WO2021132627A1 - 造血幹細胞の培養方法 - Google Patents
造血幹細胞の培養方法 Download PDFInfo
- Publication number
- WO2021132627A1 WO2021132627A1 PCT/JP2020/048917 JP2020048917W WO2021132627A1 WO 2021132627 A1 WO2021132627 A1 WO 2021132627A1 JP 2020048917 W JP2020048917 W JP 2020048917W WO 2021132627 A1 WO2021132627 A1 WO 2021132627A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- hematopoietic stem
- substituted
- optionally substituted
- atom
- Prior art date
Links
- 0 *C([C@]([C@](CCC1)([C@]2(*3)[C@]1(CC1)*=C)N)N)O*2O[C@]31N Chemical compound *C([C@]([C@](CCC1)([C@]2(*3)[C@]1(CC1)*=C)N)N)O*2O[C@]31N 0.000 description 3
- VICBVFYLZSOPLG-IYQVPCOUSA-N C[C@H](CC[C@@H]([C@H]1C)[C@@]23OO[C@](C)(CC4)O[C@H]2O[C@H]1OCCOC(C)=O)[C@@]34NC Chemical compound C[C@H](CC[C@@H]([C@H]1C)[C@@]23OO[C@](C)(CC4)O[C@H]2O[C@H]1OCCOC(C)=O)[C@@]34NC VICBVFYLZSOPLG-IYQVPCOUSA-N 0.000 description 1
- PQTJOIMKKMSDJE-KUDRULNLSA-N C[C@H]([C@@H]1CC2)[C@@H](OCCOC(C)=O)O[C@@H]3O[C@@](C)(CC4)OO[C@]13[C@@H]4[C@@H]2N Chemical compound C[C@H]([C@@H]1CC2)[C@@H](OCCOC(C)=O)O[C@@H]3O[C@@](C)(CC4)OO[C@]13[C@@H]4[C@@H]2N PQTJOIMKKMSDJE-KUDRULNLSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0647—Haematopoietic stem cells; Uncommitted or multipotent progenitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/28—Bone marrow; Haematopoietic stem cells; Mesenchymal stem cells of any origin, e.g. adipose-derived stem cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/999—Small molecules not provided for elsewhere
Definitions
- the present invention relates to a method for culturing hematopoietic stem cells.
- the present invention also relates to a method for producing hematopoietic stem cells.
- the present invention further relates to a medium for hematopoietic stem cells for growing hematopoietic stem cells in vitro.
- Hematopoietic stem cell is mainly present in the bone marrow, is defined as a blood cell having self-renewal ability and pluripotency, and is used for hematopoietic stem cell transplantation and the like.
- hematopoietic stem cell transplantation has the problem of lack of quality bone marrow source.
- it is conceivable to purify high-quality hematopoietic stem cells for example, long-term hematopoietic stem cells
- proliferate them in vitro in large quantities.
- Long-term hematopoietic stem cells Long-term HSC: LT-
- No specific marker that enables purification of HSC has been known, and a method for culturing has not been established.
- An object of the present invention is to provide a method for culturing and producing hematopoietic stem cells that can be used for hematopoietic stem cell transplantation.
- a method for culturing hematopoietic stem cells which comprises culturing a cell population containing hematopoietic stem cells in a medium containing one or more compounds represented by the formula (1) or salts thereof.
- X is OO or O
- Y is hydrogen atom, hydroxy, oxo, mercapto, carboxy, carbamoyl, cyano
- n is an integer from 0 to 10 and R 1 is an alkyl optionally substituted, a cycloalkyl optionally substituted, an aryl optionally substituted, a heteroaryl optionally substituted, or an aliphatic heterocyclic group optionally substituted.
- R 2 may be substituted alkyl, optionally substituted alkenyl, optionally substituted alkynyl, optionally substituted cycloalkyl, optionally substituted aryl, optionally substituted.
- R 3 , R 4 and R 5 are independently hydrogen atom, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted aliphatic heterocyclic group, substituted. It may be an aryl, or a heteroaryl which may be substituted, wherein R 3 and R 4 together with an adjacent nitrogen atom further form an oxygen atom, a nitrogen atom or a ring of 1 or 2.
- a 4- to 10-membered nitrogen-containing aliphatic heterocycle may be formed, which may contain a sulfur atom, and the nitrogen-containing aliphatic heterocycle may be substituted.
- X and Y are the same as the definitions in equation (1).
- Z 1 , Z 2 , Z 3 , Z 4 , Z 5 , and Z 6 are independently hydrogen atoms or C 1-6 alkyls]
- Y is hydrogen atom, hydroxy, oxo, carboxy, carbamoyl, OR 1 , SR 1 , SO 2 R 1 , COR 1 , OCOR 1 , R 2 , NR 3 R 4 , NR 5 COR 1 , NR 5 SO 2 R 1 ,
- the culture method according to any one of [1] to [5], which is a fluorine atom.
- Y is a hydrogen atom or hydroxy
- Y is hydroxy, oxo, carboxy, carbamoyl
- R 1 may be substituted with C 1-8 alkyl selected from group 1 with 1-7 substituents
- C 3- may be substituted with 1-3 groups selected from group 1.
- An oxygen atom, nitrogen which may be substituted with a 4- to 10-membered aliphatic heterocyclic group containing a heteroatom within 1 to 4 rings selected from, or 1 to 4 groups selected from group 1.
- R 2 may be substituted with a substituent of 1 to 7 selected from group 2, C 1-8 alkyl, and C 1 may be substituted with a substituent of 1 to 7 selected from group 2.
- Heteroaryl and R 3 and R 4 are independently a hydrogen atom, a C 1-6 alkyl optionally substituted with 1-7 substituents selected from Group 1, or 1-5 groups selected from Group 1.
- a good 3- to 8-membered nitrogen-containing aliphatic heterocycle may be formed, and the nitrogen-containing aliphatic heterocycle may be substituted with 1 to 3 substituents selected from the following group 1 substituents.
- Group 1> Carboxy, hydroxy, C 1-8 alkoxy, C 1-8 alkylcarbonyl, C 1-8 alkylcarbonyloxy, C 1-8 alkoxycarbonyl, mercapto, C 1-8 alkylsulfanyl, C 1-8 alkylsulfonyl, 1 or Carbamoyl optionally substituted with 2 C 1-8 alkyl, amino optionally substituted with 1 or 2 C 1-8 alkyl, C 1-8 alkylcarbonylamino, C 1-8 alkylsulfonylamino, It may be substituted with 1 to 5 groups selected from group 3, phenyl, may be substituted with 1 to 4 groups selected from group 3, and may be selected from oxygen atom, nitrogen atom and sulfur atom.
- R 1 may be C 1-4 alkyl substituted with 1-3 groups selected from group 1'
- C 3 may be substituted with 1-3 groups selected from group 1'.
- And may be substituted with a 4- to 6-membered aliphatic heterocyclic group containing a heteroatom within 1 to 3 rings selected from sulfur atoms, or 1 to 3 groups selected from group 1'. It is a 5- to 6-membered heteroaryl containing 1 to 3 heteroatoms in the ring selected from oxygen, nitrogen and sulfur atoms.
- R 2 may be C 1-4 alkyl substituted with 1-3 groups selected from group 2', C 3 may be substituted with 1-3 groups selected from group 1'. -6 cycloalkyl, phenyl optionally substituted with 1-3 groups selected from group 1', oxygen atom, nitrogen atom optionally substituted with 1-3 groups selected from group 1'. And may be substituted with a 4- to 6-membered aliphatic heterocyclic group containing a heteroatom within 1 to 3 rings selected from sulfur atoms, or 1 to 3 groups selected from group 1'. It is a 5- to 6-membered heteroaryl containing 1 to 3 heteroatoms in the ring selected from oxygen, nitrogen and sulfur atoms.
- R 3 is a hydrogen atom or C 1-3 alkyl and R 4 is a hydrogen atom, from C 1-4 alkyl, group 1'which may be substituted with 1-3 groups selected from group 1'.
- R 3 and R 4 are 3- to 8-membered nitrogen-containing aliphatic complex which may further contain an oxygen atom, a nitrogen atom or a sulfur atom constituting a ring of 1 or 2 together with adjacent nitrogen atoms.
- a ring may be formed, and the nitrogen-containing aliphatic heterocycle may be substituted with 1 to 3 groups selected from the following substituents of group 1'.
- [16-2] The culture method according to any one of [1] to [15], wherein the number of hematopoietic stem cells in the cell population after culturing is 10 times or more the number of hematopoietic stem cells in the cell population before culturing.
- [17] The culture method according to any one of [1] to [16], wherein the medium is a medium further containing UM171 or a derivative thereof.
- a method for producing hematopoietic stem cells which comprises a step of culturing a cell population containing hematopoietic stem cells, which comprises a step of culturing the cell population containing the hematopoietic stem cells by the culture method according to any one of [1] to [17].
- Hematopoietic stem cells obtained by culturing by the culturing method according to any one of [1] to [17].
- a reagent for culturing hematopoietic stem cells which comprises at least one compound represented by the formula (1) or a salt thereof.
- hematopoietic stem cells particularly long-term hematopoietic stem cells, can be cultured and proliferated while maintaining self-renewal ability and pluripotency, and long-term hematopoietic stem cells suitable for transplantation can be produced.
- the hematopoietic stem cells obtained by the culture method can be used for hematopoietic stem cell transplantation.
- results of culturing a cell population containing human hematopoietic stem cells are shown, (A) shows the number of viable cells after culturing in a medium containing only Artemethel, and (B) in a medium containing UM171 in addition to Artemeter.
- the results of culturing a cell population containing human hematopoietic stem cells are shown, (A) shows the number of CD34-positive cells after culturing in a medium containing only Artemethel, and (B) in a medium containing UM171 in addition to Artemethel.
- results of culturing a cell population containing human hematopoietic stem cells are shown.
- the method for culturing hematopoietic stem cells of the present invention comprises culturing a cell population containing hematopoietic stem cells in a medium containing one or more compounds represented by the formula (1) or salts thereof.
- a cell population containing hematopoietic stem cells is prepared from Artemether, Artemisinin, Artemimol, Artemotil, and Artesunate. Includes culturing in a medium containing one or more compounds or derivatives thereof selected from the group, or salts thereof.
- Hematopoietic stem cell is a blood cell that exists mainly in the bone marrow and has self-renewal ability (self-renewal capacity) and pluripotency that can differentiate into all blood cells (multipotency).
- self-renewal means producing cells having the same functions and properties as oneself by cell division. That is, cells produced by self-renewal of hematopoietic stem cells have pluripotency and self-renewal ability to all blood cells.
- Hematopoietic stem cells are classified into short-term hematopoietic stem cells and long-term hematopoietic stem cells according to their ability to maintain hematopoiesis.
- long-term hematopoietic stem cells refer to hematopoietic stem cells that have a longer-lasting self-renewal ability than short-term hematopoietic stem cells, and long-term hematopoietic stem cells have long-term bone marrow remodeling ability (for example, bone marrow even in secondary transplantation). Has the ability to reconstruct).
- short-term hematopoietic stem cells can reconstruct bone marrow in vitro or in primary transplantation, but cannot maintain this ability in secondary transplantation.
- the secondary transplantation means that the bone marrow-derived hematopoietic stem cells reconstructed by the primary transplantation are transplanted into another individual.
- hematopoietic stem cells refer to both short-term hematopoietic stem cells and long-term hematopoietic stem cells.
- hematopoietic stem cells Differentiation of hematopoietic stem cells means that long-term hematopoietic stem cells (long-term HSC: LT-HSC) become short-term hematopoietic stem cells, short-term hematopoietic stem cells become pluripotent hematopoietic progenitor cells, and pluripotent hematopoietic progenitor cells become pluripotent hematopoietic progenitor cells.
- long-term HSC long-term HSC: LT-HSC
- pluripotent hematopoietic progenitor cells to cells, monophasic hematopoietic progenitor cells to mature cells with unique functions, that is, mature blood cells such as erythrocytes, leukocytes, macronuclear cells, and platelets. It means to do.
- mature blood cells such as erythrocytes, leukocytes, macronuclear cells, and platelets. It means to do.
- hematopoietic stem cells only long-term hematopoietic stem cells and short-term hematopoietic stem cells have self-renewal ability, and cells downstream of pluripotent hematopoietic progenitor cells differentiate. Although it has cell division ability associated with it, it is said that it does not have self-renewal ability.
- proliferation of hematopoietic stem cells usually means that the number of hematopoietic stem cells is increased by culturing the hematopoietic stem cells in vitro.
- proliferation of hematopoietic stem cells can be achieved by self-renewal of hematopoietic stem cells having the same properties from at least some hematopoietic stem cells.
- Hematopoietic stem cells are known to self-renew in vivo, but no specific factor that induces this self-renewal has been identified, and in vitro induces hematopoietic stem cells to self-renew in the same manner as in vivo. However, it was difficult to grow it. According to the culture method of the present invention, hematopoietic stem cells can be proliferated in vitro while maintaining self-renewal ability and pluripotency.
- Hematopoietic stem cells can be identified using a marker that is expressed, preferably a marker that is specifically expressed in hematopoietic stem cells, and a marker that is not expressed in hematopoietic stem cells (negative marker) as an index.
- mouse long-term hematopoietic stem cells are identified by indexing one or more selected from the group consisting of Lineage negative, c-Kit positive, Sca-1 positive, Flk2 negative, CD34 negative or weak positive, CD150 positive and Hoxb5 positive. It may be a cell to be treated.
- mouse short-term hematopoietic stem cells are identified by indexing one or more selected from the group consisting of Lineage negative, c-Kit positive, Sca-1 positive, Flk2 negative, CD34 negative or weak positive, CD150 positive and Hoxb5 negative. It may be a cell to be treated.
- the cell lineage marker is a general term for antigens expressed in mature blood lineage cells. Examples thereof include, but are not limited to, Ter-119 (erythrocytes) and B220 (B cells).
- An example of a human cell lineage marker is, for example, Notta F et al. , Science. 2011 Jul 8; 333 (6039): 218-21, Sugimura R.M. Et al. , Nature. 2017 May 25; 545 (7655): 432-438, and Taya Y. Et al. , Science. It is reported in 2016 Dec2; 354 (6316): 1152-1155 and the like.
- Lineage-negative cells Cells that do not express all or part of the cell lineage marker may be referred to as Lineage-negative (Lin-negative or Lin-) cells.
- the mouse Lineage-negative cells may be cells that do not express Ter-119, B220, CD3, CD4, CD8a, Gr-1, CD11b, IL-7R, or the like.
- human hematopoietic stem cells are cells identified by the presence or absence of expression of one or more markers selected from the group consisting of CD34, CD38, Lineage, CD90, CD45RA, CD49f (eg, CD34 positive cells, CD34). Positive and CD38 negative cells, CD34 positive, CD38 negative, CD90 positive and CD45RA negative cells, CD34 positive, CD38 negative cells, CD90 negative and CD45RA negative cells, CD34 positive, CD38 negative, CD90 negative and CD45RA positive cells). .. It is believed that a greater proportion of long-term hematopoietic stem cells are present in the CD34-positive and CD38-negative cell populations of the CD34-positive cell population.
- the "positive” cell means a cell expressing a specific marker protein
- the “negative” cell means a cell not expressing a specific marker protein.
- CD34 positive refers to a cell expressing a CD (cluster of differentiation) 34 antigen on the cell surface
- CD38 negative refers to cells that do not express the CD38 antigen on the cell surface.
- a specific marker protein can be confirmed by a person skilled in the art using a well-known method. As one aspect, it is determined whether or not a significant increase in fluorescence intensity is observed when the cells are treated with the fluorescently labeled antibody against the marker as compared with the case where the cells are treated with the fluorescently labeled control antibody. it can.
- markers are well-known markers to those skilled in the art, but the gene sequences and amino acid sequences of various markers can be confirmed by GenBank or the like.
- GenBank Accession No. 15413 GenBank Accession No. 15413 (NC_0000076) is mentioned, and as a mouse Hoxb5 protein, GenBank Accession No. : NP_032294.2) and the like.
- commercially available products such as antibodies against various markers can be obtained, and the expression or expression level of the markers can be detected using the antibodies.
- the self-renewal ability of hematopoietic stem cells can be measured by a method known to those skilled in the art.
- human hematopoietic stem cells can be measured using an increase in the number of cells identified by the presence or absence of expression of the above-mentioned markers (eg, CD34 positive and CD38 negative) as an index.
- the self-renewal ability of mouse hematopoietic stem cells can be measured by using an increase in the number of cKit-positive Lin-negative Sca1-positive cells as an index.
- the self-renewal ability of long-term mouse hematopoietic stem cells can be measured.
- an increase in the number of Hoxb5-positive cells can be used as an index for measurement.
- the pluripotency of hematopoietic stem cells is such that the cells obtained by culturing are subjected to differentiation induction treatment into at least two or more different types of different hematopoietic progenitor cells or blood cells derived from them, and the desired hematopoietic progenitor cells or blood. It can be measured by confirming the differentiation into cells.
- a method of transplanting into an immunodeficient mouse is often used as a method for confirming the function as a human hematopoietic stem cell or a long-term hematopoietic stem cell.
- immunodeficient mice NOD.Cg-Prkdcscid Il2rgtm1Sug / Jic: hereinafter, "NOG mice”, Blood (2012), 100 (9)
- NOG mice capable of producing almost all human mature blood cells when transplanted with human hematopoietic stem cells. : 113-1124
- transplanting cultured hematopoietic stem cells into this mouse it is possible to measure the ability of the transplanted cells to engraft in the bone marrow, pluripotency, and bone marrow remodeling ability. Furthermore, by collecting the bone marrow of an immunodeficient mouse that has been transplanted once and transplanting it again (secondary transplantation) into another NOG mouse, it is possible to measure the self-renewal ability and long-term bone marrow remodeling ability of the transplanted cells. is there.
- long-term bone marrow remodeling ability is obtained by transplanting hematopoietic stem cells into immunoprotected NOG mice irradiated with a lethal dose of radiation, and for a long period of time (1 month or more after transplantation, preferably 2 months or more, 3 months or more). It can also be confirmed by the engraftment of the transplanted cells (4 months or more).
- engraftment of the transplanted cells may be confirmed by detecting cells expressing a human marker in NOG mouse blood.
- human CD45 which is a common white blood cell antigen, may be detected by an antibody thereof or the like.
- the proportion of human leukocyte cells (eg, human CD45-positive cells) in the blood at 1 month (preferably 2 months, 3 months, 4 months) after transplantation should be increased as compared with the comparison target. Just do it. That is, when the ratio is maintained or increased with respect to the comparison target, it can be confirmed that the long-term hematopoietic stem cells of the target are maintained or amplified.
- the comparison target may be, for example, a cell population obtained by a culture method in which the culture conditions are matched, or a cell population before culturing, except that the compound of the present invention is not contained.
- the proportion of human leukocyte cells (eg, human CD45-positive cells) in the blood within 1 month (preferably 2 months, 3 months, 4 months) after transplantation is large depending on experimental conditions such as the number of transplanted cells. Dependent. Therefore, the high or low ratio of human leukocyte cells (eg, human CD45 positive cells) in the blood does not matter, but for example, the ratio is 0.1% or more, 0.5% or more, 1% or more, 5%. It may be 10% or more.
- the partial replacement with donor-derived cells eg, leukocyte cells
- chimerism or donor chimerism
- the ratio of donor-derived cells (eg, leukocyte cells) is also called the chimera rate. is there.
- a cell whose function as a hematopoietic stem cell has been confirmed by this method can be said to be a cell suitable for application to an actual hematopoietic stem cell transplantation.
- the culture method according to the above [1] to [11] is also a method for culturing a cell population that increases the ability to produce mature blood cells when transplanted into a mammal.
- the culture method according to the above [1] to [11] is a method for culturing a cell population that increases at least one, preferably all, bone marrow engraftment ability, pluripotency and bone marrow remodeling ability. But also.
- the hematopoietic stem cells in the present specification may be derived from mammals, for example, hematopoietic stem cells derived from humans, monkeys, rats, mice and the like.
- Hematopoietic stem cells (cell population containing hematopoietic stem cells) can be prepared by the method described in [Manufacturing method] (step (1)).
- the term "cell population” means a population in which two or more cells of the same type or different types are present.
- the cell population is present in a medium such as a medium.
- the cell population comprises cell suspensions and cell aggregates, preferably in the form of cell suspensions or cell aggregates.
- the cell population containing hematopoietic stem cells to be cultured is not particularly limited as long as it is a population of two or more cells including hematopoietic stem cells, and is a cell population containing only hematopoietic stem cells (long-term hematopoietic stem cells and / or short-term hematopoietic stem cells). It may be a cell population containing hematopoietic stem cells and other cells.
- the term "cell population containing hematopoietic stem cells” as used herein refers to both a cell population containing only hematopoietic stem cells and a cell population containing hematopoietic stem cells and other cells.
- the hematopoietic stem cell may preferably be a long-term hematopoietic stem cell. That is, the "cell population containing hematopoietic stem cells" to be cultured in the present specification includes long-term hematopoietic stem cells.
- examples of cells other than hematopoietic stem cells that can be included in the above cell population include hematopoietic progenitor cells and mature blood cells.
- Hematopoietic progenitor cells include pluripotent hematopoietic progenitor cells, pluripotent hematopoietic progenitor cells, and monophasic hematopoietic progenitor cells.
- pluripotency, pluripotency and monoplasia is based on the degree of ability to differentiate into blood cells.
- a pluripotent hematopoietic progenitor cell is a cell having pluripotency that can differentiate into all blood cells, but cannot self-renew. Therefore, blood cells can be produced temporarily, but if all the cells are differentiated, the hematopoietic progenitor cells are depleted and the destroyed bone marrow cannot be reconstructed.
- Hypopotent hematopoietic progenitor cells are cells that can differentiate into multiple blood cells, but not all, but cannot self-replicate.
- CFU-GEMM granulocytes / monocyte progenitor cells
- CFU-GM granulocytes / macrophage colony forming cells
- GEMM stands for granulocyte, erythrocyte, monocyte, megakaryocyte.
- a monopoly hematopoietic progenitor cell is a cell that can differentiate into a specific single blood cell but does not have self-renewal ability.
- monopotent hematopoietic progenitor cells include erythroblast burst-forming cells (BFU-E), which are erythroblast progenitor cells.
- the colony method (for example, Ueda T. et al, J. Clin. Invest. (2000) 105: 101013-1021) has been used for a long time as a method for measuring hematopoietic progenitor cells by a functional method.
- the most immature colonies measured by the colony method (also referred to as CFU assay) are mixed colonies in which erythrocytes and leukocytes are mixed (hereinafter referred to as "CFU-GEMM").
- CFU-GEMM mixed colonies in which erythrocytes and leukocytes are mixed
- CFU-GEMM mixed colonies
- the culturing method according to the above [1] to [11] is also a culturing method for cells or cell populations having the ability to form mixed colonies (CFU-GEMM) derived from hematopoietic stem cells.
- Mature blood cells are a mature cell population formed from hematopoietic stem cells via hematopoietic progenitor cells. There are erythrocytes, neutrophils, monocytes, eosinophils, basophils, macrophages, platelets, mast cells, T cells, B cells, NK cells, NKT cells, etc., located upstream of hematopoietic stem cells or the mature blood cells. It can be obtained by differentiating the hematopoietic precursor cells. In addition, these mature blood cells can be identified by specific markers expressed in the cells using a known method.
- CD33 is known as a marker for monocytes, macrophages and granulocytes
- CD41 is known as a megakaryocyte / platelet marker
- CD235a is known as an erythrocyte marker
- CD19 is known as a B cell marker
- CD3 is known as a T cell marker.
- the cell population containing hematopoietic stem cells to be cultured may be, for example, a cell population collected from blood or bone marrow, and is artificially derived from pluripotent stem cells (eg, iPS cells, ES cells). It may be made.
- pluripotent stem cells eg, iPS cells, ES cells.
- the compound of the present invention is the compound of the above formula (1) or a salt thereof, preferably the formula (2), or the formula (3-1), the formula (3-2) or the formula (3-). 2), more preferably, a compound of the formula (4-1), the formula (4-2) or the formula (4-3), or a salt thereof can be mentioned.
- the compound of the present invention is a compound represented by the following chemical formula or a salt thereof. All of these compounds are sesquiterpene lactone compounds with antimalaria activity and are said to be effective against falciparum malaria with multidrug resistance (Jigan Wang et al., NATURE COMMUNICATIONS 2015 6: 10111). DOI: 10.1038).
- artemisinin is a compound separated and named from sweet wormwood (Artemisinin), which is a plant of the genus Artemisinin, which has been used as a Chinese herbal medicine for a long time.
- the compound that can be used in the culture method of the present invention is one or more compounds selected from the group consisting of artemether, artemisinin, artemimol, artemotil, and artesunate.
- the salt may be a derivative of one or more compounds selected from the group consisting of Artemether, Artemisinin, Artemimol, Artemotil, and Artesunate.
- the salt may be present. It can be said that the compound of the formula (1) is a derivative of artemether, artemisinin, artenimol, artemotil, or artesunate.
- Compound or salt thereof will be described below.
- X is OO or O
- Y is hydrogen atom, hydroxy, oxo, mercapto, carboxy, carbamoyl
- Z is bonded to the carbon at any position on the fused ring and is independently C 1-6 alkyl.
- n is an integer from 0 to 10 and R 1 is an alkyl optionally substituted, a cycloalkyl optionally substituted, an aryl optionally substituted, a heteroaryl optionally substituted, or an aliphatic heterocyclic group optionally substituted.
- R 2 may be substituted alkyl, optionally substituted alkenyl, optionally substituted alkynyl, optionally substituted cycloalkyl, optionally substituted aryl, optionally substituted.
- R 3 , R 4 and R 5 are independently hydrogen atom, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted aliphatic heterocyclic group, substituted. It may be an aryl, or a heteroaryl which may be substituted, wherein R 3 and R 4 together with an adjacent nitrogen atom further form an oxygen atom, a nitrogen atom or a ring of 1 or 2.
- a 3- to 8-membered nitrogen-containing aliphatic heterocycle may be formed, which may contain a sulfur atom, and the nitrogen-containing aliphatic heterocycle may be substituted.
- n may be an integer of 0 to 10, preferably an integer of 0 to 6, and more preferably an integer of 0 to 3.
- Equation (2) Is a single bond or a double bond
- X and Y are the same as the definitions in equation (1).
- Z 1 , Z 2 , Z 3 , Z 4 , Z 5 , and Z 6 are independently hydrogen atoms or C 1-6 alkyl. In formula (2), it is preferable that Z 1 , Z 2 , Z 3 , Z 4 , Z 5 , and Z 6 are independently hydrogen atoms or methyl.
- Eqs. (3-1), Eqs. (3-2) and Eqs. (3-3), X and Y are the same as the definitions in equation (1).
- Z 1 , Z 2 , Z 3 , Z 4 , Z 5 , and Z 6 are independently hydrogen atoms or C 1-6 alkyl.
- Z 1 , Z 2 , Z 3 , Z 4 , Z 5 , and Z 6 are independently hydrogen atoms or methyl.
- Eqs. (4-2) and Eqs. (4-3) X and Y are the same as the definitions in the equation (1).
- halogen examples include fluorine, chlorine, bromine, and iodine. Fluorine or chlorine is preferable. More preferably, it is fluorine.
- alkyl means a linear or branched saturated hydrocarbon group, and examples thereof include an alkyl having 1 to 25 carbon atoms, preferably an alkyl having 1 to 10 carbon atoms. More preferred alkyls include "C 1-8 alkyl", “C 1-6 alkyl” or "C 1-4 alkyl”.
- C 1-10 alkyl means a linear or branched saturated hydrocarbon group having 1 to 10 carbon atoms. The same applies to other numbers.
- the "C 1-10 alkyl” is preferably “C 1-8 alkyl", more preferably “C 1-6 alkyl”, and even more preferably “C 1-4 alkyl”.
- Examples of the “C 1-8 alkyl” include preferably “C 1-6 alkyl” and more preferably “C 1-4 alkyl”. As the “C 1-6 alkyl”, preferably "C 1-4 alkyl” is mentioned.
- C 1-4 alkyl examples include methyl, ethyl, propyl, 1-methylethyl, butyl, 1,1-dimethylethyl, 1-methylpropyl, 2-methylpropyl and the like.
- C 1-6 alkyl examples include, for example, 4-methylpentyl, 3-methylpentyl, 2-methylpentyl, 1-, in addition to those listed as specific examples of “C 1-4 alkyl”. Examples thereof include methylpentyl and hexyl.
- C 1-8 alkyl examples include, for example, heptyl, octyl and the like in addition to those mentioned as specific examples of "C 1-6 alkyl” described above.
- Alkenyl means a linear or branched unsaturated hydrocarbon group having one or more double bonds, preferably 1 to 3, and examples thereof include alkenyl having 2 to 7 carbon atoms.
- alkenyl include vinyl, allyl, butadienyl and the like.
- Alkynyl means a linear or branched unsaturated hydrocarbon group having one or more triple bonds, preferably 1 to 2, and examples thereof include alkynyl having 2 to 5 carbon atoms. In the present specification, examples of alkynyl include ethynyl, propynyl and the like.
- Cycloalkyl means a saturated cyclic alkyl, including those with a partially crosslinked structure. Examples of the cycloalkyl include “C 3-8 cycloalkyl” and “C 3-6 cycloalkyl”.
- the “C 3-8 cycloalkyl” means a cycloalkyl having 3 to 8 carbon atoms constituting the ring.
- the “C 3-8 cycloalkyl” is preferably “C 3-6 cycloalkyl”.
- Specific examples of “C 3-6 cycloalkyl” include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl and the like.
- Specific examples of "C 3-8 cycloalkyl” include, for example, cycloheptyl, cyclooctyl and the like in addition to those mentioned as specific examples of "C 3-6 cycloalkyl”.
- Aryl means a monocyclic or bicyclic aromatic hydrocarbon, and examples thereof include “C 6-10 aryl”.
- C 6-10 aryl means an aryl having 6 to 10 carbon atoms. Specific examples of “C 6-10 aryl” include, for example, phenyl, 1-naphthyl, 2-naphthyl and the like. As “C 6-10 aryl”, phenyl is preferably mentioned.
- heteroaryl is a monocyclic or compound ring of 1 to 4 rings independently selected from the group consisting of 1 to 4 nitrogen atoms, 1 oxygen atom and 1 sulfur atom. It means an aromatic heterocyclic group containing a heteroatom within. When heteroaryl is substituted, it may be substituted on any carbon atom or nitrogen atom as long as it is chemically stable.
- “5- to 10-membered heteroaryl” means a monocyclic or bicyclic heteroaryl composed of 5 to 10 atoms.
- the "5- to 10-membered heteroaryl” is preferably "5- to 6-membered heteroaryl", and specifically, frill, thienyl, pyrrolyl, imidazolyl, triazolyl, tetrazolyl, thiazolyl, pyridyl, pyrimidinyl, pyrazineyl.
- Examples thereof include benzoxazolyl, benzothiazolyl, benzisooxazolyl, benzisothiazolyl, benzotriazolyl, benzimidazolyl, 6,11-dihydrodibenzo [b, e] thiepinyl and the like.
- the "aliphatic heterocyclic group” is independent of the group consisting of 1 to 2 nitrogen atoms, 1 to 2 oxygen atoms and 1 to 2 sulfur atoms in addition to monocyclic or bicyclic carbon atoms. It means a saturated or unsaturated aliphatic heterocyclic group containing 1 to 4 heteroatoms selected in the above. When the aliphatic heterocyclic group is substituted, it may be substituted on any carbon atom or nitrogen atom as long as it is chemically stable.
- the "4 to 10-membered aliphatic heterocyclic group” means an aliphatic heterocyclic group composed of 4 to 10 atoms, and has a partially crosslinked structure and a partially spirolated group. , Or C 6-10 aryl or C 5-10 heteroaryl forming a fused ring.
- a 4- to 6-membered monocyclic saturated heterocyclic group is preferably mentioned.
- Specific examples of the "4- to 6-membered monocyclic saturated heterocyclic group” include azetidinyl, pyrrolidinyl, piperidinyl, piperazinyl, morpholinyl, tetrahydrofuryl, tetrahydropyranyl and the like.
- Specific examples of the "4 to 10-membered saturated heterocyclic group” include, for example, azepanyl, oxepanyl, diazepanyl, in addition to those listed as specific examples of the above-mentioned "4 to 6-membered monocyclic saturated heterocyclic group”. Homopiperidinyl and the like can be mentioned.
- Examples of the "4 to 10-membered nitrogen-containing aliphatic heterocycle” include a 4- to 10-membered nitrogen-containing aliphatic heterocycle which may further contain an oxygen atom, a nitrogen atom or a sulfur atom constituting one or two rings. Be done.
- the nitrogen-containing aliphatic heterocycle may be monocyclic or bicyclic, and may be a saturated or unsaturated nitrogen-containing aliphatic heterocycle.
- a 4- to 6-membered monocyclic saturated nitrogen-containing heterocycle is preferably mentioned.
- Specific examples of the "4- to 6-membered monocyclic saturated nitrogen-containing heterocycle” include azetidine, pyrrolidine, piperidine, piperazine, morpholine and the like.
- Specific examples of the "4- to 10-membered saturated heterocycle” include, for example, azepane, oxepannyl, diazepane, and homopiperidine, in addition to those listed as specific examples of the above-mentioned "4- to 6-membered monocyclic saturated heterocycle”. Etc.
- Alkoxy means an oxy group substituted with an alkyl, and preferred alkoxys include “C 1-8 alkoxy”, “C 1-6 alkoxy” or “C 1-4 alkoxy”. “C 1-8 alkoxy” means an oxy group substituted with the above “C 1-8 alkyl”.
- the "C 1-8 alkoxy” is preferably "C 1-6 alkoxy” or "C 1-4 alkoxy”. Specific examples of "C 1-4 alkoxy” include methoxy, ethoxy, propoxy, 1-methylethoxy, butoxy, 1,1-dimethylethoxy, 1-methylpropoxy, and 2-methylpropoxy.
- C 1-6 alkoxy examples include, for example, pentyroxy, 3-methylbutoxy, 2-methylbutoxy, and 2,2-dimethyl, in addition to those listed as specific examples of “C 1-4 alkoxy” described above.
- Propoxy, 1-ethylpropoxy, 1,1-dimethylpropoxy, hexyloxy, 4-methylpentyroxy, 3-methylpentyroxy, 2-methylpentyroxy, 1-methylpentyroxy, 3,3-dimethylbutoxy, 2,2 -Dimethylbutoxy, 1,1-dimethylbutoxy, 1,2-dimethylbutoxy and the like can be mentioned.
- Specific examples of "C 1-8 alkoxy” include, for example, heptyloxy, octyloxy, and the like, in addition to those mentioned as specific examples of "C 1-6 alkoxy” described above.
- the “C 1-8 alkyl carbonyl” means a carbonyl group substituted with the above “C 1-8 alkyl”.
- the “C 1-8 alkyl carbonyl” is preferably "C 1-6 alkyl carbonyl” or "C 1-4 alkyl carbonyl”.
- Specific examples of "C 1-4 alkylcarbonyl” include, for example, methylcarbonyl, ethylcarbonyl, propylcarbonyl, 1-methylethylcarbonyl, butylcarbonyl, 1,1-dimethylethylcarbonyl, 1-methylpropylcarbonyl, 2- Examples thereof include methylpropylcarbonyl.
- C 1-6 alkylcarbonyl include, for example, 4-methylpentylcarbonyl, 3-methylpentylcarbonyl, and 2-methyl in addition to those listed as specific examples of "C 1-4 alkylcarbonyl". Examples thereof include pentylcarbonyl, 1-methylpentylcarbonyl, hexylcarbonyl and the like.
- C 1-8 alkyl carbonyl include, for example, heptyl carbonyl, octyl carbonyl, and the like, in addition to those mentioned as specific examples of the above “C 1-6 alkyl carbonyl”.
- C 1-8 alkylcarbonyloxy means an oxy group substituted with the above “C 1-8 alkylcarbonyl".
- the "C 1-8 alkylcarbonyloxy” is preferably “C 1-6 alkylcarbonyloxy” or "C 1-4 alkylcarbonyloxy”.
- Specific examples of "C 1-4 alkylcarbonyloxy” include, for example, acetoxy, propanoyloxy, butanoyloxy, 2-methylpropanoyloxy, pentanoyloxy, 2,2-dimethylpropanoyloxy, 2 -Methylbutanoyloxy and 3-methylbutanoyloxy can be mentioned.
- C 1-6 alkylcarbonyloxy include, for example, hexanoyloxy, 3,3-dimethylbutanoyloxy, in addition to those listed as specific examples of "C 1-4 alkylcarbonyloxy”.
- C 1-8 alkylcarbonyloxy include, for example, heptylcarbonyloxy, octylcarbonyloxy and the like, in addition to those mentioned as specific examples of "C 1-6 alkylcarbonyloxy”.
- the “C 1-8 alkoxycarbonyl” means a carbonyl group substituted with the above “C 1-8 alkoxy”.
- the “C 1-8 alkoxycarbonyl” is preferably "C 1-6 alkoxycarbonyl” or "C 1-4 alkoxycarbonyl”.
- Specific examples of "C 1-4 alkoxycarbonyl” include, for example, methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl, 1-methylethoxycarbonyl, butoxycarbonyl, 1,1-dimethylethoxycarbonyl, 1-methylpropoxycarbonyl, 2- Methylpropoxycarbonyl can be mentioned.
- C 1-6 alkoxycarbonyl include, for example, pentyroxycarbonyl, 3-methylbutoxycarbonyl, and 2-methylbutoxycarbonyl in addition to those listed as specific examples of "C 1-4 alkoxycarbonyl”.
- 2,2-Dimethylpropoxycarbonyl 1,2-Dimethylpropoxycarbonyl, 1-ethylpropoxycarbonyl, 1,1-dimethylpropoxycarbonyl, hexyloxycarbonyl, 4-methylpentyroxycarbonyl, 3-methylpentyroxycarbonyl, 2-methylpentyroxycarbonyl, 1- Examples thereof include methylpentyloxycarbonyl, 3,3-dimethylbutoxycarbonyl, 2,2-dimethylbutoxycarbonyl, 1,1-dimethylbutoxycarbonyl, 1,2-dimethylbutoxycarbonyl and the like.
- C 1-8 alkoxycarbonyl include, for example, heptyloxycarbonyl, octyloxycarbonyl, and the like, in addition to those mentioned as specific examples of "C 1-6 alkoxycarbonyl".
- C 1-8 alkyl sulfanil means a sulfanil group substituted with the above “C 1-8 alkyl”.
- the "C 1-8 alkyl sulfanyl” is preferably “C 1-6 alkyl sulfanyl” or "C 1-4 alkyl sulfanyl”.
- Specific examples of "C 1-4 alkyl sulfanyl” include, for example, methyl sulfanyl, ethyl sulfanyl, propyl sulfanyl, 1-methyl ethyl sulfanyl, butyl sulfanyl, 1,1-dimethylethyl sulfanyl, 1-methylpropyl sulfanyl, 2-.
- Methylpropylsulfanyl can be mentioned.
- Specific examples of "C 1-6 alkyl sulfanyl” include, for example, pentyl sulfanyl, 3-methylbutyl sulfanyl, 2-methylbutyl sulfanyl, in addition to those listed as specific examples of "C 1-4 alkyl sulfanyl”.
- 2,2-Dimethylpropylsulfanyl 1-ethylpropylsulfanyl, 1,1-dimethylpropylsulfanyl, hexylsulfanyl, 4-methylpentylsulfanyl, 3-methylpentytylsulfanyl, 2-methylpentylsulfanyl, 1-methylpentylsulfanyl , 3,3-Dimethylbutylsulfanyl, 2,2-dimethylbutylsulfanyl, 1,1-dimethylbutylsulfanyl, 1,2-dimethylbutylsulfanyl and the like.
- C 1-8 alkyl sulfanyl include, for example, heptyl sulfanyl, octyl sulfanyl, and the like, in addition to those listed as specific examples of "C 1-6 alkyl sulfanyl”.
- C 1-8 alkyl sulfonyl means a sulfonyl group substituted with the above “C 1-8 alkyl”.
- the "C 1-8 alkyl sulfonyl” is preferably "C 1-6 alkyl sulfonyl” or "C 1-4 alkyl sulfonyl”.
- C 1-4 alkyl sulfonyl include, for example, methyl sulfonyl, ethyl sulfonyl, propyl sulfonyl, 1-methyl ethyl sulfonyl, butyl sulfonyl, 1,1-dimethyl ethyl sulfonyl, 1-methyl propyl sulfonyl, 2- Methylpropylsulfonyl may be mentioned.
- C 1-6 alkyl sulfonyl include, for example, pentyl sulfonyl, 3-methyl butyl sulfonyl, 2-methyl butyl sulfonyl, in addition to those listed as specific examples of "C 1-4 alkyl sulfonyl”.
- 2,2-Dimethylpropylsulfonyl 1-ethylpropylsulfonyl, 1,1-dimethylpropylsulfonyl, hexylsulfonyl, 4-methylpentylsulfonyl, 3-methylpentytylsulfonyl, 2-methylpentylsulfonyl, 1-methylpentylsulfonyl , 3,3-Dimethylbutylsulfonyl, 2,2-dimethylbutylsulfonyl, 1,1-dimethylbutylsulfonyl, 1,2-dimethylbutylsulfonyl and the like.
- C 1-8 alkyl sulfonyl include, for example, heptyl sulfonyl, octyl sulfonyl, and the like, in addition to those mentioned as specific examples of the above "C 1-6 alkyl sulfonyl”.
- C 1-8 alkylsulfonylamino means an amino group substituted with the above “C 1-8 alkylsulfonylamino”.
- the "C 1-8 alkyl sulfonyl amino” is preferably "C 1-6 alkyl sulfonyl amino” or "C 1-4 alkyl sulfonyl amino”.
- C 1-4 alkylsulfonylamino include, for example, methylsulfonylamino, ethylsulfonylamino, propylsulfonylamino, 1-methylethylsulfonylamino, butylsulfonylamino, 1,1-dimethylethylsulfonylamino, 1 -Methylpropylsulfonylamino and 2-methylpropylsulfonylamino can be mentioned.
- C 1-6 alkylsulfonylamino include, for example, pentylsulfonylamino, 3-methylbutylsulfonylamino, 2-, in addition to those listed as specific examples of "C 1-4 alkylsulfonylamino".
- Methylbutylsulfonylamino 2,2-dimethylpropylsulfonylamino, 1-ethylpropylsulfonylamino, 1,1-dimethylpropylsulfonylamino, hexylsulfonylamino, 4-methylpentylsulfonylamino, 3-methylpentytylsulfonylamino
- Examples include 2-methylpentylsulfonylamino, 1-methylpentylsulfonylamino, 3,3-dimethylbutylsulfonylamino, 2,2-dimethylbutylsulfonyl, 1,1-dimethylbutylsulfonyl, 1,2-dimethylbutylsulfonylamino and the like.
- C 1-8 alkyl sulfonyl amino include, for example, heptyl sulfonyl amino, octyl sulfonyl amino, and the like, in addition to those mentioned as specific examples of the above "C 1-6 alkyl sulfonyl amino".
- the “C 1-8 alkylcarbonylamino” means an amino group substituted with the above “C 1-8 alkylcarbonyl”.
- the "C 1-8 alkylcarbonylamino” is preferably "C 1-6 alkylcarbonylamino" or "C 1-4 alkylcarbonylamino".
- Specific examples of "C 1-4 alkylcarbonylamino” include, for example, acetylamino, propanoylamino, butanoylamino, 2-methylpropanoylamino, pentanoylamino, 2,2-dimethylpropanoylamino, and the like. Examples thereof include 2-methylbutanoylamino and 3-methylbutanoylamino.
- C 1-6 alkylcarbonylamino include, for example, hexanoylamino, 3,3-dimethylbutanoylamino, in addition to those listed as specific examples of "C 1-4 alkylcarbonylamino".
- C 1-8 alkylcarbonylamino include, for example, heptylcarbonylamino, octylcarbonylamino, and the like, in addition to those mentioned as specific examples of the above "C 1-6 alkylcarbonylamino".
- amino which may be substituted with 1 or 2 C 1-8 alkyl
- Aminos substituted with “" can be mentioned. Specific examples thereof include amino, methylamino, ethylamino, propylamino, 2-propylamino, butylamino, dimethylamino, diethylamino, methylethylamino, dipropylamino and the like.
- two substituents on the amino group, together with adjacent nitrogen atoms are 4-10 membered nitrogen-containing aliphatic heterocycles. It may form a ring.
- the nitrogen-containing aliphatic heterocycle is preferably piperidine, pyrrolidine, piperazine, morpholine or the like.
- carbamoyl which may be substituted with 1 or 2 C 1-8 alkyl, for example, carbamoyl, or the same or different "C 1-6 alkyl” or "C 1-4 alkyl” of 1 or 2 above.
- carbamoyl which may be substituted with 1 or 2 C 1-8 alkyl
- two substituents of the carbamoyl group are combined with adjacent nitrogen atoms to form a 4- to 10-membered nitrogen-containing aliphatic heterocycle. It may form a ring.
- the nitrogen-containing aliphatic heterocycle is preferably piperidine, pyrrolidine, piperazine, morpholine or the like.
- Eq. (1), Eq. (2), Eq. (3-1), Eq. (3-2), Eq. (3-3), Eq. (4-1), Eq. (4-2) or Eq. (4-3) ) Is hydrogen atom, hydroxy, oxo, carboxy, carbamoyl, OR 1 , SR 1 , SO 2 R 1 , COR 1 , OCOR 1 , R 2 , NR 3 R 4 , NR 5 COR 1 , NR 5 SO 2. It is preferably R 1 or a fluorine atom.
- the bond represented by is meant a single bond or a double bond.
- the above bond is a single bond when Y is an oxo group.
- Y is a hydrogen atom or hydroxy
- Y is hydroxy, oxo, carboxy, carbamoyl
- R 1 may be substituted with C 1-8 alkyl selected from group 1 with 1-7 substituents, 3-8 optionally substituted with 1-3 groups selected from group 1.
- cycloalkyl phenyl optionally substituted with 1-5 groups selected from group 1, oxygen atom, nitrogen atom and sulfur optionally substituted with 1-3 groups selected from group 1.
- R 2 may be substituted with a substituent of 1 to 7 selected from group 2, C 1-8 alkyl, and C 1 may be substituted with a substituent of 1 to 7 selected from group 2.
- a 4- to 10-membered aliphatic complex containing 1 to 4 heteroatoms in the ring selected from oxygen, nitrogen and sulfur atoms which may be substituted with 1 to 3 groups selected from group 1. It may be substituted with a ring group or 1 to 4 groups selected from group 1, and may contain 5 to 10 members including 1 to 3 heteroatoms selected from oxygen atom, nitrogen atom and sulfur atom.
- Heteroaryl, R 3 and R 4 are independently a hydrogen atom, a C 1-6 alkyl optionally substituted with 1-7 substituents selected from Group 1, or 1-5 groups selected from Group 1.
- a good 3- to 8-membered nitrogen-containing aliphatic heterocycle may be formed, and the nitrogen-containing aliphatic heterocycle may be substituted with 1 to 3 substituents selected from the following group 1 substituents.
- R 5 is a hydrogen atom or C 1-3 alkyl.
- X is OO.
- R 1 , R 3 , R 4 and R 5 when the alkyl in R 1 , R 3 , R 4 and R 5 is substituted, it is substituted with 1 to 7 selected from the following group 1 and preferably 1 to 3 substituents. May be good.
- the alkyl in R 2 if alkenyl or alkynyl is substituted, 1 to 7 selected from the group 2 or less, preferably may be substituted with 1 to 3 substituents.
- Group 1> Carboxy, hydroxy, C 1-8 alkoxy, C 1-8 alkylcarbonyl, C 1-8 alkylcarbonyloxy, C 1-8 alkoxycarbonyl, mercapto, C 1-8 alkylsulfanyl, C 1-8 alkylsulfonyl, 1 or 2 of C 1-8 amino optionally substituted by alkyl, 1 or 2 of C 1-8 alkyl optionally substituted carbamoyl, C 1-8 alkylcarbonylamino, C 1-8 alkylsulfonylamino, It may be substituted with 1 to 5 groups selected from group 3, phenyl, may be substituted with 1 to 3 groups selected from group 3, and may be selected from oxygen atom, nitrogen atom and sulfur atom.
- heteroaryl containing 1 to 4 heteroatoms in the ring
- 3-8 membered cycloalkyl optionally substituted with 1-3 groups selected from group 3, selected from group 3.
- Group 2> Carboxy, hydroxy, C 1-8 alkoxy, C 1-8 alkylcarbonyl, C 1-8 alkylcarbonyloxy, C 1-8 alkoxycarbonyl, mercapto, C 1-8 alkylsulfanyl, C 1-8 alkylsulfonyl, 1 or Carbamoyl, amino optionally substituted with C 1-8 alkyl of 2, phenyl optionally substituted with 1-5 groups selected from group 3, with 1-4 groups selected from group 3.
- 3 to 8 membered cycloalkyl which may be substituted, 1 to 4 rings selected from oxygen atom, nitrogen atom and sulfur atom which may be substituted with 1 to 3 groups selected from group 3.
- the cycloalkyl When the cycloalkyl is substituted, it may be substituted with 1-3, preferably 1-2 substituents selected from Group 1 above. Preferred examples of the substituent of the cycloalkyl group include hydroxy, C 1-8 alkoxy, and halogen.
- the aliphatic heterocyclic group When the aliphatic heterocyclic group is substituted, it may be substituted with 1 to 3, preferably 1 to 2 substituents selected from the above group 1. Preferred examples of the substituent of the aliphatic heterocyclic group include hydroxy, C 1-8 alkoxy, and halogen.
- aryl When the aryl is substituted, it may be substituted with 1 to 5, preferably 1-3 substituents selected from Group 1 above.
- Preferred examples of the aryl group substituent include hydroxy, C 1-8 alkoxy, and halogen.
- heteroaryl When the heteroaryl is substituted, it may be substituted with 1-4, preferably 1-3 substituents selected from Group 1 above. Preferred examples of the substituent of the heteroaryl group include hydroxy, C 1-8 alkoxy, and halogen.
- Group 1 and Group 3 are preferably Group 1', Group 2'and Group 3', respectively, as described below.
- a 4- to 6-membered aliphatic heterocyclic group containing a heteroatom in 1 to 3 rings ⁇ Group 2'> Carbamoyl optionally substituted with fluorine atom, hydroxy, carboxy, amino, C 1-4 alkoxy, C 2-4 alkyl carbonyloxy, C 1-4 alkoxycarbonyl, 1 or 2 C 1-8 alkyl, group 3.
- Phyl which may be substituted with 1-3 groups selected from', 3-6 membered cycloalkyl, which may be substituted with 1-3 groups selected from group 3', from group 3'.
- alkenyl or alkynyl When alkenyl or alkynyl is substituted, it may be substituted with 1-3, preferably 1-2 substituents selected from the group 3 ′′ below.
- the Z in the formula (1) is preferably C 1-4 alkyl, more preferably C 1-3 alkyl, and more preferably methyl or ethyl.
- Z may be bonded to any carbon atom constituting the fused ring, may be bonded to the same carbon atom as long as it is chemically stable, and belongs to a plurality of rings. It may be bonded on a carbon atom, and Z may be the same or different.
- the configuration of the carbon atoms constituting the ring (condensed ring) to which Z is bonded in the formula (1) is not particularly limited as long as a chemically stable structure can be obtained, but for example, the formula (2-1).
- X, Z 1 , Z 2 , Z 3 , Z 4 , Z 5 and Z 6 are the same as the definitions in formula (1) or formula (2).
- formulas (3-1'), formulas (3-2') and formulas (3-3') In the formula, X, Z 1 , Z 2 , Z 3 , Z 4 , Z 5 and Z 6 are the same as the definitions in formula (1) or formula (2)).
- the group represented by is mentioned.
- Examples of Z 1 , Z 2 , Z 3 , Z 4 , Z 5 and Z 6 in the formula (2) preferably include a hydrogen atom or C 1-4 alkyl, and more preferably a hydrogen atom or C 1-3 alkyl. , More preferably a hydrogen atom or methyl or ethyl.
- Y is oxo
- the compound of formula (2') has a tautomer represented by formula (2 ′′), and the present invention includes both of these substances as long as they can be chemically stable.
- X is the same as the definition in equation (1)
- X is O, preferably the compound represented by the above formula (2') or formula (2 ′′) can be mentioned.
- Preferred embodiments of Y include hydrogen atom, hydroxy, carboxy, carbamoyl or halogen.
- Y is a halogen, it is preferably a fluorine atom.
- R 1 is preferably C 1-4 alkyl, which may be substituted, phenyl, which may be substituted. Alternatively, cycloalkyl which may be substituted can be mentioned.
- C 1-3 alkyl which may be substituted more preferably methyl or ethyl which may be substituted respectively can be mentioned.
- substituents include halogen, carboxy, amino, C 1-4 alkoxy, C 1-4 alkyl carbonyloxy, and the like, which may be substituted with one or more substituents selected from the above group 2. , And each of them may be independently replaced with one or more than one or three.
- the halogen is preferably a fluorine atom.
- R 1 when Y is OCOR 1 examples include C 1-4 alkyl which may be substituted.
- Substituents are selected from halogen, carboxy, amino, C 1-4 alkoxy, C 1-4 alkyl carbonyloxy, which may be substituted with the same or different C 1-3 alkyl of halogen, carboxy, 1 or 2, and Group 3 above.
- Aryl and the like, which may be substituted with one or more substituents, may be mentioned, and one or more, 1 to 3 of each may be independently substituted.
- the halogen is preferably a fluorine atom.
- R 1 when Y is OCOR 1 is a carboxy-substituted C 1-4 alkyl.
- R 3 and R 4 are preferably independently hydrogen atoms or C 1-4 alkyl, or 4 to 4 with nitrogen atoms adjacent to R 3 and R 4. It may form a 10-membered nitrogen-containing aliphatic heterocycle.
- the 4- to 10-membered nitrogen-containing aliphatic heterocycle is preferably a 4- to 6-membered monocyclic saturated nitrogen-containing heterocycle, and specific examples thereof include azetidine, pyrrolidine, piperidine, piperazine, and morpholine. ..
- R 1 includes C 1-4 alkyl which may be substituted.
- substituents include halogen, carboxy, amino, C 1-4 alkoxy, C 1-4 alkyl carbonyloxy, and aryl which may be substituted with one or more substituents selected from the above group 3. , Each of them may be independently replaced with one or more than one or three.
- the halogen is preferably a fluorine atom.
- R 5 is preferably a hydrogen atom or C 1-4 alkyl, more preferably a hydrogen atom, methyl or ethyl.
- R 3 and R 4 are preferably independently hydrogen atoms, optionally substituted C 1-4 alkyl, or 1 or selected from Group 3. Examples thereof include phenyls which may be substituted with a plurality of substituents.
- the C 1-4 alkyl substituent is substituted with halogen, carboxy, amino, C 1-4 alkoxy, C 1-4 alkyl carbonyloxy, or one or more substituents selected from Group 3 above.
- Aryl may be mentioned, and one or a plurality of aryls may be independently substituted, respectively.
- the halogen is preferably a fluorine atom.
- R 3 and R 4 may be combined with adjacent nitrogen atoms to form a 4- to 10-membered nitrogen-containing aliphatic heterocycle.
- the 4- to 10-membered nitrogen-containing aliphatic heterocycle is preferably a 4- to 6-membered monocyclic saturated nitrogen-containing heterocycle, and specific examples thereof include azetidine, pyrrolidine, piperidine, piperazine, and morpholine. ..
- substituent of the C 1-4 alkyl which may be substituted, halogen, carboxy, amino, C 1-4 alkoxy, C 1-4 alkyl carbonyloxy and the like are preferably selected from the above group 2 respectively.
- Aryl, heteroaryl, cycloalkyl and aliphatic heterocyclic groups which may be substituted with one or more substituents may be mentioned, and one or a plurality of each may be independently substituted.
- the halogen is preferably a fluorine atom.
- R 5 is preferably a hydrogen atom or C 1-4 alkyl, more preferably a hydrogen atom, methyl or ethyl.
- R 2 is preferably C 1-4 alkyl, which may be substituted with one or more substituents selected from the above group 2 or group 2', the above group 3 Alternatively, phenyl, which may be substituted with one or more substituents selected from group 3', is substituted with one or more substituents selected from group 3 in [6] or [7] above. 5 or 6 member heteroaryl may be mentioned. Specific examples of the heteroaryl include furan-2-yl and triazine-1-yl, which may be substituted, respectively.
- R 2 is C 1-4 alkyl, preferably methyl or ethyl.
- Y is R 2
- R 2 one or more selected from the following group 4, preferably C 1-4 alkyl substituted with 1 to 3 substituents, preferably the following, respectively: Examples thereof include C 1-4 alkyl, methyl or ethyl substituted with 1-3 substituents selected from Group 4.
- Carbamoyl, phenyl optionally substituted with 1-5 groups selected from group 3, may be substituted with 1-4 groups selected from group 3, selected from oxygen, nitrogen and sulfur atoms 5-6 membered heteroaryl containing 1 to 3 heteroatoms in the ring, 3-6 membered cycloalkyl optionally substituted with 1-3 groups selected from Group 3, Group 3 A 4- to 8-membered aliphatic heterocyclic group containing a heteroatom in a ring of 1 to 4 selected from an oxygen atom, a nitrogen atom and a sulfur atom which may be substituted with 1 to 3 groups selected from. , Fluorine atom
- One embodiment of the compound represented by the formula (2) includes compounds having the following characteristics (A1) to (A5) or salts thereof: (A1) Z 1 , Z 3 and Z 5 are methyl, and Z 2 , Z 4 and Z 6 are hydrogen atoms; (A2) X is O or OO; (A3) Y is hydroxy, oxo, carboxy, carbamoyl, fluorine atom, OR 1 , OCOR 1 , SR 1 , SO 2 R 1 , R 2 , NHCOR 1 or NR 3 R 4 ; (A4) R 1 or R 2 may be substituted C 1-8 alkyl, optionally substituted phenyl, optionally substituted 5-6 member cycloalkyl, optionally substituted.
- R 3 and R 4 represent a methyl group or, together with adjacent nitrogen atoms, morpholine, thiomorpholine, thiomorpholine-1-oxide, thiomorpholine-1,1-dioxide, piperazine, indolin. , Forming tetrahydroisoquinoline.
- Formula (1), formula (2), formula (3-1), formula (3-2), formula (3-3), formula (4-1), formula (4-2) or formula (4-3) ) Is one embodiment of the compound having the following characteristics (B1) to (B2), or a salt thereof.
- R 1 may be substituted C 1-8 alkyl, optionally substituted phenyl, optionally substituted 5-6 member cycloalkyl, optionally substituted 5-6. It is a member heteroaryl and the substituent is selected from fluorine atom, amino, carboxy, C 1-8 alkoxy, C 1-8 alkylcarbonyloxy and phenyl;
- R 5 is a hydrogen atom.
- Equation (1), Equation (2), Equation (3-1), Equation (3-2), Equation (3-3), Equation (4-1), Equation (4-2) or Equation (4-3). ) are specific examples of the compounds of Examples 1-1 to 1-35.
- the compound of the present invention contains a salt of the compound represented by the above formula (1).
- the salt is not particularly limited as long as it does not affect the survival or differentiation of cells in cell culture, and examples thereof include salts that are acceptable as raw materials for pharmaceutical products.
- salts examples include acid addition salts and base addition salts.
- an inorganic acid salt such as hydrochloride, hydrobromide, sulfate, hydroiodide, nitrate, phosphate, or citrate, oxalate, phthalate, Fumarate, maleate, succinate, malate, acetate, formate, propionate, benzoate, trifluoroacetate, methanesulfonate, benzenesulfonate, para-toluenesulfonic acid
- organic acid salts such as salts and camphor sulfonates.
- Examples of the base addition salt include inorganic base salts such as sodium salt, potassium salt, calcium salt, magnesium salt, barium salt and aluminum salt, or trimethylamine, triethylamine, pyridine, picolin, 2,6-rutidine, ethanolamine and diethanolamine. , Triethanolamine, trimethylamine, [tris (hydroxymethyl) methylamine], tert-butylamine, cyclohexylamine, dicyclohexylamine, organic base salts such as N, N-dibenzylethylamine and the like.
- examples of the "salt” include amino acid salts with basic amino acids such as arginine, lysine, ornithine, aspartic acid, or glutamic acid, or acidic amino acids.
- the compound of the present invention when it is desired to obtain a salt of the compound of the present invention, if the compound of the present invention is obtained in the form of a salt, it may be purified as it is, or if it is obtained in the free form, it is dissolved in an appropriate organic solvent. Alternatively, it may be suspended and an acid or base may be added to form a salt by a usual method.
- Deuterium converter which converts any one or two or more of the 1 H to 2 H (D) of the compounds of the invention are also encompassed by the compounds of the present invention.
- the compound of the present invention may exist in the form of a hydrate and / or a solvate with various solvents (such as an ethanol solvate), these hydrates and / or solvates are also of the present invention. Included in the compound. Further, the present invention includes all tautomers of the compounds of the present invention, any stereoisomers present, and crystalline forms of any mode, as well as mixtures thereof.
- optical isomers based on optically active centers are optical isomers based on optically active centers, atropisomers based on axial or planar chirality generated by the constraint of intramolecular rotation, other steric isomers, tautomers, etc.
- geometric isomers and the like may exist, but all possible isomers and mixtures thereof, including these, are included within the scope of the present invention.
- the compound represented by the formula (4-3) may contain a single steric variant and a mixture of a plurality of steric isomers when it has an optically active center whose configuration is not specified.
- the optical isomer of the above and a mixture of the compound and the optical isomer thereof are also within the scope of the present invention.
- the optical isomer and the atrop isomer can be obtained as a racemate, or as an optically active substance when a starting material or an intermediate for optical activity is used.
- the corresponding raw material, intermediate or final racemate is physically separated by a known separation method such as a method using an optically active column or a fractional crystallization method. It can be divided into their optical racemates either or chemically.
- a known separation method such as a method using an optically active column or a fractional crystallization method. It can be divided into their optical racemates either or chemically.
- the diastereomer method two types of diastereomers are formed from a racemate by a reaction using an optically active dividing agent. Since these different diastereomers generally have different physical properties, they can be separated by a known method such as fractional crystallization. ⁇ Compound manufacturing method>
- the compound represented by the formula (1) is a known compound, or can be produced from a known compound by a chemical synthesis method well known to those skilled in the art.
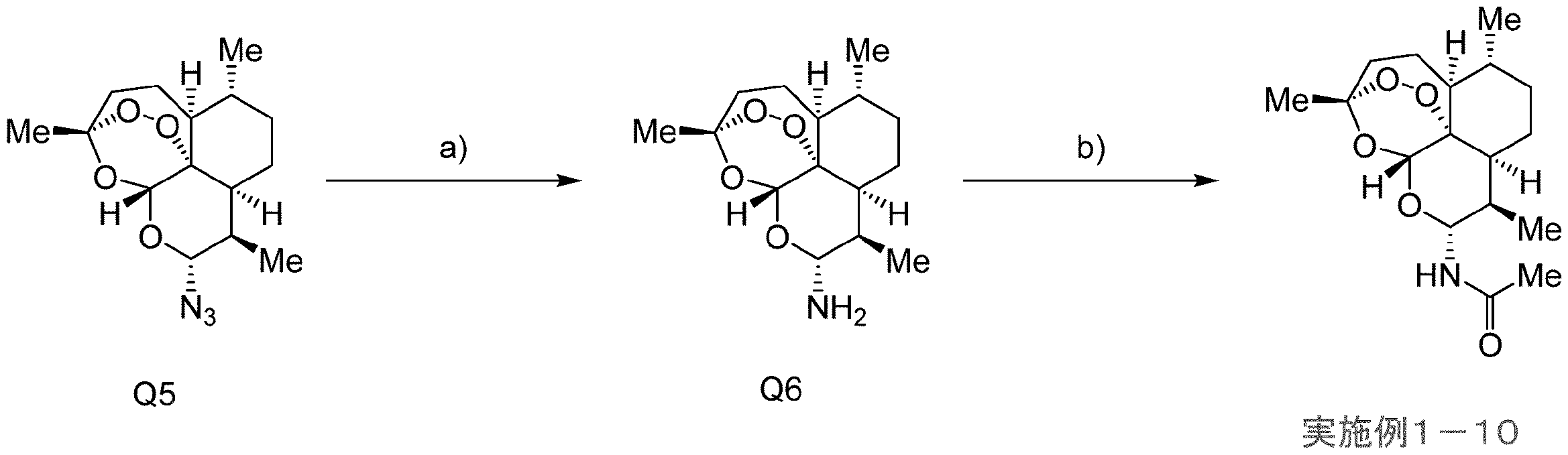
- Examples 1-33 Specific known compounds include artemisinin (Example 1-33), artemether (Example 1-30), and artenimol (Example 1-29) described in Examples of the present specification. ), Artemotil (Example 1-31), Artesunate (Example 1-32), and Examples 1-1 to 1-28, 1-34, and 1-35. Examples include compounds that are used.
- Artemether, Artenimol, Artemotil, and Artesunate are all derivatives of Artemisinin and can be semi-synthesized from Artemisinin.
- Artemether and Artemotil follow the method described in Tetrahedron Letters 2002, 43, 7235-7237, and Artesunate refers to J. et al. Med.
- Chem. Artenimoll can be synthesized according to the method described in ChemCommun 2014, 50, 1265-12655 according to the method described in 1988, 31, 645-650.
- Other compounds are also known in the literature or can be semi-synthesized from artemisinin.
- the compound represented by -3) can be produced by semisynthesis from a known compound such as artenimol or artemisinin.
- a compound in which Y is hydroxy, halogen, hydrogen atom, carboxy, COOR 1 , R 2 heteroaryl such as furan, or alkyl such as methyl
- R 1 heteroaryl such as furan, or alkyl such as methyl
- artimol, deoxydihydroartemisinin and the like are known compounds and are available.
- Artenimole can be synthesized, for example, according to the method described in ChemCommun 2014, 50, 12652-12655, and the dihydroartemisic acid described in the literature is, for example, Tetrahedron 2016, 72 (32), 4931-4937.
- Deoxydihydroartemisinin can be chemically synthesized from artemisinin according to the method described in, for example, ChemMedChem 2012, 7 (12), 2204-2226. Therefore, various a1 can be produced with reference to the methods described in these.
- Compounds A1 and A2 can be produced by reacting compound a1 with a fluorination reagent.
- the solvent is appropriately selected from the solvents exemplified below and the like, and dichloromethane is preferable.
- the fluorination reagent include N and N-diethylaminosulfur trifluoride.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually ⁇ 78 ° C. to 100 ° C., preferably ⁇ 30 ° C. to 40 ° C.
- Compound A3 can be produced by reacting compound A1 with furan in a suitable solvent in the presence of Lewis acid.
- Lewis acid includes boron trifluoride diethyl ether complex.
- the solvent is appropriately selected from the solvents exemplified below and the like, and dichloromethane is preferable.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually ⁇ 78 ° C. to 20 ° C., preferably ⁇ 78 ° C. to ⁇ 20 ° C.
- a compound in which R 2 in the formula (1) is heteroaryl can be produced.
- Compound A4 can be produced by reacting compound A3 with various periodic acids in the presence of a metal catalyst.
- the metal catalyst include ruthenium dioxide.
- Examples of periodic acid include sodium periodate and potassium periodate.
- the solvent is appropriately selected from the solvents exemplified below, and preferred examples thereof include acetonitrile, carbon tetrachloride, and water.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually ⁇ 20 ° C. to 70 ° C., preferably ⁇ 0 ° C. to 40 ° C.
- Compound A5 is produced by reacting compound A4 with the corresponding alcohol in the presence or absence of various condensing agents and / or bases in a suitable solvent, and a catalyst is used if necessary.
- a catalyst is used as the condensing agent.
- various condensing agents used in a conventional method can be used, and 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide (including hydrochloride) is preferably used.
- the base is appropriately selected from the bases exemplified below, and preferably diisopropylethylamine or triethylamine.
- the catalyst include 4-dimethylaminopyridine.
- the solvent is appropriately selected from the solvents exemplified below, and preferably contains tetrahydrofuran, dimethylformamide, or dichloromethane.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually 0 ° C. to 200 ° C., preferably 0 ° C. to 100 ° C.
- Compound A6 can be produced by reacting compound A1 with trimethylaluminum in a suitable solvent.
- the solvent is appropriately selected from the solvents exemplified below, and preferably toluene or dichloromethane.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually ⁇ 78 ° C. to 50 ° C., preferably ⁇ 40 ° C. to 30 ° C.
- a compound or the like in which R 2 in the formula (1) is alkyl can be produced by using a reagent such as an alkylating agent well known to those skilled in the art.
- Compound A7 can be produced, for example, by the methods described in Organic Letters 2007, 9, 21, 4107-4110 and ACS Catalysis 2017, 7, 3, 1998-2001, or a combination thereof.
- Compound A8 can be produced, for example, according to the method of J. Org. Chem. 2002, 67, 4, 1253-1260. That is, it is a method in which various nucleophiles are reacted with the carbonyl group of compound A7 in the presence or absence of an appropriate additive to convert the grade tertiary alcohol into a leaving group and then dehydrated.
- the nucleophile include trifluoromethyltrimethylsilane and Grignard reagent.
- the additive include tetrabutylammonium fluoride.
- the drug for converting to a leaving group include thionyl chloride.
- the solvent is appropriately selected from the solvents exemplified below, and preferred examples thereof include tetrahydrofuran and pyridine.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually ⁇ 78 ° C. to 150 ° C., preferably ⁇ 40 ° C. to 120 ° C.
- a compound in which Y is SR 1 , SOR 1 , or SO 2 R 1 can be produced by the following method. (In the equation, X, Z, n and R 1 are the same as the definition in the equation (1), and q is 1 or 2.)
- Compound B1 can be produced by reacting compound a1 with a corresponding disulfide compound in a suitable solvent in the presence of Lewis acid and a reducing agent.
- Lewis acid includes boron trifluoride diethyl ether complex.
- the reducing agent include triphenylphosphine.
- the solvent is appropriately selected from the solvents exemplified below and the like, and acetonitrile is preferable.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually ⁇ 50 ° C. to 100 ° C., preferably ⁇ 20 ° C. to 50 ° C.
- Compounds B2 and B3 can be produced by reacting compound B1 with trifluoroacetic anhydride and a urea / hydrogen peroxide reagent in a suitable solvent in the presence of a base.
- the base include sodium hydrogen carbonate.
- the solvent is appropriately selected from the solvents exemplified below and the like, and acetonitrile is preferable.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually ⁇ 40 ° C. to 50 ° C., preferably ⁇ 30 ° C. to 30 ° C.
- a compound in which Y is R 2 (aryl such as phenyl) or NR 3 R 4 can be produced by the following method.
- X, Z, n, R 3 and R 4 are the same as the definition in the formula (1), and TMS means trimethylsilyl.
- Compound c1 can be produced by reacting compound a1 with trimethylsilyl chloride in a suitable solvent in the presence of a base.
- the base is appropriately selected from the bases exemplified below, and preferably diisopropylethylamine or triethylamine.
- the solvent is appropriately selected from the solvents exemplified below and the like, and dichloromethane is preferable.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually ⁇ 50 ° C. to 100 ° C., preferably ⁇ 20 ° C. to 50 ° C.
- Compound C1 can be produced by reacting compound c1 with phenylmagnesium bromide in a suitable solvent in the presence of trimethylsilyl bromide.
- the solvent is appropriately selected from the solvents exemplified below, and is preferably tetrahydrofuran.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually ⁇ 50 ° C. to 100 ° C., preferably ⁇ 20 ° C. to 70 ° C.
- a compound or the like in which R 2 in the formula (1) is aryl can be produced by using a reagent such as arylmagnesium bromide well known to those skilled in the art.
- Compound C2 can be produced by reacting compound c1 with the corresponding amine in the presence of trimethylsilyl bromide in a suitable solvent.
- the solvent is appropriately selected from the solvents exemplified below, and preferred examples are tetrahydrofuran and dichloromethane.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually ⁇ 50 ° C. to 100 ° C., preferably ⁇ 20 ° C. to 70 ° C.
- a compound in which Y is OCOR 1 can be produced by the following method. (In the equation, X, Z, n or R 1 is the same as the definition in the equation (1).)
- compound D1 is treated with a carboxylic acid in the presence or absence of Lewis acid after obtaining an imitated compound by reacting compound a1 with an imitating reagent in the presence of various bases in a suitable solvent.
- imitating reagent used in the imitating step include trichloroacetonitrile and trifluoro-N-phenylacetimideyl chloride.
- base used in the imidylation step include organic bases such as diazabicycloundecene and triethylamine and indefinite bases such as sodium hydride and potassium carbonate, which are appropriately selected depending on the imidylation reagent used.
- the carboxylic acid used in the carboxylic acid treatment step is preferably benzoic acid or acetic acid, and more preferably benzoic acid.
- the Lewis acid used in the carboxylic acid treatment step is preferably a boron trifluoride diethyl ether complex.
- the solvent used in the imidylation step is appropriately selected from the solvents exemplified below, and is preferably a halogen-based solvent or an ether-based solvent, and more preferably dichloromethane or tetrahydrofuran.
- the reaction time is usually 5 minutes to 48 hours, preferably 10 minutes to 2 hours.
- the reaction temperature is usually ⁇ 78 ° C. to 100 ° C., preferably ⁇ 10 ° C. to 30 ° C.
- a corresponding acyl halide a corresponding carboxylic acid, a corresponding acid anhydride or the like in the presence or absence of various bases, and a condensing agent and a catalyst are used as necessary.
- the base is appropriately selected from the bases exemplified below, and preferably diisopropylethylamine or triethylamine.
- the condensing agent various condensing agents used in a conventional method can be used, and 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide (including hydrochloride) is preferably used.
- the catalyst include 4-dimethylaminopyridine.
- the solvent is appropriately selected from the solvents exemplified below, and preferably contains tetrahydrofuran, dimethylformamide, or dichloromethane.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually 0 ° C. to 200 ° C., preferably 0 ° C. to 100 ° C. This reaction can be similarly produced by using the method described in Eur. J. Org. Chem. 2002, 113-132 and the like.
- a compound in which Y is NR 5 COR 1 , NR 5 SO 2 R 1 , NR 5 CONR 3 R 4 , or 1,2,3-triazole can be produced by the following method.
- X, Z, n, R 1 , R 3 , R 4 and R 5 are the same as the definitions in the formula (1), and W is the substituent described in ⁇ Group 1> of [6]. .
- Compound e1 can be produced by reacting compound a1 with sodium azide in a suitable solvent in the presence of trimethylsilyl bromide.
- the solvent is appropriately selected from the solvents exemplified below and the like, and dichloromethane is preferable.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually ⁇ 50 ° C. to 70 ° C., preferably ⁇ 20 ° C. to 40 ° C.
- Compound e2 can be produced by reacting compound e1 with an appropriate reducing agent in an appropriate solvent.
- the reducing agent include triphenylphosphine.
- the solvent is appropriately selected from the solvents exemplified below, and preferred examples are tetrahydrofuran and water.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually ⁇ 20 ° C. to 120 ° C., preferably 0 ° C. to 80 ° C.
- Compound E1 is produced by reacting compound e2 with the corresponding acyl halide, the corresponding carboxylic acid, the corresponding carboxylic acid anhydride, etc. in the presence or absence of various bases, and if necessary, a condensing agent and a condensing agent.
- a catalyst is used.
- the base is appropriately selected from the bases exemplified below, and preferably diisopropylethylamine or triethylamine.
- the condensing agent various condensing agents used in a conventional method can be used, and 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide (including hydrochloride) is preferably used.
- the catalyst include 4-dimethylaminopyridine.
- the solvent is appropriately selected from the solvents exemplified below, and preferably contains tetrahydrofuran, dimethylformamide, or dichloromethane.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually 0 ° C. to 200 ° C., preferably 0 ° C. to 100 ° C.
- Compound E2 is produced by reacting compound e2 with the corresponding sulfonyl chloride in the presence of various bases in a suitable solvent.
- the base include triethylamine, pyridine and 2,4,6-cholidine.
- the solvent is appropriately selected from the solvents exemplified below, and preferably dichloromethane, tetrahydrofuran, and acetonitrile.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually 0 ° C. to 200 ° C., preferably 0 ° C. to 80 ° C.
- Compound E3 is treated after reacting compound e2 with a corresponding isocyanate in a suitable solvent in the presence or absence of a base, or by treating with a carbonylation reagent such as a phosgene derivative, carbonyldiimidazole, or chloroformate. It is produced by reacting with an amine.
- the solvent is appropriately selected from the solvents exemplified below, and preferably dichloromethane and tetrahydrofuran.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually 0 ° C. to 200 ° C., preferably 0 ° C. to 80 ° C.
- the base It can be produced by appropriately treating a compound in which R 3 is a hydrogen atom in compounds E1, E2 or E3 with a reactant such as R 5- Cl and R 5-Br in the presence of the compound.
- Compound E4 is a so-called Husgen cyclization reaction produced by reacting compound e1 with a corresponding alkyne compound in a suitable solvent.
- a metal catalyst to act in an appropriate solvent.
- the solvent is appropriately selected from the solvents exemplified below, and preferably dichloromethane and water.
- the metal catalyst monovalent copper is preferable, and a method of treating copper (II) sulfate with ascorbic acid to generate monovalent copper in the system is more preferable.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually 0 ° C. to 200 ° C., preferably 20 ° C. to 80 ° C.
- a compound in which Y is OR 1 can be produced by the following method. (In the equation, X, Z, n or R 1 is the same as the definition in the equation (1).)
- Compound F1 is produced by reacting compound a1 with a corresponding alcohol in a suitable solvent in the presence of various Lewis acids.
- the Lewis acid is appropriately selected from Lewis acids and the like exemplified below, and preferred examples include boron trifluoride diethyl ether complex, trimethylsilyl chloride, and silver perchlorate.
- the solvent is appropriately selected from the solvents exemplified below, and preferably dichloromethane and diethyl ether.
- the reaction time is usually 5 minutes to 48 hours, preferably 10 minutes to 24 hours.
- the reaction temperature is usually ⁇ 78 ° C. to 100 ° C., preferably ⁇ 78 ° C. to 50 ° C.
- a compound in which Y is cyano, carbamoyl, or aminomethyl can be produced by the following method.
- X, Z, n, and R 1 are the same as the definitions in the formula (1), in which case R 1 is preferably phenyl or methyl, more preferably phenyl.
- Compound g1 is produced by reacting compound D1 with various cyanation reagents in the presence or absence of various Lewis acids in a suitable solvent.
- the Lewis acid is appropriately selected from Lewis acids and the like exemplified below, and tin tetrachloride is preferable.
- the cyanation reagent include trimethylsilyl cyanide, sodium cyanide, and potassium cyanide.
- the solvent is appropriately selected from the solvents exemplified below, and preferred examples include halogen-based solvents and dimethylformamide.
- the reaction time is usually 5 minutes to 48 hours, preferably 10 minutes to 24 hours.
- the reaction temperature is usually ⁇ 78 ° C. to 100 ° C., preferably ⁇ 78 ° C. to 50 ° C.
- Compound G1 can be produced by reacting compound g1 with various bases in a suitable solvent in the presence or absence of hydrogen peroxide.
- the base is appropriately selected from the bases exemplified below, and examples thereof include potassium carbonate and potassium hydroxide.
- the solvent is appropriately selected from the solvents exemplified below, and preferred examples thereof include tetrahydrofuran, tert-butyl alcohol, and water.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually 0 ° C to 100 ° C, preferably 20 ° C to 70 ° C.
- Compound G2 can be produced by reacting compound g1 or compound G1 with various reducing agents in a suitable solvent in the presence of Lewis acid.
- Lewis acids include boron trifluoride diethyl ether complex and dodecacarbonyl triruthenium.
- the reducing agent include a hydride reducing agent and a hydrosilane-based reducing agent, and more preferably sodium borohydride and 1,1,3,3-tetramethyldisiloxane.
- the solvent is appropriately selected from the solvents exemplified below, and preferred examples are tetrahydrofuran and toluene.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually 0 ° C. to 200 ° C., preferably 20 ° C. to 100 ° C.
- a compound in which Y is mercapto can be produced by the following method. (In the equation, X, Z, or n is the same as the definition in the equation (1).)
- Compound h1 is produced by reacting compound a1 with thioacetic acid in the presence of various Lewis acids in a suitable solvent.
- the Lewis acid is appropriately selected from Lewis acids and the like exemplified below, and a boron trifluoride diethyl ether complex is preferable.
- the solvent is appropriately selected from the solvents exemplified below and the like, and dichloromethane is preferable.
- the reaction time is usually 5 minutes to 48 hours, preferably 10 minutes to 24 hours.
- the reaction temperature is usually ⁇ 78 ° C. to 100 ° C., preferably ⁇ 78 ° C. to 50 ° C.
- Compound H1 is produced by reacting compound h1 with a base in an appropriate solvent.
- the base is appropriately selected from the bases exemplified below, and sodium ethoxide is preferable.
- the solvent is appropriately selected from the solvents exemplified below, and ethanol is preferable.
- the reaction time is usually 5 minutes to 48 hours, preferably 10 minutes to 24 hours.
- the reaction temperature is usually ⁇ 78 ° C. to 150 ° C., preferably ⁇ 20 ° C. to 80 ° C.
- a compound in which Y is represented by COR 1 can be produced by the following method. (In the equation, X, Z, n and R 1 are the same as the definitions in the equation (1).)
- Compound i1 is produced by reacting compound A4 with N, O-dimethylhydroxylamine or a salt thereof in the presence or absence of various condensing agents and / or bases in a suitable solvent, and if necessary.
- a catalyst is used.
- the condensing agent various condensing agents used in a conventional method can be used, and 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide (including hydrochloride) is preferably used.
- the base is appropriately selected from the bases exemplified below, and preferably diisopropylethylamine or triethylamine.
- the catalyst include 4-dimethylaminopyridine.
- the solvent is appropriately selected from the solvents exemplified below, and preferably contains tetrahydrofuran, dimethylformamide, or dichloromethane.
- the reaction time is usually 5 minutes to 72 hours, preferably 30 minutes to 24 hours.
- the reaction temperature is usually 0 ° C. to 200 ° C., preferably 0 ° C. to 100 ° C.
- Compound I1 is produced by reacting compound i1 with a Grignard reagent in a suitable solvent.
- the solvent is appropriately selected from the solvents exemplified below, and preferred examples thereof include tetrahydrofuran and diethyl ether.
- the reaction time is usually 5 minutes to 48 hours, preferably 10 minutes to 24 hours.
- the reaction temperature is usually ⁇ 78 ° C. to 150 ° C., preferably ⁇ 20 ° C. to 80 ° C.
- the base used in each step of each of the above production methods should be selected in a timely manner depending on the reaction, the type of the raw material compound, etc., and for example, sodium hydroxide, alkali hydroxides such as potassium hydroxide, sodium carbonate, etc.
- Alkali carbonates such as potassium carbonate, sodium hydride, metal hydrides such as potassium hydride, sodium hydroxide, alkali metal hydroxides such as potassium hydroxide, sodium methoxydo, sodium t-butoxide
- Alkali metal alkoxides such as, butyllithium, organic metal bases such as lithium diisopropylamide, triethylamine, diisopropylethylamine, pyridine, 4-dimethylaminopyridine (DMAP), 1,8-diazabicyclo [5.4.0]-. Included are organic bases such as 7-undecene (DBU).
- the solvent used in each step of each of the above production methods should be selected in a timely manner depending on the reaction, the type of raw material compound, etc., and for example, alcohols such as methanol, ethanol and isopropanol, acetone, methyl ketone and the like. Ketones, halogenated hydrocarbons such as methylene chloride and chloroform, ethers such as tetrahydrofuran (THF) and dioxane, aromatic hydrocarbons such as toluene and benzene, aliphatic hydrocarbons such as hexane and heptane.
- alcohols such as methanol, ethanol and isopropanol
- acetone methyl ketone and the like.
- Ketones halogenated hydrocarbons
- ethers such as tetrahydrofuran (THF) and dioxane
- aromatic hydrocarbons such as toluene and benzene
- aliphatic hydrocarbons such
- esters such as ethyl acetate, propyl acetate, amides such as N, N-dimethylformamide (DMF), N-methyl-2-pyrrolidone, sulfoxides such as dimethyl sulfoxide (DMSO), such as acetonitrile.
- amides such as N, N-dimethylformamide (DMF), N-methyl-2-pyrrolidone
- sulfoxides such as dimethyl sulfoxide (DMSO)
- acetonitrile such as acetonitrile.
- Nitriles can be mentioned, and these solvents can be used alone or in combination of two or more. Further, depending on the type of reaction, organic bases may be used as a solvent.
- the compound of the present invention represented by the formula (1) or the like or an intermediate thereof can be separated and purified by a method known to those skilled in the art. For example, extraction, partitioning, reprecipitation, column chromatography (for example, silica gel column chromatography, ion exchange column chromatography or preparative liquid chromatography) or recrystallization can be mentioned.
- column chromatography for example, silica gel column chromatography, ion exchange column chromatography or preparative liquid chromatography
- recrystallization can be mentioned.
- recrystallization solvent examples include an alcohol solvent such as methanol, ethanol or 2-propanol, an ether solvent such as diethyl ether, an ester solvent such as ethyl acetate, an aromatic hydrocarbon solvent such as benzene or toluene, and acetone.
- a ketone solvent such as, a halogen solvent such as dichloromethane or chloroform, a hydrocarbon solvent such as hexane, an aproton solvent such as dimethylformamide or acetonitrile, water, or a mixed solvent thereof and the like can be used.
- the method described in Volume 1 of the Experimental Chemistry Course (edited by the Chemical Society of Japan, Maruzen) can be used.
- the molecular structure of the compound of the present invention is determined by a spectroscopic method such as a nuclear magnetic resonance method, an infrared absorption method, a circular dichroism spectrum analysis method, etc., with reference to the structure derived from each raw material compound. And it can be easily done by mass spectrometry.
- a spectroscopic method such as a nuclear magnetic resonance method, an infrared absorption method, a circular dichroism spectrum analysis method, etc.
- the intermediate or final product in the above production method is required to appropriately convert its functional group, and in particular, to extend various side chains from amino, hydroxyl group, carbonyl, halogen and the like, and at that time.
- a reagent to be used as a raw material is appropriately selected by a method well known to those skilled in the art, a functional group is converted, or a side chain is carefully selected. It can be manufactured by using a reagent.
- the conversion of functional groups and the extension of side chains can be carried out by common methods (see, for example, Comprehensive Organic Transitions, RC Larock, John Wiley & Sons Inc. (1999), etc.).
- protection is performed according to the method described in Protective Groups in Organic Synthesis (Theodora W. Greene, Peter G. M. Wuts, John Wiley & Sons, Inc., 1999). be able to.
- the compound of the present invention represented by the formula (1) or the like or a pharmaceutically acceptable salt thereof may have an asymmetry or may have a substituent having an asymmetric carbon.
- the compounds of the present invention also include mixtures and isolated compounds of each of these isomers and can be produced according to conventional methods. Examples of the production method include a method using a raw material having an asymmetric point, or a method of introducing an asymmetry in an intermediate stage.
- the optical isomer of the compound represented by the formula (3-1) can be produced by the method described in Angewandte Chemie International Edition 2018, 57, 8293-8296 (for example, a compound in which Y is oxo). ..
- the compounds represented by the formula (3-2) are described in Organic Process Research & Development 2007, 11, 3, 336-340, or Bioorganic & Medicinal Chemistry Letters 2010, 20, 14, 4112-4115 and the like. It can be manufactured by the method.
- the optical isomer in the case of an optical isomer, can be obtained by using an optically active raw material or performing optical resolution or the like at an appropriate stage in the manufacturing process.
- an optical division method for example, when the compound represented by the formula (1) or an intermediate thereof has a basic functional group, it is contained in an inert solvent (for example, an alcohol solvent such as methanol, ethanol or 2-propanol).
- Ether solvent such as diethyl ether, ester solvent such as ethyl acetate, hydrocarbon solvent such as toluene, aproton solvent such as acetonitrile, or a mixed solvent of two or more kinds selected from the above solvents
- optically active Acids eg, monocarboxylic acids such as mandelic acid, N-benzyloxyalanine, lactic acid, tartrate acid, dicarboxylic acids such as o-diisopropyridene tartrate, malic acid, sulfonic acids such as camphorsulfonic acid and bromocamparsulfonic acid
- a diastereomer method in which a salt is formed using a solvent can be mentioned.
- an optically active amine for example, 1-phenylethylamine, quinine, quinidine, cinchonidine, cinchonine, etc.
- Optical resolution can also be performed by forming a salt using an organic amine such as strikinine).
- the temperature at which the salt is formed is selected from the range from ⁇ 50 ° C. to the boiling point of the solvent, preferably the range from 0 ° C. to the boiling point, and more preferably the range from room temperature to the boiling point of the solvent. In order to improve the optical purity, it is desirable to raise the temperature to near the boiling point of the solvent. When the precipitated salt is collected by filtration, it can be cooled if necessary to improve the yield.
- the amount of the optically active acid or amine used is appropriately in the range of about 0.5 to about 2.0 equivalents, preferably about 1 equivalent with respect to the substrate.
- the crystals are placed in an inert solvent (for example, an alcohol solvent such as methanol, ethanol, 2-propanol; an ether solvent such as diethyl ether; an ester solvent such as ethyl acetate; a hydrocarbon solvent such as toluene; acetonitrile. It can also be recrystallized in an aproton solvent such as (or a mixed solvent of two or more kinds selected from the above solvents) to obtain a highly pure optically active salt. Further, if necessary, the optically resolved salt can be treated with an acid or a base by a usual method to obtain a free form.
- an inert solvent for example, an alcohol solvent such as methanol, ethanol, 2-propanol; an ether solvent such as diethyl ether; an ester solvent such as ethyl acetate; a hydrocarbon solvent such as toluene; acetonitrile.
- an aproton solvent such as (or a mixed solvent
- any medium suitable for maintenance culture of hematopoietic stem cells may be used.
- (Manufactured by Nipro) Ham F12 medium (Ham's Nutrition Mixture F12), DMEM (Dulvecco's Modified Eagle's Medium), RPMI1640 medium, IMDM medium (Iscover's Modified Cell), etc. It may be prepared by adding SCF (manufactured by Peprotech) and Trombopoietin (TPO, manufactured by Peprotech).
- the above medium contains a compound represented by the formula (1) or a salt thereof.
- the compound represented by the formula (1) include artemether, artemisinin, artenimol, artemotil, and artesunate.
- the concentration of each compound in the medium may be any concentration that allows hematopoietic stem cells to proliferate while maintaining self-renewal ability and pluripotency, for example, 0.01 ⁇ M to 100 ⁇ M, preferably 0.03 ⁇ M to 10 ⁇ M. It may be 0.1 ⁇ M to 10 ⁇ M, 0.1 ⁇ M to 3 ⁇ M, 0.1 ⁇ M to 1 ⁇ M.
- Artemether and artemisinin may be 0.1 ⁇ M to 10 ⁇ M, preferably 0.3 ⁇ M to 10 ⁇ M, 1 ⁇ M to 10 ⁇ M. It may be at a lower concentration for human hematopoietic stem cells.
- artemether may be 0.001 ⁇ M to 10 ⁇ M, 0.003 ⁇ M to 10 ⁇ M, 0.01 ⁇ M to 10 ⁇ M, 0.03 ⁇ M to 10 ⁇ M.
- the above medium may contain serum, fatty acids or lipids, amino acids, vitamins, growth factors, cytokines, insulin, transferrin, antioxidants, 2-mercaptoethanol, pyruvate, buffers, inorganic salts, antibiotics, as appropriate. Etc. may be contained. Serum concentrations may be 25% or less, preferably 5% to 15%, more preferably 8% to 10%.
- serum medium means a medium containing unadjusted or unpurified serum
- serum-free medium means a medium containing no adjusted or unpurified serum.
- the serum-free medium may contain a serum substitute.
- the serum substitute include those appropriately containing albumin, transferrin, fatty acid, collagen precursor, trace element, 2-mercaptoethanol or 3-thioglycerol, or an equivalent thereof.
- Commercially available products may be used as the serum substitute. For example, Knockout (trademark) Serum Research (KSR: Life Technologies), Chemically-defined Lipid connected (Life Technologies), Glutamax (trademark, Life), etc.
- the conditions for culturing hematopoietic stem cells are not particularly limited, and for example, they may be cultured in a CO 2 incubator at 37 ° C. and 5% CO 2.
- a cell population including hematopoietic stem cells may be 106 cells / mL ⁇ 1 ⁇ 10 8 cells / mL.
- it may be 100 cells / mL ⁇ 1 ⁇ 10 7 cells / mL.
- the cell population obtained by culturing by the culturing method of the present invention (in this specification, may be referred to as "cell population after culturing") is a cell population containing hematopoietic stem cells.
- cell population after culturing In the conventional culture method, most hematopoietic stem cells are eliminated by differentiation, whereas in the culture method of the present invention, at least some hematopoietic stem cells are not differentiated and maintain self-renewal ability and pluripotency. Can grow as it is.
- the ratio of the number of hematopoietic stem cells to the total number of cells in the cell population after culturing is 0.1%, 0.5%, 1% of the ratio of the number of hematopoietic stem cells to the total number of cells in the cell population before culturing. 5%, 10%, 20%, 30% or more, preferably 50% or more, more preferably 60% or more, 70% or more, 80% or more, 85% or more, 90% or more.
- the ratio of the number of long-term hematopoietic stem cells to the total number of cells in the cell population after culturing is 0.1%, 0.5%, 1% of the ratio of the number of long-term hematopoietic stem cells to the total number of cells in the cell population before culturing. 5, 5%, 10%, 20%, 30% or more, preferably 50% or more, more preferably 60% or more, 70% or more, 80% or more, 85% or more, 90% or more.
- the proportion of hematopoietic stem cells in the cell population obtained by culturing by the culture method of the present invention can change depending on the proportion of hematopoietic stem cells in the cell population before culturing.
- the proportion of long-term hematopoietic stem cells in the cell population including long-term hematopoietic stem cells is 0.1%, 0.5%, 1%, 5%, 10%, 20%, 30% or more, preferably 50 or more. % Or more, more preferably 60% or more, 70% or more, 80% or more, 85% or more, 90% or more.
- the number of hematopoietic stem cells in the cell population after culturing is 1.2 times or more, 1.5 times or more, 2 times or more, preferably 10 times or more, the number of hematopoietic stem cells in the cell population before culturing. More preferably, it is 20 times or more, 50 times or more, and 100 times or more. In one aspect, the number of hematopoietic stem cells in the cell population after culturing is 1. Compared to the number of hematopoietic stem cells in the cell population obtained by the culturing method in which the culture conditions are matched, except that the compound of the present invention is not contained.
- the number of long-term hematopoietic stem cells in the cell population after culturing is 1.2 times or more, 1.5 times or more, 2 times or more, preferably 10 times or more, the number of long-term hematopoietic stem cells in the cell population before culturing. More preferably, it is 20 times or more, 50 times or more, and 100 times or more.
- the number of long-term hematopoietic stem cells in the cell population after culturing is compared with the number of long-term hematopoietic stem cells in the cell population obtained by the culture method in which the culture conditions are matched, except that the compound of the present invention is not contained. 1.2 times or more, 1.5 times or more, 2 times or more, preferably 10 times or more, more preferably 20 times or more, 50 times or more, 100 times or more. That is, the number of hematopoietic stem cells or the number of long-term hematopoietic stem cells may be maintained or increased as compared with an appropriate comparison target.
- the number of cells of hematopoietic stem cells is 1.2 times or more, 1.5 times or more, 2 times or more, preferably 10 times or more, as compared with the above-mentioned comparison target. More preferably, it includes a method of increasing by 20 times or more, 50 times or more, and 100 times or more. Further, in the present invention, in a cell population containing long-term hematopoietic stem cells, the number of long-term hematopoietic stem cells is increased by 1.2 times or more, 1.5 times or more, 2 times or more, preferably 10 times, as compared with the above-mentioned comparison target. The above, more preferably, includes a method of increasing 20 times or more, 50 times or more, 100 times or more.
- the present invention also increases the number of hematopoietic stem cells in a cell population containing hematopoietic stem cells by 1.2 times or more, 1.5 times or more, 2 times or more, preferably 10 times or more, more preferably, as compared with the above-mentioned comparison target. , 20 times or more, 50 times or more, 100 times or more, and the ratio of hematopoietic stem cells to the total number of cells is 0.1%, 0.5%, 1%, 5%, 10%, 20%, 30%.
- the above includes a culture method for maintaining preferably 50% or more, more preferably 60% or more, 70% or more, 80% or more, 85% or more, and 90% or more.
- the number of long-term hematopoietic stem cells is increased by 1.2 times or more, 1.5 times or more, 2 times or more, preferably 10 times, as compared with the above-mentioned comparison target.
- the ratio of long-term hematopoietic stem cells to the total number of cells is 0.1%, 0.5%, 1%, 5%, 10%. , 20%, 30% or more, preferably 50% or more, more preferably 60% or more, 70% or more, 80% or more, 85% or more, 90% or more.
- the present invention also includes a culture method for amplifying cells forming CFU-GEMM colonies in a CFU assay.
- the number of CFU-GEMM colonies obtained from the cell population obtained by the culture method of the present invention is 1.2 times or more, 1.5 times or more, the number of CFU-GEMM colonies obtained from the comparison target. It includes a culture method that increases by 2 times or more, 5 times or more and 10 times or more.
- the comparison target may be, for example, a cell population obtained by a culture method in which the culture conditions are matched, or a cell population before culturing, except that the compound of the present invention is not contained. That is, the culturing method according to the above [1] to [11] is also a culturing method for cells or cell populations having the ability to form mixed colonies (CFU-GEMM) derived from hematopoietic stem cells.
- the present invention is used in a long-term (1 month or longer, preferably 2 months or longer, 3 months or longer, 4 months or longer) transplantation experiment to immunoprotected NOG mice irradiated with a lethal dose of radiation.
- a culture method that amplifies cells that can engraft.
- the proportion of human CD45-positive cells in the blood of NOG mice can be used as an index as described above, and the number of days after transplantation for carrying out the evaluation can be arbitrarily set by those skilled in the art. (Example: 1 month, 2 months, 3 months or 4 months after transplantation).
- the proportion of human CD45-positive cells when the cell population obtained by the culture method of the present invention is transplanted is 1.2 times or more the proportion of human CD45-positive cells when the comparison target is transplanted. It includes a culture method that increases 1.5 times or more, 2 times or more, and 10 times or more.
- the comparison target may be, for example, a cell population obtained by a culture method in which the culture conditions are matched, or a cell population before culturing, except that the compound of the present invention is not contained. That is, the culturing method according to the above [1] to [11] is also a culturing method for a cell population that increases the ability to produce mature blood cells when transplanted into a mammal.
- the culture method according to the above [1] to [11] is a method for culturing a cell population that increases at least one, preferably all, bone marrow engraftment ability, pluripotency and bone marrow remodeling ability. But also.
- the cell population containing hematopoietic stem cells obtained by culturing by the culture method of the present invention may be used immediately for transplantation, or may be cryopreserved if not used immediately.
- a cryopreservation method for example, when cells collected from blood are frozen, erythrocytes, plasma and leukocyte fractions are separated and removed, and a preservation solution containing 8% dimethylsulfoxide and 0.8% dextran, or HSC-BANKER GMP.
- a grade manufactured by ZENOAQ
- ZENOAQ grade
- transplantation treatment it is important that the transplanted cells function continuously in the body of the transplant recipient (recipient), and for this purpose, hematopoietic stem cells having self-renewal ability, especially long-term self-renewal ability, are used. It is important that cells containing a large amount are transplanted.
- the cell population containing hematopoietic stem cells obtained by culturing by the culture method of the present invention contains a large amount of hematopoietic stem cells, particularly long-term hematopoietic stem cells, has high self-proliferation ability, and has pluripotency. When used for transplantation, it has excellent long-term engraftment ability, pluripotency, and bone marrow remodeling ability. By transplanting an undifferentiated cell fraction such as long-term hematopoietic stem cells, a GVHD inhibitory effect can be expected.
- One aspect of the present invention is a culture of a cell population containing hematopoietic stem cells, wherein (1) a cell population containing hematopoietic stem cells obtained by culturing by the culture method of the present invention, and (2) a hematopoietic stem cell population.
- a culture containing, and the medium necessary to maintain viability is provided.
- the hematopoietic stem cells are preferably long-term hematopoietic stem cells.
- a "culture” is a liquid containing a medium and a cell population necessary for maintaining viability, and which may further contain a biological substance added or produced from the cell population.
- Biological substances include, but are not limited to, for example, cytokines, chemokines and the like.
- the proportions of hematopoietic stem cells (cells expressing the markers described above, eg, CD34-positive and CD38-negative cells) in the cell population in culture to the total number of cells are 0.1%, 0.5%, 1%. 5, 5%, 10%, 20%, 30% or more, preferably 50% or more, 60% or more, 70% or more, 80% or more, 85% or more, 90% or more.
- the ratio of long-term hematopoietic stem cell number to total cell number is 0.1%, 0.5%, 1%, 5%, 10%, 20%, 30% or more. , Preferably 50% or more, 60% or more, 70% or more, 80% or more, 85% or more, 90% or more.
- Examples of the "medium necessary for maintaining viability" in the present specification include a medium, a physiological buffer solution, etc., but are not particularly limited as long as the cell population including hematopoietic stem cells survives, and those skilled in the art can use the medium. If there is, it can be selected as appropriate.
- the above-mentioned medium can be mentioned, using a medium usually used for culturing animal cells as a basal medium.
- the medium contains fetal bovine serum, human serum, horse serum, insulin, transferrin, lactoferrin, cholesterol, ethanolamine, sodium selenate, monothioglycerol, 2-mercaptoethanol, bovine serum albumin, sodium pyruvate, polyethylene glycol.
- the medium may contain a compound represented by the formula (1) or a salt thereof.
- concentration of these compounds or derivatives thereof, or salts thereof, may be in the above-mentioned concentration range. For example, it may be 0.001 ⁇ M to 100 ⁇ M, 0.01 ⁇ M to 100 ⁇ M, preferably 0.003 ⁇ M to 100 ⁇ M, 0.03 ⁇ M to 10 ⁇ M.
- the "medium necessary for maintaining viability" in the present specification may further contain substances that act on cytokines and hematopoietic stem cells.
- Cytokines include IL-1, IL-3, IL-6, IL-11, G-CSF, GM-CSF, SCF, FlT3-L, thrombopoietin (TPO), erythropoietin, and analogs thereof. Not limited to these.
- analog includes all structural variants of cytokines and growth factors that have biological activity such as the natural form.
- UM171 As a substance that acts on hematopoietic stem cells, UM171 ((1r, 4r) -N1- (2-benzyl-7- (2-methyl-2H-tetrazol-5-yl) -9H-pyrimido [4,5-b] indol -4-yl) cyclohexane-1,4-diamine) and its derivatives, the pyrimide [4,5-b] indole derivative described in Patent Document 2 (for example, UM729 (for example, Methyl 4-((3- (piperidin-)) 1-yl) propyl) amino) -9H-pyrimido [4,5-b] indole-7-carboxylate), etc., but not limited to these.
- Patent Document 2 for example, UM729 (for example, Methyl 4-((3- (piperidin-)) 1-yl) propyl) amino) -9H-pyrimido [4,5-b] indole-7
- the medium used in the culture method of the present invention may contain UM171 and its derivatives.
- the derivative for example, Patent Document 2 (WO2013 / 110198) can be referred to.
- the method for producing a hematopoietic stem cell of the present invention is a step of preparing a cell population containing the hematopoietic stem cell and a step of culturing the cell population containing the hematopoietic stem cell, and culturing the cell population containing the hematopoietic stem cell by the culturing method of the present invention. Includes steps to be performed.
- Step of preparing a cell population containing hematopoietic stem cells includes (2) a step of culturing a cell population including the cell population, and (3) a step of collecting hematopoietic stem cells.
- the cell population containing hematopoietic stem cells can be prepared by a method well known to those skilled in the art. For example, it can be prepared by collecting from blood (peripheral blood or umbilical cord blood) or bone marrow, or by artificially producing from pluripotent stem cells (eg, iPS cells, ES cells).
- pluripotent stem cells eg, iPS cells, ES cells
- a cell population containing hematopoietic stem cells can also be obtained as a commercially available product.
- an appropriate amount of bone marrow is collected from mammals such as humans, monkeys, mice, rats, etc., and a final concentration of 2% heat-treated inactivated bovine serum is added to Ca 2+ -free and Mg 2+ -free PBS.
- a buffer solution containing (manufactured by Gibco) and a final concentration of 2 mM EDTA solution can be added to mechanically destroy bone marrow tissue to obtain a cell population containing hematopoietic stem cells.
- a cell population containing hematopoietic stem cells is obtained by collecting an appropriate amount of blood from mammals, for example, animals such as humans, monkeys, mice, and rats, and preparing a PBMC fraction according to a conventional method. be able to.
- the blood either peripheral blood or umbilical cord blood may be used.
- the blood may be mobilized blood to which a drug such as granulocyte colony stimulating factor (G-CSF) has been administered according to a conventional method.
- G-CSF granulocyte colony stimulating factor
- Hematopoietic stem cells can be mobilized into peripheral blood by administering a drug such as granulocyte colony stimulating factor (G-CSF).
- a method for collecting from umbilical cord blood a method known to those skilled in the art can be used.
- a needle for collecting the umbilical cord is inserted into a blood vessel of the umbilical cord, and umbilical cord blood is collected in a collecting bag.
- hydroxyethyl starch is added to aggregate the erythrocytes and centrifuge, and then the erythrocytes and plasma are removed to obtain a cell population containing hematopoietic stem cells.
- the cell population containing the hematopoietic stem cells may be filtered by a 100 ⁇ m, 70 ⁇ m, or 40 ⁇ m filter.
- the filtered cell population is stained with an antibody against the above-mentioned fluorescently labeled hematopoietic stem cell marker, and hematopoietic stem cell marker-positive cells are collected by MACS (Magnetic-activated cell sorting) or FACS (Flow cytometric activated cell sorter).
- MACS Magnetic-activated cell sorting
- FACS Flow cytometric activated cell sorter
- the cell types of hematopoietic stem cells, hematopoietic progenitor cells, etc. in the obtained cell population containing hematopoietic stem cells can be confirmed by performing marker expression analysis of hematopoietic stem cells, hematopoietic progenitor cells, etc. by flow cytometry or qRT-PCR. ..
- marker gene-positive cells that is, long-term hematopoietic stem cells
- the marker gene the above-mentioned gene can be used.
- the proportion of hematopoietic stem cells in the cell population containing the hematopoietic stem cells thus obtained is not limited, but the proportion is preferably high, for example, 0.1%, 0.5%, 1%, 5%, 10%. , 20%, 30% or more, 50% or more, 60% or more, 70% or more, 80% or more, 85% or more, or 90% or more of hematopoietic stem cells are preferably contained.
- mouse hematopoietic stem cells can be confirmed by analyzing the expression of hematopoietic stem cell markers by flow cytometry or qRT-PCR.
- the cell population containing the hematopoietic stem cells thus obtained preferably contains long-term hematopoietic stem cells, for example, 0.1%, 0.5%, 1%, 5%, 10%, 20%, 30% or more. , 50% or more, 60% or more, 70% or more, 80% or more, 85% or more, or 90% or more of long-term hematopoietic stem cells are preferably contained.
- Long-term hematopoietic stem cells can be identified by expression of markers such as Hoxb5, as described above.
- a known method can be used as a method for artificially preparing a cell population containing hematopoietic stem cells from pluripotent stem cells (eg, iPS cells, ES cells).
- pluripotent stem cells eg, iPS cells, ES cells.
- One embodiment includes the methods described in the following non-patent documents. Specifically, CD34-positive FLK1-positive cells are isolated after embryoid bodies are prepared from iPS cells. Then, ERG, HoxA5, HoxA9, HoxA10, LCOR, RUNX1, and SPI1 can be expressed as transcription factors to obtain a cell population containing hematopoietic stem cells (Nature. 2017 25: 545 (7655) 432-438).
- Step (1) may include a step of pre-culturing a cell population containing hematopoietic stem cells and / or a step of collecting hematopoietic stem cells (long-term hematopoietic stem cells) using a specific marker.
- the step (2) in the production method of the present invention is as described in the culturing method of the present invention. That is, the culturing step includes culturing in a medium containing the compound of the present invention. What is obtained in step (2) is a cell population containing hematopoietic stem cells (hereinafter, may be referred to as "a cell population containing hematopoietic stem cells produced by the production method of the present invention").
- the cell population containing hematopoietic stem cells produced by the production method of the present invention is the same as the cell population containing hematopoietic stem cells obtained by culturing by the culture method of the present invention.
- Culturing conditions for a cell population containing hematopoietic stem cells according to the culturing method of the present invention can be appropriately set by those skilled in the art.
- the concentration of the compound of the present invention may be set within the above-mentioned concentration range.
- the number of culture days is also not particularly limited, but may be, for example, 1 to 30 days, preferably 21 days, 14 days, 7 days, or 3 days. These culture conditions may be set based on the required state of hematopoietic stem cell amplification.
- the number of hematopoietic stem cells (which may be a cell population defined in the positive and negative states of the various markers described above) is 1.2 times or more, 1.5 times or more, 2 times or more as compared with the above-mentioned comparison target. It may be cultured until it is increased by a factor of 2 or more, preferably 10 times or more, more preferably 20 times or more, 50 times or more, or 100 times or more.
- conditions for increasing the number of CFU-GEMM colonies eg, 1.2 times or more, 1.5 times or more, 2 times or more, 10 times or more, the number of CFU-GEMM colonies with respect to the comparison target). Condition may be set.
- the medium may contain one or more selected from.
- a medium containing IL-6, SCF, FLT3-L and TPO may be used.
- it may be cultured using a medium further containing UM171 or a derivative thereof.
- the concentration of UM171 or a derivative thereof varies depending on the culture conditions, and can be appropriately set by those skilled in the art.
- the concentration of UM171 or a derivative thereof may be 1 nM to 10 ⁇ M.
- the concentration can be set using the number of hematopoietic stem cells or the number of CFU-GEMM colonies as an index.
- hematopoietic stem cells long-term hematopoietic stem cells
- a cell population containing hematopoietic stem cells obtained by culturing in step (2) may be recovered from hematopoietic stem cells or long-term hematopoietic stem cells by MACS, FACS, or the like using the above-mentioned hematopoietic stem cell markers.
- the production method of the present invention may further include a step of filling a container (such as an infusion bag) with hematopoietic stem cells collected in step (3) and / or a step of freezing the obtained cells.
- a container such as an infusion bag
- One aspect of the present invention is the formula (1), the formula (2), the formula (3-1), the formula (3-2), the formula (3-3), the formula (4-1), the formula (4-2). ) And the compound represented by any of the formula (4-3), or a reagent for culturing hematopoietic stem cells containing at least one salt thereof.
- One aspect of the present invention is (1) a medium suitable for maintenance culture of hematopoietic stem cells, and (2) formula (1), formula (2), formula (3-1), formula (3-2), formula ( A compound represented by any of 3-3), formula (4-1), formula (4-2) and formula (4-3), or a salt thereof (in the present specification, "compound of the present invention”).
- a hematopoietic stem cell culture kit containing the above is provided.
- An example of the compound represented by the formula (1) that is, a preferable example of the compound of the present invention is the following compound.
- Preferred examples of the compound represented by the formula (1) include the following compounds.
- the kit may include UM171 or a derivative thereof.
- UM171 or a derivative thereof is as described above.
- the medium suitable for the maintenance culture of hematopoietic stem cells is not particularly limited as long as it is the above-mentioned medium.
- the kit may contain antibodies for isolating hematopoietic stem cells and / or reagents for sorting cells.
- the antibody for isolating hematopoietic stem cells may be an antibody against the above-mentioned marker, and is preferably labeled with a fluorescent protein or the like.
- Reagents for sorting cells include, for example, labeled secondary antibodies used for MACS and FACS, columns for separation, and the like.
- the kit may contain (1) a medium suitable for maintenance culture of hematopoietic stem cells and (2) a compound represented by the formula (1), or a salt thereof as a single composition, or may be contained separately. It may be included as a composition.
- a composition containing the compound represented by the formula (2) (1) or a salt thereof is used as a culture assisting composition for maintaining and culturing hematopoietic stem cells, and (1) maintaining and culturing hematopoietic stem cells.
- a culture assisting composition for maintaining and culturing hematopoietic stem cells, and (1) maintaining and culturing hematopoietic stem cells.
- a cell population containing hematopoietic stem cells produced by the above-mentioned method or the like can be transplanted to a subject requiring transplantation (eg, a mammal), and the hematopoietic stem cells after transplantation live in the bone marrow of the subject (recipient). Can be dressed and produce blood. Mammals that can be targeted include, for example, humans, mice, rats, guinea pigs, hamsters, rabbits, cats, dogs, sheep, pigs, cows, horses, goats, monkeys and the like.
- the cell population containing hematopoietic stem cells is preferably prepared into a pharmaceutical composition and then transplanted.
- Transplantation of a cell population containing hematopoietic stem cells is carried out, for example, by intravenous administration (intravenous drip infusion, etc.).
- anticancer agents in order to destroy the cancer cells remaining in the body as much as possible, reduce the patient's own immunosuppression, and facilitate the survival of the transplanted cells.
- Pre-transplant treatments such as CAR-T cell therapy, total body irradiation, and treatments combined with immunosuppressants may be performed.
- One aspect of the present invention provides a pharmaceutical composition comprising an effective amount of a cell population of hematopoietic stem cells produced by the above production method.
- the pharmaceutical composition of one embodiment contains an effective amount of the above-mentioned cell population for transplantation produced by the production method of the present invention, and a pharmaceutically acceptable carrier.
- a physiological aqueous solvent physiological saline, buffer solution, serum-free medium, etc.
- the pharmaceutical composition contains a preservative, a stabilizer, a reducing agent, an tonicity agent, etc., which are usually used as sub-ingredients in a medicine containing a tissue or cell to be transplanted in transplantation medicine. May be good.
- dimethylsulfoxide, dextran, human serum albumin, sodium acetyltryptophan, sodium chloride, potassium chloride, calcium chloride hydrate, magnesium chloride, sodium hydrogencarbonate, sodium citrate hydrate, carbon dioxide and the like can be mentioned. , Not limited to this.
- an infusion bag containing a cell population of hematopoietic stem cells is provided.
- the infusion bag may contain the above-mentioned sub-ingredients.
- the bag can be diluted with physiological saline or the like before use.
- the pharmaceutical composition herein it is provided in a frozen state.
- it can be used by thawing in a water bath at 37 ° C. After thawing, it may be diluted with physiological saline or the like.
- One aspect of the present invention provides a pharmaceutical composition containing the compound of the present invention itself for treating a disease requiring regeneration or enhancement of hematopoietic stem cells, specifically, a blood cancer such as leukemia.
- One aspect of the pharmaceutical composition comprises an effective amount of a compound of the invention and a pharmaceutically acceptable carrier.
- the cell population of hematopoietic stem cells produced by the production method of the present invention is useful for transplantation medicine to supplement hematopoietic stem cells for patients who have lost the function of hematopoietic stem cells such as blood cancer. Therefore, one aspect of the present invention provides a therapeutic agent for hematopoietic stem cell replacement (eg, a therapeutic agent for blood cancer), which comprises hematopoietic stem cells produced by the production method of the present invention.
- a therapeutic agent for hematopoietic stem cell replacement eg, a therapeutic agent for blood cancer
- Blood cancer aplastic anemia, congenital hematopoietic disorders, immunodeficiency syndromes such as acquired immunodeficiency syndrome (AIDS), agammaglobulinemia, myelopathic thrombocytopenia, idiopathic thrombocytopenia that require transplantation
- a cell population containing hematopoietic stem cells produced by the production method of the present invention is transplanted into a patient suffering from a disease such as thrombocytopenia (ITP) or congenital anemia such as sickle red erythrocytes, and the hematopoietic stem cells become new blood.
- ITP thrombocytopenia
- congenital anemia such as sickle red erythrocytes
- Examples of hematological cancers include leukemia, malignant lymphoma, and multiple myeloma.
- leukemia include acute myelogenous leukemia, acute lymphocytic leukemia, chronic myelogenous leukemia, myelodysplastic syndrome, adult T-cell ATL, and chronic lymphocytic leukemia.
- malignant lymphoma include follicular lymphoma, aggressive lymphoma, Hodgkin lymphoma and the like.
- one aspect of the present invention provides a method for curing a disease requiring regeneration or enhancement of hematopoietic stem cells, specifically, a blood cancer such as leukemia, including administration of the compound of the present invention itself to a subject. ..
- Example 1-17 1-[(3R, 5aS, 6R, 8aS, 9R, 10S, 12R, 12aR) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2] Benzodioxepin-10-yl] methaneamine
- Examples 1-1 and 1-28 (3R, 5aS, 6R, 8aS, 9R, 10R, 12S, 12aR) -10-Fluoro-3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2 ]
- N, N-diethylaminosulfur trifluoride (0.6 mL) was added dropwise to a dichloromethane solution (24 mL) of Q1 (1.136 g) at 0 ° C., and the mixture was stirred at room temperature for 16 hours. After completion of the reaction, the mixture was cooled to 0 ° C., a 5% aqueous sodium carbonate solution (20 mL) was added, and the mixture was stirred at room temperature for 2 hours. The organic layer was washed with 1M aqueous hydrochloric acid solution, aqueous sodium hydrogen carbonate solution and water, and then dried over sodium sulfate.
- Example 1-7 (3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -10- (furan-2-yl) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3- j] [1,2] Benzodioxepin Boron trifluoride diethyl ether complex (16.8 mg) of the compound of Example 1-1 (285 mg) and furan (338 mg) obtained in the above experiment in a dichloromethane solution (10 mL) at ⁇ 78 ° C. under a nitrogen atmosphere. ) was added, and the mixture was stirred at ⁇ 50 ° C. for 5 hours.
- Example 1-8 (3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2] benzodioxe Pin-10-carboxylic acid
- Example 1-7 (90 mg) was dissolved in a mixture of acetonitrile (1 mL), carbon tetrachloride (1 mL) and water (1 mL). Sodium periodate (286 mg) was added, followed by rhodium (III) chloride trihydrate (7 mg), and the mixture was stirred at room temperature for 3 hours. After completion of the reaction, liquid separation extraction was performed with dichloromethane-sodium bicarbonate water, and the solvent of the organic layer was distilled off under reduced pressure. The obtained residue was purified by reverse phase column chromatography (eluting solvent; acetonitrile: water (formic acid addition)) to obtain the compound of Example 1-8 (35 mg).
- Example 1-6 (3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -3,6,9,10-Tetramethyldecahydro-12H-3,12-Epoxypyrano [4,3-j] [1,2] Benzodioxepin Tetrahedron Letter.
- the compound of Example 1-6 (11 mg) was prepared from the compound of Example 1-1 (70 mg) using the method described in 1998, 39, 1533-1536.
- Example 1-2 (3R, 5aS, 6R, 8aS, 9R, 10S, 12R, 12aR) -3,6,9-trimethyl-10-phenyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1 , 2] Production of benzodioxepine (Example 1-2) Eur. J. Org. Chem. The compound of Example 1-2 (24.2 mg) was prepared from compound Q4 (200 mg) using the method described in 2003, 2098-2114.
- Example 1-3 and Example 1-4 (3R, 5aS, 6R, 8aS, 9R, 10S, 12S, 12aR) -3,6,9-trimethyl-10- (methylsulfanyl) Decahydro-12H-3,12-epoxypyrano [4,3-j] [1 , 2] Benzodioxepin (Example 1-3) and (3R, 5aS, 6R, 8aS, 9R, 10R, 12S, 12aR) -3,6,9-trimethyl-10- (methylsulfanyl) decahydro-12H -3,12-Epoxypyrano [4,3-j] [1,2] Benzodioxepin (Example 1-4)
- Example 1-5 (3R, 5aS, 6R, 8aS, 9R, 10S, 12S, 12aR) -10- (Methanesulfonyl) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [ 1,2] Benzodioxepin J. Org. Chem.
- the compound of Example 1-5 (18.5 mg) was prepared from the compound of Example 1-3 (55 mg) using the method described in 2004,69,984-986.
- Example 1-9 4-Phenyl-1-[(3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [ 1,2] Benzodioxepin-10-yl] -1H-1,2,3-triazole
- Example 1-9 4-Phenyl-1-[(3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] ] [1,2] Benzodioxepin-10-yl] -1H-1,2,3-Triazole (Example 1-9) production Bioorg. Med. Chem. Lett. The compound of Example 1-9 (20 mg) was prepared from compound Q5 (90 mg) using the method described in 2009, 19, 382-385.
- Example 1-11 N-[(3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2] Benzodioxepin-10-yl] Methanesulfonamide Methanesulfonyl chloride (24.1 mg) and triethylamine (21.3 mg) were added to a dichloromethane solution of compound Q6 (20 mg) at 0 ° C., and the mixture was stirred at room temperature for 17 hours.
- Example 1-13 4-[(3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2] Benzodioxepin-10-yl] Morpholine Angew. Chem. Int. Ed.
- Compound Q4 (150 mg) from Example 1-13 (24 mg) was obtained from compound Q4 (150 mg) using the method described in 2006, 45, 2082-2088.
- Example 1-12 (3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -N, N, 3,6,9-Pentamethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1, 2] Benzodioxepin-10-amine
- the reaction and treatment were carried out in the same manner as in Example 1-13 using the corresponding raw material compound to obtain the compound of Example 1-12.
- Example 1-14 (3R, 5aS, 6R, 8aS, 9R, 10S, 12R, 12aR) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2] benzodioxe Pin-10-yl acetate Euro. J. Org. Chem. Compound (28 mg) of Example 1-14 was prepared from Q1 (100 mg) using the method described in 2002, 113-132.
- Example 1-15 (3R, 5aS, 6R, 8aS, 9R, 10S, 12R, 12aR) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2] benzodioxe Pin-10-carboxamide
- Compound Q3 (100 mg) from Example 1-15 (50 mg) was obtained from Compound Q3 (100 mg) using the method described in ChemmedChem 2016, 11, 1469-1479.
- Example 1-16 (3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2] benzodioxe Pin-10-carboxamide
- Compound Q3 (30 mg) from Example 1-16 (10 mg) was obtained from Compound Q3 (30 mg) using the method described in ChemmedChem 2016, 11, 1469-1479.
- Example 1-18 1-[(3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2] Benzodioxepin-10-yl] methaneamine
- a toluene solution (8 mL) of the compound of Example 1-16 (400 mg), dodecacarbonyl triruthenium (24.5 mg) and 1,1,3,3-tetramethyldisiloxane (686 mg) was stirred at 50 ° C. overnight. did. After completion of the reaction, water was added and the mixture was extracted with dichloromethane.
- Example 1-19 (3R, 5aS, 6R, 8aS, 9R, 10S, 12R, 12aR) -3,6,9-trimethyl-10-[(Propane-2-yl) oxy] Decahydro-12H-3,12-Epoxypyrano [4, 3-j] [1,2] Benzodioxepin Boron trifluoride diethyl ether complex (99.6 mg) was added to a dichloromethane solution (1 mL) of Q1 (100 mg) and isopropanol (42.1 mg) at 15 ° C., and the mixture was stirred at 15 ° C. for 16 hours.
- Example 1-20, 1-23 and 1-24 Reactions and treatments were carried out in the same manner as in Example 1-19 using the corresponding raw material compounds to obtain the compounds shown in Table 1.
- Example 1-24 1H NMR (400 MHz, CDCl 3 ): ⁇ 7.39-7.30 (5H, m), 5.49 (1H, s), 4.95-4.92 (2H, m), 4.53 (1H, d, J 12.0 Hz), 2.72- 2.68 (1H, m), 2.45-2.37 (1H, m), 2.10-2.04 (1H, m), 1.94-1.79 (3H, m), 1.67-1.62 (1H, m), 1.56-1.51 (1H, m) ), 1.49 (3H, s), 1.38-1.24 (3H, m), 0.98-0.96 (6H, m), 0.92-0.88 (1H, m).
- Example 1-22 (3R, 5aS, 6R, 8aS, 9R, 10S, 12R, 12aR) -3,6,9-trimethyl-10- (2,2,2-trifluoroethoxy) Decahydro-12H-3,12-epoxypyrano [4 , 3-j] [1,2] Benzodioxepin To a toluene solution (5 mL) of Q1 (100 mg) and 2,2,2-trifluoroethanol (351 mg), triphenylphosphine (184 mg) and diethyl azodicarboxylate (119 mg) were added at 0 ° C. to 0 ° C. Was stirred for 2 hours.
- Example 1-21 and 1-25 Reactions and treatments were carried out in the same manner as in Example 1-19 using the corresponding raw material compounds to obtain the compounds shown in Table 2.
- Example 1-21 1H NMR (400 MHz, CDCl 3 ): ⁇ 7.33-7.29 (2H, m), 7.15-7.13 (2H, m), 7.03-7.00 (1H, m), 5.54-5.52 (2H, m), 2.84-2.81 (1H, m), 2.44-2.38 (1H, m), 2.08-1.88 (4H, m), 1.75-1.70 (1H, m), 1.61-1.59 (1H, m), 1.55-1.46 (4H, m) , 1.41-1.36 (1H, m), 1.34-1.30 (1H, m), 1.04 (3H, d, J 6.0 Hz), 1.01-0.96 (4H, m).
- Example 1-25 1H NMR (400 MHz, CDCl 3 ): ⁇ 7.33-7.28 (2H, m), 7.14-7.12 (2H, m), 7.04-7.00 (1H, m), 5.51 (1H, s), 5.07 (1H, d) , J 10.0 Hz), 2.78-2.73 (1H, m), 2.47-2.39 (1H, m), 2.09-2.04 (1H, m), 1.91-1.97 (1H, m), 1.86-1.80 (1H, m) ), 1.76-1.71 (1H, m), 1.69-1.65 (1H, m), 1.56-1.49 (1H, m), 1.46 (3H, s), 1.42-1.28 (3H, m), 1.11-1.04 (1H) , m), 1.02-1.00 (6H, m).
- Example 1-26 and Example 1-27 2- ⁇ [(3R, 5aS, 6R, 8aS, 9R, 10S, 12R, 12aR) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2 ] Benzodioxepin-10-yl] oxy ⁇ ethyl acetate (Example 1-26) and 2- ⁇ [(3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -3,6,9- Trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2] benzodioxepin-10-yl] oxy ⁇ ethyl acetate (Example 1-27)
- Example 1-27 2- ⁇ [(3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2 ] Benzodioxepin-10-yl] Oxy ⁇ Ethyl Acetate
- the corresponding raw material compound Q8 was used for reaction and treatment in the same manner as in Example 1-26 to obtain the compound of Example 1-27.
- Example 1-34 (3R, 3aS, 6R, 6aS, 9S, 10aS, 10bR) -3,6,9-trimethyloctahydro-10aH-9,10b-epoxypyrano [4,3,2-jk] [2] benzoxepin-2 (3H) )-on
- Example 1-35 (3R, 5aS, 6R, 8aS, 9R, 10R, 12R, 12aR) -10-Methoxy-3,6,9-trimethyldecahydro-12H-3,12-epoxypyrano [4,3-j] [1,2 ] Benzodioxepin A compound purchased from Toronto Research Chemicals (catalog code: A777410) was used.
- Mouse Triple-mCherry Hoxb5 knock-in mouse; Non-Patent Document 1 in which a nucleic acid sequence (Hoxb5-tri-mCherry) containing a gene encoding 3 copies of mCherry fluorescent protein is inserted into the C-terminal of the mouse endogenous HoxB5 gene. It was used. Mice were bred under environmental conditions such as room temperature of 20 to 26 ° C., humidity of 40 to 70%, and light and darkness for 12 hours each (lighting: 8:00 am to 8:00 pm).
- Bone marrow cells were collected from the tibia, femur, humerus and pelvis on both sides of the mouse and collected in a mortar. Hematopoietic stem cells by adding a buffer solution containing 2% final concentration heat-treated inactivated bovine serum (manufactured by Gibco) and final concentration 2 mM EDTA to Ca 2+ -free and Mg 2+ -free PBS and destroying them with a milk stick. A cell population containing the above was obtained.
- c-Kit positive cells were stained with antibodies against the following cell surface markers.
- Cell surface markers Sca-1, Flk2, CD150, CD34, Ter-119, B220, CD3, CD4, CD8a, Gr-1, CD11b, IL-7R and CD16 / 32
- Staining with antibody was performed at 4 ° C and the cells were incubated for 30 minutes.
- Dead cell staining was performed by SYSTEMX Red Dead Cell Stein (manufactured by Life Technologies) by a method recommended by the manufacturer.
- the sorted cells were seeded on a 96-well plate.
- the cell culture medium is prepared by adding StemCell Factor (SCF, manufactured by Peprotech) and Trombopoietin (TPO, manufactured by Peprotech) to StemSpan SFEM medium (manufactured by STEMCELL Technologies), and 96-well plates for cell culture in advance. After adding 100 ⁇ L / well to (Bioscience), the cells sorted by FACS Maria II cell sorter (BD Biosciences) were seeded so as to have 10 cells / well.
- SCF StemCell Factor
- TPO Trombopoietin
- test compound was diluted with a cell culture medium to a final concentration of 1 ⁇ M, and added to the seeded cells after sorting to a final volume of 200 ⁇ L / well by medium exchange. The cells were then cultured in a CO 2 incubator at 37 ° C. and 5% CO 2 for 1 week.
- c-Kit +, Lin - , Sca-1 + or mCherry-positive fraction ratios and a cell number after proliferation was analyzed using FlowJo (BD).
- the mCherry-positive fraction is a cell fraction that expresses the Hoxb5 gene
- the KLS fraction is a c-Kit-positive, Lin-negative and Sca-1-positive cell fraction that contains hematopoietic stem cells and pluripotent progenitor cells. This is the main fraction.
- a compound having a KLS positive rate of 30% or more, an mCherry positive rate of 30% or more, and a cell number of 10 cells or more was regarded as a hit compound.
- the hit compounds were the following five compounds.
- KLS prevalence KLS to the total number of viable cells c-Kit +, Lin - , Sca-1 +
- a cell number ratio of (%) mCherry positive mCherry-positive cell number to the total number of viable cells Percentage (%).
- Example 3 Reproducibility test and concentration dependence test of hit compound
- the cells were cultured.
- the number of living cells, KLS positive rate, and mCherry positive rate were analyzed, and the results are shown in Table 3.
- the cell culture and evaluation index are the same as in Example 2.
- Example 4 Confirmation test by gene expression of mouse HoxB5
- Cells cultured in the presence of Artemether were evaluated by gene expression in mouse HoxB5.
- the cells were cultured in the same manner as in Example 3, and the cultured cells were treated with a Single Cell to CT qRT-PCR kit (manufactured by Life Technologies) as instructed by the kit. After washing the treated cells once with PBS, cell lysates are added, and after RNA extraction, they are subjected to a reverse transcription reaction for cDNA synthesis, amplified by 14 cycles of Taqman Gene Expression Assay, and 1 ⁇ TE buffered. It was diluted with a solution (pH 8.0).
- the sample was amplified using the following Taqman probe at 95 ° C. for 3 seconds and 60 ° C. for 40 cycles at 30 seconds (HoxbB5: Mm00657672 (manufactured by Thermo Fisher Scientific), Gapdh: Mm99999915-g1. (Manufactured by Thermo Fisher Scientific)). All thermal cyclers were analyzed using Quant Studio 6 (manufactured by Applied Biosystem) (Table 5).
- Example 5 Combination test with UM171
- the number of living cells, KLS positive rate, and mCherry positive rate were analyzed, and the results are shown in Table 6.
- the cell culture and evaluation index are the same as in Example 2.
- CD34-positive cells human hematopoietic stem cells
- flow cytometry the cell population containing the hematopoietic stem cells obtained above was crudely purified from a CD34-positive cell fraction using EASYSep Human Cord Blood CD34 Positive Selection Kit II (manufactured by STEMCELL Technologies).
- CD34-positive enriched cells enriched with hematopoietic stem cells and progenitor cell populations were stained with antibodies against the following cell surface markers.
- Cell surface markers CD34, CD11b, CD14, CD19, CD20, CD235ab, CD3, CD4, CD56, CD8a.
- Staining with antibody was performed at 4 ° C and the cells were incubated for 30 minutes.
- Dead cell staining was performed by a method recommended by the manufacturer by SYSTEMX Blue Dead Cell Stein (manufactured by Life Technologies).
- Flow cytometric analysis and cell sorting are performed using Lineage-negative and CD34-positive fractions as hematopoietic stem cells, using FACS Aria III cell sorter (BD Bioscience), and Flow Jo software (Tree Star). ) was used for analysis.
- the sorted cells were seeded on a 96-well plate.
- the cell culture medium is IMDM medium (ThermoFisher SCIENTIFIC), Stem Cell Factor (SCF, Peprotech), Trombopoietin (TPO, Peprotech), L-Glutamine, Pentillin Prepared by adding Transferrin-Selenium-Ethanolamine (manufactured by Thermo Fisher SCIENTIFIC), 200 ⁇ L / well was added to a 96-well plate for cell culture (manufactured by CORNING) in advance, and then sorted with a FACS Aria III cell sorter (BD Bioscience). Cells were seeded at 30 cells / well.
- Artemether or the compounds of Examples 1-23, 1-16, and 1-7, were added to the cell culture medium at final concentrations of 9 points (0.001 ⁇ M, 0.003 ⁇ M, 0.01 ⁇ M, 0.03 ⁇ M, 0.1 ⁇ M, 0.3 ⁇ M, 1 ⁇ M, 3 ⁇ M, 10 ⁇ M) were added to the sorted and seeded human cells so as to have a final volume of 200 ⁇ L / well, and the cells were allowed to act. A part of UM171 (35 nM) was added, and the presence or absence of a synergistic effect was examined. The cells were then cultured in a CO 2 incubator at 37 ° C. and 5% CO 2 for 1 week.
- the number of living cells and the number of CD34-positive cells were analyzed.
- the number of viable cells and the number of CD34-positive cells when cultured in the presence of Artemether (UM171 +/-) are shown in FIGS. 1 and 2.
- Tables 7 to 11 show the number of viable cells and the number of CD34-positive cells when cultured in the presence of various compounds.
- CD34 fraction (CD34) using a FACS Maria III cell sorter.
- the ratio of + and Lin ⁇ ) and the number of cells after proliferation were measured and analyzed using FlowJo (BD).
- the CD34 fraction is a fraction mainly composed of hematopoietic stem cells and pluripotent progenitor cells.
- CD34-positive cells were seeded one by one in 1 well of a 96-well plate with a FACS Maria III cell sorter, and Metocult H4435. Incubated in (StemCell technologies) for 2 weeks. After culturing, each well was imaged with an optical microscope (manufactured by KEYENCE), the number of colonies was visually counted, and the colony morphology was visually discriminated.
- Example 8 In vivo engraftment test using human cells
- Artemether, or the compound of Example 1-23 was prepared in a cell culture medium to a final concentration of 0.003 ⁇ M or 0.01 ⁇ M, respectively, and the above-mentioned sorted and seeded human cells (166 cell seeds per well). was added to a final volume of 200 ⁇ L / well and allowed to act. Then, for each condition, a total of 10,000 CD34-positive fraction cells were cultured in a CO 2 incubator under the conditions of 37 ° C. and 5% CO 2 for 1 week, and the number of cells was counted.
- Table 17 shows 1 month, 2 months and 3 months after transplantation
- Table 18 shows peripheral blood donor microchimerism 1 month and 2 months after transplantation.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- Hematology (AREA)
- Biotechnology (AREA)
- General Health & Medical Sciences (AREA)
- Cell Biology (AREA)
- Immunology (AREA)
- Developmental Biology & Embryology (AREA)
- Wood Science & Technology (AREA)
- Genetics & Genomics (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Microbiology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Virology (AREA)
- Epidemiology (AREA)
- Diabetes (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Plural Heterocyclic Compounds (AREA)
Abstract
Description
[1]
造血幹細胞を含む細胞集団を、式(1)で表される化合物、又はその塩を1以上含む培地中で培養することを含む、造血幹細胞の培養方法。
XはO-O又はOであり、
Yは、水素原子、ヒドロキシ、オキソ、メルカプト、カルボキシ、カルバモイル、シアノ、OR1、SR1、SOR1、SO2R1、COR1、OCOR1、R2、COOR1、NR3R4、NR5COR1、NR5SO2R1、NR5CONR3R4又はハロゲンであり、
Zは、縮合環の任意の位置の炭素に結合しており、各々独立してC1-6アルキルであり、
nは、0~10の整数であり、
R1は、置換されていてもよいアルキル、置換されていてもよいシクロアルキル、置換されていてもよいアリール、置換されていてもよいヘテロアリール、又は置換されていてもよい脂肪族複素環基であり、
R2は、置換されていてもよいアルキル、置換されていてもよいアルケニル、置換されていてもよいアルキニル、置換されていてもよいシクロアルキル、置換されていてもよいアリール、置換されていてもよいヘテロアリール、又は置換されていてもよい脂肪族複素環基であり、
R3、R4及びR5は、独立して、水素原子、置換されていてもよいアルキル、置換されていてもよいシクロアルキル、置換されていてもよい脂肪族複素環基、置換されていてもよいアリール、又は置換されていてもよいヘテロアリールであり、ここにおいてR3とR4は、隣接する窒素原子と一緒になって、更に1又は2の環を構成する酸素原子、窒素原子又は硫黄原子を含んでよい4~10員の含窒素脂肪族複素環を形成してよく、当該含窒素脂肪族複素環は置換されていてもよい]
[2]
式(1)の化合物が、式(2)で表される化合物である、[1]に記載の培養方法。
X及びYは式(1)における定義と同じであり、
Z1、Z2、Z3、Z4、Z5、及びZ6は、独立して水素原子又はC1-6アルキルである]
[3]
式(2)の化合物が、式(3-1)、(3-2)又は(3-3)で表される化合物である、[2]に記載の培養方法。
X及びYは式(1)における定義と同じであり、
Z1、Z2、Z3、Z4、Z5、及びZ6は、独立して水素原子又はC1-6アルキルである]
[4]
式(3-1)、(3-2)又は(3-3)において、Z1、Z2、Z3、Z4、Z5、及びZ6が、独立して水素原子又はメチルである、[3]に記載の培養方法。
[5]
式(1)の化合物が、式(4-1)、(4-2)又は(4-3)で表される化合物である、[1]に記載の培養方法。
X及びYは、式(1)における定義と同じである]
[6]
Yは水素原子、ヒドロキシ、オキソ、カルボキシ、カルバモイル、OR1、SR1、SO2R1、COR1、OCOR1、R2、NR3R4、NR5COR1、NR5SO2R1、又はフッ素原子である、[1]~[5]のいずれか一項に記載の培養方法。
[7]
Yは、ヒドロキシ、オキソ、カルボキシ、カルバモイル、OR1、SR1、SO2R1、OCOR1、R2、NR3R4、NR5COR1、又はフッ素原子であり、
R1が、群1から選択される1~7の置換基で置換されていてもよいC1-8アルキル、群1から選択される1~3の基で置換されていてもよいC3-8シクロアルキル、群1から選択される1~5の基で置換されていてもよいフェニル、群1から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~10員の脂肪族複素環基、又は群1から選択される1~4の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~10員のヘテロアリールであり、
R2が、群2から選択される1~7の置換基で置換されていてもよいC1-8アルキル、群2から選択される1~7の置換基で置換されていてもよいC1-8アルケニル、群1から選択される1~3の基で置換されていてもよいC3-8シクロアルキル、群1から選択される1~5の基で置換されていてもよいフェニル、群1から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~10員の脂肪族複素環基、又は群1から選択される1~4の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~10員のヘテロアリールであり、
R3及びR4は独立して、水素原子、群1から選択される1~7の置換基で置換されていてもよいC1-6アルキル、又は群1から選択される1~5の基で置換されていてもよいフェニルであり、ここにおいてR3とR4は、隣接する窒素原子と一緒になって、更に1又は2の環を構成する酸素原子、窒素原子又は硫黄原子を含んでよい3~8員の含窒素脂肪族複素環を形成してよく、当該含窒素脂肪族複素環は以下の群1の置換基から選択される1~3の置換基で置換されていてもよく、
R5が、水素原子又はC1-3アルキルである、[1]~[6]のいずれかに記載の培養方法。
<群1>
カルボキシ、ヒドロキシ、C1-8アルコキシ、C1-8アルキルカルボニル、C1-8アルキルカルボニルオキシ、C1-8アルコキシカルボニル、メルカプト、C1-8アルキルスルファニル、C1-8アルキルスルホニル、1若しくは2のC1-8アルキルで置換されていてもよいカルバモイル、1若しくは2のC1-8アルキルで置換されていてもよいアミノ、C1-8アルキルカルボニルアミノ、C1-8アルキルスルホニルアミノ、群3から選択される1~5の基で置換されていてもよいフェニル、群3から選択される1~4の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~6員のヘテロアリール、群3から選択される1~3の基で置換されていてもよいC3-8シクロアルキル、群3から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~10員の脂肪族複素環基、ハロゲン
<群2>
カルボキシ、ヒドロキシ、C1-8アルコキシ、C1-8アルキルカルボニル、C1-8アルキルカルボニルオキシ、C1-8アルコキシカルボニル、メルカプト、C1-8アルキルスルファニル、C1-8アルキルスルホニル、1若しくは2のC1-8アルキルで置換されていてもよいカルバモイル、アミノ、群3から選択される1~5の基で置換されていてもよいフェニル、群3から選択される1~4の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~6員のヘテロアリール、群3から選択される1~3の基で置換されていてもよいC3-8シクロアルキル、群3から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~8員の脂肪族複素環基、ハロゲン
<群3>
カルボキシ、ヒドロキシ、C1-8のアルキル、C1-8アルコキシ、C1-8アルキルカルボニル、C1-8アルキルカルボニルオキシ、C1-8アルコキシカルボニル、メルカプト、C1-8アルキルスルファニル、C1-8アルキルスルホニル、1若しくは2のC1-8アルキルで置換されていてもよいアミノ、1若しくは2のC1-8アルキルで置換されていてもよいカルバモイル、C1-8アルキルカルボニルアミノ、C1-8アルキルスルホニルアミノ、ハロゲン
[8]
Yは、ヒドロキシ、オキソ、カルボキシ、カルバモイル、OR1、SR1、SO2R1、OCOR1、R2、NR3R4、NR5COR1、NR5SO2R1、又はフッ素原子であり、
R1は、群1’から選択される1~3の基で置換されていてもよいC1-4アルキル、群1’から選択される1~3の基で置換されていてもよいC3-6シクロアルキル、群1’から選択される1~3の基で置換されていてもよいフェニル、群1’から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む4~6員の脂肪族複素環基、又は群1’から選択される1~3の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~6員のヘテロアリールであり、
R2は、群2’から選択される1~3の基で置換されていてもよいC1-4アルキル、群1’から選択される1~3の基で置換されていてもよいC3-6シクロアルキル、群1’から選択される1~3の基で置換されていてもよいフェニル、群1’から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む4~6員の脂肪族複素環基、又は群1’から選択される1~3の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~6員のヘテロアリールであり、
R3は、水素原子又はC1-3アルキルであり
R4は、水素原子、群1’から選択される1~3の基で置換されていてもよいC1-4アルキル、群1’から選択される1~3の基で置換されていてもよいC3-6シクロアルキル、群1’から選択される1~3の基で置換されていてもよいフェニル、群1’から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む4~6員の脂肪族複素環基、又は群1’から選択される1~3の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~6員のヘテロアリールであり、
ここにおいてR3とR4は、隣接する窒素原子と一緒になって、更に1又は2の環を構成する酸素原子、窒素原子又は硫黄原子を含んでよい3~8員の含窒素脂肪族複素環を形成してよく、当該含窒素脂肪族複素環は以下の群1’の置換基から選択される1~3の基で置換されていてもよく、
R5が、水素原子である、[1]~[7]のいずれかに記載の培養方法。
<群1’>
フッ素原子、ヒドロキシ、カルボキシ、アミノ、C1-4アルコキシ、C1-4アルキルカルボニルオキシ、群3’から選択される1~3の基で置換されていてもよいフェニル、群3’から選択される1~3の基で置換されていてもよいC3-6のシクロアルキル、群3’から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む4~6員の脂肪族複素環基
<群2’>
フッ素原子、ヒドロキシ、カルボキシ、アミノ、C1-4アルコキシ、C1-4アルキルカルボニルオキシ、C1-4アルコキシカルボニル、1若しくは2のC1-8アルキルで置換されていてもよいカルバモイル、群3’から選択される1~3の基で置換されていてもよいフェニル、群3’から選択される1~3の基で置換されていてもよいC3-6シクロアルキル、群3’から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む4~6員の脂肪族複素環基
<群3’>
フッ素原子、ヒドロキシ、C1-4のアルキル
[9]
XがO-Oである、[1]~[8]のいずれかに記載の培養方法。
[10]
式(1)の化合物が、以下の化合物からなる群から選択される化合物である、[1]に記載の培養方法。
アルテメテル(Artemether)、
アルテミシニン(Artemisinin)、
アルテニモール(Artenimol)、
アルテモチル(Artemotil)、
アルテスネイト(Artesunate)、
(3R,5aS,6R,8aS,9R,10R,12S,12aR)-10-フルオロ-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチル-10-フェニルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12S,12aR)-3,6,9-トリメチル-10-(メチルスルファニル)デカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10R,12S,12aR)-3,6,9-トリメチル-10-(メチルスルファニル)デカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12S,12aR)-10-(メタンスルホニル)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9,10-テトラメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-10-(フラン-2-イル)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボン酸、
4-フェニル-1-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]-1H-1,2,3-トリアゾール、
N-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]アセトアミド、
N-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンスルホンアミド、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-N,N,3,6,9-ペンタメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-アミン、
4-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]モルホリン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル アセテート、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボキサミド、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボキサミド、
1-[(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンアミン、
1-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンアミン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチル-10-[(プロパン-2-イル)オキシ]デカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-10-(シクロヘキシルオキシ)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチル-10-フェノキシデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチル-10-(2,2,2-トリフルオロエトキシ)デカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-10-(2-メトキシエトキシ)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-10-(ベンジルオキシ)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチル-10-フェノキシデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
2-{[(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]オキシ}エチル アセテート、
2-{[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]オキシ}エチル アセテート、
(3R,5aS,6R,8aS,12R,12aR)-3,6,9-トリメチル-3,4,5,5a,6,7,8,8a-オクタヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,3aS,6R,6aS,9S,10aS,10bR)-3,6,9-トリメチルオクタヒドロ-10aH-9,10b-エポキシピラノ[4,3,2-jk][2]ベンゾキセピン-2(3H)-オン、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-10-メトキシ-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン
[10-2]
式(1)の化合物が、以下の群から選択される化合物である、[1]に記載の培養方法。
アルテメテル(Artemether)、
アルテミシニン(Artemisinin)、
アルテニモール(Artenimol)、
アルテモチル(Artemotil)、
アルテスネイト(Artesunate)
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボキサミド、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボキサミド、
1-[(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンアミン、
1-[3R,(5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンアミン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-10-(2-メトキシエトキシ)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン
[11]
式(1)の化合物が、アルテメテル(Artemether)、アルテミシニン(Artemisinin)、アルテニモール(Artenimol)、アルテモチル(Artemotil)、及び、アルテスネイト(Artesunate)からなる群から選択される、[1]に記載の培養方法。
[12]
上記造血幹細胞が、CD34陽性細胞である、[1]~[11]のいずれかに記載の培養方法。
[13]
上記造血幹細胞が、長期造血幹細胞である、[1]~[12]のいずれかに記載の培養方法。
[14]
上記造血幹細胞が、Hoxb5陽性のマウス造血幹細胞である、[1]~[12]のいずれかに記載の培養方法。
[15]
培養後の細胞集団における総細胞数に対する造血幹細胞の割合が、培養前の細胞集団における総細胞数に対する造血幹細胞の割合の10%以上である、[1]~[14]のいずれかに記載の培養方法。
[16]
培養後の細胞集団における造血幹細胞の数が、培養前の細胞集団における造血幹細胞の数の3倍以上である、[1]~[15]のいずれかに記載の培養方法。
[16-2]
培養後の細胞集団における造血幹細胞の数が、培養前の細胞集団における造血幹細胞の数の10倍以上である、[1]~[15]のいずれかに記載の培養方法。
[17]
上記培地が、さらにUM171又はその誘導体を含む培地である、[1]~[16]のいずれかに記載の培養方法。
[18]
造血幹細胞を含む細胞集団を準備する工程と、
造血幹細胞を含む細胞集団を培養する工程であって、[1]~[17]のいずれかの培養方法によって上記造血幹細胞を含む細胞集団を培養する工程と
を含む、造血幹細胞の製造方法。
[19]
[1]~[17]のいずれかに記載の培養方法によって培養して得られた、造血幹細胞。
[20]
[18]に記載の製造方法によって製造された造血幹細胞。
[21]
式(1)で表される化合物、又はその塩を少なくとも1つ含む、造血幹細胞培養用試薬。
一態様として、本発明の造血幹細胞の培養方法は、造血幹細胞を含む細胞集団を、式(1)で表される化合物、又はその塩を1以上含む培地中で培養することを含む。
「造血幹細胞(hematopoietic stem cell:HSC)」は、主に骨髄に存在し、自己複製能(self-renewal capacity)及び、全ての血液細胞に分化できる多分化能(multipotency)を有する血液細胞である。ここで、自己複製とは、細胞分裂により自らと同じ機能・性質を有する細胞を産生することをいう。すなわち、造血幹細胞の自己複製により産生された細胞は、全ての血液細胞への多分化能及び自己複製能を有する。
本明細書における「細胞集団」とは、同一種類又は異なる種類の細胞が2以上存在している集団を意味する。好ましくは、細胞集団は培地等の媒体中に存在する。細胞集団は、細胞懸濁液及び細胞凝集体を含み、細胞懸濁液又は細胞凝集体の形態であることが好ましい。
一態様として、本発明の化合物は、上述の式(1)の化合物又はその塩であり、好ましくは式(2)、又は式(3-1)、式(3-2)若しくは式(3-2)、更に好ましくは式(4-1)、式(4-2)若しくは式(4-3)の化合物又はその塩を挙げることができる。
XはO-O又はOであり、
Yは、水素原子、ヒドロキシ、オキソ、メルカプト、カルボキシ、カルバモイル、OR1、SR1、SOR1、SO2R1、COR1、OCOR1、R2、COOR1、NR3R4、NR5COR1、NR5SO2R1、NR5CONR3R4又はハロゲンであり、
Zは、縮合環の任意の位置の炭素に結合しており、各々独立してC1-6アルキルであり、
nは、0~10の整数であり、
R1は、置換されていてもよいアルキル、置換されていてもよいシクロアルキル、置換されていてもよいアリール、置換されていてもよいヘテロアリール、又は置換されていてもよい脂肪族複素環基であり、
R2は、置換されていてもよいアルキル、置換されていてもよいアルケニル、置換されていてもよいアルキニル、置換されていてもよいシクロアルキル、置換されていてもよいアリール、置換されていてもよいヘテロアリール、又は置換されていてもよい脂肪族複素環基であり、
R3、R4及びR5は、独立して、水素原子、置換されていてもよいアルキル、置換されていてもよいシクロアルキル、置換されていてもよい脂肪族複素環基、置換されていてもよいアリール、又は置換されていてもよいヘテロアリールであり、ここにおいてR3とR4は、隣接する窒素原子と一緒になって、更に1又は2の環を構成する酸素原子、窒素原子又は硫黄原子を含んでよい3~8員の含窒素脂肪族複素環を形成してよく、当該含窒素脂肪族複素環は置換されていてもよい。
nは、0~10の整数であってよく、好ましくは0~6、更に好ましくは0~3の整数である。
X及びYは式(1)における定義と同じであり、
Z1、Z2、Z3、Z4、Z5、及びZ6は、独立して水素原子又はC1-6アルキルである。式(2)において、Z1、Z2、Z3、Z4、Z5、及びZ6が、独立して水素原子又はメチルであることが好ましい。
X及びYは式(1)における定義と同じであり、
Z1、Z2、Z3、Z4、Z5、及びZ6は、独立して水素原子又はC1-6アルキルである。ここで、Z1、Z2、Z3、Z4、Z5、及びZ6が、独立して水素原子又はメチルであることが好ましい。
式(4-1)、式(4-2)及び式(4-3)中、
X及びYは、式(1)における定義と同じである。
「C1-10アルキル」として、好ましくは「C1-8アルキル」が挙げられ、より好ましくは「C1-6アルキル」、更に好ましくは「C1-4アルキル」が挙げられる。
「C1-8アルキル」として、好ましくは「C1-6アルキル」、更に好ましくは「C1-4アルキル」が挙げられる。
「C1-6アルキル」として、好ましくは「C1-4アルキル」が挙げられる。「C1-4アルキル」の具体例としては、例えば、メチル、エチル、プロピル、1-メチルエチル、ブチル、1、1-ジメチルエチル、1-メチルプロピル、2-メチルプロピル等が挙げられる。「C1-6アルキル」の具体例としては、例えば、上記「C1-4アルキル」の具体例として挙げたものに加え、4-メチルペンチル、3-メチルペンチル、2-メチルペンチル、1-メチルペンチル、ヘキシル等が挙げられる。「C1-8アルキル」の具体例としては、例えば、上記「C1-6アルキル」の具体例として挙げたものに加え、へプチル、オクチル等が挙げられる。
「C6-10アリール」とは、炭素原子数が6~10のアリールを意味する。「C6-10アリール」の具体例としては、例えば、フェニル、1-ナフチル、2-ナフチル等が挙げられる。「C6-10アリール」として、好ましくはフェニルが挙げられる。
尚、ヘテロアリールが置換している場合、化学的に安定である限り、任意の炭素原子又は窒素原子上で置換していてもよい。
尚、脂肪族複素環基が置換している場合、化学的に安定である限り、任意の炭素原子又は窒素原子上で置換していてもよい。
「C1-8アルコキシ」とは、上記「C1-8アルキル」によって置換されたオキシ基を意味する。「C1-8アルコキシ」として、好ましくは「C1-6アルコキシ」、又は「C1-4アルコキシ」が挙げられる。「C1-4アルコキシ」の具体例としては、例えば、メトキシ、エトキシ、プロポキシ、1-メチルエトキシ、ブトキシ、1,1-ジメチルエトキシ、1-メチルプロポキシ、2-メチルプロポキシが挙げられる。「C1-6アルコキシ」の具体例としては、例えば、上記「C1-4アルコキシ」の具体例として挙げたものに加え、ペンチロキシ、3-メチルブトキシ、2-メチルブトキシ、2,2-ジメチルプロポキシ、1-エチルプロポキシ、1,1-ジメチルプロポキシ、ヘキシロキシ、4-メチルペンチロキシ、3-メチルペンチロキシ、2-メチルペンチロキシ、1-メチルペンチロキシ、3,3-ジメチルブトキシ、2,2-ジメチルブトキシ、1,1-ジメチルブトキシ、1,2-ジメチルブトキシ等が挙げられる。「C1-8アルコキシ」の具体例としては、例えば、上記「C1-6アルコキシ」の具体例として挙げたものに加え、へプチルオキシ、オクチルオキシ等が挙げられる。
1若しくは2のC1-8アルキルで置換されていてもよいアミノの一態様として、アミノ基の2つの置換基が隣接する窒素原子と一緒になって、4~10員の含窒素脂肪族複素環を形成していてもよい。当該含窒素脂肪族複素環として、好ましくは、ピペリジン、ピロリジン、ピペラジン、モルホリン等が挙げられる。
1若しくは2のC1-8アルキルで置換されていてもよいカルバモイルの一態様として、カルバモイル基の2つの置換基が隣接する窒素原子と一緒になって、4~10員の含窒素脂肪族複素環を形成していてもよい。当該含窒素脂肪族複素環として、好ましくは、ピペリジン、ピロリジン、ピペラジン、モルホリン等が挙げられる。
Yは、ヒドロキシ、オキソ、カルボキシ、カルバモイル、OR1、SR1、SO2R1、OCOR1、R2、NR3R4、NR5COR1、NR5SO2R1又はフッ素原子であり、
R1が、群1から選択される1~7の置換基で置換されていてもよいC1-8アルキル、群1から選択される1~3の基で置換されていてもよい3~8員のシクロアルキル、群1から選択される1~5の基で置換されていてもよいフェニル、群1から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~10員の脂肪族複素環基、又は群1から選択される1~4の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~10員のヘテロアリールであり、
R2が、群2から選択される1~7の置換基で置換されていてもよいC1-8アルキル、群2から選択される1~7の置換基で置換されていてもよいC1-8アルケニル、群1から選択される1~3の基で置換されていてもよい3~8員のシクロアルキル、群1から選択される1~5の基で置換されていてもよいフェニル、群1から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~10員の脂肪族複素環基、又は群1から選択される1~4の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~10員のヘテロアリールであり、
R3及びR4は独立して、水素原子、群1から選択される1~7の置換基で置換されていてもよいC1-6アルキル、又は群1から選択される1~5の基で置換されていてもよいフェニルであり、ここにおいてR3とR4は、隣接する窒素原子と一緒になって、更に1又は2の環を構成する酸素原子、窒素原子又は硫黄原子を含んでよい3~8員の含窒素脂肪族複素環を形成してよく、当該含窒素脂肪族複素環は以下の群1の置換基から選択される1~3の置換基で置換されていてもよく、
R5が、水素原子又はC1-3アルキルであることが好ましい。
本発明の一態様として、XがO-Oであることが好ましい。
カルボキシ、ヒドロキシ、C1-8アルコキシ、C1-8アルキルカルボニル、C1-8アルキルカルボニルオキシ、C1-8アルコキシカルボニル、メルカプト、C1-8アルキルスルファニル、C1-8アルキルスルホニル、1若しくは2のC1-8アルキルで置換されていてもよいアミノ、1若しくは2のC1-8アルキルで置換されていてもよいカルバモイル、C1-8アルキルカルボニルアミノ、C1-8アルキルスルホニルアミノ、群3から選択される1~5の基で置換されていてもよいフェニル、群3から選択される1~3の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む5~6員のヘテロアリール、群3から選択される1~3の基で置換されていてもよい3~8員のシクロアルキル、群3から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~10員の脂肪族複素環基、ハロゲン
<群2>
カルボキシ、ヒドロキシ、C1-8アルコキシ、C1-8アルキルカルボニル、C1-8アルキルカルボニルオキシ、C1-8アルコキシカルボニル、メルカプト、C1-8アルキルスルファニル、C1-8アルキルスルホニル、1若しくは2のC1-8アルキルで置換されていてもよいカルバモイル、アミノ、群3から選択される1~5の基で置換されていてもよいフェニル、群3から選択される1~4の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~6員のヘテロアリール、群3から選択される1~3の基で置換されていてもよい3~8員のシクロアルキル、群3から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~8員の脂肪族複素環基、ハロゲン
<群3>
カルボキシ、ヒドロキシ、C1-8のアルキル、C1-8アルコキシ、C1-8アルキルカルボニル、C1-8アルキルカルボニルオキシ、C1-8アルコキシカルボニル、メルカプト、C1-8アルキルスルファニル、C1-8アルキルスルホニル、1若しくは2のC1-8アルキルで置換されていてもよいアミノ、1若しくは2のC1-8アルキルで置換されていてもよいカルバモイル、C1-8アルキルカルボニルアミノ、C1-8アルキルスルホニルアミノ、ハロゲン
<群1’>
フッ素原子、ヒドロキシ、カルボキシ、アミノ、C1-4アルコキシ、C2-4アルキルカルボニルオキシ、群3’から選択される1~3の基で置換されていてもよいフェニル、群3’から選択される1~3の基で置換されていてもよい3~6員のシクロアルキル、群3’から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む4~6員の脂肪族複素環基
<群2’>
フッ素原子、ヒドロキシ、カルボキシ、アミノ、C1-4アルコキシ、C2-4アルキルカルボニルオキシ、C1-4アルコキシカルボニル、1若しくは2のC1-8アルキルで置換されていてもよいカルバモイル、群3’から選択される1~3の基で置換されていてもよいフェニル、群3’から選択される1~3の基で置換されていてもよい3~6員のシクロアルキル、群3’から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む4~6員の脂肪族複素環基
<群3’>
フッ素原子、ヒドロキシ、C1-4のアルキル
<群3’’>
フッ素原子
式(1)においてZは、縮合環を構成する任意の炭素原子に結合していてもよく、化学的に安定である限り同一の炭素原子上に結合していてもよく、複数の環に属する炭素原子上に結合していてもよく、Zは同一であって又は異なっていてもよい。
で表される基の具体例として、下記の式(3-1’)、式(3-2’)及び式(3-3’)
で表される基が挙げられる。
式(1)、式(2)、式(3-1)、式(3-2)、式(3-3)、式(4-1)、式(4-2)又は式(4-3)の化合物の一態様として、XがOである場合、好ましくは、上記式(2’)又は式(2’’)で表される化合物が挙げられる。
Yの好ましい態様として、水素原子、ヒドロキシ、カルボキシ、カルバモイル又はハロゲンが挙げられる。
Yがハロゲンである場合、好ましくはフッ素原子である。
YがOR1、SR1、SOR1、SO2R1、又はCOR1である場合、R1としては、好ましくは置換されていてもよいC1-4アルキル、置換されていてもよいフェニル、又は置換されていてもよいシクロアルキルが挙げられる。
また、R5は、好ましくは水素原子又はC1-4アルキル、更に好ましくは、水素原子、メチル又はエチルである。
また、R5は、好ましくは水素原子又はC1-4アルキル、更に好ましくは、水素原子、メチル又はエチルである。
カルボキシ、ヒドロキシ、アミノ、C1-4アルコキシ、C1-4アルキルカルボニル、C1-4アルキルカルボニルオキシ、C1-4アルコキシカルボニル、1若しくは2のC1-4アルキルで置換されていてもよいカルバモイル、群3から選択される1~5の基で置換されていてもよいフェニル、群3から選択される1~4の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~6員のヘテロアリール、群3から選択される1~3の基で置換されていてもよい3~6員のシクロアルキル、群3から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~8員の脂肪族複素環基、フッ素原子
(A1)Z1、Z3及びZ5がメチルであり、Z2、Z4及びZ6が水素原子であり;
(A2)XがO又はO-Oであり;
(A3)Yがヒドロキシ、オキソ、カルボキシ、カルバモイル、フッ素原子、OR1、OCOR1、SR1、SO2R1、R2、NHCOR1、又は、NR3R4であり;
(A4)R1又はR2が、置換されていてもよいC1-8アルキル、置換されていてもよいフェニル、置換されていてもよい5-6員のシクロアルキル、置換されていてもよい5-6員のヘテロアリールであり、置換基がフッ素原子、アミノ、カルボキシ、C1-8アルコキシ、C1-8アルキルカルボニルオキシ及びフェニルから選択され;
(A5)R3及びR4は、メチル基を表すか、隣接する窒素原子と一緒になって、モルホリン、チオモルホリン、チオモルホリン-1-オキシド、チオモルホリン-1,1-ジオキシド、ピペラジン、インドリン、テトラヒドロイソキノリンを形成している。
(B1)XがO-Oであり;
(B2)YがNR5SO2R1である。
式(1)、式(2)、式(3-1)、式(3-2)、式(3-3)、式(4-1)、式(4-2)又は式(4-3)で表される化合物の1つの態様としては、上記(B1)~(B2)に加えて、以下の(B3)~(B4)の特徴を有する化合物又はその塩が挙げられる:
(B3)R1が、置換されていてもよいC1-8アルキル、置換されていてもよいフェニル、置換されていてもよい5-6員のシクロアルキル、置換されていてもよい5-6員のヘテロアリールであり、置換基がフッ素原子、アミノ、カルボキシ、C1-8アルコキシ、C1-8アルキルカルボニルオキシ及びフェニルから選択され;
(B4)R5が水素原子である。
<化合物の製造方法>
造血幹細胞を含む細胞集団を培養する培地として、造血幹細胞の維持培養に適した培地であればよく、例えば、StemSpan SFEM培地(STEMCELL Technologies社製)、StemPro34(ライフテクノロジーズ社製)、HemEx-Type9A(ニプロ社製)、ハムF12培地(Ham’s Nutrient Mixture F12)、DMEM(Dulbecco’s Modified Eagle’s Medium)、RPMI1640培地、IMDM培地(Iscove’s Modified Dulbecco’s Medium)などにStem Cell Factor(SCF、Peprotech社製)とThrombopoietin(TPO、Peprotech社製)を添加して調製したものであってよい。
本発明の培養方法によって培養して得られた細胞集団(本明細書において、「培養後の細胞集団」という場合がある)は、造血幹細胞を含む細胞集団である。従来の培養方法では、殆どの造血幹細胞が分化することによってなくなるのに対して、本発明の培養方法によれば、少なくとも一部の造血幹細胞が分化せず、自己複製能及び多分化能を維持したまま増殖することができる。
本発明の一態様は、造血幹細胞を含む細胞集団の培養物であって、(1)本発明の培養方法によって培養して得られた造血幹細胞を含む細胞集団と、(2)造血幹細胞集団の生存能力を維持するために必要な媒体と、を含む、培養物を提供する。造血幹細胞は長期造血幹細胞であることが好ましい。
本発明の造血幹細胞の製造方法は、造血幹細胞を含む細胞集団を準備する工程と、造血幹細胞を含む細胞集団を培養する工程であって、本発明の培養方法によって造血幹細胞を含む細胞集団を培養する工程とを含む。
(1)造血幹細胞を含む細胞集団を準備する工程、
(2)前記細胞集団を含む細胞集団を培養する工程、及び
(3)造血幹細胞を回収する工程、を含む。
本発明の製造方法における工程(1)、すなわち、造血幹細胞を含む細胞集団を準備する工程において、造血幹細胞を含む細胞集団は、当業者であれば周知の方法を用いて準備することができる。例えば、血液(末梢血、又は、臍帯血)や骨髄から採集すること、又は多能性幹細胞(例:iPS細胞、ES細胞)から人工的に作製することによって準備することができる。また、造血幹細胞を含む細胞集団は市販品として入手することも可能である。
本発明の製造方法における工程(2)、すなわち、培養工程は、本発明の培養方法に記載の方法のとおりである。すなわち、培養工程は、本発明の化合物を含む培地中で培養することを含む。工程(2)で得られるのは、造血幹細胞を含む細胞集団(以下、「本発明の製造方法によって製造された造血幹細胞を含む細胞集団」という場合がある)である。本発明の製造方法によって製造された造血幹細胞を含む細胞集団は、本発明の培養方法によって培養して得られた造血幹細胞を含む細胞集団のとおりである。
本発明の製造方法における工程(3)、すなわち、回収工程において、工程(2)で培養して得られた造血幹細胞を含む細胞集団から、特定のマーカーを用いて造血幹細胞(長期造血幹細胞)を回収する工程である。例えば工程(2)で培養して得られた造血幹細胞を含む細胞集団を、上述した造血幹細胞のマーカーを用い、MACS又はFACSなどにより、造血幹細胞又は長期造血幹細胞を回収してもよい。
本発明の一態様は、式(1)、式(2)、式(3-1)、式(3-2)、式(3-3)、式(4-1)、式(4-2)及び式(4-3)のいずれかで表される化合物、又はその塩を少なくとも1つ含む、造血幹細胞培養用試薬を提供する。
本発明の一態様は、(1)造血幹細胞の維持培養に適した培地と、(2)式(1)、式(2)、式(3-1)、式(3-2)、式(3-3)、式(4-1)、式(4-2)及び式(4-3)のいずれかで表される化合物、又はこれらの塩(本明細書において、「本発明の化合物」という)と、を含む、造血幹細胞培養キットを提供する。
アルテメテル(Artemether)、
アルテミシニン(Artemisinin)、
アルテニモール(Artenimol)、
アルテモチル(Artemotil)、
アルテスネイト(Artesunate)、並びに、実施例1-1~1-28の化合物及び実施例1-33~1-34の化合物。
式(1)で表される化合物の好ましい例として、以下の化合物が挙げられる。
アルテメテル(Artemether)、
アルテミシニン(Artemisinin)、
アルテニモール(Artenimol)、
アルテモチル(Artemotil)、
アルテスネイト(Artesunate)
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボキサミド、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボキサミド、
1-[(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンアミン、
1-[3R,(5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンアミン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-10-(2-メトキシエトキシ)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン
上述の方法等によって製造した造血幹細胞を含む細胞集団は、移植を必要とする対象(例:哺乳動物)に移植することができ、移植後造血幹細胞は対象(レシピエント)の骨髄の中で生着し、血液を作り出すことができる。対象となりうる哺乳動物としては、例えば、ヒト、マウス、ラット、モルモット、ハムスター、ウサギ、ネコ、イヌ、ヒツジ、ブタ、ウシ、ウマ、ヤギ、サル等が挙げられる。造血幹細胞を含む細胞集団は、好ましくは医薬組成物に調製したうえ、移植する。
本発明の一態様は、上記製造方法によって製造される造血幹細胞の細胞集団の有効量を含む、医薬組成物を提供する。移植用細胞集団の有効量は、投与の目的、投与方法、及び投与対象の状況(性別、年齢、体重、病状等)によって異なるが、例えば、細胞数として、患者体重当たり1×107個~1×1010個/kgとすることができる。
本発明の製造方法によって製造される造血幹細胞の細胞集団は、血液がん等の造血幹細胞の機能が失われた患者に対して、造血幹細胞を補充する移植医療に有用である。そこで、本発明の一態様は、本発明の製造方法によって製造される造血幹細胞を含む、造血幹細胞補充のための治療薬(例:血液がんの治療薬)を提供する。移植を必要とする、血液がん、再生不良性貧血、先天性造血障害、後天性免疫不全症候群(AIDS)等の免疫不全症候群、無ガンマグロブリン血症、骨髄障害性血小板減少症、特発性血小板減少性紫斑病(ITP)、鎌状赤血球等の先天性貧血などの疾患に罹患した患者に、本発明の製造方法によって製造された造血幹細胞を含む細胞集団を移植し、当該造血幹細胞が新しい血液を作ることによって、当該患者を治療することができる。血液がんとして、白血病、悪性リンパ腫、多発性骨髄腫などが挙げられる。白血病としては、例えば急性骨髄性白血病、急性リンパ性白血病、慢性骨髄性白血病、骨髄異形成症候群、成人T細胞性ATL、慢性リンパ性白血病などが挙げられる。悪性リンパ腫としては、例えば、濾胞性リンパ腫、アグレッシブリンパ腫、ホジキンリンパ腫などが挙げられる。
また、本発明の一態様は、本発明の化合物自体を対象に投与することを含む、造血幹細胞の再生や増強が求められる疾患、具体的には白血病等の血液がんの治良方法を提供する。
1-[(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンアミン
市販のジヒドロアルテミニシン(Q1、Adams Reagent Ltd.(カタログコード01108787)、284mg)、トリクロロアセトニトリル(156mg)、ジアザビシクロウンデセン(7.58mg)のジクロロメタン溶液(5mL)を室温にて、終夜攪拌した。反応溶液に安息香酸(365mg)のジクロロメタン溶液(5mL)を添加し、室温にて2時間攪拌した。反応終了後、飽和重曹水(10mL)、水(10mL)を加え、有機層を硫酸ナトリウムで乾燥した。溶媒を減圧留去し、化合物Q2(250mg)を得た。
1H NMR (400 MHz, CDCl3): δ 8.03-8.01 (2H, m), 7.61 (1H, d, J = 7.4 Hz), 7.49 (2H, d, J = 4.6 Hz), 6.53 (1H, d, J = 3.2 Hz), 5.59 (1H, s), 2.98-2.94 (1H, m), 2.46-2.38 (1H, m), 2.10-1.89 (4H, m), 1.83-1.79 (1H, m), 1.68-1.64 (1H, m), 1.64-1.49 (5H, m), 1.45-1.35 (1H, m), 1.08-1.02 (4H, m), 0.98 (3H, d, J = 7.6 Hz).
上記の実験で得られた化合物Q2(100mg)、トリメチルシリルシアニド(76.4mg)のジクロロメタン溶液(2mL)を-78℃にて、5分攪拌した。その後、四塩化スズ(26.7mg)を加え、-78℃にて、2時間攪拌した。反応溶媒を減圧留去し、得られた残査をシリカゲルカラムクロマトグラフィー(溶出溶媒;ペンタン:酢酸エチル)で精製することにより化合物Q3(40mg)を得た。
1H NMR (400 MHz, CDCl3): δ 5.55 (1H, s), 4.79 (1H, d, J = 6.0 Hz), 2.93-2.88 (1H, m), 2.43-2.35 (1H, m), 2.12-2.06 (1H, m), 2.01-1.91 (2H, m), 1.87-1.76 (2H, m), 1.67-1.62 (1H, m), 1.56-1.43 (5H, m), 1.42-1.41 (1H, m), 1.09 (3H, d, J = 7.2 Hz), 1.10-0.99 (4H, m).
化合物Q3(100mg)、水素化ホウ素ナトリウム(64.6mg)、三フッ化ホウ素ジエチルエーテル錯体(115mg)のテトラヒドロフラン溶液(4mL)を65℃にて、2時間攪拌した。反応終了後、ジクロロメタン-重曹水で分液抽出した。有機層を飽和食塩水で洗浄し、硫酸ナトリウムで乾燥後、減圧留去した。得られた残査、二炭酸ジ-tert-ブチル(110mg)、トリエチルアミン(101mg)のテトラヒドロフラン溶液(3mL)を、室温にて2時間攪拌した。反応終了後、反応溶媒を減圧留去し、シリカゲルカラムクロマトグラフィー(溶出溶媒;ペンタン:酢酸エチル)で精製することにより成績体(45mg)を得た。その成績体(25mg)、3M塩酸-酢酸エチル(1mL)のジクロロメタン溶液(2mL)を室温にて、2時間攪拌した。反応溶媒を留去し、実施例1-17の化合物(10mg)を塩酸塩として得た。
1H NMR (400 MHz, CDCl3): δ 8.36 (3H, s), 5.56-5.36 (1H, m), 4.65-4.44 (1H, m), 3.26 (2H, s), 3.01-2.78 (1H, m), 2.43-2.11 (1H, m), 2.02-1.91 (2H, m), 1.78-1.76(1H, m), 1.66 (3H, s), 1.48 (3H, s), 1.30-1.19 (3H, m), 0.98-0.87 (7H, m).
(3R,5aS,6R,8aS,9R,10R,12S,12aR)-10-フルオロ-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン(実施例1-1)、及び(3R,5aS,6R,8aS,12R,12aR)-3,6,9-トリメチル-3,4,5,5a,6,7,8,8a-オクタヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン(実施例1-28)
実施例1-1
1H NMR (400 MHz, CDCl3): 5.60 (1H, d, J = 54.4, 2.0 Hz), 5.56 (1H, d, J = 1.6 Hz), 2.72-2.56 (1H, m), 2.38 (1H, m), 2.09-2.02 (1H, m), 1.95-1.81 (2H, m), 1.72-1.65 (1H, m), 1.62-1.58 (1H, m), 1.55-1.50 (2H, m), 1.50-1.45 (1H, m), 1.43 (3H, s), 1.40-1.32 (1H, m), 1.32-1.23 (1H, m), 0.99 (3H, d, J = 7.6 Hz), 0.94 (3H, d, J = 6.0 Hz).
実施例1-28
1H NMR (400 MHz, CDCl3): δ 6.19 (1H, d, J = 1.6 Hz), 5.54 (1H, s), 2.40 (1H, m), 2.10-2.00 (2H, m), 1.95-1.86 (1H, m), 1.73-1.62 (2H, m), 1.59 (3H, s), 1.54-1.51 (1H, m), 1.48-1.38 (5H, m), 1.26-1.03 (2H, m), 0.98 (3H, d, J = 5.6 Hz).
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-10-(フラン-2-イル)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン
1H NMR (500 MHz, CDCl3): δ 7.39 (1H, s), 6.33-6.31 (1H, m), 5.38 (1H, s), 4.46 (1H, d, J = 10.5Hz), 2.90-2.82 (1H, m), 2.39(1H, m), 2.08-2.01 (1H, m), 1.93-1.83 (1H, m), 1.77-1.71 (2H, m), 1.66-1.60 (1H, m) , 1.53-1.47 (2H, m), 1.42-1.34 (4H, m), 1.33-1.24 (1H, m), 1.11-1.02 (1H, m), 0.98 (4H, d, J = 5.5 Hz), 0.93 (3H, d, J = 7.5 Hz).
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボン酸
1H NMR (500 MHz, CDCl3): δ 5.37 (1H, s), 4.06 (1H, d, J = 11.5 Hz), 2.61-2.53 (1H, m), 2.39 (1H, m), 2.08-2.02 (1H, m), 1.95- 1.88 (1H, m), 1.80-1.73 (2H, m), 1.64-1.37 (1H, m), 1.50-1.44 (1H, m), 1.43 (3H, s), 1.39-1.25 (3H, m), 1.10-1.02 (1H, m), 0.98 (3H, d, J = 1.5 Hz), 0.97 (3H, s).
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9,10-テトラメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン
1H NMR (400 MHz, CDCl3): δ 5.36 (1H, s), 4.36-4.33 (1H, m), 2.73-2.66 (1H, m) , 2.34 (1H, m), 2.06-2.01 (1H, m), 1.93-1.78 (2H, m), 1.70-1.62 (1H, m), 1.60-1.51 (1H, m ), 1.51-1.45 (1H, m), 1.45-1.39 (4H, m), 1.36-1.28 (1H, m), 1.26-1.22 (4H, m), 0.99-0.92 (4H, m), 0.86 (3H, d, J = 7.6 Hz).
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチル-10-フェニルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン
Q1(300mg)、トリエチルアミン(132mg)のジクロロメタン溶液(4mL)に、0℃にて、クロロトリメチルシラン(142mg)を加え、0℃にて1.5時間攪拌した。反応終了後、氷水(5g)を加え、水層をジクロロメタンで抽出し、有機層を硫酸ナトリウムで乾燥した。溶媒を減圧留去した後、得られた残査をシリカゲルカラムクロマトグラフィー(溶出溶媒;ペンタン:酢酸エチル)で精製することにより化合物Q4(70mg)を得た。
1H NMR (400 MHz, CDCl3): δ 5.34 (1H, s), 4.78 (1H, d, J = 9.2 Hz), 2.42-2.31(2H, m), 2.06-2.01 (1H, m), 1.93-1.86 (1H, m), 1.79-1.67 (2H, m), 1.57-1.47 (2H, m), 1.44 (3H, s), 1.40-1.30 (3H, m), 1.05-0.95 (4H, m), 0.88 (3H, d, J = 7.2 Hz), 0.21 (9H, s).
Eur.J.Org.Chem.2003,2098-2114に記載の方法を用いて、化合物Q4(200mg)から実施例1-2の化合物(24.2mg)を製造した。
1H NMR (400 MHz, CDCl3): δ 7.34-7.31 (4H, m), 7.27-7.22 (1H, m), 5.74 (1H, d, J = 6.8 Hz), 5.60 (1H, s), 2.80-2.74 (1H, m), 2.40-2.32 (1H, m), 2.11-2.00 (2H, m), 1.91-1.85 (1H, m), 1.78-1.67 (2H, m), 1.47-1.43 (1H, m), 1.41 (3H, s), 1.37-1.26(4H, m), 1.01 (3H, d, J = 6.0 Hz), 0.53 (3H, d, J = 7.6 Hz).
(3R,5aS,6R,8aS,9R,10S,12S,12aR)-3,6,9-トリメチル-10-(メチルスルファニル)デカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン(実施例1-3)と(3R,5aS,6R,8aS,9R,10R,12S,12aR)-3,6,9-トリメチル-10-(メチルスルファニル)デカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン(実施例1-4)
実施例1-3
1H NMR (400 MHz, CDCl3): δ 5.34 (1H, s), 4.48 (1H, d, J = 5.2 Hz), 2.65-2.60 (1H, m), 2.43-2.35 (1H, m), 2.21 (3H, s), 2.07-2.01 (1H, m), 1.94-1.87 (1H, m), 1.78-1.71 (2H, m), 1.64-1.62 (1H, m), 1.56-1.48 (1H, m), 1.45 (3H, s), 1.42-1.33 (2H, m), 1.31-1.23 (1H, m), 1.07-1.03 (1H, m), 0.99-0.94 (6H, m).
実施例1-4
1H NMR (400 MHz, CDCl3): δ 5.60 (1H, s), 5.19 (1H, d, J = 5.2 Hz), 3.07-3.03 (1H, m), 2.42-2.34 (1H, m), 2.21 (3H, s), 2.08-2.02 (1H, m), 1.92-1.81 (2H, m), 1.74-1.65 (2H, m), 1.54-1.44 (2H, m), 1.40 (3H, s), 1.39-1.35 (1H, m), 1.26-1.25 (1H, m), 0.98-0.92 (7H, m).
(3R,5aS,6R,8aS,9R,10S,12S,12aR)-10-(メタンスルホニル)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン
1H NMR (400 MHz, CDCl3): δ 5.40 (1H, s), 4.35 (1H, d, J = 11.2 Hz), 2.96 (3H, s), 2.87-2.82 (1H, m), 2.42-2.34 (1H, m), 2.06-2.01 (1H, m), 1.95-1.88 (1H, m), 1.84-1.73 (2H, m), 1.50-1.40 (4H, m), 1.37-1.26 (4H, m), 1.14 (3H, d, J = 7.2 Hz), 1.10-1.03 (1H, m), 0.97 (3H, d, J = 6.0 Hz).
4-フェニル-1-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]-1H-1,2,3-トリアゾール
Bioorg.Med.Chem.Lett.2009,19,382-385に記載の方法を用いて、Q1(100mg)から化合物Q5(30mg)を製造した。
1H NMR (400 MHz, CDCl3): δ 5.41 (1H, s), 4.63 (1H, d, J = 10.0 Hz), 2.37-2.47 (2H, m), 2.03-2.09 (1H, m), 1.89-1.95 (1H, m), 1.71-1.81 (2H, m), 1.48-1.57 (1H, m), 1.47 (3H, s), 1.25-1.37 (4H, m), 0.89-1.09 (1H, m), 0.86 (3H, d, J = 4.0 Hz), 0.86 (3H, d, J = 2.4 Hz).
Bioorg. Med.Chem.Lett.2009,19,382-385に記載の方法を用いて、化合物Q5(90mg)から実施例1-9の化合物(20mg)を製造した。
1H NMR (400 MHz, CDCl3): δ 7.98 (1H, s), 7.86-7.84 (2H, m), 7.45-7.41 (2H, m), 7.36-7.32 (1H, m), 5.89 (1H, d, J = 10.4 Hz), 5.49 (1H, s), 3.63-3.60 (1H, m), 2.97-2.92 (1H, m), 2.14-1.88 (5H, m), 1.67-1.60 (4H, m), 1.35-1.15 (4H, m), 0.94 (3H, d, J=6.4 Hz), 0.85 (3H, d, J = 7.2 Hz).
N-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]アセトアミド
Bioorg.Chem.2016,66,63-71,に記載の方法を用いて、化合物Q5(100mg)から化合物Q6(87mg)を製造した。
1H-NMR (400 MHz, CDCl3) δ: 5.33 (1H, s), 4.20 (1H, d, J = 9.7Hz), 1.94-1.84 (2H, m), 1.75 (3H, m), 1.52 (2H, m), 1.46-1.41 (4H, m), 1.31-1.22 (4H, m), 0.98-0.90 (6H, m).
化合物Q6(87mg)、無水酢酸(15.6mg)と4-ジメチルアミノピリジン(44.9mg)のジクロロメタン溶液(5.0mL)を室温で15時間撹拌した。反応終了後、ジクロロメタン-5%塩酸水で分液抽出し、有機層を硫酸ナトリウムで乾燥後、減圧留去した。得られた残査をシリカゲルカラムクロマトグラフィー(溶出溶媒;ジクロロメタン:メタノール)で精製することにより実施例1-10の化合物(43mg)を得た。
1H-NMR (500 MHz, CDCl3) δ:6.01 (1H, d, J = 9.7 Hz), 5.42 (1H, s), 5.34 (1H, t, J = 10.2 Hz), 2.42-2.34 (2H, m), 2.03 (3H, s), 1.92-1.88 (1H, m), 1.80-1.77 (2H, m), 1.72 (1H, d, J = 3.3 Hz), 1.57-1.52 (1H, m), 1.44 (3H, s), 1.29 (1H, d, J = 4.2 Hz), 1.28-1.25 (2H, m), 1.13-1.04 (1H, m), 1.02 (1H, d, J = 12.2 Hz), 0.97 (3H, d, J = 6.3 Hz), 0.87 (3H, d, J = 7.2 Hz).
N-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンスルホンアミド
1H-NMR (400 MHz, CDCl3): δ 5.37 (1H, s), 5.05 (1H, d, J = 10.5 Hz), 4.78 (1H, t, J = 10.4 Hz), 3.18 (3H, s), 2.44-2.33 (2H, m), 2.08-2.01 (1H, m), 1.95-1.88 (1H, m), 1.78-1.73 (2H, m), 1.53-1.49 (2H, m), 1.42 (3H, s), 1.39-1.28 (3H, m), 1.02 (1H, d, J = 14.9 Hz), 0.96 (6H, m).
4-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]モルホリン
1H NMR (400 MHz, CDCl3): δ 5.30 (1H, s), 4.00 (1H, d, J = 10.0 Hz), 3.76-3.66 (4H, m), 3.03-2.98 (2H, m), 2.71-2.66 (2H, m), 2.63-2.57 (1H, m), 2.41-2.33 (1H, m), 2.06-2.00 (1H, m), 1.92-1.85 (1H, m), 1.77-1.69 (2H, m), 1.58-1.48 (2H, m), 1.42 (3H, s), 1.40-1.32 (2H, m), 1.26-1.22 (1H, m), 1.08-1.01 (1H, m), 0.97 (3H, d, J = 6.0 Hz), 0.84 (3H, d, J = 7.6 Hz).
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-N,N,3,6,9-ペンタメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-アミン
1H-NMR (400 MHz, CDCl3): δ 5.31 (1 H, s), 4.05 (1H, d, J = 10.2 Hz), 2.56-2.51 (1H, m), 2.42 (6H, s), 2.40-2.33 (1H, m), 2.06-2.00 (1H, m), 1.91-1.88 (1H, m), 1.76-1.76 (1H, m), 1.57-1.54 (2H, m), 1.42(3H, d, J = 8.8 Hz), 1.42-1.37 (1H, m), 1.27-1.21 (2H, m), 1.09 (1H, m), 1.04-0.98 (1H, m), 0.95 (3H, d, J = 6.3 Hz), 0.82 (3H, d, J = 7.2 Hz).
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル アセテート
1H NMR (400 MHz, CDCl3): δ 5.81 (1H, d, J = 10.0 Hz), 5.47 (1H, s), 2.62-2.56 (1H, m), 2.44-2.36 (1H, m), 2.15 (3H, s), 2.09-2.03 (1H, m), 1.95-1.88 (1H, m), 1.83-1.72 (2H, m), 1.68-1.62 (1H, m), 1.56-1.48 (1H, m), 1.46 (3H, s), 1.44-1.40 (1H, m), 1.34-1.27 (2H, m), 1.09-1.02 (1H, m), 0.99 (3H, d, J = 6.0 Hz), 0.88 (3H, d, J = 6.8 Hz).
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボキサミド
1H NMR (400 MHz, CDCl3): δ 6.58 (1H, s), 5.50-5.34 (2H, m), 4.83 (1H, d, J = 6.4 Hz), 2.94-2.88 (1H, m), 2.41-2.33 (1H, m), 2.09-1.95 (2H, m), 1.87-1.67 (3H, m), 1.43 (3H, s), 1.32-1.22 (4H, m), 1.13 (3H, d, J = 7.6 Hz), 1.00-0.97 (4H, m).
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボキサミド
1H NMR (400 MHz, CDCl3): δ 6.58 (1H, s), 5.35-5.34 (2H, m), 3.93 (1 H, d, J = 11.2 Hz), 2.56-2.50 (1H, m), 2.43-2.37 (1H, m), 2.09-2.04 (1H, m), 1.97-1.90 (1H, m), 1.81-1.74 (2H, m), 1.56-1.48 (2H, m), 1.45 (3H, s), 1.41-1.28 (3H, m), 1.12-1.05 (1H, m), 1.00-0.97 (6H, m).
1-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンアミン
1H NMR (400 MHz, CDCl3): δ 8.31 (3H, s), 5.37-5.32 (1H, m), 3.98-3.96 (1H, m), 3.35-3.34 (1H, m), 3.08-3.09 (1H, m), 240-2.33 (2H, m), 2.10-2.01 (2H, m), 1.74-1.68 (2H, m), 1.56-1.37 (7H, m), 1.30-1.26 (1H, m), 1.04-1.02 (1H, m), 0.97 (3H, m), 0.90-0.85 (3H, m).
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチル-10-[(プロパン-2-イル)オキシ]デカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン
1H NMR (400 MHz, CDCl3): 5.46 (1H, s), 4.90 (1H, d, J = 3.2 Hz), 4.03-3.97 (1H, m), 2.64-2.60 (1H, m), 2.43-2.35 (1H, m), 2.08-2.03 (1H, m), 1.93-1.82 (2H, m), 1.78-1.72 (1H, m), 1.68-1.62 (1H, m), 1.55-1.51 (1H, m), 1.46 (3H, s), 1.39-1.32 (1H, m), 1.30-1.26 (1H, m), 1.23-1.16 (4H, m), 1.10 (3H, d, J = 6.4 Hz), 0.98-0.93 (4H, m), 0.90 (3H, d, J = 7.2 Hz).
対応する原料化合物を用いて実施例1-19と同様に反応・処理し、表1に示す化合物を得た。
1H NMR (400 MHz, CDCl3): 5.47 (1H, s), 4.94 (1H, d, J = 4.0 Hz), 3.75-3.71 (1H, m), 2.65-2.60 (1H, m), 2.43-2.35 (1H, m), 2.08-2.02(1H, m), 1.96-1.86 (2H, m), 1.79-1.73 (2H, m), 1.72-1.62 (3H, m), 1.57-1.48 (2H, m), 1.46 (3H, s), 1.40-1.26 (8H, m), 0.97 (3H, d, J = 6.4 Hz), 0.95-0.85 (5H, m).
実施例1-23
1H NMR (400 MHz, CDCl3): δ 5.46 (1H, s), 4.85 (1H, d, J = 3.6 Hz), 3.99-3.94 (1H, m), 3.64-3.52 (3H, m), 3.39 (1H, s), 2.67-2.62 (1H, m), 2.43-2.35 (1H, m), 2.08-2.03 (1H, m), 1.94-1.74 (3H, m), 1.67-1.62 (1H, m), 1.51-1.46 (4H, m), 1.40-1.21 (3H, m), 0.98-0.87 (7H, m).
実施例1-24
1H NMR (400 MHz, CDCl3): δ 7.39-7.30 (5H, m), 5.49 (1H, s), 4.95-4.92 (2H, m), 4.53 (1H, d, J = 12.0 Hz), 2.72-2.68 (1H, m), 2.45-2.37 (1H, m), 2.10-2.04 (1H, m), 1.94-1.79 (3H, m), 1.67-1.62 (1H, m), 1.56-1.51 (1H, m), 1.49 (3H, s), 1.38-1.24(3H, m), 0.98-0.96 (6H, m), 0.92-0.88 (1H, m).
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチル-10-(2,2,2-トリフルオロエトキシ)デカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン
1H NMR (400 MHz, CDCl3): δ 5.43 (1H, s), 4.90 (1H, d, J = 3.2 Hz), 4.20-4.10 (1H, m), 3.95-3.85 (1H, m), 2.74-2.66 (1H, m), 2.44-2.36 (1H, m), 2.10-2.04 (1H, m), 1.95-1.88 (1H, m), 1.84-1.76 (1H, m), 1.74-1.66 (2H, m), 1.57-1.49 (2H, m), 1.46 (3H, s), 1.40-1.24 (2H, m), 0.99-0.97 (6H, m), 0.95-0.89 (1H, m).
対応する原料化合物を用いて実施例1-19と同様に反応・処理し、表2に示す化合物を得た。
1H NMR (400 MHz, CDCl3): δ 7.33-7.29 (2H, m), 7.15-7.13 (2H, m), 7.03-7.00 (1H, m), 5.54-5.52 (2H, m), 2.84-2.81 (1H, m), 2.44-2.38 (1H, m), 2.08-1.88 (4H, m), 1.75-1.70 (1H, m), 1.61-1.59 (1H, m), 1.55-1.46 (4H, m), 1.41-1.36 (1H, m), 1.34-1.30 (1H, m), 1.04 (3H, d, J = 6.0 Hz), 1.01-0.96 (4H, m).
実施例1-25
1H NMR (400 MHz, CDCl3): δ 7.33-7.28 (2H, m), 7.14-7.12 (2H, m), 7.04-7.00 (1H, m), 5.51 (1H, s), 5.07 (1H, d, J = 10.0 Hz), 2.78-2.73 (1H, m), 2.47-2.39 (1H, m), 2.09-2.04 (1H, m), 1.91-1.97 (1H, m), 1.86-1.80 (1H, m), 1.76-1.71 (1H, m), 1.69-1.65 (1H, m), 1.56-1.49 (1H, m), 1.46 (3H, s), 1.42-1.28 (3H, m), 1.11-1.04 (1H, m), 1.02-1.00 (6H, m).
2-{[(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]オキシ}エチル アセテート(実施例1-26)と
2-{[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]オキシ}エチル アセテート(実施例1-27)
2-{[(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]オキシ}エチル アセテート
化合物Q7
1H NMR (400 MHz, CDCl3) δ: 5.46 (1H, s), 4.86 (1H, d, J = 3.6 Hz), 3.93-3.88 (1H, m), 3.78-3.75 (2H, m), 3.68-3.63 (1H, m), 2.73-2.66 (1H, m), 2.39 (1H, m), 2.08-2.03 (1H, m), 1.94-1.87 (1H, m), 1.81-1.78 (2H, m), 1.75-1.71 (1H, m), 1.69-1.63 (1H, m), 1.54-1.47 (2H, m), 1.45 (3H, s), 1.39-1.30 (2H, m), 1.00-0.93 (7H, m).
化合物Q8
1H NMR (400 MHz, CDCl3) δ: 5.39 (1H, s), 4.49 (1H, d, J = 9.2 Hz), 3.89-3.87 (2H, m), 3.77-3.71 (2H, m), 2.51-2.36 (2H, m), 2.08-2.02 (1H, m), 1.95-1.88 (1H, m), 1.83-1.77 (1H, m), 1.75-1.70 (1H, m), 1.59-1.64 (1H, m), 1.53-1.49 (1H, m), 1.44 (3H, s), 1.36-1.24 (4H, m), 1.07-1.01 (1H, m), 0.98 (3H, d, J = 5.6 Hz), 0.94 (3H, d, J = 6.8 Hz).
上記の実験で得られた化合物Q7(54mg)のジクロロメタン溶液(3mL)に、無水酢酸(83.7mg)とトリエチルアミン(82.9mg)を加え、室温にて、終夜攪拌した。反応終了後、ジクロロメタン、水を加え、有機層の溶媒を減圧留去し、得られた残査をシリカゲルカラムクロマトグラフィー(溶出溶媒;ペンタン:酢酸エチル)で精製することにより実施例1-26(29.1mg)を得た。
1H NMR (400 MHz, CDCl3) δ: 5.45 (1H, s), 4.84 (1H, d, J = 3.2 Hz), 4.30-4.20 (2H, m), 4.05-4.00 (1H, m), 3.69-3.64 (1H, m), 2.69-2.62 (1H, m), 2.39 (1H, m), 2.10-2.03 (4H, m), 1.94-1.88 (1H, m), 1.86-1.75 (2H, m), 1.67-1.64 (1H, m), 1.51-1.49 (1H, m), 1.46 (3H, s), 1.34-1.24 (3H, m), 0.98-0.88 (7H, m).
2-{[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]オキシ}エチル アセテート
対応する原料化合物Q8を用いて実施例1-26と同様に反応・処理し、実施例1-27の化合物を得た。
1H NMR (400 MHz, CDCl3) δ: 5.36 (1H, s), 4.50 (1H, d, J = 9.2 Hz), 4.26 (2H, t, J = 5.0 Hz), 4.14-4.09 (1H, m), 3.76-3.70 (1H, m), 2.49-2.36 (2H, m), 2.09 (3H, s), 2.07-2.02 (1H, m), 1.94-1.87 (1H, m), 1.82-1.76 (1H, m), 1.73-1.69 (1H, m), 1.60-1.55 (1H, m), 1.52-1.51 (1H, m), 1.49 (3H, s), 1.34-1.27 (3H, m), 1.07-0.97 (4H, m), 0.90 (3H, d, J = 7.2 Hz).
