WO2015083750A1 - 神経新生に関する化合物及び医薬組成物 - Google Patents
神経新生に関する化合物及び医薬組成物 Download PDFInfo
- Publication number
- WO2015083750A1 WO2015083750A1 PCT/JP2014/082035 JP2014082035W WO2015083750A1 WO 2015083750 A1 WO2015083750 A1 WO 2015083750A1 JP 2014082035 W JP2014082035 W JP 2014082035W WO 2015083750 A1 WO2015083750 A1 WO 2015083750A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- compound
- prodrug
- pharmaceutically acceptable
- acceptable salt
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 *C(C=C(N(*)c1c2*)Sc1ccc2O*)=O Chemical compound *C(C=C(N(*)c1c2*)Sc1ccc2O*)=O 0.000 description 1
- IZNKZNXIXHLRGY-JYRVWZFOSA-N CCN1c(c2c(cc3)OCC2)c3S/C1=C\C(C)=O Chemical compound CCN1c(c2c(cc3)OCC2)c3S/C1=C\C(C)=O IZNKZNXIXHLRGY-JYRVWZFOSA-N 0.000 description 1
- NHDNXYLLAQDNEW-PXNMLYILSA-N CCN1c(c2c(cc3)[o]c(cc4)c2cc4Cl)c3S/C1=C\C(C)=O Chemical compound CCN1c(c2c(cc3)[o]c(cc4)c2cc4Cl)c3S/C1=C\C(C)=O NHDNXYLLAQDNEW-PXNMLYILSA-N 0.000 description 1
- QNQXSQQVCMPTGP-ZDLGFXPLSA-N CCN1c(c2c(cc3)[o]c4c2cc(C)c(C)c4)c3S/C1=C\C(C)=O Chemical compound CCN1c(c2c(cc3)[o]c4c2cc(C)c(C)c4)c3S/C1=C\C(C)=O QNQXSQQVCMPTGP-ZDLGFXPLSA-N 0.000 description 1
- RUQRAQTVHSATEP-PXNMLYILSA-N CCN1c(c2c(cc3)[o]c4c2ccc(Cl)c4)c3S/C1=C\C(C)=O Chemical compound CCN1c(c2c(cc3)[o]c4c2ccc(Cl)c4)c3S/C1=C\C(C)=O RUQRAQTVHSATEP-PXNMLYILSA-N 0.000 description 1
- QIAFGVQZUDBBOX-YBEGLDIGSA-N CCN1c(c2c(cc3)[o]c4ccccc24)c3S/C1=C\C(C)=O Chemical compound CCN1c(c2c(cc3)[o]c4ccccc24)c3S/C1=C\C(C)=O QIAFGVQZUDBBOX-YBEGLDIGSA-N 0.000 description 1
- NPJWZQWKOITWRE-JYRVWZFOSA-N CCN1c(c2c(cc3)[o]cc2)c3S/C1=C\C(C)=O Chemical compound CCN1c(c2c(cc3)[o]cc2)c3S/C1=C\C(C)=O NPJWZQWKOITWRE-JYRVWZFOSA-N 0.000 description 1
- KAOGHERYRZIKQY-YVLHZVERSA-N CCN1c2c(c(cc(C)cc3)c3[o]3)c3ccc2S/C1=C\C(C)=O Chemical compound CCN1c2c(c(cc(C)cc3)c3[o]3)c3ccc2S/C1=C\C(C)=O KAOGHERYRZIKQY-YVLHZVERSA-N 0.000 description 1
- WJCUXFRHZYIEOR-YVLHZVERSA-N CCN1c2c(c(ccc(C)c3)c3[o]3)c3ccc2S/C1=C\C(C)=O Chemical compound CCN1c2c(c(ccc(C)c3)c3[o]3)c3ccc2S/C1=C\C(C)=O WJCUXFRHZYIEOR-YVLHZVERSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/425—Thiazoles
- A61K31/429—Thiazoles condensed with heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/4353—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems
- A61K31/437—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom ortho- or peri-condensed with heterocyclic ring systems the heterocyclic ring system containing a five-membered ring having nitrogen as a ring hetero atom, e.g. indolizine, beta-carboline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/695—Silicon compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/02—Drugs for disorders of the nervous system for peripheral neuropathies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/20—Hypnotics; Sedatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
- A61P25/32—Alcohol-abuse
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0618—Cells of the nervous system
- C12N5/0619—Neurons
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0618—Cells of the nervous system
- C12N5/0623—Stem cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/999—Small molecules not provided for elsewhere
Definitions
- This disclosure relates to compounds and pharmaceutical compositions relating to neurogenesis.
- the present disclosure also relates to activating neurogenesis and / or activating proliferation of nerve cells.
- Patent Document 1 discloses a neurogenesis-promoting agent containing a peptide capable of promoting neurogenesis in the hippocampus of a mammalian brain.
- Patent Document 2 discloses a low molecular compound having a neurogenesis effect.
- the present disclosure in one aspect, provides a composition for activating neurogenesis, for proliferating neurons, or for suppressing differentiation of neurons.
- the present disclosure is a composition for activating neurogenesis or nerve cell proliferation, and has a DYRK inhibitory ability as an active ingredient, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
- the present invention relates to a composition containing a salt.
- the present disclosure is a composition for activating neurogenesis or nerve cell proliferation, and a compound represented by the following general formula (I) and / or (II) as an active ingredient Or a prodrug or a pharmaceutically acceptable salt thereof.
- R 1 and R 2 are each independently a hydrogen atom or a C 1-6 hydrocarbon chain
- R 3 is Z is one benzene ring, one heteroaromatic ring, one aromatic ring fused with one or more benzene rings, and one or more heteroaromatic rings together with the atoms marked with a and b.
- a condensed aromatic ring fused with one or more, a mixed condensed polycyclic condensed with one or more benzene rings and one or more heteroaromatic rings, and a ring selected from the group consisting of cycloaliphatic, ring may have a hydrogen atom, a substituent is a halogen atom or a C 1-6 alkyl group one or more, R 4 is a hydrogen atom, a halogen atom or a C 1-6 alkyl group.
- R 21 and R 23 each independently represent a hydrogen atom, a C 1-6 linear or branched or cyclic alkyl group, a benzyl or heteroarylmethyl group, a substituted or unsubstituted aryl group Or a substituted or unsubstituted heteroaryl group, wherein R 22 is —R 26 , —C ⁇ C—R 26 , —CH ⁇ CH—R 26 , and —O— (CH 2 ) n—R 26.
- N is 1 to 6
- R 27 is a hydrogen atom, C 1-6 alkyl group, a trihalomethyl group, or a hydroxyl group, 3 in the -Si (R 27) 3
- Each R 27 may be different.
- R 24 and R 25 are a hydrogen atom or
- the present disclosure relates to a method for activating neurogenesis, which, in one or more embodiments, includes administering a composition according to the present disclosure to a subject. Moreover, this indication is related with the preparation method of a nerve cell including culture
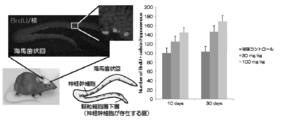
- FIG. 1 is a diagram showing that neurogenesis in the hippocampal dentate gyrus is activated by continuous oral administration of Compound 2 to an animal individual (mouse).
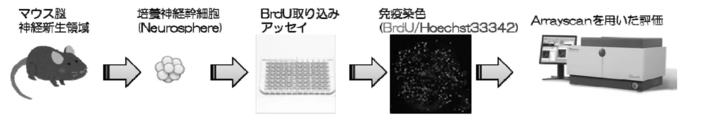
- FIG. 2 is a diagram illustrating an experimental system that demonstrates activation of proliferation of neural stem cells.
- FIG. 3 is an example of a graph showing the result of analyzing the ratio of BrdU positive cells in cultured neural stem cells administered with compounds 1 to 3 by array scan.
- FIG. 4 is an example of the result of detecting the expression of cyclin D1 in cultured stem cells administered with compounds 1 to 3 by Western blotting.
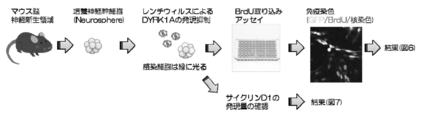
- FIG. 5 is a diagram illustrating an experimental system that demonstrates the neurogenesis activation effect by suppressing the expression of DYRK1A.
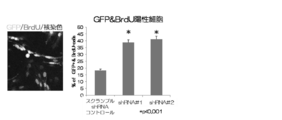
- FIG. 6 is an example of a graph showing the result of analyzing the ratio of BrdU positive cells in cultured neural stem cells administered with shRNA that suppresses the expression of DYRK1A by array scan.
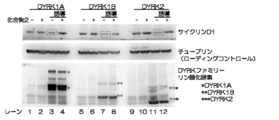
- FIG. 7 is an example of the results of detecting cyclin D1 expression in cultured stem cells by Western blotting using shRNA that suppresses DYRK1A expression.
- FIG. 8 is an example of the results of detecting the expression of cyclin D1 in neurons against the induction of DYRK family expression and the addition of Compound 2 by Western blotting.
- DYRK refers to a phosphorylase belonging to the Dual-specificity tyrosine phosphorylation-regulated kinase family.
- CLK refers to a phosphorylase belonging to the CDC-like kinase family.
- inhibitory ability refers to the ability to inhibit phosphorylase activity in one or more embodiments.
- having the ability to inhibit DYRK is having an ability to inhibit at least one of the phosphatases belonging to the DYRK family, and in one or more embodiments, the DYRK family. It has the activity which inhibits at least 1 phosphorylation activity of the phosphorylation enzyme which belongs to.
- the compound having DYRK inhibitory ability has an inhibitory ability against at least one selected from the group consisting of DYRK1A, DYRK1B, and DYRK2, and in one or more other embodiments. And at least inhibits DYRK1A.
- having a CLK inhibitory ability is an ability to inhibit at least one of the phosphorylating enzymes belonging to the CLK family, and in one or a plurality of embodiments, the CLK family. It has the activity which inhibits at least 1 phosphorylation activity of the phosphorylation enzyme which belongs to. In one or a plurality of embodiments, the compound having a CLK inhibitory ability has an inhibitory ability against at least one selected from the group consisting of CLK1, CLK2, ⁇ ⁇ CLK3, and CLK4.
- the compound having an inhibitory activity on a phosphorylating enzyme means, in one or a plurality of embodiments, when the compound is added in a known assay system for inhibiting protein phosphorylation activity in at least one of in vitro and in vivo
- the protein phosphorylation activity is, for example, 60% or less, preferably 50% or less, more preferably 40% or less, still more preferably 30% or less, and even more preferably 20% or less, compared to the control in which the compound is not added. Particularly preferably, it refers to a compound that can be inhibited to 10% or less.
- the amount of compound added is, in one or more embodiments, 0.01-10 ⁇ M.
- Protein phosphorylation activity inhibition assays include, in one or more embodiments, in vitro and / or in vivo assays disclosed in WO20100110791.
- the present disclosure is based on the finding that in one or a plurality of embodiments, a compound having DYRK inhibitory activity can activate neurogenesis and nerve cell proliferation. Therefore, the present disclosure, in one aspect, is a composition for activating neurogenesis or nerve cell proliferation, comprising a compound having DYRK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof as an active ingredient. It is related with the composition to contain.
- DYRK is thought to lead to the degradation pathway by phosphorylating cyclin D1, which positively regulates cell growth, and by the action of a compound having DYRK inhibitory activity, degradation of cyclin D1 is suppressed and the amount of cyclin D1 is reduced. Increases and promotes cell proliferation.
- the present disclosure is not limited to this mechanism and need not be interpreted.
- the active ingredient in the composition according to this aspect has a CLK inhibition ability in addition to the DYRK inhibition ability.
- the composition according to this aspect has an effect of activating neurogenesis.
- neurogenesis refers to division / proliferation of neural stem cells, production of neural progenitor cells, differentiation / maturation of the produced neural progenitor cells into neurons, or these in one or a plurality of embodiments.
- a combination of Examples of the living body or adult include mammals, humans, and mammals other than humans.
- the “neural stem cell” refers to a cell that is present in the brain and spinal cord and produces a progenitor cell that has the ability to differentiate into a nerve cell or a glial cell.
- activation of neurogenesis refers to division or proliferation of neural stem cells in a living body or an adult, production of neural progenitor cells, differentiation of produced neural progenitor cells into neural cells, in one or a plurality of embodiments. It refers to enhancement of maturity or a combination thereof.
- the composition according to this aspect is a pharmaceutical composition.
- neurogenesis can be activated, and therefore, administration to a subject can prevent central and / or peripheral nervous system diseases or disorders, Can be effective in improving, suppressing progression and / or treating.
- the disease or disorder of the central and / or peripheral nervous system is, in one or more embodiments, a disease or disorder caused by hippocampal atrophy, including intellectual disability, learning ability disorder, mood disorder, PTSD and anxiety disorder, Examples include symptomatic organic psychiatric disorders, substance-related disorders (especially alcohol-related disorders, stimulants).
- organic psychiatric disorders include trauma, infection, vascular disorders, Alzheimer's disease or other dementia caused by degenerative / metabolic disorders, Parkinson's disease, Huntington's disease, neurotraumatic disease, mild cognition.
- MCI Mild Cognitive Impairment
- post-cerebral psychiatric symptoms depressive symptoms and memory impairment
- ischemic hippocampal injury due to brief cardiac arrest
- spinal cord injury open or penetrating caused by surgery Head injury, or closed head injury caused by damage to the head region, for example.
- the present disclosure in one or more embodiments, is a pharmaceutical composition for preventing, ameliorating, suppressing progression and / or treating a disease or disorder of the central and / or peripheral nervous system, comprising an active ingredient A DYRK inhibitory compound or a prodrug thereof or a pharmaceutically acceptable salt thereof, or a compound having a DYRK inhibitory ability and a CLK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof
- an active ingredient A DYRK inhibitory compound or a prodrug thereof or a pharmaceutically acceptable salt thereof, or a compound having a DYRK inhibitory ability and a CLK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof.
- the present disclosure relates to a method for activating neurogenesis, which, in one or a plurality of embodiments, includes administering a pharmaceutical composition according to the present disclosure to a subject.
- the subject includes mammals, humans, or non-human mammals in one or more embodiments.
- the present disclosure in one or more other embodiments, includes the prevention, improvement, progression inhibition of central and / or peripheral nervous system diseases or disorders, comprising administering a pharmaceutical composition according to the present disclosure to a subject, and / Or relates to a treatment method.
- this indication is related with use of the pharmaceutical composition which concerns on this indication in the activation method of the neurogenesis based on this indication in one or some embodiment.
- the present disclosure is a pharmaceutical composition according to the present disclosure in a method for preventing, ameliorating, suppressing progression, and / or treating a disease or disorder of the central and / or peripheral nervous system of the present disclosure.
- the present disclosure in one or more embodiments, is a compound having a DYRK inhibitory ability for producing a pharmaceutical composition for activation of neurogenesis according to the present disclosure, a prodrug thereof, or a pharmaceutically acceptable product thereof.
- a compound having a DYRK inhibitory ability and a CLK inhibitory ability, a prodrug thereof, or a pharmaceutically acceptable salt thereof is a compound having a DYRK inhibitory ability and a CLK inhibitory ability, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
- the present disclosure provides a pharmaceutical composition for prevention, improvement, progression inhibition, and / or treatment of a disease or disorder of the central and / or peripheral nervous system according to the present disclosure.
- the present invention relates to the use of a compound having a DYRK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof, or a compound having a DYRK inhibitory ability and a CLK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof.
- [A1] A composition for activating neurogenesis, comprising a compound having a DYRK inhibitory ability, a prodrug thereof, or a pharmaceutically acceptable salt thereof as an active ingredient.
- [A2] The composition according to [a1], wherein the compound as an active ingredient or a prodrug thereof or a pharmaceutically acceptable salt thereof further has a CLK inhibitory ability.
- [A3] The composition according to [a1] or [a2], which is a pharmaceutical composition.
- a pharmaceutical composition for preventing, ameliorating, suppressing progression and / or treating a disease or disorder of the central and / or peripheral nervous system having a DYRK inhibitory activity as an active ingredient, or a prodrug thereof Or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition containing a compound having DYRK inhibitory ability and CLK inhibitory ability, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
- a compound having a DYRK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof, or a compound having a DYRK inhibitory ability and a CLK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof as an active ingredient A method of activating neurogenesis in a subject comprising administering to the subject a pharmaceutical composition containing.
- a compound having DYRK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof, or a compound having DYRK inhibitory ability and CLK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof as an active ingredient A method for preventing, ameliorating, suppressing progression and / or treating a disease or disorder of the central and / or peripheral nervous system, comprising administering a pharmaceutical composition containing the composition to a subject.
- a compound having a DYRK inhibitory ability or a prodrug thereof, or a pharmaceutically acceptable salt thereof, or a compound having a DYRK inhibitory ability and a CLK inhibitory ability or a prodrug thereof or the like thereof as an active ingredient in the activation of neurogenesis Use of a pharmaceutical composition containing a pharmaceutically acceptable salt.
- neural cells include neural stem cells in one or more embodiments.
- neural stem cell refers to a cell that is present in the brain and spinal cord and produces progenitor cells that have the ability to differentiate into nerve cells or glial cells.
- neural cell proliferation refers to proliferation of nerve cells or neural stem cells (hereinafter also referred to as “nerve (stem) cells”) in one or a plurality of embodiments.
- proliferation of nerve (stem) cells refers to proliferation of nerve (stem) cells in vitro, in vivo or ex vivo in one or more embodiments, or one or more embodiments.
- proliferation of cultured neural stem cells refers to a neural stem cell mass isolated and cultured from a living body in one or a plurality of embodiments.
- activation of nerve cell proliferation refers to activation of nerve (stem) cell proliferation in one or a plurality of embodiments. It means that production of progenitor cells is promoted. “Activation of nerve cell proliferation” refers to activation of nerve (stem) cell proliferation in vitro, in vivo, or ex vivo in one or more embodiments, and in one or more embodiments. , Activation of proliferation of cultured neural stem cells.
- the present disclosure provides a composition for activating neuronal cell proliferation, a compound having a DYRK inhibitory ability as an active ingredient, a prodrug thereof, or a pharmaceutically acceptable salt thereof, or DYRK
- the present invention relates to a composition comprising a compound having an inhibitory ability and a CLK inhibitory ability, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
- the composition of this aspect may be a pharmaceutical composition.
- composition of this aspect in one or a plurality of embodiments, it is possible to enhance the proliferation of cultured neural stem cells, so that the proliferation of neural stem cells existing in the brain and spinal cord can be promoted even in a living body. There is expected.
- the present disclosure is a composition for activating cultured neural stem cells, which is a compound having a DYRK inhibitory ability as an active ingredient, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
- the present invention relates to a composition containing a compound having a DYRK inhibitory ability and a CLK inhibitory ability, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
- This disclosure relates to a nerve (stem) cell growth method including, in one or a plurality of embodiments, culturing nerve (stem) cells in a medium containing the composition according to the present disclosure.
- the present disclosure relates to a method for preparing nerve (stem) cells, including culturing nerve (stem) cells in a medium containing the composition according to the present disclosure.
- this indication is related with use of the composition concerning this indication in the proliferation method of the nerve (stem) cell concerning this indication in one or some embodiment.
- the present disclosure relates to the use of the composition according to the present disclosure in the method for preparing a nerve (stem) cell according to the present disclosure.
- compositions for activating nerve cell proliferation comprising a compound having a DYRK inhibitory ability, a prodrug thereof, or a pharmaceutically acceptable salt thereof as an active ingredient.
- composition according to [b1] wherein the compound as an active ingredient or a prodrug thereof or a pharmaceutically acceptable salt thereof further has a CLK inhibitory ability.
- a composition for activating proliferation of nerve (stem) cells which is a compound having a DYRK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof, or a DYRK inhibitory ability and A composition comprising a compound having a CLK inhibitory ability, a prodrug thereof, or a pharmaceutically acceptable salt thereof.
- a compound having a DYRK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof, or a compound having a DYRK inhibitory ability and a CLK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof as an active ingredient A method for activating nerve cell proliferation, comprising culturing nerve (stem) cells in a medium containing the composition to be contained.
- a compound having a DYRK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof, or a compound having a DYRK inhibitory ability and a CLK inhibitory ability or a prodrug thereof or a pharmaceutically acceptable salt thereof as an active ingredient A method for preparing nerve (stem) cells, comprising culturing nerve (stem) cells in a medium containing the composition to be contained.
- a compound having DYRK inhibitory ability or a prodrug thereof, or a pharmaceutically acceptable salt thereof, or a compound having DYRK inhibitory ability and CLK inhibitory ability or prodrug thereof, as an active ingredient in activation of nerve cell proliferation Use of a composition containing the pharmaceutically acceptable salt thereof.
- the present disclosure relates to a compound represented by the following general formula (I), a prodrug thereof, or a pharmaceutically acceptable salt thereof.
- R 1 and R 2 are each independently a hydrogen atom or a C 1-6 hydrocarbon chain
- R 3 is Z is one benzene ring, one heteroaromatic ring, one aromatic ring fused with one or more benzene rings, and one or more heteroaromatic rings together with the atoms marked with a and b.
- a condensed aromatic ring fused with one or more, a mixed condensed polycyclic condensed with one or more benzene rings and one or more heteroaromatic rings, and a ring selected from the group consisting of cycloaliphatic, ring may have a hydrogen atom, the substituent is a halogen atom or an C 1-6 alkyl group one or more, R 4 is a hydrogen atom, halogen atoms or a C 1-6 alkyl group.
- the “prodrug” includes, in one or a plurality of embodiments, those that are easily hydrolyzed in vivo and regenerate the compound represented by the formula (I), such as a compound having a carboxyl group. If present, there may be mentioned a compound in which the carboxyl group is an alkoxycarbonyl group, a compound in which the alkylthiocarbonyl group is formed, or a compound in which the alkylaminocarbonyl group is formed.
- a compound having an amino group a compound in which the amino group is substituted with an alkanoyl group to become an alkanoylamino group, a compound in which the amino group is substituted with an alkoxycarbonyl group to become an alkoxycarbonylamino group, an acyloxymethylamino group, Or a compound that has become hydroxylamine.
- a compound having a hydroxyl group a compound in which the hydroxyl group is substituted with the acyl group to become an acyloxy group, a compound that has become a phosphate ester, or a compound that has become an acyloxymethyloxy group can be given.
- alkyl moiety of the group used for forming a prodrug examples include an alkyl group described later, and the alkyl group may be substituted (for example, with an alkoxy group having 1 to 6 carbon atoms).
- the alkyl group may be substituted (for example, with an alkoxy group having 1 to 6 carbon atoms).
- lower alkoxycarbonyl such as methoxycarbonyl, ethoxycarbonyl, methoxymethoxycarbonyl, ethoxymethoxy, etc.
- Examples include lower (eg, having 1 to 6 carbon atoms) alkoxycarbonyl substituted with an alkoxy group such as carbonyl, 2-methoxyethoxycarbonyl, 2-methoxyethoxymethoxycarbonyl, and pivaloyloxymethoxycarbonyl.
- the “C 1-6 hydrocarbon chain” refers to a monovalent group derived by removing an arbitrary hydrogen atom from an aliphatic hydrocarbon having 1 to 6 carbon atoms.
- the hydrocarbon chain may be a straight chain structure, a branched chain structure, or a cyclic structure, and examples thereof include an alkyl group, an alkenyl group, a phenyl group, and a cycloalkyl group.
- the “C 1-6 alkyl group” means, in one or more embodiments, a methyl group, an ethyl group, a 1-propyl group, a 2-propyl group, a 2-methyl-1-propyl group, a 2-methyl- 2-propyl group, 1-butyl group, 2-butyl group, 1-pentyl group, 2-pentyl group, 3-pentyl group, 2-methyl-1-butyl group, 3-methyl-1-butyl group, 2- Methyl-2-butyl group, 3-methyl-2-butyl group, 2,2-dimethyl-1-propyl group, 1-hexyl group, 2-hexyl group, 3-hexyl group, 2-methyl-1 -Pentyl group, 3-methyl-1-pentyl group, 4-methyl-1-pentyl group, 2-methyl-2-pentyl group, 3-methyl-2-pentyl group, 4-methyl-2-pentyl group, 2 -Methyl-3-pentyl
- the “heterocycle” refers to a non-aromatic ring or aromatic group that contains 1 to 2 heteroatoms in the atoms constituting the ring and may contain a double bond in the ring. Means ring of sex.
- the “heteroaromatic ring” means an aromatic heterocycle.
- the “heteroatom” means a sulfur atom, an oxygen atom or a nitrogen atom.
- cycloaliphatic means an aliphatic having a cyclic structure.
- the cycloaliphatic group include a cycloaliphatic group having 3 to 10 carbon atoms, and may be a cycloaliphatic group having a condensed ring structure composed of a plurality of rings. Specific examples include a cycloalkyl group having 3 to 10 carbon atoms, a cyclic ether group, a decahydronaphthyl group and an adamantyl group.
- cycloaliphatic group having 3 to 10 carbon atoms include cyclopropyl group, cyclobutyl group, cyclopentyl group, cyclohexyl group, cycloheptyl group and the like.
- the “pharmaceutically acceptable salt” includes a pharmacologically and / or pharmaceutically acceptable salt, for example, an inorganic acid salt, an organic acid salt, an inorganic basic salt, an organic basic salt, acidic or basic. Amino acid salts and the like.
- Preferable examples of the inorganic acid salt include hydrochloride, hydrobromide, sulfate, nitrate, phosphate and the like, and preferable examples of the organic acid salt include, for example, acetate, succinate, Examples thereof include fumarate, maleate, tartrate, citrate, lactate, stearate, benzoate, methanesulfonate, and p-toluenesulfonate.
- Preferred examples of the inorganic base salt include alkali metal salts such as sodium salt and potassium salt, alkaline earth metal salts such as calcium salt and magnesium salt, aluminum salt and ammonium salt.
- Preferable examples of the organic base salt include diethylamine salt, diethanolamine salt, meglumine salt, N, N′-dibenzylethylenediamine salt and the like.
- Preferred examples of the acidic amino acid salt include aspartate and glutamate.
- Preferable examples of the basic amino acid salt include arginine salt, lysine salt, ornithine salt and the like.
- the “salt of a compound” may include a hydrate that can be formed by absorbing moisture when the compound is left in the air. Further, in the present disclosure, the “salt of a compound” may include a solvate that can be formed by absorbing a certain kind of other solvent.
- R 1 is a C 1-6 alkyl group in one or more embodiments, and in one or more embodiments, is a methyl group, an ethyl group, or a propyl group.
- R 2 is a C 1-6 alkyl group in one or more embodiments, and is a methyl group in one or more embodiments.
- R 3 is one or more embodiments, It is. R 3 is —CH 2 —CH 2 — or —CH ⁇ CH— in one or more embodiments. Z, in one or more embodiments, forms one benzene ring with the atoms marked a and b.
- R 4 is a hydrogen atom in one or more embodiments.
- the compound represented by the general formula (I) is: It is a compound represented by these.
- the compound represented by the above general formula (I), a prodrug thereof, or a pharmaceutically acceptable salt thereof has a DYRK inhibitory ability.
- the compound represented by the general formula (I) or a prodrug thereof or a pharmaceutically acceptable salt thereof has a DYRK inhibitory ability and a CLK inhibitory ability.
- R 21 and R 23 each independently represents a hydrogen atom, a C 1-6 linear or branched or cyclic alkyl group, a benzyl or heteroarylmethyl group, a substituted or unsubstituted aryl, Or a substituted or unsubstituted heteroaryl group
- R 22 is —R 26 , —C ⁇ C—R 26 , —CH ⁇ CH—R 26 , and —O— (CH 2 ) n—R 26.
- N is 1 to 6
- R 27 is a hydrogen atom, C 1-6 alkyl group, a trihalomethyl group, or a hydroxyl group, -Si (R 27) 3 in the Three R 27 may be different from each other.
- R 24 and R 25 are a hydrogen atom or
- heteroaryl (including heteroaryl in a heteroarylmethyl group) is, in one or more embodiments, a 5- to 6-membered monocyclic group containing 1 to 2 nitrogen atoms, a nitrogen atom 5 to 6-membered monocyclic group containing 1 to 2 and one oxygen atom or one sulfur atom, a 5-membered monocyclic group containing one oxygen atom or one sulfur atom, And bicyclic groups containing 1 to 4 nitrogen atoms and fused with a 6-membered ring and a 5- or 6-membered ring.
- the aryl group include aryl groups having 10 or less carbon atoms such as a phenyl group and a naphthyl group.
- the substituents of the phenyl group, the monocyclic heteroaromatic group and the cycloaliphatic group, and the aryl group and heteroaryl (including heteroaryl in the heteroarylmethyl group) group are one or the same.
- the halogen atom includes, in one or more embodiments, fluorine, chlorine, bromine, or iodine atoms.
- the lower alkyl includes “C 1-6 alkyl group” as defined above.
- R 21 is a hydrogen atom or a C 1-3 alkyl group in one or more embodiments.
- R 22 is —R 26 or —C ⁇ C—R 26 in one or more embodiments, and R 26 is —Si (R 27 ) 3 , or is substituted or substituted in one or more embodiments.
- R 27 is a C 1-3 alkyl group in one or more embodiments.
- R 23 is a hydrogen atom or a C 1-6 alkyl group in one or more embodiments.
- R 24 and R 25 are each a hydrogen atom or a C 1-3 alkyl group in one or more embodiments.
- the compound represented by the formula (II) does not contain Harmine in one or more embodiments, and in one or more embodiments, R 21 , R 22 , R 23 , It is not a combination in which R 24 and R 25 are Harmine (a combination of R 21 is a methyl group, R 22 and R 23 are hydrogen atoms, R 24 is a methyl group, and R 25 is a hydrogen atom).
- the compound represented by the general formula (II) or a pharmaceutically acceptable salt thereof is: Or a pharmaceutically acceptable salt thereof.
- the compound represented by the general formula (II) or a prodrug thereof, or a pharmaceutically acceptable salt thereof has a DYRK inhibitory ability. In one or a plurality of embodiments of the present disclosure, the compound represented by the general formula (II) or a prodrug thereof or a pharmaceutically acceptable salt thereof has a DYRK inhibitory ability and a CLK inhibitory ability.
- the compound represented by the general formula (I) or (II) or a prodrug thereof or a pharmaceutically acceptable salt thereof has an effect of activating neurogenesis.
- activation of neurogenesis means, as described above, in one or a plurality of embodiments, neural stem cell division / proliferation, production of neural progenitor cells, and neural cells of the produced neural progenitor cells in one or more embodiments This refers to the enhancement of differentiation / maturation or a combination thereof.
- the composition according to this aspect is a pharmaceutical composition.
- the present disclosure provides a pharmaceutical composition for activating neurogenesis, wherein the compound represented by the general formula (I) or (II) or a prodrug thereof or a pharmaceutically
- the present invention relates to a pharmaceutical composition containing an acceptable salt.
- the compound represented by the general formula (I) or (II), or a prodrug thereof, or a pharmaceutically acceptable salt thereof exhibits brain transferability and oral absorption. Thereby, neurogenesis can be activated more effectively.
- neurogenesis can be activated, and therefore, administration to a subject can prevent central and / or peripheral nervous system diseases or disorders, Can be effective in improving, suppressing progression and / or treating.
- the disease or disorder of the central and / or peripheral nervous system is, in one or more embodiments, a disease or disorder caused by hippocampal atrophy, including intellectual disability, learning ability disorder, mood disorder, PTSD and anxiety disorder, Examples include symptomatic organic psychiatric disorders, substance-related disorders (especially alcohol-related disorders, stimulants).
- organic psychiatric disorders include trauma, infection, vascular disorders, Alzheimer's disease or other dementia caused by degenerative / metabolic disorders, Parkinson's disease, Huntington's disease, neurotraumatic disease, mild cognition.
- MCI Mild Cognitive Impairment
- post-cerebral psychiatric symptoms depressive symptoms and memory impairment
- ischemic hippocampal injury due to brief cardiac arrest
- spinal cord injury open or penetrating caused by surgery Head injury, or closed head injury caused by damage to the head region, for example.
- the present disclosure in one or more embodiments, is a pharmaceutical composition for preventing, ameliorating, suppressing progression and / or treating a disease or disorder of the central and / or peripheral nervous system, comprising an active ingredient
- a pharmaceutical composition containing a compound represented by the general formula (I) or (II) or a prodrug thereof or a pharmaceutically acceptable salt thereof.
- the present disclosure relates to a method for activating neurogenesis, which, in one or a plurality of embodiments, includes administering a pharmaceutical composition according to the present disclosure to a subject.
- the subject includes mammals, humans, or non-human mammals in one or more embodiments.
- the present disclosure in one or more other embodiments, includes the prevention, improvement, progression inhibition of central and / or peripheral nervous system diseases or disorders, comprising administering a pharmaceutical composition according to the present disclosure to a subject, and / Or relates to a treatment method.
- this indication is related with use of the pharmaceutical composition which concerns on this indication in the activation method of the neurogenesis based on this indication in one or some embodiment.
- the present disclosure is a pharmaceutical composition according to the present disclosure in a method for preventing, ameliorating, suppressing progression, and / or treating a disease or disorder of the central and / or peripheral nervous system of the present disclosure.
- the present disclosure is, in one or more embodiments, a compound represented by the general formula (I) or (II) for producing a pharmaceutical composition for activation of neurogenesis according to the present disclosure, or a compound thereof It relates to the use of a prodrug or a pharmaceutically acceptable salt thereof.
- the present disclosure provides a pharmaceutical composition for prevention, improvement, progression inhibition, and / or treatment of a disease or disorder of the central and / or peripheral nervous system according to the present disclosure.
- a pharmaceutical composition for prevention, improvement, progression inhibition, and / or treatment of a disease or disorder of the central and / or peripheral nervous system according to the present disclosure.
- compositions for activating neurogenesis comprising a compound represented by the general formula (I) or (II) or a prodrug thereof or a pharmaceutically acceptable salt thereof as an active ingredient, Composition.
- the compound represented by the general formula (I) is: The composition according to [c1], which is a compound represented by the formula: [C3]
- the compound represented by the general formula (II) is: The composition according to [c1], which is a compound represented by the formula: [C4]
- the compound represented by the general formula (I) or (II) or a prodrug thereof or a pharmaceutically acceptable salt thereof has a DYRK inhibitory ability, according to any one of [c1] to [c3] Composition.
- [C5] The compound represented by the general formula (I) or (II), or a prodrug thereof, or a pharmaceutically acceptable salt thereof further has a CLK inhibitory activity, and is any one of [c1] to [c4] The composition as described.
- [C6] The composition according to any one of [c1] to [c5], which is a pharmaceutical composition.
- [C7] A pharmaceutical composition for prevention, improvement, progression inhibition, and / or treatment of diseases or disorders of the central and / or peripheral nervous system, and any one of [c1] to [c5] as an active ingredient
- a pharmaceutical composition comprising the compound represented by formula (I) or (II) or a prodrug thereof or a pharmaceutically acceptable salt thereof as described in 1. above.
- [C8] Pharmaceutical composition containing a compound represented by the general formula (I) or (II) according to any one of [c1] to [c5] or a prodrug thereof or a pharmaceutically acceptable salt thereof as an active ingredient A method of activating neurogenesis in a subject comprising administering an object to the subject.
- a pharmaceutical composition containing the compound represented by the general formula (I) or (II) according to any one of [c1] to [c5] or a prodrug thereof or a pharmaceutically acceptable salt thereof as an active ingredient A method for preventing, ameliorating, suppressing progression and / or treating a disease or disorder of the central and / or peripheral nervous system, comprising administering a product to a subject.
- [C10] The compound represented by the general formula (I) or (II) according to any one of [c1] to [c5] or a prodrug thereof, or a pharmaceutically acceptable salt thereof as an active ingredient in the activation of neurogenesis Use of a pharmaceutical composition containing a salt.
- [C12] A compound represented by the general formula (I) or (II) according to any one of [c1] to [c5] or a prodrug thereof as an active ingredient in the production of a pharmaceutical composition that activates neurogenesis Or use of a pharmaceutically acceptable salt thereof.
- the “pharmaceutical composition” may be a dosage form suitable for an administration form by applying a well-known formulation technique in one or a plurality of embodiments.
- the dosage form include, but are not limited to, oral administration in a dosage form such as a tablet, capsule, granule, powder, pill, troche, syrup, and liquid.
- parenteral administration in dosage forms such as injections, liquids, aerosols, suppositories, patches, lotions, liniments, ointments, eye drops and the like can be mentioned.
- These preparations can be produced by known methods using additives such as, but not limited to, excipients, lubricants, binders, disintegrants, stabilizers, flavoring agents, and diluents.
- excipient examples include, but are not limited to, starch such as starch, potato starch, and corn starch, lactose, crystalline cellulose, calcium hydrogen phosphate, and the like.
- coating agent examples include, but are not limited to, ethyl cellulose, hydroxypropyl cellulose, hydroxypropyl methyl cellulose, shellac, talc, carnauba wax, paraffin, and the like.
- binder include, but are not limited to, polyvinyl pyrrolidone, macrogol and the same compound as the excipient.
- disintegrant examples include, but are not limited to, compounds similar to the excipients and chemically modified starch and celluloses such as croscarmellose sodium, sodium carboxymethyl starch, and crosslinked polyvinylpyrrolidone.
- stabilizer examples include, but are not limited to, paraoxybenzoates such as methylparaben and propylparaben; alcohols such as chlorobutanol, benzyl alcohol, and phenylethyl alcohol; benzalkonium chloride; phenol, cresol Mention may be made of such phenols; thimerosal; dehydroacetic acid; and sorbic acid.
- flavoring agent examples include, but are not limited to, sweeteners, acidulants, and fragrances that are commonly used.
- the solvent is not limited to these, but ethanol, phenol, chlorocresol, purified water, distilled water and the like can be used, and a surfactant or an emulsifier can also be used as necessary.
- a surfactant or an emulsifier include, but are not limited to, polysorbate 80, polyoxyl 40 stearate, lauromacrogol, and the like.
- the method of using the pharmaceutical composition according to the present disclosure may vary depending on symptoms, age, administration method, and the like.
- the method of use is not limited to these, but it is intermittent or continuous so that the concentration in the body of the compound represented by formula (I) or (II) as the active ingredient is between 100 nM and 1 mM.
- it can be administered orally, transdermally, submucosally, subcutaneously, intramuscularly, intravascularly, intracerebrally, or intraperitoneally.
- the lower limit is set to 0. 0 as the lower limit in terms of the compound represented by the general formula (I) or (II) per day for a subject (adult if human).
- the dosage may be 01 mg (preferably 0.1 mg) and the upper limit may be 2000 mg (preferably 500 mg, more preferably 100 mg) divided into one or several doses and administered according to symptoms.
- the lower limit is 0.001 mg (preferably 0.01 mg) and the upper limit is 500 mg (preferably 50 mg) per day for a subject (adult if human). Is divided into one or several times and administered according to symptoms.
- activation of nerve cell proliferation refers to activation of nerve (stem) cell proliferation in one or more embodiments as described above, and in one or more other embodiments. Furthermore, it means that production of neural progenitor cells is promoted. As described above, “activation of nerve cell proliferation” refers to activation of proliferation of nerve (stem) cells in vitro, in vivo, or ex vivo in one or more embodiments. In an embodiment of this, activation of proliferation of cultured neural stem cells.
- the present disclosure in one aspect, is a composition for activating nerve cell proliferation, which is a compound represented by the general formula (I) or (II) as an active ingredient, a prodrug thereof, or a pharmaceutically acceptable salt thereof. It relates to a composition containing an acceptable salt.
- the composition of this aspect may be a pharmaceutical composition.
- composition of this aspect in one or a plurality of embodiments, it is possible to enhance the proliferation of cultured neural stem cells, so that the proliferation of neural stem cells existing in the brain and spinal cord can be promoted even in a living body. There is expected.
- the present disclosure in one or a plurality of embodiments, is a composition for activating cultured neural stem cells, which is a compound represented by the general formula (I) or (II) or a prodrug thereof as an active ingredient Or a composition containing a pharmaceutically acceptable salt thereof.
- This disclosure relates to a nerve (stem) cell growth method including, in one or a plurality of embodiments, culturing nerve (stem) cells in a medium containing the composition according to the present disclosure.
- the present disclosure relates to a method for preparing nerve (stem) cells, including culturing nerve (stem) cells in a medium containing the composition according to the present disclosure.
- this indication is related with use of the composition concerning this indication in the proliferation method of the nerve (stem) cell concerning this indication in one or some embodiment.
- the present disclosure relates to the use of the composition according to the present disclosure in the method for preparing a nerve (stem) cell according to the present disclosure.
- [D1] A composition for activating nerve cell proliferation, comprising a compound represented by the general formula (I) or (II) or a prodrug thereof or a pharmaceutically acceptable salt thereof as an active ingredient ,Composition.
- the compound represented by the general formula (I) is: The composition of [d1] which is a compound represented by these.
- [D3] The compound represented by the general formula (II) is: The composition of [d1] which is a compound represented by these.
- the compound represented by the general formula (I) or (II), a prodrug thereof, or a pharmaceutically acceptable salt thereof has a DYRK inhibitory ability, according to any one of [d1] to [d3] Composition.
- the compound represented by the general formula (I) or (II) or a prodrug thereof or a pharmaceutically acceptable salt thereof further has a CLK inhibitory activity, and is any one of [d1] to [d4] The composition as described.
- [D6] The composition according to any one of [d1] to [d5], which is a pharmaceutical composition.
- [D7] A composition for activating proliferation of nerve (stem) cells, represented by the general formula (I) or (II) according to any one of [d1] to [d5] as an active ingredient Or a prodrug thereof or a pharmaceutically acceptable salt thereof.
- a composition comprising a compound represented by the general formula (I) or (II) according to any one of [d1] to [d5], a prodrug thereof, or a pharmaceutically acceptable salt thereof as an active ingredient
- a method for activating nerve cell proliferation comprising culturing nerve (stem) cells in a medium comprising [D9]
- a composition comprising a compound represented by the general formula (I) or (II) according to any one of [d1] to [d5] or a prodrug thereof or a pharmaceutically acceptable salt thereof as an active ingredient
- a method for preparing nerve (stem) cells comprising culturing nerve (stem) cells in a medium containing [D10]
- a compound represented by the general formula (I) or (II) according to any one of [d1] to [d5] or a prodrug thereof, or a pharmaceutically acceptable product thereof as an active ingredient in activation of nerve cell proliferation Use of a pharmaceutical composition containing a prepared salt.
- [D11] A compound represented by the general formula (I) or (II) according to any one of [d1] to [d5] as an active ingredient in the preparation of nerve (stem) cells, or a prodrug thereof, or a pharmaceutical thereof Use of a pharmaceutical composition containing an acceptable salt.
- [D12] A compound represented by the general formula (I) or (II) according to any one of [d1] to [d5] or a pro thereof as an active ingredient in the production of a pharmaceutical composition that activates proliferation of nerve cells Use of a drug or a pharmaceutically acceptable salt thereof.
- [D13] Compound represented by general formula (I) or (II) according to any one of [d1] to [d5] as an active ingredient in the manufacture of a pharmaceutical composition for the preparation of nerve (stem) cells Or use of a prodrug thereof or a pharmaceutically acceptable salt thereof.
- Production Example 1 Production of Compound 1 Compound 1 was prepared as follows.
- N-bromosuccinimide (2.31 g, 13.0 mmol, commercial product) is added to a solution of compound 1d (2.10 g, 11.9 mmol) in dichloromethane (50 mL, dehydrated, commercial product). The solution was added in small portions at ⁇ 78 ° C., and the temperature was raised to room temperature over 10 hours.
- Production Example 2 Production of Compound 2 Compound 2 was prepared as follows.
- Production Example 3 Production of Compound 3 Compound 3 was prepared as follows.
- Compound 2 prepared with carboxymethylcellulose solvent was continuously administered orally at 30 mg / kg and 100 mg / kg for 10 days or 30 days to C57BL / 6J mouse male 9-week-old body weight 25 g.
- 150 mg / kg BrdU was injected intraperitoneally, and samples were collected by perfusion fixation 24 hours later.
- a 50-um coronal section was prepared using a microtome, and after denaturation with 1.5N hydrochloric acid, BrdU was detected by the neural stem cells proliferating with anti-BrdU antibody. The sections were observed under a microscope, and the number of BrdU positive cells per hippocampus was quantified and compared in each administration group (FIG. 1).
- DYRK1A short-hairpin RNA
- FIG. 5 Cultured neural stem cells in which expression of DYRK1A was suppressed were cultured in the presence of BrdU, which is an indicator of cell proliferation, and the proliferating cells were labeled. After fixing the cells, the proportion of proliferating cells incorporating BrdU was quantitatively analyzed by staining with anti-BrdU antibody (Fig. 5, 6). Furthermore, in cultured neural stem cells that suppressed DYRK1A expression, the expression level of D1, which is a protein that positively controls cell proliferation, was detected by Western blotting (FIG. 5, 7).
- HEK293FLIP-IN cell system cell lines capable of inducing the expression of DYRK1A, DYRK1B, and DYRK2 belonging to the DYRK family by adding drugs were prepared.
- the cells were treated with drugs for 16 hours to induce DYRK expression, and then cultured for 4 hours in the presence of compound 2 (5 ⁇ M). Thereafter, the cells were collected, and the expression level of cyclin D1 was analyzed by Western blotting (FIG. 8).
- the expression level of cyclin D1 which is a positive cell growth factor, was increased by inhibiting DYRK activity by treatment with Compound 2.
- the expression level of cyclin D1 was decreased by the induction of DYRK expression.
- cyclin D1 decrease due to DYRK expression induction was corrected by inhibiting DYRK activity by treatment with Compound 2. From the above results, it was shown that the expression level of cyclin D1 was decreased by induction of DYRK expression, and the expression level of cyclin D1 was increased by inhibition of DYRK activity. That is, it can be said that DYRK activates cell proliferation by controlling the expression level of cyclin D1.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Neurology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Neurosurgery (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Genetics & Genomics (AREA)
- Wood Science & Technology (AREA)
- Epidemiology (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- General Engineering & Computer Science (AREA)
- Cell Biology (AREA)
- Psychiatry (AREA)
- Addiction (AREA)
- Developmental Biology & Embryology (AREA)
- Psychology (AREA)
- Pain & Pain Management (AREA)
- Anesthesiology (AREA)
- Hospice & Palliative Care (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/101,862 US20160303089A1 (en) | 2013-12-05 | 2014-12-03 | Compound pertaining to neuropoiesis and drug composition |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013-252443 | 2013-12-05 | ||
| JP2013252443A JP2015107945A (ja) | 2013-12-05 | 2013-12-05 | 神経新生に関する化合物及び医薬組成物 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015083750A1 true WO2015083750A1 (ja) | 2015-06-11 |
Family
ID=53273511
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/082035 Ceased WO2015083750A1 (ja) | 2013-12-05 | 2014-12-03 | 神経新生に関する化合物及び医薬組成物 |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US20160303089A1 (OSRAM) |
| JP (1) | JP2015107945A (OSRAM) |
| WO (1) | WO2015083750A1 (OSRAM) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018043674A1 (ja) * | 2016-08-31 | 2018-03-08 | 国立大学法人京都大学 | 神経新生を活性化するための組成物 |
| EP3318563A1 (en) | 2016-11-07 | 2018-05-09 | Sanofi | Substituted pyrido[3,4-b]indoles for the treatment of cartilage disorders |
| WO2018151326A1 (ja) * | 2017-02-20 | 2018-08-23 | 国立大学法人京都大学 | スプライシング異常に起因する遺伝性疾病のための医薬組成物及び治療方法 |
| WO2021201171A1 (ja) | 2020-04-01 | 2021-10-07 | 国立大学法人京都大学 | 神経炎症の抑制、そのための組成物及び方法 |
| WO2022255411A1 (ja) | 2021-06-01 | 2022-12-08 | 国立大学法人京都大学 | 自然免疫応答を増強させる方法 |
| WO2023008470A1 (ja) * | 2021-07-28 | 2023-02-02 | 住友ファーマ株式会社 | 縮環アミン誘導体 |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101716099B1 (ko) * | 2013-09-12 | 2017-03-13 | 에프. 호프만-라 로슈 아게 | 인돌-카복스아마이드 유도체 |
| WO2021153665A1 (ja) * | 2020-01-30 | 2021-08-05 | カルナバイオサイエンス株式会社 | 新規アルキン誘導体 |
| US20230365589A1 (en) * | 2020-09-18 | 2023-11-16 | Sumitomo Pharma Co., Ltd. | Novel amine derivatives |
| EP4303304A4 (en) * | 2021-03-03 | 2025-03-05 | Riken | POLYNUCLEOTIDE FOR THE TREATMENT OF A NEURODEGENERATIVE DISEASE, VECTOR, CELL, PHARMACEUTICAL COMPOSITION AND SCREENING METHOD |
| WO2023008472A1 (ja) * | 2021-07-28 | 2023-02-02 | カルナバイオサイエンス株式会社 | 新規ベンゾチアゾール誘導体 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010010797A1 (ja) * | 2008-07-23 | 2010-01-28 | 株式会社キノファーマ | Dyrkを阻害する化合物を含有する医薬組成物 |
| WO2011068990A1 (en) * | 2009-12-04 | 2011-06-09 | Medipropharma, Inc. | Compositions and methods for inhibiting dyrk1a to treat central nervous system diseases and disorders |
| JP2013505299A (ja) * | 2009-09-22 | 2013-02-14 | ニューロナセント インコーポレイテッド | ダウン症を治療するための方法および薬学的組成物 |
| WO2013026806A1 (en) * | 2011-08-19 | 2013-02-28 | Exonhit Sa | Dyrk1 inhibitors and uses thereof |
| WO2013183718A1 (ja) * | 2012-06-06 | 2013-12-12 | 国立大学法人京都大学 | スクリーニング方法、タンパク質の不安定性及び/又は安定性を誘導する物質、及び、タンパク質の活性評価 |
| WO2014021337A1 (ja) * | 2012-07-30 | 2014-02-06 | 国立大学法人京都大学 | 精神神経疾患又は悪性腫瘍に関する化合物及び医薬組成物 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004029070A2 (en) * | 2002-09-24 | 2004-04-08 | Isis Pharmaceutical, Inc. | Methods for blocking adipocyte differentiation and triglyceride accumulation with dual-specificity tyrosine- (y) - phosphorylation regulated kinase 4 (dyrk4) inhibitors |
| WO2008150837A1 (en) * | 2007-06-01 | 2008-12-11 | Smithkline Beecham Corporation | Methods of treatment |
| WO2011133795A2 (en) * | 2010-04-22 | 2011-10-27 | The Brigham And Women's Hospital, Inc. | Beta-carbolines as inhibitors of haspin and dyrk kinases |
-
2013
- 2013-12-05 JP JP2013252443A patent/JP2015107945A/ja active Pending
-
2014
- 2014-12-03 WO PCT/JP2014/082035 patent/WO2015083750A1/ja not_active Ceased
- 2014-12-03 US US15/101,862 patent/US20160303089A1/en not_active Abandoned
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010010797A1 (ja) * | 2008-07-23 | 2010-01-28 | 株式会社キノファーマ | Dyrkを阻害する化合物を含有する医薬組成物 |
| JP2013505299A (ja) * | 2009-09-22 | 2013-02-14 | ニューロナセント インコーポレイテッド | ダウン症を治療するための方法および薬学的組成物 |
| WO2011068990A1 (en) * | 2009-12-04 | 2011-06-09 | Medipropharma, Inc. | Compositions and methods for inhibiting dyrk1a to treat central nervous system diseases and disorders |
| WO2013026806A1 (en) * | 2011-08-19 | 2013-02-28 | Exonhit Sa | Dyrk1 inhibitors and uses thereof |
| WO2013183718A1 (ja) * | 2012-06-06 | 2013-12-12 | 国立大学法人京都大学 | スクリーニング方法、タンパク質の不安定性及び/又は安定性を誘導する物質、及び、タンパク質の活性評価 |
| WO2014021337A1 (ja) * | 2012-07-30 | 2014-02-06 | 国立大学法人京都大学 | 精神神経疾患又は悪性腫瘍に関する化合物及び医薬組成物 |
Non-Patent Citations (3)
| Title |
|---|
| BECKER, W.: "Activation, regulation, and inhibition of DYRK1A", FEBS JOURNAL, vol. 278, 2011, pages 246 - 256, XP055053004, DOI: doi:10.1111/j.1742-4658.2010.07956.x * |
| BOZENA, M-K. ET AL.: "Effect of Dyrk1A Activity Inhibition on Development of Neuronal ProgenitorsIsolated From Ts65Dn Mice", JOURNAL OF NEUROSCIENCE RESEARCH, vol. 90, 2012, pages 999 - 1010 * |
| YOO K.Y. ET AL.: "Epigallocatechin-3-gallate increases cell proliferation and neuroblasts in the subgranular zone of the dentate gyrus in adult mice", PHYTOTHER RES., vol. 24, no. 7, 2010, pages 1065 - 1070 * |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7054524B2 (ja) | 2016-08-31 | 2022-04-14 | 国立大学法人京都大学 | 神経新生を活性化するための組成物 |
| JPWO2018043674A1 (ja) * | 2016-08-31 | 2019-06-24 | 国立大学法人京都大学 | 神経新生を活性化するための組成物 |
| WO2018043674A1 (ja) * | 2016-08-31 | 2018-03-08 | 国立大学法人京都大学 | 神経新生を活性化するための組成物 |
| US11318126B2 (en) | 2016-08-31 | 2022-05-03 | Kyoto University | Composition for activating neurogenesis |
| EP3318563A1 (en) | 2016-11-07 | 2018-05-09 | Sanofi | Substituted pyrido[3,4-b]indoles for the treatment of cartilage disorders |
| WO2018083157A1 (en) | 2016-11-07 | 2018-05-11 | Sanofi | Substituted pyrido[3,4-b]indoles for the treatment of cartilage disorders |
| US12275733B2 (en) | 2016-11-07 | 2025-04-15 | Sanofi | Substituted pyrido[3,4-b]indoles for the treatment of cartilage disorders |
| US11130755B2 (en) | 2016-11-07 | 2021-09-28 | Sanofi | Substituted pyrido[3,4-b]indoles for the treatment of cartilage disorders |
| US11827633B2 (en) | 2016-11-07 | 2023-11-28 | Sanofi | Substituted pyrido[3,4-b]indoles for the treatment of cartilage disorders |
| JPWO2018151326A1 (ja) * | 2017-02-20 | 2019-12-12 | 国立大学法人京都大学 | スプライシング異常に起因する遺伝性疾病のための医薬組成物及び治療方法 |
| JP2022078297A (ja) * | 2017-02-20 | 2022-05-24 | 国立大学法人京都大学 | スプライシング異常に起因する遺伝性疾病のための医薬組成物及び治療方法 |
| JP7129095B2 (ja) | 2017-02-20 | 2022-09-01 | 国立大学法人京都大学 | スプライシング異常に起因する遺伝性疾病のための医薬組成物及び治療方法 |
| JP7360206B2 (ja) | 2017-02-20 | 2023-10-12 | 国立大学法人京都大学 | スプライシング異常に起因する遺伝性疾病のための医薬組成物及び治療方法 |
| US12023338B2 (en) | 2017-02-20 | 2024-07-02 | Kyoto University | Pharmaceutical composition and treatment method for genetic disease associated with splicing abnormalities |
| WO2018151326A1 (ja) * | 2017-02-20 | 2018-08-23 | 国立大学法人京都大学 | スプライシング異常に起因する遺伝性疾病のための医薬組成物及び治療方法 |
| JPWO2021201171A1 (OSRAM) * | 2020-04-01 | 2021-10-07 | ||
| WO2021201171A1 (ja) | 2020-04-01 | 2021-10-07 | 国立大学法人京都大学 | 神経炎症の抑制、そのための組成物及び方法 |
| EP4129340A4 (en) * | 2020-04-01 | 2024-08-07 | Kyoto University | SUPPRESSION OF NEUROINFLAMMATION AND RELATED COMPOSITION AND METHOD |
| JP7699831B2 (ja) | 2020-04-01 | 2025-06-30 | 国立大学法人京都大学 | 神経炎症の抑制、そのための組成物及び方法 |
| WO2022255411A1 (ja) | 2021-06-01 | 2022-12-08 | 国立大学法人京都大学 | 自然免疫応答を増強させる方法 |
| WO2023008470A1 (ja) * | 2021-07-28 | 2023-02-02 | 住友ファーマ株式会社 | 縮環アミン誘導体 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2015107945A (ja) | 2015-06-11 |
| US20160303089A1 (en) | 2016-10-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2015083750A1 (ja) | 神経新生に関する化合物及び医薬組成物 | |
| US10017524B2 (en) | Compound and pharmaceutical composition for neuropsychological disorder or malignant tumor | |
| JP5820921B2 (ja) | 1,2−二置換複素環式化合物 | |
| CN103889953B (zh) | 作为防乙型肝炎病毒感染的抗病毒剂的氨磺酰苯甲酰胺衍生物 | |
| EP0946541B1 (en) | Quinolines and their therapeutic use | |
| US6353010B1 (en) | Bicyclic aryl carboxamides and their therapeutic use | |
| KR20100010894A (ko) | Dyrk를 저해하는 화합물을 함유하는 의약 조성물 | |
| JP2018198615A (ja) | 疾病の発症又は進行の一因となる異常スプライシングを抑制できる物質のスクリーニング方法 | |
| CN103189357B (zh) | 作为kcnq2/3调节剂的取代的2-氧代-和2-硫代-二氢喹啉-3-甲酰胺 | |
| JP7054524B2 (ja) | 神経新生を活性化するための組成物 | |
| US20240174650A1 (en) | 8-(picolinamide) substituted coumarin compound, and preparation method therefor and use thereof | |
| CN105829291B (zh) | 与疼痛有关的化合物及医药组合物 | |
| TW202214574A (zh) | 經取代之(呔-1-基甲基)脲類、經取代之n-(呔-1-基甲基)醯胺類及其類似物 | |
| CN106946873B (zh) | 一种面神经损伤的治疗药物及其制备方法 | |
| WO2015147204A1 (ja) | 血管新生増殖因子を阻害する医薬組成物 | |
| KR20200078921A (ko) | N-아실유레아 유도체를 함유하는 평활근세포 증식, 부착 또는 이동 억제용 조성물 | |
| KR20160137108A (ko) | 벤조옥사졸 또는 벤조티아졸 화합물, 그의 제조, 및 용도 | |
| WO2009153897A1 (ja) | ステモナミド合成中間体ならびにがんを予防および/または治療するための医薬組成物 | |
| TW202214585A (zh) | 經取代之異喹啉基甲基醯胺類、其類似物及使用其之方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14867512 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15101862 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14867512 Country of ref document: EP Kind code of ref document: A1 |