CN104428417B - 利用针对确定抗原产生保护性体液免疫应答的重组酵母进行的接种 - Google Patents
利用针对确定抗原产生保护性体液免疫应答的重组酵母进行的接种 Download PDFInfo
- Publication number
- CN104428417B CN104428417B CN201280062015.XA CN201280062015A CN104428417B CN 104428417 B CN104428417 B CN 104428417B CN 201280062015 A CN201280062015 A CN 201280062015A CN 104428417 B CN104428417 B CN 104428417B
- Authority
- CN
- China
- Prior art keywords
- protein
- recombinant yeast
- kluyveromyces lactis
- yeast
- ibdv
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0002—Fungal antigens, e.g. Trichophyton, Aspergillus, Candida
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/16—Antivirals for RNA viruses for influenza or rhinoviruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
- A61P31/22—Antivirals for DNA viruses for herpes viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/80—Vectors or expression systems specially adapted for eukaryotic hosts for fungi
- C12N15/81—Vectors or expression systems specially adapted for eukaryotic hosts for fungi for yeasts
- C12N15/815—Vectors or expression systems specially adapted for eukaryotic hosts for fungi for yeasts for yeasts other than Saccharomyces
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/569—Immunoassay; Biospecific binding assay; Materials therefor for microorganisms, e.g. protozoa, bacteria, viruses
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6854—Immunoglobulins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/52—Bacterial cells; Fungal cells; Protozoal cells
- A61K2039/521—Bacterial cells; Fungal cells; Protozoal cells inactivated (killed)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/55—Medicinal preparations containing antigens or antibodies characterised by the host/recipient, e.g. newborn with maternal antibodies
- A61K2039/552—Veterinary vaccine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55566—Emulsions, e.g. Freund's adjuvant, MF59
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2720/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsRNA viruses
- C12N2720/00011—Details
- C12N2720/10011—Birnaviridae
- C12N2720/10034—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Virology (AREA)
- Genetics & Genomics (AREA)
- Medicinal Chemistry (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Mycology (AREA)
- Biomedical Technology (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Epidemiology (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Plant Pathology (AREA)
- Gastroenterology & Hepatology (AREA)
- Cell Biology (AREA)
- Food Science & Technology (AREA)
- Analytical Chemistry (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102011121069.9 | 2011-12-13 | ||
| DE102011121069A DE102011121069A1 (de) | 2011-12-13 | 2011-12-13 | Vakzinierung mittels rekombinanter Hefe durch Erzeugung einer protektiven humoralen Immunantwort gegen definierte Antigene |
| PCT/DE2012/001205 WO2013107436A1 (de) | 2011-12-13 | 2012-12-12 | Vakzinierung mittels rekombinanter hefe durch erzeugung einer protektiven humoralen immunantwort gegen definierte antigene |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN104428417A CN104428417A (zh) | 2015-03-18 |
| CN104428417B true CN104428417B (zh) | 2020-05-05 |
Family
ID=47681476
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201280062015.XA Active CN104428417B (zh) | 2011-12-13 | 2012-12-12 | 利用针对确定抗原产生保护性体液免疫应答的重组酵母进行的接种 |
Country Status (15)
| Country | Link |
|---|---|
| US (1) | US11065312B2 (enExample) |
| EP (1) | EP2844759B1 (enExample) |
| JP (1) | JP6313215B2 (enExample) |
| KR (1) | KR102027400B1 (enExample) |
| CN (1) | CN104428417B (enExample) |
| BR (1) | BR112014014390B1 (enExample) |
| CA (1) | CA2859231C (enExample) |
| DE (2) | DE102011121069A1 (enExample) |
| DK (1) | DK2844759T3 (enExample) |
| ES (1) | ES2690792T3 (enExample) |
| MX (1) | MX368484B (enExample) |
| PL (1) | PL2844759T3 (enExample) |
