WO2015182648A1 - 爆轟法による炭素粒子の製造方法 - Google Patents
爆轟法による炭素粒子の製造方法 Download PDFInfo
- Publication number
- WO2015182648A1 WO2015182648A1 PCT/JP2015/065220 JP2015065220W WO2015182648A1 WO 2015182648 A1 WO2015182648 A1 WO 2015182648A1 JP 2015065220 W JP2015065220 W JP 2015065220W WO 2015182648 A1 WO2015182648 A1 WO 2015182648A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- explosive
- detonation
- carbon particles
- substance
- raw material
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B32/00—Carbon; Compounds thereof
- C01B32/25—Diamond
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J3/00—Processes of utilising sub-atmospheric or super-atmospheric pressure to effect chemical or physical change of matter; Apparatus therefor
- B01J3/06—Processes using ultra-high pressure, e.g. for the formation of diamonds; Apparatus therefor, e.g. moulds or dies
- B01J3/062—Processes using ultra-high pressure, e.g. for the formation of diamonds; Apparatus therefor, e.g. moulds or dies characterised by the composition of the materials to be processed
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J3/00—Processes of utilising sub-atmospheric or super-atmospheric pressure to effect chemical or physical change of matter; Apparatus therefor
- B01J3/06—Processes using ultra-high pressure, e.g. for the formation of diamonds; Apparatus therefor, e.g. moulds or dies
- B01J3/08—Application of shock waves for chemical reactions or for modifying the crystal structure of substances
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B32/00—Carbon; Compounds thereof
- C01B32/25—Diamond
- C01B32/26—Preparation
Definitions
- the present invention relates to a method for producing carbon particles by the detonation method. More specifically, the present invention produces carbon particles containing diamond and graphitic carbon by a detonation method using a raw material containing an aromatic compound having three or more nitro groups (also called explosive raw material). On how to do.
- Nanoscale diamonds also called nanodiamonds
- nanodiamonds can be synthesized using, for example, detonation reaction of explosives.
- detonation is performed only with the explosive raw material that is a carbon source, and carbon atoms decomposed and liberated from the molecules constituting the explosive raw material by detonation reaction are generated as diamond under high temperature and high pressure during detonation It is generally called the detonation method. See, for example, Non-Patent Document 1 for the production of nanodiamonds by the detonation method.
- the present invention solves the above-mentioned conventional problems, and an object thereof is to provide a method for producing carbon particles containing nanodiamond and graphitic carbon by a detonation method using an explosive material. More specifically, the object is to provide a method for producing nanodiamonds with high yield.
- the present inventors provide an explosive substance that is liquid at room temperature and normal pressure around the explosive raw material. It has been found that carbon particles containing a larger amount of diamond can be obtained by arranging and detonating reaction than in the conventional method described above, and the present invention has been completed.
- explosive means a substance capable of detonation reaction. Explosive-based materials are included in explosives.
- An explosive substance means a substance that causes a rapid combustion reaction. Explosive substances are generally classified into solid explosives that do not have fluidity at room temperature and normal pressure, and liquid explosives that have fluidity, but in this specification, fluids flow at normal temperature and normal pressure unless otherwise specified. It means a liquid explosive with sex.
- normal temperature and normal pressure shall mean the conditions of 25 degreeC and 1 atmosphere.
- the raw material When the explosive substance detonates, the raw material is decomposed to the atomic level, and free carbon atoms are aggregated in a solid state without being oxidized, and diamond or graphitic carbon is generated.
- the raw material At the time of detonation, the raw material is brought into a high temperature and high pressure state due to a decomposition reaction, but immediately expands and is cooled.
- the process from high temperature and high pressure to vacuum cooling occurs in a much shorter time than normal combustion or deflagration, which is an explosion phenomenon slower than detonation. Carbon particles are produced.
- the present invention is a method for producing carbon particles by a detonation method, and an explosive substance that is liquid at room temperature and normal pressure around a raw material containing an aromatic compound having three or more nitro groups.
- a manufacturing method characterized by including a disposing step and a step of detonating the explosive substance.
- the raw materials used in the production method of the present invention are trinitrotoluene, cyclotrimethylenetrinitramine, cyclotetramethylenetetranitramine, pentaerythritol tetranitrate and trinitrophenylmethyl as aromatic compounds having three or more nitro groups. It is preferable to include at least one selected from the group consisting of nitramines.
- explosive substances are liquid explosives that have fluidity at room temperature and normal pressure.
- the explosive substance preferably contains at least one selected from the group consisting of a mixture of hydrazine and hydrazine nitrate, a mixture of hydrazine and ammonium nitrate, nitromethane, and a mixture of hydrazine and nitromethane.
- detonation is performed with the source material and the explosive material loaded in the chamber and / or with the coolant disposed around the source material and the explosive material in the chamber. It is preferable. At this time, if the atmosphere in the chamber does not contain oxygen gas and / or if the coolant is a substance that does not substantially generate an oxidizing substance such as oxygen or ozone, the oxidation reaction can be suppressed.
- recover carbon particles from carbon in a medium improves, and the ratio by the mass ratio which can collect
- a ratio in a mass ratio at which carbon particles can be recovered from carbon in the raw material is referred to as “carbon particle yield” which is a mass ratio of the carbon particles to the raw material.
- a ratio in a mass ratio at which diamond can be recovered from carbon in the raw material is referred to as “diamond yield” which is a mass ratio of diamond to the raw material.
- the production method of the present invention can further include a step of recovering carbon particles from the residue obtained in the detonation step.
- the recovery step for example, if classification and purification treatment is performed, carbon particles can be obtained in the form of powder having a desired particle size.
- each above-mentioned preferable embodiment may combine those arbitrary two or more.
- carbon particles containing diamond and graphitic carbon can be obtained by a detonation method using an explosive material and a liquid explosive in combination.
- This carbon particle is a carbon particle having a higher diamond content than a conventional product obtained by a detonation method using an explosive material alone or in combination with an explosive material and a solid explosive.
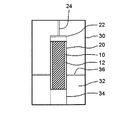
- FIG. 1 is a cross-sectional view schematically showing an example of an explosion device used in the production method of the present invention.
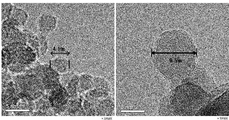
- FIG. 2 is a transmission electron micrograph of the carbon particles obtained in Experimental Example 3.
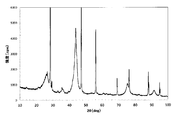
- FIG. 3 is an X-ray diffraction chart of the carbon particles obtained in Experimental Example 3.
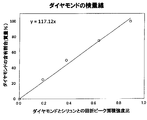
- FIG. 4 is a calibration curve graph used to determine the diamond content ratio of carbon particles.
- the production method of the present invention is a method of producing carbon particles by a detonation method, and is an explosive substance that is liquid at room temperature and pressure around a raw material containing an aromatic compound having three or more nitro groups And a step of detonating the explosive substance.
- an explosive substance that is liquid at normal temperature and normal pressure is placed around a raw material containing an aromatic compound having three or more nitro groups.
- An aromatic compound having three or more nitro groups is an explosive raw material contained in a raw material which is a carbon source for the detonation method.
- Explosive substances that are liquid at normal temperature and pressure are substances that cause stable detonation in order to generate carbon particles from raw materials.
- numerator which comprises an explosive substance contains a carbon atom
- the said explosive substance may become a carbon source with a raw material substance.
- the aromatic compound having 3 or more nitro groups is a compound having a structure in which 3 or more hydrogen atoms of an aromatic ring such as benzene, naphthalene, and anthracene are substituted with nitro groups.
- the aromatic compound may have a substituent other than a nitro group, and examples of the substituent include an alkyl group, a hydroxy group, a hydroxyalkyl group, an amino group, and a halogen group.
- positional isomers may exist, and any of these positional isomers can be used in the production method of the present invention.
- trinitrotoluene means 2,4,6-trinitrotoluene.
- aromatic compound examples include trinitrotoluene (also referred to as TNT), cyclotrimethylenetrinitramine (also referred to as RDX, hexogen), cyclotetramethylenetetranitramine (also referred to as HMX, octogen), and pentaerythritol tetranitrate (also referred to as PETN). And trinitrophenylmethylnitramine (also called tetryl). Of these aromatic compounds, TNT is particularly preferable because it is easily available.
- the aromatic compounds may be used alone or in combination of two or more.
- two or more of the aromatic compounds are used in combination include explosive explosives mainly composed of RDX and TNT, such as Composition B, cyclotol (75/25), (70/30), (65 / 30), composition B-2; mixed explosives mainly composed of HMX and TNT, such as octol (75/25); mixed explosives mainly composed of TNT and tetril, such as teritol.
- explosive explosives mainly composed of RDX and TNT such as Composition B, cyclotol (75/25), (70/30), (65 / 30), composition B-2
- mixed explosives mainly composed of HMX and TNT such as octol (75/25)
- mixed explosives mainly composed of TNT and tetril such as teritol.
- the content ratio of the aromatic compound having three or more nitro groups in the raw material is usually 50% by mass or more, preferably 80% by mass or more, more preferably 90% by mass or more, relative to the total mass of the raw material. More preferably, it is 95 mass% or more.
- the upper limit of the content of the aromatic compound having three or more nitro groups is most preferably 100% by mass, but the upper limit may be preferably about 99% by mass or 98% by mass.
- a liquid explosive having fluidity at normal temperature and normal pressure is used as the explosive substance. If a liquid explosive is used, compared with the case where a solid explosive is used, the freedom degree of a shape is high, an enlargement is easy, and operativity and safety
- the liquid explosive may be one that does not contain carbon as a constituent element. Examples of liquid explosives include a mixture of hydrazine and hydrazine nitrate, a mixture of hydrazine and ammonium nitrate, a mixture of hydrazine, hydrazine nitrate and ammonium nitrate, nitromethane, and a mixture of hydrazine and nitromethane. As used herein, hydrazine includes its hydrate, hydrazine hydrate.
- the type and the type of explosive substance are set so that the explosive speed when the explosive substance placed in the surrounding area is ignited alone is faster than the explosive speed when the raw material substance placed inside is ignited alone. It is important to select and use the composition appropriately.
- the amount of raw material and explosive substance used may be adjusted as appropriate according to the desired amount of carbon particles, and is not particularly limited.
- the ratio of explosive substance / raw material is expressed by mass ratio. , Preferably 0.1 or more, more preferably 0.2 or more, preferably 1 or less, more preferably 0.9 or less, and still more preferably 0.8 or less. If the ratio of the amount used is less than 0.1, a detonation reaction sufficient to generate carbon particles cannot be performed, and thus the yield may decrease. On the contrary, if the ratio of the amount used exceeds 1, the explosive substance is used more than necessary, which may increase the production cost.
- FIG. 1 is a cross-sectional view schematically showing an example of an explosion device used in the production method of the present invention.
- the explosive device shown in FIG. 1 is merely illustrative and is not intended to limit the present invention.
- the explosive substance 12 is arranged around the raw material substance 10.
- the high temperature and high pressure accompanying the shock wave generated by the detonation of the explosive substance 12 is applied to the raw material substance 10 as uniformly as possible, that is, in an explosive shape. It is preferable to arrange the source material 10 and the explosive material 12 symmetrically so as to ensure symmetry.
- the raw material 10 is solid and the explosive material 12 is a liquid explosive, for example, the raw material 10 is melted and pressed to produce a cylindrical molded body, and the molding is performed. After the body is placed in the center of the inside of the cylindrical container with the axial direction aligned, a liquid explosive may be injected around it.
- explosion container a container that contains a raw material and an explosive substance.
- a container made of a synthetic resin such as an acrylic resin because impurities such as metals can be prevented from being mixed.
- the explosive substance 12 is then detonated to generate carbon particles from the raw material substance 10.
- a shock wave generated by the detonation reaction of the explosive substance 12 propagates toward the raw material 10, and the shock wave is compressed by the shock wave to cause detonation, and the carbon decomposed and released from the organic molecules constituting the raw material 10. Atoms change to carbon particles containing diamond and graphitic carbon.
- Detonation may be performed in either an open system or a closed system.
- detonation may be performed inside earth or a tunnel excavated underground.
- it is preferable to perform detonation in a closed system because the residue can be prevented from scattering over a wide range.
- detonation may be performed in a state where an explosion container is loaded in, for example, a chamber.
- a chamber used for detonation is referred to as an “explosion chamber”.
- the explosion chamber may be made of metal or concrete as long as it has sufficient strength to withstand detonation. It is preferable to suspend and load the explosion container in the explosion chamber.
- the atmosphere in the explosion chamber When detonation is performed in an explosion chamber, if the atmosphere in the explosion chamber does not substantially contain oxygen during detonation, the oxidation reaction of carbon can be suppressed. Can be improved.
- the atmosphere in the explosion chamber is replaced with an inert gas such as nitrogen gas, argon gas or carbon dioxide gas, or is evacuated to about ⁇ 0.1 to ⁇ 0.01 MPaG.
- an inert gas may be injected into the explosion chamber to a weak positive pressure of about +0.000 to +0.001 MPaG.
- the symbol G attached after the pressure unit means a gauge pressure.
- the explosion container 20 may be installed in the cooling container 30 and the coolant 32 may be injected into the gap between the cooling container 30 and the explosion container 20.
- the coolant 32 is a substance that does not substantially generate an oxidizing substance such as oxygen or ozone, the oxidation reaction can be suppressed, so that the yield of carbon particles is improved.
- oxygen gas dissolved in the coolant 32 may be removed, or a coolant 32 that does not contain a constituent element that generates an oxidizing substance such as oxygen or ozone may be used.
- the coolant 32 include water, alkyl halides such as chlorofluorocarbons and carbon tetrachloride. Water is particularly preferred because it has little adverse effect on the environment.
- Explosive substance 12 is normally detonated using a detonator or explosive wire.
- an explosive agent may be interposed between the explosive substance 12 and the detonator or explosive wire.
- the explosive charge include Composition C-4 and SEP manufactured by Asahi Kasei Chemicals.
- the coolant 32 it is preferable to store the explosion container 20 in a liquid-tight container so that the coolant 32 does not enter the explosion container 20, for example.
- liquid-tight container examples include bags made of olefin-based synthetic resins such as polyethylene and polypropylene as raw materials. If the explosive substance 12 is detonated and detonated after setting in this way, carbon particles containing diamond and graphitic carbon can be obtained as the residue.
- the residue obtained in the detonation process may contain debris of an explosion container or blasting debris such as conductors / wires as impurities.
- this recovery step for example, if classification and purification treatment is performed, the carbon particles can be obtained in the form of a dry powder having a desired particle size.
- the sieve passing part is recovered.
- the residue on the sieve is classified again after crushing. Water is separated from the finally obtained sieve passage to obtain a dry powder.
- the sieve openings may be adjusted as appropriate, separation and purification treatments may be repeated, and the sieve passage having a sieve opening corresponding to the desired particle size may be used as the product.
- the recovered product is obtained by separating the water by centrifugation or the like and drying it to obtain a powder of carbon particles having a desired particle size.
- the acrylic resin when used as the explosion container 20, for example, the acrylic resin may be mixed in the carbon particles. In this case, for example, the acrylic resin may be removed by elution treatment with acetone. Further, depending on the use, it may be undesirable to mix a metal such as iron. In such a case, for example, treatment with hot concentrated nitric acid may be performed to remove metals such as iron.
- the carbon particles obtained by the production method of the present invention contain diamond and graphitic carbon. Therefore, even if it remains as it is or after any post-treatment, if the graphitic carbon remains sufficiently, the excellent characteristics of diamond and graphitic carbon can be utilized. Useful for various applications. For example, making use of diamond's excellent properties such as abrasiveness, durability, and wear resistance, such as tools, antiwear agents, lubricants, fluidized whetstones, fixed whetstones, plating / coating, durable films, lithium battery parts, etc. Useful for applications.
- graphite carbon such as conductivity, water repellency and biocompatibility, fiber materials, resin coatings that provide functionality, drug delivery systems, electronic device covers, battery electrode materials, electrical conductivity It is useful for applications such as adhesive film, reinforced rubber / water-repellent rubber, catalyst and adsorbent.
- the carbon particles may be subjected to perchloric acid treatment and / or plasma oxidation treatment to remove graphitic carbon.
- perchloric acid treatment and / or plasma oxidation treatment to remove graphitic carbon.
- diamond such as high refraction, transparency, and durability, it is particularly useful for use in optical parts such as optical lenses.
- the calibration curve for diamond uses five standard samples, and the ratio between the area intensity of the diffraction peak and the area intensity of the diffraction peak at the (220) plane and (311) plane of the silicon crystal added to each sample. From this, 4 inspections were made. The reason why the two peaks of the silicon crystal are used is to suppress the influence of the orientation of the powder silicon.
- the five standard samples are obtained by mixing the silicon crystal powder with the purified diamond so that the diamond content is 0% by mass, 25% by mass, 50% by mass, 75% by mass, or 100% by mass. .
- a calibration curve for diamond was obtained by plotting data of five standard samples with the vertical axis representing the diamond content ratio and the horizontal axis representing the area intensity ratio D220 / (Si220 + Si311) of the diffraction peak between diamond and silicon.
- the obtained calibration curve is shown in FIG.
- -X-ray diffractometer name Rigaku horizontal X-ray diffractometer SmartLab ⁇ Measuring method: ⁇ -2 ⁇ ⁇ X-ray source: Cu-K ⁇ ray ⁇ Excitation voltage-current: 45 kV-200 mA ⁇ Divergent slit: 2/3 ° ⁇ Scatter slit: 2/3 ° ⁇ Reception slit: 0.6mm
- TNT a commercially available cylindrical molded body (TNT's cylindrical filler, diameter 10 cm ⁇ length 20 cm, manufactured by Chuka Kayaku Co., Ltd.) was used.
- the mass of the TNT molded body was 2.52 kg, and the density was 1.60 g / cm 3 .
- hydrazine nitrate and hydrazine hydrate were mixed at a mass ratio of 3: 1 to prepare 0.93 kg of a hydrazine-based liquid explosive.
- detonation reaction was performed using an explosion device as shown in FIG.
- the molded body as the raw material 10 was placed in the center of the explosion container 20 having an inner diameter of 12 cm and a height of 20 cm, and the liquid explosive as the explosive substance 12 was filled around it.
- An explosive 22 (SEP), explosive wire, and No. 6 electric detonator 24 were attached to the top of the explosion container 20, covered, and stored in a liquid-tight polyethylene bag.
- a container having a capacity of 200 L was used as the cooling container 30.
- the explosion container 20 was installed in the cooling container 30. At this time, the outer bottom surface of the explosion container 20 was adjusted to be 29.5 cm in height from the inner bottom surface of the cooling container 30 by using the iron mount 34 and the iron perforated disk 36.
- distilled water was put into the cooling container 30 as the cooling material 32, and the cooling material 32 was filled in the gap between the cooling container 30 and the explosion container 20.
- a polyethylene bag containing distilled water was placed on top of the cooling vessel.
- a total of 200 L of distilled water was used.
- the cooling container 30 was covered, it was suspended from the ceiling in an explosion chamber with an internal volume of 30 m 3 using a wire sling.
- the inside of the explosion chamber was evacuated from atmospheric pressure, and the amount of remaining oxygen gas was calculated to be about 25.5 g.
- the explosive material 12 was detonated by detonating the explosive wire with the detonator. Then, about 200 L of water containing the residue was recovered from the explosion chamber and settled and separated to remove coarse rubble. At this time, since the supernatant liquid was strongly alkaline, the pH was adjusted to weak acidity by adding citric acid. The supernatant liquid that became weakly acidic was recovered as a waste liquid as it was. The precipitate was classified with a sieve having an opening of 100 ⁇ m / 16 ⁇ m using a vibrating sieve device (“KG-700-2W” manufactured by KOWA). The portion passing through the 16 ⁇ m sieve was recovered as it was.
- KG-700-2W manufactured by KOWA
- the residue on the sieve is crushed for about 5 minutes with an ultrasonic vibration device (Crest “4G-250-3-TSA”) to separate carbon from the rubble surface, and then the vibration sieve device (KOWA “KG” -700-2W "), and classified again with a sieve having an aperture of 100 ⁇ m / 32 ⁇ m / 16 ⁇ m, and a portion passing through the sieve was recovered.
- Each sieve passage was left in an 80 ° C. drier (“OF-450S” manufactured by ASONE) for 24 hours to evaporate the moisture, and then dried powder.
- Example 2 In this experimental example, as a raw material, mass 2.52Kg, density 1.60 g / cm 3 of TNT molded mass 3.82Kg, TNT molded density 1.61 g / cm 3 (of Chugokukayaku made TNT cylinder Except that it was changed to a shape-filled material (diameter 10 cm x length 30 cm) and the amount of explosive hydrazine liquid explosive was changed from 0.93 kg to 1.29 kg, the same as in Experimental Example 1. Thus, carbon particles having a 19 ⁇ m passage through a 16 ⁇ m sieve, 356.5 g through a 32 ⁇ m sieve and 222.2 g through a 100 ⁇ m sieve were obtained. The total recovered amount of carbon particles was 770.8 g. Table 1 below shows the experimental contents, the recovery amount and yield of carbon particles, and the total recovery amount and yield of diamond determined by the XRD quantitative method.
- Example 3 In this experimental example, as a raw material, mass 2.52Kg, density 1.60 g / cm 3 of TNT molded mass 6.30Kg, TNT molded density 1.59 g / cm 3 (of Chugokukayaku made TNT cylinder Changed shape filler, diameter 10cm x length 50cm), changed amount of explosive hydrazine liquid explosive from 0.93kg to 2.17kg, use of coolant distilled water Except for changing the amount from 200 L to 220 L, in the same manner as in Experimental Example 1, carbon particles of 257.4 g of 16 ⁇ m sieve passage, 531.8 g of 32 ⁇ m sieve passage, and 336.4 g of 100 ⁇ m sieve passage were obtained. The total recovered amount of carbon particles was 1125.6 g. Table 1 below shows the experimental contents, the total recovered amount and yield of carbon particles, and the total recovered amount and yield of diamond determined by the XRD quantitative method.
- FIG. 2 a transmission electron micrograph of the passage through the 16 ⁇ m sieve is shown in FIG. 2, and an X-ray diffraction chart of the passage through the 100 ⁇ m sieve is shown in FIG. From the left photograph in FIG. 2, carbon particles having a particle size of about 4.1 nm can be observed. In addition, carbon particles having a particle size of about 9.5 nm can be observed from the right photograph in FIG.
- Table 1 shows that carbon particles containing diamond and graphitic carbon can be produced by the detonation method even when the explosive raw material TNT and hydrazine liquid explosive are used in combination. Moreover, the obtained carbon particles are carbon particles having a higher diamond content than the conventional products. Actually, the yield of diamond is as high as 9.2 to 11.3%. On the other hand, according to Table 2 of Non-Patent Document 1, the diamond yield in the conventional method is 2.8% when TNT is used alone, and 4.1 to 8 when TNT and RDX are used in combination. .3%, and 3.75 to 8.2% when TNT and HMX are used in combination.
- the diamond is collected in comparison with the conventional method in which the explosive material is used alone or in combination with the explosive material and the solid explosive. It can be seen that the rate is improved.
- diamond can be produced with good yield from explosive materials by the detonation method. Therefore, the production method of the present invention makes a great contribution in various fields related to applications utilizing the excellent characteristics of diamond.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- Inorganic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Crystallography & Structural Chemistry (AREA)
- Carbon And Carbon Compounds (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15799381.7A EP3150552A4 (en) | 2014-05-30 | 2015-05-27 | Method for producing carbon particles by detonation |
| US15/313,789 US10201791B2 (en) | 2014-05-30 | 2015-05-27 | Method for producing carbon particles by detonation |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014-113057 | 2014-05-30 | ||
| JP2014113057A JP6114717B2 (ja) | 2014-05-30 | 2014-05-30 | 爆轟法による炭素粒子の製造方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015182648A1 true WO2015182648A1 (ja) | 2015-12-03 |
Family
ID=54698972
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/065220 Ceased WO2015182648A1 (ja) | 2014-05-30 | 2015-05-27 | 爆轟法による炭素粒子の製造方法 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US10201791B2 (enExample) |
| EP (1) | EP3150552A4 (enExample) |
| JP (1) | JP6114717B2 (enExample) |
| WO (1) | WO2015182648A1 (enExample) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101705943B1 (ko) * | 2014-04-08 | 2017-02-22 | 성균관대학교산학협력단 | 전기 폭발법을 이용한 다중층 그래핀이 코팅된 복합체 분말의 제조방법 |
| JP6416048B2 (ja) | 2015-07-01 | 2018-10-31 | 株式会社神戸製鋼所 | グラファイト群、該グラファイト群を含む炭素粒子 |
| JP6962907B2 (ja) * | 2016-03-18 | 2021-11-05 | 株式会社ダイセル | 硬化性樹脂組成物および光学部材 |
| RU2712551C1 (ru) * | 2019-03-11 | 2020-01-29 | Валерий Юрьевич Долматов | Способ получения детонационных наноалмазов |
| RU2711599C1 (ru) * | 2019-03-11 | 2020-01-17 | Валерий Юрьевич Долматов | Способ получения детонационных наноалмазов |
| KR102540730B1 (ko) * | 2020-07-30 | 2023-06-12 | (주)서한에프앤씨 | 진공 팩을 이용한 고체에어로졸 소화기의 점화장치 및 이를 이용한 고체에어로졸 소화기 |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH03271109A (ja) * | 1990-03-22 | 1991-12-03 | Agency Of Ind Science & Technol | ダイヤモンドの合成方法 |
| JP2005289677A (ja) * | 2004-03-31 | 2005-10-20 | Nof Corp | ダイヤモンド合成用爆薬組成物およびダイヤモンドの合成方法 |
| JP2006102656A (ja) * | 2004-10-06 | 2006-04-20 | Asahi Kasei Chemicals Corp | ダイヤモンドの製造方法 |
| WO2006075763A1 (ja) * | 2005-01-11 | 2006-07-20 | Hiroshi Ishizuka | 単結晶質ダイヤモンド微粉及びその製造方法 |
| JP2007269576A (ja) * | 2006-03-31 | 2007-10-18 | Nof Corp | クラスターダイヤモンドの合成方法、及び合成装置 |
| JP2012135718A (ja) * | 2010-12-27 | 2012-07-19 | Vision Development Co Ltd | ダイヤモンドの製造方法 |
| JP2012170913A (ja) * | 2011-02-23 | 2012-09-10 | Nippon Kayaku Co Ltd | 単結晶ナノダイヤモンド造粒粉体の製造方法 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2090239B (en) * | 1980-07-31 | 1983-06-22 | Inst Khim Fiz An Sssr | Method of obtaining diamond and/or diamond-like modification of boron nitride |
| BG49267A3 (en) * | 1990-11-09 | 1991-09-16 | Любомиров СТОЕВ Христо | Method for preparing ultradispersional diamond |
| RU2051092C1 (ru) * | 1991-12-25 | 1995-12-27 | Научно-производственное объединение "Алтай" | Алмазсодержащее вещество и способ его получения |
| JP5221953B2 (ja) * | 2005-06-29 | 2013-06-26 | 日本化薬株式会社 | 微細ダイヤモンドの製造方法及び爆薬組成物 |
| WO2007078210A1 (fr) | 2005-12-30 | 2007-07-12 | Gosudarstvennoe Uchrezhdenie 'federalnoe Agentstvo Po Pravovoi Zaschite Rezultatov Intellektualnoi Deyatelnosti Voennogo, Spetsialnogo I Dvoinogo Naznachenia' Pri Ministerstve Yustitsii Rossiyskoy Fed | Nanodiamant et procede de fabrication correspondant |
| US8506920B2 (en) * | 2007-12-21 | 2013-08-13 | Daren Normand Swanson | Method for creating diamond dust via detonation of carbon dioxide and reducing agent combinations |
-
2014
- 2014-05-30 JP JP2014113057A patent/JP6114717B2/ja active Active
-
2015
- 2015-05-27 EP EP15799381.7A patent/EP3150552A4/en not_active Withdrawn
- 2015-05-27 WO PCT/JP2015/065220 patent/WO2015182648A1/ja not_active Ceased
- 2015-05-27 US US15/313,789 patent/US10201791B2/en not_active Expired - Fee Related
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH03271109A (ja) * | 1990-03-22 | 1991-12-03 | Agency Of Ind Science & Technol | ダイヤモンドの合成方法 |
| JP2005289677A (ja) * | 2004-03-31 | 2005-10-20 | Nof Corp | ダイヤモンド合成用爆薬組成物およびダイヤモンドの合成方法 |
| JP2006102656A (ja) * | 2004-10-06 | 2006-04-20 | Asahi Kasei Chemicals Corp | ダイヤモンドの製造方法 |
| WO2006075763A1 (ja) * | 2005-01-11 | 2006-07-20 | Hiroshi Ishizuka | 単結晶質ダイヤモンド微粉及びその製造方法 |
| JP2007269576A (ja) * | 2006-03-31 | 2007-10-18 | Nof Corp | クラスターダイヤモンドの合成方法、及び合成装置 |
| JP2012135718A (ja) * | 2010-12-27 | 2012-07-19 | Vision Development Co Ltd | ダイヤモンドの製造方法 |
| JP2012170913A (ja) * | 2011-02-23 | 2012-09-10 | Nippon Kayaku Co Ltd | 単結晶ナノダイヤモンド造粒粉体の製造方法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3150552A4 * |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3150552A4 (en) | 2017-12-06 |
| JP2015227260A (ja) | 2015-12-17 |
| JP6114717B2 (ja) | 2017-04-12 |
| US20170183234A1 (en) | 2017-06-29 |
| US10201791B2 (en) | 2019-02-12 |
| EP3150552A1 (en) | 2017-04-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6114717B2 (ja) | 爆轟法による炭素粒子の製造方法 | |
| JP5973987B2 (ja) | 爆轟法による炭素粒子の製造方法 | |
| KR102046772B1 (ko) | 그래파이트군, 해당 그래파이트군을 포함하는 탄소 입자 | |
| JP6220769B2 (ja) | 爆轟法による炭素粒子の製造方法 | |
| JP6420228B2 (ja) | 被覆粒子の製造方法、並びに機能材料の製造方法 | |
| JP2007269576A (ja) | クラスターダイヤモンドの合成方法、及び合成装置 | |
| CN101208462A (zh) | 微细金刚石的制造方法及微细金刚石 | |
| JP2017013047A (ja) | 被覆粒子 | |
| JPH0659398B2 (ja) | ダイヤモンド合成用爆薬組成物 | |
| Shang et al. | Analysis crystal characteristics of diamond synthesized by different dynamic loading methods. | |
| Kudryashova et al. | Stored energy of detonation nanodiamond | |
| Ahmed et al. | Thermal and Morphological Analysis of TNT and RDX Recovered From Unserviceable Composition B Explosive for Re-Utilization | |
| AU2021241164A1 (en) | Explosive composition for diamond synthesis | |
| JP2018108904A (ja) | フッ素化グラファイト微粒子集合体およびその製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15799381 Country of ref document: EP Kind code of ref document: A1 |
|
| REEP | Request for entry into the european phase |
Ref document number: 2015799381 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2015799381 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15313789 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |