CN102159189B - 用于药物递送的纳米载体 - Google Patents
用于药物递送的纳米载体 Download PDFInfo
- Publication number
- CN102159189B CN102159189B CN2009801372511A CN200980137251A CN102159189B CN 102159189 B CN102159189 B CN 102159189B CN 2009801372511 A CN2009801372511 A CN 2009801372511A CN 200980137251 A CN200980137251 A CN 200980137251A CN 102159189 B CN102159189 B CN 102159189B
- Authority
- CN
- China
- Prior art keywords
- peg
- acid
- nano
- carrier
- polymer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 0 C***(*C)NC Chemical compound C***(*C)NC 0.000 description 3
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/0002—General or multifunctional contrast agents, e.g. chelated agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/59—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes
- A61K47/60—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes the organic macromolecular compound being a polyoxyalkylene oligomer, polymer or dendrimer, e.g. PEG, PPG, PEO or polyglycerol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6905—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a colloid or an emulsion
- A61K47/6907—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a colloid or an emulsion the form being a microemulsion, nanoemulsion or micelle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/001—Preparation for luminescence or biological staining
- A61K49/0013—Luminescence
- A61K49/0017—Fluorescence in vivo
- A61K49/0019—Fluorescence in vivo characterised by the fluorescent group, e.g. oligomeric, polymeric or dendritic molecules
- A61K49/0021—Fluorescence in vivo characterised by the fluorescent group, e.g. oligomeric, polymeric or dendritic molecules the fluorescent group being a small organic molecule
- A61K49/0032—Methine dyes, e.g. cyanine dyes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/001—Preparation for luminescence or biological staining
- A61K49/0063—Preparation for luminescence or biological staining characterised by a special physical or galenical form, e.g. emulsions, microspheres
- A61K49/0069—Preparation for luminescence or biological staining characterised by a special physical or galenical form, e.g. emulsions, microspheres the agent being in a particular physical galenical form
- A61K49/0076—Preparation for luminescence or biological staining characterised by a special physical or galenical form, e.g. emulsions, microspheres the agent being in a particular physical galenical form dispersion, suspension, e.g. particles in a liquid, colloid, emulsion
- A61K49/0082—Preparation for luminescence or biological staining characterised by a special physical or galenical form, e.g. emulsions, microspheres the agent being in a particular physical galenical form dispersion, suspension, e.g. particles in a liquid, colloid, emulsion micelle, e.g. phospholipidic micelle and polymeric micelle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/107—Emulsions ; Emulsion preconcentrates; Micelles
- A61K9/1075—Microemulsions or submicron emulsions; Preconcentrates or solids thereof; Micelles, e.g. made of phospholipids or block copolymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/51—Nanocapsules; Nanoparticles
- A61K9/5107—Excipients; Inactive ingredients
- A61K9/513—Organic macromolecular compounds; Dendrimers
- A61K9/5146—Organic macromolecular compounds; Dendrimers obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyethylene glycol, polyamines, polyanhydrides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y5/00—Nanobiotechnology or nanomedicine, e.g. protein engineering or drug delivery
