WO2015146537A1 - Gip上昇抑制剤の評価又は選択方法 - Google Patents
Gip上昇抑制剤の評価又は選択方法 Download PDFInfo
- Publication number
- WO2015146537A1 WO2015146537A1 PCT/JP2015/056603 JP2015056603W WO2015146537A1 WO 2015146537 A1 WO2015146537 A1 WO 2015146537A1 JP 2015056603 W JP2015056603 W JP 2015056603W WO 2015146537 A1 WO2015146537 A1 WO 2015146537A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- protein
- fabp4
- fabp5
- expression level
- gene
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/502—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics for testing non-proliferative effects
- G01N33/5023—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics for testing non-proliferative effects on expression patterns
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/92—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving lipids, e.g. cholesterol, lipoproteins, or their receptors
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2500/00—Screening for compounds of potential therapeutic value
- G01N2500/10—Screening for compounds of potential therapeutic value involving cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/04—Endocrine or metabolic disorders
- G01N2800/042—Disorders of carbohydrate metabolism, e.g. diabetes, glucose metabolism
Definitions
- the present invention relates to a method for evaluating or selecting a GIP elevation inhibitor.
- GIP glycose-dependent insulotropic polypeptide
- K cells secretory cells
- GIP promotes insulin secretion from pancreatic ⁇ cells in a glucose-dependent manner and contributes to the regulation of blood glucose level.
- mice with artificially increased blood GIP concentrations are suppressed in lipid burning under high fat diet intake.
- GIP receptor-deficient mice suppress the accumulation of visceral fat and subcutaneous fat due to high-fat diet loading. From these findings, it is considered that postprandial GIP regulation is effective in preventing and improving obesity.
- GIP is known to have a gastric acid secretion inhibitory action and a gastric motility inhibitory action
- GIP elevation inhibition is effective in promoting postprandial digestion and improving stomach sag. Therefore, the development of a substance that suppresses the increase in GIP is desired, and accordingly, the development of a method that can rapidly evaluate the substance's ability to suppress the increase in GIP with high sensitivity is required.
- Patent Document 1 a method using the expression of CPT1 gene or CPT1 protein in cells or the activity of CPT1 protein as an index
- Patent Document 2 a method using expression as an index
- FABPs fatty acid-binding proteins
- Fatty acids have a wide variety of functions in cells such as energy sources and metabolic control signal molecules.
- FABPs play an important role in the functional expression of fatty acids by binding to insoluble fatty acids and enabling transport to various organs within the cell.
- FABP4 and FABP5 which are isoforms of FABPs, are highly homologous in amino acid sequence and steric structure and are co-expressed in adipocytes and macrophages (Non-Patent Documents 2 to 4). Functional analysis using knockout mice has been performed for FABP4 and FABP5 (Non-Patent Documents 2 to 15).
- FABP4 accounts for 1 to 3% of the cytoplasmic protein of adipocytes and is widely used as a differentiation marker for adipocytes (Non-patent Document 5).
- FABP4 in addition to its function as a molecular chaperone in adipocytes, it has been reported that it is involved in signal transduction by lipids and organelle responses, and in macrophages it is also involved in inflammatory reactions ( Non-patent documents 2, 6, 9).
- FABP5 has been suggested to be involved in the formation of psoriasis lesions in keratinocytes (Non-patent Document 7) and has been reported to be involved in the regulation of cytokine (IL-12p70), a key molecule of innate immune response, in the pancreas. (Non-patent document 8).
- Non-patent Document 11 a mouse in which FABP4 was knocked out was found to suppress a decrease in insulin sensitivity.
- a high-fat diet was given to FABP4 knockout mice, a decrease in insulin sensitivity was suppressed as compared to the wild type, but no effect on weight gain or fatty liver was observed (Non-Patent Documents 4 and 10). Since the expression of FABP5 is increased in the adipocytes of FABP4 knockout mice, it is considered that FABP5 works compensatory (Non-patent Documents 16 and 17).
- Non-patent Document 12 A tendency similar to that of the FABP4 knockout mouse is recognized in the FABP5 knockout mouse. Based on the results of single knockout mice, analysis was performed with FABP4 / 5 double knockout. When fed with a high fat diet, diet-induced obesity, insulin resistance, type 2 diabetes, It has been reported that the induction of fatty liver is suppressed (Non-Patent Documents 13, 14, and 15).
- Non-patent Document 18 When BMS309403, an inhibitor of FABP, was orally administered to model mice with type 2 diabetes or arteriosclerosis, improvement of the disease state was observed (Non-patent Document 18).
- the present invention includes the following steps (A) to (D): (A) contacting the test substance with a tissue or cell derived from a mammal capable of expressing the FABP4 gene or FABP5 gene, or the FABP4 protein or FABP5 protein; (B) a step of measuring the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein in a tissue or cell derived from the mammal, (C) comparing the expression level or activity measured in (B) above with the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein in the control group, (D) Based on the result of (C) above, a test substance that decreases the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein, Evalu
- the present invention also includes the following steps (A ′) to (D ′): (A ′) a step of administering a test substance to a non-human mammal; (B ′) a step of measuring the expression level of the FABP4 gene or FABP5 gene, the expression level of the FABP4 protein or FABP5 protein, or the activity of the FABP4 protein or FABP5 protein in the small intestine collected from the non-human mammal; (C ′) The expression level or activity measured in (B ′) above is determined based on the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or FABP4 in the small intestine collected from a non-human mammal of the control group Comparing the activity of the protein or FABP5 protein; (D ′) Based on the result of (C ′) above, a test substance that decreases the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein
- the present invention relates to a method for evaluating or selecting a GIP elevation inhibitor.
- the present inventors inhibit FABP4 / 5 that FABP4 and FABP5 (which may be collectively referred to as FABP4 / 5 in the present specification) are expressed in enterocytes that secrete GIP. It has been found that the GIP concentration in blood is lowered by the above, and therefore, a substance that inhibits FABP4 / 5 is useful as a GIP elevation inhibitor. From these findings, the present inventors have found that it is possible to evaluate or select a GIP elevation inhibitor using the inhibitory effect on the expression or activity of FABP4 / 5 as an index.
- the GIP increase inhibitory effect of various substances can be more easily and accurately evaluated, and an excellent GIP increase inhibitor can be selected.
- the GIP elevation inhibitor selected by the method of the present invention reduces, prevents or ameliorates the onset of obesity and the like, prevents weight gain or decreases body weight, or promotes post-meal digestion and improves stomach leaning. It is useful as an active ingredient.
- FABPs are expressed in the intestine
- FABP2 mainly expressed in the intestine
- FABP2 lipid metabolism in the intestine is thought to be played by FABP2.
- the GIP increase inhibitory action of various substances is evaluated using the FABP4 / 5 inhibitory action as an index, and a GIP increase inhibitor is selected based on the evaluation result.
- “suppression of GIP elevation” means that a diet containing lipids and carbohydrates, particularly a diet rich in lipids, especially a diet rich in triacylglycerol, is taken from K cells present in the small intestine. It means to suppress the increase of secreted GIP. That is, “GIP increase suppression” in this specification refers to suppressing GIP increase mainly occurring after meals.
- GIP increase inhibitory action suppresses GIP secretion by suppressing GIP secretion by suppressing GIP secretion from K cells, and reducing GIP concentration in blood. It is a concept that includes any GIP lowering action.
- expression of FABP4 / 5 refers to expression of FABP4 gene or FABP5 gene, or expression of FABP4 protein or FABP5 protein.
- activity of FABP4 / 5 refers to the activity of FABP4 protein or FABP5 protein.
- the evaluation or selection method of the GIP elevation inhibitor of the present invention can be performed in vitro or ex vivo, or in vivo.
- the method for evaluating or selecting the GIP elevation inhibitor of the present invention includes the following steps (A) to (D).
- D A step of evaluating or selecting a test substance that decreases the expression level or activity of FABP4 / 5 as a GIP increase inhibitor based on the result of (C).
- test substance used in the method of the present invention is not particularly limited as long as it is a substance desired to be used as a GIP elevation inhibitor.
- the test substance may be a naturally occurring substance, a substance artificially synthesized by a chemical or biological method, etc., and may be a compound, a composition or a mixture. Good.
- tissues or cells derived from mammals capable of expressing FABP4 / 5 used in the above step (A) include those from mammals expressing FABP4 gene or FABP5 gene, or FABP4 protein or FABP5 protein.
- Examples include detached tissues or cells, or cultures thereof.
- the isolated tissues or cells, or cultures thereof include small intestine tissues or cells such as duodenum and jejunum collected from mammals, fat tissues or cells, thymic epithelial tissues or cells, skin epithelial tissues or cells, and Examples thereof include small intestine primary cultured cells; small intestine cultured cells such as Caco-2 cells, IEC-6 cells, IEC-18 cells, STC-1 cells, and GLUTag cells; 3T3-L1 cells, and the like.
- examples of mammal-derived tissues or cells capable of expressing FABP4 / 5 used in the above step (A) include genetically so as to express the FABP4 gene or FABP5 gene, or the FABP4 protein or FABP5 protein.
- examples include modified mammalian tissues or cells, or cultures thereof.
- the genetically modified mammalian tissue or cell, and the culture thereof, for example, can be obtained by introducing a gene encoding FABP4 protein or FABP5 protein into any tissue or cell of a mammal. It can be produced by transforming to express FABP4 / 5 or to enhance the expression of FABP4 / 5.
- Examples of a method for introducing a gene into a cell include, but are not limited to, vector introduction by electroporation or lipofection.
- the mammal from which the tissue or cell capable of expressing FABP4 / 5 used in the method of the present invention is not particularly limited, and examples thereof include humans, mice, rats, hamsters, rabbits and the like.
- the contact between a mammal-derived tissue or cell capable of expressing FABP4 / 5 and a test substance is performed by, for example, adding the test substance to the culture solution in advance to a predetermined concentration, and then adding the tissue or cell to the culture solution. Or by adding a test substance at a predetermined concentration to the culture medium on which the tissue or cells are placed.
- the tissue or cells after contact are preferably cultured at room temperature (25 ° C.) to 37 ° C., usually for about 3 to 48 hours, preferably about 6 to 24 hours.
- the concentration at the time of seeding the tissue or cells in the culture solution is not particularly limited as long as the cells can grow.
- the addition concentration of the test substance is preferably 0.00001 to 10% by mass (dry residue), particularly preferably 0.0001 to 3% by mass (dry residue).
- a conventional medium can be used, and examples thereof include 10% FBS-containing Dulbecco's Modified Eagle's Medium. It is preferable to add growth additives such as serum, growth factors, insulin, and antibacterial agents to these media during cell passage and proliferation.
- the tissue or cells are collected, and the expression level of the FABP4 gene or FABP5 gene, the expression level of the FABP4 protein or FABP5 protein, or the activity of the FABP4 protein or FABP5 protein is measured.
- the level of gene expression is detected at the mRNA level, for example, total RNA is extracted from the cells, and the real-time RT-PCR method, RNase protection assay method, Northern blot analysis method, etc. are used. This can be performed by detecting and quantifying mRNA transcribed from the FABP4 / 5 gene.
- the expression level of the FABP4 / 5 protein can be measured by a conventional immunoassay method such as RIA method, EIA method, ELISA, bioassay method, Western blot, etc.
- Western blot is inexpensive and simple and desirable.
- the activity of the FABP4 / 5 protein can be measured by measuring the amount of binding substrate bound to the FABP4 / 5 protein.
- steps (C) to (D) expression of FABP4 / 5 in a tissue or cell derived from a mammal capable of expressing FABP4 / 5 contacted with a test substance (test group) measured in step (B) above
- the amount or activity is compared with the expression level or activity of FABP4 / 5 in the control group, and a test substance that can be used as a GIP elevation inhibitor is selected based on the comparison result.
- control group examples include mammal-derived tissues or cells capable of expressing the same FABP4 / 5 as in the test group, which were not contacted with the test substance.
- tissues or cells modified so that FABP4 / 5 is not expressed include siRNA-derived FABP4 / 5 knockdown cells, tissues or cells derived from FABP4 / 5 knockout mice. Measurement of the expression level or activity of FABP4 / 5 in the control group can be carried out in the same procedure as in the test group described in connection with the above step (B).
- the expression level or activity of FABP4 / 5 in the test group is compared with the expression level or activity of FABP4 / 5 in the control group.
- the test substance is evaluated as having a GIP increase inhibitory effect, and the test substance is selected as a GIP increase inhibitor.
- the expression level or activity in the test group is statistically significantly reduced relative to the expression level or activity in the control group, the test substance is evaluated as having a GIP increase inhibitory effect.
- the expression level or activity in the control group is 100%, the expression level or activity in the test group is 90% or less, preferably 80% or less, more preferably 60% or less.
- the test substance is evaluated to have a GIP increase inhibitory effect.
- a test substance evaluated as having a GIP elevation inhibitory effect is selected as a GIP elevation inhibitor.
- the method for evaluating or selecting the GIP elevation inhibitor of the present invention includes the following steps (A ′) to (D ′).
- a ′ a step of administering a test substance to a non-human mammal
- B ′ a step of measuring the expression level or activity of FABP4 / 5 in the small intestine collected from the non-human mammal
- C ′ a step of comparing the expression level or activity measured in (B ′) above with the expression level or activity of FABP4 / 5 in the small intestine collected from a non-human mammal of the control group
- D ′ A step of evaluating or selecting a test substance that decreases the expression level or activity of FABP4 / 5 as a GIP increase inhibitor based on the result of (C ′).
- the non-human mammal used in the above step (A ′) may be any kind of animal regardless of gender and age. Examples thereof include primates such as mice, rats, hamsters, guinea pigs, rabbits, cats, dogs, and monkeys, and rodents such as rats and mice are preferred from the viewpoint of easy availability and handling.
- Examples of the method for administering the test substance to the non-human mammal include oral administration, intragastrointestinal administration, intraperitoneal administration, intravascular administration, intradermal administration, and subcutaneous administration.
- the method of oral administration is preferable from the viewpoint of simplicity and minimal invasiveness.
- GIP is secreted from K cells of the duodenum and jejunum
- a method of directly refluxing to the duodenum or jejunum using cannulation or the like is also preferable.
- the dose of the test substance is 0.0004 mg / g body weight or more, preferably 0.04 to 2 mg / g body weight.
- the number of administrations may be single or may be divided into several times at intervals. Administration is preferably performed for each meal, more preferably between 60 minutes before meal and 60 minutes after meal.
- the expression level or activity of FABP4 / 5 in the small intestine of the non-human mammal administered with the test substance is measured.
- the measurement method may be an invasive method or a non-invasive method, and is not particularly limited.
- the small intestine is collected from the non-human mammal 1 to 360 minutes, preferably 5 to 120 minutes after administration of the test substance.
- the small intestine is collected under anesthesia or immediately after euthanasia, and the lower part of the stomach from the pylorus is collected.
- the expression level or activity of FABP4 / 5 in the collected cells is measured.
- step (C ′) to (D ′) the expression level or activity of FABP4 / 5 in the small intestine (test group) of the non-human mammal administered with the test substance obtained above was not administered. Compared with the expression level or activity of the small intestine (control group) collected from the same non-human mammal.
- the test substance is evaluated as having a GIP increase inhibitory effect, and the test substance is selected as a GIP increase inhibitor.
- test substances for example, the types of test substances that can be used, the measurement procedure of the expression level or activity of FABP4 / 5, the comparison procedure between the test group and the control group, the test substance evaluation or selection procedure, This is the same as the method performed in vitro or ex vivo.
- the selected substance may be subjected to further screening.
- a test substance evaluated or selected as having an inhibitory effect on the increase in GIP by the above method is obtained from a K cell by a secretory stimulation test using a cultured cell line of a mammalian small intestine K cell model or a single administration experiment using an experimental animal.
- a test substance evaluated or selected as having an inhibitory effect on the increase in GIP by the above method is obtained from a K cell by a secretory stimulation test using a cultured cell line of a mammalian small intestine K cell model or a single administration experiment using an experimental animal.
- the GIP elevation inhibitor selected by the present invention in the above procedure reduces GIP after meals, reduces the possibility of developing obesity, prevents or improves it, suppresses weight loss or increases in body weight, or promotes digestion Used as an active ingredient to improve stomach upset.
- the present invention provides a method for evaluating or selecting an appetite suppressant comprising the above steps (A) to (D) or (A ′) to (D ′).
- the present invention provides a method for evaluating or selecting an agent for preventing and / or improving obesity comprising the above steps (A) to (D) or (A ′) to (D ′).
- the present invention provides a method for evaluating or selecting a weight gain inhibitor comprising the steps (A) to (D) or (A ′) to (D ′).
- the present invention provides a method for evaluating or selecting a digestion promoting agent or a stomach sag ameliorating agent comprising the above steps (A) to (D) or (A ′) to (D ′).
- a test substance that decreases the expression level or activity of FABP4 / 5 is added to an appetite suppressant, obesity It is evaluated or selected as a prophylactic and / or ameliorating agent, a weight gain inhibitor, a digestion promoter or a stomach sag improving agent, respectively.
- compositions, production methods, uses or methods are further disclosed herein.
- present invention is not limited to these embodiments.
- ⁇ 2> The following steps (A) to (D): (A) contacting the test substance with a tissue or cell derived from a mammal capable of expressing the FABP4 gene or FABP5 gene, or the FABP4 protein or FABP5 protein; (B) a step of measuring the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein in a tissue or cell derived from the mammal, (C) comparing the expression level or activity measured in (B) above with the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein in the control group, (D) Based on the result of (C) above, a test substance that decreases the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein is used as
- ⁇ 4> The following steps (A) to (D): (A) contacting the test substance with a tissue or cell derived from a mammal capable of expressing the FABP4 gene or FABP5 gene, or the FABP4 protein or FABP5 protein; (B) a step of measuring the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein in a tissue or cell derived from the mammal, (C) comparing the expression level or activity measured in (B) above with the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein in the control group, (D) Based on the result of (C) above, a test substance that decreases the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein Evaluating
- ⁇ 5> The following steps (A) to (D): (A) contacting the test substance with a tissue or cell derived from a mammal capable of expressing the FABP4 gene or FABP5 gene, or the FABP4 protein or FABP5 protein; (B) a step of measuring the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein in a tissue or cell derived from the mammal, (C) comparing the expression level or activity measured in (B) above with the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein in the control group, (D) A test substance that decreases the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein based on the result of (C) above as a digestion
- ⁇ 6> The following steps (A) to (D): (A) contacting the test substance with a tissue or cell derived from a mammal capable of expressing the FABP4 gene or FABP5 gene, or the FABP4 protein or FABP5 protein; (B) a step of measuring the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein in a tissue or cell derived from the mammal, (C) comparing the expression level or activity measured in (B) above with the expression level of FABP4 gene or FABP5 gene, the expression level of FABP4 protein or FABP5 protein, or the activity of FABP4 protein or FABP5 protein in the control group, (D) Based on the result of (C) above, a test substance that decreases the expression level of the FABP4 gene or FABP5 gene, the expression level of the FABP4 protein or FABP5 protein, or the activity of the FABP4 protein or FABP5 protein
- tissue or cell derived from a mammal capable of expressing the FABP4 gene or FABP5 gene, or the FABP4 protein or FABP5 protein is as follows: : (1) Small intestine tissue or cells collected from mammals, or cultures thereof; (2) Adipose tissue or cells collected from mammals, thymic epithelial tissue or cells, skin epithelial tissue or cells, or cultures thereof; (3) mammalian tissue or cells genetically modified to express the FABP4 gene or FABP5 gene, or the FABP4 protein or FABP5 protein, or a culture thereof; (4) small intestine primary cultured cells; or (5) Caco-2 cells, IEC-6 cells, IEC-18 cells, STC-1 cells, GLUTag cells, or 3T3-L1 cells.
- control group is preferably the following: (1) A mammal-derived tissue or cell capable of expressing the FABP4 gene or FABP5 gene, or FABP4 protein or FABP5 protein, which has not been contacted with the test substance; (2) a tissue or cell derived from a mammal that has no or almost no ability to express the FABP4 gene or the FABP5 gene, or the FABP4 protein or the FABP5 protein; (3) A tissue or cell derived from a mammal capable of expressing the FABP4 gene or FABP5 gene, or the FABP4 protein or FABP5 protein, modified so that the gene or protein is not expressed; or (4) the (2) Or (3) the tissue or cell contacted with the test substance.
- the test substance suppresses GIP elevation ⁇ 1>- ⁇ 8>
- the expression level or activity measured in (B) above is 90% or less, preferably 80% or less, more preferably 60% or less when the expression level or activity in the control group is 100%.
- the test substance is evaluated to have a GIP increase inhibitory effect, an appetite suppressive effect, an obesity prevention and / or improvement effect, a weight gain suppression effect, a digestion promoting effect, or a stomach sag improving effect, ⁇ 1>
- control group is preferably a small intestine collected from the non-human mammal that has not been administered a test substance.
- ⁇ 18> Preferably, when the expression level or activity measured in (B ′) is statistically significantly decreased with respect to the expression level or activity in the control group, the test substance increases GIP. Any one of ⁇ 11> to ⁇ 17>, which is evaluated as having an inhibitory effect, an appetite suppressive effect, an obesity prevention and / or improvement effect, a weight gain suppression effect, a digestion promoting effect, or a stomach sag improving effect. Method.
- the expression level or activity measured in (B ′) above is 90% or less, preferably 80% or less, more preferably 60% when the expression level or activity in the control group is 100%.
- the test substance is evaluated as having GIP increase inhibitory effect, appetite suppressive effect, obesity prevention and / or amelioration effect, weight gain inhibitory effect, digestion promoting effect, or stomach sag improving effect when ⁇ 11
- FABP4 / 5 inhibitor (FABP4 / 5 inhibitor)
- BMS309403 AstaTech, Inc.
- Compound2 J Lipid Res. 2011, 52, 646-656 was used as the FABP4 / 5 inhibitor.
- Example 1 Expression of FABP4 / 5 in intestinal cells (immunostaining)
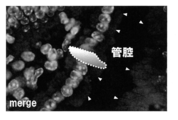
- the duodenum collected from C57BL / 6J mice (male) (Claire Japan) was fixed with a 4% Paraformaldehyde solution, frozen sections were prepared, and immunostaining was performed. Double fluorescence staining was performed using antibodies in GIP and FABP5 to examine the expression of FABP5 in intestinal cells.
- T-4340 manufactured by Peninsula
- AF1476 manufactured by R & D systems
- Alexa Fluor 488 Donkey Anti-Rabbit IgG manufactured by Invitrogen
- Alexa Fluor 568 Dnkey Anti-goat IgG manufactured by Invitrogen
- the solution was fixed in 4% Paraformaldehyde solution at 4 ° C. for 10 minutes, washed with PBS, and then reacted with a primary antibody (100-fold blocking solution: 10% Donkey Serum in PBS) at room temperature for 3 hours.
- the secondary antibody 500-fold blocking solution was reacted at room temperature for 1 hour, washed with PBS, stained with Prolong Gold antigen with DAPI (manufactured by Invitrogen) and encapsulated, and observed under a microscope. (Use of 405 nm, 488 nm, and 568 nm lasers).
- Blood was collected by orbital venous plexus blood collection before lipid emulsion administration and at 10, 30, and 60 minutes after administration. The collected blood was centrifuged at 11,000 rpm and 4 ° C. for 10 minutes to prepare plasma. The blood GIP concentration and blood triglyceride (TG) concentration were measured using GIP ELISA kit (for rat / mouse) (Millipore) and Triglyceride E-Test Wako (Wako Pure Chemical Industries, Ltd.), respectively.
- GIP ELISA kit for rat / mouse
- Triglyceride E-Test Wako Waako Pure Chemical Industries, Ltd.
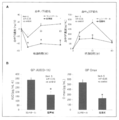
- FIG. 2 shows changes in blood TG concentration change ( ⁇ TG) and GIP concentration change ( ⁇ GIP) over time, and iAUC (0-1h) and Cmax in blood GIP concentration in mice after a single lipid emulsion administration.
- the increase in blood TG concentration and blood GIP concentration after lipid emulsion administration was statistically significantly reduced in the inhibitor-added group compared to the control group (FIG. 2A).
- iAUC and Cmax of blood GIP concentrations were also statistically significantly reduced in the inhibitor-added group compared to the control group (FIG. 2B).
- Example 3 FABP4 / 5 Inhibition Test on Small Intestine Primary Culture Cells After euthanizing 13- to 17-week-old C57BL / 6J mice by cervical dislocation under anesthesia, the upper small intestine (10 cm from immediately below the gastric pylorus) was removed and penetrated into ice-cooled L-15 medium (Sigma). . This was transferred into ice-cold PBS, fat and blood vessels adhering to the intestinal tract were carefully removed, the inside of the lumen was washed with ice-cold PBS, and the tissue was finely cut using a scalpel (small piece of 2 mm 2 or less) I made it.
- a scalpel small piece of 2 mm 2 or less
- Supernatant 1 the Collagenase solution was added in the same manner, and the mixture was incubated for 15 minutes, and the supernatant was recovered (Supernatant 2).
- Supernatant 1 and supernatant 2 were centrifuged at 100 rcf (about 800 rpm) for 3 minutes (room temperature). The supernatant was removed and suspended in 10 mL of DMEM. These were combined and centrifuged at 100 rcf for 3 minutes (room temperature).
- the supernatant was removed and suspended in 7 mL of DMEM (containing 10% FBS (Invitrogen), 1% Glutamax (Invitrogen), 1% penicillin / streptomycin (Invitrogen)). This was seeded in a 48 well plate coated with matrigel TM (BD Biosciences) (24 wells per animal) and incubated at 37 ° C., 5% CO 2 .
- DMEM containing 10% FBS (Invitrogen), 1% Glutamax (Invitrogen), 1% penicillin / streptomycin (Invitrogen)
- GIP secretion stimulation Small intestine primary cultured cells cultured for 24 hours were stimulated with stimulation medium (4.5 mM KCl, 138 mM NaCl, 4.2 mM NaHCO 3 , 1.2 mM NaH 2 PO 4 , 2.6 mM CaCl 2 , 1.2 mM MgCl 2 , 10 mM HEPES, NaOH (Adjusted to pH 7.4) once) and then incubated with stimulation medium alone or stimulation medium containing FABP4 / 5 inhibitor (10 or 25 ⁇ M).
- stimulation medium 4.5 mM KCl, 138 mM NaCl, 4.2 mM NaHCO 3 , 1.2 mM NaH 2 PO 4 , 2.6 mM CaCl 2 , 1.2 mM MgCl 2 , 10 mM HEPES, NaOH (Adjusted to pH 7.4) once
- stimulation medium alone (control), lipid micelle (PPM: stimulation medium + 500 ⁇ M Naurocholate, 200 ⁇ M oleic acid, 50 ⁇ M 2-monoolein), or PPM + FABP4 / 5 inhibitor (10 or 25 ⁇ M) was added and the medium was collected after 30 minutes.
- PPM stimulation medium + 500 ⁇ M Naurocholate, 200 ⁇ M oleic acid, 50 ⁇ M 2-monoolein
- PPM + FABP4 / 5 inhibitor 10 or 25 ⁇ M
- GIP GIP secretion rate
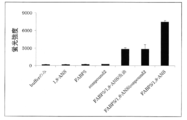
- the GIP secretion rate in the primary cultured cells of the small intestine stimulated with PPM is shown in FIG.
- PPM group the GIP secretion rate at 30 minutes after stimulation was statistically significantly increased as compared with the control group.
- PPM + inhibitor group an increase in GIP secretion rate was statistically significantly suppressed as compared to the PPM group.
- PPM stimulation + BMS309403 addition group the increase-dependent tendency of the increase in GIP secretion was recognized (FIG. 3A).
- Example 4 Screening of GIP elevation inhibitor based on FABP5 activity A GIP elevation inhibitor was screened based on FABP5 activity. The influence of the test substance on the FABP5 activity was examined by measuring FABP5 activity using the antagonistic binding of the test substance to the FABP5 protein.
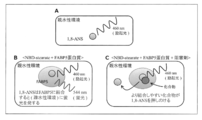
- the principle of the FABP5 activity measurement method used in this example is shown in FIG.
- 1-anilinonaphthalene-8-sulfonic acid (1,8-ANS) is a substance that has been reported to emit fluorescence by binding to FABP5 (Hum. Mol. Genet., 2014, 23 (24): 6495-6511).
- 1,8-ANS is a substance that emits fluorescence only in a hydrophobic environment, and does not emit fluorescence in a hydrophilic environment (FIG.
- test substance that suppresses the increase in fluorescence intensity by the above method is a substance that binds to FABP5 protein and inhibits its activity.
- E. coli-derived recombinant FABP5 was expressed by a conventional method and purified. Purified FABP5 protein, 1,8-ANS and test substance were added to a 96-well plate, allowed to stand at room temperature for 5 minutes, and then fluorescence intensity (excitation wavelength: 355 nm, fluorescence wavelength: 480 nm) was measured with a plate reader. It was measured.
- the FABP5 inhibitor Compound 2 having a GIP increase inhibitory action used in Example 3 and a fish oil which is an oil and fat having a GIP increase inhibitory action as shown in Reference Example 1 described later were used. The final concentration of the test substance in the evaluation system was 0.002% to 0.1% (w / v).
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Urology & Nephrology (AREA)
- Hematology (AREA)
- Organic Chemistry (AREA)
- Biotechnology (AREA)
- Physics & Mathematics (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Analytical Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Biophysics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicinal Chemistry (AREA)
- Cell Biology (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Food Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Endocrinology (AREA)
- Genetics & Genomics (AREA)
- Toxicology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/128,666 US10132795B2 (en) | 2014-03-24 | 2015-03-06 | Method for evaluating or selecting agent for suppressing GIP level elevation |
| CN201580015560.7A CN106133519B (zh) | 2014-03-24 | 2015-03-06 | Gip上升抑制剂的评价或选择方法 |
| EP15769760.8A EP3124966B1 (en) | 2014-03-24 | 2015-03-06 | Method for evaluating or selecting agent for suppressing gip level elevation |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014-060341 | 2014-03-24 | ||
| JP2014060341 | 2014-03-24 | ||
| JP2015-040187 | 2015-03-02 | ||
| JP2015040187A JP6223376B2 (ja) | 2014-03-24 | 2015-03-02 | Gip上昇抑制剤の評価又は選択方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015146537A1 true WO2015146537A1 (ja) | 2015-10-01 |
Family
ID=54195063
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/056603 Ceased WO2015146537A1 (ja) | 2014-03-24 | 2015-03-06 | Gip上昇抑制剤の評価又は選択方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US10132795B2 (enExample) |
| EP (1) | EP3124966B1 (enExample) |
| JP (1) | JP6223376B2 (enExample) |
| CN (1) | CN106133519B (enExample) |
| WO (1) | WO2015146537A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016214085A (ja) * | 2015-05-14 | 2016-12-22 | 花王株式会社 | 毛成長抑制剤の評価又は選択方法 |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114981446A (zh) * | 2020-01-17 | 2022-08-30 | 日本乐敦制药株式会社 | 组织形态、组织功能控制剂的评价/筛选方法 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002524517A (ja) * | 1998-09-17 | 2002-08-06 | ブリストル−マイヤーズ スクイブ カンパニー | aP2阻害剤およびその組成物を用いる糖尿病の治療方法 |
| JP2004065194A (ja) * | 2002-08-09 | 2004-03-04 | Sumitomo Chem Co Ltd | 脂肪細胞関連因子の分析方法 |
| JP2011080803A (ja) * | 2009-10-05 | 2011-04-21 | Kao Corp | Gip上昇抑制剤の評価又は選択方法 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU5518798A (en) | 1996-12-03 | 1998-06-29 | Trustees Of Boston University | Specific antagonists for glucose-dependent insulinotropic polypeptide (gip) |
| US7358254B2 (en) | 2001-07-13 | 2008-04-15 | Bristol-Myers Squibb Company | Method for treating atherosclerosis employing an aP2 inhibitor and combination |
| US6831102B2 (en) * | 2001-12-07 | 2004-12-14 | Bristol-Myers Squibb Company | Phenyl naphthol ligands for thyroid hormone receptor |
| EP2898898B1 (en) | 2009-03-05 | 2025-09-10 | President and Fellows of Harvard College | Secreted AP2 and methods of inhibiting same |
| WO2011007864A1 (ja) * | 2009-07-16 | 2011-01-20 | 花王株式会社 | 血中gip濃度上昇抑制剤 |
| JP5317919B2 (ja) | 2009-10-05 | 2013-10-16 | 花王株式会社 | Gip上昇抑制剤の評価又は選択方法 |
| WO2012096108A1 (ja) * | 2011-01-12 | 2012-07-19 | 花王株式会社 | 血中gip濃度上昇抑制剤、血中インスリン濃度上昇抑制剤、食後血中トリグリセリド濃度低減剤、及び血糖濃度上昇抑制剤 |
| JP6026723B2 (ja) | 2011-02-22 | 2016-11-16 | 花王株式会社 | Gip上昇抑制剤 |
| JP2012171914A (ja) | 2011-02-22 | 2012-09-10 | Kao Corp | Gip上昇抑制剤 |
| CN102961388A (zh) * | 2011-08-30 | 2013-03-13 | 三和淀粉工业株式会社 | 促进glp-1的分泌并且抑制gip的分泌的药剂 |
-
2015
- 2015-03-02 JP JP2015040187A patent/JP6223376B2/ja not_active Expired - Fee Related
- 2015-03-06 EP EP15769760.8A patent/EP3124966B1/en active Active
- 2015-03-06 WO PCT/JP2015/056603 patent/WO2015146537A1/ja not_active Ceased
- 2015-03-06 CN CN201580015560.7A patent/CN106133519B/zh not_active Expired - Fee Related
- 2015-03-06 US US15/128,666 patent/US10132795B2/en active Active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002524517A (ja) * | 1998-09-17 | 2002-08-06 | ブリストル−マイヤーズ スクイブ カンパニー | aP2阻害剤およびその組成物を用いる糖尿病の治療方法 |
| JP2004065194A (ja) * | 2002-08-09 | 2004-03-04 | Sumitomo Chem Co Ltd | 脂肪細胞関連因子の分析方法 |
| JP2011080803A (ja) * | 2009-10-05 | 2011-04-21 | Kao Corp | Gip上昇抑制剤の評価又は選択方法 |
Non-Patent Citations (8)
| Title |
|---|
| DIANE H. SONG ET AL.: "Glucose-Dependent Insulinotropic Polypeptide Enhances Adipocyte Development and Glucose Uptake in Part through Akt Activation", GASTROENTEROLOGY, vol. 3, no. 6, 13 December 2007 (2007-12-13), pages 1796 - 1805, XP022421688 * |
| FURUHASHI: "Fatty Acid-Binding Protein Family", JOURNAL OF JAPAN SOCIETY FOR THE STUDY OF OBESITY, vol. 19, no. 3, 25 December 2013 (2013-12-25), pages 167 - 174, XP008185862 * |
| HONG LAN ET AL.: "Small-molecule inhibitors of FABP4/5 ameliorate dyslipidemia but not insulin resistance in mice with diet-induced obesity", J. LIPID RES., vol. 2, no. 4, 5 April 2011 (2011-04-05), pages 646 - 656, XP055226912, ISSN: 0022-2275 * |
| K. B. COMERFORD ET AL.: "The Effects of Weight Loss on FABP4 and RBP4 in Obese Women with Metabolic Syndrome", HORM. METAB. RES., vol. 46, no. 3, 26 August 2013 (2013-08-26), pages 224 - 231, XP008184884 * |
| KIMITAKA SHIBUE ET AL.: "FABP5 is an essential modulator of fatty acid-induced GIP secretion in enteroendocrine K cells", DIABETOLOGIA, vol. 5 7, no. 1, September 2014 (2014-09-01), pages 766, XP055351043 * |
| MAS R.A.A.SYAMSUNARNO: "Fatty Acid Binding Protein-4 and -5 are Expressed in Intestinal Capillary Endothelial Cells and Regulate Lipid Absorption", CIRCULATION JOURNAL, vol. 78, no. supplement 1, 20 February 2014 (2014-02-20), pages 1737 * |
| RANDI UGLEHOLDT ET AL.: "Transgenic Rescue of Adipocyte Glucose-dependent Insulinotropic Polypeptide Receptor Expression Restores High Fat Diet-induced Body Weight Gain", J. BIOL. CHEM., vol. 6, no. 52, 28 December 2011 (2011-12-28), pages 44632 - 44645, XP055226918, ISSN: 0021-9258 * |
| SHIBUE ET AL.: "Shibosan Ketsugo Tanpaku 5 (FABP5) wa K Saibo ni Okeru Shibo Yudosei K Saibo ni Okeru Shibo Yudosei GIP Bunpi no Seigyo ni Kan'yo suru", DAI 29 KAI THE JAPANESE SOCIETY OF EXPERIMENTAL DIABETES AND OBESITY ON DIAGNOSIS OF DIABETES MELLITUS IN ANIMAL MODELS KOEN YOSHISHU, 19 February 2015 (2015-02-19), pages 48, XP008185736 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016214085A (ja) * | 2015-05-14 | 2016-12-22 | 花王株式会社 | 毛成長抑制剤の評価又は選択方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3124966B1 (en) | 2020-04-29 |
| EP3124966A1 (en) | 2017-02-01 |
| US10132795B2 (en) | 2018-11-20 |
| JP6223376B2 (ja) | 2017-11-01 |
| EP3124966A4 (en) | 2017-09-27 |
| US20170227526A1 (en) | 2017-08-10 |
| JP2015194481A (ja) | 2015-11-05 |
| CN106133519A (zh) | 2016-11-16 |
| CN106133519B (zh) | 2018-01-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Zadjali et al. | SOCS2 deletion protects against hepatic steatosis but worsens insulin resistance in high‐fat‐diet‐fed mice | |
| Shimobayashi et al. | Insulin resistance causes inflammation in adipose tissue | |
| Hansotia et al. | Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure | |
| Kim et al. | Obesity-associated improvements in metabolic profile through expansion of adipose tissue | |
| Kosteli et al. | Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue | |
| Zhao et al. | Divergent functions of endotrophin on different cell populations in adipose tissue | |
| Szatkowski et al. | Prokineticin receptor 1 as a novel suppressor of preadipocyte proliferation and differentiation to control obesity | |
| Chandra et al. | Immunoglobulin-like domain containing receptor 1 mediates fat-stimulated cholecystokinin secretion | |
| Ström et al. | Attainment of brown adipocyte features in white adipocytes of hormone-sensitive lipase null mice | |
| Lee et al. | CREB/CRTC2 controls GLP‐1‐dependent regulation of glucose homeostasis | |
| Anderson et al. | eIF2A‐knockout mice reveal decreased life span and metabolic syndrome | |
| Jain et al. | Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice | |
| WO2014144777A2 (en) | Lipids that increase insulin sensitivity and methods of using the same | |
| JP5109134B2 (ja) | 病態モデル実験動物、病態モデル実験動物の作出方法及び病態モデル実験動物の利用方法。 | |
| Zhu et al. | A disintegrin and metalloproteinase with thrombospondin motifs 18 deficiency leads to visceral adiposity and associated metabolic syndrome in mice | |
| Arivazhagan et al. | Glycation and a spark of ALEs (advanced lipoxidation end products)–igniting RAGE/Diaphanous-1 and cardiometabolic disease | |
| Arivazhagan et al. | The RAGE/DIAPH1 axis: mediator of obesity and proposed biomarker of human cardiometabolic disease | |
| JP6223376B2 (ja) | Gip上昇抑制剤の評価又は選択方法 | |
| JP6514282B2 (ja) | Gip上昇抑制剤の評価又は選択方法 | |
| US8337835B2 (en) | Use of an endogenous ligand for peroxisome proliferator activated receptor alpha to treat liver disorders | |
| Machida et al. | Pancreatic islet neuropeptide Y overexpression has minimal effect on islet morphology and β-cell adaptation to high-fat diet | |
| García-Martínez et al. | Insulin drives glucose-dependent insulinotropic peptide expression via glucose-dependent regulation of FoxO1 and LEF1/β-catenin | |
| Sun et al. | High bone microarchitecture, strength, and resistance to bone loss in MRL/MpJ mice correlates with activation of different signaling pathways and systemic factors | |
| JP2015528000A (ja) | 代謝調節のための方法および組成物 | |
| Jia et al. | Expression of Kisspeptin-GnRH system is down-regulated in hypothalamic arcuate nucleus of male rats with high-fat diet |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15769760 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15128666 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REEP | Request for entry into the european phase |
Ref document number: 2015769760 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2015769760 Country of ref document: EP |