WO2019003655A1 - セルロースナノファイバー化用パルプ繊維を製造する方法、及びセルロースナノファイバー化用パルプ繊維 - Google Patents
セルロースナノファイバー化用パルプ繊維を製造する方法、及びセルロースナノファイバー化用パルプ繊維 Download PDFInfo
- Publication number
- WO2019003655A1 WO2019003655A1 PCT/JP2018/018175 JP2018018175W WO2019003655A1 WO 2019003655 A1 WO2019003655 A1 WO 2019003655A1 JP 2018018175 W JP2018018175 W JP 2018018175W WO 2019003655 A1 WO2019003655 A1 WO 2019003655A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- ozone
- pulp fibers
- pulp fiber
- cellulose
- treatment
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21C—PRODUCTION OF CELLULOSE BY REMOVING NON-CELLULOSE SUBSTANCES FROM CELLULOSE-CONTAINING MATERIALS; REGENERATION OF PULPING LIQUORS; APPARATUS THEREFOR
- D21C9/00—After-treatment of cellulose pulp, e.g. of wood pulp, or cotton linters ; Treatment of dilute or dewatered pulp or process improvement taking place after obtaining the raw cellulosic material and not provided for elsewhere
- D21C9/10—Bleaching ; Apparatus therefor
- D21C9/147—Bleaching ; Apparatus therefor with oxygen or its allotropic modifications
- D21C9/153—Bleaching ; Apparatus therefor with oxygen or its allotropic modifications with ozone
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21C—PRODUCTION OF CELLULOSE BY REMOVING NON-CELLULOSE SUBSTANCES FROM CELLULOSE-CONTAINING MATERIALS; REGENERATION OF PULPING LIQUORS; APPARATUS THEREFOR
- D21C5/00—Other processes for obtaining cellulose, e.g. cooking cotton linters ; Processes characterised by the choice of cellulose-containing starting materials
- D21C5/02—Working-up waste paper
- D21C5/022—Chemicals therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L11/00—Methods specially adapted for refuse
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2/00—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor
- A61L2/16—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor using chemical substances
- A61L2/18—Liquid substances or solutions comprising solids or dissolved gases
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2/00—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor
- A61L2/16—Methods or apparatus for disinfecting or sterilising materials or objects other than foodstuffs or contact lenses; Accessories therefor using chemical substances
- A61L2/18—Liquid substances or solutions comprising solids or dissolved gases
- A61L2/183—Ozone dissolved in a liquid
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B09—DISPOSAL OF SOLID WASTE; RECLAMATION OF CONTAMINATED SOIL
- B09B—DISPOSAL OF SOLID WASTE NOT OTHERWISE PROVIDED FOR
- B09B3/00—Destroying solid waste or transforming solid waste into something useful or harmless
- B09B3/30—Destroying solid waste or transforming solid waste into something useful or harmless involving mechanical treatment
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B09—DISPOSAL OF SOLID WASTE; RECLAMATION OF CONTAMINATED SOIL
- B09B—DISPOSAL OF SOLID WASTE NOT OTHERWISE PROVIDED FOR
- B09B3/00—Destroying solid waste or transforming solid waste into something useful or harmless
- B09B3/30—Destroying solid waste or transforming solid waste into something useful or harmless involving mechanical treatment
- B09B3/32—Compressing or compacting
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B09—DISPOSAL OF SOLID WASTE; RECLAMATION OF CONTAMINATED SOIL
- B09B—DISPOSAL OF SOLID WASTE NOT OTHERWISE PROVIDED FOR
- B09B3/00—Destroying solid waste or transforming solid waste into something useful or harmless
- B09B3/70—Chemical treatment, e.g. pH adjustment or oxidation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29B—PREPARATION OR PRETREATMENT OF THE MATERIAL TO BE SHAPED; MAKING GRANULES OR PREFORMS; RECOVERY OF PLASTICS OR OTHER CONSTITUENTS OF WASTE MATERIAL CONTAINING PLASTICS
- B29B17/00—Recovery of plastics or other constituents of waste material containing plastics
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29B—PREPARATION OR PRETREATMENT OF THE MATERIAL TO BE SHAPED; MAKING GRANULES OR PREFORMS; RECOVERY OF PLASTICS OR OTHER CONSTITUENTS OF WASTE MATERIAL CONTAINING PLASTICS
- B29B17/00—Recovery of plastics or other constituents of waste material containing plastics
- B29B17/02—Separating plastics from other materials
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J11/00—Recovery or working-up of waste materials
- C08J11/04—Recovery or working-up of waste materials of polymers
- C08J11/10—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J11/00—Recovery or working-up of waste materials
- C08J11/04—Recovery or working-up of waste materials of polymers
- C08J11/10—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation
- C08J11/16—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with inorganic material
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J11/00—Recovery or working-up of waste materials
- C08J11/04—Recovery or working-up of waste materials of polymers
- C08J11/10—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation
- C08J11/18—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with organic material
- C08J11/22—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with organic material by treatment with organic oxygen-containing compounds
- C08J11/26—Recovery or working-up of waste materials of polymers by chemically breaking down the molecular chains of polymers or breaking of crosslinks, e.g. devulcanisation by treatment with organic material by treatment with organic oxygen-containing compounds containing carboxylic acid groups, their anhydrides or esters
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21C—PRODUCTION OF CELLULOSE BY REMOVING NON-CELLULOSE SUBSTANCES FROM CELLULOSE-CONTAINING MATERIALS; REGENERATION OF PULPING LIQUORS; APPARATUS THEREFOR
- D21C5/00—Other processes for obtaining cellulose, e.g. cooking cotton linters ; Processes characterised by the choice of cellulose-containing starting materials
- D21C5/02—Working-up waste paper
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21C—PRODUCTION OF CELLULOSE BY REMOVING NON-CELLULOSE SUBSTANCES FROM CELLULOSE-CONTAINING MATERIALS; REGENERATION OF PULPING LIQUORS; APPARATUS THEREFOR
- D21C9/00—After-treatment of cellulose pulp, e.g. of wood pulp, or cotton linters ; Treatment of dilute or dewatered pulp or process improvement taking place after obtaining the raw cellulosic material and not provided for elsewhere
- D21C9/001—Modification of pulp properties
- D21C9/007—Modification of pulp properties by mechanical or physical means
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H11/00—Pulp or paper, comprising cellulose or lignocellulose fibres of natural origin only
- D21H11/14—Secondary fibres
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B09—DISPOSAL OF SOLID WASTE; RECLAMATION OF CONTAMINATED SOIL
- B09B—DISPOSAL OF SOLID WASTE NOT OTHERWISE PROVIDED FOR
- B09B2101/00—Type of solid waste
- B09B2101/65—Medical waste
- B09B2101/67—Diapers or nappies
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29B—PREPARATION OR PRETREATMENT OF THE MATERIAL TO BE SHAPED; MAKING GRANULES OR PREFORMS; RECOVERY OF PLASTICS OR OTHER CONSTITUENTS OF WASTE MATERIAL CONTAINING PLASTICS
- B29B17/00—Recovery of plastics or other constituents of waste material containing plastics
- B29B17/02—Separating plastics from other materials
- B29B2017/0213—Specific separating techniques
- B29B2017/0293—Dissolving the materials in gases or liquids
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29L—INDEXING SCHEME ASSOCIATED WITH SUBCLASS B29C, RELATING TO PARTICULAR ARTICLES
- B29L2031/00—Other particular articles
- B29L2031/48—Wearing apparel
- B29L2031/4871—Underwear
- B29L2031/4878—Diapers, napkins
Definitions
- the present disclosure relates to a method of producing pulp fibers for cellulose nanofiberation from pulp fibers of used hygiene products, and to pulp fibers for cellulose nanofiberization derived from used hygiene products including pulp fibers and superabsorbent polymer. .
- Patent Document 1 discloses a method for producing recycled pulp which can be reused mainly as a sanitary product. Specifically, Patent Document 1 discloses a method of recovering pulp fibers from used sanitary products containing pulp fibers and a superabsorbent polymer, and producing recycled pulp that can be reused as a sanitary product. A method comprising: applying a physical force to a used sanitary product in a used sanitary product in an aqueous solution containing polyvalent metal ions or an acidic aqueous solution having a pH of 2.5 or less.
- a method which comprises the step of treating with
- Patent Document 1 the reason for treating pulp fibers with an aqueous solution containing ozone is that not only a large amount of superabsorbent polymer remains in the separated pulp fibers, but the superabsorbent polymer is oxidatively decomposed and solubilized. To remove from pulp fibers.
- Patent Document 1 discloses, as a method of treating pulp fibers with an aqueous solution containing ozone, a method of placing an aqueous solution containing ozone in a treatment tank and placing separated pulp fibers in the aqueous solution containing ozone.
- the ozone-containing aqueous solution be appropriately stirred to create a water flow during the treatment, the ozone gas be blown into the aqueous solution contained in the container, and the water flow be caused in the ozone-containing aqueous solution by rising bubbles of ozone gas. It may be generated.
- the superabsorbent polymer enlarges as it absorbs fluid such as body fluid, and the pulp fibers are involved, (ii) enlargement
- the plurality of superabsorbent polymers and the plurality of pulp fibers form a connected structure, for example, by causing gel blocking while winding up the pulp fibers into one another.
- the present disclosure is to form cellulose nanofibers from pulp fibers of used sanitary goods in which a pulp fiber for cellulose nanofiber formation having a low lignin content and a narrow distribution thereof and having excellent cellulose nanofiberation properties can be produced. It is an object of the present invention to provide a method for producing a pulp fiber for pulp.
- the present inventors are a method for producing pulp fibers for cellulose nanofibre formation from pulp fibers of used sanitary goods, which are lower than the following steps, the mixed liquid supply port, and the mixed liquid supply port.
- the ozone-containing gas is increased by the ozone-containing gas by lowering the ozone-containing gas while lowering the high-absorbent polymer and the pulp fiber.
- a step of forming a pulp fiber for forming cellulose nanofibers which is brought into contact with a fiber to dissolve at least a part of the superabsorbent polymer in the treatment solution and to form a pulp fiber for forming cellulose nanofibers from the pulp fiber;
- the processing solution discharge step of discharging the processing solution containing the pulp fiber for nanofiberization from the processing solution outlet, and the pulp fiber for cellulose nanofiber conversion having a lignin content of 0.1% by mass or less found a method characterized by having.
- the method for producing pulp fibers for cellulose nanofiber production from pulp fibers of used sanitary goods according to the present disclosure is pulp fibers for cellulose nanofiber production which is low in lignin content and narrow in distribution, and is excellent in cellulose nanofiber properties. Are manufacturable.
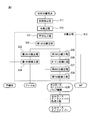
- FIG. 1 is a flowchart illustrating an embodiment of a method of the present disclosure. It is the schematic which shows the structural example of the apparatus of the ozone treatment process of FIG. It is the schematic which shows the other structural example of the apparatus of the ozone treatment process of FIG. It is the schematic which shows the other structural example of the apparatus of the ozone treatment process of FIG.
- a method of producing pulp fibers for producing cellulose nanofibers from pulp fibers of used sanitary goods which comprises the following steps: Preparing a treatment tank including a mixed liquid supply port, a treatment liquid outlet and an ozone-containing gas supply port disposed below the mixed liquid supply port; A mixed liquid supply step of supplying a mixed liquid containing super absorbent polymer and pulp fibers derived from used sanitary goods and water from the mixed liquid supply port to the processing tank, An ozone-containing gas supply step of supplying an ozone-containing gas to the treatment liquid in the treatment tank from the ozone-containing gas supply port; The ozone-containing gas is brought into contact with the high-absorbent polymer and the pulp fiber by raising the ozone-containing gas while lowering the high-absorbent polymer and the pulp fiber in the treatment tank, so that the high-absorption polymer is absorbed.
- the pulp fiber for forming cellulose nanofibers has a lignin content of 0.1% by mass or less. Characterized in that the above method.
- the superabsorbent polymer enlarges as it absorbs fluid such as body fluid, and the pulp fibers are involved, (ii) enlargement
- the plurality of superabsorbent polymers and the plurality of pulp fibers form a connected structure, for example, by causing gel blocking while winding up the pulp fibers into one another.
- the ozone-containing gas can process free pulp fibers that do not form a connected structure and decompose lignin contained in the pulp fibers, the inside of the connected structure Since it is difficult to contact the constituent pulp fibers, ie, the pulp fibers surrounded by the superabsorbent polymer, the pulp fibers constituting the connecting structure can not be treated sufficiently, ie, the superabsorbent polymer In some cases, it was difficult to decompose lignin contained in the enclosed pulp fiber. Therefore, the recycled pulp fiber produced by the method described in Patent Document 1 includes one having a narrow distribution of lignin content and a high lignin content.
- the distribution of the lignin content rate mentioned above is that the used sanitary products which are raw materials are sold by a plurality of types, for example, products of the same type (adult disposable diapers) sold by different companies and the same company There has been a tendency to be wider when it includes some different types of goods (eg, adult disposable diapers and children's disposable diapers).
- recycled pulp fibers having a high lignin content tend to be difficult to be converted into cellulose nanofibers.
- the above production method includes a predetermined cellulose fiberization pulp fiber formation step, and in the cellulose nanofiber conversion pulp fiber formation step, the ozone-containing gas is raised while lowering the superabsorbent polymer and the pulp fibers.
- the superabsorbent polymer and pulp fibers are contacted with an ozone containing gas.
- the relatively high specific gravity free superabsorbent polymer and the connecting structure settle more than the relatively low specific gravity free pulp fiber Tend to be higher.
- the ozone containing gas consumes ozone and rises while processing the highly absorbent polymer and the pulp fiber, the ozone containing gas present at the lower position is higher than the ozone containing gas present at the upper position. And the ozone content tends to be high (ie, fresh).
- the descending speed relates to the moving speed of the processing liquid 52 in the processing tank 31 downward, and generally, the first flow rate, the second flow rate, and the size of the processing tank. It is uniquely determined by
- the settling property is a property that represents the ease of falling in the vertical direction by gravity of the pulp fiber, the superabsorbent polymer and the connecting structure contained in the treatment liquid 52 in the treatment tank 31. Depending on the specific gravity and the like, each of the pulp fiber, the superabsorbent polymer and the connecting structure has different settling properties.
- the free super-absorbent polymer having a relatively high sedimentation property and the super-absorbent polymer in the connecting structure are properly oxidized and decomposed with a fresher ozone-containing gas, and the connecting structure is obtained. While being able to liberate the pulp fibers that have been constituted, the settling property is relatively low, and the ozone containing gas takes time for the free pulp fibers, which take a relatively long time to reach the treatment liquid outlet. It can be processed to break down lignin contained in free pulp fibers.
- the pulp fiber having a relatively high lignin content has a relatively high lignin content. Because it is relatively less sedimentable than low pulp fibers, the fresher ozone-containing gas can contact the pulp fibers with relatively high lignin content to break down the lignin contained therein.
- the pulp fiber for cellulose nanofiberification produced by the above production method hardly spreads the distribution of lignin content, and the pulp fiber for cellulose nanofiber formation has a predetermined low concentration of lignin content Furthermore, it has. Therefore, in the said manufacturing method, distribution of a lignin content rate is narrow and the pulp fiber for cellulose nanofiber conversion which is excellent in cellulose nanofiber conversion property can be manufactured.
- the pulp fiber for cellulose nanofiber formation has a predetermined rate of decrease in freeness, the pulp fiber for cellulose nanofiber formation is easily fluffed in the subsequent cellulose nanofiber forming step, and the cellulose nanofiber is easily formed. . Therefore, the said manufacturing method can manufacture the pulp fiber for cellulose nanofiber conversion which is excellent in cellulose nanofiber conversion property.
- the ozone-containing gas is supplied from the ozone-containing gas supply port as microbubbles or nanobubbles in the pulp fiber formation step for forming cellulose nanofibers
- the superabsorbent polymer and the pulp fibers are connected structure Even if they are formed, the microbubbles or nanobubbles give the superabsorbent polymer, the connecting structure and the pulp fibers a buoyancy, so that their settling property is reduced. Therefore, it takes a long time for the superabsorbent polymer, the connecting structure and the pulp fiber to reach the treatment solution outlet, and the ozone is a free superabsorbent polymer and the superabsorbent polymer constituting the connecting structure. Can be decomposed and the free pulp fibers and the pulp fibers that make up the connecting structure can be fully processed. Therefore, the said manufacturing method can manufacture the pulp fiber for cellulose nanofiber conversion which is excellent in cellulose nanofiber conversion property.
- Aspect 4 Aspect 3. The method according to any one of aspects 1 to 3, wherein the treatment liquid is acidic.
- the treatment liquid is acidic (for example, pH 2.5 or less). Therefore, the superabsorbent polymer to be treated can be acid-inactivated, or if the superabsorbent polymer to be treated is already inactivated, then the superabsorbent polymer is inactivated. It can hold the state.
- ozone in the ozone-containing gas removes the superabsorbent polymer constituting the connected structure While being able to act, the ozone in the ozone-containing gas acts on the pulp fibers constituting the connecting structure to reduce the lignin content of the pulp fibers (to form pulp fibers for cellulose nanofiberation with low lignin content) be able to).
- the above-mentioned manufacturing method further includes a predetermined inactivation step, ozone in the ozone-containing gas is used even when the superabsorbent polymer and the pulp fiber form a connected structure.
- a mixed solution containing superabsorbent polymer derived from sanitary goods, pulp fibers and water is supplied to the treatment tank, the superabsorbent polymer constituting the connection structure can be removed, and the ozone containing gas Ozone can act on the pulp fibers constituting the connecting structure to lower the lignin content of the pulp fibers (can form pulp fibers for cellulose nanofiberation with a low lignin content).
- the acid is an acid capable of forming a complex with the metal ion contained in the excrement
- the pulp fiber for cellulose nanofiberification to be produced is less likely to contain the metal ion. Therefore, when producing cellulose nanofibers from pulp fibers for cellulose nanofiber formation, metal ions and their precipitates are less likely to damage the equipment used for the cellulose nanofiber formation process, and pulp fibers for cellulose nanofiber formation It is difficult to inhibit miniaturization.
- Aspect 7 The method according to any one of aspects 1 to 6, wherein the pulp fiber for cellulose nanofiberification has an ash fraction of 0.65% by mass or less.
- the pulp fiber for cellulose nanofiberification produced by the above production method has a predetermined ash content. Therefore, when producing cellulose nanofibers from pulp fibers for cellulose nanofiber formation, metal ions and their precipitates are less likely to damage the equipment used for the cellulose nanofiber formation process, and pulp fibers for cellulose nanofiber formation It is difficult to inhibit miniaturization.
- Aspect 8 In the mixed liquid supply step, the mixed liquid is continuously supplied from the mixed liquid supply port to the treatment tank at a first flow rate, and in the discharged treatment liquid step, the treated liquid is discharged from the treated liquid outlet.
- Aspect 8 The method according to any one of aspects 1 to 7, wherein the method is continuously discharged at a second flow rate.
- the mixed liquid in the mixed liquid supply step, the mixed liquid is continuously supplied from the mixed liquid supply port to the treatment tank at a first flow rate, and in the treated liquid discharging step, the treated liquid is collected from the treated liquid outlet.
- the continuous discharge at a flow rate of 2 makes the treatment time of the super absorbent polymer and pulp fiber to be treated uniform, and narrows the distribution of lignin content of the cellulose nanofiber pulp fiber contained in the treatment liquid (Less variation). Therefore, the said manufacturing method can manufacture the pulp fiber for cellulose nanofiber conversion which is excellent in cellulose nanofiber conversion property.
- the said manufacturing method can manufacture cellulose nanofiber efficiently from the pulp fiber for cellulose nanofiber conversion.
- a cellulose nanofiber producing pulp fiber derived from used sanitary goods comprising pulp fibers and a superabsorbent polymer, comprising:
- the pulp fiber for forming cellulose nanofibers has a lignin content of 0.1% by mass or less.
- the pulp fiber for cellulose nanofibers characterized by the above-mentioned.
- the pulp fiber for forming cellulose nanofibers has a predetermined lignin content rate, and thus is excellent in cellulose nanofiber forming properties.
- the pulp fiber for cellulose nanofiber formation has a predetermined rate of reduction in the degree of freeness, the pulp fiber for cellulose nanofiber formation is likely to be fluffed and to be cellulose nanofiber in the subsequent cellulose nanofiber formation step.
- the pulp fiber for cellulose nanofiber formation has a predetermined ash content
- metal ions and their precipitates are used in the cellulose nanofiber formation step. It is difficult to damage the equipment used, and it is difficult to inhibit the miniaturization of cellulose nanofiber pulp fiber.
- the used sanitary goods are the sanitary goods used by the user, and include the sanitary goods in the state which absorbed the excrement of the liquid of the user, and it was used but it has not absorbed the excrement Including things, unused things, etc.
- the hygiene article comprises a topsheet, a backsheet, and an absorber disposed between the topsheet and the backsheet.
- Hygiene goods include, for example, disposable diapers, urine absorbing pads, sanitary napkins, bed sheets and pet sheets.
- a nonwoven fabric or a film As a structural member of a surface sheet, a nonwoven fabric or a film is mentioned, for example, Specifically, a liquid permeable nonwoven fabric, a synthetic resin film which has a liquid penetration hole, a composite sheet of these, etc. are mentioned.
- a structural member of a back surface sheet a nonwoven fabric or a film is mentioned, for example, Specifically, a liquid impervious nonwoven fabric, a liquid impervious synthetic resin film, and a composite sheet of these nonwoven fabrics and a synthetic resin film are mentioned. .
- the components of the absorber include an absorbent core (e.g., pulp fibers and superabsorbent polymer) and a core wrap.
- the pulp fiber is not particularly limited as long as it can be used as a sanitary product, and examples thereof include cellulose fiber.
- Examples of cellulosic fibers include wood pulp, crosslinked pulp, non-wood pulp, regenerated cellulose, semi-synthetic cellulose and the like.
- the super absorbent polymer (SAP) is not particularly limited as long as it can be used as a sanitary product, but examples thereof include polyacrylates, polysulfonates, and maleic anhydrides. Be
- One side and the other side of the absorber are respectively bonded to the top sheet and the back sheet via an adhesive.
- a portion (peripheral portion) of the top sheet extending to the outside of the absorber so as to surround the absorber is a portion on the outside of the absorber so as to surround the absorber of the back sheet. It is joined with the extended part (peripheral part) via an adhesive. Therefore, the absorber is encased within the joined body of the top sheet and the back sheet.
- the adhesive is not particularly limited as long as it can be used as a sanitary product and softened by the warm water described later to reduce the bonding strength. For example, a hot melt adhesive can be mentioned.
- a hot melt adhesive for example, a pressure-sensitive adhesive or a heat-sensitive adhesive of a rubber-based material such as styrene-ethylene-butadiene-styrene, styrene-butadiene-styrene, styrene-isoprene-styrene or an olefin-based material such as polyethylene Adhesives may be mentioned.
- FIG. 1 is a flow chart showing a material separation method for separating used sanitary goods into constituent materials.
- This material separation method is a method of separating used sanitary goods into a film, a non-woven fabric, pulp fibers and a superabsorbent polymer.
- This material separation method includes a pretreatment step S11, a decomposition step S12, and a separation step S13.
- the pretreatment step S11 the used sanitary goods are swollen with water.
- the decomposition step S12 physically shocks the swollen used sanitary goods, and the used sanitary goods are a film, a non-woven fabric, a core wrap, etc., and an absorbent core (for example, pulp fiber and super absorbent polymer). Disassemble.
- the separation step S13 separates the film, the non-woven fabric, the pulp fiber, and the superabsorbent polymer.
- the method for producing pulp fibers for cellulose nanofiberification of the present disclosure is included in the separation step S13 of the material separation method.
- the mixture of pulp fiber and super-absorbent polymer is previously obtained by any method, it is before the method for producing pulp fiber for cellulose nanofibers in the pretreatment step S11, the decomposition step S12 and the separation step S13. It is unnecessary to carry out the process of Each step will be described below.
- a plurality of used sanitary goods are recovered from the outside as they are, that is, they are not crushed, cut, etc., and are in the state of being rolled or folded. As it is, and without deactivating the superabsorbent polymer of the absorbent, it absorbs water and swells.
- hot water is absorbed and swollen in the used sanitary goods, or the absorbed water is heated after being absorbed and expanded into hot water.
- Hot water refers to water at a temperature higher than normal temperature (20 ° C. ⁇ 15 ° C. (5 to 35 ° C.): JIS Z 8703).
- the amount of liquid excrement actually absorbed in the used sanitary goods is very small compared to the maximum absorption that can be absorbed by the sanitary goods (for example, about 10 to 20% by mass of the maximum absorption) ).
- water is brought up to an amount close to the maximum absorption of the used sanitary goods (for example, 80 mass% or more of the maximum absorption) Absorb
- the whole used sanitary goods are heated to the temperature of the hot water.
- the used sanitary goods can be made to be in a state of being greatly expanded with warm water or normal temperature water (hereinafter, also simply referred to as "hot water”).
- hot water normal temperature water
- the used sanitary goods will have a very high internal pressure.
- the purpose of making water warm is mainly to weaken the adhesive strength of the adhesive as described later.
- the used sanitary goods are used by being soaked in warm water if they are in a rolled or folded state with the back sheet facing outward (with the top sheet hidden inside).
- the absorbent article of the sanitary product absorbs warm water in the warm water and expands.
- the internal pressure of the used sanitary goods is increased, and the force for opening the used sanitary goods is exerted, and the used sanitary goods in the rolled or folded state go outside. Open towards you and become generally flat. In other words, the used sanitary goods can be spread flat in hot water.
- the absorber absorbs a large amount of hot water and is greatly expanded, so either the front sheet, that is, any part of the top sheet and the back sheet that wraps the absorber is used. It seems to be easily broken. That is, by the pretreatment step S11, the used sanitary goods can be in a state where any surface is likely to be torn or broken. In addition, when the used sanitary goods are in the state expand
- the used sanitary goods are soaked in warm water and / or absorb warm water to soften the adhesive (for example, hot melt adhesive) used for bonding between respective components by the heat of warm water.
- the adhesive for example, hot melt adhesive
- the adhesive for bonding the peripheral portion of the top sheet and the peripheral portion of the back sheet can be softened by the heat of warm water to reduce the bonding strength of the adhesive.
- the adhesive for bonding the top sheet and the absorber, and the adhesive for bonding the back sheet and the absorber can be softened by the heat of warm water, and the bonding strength of these adhesives can be reduced.
- expansion of the absorber of the used sanitary product causes any part of the surface of the used sanitary product to be broken, and the bonding strength of the adhesive is reduced.
- the state can be generated.
- the used sanitary goods can be reliably disassembled in the decomposition process described later.
- the temperature of the hot water in the pretreatment step S11 is not particularly limited as long as the adhesive of the used sanitary goods can be softened, but, for example, 60 ° C. or more can be mentioned, preferably 70 ° C. or more and 98 ° C. or less.
- the adhesive for joining the component members can be further softened by the heat of the hot water, and the bonding strength of the adhesive can be further reduced.
- the temperature of the hot water is surely present as a liquid, so that the used sanitary goods can more reliably absorb the hot water.
- the expansion of the absorber and the heat of the hot water can more reliably generate a state in which the surface of the used sanitary product is likely to break and the adhesive strength of the adhesive is reduced.
- measure the temperature of hot water in a state where used sanitary goods are soaked, or the temperature 5 mm inside from the surface of used sanitary goods that absorbed water to an amount close to the maximum absorption Measure the tip of the temperature sensor.
- sterilization of constituent materials is extremely important in reuse of used sanitary goods.
- the temperature of the hot water By setting the temperature of the hot water to 70 ° C. or higher, it is possible to exert an effect of sterilizing (disinfecting) used sanitary goods, which is preferable.
- the treatment time in the pretreatment step S11 that is, the time for which the used sanitary goods are immersed in warm water is not particularly limited as long as the absorber of the used sanitary goods can expand, and is, for example, 2 to 60 minutes, Preferably, it is 4 to 30 minutes. If the time is too short, the absorbent can not expand sufficiently, and if it is too long, time is wasted and the treatment cost is unnecessarily increased.
- the amount of absorption of warm water of the absorbent in the pretreatment step S11 is not particularly limited as long as the absorbent can expand to such an extent that the used sanitary goods can be decomposed in the decomposition step described later. 80 mass% or more of the maximum absorption of is mentioned, Preferably it is 90 mass% or more.
- the used sanitary goods can be fully inflated with water. As a result, it is possible to generate an extremely high internal pressure in the absorber of the used sanitary product.
- the maximum absorption is measured by the following procedure. (1) Dry the unused sanitary goods in an atmosphere of 100 ° C. or higher, and measure the weight of the sanitary goods. (2) When an elastic material (for example, an elastic member such as around a leg, around a waist, or the like) that can form a pocket that makes it difficult for water to reach the absorber is disposed on the sanitary product, cut the elastic member Flatten the hygiene products by putting (3) In a water bath filled with sufficient tap water, immerse the hygiene products with the top sheet down and leave for 30 minutes. (4) After standing, place the hygienic article on the net with the top sheet down, drain for 20 minutes, and measure the mass of the hygienic article. And the mass difference before and behind immersion in tap water is defined as maximum absorption.
- an elastic material for example, an elastic member such as around a leg, around a waist, or the like
- the decomposition step S12 applies physical impact to the plurality of used sanitary goods expanded and swollen in the pretreatment step S11 to form the plurality of used sanitary goods into a film (back sheet), non-woven fabric (surface The sheet is broken into core wrap and an absorbent core (eg, an absorbent and a superabsorbent polymer).
- an absorbent core eg, an absorbent and a superabsorbent polymer
- the used sanitary goods are unfolded and flattened by the pretreatment step S11, and any part of the surface is likely to break off due to expansion, and in the present embodiment, the adhesive particularly due to the heat of warm water
- the bonding strength of the is reduced. Therefore, by applying physical impact to the used sanitary goods in that state in the disassembly step S12, the front sheet (non-woven fabric) and the back sheet (in particular, the bonding strength is reduced) at any part of the surface.
- the joint with the film is broken. Thereby, the joint portion can be torn off.
- the physical impact is not particularly limited, but, for example, a method of striking the used sanitary goods on a surface made of a material harder than the used sanitary goods, the used sanitary goods are disposed facing each other. There is a method of pressing from both sides while passing between a pair of rolls while passing.
- the disassembling step S12 includes the steps of: throwing a plurality of used sanitary goods that have swelled into the bottom of the rotating drum whose rotating shaft is horizontal; and rotating the rotating drum about the rotating shaft, Pulling up the plurality of used sanitary products to the top of the rotating drum and tapping on the bottom.
- the rotary drum include a rotary drum of a washing tub of a horizontal washing machine, and thus the disassembling step S12 can be performed using an existing horizontal washing machine (for example, ECO-22B manufactured by Inamoto Seisakusho Co., Ltd.).
- the size of the rotating drum is not particularly limited as long as the impact can be realized, and the inner diameter and the depth may be, for example, 50 to 150 cm and 30 to 120 cm.
- the rotational speed of the rotary drum is not particularly limited as long as the above-mentioned impact can be realized, and may be, for example, 30 times / minute to 100 times / minute.
- the temperature of used sanitary goods is kept relatively high by the warm water absorbed in the used sanitary goods, from the viewpoint of suppression of the temperature drop of the adhesive and maintenance of the effect of sterilization, 70 degreeC or more is preferable and, as for the temperature of the atmosphere in a rotating drum, 75 degreeC or more is more preferable.
- the temperature in the rotating drum is preferably 98 ° C. or less, more preferably 90 ° C. or less, from the viewpoint of handling of the used sanitary goods. It is preferred that the water in the rotating drum be as low as possible, preferably at least so low that the used sanitary products at the bottom are not below the water surface.
- the time during which the rotary drum is rotated is not particularly limited as long as the front sheet, back sheet, core wrap, etc. and the absorbent core can be disassembled, and is, for example, 2 to 40 minutes, preferably 4 to 20 minutes. It is.
- the used sanitary goods tear and tear the joint between the top sheet (non-woven fabric) and the back sheet (film) by physical impact.
- the absorbent core e.g., pulp fibers and superabsorbent polymer
- the used sanitary goods comes out (pops out) through the crevices by the internal pressure of the absorbent.
- the used sanitary goods can be more reliably decomposed into a front sheet (non-woven fabric), a back sheet (film), a core wrap, etc., and an absorbent core (eg, pulp fiber and super absorbent polymer) .
- the separation step S13 separates a plurality of films (backsheets), a plurality of nonwoven fabrics (topsheets), core wraps, and the like, and an absorbent core (for example, pulp fibers and a superabsorbent polymer).
- the non-woven fabric may be bonded to the film.
- the separation method is not particularly limited, and examples thereof include a method using a sieve that passes the absorbent core without passing through the top sheet, the back sheet, the core wrap, and the like.
- the separation step S13 includes an inactivation step S31 in which the superabsorbent polymer is inactivated with an aqueous solution containing an inactivation agent before separating the film, the nonwoven fabric, the core wrap, etc., and the absorbent core, And the first separation step S32 of separating the non-woven fabric and the mixture containing pulp fibers, the inactivated superabsorbent polymer, and the wastewater discharged from the superabsorbent polymer by inactivation.
- the top sheet (non-woven fabric), the back sheet (film) and the absorber (pulp fibers and superabsorbent polymer) can be activated with the superabsorbent polymer.
- the superabsorbent polymer adhering to the top sheet, back sheet and pulp fiber can be inactivated.
- the highly absorbent polymer in the high viscosity state can be converted to the highly absorbent polymer in the low viscosity state by inactivation dewatering before the inactivation.
- the inactivating agent is not particularly limited, and examples thereof include acids (for example, inorganic acids and organic acids), lime, calcium chloride, magnesium sulfate, magnesium chloride, aluminum sulfate, aluminum chloride and the like.
- the above acid is preferable because it does not leave ash in the pulp fiber.
- the pH is preferably 2.5 or less, and more preferably 1.3 to 2.4.
- the pH is too high, the water absorbing ability of the superabsorbent polymer can not be sufficiently reduced. In addition, the sterilization capacity may be reduced. If the pH is too low, there is a risk of corrosion of the equipment, and a large amount of alkali chemicals are required for neutralization treatment during wastewater treatment.
- examples of the inorganic acid include sulfuric acid, hydrochloric acid and nitric acid, but sulfuric acid is preferable from the viewpoint of containing no chlorine, cost and the like.
- examples of the organic acid include citric acid, tartaric acid, glycolic acid, malic acid, succinic acid, acetic acid, ascorbic acid and the like, and acids capable of forming a complex with metal ions contained in excrement, such as citric acid
- Particularly preferred are hydroxycarbonate-based organic acids such as acids, tartaric acid and gluconic acid.
- calcium ion is mentioned as a metal ion contained in excretion.
- the treatment temperature in the inactivation step S31 that is, the temperature of the aqueous solution containing the inactivating agent is not particularly limited as long as the inactivation reaction proceeds.
- the treatment temperature may be room temperature or may be higher than room temperature, and examples thereof include 15 to 30 ° C.
- the treatment time of the inactivation step S31 that is, the time for immersing the top sheet, back sheet and absorber in an aqueous solution containing an inactivating agent is not particularly limited as long as the superabsorbent polymer is inactivated and dehydrated. For example, 2 to 60 minutes can be mentioned, preferably 5 to 30 minutes.
- the amount of the aqueous solution in the inactivation step S31 is not particularly limited as long as the inactivation reaction proceeds.
- the amount of the aqueous solution is, for example, preferably 300 to 3000 parts by mass, more preferably 500 to 2500 parts by mass, and still more preferably 1000 to 2000 parts by mass with respect to 100 parts by mass of the used sanitary goods.
- the waste water is a waste water containing water released from the superabsorbent polymer by dehydration with an aqueous solution containing an inactivating agent in the inactivation step S31, that is, waste water containing excrement-derived liquid and warm water-derived water.
- the method of separating the top sheet and the back sheet, the pulp fibers, the highly absorbent polymer, and the sewage is not particularly limited.
- the product (front sheet, back sheet, pulp fiber, super absorbent polymer, sewage, etc.) produced by the inactivation step is discharged through a screen with 5 to 100 mm openings, preferably 10 to 60 mm openings. .
- pulp fibers, highly absorbent polymer and sewage can be separated in drainage while the top and back sheets remain on the screen to separate the products.
- Other large-sized objects such as non-woven fabrics and films may remain on the screen.
- the superabsorbent polymer since the superabsorbent polymer is in a highly viscous state prior to inactivation, it is not easy to separate the superabsorbent polymer attached to the top sheet, back sheet and pulp fibers. However, after deactivation, dewatering brings the superabsorbent polymer into a low viscosity state, so that the superabsorbent polymer attached to the topsheet, backsheet and pulp fibers is removed from the topsheet, backsheet and pulp fibers. It can be easily separated. Therefore, the components of the sanitary good can be efficiently separated and recovered.
- the separation step S13 may further include a second separation step S33 in which the adhesive in the joint portion is removed by a solvent that dissolves the adhesive in the joint portion between the film and the other member.
- the adhesive in each bonding portion is removed by a solvent that dissolves the adhesive in each bonding portion between the film, the non-woven fabric, and the absorber.
- the adhesive on the bonding portion between the film (back sheet) and other members is removed by a solvent.
- the film and the other members can be separated from each other without breaking or the like while maintaining the shape as it is. Therefore, components such as films of sanitary products can be efficiently recovered.
- the film and the other members can be separated without leaving an adhesive on the film, the film can be reused as a resin with high purity. Thereby, it is possible to suppress the adverse effect of the adhesive when the film is reused.
- the non-woven fabric is also similar to the film.
- the solvent used in the second separation step S33 is not particularly limited as long as it can dissolve the adhesive, but, for example, terpene containing at least one of terpene hydrocarbon, terpene aldehyde and terpene ketone It can be mentioned.
- an aqueous solution containing tempel is used, and the concentration of tkov in the aqueous solution is, for example, 0.05% by mass or more and 2% by mass or less. Preferably, it is 0.075 to 1% by mass. If the concentration of terpene is too low, it may not be possible to dissolve the adhesive at the joints. If the concentration of terpene is too high, the cost may be high.
- Tempel In addition to dissolving adhesives such as hot melt adhesives, Tempel also has an oil stain cleaning effect. Therefore, for example, when there is printing on a component of a sanitary product such as a back sheet, the tempel can also decompose and remove the printing ink.
- terpene hydrocarbons examples include myrcene, limonene, pinene, camphor, sapinene, ferandrene, paracymene, ocimene, terpinene, carene, zingiberene, caryophyllene, bisabolene, and cedrene.
- limonene, pinene, terpinene and carene are preferable.
- terpene aldehydes examples include citronellal, citral, cyclocitral, safranal, ferandral, perylaldehyde, geranial and neral.
- terpene ketones examples include camphor and tsuyoshi.
- terpene hydrocarbons are preferred, and limonene is particularly preferred.
- limonene is particularly preferred.
- the terpenes can be used alone or in combination of two or more.
- the processing temperature of the second separation step S33 that is, the temperature of the aqueous solution containing the solvent is not particularly limited as long as the dissolution of the adhesive proceeds and the used sanitary goods are decomposed into components.
- the treatment temperature may be room temperature or may be higher than room temperature, and examples thereof include 15 to 30 ° C.
- the processing time of the second separation step S33 that is, the time for immersing the top sheet, the back sheet and the absorber in an aqueous solution containing a solvent, the dissolution of the adhesive progresses and the used sanitary goods are disassembled into components.
- the treatment time is, for example, 2 to 60 minutes, preferably 5 to 30 minutes.
- the amount of the aqueous solution in the second separation step S33 is not particularly limited as long as the dissolution of the adhesive proceeds and the used sanitary goods are decomposed into components.
- the amount of the aqueous solution is, for example, preferably 300 to 3000 parts by mass, more preferably 500 to 2500 parts by mass with respect to 100 parts by mass of the used sanitary goods.
- the amount of the adhesive remaining in the film, the nonwoven fabric, the absorber and the like can be 1% by mass or less with respect to the film, the nonwoven fabric, the absorber and the like.
- the second separation step S33 may be performed in the inactivation step S31. That is, while inactivating the superabsorbent polymer attached to the top sheet, back sheet and pulp fiber, the adhesive attached to the top sheet, back sheet and pulp fiber may be dissolved. In this case, an aqueous solution containing both the deactivator and the solvent is used as an aqueous solution in which the top sheet, the back sheet, the pulp fibers and the superabsorbent polymer are immersed.
- the back sheet (film), the top sheet (non-woven fabric), and the absorbent body (pulp fibers and superabsorbent polymer) can be substantially separated in the aqueous solution.
- the back sheet (film) and the top sheet (non-woven fabric) can be separated from the absorber (pulp fiber and super absorbent polymer), and the second separation step S33 can be omitted.
- the back sheet (film) and the top sheet (non-woven fabric) are substantially separated by the removal of the adhesive.
- the film is dried by an atmosphere or hot air at a temperature higher than room temperature to further remove the first drying step S34 for removing the solvent. May be included.
- the nonwoven fabric is also dried in this step.
- the first drying step S34 a step of drying the separated film (back sheet) and the non-woven fabric (top sheet) in a high temperature atmosphere, hot air or the like is performed.
- the drying temperature is, for example, 105 to 210 ° C., preferably 110 to 190 ° C.
- the drying time is, for example, 10 to 120 minutes, preferably 15 to 100 minutes.
- the separation step S13 may include a third separation step S35 of separating pulp fibers from the separated mixture.
- the method for separating pulp fibers from the separated mixture is not particularly limited.
- the separated mixture may be Discharge while passing through a screen of 1 to 4 mm, preferably 0.15 to 2 mm.
- the superabsorbent polymer and the sewage can be separated from the mixture by leaving the pulp fibers (mainly the superabsorbent polymer remaining on the surface) on the screen in the drainage.
- this pulp fiber contains many impurities, depending on the application, it can be reused in this state.
- a high absorbency polymer is attached to the separated pulp fibers, and the separated pulp fibers and the high absorbency polymer attached to the pulp fibers are mixed with water at a predetermined ratio.
- the process proceeds to the ozone treatment step S36 as a mixed solution.
- the separation step S13 is a high absorption property in which a mixed solution containing a superabsorbent polymer and pulp fibers, their connecting structure, and water is treated with an aqueous solution containing ozone, and the pulp fibers are adhered. It includes an ozone treatment step S36 for reducing the molecular weight, solubilizing and removing the polymer.
- the superabsorbent polymer enlarges as it absorbs fluid such as body fluid, and the pulp fibers are involved, (ii) enlargement
- the plurality of superabsorbent polymers and the plurality of pulp fibers form a connected structure, for example, by causing gel blocking while winding up the pulp fibers into one another.
- the liquid mixture includes free pulp fibers and free super absorbent polymer, as well as a connecting structure composed of a plurality of super absorbent polymers and a plurality of pulp fibers.
- the superabsorbent polymer contained in the mixed liquid (treatment liquid) is oxidatively decomposed with ozone in the aqueous solution and removed by solubilization in the aqueous solution.
- the state in which the superabsorbent polymer is oxidatively decomposed and solubilized in an aqueous solution refers to the state in which the superabsorbent polymer and the connecting structure pass through a 2 mm screen.
- impurities such as superabsorbent polymers can be removed from the mixed solution (treatment solution) to produce pulp fibers with high purity.
- secondary sterilization, bleaching and deodorization of pulp fibers can be performed by ozone treatment.
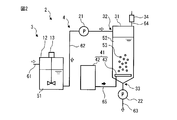
- FIG. 2 is schematic which shows an example of a structure of the apparatus 2 which performs ozone treatment process S36.
- the device 2 includes a mixed liquid storage unit 3 for storing a mixed liquid 51 containing water, pulp fibers separated in the third separation step S35, and a superabsorbent polymer, and high absorption contained in the mixed liquid 51. And an ozonized portion 4 for oxidatively decomposing and removing from the pulp fiber.
- the liquid mixture storage unit 3 includes a liquid mixture tank 12 and a stirrer 13.
- the liquid mixture tank 12 stores the liquid mixture 51 supplied through the pipe 61.
- the stirrer 13 stirs the mixed solution 51 in the mixed solution tank 12 so that the pulp fibers and the superabsorbent polymer in the mixed solution 51 are not separated from water and sink below the mixed solution 51.
- the ozone processing unit 4 includes a supply pump 21, a treatment tank 31, an ozone supply device 41, a delivery pump 22, and an ozone decomposition device 34.
- the treatment tank 31 has an acidic aqueous solution as the treatment liquid 52.
- the processing tank 31 includes a mixed liquid supply port 32, a processing liquid outlet 33, and an ozone-containing gas supply port 43.
- the mixed solution supply port 32 is disposed above the mixing tank 31 and supplies the mixed solution 51 to the processing tank 31.
- the treatment liquid outlet 33 is disposed below the mixing tank 31 and discharges the treatment liquid 52.
- the ozone-containing gas supply port 43 is disposed in the lower part of the mixing tank 31, specifically, in the upper part of the processing liquid outlet 33, and delivers the ozone-containing gas 53 into the processing tank 31.
- the supply pump 21 continuously supplies the mixed solution 51 of the mixed solution tank 12 through the pipe 62 from the mixed solution supply port 32 into the processing tank 31 at a first flow rate.
- the ozone supply device 41 supplies the ozone containing gas 53 to the treatment tank 31.
- Examples of the ozone generator 42 of the ozone supply device 41 include an ozone water exposure tester ED-OWX-2 manufactured by Ecodesign Co., Ltd., an ozone generator OS-25V manufactured by Mitsubishi Electric Corporation, and the like.
- the ozone containing gas 53 is another kind of gas containing ozone, for example, oxygen gas containing ozone.
- the ozone-containing gas supply port 43 sends the ozone-containing gas 53 supplied to the treatment tank 31 through the pipe 65 into the treatment tank 31 and is disposed at the lower portion (preferably the bottom) of the treatment tank 31.
- the ozone-containing gas supply port 43 continuously supplies the ozone-containing gas 53 into the treatment liquid 52 as a plurality of fine bubbles from the lower part to the upper part of the treatment liquid 52 (treatment tank 31).
- the delivery pump 22 continuously discharges the treatment liquid 52 in the treatment tank 31 through the pipe 63 from the treatment liquid outlet 33 to the outside of the treatment tank 31 at a second flow rate.
- the ozonolysis device 34 receives the ozone-containing gas 53 accumulated in the upper portion of the treatment tank 31 via the pipe 64, detoxifies ozone, and discharges the ozone to the outside.
- the treatment liquid 52 in the treatment tank 31 is only the treatment liquid 52 before the start of the ozone treatment step S36, and after the start, it becomes a liquid in which the treatment liquid 52 and the liquid mixture 51 are mixed.
- the liquid in the processing tank 31 is defined as the processing liquid 52 including the liquid in which the processing liquid 52 and the mixed liquid 51 are mixed.
- the pulp fibers and the superabsorbent polymer separated in the third separation step S35 are mixed with water so as to have a concentration set in advance, and become the mixed liquid 51.
- the concentration of pulp fibers of the mixed solution 51 is set so as to be a preset concentration in a state of being introduced into the treatment tank 31 and mixed with the treatment liquid 52.
- the mixed solution 51 is supplied to the mixed solution tank 12 via the pipe 61 and stored. Since the specific gravity of the pulp fiber and the superabsorbent polymer is larger than 1, the mixed solution 51 is stirred by the stirrer 13 in the mixed solution tank 12 so that the pulp fiber and the superabsorbent polymer are not separated from water.
- the flow rate of the mixed liquid 51 in the mixed liquid tank 12 is controlled by the supply pump 21 and continuously supplied from the mixed liquid supply port 32 to the processing tank 31 through the pipe 62 at a first flow rate.
- the treatment liquid 52 is an acidic aqueous solution and has a specific gravity of approximately 1. Thus, the pulp fibers and the superabsorbent polymer settle from the top to the bottom of the treatment liquid 52.
- the ozone-containing gas 53 generated by the ozone generator 42 is supplied to the treatment tank 31 through the pipe 65, and fine bubbles (for example, micro bubbles) in the treatment liquid 52 from the ozone-containing gas supply port 43 of the treatment tank 31.
- fine bubbles for example, micro bubbles
- the ozone-containing gas 53 rises from the lower part to the upper part of the treatment liquid 52.
- the ozone-containing gas 53 adheres to the surface of the pulp fiber and the superabsorbent polymer, and the connecting structure.
- the ozone in the ozone-containing gas 53 oxidatively decomposes the free super-absorbent polymer and dissolves it in the treatment liquid 52.
- the superabsorbent polymer on the pulp fibers is thereby removed from the pulp fibers.
- the pulp fiber descends to the bottom of the treatment tank 31, and the ozone-containing gas 53 escapes to the space above the treatment tank 31.
- the relatively high specific gravity free superabsorbent polymer and the connected structure containing the superabsorbent polymer have relatively low specific gravity It tends to be more settleable than free pulp fibers.
- the ozone containing gas consumes ozone and rises while processing the highly absorbent polymer and the pulp fiber, the ozone containing gas present at the lower position is higher than the ozone containing gas present at the upper position. And the ozone content tends to be high (ie, fresh).
- the free superabsorbent polymer and the connecting structure having a relatively rapid downward movement can be appropriately oxidatively decomposed with a fresher ozone-containing gas to form free pulp fibers.
- the ozone-containing gas can process the free pulp fibers as well as the pulp fibers for forming cellulose nanofibers over time.
- the lignin of the pulp fiber (as well as the pulp fiber for cellulose nanofiber formation) can be decomposed by the ozone in the ozone-containing gas colliding against the pulp fiber while colliding with it.
- the processing liquid 52 (including pulp fiber for cellulose nanofiber formation) at the bottom of the processing tank 31 is treated with the flow control of the delivery pump 22 from the processing liquid outlet 33 of the processing tank 31 through the pipe 63. Out continuously at a second flow rate.
- the ozone of the ozone-containing gas 53 accumulated in the upper part of the treatment tank 31 is detoxified by the ozonolysis device 34 and released to the outside.
- the mixed solution 51 is continuously supplied from the upper portion of the processing tank 31 into the processing tank 31 at the first flow rate, and the processing liquid 52 is discharged from the lower portion (bottom) of the processing tank 31 to the outside of the processing tank 31. It is discharged continuously at the second flow rate.
- a continuous and stable flow of fluid (including pulp fibers) from top to bottom can be forcibly generated in the treatment tank 31.
- the treatment liquid 52 discharged from the treatment tank 31 contains pulp fiber for cellulose nanofiberification from which the superabsorbent polymer has been removed, and contains low molecular weight organic matter generated by oxidative decomposition of the superabsorbent polymer. It is.
- the pulp fiber for forming cellulose nanofibers is recovered at a step downstream of the delivery pump 22, for example, at a fourth separation step S37 described later.
- the method continuously supplies, at a first flow rate, a mixed solution 51 containing at least a pulp fiber and a superabsorbent polymer into a treatment tank 31 having a treatment liquid 52 capable of dissolving the superabsorbent polymer. While containing the pulp fiber for cellulose nanofiber formation from which the super-absorbent polymer has been removed, the treatment liquid 52 containing the low-molecular-weight organic substance produced by the oxidative decomposition of the super-absorbent polymer is provided outside the treatment tank 31. , Discharge continuously at a second flow rate.
- a continuous and stable fluid (pulp fibers) is discharged from the mixed liquid supply port 32 for supplying the mixed liquid 51 in the processing tank 31 to the treated liquid outlet 33 for discharging the treated liquid 52.
- the fluid flow i.e., the water flow, allows the treatment of the superabsorbent polymer (solubilization) and the treatment of the pulp fibers, even at high throughputs of pulp fibers and superabsorbent polymer.
- the first flow rate and the second flow rate be the same.
- the amount of the treatment liquid 52 in the treatment tank 31 can be kept constant, and stable continuous treatment is possible.
- the first flow rate and the first flow rate may change with time. That is, the first flow rate and the second flow rate do not always have to be completely the same, and may be substantially the same on average over time.
- substantially identical means that the difference between the first flow rate and the second flow rate is within 5% by mass. Also in this case, stable and continuous processing is possible.
- the concentration of ozone in the treatment liquid 52 is not particularly limited as long as it is a concentration capable of oxidatively decomposing the superabsorbent polymer, for example, 1 to 50 mass ppm And preferably 2 to 40 mass ppm, more preferably 3 to 30 mass ppm. If the ozone concentration in the treatment liquid 52 is too low, the superabsorbent polymer can not be completely solubilized, and there is a possibility that the superabsorbent polymer may remain in the pulp fiber. Conversely, if the ozone concentration in the treatment liquid 52 is too high, the oxidizing power is also increased, which may damage the pulp fibers, and may also cause safety problems.
- the ozone treatment temperature is not particularly limited as long as it is a temperature at which the superabsorbent polymer can be oxidatively decomposed, and may be, for example, room temperature or may be higher than room temperature.
- the concentration of ozone in the treatment liquid 52 is measured by the following method.
- (1) 85 mL of the processing solution 52 in which ozone is dissolved is reacted with 100 mL graduated cylinder containing about 0.15 g of potassium iodide and 5 mL of 10% citric acid solution.
- (3) The concentration of ozone in the aqueous solution is calculated using the following equation.
- the ozone concentration in the ozone-containing gas 53 is preferably 40 to 200 g / m 3 , more preferably 40 to 150 g / m 3 , and still more preferably 40 to 100 g / m 3 . If the ozone concentration in the ozone-containing gas 53 is too low, the superabsorbent polymer can not be completely solubilized, and the superabsorbent polymer may remain. If the concentration in the ozone-containing gas 53 is too high, it may lead to damage to pulp fibers, a decrease in safety, and an increase in manufacturing costs.
- the ozone concentration in the ozone-containing gas 53 can be measured, for example, by an ultraviolet absorption type ozone densitometer (for example, an ozone monitor OZM-5000G manufactured by Ecodesign Co., Ltd.).
- the concentration of the pulp fiber and the superabsorbent polymer in the treatment liquid 52 is not particularly limited as long as it is a concentration capable of oxidatively decomposing the superabsorbent polymer by ozone in the treatment liquid 52, for example, 0.1 to 20% by mass is mentioned, preferably 0.2 to 10% by mass, and more preferably 0.3 to 5% by mass. If the concentration of pulp fiber is too high, the superabsorbent polymer can not be completely solubilized, and the superabsorbent polymer may remain in the pulp fiber. Conversely, if the concentration of pulp fibers is too low, the oxidizing power is also increased, which may damage the pulp fibers and may cause safety problems.
- the concentrations of the pulp fibers and the superabsorbent polymer in the mixed solution 51 are appropriately set based on the concentrations of the pulp fibers and the highly absorbent polymer in the treatment solution 52 and the amount of the treatment solution 52.
- the treatment liquid 52 is preferably acidic. More preferably, the pH of the treatment liquid 52 is more than 0 and 5.0 or less, and more preferably 1.5 to 2.5. By treating in an acidic state, the deactivation of ozone is suppressed, the oxidative decomposition effect of the highly absorbent polymer by ozone is improved, and the highly absorbent polymer can be oxidatively decomposed in a short time.
- the pH of the mixed solution 51 may be made the same as the pH of the treatment liquid 52, and the mixed solution 51 may be supplied to the treatment tank 31.
- the pH of the processing solution 52 may be monitored by a pH sensor, and a predetermined acidic solution may be added to the processing solution 52 by an amount according to the fluctuation range when the pH changes to the neutral side.
- the volume V of the processing tank 31 is not particularly limited, and may be, for example, 50 to 80 L.
- the flow rate R O (unit: L / min) of the ozone-containing gas and the volume V (unit: L) of the treatment liquid 52 in the treatment tank 31 satisfy 0.01 ⁇ R O /V ⁇ 1.25 preferable. More preferably, 0.03 ⁇ R O /V ⁇ 1.0, and still more preferably, 0.06 ⁇ R O /V ⁇ 0.75. If R O / V is too small, the superabsorbent polymer can not be completely solubilized, and the superabsorbent polymer may remain in the pulp fiber. If R O / V is too large, it may lead to pulp fiber damage, reduced safety and increased production costs.
- As the flow rate R O of the ozone-containing gas is not particularly limited, for example, 3 ⁇ 6L / min.
- the time is not particularly limited as long as The in-tank treatment time may be short if the ozone concentration of the treatment liquid 52 is high, and it will be long if the ozone concentration of the treatment liquid 52 is low.
- the in-tank treatment time may be, for example, 2 minutes to 60 minutes, preferably 5 minutes to 30 minutes.
- the product (hereinafter also referred to as “CT value”) of the ozone concentration (mass ppm) in the treatment liquid 52 and the treatment time (minute) in the tank is preferably 100 to 6000 ppm ⁇ min, more preferably 200 to 4000 ppm And more preferably 300 to 2000 ppm ⁇ minute. If the CT value is too small, the superabsorbent polymer can not be completely solubilized, and there is a possibility that the superabsorbent polymer may remain in the recovered pulp fiber. If the CT value is too large, it may lead to pulp fiber damage, reduced safety and increased production costs.
- the superabsorbent polymer is oxidatively decomposed into low molecular weight components by ozone and dissolved in the treatment liquid 52.
- the low molecular weight components dissolved in the treatment liquid 52 are discharged together with the treatment liquid 52.
- the disinfecting action of ozone primarily disinfects used sanitary goods.
- the ozone treatment step S36 continuously supplies the mixed solution 51 from the upper part of the treatment tank 31 while the treatment liquid 52 is added from the lower part of the treatment tank 31. And a step of discharging continuously. Since the specific gravity of the pulp fibers and the superabsorbent polymer in the mixed solution 51 is larger than the specific gravity of the water of the treatment solution 52, the pulp fibers and the highly absorbent polymer, and the connecting structure naturally settle.
- the treatment liquid 52 capable of dissolving the superabsorbent polymer is an aqueous solution containing an ozone-containing gas capable of dissolving and oxidizing the superabsorbent polymer.
- the ozone treatment step S36 (continuous treatment step) further includes a delivery step of continuously delivering a plurality of bubbles of the ozone-containing gas from the lower portion to the upper portion of the treatment liquid 52.
- the ozone-containing gas is increased, and the pulp fibers and the superabsorbent polymer are lowered, that is, countercurrent.
- the contact probability of the pulp fiber and the superabsorbent polymer with the ozone-containing gas can be increased.
- the deeper the pulp fibers and superabsorbent polymer settle the more it can be in contact with higher concentrations of ozone containing gas. Therefore, the superabsorbent polymer which can not be completely dissolved in the processing solution 52 only by the ozone containing gas which is in contact with the processing solution 52 at a shallow position can be contacted with the high concentration ozone containing gas in the processing solution 52 deep.
- the superabsorbent polymer can be reliably dissolved in the treatment liquid 52.
- the superabsorbent polymer can be reliably dissolved in the treatment solution and removed from the fibers.
- the above-mentioned delivery step includes the step of delivering the ozone-containing gas in the form of microbubbles or nanobubbles.
- the microbubbles are bubbles having a diameter of about 1 to 1000 ⁇ m, preferably about 10 to 500 ⁇ m, and the nanobubbles refer to a bubble having a diameter of about 100 to 1000 nm, preferably about 100 to 500 nm.
- the microbubbles or nanobubbles are such fine bubbles, and have a property that the surface area per unit volume is large and the rising speed in the liquid is slow. Therefore, in the present method, as a preferable embodiment, the ozone-containing gas of such fine bubbles is sent from the lower part to the upper part of the processing liquid 52 of the processing tank 31.
- pulp fibers and superabsorbent polymers move from the top to the bottom.
- the fine bubbles rise at a low speed, the probability of the bubbles contacting the pulp fibers can be increased.
- the fine bubbles have a narrow footprint on the surface of the pulp fibers, more bubbles can contact the surface of the pulp fibers. Thereby, it is possible to uniformly wrap the pulp fibers and the superabsorbent polymer, and the connecting structure with fine bubbles, and to further increase the contact area between them and the ozone-containing gas.
- the more bubbles contact the surface of the pulp fibers the buoyancy of the bubbles reduces the settling of the pulp fibers and superabsorbent polymer, and the connecting structure, and the contact time between them and the ozone-containing gas Can be increased more.
- the superabsorbent polymer can be more reliably dissolved in the treatment liquid 52 and removed from the pulp fibers.
- the treatment liquid 52 is an acidic aqueous solution, for example, an acidic aqueous solution having a pH of 2.5 or less.
- an acidic aqueous solution having a pH of 2.5 or less even when the water absorbing ability partially remains in the superabsorbent polymer in the mixed solution 51, it is possible to suppress the water absorption and expansion of the superabsorbent polymer. Thereby, the superabsorbent polymer can be dissolved in the treatment liquid 52 in a short time, and the superabsorbent polymer can be more reliably removed.
- the treatment liquid 52 is an ozone-containing aqueous solution
- the ozone in the ozone-containing aqueous solution can be hardly inactivated, so that the superabsorbent polymer can be oxidized and decomposed in a shorter time, and can be dissolved. It can be more reliably removed from the fiber.
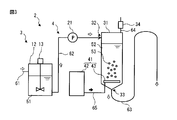
- FIG. 3 is a schematic view showing another configuration example of the apparatus 2 of the ozone treatment process of FIG.
- the pipe 63 of the ozone processing unit 4 has a continuous U-shaped pipe structure in which two U-shaped pipes are connected in reverse and continuously to each other, as compared with the apparatus 2 of FIG. The difference is that the delivery pump 22 is omitted.
- the pipe 63 is filled with the processing liquid 52, and the level of the liquid surface of the processing liquid 52 in the processing tank 31 is higher than the level of the liquid level in the tank of the next step connected by the pipe 63.
- the treatment liquid 52 is discharged to the tank of the next process through the pipe 63 by the principle of siphon. Therefore, if the height of the liquid surface of the processing liquid 52 in the processing tank 31 and the height of the liquid surface of the liquid in the next step are made the same initially before the start of the processing, the processing is started by the start of the processing.
- the level of the liquid level in the tank of the next step the height before the start of the treatment is maintained during the treatment. In this case, the delivery pump 22 is unnecessary, and control of the second flow rate of the delivery pump 22 is unnecessary.
- the separation step S13 further includes a fourth separation step S37 for separating pulp fibers from the treatment liquid 52 discharged from the treatment tank 31, and a second drying step for drying the separated pulp fibers. And S38 may be included.
- the method for separating pulp fibers from the processing solution 52 discharged from the processing tank 31 is not particularly limited.
- the processing solution 52 containing pulp fibers for forming cellulose nanofibers may be, for example, And a method of passing a screen mesh having an opening of 0.15 to 2 mm.
- waste water containing a product of oxidative decomposition of the superabsorbent polymer passes through the screen.
- pulp fibers for cellulose nanofiberation remain on the screen.
- the separated pulp fibers are dried in a high temperature atmosphere, hot air or the like.
- the drying temperature is, for example, 105 to 210 ° C., preferably 110 to 190 ° C.
- the drying time is, for example, 10 to 120 minutes, preferably 15 to 100 minutes.
- pulp fibers can be disinfected (disinfected) in a high temperature atmosphere or hot air.
- pulp fibers for cellulose nanofiberation have a lignin content of 0.1 mass% or less, and preferably lignin content of 0.08 mass% or less, and more preferably 0.06 mass% or less Have a rate. By doing so, the pulp fiber for cellulose nanofiberation is excellent in cellulose nanofiberability.
- lignin content rate of pulp fiber for cellulose nanofibre conversion is described on pages 85 to 87 of “Method for Guide to Soil Diagnosis” published in March 1988, from the Agriculture Guidance Division, Ehime Prefecture Agriculture, Forestry and Fisheries Division. It can be measured according to the method of We will post the summary below.
- the pulp fiber for cellulose nanofiberification preferably has a beating speed reduction rate of 300 mL or more, more preferably 320 mL or more, more preferably 340 mL or more, and still more preferably 360 mL or more.
- the pulp fiber for cellulose nanofiberation becomes easy to be fluffed and to be easily celluloseified in the subsequent cellulose nanofiber formation step.
- the pulp fiber for cellulose nanofiberification preferably has a beating speed reduction rate of 990 mL or less, more preferably 800 mL or less, more preferably 700 mL or less, and still more preferably 600 mL or less.
- a beating speed reduction rate of 990 mL or less, more preferably 800 mL or less, more preferably 700 mL or less, and still more preferably 600 mL or less.
- the above freeness reduction rate is measured in accordance with the following freeness reduction test.
- ⁇ Degrading degree reduction test> Pulp fiber for cellulose nanofibrillation is beaten for 1 hour or more, preferably 2 hours, according to JIS P 8221-1: 1998.
- samples are taken every 20 minutes, and the pulp-freeness test method of JIS P 8121-2: 2012-Part 2: Beating of each sample according to the Canadian Standard Freeness Method ( Measure Canadian Standard freeness.
- the time (h) is plotted on the horizontal axis and the beating degree (mL) is plotted on the vertical axis, approximated to a linear function by the least squares method, and the absolute value of the slope is taken as the beating degree reduction rate (mL / m) adopt.
- the rate of decrease in the degree of freeness means that the greater the value, the faster the decrease in the degree of freeness per unit time, that is, the pulp fibers for forming cellulose nanofibers are more likely to be beaten (fuzzy).
- cellulose nanofiber pulp fibers preferably have a water contact angle of 20 ° or less, more preferably 15 ° or less, and still more preferably 10 ° or less.
- the pulp fiber for producing cellulose nanofibers produced by the above-mentioned production method can be easily dispersed in an aqueous solution for the production of cellulose nanofibers after being dried and stored.
- the water contact angle of the pulp fiber for forming cellulose nanofibers may be 0 °.
- the water contact angle of the cellulose nanofiber pulp fiber can be measured as follows. (1) Temperature: 20 ⁇ 5 ° C and humidity: 65 ⁇ 5% RH in an aluminum ring (outside diameter: 43 mm, inside diameter: 40 mm, height: 5 mm) and dried at 120 ° C for 60 minutes Prepare the saccharified pulp fiber and leave it for 24 hours. (2) Fill 1.5 g of pulp fibers for cellulose nanofibre evenly into an aluminum ring, and use 3 Mpa of pulp fibers for cellulose nanofibre conversion together with aluminum rings using a smooth press machine on the bottom. Compress at pressure for 1 minute to smooth the surface of cellulose nanofiber pulp fiber.
- the water contact angle of the compressed pulp fiber for cellulose nanofiber formation was measured in accordance with JIS R 3257: 1999 “Test method for wettability of substrate glass surface”. Measure according to the static drop method.
- An example of the contact angle measuring device is an automatic contact angle meter CA-V manufactured by Kyowa Interface Science Co., Ltd.
- the water contact angle means a value after 200 ms after dropping deionized water.
- pulp fibers for cellulose nanofiberation are preferably 0.65% by mass or less, more preferably 0.50% by mass or less, still more preferably 0.30% by mass, and still more preferably 0.20% by mass. It has an ash fraction of less than%.
- the above-mentioned ash fraction can be lowered by selecting an acid capable of forming a complex with a metal ion contained in the excrement as an inactivating agent, particularly citric acid, in an inactivating step S31 of inactivating the superabsorbent polymer .
- the ash content refers to the amount of mineral or non-combustible residue left after the organic matter is incinerated, and the ash content refers to the ratio (mass ratio) of the ash content contained in the material to be promoted.
- the said ash fraction is measured according to "5. ash content test method” of "2. General test method” of a physiological treatment article material specification. Specifically, the ash fraction is measured as follows. (1) A platinum, quartz or porcelain crucible is ignited in advance at 500 to 550 ° C. for 1 hour, allowed to cool, and its mass accurately measured. (2) Take 2 to 4 g of pulp fiber for cellulose nanofibers dried at 120 ° C.
- the mixture before the ozone treatment step S36 (continuous treatment step), the mixture is treated with an aqueous solution capable of inactivating the water absorption performance of the superabsorbent polymer to achieve high absorption in the mixture.
- the ozone treatment in the subsequent step is performed.
- the superabsorbent polymer can be more easily dissolved in the treatment liquid 52 in a short time.
- the aqueous solution capable of inactivating the water absorption performance of the superabsorbent polymer in the inactivation step S31 is an acidic aqueous solution, for example, an acidic aqueous solution having a pH of 2.5 or less.
- the aqueous solution capable of inactivating the water absorption performance of the superabsorbent polymer is an acidic aqueous solution, the superabsorbent polymer is more easily inactivated, whereby the step of the inactivation step S31.
- the water absorption performance of the superabsorbent polymer can be suppressed more reliably.
- the superabsorbent polymer can be more easily dissolved in the treatment liquid for a short period of time in the later stage of the ozone treatment step S36 (continuous treatment step).
- the processing tank 31 may include at least a first processing tank 31-1 and a second processing tank 31-2 connected in series with each other.
- FIG. 4 is a schematic view showing another configuration example of the apparatus 2 of the ozone treatment process of FIG. As compared with the apparatus 2 of FIG. 2, the apparatus 2 of FIG. 4 has two ozone processing units 4 joined in series, in other words, the first processing tank 31-1 and the second processing tank 31. -2 differs in that they are joined in series.
- the first processing tank 31-1 is supplied with the mixed solution 51, and the first processed liquid (the processing liquid 52-1 of the first processing tank 31-1) is discharged, and the second processing The processing tank 31-2 is supplied with the first processed liquid, and the mixed liquid 51 is discharged so that the second processed liquid (the processing liquid 52-2 of the second processing tank 31-2) is discharged. It is processed in multiple stages. In that case, treatment is performed with the new treatment solutions 52-1 and 52-2 for each of the first and second treatment vessels 31-1 and 31-2, as compared to the case where one treatment vessel 31 having a large capacity is provided.
- the superabsorbent polymer which can not be dissolved in the first treatment tank (first treatment tank) 31-1 can be easily dissolved in the second treatment tank (second treatment tank) 31-2. Etc.
- the superabsorbent polymer can be more reliably dissolved and removed from the fibers.
- the used sanitary goods are in the form as they are without breaking, etc., and the superabsorbent polymer is not inactivated.
- a very high internal pressure can be generated in the used sanitary product, and any part of the surface can be in a state of being likely to break up.
- the disassembling step S12 by applying a physical impact to the used sanitary goods in such a state, any part of the surface is split to eject the inner absorbent core to the outside. be able to.
- the used sanitary goods can be decomposed into at least a film (back sheet) and an absorbent core.
- the film since the film generally maintains its original shape, it can be easily separated from the absorbent core in the subsequent separation step S13. As a result, a component such as a film can be separated from other components while maintaining its shape without breaking or the like. Therefore, components such as films of sanitary goods can be efficiently recovered.
- the sanitary goods can be easily and cleanly separated, the pulp fibers and the superabsorbent polymer can be separated from the sanitary goods, and the non-woven fabric and the film can be separated separately from each other while leaving the form of the member. That is, pulp fibers, films and non-woven fabrics can be easily recovered separately without crushing sanitary products or going through complicated separation steps.
- limonene When limonene is used as a terpene, it has a refreshing citrus odor as a secondary effect of limonene, so it can cover up the odor derived from excrement to some extent and reduce the burden of odor on workers and the influence of odor on nearby areas. . Because limonene is a monoterpene and similar in structure to styrene, it can dissolve styrenic hot melt adhesives commonly used in hygiene products. Since cleaning of sanitary goods can be performed at normal temperature, energy costs can be reduced and generation and diffusion of odor can be suppressed.
- Terpene has a high oil stain cleaning effect, and in addition to the dissolution effect of the hot melt adhesive, when there is printing on the film, its printing ink can be decomposed and removed, and the printed film can be recovered as a high purity plastic material It is.
- the manufacturing method of the present disclosure can further include a cellulose nanofiber forming step of cellulose nanofiberizing pulp fibers for cellulose nanofiberization after the treatment liquid discharging step.
- the above-mentioned cellulose nanofiber formation step is not particularly limited, and includes cellulose nanofiber formation methods known in the art. Examples of the method for forming cellulose nanofibers include the methods described in JP-A-2010-235681, JP-A-2010-254726, and the like.
- the dried cellulose nanofiber pulp fiber obtained in the second drying step S38 can be subjected to the cellulose nanofiber forming step, or
- the undried pulp fiber for cellulose nanofiberification obtained in the separation step S37 of 4 may be subjected to the cellulose nanofiber formation step or the pulp fiber for cellulose nanofiberification obtained in the ozonation step S36
- the treatment liquid 52 containing can be provided to a cellulose nanofiber formation step.
- Production Example 1 The inactivation step S31 is performed with citric acid of pH 2.0, the ozone treatment step S36 is performed under the above conditions, and the obtained pulp fiber for cellulose nanofiber formation is dried at 120 ° C. for 60 minutes, and used for cellulose nanofiber formation Pulp fiber No. I got one.
- Production Example 2 Pulp fiber No. 1 for cellulose nanofibers is produced in the same manner as in Production Example 1 except that the inactivation step S31 is carried out with calcium hydroxide. I got two.
- Pulp fiber No. 1 for cellulose nanofibers 1 to No. The lignin content (mass%), water contact angle (°) and ash fraction (mass%) of 5 were measured according to the method described herein. The results are shown in Table 1.
- pulp fiber No. 1 for cellulose nanofibers. 1, No. 2 and No. The beating degree reduction rate (mL / h) of 5 and the beating degree (mL) after beating for a predetermined time in the beating degree reduction test was measured according to the beating degree reduction test described in the present specification. The results are shown together in Table 1.
- treatment tank 32 mixed liquid supply port 33 treated liquid outlet 43 ozone containing gas supply port 51 mixed liquid 52 treated liquid 53 ozone contained gas S36 ozonation process
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Environmental & Geological Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Health & Medical Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Toxicology (AREA)
- Mechanical Engineering (AREA)
- Organic Chemistry (AREA)
- Polymers & Plastics (AREA)
- Medicinal Chemistry (AREA)
- Sustainable Development (AREA)
- Wood Science & Technology (AREA)
- Absorbent Articles And Supports Therefor (AREA)
- Apparatus For Disinfection Or Sterilisation (AREA)
- Processing Of Solid Wastes (AREA)
- Separation, Recovery Or Treatment Of Waste Materials Containing Plastics (AREA)
- Paper (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201880040344.1A CN110770394B (zh) | 2017-06-28 | 2018-05-10 | 制造纤维素纳米纤维化用浆粕纤维的方法及纤维素纳米纤维化用浆粕纤维 |
| US16/626,983 US20200131702A1 (en) | 2017-06-28 | 2018-05-10 | Method for producing pulp fibers for cellulose nanofiberization, and pulp fibers for cellulose nanofiberization |
| EP18824513.8A EP3626882B1 (en) | 2017-06-28 | 2018-05-10 | Method for producing pulp fibers for cellulose nanofiberization, and pulp fibers for cellulose nanofiberization |
| US18/153,410 US11987932B2 (en) | 2017-06-28 | 2023-01-12 | Method for producing pulp fibers for cellulose nanofiberization, and pulp fibers for cellulose nanofiberization |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017-126793 | 2017-06-28 | ||
| JP2017126793A JP6669694B2 (ja) | 2017-06-28 | 2017-06-28 | セルロースナノファイバー化用パルプ繊維を製造する方法、及びセルロースナノファイバー化用パルプ繊維 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/626,983 A-371-Of-International US20200131702A1 (en) | 2017-06-28 | 2018-05-10 | Method for producing pulp fibers for cellulose nanofiberization, and pulp fibers for cellulose nanofiberization |
| US18/153,410 Division US11987932B2 (en) | 2017-06-28 | 2023-01-12 | Method for producing pulp fibers for cellulose nanofiberization, and pulp fibers for cellulose nanofiberization |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019003655A1 true WO2019003655A1 (ja) | 2019-01-03 |
Family
ID=64741517
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/018175 Ceased WO2019003655A1 (ja) | 2017-06-28 | 2018-05-10 | セルロースナノファイバー化用パルプ繊維を製造する方法、及びセルロースナノファイバー化用パルプ繊維 |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US20200131702A1 (enExample) |
| EP (1) | EP3626882B1 (enExample) |
| JP (1) | JP6669694B2 (enExample) |
| CN (1) | CN110770394B (enExample) |
| WO (1) | WO2019003655A1 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021095476A (ja) * | 2019-12-16 | 2021-06-24 | ユニ・チャーム株式会社 | セルロースナノファイバーの製造方法及びセルロースナノファイバー |
| WO2021152958A1 (ja) * | 2020-01-31 | 2021-08-05 | ユニ・チャーム株式会社 | カルボキシル化セルロースナノファイバー化用パルプ繊維を製造する方法、及びカルボキシル化セルロースナノファイバー化用パルプ繊維 |
| WO2021213740A1 (de) * | 2020-04-23 | 2021-10-28 | Messer Austria Gmbh | Verfahren und vorrichtung zur herstellung von gebleichtem zellstoff |
| US12410557B2 (en) | 2020-04-23 | 2025-09-09 | Messer Austria Gmbh | Process and apparatus for white liquor oxidation |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3862484B1 (en) * | 2018-11-09 | 2024-05-15 | Unicharm Corporation | Method for producing recycled pulp fibers, recycled pulp fibers, and use for ozone |
| JP6643455B1 (ja) * | 2018-11-09 | 2020-02-12 | ユニ・チャーム株式会社 | セルロースナノファイバー化用パルプ繊維を製造する方法 |
| CN114269535B (zh) * | 2019-08-30 | 2025-03-11 | 尤妮佳股份有限公司 | 制造纤维复合增强材料用的浆粕纤维原料的方法、纤维复合增强材料用的浆粕纤维原料、纤维复合增强材料、粒料、薄膜、纤维、无纺布 |
| JP2023020704A (ja) * | 2021-07-30 | 2023-02-09 | ユニ・チャーム株式会社 | 使用済み吸収性物品の前処理方法及び前処理装置 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000084533A (ja) * | 1998-09-16 | 2000-03-28 | Takeshi Cho | 使用済み紙おむつの使用材料の再生処理方法 |
| JP2010235681A (ja) | 2009-03-30 | 2010-10-21 | Nippon Paper Industries Co Ltd | セルロースナノファイバーの製造方法 |
| JP2010254726A (ja) | 2009-04-21 | 2010-11-11 | Oji Paper Co Ltd | 微細繊維状セルロースの製造方法 |
| WO2015190140A1 (ja) * | 2014-06-12 | 2015-12-17 | ユニ・チャーム株式会社 | 使用済み衛生用品からリサイクルパルプを製造する方法 |
| JP2017100133A (ja) * | 2013-04-10 | 2017-06-08 | ユニ・チャーム株式会社 | 使用済み衛生用品の高分子吸水材を処理する方法および使用済み衛生用品からパルプ繊維を回収する方法 |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0216881A (ja) | 1988-07-05 | 1990-01-19 | Sony Corp | スーパーインポーズ装置 |
| US6238516B1 (en) * | 1991-02-14 | 2001-05-29 | Dana L. Watson | System and method for cleaning, processing, and recycling materials |
| JP3641690B2 (ja) * | 2001-12-26 | 2005-04-27 | 関西ティー・エル・オー株式会社 | セルロースミクロフィブリルを用いた高強度材料 |
| US7713422B2 (en) | 2004-09-07 | 2010-05-11 | K.I. System Co., Ltd. | Black liquor treatment method |
| JP4958097B2 (ja) | 2006-07-19 | 2012-06-20 | 国立大学法人京都大学 | ナノファイバーシート及びその製造方法並びに繊維強化複合材料 |
| EP2183428A4 (en) * | 2007-07-24 | 2012-05-30 | Herbert Gunther Joachim Langner | METHOD AND DEVICE FOR SEPARATING WASTE FROM CELLULOSE FIBERS IN THE TREATMENT OF ALTPAPIER |
| WO2011088889A1 (en) * | 2010-01-19 | 2011-07-28 | Södra Skogsägarna Ekonomisk Förening | Process for production of oxidised cellulose pulp |
| US9586244B2 (en) | 2010-05-01 | 2017-03-07 | Red Bag Solutions | System and method for processing waste material |
| JP6094285B2 (ja) * | 2013-03-14 | 2017-03-15 | 王子ホールディングス株式会社 | 吸収体用基材 |
| EP3054228A4 (en) * | 2013-10-02 | 2017-08-16 | Toray Industries, Inc. | Heat exchange element and heat exchanger |
| JP6038001B2 (ja) | 2013-10-30 | 2016-12-07 | ユニ・チャーム株式会社 | 使用済み衛生用品からリサイクルパルプを製造する方法 |
| JP6646924B2 (ja) * | 2014-09-22 | 2020-02-14 | ユニ・チャーム株式会社 | 使用済み衛生用品からパルプ繊維を回収する方法およびその方法により得られる再生パルプ |
| JP6203158B2 (ja) * | 2014-10-15 | 2017-09-27 | ユニ・チャーム株式会社 | 使用済み衛生用品からリサイクルパルプを製造する方法 |
| JP6161669B2 (ja) * | 2014-12-26 | 2017-07-12 | ユニ・チャーム株式会社 | 使用済み吸収性物品のリサイクル方法 |
| WO2017015467A1 (en) * | 2015-07-22 | 2017-01-26 | Glucan Biorenewables, Llc | High purity cellulose compositions and production methods |
-
2017
- 2017-06-28 JP JP2017126793A patent/JP6669694B2/ja active Active
-
2018
- 2018-05-10 US US16/626,983 patent/US20200131702A1/en not_active Abandoned
- 2018-05-10 WO PCT/JP2018/018175 patent/WO2019003655A1/ja not_active Ceased
- 2018-05-10 CN CN201880040344.1A patent/CN110770394B/zh active Active
- 2018-05-10 EP EP18824513.8A patent/EP3626882B1/en active Active
-
2023
- 2023-01-12 US US18/153,410 patent/US11987932B2/en active Active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000084533A (ja) * | 1998-09-16 | 2000-03-28 | Takeshi Cho | 使用済み紙おむつの使用材料の再生処理方法 |
| JP2010235681A (ja) | 2009-03-30 | 2010-10-21 | Nippon Paper Industries Co Ltd | セルロースナノファイバーの製造方法 |
| JP2010254726A (ja) | 2009-04-21 | 2010-11-11 | Oji Paper Co Ltd | 微細繊維状セルロースの製造方法 |
| JP2017100133A (ja) * | 2013-04-10 | 2017-06-08 | ユニ・チャーム株式会社 | 使用済み衛生用品の高分子吸水材を処理する方法および使用済み衛生用品からパルプ繊維を回収する方法 |
| WO2015190140A1 (ja) * | 2014-06-12 | 2015-12-17 | ユニ・チャーム株式会社 | 使用済み衛生用品からリサイクルパルプを製造する方法 |
| JP2016000881A (ja) | 2014-06-12 | 2016-01-07 | ユニ・チャーム株式会社 | 使用済み衛生用品からリサイクルパルプを製造する方法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3626882A4 |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114829706A (zh) * | 2019-12-16 | 2022-07-29 | 尤妮佳股份有限公司 | 纤维素纳米纤维的制造方法及纤维素纳米纤维 |
| WO2021124655A1 (ja) * | 2019-12-16 | 2021-06-24 | ユニ・チャーム株式会社 | セルロースナノファイバーの製造方法及びセルロースナノファイバー |
| JP7517817B2 (ja) | 2019-12-16 | 2024-07-17 | ユニ・チャーム株式会社 | セルロースナノファイバーの製造方法及びセルロースナノファイバー |
| EP4056597A4 (en) * | 2019-12-16 | 2023-01-18 | Unicharm Corporation | CELLULOSE MANUFACTURING PROCESS AND CELLULOSE NANOFIBER |
| JP2021095476A (ja) * | 2019-12-16 | 2021-06-24 | ユニ・チャーム株式会社 | セルロースナノファイバーの製造方法及びセルロースナノファイバー |
| CN115038837A (zh) * | 2020-01-31 | 2022-09-09 | 尤妮佳股份有限公司 | 制造羧基化纤维素纳米纤维化用浆粕纤维的方法以及羧基化纤维素纳米纤维化用浆粕纤维 |
| JP2021123807A (ja) * | 2020-01-31 | 2021-08-30 | ユニ・チャーム株式会社 | カルボキシル化セルロースナノファイバー化用パルプ繊維を製造する方法、及びカルボキシル化セルロースナノファイバー化用パルプ繊維 |
| CN115038837B (zh) * | 2020-01-31 | 2023-11-10 | 尤妮佳股份有限公司 | 制造羧基化纤维素纳米纤维化用浆粕纤维的方法以及羧基化纤维素纳米纤维化用浆粕纤维 |
| JP7458802B2 (ja) | 2020-01-31 | 2024-04-01 | ユニ・チャーム株式会社 | カルボキシル化セルロースナノファイバー化用パルプ繊維を製造する方法、及びカルボキシル化セルロースナノファイバー化用パルプ繊維 |
| JP2024060102A (ja) * | 2020-01-31 | 2024-05-01 | ユニ・チャーム株式会社 | カルボキシル化セルロースナノファイバー化用パルプ繊維を製造する方法、及びカルボキシル化セルロースナノファイバー化用パルプ繊維 |
| WO2021152958A1 (ja) * | 2020-01-31 | 2021-08-05 | ユニ・チャーム株式会社 | カルボキシル化セルロースナノファイバー化用パルプ繊維を製造する方法、及びカルボキシル化セルロースナノファイバー化用パルプ繊維 |
| WO2021213740A1 (de) * | 2020-04-23 | 2021-10-28 | Messer Austria Gmbh | Verfahren und vorrichtung zur herstellung von gebleichtem zellstoff |
| US20230203751A1 (en) * | 2020-04-23 | 2023-06-29 | Messer Austria Gmbh | Process and apparatus for producing bleached cellulose |
| US12410557B2 (en) | 2020-04-23 | 2025-09-09 | Messer Austria Gmbh | Process and apparatus for white liquor oxidation |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3626882B1 (en) | 2025-04-23 |
| CN110770394B (zh) | 2022-10-25 |
| US20200131702A1 (en) | 2020-04-30 |
| JP6669694B2 (ja) | 2020-03-18 |
| EP3626882A4 (en) | 2020-05-13 |
| EP3626882A1 (en) | 2020-03-25 |
| US20230160142A1 (en) | 2023-05-25 |
| US11987932B2 (en) | 2024-05-21 |
| JP2019005733A (ja) | 2019-01-17 |
| CN110770394A (zh) | 2020-02-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12364629B2 (en) | Method for producing recycled pulp fibers, and recycled pulp fibers | |
| WO2019003655A1 (ja) | セルロースナノファイバー化用パルプ繊維を製造する方法、及びセルロースナノファイバー化用パルプ繊維 | |
| JP6643455B1 (ja) | セルロースナノファイバー化用パルプ繊維を製造する方法 | |
| JP7778191B2 (ja) | パルプ繊維原料を製造する方法、及びセルロース原料としてのパルプ繊維原料 | |
| WO2019003657A1 (ja) | リサイクル繊維を製造する方法及びリサイクル繊維 | |
| WO2019003656A1 (ja) | 糖化用パルプ繊維を製造する方法、及び糖化用パルプ繊維水溶液 | |
| JP7179077B2 (ja) | 繊維複合強化材料用のパルプ繊維原料を製造する方法、繊維複合強化材料用のパルプ繊維原料、繊維複合強化材料、ペレット、フィルム、繊維、不織布 | |
| JP2020108346A (ja) | 培地用パルプ繊維を製造する方法、培地用パルプ繊維、及び培地用パルプ繊維の使用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18824513 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2018824513 Country of ref document: EP Effective date: 20191220 |
|
| WWG | Wipo information: grant in national office |
Ref document number: 2018824513 Country of ref document: EP |