WO2015083426A1 - 非水系分散剤、カラーフィルタ用色材分散液、カラーフィルタ、液晶表示装置及び有機発光表示装置 - Google Patents
非水系分散剤、カラーフィルタ用色材分散液、カラーフィルタ、液晶表示装置及び有機発光表示装置 Download PDFInfo
- Publication number
- WO2015083426A1 WO2015083426A1 PCT/JP2014/076462 JP2014076462W WO2015083426A1 WO 2015083426 A1 WO2015083426 A1 WO 2015083426A1 JP 2014076462 W JP2014076462 W JP 2014076462W WO 2015083426 A1 WO2015083426 A1 WO 2015083426A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- general formula
- hydrogen atom
- structural unit
- unit represented
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B67/00—Influencing the physical, e.g. the dyeing or printing properties of dyestuffs without chemical reactions, e.g. by treating with solvents grinding or grinding assistants, coating of pigments or dyes; Process features in the making of dyestuff preparations; Dyestuff preparations of a special physical nature, e.g. tablets, films
- C09B67/0071—Process features in the making of dyestuff preparations; Dehydrating agents; Dispersing agents; Dustfree compositions
- C09B67/0084—Dispersions of dyes
- C09B67/0085—Non common dispersing agents
- C09B67/009—Non common dispersing agents polymeric dispersing agent
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F265/00—Macromolecular compounds obtained by polymerising monomers on to polymers of unsaturated monocarboxylic acids or derivatives thereof as defined in group C08F20/00
- C08F265/04—Macromolecular compounds obtained by polymerising monomers on to polymers of unsaturated monocarboxylic acids or derivatives thereof as defined in group C08F20/00 on to polymers of esters
- C08F265/06—Polymerisation of acrylate or methacrylate esters on to polymers thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F290/00—Macromolecular compounds obtained by polymerising monomers on to polymers modified by introduction of aliphatic unsaturated end or side groups
- C08F290/02—Macromolecular compounds obtained by polymerising monomers on to polymers modified by introduction of aliphatic unsaturated end or side groups on to polymers modified by introduction of unsaturated end groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F297/00—Macromolecular compounds obtained by successively polymerising different monomer systems using a catalyst of the ionic or coordination type without deactivating the intermediate polymer
- C08F297/02—Macromolecular compounds obtained by successively polymerising different monomer systems using a catalyst of the ionic or coordination type without deactivating the intermediate polymer using a catalyst of the anionic type
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F8/00—Chemical modification by after-treatment
- C08F8/40—Introducing phosphorus atoms or phosphorus-containing groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/17—Amines; Quaternary ammonium compounds
- C08K5/18—Amines; Quaternary ammonium compounds with aromatically bound amino groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L53/00—Compositions of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B11/00—Diaryl- or thriarylmethane dyes
- C09B11/04—Diaryl- or thriarylmethane dyes derived from triarylmethanes, i.e. central C-atom is substituted by amino, cyano, alkyl
- C09B11/10—Amino derivatives of triarylmethanes
- C09B11/12—Amino derivatives of triarylmethanes without any OH group bound to an aryl nucleus
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B11/00—Diaryl- or thriarylmethane dyes
- C09B11/04—Diaryl- or thriarylmethane dyes derived from triarylmethanes, i.e. central C-atom is substituted by amino, cyano, alkyl
- C09B11/10—Amino derivatives of triarylmethanes
- C09B11/24—Phthaleins containing amino groups ; Phthalanes; Fluoranes; Phthalides; Rhodamine dyes; Phthaleins having heterocyclic aryl rings; Lactone or lactame forms of triarylmethane dyes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B57/00—Other synthetic dyes of known constitution
- C09B57/14—Benzoxanthene dyes; Benzothioxanthene dyes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B63/00—Lakes
- C09B63/005—Metal lakes of dyes
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/20—Filters
- G02B5/201—Filters in the form of arrays
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/20—Filters
- G02B5/22—Absorbing filters
- G02B5/223—Absorbing filters containing organic substances, e.g. dyes, inks or pigments
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K59/00—Integrated devices, or assemblies of multiple devices, comprising at least one organic light-emitting element covered by group H10K50/00
- H10K59/30—Devices specially adapted for multicolour light emission
- H10K59/38—Devices specially adapted for multicolour light emission comprising colour filters or colour changing media [CCM]
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K71/00—Manufacture or treatment specially adapted for the organic devices covered by this subclass
- H10K71/10—Deposition of organic active material
- H10K71/12—Deposition of organic active material using liquid deposition, e.g. spin coating
- H10K71/15—Deposition of organic active material using liquid deposition, e.g. spin coating characterised by the solvent used
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/141—Organic polymers or oligomers comprising aliphatic or olefinic chains, e.g. poly N-vinylcarbazol, PVC or PTFE
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/151—Copolymers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
- H10K85/633—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine comprising polycyclic condensed aromatic hydrocarbons as substituents on the nitrogen atom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6574—Polycyclic condensed heteroaromatic hydrocarbons comprising only oxygen in the heteroaromatic polycondensed ring system, e.g. cumarine dyes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/0008—Organic ingredients according to more than one of the "one dot" groups of C08K5/01 - C08K5/59
- C08K5/0041—Optical brightening agents, organic pigments
-
- G—PHYSICS
- G02—OPTICS
- G02F—OPTICAL DEVICES OR ARRANGEMENTS FOR THE CONTROL OF LIGHT BY MODIFICATION OF THE OPTICAL PROPERTIES OF THE MEDIA OF THE ELEMENTS INVOLVED THEREIN; NON-LINEAR OPTICS; FREQUENCY-CHANGING OF LIGHT; OPTICAL LOGIC ELEMENTS; OPTICAL ANALOGUE/DIGITAL CONVERTERS
- G02F1/00—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics
- G02F1/01—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour
- G02F1/13—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour based on liquid crystals, e.g. single liquid crystal display cells
- G02F1/133—Constructional arrangements; Operation of liquid crystal cells; Circuit arrangements
- G02F1/1333—Constructional arrangements; Manufacturing methods
- G02F1/1335—Structural association of cells with optical devices, e.g. polarisers or reflectors
- G02F1/133509—Filters, e.g. light shielding masks
- G02F1/133514—Colour filters
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/626—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene containing more than one polycyclic condensed aromatic rings, e.g. bis-anthracene
Definitions
- the present invention relates to a non-aqueous dispersant, a color material dispersion for a color filter, a color filter, a liquid crystal display device and an organic light emitting display device.
- a backlight is used as a light source, the amount of light is controlled by electrically driving the liquid crystal, and color expression is performed by the light passing through a color filter. Therefore, a color filter must be present in the color representation of a liquid crystal television and plays a major role in determining the performance of the display.
- a color filter In the organic light emitting display device, when a color filter is used for the white light emitting organic light emitting element, a color image is formed as in the liquid crystal display device.
- An image display device including a color filter affects the design and performance of a mobile terminal in order to be directly linked to the usable time and charging frequency of the mobile terminal.
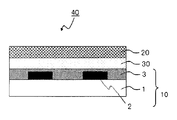
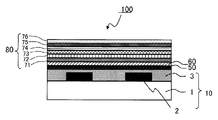
- the color filter is generally formed on a transparent substrate, a transparent layer formed on the transparent substrate, and composed of a colored layer of three primary colors of red, green, and blue, and on the transparent substrate so as to partition each colored pattern. And a light shielding portion formed.
- a pigment dispersion method using a pigment having excellent heat resistance and light resistance as a coloring material has been widely used.
- color filters using pigments it has become difficult to achieve the current demand for higher brightness.
- a colored resin composition for a color filter using a dye As a means for achieving high brightness, a colored resin composition for a color filter using a dye has been studied. Dyes generally have a higher transmittance than pigments and can produce high-intensity color filters, but they have poor heat resistance and have a problem that their chromaticity tends to change during high-temperature heating in the color filter production process. It was. In addition, the colored resin composition for color filters is also required to have improved alkali resistance so that the coating film does not peel off in an alkaline aqueous solution in the production process.
- Patent Document 1 discloses a colored photosensitive composition containing a dyed lake pigment, and examples of the dyed lake pigment include known pigments with color index numbers.
- Patent Document 2 discloses a color having a colorant composed of a copper phthalocyanine blue pigment and a metal lake pigment of a xanthene basic dye as a blue coloring composition for a color filter that enables high brightness and a wide color reproduction range. A blue coloring composition for a filter is disclosed. However, the colored layer using the coloring composition described in these materials has insufficient heat resistance of the lake pigment.
- Patent Document 3 discloses at least one selected from the group consisting of dyes and lake pigments as a coloring composition that is exhibited without losing the excellent chromaticity characteristics of the dyes and lake pigments even after a high-temperature heating step.
- a coloring composition characterized in that it contains a crosslinking agent characterized in that it contains a crosslinking agent.
- the technique of Patent Document 3 has a problem that the dispersibility of the lake pigment is poor, or the heat resistance of the lake pigment is insufficient and the alkali resistance is poor, as shown in a comparative example described later.
- the present invention has been made in view of the above circumstances, and is excellent in dispersibility and non-aqueous dispersant capable of forming a resin layer excellent in alkali resistance, dispersibility and heat resistance of a metal lake color material, Colorant dispersion for color filters capable of forming a coating film with high brightness and excellent alkali resistance, a color filter excellent in heat resistance, high brightness and excellent in alkali resistance, a liquid crystal display device having the color filter, and organic An object is to provide a light-emitting display device.
- the non-aqueous dispersant according to the present invention is represented by the following general formula (II) and at least one selected from the structural unit represented by the following general formula (I) and the structural unit represented by the following general formula (I ′). Or at least one selected from a structural unit represented by the following general formula (I) and a structural unit represented by the following general formula (I ′): It is a block copolymer having a block part and a block part containing a structural unit represented by the following general formula (III).
- L 1 is a direct bond or a divalent linking group
- R 1 is a hydrogen atom or a methyl group
- R 2 is a hydrocarbon group, — [CH ( R 6 ) —CH (R 7 ) —O] x1 —R 8 , or a monovalent group represented by — [(CH 2 ) y1 —O] z1 —R 8
- R 6 and R 7 are each independently A hydrogen atom or a methyl group
- R 8 is a hydrogen atom, a hydrocarbon group, —CHO, —CH 2 CHO, —CO—CH ⁇ CH 2 , —CO—C (CH 3 ) ⁇ CH 2 or —CH 2 COOR 9
- R 9 is a hydrogen atom or an alkyl group having 1 to 5 carbon atoms, and the hydrocarbon group may have a substituent
- x1 is an integer of 1 to 18, y1 represents an integer of 1 to 5, and
- z1 represents an integer
- X + represents an organic cation.
- L 2 is a direct bond or a divalent linking group
- R 3 is a hydrogen atom or a methyl group
- Polymer is a structural unit represented by the following general formula (IV) and a general formula (V ) Represents a polymer chain having one or more selected from structural units represented by:
- R 4 is a hydrogen atom or a methyl group
- R 5 is a hydrocarbon group, — [CH (R 10 ) —CH (R 11 ) —O] x2 —R 12 , — [(CH 2 ) y2 —O] z2 —R 12 , — [CO— (CH 2 ) y2 —O] z2 —R 12 , —CO—O—R 12 ′ or —O—CO—R 12 ′′
- R 10 and R 11 are each independently a hydrogen atom or a methyl group
- R 10 and R 11 are each independently a hydrogen atom or
- R 14 is a hydrogen atom or a methyl group
- R 15 is a hydrocarbon group, — [CH (R 16 ) —CH (R 17 ) —O] x3 — R 18 , — [(CH 2 ) y 3 —O] z 3 —R 18 , — [CO— (CH 2 ) y 3 —O] z 3 —R 18 , —CO—O—R 19 or —O—CO—R 20
- R 16 and R 17 are each independently a hydrogen atom or a methyl group
- R 18 is a hydrogen atom, a hydrocarbon group, —CHO, —CH 2 CHO or —CH 2 COOR 21
- R 19 is a hydrocarbon group, — [CH (R 16 ) —CH (R 17 ) —O] x4 —R 18 , — [(CH 2 ) y4 —O] z4 —R 18 ,
- n and n ′ represent an integer of 5 to 200.
- x3 and x4 are integers of 1 to 18, y3 and y4 are integers of 1 to 5, and z3 and z4 are integers of 1 to 18.
- a color material dispersion for a color filter according to the present invention contains (A) a color material, (B) a dispersant, and (C) a solvent.
- the (A) color material is a metal lake of a basic dye.
- a coloring material is contained, and the (B) dispersant is at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′) and the general formula ( II) or selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′). It is a block copolymer having a block part containing at least one kind and a block part containing a structural unit represented by the general formula (III).
- the color material dispersion for a color filter according to the present invention is particularly preferably used when the metal lake color material of the basic dye is a metal lake color material of a xanthene-based basic dye.
- the metal lake color material of the basic dye is a metal lake color material of a xanthene-based basic dye.
- a metal lake color material of xanthene-based basic dye such as CI Pigment Red 81
- the lake color material is likely to sublime in a high-temperature heating process at the time of manufacturing a color filter, as shown in a comparative example described later.
- sublimation in a high-temperature heating step of a metal lake color material of a xanthene-based basic dye can be suppressed.
- the color material (A) contains a color material (A-1) represented by the following general formula (VI), and the color material (A-1)
- the inside anion is preferably a polyacid anion containing at least tungsten from the viewpoint of high brightness and excellent heat resistance.
- A is an a-valent organic group in which the carbon atom directly bonded to N has no ⁇ bond, and the organic group is saturated aliphatic carbonized at least at the terminal directly bonded to N.
- B c- represents a polyvalent polyvalent R i to R v each independently represents a hydrogen atom, an optionally substituted alkyl group or an optionally substituted aryl group, R ii and R iii , R good .
- Ar 1 be iv and R v are bonded to form a ring structure represents a divalent aromatic group which may have a substituent.
- R i ⁇ R v and Ar 1 each They may be the same or different.
- a and c represent an integer of 2 or more, and b and d represent an integer of 1 or more.
- e is 0 or 1, and when e is 0, there is no bond.
- a plurality of e may be the same or different.
- the color filter according to the present invention is a color filter including at least a transparent substrate and a colored layer provided on the transparent substrate, wherein at least one of the colored layers includes (A) a color material, and (B). And (A) the coloring material contains a metal lake coloring material of a basic dye, and the (B) dispersing agent comprises the structural unit represented by the general formula (I) and the general It is a graft copolymer having at least one selected from the structural units represented by the formula (I ′) and the structural units represented by the general formula (II), or represented by the general formula (I). Block having at least one selected from the structural unit represented by formula (I ′) and the block unit comprising the structural unit represented by formula (III) It is a combination.
- the liquid crystal display device includes the color filter according to the present invention, a counter substrate, and a liquid crystal layer formed between the color filter and the counter substrate.
- the organic light emitting display device includes the color filter according to the present invention and an organic light emitter.
- the non-aqueous dispersant capable of forming a resin layer having excellent dispersibility and excellent alkali resistance, excellent dispersibility and heat resistance of the metal lake color material, high brightness, and excellent alkali resistance.
- a color material dispersion for a color filter capable of forming a coating film, a color filter having excellent heat resistance, high brightness, and excellent alkali resistance, a liquid crystal display device having the color filter, and an organic light emitting display device can be provided. .
- light includes electromagnetic waves having wavelengths in the visible and invisible regions, and further includes radiation, and the radiation includes, for example, microwaves and electron beams. Specifically, it means an electromagnetic wave having a wavelength of 5 ⁇ m or less and an electron beam.
- (meth) acryl represents each of acryl and methacryl
- (meth) acrylate represents each of acrylate and methacrylate.
- the organic group means a group having one or more carbon atoms.
- Non-aqueous dispersant includes at least one selected from a structural unit represented by the following general formula (I) and a structural unit represented by the following general formula (I ′) and the following general formula ( II) or a graft copolymer having a structural unit represented by the following general formula (I) and a structural unit represented by the following general formula (I ′) It is a block copolymer having a block part containing at least one kind and a block part containing a structural unit represented by the following general formula (III).
- L 1 is a direct bond or a divalent linking group
- R 1 is a hydrogen atom or a methyl group
- R 2 is a hydrocarbon group, — [CH ( R 6 ) —CH (R 7 ) —O] x1 —R 8 , or a monovalent group represented by — [(CH 2 ) y1 —O] z1 —R 8
- R 6 and R 7 are each independently A hydrogen atom or a methyl group
- R 8 is a hydrogen atom, a hydrocarbon group, —CHO, —CH 2 CHO, —CO—CH ⁇ CH 2 , —CO—C (CH 3 ) ⁇ CH 2 or —CH 2 COOR 9
- R 9 is a hydrogen atom or an alkyl group having 1 to 5 carbon atoms, and the hydrocarbon group may have a substituent
- x1 is an integer of 1 to 18, y1 represents an integer of 1 to 5, and
- z1 represents an integer
- X + represents an organic cation.
- L 2 is a direct bond or a divalent linking group
- R 3 is a hydrogen atom or a methyl group
- Polymer is a structural unit represented by the following general formula (IV) and a general formula (V ) Represents a polymer chain having one or more selected from structural units represented by:
- R 4 is a hydrogen atom or a methyl group
- R 5 is a hydrocarbon group, — [CH (R 10 ) —CH (R 11 ) —O] x2 —R 12 , — [(CH 2 ) y2 —O] z2 —R 12 , — [CO— (CH 2 ) y2 —O] z2 —R 12 , —CO—O—R 12 ′ or —O—CO—R 12 ′′
- R 10 and R 11 are each independently a hydrogen atom or a methyl group
- R 10 and R 11 are each independently a hydrogen atom or
- R 14 is a hydrogen atom or a methyl group
- R 15 is a hydrocarbon group, — [CH (R 16 ) —CH (R 17 ) —O] x3 — R 18 , — [(CH 2 ) y 3 —O] z 3 —R 18 , — [CO— (CH 2 ) y 3 —O] z 3 —R 18 , —CO—O—R 19 or —O—CO—R 20
- R 16 and R 17 are each independently a hydrogen atom or a methyl group
- R 18 is a hydrogen atom, a hydrocarbon group, —CHO, —CH 2 CHO or —CH 2 COOR 21
- R 19 is a hydrocarbon group, — [CH (R 16 ) —CH (R 17 ) —O] x4 —R 18 , — [(CH 2 ) y4 —O] z4 —R 18 ,
- n and n ′ represent an integer of 5 to 200.
- x3 and x4 are integers of 1 to 18, y3 and y4 are integers of 1 to 5, and z3 and z4 are integers of 1 to 18.
- the non-aqueous dispersant according to the present invention contains at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the following general formula (I ′), whereby particles such as a coloring material It is possible to form a resin layer with improved dispersibility and excellent alkali resistance.
- the non-aqueous dispersant according to the present invention has an effect of improving the heat resistance of a colored layer using the color material dispersion, particularly when a color material having low heat resistance such as a rake color material is dispersed. Even under high temperature heating such as post-baking, the change in chromaticity of the coloring material can be suppressed.
- a metal lake color material of xanthene-based basic dye such as CI Pigment Red 81
- the lake color material is likely to sublime in a high-temperature heating process at the time of manufacturing a color filter, as shown in a comparative example described later.
- a metal lake pigment of xanthene basic dye is used as a coloring material, although the spectral characteristics of the color filter are excellent, color transfer occurs in a furnace such as an oven during post-baking, and the interior of the furnace is contaminated, or another There has been a problem that the color material that has been transferred to the furnace during the formation of the colored layer is further transferred to the other colored layer, thereby reducing the luminance of the colored layer.
- non-aqueous dispersant according to the present invention when used in combination with a metal lake color material of xanthene basic dye, sublimation of the metal lake color material of xanthene basic dye is suppressed, and the above-mentioned color transfer is suppressed. Is done.
- the non-aqueous dispersant according to the present invention has high dispersibility, at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the following general formula (I ′).
- Coloring material in which acidic phosphorus compound group (—P ( ⁇ O) (— R 2 ) (OH)) and a salt thereof (—P ( ⁇ O) (— R 2 ) (O ⁇ X + )) are dispersed It is presumed that the adsorbing force on the particle surface is strong, while the graft chains and other block parts are highly soluble in non-aqueous solvents.
- the non-aqueous dispersant according to the present invention can form a resin layer excellent in alkali resistance because it has a structure in which a carbon atom is directly bonded to a phosphorus atom and is not easily hydrolyzed. Further, the non-aqueous dispersant according to the present invention has the effect of improving the heat resistance of a color material having a low heat resistance such as a lake color material.
- the graft copolymer has at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′) and the structure represented by the general formula (II). With units. ⁇ Structural Unit Represented by General Formula (I) and Structural Unit Represented by General Formula (I ′)>
- L 1 is a direct bond or a divalent linking group.
- the direct bond means that the phosphorus atom is directly bonded to the carbon atom of the main chain skeleton without a linking group.
- the divalent linking group in L 1 is not particularly limited as long as it can link the carbon atom of the main chain skeleton and the phosphorus atom.
- Examples of the divalent linking group for L 1 include a linear, branched or cyclic alkylene group, a linear, branched or cyclic alkylene group having a hydroxyl group, an arylene group, a —CONH— group, a —COO— group, And —NHCOO— group, ether group (—O— group), thioether group (—S— group), and combinations thereof.
- the direction of bonding of the divalent linking group is arbitrary.
- —CO when the divalent linking group includes —CONH—, —CO may be on the carbon atom side of the main chain and —NH may be on the phosphorus atom side of the side chain, and on the contrary, —NH may be on the main chain. On the carbon atom side, —CO may be on the phosphorus atom side of the side chain.
- L 1 in formulas (I) and (I ′) is preferably a divalent linking group containing a —CONH— group or a —COO— group.
- L 1 is a divalent linking group containing a —COO— group
- the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′) are each represented by the following formula (I And a structure represented by the following formula (I′-1).
- R 1 and R 2 are the same as in general formula (I) and general formula (I ′), and X + represents general formula (I ′) is the same, and L 1 ′ is an alkylene group having 1 to 8 carbon atoms which may have a hydroxyl group, — [CH (R a ) —CH (R b ) —O] x —, Or — [(CH 2 ) y —O] z — (CH 2 ) y —O—, — [CH (R c )] w —O—, wherein R a , R b and R c are each independently Is a hydrogen atom, a methyl group or a hydroxyl group, x is an integer of 1 to 18, y is an integer of 1 to 5, z is an integer of 1 to 18, and w is an integer of 1 to 18.)
- the alkylene group having 1 to 8 carbon atoms in L 1 ′ may be linear, branched, or cyclic, and examples thereof include a methylene group, an ethylene group, a trimethylene group, a propylene group, various butylene groups, and various types.
- a pentylene group, various hexylene groups, various octylene groups, and the like, and some hydrogens may be substituted with hydroxyl groups.
- x is an integer of 1 to 18, preferably an integer of 1 to 4, more preferably an integer of 1 to 2
- y is an integer of 1 to 5, preferably an integer of 1 to 4, more preferably 2 or 3. is there.
- z is an integer of 1 to 18, preferably an integer of 1 to 4, more preferably an integer of 1 to 2.
- w is an integer of 1 to 18, preferably an integer of 1 to 4.
- L 1 in the general formula (I) and the general formula (I ′) include, for example, —COO—CH 2 CH (OH) CH 2 —O—, —COO—CH 2 CH 2 —O—.
- Examples include, but are not limited to, CH 2 CH (OH) CH 2 —O—, —COO—CH 2 C (CH 2 CH 3 ) (CH 2 OH) CH 2 —O—, and the like.
- Examples of the hydrocarbon group for R 2 include an alkyl group having 1 to 18 carbon atoms, an alkenyl group having 2 to 18 carbon atoms, an aralkyl group, and an aryl group.
- the alkyl group having 1 to 18 carbon atoms may be linear, branched or cyclic, for example, methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, cyclopentyl group. Cyclohexyl group, bornyl group, isobornyl group, dicyclopentanyl group, adamantyl group, lower alkyl group-substituted adamantyl group and the like.
- the alkenyl group having 2 to 18 carbon atoms may be linear, branched or cyclic.
- Examples of such an alkenyl group include a vinyl group, an allyl group, and a propenyl group.
- the position of the double bond of the alkenyl group is not limited, but from the viewpoint of the reactivity of the polymer obtained, it is preferable that there is a double bond at the terminal of the alkenyl group.
- the aryl group include a phenyl group, a biphenyl group, a naphthyl group, a tolyl group, and a xylyl group, and may further have a substituent.
- the aryl group preferably has 6 to 24 carbon atoms, more preferably 6 to 12 carbon atoms.
- a benzyl group, a phenethyl group, a naphthylmethyl group, a biphenylmethyl group, etc. are mentioned, Furthermore, you may have a substituent.
- the aralkyl group preferably has 7 to 20 carbon atoms, more preferably 7 to 14 carbon atoms.
- the alkyl group or alkenyl group may have a substituent, and examples of the substituent include halogen atoms such as F, Cl, and Br, and nitro groups.
- examples of the substituent of the aromatic ring such as the aryl group and the aralkyl group include straight chain and branched alkyl groups having 1 to 4 carbon atoms, alkenyl groups, nitro groups, and halogen atoms. .
- the preferred carbon number does not include the carbon number of the substituent.
- x1 is the same as x
- y1 is the same as y
- z1 is the same as z.
- examples of the hydrocarbon group for R 8 include the same hydrocarbon groups as those described above for R 2 .
- R 2 in the general formula (I) is a methyl group, an ethyl group, an aryl group or an aralkyl group which may have a substituent, a vinyl group,
- An allyl group a monovalent group represented by — [CH (R 6 ) —CH (R 7 ) —O] x1 —R 8 , or — [(CH 2 ) y1 —O] z1 —R 8 , R 6 and Dispersibility and dispersion stability of particles in which R 7 is each independently a hydrogen atom or a methyl group, and R 8 is —CO—CH ⁇ CH 2 or —CO—C (CH 3 ) ⁇ CH 2 It is preferable from the point which is excellent in property. Among these, an aryl group which may have a substituent is more preferable from the viewpoint of dispersibility.
- the structural unit represented by the general formula (I) may be contained alone or in combination of two or more in the poly
- X + represents an organic cation.
- An organic cation refers to one containing a carbon atom in the cation moiety.
- Examples of the organic cation include imidazolium cation, pyridinium cation, aminidium cation, piperidinium cation, pyrrolidinium cation, ammonium cation such as tetraalkylammonium cation and trialkylammonium cation, and sulfonium cation such as trialkylsulfonium cation.
- phosphonium cations such as tetraalkylphosphonium cations.
- a protonated nitrogen-containing organic cation is preferable from the viewpoint of dispersibility and alkali developability.
- an organic cation has an ethylenically unsaturated double bond, it is preferable from the point which can provide sclerosis
- R 2 in the general formula (I ′) is a methyl group, an ethyl group, an aryl group or an aralkyl group which may have a substituent, vinyl.
- Group, an allyl group, a monovalent group represented by — [CH (R 6 ) —CH (R 7 ) —O] x1 —R 8 , or — [(CH 2 ) y1 —O] z1 —R 8 , R 6 and R 7 are each independently a hydrogen atom or a methyl group
- R 8 is —CO—CH ⁇ CH 2 or —CO—C (CH 3 ) ⁇ CH 2
- X + is a protonated nitrogen-containing nitrogen organic cations, those which are inter alia protonated imidazolium cation, preferably from the viewpoint of excellent dispersibility and dispersion stability and alkali developability of the particles to be dispersed, among others, be R 2 is
- the non-aqueous dispersant of the present invention may contain both the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′).
- both of the structural units are included, there is no particular limitation as long as good dispersibility and dispersion stability are exhibited, and the ratio of the number of structural units represented by the general formula (I ′) is:
- the content is preferably 0 to 50 mol% based on the total number of structural units of the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′).
- L 2 is a direct bond or a divalent linking group.
- the divalent linking group in L 2 is not particularly limited as long as it can link a carbon atom derived from an ethylenically unsaturated double bond and a polymer chain.
- Examples of the divalent linking group for L 2 include the same divalent linking groups as those described above for L 1 .
- Polymer represents a polymer chain having at least one structural unit represented by the general formula (IV) or the general formula (V).
- R 14 is a hydrogen atom or a methyl group
- R 15 is a hydrocarbon group
- — [CH (R 16 ) —CH (R 17 ) —O] x3 —R 18 , — [(CH 2 ) Y3 —O] z3 —R 18 , — [CO— (CH 2 ) y3 —O] z3 —R 18 , —CO—O—R 19 or —O—CO—R 20 is there.
- the hydrocarbon group for R 15 is preferably an alkyl group having 1 to 18 carbon atoms, an alkenyl group having 2 to 18 carbon atoms, an aralkyl group, or an aryl group. These include, for example, those similar to R 2 described above.
- R 18 is a hydrogen atom or a monovalent group represented by an alkyl group having 1 to 18 carbon atoms, an aralkyl group, an aryl group, —CHO, —CH 2 CHO, or —CH 2 COOR 21
- R 19 is An alkyl group having 1 to 18 carbon atoms, an aralkyl group, an aryl group, — [CH (R 16 ) —CH (R 17 ) —O] x4 —R 18 , — [(CH 2 ) y4 —O] z4 —R 18 , — [CO— (CH 2 ) y4 —O] z4 —R 18 is a monovalent group.
- R 20 represents an alkyl group having 1 to 18 carbon atoms
- R 21 represents a hydrogen atom or an alkyl group having 1 to 5 carbon atoms.
- the alkyl group, aralkyl group, and aryl group having 1 to 18 carbon atoms are as described above for R 2 .
- the alkyl group in R 20 and R 21 is as shown for R 2 above.
- R 15 , R 18 , R 19 and R 20 are groups having an aromatic ring, the aromatic ring may further have a substituent.
- substituents examples include linear, branched, and cyclic alkyl groups having 1 to 5 carbon atoms, as well as halogen atoms such as alkenyl groups, nitro groups, F, Cl, and Br.
- the preferred carbon number does not include the carbon number of the substituent.
- x3 and x4 are the same as x
- y3 and y4 are the same as y
- z3 and z4 are the same as z.
- R 15 , R 18 , R 19 , and R 20 are within a range that does not hinder the dispersion performance of the graft copolymer, and further, an alkoxy group, a hydroxyl group, a carboxyl group, an amino group, an epoxy group, and an isocyanate group. Or a substituent such as a hydrogen bond-forming group. Moreover, after synthesizing a graft copolymer having these substituents, a compound having a functional group that reacts with the substituent and a polymerizable group may be reacted to add a polymerizable group.
- adding a polymerizable group by reacting a graft copolymer having a carboxyl group with glycidyl (meth) acrylate, or reacting a graft copolymer having an isocyanate group with hydroxyethyl (meth) acrylate.
- a polymerizable group by reacting a graft copolymer having a carboxyl group with glycidyl (meth) acrylate, or reacting a graft copolymer having an isocyanate group with hydroxyethyl (meth) acrylate.
- the polymer chain contained in the structural unit represented by the general formula (IV) includes methyl (meth) acrylate, ethyl (meth) acrylate, isopropyl (meth) acrylate, n-butyl (meth) acrylate, among the structural units described above.
- Those having a structural unit derived from (meth) acrylate, dicyclopentanyl (meth) acrylate, adamantyl (meth) acrylate, styrene, ⁇ -methylstyrene, vinylcyclohexane and the like are preferable. However, it is not limited to these.
- m is an integer of 1 to 5, preferably an integer of 2 to 5, more preferably an integer of 4 or 5.
- the number of units n and n ′ of the structural units of the polymer chain may be an integer of 5 to 200, and is not particularly limited, but is preferably within the range of 5 to 100.
- R 15 and R 19 those having excellent solubility with an organic solvent described later are preferably used, and can be appropriately selected according to the organic solvent used in the colorant dispersion. good.
- the organic solvent is an organic solvent such as an ether alcohol acetate type, an ether type, an ester type or the like generally used as an organic solvent for a colorant dispersion
- a methyl group, an ethyl group, Group, isobutyl group, n-butyl group, 2-ethylhexyl group, benzyl group and the like are preferable.
- the reason for setting the R 15 and R 19 in this way is that the structural unit containing the R 15 and R 19 has solubility in the organic solvent, and the acidic phosphorus compound group of the monomer and This is because the dispersibility and stability of the particles such as the color material can be made particularly excellent because the salt portion has high adsorptivity to the particles such as the color material.
- the mass average molecular weight Mw of the polymer chain in Polymer is preferably in the range of 500 to 15000, and more preferably in the range of 1000 to 8000.
- the polymer chain in Polymer preferably has a solubility at 23 ° C. of 50 (g / 100 g solvent) or more with respect to the organic solvent used in combination.
- the solubility of the polymer chain can be based on the fact that the raw material into which the polymer chain is introduced when preparing the graft copolymer has the solubility. For example, when a polymerizable oligomer containing a polymer chain and a group having an ethylenically unsaturated double bond at its end is used to introduce a polymer chain into the graft copolymer, the polymerizable oligomer has the solubility. Just do it.
- a polymer chain containing a reactive group capable of reacting with a reactive group contained in the copolymer is used.
- the polymer chain containing the reactive group may have the solubility.
- the polymer chain may be a homopolymer or a copolymer. Moreover, the polymer chain contained in the structural unit represented by the general formula (II) may be used alone or in combination of two or more in the graft copolymer.
- the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′) are included in a proportion of 3 to 80% by mass. It is preferably 5 to 50% by mass, more preferably 10 to 40% by mass. If the total content of the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′) in the graft copolymer is within the above range, the particles in the graft copolymer Since the ratio of the affinity part is appropriate and the decrease in solubility in an organic solvent can be suppressed, the adsorptivity to particles such as a coloring material is improved, and excellent dispersibility and dispersion stability are obtained.
- the structural unit represented by the general formula (II) is preferably contained in a proportion of 20 to 97% by mass, more preferably 50 to 95% by mass, and 60 to 90 mass% is more preferable.
- the content ratio of the structural unit is represented by at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′), and the general formula (II). It is calculated from the charged amount when synthesizing the graft copolymer having the structural unit.
- the mass average molecular weight Mw of the graft copolymer is preferably in the range of 1000 to 100,000, more preferably in the range of 3000 to 30000, and further in the range of 5000 to 20000. preferable. By being in the above range, particles such as a coloring material can be uniformly dispersed.
- the mass average molecular weight Mw is a value measured by GPC (gel permeation chromatography).
- HLC-8120GPC manufactured by Tosoh was used, the elution solvent was N-methylpyrrolidone to which 0.01 mol / liter of lithium bromide was added, and the polystyrene standards for calibration curves were Mw377400, 210500, 96000, 50400, 20650, 10850, 5460, 2930, 1300, 580 (Easy PS-2 series manufactured by Polymer Laboratories) and Mw1090000 (manufactured by Tosoh), and the measurement column was TSK-GEL ALPHA-M ⁇ 2 (manufactured by Tosoh) It is.
- the graft copolymer used in the present invention has a constitutional unit represented by the general formula (I), a constitutional unit represented by the general formula (I ′), and a constitution represented by the general formula (II). In addition to the units, other structural units may be included. It is copolymerizable with an ethylenically unsaturated double bond-containing monomer or the like that derives at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′). An ethylenically unsaturated double bond-containing monomer can be appropriately selected and copolymerized to introduce other structural units.
- the method for producing the graft copolymer includes at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′), and the general There is no particular limitation as long as it is a method capable of producing a graft copolymer having a structural unit represented by formula (II).
- a graft copolymer having the structural unit represented by the general formula (I) and the structural unit represented by the general formula (II) for example, a monomer represented by the following general formula (Ia) And a polymerizable oligomer comprising a polymer chain having at least one structural unit represented by the general formula (IV) or the general formula (V) and a group having an ethylenically unsaturated double bond at its terminal.
- an organic phosphonic acid (R 2 P ( ⁇ O) (OH) 2 ) having a desired structure is added to a structural unit derived from a monomer having a reactive group, whereby the general formula (I)
- the structure represented by Examples of the unit include a unit.
- the graft copolymer which has a structural unit represented by the said general formula (I '), and a structural unit represented by the said general formula (II), it represents with the said general formula (I).
- a salt forming agent including an organic cation is further added, and the mixture is heated and stirred as necessary.
- the salt forming agent may be appropriately selected according to the organic cation to be introduced.
- the addition amount of the salt forming agent is not particularly limited as long as good dispersibility and dispersion stability are exhibited, and may be appropriately adjusted according to the proportion of the structural unit to be introduced, but is generally represented by the general formula (I).
- the amount is about 0.05 to 1.00 molar equivalent, preferably 0.3 to 0.5 molar equivalent, relative to the phosphorus moiety contained in the structural unit. If necessary, other monomers can also be used to produce a graft copolymer using a known polymerization means.
- a polymer chain may be introduced using a polymer chain.
- a functional group that reacts with the substituent is added.
- the polymer chain may be introduced by reacting with the polymer chain contained therein.
- a copolymer having a glycidyl group at the side chain is reacted with a polymer chain having a carboxyl group at the terminal, or a copolymer having an isocyanate group at the side chain is reacted with a polymer chain having a hydroxy group at the terminal.
- polymer chains can be introduced.
- additives generally used in the polymerization for example, a polymerization initiator, a dispersion stabilizer, a chain transfer agent and the like may be used.
- Examples of the method for producing the monomer represented by the general formula (Ia) include an organic phosphonic acid compound having a desired structure, a glycidyl group, an alicyclic epoxy group, an oxetane group, a hydroxyl group, and the like, and an ethylenically unsaturated group. The method of making it react with the compound which has a double bond is mentioned.
- the block copolymer includes a block part containing at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′), and the general formula (III). And a block portion including a structural unit represented.
- the block copolymer has a block part containing at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′). Since the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′) are as described above, description thereof is omitted here.
- the structural unit represented by the general formula (I) and the general formula (I ′) are as described above, description thereof is omitted here.
- the structural unit represented by the general formula (I) and the general formula ( The total number of structural units represented by I ′) is preferably 3 or more.
- At least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′) is only required to function as a colorant affinity site, and is composed of one type.
- two or more structural units may be included.
- the block part containing at least one kind selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′) has two or more kinds in the block portion.
- the structural units may be arranged at random.
- the total content of the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′) is 100 masses of all the structural units of the block copolymer.
- the content rate of the said structural unit is computed from the preparation amount at the time of synthesize
- the block copolymer has a block portion containing the structural unit represented by the general formula (III), thereby improving the solvent affinity, the dispersibility and dispersion stability of the coloring material, and the heat resistance. The property is also good.
- R 5 is a hydrocarbon group, — [CH (R 10 ) —CH (R 11 ) —O] x2 —R 12 , — [(CH 2 ) y2 —O] z2 —R 12 , — [CO— (CH 2 ) y2 —O] z2 —R 12 , —CO—O—R 12 ′, or —O—CO—R 12 ′′ .
- the hydrocarbon group for R 5 can be the same as that shown for R 2 above.
- R 12 represents a hydrogen atom, a hydrocarbon group, a monovalent group represented by —CHO, —CH 2 CHO, or —CH 2 COOR 13
- R 12 ′ represents a hydrocarbon group, — [CH ( R 10 ) —CH (R 11 ) —O] x2 ′ —R 12 , — [(CH 2 ) y2 ′ —O] z2 ′ —R 12 , — [CO— (CH 2 ) y2 ′ —O] z2 ′ —R 12 is a monovalent group
- R 12 ′′ is an alkyl group having 1 to 18 carbon atoms
- R 13 is a hydrogen atom or an alkyl group having 1 to 5 carbon atoms
- the hydrocarbon group is substituted It may have a group.
- the hydrocarbon group for R 12 can be the same as that shown for R 2 .
- x2 and x2 ′ are the same as x
- y2 and y2 ′ are the same as y
- z2 and z2 ′ are the same as z.
- R 5 in the structural unit represented by the general formula (III) may be the same or different from each other.
- R 5 it is preferable to use a material having excellent solubility with a solvent described later, and examples thereof include those similar to R 15 .
- R 5 is substituted with a substituent such as an alkoxy group, a hydroxyl group, a carboxyl group, an amino group, an epoxy group, an isocyanate group, or a hydrogen bond-forming group as long as it does not hinder the dispersion performance of the block copolymer.
- the substituent may be added by reacting with the compound having the substituent.
- a compound having a functional group that reacts with the substituent and a polymerizable group may be reacted to add a polymerizable group.
- adding a polymerizable group by reacting a block copolymer having a glycidyl group with (meth) acrylic acid or reacting a block copolymer having an isocyanate group with hydroxyethyl (meth) acrylate. Can do.
- the number of structural units constituting the block part including the structural unit represented by the general formula (III) is not particularly limited, but the solvent affinity part and the colorant affinity part effectively act, and the colorant dispersion liquid From the viewpoint of improving dispersibility, it is preferably 10 to 200, more preferably 10 to 100, and even more preferably 10 to 70.
- the content of the structural unit represented by the general formula (III) is 40 to 95% by mass when the total structural unit of the block copolymer is 100% by mass. Preferably, it is 60 to 90% by mass or more.
- the content rate of the said structural unit is computed from the preparation amount at the time of synthesize
- the block part including the structural unit represented by the general formula (III) may be selected so as to function as a solvent affinity site, and the structural unit represented by the general formula (III) is composed of one kind. There may be two or more structural units. In the present invention, when the structural unit represented by the general formula (III) includes two or more structural units, the block unit including the structural unit represented by the general formula (III) has two or more structural units. The units may be arranged at random.
- the number of units of the structural unit of the block part containing at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′) is preferably in the range of 0.01 to 1, 0.05 It is more preferable that it is in the range of -0.7 from the viewpoint of the dispersibility and dispersion stability of the color material.
- a block part containing at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′) and the general formula ( III) is not particularly limited as long as it can stably disperse the colorant, and the structural unit represented by the general formula (I) and The block part containing at least one selected from the structural unit represented by the general formula (I ′) is bonded to only one end of the block copolymer, and is excellent in interaction with the colorant and dispersed. It is preferable from the point which can suppress effectively aggregation of agents.
- the mass average molecular weight of the block copolymer is not particularly limited, but is preferably 2500 to 20000, more preferably 3000 to 12000, from the viewpoint of good dispersibility and excellent heat resistance. Further, it is more preferably 5000 to 10,000.
- the block unit containing at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′).
- a block copolymer having a block part containing the structural unit represented by the general formula (III) may be used, and the method is not particularly limited.
- a monomer having a reactive group such as a cyclic epoxy group, an oxetane group or a hydroxyl group and an ethylenically unsaturated double bond
- a monomer for deriving the structural unit represented by the general formula (III) can be used for living polymerization, etc.
- an organic phosphonic acid (R 2 P ( ⁇ O) (OH) 2 ) having a desired structure is derived from the monomer having the reactive group.
- Composition unit A method of forming the structural unit represented by the general formula (I) by adding to the above can be mentioned.
- a salt forming agent including an organic cation is further added, and the mixture is heated and stirred as necessary.
- a salt between the acidic phosphorus compound group of the structural unit represented by the general formula (I) and the organic cation to obtain the structural unit represented by the general formula (I ′) Is mentioned.
- the salt forming agent the same salt forming agent as that used in the production of the graft copolymer can be used, and the content ratio of the salt forming agent is the same as that in the production of the graft copolymer. be able to.
- the particles dispersible with the dispersant according to the present invention are not limited to the color materials described below.
- the dispersant of the present invention can suitably disperse, for example, metal particles, metal oxides, pigments and the like.
- the dispersant of the present invention has a high affinity for the constituent unit represented by the general formula (I) and the constituent unit represented by the general formula (I ′) with the dispersed particles.
- the color material is dispersed, the color material is excellent in dispersibility and dispersion stability, and fading due to oxidation can be prevented.
- the color material dispersion for color filter according to the present invention contains (A) a color material, (B) a dispersant, and (C) a solvent.
- a basic dye metal lake colorant, and the dispersant (B) is selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′). It is a graft copolymer having at least one kind and a structural unit represented by the general formula (II), or represented by the structural unit represented by the general formula (I) and the general formula (I ′). It is a block copolymer having a block part containing at least one kind selected from structural units and a block part containing a structural unit represented by the general formula (III).
- the color material dispersion for a color filter according to the present invention is excellent in dispersibility and heat resistance of a metal lake color material, and can form a coating film having high brightness and excellent alkali resistance.
- the color material dispersion for a color filter according to the present invention is selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′) of the specific (B) dispersant.
- the color material dispersion for a color filter (A) the color material is used by being dispersed in (C) a solvent with (B) a dispersant.
- the (A) coloring material used in the present invention contains a metal lake coloring material of a basic dye.
- the basic dye metal lake color material is composed of a cation portion of a basic dye and an anion portion of a metal lake agent.
- the basic dye is an ionic dye having a cation moiety as a chromophore, such as an azine dye, an oxazine dye, a thiazine dye, an azo dye, an anthraquinone dye, a xanthene dye, or a triarylmethane dye.
- dyes phthalocyanine dyes, auramine dyes, acridine dyes, methine dyes, and the like. Specific examples include those with the following color index (CI) names.
- Phthalocyanine dyes such as Basic Blue 140; C. I. Auramine dyes such as basic yellow 2, 3, 37; C. I. Basic Yellow 5, 6, 7, 9, C.I. I. Acridine dyes such as Basic Orange 4, 5, 14, 15, 16, 17, 18, 19, 2; C. I. Basic Red 12, 13, 14, 15, 27, 28, 37, 52, 90, C.I. I. Basic Blue 62, 63, C.I. I. Basic yellow 11, 13, 21, 22, 28, 29, 49, 51, 52, 53, C.I. I. Methine dyes such as Basic Violet 7, 15, 16, 20, 21, 22 etc.
- the metal lake agent is not particularly limited as long as a metal is contained in the anion portion, and an anion of an oxo acid such as chromate ion, tungstate ion (WO 4 2 ⁇ ), molybdate ion (MoO 4 2 ⁇ ) or the like.
- polyacid anions such as chromate ion, tungstate ion (WO 4 2 ⁇ ), molybdate ion (MoO 4 2 ⁇ ) or the like.
- polyacid anions is preferable from the viewpoint of improving heat resistance and light resistance.
- the polyacid is an acid obtained by condensing a plurality of oxo acids.
- the polyacid anion may be an isopolyacid ion (M m O n ) d- or a heteropoly acid ion (X 1 M m O n ) d- .
- M represents a poly atom
- X represents a hetero atom
- m represents a composition ratio of the poly atom
- n represents a composition ratio of the oxygen atom.
- the poly atom M include Mo, W, V, Ti, and Nb.
- the hetero atom X include Si, P, As, S, Fe, and Co.
- a counter cation such as Na + or H + may be partially included.
- a polyacid anion containing at least one of tungsten (W) and molybdenum (Mo) is preferably used.
- polyacid anions include tungstate ions [W 10 O 32 ] 4 ⁇ , phosphotungstate ions [PW 12 O 40 ] 3 ⁇ , [P 2 W 18 O 62 ] 6 ⁇ , tungsten carbide.
- C. I. Pigment red 81 C.I. I. Pigment red 81: 1, C.I. I. Pigment red 81: 2, C.I. I. Pigment red 81: 3, C.I. I. Pigment red 81: 4, C.I. I. Pigment red 81: 5, C.I. I. Pigment red 82, C.I. I. Pigment red 169, C.I. I. Pigment violet 1, C.I. I. Pigment violet 2, C.I. I. Xanthene-based metal lake colorant such as CI Pigment Violet 2: 1; C. I. Pigment blue 1, C.I. I.
- Pigment blue 1 2, C.I. I. Pigment blue 2, C.I. I. Pigment blue 3, C.I. I. Pigment blue 8, C.I. I. Pigment blue 9, C.I. I. Pigment blue 10, C.I. I. Pigment blue 11, C.I. I. Pigment blue 12, C.I. I. Pigment blue 14, C.I. I. Pigment blue 53, C.I. I. Pigment blue 62, C.I. I. Pigment violet 3, C.I. I. Pigment violet 3: 1, C.I. I. Pigment violet 3: 3, C.I. I. Pigment violet 27, C.I. I. Pigment violet 39, C.I. I. Pigment green 1, C.I. I. Pigment green 2, C.I. I. Pigment green 3, C.I. I. Triarylmethane-based metal lake colorant such as CI Pigment Green 4.
- a metal lake color material using a triarylmethane dye or a xanthene basic dye as a basic dye is preferably used from the viewpoint of achieving high brightness of the colored layer.
- the metal lake color of the xanthene-based basic dye can be suppressed because sublimation of the color material can be suppressed.
- a material is preferably used.
- a color material (A-2) represented by the following general formula (VII) is preferable.
- B ′ c′— represents a c′- valent polyacid anion.
- R vi to R ix each independently represents a hydrogen atom, an alkyl group which may have a substituent, or a substituted group.
- X 1 and X 2 each independently have a hydrogen atom, a halogen atom or a substituent.
- Y represents an optionally substituted alkyl group, and Y represents a hydrogen atom, an optionally substituted alkyl group, an optionally substituted aryl group, or an optionally substituted aralkyl.
- c ′ represents a number of 2 or more
- f and g represent a number of 1 or more.
- Examples of the alkyl group in R vi to R ix include a linear or branched alkyl group having 1 to 12 carbon atoms, and among them, a linear or branched alkyl group having 1 to 8 carbon atoms. A linear or branched alkyl group having 1 to 5 carbon atoms is more preferable from the viewpoint of luminance and heat resistance.
- the alkyl group in R vi to R ix is particularly preferably an ethyl group or a methyl group.
- the substituent that the alkyl group may have is not particularly limited, and examples thereof include an aryl group, a halogen atom, and a hydroxyl group, and examples of the substituted alkyl group include a benzyl group.
- Examples of the aryl group in R vi to R ix include an aryl group having 6 to 12 carbon atoms, and specific examples of the aryl group include a phenyl group and a naphthyl group. Examples of the substituent that the aryl group may have include an alkyl group and a halogen atom. Examples of the aralkyl group in R vi to R ix include an aralkyl group having 7 to 16 carbon atoms, and specific examples include a benzyl group, a phenethyl group, a naphthylmethyl group, and a biphenylmethyl group.
- R vi and R vii , R viii and R ix are combined to form a ring structure.
- R vi and R vii , and R viii and R ix form a ring structure via a nitrogen atom.
- the ring structure is not particularly limited, and examples thereof include a pyrrolidine ring, a piperidine ring, and a morpholine ring.
- the alkyl group in X 1 and X 2 can be the same as the alkyl group in R vi to R ix .
- a halogen atom in X ⁇ 1 > and X ⁇ 2 > a fluorine atom, a chlorine atom, and a bromine atom are mentioned.
- the alkyl group, aryl group and aralkyl group in Y can be the same as those in R vi to R ix .
- the substitution position of the —COOY group of the benzene ring bonded to the xanthene skeleton is not particularly limited, but is preferably in the ortho or para position with respect to the xanthene skeleton, It is preferable from the viewpoint of heat resistance and light resistance that the COOY group is substituted in the ortho position with respect to the xanthene skeleton.
- the -COOY group when it is in the ortho position, it can resonate with the carbon atom of the xanthene skeleton to which the benzene ring is bonded to form a ring structure, which improves heat resistance and light resistance. It is estimated that.
- cation skeleton in the general formula (VII) include C.I. I. Basic Red 1 (Rhodamine 6G), C.I. I. Basic Red 1: 1, C.I. I. Basic Red 3, C.I. I. Basic Red 4, C.I. I. Basic Red 8, C.I. I. Basic Violet 10 (Rhodamine B), C.I. I. Basic Violet 11 (Rhodamine 3B), C.I. I. Basic violet 11: 1 (rhodamine A) and the like can be mentioned, and rhodamine 6G, rhodamine A and rhodamine B are preferable from the viewpoint of luminance and heat resistance.
- the polyacid anion as described above can be appropriately used.
- a polyacid anion containing at least tungsten is preferable.
- the molar ratio of tungsten to molybdenum is preferably 100: 0 to 85:15 from the viewpoint of heat resistance and light resistance. Of these, 100: 0 to 90:10 is preferable from the viewpoint of heat resistance.
- the polyacid anion in the colorant (A-2) can be used alone or in combination of two or more of the above-mentioned polyacid anions.
- the molar ratio of tungsten and molybdenum may be within the above range. Further, in order to adjust the molar ratio of tungsten to molybdenum, phosphomolybdate ions, silicomolybdate ions, etc. that do not contain tungsten may be used in combination.
- the color material (A) contains a color material (A-1) represented by the following general formula (VI), and the anion in the color material (A-1) is at least A polyacid anion containing tungsten is preferable from the viewpoint of increasing the brightness of the color filter and heat resistance.
- a color material (A-1) represented by the following general formula (VI) the anion in the color material (A-1) is at least A polyacid anion containing tungsten is preferable from the viewpoint of increasing the brightness of the color filter and heat resistance.
- A is an a-valent organic group in which the carbon atom directly bonded to N has no ⁇ bond, and the organic group is saturated aliphatic carbonized at least at the terminal directly bonded to N.
- B c- represents a polyvalent polyvalent R i to R v each independently represents a hydrogen atom, an optionally substituted alkyl group or an optionally substituted aryl group, R ii and R iii , R good .
- Ar 1 be iv and R v are bonded to form a ring structure represents a divalent aromatic group which may have a substituent.
- R i ⁇ R v and Ar 1 each They may be the same or different.
- a and c represent an integer of 2 or more, and b and d represent an integer of 1 or more.
- e is 0 or 1, and when e is 0, there is no bond.
- a plurality of e may be the same or different.
- the color material (A-1) contains a divalent or higher valent anion and a divalent or higher cation, in the aggregate of the color material (A-1), the anion and the cation are simply one molecule to one molecule. It is presumed that they form a molecular aggregate in which a plurality of molecules are associated via ionic bonds, rather than ionic bonds. Therefore, the apparent molecular weight of the coloring material (A-1) is remarkably increased as compared with the molecular weight of the conventional lake pigment. It is presumed that the formation of such molecular aggregates further increases the cohesive force in the solid state, lowers the thermal motion, suppresses dissociation of ion pairs and decomposition of the cation moiety, and improves heat resistance and light resistance.
- a in the general formula (VI) is an a-valent organic group in which the carbon atom directly bonded to N (nitrogen atom) does not have a ⁇ bond, and the organic group is saturated at least at the terminal directly bonded to N.
- An aliphatic hydrocarbon group having an aliphatic hydrocarbon group or an aromatic group having the aliphatic hydrocarbon group is represented, and O (oxygen atom), S (sulfur atom), and N (nitrogen atom) are present in the carbon chain. It may be included. Since the carbon atom directly bonded to N does not have a ⁇ bond, the color characteristics such as the color tone and transmittance of the cationic coloring portion are not affected by the linking group A and other coloring portions, Similar colors can be retained.
- an aliphatic hydrocarbon group having a saturated aliphatic hydrocarbon group at the terminal directly bonded to N is linear, branched or cyclic unless the terminal carbon atom directly bonded to N has a ⁇ bond.
- the carbon atom other than the terminal may have an unsaturated bond, may have a substituent, and the carbon chain contains O, S, and N. Also good.
- a carbonyl group, a carboxy group, an oxycarbonyl group, an amide group or the like may be contained, and a hydrogen atom may be further substituted with a halogen atom or the like.
- the aromatic group having an aliphatic hydrocarbon group in A is a monocyclic or polycyclic aromatic group having an aliphatic hydrocarbon group having a saturated aliphatic hydrocarbon group at the terminal directly bonded to N. And may have a substituent, and may be a heterocyclic ring containing O, S, and N. Especially, it is preferable that A contains a cyclic
- a bridged alicyclic hydrocarbon group is preferable from the viewpoint of skeleton fastness.
- the bridged alicyclic hydrocarbon group means a polycyclic aliphatic hydrocarbon group having a bridged structure in the aliphatic ring and having a polycyclic structure, for example, norbornane, bicyclo [2,2,2]. Examples include octane and adamantane.
- norbornane is preferable.
- the group containing a benzene ring and a naphthalene ring is mentioned, for example, Among these, the group containing a benzene ring is preferable. From the viewpoint of easy availability of raw materials, A is preferably divalent.
- A is a divalent organic group, a linear, branched or cyclic alkylene group having 1 to 20 carbon atoms, or an aromatic group substituted with two alkylene groups having 1 to 20 carbon atoms such as a xylylene group Etc.
- the alkyl group for R i to R v is not particularly limited. Examples thereof include straight-chain or branched alkyl groups having 1 to 20 carbon atoms. Among them, straight-chain or branched alkyl groups having 1 to 8 carbon atoms are preferable, and straight chain having 1 to 5 carbon atoms. A chain or branched alkyl group is more preferable from the viewpoint of luminance and heat resistance. Of these, the alkyl group in R i to R v is particularly preferably an ethyl group or a methyl group.
- the substituent that the alkyl group may have is not particularly limited, and examples thereof include an aryl group, a halogen atom, and a hydroxyl group, and examples of the substituted alkyl group include a benzyl group.
- the aryl group in R i to R v is not particularly limited. For example, a phenyl group, a naphthyl group, etc. are mentioned. Examples of the substituent that the aryl group may have include an alkyl group and a halogen atom.
- R ii and R iii , and R iv and R v are combined to form a ring structure when R vi and R vii , and R viii and R ix are combined to form a ring structure. It is the same.
- R i to R v are each independently a hydrogen atom, an alkyl group having 1 to 5 carbon atoms, a phenyl group, or R ii and R iii , R iv and R v. It is preferable that they are bonded to form a pyrrolidine ring, a piperidine ring, or a morpholine ring.
- R i to R v can each independently have the above structure, and among these, R i is preferably a hydrogen atom from the viewpoint of color purity, and R ii to R ii from the viewpoint of ease of production and raw material procurement. More preferably, R v are all the same.
- the divalent aromatic group in Ar 1 is not particularly limited.
- Ar 1 the same aromatic groups as those described for the aromatic group for A can be used.
- Ar 1 is preferably an aromatic group having 6 to 20 carbon atoms, more preferably an aromatic group composed of a condensed polycyclic carbocycle having 10 to 14 carbon atoms.
- a phenylene group or a naphthylene group is more preferable because the structure is simple and the raw material is inexpensive.
- the substituent that the aromatic group may have include an alkyl group having 1 to 5 carbon atoms and a halogen atom.
- a plurality of R i to R v and Ar 1 in one molecule may be the same or different.
- the color development site exhibits the same color development, so that the same color as the single color development site can be reproduced, which is preferable from the viewpoint of color purity.
- at least one of R i to R v and Ar 1 is a different substituent, a color obtained by mixing a plurality of types of monomers can be reproduced and adjusted to a desired color. it can.
- the anion moiety (B c ⁇ ) is a c-valent polyacid anion.
- the anion (B c ⁇ ) of the color material (A-1) is preferably a polyacid anion containing at least tungsten having a valence of 2 or more, and is a polyacid anion containing at least tungsten and may contain molybdenum. Is more preferable from the viewpoint of heat resistance.
- Examples of the polyacid anion containing at least tungsten include the same ones as mentioned above.
- the content ratio of tungsten and molybdenum is not particularly limited, but the molar ratio of tungsten to molybdenum is preferably 100: 0 to 85:15 from the viewpoint of excellent heat resistance. 100: 0 to 90:10 is more preferable.
- the polyacid anion (B c ⁇ ) the above-mentioned polyacid anions can be used singly or in combination of two or more, and when used in combination of two or more, tungsten and molybdenum in the entire polyacid anion And the molar ratio is preferably within the above range.
- b represents the number of cations
- d represents the number of anions in the molecular aggregate
- b and d represent 1 or more.
- a plurality of cations in the molecular aggregate may be one kind alone, or two or more kinds may be combined.
- the anions present in the molecular aggregate may be one kind or a combination of two or more kinds.
- E in the general formula (VI) is an integer of 0 or 1.
- a plurality of e may be the same or different. That is, for example, it may be a cation moiety having only a triarylmethane skeleton or a plurality of xanthene skeletons, or may be a cation moiety containing both a triarylmethane skeleton and a xanthene skeleton in one molecule. From the viewpoint of color purity, an anion portion having only the same skeleton is preferable.
- the color material represented by the general formula (VI) can be adjusted to a desired color.
- the method for producing the color material (A-2) represented by the general formula (VII) and the color material (A-1) represented by the general formula (VI) is appropriately selected from conventionally known methods. That's fine.
- a method for producing the coloring material (A-1) for example, it can be obtained by the production method described in International Publication No. 2012/144520 pamphlet.
- the color material may further contain another color material for the purpose of controlling the color tone within a range not impairing the effects of the present invention.
- the other coloring material include known pigments and dyes, and are not particularly limited as long as the effects of the present invention are not impaired, and are the same as those used in the color resin coloring resin composition described later. can do.
- the average dispersed particle size of the color material (A) used in the present invention is not particularly limited as long as it can produce a desired color when it is used as a colored layer of a color filter. From the viewpoint of excellent heat resistance and light resistance, it is preferably in the range of 10 to 200 nm, more preferably in the range of 20 to 160 nm. (A) Since the average dispersed particle size of the color material is in the above range, the liquid crystal display device and the organic light emitting display device manufactured using the colored resin composition for a color filter of the present invention have high contrast and high quality. Can be.
- the average dispersed particle size of the (A) color material in the color material dispersion is the dispersed particle size of the color material particles dispersed in a dispersion medium containing at least a solvent, and is measured by a laser light scattering particle size distribution meter. It is what is done.
- the particle size can be measured with a laser light scattering particle size distribution meter by appropriately diluting the color material dispersion liquid to a concentration that can be measured with a laser light scattering particle size distribution meter (for example, 1000 times). Etc.) and can be measured at 23 ° C.
- the average dispersed particle size here is a volume average particle size.
- the content of the color material is not particularly limited, but from the viewpoint of dispersibility and dispersion stability, it is 5 to 40% by mass, and further It is preferably in the range of 10 to 20% by mass.
- the color material (A-1) and the metal lake color material of the xanthene-based basic dye may be mixed and used.
- the mixing ratio between the color material (A-1) and the metal lake color material of the xanthene-based basic dye may be appropriately set in order to adjust to a desired color tone, and is not particularly limited.
- the mass ratio of the color material (A-1) to the metal lake color material of the xanthene-based basic dye is preferably 50:50 to 99: 1, and 70:30 to 95: 5 is more preferable.
- the solvent (C) is a solvent capable of dissolving or dispersing these without reacting with the components in the color material dispersion for a color filter according to the present invention or the later-described colored resin composition.
- Organic solvents such as alcohols, ether alcohols, esters, ketones, ether alcohol acetates, ethers, aprotic amides, lactones, unsaturated hydrocarbons, saturated hydrocarbons are used.
- Preferred ester solvents include, for example, methyl methoxypropionate, ethyl ethoxypropionate, methoxyethyl acetate, propylene glycol monomethyl ether acetate, 3-methoxy-3-methyl-1-butyl acetate, 3-methoxybutyl acetate, methoxybutyl Acetate, ethoxyethyl acetate, ethyl cellosolve acetate, dipropylene glycol methyl ether acetate, propylene glycol diacetate, 1,3-butylene glycol diacetate, cyclohexanol acetate, 1,6-hexanediol diacetate, diethylene glycol monoethyl ether acetate, Examples include diethylene glycol monobutyl ether acetate.
- propylene glycol monomethyl ether acetate PGMEA
- PGMEA propylene glycol monomethyl ether acetate
- solvents may be used alone or in combination of two or more.
- the color material dispersion for a color filter according to the present invention is prepared using (C) the solvent in a proportion of usually 50 to 95% by mass, preferably 60 to 85% by mass, based on the total amount of the color material dispersion. To do. When there is too little solvent, a viscosity will rise and a dispersibility will fall easily. Moreover, when there are too many solvents, a coloring material density
- the dispersion auxiliary resin examples include alkali-soluble resins exemplified by a color resin composition for color filters described later.
- the steric hindrance of the alkali-soluble resin makes it difficult for the colorant particles to come into contact with each other, and may have the effect of stabilizing the dispersion or reducing the dispersant due to the dispersion stabilizing effect.
- Other components include, for example, surfactants for improving wettability, silane coupling agents for improving adhesion, antifoaming agents, repellency inhibitors, antioxidants, anti-aggregation agents, and UV absorbers. Etc.
- the color material dispersion for color filter according to the present invention is used as a preliminary preparation for preparing a colored resin composition for color filter described later. That is, the color material dispersion is preliminarily prepared in the pre-stage of preparing a colored resin composition described later, (color material component mass in the composition) / (solid content mass other than the color material component in the composition) ) Colorant dispersion with a high ratio. Specifically, the ratio of (mass of color material component in composition) / (mass of solid content other than color material component in composition) is usually 1.0 or more.
- the solid content means all components other than the solvent.
- a method for producing a color material dispersion for a color filter includes (A) a color material, (B) a dispersant, (C) a solvent, and various additive components used as desired.
- the color material may be any method that can be uniformly dispersed in the solvent (C) with the dispersant (B), and can be uniformly dispersed by mixing using a known mixing means.
- a dispersant is mixed and stirred in a solvent (C) to prepare a dispersant solution, and then the (A) color material is added to the dispersant solution.
- other components as necessary and dispersed using a known stirrer or disperser.
- a color material dispersion liquid in which the color material (A-1) is dispersed and a color material dispersion liquid in which the color material (A-2) which is a metal lake color material of a xanthene-based basic dye is dispersed separately. It is good also as the color material dispersion liquid for color filters which concerns on this invention by preparing and mixing these.
- the dispersing machine for performing the dispersion treatment examples include a roll mill such as a two-roll or a three-roll, a ball mill such as a ball mill or a vibration ball mill, a bead mill such as a paint conditioner, a continuous disk type bead mill, or a continuous annular type bead mill.
- the bead diameter to be used is preferably 0.03 to 2.00 mm, more preferably 0.10 to 1.0 mm.
- preliminary dispersion is performed with 2 mm zirconia beads having a relatively large bead diameter, and the main dispersion is further performed with 0.1 mm zirconia beads having a relatively small bead diameter. Further, after dispersion, it is preferably filtered through a membrane filter of 0.5 to 5.0 ⁇ m.
- the viscosity of the color material dispersion for the color filter according to the present invention is not particularly limited, but the shear viscosity when the shear rate is 60 rpm is 7 mPa ⁇ s or less from the viewpoint of good dispersibility and good handleability. It is preferably 5 mPa ⁇ s or less.
- the shear viscosity can be measured using a known viscoelasticity measuring apparatus and is not particularly limited. For example, the shear viscosity can be measured using “Rheometer MCR301” manufactured by Anton Paar.
- the color filter color resin composition may be obtained by adding (D) a binder component to the color filter colorant dispersion according to the present invention. That is, it contains (A) a color material, (B) a dispersant, (C) a solvent, and (D) a binder component, and (A) the color material contains a metal lake color material of a basic dye.
- the dispersant (B) is represented by the general formula (II) and at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′). Or a block containing at least one selected from the structural unit represented by the general formula (I) and the structural unit represented by the general formula (I ′).
- the colored resin composition for color filters which is a block copolymer which has a part and a block part containing the structural unit represented by the said general formula (III).
- the colored resin composition for a color filter is excellent in dispersibility, heat resistance and alkali resistance of a metal lake color material, and can form a high-luminance colored layer.
- the colored resin composition contains (A) a color material, (B) a dispersant, (C) a solvent, and (D) a binder component, and contains other components as necessary. It may be.
- a colored resin composition for a color filter will be described.
- the color material, (B) the dispersant, and (C) the solvent the color material dispersion for color filter according to the present invention and Since it can be the same, the description here is omitted.
- the colored resin composition for a color filter contains a binder component in order to impart film formability and adhesion to the surface to be coated. In order to impart sufficient hardness to the coating film, it is preferable to contain a curable binder component. It does not specifically limit as a curable binder component, The curable binder component used in forming the coloring layer of a conventionally well-known color filter can be used suitably. Examples of the curable binder component include a photocurable binder component containing a photocurable resin that can be polymerized and cured by visible light, ultraviolet light, electron beam, and the like, and a thermosetting resin that can be polymerized and cured by heating. What contains the thermosetting binder component to contain can be used.
- thermosetting binder component when the colored resin composition for color filter is used in an ink jet system, a curable binder component needs to be developable when a colored layer can be formed by selectively adhering to the substrate in a pattern. Absent.
- a well-known thermosetting binder component, a photosensitive binder component, etc. which are used when forming a color filter colored layer by an inkjet system etc. can be used suitably.
- thermosetting binder a combination of a compound having two or more thermosetting functional groups in one molecule and a curing agent is usually used, and a catalyst capable of promoting a thermosetting reaction may be added.
- thermosetting functional group examples include an epoxy group, an oxetanyl group, an isocyanate group, and an ethylenically unsaturated bond.
- An epoxy group is preferably used as the thermosetting functional group.
- Specific examples of the thermosetting binder component include those described in International Publication No. 2012/144521 pamphlet.
- the photosensitive binder component which has alkali developability is used suitably.
- a curable binder component is not limited to these.
- a thermosetting binder component that can be polymerized and cured by heating such as an epoxy resin may be further used.
- Examples of the photosensitive binder component include a positive photosensitive binder component and a negative photosensitive binder component.
- Examples of the positive photosensitive binder component include a system containing an alkali-soluble resin and an o-quinonediazide group-containing compound as a photosensitizing component.
- the negative photosensitive binder component a system containing at least an alkali-soluble resin, a polyfunctional monomer, and a photoinitiator is preferably used.
- a negative photosensitive binder component is preferable because a pattern can be easily formed by an existing process by a photolithography method.
- the alkali-soluble resin, the polyfunctional monomer, and the photoinitiator constituting the negative photosensitive binder component will be specifically described.
- the alkali-soluble resin in the present invention has an acidic group, acts as a binder resin, and is appropriately selected and used as long as it is soluble in a developer used for pattern formation, particularly preferably an alkali developer. be able to.
- the preferred alkali-soluble resin in the present invention is preferably a resin having a carboxyl group as an acidic group. Specifically, an acrylic copolymer having a carboxyl group, an epoxy (meth) acrylate resin having a carboxyl group, etc. Can be mentioned.
- acrylic copolymers and epoxy acrylate resins may be used as a mixture of two or more.
- the acrylic copolymer having a carboxyl group is obtained by copolymerizing a carboxyl group-containing ethylenically unsaturated monomer and an ethylenically unsaturated monomer.
- the acrylic copolymer having a carboxyl group may further contain a structural unit having an aromatic carbocyclic ring.
- the aromatic carbocycle functions as a component that imparts coating properties to the colored resin composition for color filters.
- the acrylic copolymer having a carboxyl group may further contain a structural unit having an ester group.
- the structural unit having an ester group not only functions as a component that suppresses alkali solubility of the colored resin composition for a color filter, but also functions as a component that improves the solubility in a solvent and further the solvent resolubility.
- acrylic copolymer having a carboxyl group examples include those described in International Publication No. 2012/144521 pamphlet. Specifically, for example, methyl (meth) acrylate, ethyl ( Examples thereof include a copolymer composed of a monomer having no carboxyl group, such as (meth) acrylate, and one or more selected from (meth) acrylic acid and anhydrides thereof.
- a polymer having an ethylenically unsaturated bond introduced by adding an ethylenically unsaturated compound having a reactive functional group such as a glycidyl group or a hydroxyl group to the above copolymer can be exemplified, but the present invention is not limited thereto. Is not to be done.
- a polymer having an ethylenically unsaturated bond introduced, for example, by adding an ethylenically unsaturated compound having a glycidyl group or a hydroxyl group to the copolymer is polymerized with a polyfunctional monomer described later at the time of exposure. This is particularly suitable in that the colored layer becomes more stable.
- the copolymerization ratio of the carboxyl group-containing ethylenically unsaturated monomer in the carboxyl group-containing copolymer is usually 5 to 50% by mass, preferably 10 to 40% by mass.
- the copolymerization ratio of the carboxyl group-containing ethylenically unsaturated monomer is less than 5% by mass, the solubility of the resulting coating film in an alkaline developer is lowered, and pattern formation becomes difficult.
- the copolymerization ratio exceeds 50% by mass, there is a tendency that the formed pattern is easily detached from the substrate or the pattern surface is roughened during development with an alkali developer.
- the preferred molecular weight of the carboxyl group-containing copolymer is preferably in the range of 1,000 to 500,000, more preferably 3,000 to 200,000. If it is less than 1,000, the binder function after curing is remarkably lowered, and if it exceeds 500,000, pattern formation may be difficult during development with an alkaline developer.
- Acrylate compounds are suitable.
- the epoxy compound, unsaturated group-containing monocarboxylic acid, and acid anhydride can be appropriately selected from known ones. Specific examples include those described in International Publication No. 2012/144521 pamphlet. Each of the epoxy compound, the unsaturated group-containing monocarboxylic acid, and the acid anhydride may be used alone or in combination of two or more.
- the alkali-soluble resin used in the colored resin composition for color filters may be used alone or in combination of two or more, and the content thereof is a color contained in the colored resin composition. It is usually in the range of 10 to 1000 parts by weight, preferably in the range of 20 to 500 parts by weight, with respect to 100 parts by weight of the material. If the content of the alkali-soluble resin is too small, sufficient alkali developability may not be obtained, and if the content of the alkali-soluble resin is too large, the ratio of the coloring material is relatively low, which is sufficient. The coloring density may not be obtained.
- the polyfunctional monomer used in the colored resin composition for a color filter is not particularly limited as long as it can be polymerized by a photoinitiator described later, and usually has two or more ethylenically unsaturated double bonds.
- polyfunctional (meth) acrylates having two or more acryloyl groups or methacryloyl groups are preferable.
- Such polyfunctional (meth) acrylate may be appropriately selected from conventionally known ones. Specific examples include those described in International Publication No. 2012/144521 pamphlet.
- polyfunctional (meth) acrylates may be used individually by 1 type, and may be used in combination of 2 or more type.
- the polyfunctional monomer has three (trifunctional) or more polymerizable double bonds.
- the content of the polyfunctional monomer used in the colored resin composition for a color filter is not particularly limited, but is usually about 5 to 500 parts by mass, preferably 20 to 300 parts by mass with respect to 100 parts by mass of the alkali-soluble resin. Part range. If the content of the polyfunctional monomer is less than the above range, the photocuring may not sufficiently proceed and the exposed part may be eluted, and if the content of the polyfunctional monomer is more than the above range, the alkali developability may be deteriorated. There is.
- Photoinitiator There is no restriction
- the content of the photoinitiator used in the colored resin composition for a color filter is usually about 0.01 to 100 parts by mass, preferably 5 to 60 parts by mass with respect to 100 parts by mass of the polyfunctional monomer. When this content is less than the above range, the polymerization reaction cannot be sufficiently caused, so that the hardness of the colored layer may not be sufficient. In some cases, the content of the coloring material or the like in the solid content is relatively small, and a sufficient coloring density cannot be obtained.
- the colored resin composition for color filters may contain other color materials and various additives as necessary.
- a conventionally known pigment or dye can be selected according to the purpose, and one or more kinds can be used.
- Specific examples of other coloring materials include C.I. I. Pigment violet 19, C.I. I. Pigment violet 23; C.I. I. Pigment blue 15, C.I. I. Pigment blue 15: 3, C.I. I. Pigment blue 15: 4, C.I. I. Pigment blue 15: 6, C.I. I. And pigments such as CI Pigment Blue 60 and dyes such as Acid Red.
- the blending amount is not particularly limited, but from the viewpoint of the transmittance, heat resistance, light resistance, etc. of the colored layer, (A) other color materials with respect to 100 parts by mass of the total color material Is preferably 40 parts by mass or less, and more preferably 20 parts by mass or less.
- the colored resin composition for a color filter preferably further contains an antioxidant from the viewpoint of heat resistance and light resistance.
- the antioxidant may be appropriately selected from conventionally known antioxidants. Specific examples of antioxidants include, for example, hindered phenol antioxidants, amine antioxidants, phosphorus antioxidants, sulfur antioxidants, hydrazine antioxidants, and the like. From the viewpoint, it is preferable to use a hindered phenol-based antioxidant.
- the hindered phenol antioxidant contains at least one phenol structure, and has a structure in which a substituent having 4 or more carbon atoms is substituted on at least one of the 2-position and 6-position of the hydroxyl group of the phenol structure. Means an antioxidant.
- the amount of the antioxidant is not particularly limited as long as the effect of the present invention is not impaired.
- the blending amount of the antioxidant is preferably 0.1 to 5.0 parts by mass with respect to 100 parts by mass of the total solid content in the colored resin composition, and preferably 0.5 to 4. More preferably, it is 0 parts by mass. If it is more than the said lower limit, it is excellent in heat resistance. On the other hand, if it is below the said upper limit, a colored resin composition can be used as a highly sensitive photosensitive resin composition.
- antioxidant additives in addition to the antioxidants, for example, polymerization terminators, chain transfer agents, leveling agents, plasticizers, surfactants, antifoaming agents, silane coupling agents, ultraviolet absorbers, adhesion promoters, etc. Is mentioned. Specific examples of the surfactant and the plasticizer include those described in International Publication No. 2012/144521 pamphlet.
- the total content of the color material (A) is preferably 3 to 65% by mass, more preferably 4 to 55% by mass, based on the total solid content of the colored resin composition. If it is at least the lower limit, the colored layer has a sufficient color density when the colored resin composition is applied to a predetermined film thickness (usually 1.0 to 5.0 ⁇ m). Moreover, if it is below the said upper limit, while being excellent in the dispersibility and dispersion stability, the colored layer which has sufficient hardness and adhesiveness with a board
- the solid content is everything except the above-mentioned solvent, and includes a liquid polyfunctional monomer.
- the content of the (B) dispersant is not particularly limited as long as it can uniformly disperse the color material (A).
- the content of the solid content of the colored resin composition is not limited. On the other hand, 3 to 40% by mass can be used. Further, it is preferably blended in a proportion of 5 to 35% by weight, particularly preferably 5 to 25% by weight, based on the total solid content of the colored resin composition. If it is more than the said lower limit, it is excellent in the dispersibility and dispersion stability of (A) coloring material, and is excellent in storage stability. Moreover, if it is below the said upper limit, developability will become favorable.
- the total amount of the binder component is 10 to 92% by mass, preferably 15 to 87% by mass, based on the total solid content of the colored resin composition. If it is more than the said lower limit, the colored layer which has sufficient hardness and adhesiveness with a board
- the coating property can be excellent.
- the method for producing a colored resin composition for a color filter includes (A) a color material, (B) a dispersant, (C) a solvent, (D) a binder component, and various additive components used as desired.
- the coloring material may be any method that can be uniformly dispersed in the solvent (C) from the dispersing agent (B), and is not particularly limited, and can be prepared by mixing using a known mixing means. it can.
- Examples of the method for preparing the resin composition include (1) a method of mixing (D) the binder component and various additive components used as desired in the color material dispersion for color filter according to the present invention; (2) (C) A method in which (A) a color material, (B) a dispersant, (D) a binder component, and various additive components used as desired are simultaneously added to a solvent and mixed; (3) (C ) A method in which (B) a dispersant, (D) a binder component, and various additive components used as desired are added and mixed in a solvent, and then (A) a color material is added and mixed. be able to.
- the method (1) is preferable because it can effectively prevent aggregation of the color material and can be uniformly dispersed.
- Color filter is a color filter comprising at least a transparent substrate and a colored layer provided on the transparent substrate, wherein at least one of the colored layers comprises (A) a colorant, ( B) a dispersant, the (A) colorant contains a metal lake colorant of a basic dye, and the (B) dispersant is a structural unit represented by the general formula (I) and It is a graft copolymer having at least one selected from the structural unit represented by the general formula (I ′) and the structural unit represented by the general formula (II), or the general formula (I) A block having at least one selected from the structural unit represented by formula (I) and the structural unit represented by the general formula (I ′) and a block unit comprising the structural unit represented by the general formula (III). It is a copolymer To.
- FIG. 1 is a schematic sectional view showing an example of the color filter of the present invention.
- the color filter 10 of the present invention has a transparent substrate 1, a light shielding part 2, and a colored layer 3.
- At least one of the colored layers used in the color filter of the present invention contains the (A) color material and the (B) dispersant. Since the (A) color material and the (B) dispersant are as described above, description thereof is omitted here.
- the colored layer is usually formed in an opening of a light shielding part on a transparent substrate, which will be described later, and is usually composed of three or more colored patterns.
- the arrangement of the colored layers is not particularly limited, and for example, a general arrangement such as a stripe type, a mosaic type, a triangle type, or a four-pixel arrangement type can be used.
- variety, area, etc. of a colored layer can be set arbitrarily.
- the thickness of the colored layer is appropriately controlled by adjusting the coating method, the solid content concentration, the viscosity, and the like of the colored resin composition for a color filter, but is usually preferably in the range of 1 to 5 ⁇ m.
- the colored layer used in the color filter of the present invention is the above-described (A) color material, (B) a dispersant, (C) a solvent, and (D) a colored resin composition for a color filter containing a binder component.
- the colored resin composition for a color filter is preferably a cured product.
- the colored layer can be formed by the following method. First, a colored resin composition for a color filter is applied on a transparent substrate, which will be described later, using a coating means such as a spray coating method, a dip coating method, a bar coating method, a call coating method, a spin coating method, and the like. To form.
- an alkali-soluble resin and a polyfunctional monomer are photopolymerized.
- a photosensitive coating film is used.
- the light source used for exposure include ultraviolet rays such as a low-pressure mercury lamp, a high-pressure mercury lamp, and a metal halide lamp, and an electron beam.
- the exposure amount is appropriately adjusted depending on the light source used, the thickness of the coating film, and the like.
- you may heat-process. The heating conditions are appropriately selected depending on the blending ratio of each component in the colored resin composition to be used, the thickness of the coating film, and the like.
- a coating film is formed with a desired pattern by melt
- a solution in which an alkali is dissolved in water or a water-soluble solvent is usually used.
- An appropriate amount of a surfactant or the like may be added to the alkaline solution.
- a general method can be adopted as the developing method.
- the developer is usually washed and the cured coating film of the colored resin composition is dried to form a colored layer.
- the heating conditions are not particularly limited and are appropriately selected depending on the application of the coating film.
- the light shielding part in the color filter of the present invention is formed in a pattern on a transparent substrate described later, and can be the same as that used as a light shielding part in a general color filter.
- the pattern shape of the light shielding portion is not particularly limited, and examples thereof include a stripe shape and a matrix shape.
- Examples of the light-shielding portion include those obtained by dispersing or dissolving a black pigment in a binder resin, and metal thin films such as chromium and chromium oxide.
- the metal thin film may be a CrO x film (x is an arbitrary number) and a laminate of two Cr films, and a CrO x film (x is an arbitrary number) with a reduced reflectance.
- the light shielding part is a material in which a black color material is dispersed or dissolved in a binder resin
- the light shielding part can be formed by any method that can pattern the light shielding part, and is not particularly limited. For example, a photolithography method, a printing method, an ink jet method and the like using the colored resin composition for the light shielding part can be exemplified.
- the thickness of the light shielding portion is set to about 0.2 to 0.4 ⁇ m in the case of a metal thin film, and about 0.5 to 2 ⁇ m in the case where a black color material is dispersed or dissolved in a binder resin. Is set.
- the transparent substrate in the color filter of the present invention is not particularly limited as long as it is a base material transparent to visible light, and a transparent substrate used for a general color filter can be used. Specifically, a transparent rigid material having no flexibility such as quartz glass, alkali-free glass, or synthetic quartz plate, or a flexible or flexible resin such as a transparent resin film, an optical resin plate, or flexible glass. A transparent flexible material is mentioned.
- the thickness of the transparent substrate is not particularly limited, but for example, a thickness of about 100 ⁇ m to 1 mm can be used according to the use of the color filter of the present invention.
- the color filter of the present invention may be one in which, for example, an overcoat layer, a transparent electrode layer, an alignment film, a columnar spacer, or the like is formed in addition to the transparent substrate, the light shielding portion, and the colored layer. .
- FIG. 2 is a schematic view showing an example of the liquid crystal display device of the present invention.
- the liquid crystal display device 40 of the present invention includes a color filter 10, a counter substrate 20 having a TFT array substrate and the like, and a liquid crystal layer formed between the color filter 10 and the counter substrate 20. 30.
- the liquid crystal display device of the present invention is not limited to the configuration shown in FIG. 2, but can be a configuration generally known as a liquid crystal display device using a color filter.
- the driving method of the liquid crystal display device of the present invention is not particularly limited, and a driving method generally used for a liquid crystal display device can be employed. Examples of such a drive method include a TN method, an IPS method, an OCB method, and an MVA method. In the present invention, any of these methods can be preferably used. Further, the counter substrate can be appropriately selected and used according to the driving method of the liquid crystal display device of the present invention. Furthermore, as the liquid crystal constituting the liquid crystal layer, various liquid crystals having different dielectric anisotropy and mixtures thereof can be used according to the driving method of the liquid crystal display device of the present invention.
- a method generally used as a method for manufacturing a liquid crystal cell can be used, and examples thereof include a vacuum injection method and a liquid crystal dropping method.
- a vacuum injection method for example, a liquid crystal cell is prepared in advance using a color filter and a counter substrate, and the liquid crystal is heated to obtain an isotropic liquid, and the liquid crystal is applied to the liquid crystal cell using the capillary effect.
- the liquid crystal layer can be formed by injecting in this state and sealing with an adhesive. Thereafter, the sealed liquid crystal can be aligned by slowly cooling the liquid crystal cell to room temperature.
- liquid crystal dropping method for example, a sealant is applied to the periphery of the color filter, the color filter is heated to a temperature at which the liquid crystal becomes isotropic, and the liquid crystal is dropped in an isotropic liquid state using a dispenser or the like.
- the liquid crystal layer can be formed by overlapping the color filter and the counter substrate under reduced pressure and bonding them with a sealant. Thereafter, the sealed liquid crystal can be aligned by slowly cooling the liquid crystal cell to room temperature.
- FIG. 3 is a schematic view illustrating an example of the organic light emitting display device of the present invention.
- the organic light emitting display device 100 of the present invention includes a color filter 10 and an organic light emitter 80.
- An organic protective layer 50 and an inorganic oxide film 60 may be provided between the color filter 10 and the organic light emitter 80.
- the transparent anode 71, the hole injection layer 72, the hole transport layer 73, the light emitting layer 74, the electron injection layer 75, and the cathode 76 are sequentially formed on the upper surface of the color filter. Examples thereof include a method and a method in which an organic light emitter 80 formed on another substrate is bonded onto the inorganic oxide film 60.
- the transparent anode 71, the hole injection layer 72, the hole transport layer 73, the light emitting layer 74, the electron injection layer 75, the cathode 76, and other configurations in the organic light emitting body 80 known structures can be appropriately used.
- the organic light emitting display device 100 manufactured as described above can be applied to, for example, a passive drive type organic EL display or an active drive type organic EL display.
- the organic light emitting display device of the present invention is not limited to the configuration shown in FIG. 3, and may be a known configuration as an organic light emitting display device that generally uses a color filter.
- AIBN bisisobutyronitrile
- macromonomer MM-1 4 parts by mass of macromonomer MM-1 was obtained by adding 3 parts by mass and 10 parts by mass of PGMEA and stirring for 3 hours.
- the obtained macromonomer MM-1 was confirmed by GPC (gel permeation chromatography) under the conditions of N-methylpyrrolidone, 0.01 mol / L lithium bromide added / polystyrene standard, and the mass average molecular weight (Mw 4010, number average molecular weight (Mn) 1910, molecular weight distribution (Mw / Mn) was 2.10.
- a mixed solution of 0 parts by mass and 0.5 parts by mass of AIBN was added dropwise over 1.5 hours, heated and stirred for 3 hours, and then a mixed solution of AIBN 0.10 parts by mass and PGMEA 10.0 parts by mass was added dropwise over 10 minutes. Further, by aging at the same temperature for 1 hour, a 25.0% by mass solution of graft copolymer A was obtained. As a result of GPC measurement, the obtained graft copolymer A had a mass average molecular weight (Mw) of 10570, a number average molecular weight (Mn) of 4370, and a molecular weight distribution (Mw / Mn) of 2.42.
- Mw mass average molecular weight
- Mn number average molecular weight
- Mn molecular weight distribution
- the obtained phosphorus-based graft copolymer C had a mass average molecular weight (Mw) of 5950, a number average molecular weight (Mn) of 2930, and a molecular weight distribution (Mw / Mn) of 2.03.
- the acid value was 54 mgKOH / g.
- a part by mass of the mixed solution was added dropwise over 60 minutes. The temperature was kept below 40 ° C. by cooling the reactor with an ice bath. After 1 hour, 25.0 parts by mass of glycidyl methacrylate was added dropwise over 20 minutes. After reacting for 1 hour, 1 part by mass of methanol was added to stop the reaction. 180.0 mass parts of PGMEA was added to the THF solution of the obtained block copolymer A, and solvent substitution was performed by evaporation to obtain a 40.0 mass% PGMEA solution of the block copolymer A.
- the obtained block copolymer A had a mass average molecular weight (Mw) of 9470, a number average molecular weight (Mn) of 7880, and a molecular weight distribution (Mw / Mn) of 1.20.
- the obtained binder resin A had a mass average molecular weight (Mw) of 8500, a number average molecular weight (Mn) of 4200, a molecular weight distribution (Mw / Mn) of 2.02, and an acid value of 85 mgKOH / g.
- the obtained binder resin B had a mass average molecular weight (Mw) of 4950, a number average molecular weight (Mn) of 2240, a molecular weight distribution (Mw / Mn) of 2.21, and an acid value of 64 mgKOH / g.
- Binder Composition B (Comparative Synthesis Example 2 Preparation of Binder Composition B) PGMEA 11.33 parts by mass, binder resin B of comparative synthesis example 1 (solid content 30% by mass) 26.67 parts by mass, Aronix M403 8.00 parts by mass, Irgacure 907 3.00 parts by mass, Kayacure DETX-S 1.00 Binder composition B (solid content 40% by mass) was prepared by mixing parts by mass.
- Example 1 (1) Production of Color Material Dispersion Liquid 9.10 parts by mass of triarylmethane-based lake color material A of Synthesis Example 3 and 3.90 parts by mass of xanthene-based lake color material A of Synthesis Example 1 phosphorus prepared in Production Example 1 20.80 parts by mass of the graft copolymer A solution (solid content 5.20 parts by mass), 11.82 parts by mass of binder resin A of Synthesis Example 7 (solid content 5.20 parts by mass), 54.38 parts by mass of PGMEA Were mixed with a paint shaker (manufactured by Asada Tekko) as a pre-dispersion for 2 hours with 2 mm zirconia beads, and further as a main dispersion for 6 hours with 0.1 mm zirconia beads to obtain a colorant dispersion A.
- a paint shaker manufactured by Asada Tekko
- Example 1 (1) Production of Color Material Dispersion Solution
- the color material, the dispersant, and the solvent were changed to those shown in Table 1 and Table 2, respectively (1) in Example 1
- Colorant dispersions B to AA were obtained in the same manner as colorant dispersion A).
- the binder resin A of Synthesis Example 7 is also included in the color material dispersions B to AA in the same amount as the color material dispersion A.
- Disperbyk-161 and Disperbyk-170 are urethane dispersants
- Disperbyk-111 is a phosphate ester dispersant
- BYK-LPN21116 is a quaternary ammonium salt-containing acrylate dispersant
- All are dispersants manufactured by Big Chemie Japan.
- the numerical value represents part by mass, and in the case of a dispersant, the part by mass of the dispersant solution and the part by mass of the solid content in parentheses.
- Example 1 (2) Production of Colored Resin Composition
- the color material dispersions B to AA obtained as described above were used.
- blue colored resin compositions of Examples 2 to 17 and Comparative Examples 1 to 6 and Comparative Examples 8 to 11 were obtained.
- Comparative Example 7 The blue colored resin of Comparative Example 7 was the same as Comparative Example 6 except that the binder composition B obtained in Comparative Synthesis Example 2 was used instead of the binder composition A in (2) of Comparative Example 6. A composition was obtained.
- the chromaticity (x, y), luminance (Y), L, a, b (L 0 , a 0 , b 0 ) of the colored substrate thus obtained was changed to “Microspectrophotometer OSP-SP200” manufactured by Olympus. And measured.
- the substrate on which the colored film is formed is post-baked for 60 minutes in a clean oven at 230 ° C., and the chromaticity (x, y), luminance (Y), and L, a, b (L 1 ) of the obtained colored film. , A 1 , b 1 ) were measured again.
- the color difference ( ⁇ Eab) before and after post-baking was calculated from the following formula.
- ⁇ Eab ⁇ (L 1 ⁇ L 0 ) 2 + (a 1 ⁇ a 0 ) 2 + (b 1 ⁇ b 0 ) 2 ⁇ 1/2
- Tables 3 to 5 show the chromaticity (x, y) and luminance (Y) of the colored film after post-baking, and the color difference ( ⁇ Eab) before and after post-baking.
- Post-baking was performed for 30 minutes in a clean oven.
- the obtained glass substrate on which the colored pattern was formed was immersed in a 5.0% by mass aqueous sodium hydroxide solution maintained at 40 ° C., and the time until the colored pattern peeled from the glass substrate was measured.
- the measurement results are shown in Tables 3-5.
- the glass plate on which the colored layer was formed was shower-developed for 60 seconds using a 0.05% by weight aqueous potassium hydroxide solution as an alkaline developer, and further washed with ultrapure water for 60 seconds.
- the glass substrate was arrange
- the color transfer was evaluated according to the following evaluation criteria. The evaluation results are shown in Tables 3-4. [Evaluation criteria] A: No coloring, B: Light coloring, C: Dark coloring
- Tables 3 and 4 show evaluations of Examples and Comparative Examples in which a triarylmethane-based lake color material A that is a blue color material and a xanthene-based lake color material A or B that is a purple color material are used as color materials. Results are shown.
- a xanthene rake color material is used as the color material, there has conventionally been a problem of color transfer of the color material due to high-temperature heating.
- Examples 1 to 3, 6 to 9 using the specific graft copolymer among the dispersant (B) specified in the present invention as the dispersant and 11 and Examples 4, 5 and 10 using the specific block copolymer had no color transfer upon high-temperature heating, were excellent in dispersion performance and heat resistance, and were excellent in alkali resistance.
- Comparative Examples 1 to 9 using a dispersant other than the dispersant (B) specified in the present invention were carried out using the same coloring material in at least one of dispersion performance, heat resistance and alkali resistance. It was inferior to the example.
- Comparative Example 4 is an example using a commercially available phosphate ester-based dispersant, but since the dispersibility of the coloring material was particularly poor and gelled, evaluation items other than the dispersion performance could not be evaluated.
- Comparative Example 5 a polymer having a phosphonooxy group was used as a dispersant, but the dispersibility of the coloring material was poor and the alkali resistance was also poor.
- Comparative Examples 1 to 3 and 6 to 9 had better dispersibility than Comparative Examples 4 and 5, but had poor heat resistance and caused color transfer due to high-temperature heating.
- Comparative Example 7 a polymer having a phosphonooxy group was contained as a binder resin.
- Examples 12 to 15 using the specific graft copolymer and Examples 16 to 17 using the specific block copolymer are as follows: Compared to Comparative Example 10 using a salt-type amine block copolymer, it was excellent in heat resistance. Comparative Example 11 is an example in which a polymer having a phosphonooxy group was used as a dispersant, but the dispersibility of the coloring material was poor and the alkali resistance was also poor.
Landscapes
- Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Engineering & Computer Science (AREA)
- Nonlinear Science (AREA)
- Inorganic Chemistry (AREA)
- Materials Engineering (AREA)
- Manufacturing & Machinery (AREA)
- General Chemical & Material Sciences (AREA)
- Mathematical Physics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Optical Filters (AREA)
- Emulsifying, Dispersing, Foam-Producing Or Wetting Agents (AREA)
- Macromonomer-Based Addition Polymer (AREA)
- Liquid Crystal (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Graft Or Block Polymers (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Electroluminescent Light Sources (AREA)
- Pigments, Carbon Blacks, Or Wood Stains (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020167014472A KR102210034B1 (ko) | 2013-12-05 | 2014-10-02 | 비수계 분산제, 컬러 필터용 색재 분산액, 컬러 필터, 액정 표시 장치 및 유기 발광 표시 장치 |
| US15/101,360 US10570287B2 (en) | 2013-12-05 | 2014-10-02 | Non-aqueous dispersant, color material dispersion liquid for color filter, color filter, liquid crystal display device and organic light-emitting display device |
| CN201480065528.5A CN105792921B (zh) | 2013-12-05 | 2014-10-02 | 非水系分散剂、彩色滤光片用色料分散液、彩色滤光片、液晶显示装置及有机发光显示装置 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013-251876 | 2013-12-05 | ||
| JP2013251876A JP5895925B2 (ja) | 2013-12-05 | 2013-12-05 | 非水系分散剤、カラーフィルタ用色材分散液、カラーフィルタ、液晶表示装置及び有機発光表示装置 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015083426A1 true WO2015083426A1 (ja) | 2015-06-11 |
Family
ID=53273203
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/076462 Ceased WO2015083426A1 (ja) | 2013-12-05 | 2014-10-02 | 非水系分散剤、カラーフィルタ用色材分散液、カラーフィルタ、液晶表示装置及び有機発光表示装置 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US10570287B2 (enExample) |
| JP (1) | JP5895925B2 (enExample) |
| KR (1) | KR102210034B1 (enExample) |
| CN (2) | CN107266634B (enExample) |
| TW (1) | TWI634149B (enExample) |
| WO (1) | WO2015083426A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11084934B2 (en) * | 2016-06-28 | 2021-08-10 | Dai Nippon Printing Co., Ltd. | Color material dispersion liquid, color resin composition, color filter, liquid crystal display device, and light-emitting display device |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6943558B2 (ja) * | 2016-01-20 | 2021-10-06 | 東友ファインケム株式会社Dongwoo Fine−Chem Co., Ltd. | 着色硬化性樹脂組成物、カラーフィルタ、及び表示装置 |
| CN109415298B (zh) * | 2016-06-28 | 2022-03-29 | 大日本印刷株式会社 | 化合物及其中间体 |
| JP6872875B2 (ja) * | 2016-09-28 | 2021-05-19 | 理想科学工業株式会社 | 着色樹脂粒子分散体 |
| KR102503367B1 (ko) * | 2016-11-16 | 2023-02-24 | 동우 화인켐 주식회사 | 착색 감광성 수지 조성물, 이를 이용하여 제조된 컬러필터 및 화상표시장치 |
| JP6953851B2 (ja) * | 2017-07-14 | 2021-10-27 | 大日本印刷株式会社 | 高分子分散剤及びその製造方法、色材分散液、着色樹脂組成物、カラーフィルタ、液晶表示装置、及び発光表示装置 |
| CN108948384B (zh) * | 2018-07-31 | 2021-02-09 | 惠州莹光塑胶颜料有限公司 | 一种应用于遥控接收面板的黑色色料及其制备方法 |
| CN114051519B (zh) * | 2019-07-09 | 2023-10-13 | Dnp精细化工股份有限公司 | 色材分散液、分散剂、感光性着色树脂组合物、固化物、滤色器、显示设备 |
| JP7731799B2 (ja) * | 2019-11-08 | 2025-09-01 | 株式会社Dnpファインケミカル | 色材分散液、分散剤、感光性着色樹脂組成物、硬化物、カラーフィルタ、表示装置 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0445456A (ja) * | 1990-06-13 | 1992-02-14 | Fuji Photo Film Co Ltd | 静電写真用液体現像剤 |
| JP2000104005A (ja) * | 1998-09-28 | 2000-04-11 | Dainippon Printing Co Ltd | 顔料分散剤、感光性着色組成物及び遮光層用組成物 |
| JP2010083923A (ja) * | 2008-09-29 | 2010-04-15 | Dainippon Printing Co Ltd | 顔料分散剤、カラーフィルタ用ネガ型レジスト組成物、カラーフィルタ及び液晶表示装置 |
| JP2012252319A (ja) * | 2011-05-11 | 2012-12-20 | Jsr Corp | 着色組成物、カラーフィルタ及び表示素子 |
| JP2013230416A (ja) * | 2012-04-27 | 2013-11-14 | Ricoh Co Ltd | 金属粒子分散剤、金属粒子分散インク及び導電性パターン形成方法 |
| WO2014061750A1 (ja) * | 2012-10-18 | 2014-04-24 | 大日本印刷株式会社 | 分散剤、導電性基板用金属粒子分散体、及び導電性基板の製造方法 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5114822A (en) * | 1990-01-31 | 1992-05-19 | Fuji Photo Film Co., Ltd. | Liquid developer for electrostatic photography |
| JP2001081348A (ja) | 1999-07-14 | 2001-03-27 | Nippon Kayaku Co Ltd | 着色感光性組成物 |
| TWI388926B (zh) * | 2003-02-07 | 2013-03-11 | Mitsubishi Chem Corp | 著色樹脂組成物、彩色濾光片及液晶顯示裝置 |
| JP5251329B2 (ja) | 2008-07-22 | 2013-07-31 | 東洋インキScホールディングス株式会社 | カラーフィルタ用青色着色組成物、カラーフィルタおよびカラー表示装置 |
| JP5223980B2 (ja) | 2011-04-21 | 2013-06-26 | 大日本印刷株式会社 | 色材分散液、カラーフィルター用着色樹脂組成物、カラーフィルター、液晶表示装置及び有機発光表示装置 |
| JP5221787B2 (ja) | 2011-04-21 | 2013-06-26 | 大日本印刷株式会社 | 色材、及びその製造方法 |
-
2013
- 2013-12-05 JP JP2013251876A patent/JP5895925B2/ja active Active
-
2014
- 2014-10-02 CN CN201710386740.7A patent/CN107266634B/zh active Active
- 2014-10-02 KR KR1020167014472A patent/KR102210034B1/ko active Active
- 2014-10-02 US US15/101,360 patent/US10570287B2/en active Active
- 2014-10-02 CN CN201480065528.5A patent/CN105792921B/zh active Active
- 2014-10-02 WO PCT/JP2014/076462 patent/WO2015083426A1/ja not_active Ceased
- 2014-12-04 TW TW103142121A patent/TWI634149B/zh active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0445456A (ja) * | 1990-06-13 | 1992-02-14 | Fuji Photo Film Co Ltd | 静電写真用液体現像剤 |
| JP2000104005A (ja) * | 1998-09-28 | 2000-04-11 | Dainippon Printing Co Ltd | 顔料分散剤、感光性着色組成物及び遮光層用組成物 |
| JP2010083923A (ja) * | 2008-09-29 | 2010-04-15 | Dainippon Printing Co Ltd | 顔料分散剤、カラーフィルタ用ネガ型レジスト組成物、カラーフィルタ及び液晶表示装置 |
| JP2012252319A (ja) * | 2011-05-11 | 2012-12-20 | Jsr Corp | 着色組成物、カラーフィルタ及び表示素子 |
| JP2013230416A (ja) * | 2012-04-27 | 2013-11-14 | Ricoh Co Ltd | 金属粒子分散剤、金属粒子分散インク及び導電性パターン形成方法 |
| WO2014061750A1 (ja) * | 2012-10-18 | 2014-04-24 | 大日本印刷株式会社 | 分散剤、導電性基板用金属粒子分散体、及び導電性基板の製造方法 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11084934B2 (en) * | 2016-06-28 | 2021-08-10 | Dai Nippon Printing Co., Ltd. | Color material dispersion liquid, color resin composition, color filter, liquid crystal display device, and light-emitting display device |
| US11655371B2 (en) | 2016-06-28 | 2023-05-23 | Dai Nippon Printing Co., Ltd. | Color material dispersion liquid, color resin composition, color filter, liquid crystal display device, and light-emitting display device |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107266634B (zh) | 2019-09-24 |
| JP5895925B2 (ja) | 2016-03-30 |
| TW201522477A (zh) | 2015-06-16 |
| JP2015107471A (ja) | 2015-06-11 |
| KR102210034B1 (ko) | 2021-02-01 |
| CN105792921B (zh) | 2017-06-09 |
| US10570287B2 (en) | 2020-02-25 |
| KR20160096232A (ko) | 2016-08-12 |
| TWI634149B (zh) | 2018-09-01 |
| CN105792921A (zh) | 2016-07-20 |
| CN107266634A (zh) | 2017-10-20 |
| US20160376443A1 (en) | 2016-12-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5895925B2 (ja) | 非水系分散剤、カラーフィルタ用色材分散液、カラーフィルタ、液晶表示装置及び有機発光表示装置 | |
| JP5403175B2 (ja) | カラーフィルタ用色材分散液、カラーフィルタ用着色樹脂組成物、カラーフィルタ、並びに、液晶表示装置及び有機発光表示装置 | |
| JP6217307B2 (ja) | 色材、色材分散液、カラーフィルタ用着色樹脂組成物、カラーフィルタ、液晶表示装置、及び有機発光表示装置 | |
| JP5772919B2 (ja) | カラーフィルタ用色材分散液、色材、カラーフィルタ、液晶表示装置、及び、有機発光表示装置 | |
| JPWO2011108496A1 (ja) | 顔料分散液、カラーフィルタ用ネガ型レジスト組成物、カラーフィルタ、並びに、液晶表示装置及び有機発光表示装置 | |
| WO2016103864A1 (ja) | カラーフィルタ用色材分散液、カラーフィルタ用着色樹脂組成物、色材、カラーフィルタ、液晶表示装置及び発光表示装置 | |
| JP2018003013A (ja) | 色材分散液、着色樹脂組成物、カラーフィルター、液晶表示装置、及び、発光表示装置 | |
| JP6152879B2 (ja) | 色材分散液、カラーフィルタ用着色樹脂組成物、カラーフィルタの製造方法、液晶表示装置の製造方法、及び発光表示装置の製造方法 | |
| JP6078999B2 (ja) | カラーフィルタ用赤色顔料分散液及びその製造方法、カラーフィルタ用赤色感光性樹脂組成物及びその製造方法、カラーフィルタ、並びに、液晶表示装置及び有機発光表示装置 | |
| JP6056844B2 (ja) | カラーフィルタ用色材分散液、カラーフィルタ用着色樹脂組成物、色材、カラーフィルタ、液晶表示装置及び発光表示装置 | |
| JP2017037302A (ja) | 感光性着色樹脂組成物、カラーフィルタ及びその製造方法、液晶表示装置、並びに発光表示装置 | |
| JP6256126B2 (ja) | 色材分散組成物、カラーフィルタ、液晶表示装置、及び有機発光表示装置 | |
| WO2018025498A1 (ja) | 着色組成物、カラーフィルタ及びその製造方法、液晶表示装置、並びに、発光表示装置 | |
| JP6295600B2 (ja) | 色材、色材分散液、カラーフィルタ用着色樹脂組成物、カラーフィルタ、液晶表示装置、及び有機発光表示装置 | |
| JP6319205B2 (ja) | カラーフィルタ用色材分散液、色材、カラーフィルタ、液晶表示装置、及び、有機発光表示装置 | |
| JP6953851B2 (ja) | 高分子分散剤及びその製造方法、色材分散液、着色樹脂組成物、カラーフィルタ、液晶表示装置、及び発光表示装置 | |
| JP6582944B2 (ja) | 色材分散液、カラーフィルタ用着色樹脂組成物、カラーフィルタ及びその製造方法、液晶表示装置、並びに発光表示装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14868449 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20167014472 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15101360 Country of ref document: US |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14868449 Country of ref document: EP Kind code of ref document: A1 |