BR112014032974B1 - Método para detecção, identificação e/ou quantificação de um ou mais carboidratos e uso de um oligotiofeno conjugado luminescente - Google Patents
Método para detecção, identificação e/ou quantificação de um ou mais carboidratos e uso de um oligotiofeno conjugado luminescente Download PDFInfo
- Publication number
- BR112014032974B1 BR112014032974B1 BR112014032974-5A BR112014032974A BR112014032974B1 BR 112014032974 B1 BR112014032974 B1 BR 112014032974B1 BR 112014032974 A BR112014032974 A BR 112014032974A BR 112014032974 B1 BR112014032974 B1 BR 112014032974B1
- Authority
- BR
- Brazil
- Prior art keywords
- phta
- carbohydrate
- carbohydrates
- lco
- signal
- Prior art date
Links
- 235000014633 carbohydrates Nutrition 0.000 title claims abstract description 235
- 150000001720 carbohydrates Chemical class 0.000 title claims abstract description 234
- 238000001514 detection method Methods 0.000 title claims abstract description 61
- 238000000034 method Methods 0.000 title claims abstract description 57
- 238000011002 quantification Methods 0.000 title claims abstract description 15
- 238000011065 in-situ storage Methods 0.000 claims abstract description 7
- 239000001913 cellulose Substances 0.000 claims description 61
- 229920002678 cellulose Polymers 0.000 claims description 61
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 17
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 claims description 16
- 229960002897 heparin Drugs 0.000 claims description 16
- 229920000856 Amylose Polymers 0.000 claims description 15
- 229920002101 Chitin Polymers 0.000 claims description 15
- 229920002527 Glycogen Polymers 0.000 claims description 15
- 229940096919 glycogen Drugs 0.000 claims description 15
- 239000008103 glucose Substances 0.000 claims description 13
- 229920000669 heparin Polymers 0.000 claims description 13
- SQDAZGGFXASXDW-UHFFFAOYSA-N 5-bromo-2-(trifluoromethoxy)pyridine Chemical compound FC(F)(F)OC1=CC=C(Br)C=N1 SQDAZGGFXASXDW-UHFFFAOYSA-N 0.000 claims description 11
- 229920001287 Chondroitin sulfate Polymers 0.000 claims description 11
- 229940059329 chondroitin sulfate Drugs 0.000 claims description 11
- 239000000758 substrate Substances 0.000 claims description 11
- 229920002498 Beta-glucan Polymers 0.000 claims description 10
- 150000004676 glycans Chemical class 0.000 claims description 9
- 230000003287 optical effect Effects 0.000 claims description 9
- 239000000178 monomer Substances 0.000 claims description 8
- 229920001282 polysaccharide Polymers 0.000 claims description 8
- 239000005017 polysaccharide Substances 0.000 claims description 8
- 238000001727 in vivo Methods 0.000 claims description 7
- 238000000338 in vitro Methods 0.000 claims description 6
- 150000002772 monosaccharides Chemical class 0.000 claims description 6
- 150000003862 amino acid derivatives Chemical class 0.000 claims description 4
- 150000001413 amino acids Chemical class 0.000 claims description 4
- 230000002503 metabolic effect Effects 0.000 claims description 4
- 239000002858 neurotransmitter agent Substances 0.000 claims description 4
- 150000007523 nucleic acids Chemical class 0.000 claims description 4
- 102000039446 nucleic acids Human genes 0.000 claims description 4
- 108020004707 nucleic acids Proteins 0.000 claims description 4
- 238000003860 storage Methods 0.000 claims description 4
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 claims description 3
- 229920002683 Glycosaminoglycan Polymers 0.000 claims description 3
- 229940072056 alginate Drugs 0.000 claims description 3
- 235000010443 alginic acid Nutrition 0.000 claims description 3
- 229920000615 alginic acid Polymers 0.000 claims description 3
- FYGDTMLNYKFZSV-URKRLVJHSA-N (2s,3r,4s,5s,6r)-2-[(2r,4r,5r,6s)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(2r,4r,5r,6s)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1[C@@H](CO)O[C@@H](OC2[C@H](O[C@H](O)[C@H](O)[C@H]2O)CO)[C@H](O)[C@H]1O FYGDTMLNYKFZSV-URKRLVJHSA-N 0.000 claims description 2
- 239000012084 conversion product Substances 0.000 claims 1
- 150000001875 compounds Chemical class 0.000 abstract description 9
- 239000000523 sample Substances 0.000 description 115
- 235000010980 cellulose Nutrition 0.000 description 60
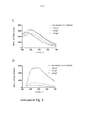
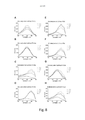
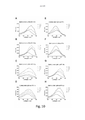
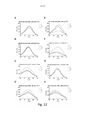
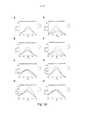
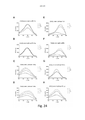
- 230000005284 excitation Effects 0.000 description 38
- 238000000695 excitation spectrum Methods 0.000 description 23
- 238000010790 dilution Methods 0.000 description 21
- 239000012895 dilution Substances 0.000 description 21
- 238000004458 analytical method Methods 0.000 description 19
- 230000008859 change Effects 0.000 description 18
- 238000003556 assay Methods 0.000 description 16
- 230000003595 spectral effect Effects 0.000 description 15
- 239000000725 suspension Substances 0.000 description 15
- 241001354013 Salmonella enterica subsp. enterica serovar Enteritidis Species 0.000 description 13
- 239000000306 component Substances 0.000 description 13
- 238000000295 emission spectrum Methods 0.000 description 13
- 238000004519 manufacturing process Methods 0.000 description 13
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 12
- 239000000661 sodium alginate Substances 0.000 description 12
- 235000010413 sodium alginate Nutrition 0.000 description 12
- 229940005550 sodium alginate Drugs 0.000 description 12
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 11
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 11
- 230000001580 bacterial effect Effects 0.000 description 11
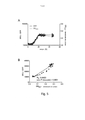
- 230000032770 biofilm formation Effects 0.000 description 11
- 210000002744 extracellular matrix Anatomy 0.000 description 11
- 239000000047 product Substances 0.000 description 11
- 239000000243 solution Substances 0.000 description 11
- 230000015572 biosynthetic process Effects 0.000 description 10
- 238000010219 correlation analysis Methods 0.000 description 10
- 235000013305 food Nutrition 0.000 description 9
- 150000002431 hydrogen Chemical class 0.000 description 9
- 229910052739 hydrogen Inorganic materials 0.000 description 9
- 239000001257 hydrogen Substances 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- 235000018102 proteins Nutrition 0.000 description 9
- 108090000623 proteins and genes Proteins 0.000 description 9
- 102000004169 proteins and genes Human genes 0.000 description 9
- -1 compound monosaccharide Chemical class 0.000 description 8
- 230000012010 growth Effects 0.000 description 8
- 210000004027 cell Anatomy 0.000 description 7
- 239000003814 drug Substances 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 210000001519 tissue Anatomy 0.000 description 7
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical compound C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 6
- 239000008358 core component Substances 0.000 description 6
- 239000013642 negative control Substances 0.000 description 6
- 238000011160 research Methods 0.000 description 6
- 229930192474 thiophene Natural products 0.000 description 6
- SMJRBWINMFUUDS-UHFFFAOYSA-N 2-thienylacetic acid Chemical compound OC(=O)CC1=CC=CS1 SMJRBWINMFUUDS-UHFFFAOYSA-N 0.000 description 5
- 239000012472 biological sample Substances 0.000 description 5
- 238000013461 design Methods 0.000 description 5
- 239000000203 mixture Substances 0.000 description 5
- 238000012544 monitoring process Methods 0.000 description 5
- 230000035772 mutation Effects 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 238000005406 washing Methods 0.000 description 5
- 229920001503 Glucan Polymers 0.000 description 4
- WQZGKKKJIJFFOK-DVKNGEFBSA-N alpha-D-glucose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-DVKNGEFBSA-N 0.000 description 4
- 235000001014 amino acid Nutrition 0.000 description 4
- 238000011088 calibration curve Methods 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- IQFVPQOLBLOTPF-HKXUKFGYSA-L congo red Chemical compound [Na+].[Na+].C1=CC=CC2=C(N)C(/N=N/C3=CC=C(C=C3)C3=CC=C(C=C3)/N=N/C3=C(C4=CC=CC=C4C(=C3)S([O-])(=O)=O)N)=CC(S([O-])(=O)=O)=C21 IQFVPQOLBLOTPF-HKXUKFGYSA-L 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 238000012921 fluorescence analysis Methods 0.000 description 4
- 238000001917 fluorescence detection Methods 0.000 description 4
- 239000003068 molecular probe Substances 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 230000035945 sensitivity Effects 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 150000003577 thiophenes Chemical class 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 108010048112 Amyloidogenic Proteins Proteins 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 229920002971 Heparan sulfate Polymers 0.000 description 3
- 238000002835 absorbance Methods 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 238000001506 fluorescence spectroscopy Methods 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 238000011194 good manufacturing practice Methods 0.000 description 3
- 239000000543 intermediate Substances 0.000 description 3
- 238000004949 mass spectrometry Methods 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 238000011895 specific detection Methods 0.000 description 3
- 239000013589 supplement Substances 0.000 description 3
- 230000002123 temporal effect Effects 0.000 description 3
- 239000002023 wood Substances 0.000 description 3
- 208000024827 Alzheimer disease Diseases 0.000 description 2
- 102000009091 Amyloidogenic Proteins Human genes 0.000 description 2
- 230000005526 G1 to G0 transition Effects 0.000 description 2
- 108090000288 Glycoproteins Proteins 0.000 description 2
- 102000003886 Glycoproteins Human genes 0.000 description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 2
- 206010028980 Neoplasm Diseases 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- 230000003698 anagen phase Effects 0.000 description 2
- 125000000129 anionic group Chemical group 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 235000013361 beverage Nutrition 0.000 description 2
- 201000011510 cancer Diseases 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 125000002091 cationic group Chemical group 0.000 description 2
- 238000004737 colorimetric analysis Methods 0.000 description 2
- 235000013409 condiments Nutrition 0.000 description 2
- 238000001218 confocal laser scanning microscopy Methods 0.000 description 2
- 230000000875 corresponding effect Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 238000007865 diluting Methods 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 229920002521 macromolecule Polymers 0.000 description 2
- 230000000813 microbial effect Effects 0.000 description 2
- 229940016286 microcrystalline cellulose Drugs 0.000 description 2
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 2
- 239000008108 microcrystalline cellulose Substances 0.000 description 2
- 238000000386 microscopy Methods 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 230000001537 neural effect Effects 0.000 description 2
- 210000001178 neural stem cell Anatomy 0.000 description 2
- 238000002135 phase contrast microscopy Methods 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 230000004845 protein aggregation Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 230000011664 signaling Effects 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 210000000130 stem cell Anatomy 0.000 description 2
- 239000003765 sweetening agent Substances 0.000 description 2
- 238000012795 verification Methods 0.000 description 2
- RFYMZEYIFGPBJS-UHFFFAOYSA-N 1h-imidazole;thiophene Chemical compound C=1C=CSC=1.C1=CNC=N1 RFYMZEYIFGPBJS-UHFFFAOYSA-N 0.000 description 1
- VMJOFTHFJMLIKL-UHFFFAOYSA-N 2-thiophen-2-ylethanol Chemical compound OCCC1=CC=CS1 VMJOFTHFJMLIKL-UHFFFAOYSA-N 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 108010094108 Amyloid Proteins 0.000 description 1
- 102000001049 Amyloid Human genes 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- SGHZXLIDFTYFHQ-UHFFFAOYSA-L Brilliant Blue Chemical compound [Na+].[Na+].C=1C=C(C(=C2C=CC(C=C2)=[N+](CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=2C(=CC=CC=2)S([O-])(=O)=O)C=CC=1N(CC)CC1=CC=CC(S([O-])(=O)=O)=C1 SGHZXLIDFTYFHQ-UHFFFAOYSA-L 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- MSFSPUZXLOGKHJ-UHFFFAOYSA-N Muraminsaeure Natural products OC(=O)C(C)OC1C(N)C(O)OC(CO)C1O MSFSPUZXLOGKHJ-UHFFFAOYSA-N 0.000 description 1
- 108010013639 Peptidoglycan Proteins 0.000 description 1
- 208000024777 Prion disease Diseases 0.000 description 1
- 229940127321 Structural Macromolecules Drugs 0.000 description 1
- ZHXRHTSXFGTQLL-XRIGFGBMSA-N acetic acid;(2s)-2,6-diaminohexanoic acid;thiophene Chemical compound CC(O)=O.C=1C=CSC=1.NCCCC[C@H](N)C(O)=O ZHXRHTSXFGTQLL-XRIGFGBMSA-N 0.000 description 1
- ACVPKUOJYOGVBN-QTNFYWBSSA-N acetic acid;(2s)-2-aminopentanedioic acid;thiophene Chemical compound CC(O)=O.C=1C=CSC=1.OC(=O)[C@@H](N)CCC(O)=O ACVPKUOJYOGVBN-QTNFYWBSSA-N 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 239000003146 anticoagulant agent Substances 0.000 description 1
- 229940127219 anticoagulant drug Drugs 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 239000008122 artificial sweetener Substances 0.000 description 1
- 235000021311 artificial sweeteners Nutrition 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 244000052616 bacterial pathogen Species 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- AVJBPWGFOQAPRH-MMPMEFKSSA-N beta-D-GlcpA-(1->3)-beta-D-GalpNAc4S Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@H](OS(O)(=O)=O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](C(O)=O)O1 AVJBPWGFOQAPRH-MMPMEFKSSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 239000002551 biofuel Substances 0.000 description 1
- 230000009141 biological interaction Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 238000004061 bleaching Methods 0.000 description 1
- 238000012511 carbohydrate analysis Methods 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000003636 chemical group Chemical group 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 229920001940 conductive polymer Polymers 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- ZXJXZNDDNMQXFV-UHFFFAOYSA-M crystal violet Chemical compound [Cl-].C1=CC(N(C)C)=CC=C1[C+](C=1C=CC(=CC=1)N(C)C)C1=CC=C(N(C)C)C=C1 ZXJXZNDDNMQXFV-UHFFFAOYSA-M 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 235000015872 dietary supplement Nutrition 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 230000009881 electrostatic interaction Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 229940031098 ethanolamine Drugs 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 238000002866 fluorescence resonance energy transfer Methods 0.000 description 1
- 238000012632 fluorescent imaging Methods 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 244000053095 fungal pathogen Species 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 229960001235 gentian violet Drugs 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 239000000852 hydrogen donor Substances 0.000 description 1
- 230000003100 immobilizing effect Effects 0.000 description 1
- 238000011503 in vivo imaging Methods 0.000 description 1
- 238000010249 in-situ analysis Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 239000011147 inorganic material Substances 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 238000012417 linear regression Methods 0.000 description 1
- 230000008376 long-term health Effects 0.000 description 1
- 230000001320 lysogenic effect Effects 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 238000007431 microscopic evaluation Methods 0.000 description 1
- HZAXFHJVJLSVMW-UHFFFAOYSA-N monoethanolamine hydrochloride Natural products NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 1
- 231100000065 noncytotoxic Toxicity 0.000 description 1
- 230000002020 noncytotoxic effect Effects 0.000 description 1
- 238000006384 oligomerization reaction Methods 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 150000002482 oligosaccharides Chemical class 0.000 description 1
- 238000012634 optical imaging Methods 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 235000021485 packed food Nutrition 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 108010001062 polysaccharide-K Proteins 0.000 description 1
- 150000004804 polysaccharides Polymers 0.000 description 1
- 229920000123 polythiophene Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 238000001303 quality assessment method Methods 0.000 description 1
- 238000000275 quality assurance Methods 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 235000021487 ready-to-eat food Nutrition 0.000 description 1
- 238000010223 real-time analysis Methods 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000004621 scanning probe microscopy Methods 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000010183 spectrum analysis Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 235000021092 sugar substitutes Nutrition 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000004809 thin layer chromatography Methods 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- 238000004065 wastewater treatment Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
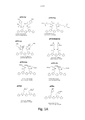
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/06—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to the ring carbon atoms
- C07D333/24—Radicals substituted by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/5308—Immunoassay; Biospecific binding assay; Materials therefor for analytes not provided for elsewhere, e.g. nucleic acids, uric acid, worms, mites
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing three or more hetero rings
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/52—Use of compounds or compositions for colorimetric, spectrophotometric or fluorometric investigation, e.g. use of reagent paper and including single- and multilayer analytical elements
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/58—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving labelled substances
- G01N33/582—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving labelled substances with fluorescent label
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/06—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to the ring carbon atoms
- C07D333/14—Radicals substituted by singly bound hetero atoms other than halogen
- C07D333/20—Radicals substituted by singly bound hetero atoms other than halogen by nitrogen atoms
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2400/00—Assays, e.g. immunoassays or enzyme assays, involving carbohydrates
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2400/00—Assays, e.g. immunoassays or enzyme assays, involving carbohydrates
- G01N2400/10—Polysaccharides, i.e. having more than five saccharide radicals attached to each other by glycosidic linkages; Derivatives thereof, e.g. ethers, esters
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2400/00—Assays, e.g. immunoassays or enzyme assays, involving carbohydrates
- G01N2400/10—Polysaccharides, i.e. having more than five saccharide radicals attached to each other by glycosidic linkages; Derivatives thereof, e.g. ethers, esters
- G01N2400/12—Homoglycans, i.e. polysaccharides having a main chain consisting of one single sugar
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2400/00—Assays, e.g. immunoassays or enzyme assays, involving carbohydrates
- G01N2400/10—Polysaccharides, i.e. having more than five saccharide radicals attached to each other by glycosidic linkages; Derivatives thereof, e.g. ethers, esters
- G01N2400/38—Heteroglycans, i.e. polysaccharides having more than one sugar residue in the main chain in either alternating or less regular sequence, e.g. gluco- or galactomannans, Konjac gum, Locust bean gum or Guar gum
- G01N2400/40—Glycosaminoglycans, i.e. GAG or mucopolysaccharides, e.g. chondroitin sulfate, dermatan sulfate, hyaluronic acid, heparin, heparan sulfate, and related sulfated polysaccharides
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Hematology (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Urology & Nephrology (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Cell Biology (AREA)
- Food Science & Technology (AREA)
- Biotechnology (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Microbiology (AREA)
- Pathology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Investigating Or Analysing Materials By The Use Of Chemical Reactions (AREA)