| RU (1) | RU2630620C2 (enExample) |
| WO (1) | WO2013107436A1 (enExample) |
| ZA (1) | ZA201405034B (enExample) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102017012109A1 (de) * | 2017-12-27 | 2019-06-27 | Martin-Luther-Universität Halle-Wittenberg | Optimiertes Wirts-/Vektorsystem zur Erzeugung protektiver mono- und multivalenter subunit-Vakzine auf Basis der Hefe Kluyveromyces lactis |
| CN109467606A (zh) * | 2018-11-15 | 2019-03-15 | 大连理工大学 | 一种大肠杆菌肠毒素STa-LTB-STb融合蛋白及其编码基因和应用 |
| JP7372647B2 (ja) * | 2019-06-27 | 2023-11-01 | 国立研究開発法人農業・食品産業技術総合研究機構 | α‐リノレン酸産生能が向上した酵母またはその培養物 |
| CN110646614B (zh) * | 2019-07-15 | 2023-05-23 | 新乡学院 | Ibdv抗体的elisa试剂盒和测试方法、有效抗体效价确定方法 |
| CN114870006A (zh) * | 2022-04-13 | 2022-08-09 | 河北农业大学 | 一种etec疫苗及其应用 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20110293659A1 (en) * | 2008-11-14 | 2011-12-01 | Karin Breunig | Method for the oral/mucosal vaccination by means of recombinant yeasts |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0474676A4 (en) * | 1989-05-30 | 1993-02-24 | The Commonwealth Scientific And Industrial Research Organization | Production of ibdv vp2 in highly immunogenic form |
| US5830463A (en) | 1993-07-07 | 1998-11-03 | University Technology Corporation | Yeast-based delivery vehicles |
| US6234977B1 (en) | 2000-01-28 | 2001-05-22 | Michael Christy | Variable-force monofilament sensory device and methods of using same |
| US7083787B2 (en) | 2000-11-15 | 2006-08-01 | Globeimmune, Inc. | Yeast-dendritic cell vaccines and uses thereof |
| JP4646903B2 (ja) * | 2003-03-24 | 2011-03-09 | インターベツト・インターナシヨナル・ベー・ベー | 伝染性ファブリキウス嚢病ウイルス(ibdv)の変異株およびワクチン |
| ES2242542B1 (es) * | 2004-04-30 | 2006-12-16 | Consejo Superior De Investigaciones Cientificas | Procedimiento para la produccion en levaduras de capsidas virales vacias compuestas por proteinas derivadas de pvp2 del virus causante de la enfermedad de la bursitis infecciosa (ibdv). |
| EP1784211A4 (en) * | 2004-07-29 | 2010-06-30 | Novartis Vaccines & Diagnostic | IMMUNOGENIC COMPOSITIONS FOR GRAMPOSITIVE BACTERIA SUCH AS STREPTOCOCCUS AGALACTIAE |
| AU2005295317B2 (en) | 2004-10-18 | 2011-10-13 | Globeimmune, Inc. | Yeast-based therapeutic for chronic hepatitis C infection |
| MX2008009929A (es) | 2006-02-02 | 2008-10-01 | Globeimmune Inc | Vacuna a base de levadura para inducir una respuesta inmune. |
| WO2008153339A2 (en) * | 2007-06-12 | 2008-12-18 | Univ Sungkyunkwan Found | A vaccine for treating or preventing pasteurellosis using a yeast surface-expression system |
| CN101638824B (zh) | 2009-03-16 | 2012-08-08 | 浙江双友物流器械股份有限公司 | 一种耐磨织带 |
| JP5913103B2 (ja) | 2009-09-14 | 2016-04-27 | ザ リージェンツ オブ ザ ユニバーシティ オブ コロラド,ア ボディー コーポレイトTHE REGENTS OF THE UNIVERSITY OF COLORADO,a body corporate | 酵母ベースの免疫療法組成物を含む組合せ物及び被験者のスクリーニング方法 |
-
2011
- 2011-12-13 DE DE102011121069A patent/DE102011121069A1/de not_active Ceased
-
2012
- 2012-12-12 DE DE112012005213.7T patent/DE112012005213A5/de not_active Withdrawn
- 2012-12-12 ES ES12823073.7T patent/ES2690792T3/es active Active
- 2012-12-12 CA CA2859231A patent/CA2859231C/en active Active
- 2012-12-12 US US14/364,238 patent/US11065312B2/en active Active
- 2012-12-12 KR KR1020147019123A patent/KR102027400B1/ko active Active
- 2012-12-12 WO PCT/DE2012/001205 patent/WO2013107436A1/de not_active Ceased
- 2012-12-12 RU RU2014127435A patent/RU2630620C2/ru active
- 2012-12-12 BR BR112014014390-0A patent/BR112014014390B1/pt active IP Right Grant
- 2012-12-12 CN CN201280062015.XA patent/CN104428417B/zh active Active
- 2012-12-12 PL PL12823073T patent/PL2844759T3/pl unknown
- 2012-12-12 MX MX2014007183A patent/MX368484B/es active IP Right Grant
- 2012-12-12 DK DK12823073.7T patent/DK2844759T3/en active
- 2012-12-12 JP JP2014546315A patent/JP6313215B2/ja active Active
- 2012-12-12 EP EP12823073.7A patent/EP2844759B1/de active Active
-
2014
- 2014-07-10 ZA ZA2014/05034A patent/ZA201405034B/en unknown
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20110293659A1 (en) * | 2008-11-14 | 2011-12-01 | Karin Breunig | Method for the oral/mucosal vaccination by means of recombinant yeasts |
Non-Patent Citations (2)
| Title |
|---|
| Development and large-scale use of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens;Jacob Pitcovski et al;《Vaccine》;20031231;第21卷;4736-4743 * |
| Infectious bursal disease virus isolate D78 segment A polyprotein gene, partial cds;Jackwood,D.J et al;《GenBank: EU162087.1》;20080709;ORIGIN * |
Also Published As
| Publication number | Publication date |
|---|---|
| MX2014007183A (es) | 2014-11-25 |
| ZA201405034B (en) | 2015-12-23 |
| EP2844759B1 (de) | 2018-07-18 |
| JP6313215B2 (ja) | 2018-04-18 |
| CA2859231C (en) | 2019-04-30 |
| CA2859231A1 (en) | 2013-07-25 |
| MX368484B (es) | 2019-10-04 |
| BR112014014390B1 (pt) | 2022-08-09 |
| US20150190486A1 (en) | 2015-07-09 |
| KR102027400B1 (ko) | 2019-10-04 |
| WO2013107436A1 (de) | 2013-07-25 |
| US11065312B2 (en) | 2021-07-20 |
| DK2844759T3 (en) | 2018-11-05 |
| RU2014127435A (ru) | 2016-02-10 |
| WO2013107436A8 (de) | 2015-01-15 |
| EP2844759A1 (de) | 2015-03-11 |
| ES2690792T3 (es) | 2018-11-22 |
| KR20140105821A (ko) | 2014-09-02 |
| CN104428417A (zh) | 2015-03-18 |
| DE112012005213A5 (de) | 2014-11-13 |
| BR112014014390A2 (pt) | 2020-12-08 |
| PL2844759T3 (pl) | 2019-01-31 |
| RU2630620C2 (ru) | 2017-09-11 |
| JP2015507472A (ja) | 2015-03-12 |
| DE102011121069A1 (de) | 2013-06-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
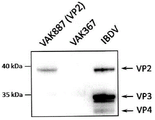
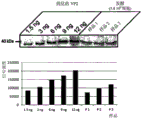
| Arnold et al. | Protective vaccination against infectious bursal disease virus with whole recombinant Kluyveromyces lactis yeast expressing the viral VP2 subunit | |
| JP5508252B2 (ja) | 鳥インフルエンザ遺伝子を含有する組み換え七面鳥ヘルペスウイルス | |
| JP2010187679A (ja) | 新規な組換えヘルペスウイルスおよび変異体ヘルペスウイルス | |
| CN104428417B (zh) | 利用针对确定抗原产生保护性体液免疫应答的重组酵母进行的接种 | |
| US11696947B2 (en) | H52 IBV vaccine with heterologous spike protein | |
| US12473562B2 (en) | Optimized host/vector system for producing protective mono-and multivalent subunit vaccines on the basis of the yeast Kluyveromyces lactis | |
| JP2017527306A (ja) | rDNA NTS基盤の遺伝子多重挿入カセットセット、及びそれを用いたGRAS等級の組換え酵母菌株 | |
| KR101526053B1 (ko) | 재조합 백신 개발을 위한 효모 야로위아 리폴리티카에서 코돈 최적화된 원형 및 n 단편-절단 형태의 노다 캡시드 단백질 생산 방법 | |
| Gebauer et al. | Subunit vaccines based on recombinant yeast protect against influenza A virus in a one-shot vaccination scheme | |
| US20220023413A1 (en) | Recombinant mumps virus vaccine expressing genotype g fusion and hemagglutinin-neuraminidase proteins | |
| CN102741414B (zh) | 通过重组酵母口服/粘膜接种疫苗的方法 | |
| CN116492455B (zh) | 非洲猪瘟病毒k421r基因及利用其制备的复制缺陷型非洲猪瘟疫苗 | |
| CN109750006A (zh) | 一种犬瘟热病毒复制缺陷毒株及其构建方法 | |
| Slibinskas et al. | Synthesis of mumps virus nucleocapsid protein in yeast Pichia pastoris | |
| KR20230119635A (ko) | 백신 제조를 위한 효모 플랫폼 | |
| NL2030423B1 (en) | Replication-deficient strain of canine distemper virus and construction method thereof | |
| Goudarzi et al. | Expression of the VP2 gene of classical D78 infectious bursal disease virus in the methylotrophic yeast Pichia pastoris as a secretory protein | |
| HK40037655A (en) | Live-attenuated flaviruses with heterologous antigens | |
| EA045050B1 (ru) | Оптимизированная система вектор-хозяин для получения защитной моно- и поливалентной субъединичной вакцины на основе дрожжей kluyveromyces lactis | |
| KR20150021344A (ko) | 비병원성 전염성낭병 변이 바이러스 및 이의 백신으로써의 용도 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| TA01 | Transfer of patent application right |
Effective date of registration: 20190930 Address after: Harley Applicant after: Vero vaccine Co., Ltd. Address before: Harley Applicant before: Martin-luther-universitaet Halle-wittenberg |
|
| TA01 | Transfer of patent application right | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant |