WO2018199165A1 - 空気浄化システム - Google Patents
空気浄化システム Download PDFInfo
- Publication number
- WO2018199165A1 WO2018199165A1 PCT/JP2018/016796 JP2018016796W WO2018199165A1 WO 2018199165 A1 WO2018199165 A1 WO 2018199165A1 JP 2018016796 W JP2018016796 W JP 2018016796W WO 2018199165 A1 WO2018199165 A1 WO 2018199165A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- space
- carbon dioxide
- air
- room
- water vapor
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/26—Drying gases or vapours
- B01D53/268—Drying gases or vapours by diffusion
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/22—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by diffusion
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/22—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols by diffusion
- B01D2053/221—Devices
- B01D2053/223—Devices with hollow tubes
- B01D2053/224—Devices with hollow tubes with hollow fibres
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/50—Carbon oxides
- B01D2257/504—Carbon dioxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/80—Water
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2258/00—Sources of waste gases
- B01D2258/06—Polluted air
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2259/00—Type of treatment
- B01D2259/45—Gas separation or purification devices adapted for specific applications
- B01D2259/4508—Gas separation or purification devices adapted for specific applications for cleaning air in buildings
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2259/00—Type of treatment
- B01D2259/45—Gas separation or purification devices adapted for specific applications
- B01D2259/4566—Gas separation or purification devices adapted for specific applications for use in transportation means
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2259/00—Type of treatment
- B01D2259/45—Gas separation or purification devices adapted for specific applications
- B01D2259/4566—Gas separation or purification devices adapted for specific applications for use in transportation means
- B01D2259/4575—Gas separation or purification devices adapted for specific applications for use in transportation means in aeroplanes or space ships
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02C—CAPTURE, STORAGE, SEQUESTRATION OR DISPOSAL OF GREENHOUSE GASES [GHG]
- Y02C20/00—Capture or disposal of greenhouse gases
- Y02C20/40—Capture or disposal of greenhouse gases of CO2
Definitions
- the present invention relates to an air purification system that purifies air in a room.
- Ventilation is carried out for rooms that accommodate people in buildings and transport equipment in order to suppress the increase in carbon dioxide concentration.
- the room is provided with an air conditioner for heating or cooling.
- the ventilation volume for suppressing the above-mentioned increase in carbon dioxide concentration is relatively large, so the load on the air conditioner increases accordingly.
- Patent Document 1 discloses an air purification system mounted on a railway vehicle.
- a carbon dioxide removing device is provided in a circulation path for circulating the air in the room.
- the carbon dioxide removing device includes a separation membrane that selectively permeates carbon dioxide.
- the carbon dioxide removing device has a permeation side in order to give a pressure difference between the circulation path side of the separation membrane (one of the spaces partitioned by the separation membrane) and the permeation side (the other of the spaces partitioned by the separation membrane).
- a vacuum pump for evacuating is connected.
- the pressure on the permeation side of the separation membrane is set to about 20 kPa, and a pressure difference of about 80 kPa is generated between the circulation path side and the permeation side of the separation membrane, so that carbon dioxide in the circulated air is reduced. It is described that it selectively permeates the separation membrane.
- the partial pressure of carbon dioxide in the air is about 0.1 kPa when the carbon dioxide concentration is 1000 ppm. Therefore, the pressure of 20 kPa is considerably higher than the partial pressure of carbon dioxide in the air, and if the separation membrane selectively permeates only carbon dioxide, the partial pressure of carbon dioxide on the permeate side immediately increases.
- some separation membranes selectively allow not only carbon dioxide but also water vapor.
- the carbon dioxide permeability and water vapor permeability of the separation membrane are similar, if the total pressure on the permeate side is 20 kPa, the carbon dioxide partial pressure on the permeate side is higher than the carbon dioxide partial pressure in the air. Even in this case, the carbon dioxide partial pressure on the permeate side immediately increases, so the amount of carbon dioxide that permeates is extremely small, or a large amount of air is combined with carbon dioxide to obtain a realistic carbon dioxide permeation amount. The transmission becomes necessary and the device becomes unrealistic. That is, in the degree of vacuum described in the cited document 1, it is not realistic to remove carbon dioxide from the air in the room.
- an object of the present invention is to realize an air purification system capable of efficiently removing carbon dioxide using a separation membrane and a vacuum pump.
- an air purification system of the present invention is an air purification system for purifying air in a room, and includes a first space and a second space partitioned by a separation membrane that selectively transmits carbon dioxide.
- a carbon dioxide removing device including a space, a feed path for guiding air in the room to the first space, a return path for guiding clean air from which carbon dioxide has been removed from the first space to the room, and the second And a decompression pump that evacuates the second space so that the partial pressure of carbon dioxide in the space is lower than the partial pressure of carbon dioxide in the air in the room.
- the carbon dioxide partial pressure in the second space of the carbon dioxide removing device is lower than the carbon dioxide partial pressure in the first space, so that carbon dioxide continues to selectively permeate the separation membrane. Therefore, carbon dioxide can be efficiently removed from the air in the room.
- the separation membrane selectively permeates water vapor as well as carbon dioxide
- the vacuum pump is configured such that the total pressure in the second space is lower than the partial pressure of water vapor in the air in the room.
- the second space may be evacuated.
- the separation membrane selectively transmits only carbon dioxide, that is, when the second space is only carbon dioxide, the pressure in the second space is less than the partial pressure of carbon dioxide in the first space. Need to be evacuated. For example, when the carbon dioxide concentration in the room air is 1000 ppm, the pressure in the second space needs to be less than 0.1 kPa.
- the separation membrane selectively permeates not only carbon dioxide but also water vapor, since there is more water vapor than carbon dioxide in the room air, carbon dioxide also in the second space. The amount of water vapor can be increased. Therefore, if the total pressure in the second space is set to be lower than the partial pressure of water vapor in the first space, it is possible to suppress an increase in the partial pressure of carbon dioxide in the second space, which is necessary for the actual scale of the apparatus. It is possible to obtain a carbon permeation amount.
- the air purification system may further include a water vapor supply device that supplies water vapor to the second space such that a water vapor partial pressure in the second space is substantially equal to a water vapor partial pressure in the first space. .
- a water vapor supply device that supplies water vapor to the second space such that a water vapor partial pressure in the second space is substantially equal to a water vapor partial pressure in the first space.
- carbon dioxide can be efficiently removed using a separation membrane and a vacuum pump.
- FIG. 1 is a schematic configuration diagram of an air purification system according to a first embodiment of the present invention. It is a schematic block diagram of the air purification system which concerns on 2nd Embodiment of this invention.
- FIG. 1 shows an air purification system 1A according to the first embodiment of the present invention.
- This air purification system 1A purifies the air in the room 2 that accommodates people.
- the room 2 may be a room of a building such as an office building, or may be a room of transportation equipment such as a railway vehicle or an aircraft (so-called cabin).
- the room 2 may be a space station, a submarine, a disaster evacuation facility, or the like.
- the air purification system 1A includes a carbon dioxide removing device 4.
- the carbon dioxide removing device 4 is arranged outside the room 2, but the carbon dioxide removing device 4 may be arranged inside the room 2.
- the carbon dioxide removing device 4 includes a first space 41 and a second space 42 partitioned by a separation membrane 43.
- the separation membrane 43 selectively transmits carbon dioxide from the first space 41 toward the second space 42.
- the separation membrane 43 selectively transmits water vapor as well as carbon dioxide.
- the carbon dioxide permeability and water vapor permeability of the separation membrane 43 are desirably 1000 times or more of the nitrogen permeability and the oxygen permeability.
- the separation membrane 43 is a hollow fiber membrane.
- many hollow fiber membranes may comprise one membrane module, and the carbon dioxide removal apparatus 4 may contain many membrane modules.
- the separation membrane 43 is a hollow fiber membrane, the inside of the hollow fiber membrane is the first space 41 and the outside of the hollow fiber membrane is the second space 42.
- Air in the room 2 is guided to the first space 41 of the carbon dioxide removal device 4 through the feed path 51, and clean air from which carbon dioxide has been removed is guided to the room 2 through the return path 52 from the first space 41. It is burned.
- the blower 53 is provided in the feed path 51, but the blower 53 may be provided in the return path 52.
- the blower 53 may be a blower or a fan (the same applies to a blower described later).
- a suction path 61 is connected to the second space 42 of the carbon dioxide removal device 4.
- the downstream end of the suction path 61 is open to the atmosphere.
- a vacuum pump 62 is provided in the suction path 61.
- the decompression pump 62 evacuates the second space 42 so that the partial pressure of carbon dioxide in the second space 42 is lower than the partial pressure of carbon dioxide in the air in the room 2.
- the carbon dioxide partial pressure is about 0.1 kPa.
- the separation membrane 43 transmits not only carbon dioxide but also water vapor, even if the total pressure in the second space 42 is about 1.0 kPa, the carbon dioxide partial pressure in the second space 42 is the first. It can be suppressed to less than the partial pressure of carbon dioxide in the space 41.
- the energy required for driving the decompression pump 62 can be reduced, although the size of the carbon dioxide removing device 4 is increased.
- the total pressure in the second space 42 which is a target value for controlling the decompression pump 62, may be, for example, 0.5 kPa or less. Alternatively, the total pressure in the second space 42 may be changed according to the relative humidity in the room 2.
- the room 2 is provided with an air conditioner 3 for heating and cooling.
- the room 2 is connected to a first ventilation path 71 and a second ventilation path 72.
- Blowers 73 and 74 are provided in the first ventilation path 71 and the second ventilation path 72, respectively.
- the carbon dioxide partial pressure in the second space 42 of the carbon dioxide removing device 4 is lower than the carbon dioxide partial pressure in the first space 41, so carbon dioxide is Continue to selectively permeate the separation membrane 43. Therefore, carbon dioxide can be efficiently removed from the air in the room 2.
- the separation membrane 43 selectively transmits water vapor as well as carbon dioxide.
- the separation membrane 43 selectively transmits only carbon dioxide, that is, when the second space 42 is only carbon dioxide, the second space 42 is less than the partial pressure of carbon dioxide in the first space 41. It is necessary to evacuate the vacuum so that For example, when the carbon dioxide concentration in the room air is 1000 ppm, the pressure in the second space 42 needs to be less than 0.1 kPa.
- the separation membrane 43 selectively permeates not only carbon dioxide but also water vapor as in the present embodiment, since there is more water vapor than carbon dioxide in the room air, Even in the second space 42, the amount of water vapor can be made larger than that of carbon dioxide.
- the total pressure in the second space 42 is set to be lower than the partial pressure of water vapor in the first space 41, it is possible to suppress an increase in the partial pressure of carbon dioxide in the second space 42, which is necessary depending on a realistic apparatus scale. It becomes possible to obtain the carbon dioxide permeation amount.
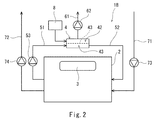
- FIG. 2 shows an air purification system 1B according to the second embodiment of the present invention.
- the same components as those in the first embodiment are denoted by the same reference numerals, and a duplicate description is omitted.
- the air purification system 1B of the present embodiment is obtained by adding a water vapor supply device 8 to the air purification system 1A of the first embodiment.
- the water vapor supply device 8 supplies water vapor to the second space 42 such that the water vapor partial pressure in the second space 42 of the carbon dioxide removing device 4 is substantially equal to the water vapor partial pressure in the first space 41.
- the water vapor partial pressure in the second space 42 of the carbon dioxide removing device 4 is preferably maintained within a range of ⁇ 10% of the water vapor partial pressure in the first space 41.
- water vapor is prevented from passing through the separation membrane 43 from the first space 41 toward the second space 42. Accordingly, since the suction amount of the decompression pump 62 is reduced, the load on the decompression pump 62 can be reduced.
- the present invention is not limited to the above-described embodiments, and various modifications can be made without departing from the gist of the present invention.
- the air conditioner 3 is not necessarily provided in the room 2.
- the component that selectively permeates through the separation membrane 43 together with carbon dioxide is not necessarily water vapor, and may be nitrogen or oxygen. That is, the separation membrane 43 may selectively transmit not only carbon dioxide but also nitrogen or oxygen.
- the separation membrane 43 may selectively transmit only carbon dioxide.
- the decompression pump 62 may evacuate the second space 42 so that the pressure in the second space 42 is less than 0.1 kPa.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Analytical Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Separation Using Semi-Permeable Membranes (AREA)
- Gas Separation By Absorption (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16/608,913 US20200246749A1 (en) | 2017-04-27 | 2018-04-25 | Air purifying system |
| CN201880025114.8A CN110536737A (zh) | 2017-04-27 | 2018-04-25 | 空气净化系统 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017087808A JP6877229B2 (ja) | 2017-04-27 | 2017-04-27 | 空気浄化システム |
| JP2017-087808 | 2017-04-27 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018199165A1 true WO2018199165A1 (ja) | 2018-11-01 |
Family
ID=63919845
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/016796 Ceased WO2018199165A1 (ja) | 2017-04-27 | 2018-04-25 | 空気浄化システム |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US20200246749A1 (enExample) |
| JP (1) | JP6877229B2 (enExample) |
| CN (1) | CN110536737A (enExample) |
| WO (1) | WO2018199165A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109276194A (zh) * | 2018-11-28 | 2019-01-29 | 贵州工程应用技术学院 | 一种智能工业清洁机器人 |
| EP3922344A4 (en) * | 2019-02-05 | 2022-10-26 | Kawasaki Jukogyo Kabushiki Kaisha | AIR PURIFICATION SYSTEM |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021102198A (ja) * | 2019-12-25 | 2021-07-15 | 株式会社クラレ | 二酸化炭素分離デバイス、空気清浄機、エアーコンディショナ、及び二酸化炭素濃縮装置 |
| JP7773852B2 (ja) * | 2020-11-10 | 2025-11-20 | 日産自動車株式会社 | 車両用空調方法及び車両用空調システム |
| KR102556881B1 (ko) * | 2021-06-03 | 2023-07-19 | 화인클린(주) | 미세먼지와 이산화탄소 제거를 위한 공기청정기 |
| US20220401878A1 (en) * | 2021-06-21 | 2022-12-22 | Air Products And Chemicals, Inc. | Reducing energy consumption for marine and offshore membrane applications |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014065387A1 (ja) * | 2012-10-22 | 2014-05-01 | 住友化学株式会社 | 共重合体及び炭酸ガス分離膜 |
| JP5738704B2 (ja) * | 2011-07-26 | 2015-06-24 | 富士フイルム株式会社 | 二酸化炭素分離装置および二酸化炭素分離方法 |
| JP5743639B2 (ja) * | 2011-03-29 | 2015-07-01 | 新日鉄住金エンジニアリング株式会社 | 二酸化炭素ガス分離システム |
| JP2015192927A (ja) * | 2014-03-31 | 2015-11-05 | Tdk株式会社 | ガス分離膜およびガス分離装置 |
| JP2017006820A (ja) * | 2015-06-18 | 2017-01-12 | 住友化学株式会社 | 二酸化炭素分離方法及び二酸化炭素分離装置 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5799652A (en) * | 1995-05-22 | 1998-09-01 | Hypoxico Inc. | Hypoxic room system and equipment for Hypoxic training and therapy at standard atmospheric pressure |
| JP2003025991A (ja) * | 2001-07-13 | 2003-01-29 | Hitachi Ltd | 換気装置 |
| EP2411122A4 (en) * | 2009-03-26 | 2012-08-22 | Eco Bio Technologies Pty Ltd | METHOD FOR SEPARATING GASES |
-
2017
- 2017-04-27 JP JP2017087808A patent/JP6877229B2/ja active Active
-
2018
- 2018-04-25 CN CN201880025114.8A patent/CN110536737A/zh active Pending
- 2018-04-25 WO PCT/JP2018/016796 patent/WO2018199165A1/ja not_active Ceased
- 2018-04-25 US US16/608,913 patent/US20200246749A1/en not_active Abandoned
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5743639B2 (ja) * | 2011-03-29 | 2015-07-01 | 新日鉄住金エンジニアリング株式会社 | 二酸化炭素ガス分離システム |
| JP5738704B2 (ja) * | 2011-07-26 | 2015-06-24 | 富士フイルム株式会社 | 二酸化炭素分離装置および二酸化炭素分離方法 |
| WO2014065387A1 (ja) * | 2012-10-22 | 2014-05-01 | 住友化学株式会社 | 共重合体及び炭酸ガス分離膜 |
| JP2015192927A (ja) * | 2014-03-31 | 2015-11-05 | Tdk株式会社 | ガス分離膜およびガス分離装置 |
| JP2017006820A (ja) * | 2015-06-18 | 2017-01-12 | 住友化学株式会社 | 二酸化炭素分離方法及び二酸化炭素分離装置 |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109276194A (zh) * | 2018-11-28 | 2019-01-29 | 贵州工程应用技术学院 | 一种智能工业清洁机器人 |
| EP3922344A4 (en) * | 2019-02-05 | 2022-10-26 | Kawasaki Jukogyo Kabushiki Kaisha | AIR PURIFICATION SYSTEM |
| US11872521B2 (en) | 2019-02-05 | 2024-01-16 | Kawasaki Jukogyo Kabushiki Kaisha | Air purifying system |
Also Published As
| Publication number | Publication date |
|---|---|
| US20200246749A1 (en) | 2020-08-06 |
| JP6877229B2 (ja) | 2021-05-26 |
| CN110536737A (zh) | 2019-12-03 |
| JP2018183750A (ja) | 2018-11-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2018199165A1 (ja) | 空気浄化システム | |
| WO2018199164A1 (ja) | 空気浄化システム | |
| US7601202B2 (en) | Method and device for reducing the carbon dioxide concentration in air | |
| CN113218023B (zh) | 空气组成调节装置 | |
| WO2019065889A1 (ja) | 庫内空気調節装置 | |
| MX2024014574A (es) | Sistema de captura directa de aire con panel movil de alto rendimiento | |
| JP6164682B2 (ja) | ガス分離装置及びそれを用いた酸性ガスの分離方法 | |
| JP2018502303A5 (enExample) | ||
| JP6953764B2 (ja) | バイオガス濃縮システムおよびバイオガス濃縮方法 | |
| WO2021168605A1 (zh) | 隧道式复合消毒方舱 | |
| JP2002263433A (ja) | 保存庫内の気体組成の制御方法と保存庫 | |
| EP4108315A1 (en) | Reducing energy consumption for marine and offshore membrane applications | |
| JP4810748B2 (ja) | 除湿システム | |
| JP6609386B1 (ja) | 空気浄化システム | |
| JP2003089508A (ja) | オゾン発生システム | |
| JP2022076664A (ja) | 車両用空調方法及び車両用空調システム | |
| JPH0730927B2 (ja) | 空気調和機 | |
| KR101514449B1 (ko) | 육불화황(sf6) 농축 및 열분해장치 | |
| JP2012034781A (ja) | 除染装置 | |
| CN110559850A (zh) | 一种空气净化器 | |
| JP2025031375A (ja) | 二酸化炭素回収方法及び二酸化炭素回収装置 | |
| JP2025001702A (ja) | 二酸化炭素分離システム及び二酸化炭素分離方法 | |
| WO2024190846A1 (ja) | ガス分離システム及び混合ガスの分離方法 | |
| JP2018051505A (ja) | 吸着体の製造方法、及び、二酸化炭素の除去方法 | |
| JP2025097346A (ja) | 除害装置、除害方法及び半導体製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18790124 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 18790124 Country of ref document: EP Kind code of ref document: A1 |