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nanotechnology (AREA)
- Biomedical Technology (AREA)
- Dispersion Chemistry (AREA)
- Biophysics (AREA)
- Molecular Biology (AREA)
- Optics & Photonics (AREA)
- Physics & Mathematics (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Medical Informatics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US9927208P | 2008-09-23 | 2008-09-23 | |
| US61/099,272 | 2008-09-23 | ||
| PCT/US2009/057852 WO2010039496A2 (en) | 2008-09-23 | 2009-09-22 | Nanocarriers for drug delivery |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN102159189A CN102159189A (zh) | 2011-08-17 |
| CN102159189B true CN102159189B (zh) | 2013-06-12 |
Family
ID=41508806
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
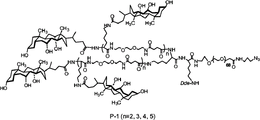
| CN2009801372511A Active CN102159189B (zh) | 2008-09-23 | 2009-09-22 | 用于药物递送的纳米载体 |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US9579400B2 (cg-RX-API-DMAC7.html) |
| EP (1) | EP2344134B1 (cg-RX-API-DMAC7.html) |
| JP (1) | JP5539993B2 (cg-RX-API-DMAC7.html) |
| CN (1) | CN102159189B (cg-RX-API-DMAC7.html) |
| WO (1) | WO2010039496A2 (cg-RX-API-DMAC7.html) |
Families Citing this family (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090136937A1 (en) | 2007-05-09 | 2009-05-28 | Coleman Matthew A | Methods and systems for monitoring production of a target protein in a nanolipoprotein particle |
| US10106650B2 (en) | 2011-05-13 | 2018-10-23 | The Regents Of The University Of California | Reversibly crosslinked micelle systems |
| EP3473273A1 (en) | 2011-07-29 | 2019-04-24 | Avelas Biosciences, Inc. | Selective delivery molecules and methods of use |
| US9644038B2 (en) | 2011-12-21 | 2017-05-09 | The Regents Of The University Of California | Apolipoprotein nanodiscs with telodendrimer |
| US8895055B2 (en) * | 2011-12-21 | 2014-11-25 | The Regents Of The University Of California | Telodendrimer nanodiscs without apolipoprotein |
| US10406233B2 (en) * | 2011-12-21 | 2019-09-10 | The Regents Of The University Of California | Telodendrimers with enhanced drug delivery |
| CA2873572C (en) * | 2012-05-16 | 2021-07-06 | Mewa Singh | Pharmaceutical compositions for the delivery of substantially water-insoluble drugs |
| US9642916B2 (en) * | 2012-12-12 | 2017-05-09 | The Regents Of The University Of California | Porphyrin modified telodendrimers |
| HK1217720A1 (zh) | 2013-01-30 | 2017-01-20 | Avelas Acquisition Corporation | 选择性递送分子及使用方法 |
| US11406714B2 (en) | 2013-08-21 | 2022-08-09 | The Research Foundation For The State University Of New York | Telodendrimers and nanocarriers and methods of using same |
| EP3204048A4 (en) | 2014-10-07 | 2018-06-27 | The Research Foundation for the State University of New York | Functional segregated telodendrimers and nanocarriers and methods of making and using same |
| WO2016168607A1 (en) * | 2015-04-15 | 2016-10-20 | University Of Tennessee Research Foundation | Compositions and methods of muc13 antibodies for cancer treatment and diagnosis |
| TWI614023B (zh) * | 2015-05-20 | 2018-02-11 | 免疫功坊股份有限公司 | 具有標的部分及效應部分的胜肽核多臂接合物 |
| KR102081192B1 (ko) * | 2015-07-20 | 2020-02-25 | 에스티팜 주식회사 | 경구투여가 어려운 약물의 경구투여를 위한 신규한 약물 전달체 및 이의 제조방법 |
| US12226529B2 (en) | 2015-08-25 | 2025-02-18 | Lawrence Livermore National Security, Llc | Stable nanolipoprotein particles and related compositions methods and systems |
