 claims description 14
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Natural products NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 13
- 101001137987 Homo sapiens Lymphocyte activation gene 3 protein Proteins 0.000 claims description 13
- 230000001093 anti-cancer Effects 0.000 claims description 12
- 238000000338 in vitro Methods 0.000 claims description 12
- 208000014018 liver neoplasm Diseases 0.000 claims description 12
- 102200100726 rs61752874 Human genes 0.000 claims description 12
- 101150069255 KLRC1 gene Proteins 0.000 claims description 11
- 101100404845 Macaca mulatta NKG2A gene Proteins 0.000 claims description 11
- 102100022682 NKG2-A/NKG2-B type II integral membrane protein Human genes 0.000 claims description 11
- 101001109501 Homo sapiens NKG2-D type II integral membrane protein Proteins 0.000 claims description 10
- 101000831007 Homo sapiens T-cell immunoreceptor with Ig and ITIM domains Proteins 0.000 claims description 10
- 102100022680 NKG2-D type II integral membrane protein Human genes 0.000 claims description 10
- 102100024834 T-cell immunoreceptor with Ig and ITIM domains Human genes 0.000 claims description 10
- 238000001727 in vivo Methods 0.000 claims description 10
- 239000008194 pharmaceutical composition Substances 0.000 claims description 10
- 101000801234 Homo sapiens Tumor necrosis factor receptor superfamily member 18 Proteins 0.000 claims description 9
- 102100033728 Tumor necrosis factor receptor superfamily member 18 Human genes 0.000 claims description 9
- 102000004127 Cytokines Human genes 0.000 claims description 8
- 108090000695 Cytokines Proteins 0.000 claims description 8
- 206010014733 Endometrial cancer Diseases 0.000 claims description 8
- 206010014759 Endometrial neoplasm Diseases 0.000 claims description 8
- 101001002657 Homo sapiens Interleukin-2 Proteins 0.000 claims description 8
- 208000008839 Kidney Neoplasms Diseases 0.000 claims description 8
- 206010038389 Renal cancer Diseases 0.000 claims description 8
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 8
- 206010017758 gastric cancer Diseases 0.000 claims description 8
- 201000010982 kidney cancer Diseases 0.000 claims description 8
- 201000011549 stomach cancer Diseases 0.000 claims description 8
- 239000004471 Glycine Substances 0.000 claims description 7
- 102100034458 Hepatitis A virus cellular receptor 2 Human genes 0.000 claims description 7
- 101001023379 Homo sapiens Lysosome-associated membrane glycoprotein 1 Proteins 0.000 claims description 7
- 102100035133 Lysosome-associated membrane glycoprotein 1 Human genes 0.000 claims description 7
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 claims description 7
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 claims description 7
- 239000005557 antagonist Substances 0.000 claims description 7
- 230000037430 deletion Effects 0.000 claims description 7
- 238000012217 deletion Methods 0.000 claims description 7
- 239000003937 drug carrier Substances 0.000 claims description 7
- 206010041823 squamous cell carcinoma Diseases 0.000 claims description 7
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical group COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 claims description 6
- 108700004922 F42A Proteins 0.000 claims description 6
- 101001068133 Homo sapiens Hepatitis A virus cellular receptor 2 Proteins 0.000 claims description 6
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 claims description 6
- 101000851370 Homo sapiens Tumor necrosis factor receptor superfamily member 9 Proteins 0.000 claims description 6
- 108010004222 Natural Cytotoxicity Triggering Receptor 3 Proteins 0.000 claims description 6
- 102100032852 Natural cytotoxicity triggering receptor 3 Human genes 0.000 claims description 6
- 102220498123 Protein LRATD2_E95S_mutation Human genes 0.000 claims description 6
- 102220495631 Putative uncharacterized protein LOC645739_F42A_mutation Human genes 0.000 claims description 6
- 101710187830 Tumor necrosis factor receptor superfamily member 1B Proteins 0.000 claims description 6
- 102100033733 Tumor necrosis factor receptor superfamily member 1B Human genes 0.000 claims description 6
- 102100036856 Tumor necrosis factor receptor superfamily member 9 Human genes 0.000 claims description 6
- 239000013604 expression vector Substances 0.000 claims description 6
- 238000001959 radiotherapy Methods 0.000 claims description 6
- 238000002271 resection Methods 0.000 claims description 6
- 206010005003 Bladder cancer Diseases 0.000 claims description 5
- 206010006187 Breast cancer Diseases 0.000 claims description 5
- 208000026310 Breast neoplasm Diseases 0.000 claims description 5
- 206010008342 Cervix carcinoma Diseases 0.000 claims description 5
- 206010009944 Colon cancer Diseases 0.000 claims description 5
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims description 5
- 206010058467 Lung neoplasm malignant Diseases 0.000 claims description 5
- 206010033128 Ovarian cancer Diseases 0.000 claims description 5
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 5
- 206010039491 Sarcoma Diseases 0.000 claims description 5
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 claims description 5
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 claims description 5
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 claims description 5
- 201000010881 cervical cancer Diseases 0.000 claims description 5
- 201000007270 liver cancer Diseases 0.000 claims description 5
- 201000005202 lung cancer Diseases 0.000 claims description 5
- 208000020816 lung neoplasm Diseases 0.000 claims description 5
- 201000001441 melanoma Diseases 0.000 claims description 5
- 244000309459 oncolytic virus Species 0.000 claims description 5
- 201000005112 urinary bladder cancer Diseases 0.000 claims description 5
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 claims description 4
- 208000002008 AIDS-Related Lymphoma Diseases 0.000 claims description 4
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 claims description 4
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 claims description 4
- 208000003950 B-cell lymphoma Diseases 0.000 claims description 4
- 206010004146 Basal cell carcinoma Diseases 0.000 claims description 4
- 206010005949 Bone cancer Diseases 0.000 claims description 4
- 208000018084 Bone neoplasm Diseases 0.000 claims description 4
- 208000006332 Choriocarcinoma Diseases 0.000 claims description 4
- 208000000461 Esophageal Neoplasms Diseases 0.000 claims description 4
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 claims description 4
- 206010019695 Hepatic neoplasm Diseases 0.000 claims description 4
- 208000017604 Hodgkin disease Diseases 0.000 claims description 4
- 208000021519 Hodgkin lymphoma Diseases 0.000 claims description 4
- 208000010747 Hodgkins lymphoma Diseases 0.000 claims description 4
- 206010025312 Lymphoma AIDS related Diseases 0.000 claims description 4
- 206010025323 Lymphomas Diseases 0.000 claims description 4
- 208000025205 Mantle-Cell Lymphoma Diseases 0.000 claims description 4
- 208000003445 Mouth Neoplasms Diseases 0.000 claims description 4
- 206010029260 Neuroblastoma Diseases 0.000 claims description 4
- 206010030155 Oesophageal carcinoma Diseases 0.000 claims description 4
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 4
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 4
- 206010060862 Prostate cancer Diseases 0.000 claims description 4
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 4
- 208000015634 Rectal Neoplasms Diseases 0.000 claims description 4
- 201000000582 Retinoblastoma Diseases 0.000 claims description 4
- 208000004337 Salivary Gland Neoplasms Diseases 0.000 claims description 4
- 206010061934 Salivary gland cancer Diseases 0.000 claims description 4
- 208000000453 Skin Neoplasms Diseases 0.000 claims description 4
- 206010041067 Small cell lung cancer Diseases 0.000 claims description 4
- 208000024313 Testicular Neoplasms Diseases 0.000 claims description 4
- 206010057644 Testis cancer Diseases 0.000 claims description 4
- 208000024770 Thyroid neoplasm Diseases 0.000 claims description 4
- 208000002495 Uterine Neoplasms Diseases 0.000 claims description 4
- 206010047741 Vulval cancer Diseases 0.000 claims description 4
- 208000004354 Vulvar Neoplasms Diseases 0.000 claims description 4
- 201000009036 biliary tract cancer Diseases 0.000 claims description 4
- 208000020790 biliary tract neoplasm Diseases 0.000 claims description 4
- 201000000220 brain stem cancer Diseases 0.000 claims description 4
- 201000007455 central nervous system cancer Diseases 0.000 claims description 4
- 230000001684 chronic effect Effects 0.000 claims description 4
- 201000010918 connective tissue cancer Diseases 0.000 claims description 4
- 201000004101 esophageal cancer Diseases 0.000 claims description 4
- 208000024519 eye neoplasm Diseases 0.000 claims description 4
- 230000003325 follicular Effects 0.000 claims description 4
- 201000003444 follicular lymphoma Diseases 0.000 claims description 4
- 208000005017 glioblastoma Diseases 0.000 claims description 4
- 201000009277 hairy cell leukemia Diseases 0.000 claims description 4
- 201000010536 head and neck cancer Diseases 0.000 claims description 4
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 4
- 208000020082 intraepithelial neoplasia Diseases 0.000 claims description 4
- 208000012987 lip and oral cavity carcinoma Diseases 0.000 claims description 4
- 201000005249 lung adenocarcinoma Diseases 0.000 claims description 4
- 210000004698 lymphocyte Anatomy 0.000 claims description 4
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 4
- 208000025113 myeloid leukemia Diseases 0.000 claims description 4
- 201000000050 myeloid neoplasm Diseases 0.000 claims description 4
- 229960003301 nivolumab Drugs 0.000 claims description 4
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 4
- 201000008106 ocular cancer Diseases 0.000 claims description 4
- 201000002528 pancreatic cancer Diseases 0.000 claims description 4
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 4
- 201000002628 peritoneum cancer Diseases 0.000 claims description 4
- 230000004962 physiological condition Effects 0.000 claims description 4
- 206010038038 rectal cancer Diseases 0.000 claims description 4
- 201000001275 rectum cancer Diseases 0.000 claims description 4
- 201000009410 rhabdomyosarcoma Diseases 0.000 claims description 4
- 201000000849 skin cancer Diseases 0.000 claims description 4
- 208000000587 small cell lung carcinoma Diseases 0.000 claims description 4
- 201000003120 testicular cancer Diseases 0.000 claims description 4
- 239000004308 thiabendazole Substances 0.000 claims description 4
- 201000002510 thyroid cancer Diseases 0.000 claims description 4
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 4
- 206010046766 uterine cancer Diseases 0.000 claims description 4
- 201000005102 vulva cancer Diseases 0.000 claims description 4
- 208000010507 Adenocarcinoma of Lung Diseases 0.000 claims description 3
- 102220638986 Beta-enolase_H16S_mutation Human genes 0.000 claims description 3
- 102100038078 CD276 antigen Human genes 0.000 claims description 3
- 102220603939 Clathrin heavy chain 1_P65N_mutation Human genes 0.000 claims description 3
- 102220606752 Gap junction beta-1 protein_H16P_mutation Human genes 0.000 claims description 3
- 101150112082 Gpnmb gene Proteins 0.000 claims description 3
- 102100035943 HERV-H LTR-associating protein 2 Human genes 0.000 claims description 3
- 101000884279 Homo sapiens CD276 antigen Proteins 0.000 claims description 3
- 101000889276 Homo sapiens Cytotoxic T-lymphocyte protein 4 Proteins 0.000 claims description 3
- 101001021491 Homo sapiens HERV-H LTR-associating protein 2 Proteins 0.000 claims description 3
- 101000955999 Homo sapiens V-set domain-containing T-cell activation inhibitor 1 Proteins 0.000 claims description 3
- 101000666896 Homo sapiens V-type immunoglobulin domain-containing suppressor of T-cell activation Proteins 0.000 claims description 3
- 102220604965 Killer cell immunoglobulin-like receptor 3DL1_M23V_mutation Human genes 0.000 claims description 3
- 102220608429 Mitogen-activated protein kinase kinase kinase kinase 1_E95V_mutation Human genes 0.000 claims description 3
- 102220505697 Palmitoyl-protein thioesterase 1_Y45F_mutation Human genes 0.000 claims description 3
- 102220503469 Superoxide dismutase [Cu-Zn]_D84A_mutation Human genes 0.000 claims description 3
- 102220610852 Thialysine N-epsilon-acetyltransferase_L72G_mutation Human genes 0.000 claims description 3
- 102100038929 V-set domain-containing T-cell activation inhibitor 1 Human genes 0.000 claims description 3
- 102100038282 V-type immunoglobulin domain-containing suppressor of T-cell activation Human genes 0.000 claims description 3
- 102220359607 c.194C>A Human genes 0.000 claims description 3
- 229940127089 cytotoxic agent Drugs 0.000 claims description 3
- IJJVMEJXYNJXOJ-UHFFFAOYSA-N fluquinconazole Chemical compound C=1C=C(Cl)C=C(Cl)C=1N1C(=O)C2=CC(F)=CC=C2N=C1N1C=NC=N1 IJJVMEJXYNJXOJ-UHFFFAOYSA-N 0.000 claims description 3
- 229960005386 ipilimumab Drugs 0.000 claims description 3
- 102220234588 rs1114167484 Human genes 0.000 claims description 3
- 102200000390 rs121917859 Human genes 0.000 claims description 3
- 102220308083 rs1462326368 Human genes 0.000 claims description 3
- 102200150061 rs1553765909 Human genes 0.000 claims description 3
- 102200105319 rs397514675 Human genes 0.000 claims description 3
- 229960000074 biopharmaceutical Drugs 0.000 claims description 2
- 239000000710 homodimer Substances 0.000 claims description 2
- 101710089372 Programmed cell death protein 1 Proteins 0.000 claims 23
- 102100040678 Programmed cell death protein 1 Human genes 0.000 claims 23
- 108010074708 B7-H1 Antigen Proteins 0.000 claims 3
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 claims 3
- 108040006849 interleukin-2 receptor activity proteins Proteins 0.000 claims 3
- 102100029193 Low affinity immunoglobulin gamma Fc region receptor III-A Human genes 0.000 claims 2
- 208000002699 Digestive System Neoplasms Diseases 0.000 claims 1
- 101150046249 Havcr2 gene Proteins 0.000 claims 1
- 206010043515 Throat cancer Diseases 0.000 claims 1
- 102000004060 Transforming Growth Factor-beta Type II Receptor Human genes 0.000 claims 1
- 108010082684 Transforming Growth Factor-beta Type II Receptor Proteins 0.000 claims 1
- 229960003852 atezolizumab Drugs 0.000 claims 1
- 230000001413 cellular effect Effects 0.000 claims 1
- 208000024558 digestive system cancer Diseases 0.000 claims 1
- 229950009791 durvalumab Drugs 0.000 claims 1
- 201000010231 gastrointestinal system cancer Diseases 0.000 claims 1
- 201000005243 lung squamous cell carcinoma Diseases 0.000 claims 1
- 230000002934 lysing effect Effects 0.000 claims 1
- 208000016847 malignant urinary system neoplasm Diseases 0.000 claims 1
- 230000000241 respiratory effect Effects 0.000 claims 1
- 201000004435 urinary system cancer Diseases 0.000 claims 1
- 108010038453 Interleukin-2 Receptors Proteins 0.000 abstract description 49
- 102000010789 Interleukin-2 Receptors Human genes 0.000 abstract description 49
- 230000002829 reductive effect Effects 0.000 abstract description 30
- 102000000588 Interleukin-2 Human genes 0.000 abstract description 6
- 125000003275 alpha amino acid group Chemical group 0.000 description 83
- 108020001507 fusion proteins Proteins 0.000 description 74
- 102000037865 fusion proteins Human genes 0.000 description 74
- 235000001014 amino acid Nutrition 0.000 description 65
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 63
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 63
- 230000014509 gene expression Effects 0.000 description 50
- 230000000694 effects Effects 0.000 description 49
- 210000003289 regulatory T cell Anatomy 0.000 description 46
- 230000035755 proliferation Effects 0.000 description 39
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 38
- 108010034529 leucyl-lysine Proteins 0.000 description 36
- BKZFBJYIVSBXCO-KKUMJFAQSA-N Asn-Phe-His Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CNC=N1)C(O)=O BKZFBJYIVSBXCO-KKUMJFAQSA-N 0.000 description 33
- JNCRAQVYJZGIOW-QSFUFRPTSA-N Asn-Val-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O JNCRAQVYJZGIOW-QSFUFRPTSA-N 0.000 description 33
- XDGBFDYXZCMYEX-NUMRIWBASA-N Asp-Glu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC(=O)O)N)O XDGBFDYXZCMYEX-NUMRIWBASA-N 0.000 description 33
- XLLSMEFANRROJE-GUBZILKMSA-N Cys-Leu-Glu Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CS)N XLLSMEFANRROJE-GUBZILKMSA-N 0.000 description 33
- KPNWAJMEMRCLAL-GUBZILKMSA-N Gln-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)N)N KPNWAJMEMRCLAL-GUBZILKMSA-N 0.000 description 33
- MUSGDMDGNGXULI-DCAQKATOSA-N Glu-Glu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O MUSGDMDGNGXULI-DCAQKATOSA-N 0.000 description 33
- HVKAAUOFFTUSAA-XDTLVQLUSA-N Glu-Tyr-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(O)=O HVKAAUOFFTUSAA-XDTLVQLUSA-N 0.000 description 33
- WXLYNEHOGRYNFU-URLPEUOOSA-N Ile-Thr-Phe Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N WXLYNEHOGRYNFU-URLPEUOOSA-N 0.000 description 33
- 108010078274 isoleucylvaline Proteins 0.000 description 33
- GYBVHTWOQJMYAM-HRCADAONSA-N Tyr-Met-Pro Chemical compound CSCC[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)N GYBVHTWOQJMYAM-HRCADAONSA-N 0.000 description 32
- FSXRLASFHBWESK-UHFFFAOYSA-N dipeptide phenylalanyl-tyrosine Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FSXRLASFHBWESK-UHFFFAOYSA-N 0.000 description 32
- 230000008685 targeting Effects 0.000 description 32
- QNNBHTFDFFFHGC-KKUMJFAQSA-N Asn-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O QNNBHTFDFFFHGC-KKUMJFAQSA-N 0.000 description 31
- RKDFEMGVMMYYNG-WDCWCFNPSA-N Thr-Gln-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O RKDFEMGVMMYYNG-WDCWCFNPSA-N 0.000 description 31
- WKPXXXUSUHAXDE-SRVKXCTJSA-N Arg-Pro-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O WKPXXXUSUHAXDE-SRVKXCTJSA-N 0.000 description 30
- CQMQJWRCRQSBAF-BPUTZDHNSA-N Asn-Arg-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(=O)N)N CQMQJWRCRQSBAF-BPUTZDHNSA-N 0.000 description 30
- AWXDRZJQCVHCIT-DCAQKATOSA-N Asn-Pro-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC(N)=O AWXDRZJQCVHCIT-DCAQKATOSA-N 0.000 description 30
- IVGJYOOGJLFKQE-AVGNSLFASA-N Glu-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N IVGJYOOGJLFKQE-AVGNSLFASA-N 0.000 description 30
- QNJNPKSWAHPYGI-JYJNAYRXSA-N Glu-Phe-Leu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=CC=C1 QNJNPKSWAHPYGI-JYJNAYRXSA-N 0.000 description 30
- CAQXJMUDOLSBPF-SUSMZKCASA-N Glu-Thr-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CAQXJMUDOLSBPF-SUSMZKCASA-N 0.000 description 30
- VIPDPMHGICREIS-GVXVVHGQSA-N Glu-Val-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O VIPDPMHGICREIS-GVXVVHGQSA-N 0.000 description 30
- DGKBSGNCMCLDSL-BYULHYEWSA-N Gly-Ile-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)CN DGKBSGNCMCLDSL-BYULHYEWSA-N 0.000 description 30
- UROVZOUMHNXPLZ-AVGNSLFASA-N His-Leu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CN=CN1 UROVZOUMHNXPLZ-AVGNSLFASA-N 0.000 description 30
- TWYOYAKMLHWMOJ-ZPFDUUQYSA-N Ile-Leu-Asn Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O TWYOYAKMLHWMOJ-ZPFDUUQYSA-N 0.000 description 30
- RQJUKVXWAKJDBW-SVSWQMSJSA-N Ile-Ser-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N RQJUKVXWAKJDBW-SVSWQMSJSA-N 0.000 description 30
- PDQDCFBVYXEFSD-SRVKXCTJSA-N Leu-Leu-Asp Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O PDQDCFBVYXEFSD-SRVKXCTJSA-N 0.000 description 30
- ZDJQVSIPFLMNOX-RHYQMDGZSA-N Leu-Thr-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N ZDJQVSIPFLMNOX-RHYQMDGZSA-N 0.000 description 30
- KNKHAVVBVXKOGX-JXUBOQSCSA-N Lys-Ala-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KNKHAVVBVXKOGX-JXUBOQSCSA-N 0.000 description 30
- FHIAJWBDZVHLAH-YUMQZZPRSA-N Lys-Gly-Ser Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O FHIAJWBDZVHLAH-YUMQZZPRSA-N 0.000 description 30
- XDGFFEZAZHRZFR-RHYQMDGZSA-N Met-Leu-Thr Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XDGFFEZAZHRZFR-RHYQMDGZSA-N 0.000 description 30
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 30
- VHDNDCPMHQMXIR-IHRRRGAJSA-N Phe-Met-Cys Chemical compound SC[C@@H](C(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC1=CC=CC=C1 VHDNDCPMHQMXIR-IHRRRGAJSA-N 0.000 description 30
- YMEXHZTVKDAKIY-GHCJXIJMSA-N Ser-Asn-Ile Chemical compound CC[C@H](C)[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO)C(O)=O YMEXHZTVKDAKIY-GHCJXIJMSA-N 0.000 description 30
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 30
- XYFISNXATOERFZ-OSUNSFLBSA-N Thr-Ile-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N XYFISNXATOERFZ-OSUNSFLBSA-N 0.000 description 30
- MGJLBZFUXUGMML-VOAKCMCISA-N Thr-Lys-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)O)N)O MGJLBZFUXUGMML-VOAKCMCISA-N 0.000 description 30
- LYERIXUFCYVFFX-GVXVVHGQSA-N Val-Leu-Glu Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N LYERIXUFCYVFFX-GVXVVHGQSA-N 0.000 description 30
- 201000010099 disease Diseases 0.000 description 30
- 238000011282 treatment Methods 0.000 description 30
- PNHQRQTVBRDIEF-CIUDSAMLSA-N Asn-Leu-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)N)N PNHQRQTVBRDIEF-CIUDSAMLSA-N 0.000 description 29
- 108010044940 alanylglutamine Proteins 0.000 description 29
- QKCZZAZNMMVICF-DCAQKATOSA-N Gln-Leu-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O QKCZZAZNMMVICF-DCAQKATOSA-N 0.000 description 28
- 108090000623 proteins and genes Proteins 0.000 description 28
- AXZGZMGRBDQTEY-SRVKXCTJSA-N Leu-Gln-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O AXZGZMGRBDQTEY-SRVKXCTJSA-N 0.000 description 27
- 238000000684 flow cytometry Methods 0.000 description 26
- KLXQWABNAWDRAY-ACRUOGEOSA-N Phe-Lys-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 KLXQWABNAWDRAY-ACRUOGEOSA-N 0.000 description 25
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 24
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 24
- 102100026878 Interleukin-2 receptor subunit alpha Human genes 0.000 description 24
- 235000018102 proteins Nutrition 0.000 description 24
- 102000004169 proteins and genes Human genes 0.000 description 24
- RQHLMGCXCZUOGT-ZPFDUUQYSA-N Asp-Leu-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O RQHLMGCXCZUOGT-ZPFDUUQYSA-N 0.000 description 23
- FFZJHQODAYHGPO-KZVJFYERSA-N Ala-Pro-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N FFZJHQODAYHGPO-KZVJFYERSA-N 0.000 description 22
- HHABWQIFXZPZCK-ACZMJKKPSA-N Cys-Gln-Ser Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CS)N HHABWQIFXZPZCK-ACZMJKKPSA-N 0.000 description 22
- 101000835093 Homo sapiens Transferrin receptor protein 1 Proteins 0.000 description 22
- 102100026144 Transferrin receptor protein 1 Human genes 0.000 description 22
- 239000011324 bead Substances 0.000 description 19
- 230000037396 body weight Effects 0.000 description 19
- 239000000203 mixture Substances 0.000 description 19
- HFNPOYOKIPGAEI-SRVKXCTJSA-N Pro-Leu-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1 HFNPOYOKIPGAEI-SRVKXCTJSA-N 0.000 description 18
- 230000000284 resting effect Effects 0.000 description 16
- 230000004913 activation Effects 0.000 description 15
- 230000001225 therapeutic effect Effects 0.000 description 14
- 239000013598 vector Substances 0.000 description 14
- 230000006044 T cell activation Effects 0.000 description 13
- 230000004048 modification Effects 0.000 description 13
- 238000012986 modification Methods 0.000 description 13
- 102000005962 receptors Human genes 0.000 description 12
- 108020003175 receptors Proteins 0.000 description 12
- 230000009467 reduction Effects 0.000 description 12
- 239000013074 reference sample Substances 0.000 description 12
- 230000011664 signaling Effects 0.000 description 12
- OTZMRMHZCMZOJZ-SRVKXCTJSA-N Arg-Leu-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O OTZMRMHZCMZOJZ-SRVKXCTJSA-N 0.000 description 11
- 210000001519 tissue Anatomy 0.000 description 11
- FMDHKPRACUXATF-ACZMJKKPSA-N Ser-Gln-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O FMDHKPRACUXATF-ACZMJKKPSA-N 0.000 description 10
- 230000004071 biological effect Effects 0.000 description 10
- 239000003550 marker Substances 0.000 description 10
- 108010051242 phenylalanylserine Proteins 0.000 description 10
- 239000000539 dimer Substances 0.000 description 9
- 102000040430 polynucleotide Human genes 0.000 description 9
- 108091033319 polynucleotide Proteins 0.000 description 9
- 239000002157 polynucleotide Substances 0.000 description 9
- 239000000523 sample Substances 0.000 description 9
- IPZQNYYAYVRKKK-FXQIFTODSA-N Ala-Pro-Ala Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O IPZQNYYAYVRKKK-FXQIFTODSA-N 0.000 description 8
- 208000035475 disorder Diseases 0.000 description 8
- 238000002474 experimental method Methods 0.000 description 8
- 238000009472 formulation Methods 0.000 description 8
- 108010049041 glutamylalanine Proteins 0.000 description 8
- 230000002401 inhibitory effect Effects 0.000 description 8
- 239000004570 mortar (masonry) Substances 0.000 description 8
- 239000002773 nucleotide Substances 0.000 description 8
- 125000003729 nucleotide group Chemical group 0.000 description 8
- 230000004044 response Effects 0.000 description 8
- 239000000126 substance Substances 0.000 description 8
- 102100026120 IgG receptor FcRn large subunit p51 Human genes 0.000 description 7
- 101710177940 IgG receptor FcRn large subunit p51 Proteins 0.000 description 7
- ATNKHRAIZCMCCN-BZSNNMDCSA-N Lys-Lys-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)N ATNKHRAIZCMCCN-BZSNNMDCSA-N 0.000 description 7
- HEUVHBXOVZONPU-BJDJZHNGSA-N Ser-Leu-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HEUVHBXOVZONPU-BJDJZHNGSA-N 0.000 description 7
- 125000000539 amino acid group Chemical group 0.000 description 7
- 239000000872 buffer Substances 0.000 description 7
- 238000006471 dimerization reaction Methods 0.000 description 7
- 230000001404 mediated effect Effects 0.000 description 7
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 7
- 108020004414 DNA Proteins 0.000 description 6
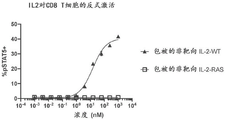
- 102000001712 STAT5 Transcription Factor Human genes 0.000 description 6
- 108010029477 STAT5 Transcription Factor Proteins 0.000 description 6
- 238000003556 assay Methods 0.000 description 6
- 238000010790 dilution Methods 0.000 description 6
- 239000012895 dilution Substances 0.000 description 6
- 239000012636 effector Substances 0.000 description 6
- 230000006870 function Effects 0.000 description 6
- 230000006698 induction Effects 0.000 description 6
- 230000005764 inhibitory process Effects 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- 230000009870 specific binding Effects 0.000 description 6
- 208000024891 symptom Diseases 0.000 description 6
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 6
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 5
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 5
- 210000002744 extracellular matrix Anatomy 0.000 description 5
- 210000002950 fibroblast Anatomy 0.000 description 5
- 239000003446 ligand Substances 0.000 description 5
- 210000004072 lung Anatomy 0.000 description 5
- 238000009099 neoadjuvant therapy Methods 0.000 description 5
- 235000000346 sugar Nutrition 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical group N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- 206010061818 Disease progression Diseases 0.000 description 4
- 108010087819 Fc receptors Proteins 0.000 description 4
- 102000009109 Fc receptors Human genes 0.000 description 4
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 description 4
- 108010073807 IgG Receptors Proteins 0.000 description 4
- 102000009490 IgG Receptors Human genes 0.000 description 4
- 108060003951 Immunoglobulin Proteins 0.000 description 4
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 description 4
- OIQSIMFSVLLWBX-VOAKCMCISA-N Lys-Leu-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OIQSIMFSVLLWBX-VOAKCMCISA-N 0.000 description 4
- 241000124008 Mammalia Species 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 4
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 4
- 230000003213 activating effect Effects 0.000 description 4
- 238000013459 approach Methods 0.000 description 4
- 230000000903 blocking effect Effects 0.000 description 4
- 210000004671 cell-free system Anatomy 0.000 description 4
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 4
- 230000001086 cytosolic effect Effects 0.000 description 4
- 230000001419 dependent effect Effects 0.000 description 4
- 230000005750 disease progression Effects 0.000 description 4
- 210000003527 eukaryotic cell Anatomy 0.000 description 4
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 4
- 230000028993 immune response Effects 0.000 description 4
- 102000018358 immunoglobulin Human genes 0.000 description 4
- 238000002955 isolation Methods 0.000 description 4
- 210000003734 kidney Anatomy 0.000 description 4
- 230000000670 limiting effect Effects 0.000 description 4
- 210000004962 mammalian cell Anatomy 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 210000000130 stem cell Anatomy 0.000 description 4
- 150000008163 sugars Chemical group 0.000 description 4
- 238000001356 surgical procedure Methods 0.000 description 4
- 230000004083 survival effect Effects 0.000 description 4
- 210000004881 tumor cell Anatomy 0.000 description 4
- VNFSAYFQLXPHPY-CIQUZCHMSA-N Ala-Thr-Ile Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O VNFSAYFQLXPHPY-CIQUZCHMSA-N 0.000 description 3
- OISWSORSLQOGFV-AVGNSLFASA-N Arg-Met-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CCCN=C(N)N OISWSORSLQOGFV-AVGNSLFASA-N 0.000 description 3
- BVLIJXXSXBUGEC-SRVKXCTJSA-N Asn-Asn-Tyr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O BVLIJXXSXBUGEC-SRVKXCTJSA-N 0.000 description 3
- PBSQFBAJKPLRJY-BYULHYEWSA-N Asn-Gly-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC(=O)N)N PBSQFBAJKPLRJY-BYULHYEWSA-N 0.000 description 3
- 101100510617 Caenorhabditis elegans sel-8 gene Proteins 0.000 description 3
- 102000014914 Carrier Proteins Human genes 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- YPMDZWPZFOZYFG-GUBZILKMSA-N Gln-Leu-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YPMDZWPZFOZYFG-GUBZILKMSA-N 0.000 description 3
- QJCKNLPMTPXXEM-AUTRQRHGSA-N Glu-Glu-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O QJCKNLPMTPXXEM-AUTRQRHGSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- PFPUFNLHBXKPHY-HTFCKZLJSA-N Ile-Ile-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)O)N PFPUFNLHBXKPHY-HTFCKZLJSA-N 0.000 description 3
- JODPUDMBQBIWCK-GHCJXIJMSA-N Ile-Ser-Asn Chemical compound [H]N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O JODPUDMBQBIWCK-GHCJXIJMSA-N 0.000 description 3
- IBMVEYRWAWIOTN-UHFFFAOYSA-N L-Leucyl-L-Arginyl-L-Proline Natural products CC(C)CC(N)C(=O)NC(CCCN=C(N)N)C(=O)N1CCCC1C(O)=O IBMVEYRWAWIOTN-UHFFFAOYSA-N 0.000 description 3
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 3
- 206010023825 Laryngeal cancer Diseases 0.000 description 3
- IBMVEYRWAWIOTN-RWMBFGLXSA-N Leu-Arg-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(O)=O IBMVEYRWAWIOTN-RWMBFGLXSA-N 0.000 description 3
- IGUOAYLTQJLPPD-DCAQKATOSA-N Leu-Asn-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N IGUOAYLTQJLPPD-DCAQKATOSA-N 0.000 description 3
- JKGHDYGZRDWHGA-SRVKXCTJSA-N Leu-Asn-Leu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O JKGHDYGZRDWHGA-SRVKXCTJSA-N 0.000 description 3
- VQPPIMUZCZCOIL-GUBZILKMSA-N Leu-Gln-Ala Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O VQPPIMUZCZCOIL-GUBZILKMSA-N 0.000 description 3
- LVTJJOJKDCVZGP-QWRGUYRKSA-N Leu-Lys-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O LVTJJOJKDCVZGP-QWRGUYRKSA-N 0.000 description 3
- YNNPKXBBRZVIRX-IHRRRGAJSA-N Lys-Arg-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O YNNPKXBBRZVIRX-IHRRRGAJSA-N 0.000 description 3
- NCTDKZKNBDZDOL-GARJFASQSA-N Lys-Asn-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCCCN)N)C(=O)O NCTDKZKNBDZDOL-GARJFASQSA-N 0.000 description 3
- OWRUUFUVXFREBD-KKUMJFAQSA-N Lys-His-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(O)=O OWRUUFUVXFREBD-KKUMJFAQSA-N 0.000 description 3
- XOQMURBBIXRRCR-SRVKXCTJSA-N Lys-Lys-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCCN XOQMURBBIXRRCR-SRVKXCTJSA-N 0.000 description 3
- 206010027476 Metastases Diseases 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 241000288906 Primates Species 0.000 description 3
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 3
- GZBKRJVCRMZAST-XKBZYTNZSA-N Ser-Glu-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GZBKRJVCRMZAST-XKBZYTNZSA-N 0.000 description 3
- LHEZGZQRLDBSRR-WDCWCFNPSA-N Thr-Glu-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O LHEZGZQRLDBSRR-WDCWCFNPSA-N 0.000 description 3
- VRUFCJZQDACGLH-UVOCVTCTSA-N Thr-Leu-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VRUFCJZQDACGLH-UVOCVTCTSA-N 0.000 description 3
- BDYBHQWMHYDRKJ-UNQGMJICSA-N Thr-Phe-Met Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCSC)C(=O)O)N)O BDYBHQWMHYDRKJ-UNQGMJICSA-N 0.000 description 3
- FOADDSDHGRFUOC-DZKIICNBSA-N Val-Glu-Phe Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N FOADDSDHGRFUOC-DZKIICNBSA-N 0.000 description 3
- HGJRMXOWUWVUOA-GVXVVHGQSA-N Val-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N HGJRMXOWUWVUOA-GVXVVHGQSA-N 0.000 description 3
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 3
- 238000009098 adjuvant therapy Methods 0.000 description 3
- 108700025316 aldesleukin Proteins 0.000 description 3
- 230000004075 alteration Effects 0.000 description 3
- 239000012491 analyte Substances 0.000 description 3
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 3
- 230000000890 antigenic effect Effects 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 108091008324 binding proteins Proteins 0.000 description 3
- 239000003124 biologic agent Substances 0.000 description 3
- 210000000988 bone and bone Anatomy 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 238000002512 chemotherapy Methods 0.000 description 3
- 230000004540 complement-dependent cytotoxicity Effects 0.000 description 3
- 210000004443 dendritic cell Anatomy 0.000 description 3
- 210000002249 digestive system Anatomy 0.000 description 3
- 238000010494 dissociation reaction Methods 0.000 description 3
- 230000005593 dissociations Effects 0.000 description 3
- 239000000975 dye Substances 0.000 description 3
- 210000002889 endothelial cell Anatomy 0.000 description 3
- 239000012634 fragment Substances 0.000 description 3
- 230000004927 fusion Effects 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 238000005734 heterodimerization reaction Methods 0.000 description 3
- 230000001976 improved effect Effects 0.000 description 3
- 210000002602 induced regulatory T cell Anatomy 0.000 description 3
- 230000001939 inductive effect Effects 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 206010023841 laryngeal neoplasm Diseases 0.000 description 3
- 150000002632 lipids Chemical class 0.000 description 3
- 201000000564 macroglobulinemia Diseases 0.000 description 3
- 210000002540 macrophage Anatomy 0.000 description 3
- 210000004882 non-tumor cell Anatomy 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 238000004806 packaging method and process Methods 0.000 description 3
- 230000026731 phosphorylation Effects 0.000 description 3
- 238000006366 phosphorylation reaction Methods 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 230000002062 proliferating effect Effects 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 210000002345 respiratory system Anatomy 0.000 description 3
- 230000000717 retained effect Effects 0.000 description 3
- 108010048818 seryl-histidine Proteins 0.000 description 3
- 230000009450 sialylation Effects 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 238000001890 transfection Methods 0.000 description 3
- 108010051110 tyrosyl-lysine Proteins 0.000 description 3
- 230000002485 urinary effect Effects 0.000 description 3
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- QMOQBVOBWVNSNO-UHFFFAOYSA-N 2-[[2-[[2-[(2-azaniumylacetyl)amino]acetyl]amino]acetyl]amino]acetate Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(O)=O QMOQBVOBWVNSNO-UHFFFAOYSA-N 0.000 description 2
- KZMAWJRXKGLWGS-UHFFFAOYSA-N 2-chloro-n-[4-(4-methoxyphenyl)-1,3-thiazol-2-yl]-n-(3-methoxypropyl)acetamide Chemical compound S1C(N(C(=O)CCl)CCCOC)=NC(C=2C=CC(OC)=CC=2)=C1 KZMAWJRXKGLWGS-UHFFFAOYSA-N 0.000 description 2
- KMFPQTITXUKJOV-DCAQKATOSA-N Arg-Ser-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O KMFPQTITXUKJOV-DCAQKATOSA-N 0.000 description 2
- DONWIPDSZZJHHK-HJGDQZAQSA-N Asp-Lys-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)N)O DONWIPDSZZJHHK-HJGDQZAQSA-N 0.000 description 2
- 241000251730 Chondrichthyes Species 0.000 description 2
- 101100421450 Drosophila melanogaster Shark gene Proteins 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 108010021468 Fc gamma receptor IIA Proteins 0.000 description 2
- 108010021472 Fc gamma receptor IIB Proteins 0.000 description 2
- 241000238631 Hexapoda Species 0.000 description 2
- HZWWOGWOBQBETJ-CUJWVEQBSA-N His-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N)O HZWWOGWOBQBETJ-CUJWVEQBSA-N 0.000 description 2
- 101000935587 Homo sapiens Flavin reductase (NADPH) Proteins 0.000 description 2
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 description 2
- 102000037978 Immune checkpoint receptors Human genes 0.000 description 2
- 108091008028 Immune checkpoint receptors Proteins 0.000 description 2
- KLSUAWUZBMAZCL-RHYQMDGZSA-N Leu-Thr-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(O)=O KLSUAWUZBMAZCL-RHYQMDGZSA-N 0.000 description 2
- 102100029205 Low affinity immunoglobulin gamma Fc region receptor II-b Human genes 0.000 description 2
- 241000282567 Macaca fascicularis Species 0.000 description 2
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 description 2
- CGBYDGAJHSOGFQ-LPEHRKFASA-N Pro-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2 CGBYDGAJHSOGFQ-LPEHRKFASA-N 0.000 description 2
- HWLKHNDRXWTFTN-GUBZILKMSA-N Pro-Pro-Cys Chemical compound C1C[C@H](NC1)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CS)C(=O)O HWLKHNDRXWTFTN-GUBZILKMSA-N 0.000 description 2
- 108020004511 Recombinant DNA Proteins 0.000 description 2
- 241000283984 Rodentia Species 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- 108010090804 Streptavidin Proteins 0.000 description 2
- 230000005867 T cell response Effects 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 229960005310 aldesleukin Drugs 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 210000004102 animal cell Anatomy 0.000 description 2
- 238000005349 anion exchange Methods 0.000 description 2
- 230000000259 anti-tumor effect Effects 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- 108010047857 aspartylglycine Proteins 0.000 description 2
- 230000003115 biocidal effect Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 210000000481 breast Anatomy 0.000 description 2
- 238000005341 cation exchange Methods 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 238000002648 combination therapy Methods 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 108010060199 cysteinylproline Proteins 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 238000003745 diagnosis Methods 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 230000003511 endothelial effect Effects 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 230000017188 evasion or tolerance of host immune response Effects 0.000 description 2
- 230000007717 exclusion Effects 0.000 description 2
- 230000002538 fungal effect Effects 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 238000004191 hydrophobic interaction chromatography Methods 0.000 description 2
- 206010020718 hyperplasia Diseases 0.000 description 2
- 210000002865 immune cell Anatomy 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 238000011221 initial treatment Methods 0.000 description 2
- 230000037041 intracellular level Effects 0.000 description 2
- 210000001165 lymph node Anatomy 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 108020004999 messenger RNA Proteins 0.000 description 2
- 230000009401 metastasis Effects 0.000 description 2
- 238000010369 molecular cloning Methods 0.000 description 2
- 210000001616 monocyte Anatomy 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 210000004985 myeloid-derived suppressor cell Anatomy 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- 210000000440 neutrophil Anatomy 0.000 description 2
- 231100000252 nontoxic Toxicity 0.000 description 2
- 230000003000 nontoxic effect Effects 0.000 description 2
- 230000000174 oncolytic effect Effects 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 210000003668 pericyte Anatomy 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- 239000013641 positive control Substances 0.000 description 2
- 208000017805 post-transplant lymphoproliferative disease Diseases 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 210000001236 prokaryotic cell Anatomy 0.000 description 2
- 108010020755 prolyl-glycyl-glycine Proteins 0.000 description 2
- 108010077112 prolyl-proline Proteins 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 230000009257 reactivity Effects 0.000 description 2
- 238000004064 recycling Methods 0.000 description 2
- 210000001995 reticulocyte Anatomy 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 210000002536 stromal cell Anatomy 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 208000011580 syndromic disease Diseases 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 229950007217 tremelimumab Drugs 0.000 description 2
- 210000003171 tumor-infiltrating lymphocyte Anatomy 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 210000005166 vasculature Anatomy 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 102100022464 5'-nucleotidase Human genes 0.000 description 1
- 239000005660 Abamectin Substances 0.000 description 1
- 206010069754 Acquired gene mutation Diseases 0.000 description 1
- MVBWLRJESQOQTM-ACZMJKKPSA-N Ala-Gln-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O MVBWLRJESQOQTM-ACZMJKKPSA-N 0.000 description 1
- DPNZTBKGAUAZQU-DLOVCJGASA-N Ala-Leu-His Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N DPNZTBKGAUAZQU-DLOVCJGASA-N 0.000 description 1
- BLTRAARCJYVJKV-QEJZJMRPSA-N Ala-Lys-Phe Chemical compound C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(O)=O BLTRAARCJYVJKV-QEJZJMRPSA-N 0.000 description 1
- OINVDEKBKBCPLX-JXUBOQSCSA-N Ala-Lys-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OINVDEKBKBCPLX-JXUBOQSCSA-N 0.000 description 1
- DEWWPUNXRNGMQN-LPEHRKFASA-N Ala-Met-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CCSC)C(=O)N1CCC[C@@H]1C(=O)O)N DEWWPUNXRNGMQN-LPEHRKFASA-N 0.000 description 1
- 239000012114 Alexa Fluor 647 Substances 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 244000303258 Annona diversifolia Species 0.000 description 1
- 235000002198 Annona diversifolia Nutrition 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- 101001007348 Arachis hypogaea Galactose-binding lectin Proteins 0.000 description 1
- OTCJMMRQBVDQRK-DCAQKATOSA-N Arg-Asp-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O OTCJMMRQBVDQRK-DCAQKATOSA-N 0.000 description 1
- ZJBUILVYSXQNSW-YTWAJWBKSA-N Arg-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O ZJBUILVYSXQNSW-YTWAJWBKSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- SRUUBQBAVNQZGJ-LAEOZQHASA-N Asn-Gln-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC(=O)N)N SRUUBQBAVNQZGJ-LAEOZQHASA-N 0.000 description 1
- CTQIOCMSIJATNX-WHFBIAKZSA-N Asn-Gly-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](C)C(O)=O CTQIOCMSIJATNX-WHFBIAKZSA-N 0.000 description 1
- WQLJRNRLHWJIRW-KKUMJFAQSA-N Asn-His-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CC2=CN=CN2)NC(=O)[C@H](CC(=O)N)N)O WQLJRNRLHWJIRW-KKUMJFAQSA-N 0.000 description 1
- HNXWVVHIGTZTBO-LKXGYXEUSA-N Asn-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O HNXWVVHIGTZTBO-LKXGYXEUSA-N 0.000 description 1
- CUQDCPXNZPDYFQ-ZLUOBGJFSA-N Asp-Ser-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O CUQDCPXNZPDYFQ-ZLUOBGJFSA-N 0.000 description 1
- JSNWZMFSLIWAHS-HJGDQZAQSA-N Asp-Thr-Leu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)O)NC(=O)[C@H](CC(=O)O)N)O JSNWZMFSLIWAHS-HJGDQZAQSA-N 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 108091008875 B cell receptors Proteins 0.000 description 1
- 230000003844 B-cell-activation Effects 0.000 description 1
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 1
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 1
- 229940125565 BMS-986016 Drugs 0.000 description 1
- 102100026189 Beta-galactosidase Human genes 0.000 description 1
- 229920002799 BoPET Polymers 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 102000049320 CD36 Human genes 0.000 description 1
- 108010045374 CD36 Antigens Proteins 0.000 description 1
- 241000282832 Camelidae Species 0.000 description 1
- 241000282465 Canis Species 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 description 1
- 108010077544 Chromatin Proteins 0.000 description 1
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 1
- 108091035707 Consensus sequence Proteins 0.000 description 1
- GMXSSZUVDNPRMA-FXQIFTODSA-N Cys-Arg-Asp Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O GMXSSZUVDNPRMA-FXQIFTODSA-N 0.000 description 1
- OHLLDUNVMPPUMD-DCAQKATOSA-N Cys-Leu-Val Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CS)N OHLLDUNVMPPUMD-DCAQKATOSA-N 0.000 description 1
- NDNZRWUDUMTITL-FXQIFTODSA-N Cys-Ser-Val Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NDNZRWUDUMTITL-FXQIFTODSA-N 0.000 description 1
- LEVWYRKDKASIDU-QWWZWVQMSA-N D-cystine Chemical compound OC(=O)[C@H](N)CSSC[C@@H](N)C(O)=O LEVWYRKDKASIDU-QWWZWVQMSA-N 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 206010058314 Dysplasia Diseases 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 241000976823 Embernagra platensis Species 0.000 description 1
- 241000283073 Equus caballus Species 0.000 description 1
- 241000282324 Felis Species 0.000 description 1
- 102100037362 Fibronectin Human genes 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- 229910052688 Gadolinium Inorganic materials 0.000 description 1
- NVEASDQHBRZPSU-BQBZGAKWSA-N Gln-Gln-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O NVEASDQHBRZPSU-BQBZGAKWSA-N 0.000 description 1
- ZEEPYMXTJWIMSN-GUBZILKMSA-N Gln-Lys-Ser Chemical compound NCCCC[C@@H](C(=O)N[C@@H](CO)C(O)=O)NC(=O)[C@@H](N)CCC(N)=O ZEEPYMXTJWIMSN-GUBZILKMSA-N 0.000 description 1
- DOMHVQBSRJNNKD-ZPFDUUQYSA-N Gln-Met-Ile Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O DOMHVQBSRJNNKD-ZPFDUUQYSA-N 0.000 description 1
- XUMFMAVDHQDATI-DCAQKATOSA-N Gln-Pro-Arg Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O XUMFMAVDHQDATI-DCAQKATOSA-N 0.000 description 1
- HMIXCETWRYDVMO-GUBZILKMSA-N Gln-Pro-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O HMIXCETWRYDVMO-GUBZILKMSA-N 0.000 description 1
- CKOFNWCLWRYUHK-XHNCKOQMSA-N Glu-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)O)N)C(=O)O CKOFNWCLWRYUHK-XHNCKOQMSA-N 0.000 description 1
- HUFCEIHAFNVSNR-IHRRRGAJSA-N Glu-Gln-Tyr Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HUFCEIHAFNVSNR-IHRRRGAJSA-N 0.000 description 1
- IRXNJYPKBVERCW-DCAQKATOSA-N Glu-Leu-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O IRXNJYPKBVERCW-DCAQKATOSA-N 0.000 description 1
- AAJHGGDRKHYSDH-GUBZILKMSA-N Glu-Pro-Gln Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CCC(=O)O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O AAJHGGDRKHYSDH-GUBZILKMSA-N 0.000 description 1
- BPCLDCNZBUYGOD-BPUTZDHNSA-N Glu-Trp-Glu Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)N)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=CNC2=C1 BPCLDCNZBUYGOD-BPUTZDHNSA-N 0.000 description 1
- ZYRXTRTUCAVNBQ-GVXVVHGQSA-N Glu-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZYRXTRTUCAVNBQ-GVXVVHGQSA-N 0.000 description 1