(3R,3aS,6R,6aS,9S,10aS,10bR)-3,6,9-トリメチルオクタヒドロ-10aH-9,10b-エポキシピラノ[4,3,2-jk][2]ベンゾキセピン-2(3H)-オン
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-10-メトキシ-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン
マウス内在性HoxB5遺伝子のC末端に、3コピーのmCherry蛍光タンパク質をコードする遺伝子を含む核酸配列(Hoxb5-tri-mCherry)を挿入したマウス(triple-mCherry Hoxb5 knock-in mouse;非特許文献1)を使用した。マウスの飼育は、室温20~26℃、湿度40~70%、明暗各12時間(照明:午前8時~午後8時)の環境条件で飼育した。
骨髄細胞は、上記マウスの両側の脛骨、大腿骨、上腕骨及び骨盤から採集し、乳鉢に集めた。Ca2+フリー及びMg2+フリーのPBSに、終濃度2%熱処理不活化牛血清(Gibco社製)及び終濃度2mM EDTAを添加した緩衝液を添加し、乳棒でこれらを破壊することによって、造血幹細胞を含む細胞集団を得た。
まず上記で得られた造血幹細胞を含む細胞集団を、100μm、70μm、40μmのろ過機(メッシュ)に通した。造血幹細胞及び前駆体細胞集団を富化させるため、細胞集団をAPC結合抗c-Kit(2B8)により染色し、抗APC磁力ビーズ及びLSカラム(両方ともMiltenyi Biotec社製)を用いて分画した。
細胞表面マーカー:Sca-1、Flk2、CD150、CD34、Ter-119、B220、CD3、CD4、CD8a、Gr-1、CD11b、IL-7R及びCD16/32
被検化合物は、10mMのDMSO溶液を、細胞培養用培地で終濃度1μMとなるよう希釈して、上記仕分け後播種した細胞に、培地交換によって終容量200μL/ウェルとなるように添加した。その後、細胞はCO2インキュベーターで、37℃、5%CO2の条件で1週間培養した。
5つのヒット化合物のDMSO溶液を、細胞培養用培地で終濃度5点(0.1μM、0.3μM、1μM、3μM、10μM)に調整し、n=2で実施例1と同様の方法にて細胞の培養を行った。生細胞数、KLS陽性率、mCherry陽性率を解析し、その結果を表3に示す。細胞の培養と評価指標は、実施例2と同じである。
Artemetherの存在下で培養した細胞は、マウスHoxB5の遺伝子発現によって評価した。実施例3と同様に細胞を培養し、培養後の細胞を、Single Cell to CT qRT-PCRキット(Life Technologies社製)によって、同キットの指示通り処理した。処理後の細胞をPBSで1回洗浄した後に、細胞溶解液を添加し、RNA抽出後にcDNA合成のための逆転写反応に付し、14サイクルのTaqman Gene Expression Assayによって増幅し、1×TE緩衝液(pH8.0)にて希釈した。リアルタイムPCRのためには、以下のTaqmanプローブを用いて、95℃、3秒及び60℃、30秒で40サイクル行い、サンプルを増幅した(HoxbB5:Mm00657672(ThermoFisherScientific社製)、Gapdh:Mm99999915-g1(ThermoFisherScientific社製))。すべてのサーマルサイクラーは、QuantStadio6(Applied Biosystem社製)を使用して、解析した(表5)。
アルテメテル(Artemether)のDMSO溶液を、細胞培養用培地で終濃度3点(0.1μM、1μM、10μM)、さらにUM171のDMSO溶液を、細胞培養用培地で終濃度4点(0.1μM、0.3μM、1μM、3μM)に調整し、n=2で実施例1と同様の方法にて細胞の培養を行った。生細胞数、KLS陽性率、mCherry陽性率を解析し、その結果を表6に示す。細胞の培養と評価指標は、実施例2と同じである。
<ヒト臍帯血由来単核細胞の採集>
ヒト臍帯血由来単核細胞は、EasySep RBC Depletion Reagent(STEMCELL Technologies社製)を用い赤血球除去、濃縮処理を行い、造血幹細胞を含む細胞集団を得た。
まず上記で得られた造血幹細胞を含む細胞集団を、EASYSep Human Cord Blood CD34 Positive Selection Kit II(STEMCELL Technologies社製)を用い、CD34陽性細胞分画を粗精製した。造血幹細胞及び前駆体細胞集団を富化したCD34陽性濃縮細胞に対し以下の細胞表面マーカーに対する抗体を用いて染色した。
細胞表面マーカー:CD34、CD11b、CD14、CD19、CD20、CD235ab、CD3、CD4、CD56、CD8a。
アルテメテル(Artemether)存在下(UM171+/-)で培養した場合の生細胞数及びCD34陽性細胞数を、図1及び図2に示す。また、各種化合物存在下で培養した場合の生細胞数及びCD34陽性細胞数を表7~11に示す。
アルテメテル(Artemether)、または実施例1-23、実施例1-16、及び実施例1-7化合物を細胞培養用培地で終濃度9点(0.001μM、0.003μM、0.01μM、0.03μM、0.1μM、0.3μM、1μM、3μM、10μM)に調製し、上記仕分けして播種したヒト細胞に、終容量200μL/ウェルとなるように添加し、作用させた。一部、UM171 35nMを添加し、相乗効果の有無を検討した。その後、細胞はCO2インキュベーターで、37℃、5%CO2の条件で1週間培養し回収した後、96ウェルプレートの1ウェルにCD34陽性細胞を1細胞ずつFACS AriaIIIセルソーターで播種し、Methocult H4435(StemCell technologies社)で2週間培養した。培養後、各ウェルを光学顕微鏡(KEYENCE社製)にて撮像し、コロニー数を目視にてカウントし、コロニー形態を目視にて判別した。コロニーの形態から、骨髄系前駆細胞由来のColonyFormationUnit-Granulocyte、Erythroid、Macrophage、Megakaryocyte(CFU-GEMM)及び、赤芽球バーストコロニー形成細胞BurstFormingUnit-Erythroid(BFU-E)、顆粒球・マクロファージコロニー形成細胞ColonyFormationgUnit-Granulocyte、Macrophage(CFU-GM)を判別しカウントした。
アルテメテル(Artemether)、または実施例1-23の化合物を、細胞培養用培地でそれぞれ終濃度0.003μM又は0.01μMに調製し、上記仕分けして播種したヒト細胞(1ウェルあたり166細胞播種)に、終容量200μL/ウェルとなるように添加し、作用させた。その後、それぞれの条件について、トータル10000個のCD34陽性画分の細胞をCO2インキュベーターで、37℃、5%CO2の条件で1週間培養し、細胞数をカウントした。培養後の細胞のうち、培養前と同数の10000個の細胞を、致死量の放射線照射した免疫無防備状態のNOGマウスに移植した。比較対象として、10000個の新鮮臍帯血由来CD34陽性細胞(無培養)を移植した。移植後の示された時点での末梢血ドナーキメリズムを、ヒト特異的CD45抗体とマウス特異的CD45抗体を用いてヒトCD45陽性細胞数とマウスCD45陽性細胞数を測定し、ヒトCD45陽性細胞とマウスCD45陽性細胞の合計値に対するヒトCD45陽性細胞の割合(キメラ率)で示した。
Claims (21)
- 造血幹細胞を含む細胞集団を、式(1)で表される化合物、又はその塩を1以上含む培地中で培養することを含む、造血幹細胞の培養方法。
XはO-O又はOであり、
Yは、水素原子、ヒドロキシ、オキソ、メルカプト、カルボキシ、カルバモイル、シアノ、OR1、SR1、SOR1、SO2R1、COR1、OCOR1、R2、COOR1、NR3R4、NR5COR1、NR5SO2R1、NR5CONR3R4又はハロゲンであり、
Zは、縮合環の任意の位置の炭素に結合しており、各々独立してC1-6アルキルであり、
nは、0~10の整数であり、
R1は、置換されていてもよいアルキル、置換されていてもよいシクロアルキル、置換されていてもよいアリール、置換されていてもよいヘテロアリール、又は置換されていてもよい脂肪族複素環基であり、
R2は、置換されていてもよいアルキル、置換されていてもよいアルケニル、置換されていてもよいアルキニル、置換されていてもよいシクロアルキル、置換されていてもよいアリール、置換されていてもよいヘテロアリール、又は置換されていてもよい脂肪族複素環基であり、
R3、R4及びR5は、独立して、水素原子、置換されていてもよいアルキル、置換されていてもよいシクロアルキル、置換されていてもよい脂肪族複素環基、置換されていてもよいアリール、又は置換されていてもよいヘテロアリールであり、ここにおいてR3とR4は、隣接する窒素原子と一緒になって、更に1又は2の環を構成する酸素原子、窒素原子又は硫黄原子を含んでよい4~10員の含窒素脂肪族複素環を形成してよく、当該含窒素脂肪族複素環は置換されていてもよい] - 式(3-1)、(3-2)又は(3-3)において、Z1、Z2、Z3、Z4、Z5、及びZ6が、独立して水素原子又はメチルである、請求項3に記載の培養方法。
- Yは水素原子、ヒドロキシ、オキソ、カルボキシ、カルバモイル、OR1、SR1、SO2R1、COR1、OCOR1、R2、NR3R4、NR5COR1、NR5SO2R1、又はフッ素原子である、請求項1~5のいずれか一項に記載の培養方法。
-
Yは、ヒドロキシ、オキソ、カルボキシ、カルバモイル、OR1、SR1、SO2R1、OCOR1、R2、NR3R4、NR5COR1、又はフッ素原子であり、
R1が、群1から選択される1~7の置換基で置換されていてもよいC1-8アルキル、群1から選択される1~3の基で置換されていてもよいC3-8シクロアルキル、群1から選択される1~5の基で置換されていてもよいフェニル、群1から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~10員の脂肪族複素環基、又は群1から選択される1~4の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~10員のヘテロアリールであり、
R2が、群2から選択される1~7の置換基で置換されていてもよいC1-8アルキル、群2から選択される1~7の置換基で置換されていてもよいC1-8アルケニル、群1から選択される1~3の基で置換されていてもよいC3-8シクロアルキル、群1から選択される1~5の基で置換されていてもよいフェニル、群1から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~10員の脂肪族複素環基、又は群1から選択される1~4の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~10員のヘテロアリールであり、
R3及びR4は独立して、水素原子、群1から選択される1~7の置換基で置換されていてもよいC1-6アルキル、又は群1から選択される1~5の基で置換されていてもよいフェニルであり、ここにおいてR3とR4は、隣接する窒素原子と一緒になって、更に1又は2の環を構成する酸素原子、窒素原子又は硫黄原子を含んでよい3~8員の含窒素脂肪族複素環を形成してよく、当該含窒素脂肪族複素環は以下の群1の置換基から選択される1~3の置換基で置換されていてもよく、
R5が、水素原子又はC1-3アルキルである、請求項1~6のいずれか一項に記載の培養方法。
<群1>
カルボキシ、ヒドロキシ、C1-8アルコキシ、C1-8アルキルカルボニル、C1-8アルキルカルボニルオキシ、C1-8アルコキシカルボニル、メルカプト、C1-8アルキルスルファニル、C1-8アルキルスルホニル、1若しくは2のC1-8アルキルで置換されていてもよいカルバモイル、1若しくは2のC1-8アルキルで置換されていてもよいアミノ、C1-8アルキルカルボニルアミノ、C1-8アルキルスルホニルアミノ、群3から選択される1~5の基で置換されていてもよいフェニル、群3から選択される1~4の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~6員のヘテロアリール、群3から選択される1~3の基で置換されていてもよいC3-8シクロアルキル、群3から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~10員の脂肪族複素環基、ハロゲン
<群2>
カルボキシ、ヒドロキシ、C1-8アルコキシ、C1-8アルキルカルボニル、C1-8アルキルカルボニルオキシ、C1-8アルコキシカルボニル、メルカプト、C1-8アルキルスルファニル、C1-8アルキルスルホニル、1若しくは2のC1-8アルキルで置換されていてもよいカルバモイル、アミノ、群3から選択される1~5の基で置換されていてもよいフェニル、群3から選択される1~4の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~6員のヘテロアリール、群3から選択される1~3の基で置換されていてもよいC3-8シクロアルキル、群3から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~4個の環内のヘテロ原子を含む4~8員の脂肪族複素環基、ハロゲン
<群3>
カルボキシ、ヒドロキシ、C1-8のアルキル、C1-8アルコキシ、C1-8アルキルカルボニル、C1-8アルキルカルボニルオキシ、C1-8アルコキシカルボニル、メルカプト、C1-8アルキルスルファニル、C1-8アルキルスルホニル、1若しくは2のC1-8アルキルで置換されていてもよいアミノ、1若しくは2のC1-8アルキルで置換されていてもよいカルバモイル、C1-8アルキルカルボニルアミノ、C1-8アルキルスルホニルアミノ、ハロゲン -
Yは、ヒドロキシ、オキソ、カルボキシ、カルバモイル、OR1、SR1、SO2R1、OCOR1、R2、NR3R4、NR5COR1、NR5SO2R1、又はフッ素原子であり、
R1は、群1’から選択される1~3の基で置換されていてもよいC1-4アルキル、群1’から選択される1~3の基で置換されていてもよいC3-6シクロアルキル、群1’から選択される1~3の基で置換されていてもよいフェニル、群1’から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む4~6員の脂肪族複素環基、又は群1’から選択される1~3の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~6員のヘテロアリールであり、
R2は、群2’から選択される1~3の基で置換されていてもよいC1-4アルキル、群1’から選択される1~3の基で置換されていてもよいC3-6シクロアルキル、群1’から選択される1~3の基で置換されていてもよいフェニル、群1’から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む4~6員の脂肪族複素環基、又は群3’から選択される1~3の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~6員のヘテロアリールであり、
R3は、水素原子又はC1-3アルキルであり
R4は、水素原子、群1’から選択される1~3の基で置換されていてもよいC1-4アルキル、群1’から選択される1~3の基で置換されていてもよいC3-6シクロアルキル、群1’から選択される1~3の基で置換されていてもよいフェニル、群1’から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む4~6員の脂肪族複素環基、又は群1’から選択される1~3の基で置換されていてもよく酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む5~6員のヘテロアリールであり、
ここにおいてR3とR4は、隣接する窒素原子と一緒になって、更に1又は2の環を構成する酸素原子、窒素原子又は硫黄原子を含んでよい3~8員の含窒素脂肪族複素環を形成してよく、当該含窒素脂肪族複素環は以下の群1’の置換基から選択される1~3の基で置換されていてもよく、
R5が、水素原子である、請求項1~7のいずれか一項に記載の培養方法。
<群1’>
フッ素原子、ヒドロキシ、カルボキシ、アミノ、C1-4アルコキシ、C1-4アルキルカルボニルオキシ、群3’から選択される1~3の基で置換されていてもよいフェニル、群3’から選択される1~3の基で置換されていてもよいC3-6のシクロアルキル、群3’から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む4~6員の脂肪族複素環基
<群2’>
フッ素原子、ヒドロキシ、カルボキシ、アミノ、C1-4アルコキシ、C1-4アルキルカルボニルオキシ、C1-4アルコキシカルボニル、1若しくは2のC1-8アルキルで置換されていてもよいカルバモイル、群3’から選択される1~3の基で置換されていてもよいフェニル、群3’から選択される1~3の基で置換されていてもよいC3-6シクロアルキル、群3’から選択される1~3の基で置換されていてもよい酸素原子、窒素原子及び硫黄原子から選択される1~3個の環内のヘテロ原子を含む4~6員の脂肪族複素環基
<群3’>
フッ素原子、ヒドロキシ、C1-4のアルキル - XがO-Oである、請求項1~8のいずれか一項に記載の培養方法。
- 式(1)の化合物が、以下の化合物からなる群から選択される化合物である、、請求項1に記載の培養方法。
アルテメテル(Artemether)、
アルテミシニン(Artemisinin)、
アルテニモール(Artenimol)、
アルテモチル(Artemotil)、
アルテスネイト(Artesunate)、
(3R,5aS,6R,8aS,9R,10R,12S,12aR)-10-フルオロ-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチル-10-フェニルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12S,12aR)-3,6,9-トリメチル-10-(メチルスルファニル)デカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10R,12S,12aR)-3,6,9-トリメチル-10-(メチルスルファニル)デカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12S,12aR)-10-(メタンスルホニル)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9,10-テトラメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-10-(フラン-2-イル)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボン酸、
4-フェニル-1-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]-1H-1,2,3-トリアゾール、
N-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]アセトアミド、
N-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンスルホンアミド、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-N,N,3,6,9-ペンタメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-アミン、
4-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]モルホリン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル アセテート、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボキサミド、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-カルボキサミド、
1-[(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンアミン、
1-[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]メタンアミン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチル-10-[(プロパン-2-イル)オキシ]デカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-10-(シクロヘキシルオキシ)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチル-10-フェノキシデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチル-10-(2,2,2-トリフルオロエトキシ)デカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-10-(2-メトキシエトキシ)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10S,12R,12aR)-10-(ベンジルオキシ)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチル-10-フェノキシデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
2-{[(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]オキシ}エチル アセテート、
2-{[(3R,5aS,6R,8aS,9R,10R,12R,12aR)-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン-10-イル]オキシ}エチル アセテート、
(3R,5aS,6R,8aS,12R,12aR)-3,6,9-トリメチル-3,4,5,5a,6,7,8,8a-オクタヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン、
(3R,3aS,6R,6aS,9S,10aS,10bR)-3,6,9-トリメチルオクタヒドロ-10aH-9,10b-エポキシピラノ[4,3,2-jk][2]ベンゾキセピン-2(3H)-オン、
(3R,5aS,6R,8aS,9R,10R,12R,12aR)-10-メトキシ-3,6,9-トリメチルデカヒドロ-12H-3,12-エポキシピラノ[4,3-j][1,2]ベンゾジオキセピン - 式(1)の化合物が、アルテメテル(Artemether)、アルテミシニン(Artemisinin)、アルテニモール(Artenimol)、アルテモチル(Artemotil)、及び、アルテスネイト(Artesunate)からなる群から選択される、請求項1に記載の培養方法。
- 前記造血幹細胞が、CD34陽性細胞である、請求項1~11のいずれか一項に記載の培養方法。
- 前記造血幹細胞が、長期造血幹細胞である、請求項1~12のいずれか一項に記載の培養方法。
- 前記造血幹細胞が、Hoxb5陽性のマウス造血幹細胞である、請求項1~12のいずれか一項に記載の培養方法。
- 培養後の細胞集団における総細胞数に対する造血幹細胞の割合が、培養前の細胞集団における総細胞数に対する造血幹細胞の割合の10%以上である、請求項1~14のいずれか一項に記載の培養方法。
- 培養後の細胞集団における造血幹細胞の数が、培養前の細胞集団における造血幹細胞の数の3倍以上である、請求項1~15のいずれか一項に記載の培養方法。
- 前記培地が、さらにUM171又はその誘導体を含む培地である、請求項1~16のいずれか一項に記載の培養方法。
- 造血幹細胞を含む細胞集団を準備する工程と、
造血幹細胞を含む細胞集団を培養する工程であって、請求項1~17のいずれか一項に記載の培養方法によって前記造血幹細胞を含む細胞集団を培養する工程と
を含む、造血幹細胞の製造方法。 - 請求項1~17のいずれか一項に記載の培養方法によって培養して得られた、造血幹細胞。
- 請求項18に記載の製造方法によって製造された造血幹細胞。
- 式(1)で表される化合物、又はその塩を少なくとも1つ含む、造血幹細胞培養用試薬。
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020227023892A KR20220122667A (ko) | 2019-12-26 | 2020-12-25 | 조혈 줄기세포의 배양 방법 |
| EP20906474.0A EP4071239A1 (en) | 2019-12-26 | 2020-12-25 | Method for culturing hematopoietic stem cells |
| JP2021567704A JPWO2021132627A1 (ja) | 2019-12-26 | 2020-12-25 | |
| US17/788,423 US20230094952A1 (en) | 2019-12-26 | 2020-12-25 | Method for Culturing Hematopoietic Stem Cells |
| AU2020411454A AU2020411454A1 (en) | 2019-12-26 | 2020-12-25 | Method for culturing hematopoietic stem cells |
| CA3165443A CA3165443A1 (en) | 2019-12-26 | 2020-12-25 | Method for culturing hematopoietic stem cells |
| CN202080090191.9A CN114901810A (zh) | 2019-12-26 | 2020-12-25 | 造血干细胞的培养方法 |
| IL294152A IL294152A (en) | 2019-12-26 | 2022-06-21 | A method for culturing hematopoietic stem cells |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019237030 | 2019-12-26 | ||
| JP2019-237030 | 2019-12-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2021132627A1 true WO2021132627A1 (ja) | 2021-07-01 |
Family
ID=76573084
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2020/048917 WO2021132627A1 (ja) | 2019-12-26 | 2020-12-25 | 造血幹細胞の培養方法 |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US20230094952A1 (ja) |
| EP (1) | EP4071239A1 (ja) |
| JP (1) | JPWO2021132627A1 (ja) |
| KR (1) | KR20220122667A (ja) |
| CN (1) | CN114901810A (ja) |
| AU (1) | AU2020411454A1 (ja) |
| CA (1) | CA3165443A1 (ja) |
| IL (1) | IL294152A (ja) |
| TW (1) | TW202137978A (ja) |
| WO (1) | WO2021132627A1 (ja) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111235098A (zh) * | 2020-01-17 | 2020-06-05 | 康妍葆(北京)干细胞科技有限公司 | 青蒿素在制备培养干细胞的产品中的应用、培养基及培养方法 |
| WO2022230793A1 (ja) * | 2021-04-26 | 2022-11-03 | 住友ファーマ株式会社 | オキセピン誘導体 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH04506669A (ja) * | 1990-03-23 | 1992-11-19 | エス・アール・アイ・インターナシヨナル | アルテミシニンの抗マラリア性同族体 |
| WO2013110198A1 (en) | 2012-01-27 | 2013-08-01 | Université de Montréal | Pyrimido[4,5-b]indole derivatives and use thereof in the expansion of hematopoietic stem cells |
| US20170350879A1 (en) | 2016-06-07 | 2017-12-07 | The Board Of Trustees Of The Leland Stanford Junior University | Long term hematopoietic stem cell specific reporter mouse and uses thereof |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003048167A1 (en) * | 2001-12-06 | 2003-06-12 | Ufc Limited | Trioxane derivatives |
-
2020
- 2020-12-25 WO PCT/JP2020/048917 patent/WO2021132627A1/ja unknown
- 2020-12-25 CN CN202080090191.9A patent/CN114901810A/zh active Pending
- 2020-12-25 EP EP20906474.0A patent/EP4071239A1/en not_active Withdrawn
- 2020-12-25 JP JP2021567704A patent/JPWO2021132627A1/ja active Pending
- 2020-12-25 KR KR1020227023892A patent/KR20220122667A/ko unknown
- 2020-12-25 TW TW109146309A patent/TW202137978A/zh unknown
- 2020-12-25 US US17/788,423 patent/US20230094952A1/en active Pending
- 2020-12-25 AU AU2020411454A patent/AU2020411454A1/en active Pending
- 2020-12-25 CA CA3165443A patent/CA3165443A1/en active Pending
-
2022
- 2022-06-21 IL IL294152A patent/IL294152A/en unknown
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH04506669A (ja) * | 1990-03-23 | 1992-11-19 | エス・アール・アイ・インターナシヨナル | アルテミシニンの抗マラリア性同族体 |
| WO2013110198A1 (en) | 2012-01-27 | 2013-08-01 | Université de Montréal | Pyrimido[4,5-b]indole derivatives and use thereof in the expansion of hematopoietic stem cells |
| JP2015504902A (ja) * | 2012-01-27 | 2015-02-16 | ユニヴェルスィテ・ドゥ・モントリオール | ピリミド[4,5−b]インドール誘導体及び造血幹細胞の増殖におけるその使用 |
| US20170350879A1 (en) | 2016-06-07 | 2017-12-07 | The Board Of Trustees Of The Leland Stanford Junior University | Long term hematopoietic stem cell specific reporter mouse and uses thereof |
Non-Patent Citations (34)
| Title |
|---|
| "Angew. Chem. Int. Ed.", vol. 45, 2006, pages: 2082 - 2088 |
| "GenBank", Database accession no. NC _ 000077.6 |
| "NOG mouse", BLOOD, vol. 100, no. 9, 2012, pages 1113 - 1124 |
| ACS CATALYSIS, vol. 7, no. 3, 2017, pages 1998 - 2001 |
| ANGEWANDTE CHEMIE INTERNATIONAL EDITION, vol. 57, 2018, pages 8293 - 8296 |
| AVERY, MITCHELL A. ET AL.: "Structure-Activity Relationships of the Antimalarial Agent Artemisinin. 1. Synthesis and Comparative Molecular Field Analysis of C-9 Analogs of Artemisinin and 10-Deoxoartemisinin", J. MED. CHEM., vol. 36, 1993, pages 4264 - 4275, XP002126172 * |
| BIOORG. CHEM., vol. 66, 2016, pages 63 - 71 |
| BIOORG. MED. CHEM. LETT., vol. 19, 2009, pages 382 - 385 |
| BIOORGANIC & MEDICINAL CHEMISTRY LETTERS, vol. 20, no. 14, 2010, pages 4112 - 4115 |
| CHEM COMMUN, vol. 50, 2014, pages 12652 - 12655 |
| CHEM MED CHE, vol. 11, 2016, pages 1469 - 1479 |
| CHEM MED CHEM, vol. 11, 2016, pages 1469 - 1479 |
| CHEMMEDCHEM, vol. 7, no. 12, 2012, pages 2204 - 2226 |
| EUR. J. ORG. CHEM., 2002, pages 113 - 132 |
| EUR. J. ORG. CHEM., 2003, pages 2098 - 2114 |
| FARES I ET AL., SCIENCE, vol. 345, 2014, pages 1509 - 1512 |
| FARES, IMAN ET AL.: "Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal", SCIENCE, vol. 345, 2014, pages 1509 - 1512, XP055370388 * |
| J. MED. CHEM., vol. 31, 1988, pages 645 - 650 |
| J. ORG. CHEM., vol. 67, no. 4, 2002, pages 1253 - 1260 |
| J. ORG. CHEM., vol. 69, 2004, pages 984 - 986 |
| JAMES Y. CHEN ET AL.: "Hoxb5 marks long-term haematopoietic stem cells and reveals ahomogenous perivascular niche", NATURE, vol. 530, 2016, pages 223 - 227, XP055509455 |
| JIGANG WANG ET AL., NATURE COMMUNICATIONS, vol. 6, 2015, pages 10111 |
| LETTERS, vol. 9, no. 21, 2007, pages 4107 - 4110 |
| NATURE, vol. 25, no. 7655, 2017, pages 432 - 438 |
| NOTTA F ET AL., SCIENCE, vol. 333, no. 6039, 8 July 2011 (2011-07-08), pages 218 - 21 |
| ORGANIC PROCESS RESEARCH & DEVELOPMENT, vol. 11, no. 3, 2007, pages 336 - 340 |
| SUGIMURA R. ET AL., NATURE, vol. 545, no. 7655, 25 May 2017 (2017-05-25), pages 432 - 438 |
| TAYA Y. ET AL., SCIENCE, vol. 354, no. 6316, 2 December 2016 (2016-12-02), pages 1152 - 1155 |
| TETRAHEDRON LETT., vol. 39, 1998, pages 1533 - 1536 |
| TETRAHEDRON LETTERS, vol. 43, 2002, pages 7235 - 7237 |
| TETRAHEDRON, vol. 72, no. 32, 2016, pages 4931 - 4937 |
| THEODORA W. GREENEPETER G. M. WUTS: "Protective Groups in Organic Synthesis", vol. 1, 1999, MARUZEN PUBLISHING CO., LTD. |
| UEDA T. ET AL., J. CLIN. INVEST., vol. 105, 2000, pages 1013 - 1021 |
| YIN KWAN WONG ET AL., MED RES REV, vol. 37, 2017, pages 1492 - 1517 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111235098A (zh) * | 2020-01-17 | 2020-06-05 | 康妍葆(北京)干细胞科技有限公司 | 青蒿素在制备培养干细胞的产品中的应用、培养基及培养方法 |
| WO2022230793A1 (ja) * | 2021-04-26 | 2022-11-03 | 住友ファーマ株式会社 | オキセピン誘導体 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20230094952A1 (en) | 2023-03-30 |
| CA3165443A1 (en) | 2021-07-01 |
| JPWO2021132627A1 (ja) | 2021-07-01 |
| IL294152A (en) | 2022-08-01 |
| TW202137978A (zh) | 2021-10-16 |
| CN114901810A (zh) | 2022-08-12 |
| AU2020411454A1 (en) | 2022-07-14 |
| EP4071239A1 (en) | 2022-10-12 |
| KR20220122667A (ko) | 2022-09-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN110785419B (zh) | 芳烃受体拮抗剂及其用途 | |
| KR102098122B1 (ko) | 피리미도[4,5-b]인돌 유도체 및 조혈 줄기 세포의 증식에서의 이의 용도 | |
| WO2021132627A1 (ja) | 造血幹細胞の培養方法 | |
| EP2836214B1 (en) | Bruton's tyrosine kinase inhibitors for hematopoietic mobilization | |
| US20240158747A1 (en) | Composition and methods for inducing myeloid suppressive cells and use thereof | |
| JP7168552B2 (ja) | 造血幹細胞・前駆細胞の作製、増殖および分化のための置換アゾール誘導体 | |
| US9518250B2 (en) | Method for the ex vivo expansion of hematopoietic stem and progenitor cells | |
| WO2012102937A2 (en) | Compounds that expand hematopoietic stem cells | |
| JP2022517342A (ja) | 幹細胞機能を向上させるための薬剤及び方法 | |
| CN101063110B (zh) | 成体干细胞的培养基和培养方法 | |
| WO2022230793A1 (ja) | オキセピン誘導体 | |
| TWI764896B (zh) | 有效率的nkt細胞活化技術 | |
| KR101169835B1 (ko) | Cd34 양성 조혈모세포를 거핵구 및 혈소판으로분화유도하는 방법 | |
| Rollini et al. | Phenotypic and functional analysis of human fetal liver hematopoietic stem cells in culture | |
| Kim et al. | Generation of CD34+ cells from human embryonic stem cells using a clinically applicable methodology and engraftment in the fetal sheep model | |
| Spangrude et al. | Paradigm shifts in stem-cell biology | |
| Nobuhisa et al. | Identification of a population of cells with hematopoietic stem cell properties in mouse aorta–gonad–mesonephros cultures | |
| DeLuca et al. | Production of human B cells from CD34+ CD38− T− B− progenitors in organ culture by sequential cytokine stimulation | |
| JP2024516187A (ja) | 幹細胞機能を向上させるためのウロリチン | |
| KR20100109542A (ko) | 생착능이 증가된 조혈줄기세포의 제조방법 | |
| Arora | Developmental Maturation within the Hematopoietic System | |
| Mayani et al. | B zyxwvutsrqponmlk | |
| Zúñiga-Pflücker et al. | Notch signals are required for in vitro but not in vivo maintenance of |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 20906474 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2021567704 Country of ref document: JP Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 3165443 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2020906474 Country of ref document: EP Effective date: 20220704 |
|
| ENP | Entry into the national phase |
Ref document number: 20227023892 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2020411454 Country of ref document: AU Date of ref document: 20201225 Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |