WO2020158030A1 - 親水性部材並びにこれを用いたレンズ、車載用カメラ、樹脂フィルム及び窓 - Google Patents
親水性部材並びにこれを用いたレンズ、車載用カメラ、樹脂フィルム及び窓 Download PDFInfo
- Publication number
- WO2020158030A1 WO2020158030A1 PCT/JP2019/034150 JP2019034150W WO2020158030A1 WO 2020158030 A1 WO2020158030 A1 WO 2020158030A1 JP 2019034150 W JP2019034150 W JP 2019034150W WO 2020158030 A1 WO2020158030 A1 WO 2020158030A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- layer
- silicon dioxide
- outermost surface
- base material
- film
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B1/00—Optical elements characterised by the material of which they are made; Optical coatings for optical elements
- G02B1/10—Optical coatings produced by application to, or surface treatment of, optical elements
- G02B1/11—Anti-reflection coatings
- G02B1/113—Anti-reflection coatings using inorganic layer materials only
- G02B1/115—Multilayers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J21/00—Catalysts comprising the elements, oxides, or hydroxides of magnesium, boron, aluminium, carbon, silicon, titanium, zirconium, or hafnium
- B01J21/06—Silicon, titanium, zirconium or hafnium; Oxides or hydroxides thereof
- B01J21/063—Titanium; Oxides or hydroxides thereof
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/30—Catalysts, in general, characterised by their form or physical properties characterised by their physical properties
- B01J35/39—Photocatalytic properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B17/00—Layered products essentially comprising sheet glass, or glass, slag, or like fibres
- B32B17/06—Layered products essentially comprising sheet glass, or glass, slag, or like fibres comprising glass as the main or only constituent of a layer, next to another layer of a specific material
- B32B17/10—Layered products essentially comprising sheet glass, or glass, slag, or like fibres comprising glass as the main or only constituent of a layer, next to another layer of a specific material of synthetic resin
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C17/00—Surface treatment of glass, not in the form of fibres or filaments, by coating
- C03C17/34—Surface treatment of glass, not in the form of fibres or filaments, by coating with at least two coatings having different compositions
- C03C17/3411—Surface treatment of glass, not in the form of fibres or filaments, by coating with at least two coatings having different compositions with at least two coatings of inorganic materials
- C03C17/3417—Surface treatment of glass, not in the form of fibres or filaments, by coating with at least two coatings having different compositions with at least two coatings of inorganic materials all coatings being oxide coatings
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/06—Glass compositions containing silica with more than 90% silica by weight, e.g. quartz
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/12—Silica-free oxide glass compositions
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/12—Silica-free oxide glass compositions
- C03C3/14—Silica-free oxide glass compositions containing boron
- C03C3/15—Silica-free oxide glass compositions containing boron containing rare earths
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B1/00—Optical elements characterised by the material of which they are made; Optical coatings for optical elements
- G02B1/10—Optical coatings produced by application to, or surface treatment of, optical elements
- G02B1/18—Coatings for keeping optical surfaces clean, e.g. hydrophobic or photo-catalytic films
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B7/00—Mountings, adjusting means, or light-tight connections, for optical elements
- G02B7/02—Mountings, adjusting means, or light-tight connections, for optical elements for lenses
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03B—APPARATUS OR ARRANGEMENTS FOR TAKING PHOTOGRAPHS OR FOR PROJECTING OR VIEWING THEM; APPARATUS OR ARRANGEMENTS EMPLOYING ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ACCESSORIES THEREFOR
- G03B30/00—Camera modules comprising integrated lens units and imaging units, specially adapted for being embedded in other devices, e.g. mobile phones or vehicles
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C2201/00—Glass compositions
- C03C2201/06—Doped silica-based glasses
- C03C2201/30—Doped silica-based glasses containing metals
- C03C2201/50—Doped silica-based glasses containing metals containing alkali metals
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C2217/00—Coatings on glass
- C03C2217/40—Coatings comprising at least one inhomogeneous layer
- C03C2217/43—Coatings comprising at least one inhomogeneous layer consisting of a dispersed phase in a continuous phase
- C03C2217/44—Coatings comprising at least one inhomogeneous layer consisting of a dispersed phase in a continuous phase characterized by the composition of the continuous phase
- C03C2217/45—Inorganic continuous phases
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C2217/00—Coatings on glass
- C03C2217/40—Coatings comprising at least one inhomogeneous layer
- C03C2217/43—Coatings comprising at least one inhomogeneous layer consisting of a dispersed phase in a continuous phase
- C03C2217/46—Coatings comprising at least one inhomogeneous layer consisting of a dispersed phase in a continuous phase characterized by the dispersed phase
- C03C2217/47—Coatings comprising at least one inhomogeneous layer consisting of a dispersed phase in a continuous phase characterized by the dispersed phase consisting of a specific material
- C03C2217/475—Inorganic materials
- C03C2217/477—Titanium oxide
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C2217/00—Coatings on glass
- C03C2217/40—Coatings comprising at least one inhomogeneous layer
- C03C2217/43—Coatings comprising at least one inhomogeneous layer consisting of a dispersed phase in a continuous phase
- C03C2217/46—Coatings comprising at least one inhomogeneous layer consisting of a dispersed phase in a continuous phase characterized by the dispersed phase
- C03C2217/47—Coatings comprising at least one inhomogeneous layer consisting of a dispersed phase in a continuous phase characterized by the dispersed phase consisting of a specific material
- C03C2217/475—Inorganic materials
- C03C2217/478—Silica
Definitions
- the present invention relates to a hydrophilic member, a lens using the hydrophilic member, a vehicle-mounted camera, a resin film, and a window.
- Patent Document 1 discloses a vehicle-mounted camera in which a hydrophilic film is formed in which a water film is easily formed even if a small water droplet slightly adheres to the lens surface, and the surface of the lens has an average particle diameter within a predetermined range. Silicon particles, and a hydrophilic film comprising a binder containing silicon dioxide as a main component is provided, and among the silicon dioxide particles dispersed in the hydrophilic film, one in which the average particle size of the silicon dioxide particles on the lens surface side is increased is disclosed. There is.
- Patent Document 2 discloses a barrier layer containing at least one of Al 2 O 3 , SiO 2 , and MgF 2 on a transparent glass substrate from the viewpoint of suppressing the occurrence of colored interference colors and colored double images.
- An anti-fogging element or the like having the respective layers each having a predetermined film thickness is disclosed.
- Patent Document 3 discloses a photocatalyst coated body in which a photocatalyst layer is provided on an organic base material which is a base material, and the photocatalyst layer includes titanium oxide particles which are photocatalyst particles and silica particles which are inorganic oxide particles. It is disclosed that the photocatalyst layer has a gap between the particles in the layer and comprises an intermediate layer containing a silicone-modified resin or the like between the substrate and the photocatalyst layer.
- Patent Document 4 discloses a photocatalyst layer-forming composition which exhibits photocatalytic activity such as decomposition and removal of harmful substances, deodorization, and antifouling both in rain and in fine weather, and which is excellent in transparency. Disclosed is one containing a certain predetermined titanium dioxide and a silica compound, and having a certain characteristic. Patent Document 4 discloses that a transparent substrate with a photocatalyst layer is formed by interposing a substrate protective layer that protects the substrate from the photocatalytic action of the photocatalyst layer between the substrate and the photocatalyst layer. Examples of silica compounds As an alcoholic silica sol.
- Patent Document 1 does not describe a countermeasure when organic matter adheres, so it is considered that there is room for improvement in securing hydrophilicity for a long time.
- Patent Documents 2 to 4 describe a member having a film having hydrophilicity and antifouling property using titanium oxide as a photocatalyst, but the hardness of the film is not described in detail.
- An object of the present invention is to provide a member which has high hydrophilicity, antifouling property, transparency and abrasion resistance, and is less likely to cause decomposition of the base material due to radicals generated even when the base material is a resin. ..
- the hydrophilic member of the present invention includes a base material, a base layer, and an outermost surface layer, the base layer is disposed between the base material and the outermost surface layer, and the outermost surface layer is made of carbon dioxide. It contains silicon particles, titanium dioxide particles and a silicon dioxide binder, and the underlayer contains silicon dioxide.
- the present invention it is possible to provide a member having high hydrophilicity, antifouling property, transparency and abrasion resistance, and even if the base material is a resin, the base material is unlikely to be decomposed by radicals generated. ..
- FIG. 6 is a schematic cross-sectional view showing another example of the hydrophilic member of the present invention, which has a configuration in which a resin film is attached to a glass plate.
- FIG. 6 is a perspective view showing another example of the hydrophilic member of the present invention, in which an antireflection film is formed on the surface of a base material. It is a schematic cross section which shows the vehicle-mounted camera of this invention.
- the present invention relates to a hydrophilic member in which a film having hydrophilicity and antifouling property is provided on the surface of a substrate, specifically, a hydrophilic member using a glass plate, a resin plate, a resin film or the like as a substrate.
- the base material may be a hard plate-like member that is difficult to bend or a film-like member that has flexibility.
- the hydrophilic member is used for lenses, windows and the like. Further, it is also used for a vehicle-mounted camera having a lens, a window, etc., a sensor, etc.
- the hydrophilic member of the present invention includes a base material, a base layer, and an outermost surface layer.
- the base layer is arranged between the base material and the outermost surface layer.
- the outermost surface layer comprises silicon dioxide particles, titanium dioxide particles and silicon dioxide binder.
- the underlayer contains silicon dioxide.
- the average particle size of silicon dioxide particles in the outermost surface layer is preferably 10 to 50 nm.
- the average particle size of titanium dioxide particles in the outermost surface layer is preferably 7 to 35 nm.
- the mass ratio of silicon dioxide and titanium dioxide in the outermost surface layer is preferably 4:6 to 7:3.
- the thickness of the outermost surface layer is preferably 40 to 220 nm.
- the arithmetic mean roughness of the outermost surface layer is 1 to 4 nm.
- the thickness of the underlayer is preferably 15 to 90 nm.
- the base material may be formed of glass or resin.
- the base material may be formed of silicon dioxide glass, lanthanum-boron glass or tantalum glass.
- the base material may be made of polycarbonate resin or acrylic resin.
- the lens of the present invention may be the hydrophilic member itself. It may also include a hydrophilic member.
- An antireflection film may be arranged between the base layer and the base material.
- the antireflection film has a dialuminum trioxide layer and a zirconium dioxide layer formed in this order on the surface of the substrate.
- the camera of the present invention may be provided on the outer peripheral portion of the vehicle, and includes the above lens. That is, it is a vehicle-mounted camera.
- the resin film of the present invention is preferably a substantially transparent one that transmits light in the visible region.
- the resin film may be the hydrophilic member itself. It may also include a hydrophilic member.
- the base layer and the outermost surface layer may be provided on one surface of the base material, and the adhesive layer may be provided on the other surface.
- the window of the present invention is preferably visible from the inside of a building or vehicle.
- This window may be the hydrophilic member itself. It may also include a hydrophilic member.
- the base layer and the outermost surface layer may be provided on the inner or outer surface of the base material.
- the window may have a structure in which a resin film is attached to the base material.
- the base layer and the outermost surface layer are preferably provided on the surface of the resin forming the resin film.
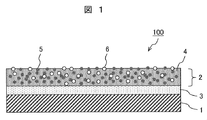
- FIG. 1 schematically shows a cross section of the hydrophilic member of the present invention.
- the hydrophilic member 100 has a film having a two-layer structure on the surface of the base material 1.
- the film has a photocatalyst layer 2 (upper layer film) that constitutes the outermost surface, and a base layer 3 (lower layer film) provided between the base material 1 and the photocatalyst layer 2.
- the photocatalyst layer 2 has a structure in which silicon dioxide particles 5 and titanium dioxide particles 6 are dispersed in a binder 4 containing silicon dioxide as a substantial basic component.
- the underlayer 3 is a thin film of silicon dioxide and may be formed by applying a binder 4 as described later.
- the upper layer film is also referred to as “outermost surface layer”.
- Underlayer 3 (underlayer film) a) Structure of the film
- This film is a thin film of silicon dioxide.
- This film can also be formed by a vacuum process such as sputtering or vapor deposition.
- a silicon dioxide film formed by applying and thermosetting is suitable. This is because a large number of angstrom-order gaps are formed on the surface of the film thus formed, and the binder of the coating film forming the outermost surface penetrates into these gaps and hardens, resulting in a strong anchor effect. Because you can expect
- the thickness of the film needs to be at least 15 nm so that the anchor effect can be exhibited. Further, since this film contains many fine gaps in the film, if it is too thick, the physical strength for withstanding an impact such as rubbing decreases. In order to prevent this, it is desirable that the thickness of the film is suppressed to 90 nm at the maximum.
- the base material is a resin film that can be easily deformed
- the lower layer film may crack when it is rolled to an internal diameter of about 10 cm for storage.
- the thickness of the lower layer film is 50 nm or less, the result is obtained that the lower layer film is hardly cracked even when the base material is rolled. Therefore, when the base material is a resin film, the thickness of the lower layer film is preferably 15 to 50 nm.
- silica sol uses water or alcohol such as ethanol, 1-propanol, 2-propanol and 1-butanol as a solvent. Among these, alcohol is preferable since it has a low surface tension and is hard to be repelled by the resin base material. Thermal curing is performed at a temperature that the substrate can withstand.
- Photocatalyst layer 2 (upper layer film) a) Structure of the film As described above, this film has a structure in which the silicon dioxide particles 5 and the titanium dioxide particles 6 are dispersed in the binder 4 having silicon dioxide as a substantial basic component (FIG. 1). Details of each composition are as follows.
- Binder Material The binder is preferably silica sol used when forming the lower layer film.
- the hydrophilic member of the present invention is considered to be mainly applied when it is used for a lens, a window, or the like, that is, when it is used as a member that at least needs to transmit visible light to some extent. It should be noted that it may be a member that transmits other than visible light. For example, it may be a member that transmits infrared rays, ultraviolet rays, and the like. Of course, the present invention is also applicable when it is used as a member that does not attach importance to light transmittance.
- the average particle diameter of the silicon dioxide particles needs to be 50 nm or less.
- the average particle diameter is preferably 10 nm or more.
- the average particle size of silicon dioxide particles is preferably 10 to 50 nm.
- Titanium dioxide particles Like the above-mentioned silicon dioxide particles, if the size of the titanium dioxide particles is too large, the transmittance is lowered due to scattering. Further, since titanium dioxide has a refractive index of about 2.6, it has a high reflectance. Therefore, the refractive index is higher than that of silicon dioxide having a refractive index of about 1.5, and the effect is larger than that of silicon dioxide even with a small diameter. Therefore, the average particle diameter needs to be 35 nm or less.
- the specific gravity of titanium dioxide (specific gravity is about 3.9) is higher than that of silicon dioxide.
- the lower limit of the particle size is smaller than that of silicon dioxide, and it is possible to handle the particles up to 7 nm in the same manner as silicon dioxide particles having an average particle diameter of 10 nm (specific gravity is about 2.2).
- the average particle size of titanium dioxide is preferably 7 to 35 nm.
- the film thickness is somewhat different depending on the size of silicon dioxide or titanium dioxide used, but is preferably about 40 to 220 nm.
- the film has a particle size of about 1.2 times the particle size, the film becomes a film of almost single particles.
- the surface roughness here, represented by arithmetic average roughness (Ra)
- Ra arithmetic average roughness
- Ra is 5 nm when the lower limit of the average particle diameter of silicon dioxide is 10 nm.
- the film is thickened to about 4 times the particle size, the film becomes flat and Ra becomes small.
- the lower limit of the film thickness is preferably 40 nm, which is about four times the lower limit of the size of the silicon dioxide particles used.
- titanium dioxide has a refractive index of about 2.5, which is higher than that of general-purpose transparent substrates such as silicon dioxide (refractive index of about 1.5) and acrylic resin (refractive index of about 1.49). .. Therefore, if this film becomes too thick, the reflectance in the visible region becomes high, and the transmittance will decrease. In order to secure the visibility of the camera lens and the vehicle, it is considered that the transmittance needs to be 85% in the visible region of 400 to 700 nm. Addition of titanium dioxide particles, silicon dioxide particles, etc. tends to lower the transmittance in the short wavelength region even in the visible region, specifically, in the vicinity of 400 nm due to Rayleigh scattering. Therefore, it is necessary to secure the transmittance at 400 nm of 85% or more.
- the film thickness needs to be 220 nm or less. Further, when the film thickness is about this level, the film becomes considerably flat. However, since it contains particles of silicon dioxide and titanium dioxide, it will differ depending on the content of each particle, but if you try to maintain the content of titanium dioxide necessary to exert the antifouling function by the photocatalytic action, When the film thickness is 220 nm, Ra is about 1 nm.
- the thickness of the film is preferably 40 to 220 nm.
- the arithmetic surface roughness Ra of the film is preferably 1 to 4 nm.
- the upper layer film may crack when it is rolled to an inner diameter of about 10 cm for storage. In order to suppress it, it is desired to make the upper layer film as thin as possible. Specifically, when the thickness of the upper layer film is set to 80 nm or less, the result shows that the upper layer film is hardly cracked even when the base material is rolled. Therefore, when the substrate is a resin film, the thickness of the upper layer film is preferably 40 to 80 nm. It is speculated that the reason why the upper layer film can be thicker than the lower layer film is that the flexibility of the film is improved because it contains particles of titanium dioxide and silicon dioxide.
- the ratio of the ratio of silicon dioxide to titanium dioxide in the film is determined in consideration of photocatalysis, hydrophilicity, physical strength of the film, etc.
- increasing the proportion of silicon dioxide binder increases the physical strength of the film.
- titanium dioxide which has a higher specific gravity than silicon dioxide, is localized on the lower layer side rather than the surface side of the layer. Then, it becomes difficult for oxygen radicals and OH radicals for decomposing organic substances on the surface to reach the surface. In particular, the thicker the film, the greater its effect, so the proportion of titanium dioxide must be increased.
- the proportion of titanium dioxide added was increased at any film thickness.
- the pencil hardness was 3H or more.
- the proportion of titanium dioxide was increased from this, the pencil hardness was significantly reduced.
- the film having titanium dioxide particles of 73% by mass had a pencil hardness of H or less even when the ratio of silicon dioxide particles was reduced to almost zero.
- the pencil hardness of the film in which the titanium dioxide particles were 77% by mass decreased to B.
- the upper limit of the addition ratio of titanium dioxide particles is 70%.
- the ratio of titanium dioxide particles in the film is preferably 30 to 70% by mass.
- the rest is silicon dioxide binder and silicon dioxide particles.
- silicon dioxide particles are added to improve the hydrophilicity of the film. By adding these particles, the ratio of fine gaps inside the film increases, so that water easily penetrates due to the capillary phenomenon and the hydrophilicity of the film surface is improved. However, if the proportion of silicon dioxide particles is increased, the proportion of silicon dioxide binder is reduced, so that the physical strength such as abrasion resistance is reduced.
- the ratio of the silicon dioxide binder needs to be at least 20% by mass in order to secure the pencil hardness of 3H. It was also found that in order to obtain hydrophilicity with a contact angle with water of 10° or less, the silicon dioxide particles should be 10% or more on a mass basis.
- the ratio of silicon dioxide particles in the film is preferably 10% by mass or more.
- a film is formed by applying a coating material in which the above-mentioned titanium dioxide particles, silicon dioxide particles and silica sol are mixed in a solvent to a base material and thermosetting.
- the solvent is not particularly limited as long as the silica sol can be dissolved, but when applied to a substrate such as a resin film, the solvent having a high surface tension may be repelled after application and a uniform film may not be formed. is there. Therefore, solvents such as water and ethylene glycol having high surface tension are not preferable.
- the base material is a polycarbonate resin, an acrylic resin, or the like
- the base material is dissolved in a ketone solvent or an ester solvent, which is not preferable. Therefore, alcohols such as ethanol, 1-propanol, 2-propanol and 1-butanol, which have a surface tension smaller than that of water and ethylene glycol, and which do not dissolve polycarbonate resin and acrylic resin, are suitable.
- the concentration of solids in the paint also varies depending on the coating method.
- the coating method may be an ordinary coating method such as spin coating, dip coating, flow coating, bar coating or roll coating.
- silicon dioxide particles and titanium dioxide particles are dispersed in the paint, and the silica sol exists in a dissolved state.
- Silicon dioxide has a specific gravity of about 2.2, which is larger than that of an alcohol solvent having a specific gravity of about 0.8.
- the particle size is 50 nm at the maximum, it has a large surface area and can be easily dispersed in the paint by an ordinary stirring operation using an overhead stirrer or a stirring bar.
- titanium dioxide particles are as high as about 3.9, it is possible to disperse them in the paint by stirring with a device such as a planetary ball mill or homogenizer.
- examples of the organic dispersant include ethylene glycol monoalkyl ethers and diethylene glycol monoalkyl ethers that are volatilized by thermosetting during film formation.
- thermosetting After applying the coating material to the base material by the above-mentioned application method, thermosetting it to form a film.
- the base material is a resin such as a polycarbonate resin or an acrylic resin
- the heating temperature is too high, the base material is deformed. Therefore, it is desirable to set the heating temperature to the maximum temperature that the substrate can withstand.
- the substrate of the present invention is a glass plate, a resin plate, a lens or the like having high visibility. Since the above-mentioned two-layer film formed on the surface transmits approximately 85% or more of light in the visible region, the visibility of the base material can be secured.
- the base material is a window glass plate of a building or a window glass of a vehicle
- the above-mentioned two-layer film is formed on glass in which sodium, potassium, etc. are added to silicon dioxide. To take.
- the base material is a resin plate or resin film
- the lower layer film is too thin, oxygen radicals, OH radicals, etc. generated from the surface film may decompose the surface of the resin plate or resin film. Therefore, it is desirable that this film has a thickness of about 40 to 90 nm. By making this film thick, oxygen radicals, OH radicals, etc. are prevented from reaching the surface of the resin plate or resin film.
- the coating liquid may be repelled and you may not be able to form a uniform film.
- the surface to be coated is irradiated with oxygen plasma or placed in an ozone atmosphere to improve the wettability of the surface and prevent the coating liquid from splashing.
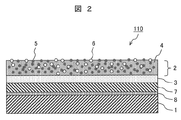
- FIG. 2 is another example of the hydrophilic member of the present invention, schematically showing a cross section of a glass plate to which a resin film is attached.
- the hydrophilic member 110 shown in this figure is obtained by sticking a resin film 7 having a double-layered film and an adhesive layer 8 on the surface of a substrate 1 formed of a glass plate.
- the membrane has a bilayer structure similar to that shown in FIG. That is, it has the photocatalyst layer 2 which constitutes the outermost surface, and the underlayer 3 provided between the resin film 7 and the photocatalyst layer 2.
- the resin film itself provided with the photocatalyst layer 2 and the base layer 3 is also an example of the hydrophilic member of the present invention using a resin as a base material.
- the resin film preferably has flexibility.
- the adhesive layer 8 is formed on the back surface of the resin film 7, and the adhesive layer 8 is applied to a glass plate and is simply affixed without undergoing a step of heat curing, whereby the hydrophilic property of the present invention is improved.
- a glass plate which is one of the flexible members, can be formed.
- an antireflection film may be formed on the surface of the base material.
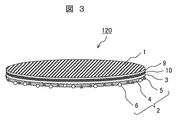
- FIG. 3 is a perspective view showing another example of the hydrophilic member of the present invention, in which an antireflection film is formed on the surface of a base material.
- a dialuminum trioxide layer 9 and a zirconium dioxide layer 10 forming an antireflection film are provided on the lower surface of the substrate 1.
- the underlying layer 3 is provided on the surface of the zirconium dioxide layer 10, and the photocatalytic layer 2 containing the binder 4, the silicon dioxide particles 5 and the titanium dioxide particles 6 is provided on the surface of the underlying layer 3. That is, this is a configuration in which the above-mentioned two-layer film is formed on the surface of the antireflection film.
- the antireflection film there is one in which a zirconium dioxide layer is provided without providing the dialuminum trioxide layer.
- the underlying layer 3 is provided on the surface of the zirconium dioxide layer, and the photocatalyst layer 2 (outermost surface layer) is provided.
- the thickness of the outermost surface layer is 50 nm or less. This is desirable because it reduces the interference of light in the visible wavelength range.
- the material of the lens is generally silicon dioxide glass that contains a small amount of sodium, potassium, etc., but when it is necessary to secure a high viewing angle like a car-mounted camera, it has a higher refractive index than silicon dioxide.
- Substrate that is substantially transparent in the visible region specifically, lanthanum-boron-based glass, tantalum-based glass, or the like is used.
- the lanthanum-boron type glass is a glass containing lanthanum oxide (La 2 O 3 ) and boron oxide (B 2 O 3 ).
- the tantalum-based glass is glass containing tantalum oxide (Ta 2 O 5 ).
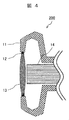
- FIG. 4 is a schematic sectional view showing an example of the vehicle-mounted camera of the present invention.
- the camera 200 has a lens 13 attached to a housing 11 thereof via a packing 12.
- a CCD element 14 is provided inside the lens 13.
- the light entering through the lens 13 is converted into electrical image information by the CCD element 14 and sent to an image processing device (not shown).
- a film having hydrophilicity and antifouling property is provided on the surface of the lens 13. That is, the lens 13 is the hydrophilic member of the present invention.
- the lens of the present invention has a layer containing titanium dioxide particles and silicon dioxide particles, it has a slightly higher haze than a transparent lens.
- the CCD element can be arranged as close as possible to the surface of the lens to suppress the reduction in transmittance due to turbidity as much as possible.
- the haze of the lens used in the vehicle-mounted camera of the present invention is preferably 1 or less.
- the amount of light to be input is reduced by about 2% from the amount of light entering from the lens by 3 mm.
- the difference in light transmittance between the case where the distance between the CCD element and the lens is 3 mm and the case where the lens is brought into contact with the lens is 0.5% or less. Therefore, the distance between the CCD element and the lens is preferably 3 mm or less.
- Tetraethoxysilane 70 parts by mass was dissolved in ethanol (930 parts by mass), a very small amount of nitric acid was added, and the mixture was heated at 50°C for about 1 hour. Through this process, the solvent was volatilized at a silicon concentration of about 1% by mass to obtain a silica sol liquid (1000 parts by mass) having a silicon dioxide concentration after thermal curing of about 2% by mass.
- This liquid is a liquid containing a compound having a silicon dioxide structure and an alkoxysilane site. This liquid is referred to as a coating liquid A.
- Titanium dioxide particles having an average particle diameter of 7 nm, silicon dioxide particles having an average particle diameter of 10 nm, the coating liquid A, and ethanol were mixed and stirred using a planetary ball mill.
- the mixing ratios of coating liquid A, titanium dioxide particles, silicon dioxide particles, and ethanol as a solvent are as shown in Table 1.
- the coating solution A was applied to a slide glass made of soda lime glass by a spin coating method (rotation speed: 2000 rpm, rotation time: 30 seconds). Then, by heating at 150° C. for 10 minutes, a silicon dioxide layer was formed on the surface of the slide glass.
- the coating solution B is applied to the slide glass made of soda lime glass by the spin coating method (rotation speed: 2000 rpm, rotation time: 30 seconds). Then, by heating at 150° C. for 10 minutes, a silicon dioxide layer containing titanium dioxide particles and silicon dioxide particles was formed on the surface of the slide glass.
- the proportion of titanium dioxide in the upper layer film is preferably 30 to 70%.
- Example 2 a film was formed and evaluated in the same manner as in Example 1 except that the rotation speed during spin coating was changed.
- Table 2 shows the number of rotations during spin coating and the properties of the formed upper layer film.
- the film thickness of the upper layer film was 40 nm or more.
- the arithmetic average roughness of the surface was 4 nm or less, and the pencil hardness was 3H or more.
- the rotation speed during spin coating was set to 5000 rpm, the arithmetic mean roughness became 5 nm or more, and the pencil hardness fell to H or less.
- the thickness of the upper layer film is preferably 40 nm or more. It was also found that the arithmetic average roughness of the surface is preferably 4 nm or less.
- Example 2 a film was formed and evaluated in the same manner as in Example 1 except that the rotation speed during spin coating was changed.
- Table 3 shows the number of rotations during spin coating, the properties of the formed upper layer film, and the like.
- the film thickness of the upper layer film was 200 to 210 nm, and in this case, the light transmittance at 400 nm was 86% or more. However, when the rotation speed during spin coating was 700 rpm, the light transmittance at 400 nm was 83% or less. The higher the light transmittance, the more preferable from the viewpoint of ensuring the visibility.
- the upper layer film thickness is preferably 220 nm or less.
- Example 2 not only titanium dioxide having an average particle diameter of 7 nm but also titanium dioxide having an average particle diameter of 25 nm, 33 nm, and 40 nm was used, and the composition of the upper layer film was adjusted so that both titanium dioxide and silicon dioxide were 50% by mass. .. Except for this, the same experiment as in Example 1 was performed.
- Table 4 shows a summary of the results.
- the transmittance of 400 nm light was 86% or more when the titanium dioxide used had an average particle diameter of 33 nm or less, but decreased to 81% when the average particle diameter was 40 nm.
- the average particle size of titanium dioxide is 35 nm or less, the transmittance of about 85% or more is secured. I understand. From this, it was determined that the average particle size of titanium dioxide is preferably 35 nm or less.
- Example 2 not only those having an average particle diameter of silicon dioxide of 10 nm but also those having an average particle diameter of 30 nm, 47 nm and 55 nm were used, and the composition of the upper layer film was adjusted to be 50 mass% for both titanium dioxide and silicon dioxide. .. Except for this, the same experiment as in Example 1 was performed.
- the transmittance of 400 nm light was 86% or more when the average particle size of silicon dioxide used was 47 nm or less, but decreased to 81% when the average particle size was 55 nm.
- the average particle diameter of silicon dioxide is 50 nm or less, the transmittance of about 85% or more is secured. I understand. From this, it was determined that the average particle diameter of silicon dioxide is preferably 50 nm or less.
Landscapes
- Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Geochemistry & Mineralogy (AREA)
- General Physics & Mathematics (AREA)
- Life Sciences & Earth Sciences (AREA)
- Optics & Photonics (AREA)
- Inorganic Chemistry (AREA)
- Laminated Bodies (AREA)
- Catalysts (AREA)
- Lens Barrels (AREA)
- Surface Treatment Of Optical Elements (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201980090434.6A CN113348219B (zh) | 2019-01-28 | 2019-08-30 | 亲水性构件及使用其的透镜、车载用相机、树脂膜和窗户 |
| EP19912591.5A EP3919270A4 (en) | 2019-01-28 | 2019-08-30 | HYDROPHILIC ELEMENT, LENS IN WHICH SUCH HYDROPHILIC ELEMENT IS INSTALLED, ON-BOARD CAMERA, RESIN FILM AND WINDOW |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019011846A JP7376239B2 (ja) | 2019-01-28 | 2019-01-28 | 親水性部材並びにこれを用いたレンズ、車載用カメラ、樹脂フィルム及び窓 |
| JP2019-011846 | 2019-01-28 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020158030A1 true WO2020158030A1 (ja) | 2020-08-06 |
Family
ID=71841338
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/034150 Ceased WO2020158030A1 (ja) | 2019-01-28 | 2019-08-30 | 親水性部材並びにこれを用いたレンズ、車載用カメラ、樹脂フィルム及び窓 |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP3919270A4 (enExample) |
| JP (1) | JP7376239B2 (enExample) |
| CN (1) | CN113348219B (enExample) |
| WO (1) | WO2020158030A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12152153B2 (en) * | 2021-12-03 | 2024-11-26 | Canon Kabushiki Kaisha | Light transmitting member, transparent protective cover, and image pickup system |
| CN119241893A (zh) * | 2024-09-30 | 2025-01-03 | 比亚迪股份有限公司 | 板件、空调和车辆 |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020148845A (ja) * | 2019-03-12 | 2020-09-17 | マクセル株式会社 | レンズ、レンズの製造方法、および、レンズユニット |
| JP2023148631A (ja) * | 2022-03-30 | 2023-10-13 | デクセリアルズ株式会社 | 光触媒部材 |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001047581A (ja) * | 1999-08-05 | 2001-02-20 | Toto Ltd | 光触媒コートポリエステルフィルム |
| JP2002148402A (ja) * | 2000-11-13 | 2002-05-22 | Seiko Epson Corp | 光学物品及びその製造方法 |
| WO2006137446A1 (ja) * | 2005-06-22 | 2006-12-28 | Ube-Nitto Kasei Co., Ltd. | 防汚性印刷シート |
| JP2008132474A (ja) * | 2006-10-30 | 2008-06-12 | Fujifilm Corp | 親水性部材及び親水性部材用基板 |
| JP2009262139A (ja) * | 2008-03-31 | 2009-11-12 | Toto Ltd | 光触媒塗装体およびそのための光触媒コーティング液 |
| CN104340983A (zh) * | 2013-08-01 | 2015-02-11 | 京程科技股份有限公司 | 二氧化硅-二氧化钛溶胶的制法及应用 |
| JP2016037584A (ja) * | 2014-08-08 | 2016-03-22 | キヤノン株式会社 | 遮光塗料、遮光塗料セット、遮光膜、光学素子、遮光膜の製造方法及び光学素子の製造方法 |
| WO2016147573A1 (ja) * | 2015-03-19 | 2016-09-22 | パナソニックIpマネジメント株式会社 | 親水レンズ |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000271491A (ja) * | 1999-01-22 | 2000-10-03 | Nissan Motor Co Ltd | 光触媒膜及びその製造方法 |
| JP5680537B2 (ja) * | 2009-08-17 | 2015-03-04 | 日本板硝子株式会社 | 光触媒膜を備えたガラス物品 |
-
2019
- 2019-01-28 JP JP2019011846A patent/JP7376239B2/ja active Active
- 2019-08-30 CN CN201980090434.6A patent/CN113348219B/zh active Active
- 2019-08-30 EP EP19912591.5A patent/EP3919270A4/en active Pending
- 2019-08-30 WO PCT/JP2019/034150 patent/WO2020158030A1/ja not_active Ceased
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001047581A (ja) * | 1999-08-05 | 2001-02-20 | Toto Ltd | 光触媒コートポリエステルフィルム |
| JP2002148402A (ja) * | 2000-11-13 | 2002-05-22 | Seiko Epson Corp | 光学物品及びその製造方法 |
| WO2006137446A1 (ja) * | 2005-06-22 | 2006-12-28 | Ube-Nitto Kasei Co., Ltd. | 防汚性印刷シート |
| JP2008132474A (ja) * | 2006-10-30 | 2008-06-12 | Fujifilm Corp | 親水性部材及び親水性部材用基板 |
| JP2009262139A (ja) * | 2008-03-31 | 2009-11-12 | Toto Ltd | 光触媒塗装体およびそのための光触媒コーティング液 |
| CN104340983A (zh) * | 2013-08-01 | 2015-02-11 | 京程科技股份有限公司 | 二氧化硅-二氧化钛溶胶的制法及应用 |
| JP2016037584A (ja) * | 2014-08-08 | 2016-03-22 | キヤノン株式会社 | 遮光塗料、遮光塗料セット、遮光膜、光学素子、遮光膜の製造方法及び光学素子の製造方法 |
| WO2016147573A1 (ja) * | 2015-03-19 | 2016-09-22 | パナソニックIpマネジメント株式会社 | 親水レンズ |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12152153B2 (en) * | 2021-12-03 | 2024-11-26 | Canon Kabushiki Kaisha | Light transmitting member, transparent protective cover, and image pickup system |
| CN119241893A (zh) * | 2024-09-30 | 2025-01-03 | 比亚迪股份有限公司 | 板件、空调和车辆 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN113348219A (zh) | 2021-09-03 |
| JP7376239B2 (ja) | 2023-11-08 |
| EP3919270A4 (en) | 2022-11-16 |
| JP2020116897A (ja) | 2020-08-06 |
| CN113348219B (zh) | 2023-09-01 |
| EP3919270A1 (en) | 2021-12-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2020158030A1 (ja) | 親水性部材並びにこれを用いたレンズ、車載用カメラ、樹脂フィルム及び窓 | |
| US9028958B2 (en) | Superhydrophilic nanostructure | |
| Aegerter et al. | Coatings made by sol–gel and chemical nanotechnology | |
| JP4995428B2 (ja) | 酸化チタン塗膜形成方法 | |
| US8048511B2 (en) | Titanium oxide coating agent and titanium oxide film forming method | |
| Shahzadi et al. | Transparent, self-cleaning, scratch resistance and environment friendly coatings for glass substrate and their potential applications in outdoor and automobile industry | |
| JP5761346B2 (ja) | 無機親水性コート液、それから得られる親水性被膜及びこれを用いた部材 | |
| KR100706928B1 (ko) | 친수성-방담방오성 막의 제조방법 및 그 막을 갖는 친수성 미러의 제조방법 | |
| WO2011040405A1 (ja) | 光触媒塗装体およびそのための光触媒コーティング液 | |
| EP2202000A1 (en) | Photocatalytic film, method for production of photocatalytic film, article, and hydrophilization method | |
| WO2014061606A1 (ja) | 防汚性反射防止膜、物品およびその製造方法 | |
| KR20010032701A (ko) | 광촉매성 산화물 함유 조성물, 박막 및 복합체 | |
| JPH0956549A (ja) | 防曇性鏡 | |
| CN101605606A (zh) | 光催化薄膜、形成光催化薄膜的方法以及覆盖有光催化薄膜的制品 | |
| CN101050064A (zh) | 涂敷有红外屏蔽膜的玻璃板及其制造方法 | |
| CN108572404B (zh) | 光学构件、摄像设备和光学构件的制造方法 | |
| JP2002346393A (ja) | 光触媒体およびその製造方法 | |
| JP5942140B2 (ja) | 被覆部材 | |
| JP4501562B2 (ja) | 積層膜付き基材およびその製造方法 | |
| JP6736049B2 (ja) | ガラス複合体、それを備えた透明スクリーン、およびそれを備えた映像投影システム | |
| WO2011122615A1 (ja) | 積層構造及び積層体 | |
| Tanaka et al. | Coatings with photocatalyst on architectural glass | |
| JP2013185043A (ja) | 光触媒塗工液、太陽光発電システム用カバー、及びその製造方法 | |
| JP6695417B2 (ja) | 光触媒構造体及びその製造方法 | |
| JP2010115608A (ja) | 光触媒膜およびそれを有する物品 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19912591 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2019912591 Country of ref document: EP Effective date: 20210830 |