- WGYHAAXZWPEBDQ-IFFSRLJSSA-N Glu-Val-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WGYHAAXZWPEBDQ-IFFSRLJSSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- BUEFQXUHTUZXHR-LURJTMIESA-N Gly-Gly-Pro zwitterion Chemical compound NCC(=O)NCC(=O)N1CCC[C@H]1C(O)=O BUEFQXUHTUZXHR-LURJTMIESA-N 0.000 description 1
- GMTXWRIDLGTVFC-IUCAKERBSA-N Gly-Lys-Glu Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O GMTXWRIDLGTVFC-IUCAKERBSA-N 0.000 description 1
- JSLVAHYTAJJEQH-QWRGUYRKSA-N Gly-Ser-Phe Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 JSLVAHYTAJJEQH-QWRGUYRKSA-N 0.000 description 1
- SYOJVRNQCXYEOV-XVKPBYJWSA-N Gly-Val-Glu Chemical compound [H]NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O SYOJVRNQCXYEOV-XVKPBYJWSA-N 0.000 description 1
- 208000002927 Hamartoma Diseases 0.000 description 1
- FYVHHKMHFPMBBG-GUBZILKMSA-N His-Gln-Asp Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N FYVHHKMHFPMBBG-GUBZILKMSA-N 0.000 description 1
- YAALVYQFVJNXIV-KKUMJFAQSA-N His-Leu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CN=CN1 YAALVYQFVJNXIV-KKUMJFAQSA-N 0.000 description 1
- 101000678236 Homo sapiens 5'-nucleotidase Proteins 0.000 description 1
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 1
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 1
- 101100005713 Homo sapiens CD4 gene Proteins 0.000 description 1
- 101001103039 Homo sapiens Inactive tyrosine-protein kinase transmembrane receptor ROR1 Proteins 0.000 description 1
- 101000998120 Homo sapiens Interleukin-3 receptor subunit alpha Proteins 0.000 description 1
- 101000878605 Homo sapiens Low affinity immunoglobulin epsilon Fc receptor Proteins 0.000 description 1
- 101000946889 Homo sapiens Monocyte differentiation antigen CD14 Proteins 0.000 description 1
- 101001103036 Homo sapiens Nuclear receptor ROR-alpha Proteins 0.000 description 1
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 1
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- MKWSZEHGHSLNPF-NAKRPEOUSA-N Ile-Ala-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)O)N MKWSZEHGHSLNPF-NAKRPEOUSA-N 0.000 description 1
- DFJJAVZIHDFOGQ-MNXVOIDGSA-N Ile-Glu-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N DFJJAVZIHDFOGQ-MNXVOIDGSA-N 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 102100039615 Inactive tyrosine-protein kinase transmembrane receptor ROR1 Human genes 0.000 description 1
- 102100033493 Interleukin-3 receptor subunit alpha Human genes 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 1
- 101150030213 Lag3 gene Proteins 0.000 description 1
- DLCOFDAHNMMQPP-SRVKXCTJSA-N Leu-Asp-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O DLCOFDAHNMMQPP-SRVKXCTJSA-N 0.000 description 1
- UHNQRAFSEBGZFZ-YESZJQIVSA-N Leu-Phe-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N UHNQRAFSEBGZFZ-YESZJQIVSA-N 0.000 description 1
- DPURXCQCHSQPAN-AVGNSLFASA-N Leu-Pro-Pro Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 DPURXCQCHSQPAN-AVGNSLFASA-N 0.000 description 1
- XOWMDXHFSBCAKQ-SRVKXCTJSA-N Leu-Ser-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C XOWMDXHFSBCAKQ-SRVKXCTJSA-N 0.000 description 1
- SBANPBVRHYIMRR-UHFFFAOYSA-N Leu-Ser-Pro Natural products CC(C)CC(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O SBANPBVRHYIMRR-UHFFFAOYSA-N 0.000 description 1
- DAYQSYGBCUKVKT-VOAKCMCISA-N Leu-Thr-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(O)=O DAYQSYGBCUKVKT-VOAKCMCISA-N 0.000 description 1
- AIQWYVFNBNNOLU-RHYQMDGZSA-N Leu-Thr-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O AIQWYVFNBNNOLU-RHYQMDGZSA-N 0.000 description 1
- VUBIPAHVHMZHCM-KKUMJFAQSA-N Leu-Tyr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CC=C(O)C=C1 VUBIPAHVHMZHCM-KKUMJFAQSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 102000019298 Lipocalin Human genes 0.000 description 1
- 108050006654 Lipocalin Proteins 0.000 description 1
- 102100038007 Low affinity immunoglobulin epsilon Fc receptor Human genes 0.000 description 1
- 102100029204 Low affinity immunoglobulin gamma Fc region receptor II-a Human genes 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- KCXUCYYZNZFGLL-SRVKXCTJSA-N Lys-Ala-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O KCXUCYYZNZFGLL-SRVKXCTJSA-N 0.000 description 1
- WSXTWLJHTLRFLW-SRVKXCTJSA-N Lys-Ala-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O WSXTWLJHTLRFLW-SRVKXCTJSA-N 0.000 description 1
- LUTDBHBIHHREDC-IHRRRGAJSA-N Lys-Pro-Lys Chemical compound NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O LUTDBHBIHHREDC-IHRRRGAJSA-N 0.000 description 1
- GILLQRYAWOMHED-DCAQKATOSA-N Lys-Val-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN GILLQRYAWOMHED-DCAQKATOSA-N 0.000 description 1
- 241000282560 Macaca mulatta Species 0.000 description 1
- 206010027145 Melanocytic naevus Diseases 0.000 description 1
- RKIIYGUHIQJCBW-SRVKXCTJSA-N Met-His-Glu Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(O)=O RKIIYGUHIQJCBW-SRVKXCTJSA-N 0.000 description 1
- FWAHLGXNBLWIKB-NAKRPEOUSA-N Met-Ile-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCSC FWAHLGXNBLWIKB-NAKRPEOUSA-N 0.000 description 1
- 108020005196 Mitochondrial DNA Proteins 0.000 description 1
- 102100035877 Monocyte differentiation antigen CD14 Human genes 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 101000574441 Mus musculus Alkaline phosphatase, germ cell type Proteins 0.000 description 1
- 101100425758 Mus musculus Tnfrsf1b gene Proteins 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 239000005041 Mylar™ Substances 0.000 description 1
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 1
- 230000006051 NK cell activation Effects 0.000 description 1
- 208000003788 Neoplasm Micrometastasis Diseases 0.000 description 1
- 208000007256 Nevus Diseases 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 108010038807 Oligopeptides Proteins 0.000 description 1
- 102000015636 Oligopeptides Human genes 0.000 description 1
- 238000012408 PCR amplification Methods 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- JOXIIFVCSATTDH-IHPCNDPISA-N Phe-Asn-Trp Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)N JOXIIFVCSATTDH-IHPCNDPISA-N 0.000 description 1
- 241000276498 Pollachius virens Species 0.000 description 1
- 208000037062 Polyps Diseases 0.000 description 1
- IHCXPSYCHXFXKT-DCAQKATOSA-N Pro-Arg-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O IHCXPSYCHXFXKT-DCAQKATOSA-N 0.000 description 1
- GMJDSFYVTAMIBF-FXQIFTODSA-N Pro-Ser-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O GMJDSFYVTAMIBF-FXQIFTODSA-N 0.000 description 1
- KHRLUIPIMIQFGT-AVGNSLFASA-N Pro-Val-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O KHRLUIPIMIQFGT-AVGNSLFASA-N 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 208000006265 Renal cell carcinoma Diseases 0.000 description 1
- 108700008625 Reporter Genes Proteins 0.000 description 1
- HZWAHWQZPSXNCB-BPUTZDHNSA-N Ser-Arg-Trp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O HZWAHWQZPSXNCB-BPUTZDHNSA-N 0.000 description 1
- VAUMZJHYZQXZBQ-WHFBIAKZSA-N Ser-Asn-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O VAUMZJHYZQXZBQ-WHFBIAKZSA-N 0.000 description 1
- GVIGVIOEYBOTCB-XIRDDKMYSA-N Ser-Leu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CO)CC(C)C)C(O)=O)=CNC2=C1 GVIGVIOEYBOTCB-XIRDDKMYSA-N 0.000 description 1
- RHAPJNVNWDBFQI-BQBZGAKWSA-N Ser-Pro-Gly Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O RHAPJNVNWDBFQI-BQBZGAKWSA-N 0.000 description 1
- HNDMFDBQXYZSRM-IHRRRGAJSA-N Ser-Val-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O HNDMFDBQXYZSRM-IHRRRGAJSA-N 0.000 description 1
- 108020004459 Small interfering RNA Proteins 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 1
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 1
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 1
- ZESGVALRVJIVLZ-VFCFLDTKSA-N Thr-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@@H]1C(=O)O)N)O ZESGVALRVJIVLZ-VFCFLDTKSA-N 0.000 description 1
- 101710120037 Toxin CcdB Proteins 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- XGFGVFMXDXALEV-XIRDDKMYSA-N Trp-Leu-Asn Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N XGFGVFMXDXALEV-XIRDDKMYSA-N 0.000 description 1
- QYSBJAUCUKHSLU-JYJNAYRXSA-N Tyr-Arg-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O QYSBJAUCUKHSLU-JYJNAYRXSA-N 0.000 description 1
- GZUIDWDVMWZSMI-KKUMJFAQSA-N Tyr-Lys-Cys Chemical compound NCCCC[C@@H](C(=O)N[C@@H](CS)C(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 GZUIDWDVMWZSMI-KKUMJFAQSA-N 0.000 description 1
- SQUMHUZLJDUROQ-YDHLFZDLSA-N Tyr-Val-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O SQUMHUZLJDUROQ-YDHLFZDLSA-N 0.000 description 1
- BMGOFDMKDVVGJG-NHCYSSNCSA-N Val-Asp-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N BMGOFDMKDVVGJG-NHCYSSNCSA-N 0.000 description 1
- OACSGBOREVRSME-NHCYSSNCSA-N Val-His-Asn Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(N)=O)C(O)=O OACSGBOREVRSME-NHCYSSNCSA-N 0.000 description 1
- KISFXYYRKKNLOP-IHRRRGAJSA-N Val-Phe-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)O)N KISFXYYRKKNLOP-IHRRRGAJSA-N 0.000 description 1
- KRAHMIJVUPUOTQ-DCAQKATOSA-N Val-Ser-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N KRAHMIJVUPUOTQ-DCAQKATOSA-N 0.000 description 1
- NZYNRRGJJVSSTJ-GUBZILKMSA-N Val-Ser-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NZYNRRGJJVSSTJ-GUBZILKMSA-N 0.000 description 1
- BGTDGENDNWGMDQ-KJEVXHAQSA-N Val-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](C(C)C)N)O BGTDGENDNWGMDQ-KJEVXHAQSA-N 0.000 description 1
- ZLNYBMWGPOKSLW-LSJOCFKGSA-N Val-Val-Asp Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLNYBMWGPOKSLW-LSJOCFKGSA-N 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 238000002679 ablation Methods 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 210000005006 adaptive immune system Anatomy 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 1
- 229960000548 alemtuzumab Drugs 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 210000004381 amniotic fluid Anatomy 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 238000005571 anion exchange chromatography Methods 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000692 anti-sense effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 230000009831 antigen interaction Effects 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 108010038633 aspartylglutamate Proteins 0.000 description 1
- RRZXIRBKKLTSOM-XPNPUAGNSA-N avermectin B1a Chemical compound C1=C[C@H](C)[C@@H]([C@@H](C)CC)O[C@]11O[C@H](C\C=C(C)\[C@@H](O[C@@H]2O[C@@H](C)[C@H](O[C@@H]3O[C@@H](C)[C@H](O)[C@@H](OC)C3)[C@@H](OC)C2)[C@@H](C)\C=C\C=C/2[C@]3([C@H](C(=O)O4)C=C(C)[C@@H](O)[C@H]3OC\2)O)C[C@H]4C1 RRZXIRBKKLTSOM-XPNPUAGNSA-N 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- 239000012472 biological sample Substances 0.000 description 1
- 239000000090 biomarker Substances 0.000 description 1
- 230000002051 biphasic effect Effects 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 238000002619 cancer immunotherapy Methods 0.000 description 1
- 208000035269 cancer or benign tumor Diseases 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000005277 cation exchange chromatography Methods 0.000 description 1
- 230000020411 cell activation Effects 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 210000003483 chromatin Anatomy 0.000 description 1
- 238000012761 co-transfection Methods 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 238000004737 colorimetric analysis Methods 0.000 description 1
- 238000007398 colorimetric assay Methods 0.000 description 1
- 230000009137 competitive binding Effects 0.000 description 1
- 108091008034 costimulatory receptors Proteins 0.000 description 1
- 238000000315 cryotherapy Methods 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 1
- 229960003067 cystine Drugs 0.000 description 1
- 230000016396 cytokine production Effects 0.000 description 1
- 102000003675 cytokine receptors Human genes 0.000 description 1
- 108010057085 cytokine receptors Proteins 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 230000002222 downregulating effect Effects 0.000 description 1
- 230000007783 downstream signaling Effects 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 210000003162 effector t lymphocyte Anatomy 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 210000001163 endosome Anatomy 0.000 description 1
- 108010048367 enhanced green fluorescent protein Proteins 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 210000003754 fetus Anatomy 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000009459 flexible packaging Methods 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 102000006815 folate receptor Human genes 0.000 description 1
- UIWYJDYFSGRHKR-UHFFFAOYSA-N gadolinium atom Chemical compound [Gd] UIWYJDYFSGRHKR-UHFFFAOYSA-N 0.000 description 1
- 230000005021 gait Effects 0.000 description 1
- 230000030279 gene silencing Effects 0.000 description 1
- 210000004602 germ cell Anatomy 0.000 description 1
- 108010013768 glutamyl-aspartyl-proline Proteins 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 108010010147 glycylglutamine Proteins 0.000 description 1
- 108010015792 glycyllysine Proteins 0.000 description 1
- 230000003862 health status Effects 0.000 description 1
- 210000002216 heart Anatomy 0.000 description 1
- 230000002489 hematologic effect Effects 0.000 description 1
- 210000003494 hepatocyte Anatomy 0.000 description 1
- 230000013632 homeostatic process Effects 0.000 description 1
- 230000028996 humoral immune response Effects 0.000 description 1
- 210000004408 hybridoma Anatomy 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000003053 immunization Effects 0.000 description 1
- 238000002649 immunization Methods 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 108091008042 inhibitory receptors Proteins 0.000 description 1
- 210000005007 innate immune system Anatomy 0.000 description 1
- 210000002570 interstitial cell Anatomy 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 238000012004 kinetic exclusion assay Methods 0.000 description 1
- 229910052747 lanthanoid Inorganic materials 0.000 description 1
- 150000002602 lanthanoids Chemical class 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 1
- 108010091871 leucylmethionine Proteins 0.000 description 1
- 108010057821 leucylproline Proteins 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 238000012417 linear regression Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 230000001926 lymphatic effect Effects 0.000 description 1
- 230000000527 lymphocytic effect Effects 0.000 description 1
- 210000005210 lymphoid organ Anatomy 0.000 description 1
- 108010003700 lysyl aspartic acid Proteins 0.000 description 1
- 108010064235 lysylglycine Proteins 0.000 description 1
- 230000008774 maternal effect Effects 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000005541 medical transmission Effects 0.000 description 1
- 210000003071 memory t lymphocyte Anatomy 0.000 description 1
- 210000002901 mesenchymal stem cell Anatomy 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 108091070501 miRNA Proteins 0.000 description 1
- 230000000116 mitigating effect Effects 0.000 description 1
- 238000012434 mixed-mode chromatography Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 229950001907 monalizumab Drugs 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 230000006654 negative regulation of apoptotic process Effects 0.000 description 1
- 238000011227 neoadjuvant chemotherapy Methods 0.000 description 1
- 238000002515 oligonucleotide synthesis Methods 0.000 description 1
- 210000000963 osteoblast Anatomy 0.000 description 1
- 229940127084 other anti-cancer agent Drugs 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 239000003002 pH adjusting agent Substances 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 210000003800 pharynx Anatomy 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 108010073101 phenylalanylleucine Proteins 0.000 description 1
- 108010073025 phenylalanylphenylalanine Proteins 0.000 description 1
- 229950010773 pidilizumab Drugs 0.000 description 1
- 210000002826 placenta Anatomy 0.000 description 1
- 210000002381 plasma Anatomy 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 230000023603 positive regulation of transcription initiation, DNA-dependent Effects 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- 238000011533 pre-incubation Methods 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 229940087463 proleukin Drugs 0.000 description 1
- 108010070643 prolylglutamic acid Proteins 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical group [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 238000012797 qualification Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 229930002330 retinoic acid Natural products 0.000 description 1
- 230000001177 retroviral effect Effects 0.000 description 1
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 1
- 238000009738 saturating Methods 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 108010048397 seryl-lysyl-leucine Proteins 0.000 description 1
- 231100000004 severe toxicity Toxicity 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 210000002027 skeletal muscle Anatomy 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 210000000813 small intestine Anatomy 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000012064 sodium phosphate buffer Substances 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 230000037439 somatic mutation Effects 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 239000012192 staining solution Substances 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 1
- 210000001179 synovial fluid Anatomy 0.000 description 1
- 210000002437 synoviocyte Anatomy 0.000 description 1
- 210000001550 testis Anatomy 0.000 description 1
- 108010071097 threonyl-lysyl-proline Proteins 0.000 description 1
- 238000000954 titration curve Methods 0.000 description 1
- 125000002088 tosyl group Chemical group [H]C1=C([H])C(=C([H])C([H])=C1C([H])([H])[H])S(*)(=O)=O 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000003146 transient transfection Methods 0.000 description 1
- 238000011277 treatment modality Methods 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 239000013638 trimer Substances 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 231100000402 unacceptable toxicity Toxicity 0.000 description 1
- 230000004222 uncontrolled growth Effects 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 210000004291 uterus Anatomy 0.000 description 1
- 238000002255 vaccination Methods 0.000 description 1
- 108010052774 valyl-lysyl-glycyl-phenylalanyl-tyrosine Proteins 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 108010037501 vesicular transport factor p115 Proteins 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/52—Cytokines; Lymphokines; Interferons
- C07K14/54—Interleukins [IL]
- C07K14/55—IL-2
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2812—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD4
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2815—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD8
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2851—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the lectin superfamily, e.g. CD23, CD72
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2878—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2896—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against molecules with a "CD"-designation, not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Peptides Or Proteins (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201962789075P | 2019-01-07 | 2019-01-07 | |
| US62/789,075 | 2019-01-07 | ||
| PCT/US2020/012296 WO2020146221A1 (en) | 2019-01-07 | 2020-01-06 | Polypeptides comprising modified il-2 polypeptides and uses thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN113924311A true CN113924311A (zh) | 2022-01-11 |
Family
ID=69374426
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202080018011.6A Pending CN113924311A (zh) | 2019-01-07 | 2020-01-06 | 包含修饰的il-2多肽的多肽及其用途 |
Country Status (15)
| Country | Link |
|---|---|
| US (1) | US20220089667A1 (enExample) |
| EP (1) | EP3908596A1 (enExample) |
| JP (2) | JP7594161B2 (enExample) |
| KR (1) | KR20210113265A (enExample) |
| CN (1) | CN113924311A (enExample) |
| AR (1) | AR117770A1 (enExample) |
| AU (1) | AU2020206672B2 (enExample) |
| BR (1) | BR112021012294A2 (enExample) |
| CA (1) | CA3125529A1 (enExample) |
| CL (1) | CL2021001779A1 (enExample) |
| IL (1) | IL284633A (enExample) |
| MX (1) | MX2021008147A (enExample) |
| SG (1) | SG11202106700WA (enExample) |
| TW (1) | TWI874345B (enExample) |
| WO (1) | WO2020146221A1 (enExample) |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SG11202000939PA (en) | 2017-08-03 | 2020-02-27 | Synthorx Inc | Cytokine conjugates for the treatment of proliferative and infectious diseases |
| EP3746095A4 (en) | 2018-02-01 | 2021-04-21 | Nkmax Co., Ltd. | Method of producing natural killer cells and composition for treating cancer |
| AU2019376076B9 (en) | 2018-11-08 | 2025-06-19 | Synthorx, Inc. | Interleukin 10 conjugates and uses thereof |
| WO2021041206A1 (en) | 2019-08-23 | 2021-03-04 | Synthorx, Inc. | Il-15 conjugates and uses thereof |
| TW202124385A (zh) | 2019-09-10 | 2021-07-01 | 美商欣爍克斯公司 | 治療自體免疫疾病之il-2接合物及使用方法 |
| JP2023531876A (ja) * | 2020-06-30 | 2023-07-26 | ジーアイ イノベーション, インコーポレイテッド | 抗lag-3抗体とil-2とを含む融合タンパク質及びその使用 |
| MX2022016532A (es) * | 2020-07-02 | 2023-04-12 | Inhibrx Inc | Polipeptidos que comprenden polipeptidos de il-2 modificados y usos de los mismos. |
| IL302276A (en) | 2020-10-23 | 2023-06-01 | Asher Biotherapeutics Inc | Fusions with cd8 antigen binding molecules for modulating immune cell function |
| US20220144956A1 (en) * | 2020-11-06 | 2022-05-12 | Xencor, Inc. | HETERODIMERIC ANTIBODIES THAT BIND TGFbetaRII |
| CA3196844A1 (en) | 2020-11-25 | 2022-06-02 | Raphael Rozenfeld | Tumor-specific cleavable linkers |
| EP4259213A4 (en) * | 2020-12-09 | 2024-11-20 | Asher Biotherapeutics, Inc. | TARGETED CYTOKINE CONSTRUCT FOR MANIPULATED CELL THERAPY |
| CN112979782B (zh) * | 2021-03-08 | 2023-07-18 | 深圳市乐土生物医药有限公司 | 一种多肽及其用途 |
| AU2022252307A1 (en) * | 2021-03-31 | 2023-10-12 | Anwita Biosciences, Inc. | Fusion proteins, pharmaceutical compositions, and therapeutic applications |
| WO2023004305A1 (en) * | 2021-07-20 | 2023-01-26 | Inhibrx, Inc. | Cd8-targeted modified il-2 polypeptides and uses thereof |
| CA3225092A1 (en) * | 2021-07-20 | 2023-01-26 | John C. Timmer | Cd8-binding polypeptides and uses thereof |
| WO2023034740A1 (en) * | 2021-08-30 | 2023-03-09 | Inhibrx, Inc. | Nkp46-binding polypeptides and uses thereof |
| TW202328171A (zh) * | 2021-08-30 | 2023-07-16 | 美商英伊布里克斯公司 | 靶向NKp46之經修飾之IL-2多肽及其用途 |
| CN118019849A (zh) * | 2021-09-26 | 2024-05-10 | 上海药明生物技术有限公司 | Il-2变体及其融合蛋白 |
| TW202334193A (zh) * | 2022-01-05 | 2023-09-01 | 美商英伊布里克斯公司 | 靶向γδ T細胞之經修飾IL-2多肽及其用途 |
| JP2025528019A (ja) * | 2022-07-28 | 2025-08-26 | プロヴィヴァ セラピューティクス (ホン コン) リミテッド | Il-2プロサイトカイン抗体融合タンパク質 |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005086798A2 (en) * | 2004-03-05 | 2005-09-22 | Chiron Corporation | Improved interleukin-2 muteins |
| CN101426916A (zh) * | 2004-03-05 | 2009-05-06 | 诺华疫苗和诊断公司 | 改进的白介素-2突变蛋白 |
| CN102101885A (zh) * | 2010-09-01 | 2011-06-22 | 宋波 | 低诱导抑制性t细胞的人白细胞介素ⅱ突变体及其用途 |
| CN103492411A (zh) * | 2011-02-10 | 2014-01-01 | 罗切格利卡特公司 | 突变体白介素-2多肽 |
| CN105980410A (zh) * | 2014-02-06 | 2016-09-28 | 豪夫迈·罗氏有限公司 | 白介素-2融合蛋白和其用途 |
| WO2018217989A1 (en) * | 2017-05-24 | 2018-11-29 | Pandion Therapeutics, Inc. | Targeted immunotolerance |
| CN109071623A (zh) * | 2016-05-04 | 2018-12-21 | 美国安进公司 | 用于扩增t调节性细胞的白细胞介素-2突变蛋白 |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US6548640B1 (en) | 1986-03-27 | 2003-04-15 | Btg International Limited | Altered antibodies |
| EP0940468A1 (en) | 1991-06-14 | 1999-09-08 | Genentech, Inc. | Humanized antibody variable domain |
| BR9813365A (pt) | 1997-12-05 | 2004-06-15 | Scripps Research Inst | Método para produção e humanização de um anticorpo monoclonal de rato |
| DK1454138T3 (da) * | 2001-12-04 | 2012-02-13 | Merck Patent Gmbh | Immunocytokiner med moduleret selektivitet |
| US7217797B2 (en) | 2002-10-15 | 2007-05-15 | Pdl Biopharma, Inc. | Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis |
| WO2005040395A1 (en) | 2003-10-22 | 2005-05-06 | Keck Graduate Institute | Methods of synthesizing heteromultimeric polypeptides in yeast using a haploid mating strategy |
| MXPA06011199A (es) | 2004-03-31 | 2007-04-16 | Genentech Inc | Anticuerpos anti-tgf-beta humanizados. |
| KR102677704B1 (ko) * | 2012-05-30 | 2024-06-21 | 추가이 세이야쿠 가부시키가이샤 | 표적 조직 특이적 항원 결합 분자 |
| AU2017206618A1 (en) * | 2016-01-11 | 2018-07-05 | Universität Zürich | Combination therapy comprising a superagonistic antibody against interleukin-2 and a checkpoint blockade agent |
| KR102619015B1 (ko) * | 2017-03-15 | 2023-12-28 | 큐 바이오파마, 인크. | 면역 반응을 조절하는 방법 |
| WO2018184965A1 (en) * | 2017-04-03 | 2018-10-11 | F. Hoffmann-La Roche Ag | Immunoconjugates of il-2 with an anti-pd-1 and tim-3 bispecific antibody |
| FI3606955T3 (fi) * | 2017-04-05 | 2025-01-08 | Hoffmann La Roche | Pd1:een ja lag3:een spesifisesti sitoutuvia bispesifisiä vasta-aineita |
| US11542312B2 (en) * | 2017-06-19 | 2023-01-03 | Medicenna Therapeutics, Inc. | IL-2 superagonists in combination with anti-PD-1 antibodies |
-
2020
- 2020-01-06 CA CA3125529A patent/CA3125529A1/en active Pending
- 2020-01-06 WO PCT/US2020/012296 patent/WO2020146221A1/en not_active Ceased
- 2020-01-06 AU AU2020206672A patent/AU2020206672B2/en active Active
- 2020-01-06 EP EP20702547.9A patent/EP3908596A1/en active Pending
- 2020-01-06 US US17/418,458 patent/US20220089667A1/en active Pending
- 2020-01-06 AR ARP200100028A patent/AR117770A1/es unknown
- 2020-01-06 JP JP2021538982A patent/JP7594161B2/ja active Active
- 2020-01-06 BR BR112021012294-0A patent/BR112021012294A2/pt unknown
- 2020-01-06 MX MX2021008147A patent/MX2021008147A/es unknown
- 2020-01-06 SG SG11202106700WA patent/SG11202106700WA/en unknown
- 2020-01-06 TW TW109100336A patent/TWI874345B/zh active
- 2020-01-06 KR KR1020217024431A patent/KR20210113265A/ko active Pending
- 2020-01-06 CN CN202080018011.6A patent/CN113924311A/zh active Pending
-
2021
- 2021-07-05 IL IL284633A patent/IL284633A/en unknown
- 2021-07-05 CL CL2021001779A patent/CL2021001779A1/es unknown
-
2024
- 2024-10-22 JP JP2024185692A patent/JP2025013911A/ja active Pending
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005086798A2 (en) * | 2004-03-05 | 2005-09-22 | Chiron Corporation | Improved interleukin-2 muteins |
| CN101426916A (zh) * | 2004-03-05 | 2009-05-06 | 诺华疫苗和诊断公司 | 改进的白介素-2突变蛋白 |
| CN102101885A (zh) * | 2010-09-01 | 2011-06-22 | 宋波 | 低诱导抑制性t细胞的人白细胞介素ⅱ突变体及其用途 |
| CN103492411A (zh) * | 2011-02-10 | 2014-01-01 | 罗切格利卡特公司 | 突变体白介素-2多肽 |
| CN105980410A (zh) * | 2014-02-06 | 2016-09-28 | 豪夫迈·罗氏有限公司 | 白介素-2融合蛋白和其用途 |
| CN109071623A (zh) * | 2016-05-04 | 2018-12-21 | 美国安进公司 | 用于扩增t调节性细胞的白细胞介素-2突变蛋白 |
| WO2018217989A1 (en) * | 2017-05-24 | 2018-11-29 | Pandion Therapeutics, Inc. | Targeted immunotolerance |
Also Published As
| Publication number | Publication date |
|---|---|
| AR117770A1 (es) | 2021-08-25 |
| BR112021012294A2 (pt) | 2021-09-08 |
| KR20210113265A (ko) | 2021-09-15 |
| US20220089667A1 (en) | 2022-03-24 |
| TW202043259A (zh) | 2020-12-01 |
| MX2021008147A (es) | 2021-08-11 |
| WO2020146221A1 (en) | 2020-07-16 |
| JP2025013911A (ja) | 2025-01-28 |
| JP2022516557A (ja) | 2022-02-28 |
| JP7594161B2 (ja) | 2024-12-04 |
| AU2020206672B2 (en) | 2025-10-02 |
| TWI874345B (zh) | 2025-03-01 |
| CA3125529A1 (en) | 2020-07-16 |
| SG11202106700WA (en) | 2021-07-29 |
| AU2020206672A1 (en) | 2021-07-15 |
| IL284633A (en) | 2021-08-31 |
| EP3908596A1 (en) | 2021-11-17 |
| CL2021001779A1 (es) | 2022-03-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7594161B2 (ja) | 修飾il-2ポリペプチドを含むポリペプチド及びその使用 | |
| CN116615440A (zh) | 包含修饰的il-2多肽的多肽及其用途 | |
| JP7462611B2 (ja) | Ox40結合性ポリペプチド及びその使用 | |
| CN114127115A (zh) | 结合CLEC12a的多肽及其用途 | |
| JP2024526835A (ja) | Cd8結合ポリペプチド及びその使用 | |
| JP2024534838A (ja) | NKp46結合ポリペプチド及びその使用 | |
| CN114040927A (zh) | 结合cd33的多肽及其用途 | |
| WO2023004305A1 (en) | Cd8-targeted modified il-2 polypeptides and uses thereof | |
| TW202328171A (zh) | 靶向NKp46之經修飾之IL-2多肽及其用途 | |
| JP2025504363A (ja) | γδT細胞結合ポリペプチド及びその使用 | |
| RU2849624C2 (ru) | Полипептиды, содержащие модифицированный полипептид il-2, и их применения | |
| TW202334193A (zh) | 靶向γδ T細胞之經修飾IL-2多肽及其用途 | |
| HK40050757A (en) | Ox40-binding polypeptides and uses thereof | |
| CN117980335A (zh) | Cd8结合多肽及其用途 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| TA01 | Transfer of patent application right | ||
| TA01 | Transfer of patent application right |
Effective date of registration: 20240926 Address after: California, USA Applicant after: Yinxibi Bioscience Co.,Ltd. Country or region after: U.S.A. Address before: California, USA Applicant before: INHIBRX L.P. Country or region before: U.S.A. |