WO2015190344A1 - マイクロニードルシート及び経皮投与用貼付剤 - Google Patents
マイクロニードルシート及び経皮投与用貼付剤 Download PDFInfo
- Publication number
- WO2015190344A1 WO2015190344A1 PCT/JP2015/065842 JP2015065842W WO2015190344A1 WO 2015190344 A1 WO2015190344 A1 WO 2015190344A1 JP 2015065842 W JP2015065842 W JP 2015065842W WO 2015190344 A1 WO2015190344 A1 WO 2015190344A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- skin
- sheet
- microneedle
- stratum corneum
- base material
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0021—Intradermal administration, e.g. through microneedle arrays, needleless injectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/70—Web, sheet or filament bases ; Films; Fibres of the matrix type containing drug
- A61K9/7023—Transdermal patches and similar drug-containing composite devices, e.g. cataplasms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M2037/0007—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin having means for enhancing the permeation of substances through the epidermis, e.g. using suction or depression, electric or magnetic fields, sound waves or chemical agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
- A61M2037/0023—Drug applicators using microneedles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
- A61M2037/0061—Methods for using microneedles
Definitions
- the present invention relates to a microneedle sheet and a patch for transdermal administration for introducing a target substance into the horny layer of skin using a plurality of microneedles.
- transdermal administration using a patch for transdermal administration has been performed as one means for non-invasively administering a drug or the like from the surface of a living body such as skin or mucous membrane. Then, in order to efficiently absorb the drug from the patch for transdermal administration into the body, the drug is adsorbed on a microneedle having a high aspect ratio called a so-called microneedle, and the microneedle is arrayed on a sheet.
- Formulations called arranged microneedle sheets or microneedle patches have been developed.
- Patent Document 1 Japanese Patent No. 5472771 discloses a technique for introducing a drug into the skin deeper than the stratum corneum by holding the drug in the microneedle and perforating the stratum corneum with the microneedle. Is disclosed.
- An object of the present invention is to provide a patch for transdermal administration and a microneedle sheet capable of efficiently introducing a target substance into the stratum corneum and suppressing a decrease in protective ability of the stratum corneum.
- a microneedle sheet according to an aspect of the present invention includes a target substance to be introduced into a horny layer of skin, and a plurality of microneedles formed on the sheet-like base material and in contact with the horny layer of the skin Each of the plurality of microneedles has a skin raised from the sheet-like base material in order to expand the stratum corneum by pressing the stratum corneum surface without piercing the stratum corneum surface of the skin.
- the skin stretching portion has a distal end surface having an area of 1 ⁇ 10 ⁇ 3 mm 2 or more and a height from the surface of the sheet-like substrate of 30 ⁇ m to 300 ⁇ m, and adjacent to each other. It arrange
- the distal end surface of the skin extension portion does not pierce the keratinous surface and presses the horny surface to extend the stratum corneum. Can be prevented, and the deterioration of the protective function of the stratum corneum can be suppressed.
- the target substance can be efficiently introduced from the sheet-like base material or the skin stretch portion into the stratum corneum stretched by the skin stretch portion.
- a microneedle sheet includes a target substance to be introduced into the horny layer of the skin, and a plurality of microneedles formed on the sheet-like base material and abutted against the horny layer of the skin
- Each of the plurality of microneedles has a tip surface for extending the stratum corneum by pressing the stratum corneum surface without piercing the stratum corneum surface of the skin. It has a skin extension part raised from the sheet-like base material, and a spinous protrusion part formed on the distal end surface of the skin extension part, having a shape that pierces the surface of the stratum corneum and the top part stays inside the stratum corneum.
- the stratum corneum is stretched by pressing the stratum corneum surface without the distal end surface of the skin stretching portion piercing the stratum corneum surface.
- the root of the remains on the surface of the stratum corneum.
- the spinous protrusion can be pierced into the stratum corneum so that the apex stays inside the stratum corneum, and the stratum corneum is prevented from being pierced by the microneedle. Therefore, while the fall of the protective function of a stratum corneum can be suppressed, the target substance can be efficiently introduced from the sheet-like base material or the skin stretch portion into the stratum corneum stretched by the skin stretch portion. Furthermore, the target substance can be efficiently introduced into the inside of the stratum corneum from the spinous projections stuck on the surface of the stratum corneum.
- the spinous process portion preferably has an area of less than 5 ⁇ 10 ⁇ 4 mm 2 in plan view and a height of 1 ⁇ m or more and 20 ⁇ m or less.
- the plurality of microneedles are arranged at a density such that the area occupied by the spinous protrusions per unit area of the microneedle sheet is 0.2% or less.
- the plurality of microneedles are preferably arranged at a density such that the area occupied by the tip surface per unit area of the microneedle sheet is 0.3% or more.
- the skin stretched part is configured so that the length from the root of the spinous process part to the end part of the distal end surface is 8 ⁇ m or more and 20 ⁇ m or less.
- the skin stretch portion configured as described above a portion from the root of the spinous projection portion to the end portion of the distal end surface is sufficiently caught, and the distal end surface of the skin stretch portion hardly enters the stratum corneum.
- the patch for transdermal administration includes the above-described microneedle sheet and a pressing portion that maintains a state in which the microneedle sheet is pressed against the skin.
- the transdermal patch having a pressing part can realize a state in which the stratum corneum is stably stretched by pressing the stratum corneum without the tip surface of the skin stretching part piercing the stratum corneum.
- the state in which the target substance is efficiently introduced from the sheet-like base material or the skin stretched part into the stratum corneum stretched by the stretched part can be stably maintained for a relatively long period of time.
- the distal end surface of the skin stretching portion can push the stratum corneum of the skin to stretch the stratum corneum, and the target substance is efficiently transferred from the microneedle to the stratum corneum. Can be introduced.
- the distal end surface of the skin stretched portion hardly breaks the stratum corneum of the skin, so that a decrease in the protective ability of the stratum corneum can be suppressed.
- the typical sectional view showing the structure outline of the patch for transdermal administration concerning a 1st embodiment.
- the typical expanded side view for demonstrating the microneedle described in FIG. The typical partial expanded side view of the microneedle of FIG.
- the expanded perspective view which shows a microneedle sheet
- FIG. 6C is a schematic cross-sectional view for explaining the relationship between the spinous protrusions
- FIG. 6C is a schematic partially enlarged perspective view for explaining the relationship between the microneedle and the skin shown in FIG. .
- A Schematic sectional view for explaining the relationship between the skin and the spinous protrusion when the interval between adjacent microneedles is too narrow
- Typical sectional drawing for demonstrating the relationship between a spinous process part. The figure for demonstrating the relationship between the space
- seat of FIG. Typical sectional drawing for demonstrating the relationship between the microneedle which concerns on 2nd Embodiment, and skin.
- FIG. 1 schematically shows a cross-sectional structure of a transdermal patch.
- a transdermal administration patch 10 shown in FIG. 1 includes a microneedle sheet 20 and a moisture-permeable sheet 30.
- an adhesive layer 32 is formed by applying an adhesive to the entire surface of the moisture-permeable sheet 30.
- the moisture-permeable sheet 30 serves as a support member for maintaining the microneedle sheet 20 in a state of being attached to the skin.
- the moisture-permeable sheet 30 is formed to be slightly larger than the microneedle sheet 20 so that the function as a support member can be sufficiently exhibited.
- the moisture permeable sheet 30 protrudes from the end of the microneedle sheet 20 over the entire circumference of the microneedle sheet 20.
- the adhesive 10 applied to the region Ar1 that protrudes out of the moisture permeable sheet 30 sticks to the skin, whereby the patch 10 for transdermal administration is brought into contact with the skin. Affixed to the skin.
- the microneedle sheet 20 includes a sheet-like base material 21 and a plurality of microneedles 50.
- the back surface of the sheet-like substrate 21 is attached to the adhesive layer 32, and a plurality of microneedles 50 are formed on the surface of the sheet-like substrate 21.
- the sheet-like base material 21 is formed into various planar shapes suitable for the site of the skin to be attached.
- the thickness of the sheet-like base material 21 is relatively thin, for example, several hundred ⁇ m.
- the sheet-like base material 21 may have a single layer structure made of a single material or a multilayer structure made of different materials.
- the material of the surface layer of the sheet-like substrate 21 is preferably the same as the material of the microneedles 50, but any material that can support a plurality of microneedles 50 in a standing state on the surface of the sheet-like substrate 21. That's fine.
- the surface layer of the sheet-like substrate 21 is formed of a polymer material that is harmless to a living body.
- the macromolecules that are harmless to the living body include resins that are harmless to the living body, polysaccharides that are harmless to the living body, proteins that are harmless to the living body, and compounds that are harmless to the living body derived therefrom.
- harmless to a living body means that it can be applied to medical, cosmetic or veterinary purposes when the amount introduced from the skin is appropriately adjusted by an appropriate method of use.
- the microneedle 50 is preferably formed of the same material as that of the surface layer of the sheet-like substrate 21, but a material suitable for introducing the target substance into the stratum corneum is selected as the material of the microneedle 50.
- the material of the microneedle 50 is also formed of a polymer material that is harmless to the living body.
- the polymer substance as the material of the microneedle 50 includes, for example, a resin harmless to the living body, a polysaccharide harmless to the living body, a protein harmless to the living body, and a compound harmless to the living body derived therefrom.
- the polymer material that is harmless to the living body as the material of the microneedle 50 preferably has at least one of the properties of in vivo solubility and in vivo degradability.
- the in vivo solubility is a property that dissolves in vivo
- the in vivo degradability is a property that decomposes in vivo.
- the in-vivo-soluble and / or bio-degradable polymer substance forming the microneedle 50 is water-soluble. If the microneedle 50 that has penetrated into the stratum corneum of the skin is a water-soluble polymer substance, the microneedle 50 is dissolved by the water in the stratum corneum, so that the target substance is smoothly introduced into the stratum corneum using the microneedle 50. It becomes easy to do.
- Polysaccharides that are water-soluble and have at least one of in vivo solubility and biodegradability and are harmless to the living body and compounds that are harmless to the living body include, for example, maltose, dextran, water-soluble chitosan, Examples include pullulan, sodium chondroitin sulfate, sodium hyaluronate and glycogen.
- proteins that are water-soluble and have at least one of in vivo solubility and biodegradability and are harmless to the living body and compounds that are harmless to the living body include serum albumin and serum ⁇ -acid glycoprotein. Can be mentioned.
- Examples of a resin that is water-soluble and has at least one of in vivo solubility and biodegradability and is harmless to a living body and a compound that is harmless to a living body include, for example, biodegradation that is harmless to a water-soluble living body And polymers derived therefrom.
- Examples of the water-soluble biodegradable polymer harmless to the living body and compounds derived therefrom include, for example, carboxyvinyl polymer, and water-soluble and biocompatible polymer polyethylene glycol (PEG) and lactic acid / glycolic acid-copolymer Examples thereof include block polymers having water solubility and biodegradability obtained by block copolymerization of (PLGA), polycaprolactone (PCL), or polylactic acid (PLA).
- a non-water-soluble resin having at least one of in vivo solubility and biodegradability a harmless resin that is harmless to a living body and a compound that is harmless to the living body, for example, polylactic acid, polyglycolic acid And polydioxanone.
- the material used for the above-described microneedle 50 can be used as the material for the sheet-like base material 21 as it is.
- the target substance introduced into the stratum corneum may be the same as the above-mentioned material, but may be different from the above-mentioned material.
- the target substance to be introduced into the stratum corneum include biologically active substances used for the treatment and diagnosis and prevention of wounds, beauty, and veterinary purposes. Such biologically active substances include, for example, drugs, nutrients and cosmetics.
- the target substance is particularly preferably a bioactive substance that acts on the beauty of the stratum corneum or the wound disease of the stratum corneum. For example, sodium hyaluronate that is effective for the beauty of the stratum corneum Is mentioned.
- the microneedle sheet 20 according to the first embodiment is entirely made of sodium hyaluronate, and the microneedle 50 is also made of, for example, sodium hyaluronate.
- the patch 10 for transdermal administration according to the first embodiment is, for example, a cosmetic. It is applied to the face to introduce sodium hyaluronate into the stratum corneum of the facial skin. The shape of the microneedle 50 will be described in detail later.
- the base material of the moisture-permeable sheet 30 is, for example, a large number (a plurality) of vapor-permeable holes (not shown) having a pore diameter of 0.1 to 100 ⁇ m, preferably 10 to 30 ⁇ m, through which water vapor is transmitted. It is formed with the polyurethane film 31 which has.
- the thickness of the moisture permeable sheet 30 is, for example, about several tens of ⁇ m.
- the moisture-permeable sheet 30 has an adhesive layer 32 for attaching to the skin.
- the moisture-permeable sheet 30 is configured to transmit water vapor from the vapor transmission holes of the polyurethane film 31 and the pressure-sensitive adhesive layer 32 so that the skin where the moisture-permeable sheet 30 is attached does not get steamed.
- the adhesive layer 32 is sparsely applied so as to reduce the application area so as not to block all the vapor transmission holes.
- FIG. 2 is a partially enlarged side view in which one microneedle and its periphery are partially enlarged.
- FIG. 3 is a partially enlarged perspective view in which one microneedle and a peripheral portion thereof are enlarged.
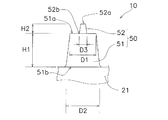
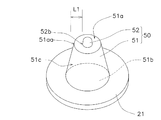
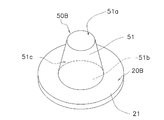
- the microneedle 50 has a skin extension 51 and a spinous protrusion 52.
- the skin stretched portion 51 is a portion that protrudes from the sheet-like base material 21 and is formed integrally with the sheet-like base material 21.
- the skin stretched part 51 and the sheet-like base material 21 are entirely formed of, for example, sodium hyaluronate.
- the skin extension 51 is a truncated cone, and the diameter D1 of the front end surface 51a is set within a range of 40 ⁇ m to 250 ⁇ m, the diameter D2 of the rear cross section 51b is set within a range of 120 ⁇ m to 700 ⁇ m, and the front cross section 51b
- the height H1 to the surface 51a is set within a range of 30 ⁇ m to 300 ⁇ m.
- the diameter D1 of the distal end surface 51a is preferably set within a range of 60 ⁇ m to 80 ⁇ m so that the skin is easily deformed.
- the distal end surface including the formation region of the spinous protrusions 52 is included.
- the area of 51a is preferably 1 ⁇ 10 ⁇ 3 mm 2 or more.
- the spinous protrusion 52 is formed on the distal end surface 51 a of the skin extension 51.
- the spinous protrusion 52 has a conical shape with a thin tip, the diameter D3 of the root 52b is set within a range of 1 ⁇ m to 24 ⁇ m, and the height H2 from the root 52b to the apex 52a is 1 ⁇ m to It is set within the range of 20 ⁇ m.
- the diameter D3 of the root portion 52b is set within the range of 5 ⁇ m to 18 ⁇ m, and the height H2 from the root portion 52b to the top portion 52a is 5 ⁇ m to 18 ⁇ m. It is preferable to set within the range.
- the shape of the horizontal cross section (the cross section parallel to the surface of the sheet-like base material 21) of the root portion 52b of the spinous protrusion 52 is at least the length from the root portion 52b to the end portion 51aa of the distal end surface 51a of the skin extension portion 51.
- the size is set so that L1 falls within the range of 8 ⁇ m to 20 ⁇ m.
- the number of spinous protrusions 52 formed on one distal end surface 51a may be plural, but one is preferable in order to surely enter the stratum corneum.
- the pressure applied to the spinous protrusion 52 is increased so that the apex 52a can easily enter the stratum corneum.
- the area in plan view is preferably less than 5 ⁇ 10 ⁇ 4 mm 2 .
- the skin stretch portion 51 set to the above-described size has a distal end surface 51a of the skin stretch portion 51 when the transdermal administration patch 10 is attached to normal human skin by the adhesive layer 32. Does not enter the stratum corneum of the skin. In other words, if the shape of the skin stretch portion 51 is set to the size as described above, the skin around the skin stretch portion 51 stretches when the transdermal administration patch 10 is applied to the skin. This means that the skin surface reaches the surface of the sheet-like substrate 21.
- the spinous protrusions 52 set to have the above-described size are formed when the patch 10 for transdermal administration is attached to normal human skin by the adhesive layer 32.
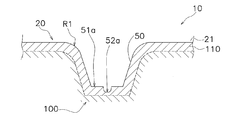
- FIG. 4 schematically shows the relationship between one microneedle 50 and the skin 100 when the transdermal patch 10 is affixed to the human skin 100.
- the skin stretched portion 51 does not enter the stratum corneum 110, and only the spinous protrusion 52 enters the inside of the stratum corneum 110.
- the top 52 a of the spinous protrusion 52 is inside the stratum corneum 110.
- the plurality of microneedles 50 are arranged in a lattice pattern as shown in FIG. 5, for example.
- the arrangement of the plurality of microneedles 50 is not limited to the lattice shape, but it is preferable that they are arranged uniformly throughout. This is because when the arrangement of the microneedles 50 is biased, a portion where a relatively high pressure is applied and a portion where a relatively low pressure is applied are formed. If the pressure difference between the relatively high pressure portion and the relatively low pressure portion becomes too large, a region where the spinous protrusion 52 does not pierce the stratum corneum occurs, or conversely, the skin extension portion 51 pierces the stratum corneum.
- each spine A high pressure is preferably applied to the protrusion 52.
- the total area of the root portions 52b arranged per unit area is preferably 0.2% or less (in FIG. 5, 0.002 ⁇ UL 2 or less).
- the root portion 52b is circular and substantially flat and the diameter D3 is 24 ⁇ m, 400 or less may be arranged per 1 cm 2 .
- the spinous protrusion 52 contacts the stratum corneum 110 of the skin 100. Since the front end surface 51a of the skin stretch portion 51 hits, it is preferable to distribute the pressure when the skin is deformed to as many skin stretch portions 51 as possible. Specifically, it is preferable that the total area of the tip surface 51a per unit area is 0.3% or more of the unit area (for example, 0.003 ⁇ UL 2 or more in FIG. 5). . For example, if the tip surface 51a is circular and substantially flat and the diameter D1 is 40 ⁇ m, 250 or more may be arranged per 1 cm 2 .
- the diameter D1 of the distal end surface 51a is set within the range of 40 ⁇ m to 250 ⁇ m.
- the height H1 from the rear cross section 51b to the tip surface 51a is set within a range of 30 ⁇ m to 300 ⁇ m.
- the area Ar2 surrounded by the one-dot chain circle in FIG. 5 and the rear cross section 51b of the microneedle 50 is an area that does not come into contact with the skin 100 when the transdermal patch 10 is applied to the skin 100.
- the non-contact region Ar2 has a pressure of 0.5 MPa.
- the total area is preferably 0.3% or less of the unit area (for example, 0.003 ⁇ UL 2 or less in FIG. 5).
- the gap between the adjacent skin extension portions and the sheet-like base material is arranged so that the distance between the adjacent squares of the skin extension portions is larger than the value obtained by dividing 100 by the square of the height of the adjacent skin extension portions. It is preferable. This point will be described in detail later with reference to FIGS. Moreover, it is preferable to make the inclination of the side surface 51c of the skin extension part 51 gentle, and it is preferable that the difference in the diameter D2 of the rear cross section 51b and the diameter D1 of the front end face 51a is 100 ⁇ m or more.
- the microneedles 50 are shaped so that the surface of the skin 100 is in close contact with the surfaces of the microneedles 50 and the sheet-like substrate 21 as shown in FIG. Is formed.
- the curvature radius R1 of the skirt portion of the rear cross section 51b is preferably 0.1 mm or less and 0.01 mm or more, and more preferably 0.05 mm or less.
- microneedle 50 and sheet-like base material 21 are made of the same material. Preferably, it is configured.
- microneedle sheet 20 is dried by pouring an aqueous solution of sodium hyaluronate into a stamper (not shown) which is a mold in which the shape of the plurality of microneedles 50 is carved. Manufactured. If sodium hyaluronate is a target substance for administration, the microneedle sheet 20 may be formed using an aqueous solution of sodium hyaluronate as a main component.
- the microneedle sheet 20 is made such that an aqueous solution of sodium hyaluronate to which another biologically active substance is added is poured into a stamper and dried. May be manufactured. Furthermore, substances other than the target substance such as a stabilizer for the target substance may be added. In addition, as a method for adding other biologically active substance of sodium hyaluronate, a method other than the above may be used, and for example, it may be added by applying after drying. By the above manufacturing method, for example, the microneedle sheet 20 in which 250 microneedles 50 made of sodium hyaluronate are formed per 1 cm can be manufactured.
- the microneedle sheet 20 has a skin extension 51 having a diameter D1 of the front end surface 51a of 68 ⁇ m, a diameter D2 of the rear cross section 51b of 200 ⁇ m, and a height H1 of 100 ⁇ m, and a diameter D3 of the root 52b of 20 ⁇ m and a height H2. And a spinous protrusion 52 having a length L1 of 8 ⁇ m.
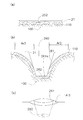
- FIG. 6A is a schematic cross-sectional view showing a state immediately before the spinous protrusions 252 formed on the sheet-like base material 21 are brought into contact with the skin 100.
- the unevenness of the skin 100 can be achieved only by the very fine spinous protrusions 252. Since the skin 100 is stretched due to undulation, skin structure, etc., there are cases where the stratum corneum 110 is not pierced well or where the surface of the skin 100 is not touched by unevenness or undulation. On the other hand, when there is the skin stretched portion 51 shown in FIG.
- the skin 100 is stretched by the skin stretched portion 51 and the skin 100 is stretched more than before the transdermal administration patch 10 is applied. Since the rate decreases, the top 52a of the fine spinous protrusion 52 having a height of 1 ⁇ m to 20 ⁇ m penetrates into the horny layer 110.
- the hyaluronic acid from the spinous protrusion 52 to the stratum corneum 110.
- the amount of sodium hyaluronate supplied to the stratum corneum 110 can be increased as compared with the case where only the skin stretched portion 51 without the spinous protrusion 52 is supplied with sodium acid.
- the transdermal patch 10 when used, for example, a situation frequently occurs in which the transdermal patch 10 is left on the skin with the adhesive layer 32 (an example of a pressing portion) even during sleep. To do. During sleep, a user using the transdermal patch 10 unconsciously applies the transdermal patch 10 to bedding or surrounding equipment, and the high pressure is applied to the transdermal patch 10. It is expected to take. As shown in FIG. 6B and FIG. 6C, a region where the skin 100 does not contact the surface of the skin extension 261 or the sheet-like base material 21 if the skin extension 261 is excessively raised.
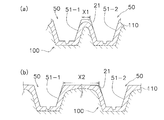
- the microneedle 50 As shown in FIG. 7B, the microneedle 50 according to the first embodiment described above has an interval between the boundaries between the skin extension portions 51-1 and 51-2 adjacent to each other and the sheet-like base material 21.
- X2 is arranged so as to be separated from a value obtained by dividing the square of the height H1 of adjacent skin stretched parts by 100, that is, X2 ⁇ (H1 ⁇ H1) ⁇ 100.
- the non-contact region Ar2 where the stratum corneum 110 does not contact the sheet-like base material 21 has a total area of 0.3% or less of the unit area when a pressure of 500 gf / cm 2 is applied to the microneedle sheet 20 (for example, In FIG. 5, it is configured to be 0.003 ⁇ UL 2 or less.
- the difference between the diameter D2 of the rear section 51b and the diameter D1 of the front end surface 51a is set to 132 ⁇ m ( ⁇ 100 ⁇ m). If the interval X1 ( ⁇ X2) between the boundaries between the skin extension portions 51-1 and 51-2 and the sheet-like base material 21 is small as shown in FIG.
- FIG. 8 shows an example of the relationship between the shape of the microneedle 50 and the adhesion of the microneedle sheet 20 to the horny layer 110 of the skin 100 with respect to the microneedle sheet 20 formed of sodium hyaluronate.
- “ ⁇ ” described in the column “Close contact with skin” indicates that the contact with the skin is good, and “X” indicates that the contact with the skin is poor. “ ⁇ ” indicates that the contact with the skin is not so good.
- needle shape NS4, NS5, NS6 shown by FIG. 8 what the skin expansion
- the needle shapes NS4, NS5, NS6 is described as an example of poor adhesion.
- the height of the skin stretched portion 51 is “0 ⁇ m”
- the sheet-like base material 21 is in close contact with the stratum corneum 110, and therefore, as shown in the graph of FIG.
- the straight line LL is considered to pass through the origin of the graph.
- the distance X2 between the boundaries is the distance X2 between the boundaries.
- the height of the microneedles 50 is increased and the space between them is expanded. It is preferable to reduce the height to narrow the space between them. From this point of view, it can be considered that the square of the height of the microneedle 50 is preferably 40000 or less from FIG. Moreover, when the heights of the microneedles 50 adjacent to each other are different and there are high and low ones, they are adjusted to the high ones.
- the degree of contact between the stratum corneum 110 and the microneedle sheet 20, the damage of the stratum corneum 110 by the skin extension 51, and the intrusion of the spinous protrusions 52 into the stratum corneum 110 are caused by the microscopic area of the portion actually attached to the skin.
- the needle sheet 20 was evaluated by observation using an enlarged image with an optical observation device.
- the surface of the skin 100 is substantially microneedle 50 and sheet-like when a pressure of about 0.13 MPa is applied.
- the microneedle 50 is formed in a shape that is in close contact with the surface of the substrate 21, and the close contact with the skin 100 is improved.
- the microneedle 50 is formed in a shape that is in close contact with the surface of the microneedle 50 and the sheet-like substrate 21. Since the microneedle 50 has such a shape, even when a pressure higher than 0.13 MPa is applied to the microneedle sheet 20, most of the pressure is applied to the sheet-like base material 21, and stress is concentrated on the tip surface 51a. Therefore, the stratum corneum 110 is prevented from being broken at the tip surface 51a. Further, when the portion where the skin 100 is in close contact with the sheet-like substrate 21 is increased, the microneedle 50 and the sheet-like substrate 21 are made of the same material in consideration of transdermal administration from the sheet-like substrate 21 to the skin 100. It is preferable that it is comprised. For example, when it is desired to administer as much sodium hyaluronate as possible to the skin 100 for cosmetic purposes, it is effective that the sheet-like substrate 21 is formed of sodium hyaluronate.
- the microneedle sheet 20 is formed of sodium hyaluronate has been described as an example.
- the microneedle sheet 20 may be formed of a thermoplastic different from that of the first embodiment, for example. it can.
- the microneedle sheet in such a case may be manufactured by injection molding into a mold in which the same shape as the plurality of microneedles 50 is carved.
- the target substance is not a thermoplastic, if the target substance is not denatured by heat, it can be mixed with the thermoplastic and injection molded. After the injection molding, the target substance is heated after the molding by means such as coating. It may be added to a plastic plastic.
- the cost for adding the target substance can be reduced by applying it after injection molding.
- the sheet-like base material 21A and the microneedles 50A are formed of, for example, polylactic acid which is a thermoplastic plastic, May be coated with a layer 53 of sodium hyaluronate.
- the skin extension 51 and the spinous protrusion 52 of the microneedle 50A are also formed of polylactic acid.
- the shape of the microneedle 50A can be maintained even if it is attached to the skin 100.
- the pressing portion is not limited to such a pressure-sensitive adhesive.
- the pressure-sensitive adhesive or adhesive formed on the surface of the sheet-like base material 21 of the microneedle sheet 20, that is, the surface on which the microneedles 50 are formed can be used as the pressing portion.
- the pressing portion may be a bandage that wraps the microneedle sheet 20 around the human body in a state where the microneedle sheet 20 is in close contact with the skin 100.
- a cushion member may be provided at a portion that contacts the microneedle sheet 20 to increase the pressing force.
- a string-like member such as a string or rubber is attached to both ends of the cloth, like an eyepatch, and the cloth part of the cloth that is attached to the body with the string-like member or hooked on the body with a string-like member
- the needle seat 20 may be attached.
- FIG. 11 is a partially enlarged side view in which one microneedle and its periphery are partially enlarged.
- FIG. 12 is a partially enlarged perspective view in which one microneedle and a peripheral portion thereof are enlarged.
- the microneedle 50 ⁇ / b> B has a skin stretching portion 51.
- a spinous protrusion is not formed on the distal end surface 51a of the skin extension 51.
- the transdermal patch 10B according to the second embodiment can be configured in the same manner as the transdermal patch 10 of the first embodiment except for the shape and arrangement of the plurality of microneedles 50B.
- the shape and arrangement of the microneedles 50B of the transdermal patch 10B according to the embodiment will be described, and other descriptions will be omitted.
- the microneedle 50B has a skin extension 51 protruding from the sheet-like substrate 21, and the sheet-like substrate. 21 is formed integrally.
- the skin stretched part 51 and the sheet-like base material 21 are entirely formed of, for example, sodium hyaluronate.
- the skin extension 51 is a truncated cone, and the diameter D1 of the front end surface 51a is set within a range of 40 ⁇ m to 250 ⁇ m, the diameter D2 of the rear cross section 51b is set within a range of 120 ⁇ m to 700 ⁇ m, and the front cross section 51b
- the height H1 to the surface 51a is set within a range of 30 ⁇ m to 300 ⁇ m.
- the diameter D1 of the distal end surface 51a is preferably set within a range of 60 ⁇ m to 80 ⁇ m so that the skin is easily deformed. Expressing this in terms of the area of the tip surface 51a, in order to reduce the pressure applied to the tip surface 51a and make it difficult for the tip surface 51a to enter the stratum corneum, the area of the tip surface 51a is 1 ⁇ 10 ⁇ 3 mm 2. This is preferable.
- the skin stretched part 51 set to the above size is such that when the transdermal patch 10B is attached to normal human skin, the distal end surface 51a of the skin stretched part 51 is the stratum corneum of the skin. It is the structure which does not penetrate in. In other words, if the shape of the skin stretch portion 51 is set to the size as described above, the skin around the skin stretch portion 51 stretches when the transdermal administration patch 10B is applied to the skin. This means that the skin surface reaches the surface of the sheet-like substrate 21.
- FIG. 13 schematically shows a relationship between one microneedle 50 ⁇ / b> B and the skin 100 when the transdermal patch 10 ⁇ / b> B is attached to the human skin 100. As can be seen from the cross section shown in FIG. 13, the skin stretched portion 51 does not enter the stratum corneum 110, and only the stratum corneum 110 around it is stretched.
- the plurality of microneedles 50B are arranged in a lattice like the microneedles 50 shown in FIG. 5, for example.
- the arrangement of the plurality of microneedles 50B is not limited to the lattice shape, but it is preferable that they are arranged uniformly throughout. This is because if the arrangement of the microneedles 50B is biased, a portion where a relatively high pressure is applied and a portion where a relatively low pressure is applied are formed. This is because if the pressure difference between the relatively high pressure portion and the relatively low pressure portion becomes too large, a region where the skin stretched portion 51 is stuck in the stratum corneum may occur.
- the tip surface 51a of the skin stretching portion 51 hits the stratum corneum 110 of the skin 100.
- the total area of the front end surface 51a per unit area is preferably arranged such that (0.003 ⁇ UL 2 or more in for example FIG. 5) of 0.3% or more per unit area .
- the non-contact region Ar2 in which the stratum corneum 110 does not contact the sheet-like base material 21 has a total area of 0 when the pressure of 500 gf / cm 2 is applied to the microneedle sheet 20. 3% or less (for example, 0.003 ⁇ UL 2 or less in FIG. 5).
- the stratum corneum 110 can be prevented from being damaged by the skin stretched portion 51.
- the difference between the diameter D2 of the rear section 51b and the diameter D1 of the front end surface 51a is set to 132 ⁇ m ( ⁇ 100 ⁇ m).
- the surface of the skin 100 is substantially microneedle 50B and the sheet-like base material when a pressure of about 0.13 MPa is applied.
- the microneedle 50 ⁇ / b> B is formed in a shape that is in close contact with the surface of 21, and the close contact with the skin 100 is improved.
- the plurality of microneedles 50B of the second embodiment are formed in a shape in which the surface of the skin 100 is in close contact with the surfaces of the microneedles 50B and the sheet-like substrate 21 when a pressure of about 0.13 MPa is applied. . Since the microneedle 50B has such a shape, even if a pressure higher than 0.13 MPa is applied to the microneedle sheet 20B, most of the pressure is applied to the sheet-like base material 21, and stress is concentrated on the tip surface 51a. Therefore, the stratum corneum 110 is prevented from being broken at the tip surface 51a.
- microneedle 50B and sheet-like base material 21 are made of the same material. Preferably, it is configured.
- the microneedle 50B of the second embodiment described above is when a force of 10 N is applied to a disk having a diameter of 10 mm on which a plurality of microneedles 50B are formed, in other words, when a pressure of about 0.13 MPa is applied.
- the microneedles 50B are formed in a shape in which the surface of the skin 100 is in close contact with the surfaces of the microneedles 50B and the sheet-like base material 21. Since the microneedle 50B has such a shape, even if a pressure higher than 0.13 MPa is applied to the microneedle sheet 20, most of the pressure is applied to the sheet-like base material 21, and stress is concentrated on the tip surface 51a.
- the stratum corneum 110 is prevented from being broken at the tip surface 51a.
- the microneedle 50B and the sheet-like substrate 21 are made of the same material in consideration of transdermal administration from the sheet-like substrate 21 to the skin 100. It is preferable that it is comprised. For example, when it is desired to administer as much sodium hyaluronate as possible to the skin 100 for cosmetic purposes, it is effective that the sheet-like substrate 21 is formed of sodium hyaluronate.
- the microneedle sheet 20B on which the skin stretched part 51 is formed and the sheet consisting only of the sheet-like base material 21 are affixed to the skin and compared, Since the sodium hyaluronate is supplied to the stratum corneum 110 from the 51a and the side surface 51c, the microneedle sheet is more than the one composed only of the sheet-like base material 21 from which the sodium hyaluronate is supplied to the stratum corneum 110 only from the sheet base material 20B increases the amount of sodium hyaluronate supplied to the stratum corneum 110.
- the microneedle sheet 20B may be formed of, for example, a thermoplastic different from the first embodiment. it can.
- the microneedle sheet in such a case may be manufactured by injection molding into a mold having the same shape as the plurality of microneedles 50B.
- the target substance is not a thermoplastic, if the target substance is not denatured by heat, it can be mixed with the thermoplastic and injection molded. After the injection molding, the target substance is heated after the molding by means such as coating. It may be added to a plastic plastic. If the target substance is sensitive to heat or expensive, for example, the cost for adding the target substance can be reduced by applying it after injection molding.
- the spinous protrusion 52 can be removed from the transdermal patch 10A shown in FIG.
- the pressing portion is not limited to such a pressure-sensitive adhesive.
- a pressure-sensitive adhesive or adhesive formed on the surface of the sheet-like substrate 21 of the microneedle sheet 20B, that is, the surface on which the microneedle 50B is formed can be used as the pressing portion.
- the pressing portion may be a bandage that wraps the microneedle sheet 20B around the human body in a state where the microneedle sheet 20B is in close contact with the skin 100.
- a cushion member may be provided in a portion that contacts the microneedle sheet 20B to increase the pressing force.
- a string-like member such as a string or rubber is attached to both ends of the cloth, like an eyepatch, and the cloth part of the cloth that is attached to the body with the string-like member or hooked on the body with a string-like member You may make it attach with the needle seat 20B.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Dermatology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Heart & Thoracic Surgery (AREA)
- Anesthesiology (AREA)
- Medical Informatics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Medicinal Preparation (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/317,906 US20170106180A1 (en) | 2014-06-10 | 2015-06-02 | Microneedle sheet and transdermal patch |
| KR1020167034984A KR102167464B1 (ko) | 2014-06-10 | 2015-06-02 | 마이크로 니들 시트 및 경피 투여용 첩부제 |
| CN201580031084.8A CN106456955B (zh) | 2014-06-10 | 2015-06-02 | 微针片以及经皮给药贴附剂 |
| EP15806520.1A EP3156097A1 (en) | 2014-06-10 | 2015-06-02 | Microneedle sheet and dermal administration plaster |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014-119370 | 2014-06-10 | ||
| JP2014119370A JP6023752B2 (ja) | 2014-06-10 | 2014-06-10 | マイクロニードルシート及び経皮投与用貼付剤 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015190344A1 true WO2015190344A1 (ja) | 2015-12-17 |
Family
ID=54833440
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/065842 Ceased WO2015190344A1 (ja) | 2014-06-10 | 2015-06-02 | マイクロニードルシート及び経皮投与用貼付剤 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20170106180A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP3156097A1 (cg-RX-API-DMAC7.html) |
| JP (1) | JP6023752B2 (cg-RX-API-DMAC7.html) |
| KR (1) | KR102167464B1 (cg-RX-API-DMAC7.html) |
| CN (1) | CN106456955B (cg-RX-API-DMAC7.html) |
| WO (1) | WO2015190344A1 (cg-RX-API-DMAC7.html) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020044853A1 (ja) * | 2018-08-28 | 2020-03-05 | Nissha株式会社 | 肌改質シート |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020092229A1 (en) * | 2018-10-31 | 2020-05-07 | The Regents Of The University Of California | Biodegradable microneedles for transdermal therapeutic agent delivery |
| CN111408032A (zh) * | 2020-03-05 | 2020-07-14 | 优微(珠海)生物科技有限公司 | 一种透气透水微针贴片及其使用方法 |
| CN112618945B (zh) * | 2020-12-14 | 2022-06-21 | 北京航空航天大学 | 中空封闭型微针及其制备方法和包括该微针的操作装置 |
| GB2622416B (en) * | 2022-09-15 | 2025-04-02 | Ndm Tech Ltd | Skin preparation device |
| TWI824724B (zh) * | 2022-09-16 | 2023-12-01 | 達運精密工業股份有限公司 | 微針貼片、其製造模具、以及其製造方法 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007089792A (ja) * | 2005-09-28 | 2007-04-12 | Nano Device & System Research Inc | 経皮投与装置 |
| JP5439633B1 (ja) * | 2012-04-25 | 2014-03-12 | 帝人株式会社 | マイクロニードルおよびマイクロニードルアレイ |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| PT2563450T (pt) * | 2010-04-28 | 2017-08-28 | Kimberly Clark Co | Dispositivo para entrega de medicação para a artrite reumatóide |
| JP6121734B2 (ja) * | 2012-02-09 | 2017-04-26 | 久光製薬株式会社 | マイクロニードル用ゾルミトリプタン含有コーティング組成物及びマイクロニードルデバイス |
| JP5472771B1 (ja) * | 2012-09-28 | 2014-04-16 | コスメディ製薬株式会社 | 段差に薬物を保持したマイクロニードル |
| CN203355123U (zh) * | 2013-06-13 | 2013-12-25 | 陈彦彪 | 一种超微针片 |
-
2014
- 2014-06-10 JP JP2014119370A patent/JP6023752B2/ja active Active
-
2015
- 2015-06-02 CN CN201580031084.8A patent/CN106456955B/zh active Active
- 2015-06-02 WO PCT/JP2015/065842 patent/WO2015190344A1/ja not_active Ceased
- 2015-06-02 US US15/317,906 patent/US20170106180A1/en not_active Abandoned
- 2015-06-02 EP EP15806520.1A patent/EP3156097A1/en not_active Withdrawn
- 2015-06-02 KR KR1020167034984A patent/KR102167464B1/ko active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007089792A (ja) * | 2005-09-28 | 2007-04-12 | Nano Device & System Research Inc | 経皮投与装置 |
| JP5439633B1 (ja) * | 2012-04-25 | 2014-03-12 | 帝人株式会社 | マイクロニードルおよびマイクロニードルアレイ |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020044853A1 (ja) * | 2018-08-28 | 2020-03-05 | Nissha株式会社 | 肌改質シート |
| JPWO2020044853A1 (ja) * | 2018-08-28 | 2020-10-22 | Nissha株式会社 | 肌改質シート |
| JP2021045562A (ja) * | 2018-08-28 | 2021-03-25 | Nissha株式会社 | 肌改質シート |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3156097A1 (en) | 2017-04-19 |
| CN106456955B (zh) | 2018-09-25 |
| CN106456955A (zh) | 2017-02-22 |
| KR102167464B1 (ko) | 2020-10-19 |
| JP2015231466A (ja) | 2015-12-24 |
| US20170106180A1 (en) | 2017-04-20 |
| JP6023752B2 (ja) | 2016-11-09 |
| KR20170019357A (ko) | 2017-02-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6023752B2 (ja) | マイクロニードルシート及び経皮投与用貼付剤 | |
| KR101724655B1 (ko) | 마이크로 니들 패치 및 그의 제조 방법 | |
| KR102265808B1 (ko) | 치료제의 전달을 위한 마이크로어레이 및 그것의 사용방법 | |
| JP5966156B2 (ja) | 剣山型マイクロニードルのアプリケータデバイス | |
| US20080200883A1 (en) | Micro-needle and micro-needle patch | |
| KR20180096610A (ko) | 마이크로 니들 및 마이크로 니들 패치 | |
| JP2016511014A5 (cg-RX-API-DMAC7.html) | ||
| JP2016189844A (ja) | マイクロニードルパッチ | |
| US20180344998A1 (en) | Microneedle and method for producing same | |
| KR102497984B1 (ko) | 마이크로 니들 제제 및 마이크로 니들 제제의 제조 방법 | |
| KR102234331B1 (ko) | 마이크로 니들 시트 | |
| KR101833821B1 (ko) | 패치 | |
| US20220008353A1 (en) | Multifunctional microstructure patch | |
| KR101724654B1 (ko) | 마이크로 니들 패치 및 그의 제조 방법 | |
| US20130323296A1 (en) | Carrier for oromucosal, especially sublingual administration of physiologically active substances | |
| JP2016189845A (ja) | マイクロニードルシート | |
| KR20210029581A (ko) | 마이크로 니들 패치 및 마이크로 니들 시스템 | |
| CN102274560B (zh) | 注射针 | |
| JP6943517B2 (ja) | マイクロニードル及びマイクロニードルアレイ | |
| JP2022132921A (ja) | マイクロニードル及びマイクロニードルアレイ | |
| KR200499729Y1 (ko) | 마이크로니들 패치 및 이를 이용한 피부미용 마사지기 | |
| US20240226521A1 (en) | Transdermal microneedle patch containing poly-lactic acid needles that are easily absorbable into skin | |
| KR20250083270A (ko) | 마이크로니들 패치 구조체 | |
| WO2023032118A1 (ja) | マイクロニードル、マイクロニードルアレイ及びマイクロニードルパッチ並びにマイクロニードルアレイの製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15806520 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15317906 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20167034984 Country of ref document: KR Kind code of ref document: A |
|
| REEP | Request for entry into the european phase |
Ref document number: 2015806520 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2015806520 Country of ref document: EP |