| JP2018527350A (ja) * | 2015-09-01 | 2018-09-20 | イミュンワーク インク.Immunwork Inc. | 血餅形成の予防及び/又は血栓症の治療のための分子構築物 |
| US11279749B2 (en) | 2015-09-11 | 2022-03-22 | Lawrence Livermore National Security, Llc | Synthetic apolipoproteins, and related compositions methods and systems for nanolipoprotein particles formation |
| CN108601949A (zh) * | 2016-01-14 | 2018-09-28 | 加利福尼亚大学董事会 | 用llp2a-二膦酸盐化合物治疗骨坏死的方法 |
| CN110392583B (zh) * | 2016-09-15 | 2023-01-03 | 加利福尼亚大学董事会 | 改进的杂化的末端树枝状聚合物 |
| CN108159422B (zh) * | 2016-12-07 | 2020-09-15 | 上海时莱生物技术有限公司 | 一种自组装载药系统及其复合制剂的制备方法 |
| WO2018136778A1 (en) | 2017-01-19 | 2018-07-26 | The Research Foundation For The State University Of New York | Telodendrimers with riboflavin moieties and nanocarriers and methods of making and using same |
| WO2018160759A1 (en) * | 2017-02-28 | 2018-09-07 | The Research Foundation For The State University Of New York | Zwitterionic dendritic amphiphiles, zwitterionic dendrimers, zwitterionic telodendrimers, nanocarriers comprising same, and methods of making and using same |
| WO2018204421A2 (en) | 2017-05-02 | 2018-11-08 | Lawrence Livermore National Security, Llc | Momp telonanoparticles, and related compositions, methods and systems |
| WO2018204495A1 (en) | 2017-05-02 | 2018-11-08 | Synthetic Genomics, Inc. | Nanolipoprotein particles and related compositions methods and systems for loading rna |
| WO2019079277A1 (en) * | 2017-10-16 | 2019-04-25 | Dynamic Biologics Inc. | POLYMERIC COMPOSITIONS OF MONOMETHYL FUMARATE FOR THE TREATMENT OF RECURRENT-REMEDY PLAQUE SCLEROSIS AND PSORIASIS |
| CN107955153B (zh) * | 2017-11-29 | 2020-12-15 | 南京拉克森生物医药科技有限公司 | 一种聚乙二醇-去氧胆酸及其衍生物的制备方法和应用 |
| CA3150959A1 (en) * | 2019-08-14 | 2021-02-18 | The Regents Of The University Of California | Smart peptides and transformable nanoparticles for cancer immunotherapy |
| CA3164919A1 (en) * | 2019-12-17 | 2021-06-24 | The Regents Of The University Of California | Sequential targeting in crosslinking nano-theranostics for treating brain tumors |
| CN111189747B (zh) * | 2020-01-05 | 2022-03-29 | 天津大学 | 一种针对颗粒聚结成球技术的三元溶剂体系筛选方法 |
| KR20210122492A (ko) * | 2020-04-01 | 2021-10-12 | 에스티팜 주식회사 | 항암 요법을 위한 헤파린-담즙산 올리고머 컨쥬게이트 |
| CN112679504B (zh) * | 2020-12-24 | 2022-04-19 | 河北工业大学 | 两亲性共轭寡聚物及其制备及利用其自组装制备得到的载药纳米粒子 |
| CN119661825B (zh) * | 2023-09-19 | 2025-11-14 | 上海交通大学 | 聚两性离子-胆酸及其合成以及其在药物递送中的应用 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1310612A (zh) * | 1998-05-20 | 2001-08-29 | 利普索姆公司 | 新型颗粒制剂 |
| US6365191B1 (en) * | 1999-02-17 | 2002-04-02 | Dabur Research Foundation | Formulations of paclitaxel, its derivatives or its analogs entrapped into nanoparticles of polymeric micelles, process for preparing same and the use thereof |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020041898A1 (en) | 2000-01-05 | 2002-04-11 | Unger Evan C. | Novel targeted delivery systems for bioactive agents |
| AU2002953073A0 (en) | 2002-11-21 | 2003-01-16 | Access Pharmaceuticals Australia Pty Limited | Amplification of biotin-mediated targeting |
| KR100578382B1 (ko) | 2004-07-16 | 2006-05-11 | 나재운 | 항암제의 전달체용 수용성 키토산 나노입자 및 그 제조방법 |
| US7846893B2 (en) | 2005-02-16 | 2010-12-07 | Rutgers, The State University Of New Jersey | Drug-polymer conjugates coupled to a peptidic carrier |
| WO2007084126A1 (en) * | 2006-01-19 | 2007-07-26 | Allexcel, Inc. | Solubilization and targeted delivery of drugs with self-assembling amphiphilic polymers |
| BRPI0716808A2 (pt) | 2006-09-15 | 2013-11-05 | Enzon Pharmaceuticals Inc | Ligantes poliméricos baseados em lisina |