- Investigating, Analyzing Materials By Fluorescence Or Luminescence (AREA)
- Heterocyclic Compounds Containing Sulfur Atoms (AREA)
- Investigating Or Analyzing Non-Biological Materials By The Use Of Chemical Means (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SE1250751-3 | 2012-07-02 | ||
| SE1250751A SE536793C2 (sv) | 2012-07-02 | 2012-07-02 | Detektion av kolhydrater |
| PCT/SE2013/050810 WO2014007730A1 (en) | 2012-07-02 | 2013-06-27 | Carbohydrate detection |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| BR112014032974A2 BR112014032974A2 (pt) | 2017-06-27 |
| BR112014032974B1 true BR112014032974B1 (pt) | 2021-09-14 |
Family
ID=49882344
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| BR112014032974-5A BR112014032974B1 (pt) | 2012-07-02 | 2013-06-27 | Método para detecção, identificação e/ou quantificação de um ou mais carboidratos e uso de um oligotiofeno conjugado luminescente |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US9958439B2 (enExample) |
| EP (1) | EP2867673B1 (enExample) |
| JP (1) | JP6317342B2 (enExample) |
| AU (1) | AU2013285614B2 (enExample) |
| BR (1) | BR112014032974B1 (enExample) |
| CA (1) | CA2877470C (enExample) |
| IN (1) | IN2014DN10649A (enExample) |
| NO (1) | NO2867673T3 (enExample) |
| PL (1) | PL2867673T3 (enExample) |
| SE (1) | SE536793C2 (enExample) |
| WO (1) | WO2014007730A1 (enExample) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6982319B2 (ja) * | 2015-10-01 | 2021-12-17 | リヒター ライフ サイエンス ディヴェロップメント アクチエボラグRichter Life Science Development AB | 微生物ペプチドの検出 |
| FR3050271B1 (fr) | 2016-04-18 | 2018-04-13 | Centre National De La Recherche Scientifique | Procede de sequencage d'oligosaccharides |
| DE102016113166B4 (de) * | 2016-07-18 | 2022-02-24 | Joanneum Research Forschungsgesellschaft Mbh | Verfahren zum Nachweis eines Biofilms und Mittel zum Nachweis eines Biofilms |
| SE543571C2 (en) * | 2019-02-07 | 2021-03-30 | Christian Strietzel | Conducting redox oligomers |
| JP2020165847A (ja) * | 2019-03-29 | 2020-10-08 | 株式会社島津製作所 | 食品の品質判定方法、及び、食品品質判定装置 |
| WO2021211873A1 (en) * | 2020-04-15 | 2021-10-21 | The Penn State Research Foundation | Biodegradable dna-alginate conjugate for reversible protein and cell labeling and imaging |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005045065A1 (en) | 2003-10-31 | 2005-05-19 | Immunetics, Inc. | Rapid peptidoglycan-based assay for detection of bacterial contamination of platelets |
| WO2007075595A2 (en) | 2005-12-20 | 2007-07-05 | Vertex Pharmacueticals Incorporated | Biofilm assay |
| CN102256959B (zh) | 2008-10-17 | 2014-01-08 | 比奥克罗密克斯药业有限公司 | 用于治疗的噻吩化合物 |
| WO2011102789A1 (en) | 2010-02-16 | 2011-08-25 | Oboe Ipr Ab | Oligothiophene derivate as molecular probes |
| GB201020236D0 (en) | 2010-11-30 | 2011-01-12 | Convatec Technologies Inc | A composition for detecting biofilms on viable tissues |
-
2012
- 2012-07-02 SE SE1250751A patent/SE536793C2/sv not_active IP Right Cessation
-
2013
- 2013-06-27 EP EP13813945.6A patent/EP2867673B1/en active Active
- 2013-06-27 BR BR112014032974-5A patent/BR112014032974B1/pt active IP Right Grant
- 2013-06-27 AU AU2013285614A patent/AU2013285614B2/en active Active
- 2013-06-27 PL PL13813945T patent/PL2867673T3/pl unknown
- 2013-06-27 NO NO13813945A patent/NO2867673T3/no unknown
- 2013-06-27 US US14/408,440 patent/US9958439B2/en active Active
- 2013-06-27 JP JP2015520123A patent/JP6317342B2/ja active Active
- 2013-06-27 IN IN10649DEN2014 patent/IN2014DN10649A/en unknown
- 2013-06-27 WO PCT/SE2013/050810 patent/WO2014007730A1/en not_active Ceased
- 2013-06-27 CA CA2877470A patent/CA2877470C/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| PL2867673T3 (pl) | 2018-07-31 |
| CA2877470C (en) | 2022-07-19 |
| EP2867673A1 (en) | 2015-05-06 |
| JP2015524552A (ja) | 2015-08-24 |
| AU2013285614A1 (en) | 2015-01-22 |
| SE536793C2 (sv) | 2014-08-19 |
| AU2013285614B2 (en) | 2018-10-18 |
| NO2867673T3 (enExample) | 2018-05-05 |
| JP6317342B2 (ja) | 2018-04-25 |
| IN2014DN10649A (enExample) | 2015-09-11 |
| WO2014007730A1 (en) | 2014-01-09 |
| CA2877470A1 (en) | 2014-01-09 |
| US9958439B2 (en) | 2018-05-01 |
| SE1250751A1 (sv) | 2014-01-03 |
| US20150212076A1 (en) | 2015-07-30 |
| EP2867673A4 (en) | 2016-03-02 |
| BR112014032974A2 (pt) | 2017-06-27 |
| EP2867673B1 (en) | 2017-12-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| BR112014032974B1 (pt) | Método para detecção, identificação e/ou quantificação de um ou mais carboidratos e uso de um oligotiofeno conjugado luminescente | |
| Mudliar et al. | An efficient J-aggregate based fluorescence turn-on and ratiometric sensor for heparin | |
| Si et al. | Rapid and accurate detection of Escherichia coli growth by fluorescent pH-sensitive organic nanoparticles for high-throughput screening applications | |
| Wu et al. | A novel colorimetric aptasensor for detection of chloramphenicol based on lanthanum ion–assisted gold nanoparticle aggregation and smartphone imaging | |
| EP2217927B1 (en) | Visual glucose sensor and methods of use thereof | |
| Gonzalez-Torres et al. | Evaluation of biochemical algal floc properties using Reflectance Fourier-Transform Infrared Imaging | |
| CN102460172A (zh) | 用于抗微生物剂抗性测定的方法 | |
| Si et al. | Carboxylate-containing two-photon probe for the simultaneous detection of extra-and intracellular pH values in colon cancer tissue | |
| Hou et al. | Pyrene-porphyrin based ratiometric fluorescent sensor array for discrimination of glycosaminoglycans | |
| Jiang et al. | Near-Infrared Fluorescent Lysosomal Viscosity Probe with Strong Solid Fluorescence for Rapid Imaging of Rheumatoid Arthritis | |
| Jiang et al. | Simple and fast colorimetric detection of lipopolysaccharide based on aptamer and SYBR Green I mediated aggregation of gold nanoparticles | |
| Richter-Dahlfors et al. | Fluorescent optotracers for bacterial and biofilm detection and diagnostics | |
| Ge et al. | Utilizing hyaluronic acid as a versatile platform for fluorescence resonance energy transfer-based glucose sensing | |
| US20140024813A1 (en) | Binding of pathological forms of proteins using conjugated polyelectrolytes | |
| Francoia et al. | A KISS (keep it simple, sensor) array for glycosaminoglycans | |
| Jia et al. | Dyes inspired sensor arrays for discrimination of glycosaminoglycans | |
| Xu et al. | Metabolic Activity Profiling of High-Temperature Daqu Microbiota Using Single-Cell Raman Spectroscopy and Deuterium Isotope Probing | |
| Pihlasalo et al. | Luminometric nanoparticle-based assay for high sensitivity detection of β-amyloid aggregation | |
| CN107941773B (zh) | 一种基于荧光分子的内毒素的检测方法 | |
| Du et al. | Significantly different fluorescent responses of two aggregation-induced emission probes to heparin | |
| Baibek et al. | A Long Fluorescence Lifetime Probe for Labeling of Gram-Negative Bacteria | |
| Meng et al. | A Multiple‐Stimulus‐Responsive Biomimetic Assembly Based on a Polyisocyanopeptide and Conjugated Polymer | |
| Subedi et al. | Ratiometric fluorescent detection of heparan sulfate in human plasma and serum using peptide-based fluorescent probes | |
| Nesměrák et al. | Spectrometric methods in pharmaceutical analysis of glycosaminoglycans: the state-of-the-art | |
| Hong et al. | Fluorescent reporters for antimicrobial peptides |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| B06F | Objections, documents and/or translations needed after an examination request according [chapter 6.6 patent gazette] | ||
| B06F | Objections, documents and/or translations needed after an examination request according [chapter 6.6 patent gazette] | ||
| B06I | Publication of requirement cancelled [chapter 6.9 patent gazette] |
Free format text: ANULADA A PUBLICACAO CODIGO 6.6.1 NA RPI NO 2462 DE 13/03/2018 POR TER SIDO INDEVIDA. |
|
| B06U | Preliminary requirement: requests with searches performed by other patent offices: procedure suspended [chapter 6.21 patent gazette] | ||
| B09A | Decision: intention to grant [chapter 9.1 patent gazette] | ||
| B16A | Patent or certificate of addition of invention granted [chapter 16.1 patent gazette] |
Free format text: PRAZO DE VALIDADE: 20 (VINTE) ANOS CONTADOS A PARTIR DE 27/06/2013, OBSERVADAS AS CONDICOES LEGAIS. |