WO2018092479A1 - 水素製造装置および水素製造装置の運転方法 - Google Patents
水素製造装置および水素製造装置の運転方法 Download PDFInfo
- Publication number
- WO2018092479A1 WO2018092479A1 PCT/JP2017/037162 JP2017037162W WO2018092479A1 WO 2018092479 A1 WO2018092479 A1 WO 2018092479A1 JP 2017037162 W JP2017037162 W JP 2017037162W WO 2018092479 A1 WO2018092479 A1 WO 2018092479A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- hydrogen
- reformer
- fuel cell
- storage container
- containing gas
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/04—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids
- H01M8/04223—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids during start-up or shut-down; Depolarisation or activation, e.g. purging; Means for short-circuiting defective fuel cells
- H01M8/04225—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids during start-up or shut-down; Depolarisation or activation, e.g. purging; Means for short-circuiting defective fuel cells during start-up
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/08—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/08—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor
- B01J19/087—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy
- B01J19/088—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy giving rise to electric discharges
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/24—Stationary reactors without moving elements inside
- B01J19/2475—Membrane reactors
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J7/00—Apparatus for generating gases
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B3/00—Hydrogen; Gaseous mixtures containing hydrogen; Separation of hydrogen from mixtures containing it; Purification of hydrogen
- C01B3/02—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen
- C01B3/04—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen by decomposition of inorganic compounds, e.g. ammonia
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B3/00—Hydrogen; Gaseous mixtures containing hydrogen; Separation of hydrogen from mixtures containing it; Purification of hydrogen
- C01B3/02—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen
- C01B3/04—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen by decomposition of inorganic compounds, e.g. ammonia
- C01B3/047—Decomposition of ammonia
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B3/00—Hydrogen; Gaseous mixtures containing hydrogen; Separation of hydrogen from mixtures containing it; Purification of hydrogen
- C01B3/02—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen
- C01B3/22—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen by decomposition of gaseous or liquid organic compounds
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B3/00—Hydrogen; Gaseous mixtures containing hydrogen; Separation of hydrogen from mixtures containing it; Purification of hydrogen
- C01B3/50—Separation of hydrogen or hydrogen containing gases from gaseous mixtures, e.g. purification
- C01B3/501—Separation of hydrogen or hydrogen containing gases from gaseous mixtures, e.g. purification by diffusion
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/04—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/04—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids
- H01M8/04298—Processes for controlling fuel cells or fuel cell systems
- H01M8/043—Processes for controlling fuel cells or fuel cell systems applied during specific periods
- H01M8/04302—Processes for controlling fuel cells or fuel cell systems applied during specific periods applied during start-up
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/04—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids
- H01M8/04298—Processes for controlling fuel cells or fuel cell systems
- H01M8/04313—Processes for controlling fuel cells or fuel cell systems characterised by the detection or assessment of variables; characterised by the detection or assessment of failure or abnormal function
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/04—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids
- H01M8/04298—Processes for controlling fuel cells or fuel cell systems
- H01M8/04694—Processes for controlling fuel cells or fuel cell systems characterised by variables to be controlled
- H01M8/04746—Pressure; Flow
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/04—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids
- H01M8/04298—Processes for controlling fuel cells or fuel cell systems
- H01M8/04694—Processes for controlling fuel cells or fuel cell systems characterised by variables to be controlled
- H01M8/04746—Pressure; Flow
- H01M8/04776—Pressure; Flow at auxiliary devices, e.g. reformer, compressor, burner
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/04—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids
- H01M8/04298—Processes for controlling fuel cells or fuel cell systems
- H01M8/04694—Processes for controlling fuel cells or fuel cell systems characterised by variables to be controlled
- H01M8/04858—Electric variables
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/04—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids
- H01M8/04298—Processes for controlling fuel cells or fuel cell systems
- H01M8/04694—Processes for controlling fuel cells or fuel cell systems characterised by variables to be controlled
- H01M8/04858—Electric variables
- H01M8/04925—Power, energy, capacity or load
- H01M8/04932—Power, energy, capacity or load of the individual fuel cell
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/06—Combination of fuel cells with means for production of reactants or for treatment of residues
- H01M8/0606—Combination of fuel cells with means for production of reactants or for treatment of residues with means for production of gaseous reactants
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/06—Combination of fuel cells with means for production of reactants or for treatment of residues
- H01M8/0606—Combination of fuel cells with means for production of reactants or for treatment of residues with means for production of gaseous reactants
- H01M8/0612—Combination of fuel cells with means for production of reactants or for treatment of residues with means for production of gaseous reactants from carbon-containing material
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/06—Combination of fuel cells with means for production of reactants or for treatment of residues
- H01M8/0606—Combination of fuel cells with means for production of reactants or for treatment of residues with means for production of gaseous reactants
- H01M8/0612—Combination of fuel cells with means for production of reactants or for treatment of residues with means for production of gaseous reactants from carbon-containing material
- H01M8/0618—Reforming processes, e.g. autothermal, partial oxidation or steam reforming
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/06—Combination of fuel cells with means for production of reactants or for treatment of residues
- H01M8/0606—Combination of fuel cells with means for production of reactants or for treatment of residues with means for production of gaseous reactants
- H01M8/0612—Combination of fuel cells with means for production of reactants or for treatment of residues with means for production of gaseous reactants from carbon-containing material
- H01M8/0625—Combination of fuel cells with means for production of reactants or for treatment of residues with means for production of gaseous reactants from carbon-containing material in a modular combined reactor/fuel cell structure
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/22—Fuel cells in which the fuel is based on materials comprising carbon or oxygen or hydrogen and other elements; Fuel cells in which the fuel is based on materials comprising only elements other than carbon, oxygen or hydrogen
- H01M8/222—Fuel cells in which the fuel is based on compounds containing nitrogen, e.g. hydrazine, ammonia
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/08—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor
- B01J2219/0801—Controlling the process
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/08—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor
- B01J2219/0803—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy
- B01J2219/0805—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy giving rise to electric discharges
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/08—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor
- B01J2219/0803—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy
- B01J2219/0805—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy giving rise to electric discharges
- B01J2219/0807—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy giving rise to electric discharges involving electrodes
- B01J2219/0809—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy giving rise to electric discharges involving electrodes employing two or more electrodes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/08—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor
- B01J2219/0803—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy
- B01J2219/0805—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy giving rise to electric discharges
- B01J2219/0807—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor employing electric or magnetic energy giving rise to electric discharges involving electrodes
- B01J2219/0824—Details relating to the shape of the electrodes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/08—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor
- B01J2219/0869—Feeding or evacuating the reactor
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/08—Processes employing the direct application of electric or wave energy, or particle radiation; Apparatus therefor
- B01J2219/0894—Processes carried out in the presence of a plasma
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/02—Processes for making hydrogen or synthesis gas
- C01B2203/0266—Processes for making hydrogen or synthesis gas containing a decomposition step
- C01B2203/0272—Processes for making hydrogen or synthesis gas containing a decomposition step containing a non-catalytic decomposition step
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/04—Integrated processes for the production of hydrogen or synthesis gas containing a purification step for the hydrogen or the synthesis gas
- C01B2203/0405—Purification by membrane separation
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/04—Integrated processes for the production of hydrogen or synthesis gas containing a purification step for the hydrogen or the synthesis gas
- C01B2203/0405—Purification by membrane separation
- C01B2203/041—In-situ membrane purification during hydrogen production
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/06—Integration with other chemical processes
- C01B2203/066—Integration with other chemical processes with fuel cells
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/08—Methods of heating or cooling
- C01B2203/0805—Methods of heating the process for making hydrogen or synthesis gas
- C01B2203/0861—Methods of heating the process for making hydrogen or synthesis gas by plasma
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/16—Controlling the process
- C01B2203/1642—Controlling the product
- C01B2203/1647—Controlling the amount of the product
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/16—Controlling the process
- C01B2203/1685—Control based on demand of downstream process
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M2008/1095—Fuel cells with polymeric electrolytes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M2250/00—Fuel cells for particular applications; Specific features of fuel cell system
- H01M2250/10—Fuel cells in stationary systems, e.g. emergency power source in plant
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02B—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO BUILDINGS, e.g. HOUSING, HOUSE APPLIANCES OR RELATED END-USER APPLICATIONS
- Y02B90/00—Enabling technologies or technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02B90/10—Applications of fuel cells in buildings
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/36—Hydrogen production from non-carbon containing sources, e.g. by water electrolysis
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/50—Fuel cells
Definitions
- the present invention relates to a hydrogen production apparatus.
- the present invention relates to a hydrogen production apparatus capable of starting without using an external energy supply means and continuing the production of hydrogen.
- Hydrogen tends to be expensive for transportation and storage compared to other fuels. For this reason, there is a potential demand to produce hydrogen on-site by installing a hydrogen production device adjacent to a device that uses hydrogen or by incorporating a hydrogen production device in a facility that uses hydrogen. To do.
- a method of producing hydrogen by decomposing a hydrogen source such as ammonia, urea, or a hydrocarbon-based gas
- An apparatus used for decomposing a hydrogen source is generally called a reformer.
- the reformer needs to supply energy from the outside at the time of start-up, and the hydrogen production apparatus in a normal time supplies the energy at the start-up to the reformer by connecting to an external power source, for example.
- an external power source for example.
- a storage battery is known as means for supplying energy necessary for starting up a hydrogen production apparatus in an emergency.
- a storage battery having a capacity that is large enough to enable the hydrogen production apparatus to start up is large and expensive, which is a cause of increasing the size and cost of the entire hydrogen production apparatus.
- the capacity of the storage battery gradually decreases by repeating charging and discharging, and there is a possibility that necessary power cannot be supplied after a certain period of use.
- Patent Document 1 discloses a technique for suppressing power consumption during startup of a hydrogen generator equipped with a self-starting power source.
- Patent Document 2 discloses a technique for preventing the start-up process of the hydrogen generator from overlapping the power outage period based on power outage information acquired in advance.
- a hydrogen production apparatus using a conventional reformer could not be started unless energy such as electric energy was supplied from the outside. For this reason, it has been a problem that it cannot be activated independently during a power failure or disaster. Therefore, there has been a demand for a technique that can easily and reliably start the hydrogen production apparatus even when external energy supply cannot be obtained.
- the present invention provides a hydrogen production apparatus that can be activated without receiving external energy supply.
- the hydrogen production apparatus of the invention is connected to a hydrogen source, an input unit for introducing a raw material containing hydrogen, a reformer for decomposing the raw material introduced by the input unit to produce a hydrogen-containing gas, and reforming
- a hydrogen storage container that temporarily stores the hydrogen-containing gas produced by the reactor, a measurement unit that measures the amount of hydrogen-containing gas stored in the hydrogen storage container, and power generation using the hydrogen produced by the reformer for reforming
- a fuel cell for supplying electric power to the reformer, a hydrogen supply passage for fuel for supplying at least a part of the hydrogen produced by the reformer to the fuel cell, and an external for supplying a part of the hydrogen produced by the reformer to the outside
- a control unit that receives the measurement data of the supply path and the measurement unit and controls the production amount of the hydrogen-containing gas in the reformer, the storage amount of the hydrogen-containing gas in the hydrogen storage container, and the power generation amount of the fuel cell
- control unit of the hydrogen production apparatus of the present invention stores a threshold value of measurement data corresponding to the minimum amount of the hydrogen-containing gas necessary for starting the fuel cell. When the measured data falls below the threshold value, control is performed to increase the storage amount of the hydrogen storage container. And the fuel cell at the time of starting generates electric power using the hydrogen stored in the hydrogen storage container, and supplies the electric power to the reformer.
- the hydrogen production apparatus of the present invention is equipped with a fuel cell that generates power by hydrogen and oxygen as fuels through these chemical reactions.
- the hydrogen storage container which stores the hydrogen-containing gas which a reformer supplies always stores the quantity required for starting of a fuel cell at the time of steady operation.
- the fuel cell starts power generation by supplying the hydrogen-containing gas stored in the fuel cell from the hydrogen storage container.
- the reformer starts producing hydrogen.
- the fuel cell can continue to generate power using hydrogen produced by the reformer, and can stably supply the necessary energy to the reformer.
- the output power of the fuel cell provided in the hydrogen production apparatus of the present invention is preferably larger than the power consumed by the reformer.
- the operating temperature of the fuel cell is preferably equal to or higher than the operating temperature of the reformer.
- the reformer provided in the hydrogen production apparatus of the present invention includes a plasma reaction vessel having a raw material supply port and a hydrogen outlet for decomposing the raw material into plasma, and plasma generation receiving power supplied from the fuel cell. And a hydrogen separation section that partitions the hydrogen outlet side of the plasma reaction vessel, the hydrogen separation portion separates hydrogen from the raw material that is plasma in the plasma reaction vessel, and the hydrogen outlet side It is preferable to pass through.
- the hydrogen production apparatus of the present invention is a hydrogen separation membrane in which a hydrogen separation unit is connected to a plasma generation power source, and the hydrogen separation membrane functions as a high voltage electrode by being supplied with power, It is preferable that the raw material is turned into plasma by discharging between them.
- the raw material containing hydrogen is ammonia or urea.
- the present invention also provides a method for operating the hydrogen production apparatus.
- the operation method of the present invention includes an input unit that is connected to a hydrogen source, introduces a raw material containing hydrogen, a reformer that decomposes the raw material introduced by the input unit to produce a hydrogen-containing gas, and a reformer
- a hydrogen storage container that temporarily stores the hydrogen-containing gas produced by the reactor, a measurement unit that measures the amount of hydrogen-containing gas stored in the hydrogen storage container, and power generation using the hydrogen produced by the reformer for reforming
- a fuel cell for supplying electric power to the reformer, a hydrogen supply passage for fuel for supplying at least a part of the hydrogen produced by the reformer to the fuel cell, and an external for supplying a part of the hydrogen produced by the reformer to the outside
- a control unit that receives the measurement data of the supply path and the measurement unit and controls the production amount of the hydrogen-containing gas in the reformer, the storage amount of the hydrogen-containing gas in the hydrogen storage container, and the power generation amount of the fuel cell
- the control unit stores a threshold value of measurement data corresponding to the minimum amount of the hydrogen-containing gas necessary for starting the fuel cell, and the received measurement data is the threshold value. Control is performed to increase the storage amount of the hydrogen storage container when the measured data falls below the threshold value by comparing with the value. And, at the time of start-up, a step in which the control unit supplies hydrogen from the hydrogen storage container to the fuel cell, a step in which the fuel cell starts power generation with the supplied hydrogen, and a step in which the electric power generated by the fuel cell is supplied to the reformer And a step of producing hydrogen by decomposing the raw material into plasma by the reformer, and a step of supplying the produced hydrogen to the fuel cell and continuing power generation.
- the hydrogen production apparatus of the present invention can produce hydrogen by starting up independently without receiving external energy supply such as electric energy. Moreover, the hydrogen production apparatus of the present invention can produce hydrogen by starting independently without providing a storage battery for starting the reformer.
- the output power of the fuel cell is larger than the power consumed by the reformer, so that the reformer can be reliably started only by the power supplied from the fuel cell. .
- the reformer once started can stably supply hydrogen necessary for the fuel cell to continue to operate, and the fuel cell continues to generate power. That is, the hydrogen production apparatus of the present invention can be operated independently in addition to the independent activation by the electric power supplied by the fuel cell which is a component.
- the hydrogen production apparatus of the present invention does not require a temperature raising means for the reformer and a means for cooling the hydrogen-containing gas supplied from the reformer because the operating temperature of the fuel cell is equal to or higher than the operating temperature of the reformer. It becomes. Thereby, the whole hydrogen production apparatus can be comprised more simply, and electric power consumption can be reduced. Moreover, the conditions of an installation location can be eased.
- the reformer of the hydrogen production apparatus of the present invention is a plasma reformer provided with a plasma reaction vessel, a plasma generation power source, and a hydrogen separator, so that a hydrogen separation membrane can be obtained under normal temperature and atmospheric pressure conditions.
- a hydrogen-containing gas can be produced by performing a discharge between the electrode and the ground electrode and using hydrogen as a raw material as plasma. Since the plasma reformer of the present invention operates at room temperature, the combination with a polymer electrolyte fuel cell having an operating temperature of 100 ° C. or less eliminates the need for temperature raising means and cooling means, and the thermal design of the entire hydrogen production apparatus It becomes easy and the control of the reformer becomes easy.
- the hydrogen production apparatus can be reduced in price and size.
- the self-sustained activation of the hydrogen production apparatus in the present invention means that the reformer and the fuel cell are activated without receiving supply of electric energy or equivalent energy from the outside, and the production of hydrogen is started to the outside. Says to supply hydrogen.
- the hydrogen source is a means for storing a raw material containing hydrogen and supplying this substance as a raw material to the hydrogen production apparatus of the present invention. More specifically, it refers to a storage container for raw materials containing hydrogen or a supply pipe communicating with the storage container.
- the substance stored or supplied by the hydrogen source is a hydrocarbon gas such as ammonia, urea, or methane.
- the reformer refers to an apparatus for producing hydrogen using a material containing hydrogen as a raw material.
- the most preferred form of reformer comprises a plasma reaction vessel, a plasma generating power source, a hydrogen separator functioning as a high voltage electrode, and a ground electrode, and a substance containing hydrogen by discharge between the electrodes.
- a plasma reformer that uses plasma as a plasma and separates it by allowing only hydrogen to pass through in a hydrogen separator.
- a reformer conforming to the plasma reformer a reformer that decomposes a hydrogen-containing substance by using a catalyst to extract hydrogen, and a reformer that combines a plasma reaction and a reaction by a catalyst are applicable. It is.
- the hydrogen-containing gas produced by the plasma reformer is a gas containing hydrogen as a main component, particularly high-purity hydrogen having a hydrogen concentration of 99.9% or more.
- the control unit performs the following control during steady operation. -Control of the amount of raw material containing hydrogen to the input section. -Start and stop of the reformer, and control of the amount of hydrogen-containing gas produced during operation. -Monitoring and control of the storage amount of the hydrogen-containing gas in the hydrogen storage container using the comparison result between the storage amount of the hydrogen storage container detected by the detection unit and the stored threshold value. -Control of oxygen supply to the fuel cell.
- the control unit confirms the storage amount of the hydrogen storage container and stops the production of hydrogen when an abnormality such as a power failure or a disaster occurs or when a stop command is received from the outside.
- the control unit When the control unit receives an instruction to start hydrogen production from the outside, and when a pre-planned time is reached, the control unit performs a startup procedure of the hydrogen production apparatus.
- a pressure gauge for measuring the pressure of the hydrogen storage container can be applied to the measurement unit.
- a scale that measures the weight of gas stored in the hydrogen storage container can be applied.
- the fuel cell most preferably used in the hydrogen production apparatus of the present invention is a polymer electrolyte fuel cell. In addition, various fuel cells can be applied.
- the hydrogen production apparatus 1 shown in FIG. 1 includes an input unit 11, a reformer 12, a hydrogen storage container 13, a measurement unit 14, a fuel cell 15, a control unit 18, and an oxygen supply unit 43. ing.
- the fuel cell 15 is connected to a power supply path 30 that supplies the generated power to the reformer 12.
- the hydrogen storage container 13 is provided with two pipes for deriving hydrogen, and each is provided with an on-off valve.
- the first output pipe is a fuel hydrogen supply passage 16 communicating with the fuel cell 15, and is provided with an on-off valve 19.
- the second output pipe is an external supply path 17 for supplying hydrogen to the outside, and an on-off valve 20 is provided.
- the control unit 18 is connected to the input unit 11, the reformer 12, the measurement unit 14, the fuel cell 15, the oxygen supply unit 43, and the on-off valves 19 and 20 in a communicable state. .
- the input unit 11 is connected to a hydrogen source 41 that stores and supplies a raw material containing hydrogen, and introduces the raw material received from the hydrogen source 41 into the reformer 12 via the raw material introduction path 29.
- the input unit 11 is preferably composed of a solenoid valve.
- the control unit 18 controls the opening / closing amount of the input unit 11 to control the introduction amount of the raw material, and to control the production amount of the hydrogen-containing gas in the reformer 12.
- the reformer 12 decomposes a predetermined amount of raw material introduced through the raw material introduction passage 29 to produce a hydrogen-containing gas.
- the produced hydrogen-containing gas is temporarily stored in the hydrogen storage container 13 via the hydrogen supply path 21.
- a measuring unit 14 is connected to the hydrogen storage container 13 and measures the storage amount of the hydrogen-containing gas in the hydrogen storage container 13.
- the measurement unit 14 is preferably a pressure gauge and measures the pressure in the hydrogen storage container 13. The measured pressure is input to the control unit 18.
- the hydrogen storage container 13 is provided with a fuel hydrogen supply path 16 and an external supply path 17 as pipes for deriving hydrogen.
- An on-off valve 19 is provided in the fuel hydrogen supply passage 16 communicating with the fuel cell 15.
- the control unit 18 controls the amount of hydrogen-containing gas supplied to the fuel cell 15 by controlling the opening / closing amount of the opening / closing valve 19.
- the control unit 18 also controls the opening / closing amount of the on-off valve 20 provided in the external supply path 17 to control the amount of hydrogen supplied to the outside and the storage amount of the hydrogen storage container 13.
- the on-off valves 19 and 20 are preferably constituted by electromagnetic valves.
- the fuel cell 15 generates power using the hydrogen-containing gas supplied from the hydrogen storage container 13 and oxygen contained in the air supplied from the oxygen supply means 43.
- a polymer electrolyte fuel cell having an operating temperature of 100 ° C. or less is most preferably applied.
- the fuel cell 15 supplies the power generated via the power supply path 30 to the reformer 12.
- the control unit 18 monitors the power generation amount of the fuel cell 15 and controls the opening / closing amount of the on-off valve 19 and the oxygen supply amount of the oxygen supply means 43 in order to control a necessary amount of power generation.
- a normal blower (fan) is preferably used for the oxygen supply means 43.
- the control unit 18 at the time of steady operation is necessary for achieving two purposes of securing an external supply amount of hydrogen and storing a hydrogen-containing gas necessary for starting the fuel cell 15 in the hydrogen storage container 13. I do.
- the control unit 18 thresholds the pressure inside the hydrogen storage container 13 when storing the minimum amount of hydrogen-containing gas necessary for starting the fuel cell 15 (hereinafter, this storage amount is referred to as “starting hydrogen amount”). It is stored as a value.
- the control part 18 receives the measurement data of the measurement part 14, and compares it with a threshold value. As a result of the comparison, when it is determined that the stored hydrogen-containing gas is lower than the startup hydrogen amount, the control unit 18 controls the input unit 11 to increase the amount of raw material supplied to the reformer 12. Then, the production amount of the hydrogen-containing gas is increased, and the storage amount of the hydrogen storage container 13 is increased to be equal to or greater than the startup hydrogen amount.
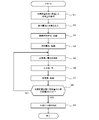
- a method for stopping the hydrogen production apparatus 1 will be described with reference to FIG.
- a series of stop processes of the hydrogen production apparatus 1 starts when a stop command is input to the control unit 18, and is all performed by the control of the control unit 18.
- the control means 18 closes the on-off valve 20 and stops the hydrogen supply to the outside (step S11).
- the control part 18 confirms the measurement data of the measurement part 14, and confirms that the hydrogen amount for starting is stored in the hydrogen storage container 13 (step S12).
- the input unit 11 is closed (step S13), and the reformer 12 is stopped (step S14).
- the control means 18 closes the on-off valve 19 to seal the hydrogen storage container 13, and stops the supply of hydrogen to the fuel cell 15 ( Step S16). Further, the oxygen supply is stopped (step S17), and finally the fuel cell is stopped (step S18).
- the hydrogen production apparatus 1 stops in a state where the hydrogen storage container 13 stores a hydrogen-containing gas that is equal to or more than the amount of hydrogen for activation.
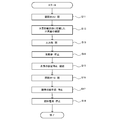
- a method for starting the hydrogen production apparatus 1 will be described with reference to FIG. All the activations are performed under the control of the control unit 18.
- the control part 18 confirms the measurement data of the measurement part 14, and confirms that the hydrogen amount for starting is stored in the hydrogen storage container 13 (step S1).
- the on-off valve 19 is opened, and hydrogen is supplied from the hydrogen storage container 13 to the fuel cell 15 (step S2).
- the oxygen supply means 43 is activated to supply oxygen to the fuel cell 15 (step S3), and the fuel cell 15 is activated to start power generation (step S4).
- the generated power is supplied to the reformer 12 via the power supply path 30, and the reformer 12 is activated (step S5).
- the control means 18 opens the input unit 11 and supplies a raw material containing hydrogen to the reformer 12 (step S6).
- step S7 When the electric power and the raw material are supplied, the reformer 12 starts producing hydrogen (step S7).
- the control unit 18 sequentially confirms the measurement data of the measurement unit 14 and confirms that the startup hydrogen amount is stored in the hydrogen storage container 13 (step S8).
- step S8 becomes yes and supply of hydrogen to the outside is started (step S9).
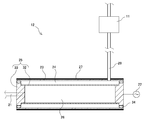
- the reformer 12 preferably used in the present embodiment will be described with reference to FIG.
- the reformer 12 includes a plasma reactor 23, a high voltage electrode 25 accommodated in the plasma reactor 23, and a ground electrode 27 disposed in contact with the outside of the plasma reactor 23.
- the plasma reactor 23 is made of quartz and has a cylindrical shape.
- the high voltage electrode 25 includes a cylindrical hydrogen separation membrane 32 and a disk-like support 33 that supports both ends of the hydrogen separation membrane 32.
- a suitable material for the hydrogen separation membrane 32 is a palladium alloy thin film.
- the high voltage electrode 25 is connected to a high voltage pulse power source 22 connected to the fuel cell 15 via the power supply path 30, and a high voltage is applied thereto.
- An O-ring 34 is fitted between the plasma reactor 23 and the support 33 so that the hydrogen separation membrane 32 is concentrically arranged with respect to the inner wall of the plasma reactor 23.
- a discharge space 24 is formed between the inner wall of the plasma reactor 23 and the hydrogen separation membrane 32 so as to maintain a constant distance.
- an inner chamber 26 that is surrounded by the hydrogen separation membrane 32 and the support 33 and is a closed space is formed inside the hydrogen separation membrane 32.
- the ground electrode 27 is arranged concentrically with the plasma reactor 23 and the hydrogen separation membrane 32.
- the most suitable raw material supplied from the hydrogen source 41 via the input unit 11 and the raw material introduction path 29 is ammonia gas.
- the ammonia gas is supplied to the discharge space 24 of the reformer 12.
- the plasma reactor 23 functions as a dielectric, and the hydrogen separation membrane of the high voltage electrode 25 is provided.
- a power source 22 that applies a high voltage to the high voltage electrode 25 applies a voltage having a waveform holding time as short as 10 ⁇ s.
- ammonia gas is supplied to the discharge space at a predetermined flow rate, and a dielectric barrier discharge is generated between the hydrogen separation membrane 32 of the high voltage electrode 25 and the ground electrode 27. This is performed by generating an atmospheric pressure non-equilibrium plasma of ammonia in the discharge space 24. Hydrogen gas generated from the atmospheric pressure non-equilibrium plasma of ammonia passes through the hydrogen separation membrane 32, moves to the inner chamber 26, and is separated. Hydrogen generated from the atmospheric pressure non-equilibrium plasma of ammonia is adsorbed on the hydrogen separation membrane 32 in the form of hydrogen atoms, passes through the hydrogen separation membrane 32 while diffusing, and recombines after passing through the hydrogen separation membrane 32. It moves to the inner chamber 26 as hydrogen molecules. In this way, only hydrogen is separated. The hydrogen moved to the inner chamber 26 is stored in the hydrogen storage container 13 via the hydrogen supply path 21 as high-purity hydrogen having a hydrogen concentration of 99.9% or more.
- a pressure gauge is used as the measuring unit 14 for measuring the hydrogen-containing gas storage amount in the hydrogen storage container 13.
- the control unit 18 stores a pressure threshold corresponding to the amount of the hydrogen-containing gas necessary for starting the fuel cell 15.
- the control unit 18 monitors the measurement result of the measurement unit 14 while hydrogen is being produced, and uses the comparison result between the stored threshold value and the measurement result, and thereby the hydrogen content of the reformer 12 is included.
- the amount of gas produced and the amount stored in the hydrogen storage container 13 are feedback-controlled, and a hydrogen-containing gas corresponding to the amount of 50 liters of hydrogen necessary for starting the fuel cell 15 is always stored in the hydrogen storage container 13.
- the reformer 12 of this embodiment includes a plasma reactor 23, a high voltage electrode 25 accommodated in the plasma reactor 23, and a ground electrode 27 disposed in contact with the outside of the plasma reactor 23. It is a plasma reformer provided.
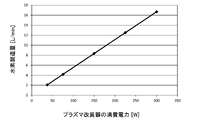
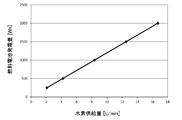
- An example of the relationship between the power consumption of the reformer 12 and the amount of hydrogen produced is shown in Table 1 and FIG.
- the volume of hydrogen shown below is a volume when converted in a standard state (1 atm, 0 ° C.).
- the plasma reformer 12 of this example can produce hydrogen in proportion to the supplied power. Specifically, when ammonia as a raw material is supplied at a rate of 1.39 liters per minute (volume converted in a standard state), 2.09 liters of hydrogen per minute is produced per 37.5 W of power consumption.
- the electric power generated by the fuel cell 15 is supplied from the power supply path 30 to the reformer 12.
- the reformer 12 is activated upon receiving power supply, and a dielectric barrier discharge is performed between the hydrogen separation membrane 32 of the high voltage electrode 25 to which a high voltage is applied from the high voltage pulse power supply 22 and the ground electrode 27. Then, production of hydrogen is started.
- the reformer 12 can produce 5.57 liters of hydrogen per minute with 100 W of power.
- the control unit 18 stores the produced hydrogen in the hydrogen storage container 13. Then, a part of the stored hydrogen is supplied from the fuel hydrogen supply path 16 to the fuel cell 15 to cause the fuel cell 15 to continue power generation. In this way, the fuel cell 15 and the reformer 12 are started up and the power is stably supplied, so that the production of hydrogen can be continued.
- the configuration of the hydrogen production apparatus 1 described in the present embodiment and the operation method thereof can be changed as appropriate.
- a cylindrical hydrogen separation membrane 32 housed in the plasma reactor 23 is grounded, and an electrode disposed in contact with the outside of the plasma reactor 23 is replaced with a high voltage pulse power source. 22 can be connected.
- the hydrogen separation membrane 32 functions as a ground electrode and can generate a dielectric barrier discharge as in the embodiment. Even in this case, the hydrogen separation membrane 32 can separate hydrogen by being exposed to plasma.
- the hydrogen storage container 13 and the on-off valves 19 and 20 are arranged at positions separated from each other.
- the on-off valves 19 and 20 are integrated with the hydrogen storage container 13, It can arrange
- the measuring unit 14 that measures the storage amount of the hydrogen storage container 13 can be replaced with a measuring device other than the pressure gauge. For example, a scale for measuring the weight of hydrogen can be applied.
- the wiring of the power supply path 30 for supplying power from the fuel cell 15 to the reformer 12 and the current / voltage control means can be appropriately changed according to the arrangement and functions of the entire device.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Sustainable Development (AREA)
- Manufacturing & Machinery (AREA)
- Electrochemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Sustainable Energy (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Health & Medical Sciences (AREA)
- Inorganic Chemistry (AREA)
- Combustion & Propulsion (AREA)
- Toxicology (AREA)
- Fuel Cell (AREA)
- Hydrogen, Water And Hydrids (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201780056033.XA CN109790015A (zh) | 2016-11-18 | 2017-10-13 | 制氢装置及制氢装置的操作方法 |
| DE112017005827.9T DE112017005827T5 (de) | 2016-11-18 | 2017-10-13 | Wasserstofferzeugungsvorrichtung und Betriebsverfahren einer Wasserstofferzeugungsvorrichtung |
| US16/333,971 US20190252700A1 (en) | 2016-11-18 | 2017-10-13 | Hydrogen-Producing Device and Operation Method of Hydrogen-Producing Device |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016224912A JP6929045B2 (ja) | 2016-11-18 | 2016-11-18 | 水素製造装置および水素製造装置の運転方法 |
| JP2016-224912 | 2016-11-18 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018092479A1 true WO2018092479A1 (ja) | 2018-05-24 |
Family
ID=62145609
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/037162 Ceased WO2018092479A1 (ja) | 2016-11-18 | 2017-10-13 | 水素製造装置および水素製造装置の運転方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20190252700A1 (enExample) |
| JP (1) | JP6929045B2 (enExample) |
| CN (1) | CN109790015A (enExample) |
| DE (1) | DE112017005827T5 (enExample) |
| WO (1) | WO2018092479A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020118736A1 (zh) * | 2018-12-14 | 2020-06-18 | 中国科学院大连化学物理研究所 | 一种基于广谱燃料的燃料电池系统及其运行方法 |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11148116B2 (en) * | 2017-08-21 | 2021-10-19 | Hychar Energy, Llc | Methods and apparatus for synthesizing compounds by a low temperature plasma dual-electric field aided gas phase reaction |
| CN110255496B (zh) * | 2019-06-27 | 2022-03-25 | 大连民族大学 | 一种阵列式非平衡等离子体重整器 |
| US11994061B2 (en) | 2021-05-14 | 2024-05-28 | Amogy Inc. | Methods for reforming ammonia |
| US11724245B2 (en) | 2021-08-13 | 2023-08-15 | Amogy Inc. | Integrated heat exchanger reactors for renewable fuel delivery systems |
| EP4352008A4 (en) | 2021-06-11 | 2025-07-02 | Amogy Inc | AMMONIA TREATMENT SYSTEMS AND METHODS |
| US11539063B1 (en) | 2021-08-17 | 2022-12-27 | Amogy Inc. | Systems and methods for processing hydrogen |
| WO2023098619A1 (zh) * | 2021-11-30 | 2023-06-08 | 上海慕帆动力科技有限公司 | 发电系统、发电系统动态调节方法及发电系统控制方法 |
| CN114352369B (zh) * | 2021-11-30 | 2023-03-14 | 上海慕帆动力科技有限公司 | 氨分解制氢的燃气轮机-汽轮机联合发电系统及控制方法 |
| CN114352412B (zh) * | 2021-11-30 | 2023-08-29 | 上海慕帆动力科技有限公司 | 一种基于氨分解制氢的发电系统及动态调节方法 |
| CN114718702B (zh) * | 2022-03-02 | 2023-02-28 | 武汉理工大学 | 一种发动机的催化辅助系统及方法、设备 |
| WO2023225617A2 (en) * | 2022-05-20 | 2023-11-23 | Plasmerica, Llc | Apparatus and method for hydrogen generation |
| US11840447B1 (en) | 2022-10-06 | 2023-12-12 | Amogy Inc. | Systems and methods of processing ammonia |
| US11866328B1 (en) | 2022-10-21 | 2024-01-09 | Amogy Inc. | Systems and methods for processing ammonia |
| US11795055B1 (en) | 2022-10-21 | 2023-10-24 | Amogy Inc. | Systems and methods for processing ammonia |
| WO2024107662A1 (en) * | 2022-11-14 | 2024-05-23 | Plasmerica, Llc | Apparatus and method for propogation reaction of hydrocarbons |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003163025A (ja) * | 2001-11-28 | 2003-06-06 | Tokyo Gas Co Ltd | 水素製造、貯蔵システム |
| JP2004095363A (ja) * | 2002-08-30 | 2004-03-25 | Honda Motor Co Ltd | 水素供給装置 |
| WO2014054277A1 (ja) * | 2012-10-02 | 2014-04-10 | 国立大学法人岐阜大学 | 水素生成装置及び水素生成装置を備えた燃料電池システム |
-
2016
- 2016-11-18 JP JP2016224912A patent/JP6929045B2/ja active Active
-
2017
- 2017-10-13 CN CN201780056033.XA patent/CN109790015A/zh not_active Withdrawn
- 2017-10-13 US US16/333,971 patent/US20190252700A1/en not_active Abandoned
- 2017-10-13 DE DE112017005827.9T patent/DE112017005827T5/de not_active Withdrawn
- 2017-10-13 WO PCT/JP2017/037162 patent/WO2018092479A1/ja not_active Ceased
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003163025A (ja) * | 2001-11-28 | 2003-06-06 | Tokyo Gas Co Ltd | 水素製造、貯蔵システム |
| JP2004095363A (ja) * | 2002-08-30 | 2004-03-25 | Honda Motor Co Ltd | 水素供給装置 |
| WO2014054277A1 (ja) * | 2012-10-02 | 2014-04-10 | 国立大学法人岐阜大学 | 水素生成装置及び水素生成装置を備えた燃料電池システム |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020118736A1 (zh) * | 2018-12-14 | 2020-06-18 | 中国科学院大连化学物理研究所 | 一种基于广谱燃料的燃料电池系统及其运行方法 |
| US12021281B2 (en) | 2018-12-14 | 2024-06-25 | Dalian Institute Of Chemical Physics, Chinese Academy Of Sciences | Multi-fuel fuel cell system and operation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN109790015A (zh) | 2019-05-21 |
| US20190252700A1 (en) | 2019-08-15 |
| DE112017005827T5 (de) | 2019-08-08 |
| JP2018080093A (ja) | 2018-05-24 |
| JP6929045B2 (ja) | 2021-09-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6929045B2 (ja) | 水素製造装置および水素製造装置の運転方法 | |
| JP6789080B2 (ja) | 燃料電池システムおよび燃料電池システムの運転方法 | |
| JP6582011B2 (ja) | 燃料電池システムを備えた輸送機器 | |
| JP5276318B2 (ja) | 蒸気パージによる燃料電池の停止 | |
| CN104661955B (zh) | 氢生成装置及设置有氢生成装置的燃料电池系统 | |
| EP2808298B1 (en) | Method for operating hydrogen generation device and method for operating a fuel cell system | |
| TWI243863B (en) | Hydrogen/oxygen supply system | |
| JP5037214B2 (ja) | 改質器システム、燃料電池システム、及びその運転方法 | |
| US11332835B2 (en) | Hydrogen system | |
| JP2018081870A5 (enExample) | ||
| WO2018096713A1 (ja) | 再生型燃料電池システム及び水電解システム | |
| US7205058B2 (en) | Residual stack shutdown energy storage and usage for a fuel cell power system | |
| TWI787831B (zh) | 產氫燃料電池系統和操作用於備用電源運作的產氫燃料電池系統的方法 | |
| US20070122666A1 (en) | Method of operating fuel cell system and fuel cell system | |
| US8053128B2 (en) | Apparatus for solid-oxide fuel cell shutdown having a timing circuit and a reservoir | |
| JPS6282660A (ja) | りん酸型燃料電池の停止方法 | |
| TW202337556A (zh) | 多個輸出的集管 | |
| US12283727B2 (en) | Hydrogen-producing fuel cell systems and methods of operating the same | |
| JPWO2017154138A1 (ja) | 水素漏えい防止システム、水素漏えい防止方法水素製造システム、電力貯蔵システム | |
| JP2003217640A (ja) | 燃料電池発電システム | |
| US20230078714A1 (en) | Hydrogen system and method for operating hydrogen system | |
| JP2024154104A (ja) | 電解合成システム | |
| HK40087745A (zh) | 产氢燃料电池系统和操作用於备用电源运作的产氢燃料电池系统的方法 | |
| JP2007220446A (ja) | 送液装置、改質原料供給装置および燃料電池システム | |
| JP2008251356A (ja) | 燃料電池システム及びその運転停止方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17872470 Country of ref document: EP Kind code of ref document: A1 |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 17872470 Country of ref document: EP Kind code of ref document: A1 |