-
2009
- 2009-09-22 JP JP2011528068A patent/JP5539993B2/ja active Active
- 2009-09-22 US US13/120,140 patent/US9579400B2/en active Active
- 2009-09-22 WO PCT/US2009/057852 patent/WO2010039496A2/en not_active Ceased
- 2009-09-22 EP EP09740775.3A patent/EP2344134B1/en active Active
- 2009-09-22 CN CN2009801372511A patent/CN102159189B/zh active Active
-
2017
- 2017-01-23 US US15/412,912 patent/US10556021B2/en active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1310612A (zh) * | 1998-05-20 | 2001-08-29 | 利普索姆公司 | 新型颗粒制剂 |
| US6365191B1 (en) * | 1999-02-17 | 2002-04-02 | Dabur Research Foundation | Formulations of paclitaxel, its derivatives or its analogs entrapped into nanoparticles of polymeric micelles, process for preparing same and the use thereof |
Non-Patent Citations (4)
| Title |
|---|
| DUNCAN R.THE DAWNING ERA OF POLYMER THERAPEUTICS.《NATURE REVIEWS. DRUG DISCOVERY》.2003,第2卷(第1期),347-360. * |
| RUXANDRA GREF ET AL.Biodegradable Long-Circulating Polymeric Nanospheres.《SCIENCE》.1994,第263卷(第5153期),1600-1603. * |
| VIJAYALAKSHMI N ET AL.A simple construction of a bile acid based dendritic light harvesting system.《ORGANIC LETTERS》.2005,第7卷(第13期),2727-2730. * |
| YILONG CHEN ET AL.Fluorescence study of inclusion complexes between star-shaped cholic acid derivatives and polycyclic aromatic fluorescent probes and the size effects of host.《JOURNAL OF PHYSICAL CHEMISTRY B》.2008,第112卷(第11期),3402-3409. * |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5539993B2 (ja) | 2014-07-02 |
| WO2010039496A3 (en) | 2010-07-01 |
| US20170290921A1 (en) | 2017-10-12 |
| US9579400B2 (en) | 2017-02-28 |
| CN102159189A (zh) | 2011-08-17 |
| JP2012503603A (ja) | 2012-02-09 |
| EP2344134B1 (en) | 2017-11-08 |
| US10556021B2 (en) | 2020-02-11 |
| WO2010039496A2 (en) | 2010-04-08 |
| US20110286915A1 (en) | 2011-11-24 |
| EP2344134A2 (en) | 2011-07-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102159189B (zh) | 用于药物递送的纳米载体 | |
| US11192978B2 (en) | Reversibly crosslinked micelle systems | |
| US9642916B2 (en) | Porphyrin modified telodendrimers | |
| Zhang et al. | A pH-sensitive nanosystem based on carboxymethyl chitosan for tumor-targeted delivery of daunorubicin | |
| Mohammadifar et al. | Polyamidoamine and polyglycerol; their linear, dendritic and linear–dendritic architectures as anticancer drug delivery systems | |
| US10406233B2 (en) | Telodendrimers with enhanced drug delivery | |
| Gheybi et al. | Supramolecular anticancer drug delivery systems based on linear–dendritic copolymers | |
| EP3347053B1 (en) | Functional, segregated, charged telodendrimers and nanocarriers and methods of making and using same | |
| KR20140041522A (ko) | 약물 전달용 중합체 나노입자 | |
| EP2725053A1 (en) | Branched amphipathic block polymer and molecular aggregate and drug delivery system using same | |
| Jin et al. | Amphipathic dextran-doxorubicin prodrug micelles for solid tumor therapy | |
| Gatti et al. | Hydrazone linked doxorubicin-PLA prodrug nanoparticles with high drug loading | |
| Zhou et al. | HPMA polymeric nanocarriers for anticancer drugs with tumor microenvironment-responsive extracellular biodegradation and intracellular drug release | |
| Minko et al. | Multifunctional nanotherapeutics for cancer | |
| TR2022021916A2 (tr) | Nano-i̇laç taşima si̇stemi̇ | |
| Ali et al. | pH-responsive polymeric micelles for drug delivery | |
| JP6235460B6 (ja) | 可逆的に架橋されたミセルsystems |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant |