WO2019123972A1 - ニッケル粉の製造方法 - Google Patents
ニッケル粉の製造方法 Download PDFInfo
- Publication number
- WO2019123972A1 WO2019123972A1 PCT/JP2018/043216 JP2018043216W WO2019123972A1 WO 2019123972 A1 WO2019123972 A1 WO 2019123972A1 JP 2018043216 W JP2018043216 W JP 2018043216W WO 2019123972 A1 WO2019123972 A1 WO 2019123972A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- nickel
- solution
- nickel powder
- reduction
- powder
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/16—Making metallic powder or suspensions thereof using chemical processes
- B22F9/18—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds
- B22F9/24—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds starting from liquid metal compounds, e.g. solutions
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F3/00—Manufacture of workpieces or articles from metallic powder characterised by the manner of compacting or sintering; Apparatus specially adapted therefor ; Presses and furnaces
- B22F3/12—Both compacting and sintering
- B22F3/16—Both compacting and sintering in successive or repeated steps
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/16—Making metallic powder or suspensions thereof using chemical processes
- B22F9/18—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds
- B22F9/24—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds starting from liquid metal compounds, e.g. solutions
- B22F9/26—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds starting from liquid metal compounds, e.g. solutions using gaseous reductors
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B23/00—Obtaining nickel or cobalt
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B23/00—Obtaining nickel or cobalt
- C22B23/04—Obtaining nickel or cobalt by wet processes
- C22B23/0407—Leaching processes
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B3/00—Extraction of metal compounds from ores or concentrates by wet processes
- C22B3/04—Extraction of metal compounds from ores or concentrates by wet processes by leaching
- C22B3/06—Extraction of metal compounds from ores or concentrates by wet processes by leaching in inorganic acid solutions, e.g. with acids generated in situ; in inorganic salt solutions other than ammonium salt solutions
- C22B3/08—Sulfuric acid, other sulfurated acids or salts thereof
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B3/00—Extraction of metal compounds from ores or concentrates by wet processes
- C22B3/04—Extraction of metal compounds from ores or concentrates by wet processes by leaching
- C22B3/12—Extraction of metal compounds from ores or concentrates by wet processes by leaching in inorganic alkaline solutions
- C22B3/14—Extraction of metal compounds from ores or concentrates by wet processes by leaching in inorganic alkaline solutions containing ammonia or ammonium salts
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B3/00—Extraction of metal compounds from ores or concentrates by wet processes
- C22B3/20—Treatment or purification of solutions, e.g. obtained by leaching
- C22B3/22—Treatment or purification of solutions, e.g. obtained by leaching by physical processes, e.g. by filtration, by magnetic means, or by thermal decomposition
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B3/00—Extraction of metal compounds from ores or concentrates by wet processes
- C22B3/20—Treatment or purification of solutions, e.g. obtained by leaching
- C22B3/44—Treatment or purification of solutions, e.g. obtained by leaching by chemical processes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2301/00—Metallic composition of the powder or its coating
- B22F2301/15—Nickel or cobalt
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2998/00—Supplementary information concerning processes or compositions relating to powder metallurgy
- B22F2998/10—Processes characterised by the sequence of their steps
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2999/00—Aspects linked to processes or compositions used in powder metallurgy
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P10/00—Technologies related to metal processing
- Y02P10/20—Recycling
Definitions
- the present invention relates to a method for obtaining a high purity nickel powder of low sulfur grade and a briquette obtained by solidifying it from a solution of nickel sulfate ammonium complex.
- it can be applied to the treatment of an intermediate product solution in the process generated in the wet nickel smelting process.
- Non-Patent Document 1 describes a manufacturing process of a nickel powder in which an iron compound is added as seed crystals during reduction reaction to precipitate nickel on the iron compound, but iron contamination derived from seed crystals in a product is I have problems with the points that arise.
- Patent Document 1 is inexpensive and excellent in weather resistance, has low electric resistance in the state of being kneaded with a resin, reduces initial electric resistance and electric resistance during use, and can be used stably for a long period of time, conductive Disclosed are nickel powders suitable as conductive particles for pastes and conductive resins, and methods of providing the same.
- the nickel powder disclosed in Patent Document 1 is a nickel powder containing 1 to 20% by mass of cobalt, the balance being nickel and an unavoidable impurity, and comprising secondary particles in which primary particles are aggregated, The amount is 0.8% by mass or less. It is said that it is preferable that cobalt be contained only in the surface layer portion of the secondary particles, and the cobalt content in the surface layer portion be 1 to 40% by mass. When it is intended to obtain this nickel powder according to the disclosed manufacturing method, cobalt will co-exist, and nickel and cobalt co-exist as in nickel oxide ore, for example. It is not suitable for purity and economical recovery applications.
- Patent Document 2 provides a method of producing a metal powder by a liquid phase reduction method, which is improved so as not to easily generate particle aggregates.
- This manufacturing method is carried out by dissolving the metal compound, the reducing agent, the complexing agent, and the dispersing agent to thereby adjust the pH of the aqueous solution by the first step of preparing an aqueous solution containing metal ions derived from the metal compound. And a second step of depositing metal powder by reducing metal ions with a reducing agent.
- this production method is expensive using expensive chemicals, and it is not economically advantageous to apply to the above-mentioned large scale operation as nickel smelting.
- the first invention of the present invention for solving the above-mentioned problems is characterized in that nickel is characterized in that the processing steps shown in the following (1) to (6) are carried out in the production step of producing nickel powder from a nickel sulfate solution. It is a manufacturing method of powder.
- the recovered nickel powder is repeated in either or both of the complexing step (2) and the reduction step (3), and a sulfurizing agent is added to the recovered reduction solution, nickel sulfide And solid-liquid separation, nickel sulfide and nickel recovery step to form a solution after nickel recovery.
- a nickel regeneration step in which the nickel sulfide obtained in the nickel recovery step (5) is oxidized and leached, and the obtained nickel sulfate solution is repeated in the hydroxylation step (1).
- the nickel powder recovered in the solid-liquid separation step (4) in the first aspect of the present invention is sieved according to the particle size, and nickel powder smaller than the preset particle size is sorted to form seed crystals. And adding repeatedly to either or both of the complexing step (2) and the reduction step (3) to obtain nickel powder coarser than the particle diameter of the seed crystal nickel powder. It is a manufacturing method of nickel powder.
- the average particle diameter of the seed crystals added to either or both of the complexing step (2) and the reduction step (3) in the second aspect is 0.1 to 100 ⁇ m.
- the mixed slurry Is a method of producing a nickel powder further comprising adding a dispersant.
- the amount of the seed crystals added in the complexing step (2) in the first to fourth aspects is 1 to 100% with respect to the weight of nickel in the nickel sulfate ammonium complex solution. It is a manufacturing method of nickel powder characterized by being.
- the sixth invention of the present invention sifts off the reducing slurry in the first to fifth inventions, and the sieving reduction slurry of the nickel powder under reduction and the reduction end solution is used as the reduction end of the complexing step (2).
- a method for producing a nickel powder characterized in that it is used repeatedly as part of a liquid and a seed nickel powder.
- the complexing step (2) in the sixth invention comprises a dissolving step of adding a reduction end solution to obtain a nickel sulfate ammonium complex solution, nickel powder or nickel powder, and a reduction end solution. It is a manufacturing method of the nickel powder characterized by comprising two steps of a seed crystal addition process of adding mixed slurry containing.
- the nickel sulfate solution in the first invention is a mixed sulfide of nickel and cobalt recovered by leaching nickel oxide ore, nickel sulfide, crude nickel sulfate, nickel oxide, nickel hydroxide It is a manufacturing method of nickel powder characterized by being obtained by dissolving at least one sort of powder of nickel carbonate and metallic nickel in sulfuric acid acid solution.
- the ninth invention of the present invention relates to a leaching step in which the nickel sulfate solution in the first invention dissolves a nickel-containing substance containing cobalt as an impurity, and to adjust the pH of the leachate containing nickel and cobalt obtained in the leaching step.
- the nickel powder solution is a nickel sulfate solution obtained through a solvent extraction step of separating into a nickel sulfate solution and a cobalt recovery solution by a solvent extraction method.
- the ammonium sulfate concentration in the nickel sulfate ammonium complex solution in the first invention is 100 to 500 g / L, and the ammonium concentration is 1 in molar ratio to the nickel concentration in the complex solution. It is a manufacturing method of nickel powder characterized by being .9 or more.
- the blowing of hydrogen gas in the reduction step (3) in the first aspect is performed while maintaining the temperature in the range of 100 to 200 ° C. and the pressure in the range of 0.8 to 4.0 MPa. It is a manufacturing method of nickel powder characterized by the above.
- the twelfth invention of the present invention is the method for producing nickel powder, wherein the dispersant in the fourth invention contains polyacrylate.
- a thirteenth invention of the present invention provides a nickel powder ore process for processing nickel powder obtained through the reduction process (3) according to the first invention into massive nickel briquettes using a briquette machine, A nickel powder comprising a briquette sintering step of sintering the massive nickel briquette under a holding condition at a temperature of 500 to 1200 ° C. in a hydrogen atmosphere to form a nickel briquette of a sintered body Manufacturing method.
- the fourteenth invention of the present invention is characterized by comprising an ammonium sulfate recovery step of concentrating the reduction final solution in the solid-liquid separation step (4) in the first invention, crystallizing ammonium sulfate to recover ammonium sulfate crystals. It is a manufacturing method of nickel powder.
- the fifteenth invention of the present invention is characterized in that it includes an ammonia recovery step of adding alkali to the final solution after reduction in the solid-liquid separation step (4) of the first invention and heating it to volatilize and recover ammonia gas. It is a manufacturing method of nickel powder.
- the steps shown in the following (1) to (6) are applied to the process liquid of the wet smelting process to obtain a nickel sulfate ammonium chloride complex solution It is characterized in that high purity nickel powder with few impurities is produced.
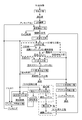
- the method for producing high purity nickel powder of the present invention will be described with reference to the production flow chart of the high purity nickel powder of the present invention shown in FIG.
- the leaching step is an industrial intermediate or the like consisting of one or a mixture of nickel and cobalt mixed sulfide, crude nickel sulfate, nickel oxide, nickel hydroxide, nickel carbonate, nickel powder, etc., as a starting material.
- (1) Hydroxylation Step an alkali is added to the nickel sulfate solution obtained through the above-described steps, etc. to form a nickel hydroxide precipitate, and the solid component precipitation and the liquid component are separated.
- the alkali to be added it is preferable to use sodium hydroxide, calcium hydroxide, etc. which are industrially inexpensive and can be procured in large quantities.
- this complexation step consists of two steps of a dissolution step and a seed crystal addition step.
- the nickel hydroxide of the precipitate obtained in the hydroxylation step (1) is Ammonia in the form of a reduced end solution obtained by solid-liquid separation of the reduced slurry obtained in the reduction step (3) is added to form a mixed solution of nickel hydroxide and the reduced end solution to form a complex. It is a process of forming a nickel sulfate ammonium complex which is an ammonium complex of nickel by forming a nickel ammonium sulfate complex solution.
- the ammonia concentration can be adjusted by adding ammonia gas or ammonia water.
- Ammonia is added so that the ammonium concentration at that time is 1.9 or more in molar ratio with respect to the nickel concentration in the solution.
- the ammonium concentration of ammonia to be added is less than 1.9, nickel does not form an ammine complex, and a nickel hydroxide precipitate is formed.
- ammonium sulfate can be added in this step to adjust the ammonium sulfate concentration.
- the ammonium sulfate concentration is preferably 100 to 500 g / L, and if it exceeds 500 g / L, solubility will be exceeded and crystals will precipitate, making it difficult to achieve less than 100 g / L in terms of metal balance of the process. is there.
- recovery process mentioned later can also be used for the ammonia gas or ammonia water used at this process.
- nickel powder having an average particle diameter of 0.1 to 100 ⁇ m is added as seed crystals in the form of a nickel powder slurry to the generated nickel sulfate ammonium complex solution, seed crystals and nickel sulfate ammonium complex solution
- a seeding step is performed to form a mixed slurry comprising nickel hydroxide.
- the weight of the seed crystals added at this time is preferably 1 to 100% with respect to the weight of nickel in the nickel sulfate ammonium complex solution. If it is less than 1%, the reaction efficiency at the time of reduction in the next step is significantly reduced. On the other hand, if it exceeds 100%, the amount used is large, so that production of seed crystals is expensive and not economical.
- the dispersant to be used here is not particularly limited as long as it has a sulfonate, but lignin sulfonate is preferable as one which can be obtained at low cost industrially.
- reaction temperature is preferably 100 to 200 ° C.
- the temperature is less than 100 ° C., more preferably less than 150 ° C., the reduction efficiency decreases, and even if it exceeds 200 ° C., the reaction is not affected and the loss of heat energy and the like increases.
- the pressure at the time of reaction is preferably 0.8 to 4.0 MPa. If the pressure is less than 0.8 MPa, the reaction efficiency decreases, and if the pressure exceeds 4.0 MPa, the reaction is not affected, and the loss of hydrogen gas increases.
- magnesium ion, sodium ion, calcium ion, sulfate ion and ammonium ion mainly exist as impurities in the liquid of the obtained mixed slurry, they remain in the solution, and therefore, high purity nickel powder Can be generated.
- nickel hydroxide in the mixed slurry solution reacts with ammonium ions generated by reduction reaction, dissolves in a solution as a nickel ammine complex, and is reduced by reacting with hydrogen gas, and nickel is reduced to seed crystals. It precipitates.
- Solid-Liquid Separation Step Solid-liquid separation is performed on the reduced slurry generated in the previous reduction step (3) to recover high purity nickel powder and reduced final solution with few impurities, respectively, and complex the high purity nickel powder In 2), as the seed crystals, nickel powder to be subjected to particle growth in the reduction step (3) is repeatedly supplied to either or both of the steps.
- the recovered reduction final solution is a step which is repeated as a substitute for ammonia water in the complexing step (2).
- the nickel sulfate is further added to the nickel sulfate ammonium complex solution obtained in the complexing step (2), and in the reduction step (3), by supplying hydrogen gas, nickel is further reduced on high purity nickel powder.
- the particles can be grown to precipitate. Further, by repeating the supply to the reduction step a plurality of times, it is also possible to produce high purity nickel powder having higher bulk density and larger particle size.
- the obtained high-purity nickel powder may be finished into the shape of a briquette that is coarser, less likely to be oxidized, and easy to handle, through the following nickel powder group ore process or briquette firing process. Furthermore, an ammonia recovery step may be provided.
- the high purity nickel powder produced according to the present invention is, after being dried, formed and processed by means of a ball mill or the like as a product form to obtain massive nickel briquettes. Moreover, in order to improve the formability to the briquette, in some cases, a substance that does not contaminate the product quality such as water may be added to the nickel powder as a binder.
- the nickel briquettes produced in the briquette process are roasted and sintered in a hydrogen atmosphere to produce briquettes.
- the strength is increased, and a slight amount of residual ammonia and sulfur components are removed, and the roasting / sintering temperature is preferably 500 to 1200.degree. If it is less than 500 ° C., sintering is insufficient, and even if it exceeds 1200 ° C., the efficiency hardly changes and the energy loss increases.
- Nickel recovery step Nickel remains in the reduction end solution generated in the solid-liquid separation step (4), and when there is a large amount of residual nickel, ammonium sulfate crystals produced in the ammonium sulfate recovery step of the next step It may contaminate and contaminate the quality. Therefore, it is also necessary to remove in advance.
- the sulfiding agent used here may be any industrially used sulfiding agent such as hydrogen sulfide gas or sodium hydrosulfide, but in order to further improve the quality of ammonium sulfate crystals, it is preferable to use hydrogen sulfide gas. .
- Nickel sulfide precipitated by adding a sulfiding agent can be separated by solid-liquid separation and recovered, and then leached again and repeated in the system.
- This leaching is preferred because the nickel sulfide recovered in the previous nickel recovery step is exclusively leached exclusively for the purpose and there are few problems such as impurities, so it is efficient and preferable.
- Ammonium sulfate recovery process Ammonium sulfate and ammonia are contained in the solution after nickel recovery generated by the above [nickel recovery step]. Thus, ammonium sulfate can be recovered as ammonium sulfate crystals by crystallizing ammonium sulfate by heating and concentrating the solution after reaction by performing an ammonium sulfate recovery step.
- ammonia recovery process Further, ammonia can be recovered by volatilizing the ammonia gas by heating after adding an alkali to the reduction end solution to adjust the pH to 10 to 13.
- the alkali used here is not particularly limited, caustic soda, slaked lime, etc. are preferable because they are industrially inexpensive.
- the recovered ammonia gas can be brought into contact with water to produce ammonia water, and the obtained ammonia water can be repeatedly used in the process.
- nickel hydroxide By adding 800 ml of slaked lime adjusted to a slurry concentration of 200 g / L to 1000 ml of a nickel sulfate solution having a nickel concentration of 120 g / L, 116 g of nickel hydroxide was obtained.
- the nickel hydroxide is charged into 1700 ml of a mixed solution of a nickel sulfate solution having a nickel concentration of 30 g / L and an ammonium sulfate solution having an ammonia concentration of 40 g / L together with 12.8 g of nickel powder having an average particle diameter of 2 ⁇ m as seed crystals and stirring. A mixed slurry was made.
- the mixed slurry is heated to 185 ° C. while stirring in an autoclave, and hydrogen gas is blown into the autoclave so that the pressure in the autoclave becomes 3.5 MPa, and a reduction step is performed after the solid-liquid separation step by filtration. Then, the grain-grown nickel powder was recovered. At this time, the collected nickel powder had an average particle diameter of 65 ⁇ m and a collected amount of 119 g. Furthermore, after washing
- the prepared mixed slurry is heated to 120 ° C. while stirring in an autoclave, hydrogen gas is blown into the autoclave so that the pressure in the autoclave is 3.5 MPa, and supplied to reduce the nickel powder production process.
- hydrogen gas is blown into the autoclave so that the pressure in the autoclave is 3.5 MPa, and supplied to reduce the nickel powder production process.
- One hour after the supply of hydrogen gas the supply of hydrogen gas was stopped and the autoclave was cooled.
- the reduced slurry obtained after cooling was subjected to solid-liquid separation treatment by filtration to recover a high purity small diameter nickel powder.
- the amount of nickel powder recovered at this time was 70 g.
- 116 g of nickel hydroxide was added to the final solution after reduction after solid-liquid separation to prepare a slurry, and the high-purity small diameter nickel powder collected in the slurry was added to the entire amount to prepare a mixed slurry.
- the mixed slurry was heated to 120 ° C. while being stirred by an autoclave, and hydrogen gas was blown into the autoclave so that the pressure in the autoclave was 3.5 MPa and supplied.
- One hour after the supply of hydrogen gas the supply of hydrogen gas was stopped and the autoclave was cooled.
- the slurry obtained after cooling was subjected to solid-liquid separation treatment by filtration to recover high-purity grain-grown nickel powder.
- a reduced slurry obtained in the solid-liquid separation step of Example 1 is used as part of an ammonia source to prepare a mixed slurry, subjected to a reduction step under the same conditions as in Example 1, and subjected to the solid-liquid separation step.
- the grown nickel powder was recovered.
- the same nickel powder as in Example 1 was recovered.
- a nickel powder prepared under the same conditions as in Example 1 was added to a solution containing 336 g of nickel sulfate and 330 g of ammonium sulfate, 191 ml of 25% ammonia water, and adjusted to a total liquid volume of 1000 ml.
- grain-grown nickel powder was produced.
- the same operation was repeated ten times using the produced nickel powder, to thereby grow particles of the nickel powder.
- the average particle diameter of the recovered nickel powder was 111 ⁇ m, and the particles had grown to 1.7 times the size of the nickel powder of Example 1.
- the sulfur grade in the nickel powder obtained by this repeated operation was 0.04%. Also, sodium and magnesium were below the lower limit of quantification as in Table 1 above. Furthermore, the obtained nickel powder was heated to 1000 ° C. in a 2% hydrogen atmosphere and held for 60 minutes. The sulfur grade in the obtained nickel powder after holding was 0.008%, and the sulfur grade could be further reduced by roasting.
- nickel hydroxide to the nickel sulfate ammonium complex solution prepared by mixing 135 g of nickel sulfate hexahydrate, 191 ml of 25% ammonia water, 169 g of ammonium sulfate and pure water, and add the pure water to a solution volume of 1000 ml.
- 15 g of nickel powder having an average particle diameter of 1 ⁇ m was used as a seed crystal to prepare a mixed slurry.
- the mixed slurry was heated to 100 ° C. while being stirred by an autoclave, and a hydrogen gas was supplied so that the pressure in the autoclave was 3.5 MPa to carry out a nickel powder production treatment.
- a hydrogen gas was supplied so that the pressure in the autoclave was 3.5 MPa to carry out a nickel powder production treatment.
- One hour after the supply of hydrogen gas the supply of hydrogen gas was stopped and the autoclave was cooled.
- the reduced slurry obtained after cooling was subjected to solid-liquid separation treatment by filtration to recover a high purity small diameter nickel powder.
- the nickel reduction rate at this time was 58%.
- Example 6 Using the same mixed slurry as in Example 6, the same operation as in Example 6 was performed under the conditions of a temperature of 100 ° C and a pressure in the autoclave of 0.8 MPa. The nickel reduction rate at this time was 56%.
- Example 6 Using the same mixed slurry as in Example 6, the same operation as in Example 6 was performed under the conditions of a temperature of 120 ° C and a pressure in the autoclave of 3.5 MPa. The nickel reduction rate at this time was 74%.
- Example 6 Using the same mixed slurry as in Example 6, the same operation as in Example 6 was performed under the conditions of a temperature of 120 ° C and a pressure in the autoclave of 2.0 MPa. The nickel reduction rate at this time was 74%.
- Example 6 The same operation as in Example 6 was performed using the same mixed slurry as in Example 6 under the conditions of a temperature of 120 ° C. and a pressure in the autoclave of 1.5 MPa. The nickel reduction rate at this time was 74%.
- Example 1 A solution prepared by adding 191 ml of 25% ammonia water to a solution containing nickel sulfate solution containing 75 g of nickel and 330 g of ammonium sulfate without performing the hydroxylation step in Example 1 so that the total liquid volume is 1000 ml. Then, a nickel powder was produced under the same conditions as in Example 1 except that 7.5 g was added as seed crystals of nickel powder having an average particle diameter of 1 ⁇ m to prepare a mixed slurry. After washing the recovered nickel powder with pure water, the impurity grade of the nickel powder was analyzed. The results are shown in Table 4. The mixing of Mg and Na into the nickel powder resulted in more than that of Example 1. The average particle diameter and the recovery amount were almost the same as in Example 1.
- Example 2 (Comparative example 2) Using the same method as in Comparative Example 1 above, a nickel powder was produced without being subjected to the hydroxylation step. This nickel powder was repeated ten times in the same manner as in Example 3 to grow grains. The sulfur grade in the nickel powder obtained by this repeated operation was 0.1%, and it was not possible to obtain a high purity nickel powder having a sulfur grade as high as 0.04% obtained in Example 3 of the present invention .
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Mechanical Engineering (AREA)
- Organic Chemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Environmental & Geological Engineering (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Geochemistry & Mineralogy (AREA)
- Geology (AREA)
- Inorganic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
- Manufacture And Refinement Of Metals (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA3085986A CA3085986A1 (en) | 2017-12-21 | 2018-11-22 | Method for producing nickel powder |
| US16/954,357 US20200376564A1 (en) | 2017-12-21 | 2018-11-22 | Method for producing nickel powder |
| EP18892385.8A EP3730238A1 (en) | 2017-12-21 | 2018-11-22 | Method for producing nickel powder |
| CN201880080390.4A CN111479643A (zh) | 2017-12-21 | 2018-11-22 | 镍粉的制造方法 |
| AU2018392525A AU2018392525B2 (en) | 2017-12-21 | 2018-11-22 | Method for producing nickel powder |
| PH12020550922A PH12020550922A1 (en) | 2017-12-21 | 2020-06-16 | Method for producing nickel powder |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017-245631 | 2017-12-21 | ||
| JP2017245631A JP6624464B2 (ja) | 2017-12-21 | 2017-12-21 | ニッケル粉の製造方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019123972A1 true WO2019123972A1 (ja) | 2019-06-27 |
Family
ID=66993266
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/043216 Ceased WO2019123972A1 (ja) | 2017-12-21 | 2018-11-22 | ニッケル粉の製造方法 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US20200376564A1 (enExample) |
| EP (1) | EP3730238A1 (enExample) |
| JP (1) | JP6624464B2 (enExample) |
| CN (1) | CN111479643A (enExample) |
| AU (1) | AU2018392525B2 (enExample) |
| CA (1) | CA3085986A1 (enExample) |
| PH (1) | PH12020550922A1 (enExample) |
| WO (1) | WO2019123972A1 (enExample) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN118302547A (zh) * | 2021-11-30 | 2024-07-05 | 尤米科尔公司 | 选择性浸出 |
| AU2024213150B2 (en) * | 2023-08-25 | 2025-06-26 | Kemco | All-in-one nickel recovering method for nickel metal recovery from raw materials containing nickel |
| EP4538399A4 (en) * | 2023-08-25 | 2025-11-19 | Korea Zinc Co Ltd | ALL-IN-ONE NICKEL METALLURGICAL METALLURGICAL METALLING PROCESS FOR NICKEL RECOVERY FROM NICKEL-CONTAINING RAW MATERIALS |
| KR102789618B1 (ko) * | 2023-08-25 | 2025-04-03 | 고려아연 주식회사 | 니켈을 함유하는 원료로부터 니켈을 회수하기 위한 올인원 니켈 제련 방법 |
| CN120513310A (zh) * | 2023-08-25 | 2025-08-19 | 高丽亚铅株式会社 | 从含镍原料制备硫酸镍水溶液的方法 |
| JP2025530955A (ja) * | 2023-08-25 | 2025-09-19 | コリア・ジンク・カンパニー・リミテッド | ニッケルを含有する原料からニッケル水酸化物を回収するためのオールインワンニッケル製錬方法 |
| CN120513308A (zh) * | 2023-08-25 | 2025-08-19 | 高丽亚铅株式会社 | 从含镍原料中回收氧化镍的一体式镍冶炼方法 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005240164A (ja) | 2004-02-27 | 2005-09-08 | Sumitomo Metal Mining Co Ltd | ニッケル粉およびその製造方法 |
| JP2005350766A (ja) | 2004-05-13 | 2005-12-22 | Sumitomo Metal Mining Co Ltd | ニッケル酸化鉱石の湿式製錬方法 |
| JP2010242143A (ja) | 2009-04-02 | 2010-10-28 | Sumitomo Electric Ind Ltd | 金属粉末および金属粉末製造方法、導電性ペースト、並びに積層セラミックコンデンサ |
| JP2015140480A (ja) * | 2014-01-30 | 2015-08-03 | 国立大学法人高知大学 | ニッケル粉の製造方法 |
| WO2015125650A1 (ja) * | 2014-02-21 | 2015-08-27 | 国立大学法人高知大学 | ニッケル粉の製造方法 |
| JP2017155319A (ja) * | 2016-03-04 | 2017-09-07 | 住友金属鉱山株式会社 | ニッケル粉の製造方法 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA950681A (en) * | 1971-06-30 | 1974-07-09 | Donald R. Weir | Production of nickel powder from basic nickel carbonate |
| JP6188222B2 (ja) * | 2013-12-26 | 2017-08-30 | 日本放送協会 | トピック抽出装置、及びプログラム |
| JP6099601B2 (ja) * | 2014-02-17 | 2017-03-22 | 国立大学法人高知大学 | ニッケル粉の製造方法 |
| JP6610425B2 (ja) * | 2015-08-31 | 2019-11-27 | 住友金属鉱山株式会社 | ニッケル粉の製造方法 |
| JP6726396B2 (ja) * | 2016-02-22 | 2020-07-22 | 住友金属鉱山株式会社 | ニッケル粉の製造方法 |
| CN107116228B (zh) * | 2017-06-20 | 2019-01-04 | 中南大学 | 一种固相还原制备超细镍粉的方法 |
-
2017
- 2017-12-21 JP JP2017245631A patent/JP6624464B2/ja active Active
-
2018
- 2018-11-22 AU AU2018392525A patent/AU2018392525B2/en not_active Expired - Fee Related
- 2018-11-22 CN CN201880080390.4A patent/CN111479643A/zh active Pending
- 2018-11-22 US US16/954,357 patent/US20200376564A1/en not_active Abandoned
- 2018-11-22 EP EP18892385.8A patent/EP3730238A1/en not_active Withdrawn
- 2018-11-22 CA CA3085986A patent/CA3085986A1/en not_active Abandoned
- 2018-11-22 WO PCT/JP2018/043216 patent/WO2019123972A1/ja not_active Ceased
-
2020
- 2020-06-16 PH PH12020550922A patent/PH12020550922A1/en unknown
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005240164A (ja) | 2004-02-27 | 2005-09-08 | Sumitomo Metal Mining Co Ltd | ニッケル粉およびその製造方法 |
| JP2005350766A (ja) | 2004-05-13 | 2005-12-22 | Sumitomo Metal Mining Co Ltd | ニッケル酸化鉱石の湿式製錬方法 |
| JP2010242143A (ja) | 2009-04-02 | 2010-10-28 | Sumitomo Electric Ind Ltd | 金属粉末および金属粉末製造方法、導電性ペースト、並びに積層セラミックコンデンサ |
| JP2015140480A (ja) * | 2014-01-30 | 2015-08-03 | 国立大学法人高知大学 | ニッケル粉の製造方法 |
| WO2015125650A1 (ja) * | 2014-02-21 | 2015-08-27 | 国立大学法人高知大学 | ニッケル粉の製造方法 |
| JP2017155319A (ja) * | 2016-03-04 | 2017-09-07 | 住友金属鉱山株式会社 | ニッケル粉の製造方法 |
Non-Patent Citations (1)
| Title |
|---|
| POWDER METALLURGY, 1958, pages 40 - 52 |
Also Published As
| Publication number | Publication date |
|---|---|
| CA3085986A1 (en) | 2019-06-27 |
| PH12020550922A1 (en) | 2021-05-31 |
| AU2018392525A1 (en) | 2020-06-25 |
| AU2018392525B2 (en) | 2020-09-03 |
| CN111479643A (zh) | 2020-07-31 |
| JP6624464B2 (ja) | 2019-12-25 |
| EP3730238A1 (en) | 2020-10-28 |
| JP2019112661A (ja) | 2019-07-11 |
| US20200376564A1 (en) | 2020-12-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5828923B2 (ja) | ニッケル粉の製造方法 | |
| JP6610425B2 (ja) | ニッケル粉の製造方法 | |
| JP6624464B2 (ja) | ニッケル粉の製造方法 | |
| JP2017150063A5 (enExample) | ||
| CN107406910B (zh) | 钴粉的制造方法 | |
| JP6726396B2 (ja) | ニッケル粉の製造方法 | |
| JP6531913B2 (ja) | ニッケル粉の製造方法 | |
| JP2017150002A5 (enExample) | ||
| WO2017038589A1 (ja) | ニッケル粉の製造方法 | |
| JP2019065345A (ja) | ニッケル粉の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18892385 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 3085986 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2018392525 Country of ref document: AU Date of ref document: 20181122 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2018892385 Country of ref document: EP Effective date: 20200721 |