WO2014208740A1 - 含窒素カーボンアロイの製造方法、含窒素カーボンアロイ及び燃料電池触媒 - Google Patents
含窒素カーボンアロイの製造方法、含窒素カーボンアロイ及び燃料電池触媒 Download PDFInfo
- Publication number
- WO2014208740A1 WO2014208740A1 PCT/JP2014/067220 JP2014067220W WO2014208740A1 WO 2014208740 A1 WO2014208740 A1 WO 2014208740A1 JP 2014067220 W JP2014067220 W JP 2014067220W WO 2014208740 A1 WO2014208740 A1 WO 2014208740A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- nitrogen
- carbon alloy
- group
- carbon
- containing carbon
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 CC=CC(OCc1nc(COc2ccncc2)nc(COC(C=CC)=CC=C=C)n1)=CC=* Chemical compound CC=CC(OCc1nc(COc2ccncc2)nc(COC(C=CC)=CC=C=C)n1)=CC=* 0.000 description 3
- VXVXLUNBDNNDAF-UHFFFAOYSA-N C(c1ccccc1)c1nc(Cc2ccncc2)nc(Cc2ccncc2)n1 Chemical compound C(c1ccccc1)c1nc(Cc2ccncc2)nc(Cc2ccncc2)n1 VXVXLUNBDNNDAF-UHFFFAOYSA-N 0.000 description 1
- HHDSCPCUTZSXOW-CYMBIFCZSA-N C/C=C\C(\C1=NC(c2ccncc2)=[I]C(C)=N1)=C/C=C Chemical compound C/C=C\C(\C1=NC(c2ccncc2)=[I]C(C)=N1)=C/C=C HHDSCPCUTZSXOW-CYMBIFCZSA-N 0.000 description 1
- ZUSRBHIHGVVFKN-UHFFFAOYSA-N CC1(C(Nc2cnccc2)=O)N=C(C(Nc2cnccc2)=O)N=C(C(Nc2cnccc2)=O)N1 Chemical compound CC1(C(Nc2cnccc2)=O)N=C(C(Nc2cnccc2)=O)N=C(C(Nc2cnccc2)=O)N1 ZUSRBHIHGVVFKN-UHFFFAOYSA-N 0.000 description 1
- LIESXEPMSSBHNG-UHFFFAOYSA-N O=C(c1cc(C(Nc2cccnc2)=O)cc(C(Nc2cnccc2)=O)c1)Nc1cnccc1 Chemical compound O=C(c1cc(C(Nc2cccnc2)=O)cc(C(Nc2cnccc2)=O)c1)Nc1cnccc1 LIESXEPMSSBHNG-UHFFFAOYSA-N 0.000 description 1
- PFHCOOYBRXRFAM-UHFFFAOYSA-N O=C(c1cc(C(Nc2ccncc2)=O)cc(C(Nc2ccncc2)=O)c1)Nc1ccncc1 Chemical compound O=C(c1cc(C(Nc2ccncc2)=O)cc(C(Nc2ccncc2)=O)c1)Nc1ccncc1 PFHCOOYBRXRFAM-UHFFFAOYSA-N 0.000 description 1
- IYALGNJOJQVNEL-UHFFFAOYSA-N O=C(c1cc(C(Nc2ccncc2)=O)nc(C(Nc2ccncc2)=O)c1)Nc1ccncc1 Chemical compound O=C(c1cc(C(Nc2ccncc2)=O)nc(C(Nc2ccncc2)=O)c1)Nc1ccncc1 IYALGNJOJQVNEL-UHFFFAOYSA-N 0.000 description 1
- RZAGTEOPJCTJMA-UHFFFAOYSA-N O=C(c1nc(C(Nc2ccncc2)=O)nc(C(Nc2ccncc2)=O)c1)Nc1ccncc1 Chemical compound O=C(c1nc(C(Nc2ccncc2)=O)nc(C(Nc2ccncc2)=O)c1)Nc1ccncc1 RZAGTEOPJCTJMA-UHFFFAOYSA-N 0.000 description 1
- WPPCVRQXHZNCQA-UHFFFAOYSA-N O=C(c1nc(C(Nc2ccncc2)=O)nc(C(Nc2ccncc2)=O)n1)Nc1ccncc1 Chemical compound O=C(c1nc(C(Nc2ccncc2)=O)nc(C(Nc2ccncc2)=O)n1)Nc1ccncc1 WPPCVRQXHZNCQA-UHFFFAOYSA-N 0.000 description 1
- CBAHNJPYGBVJLV-UHFFFAOYSA-N O=C(c1nc(C(Nc2cnccc2)=O)nc(C(Nc2cnccc2)=O)c1)Nc1cccnc1 Chemical compound O=C(c1nc(C(Nc2cnccc2)=O)nc(C(Nc2cnccc2)=O)c1)Nc1cccnc1 CBAHNJPYGBVJLV-UHFFFAOYSA-N 0.000 description 1
- UDVTWJJQTPNZAG-UHFFFAOYSA-N OC(c1cc(C(Nc2cnccc2)=O)cc(C(Nc2cnccc2)=O)n1)Nc1cnccc1 Chemical compound OC(c1cc(C(Nc2cnccc2)=O)cc(C(Nc2cnccc2)=O)n1)Nc1cnccc1 UDVTWJJQTPNZAG-UHFFFAOYSA-N 0.000 description 1
- KEFOGWOYGLVUKA-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c2ccncc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c2ccccc2)nc(-c2ccncc2)n1 KEFOGWOYGLVUKA-UHFFFAOYSA-N 0.000 description 1
- VACMVCGPXXITOG-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ccncc2)nc(-c2ccncc2)n1 Chemical compound c(cc1)ccc1-c1nc(-c2ccncc2)nc(-c2ccncc2)n1 VACMVCGPXXITOG-UHFFFAOYSA-N 0.000 description 1
- YTGOYWZAGSWPOK-UHFFFAOYSA-N c(cc1)ccc1-c1nc(-c2ccncc2)ncn1 Chemical compound c(cc1)ccc1-c1nc(-c2ccncc2)ncn1 YTGOYWZAGSWPOK-UHFFFAOYSA-N 0.000 description 1
- JJUHYOUNRYJQOE-UHFFFAOYSA-N c1cc(-c2nc(-c3ccncc3)ncn2)cnc1 Chemical compound c1cc(-c2nc(-c3ccncc3)ncn2)cnc1 JJUHYOUNRYJQOE-UHFFFAOYSA-N 0.000 description 1
- CMBFWILAGNQSCO-UHFFFAOYSA-N c1cc(-c2nc(-c3ccncc3)ncn2)ncc1 Chemical compound c1cc(-c2nc(-c3ccncc3)ncn2)ncc1 CMBFWILAGNQSCO-UHFFFAOYSA-N 0.000 description 1
- IZPIJKHBNCGHTI-UHFFFAOYSA-N c1ccnc(-c2nc(-c3ccncc3)nc(-c3ccncc3)n2)c1 Chemical compound c1ccnc(-c2nc(-c3ccncc3)nc(-c3ccncc3)n2)c1 IZPIJKHBNCGHTI-UHFFFAOYSA-N 0.000 description 1
- OFDVABAUFQJWEZ-UHFFFAOYSA-N c1cncc(-c2cnccc2)c1 Chemical compound c1cncc(-c2cnccc2)c1 OFDVABAUFQJWEZ-UHFFFAOYSA-N 0.000 description 1
- CBMYFVSIIYILRH-UHFFFAOYSA-N c1cnccc1-c1nc(-c2ccncc2)nc(-c2ccncc2)n1 Chemical compound c1cnccc1-c1nc(-c2ccncc2)nc(-c2ccncc2)n1 CBMYFVSIIYILRH-UHFFFAOYSA-N 0.000 description 1
- FDGOAUPCDXMTPA-UHFFFAOYSA-N c1cnccc1-c1nc(-c2ccncc2)ncn1 Chemical compound c1cnccc1-c1nc(-c2ccncc2)ncn1 FDGOAUPCDXMTPA-UHFFFAOYSA-N 0.000 description 1
- OAOYLHDSLIUMRV-UHFFFAOYSA-N c1cnccc1-c1ncncn1 Chemical compound c1cnccc1-c1ncncn1 OAOYLHDSLIUMRV-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/86—Inert electrodes with catalytic activity, e.g. for fuel cells
- H01M4/90—Selection of catalytic material
- H01M4/9008—Organic or organo-metallic compounds
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B32/00—Carbon; Compounds thereof
- C01B32/05—Preparation or purification of carbon not covered by groups C01B32/15, C01B32/20, C01B32/25, C01B32/30
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
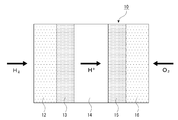
- H01M2008/1095—Fuel cells with polymeric electrolytes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/50—Fuel cells
Definitions
- the present invention relates to a method for producing a nitrogen-containing carbon alloy, a nitrogen-containing carbon alloy, and a fuel cell catalyst. Specifically, the present invention relates to a method for producing a nitrogen-containing carbon alloy including a step of firing a precursor containing a heteroaromatic ring compound having a nitrogen-containing aromatic group and an inorganic metal salt, a nitrogen-containing carbon alloy, and a nitrogen-containing compound. Fuel cell catalyst using carbon alloy
- a noble metal catalyst using platinum (Pt), palladium (Pd), etc. is used as a catalyst having a high oxygen reduction activity, for example, in a solid polymer electrolyte fuel cell used in automobiles, household electric heat supply systems, etc.
- platinum palladium
- noble metal-based catalysts are expensive, it is difficult to further spread them. For this reason, technological development of a catalyst in which platinum is greatly reduced or a catalyst formed without using platinum is being promoted.
- Patent Document 1 discloses a polymer electrolyte fuel cell catalyst comprising a composite of an s-triazine ring derivative and a metal.
- Patent Document 2 discloses a nitrogen-containing carbon alloy catalyst produced by firing a nitrogen-containing heterocyclic compound having a molecular weight of 60 to 2000 and an inorganic metal or inorganic metal salt.
- Non-Patent Document 1 discloses a non-platinum catalyst for a fuel cell prepared by heating and firing a mixture of 2- pyridyltriazine Fe complex [Fe (TPTZ) 2 ] supported on carbon.
- TPTZ 2- pyridyltriazine Fe complex
- it has been proposed to fire a mixture of 2-pyridyltriazine and a metal complex, and there is no description about using an inorganic metal or firing an inorganic metal.
- the nitrogen-containing carbon alloy catalyst containing a nitrogen-containing compound can exhibit catalytic activity without using platinum.
- recent applications such as fuel cells are required to have higher oxygen reduction activity, and the oxygen reduction activity of conventional carbon catalysts may be insufficient. For this reason, it has been desired to produce a nitrogen-containing carbon alloy that can exhibit higher oxygen reduction activity.
- the yield is poor, and further improvement in productivity has been demanded.
- the present inventors proceeded with studies for the purpose of producing a nitrogen-containing carbon alloy having higher oxygen reduction activity. Furthermore, the present inventors have studied for the purpose of increasing the yield of nitrogen-containing carbon alloy and increasing productivity.
- the present inventors calcined a precursor containing a heteroaromatic ring compound having a nitrogen-containing aromatic group having a specific structure and an inorganic metal salt, It has been found that oxygen reduction activity can be sufficiently enhanced by producing a nitrogen-containing carbon alloy. Furthermore, the present inventors succeeded in increasing the yield of nitrogen-containing carbon alloy, and completed the present invention. Specifically, the present invention has the following configuration.
- a precursor containing an inorganic metal salt and at least one selected from a heteroaromatic ring compound having a nitrogen-containing aromatic group represented by the following general formula (1), a salt thereof and a hydrate thereof is calcined.
- a method for producing a nitrogen-containing carbon alloy comprising a step;
- A represents an atomic group composed of a 5- to 11-membered non-fused heteroaromatic ring
- L represents a single bond or a (x + 1) -valent linking group
- B represents a hydrogen atom, a substituted or Represents an unsubstituted aromatic group, or a substituted or unsubstituted nitrogen-containing aromatic group, and at least one B is a substituted or unsubstituted nitrogen-containing aromatic group, and at least one of the nitrogen-containing aromatic groups ,
- Any one or both of the ring skeleton constituent atoms at the 3-position and the 4-position with respect to the bonding site with L are nitrogen atoms.
- the number of heteroatoms in the non-fused heteroaromatic ring of A is the same as or more than the number of heteroatoms per one nitrogen-containing aromatic group of B.
- X and y each independently represents an integer of 1 or more.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group represented by the general formula (1) is a nitrogen-containing carbon alloy according to [1] or [2] represented by the following general formula (2).
- Q 1 to Q 3 each independently represent a hetero atom or a carbon atom, at least one of Q 1 to Q 3 is a nitrogen atom, b1 to b3 are each independently a hydrogen atom, Represents a substituted or unsubstituted aromatic group, or a substituted or unsubstituted nitrogen-containing aromatic group, and at least one of b1 to b3 is a substituted or unsubstituted nitrogen-containing aromatic group, and a nitrogen-containing aromatic group
- one or both of the ring skeleton constituent atoms at the 3-position and the 4-position with respect to the binding site are nitrogen atoms.
- the number of heteroatoms in the non-condensed heteroaromatic ring containing Q 1 to Q 3 is the same as or larger than the number of heteroatoms per one nitrogen-containing aromatic group of b1 to b3.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group is a compound represented by the following formula (3) or (4): the nitrogen-containing carbon alloy according to any one of [1] to [6] Production method.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group represented by the general formula (1) is a nitrogen-containing carbon alloy according to [1] or [2] represented by the following general formula (5).
- A represents an atomic group composed of a 5- to 11-membered non-fused heteroaromatic ring
- L represents a single bond or a (x + 1) -valent linking group
- B represents a hydrogen atom, substituted Or an unsubstituted aromatic group or a substituted or unsubstituted nitrogen-containing aromatic group, and at least one B is a substituted or unsubstituted nitrogen-containing aromatic group, and at least one of the nitrogen-containing aromatic groups
- any one or both of the ring skeleton constituting atoms at the 3rd and 4th positions relative to the bonding site with L are nitrogen atoms.
- the number of heteroatoms in the non-fused heteroaromatic ring of A is the same as or more than the number of heteroatoms per one nitrogen-containing aromatic group of B.
- X represents an integer of 1 or more.
- the method for producing a nitrogen-containing carbon alloy according to [1] or [2], wherein the heteroaromatic ring compound having a nitrogen-containing aromatic group is a compound selected from the following compound group.
- [16] The method for producing a nitrogen-containing carbon alloy according to [14] or [15], wherein the organometallic complex is an acetylacetone iron (II) complex.
- the firing step is a step of firing the precursor at 400 ° C. or higher.
- a nitrogen-containing carbon alloy having a sufficiently high oxygen reduction activity can be obtained.
- the nitrogen-containing carbon alloy obtained by the production method of the present invention can be used as a carbon catalyst, and such a carbon catalyst is preferably used for a fuel cell or an environmental catalyst.
- the yield of a nitrogen-containing carbon alloy can be raised and productivity can be improved.
- a numerical range expressed using “to” means a range including numerical values described before and after “to” as a lower limit value and an upper limit value.
- the present invention includes a step of firing a precursor containing an inorganic metal salt and at least one selected from a heteroaromatic ring compound having a nitrogen-containing aromatic group having a specific structure, a salt thereof and a hydrate thereof.
- the present invention relates to a method for producing a nitrogen carbon alloy.
- the hetero aromatic ring compound which has a nitrogen-containing aromatic group, an inorganic metal salt, etc. are demonstrated in detail.
- Heteroaromatic ring compound having nitrogen-containing aromatic group used in the present invention is represented by the following general formula (1). Note that the heteroaromatic ring compound having a nitrogen-containing aromatic group includes salts thereof or hydrates thereof.
- A represents an atomic group composed of a 5- to 11-membered non-condensed heteroaromatic ring, and L represents a single bond or a (x + 1) -valent linking group.
- B represents a hydrogen atom, a substituted or unsubstituted aromatic group, or a substituted or unsubstituted nitrogen-containing aromatic group, and at least one B is a substituted or unsubstituted nitrogen-containing aromatic group, In at least one of the group groups, either one or both of the ring skeleton constituent atoms at the 3rd and 4th positions relative to the bonding site with L is a nitrogen atom.
- the number of heteroatoms in the non-fused heteroaromatic ring of A is the same as or more than the number of heteroatoms per one nitrogen-containing aromatic group of B.
- X and y each independently represents an integer of 1 or more.
- A represents an atomic group composed of a 5- to 11-membered non-condensed heteroaromatic ring.
- the non-fused heteroaromatic ring is a heteroaromatic ring having no fused ring, and the atomic group composed of such a non-fused heteroaromatic ring has at least one non-fused heteroaromatic ring.
- the atomic group composed of a non-fused heteroaromatic ring may be composed of two or more non-fused heteroaromatic rings, but is preferably composed of one non-fused heteroaromatic ring.
- the number of ring members in one non-fused heteroaromatic ring may be 5 to 11, preferably 5 to 10, more preferably 5 to 8, and still more preferably 5 or 6.
- hetero atom constituting the 5- to 11-membered non-condensed heteroaromatic ring examples include 1 to 3 heteroatoms selected from the group consisting of a nitrogen atom, an oxygen atom and a sulfur atom.
- the non-fused heteroaromatic ring examples include a pyridine ring, a pyrimidine ring, a triazine ring, an imidazole ring, a pyrroline ring, an imidazole group, a furan ring, and a thiophene ring.
- a pyridine ring, a pyrimidine ring, and a triazine ring Preferred examples include an imidazole ring and a pyrroline ring.
- L represents a single bond or a (x + 1) -valent linking group.
- the (x + 1) -valent linking group includes a substituted or unsubstituted aromatic group, a substituted or unsubstituted alkylene group, a substituted or unsubstituted alkenylene group, a substituted or unsubstituted alkynylene group, a substituted or unsubstituted cycloalkylene X ⁇ 1 groups from the group, —CO—, —CO 2 —, —O—, —NH—, —SO—, —SO 2 —, —S—, —CONH—, —NHCO—, or combinations thereof.
- the aromatic group preferably has 6 to 20 carbon atoms, more preferably a phenylene group, a biphenylene group, or a naphthylene group, further preferably a phenylene group or a biphenylene group, and more preferably a phenylene group. Particularly preferred.
- the alkylene group preferably has 1 to 20 carbon atoms, more preferably 1 to 15 carbon atoms, still more preferably 1 to 10 carbon atoms, and particularly preferably 1 to 6 carbon atoms.
- the alkenylene group preferably has 2 to 20 carbon atoms, more preferably 2 to 15 carbon atoms, still more preferably 2 to 10 carbon atoms, and particularly preferably 2 to 6 carbon atoms.
- the alkynylene group preferably has 2 to 20 carbon atoms, more preferably 2 to 15 carbon atoms, still more preferably 2 to 10 carbon atoms, and particularly preferably 2 to 6 carbon atoms.
- the cycloalkylene group preferably has 4 to 20 carbon atoms, more preferably 4 to 15 carbon atoms, still more preferably 5 to 12 carbon atoms, and particularly preferably 5 to 10 carbon atoms.
- the substituent When having a substituent, the substituent includes a halogen atom (fluorine atom, chloro atom, bromine atom or iodine atom), hydroxy group, cyano group, aliphatic group (aralkyl group, cycloalkyl group, active methine group, etc.
- halogen atom fluorine atom, chloro atom, bromine atom or iodine atom
- hydroxy group cyano group
- aliphatic group aralkyl group, cycloalkyl group, active methine group, etc.
- a halogen atom (a fluorine atom, a chloro atom, a bromine atom or an iodine atom), a vinyl group, an allyl group, an acetylenyl group, an aryl group (regarding the position of substitution) and an amino group are preferable as a substituent.
- L represents a single bond or a divalent linking group
- L represents a trivalent linking group.
- divalent linking group examples include a substituted or unsubstituted alkylene group, a substituted or unsubstituted alkenylene group, an alkynylene group, a substituted or unsubstituted cycloalkylene group, a substituted or unsubstituted phenylene group, a substituted or unsubstituted group.
- Examples of the trivalent linking group include:>CH—,> N—, a group obtained by removing one hydrogen atom from a group having a substituent among the groups listed as examples of the divalent linking group, and the like. Can be mentioned.
- L may be a group obtained by combining two or more of the above linking groups. For example, a combination of two or more substituted or unsubstituted alkylene groups, alkenylene groups, and alkynylene groups, or a combination of two or more substituted or unsubstituted alkylene groups, alkenylene groups, alkynylene groups, and substituted or unsubstituted phenylene groups. It can be based.
- the plurality of L may be the same or different.
- the (x + 1) -valent linking group include a phenylene group, an alkylene group having 1 to 6 carbon atoms, an alkenylene group having 2 to 6 carbon atoms, an alkynylene group having 2 to 6 carbon atoms, —CO 2 —, —CONH—, — O- or a combination of x + 1 valences in which x-1 arbitrary hydrogen atoms are removed from a combination thereof, phenylene group, alkylene group having 1 to 4 carbon atoms, alkenylene group having 2 to 6 carbon atoms Further, an alkynylene group having 2 to 6 carbon atoms, —CONH—, or a combination of x + 1 valences in which any hydrogen atom of x-1 is removed from a combination thereof is more preferable.
- B represents a hydrogen atom, a substituted or unsubstituted aromatic group, or a substituted or unsubstituted nitrogen-containing aromatic group, and at least one B is a substituted or unsubstituted nitrogen-containing aromatic group
- the nitrogen-containing aromatic group means a group having a nitrogen atom as a constituent atom of the aromatic group.
- at least one of the Bs may be a substituted or unsubstituted nitrogen-containing aromatic group, but it is preferable that all Bs are substituted or unsubstituted nitrogen-containing aromatic groups.
- the nitrogen-containing aromatic group is preferably a pyridyl group, a pyrimidyl group, a triazyl group, or an imidazolyl group.
- a plurality of B may be the same or different.
- B is preferably a hydrogen atom, a phenyl group, a pyridyl group, a pyrimidyl group, a triazine group, or an imidazolyl group.
- the number of carbon atoms is preferably 6 to 14, more preferably 6 to 10, and particularly preferably a phenyl group.
- At least one B is a nitrogen-containing aromatic group, and in this case, it is preferably 5 to 11 members, more preferably 5 to 8 members, more preferably 5 or 6 members, and a pyridyl group , A pyrimidyl group, a triazine group, or an imidazolyl group is particularly preferable.
- aromatic group or nitrogen-containing aromatic group represented by B has a substituent
- preferable substituents include a halogen atom (a fluorine atom, a chloro atom, a bromine atom or an iodine atom), a hydroxy group, and a cyano group.
- Aliphatic groups including aralkyl groups, cycloalkyl groups, active methine groups, etc.
- vinyl groups including aralkyl groups, cycloalkyl groups, active methine groups, etc.
- vinyl groups allyl groups, acetylenyl groups, aryl groups (regardless of the position of substitution), acyl groups, aliphatic oxy groups (alkoxy groups) Or an aryloxy group, a heterocyclic oxy group, an aliphatic carbonyl group, an arylcarbonyl group, a heterocyclic carbonyl group, an aliphatic oxycarbonyl group.
- Substituents containing unsaturated groups are more preferred, vinyl group, allyl group, acetylenyl group, aryl group (phenyl group, naphthyl group, phenanthrene group, anthracenyl group, triphenyl group, pyrenyl group, perylenyl group, benzhydryl group, benzyl group. Group, cinamyl group, cumenyl group, methicyl group, phenylethyl group, styryl group, tolyl group, trityl group, xylyl group). These substituent groups may be further substituted, and examples of the further substituent include groups selected from the substituents and heteroaromatic groups described above (regardless of the position of substitution).
- either one or both of the ring skeleton constituting atoms at the 3-position and the 4-position with respect to the bonding site with L are nitrogen atoms.
- either one or both of the ring skeleton constituting atoms at the 3-position and the 4-position with respect to the bonding site with L may be a nitrogen atom.
- it is more preferable that either one or both of the ring skeleton constituent atoms at the 3-position and the 4-position with respect to the bonding site with L is a nitrogen atom.
- the ring skeleton constituting atom at the 3rd or 4th position with respect to the bonding position with L is a nitrogen atom, but the ring skeleton constituting atom other than the corresponding site is a nitrogen atom. May be.
- the ring skeleton constituent atoms at the 3rd and 1st positions relative to the bond position with L are nitrogen atoms
- the ring skeleton constituent atoms at the 3rd and 2nd positions are nitrogen atoms
- the ring skeleton constituent atom may be a nitrogen atom.
- the ring skeleton constituting atom at the 3rd or 4th position is a nitrogen atom, for example, it is preferred that one or both of the ring skeleton constituting atoms at the 3rd and 4th positions are nitrogen atoms.
- the atomic group composed of the non-condensed heteroaromatic ring includes a triazine ring, it is preferable that the ring skeleton constituent atom at the 2-position in the nitrogen-containing aromatic group is not a nitrogen atom.
- the number of heteroatoms in the non-condensed heteroaromatic ring of A is the same as or larger than the number of heteroatoms per one nitrogen-containing aromatic group of B.
- the number of heteroatoms per all nitrogen-containing aromatic groups satisfies the above condition.
- the atomic group of A is composed of two or more non-fused heteroaromatic rings
- the number of heteroatoms in the non-fused heteroaromatic ring of A represents the number of heteroatoms per one non-fused heteroaromatic ring. .
- x and y each independently represent an integer of 1 or more.
- x is preferably an integer of 1 to 6, more preferably an integer of 1 to 5, more preferably an integer of 1 to 4, and still more preferably an integer of 1 to 3. 1 or 2 is particularly preferable.
- y is preferably an integer of 1 to 8, more preferably an integer of 1 to 6, more preferably an integer of 1 to 5, and more preferably an integer of 1 to 3. Further preferred.
- the nitrogen-containing aromatic group is coordinated with an inorganic metal or a salt thereof to form a porous structure.
- the central atomic group of the heteroaromatic ring compound is thermally decomposed to form an oxygen reduction reaction (ORR) active site, and a highly active nitrogen-containing carbon alloy can be obtained.
- ORR oxygen reduction reaction
- the thermal decomposition of the non-fused heteroaromatic part (central atomic group) can be easily advanced. . Thereby, formation of an oxygen reduction reaction (ORR) active site can be promoted, and a higher activity nitrogen-containing carbon alloy can be obtained.
- the coordination between the inorganic metal and the nitrogen-containing aromatic group is such that either one or both of the ring skeleton constituent atoms at the 3-position and the 4-position with respect to the bonding position with L in the nitrogen-containing aromatic group are nitrogen atoms. In some cases, it becomes easier to proceed. This is because, when the ring skeleton constituent atom at the 2-position is a nitrogen atom, the distance between the hetero atom of the central atomic group and the nitrogen atom of the nitrogen-containing aromatic group is reduced, so that the inorganic metal is the central atomic group. This is thought to be due to coordination with a heteroatom to form a complex.
- Such coordination is not a preferred coordination mode of the inorganic metal and the nitrogen-containing aromatic group, and causes the thermal decomposition of the central atomic group to hardly proceed. For this reason, it is considered that when the ring skeleton constituent atom at the 2-position is a nitrogen atom, formation of an oxygen reduction reaction (ORR) active site is suppressed, and the catalytic activity of the nitrogen-containing carbon alloy is lowered.
- ORR oxygen reduction reaction
- the heteroaromatic ring compound having a nitrogen-containing aromatic group represented by the general formula (1) is preferably represented by the following general formula (2).
- Q 1 to Q 3 each independently represent a hetero atom or a carbon atom, at least one of Q 1 to Q 3 is a nitrogen atom, b1 to b3 are each independently a hydrogen atom, Represents a substituted or unsubstituted aromatic group, or a substituted or unsubstituted nitrogen-containing aromatic group, and at least one of b1 to b3 is a substituted or unsubstituted nitrogen-containing aromatic group, In at least one, either one or both of the ring skeleton constituent atoms at the 3-position and the 4-position with respect to the binding site are nitrogen atoms.
- the number of heteroatoms in the non-condensed heteroaromatic ring containing Q 1 to Q 3 is the same as or larger than the number of heteroatoms per one nitrogen-containing aromatic group of b1 to b3.
- the binding site of b1 to b3 refers to a binding site of b1 to b3 and a linking group between non-condensed heteroaromatic rings including Q 1 to Q 3 .
- Q 1 to Q 3 each independently represents a hetero atom or a carbon atom, and at least one of Q 1 to Q 3 represents a nitrogen atom.
- Q 1 to Q 3 are each independently preferably a nitrogen atom, a sulfur atom, or a carbon atom, particularly preferably a nitrogen atom or a carbon atom, and any one is a nitrogen atom.
- the atoms in Q 1 to Q 3 may be ionized.
- it is preferable that at least one of Q 1 ⁇ Q 3 is a nitrogen atom, and more preferably all of Q 1 ⁇ Q 3 is a nitrogen atom.
- B1 to b3 in the general formula (2) have the same meaning as B in the general formula (1), and the preferred range is also the same.
- b1 to b3 preferably represent a substituted or unsubstituted nitrogen-containing aromatic group, and b1 to b3 are preferably 5 or 6 membered rings, preferably 6 membered rings. Is more preferable.
- substituents that can be taken by b1 to b3 include the same substituents as those that can be taken by B in the general formula (1).
- b1 to b3 are preferably a pyridyl group, a pyrimidyl group or a triazyl group, and more preferably a pyridyl group or a pyrimidyl group.
- either one or both of the ring skeleton constituting atoms at the 3-position and the 4-position with respect to the bonding site with L are nitrogen atoms.
- the ring skeleton constituting atom at the 2-position with respect to the binding site is not a nitrogen atom.
- Q 1 and Q 3 are preferably carbon atoms
- Q 1 And Q 2 are preferably carbon atoms.
- Q 2 and Q 3 are preferably carbon atoms. That is, the distance between the nitrogen atom constituting the central atomic group and the nitrogen atom of the nitrogen-containing aromatic group represented by b1 to b3 is preferably a certain distance or more, and the distance between the nitrogen atoms of the nitrogen-containing aromatic group Is preferably 4 atoms or more.
- the number of heteroatoms in the non-condensed heteroaromatic ring containing Q 1 to Q 3 is the same as or larger than the number of heteroatoms per one nitrogen-containing aromatic group of b1 to b3.
- the number of heteroatoms per one nitrogen-containing aromatic group satisfies the above condition.
- the number of heteroatoms in the non-fused heteroaromatic ring represents the number of heteroatoms per one non-fused heteroaromatic ring.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group represented by the general formula (1) is preferably a compound represented by the following formula (3) or (4).
- the heteroaromatic ring compound having a nitrogen-containing aromatic group may be a compound represented by the following general formula (5).
- A represents an atomic group composed of a 5- to 11-membered non-fused heteroaromatic ring
- L represents a single bond or a (x + 1) -valent linking group
- B represents a hydrogen atom, substituted Or an unsubstituted aromatic group or a substituted or unsubstituted nitrogen-containing aromatic group, and at least one B is a substituted or unsubstituted nitrogen-containing aromatic group, and at least one of the nitrogen-containing aromatic groups
- any one or both of the ring skeleton constituting atoms at the 3rd and 4th positions relative to the bonding site with L are nitrogen atoms.
- the number of heteroatoms in the non-fused heteroaromatic ring of A is the same as or more than the number of heteroatoms per one nitrogen-containing aromatic group of B.
- X represents an integer of 1 or more.
- a in the general formula (5) is the same as A in the general formula (1), and the preferred range is also the same.
- L in General formula (5) is the same as L in General formula (1), and its preferable range is also the same.
- B represents a hydrogen atom, a substituted or unsubstituted aromatic group, or a substituted or unsubstituted nitrogen-containing aromatic group, and at least one B is a substituted or unsubstituted nitrogen-containing aromatic group Represents a group.
- the nitrogen-containing aromatic group means a group having a nitrogen atom as a constituent atom of the aromatic group.
- B in the general formula (5) is the same as B in the general formula (1), and the preferred range is also the same.
- at least one of B may be a substituted or unsubstituted nitrogen-containing aromatic group, but it is preferable that all B are substituted or unsubstituted nitrogen-containing aromatic groups.
- the nitrogen-containing aromatic group is preferably a pyridyl group, a pyrimidyl group, a triazyl group, or an imidazolyl group.
- one or both of the ring skeleton constituting atoms at the 3-position and 4-position with respect to the bonding site with L are nitrogen atoms. is there.
- the substituted or unsubstituted nitrogen-containing aromatic group represented by at least one B one or both of the ring skeleton constituting atoms at the 3-position and the 4-position with respect to the bonding site with L are nitrogen atoms.
- the preferred configuration is the same as B in the general formula (1).
- the number of heteroatoms in the non-condensed heteroaromatic ring of A is the same as or larger than the number of heteroatoms per one nitrogen-containing aromatic group of B.
- the number of heteroatoms per all nitrogen-containing aromatic groups satisfies the above condition.
- x represents an integer of 1 or more.
- x is preferably an integer of 1 to 6, more preferably an integer of 1 to 5, more preferably an integer of 1 to 4, and still more preferably an integer of 1 to 3. 1 or 2 is particularly preferable.
- heteroaromatic ring compound having a nitrogen-containing aromatic group represented by the general formula (1) include the following compounds. However, the present invention is not limited to the following specific examples.
- the nitrogen-containing aromatic group of a terminal part corresponds to the non-condensed heteroaromatic ring represented by A of General formula (1), and the connection part containing a benzene ring is common. It corresponds to the linking group represented by L in formula (1).
- heteroaromatic ring compound having a nitrogen-containing aromatic group represented by the general formula (1) the following compounds are preferably used among the above compounds.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group described above forms a crystal structure by two or more bonds or interactions selected from ⁇ - ⁇ interaction, coordination bond, charge transfer interaction and hydrogen bond. It is preferable. This is because by using a low molecular weight compound having a crystal structure, the intermolecular interaction can be improved and vaporization during firing when obtaining a nitrogen-containing carbon alloy can be suppressed.
- the crystal structure here refers to the arrangement and arrangement of molecules in the crystal. In other words, the crystal structure consists of a repeating structure of unit cell, and the molecules are arranged at any position in the unit cell and oriented. In the crystal, the molecules have a uniform appearance.
- each molecular interaction is the same inside or outside the unit cell.
- a heteroaromatic compound having a nitrogen-containing aromatic group having a laminated structure an aromatic ring, a heterocyclic ring, a condensed polycyclic ring, a condensed heterocyclic polycyclic ring, an unsaturated group (nitrile group, vinyl group, allyl group, acetylene) Group) and the like (for example, an aromatic ring is a face-to-face ⁇ - ⁇ interaction ( ⁇ - ⁇ stack)).
- Stacking is performed by SP 2 orbits of carbons derived from unsaturated bonds in these rings and groups and regularly overlapping at equal intervals between molecules to form a stacked column structure.
- the adjacent stacked columns have a uniform structure in which the intermolecular distance is defined by hydrogen bonding or van der Waals interaction. For this reason, it has the effect that the heat transfer in a crystal
- the heteroaromatic ring compound having a nitrogen-containing aromatic group used in the present invention preferably has crystallinity.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group is preferable because the crystallinity is crystallinity, and the compound can be controlled in orientation during firing, so that it becomes a uniform carbon material.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group preferably further has a melting point of 25 ° C. or higher.
- the melting point is 25 ° C. or higher, there is an air layer that contributes to heat resistance during firing, boiling or bumping can be prevented from the relationship between temperature and vapor pressure, and a carbon material can be easily obtained.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group is not particularly limited in molecular weight, but in the case of a low molecular weight compound or oligomer, it is preferably 60 to 2000, more preferably 100 to 1500, and 130 to 1000. It is particularly preferred that By setting it within the above range, purification before firing becomes easy.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group is a polymer
- the number average molecular weight is preferably from 2,000 to 1,000,000, and more preferably from 2,000 to 100,000.
- the molecular weight distribution (dispersion degree weight average molecular weight / number average molecular weight) is not particularly limited, but is preferably 3.0 or less, more preferably 2.0 or less, and even more preferably 1.5 or less.
- the electroconductivity of the carbon material obtained by setting it as the said range improves, it is preferable.
- JIS K 7252 shows to obtain the average molecular weight and molecular weight distribution of a polymer by plastic size exclusion chromatography
- SEC size exclusion chromatography
- the heteroaromatic ring compound having a nitrogen-containing aromatic group may be a polymer such as Poly (4-VinylPyridine), a low molecular compound or an oligomer in the above exemplary compounds.
- the degree of polymerization n is preferably 20 to 1.0 ⁇ 10 4 , more preferably 20 to 1.0 ⁇ 10 3 , and further preferably 20 to 1.0 ⁇ 10 2. preferable.
- the degree of polymerization n is preferably 2 to 20, and more preferably 4 to 10.
- the heteroaromatic compound having a nitrogen-containing aromatic group is a polymer
- the nitrogen-containing aromatic group is arranged by a covalent bond, by setting the degree of polymerization within the above range, adjacent carbons are bonded together during firing. This is preferable because it is easy to be performed and conductivity is improved.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group is a low molecular weight compound or oligomer
- the nitrogen-containing aromatic group is bonded by a covalent bond.
- the nitrogen aromatic group is preferred because it is oriented with the metal, the nitrogen-containing aromatic group is easily arranged, and vacancies are easily formed.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group is preferably contained in an amount of more than 0.1% by mass, and 0.5 to 99% by mass with respect to the total mass in the precursor. Is more preferable, and 5 to 90% by mass is more preferable.
- a carbon alloy having higher oxygen reduction activity can be generated.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group may be used alone or in combination of two or more. Moreover, it is preferable that the metal content in the hetero aromatic ring compound which has nitrogen-containing aromatic groups other than the inorganic metal salt mentioned later is 10 ppm or less.
- the nitrogen content of the heteroaromatic ring compound having a nitrogen-containing aromatic group is preferably 0.1% by mass to 55% by mass, and preferably 1% by mass to 30% by mass with respect to the total mass of the precursor. Is more preferable, and further 4 to 20% by mass is particularly preferable.
- N nitrogen atom
- the nitrogen atom and metal are regularly positioned uniformly on the crystal edge, and the nitrogen and metal are It becomes easy to interact. Thereby, the composition ratio of nitrogen atom and metal can be a composition ratio having higher oxygen reduction activity.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group is preferably a hardly volatile compound having a ⁇ TG of ⁇ 95% to ⁇ 0.1% at 400 ° C. in a nitrogen atmosphere, and is ⁇ 95% to ⁇ It is more preferably a hardly volatile compound of 1%, particularly preferably -90% to -5%.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group is preferably a hardly volatile compound that is carbonized without being vaporized during firing.
- ⁇ TG is increased from 30 ° C. to 1000 ° C.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group is also preferably a pigment having a structure represented by the general formula (1).
- the pigment forms a stacked column structure by ⁇ - ⁇ interaction between molecules, and has a uniform structure with a defined intermolecular distance by hydrogen bonds or van der Waals interactions between the stacked columns. It has the effect that heat transfer is easily achieved. In addition, it has crystallinity, and is vibration-reduced and heat-resistant by phonon (quantized lattice vibration) against heat. Therefore, the decomposition temperature is maintained up to the carbonization temperature, and there is an effect that the vaporization of the decomposition product is reduced and the carbonization is achieved.
- isoindoline pigments isoindolinone pigments, diketopyrrolopyrrole pigments, quinacridone pigments, oxazine pigments, phthalocyanine pigments, quinophthalone pigments, and latent pigments or dyes obtained by converting the above pigments into
- a pigment such as a lake pigment pigmented with a metal ion is preferred, and a diketopyrrolopyrrole pigment, a quinacridone pigment, an isoindoline pigment, an isoindolinone pigment, a quinophthalone pigment, and a latent pigment obtained by laminating the above pigment ( (Described later) is more preferable.
- An inorganic metal salt is used for the preparation of the precursor described above.
- the inorganic metal salt is not particularly limited, but may be a hydroxide, oxide, nitride, sulfite, sulfide, sulfonate, carbonylate, nitrate, nitrite, halide or the like.
- the counter ion is a halogen ion or a nitrate ion.
- the counter ion is a halide or nitrate that is a halogen ion, nitrate ion or sulfate ion, it is preferable because it can bind to carbon on the surface of the carbon produced during thermal decomposition and increase the specific surface area.
- the inorganic metal salt is preferably a halide, and particularly preferably an inorganic metal chloride.
- the inorganic metal salt can contain crystal water, and the inorganic metal salt is preferably a hydrated salt. Since the inorganic metal salt contains crystal water, the thermal conductivity is improved, which is preferable in that it can be uniformly fired.
- the inorganic metal salt containing crystal water for example, cobalt chloride (III) hydrate salt, iron chloride (III) hydrate salt, cobalt chloride (II) hydrate salt, iron chloride (II) hydrate salt is preferably used. it can.
- the metal species of the inorganic metal salt is preferably at least one of Fe, Co, Ni, Mn, and Cr, and more preferably Fe or Co.
- Fe, Co, Ni, Mn, and Cr salts are excellent in forming a nano-sized shell structure that improves the catalytic activity of the carbon catalyst, and in particular, Co and Fe form a nano-sized shell structure. It is preferable because it is particularly excellent.
- Co and Fe contained in the carbon catalyst can improve the oxygen reduction activity of the catalyst in the carbon catalyst. Most preferably, it is Fe as a transition metal.
- the Fe-containing nitrogen-containing carbon alloy has a high rising potential, has a higher number of reaction electrons than Co, and can relatively improve the durability of the fuel cell.
- elements other than transition metals for example, B, alkali metals (Na, K, Cs), alkaline earths (Mg, Ca, Ba), lead, tin, indium, thallium, etc. ) May be included in one or more types.
- the precursor includes the total of the heteroaromatic ring compound having a nitrogen-containing aromatic group and the inorganic metal salt contained in the precursor (however, the total includes the mass of hydrated water), It is preferable that the inorganic metal salt (however, the inorganic metal salt here includes the mass of hydrated water) exceeds 5 mass%.
- the carbon alloy which has higher oxygen reduction activity can be produced
- the decomposition product interacts with the metal (forms a complex), and the performance of the nitrogen-containing carbon alloy catalyst is further improved. Further, due to the catalytic action of a specific transition metal compound added to a heteroaromatic ring compound having a nitrogen-containing aromatic group containing a nitrogen atom (N) as a constituent element, the nitrogen atom (N) is increased on the carbon catalyst surface. It is preferable to form carbon fine particles containing a transition metal compound which forms a nitrogen-containing carbon alloy fixed at a concentration and interacts with the nitrogen atom (N). In addition, the transition metal compound which interacted with some nitrogen atoms (N) by the acid treatment mentioned later may drop off.
- the nitrogen-containing carbon alloy obtained in the present invention is based on the total of the heteroaromatic ring compound having a nitrogen-containing aromatic group and the inorganic metal salt contained in the precursor (however, the total includes the mass of hydrated water)
- the inorganic metal salt (however, the inorganic metal salt here includes the mass of hydrated water) exceeds 0.1% by mass, and exceeds 0.5% by mass and is 85% by mass or less. More preferably, it is contained more than 1 mass% and 70 mass% or less. By setting it within this range, a carbon alloy having high oxygen reduction reaction activity (ORR activity) can be generated.
- the ORR activity can be measured as an ORR activity value by obtaining a potential by the method described in detail in Examples.
- the value of potential at the time of oxygen reduction is high, specifically, the potential of the current density values -1 mA / cm 2 in the electrode coating weight of 0.05 mg / cm 2 is 0 .39V or more is preferable, 0.43V or more is more preferable, and 0.45V or more is more preferable.
- the potential of the current density values -1 mA / cm 2 in the electrode coating amount of 0.5 mg / cm 2 is preferably not less than 0.70 V, more preferably at least 0.72V, and even more preferably 0.74 V.
- the coating amount and the current density increase linearly, but as the coating amount increases, the current density decreases from the assumed straight line due to an increase in resistance between carbon alloy particles, an increase in diffusion resistance of oxygen and water, and the like. According to Ohm's law, the application amount and the potential are similarly deviated from linear and lower in the relationship between the application amount and the potential.
- the value of the potential at 0.5 mg / cm 2 is a value that takes into account the catalytic activity and the conductivity of the carbon alloy shown at 0.05 mg / cm 2 , and is particularly preferable because of its excellent conductivity.
- the heteroaromatic ring compound having a nitrogen-containing aromatic group and the inorganic metal salt do not need to be uniformly dispersed in the organic material before firing. That is, when a heteroaromatic ring compound having a nitrogen-containing aromatic group undergoes calcination decomposition, if the decomposition product is in contact with a vaporized product such as an inorganic metal salt, an active species having oxygen reduction reaction activity is formed. Therefore, the oxygen reduction reaction activity of the carbon alloy is not affected by the mixed state of the heteroaromatic ring compound and the inorganic metal salt at room temperature.
- the particle diameter of the inorganic metal salt is preferably 0.001 to 100 ⁇ m. More preferably, it is 0.01 to 10 ⁇ m. By making the particle size of the inorganic metal salt within this range, it becomes possible to uniformly mix with the heteroaromatic ring compound having a nitrogen-containing aromatic group, and the heteroaromatic ring compound is likely to form a complex when it is decomposed. .
- the precursor preferably further contains at least one organometallic complex.
- organometallic complexes include compounds described in the Basic Complex Engineering Study Group, Coordination Chemistry-Fundamentals and Latest Topics, and Kodansha Scientific (1994).
- a compound in which a ligand is coordinated can be preferably exemplified, and a metal acetate complex or a ⁇ -diketone metal complex can be preferably used.
- the organometallic complex can take the coordination number of various ligands, may be a coordination geometric isomer, and may have different valences of metal ions.
- the organometallic complex may be an organometallic compound having a metal-carbon bond.
- Preferable metal ions are Fe, Co, Ni, Mn and Cr ions.
- Preferable ligands include monodentate ligands (halide ions, cyanide ions, ammonia, pyridine (py), triphenylphosphine, carboxylic acid, etc.), bidentate ligands (ethylenediamine (en), ⁇ - Diketonate (acetylacetonate (acac), pivaloylmethane (DPM), diisobutoxymethane (DIBM), isobutoxypivaloylmethane (IBPM), tetramethyloctadione (TMOD)), trifluoroacetylacetonate (TFA), bipyridine (Bpy), phenanthrene (phen), etc.), multidentate ligands (ethylenediaminetetraacetate ion (edta), etc.).
- ⁇ -diketone metal complexes bis (acetylacetonato) iron (II) [Fe (acac) 2 ], tris (acetylacetonato) iron (III) [Fe ( acac) 3 ], bis (acetylacetonato) cobalt (II) [Co (acac) 2 ], tris (acetylacetonato) cobalt (III) [Co (acac) 3 ], bis (dipivaloylmethane) iron (II) [Fe (DPM) 2 ], tris (dipivaloylmethane) iron (III) [Fe (DPM) 3 ], tris (dipivaloylmethane) cobalt (III) [Co (DPM) 3 ] , bis (diisobutoxyphenyl methane) iron (II) [Fe (DIBM) 2], tris (diisobutoxyphen
- ⁇ -diketone metal complexes bis (acetylacetonato) iron (II) [Fe (acac) 2 ], tris (acetylacetonato) iron (III) [Fe (acac) 3 ], bis (dipivaloyl) Methane) iron (II) [Fe (DPM) 2 ], bis (diisobutoxymethane) iron (II) [Fe (DIBM) 2 ], bis (isobutoxypivaloylmethane) iron (II) [Fe (IBPM) 2 ], bis (tetramethyloctadione) iron (II) [Fe (TMOD) 2 ]), N, N′-ethylenediaminebis (salicylideneaminato) iron (II) [Fe (salen)], tris (2,2′-bipyridine) iron (II) chloride [Fe (bpy) 3 ] Cl 2
- the organometallic complex preferably contains a ⁇ -diketone metal complex.
- a ⁇ -diketone metal complex may be used alone, or a ⁇ -diketone metal complex and another organometallic complex may be mixed and used.
- the ⁇ -diketone metal complex represents a compound represented by the following general formula (6) and tautomers thereof.
- M represents a metal
- R 1 and R 3 each independently represents a hydrocarbon group which may have a substituent
- R 2 has a hydrogen atom or a substituent.
- the hydrocarbon group which may be made is shown.
- R 1 , R 2 and R 3 may be bonded to each other to form a ring.
- n represents an integer of 0 or more
- m represents an integer of 1 or more.
- the ⁇ -diketone or its ion is coordinated or bonded to the atom or ion of the metal M.
- Preferred metals include Fe, Co, Ni, Mn and Cr, more preferably Fe and Co, and still more preferably Fe.
- Examples of the “hydrocarbon group” in the hydrocarbon group optionally having a substituent for R 1 , R 2 , and R 3 include an aliphatic hydrocarbon group, an alicyclic hydrocarbon group, and an aromatic hydrocarbon group. , Heterocyclic (heterocyclic) hydrocarbon groups, and groups in which a plurality of these are bonded.
- Examples of the aliphatic hydrocarbon group include alkyl groups such as methyl, ethyl, propyl, isopropyl, butyl, isobutyl, s-butyl, t-butyl, and hexyl groups (C 1-6 alkyl groups and the like); An alkenyl group ( C2-6 alkenyl group etc.) etc.
- Examples of the alicyclic hydrocarbon group include cycloalkyl groups such as cyclopentyl and cyclohexyl groups (3 to 15-membered cycloalkyl groups and the like); cycloalkenyl groups such as cyclohexenyl groups (3 to 15-membered cycloalkenyl groups and the like) ); A bridged carbocyclic group such as an adamantyl group (such as a bridged carbocyclic group having about 6 to 20 carbon atoms).
- Examples of the aromatic hydrocarbon group include an aromatic hydrocarbon group (aryl group) having about 6 to 20 carbon atoms such as a phenyl group and a naphthyl group.
- Heterocyclic (heterocyclic) hydrocarbon groups include, for example, nitrogen-containing five-membered hydrocarbon groups such as pyrrolyl, imidazolyl and pyrazolyl groups; nitrogen-containing six-membered pyridyl, pyrazinyl, pyrimidinyl and pyridazinyl groups Member ring hydrocarbon group: pyrrolidinyl group, indolizinyl group, isoindolyl group, isoinindolinyl group, indolyl group, indazolyl group, purinyl group, quinolidinyl group, quinolinyl group, naphthyridinyl group, phthalazinyl group, quinoxalinyl group, cinnolinyl group, pteridinyl group Nitrogen-containing condensed bicyclic hydrocarbon groups such as carbazolyl group, ⁇ -carbolinyl group, phenanthridinyl group, a
- Examples of the substituent that the hydrocarbon group may have include, for example, a halogen atom such as fluorine, chlorine, bromine atom; an alkoxy group such as methoxy, ethoxy, propoxy, isopropyloxy, butoxy, isobutyloxy, t-butyloxy group (C 1-4 alkoxy group etc.); hydroxy group; alkoxycarbonyl groups such as methoxycarbonyl and ethoxycarbonyl groups (C 1-4 alkoxy-carbonyl group etc.); acyl groups such as acetyl, propionyl and benzoyl groups (C 1- 10 acyl group); cyano group; nitro group and the like.
- a halogen atom such as fluorine, chlorine, bromine atom
- an alkoxy group such as methoxy, ethoxy, propoxy, isopropyloxy, butoxy, isobutyloxy, t-butyloxy group (C 1-4 alkoxy group etc
- Examples of the ring formed by combining R 1 , R 2 , and R 3 with each other include, for example, a 5- to 15-membered cycloalkane ring or cycloalkene ring such as a cyclopentane ring, cyclopentene ring, cyclohexane ring, cyclohexene ring, etc. Is mentioned.
- R 1 and R 3 are an alkyl group (C 1-6 alkyl group etc.), an alkenyl group (C 2-6 alkenyl group etc.), a cycloalkyl group (3-15 membered cycloalkyl group etc.), a cycloalkenyl group (3- to 15-membered cycloalkenyl group etc.), aryl group (C 6-15 aryl group etc.), aryl group having a substituent (C 6 having a substituent such as p-methylphenyl group, p-hydroxyphenyl group) -15 aryl group).
- R 2 is a hydrogen atom, an alkyl group (C 1-6 alkyl group etc.), an alkenyl group (C 2-6 alkenyl group etc.), a cycloalkyl group (3-15 membered cycloalkyl group etc.), a cycloalkenyl group (3- to 15-membered cycloalkenyl group etc.), aryl group (C 6-15 aryl group etc.), aryl group having a substituent (C 6 having a substituent such as p-methylphenyl group, p-hydroxyphenyl group) -15 aryl group).
- the valence n of the metal may be any of 0, 1, 2, 3 and the like, but is usually divalent or trivalent.
- the ⁇ -diketone coordinates as the corresponding anion, ⁇ -diketonate.
- the coordination number m is usually the same.
- a solvent or the like may be axially coordinated with the metal. In that case, the valence n and the coordination number m of the metal may be different. Examples of the solvent that may be axially coordinated include pyridine, acetonitrile, alcohol, and the like, but any solvent that can be axially coordinated may be used.
- ⁇ -diketone iron complex As the ⁇ -diketone iron complex, a commercially available product may be used as it is or after purification, or it may be prepared and used. It can also be generated and used in a reaction system. When it is generated in the reaction system, for example, iron chloride, hydroxide and ⁇ -diketone such as acetylacetone may be added. At this time, a base such as ammonia, amines, alkali metal or alkaline earth metal hydroxides, carbonates or carboxylates can be added as necessary.
- a base such as ammonia, amines, alkali metal or alkaline earth metal hydroxides, carbonates or carboxylates can be added as necessary.
- the amount of ⁇ -diketone iron complex added is usually about 0.001 to 50 mol%, preferably 0.01 to 20 mol%, particularly preferably about 0.1 to 10 mol%.
- a conductive assistant may be added to the precursor and fired, or may be added to the carbon alloy. Since the conductive auxiliary agent is uniformly dispersed, it is preferable to add a conductive auxiliary agent and fire.
- a conductive support agent For example, Norit (made by NORIT), Ketjen black (made by Lion), Vulcan (made by Cabot), black pearl (made by Cabot), acetylene black (Chevron) Carbon black such as (manufactured) (all are trade names), graphite, and carbon materials such as fullerenes such as C60 and C70, carbon nanotubes, carbon nanohorns, and carbon fibers.
- the addition ratio of the conductive auxiliary agent is preferably 0.01% by mass to 50% by mass, more preferably 0.1% by mass to 20% by mass with respect to the total mass of the precursor. More preferably, the content is from 10% to 10% by mass. If too much conductive additive is added, the aggregation / growth of the metal produced from the inorganic metal salt in the system becomes non-uniform and the desired porous nitrogen-containing carbon cannot be obtained, which is not suitable.
- the step of firing the precursor is as follows: (1) A step of preparing a precursor by mixing a heteroaromatic ring compound having a nitrogen-containing aromatic group and an inorganic metal salt containing one or more of Fe, Co, Ni, Mn and Cr; (2) a temperature raising step of raising the temperature of the precursor from 1 to 2000 ° C. per minute from room temperature to the carbonization temperature under an inert atmosphere; (3) a carbonization step of holding at 400 to 2000 ° C. for 0.1 to 100 hours; (4) It is preferable to include a cooling step of cooling from the carbonization temperature to room temperature.
- the manufacturing method of the nitrogen-containing carbon alloy of the present invention is the firing step, (6) It preferably includes a step of washing the baked nitrogen-containing carbon alloy with an acid, (7) More preferably, after the acid cleaning step, a step of refiring the acid-cleaned nitrogen-containing carbon alloy is included.
- the steps (1) to (7) will be described in order with respect to the method for producing a nitrogen-containing carbon alloy of the present invention.
- Precursor preparation step In the precursor preparation step, the above-described heteroaromatic ring compound having a nitrogen-containing aromatic group and the above-described inorganic metal salt are mixed to prepare a precursor.
- the precursor adjusted in the manufacturing process of a nitrogen-containing carbon alloy is baked after that, it is preferable to further include the process of grind
- the production method of the present invention includes a heteroaromatic ring compound having a nitrogen-containing aromatic group having a specific structure and an inorganic metal salt. It is preferable that the precursor is heated to the carbonization temperature and cooled to room temperature after the heat treatment.
- the temperature may be raised in multiple stages in the temperature raising process of the temperature raising process and the re-baking process described later.
- the latter stage of the temperature raising process may be carried out by holding the temperature after the completion of the preceding temperature raising process or by raising the temperature as it is.
- the temperature may be raised and a subsequent temperature increase process may be performed.
- the sample after a process may be grind
- the metal may be removed by acid cleaning of the sample after the treatment in (6) acid cleaning step described later.
- the sample before treatment may be inserted into a carbonization device and then heated from room temperature to a predetermined temperature, or may be increased by inserting the sample before treatment into a carbonization device or the like at a predetermined temperature. May be warm.
- the temperature of the sample before processing is raised from room temperature to a predetermined temperature.
- the temperature rising rate is preferably 1 to 2000 ° C./min, more preferably 1 to 1000 ° C./min, and 1 to 500 ° C./min. Is more preferable.
- Preliminary carbide In order to obtain a pre-carbide having pores formed, it is preferable to perform the previous treatment of the organic material containing a heteroaromatic ring compound having a nitrogen-containing aromatic group and an inorganic metal salt at a relatively low temperature. In such a low temperature treatment, a constant temperature may be maintained. By doing so, only a heat-stable structure can be maintained, and unstable impurity components, solvents, and the like can be removed.
- the temperature raising treatment performed at a relatively low temperature it is preferable to raise the temperature of an organic material containing a heteroaromatic ring compound having a nitrogen-containing aromatic group and an inorganic metal salt to 100 ° C. to 1500 ° C., preferably 150 ° C. to 1050 ° C. It is more preferable to raise the temperature to 200 ° C to 1000 ° C. By doing so, a uniform preliminary carbide is obtained.
- the inert atmosphere refers to a gas atmosphere such as a nitrogen gas or a rare gas atmosphere. Note that even if oxygen is contained, the atmosphere may be any atmosphere in which the amount of oxygen is limited to such an extent that the workpiece is not combusted.
- the inert atmosphere may be either a closed system or a distribution system that distributes a new gas, and is preferably a distribution system.
- the gas flow rate is preferably 0.01 to 2.0 liters / min per 36 mm ⁇ inner diameter, and the gas flow rate is 0.05 to 1.0 per 36 mm ⁇ inner diameter. It is more preferable to circulate a liter / min gas, and it is particularly preferable that the gas flow rate is 0.1 to 0.5 liter / min gas per an inner diameter of 36 mm ⁇ .
- the temperature holding time is 0.1 to 100 hours, preferably 0.2 to 10 hours, and more preferably 0.5 to 5 hours. Even if the carbonization treatment is performed for more than 100 hours, an effect corresponding to the treatment time may not be obtained.
- the heating device used in the above temperature raising treatment is not particularly limited, but is a tubular furnace (Kantar wire furnace, imaging furnace), muffle furnace, vacuum gas replacement furnace, rotary furnace (rotary kiln), roller hearth kiln, pusher kiln, multistage It is preferable to use a furnace, a tunnel furnace, a fluidized firing furnace, etc., more preferably a tubular furnace (a cantal wire furnace, an imaging furnace), a muffle furnace, a rotary furnace (rotary kiln), a fluidized firing furnace, a tubular furnace (a cantal wire) Furnaces, imaging furnaces) and muffle furnaces are particularly preferred.
- the portions of the temperature raising treatment are collectively infusible.
- the subsequent temperature increase treatment is continued following the temperature increase treatment of the precursor containing the heteroaromatic ring compound having a nitrogen-containing aromatic group having the specific structure in the previous stage and the inorganic metal salt. It is preferable to do so.
- the residual heat of the previous stage can be utilized, the decomposition reaction and the carbonization reaction of the organic material can be continuously performed, and the decomposition product and the metal interact with each other, so that the metal is more active. It can be stabilized with.
- the subsequent temperature increase treatment is preferably performed in an inert atmosphere
- the inert atmosphere may be either a closed system or a distribution system for circulating a new gas, and is preferably a distribution system.
- the gas flow rate is preferably 0.01 ml to 2.0 liters / min per 36 mm ⁇ inside diameter, and the gas flow rate is 0.02 ml to 1 per 36 mm ⁇ inside diameter. More preferably, a gas of 0.0 liter / min is circulated, and the flow rate of the gas is particularly preferably 0.05 to 0.5 liter / min.
- the downstream gas flow rate may be different from the upstream gas flow rate.
- the temperature holding time is 0.1 to 100 hours, preferably 0.2 to 10 hours, and more preferably 0.5 to 5 hours. Even if the carbonization treatment is performed for more than 100 hours, an effect corresponding to the treatment time may not be obtained.
- the heating device used in the above temperature raising treatment is not particularly limited, but is a tubular furnace (Kantar wire furnace, imaging furnace), muffle furnace, vacuum gas replacement furnace, rotary furnace (rotary kiln), roller hearth kiln, pusher kiln, multistage It is preferable to use a furnace, a tunnel furnace, a fluidized firing furnace or the like, and it is more preferable to use a tubular furnace (a cantal wire furnace, an imaging furnace), a muffle furnace, a rotary furnace (rotary kiln), a fluidized firing furnace, a tubular furnace (a cantal wire) Furnaces, imaging furnaces) and muffle furnaces are particularly preferred.
- the firing temperature of the carbonization treatment of the precursor containing a heteroaromatic ring compound having a nitrogen-containing aromatic group having a specific structure and an inorganic metal salt is such that the heteroaromatic ring compound having a nitrogen-containing aromatic group is thermally decomposed and carbonized.
- the upper limit of the carbonization temperature needs to be 2000 ° C., although it is not particularly limited as long as it is a temperature to be converted.
- the lower limit of the reaction temperature is preferably 400 ° C, more preferably 500 ° C, even more preferably 600 ° C, and even more preferably 700 ° C. .
- reaction temperature is 2000 degrees C or less, nitrogen will remain in carbon skeleton and it can be set as desired N / C atomic ratio, and sufficient oxygen reduction reaction activity will be obtained.
- the firing temperature is preferably 700 to 1200 ° C, particularly preferably 700 to 1000 ° C.
- carbonization treatment is performed within this range, the yield of carbon alloy may be reduced, but the crystallite size of the obtained carbon alloy is uniform, so that the metal is uniformly distributed and the state of high activity is maintained. The As a result, it becomes possible to produce a carbon alloy having excellent oxygen reduction performance. Further, by performing the carbonization treatment within the above range, nitrogen easily remains in the carbon skeleton due to the action of the generated inorganic metal, and the oxygen reduction reaction activity can be enhanced.
- the temperature raising treatment is preferably performed under a flow of an inert gas or a non-oxidizing gas, and such an atmosphere may be either a closed system or a flow system for flowing a new gas, preferably a flow system. It is.
- the gas flow rate is preferably 0.01 to 2.0 liters / min per 36 mm ⁇ inner diameter, and 0.02 to 1.0 liter / min per 36 mm ⁇ inner diameter. More preferably, it is particularly preferable to circulate a gas of 0.05 ml to 0.5 liter / min per 36 mm ⁇ inside diameter. It is preferable for the flow rate to be within this range because the desired nitrogen-containing carbon alloy can be suitably obtained.
- the treatment time for the carbonization treatment is 0.1 to 100 hours, preferably 0.2 to 10 hours, and more preferably 0.5 to 5 hours. Even if the carbonization treatment is performed for more than 100 hours, an effect corresponding to the treatment time may not be obtained.
- the heating device used in the above temperature raising treatment is not particularly limited, but is a tubular furnace (Kantar wire furnace, imaging furnace), muffle furnace, vacuum gas replacement furnace, rotary furnace (rotary kiln), roller hearth kiln, pusher kiln, multistage It is preferable to use a furnace, a tunnel furnace, a fluidized firing furnace or the like, and it is more preferable to use a tubular furnace (a cantal wire furnace, an imaging furnace), a muffle furnace, a rotary furnace (rotary kiln), a fluidized firing furnace, a tubular furnace (a cantal wire) Furnaces, imaging furnaces) and muffle furnaces are particularly preferred.
- the carbon alloy may be cooled to room temperature and then crushed.
- the pulverization treatment can be performed by any method known to those skilled in the art.
- the pulverization can be performed using a ball mill, agate pulverization, mechanical pulverization, or the like.
- the method for producing a nitrogen-containing carbon alloy of the present invention may include an acid washing process for washing the fired nitrogen-containing carbon alloy with an acid after the firing process.
- the ORR activity can be improved by acid cleaning of the metal on the surface of the produced carbon alloy catalyst. Without being bound by any theory, it is expected that a porous nitrogen-containing carbon alloy having optimum porosity can be obtained by this acid cleaning treatment.
- any aqueous Bronsted (proton) acid including a strong acid or a weak acid having a pH of 7 or less can be used in the acid cleaning step.
- an inorganic acid (mineral acid) or an organic acid can be used.
- Suitable acids include HCI, HBr, HI, H 2 SO 4 , H 2 SO 3 , HNO 3 , HClO 4 , [HSO 4 ] ⁇ , [HSO 3 ] ⁇ , [H 3 O] + , H 2 [C 2 O 4 ], HCO 2 H, HCIO 3 , HBrO 3 , HBrO 4 , HIO 3 , HIO 4 , FSO 3 H, CF 3 SO 3 H, CF 3 CO 2 H, CH 3 CO 2 H, B (OH) 3 , etc. (including any combination thereof), but are not limited to these. Further, the method described in JP-T-2010-524195 can also be used in the present invention.
- the manufacturing method of the nitrogen-containing carbon alloy of this invention further includes the process of grind
- the calcining temperature in the refiring process is preferably 500 to 2000 ° C, and preferably 600 to 1500 ° C. Is more preferable, and a temperature of 1000 to 1500 ° C. is even more preferable.
- firing may be performed in a pressurized state during the reaction.
- the gas discharge port may be trapped with water and fired in a state where back pressure is applied.
- the pressure in the carbonization step is 0.01 to 5 MPa, preferably 0.05 to 1 MPa, more preferably 0.08 to 0.3 MPa, and particularly preferably 0.09 to 0.15 MPa.
- High-pressure treatment is not preferable because it has a diamond structure constituted by sp 3 orbitals.
- the method of the refiring step is not particularly limited, but preferably a tubular furnace, a rotary furnace (rotary kiln), a roller hearth kiln, a pusher kiln, a multi-stage furnace, a vacuum gas replacement furnace, a tunnel furnace, a fluidized firing furnace, and the like are more preferable.
- a rotary furnace rotary kiln
- a vacuum gas replacement furnace a vacuum gas replacement rotary furnace (rotary kiln)
- a tunnel furnace and a fluidized firing furnace
- a vacuum gas replacement rotary furnace vacuum gas replacement rotary furnace
- the apparatus used for deaeration is not particularly limited as long as it can be deaerated, but it is preferable to use a vacuum gas replacement furnace or a vacuum gas replacement rotary furnace (rotary kiln).
- the pressure during vacuum degassing is not particularly limited, but is preferably 4 ⁇ 10 4 Pa or less, more preferably 4 ⁇ 10 3 Pa or less, and particularly preferably 2 ⁇ 10 2 Pa or less.
- the apparatus used at this time is not particularly limited as long as it can flow the carbon alloy, but it is preferable to use a rotary furnace (rotary kiln), a vacuum gas replacement rotary furnace (rotary kiln), or a fluidized firing furnace.
- a rotary furnace rotary kiln
- a vacuum gas replacement rotary furnace rotary kiln
- the sample tube is rotated at the time of firing, but is not limited to the rotation speed, speed change, and the like.
- the rotation speed is preferably 10 rpm or less, more preferably 5 rpm or less.
- the method for producing a nitrogen-containing carbon alloy of the present invention it is preferable to perform a carbonization treatment in the presence of an activator (activation step).
- an activator activation step
- the surface area of the carbide can be measured by the N 2 adsorption amount.
- the activator that can be used is not particularly limited.
- carbon dioxide, ammonia gas, water vapor, air, oxygen gas, hydrogen gas, carbon monoxide gas, methane gas, alkali metal hydroxide, zinc chloride, and phosphoric acid At least one selected from the group consisting of carbon dioxide, ammonia gas, water vapor, air, and oxygen gas can be used, more preferably at least one selected from the group consisting of:
- the gas activator is preferably diluted with an inert gas.
- the inert gas to be diluted include nitrogen gas and rare gases (for example, argon gas, helium gas, and neon gas).
- the gas activator may be contained in the atmosphere of carbonization treatment in an amount of 2 to 80 mol%, preferably 10 to 60 mol%. If it is 2 mol% or more, a sufficient activation effect can be obtained, while if it exceeds 80 mol%, the activation effect becomes remarkable and the yield of carbide is remarkably reduced, making it impossible to produce carbide efficiently. There is a fear.
- the solid activator such as alkali metal hydroxide may be mixed with the carbonized substance in a solid state, or after being dissolved or diluted with a solvent such as water, impregnated with the carbonized substance or in a slurry state. And may be kneaded into the article to be carbonized.
- the liquid activator may be diluted with water or the like and then impregnated with the carbonized material or kneaded into the carbonized material.
- the pressure in the gas phase may be any of normal pressure, pressurization, and depressurization, but is preferably pressurized at a high temperature.
- the gas may be stationary or distributed, but is preferably distributed from the viewpoint of discharging generated impurities.
- Nitrogen atoms can be introduced after carbonization.
- a liquid phase doping method a gas phase doping method, or a gas phase-liquid phase doping method can be used.
- nitrogen atoms can be introduced into the surface of the carbon catalyst by heat treatment by maintaining the carbon alloy in an ammonia atmosphere as a nitrogen source at 200 to 1200 ° C. for 5 to 180 minutes.
- the nitrogen-containing carbon alloy of the present invention is produced by the above-described method for producing a nitrogen-containing carbon alloy.
- the nitrogen-containing carbon alloy of the present invention obtained by firing the precursor is a nitrogen-containing carbon alloy into which nitrogen has been introduced.
- the nitrogen-containing carbon alloy of the present invention preferably contains graphene, which is an aggregate of carbon atoms having a hexagonal network structure in which carbon is chemically bonded by sp 2 hybrid orbitals and spreads in two dimensions.
- the content of surface nitrogen atoms in the carbon catalyst is more preferably 0.02 to 0.3 in terms of atomic ratio (N / C) to surface carbon.
- the atomic ratio (N / C) of the nitrogen atom to the carbon atom is less than 0.02, the number of effective nitrogen atoms bonded to the metal is reduced, and sufficient oxygen reduction catalyst characteristics cannot be obtained.
- the atomic ratio (N / C) of nitrogen atoms to carbon atoms exceeds 0.3, the strength of the carbon skeleton of the carbon alloy is lowered, and the electrical conductivity is lowered.
- the skeleton of the carbon alloy may be formed of at least carbon atoms and nitrogen atoms, and may contain hydrogen atoms, oxygen atoms, and the like as other atoms.
- the atomic ratio ((other atoms) / (C + N)) of other atoms to carbon atoms and nitrogen atoms is preferably 0.3 or less.
- carbon alloy is placed in a predetermined container, cooled to liquid nitrogen temperature (-196 ° C), nitrogen gas is introduced into the container and adsorbed, and the adsorption amount of single molecules and adsorption parameters are determined from the adsorption isotherm.
- a BET Brunauer-Emmett-Teller
- the pore shape of the carbon alloy is not particularly limited, and for example, pores may be formed only on the surface, or pores may be formed not only on the surface but also inside.
- pores may be tunnel-shaped, and it has a shape in which polygonal cavities such as spherical or hexagonal columns are connected to each other. It may be.
- the specific surface area of carbon alloy is preferably at 90m 2 / g or more, more preferably 350 meters 2 / g or more, particularly preferably 670m 2 / g or more.
- the catalytically active site metal coordination product or configuration space (field) having at least C, N, and metal ions as constituents
- the specific surface area of the carbon alloy is preferably 3000 m 2 / g or less, and preferably 2000 m 2 / g or less. More preferred is 1500 m 2 / g or less.
- the shape of the nitrogen-containing carbon alloy of the present invention is not particularly limited as long as it has oxygen reduction reaction activity.
- a large distorted structure such as a sheet shape, a fiber shape, a plate shape, a column shape, a block shape, a particle shape, many ellipses other than a spherical shape, a flat shape, a square shape, and the like can be given.
- it is preferably a block shape or a particle shape.
- a slurry described later is applied and dried, it is preferably a fiber shape, a plate shape, or a column shape from the viewpoint of imparting thixotropy.
- the slurry containing a carbon alloy can be produced by dispersing the nitrogen-containing carbon alloy of the present invention in a solvent.
- a slurry in which a carbon alloy is dispersed in a solvent is applied to a support material, baked, and dried, so that an arbitrary shape is obtained. It is possible to form a carbon catalyst that has been processed into Thus, by making a carbon alloy into a slurry, the workability of the carbon catalyst is improved, and it can be easily used as an electrode catalyst or an electrode material.
- the carbon alloy catalyst for fuel cells of the present invention preferably has a coating amount after drying of the nitrogen-containing carbon alloy of 0.01 mg / cm 2 or more, more preferably 0.02 to 100 mg / cm 2 , Particularly preferred is 0.05 to 10 mg / cm 2 .
- the solvent a solvent used when producing an electrode catalyst for a fuel cell or an electrode material for a power storage device can be appropriately selected and used.
- a solvent used for producing an electrode material for a power storage device diethyl carbonate (DEC), dimethyl carbonate (DMC), 1,2-dimethoxyethane (DME), ethylene carbonate (EC), ethyl methyl carbonate (EMC) ), N-methyl-2-pyrrolidone (NMP), propylene carbonate (PC), ⁇ -butyrolactone (GBL), etc.
- DEC diethyl carbonate
- DMC dimethyl carbonate
- DME 1,2-dimethoxyethane
- EC ethylene carbonate
- EMC ethyl methyl carbonate
- NMP N-methyl-2-pyrrolidone
- PC propylene carbonate
- GBL ⁇ -butyrolactone
- the use of the nitrogen-containing carbon alloy of the present invention is not particularly limited to structural materials, electrode materials, filtration materials, catalyst materials, etc., but is preferably used as electrode materials for power storage devices such as capacitors and lithium secondary batteries. More preferably, it is used as a carbon catalyst for a fuel cell, zinc-air cell, lithium-air cell or the like having reactive activity.
- the catalyst may be included in the catalyst layer. Furthermore, the electrode membrane assembly can be provided in a fuel cell.
- FIG. 1 shows a schematic configuration diagram of a fuel cell 10 using a carbon catalyst made of a nitrogen-containing carbon alloy of the present invention.
- the carbon catalyst is applied to the anode electrode and the cathode electrode.
- the fuel cell 10 includes a separator 12, an anode electrode catalyst (fuel electrode) 13, a cathode electrode catalyst (oxidant electrode) 15, and a separator 16 that are disposed so as to sandwich the solid polymer electrolyte 14.
- a fluorine-based cation exchange resin membrane represented by a perfluorosulfonic acid resin membrane is used.
- the fuel cell 10 provided with the carbon catalyst in the anode electrode catalyst 13 and the cathode electrode catalyst 15 is configured by bringing the carbon catalyst into contact with both of the solid polymer electrolyte 14 as the anode electrode catalyst 13 and the cathode electrode catalyst 15.
- The By forming the carbon catalyst described above on both sides of the solid polymer electrolyte, and adhering the anode electrode catalyst 13 and the cathode electrode catalyst 15 to both main surfaces of the solid polymer electrolyte 14 on the electrode reaction layer side by hot pressing, It integrates as MEA (Membrane Electrode Assembly).
- a gas diffusion layer made of a porous sheet (for example, carbon paper) that also functions as a current collector is interposed between the separator and the anode and cathode electrode catalyst.
- a carbon catalyst having a large specific surface area and high gas diffusibility can be used as the anode and cathode electrode catalyst.
- the separators 12 and 16 support the anode and cathode electrode catalyst layers 13 and 15 and supply and discharge reaction gases such as fuel gas H 2 and oxidant gas O 2 .
- a reaction gas is supplied to each of the anode and cathode electrode catalysts 13 and 15, a gas phase (reaction gas) and a liquid phase (solid) are formed at the boundary between the carbon catalyst provided on both electrodes and the solid polymer electrolyte 14.
- a three-phase interface of a polymer electrolyte membrane) and a solid phase (a catalyst possessed by both electrodes) is formed. And direct-current power generate
- FIG. 2 shows a schematic configuration diagram of an electric double layer capacitor 20 using a carbon catalyst made of nitrogen-containing carbon alloy and having an excellent storage capacity.
- the first electrode 21 and the second electrode 22 which are polarizable electrodes are opposed to each other through the separator 23, and are accommodated in the outer lid 24a and the outer case 24b. ing.
- the first electrode 21 and the second electrode 22 are connected to the exterior lid 24a and the exterior case 24b via current collectors 25, respectively.
- the separator 23 is impregnated with an electrolytic solution.
- the electric double layer capacitor 20 is configured by caulking and sealing the outer lid 24a and the outer case 24b while being electrically insulated via the gasket 26.
- the carbon catalyst made of the above-described nitrogen-containing carbon alloy can be applied to the first electrode 21 and the second electrode 22.
- the electric double layer capacitor by which the carbon catalyst was applied to the electrode material can be comprised.
- the above-described carbon catalyst has a fibrous structure in which nanoshell carbon is aggregated, and furthermore, since the fiber diameter is in a nanometer unit, the specific surface area is large, and the electrode interface where charges are accumulated in the capacitor is large.
- the above-mentioned carbon catalyst is electrochemically inactive with respect to the electrolytic solution, and has appropriate electrical conductivity. For this reason, the electrostatic capacitance per unit volume of an electrode can be improved by applying as an electrode of a capacitor.
- the above-described carbon catalyst can be applied as an electrode material composed of a carbon material, such as a negative electrode material of a lithium ion secondary battery. And since the specific surface area of a carbon catalyst is large, a secondary battery with a large electrical storage capacity can be comprised.
- the nitrogen-containing carbon alloy of the present invention is used as a substitute for an environmental catalyst containing a noble metal such as platinum.
- a catalyst for exhaust gas purification for removing pollutants contained in polluted air (mainly gaseous substances) etc. by decomposition treatment a catalyst material composed of noble metal materials such as platinum alone or in combination Environmental catalysts are used.
- the above-mentioned carbon catalyst can be used as an alternative to these exhaust gas purifying catalysts containing noble metals such as platinum. Since the above-described carbon catalyst is provided with an oxygen reduction reaction catalytic action, it has a function of decomposing substances to be treated such as pollutants.
- a low-cost environmental catalyst can be provided.
- the specific surface area is large, the treatment area for decomposing the material to be treated per unit volume can be increased, and an environmental catalyst having an excellent decomposition function per unit volume can be constituted.
- a noble metal-based material such as platinum used in conventional environmental catalysts is carried alone or in a composite, so that an environmental catalyst with more excellent catalytic action such as a decomposition function can be obtained.
- the environmental catalyst provided with the above-mentioned carbon catalyst can also be used as a purification catalyst for water treatment as well as the above-described exhaust gas purification catalyst.
- the nitrogen-containing carbon alloy of the present invention can be widely used as a catalyst for chemical reaction, and in particular, can be used as a substitute for a platinum catalyst. That is, the above-mentioned carbon catalyst can be used as a substitute for a general process catalyst for the chemical industry containing a noble metal such as platinum. For this reason, according to the above-mentioned carbon catalyst, a low-cost chemical reaction process catalyst can be provided without using expensive noble metals such as platinum. Furthermore, since the above-mentioned carbon catalyst has a large specific surface area, it can constitute a chemical reaction process catalyst excellent in chemical reaction efficiency per unit volume.
- Such a carbon catalyst for chemical reaction is applied to, for example, a hydrogenation reaction catalyst, a dehydrogenation reaction catalyst, an oxidation reaction catalyst, a polymerization reaction catalyst, a reforming reaction catalyst, and a steam reforming catalyst. be able to. More specifically, it is possible to apply a carbon catalyst to each chemical reaction with reference to a catalyst related literature such as “Catalyst Preparation (Kodansha) by Takaho Shirasaki and Naoyuki Todo, 1975”.
- ⁇ Method for evaluating properties of nitrogen-containing carbon alloy> (Specific surface area measurement by BET method) About nitrogen-containing carbon alloy sample before acid cleaning and nitrogen-containing carbon alloy sample isolated after acid cleaning, using a sample pretreatment device (BELPREP-flow (trade name) manufactured by Nippon Bell Co., Ltd.), nitrogen-containing carbon alloy The sample was dried under vacuum at 200 ° C. for 3 hours. The specific surface area of the nitrogen-containing carbon alloy was measured under simple measurement conditions using an automatic specific surface area / pore distribution measuring device (BELSORP-mini II (trade name) manufactured by Nippon Bell Co., Ltd.). The specific surface area was determined by the BET (Brunauer-Emmett-Teller) method using an analysis program installed in the apparatus.
- BET Brunauer-Emmett-Teller
- Example 1 Carbon Material Synthesis of Iron (II) Chloride Tetrahydrate Addition (4-Py) 3 -TAz Mixture (1C)> (Preparation of (4-Py) 3 -TAz) New J.M. Chem. , 2006, 30, 1276-1281. (4-Py) 3 -TAz was adjusted with reference to FIG. 4-Cyanopyridine 10 g, 18-crown-6 10 g, KOH 225 mg, and decalin 10 mL were mixed and stirred at 200 ° C. for 5 hours. After air cooling to room temperature, the reaction product was filtered and washed by boiling with pyridine. The obtained solid was dissolved in 1N hydrochloric acid, and the solid was precipitated with aqueous ammonia, filtered, washed with water and dried to obtain 7.1 g of (4-Py) 3 -TAz.
- Carbon material (1B) was pulverized in an agate mortar to obtain an acid-free carbon material.
- the result of measuring the specific surface area of the obtained acid-free carbon material by the BET method is shown in the column before acid cleaning in Table 1 below.
- the acid-free washed carbon material obtained by pulverizing the carbon material (1B) in an agate mortar was repeatedly washed with concentrated hydrochloric acid, centrifuged, and the supernatant was removed until no coloration occurred. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C.
- Oxygen reduction reaction (ORR) activity of carbon alloy coated electrode preparation of carbon alloy coated electrode
- ORR Oxygen reduction reaction activity of carbon alloy coated electrode
- Nafion solution 5% alcohol aqueous solution
- IPA 1-propanol
- the nitrogen-containing carbon alloy dispersion is placed on the carbon electrode so that the nitrogen-containing carbon alloy is 0.05 mg / cm 2. It was applied and dried at room temperature to obtain a carbon alloy coated electrode.
- Example 2 ⁇ Cobalt (II) chloride hexahydrate added (4-Py) 3 -TAz mixture carbon material synthesis (2C)> After adding 1.60 g of the above (4-Py) 3 -TAz and 1.60 g of cobalt (II) hexahydrate, mechanically pulverized and mixed to add cobalt chloride (II) hexahydrate (4-Py) A 3- TAz mixture (2A) was obtained.
- Cobalt (II) chloride hexahydrate added (4-Py) 3 -TAz mixture (2A) 3.1559 g was weighed into a quartz boat and inserted into a tubular furnace with 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz It was installed in the center of the tube and nitrogen was circulated at 300 mL per minute for 30 minutes at room temperature. The temperature was raised from 30 ° C. to 700 ° C. at a rate of 5 ° C. per minute and held at 700 ° C. for 1 hour. Then, it cooled to room temperature over 3 hours, and obtained carbon-material (2B) 1.0901g.
- the carbon material (2B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant liquid were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and left overnight to obtain 0.6635 g of acid-washed carbon material (2C). The obtained acid cleaned carbon material (2C) was used as the nitrogen-containing carbon alloy of Example 2. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- the carbon material (3B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and left overnight as it was to obtain 0.4564 g of acid-cleaned carbon material (3C). The obtained acid cleaned carbon material (3C) was used as the nitrogen-containing carbon alloy of Example 3. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 4 Cobalt (II) chloride hexahydrate added (3-Py) 3 -TAz mixture carbon material synthesis (4C)> (Preparation of cobalt (II) chloride hexahydrate (3-Py) 3 -TAz mixture) After adding 6.30 g of the above (3-Py) 3 -TAz and 6.30 g of cobalt (II) chloride hexahydrate, mechanically pulverized and mixed to add cobalt (II) chloride hexahydrate (3-Py ) 3 -TAz mixture (4A) was obtained.
- the carbon material (4B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.6443 g of acid-cleaned carbon material (4C). The obtained acid-washed carbon material (4C) was used as the nitrogen-containing carbon alloy of Example 4. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 5 ⁇ FeAA2, iron (II) chloride tetrahydrate added (4-Py) 3 -TAz mixture carbon material synthesis (5C)> (FeAA2, iron (II) chloride tetrahydrate added (4-Py) 3 -TAz mixture preparation) After adding the above (4-Py) 3 -TAz 6.30 g, FeAA2 0.403 g and iron (II) chloride tetrahydrate 6.30 g, mechanically pulverized and mixed, FeAA2 and iron (II) chloride 4 water A Japanese addition (4-Py) 3 -TAz mixture (5A) was obtained.
- the carbon material (5B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. The obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.6562 g of acid-washed carbon material (5C). The obtained acid cleaned carbon material (5C) was used as the nitrogen-containing carbon alloy of Example 5. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 6 ⁇ FeAA2, iron (II) chloride tetrahydrate added (4-Py) 3 -TAz mixture carbon material synthesis (6C)> (Infusibilization and carbonization treatment)
- the above-described FeAA2 and iron chloride (II) tetrahydrate added (4-Py) 3 -TAz mixture (5A) (3.1769 g) was weighed into a quartz boat and inserted into a tubular furnace at 4.0 cm ⁇ (inner diameter 3. 6 cm ⁇ ) was installed in the center of the quartz tube, and nitrogen was circulated at 300 mL per minute for 30 minutes at room temperature. The temperature was raised from 30 ° C. to 800 ° C. by 5 ° C. per minute and held at 800 ° C. for 1 hour. Then, it cooled to room temperature over 3 hours, and obtained carbon-material (6B) 0.8955g.
- the carbon material (6B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.5988 g of the acid-washed carbon material (6C). The obtained acid cleaned carbon material (6C) was used as the nitrogen-containing carbon alloy of Example 6. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 7 ⁇ FeAA2, iron (II) chloride tetrahydrate added (4-Py) 3 -TAz mixture carbon material synthesis (7C)> (Infusibilization and carbonization treatment)
- the above-mentioned FeAA2, iron chloride (II) tetrahydrate added (4-Py) 3 -TAz mixture (5A) 3.1134 g was weighed into a quartz boat and inserted into a tubular furnace, 4.0 cm ⁇ (inner diameter 3. 6 cm ⁇ ) was installed in the center of the quartz tube, and nitrogen was circulated at 300 mL per minute for 30 minutes at room temperature. The temperature was raised from 30 ° C to 900 ° C at 5 ° C per minute and held at 900 ° C for 1 hour. Then, it cooled to room temperature over 3 hours, and obtained carbon-material (7B) 0.8122g.
- the carbon material (7B) was pulverized in an agate mortar, washed with water, filtered and air-dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and left overnight as it was to obtain a non-acid cleaned carbon material (7C).
- the obtained non-acid cleaned carbon material (7C) was used as the nitrogen-containing carbon alloy of Example 7.
- the specific surface area was measured by the BET method. The results are shown in the column before acid cleaning in Table 1 below.
- the non-acid-cleaned carbon material (7C) was pulverized in an agate mortar, and concentrated hydrochloric acid cleaning, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and left overnight as it was to obtain 0.5040 g of acid-cleaned carbon material (8C). The obtained acid-washed carbon material (8C) was used as the nitrogen-containing carbon alloy of Example 8. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 9 ⁇ FeAA2, iron (II) chloride tetrahydrate added (4-Py) 3 -TAz mixture carbon material synthesis (9C)> (Infusibilization and carbonization treatment)
- the above-mentioned FeAA2, iron chloride (II) tetrahydrate added (4-Py) 3 -TAz mixture (5A) 3.2067 g was weighed into a quartz boat, and inserted into a tubular furnace, 4.0 cm ⁇ (inner diameter 3. 6 cm ⁇ ) was installed in the center of the quartz tube, and nitrogen was circulated at 300 mL per minute for 30 minutes at room temperature. The temperature was raised from 30 ° C. to 1000 ° C. at 5 ° C. per minute and held at 1000 ° C. for 1 hour. Then, it cooled to room temperature over 3 hours, and obtained carbon-material (9B) 0.8220g.
- the carbon material (9B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. The obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.5407 g of acid-washed carbon material (9C). The obtained acid-washed carbon material (9C) was used as the nitrogen-containing carbon alloy of Example 9. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 10 ⁇ FeAA2, iron chloride (II) tetrahydrate added (3-Py) 3 -TAz mixture carbon material synthesis (10C)> (FeAA2, iron (II) chloride tetrahydrate added (3-Py) 3 -TAz mixture preparation) (3-Py) 3 -TAz 6.30 g, FeAA2 0.403 g, iron (II) chloride tetrahydrate 6.30 g was added, then mechanically pulverized and mixed, and FeAA2 and iron (II) chloride tetrahydrate added A (3-Py) 3 -TAz mixture (10A) was obtained.
- the carbon material (10B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until no coloration occurred. After washing with water, it was filtered and air dried. Furthermore, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.6234 g of acid-cleaned carbon material (10C). The obtained acid-washed carbon material (10C) was used as the nitrogen-containing carbon alloy of Example 10. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 11 ⁇ FeAA2, Fe (II) chloride tetrahydrate added (3-Py) 3 -TAz mixture carbon material synthesis (11C)> (Infusibilization and carbonization treatment)
- the above-described FeAA2, iron chloride (II) tetrahydrate added (3-Py) 3 -TAz mixture (10A) (3.0646 g) was weighed into a quartz boat and inserted into a tubular furnace at 4.0 cm ⁇ (inner diameter 3. 6 cm ⁇ ) was installed in the center of the quartz tube, and nitrogen was circulated at 300 mL per minute for 30 minutes at room temperature. The temperature was raised from 30 ° C. to 800 ° C. by 5 ° C. per minute and held at 800 ° C. for 1 hour. Then, it cooled to room temperature over 3 hours, and obtained carbon-material (11B) 0.8184g.
- the carbon material (11B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and left overnight as it was to obtain 0.5646 g of acid-washed carbon material (11C). The obtained acid-washed carbon material (11C) was used as the nitrogen-containing carbon alloy of Example 11. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 12 ⁇ FeAA2, Fe (II) chloride tetrahydrate added (3-Py) 3 -TAz mixture carbon material synthesis (12C)> (Infusibilization and carbonization treatment)
- the above-described FeAA2, iron chloride (II) tetrahydrate added (3-Py) 3 -TAz mixture (10A) (3.0131 g) was weighed into a quartz boat and inserted into a tubular furnace at 4.0 cm ⁇ (inner diameter 3. 6 cm ⁇ ) was installed in the center of the quartz tube, and nitrogen was circulated at 300 mL per minute for 30 minutes at room temperature. The temperature was raised from 30 ° C to 900 ° C at 5 ° C per minute and held at 900 ° C for 1 hour. Then, it cooled to room temperature over 3 hours, and obtained carbon-material (12B) 0.7353g.
- the carbon material (12B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant liquid were repeated until there was no coloration. After washing with water, it was filtered and air dried. The obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.5141 g of acid-washed carbon material (12C). The obtained acid cleaned carbon material (12C) was used as the nitrogen-containing carbon alloy of Example 12. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 13 ⁇ FeAA2, Fe (II) chloride tetrahydrate added (3-Py) 3 -TAz mixture carbon material synthesis (13C)> (Infusibilization and carbonization treatment)
- the above-described FeAA2, iron chloride (II) tetrahydrate added (3-Py) 3 -TAz mixture (10A) (3.0748 g) was weighed into a quartz boat and inserted into a tubular furnace at 4.0 cm ⁇ (inner diameter 3. 6 cm ⁇ ) was installed in the center of the quartz tube, and nitrogen was circulated at 300 mL per minute for 30 minutes at room temperature. The temperature was raised from 30 ° C. to 1000 ° C. at 5 ° C. per minute and held at 1000 ° C. for 1 hour. Then, it cooled to room temperature over 3 hours, and obtained carbon-material (13B) 0.8436g.
- the carbon material (13B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. The obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.5610 g of acid-cleaned carbon material (13C). The obtained acid cleaned carbon material (13C) was used as the nitrogen-containing carbon alloy of Example 13. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 14 ⁇ FeAA2, iron chloride (II) tetrahydrate added (4-Py) 3 -Py mixture carbon material synthesis (14C)> (Preparation of (4-Py) 3 -Py) CrystEngCom, 2011, 13, 6864-6870.
- (4-Py) 3 -Py was adjusted with reference to FIG. 1.21 g of 4-acetylpyridine and 0.54 g of pyridine-4-carboxaldehyde were dissolved in 40 mL of ethanol, 0.62 g of KOH and 20 mL of 32% aqueous ammonia were added, and the mixture was stirred for 24 hours at room temperature. The precipitated solid was filtered, washed with water and ethanol, dried and recrystallized with ethanol to obtain 1.00 g of (4-Py) 3 -Py.
- the carbon material (14B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.8111 g of acid-washed carbon material (14C). The obtained acid-washed carbon material (14C) was used as the nitrogen-containing carbon alloy of Example 14. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 15 ⁇ FeAA2, iron chloride (II) tetrahydrate added (4-MePy) 3 -TAz / I mixture carbon material synthesis (15C)> (Preparation of FeAA2, (4-MePy) 3 -TAz / I) Liebigs Analder der Chemie, 1991, 10, 1021-1028. (4-MePy) 3 -TAz / I was adjusted with reference to FIG. (4-Py) 3 -TAz 0.30 g, MeI 0.54 g, and DMF 5 mL described in Example 1 were placed in an autoclave and stirred at 90 ° C. for 4 days. After air cooling, the precipitate was filtered, washed with acetone and dried to obtain 0.55 g of (4-MePy) 3 -TAz / I.
- FeAA2, iron chloride (II) tetrahydrate added (4-MePy) 3 -TAz / I mixture preparation After adding the above (4-MePy) 3 -TAz / I 6.30 g, FeAA2 0.403 g, iron (II) chloride tetrahydrate 6.30 g, mechanically pulverized and mixed, FeAA2, iron chloride (II) Tetrahydrate added (4-MePy) 3 -TAz / I mixture (15A) was obtained.
- the carbon material (15B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.3096 g of acid-washed carbon material (15C). The obtained acid cleaned carbon material (15C) was used as the nitrogen-containing carbon alloy of Example 15. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 16 ⁇ FeAA2, iron chloride (II) tetrahydrate added (4-Py) 3 -TAz mixture carbon material re-firing and acid treatment (16C)>
- Infusibilization and carbonization treatment firing method A 0.5179 g of the acid-cleaned carbon material (5C) of Example 5 was weighed into a quartz boat and placed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a tubular furnace. The mixture was circulated for 200 minutes for 30 minutes at room temperature.
- the baking method A is a baking method which implements only substitution by nitrogen circulation. Nitrogen was lowered to 20 mL / min during the temperature increase, and the temperature was increased from 30 ° C. to 1000 ° C. at 5 ° C./min and held at 1000 ° C. for 1 hour. Then, it cooled to room temperature over 3 hours, and obtained carbon-material (16B) 0.4254g.
- the carbon material (16B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.3645 g of acid-washed carbon material (16C). The obtained acid cleaned carbon material (16C) was used as the nitrogen-containing carbon alloy of Example 16. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 17 ⁇ FeAA2, iron chloride (II) tetrahydrate added (4-Py) 3 -TAz mixture carbon material refiring and acid treatment (17C)> (Infusibilization and carbonization treatment, firing method B, vacuum gas replacement) 0.5095 g of the acid-washed carbon material (5C) of Example 5 was weighed into a quartz boat and placed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement furnace, and nitrogen was added. Was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the firing method B is a firing method in which the operation of deaeration with a vacuum pump and then nitrogen replacement is repeated three times. Thereafter, nitrogen was lowered to 20 mL / min during the temperature rise, and the temperature was raised from 30 ° C. to 1000 ° C. at 5 ° C./min and held at 1000 ° C. for 1 hour. Then, it cooled to room temperature over 3 hours, and obtained carbon-material (17B) 0.3915g.
- the carbon material (17B) was pulverized with an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until no coloration occurred. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.3677 g of acid-washed carbon material (17C). The obtained acid-washed carbon material (17C) was used as the nitrogen-containing carbon alloy of Example 17. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 18 ⁇ FeAA2, iron chloride (II) tetrahydrate added (4-Py) 3 -TAz mixture carbon material refiring and acid treatment (18C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace) Weighing 0.21658 g of the acid-cleaned carbon material of Example 5 (5C) into a quartz boat and installing it in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas displacement rotary furnace, Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the firing method C is a firing method in which after degassing with a vacuum pump, the operation of replacing nitrogen is repeated three times, and the sample tube is rotated at 1.3 rpm during firing in a rotary furnace. Thereafter, nitrogen was lowered to 20 mL / min, the temperature was raised from 30 ° C. to 1000 ° C. at 5 ° C./min, and held at 1000 ° C. for 1 hour, and the quartz tube was rotated at 1.3 rpm per minute. Then, it cooled to room temperature over 3 hours, and obtained carbon-material (18B) 0.1704g.
- the carbon material (18B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant liquid were repeated until there was no coloration. After washing with water, it was filtered and air dried. The obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.1462 g of acid-washed carbon material (18C). The obtained acid-washed carbon material (18C) was used as the nitrogen-containing carbon alloy of Example 18. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 19 ⁇ Addition of FeAA2, iron chloride (II) tetrahydrate (4-Py) 3 -TAz mixture carbon material re-firing and acid treatment (19C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace) Weigh out 0.2327 g of the acid-cleaned carbon material (6C) of Example 6 in a quartz boat and install it in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas displacement rotary furnace. Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (19B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.1677 g of acid-washed carbon material (19C). The obtained acid cleaned carbon material (19C) was used as the nitrogen-containing carbon alloy of Example 19. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 20 ⁇ FeAA2, iron (II) chloride tetrahydrate added (4-Py) 3 -TAz mixture carbon material refiring and acid treatment (20C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace) 0.3761 g of the acid-cleaned carbon material (8C) of Example 8 was measured on a quartz boat and installed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement rotary furnace. Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (20B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and left overnight as it was to obtain 0.2887 g of the acid-washed carbon material (20C). The obtained acid-washed carbon material (20C) was used as the nitrogen-containing carbon alloy of Example 20. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 21 ⁇ FeAA2, iron chloride (II) tetrahydrate added (4-Py) 3 -TAz mixture carbon material re-firing and acid treatment (21C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace)
- the acid-cleaned carbon material (9C) of Example 9 (0.3588 g) was weighed into a quartz boat and installed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement rotary furnace. Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (21B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.3264 g of acid-washed carbon material (21C). The obtained acid cleaned carbon material (21C) was used as the nitrogen-containing carbon alloy of Example 21. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 22 ⁇ FeAA2, iron (II) chloride tetrahydrate added (3-Py) 3 -TAz mixture carbon material refiring and acid treatment (22C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace) 0.4830 g of the acid-cleaned carbon material (10C) of Example 10 was measured in a quartz boat and installed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement rotary furnace. Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (22B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. The obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.3832 g of acid-cleaned carbon material (22C). The obtained acid cleaned carbon material (22C) was used as the nitrogen-containing carbon alloy of Example 22. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 23 ⁇ FeAA2, iron (II) chloride tetrahydrate added (3-Py) 3 -TAz mixture carbon material re-firing and acid treatment (23C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace) 0.4239 g of the acid-cleaned carbon material (11C) of Example 11 was weighed into a quartz boat and installed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement rotary furnace. Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (23B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and left overnight as it was to obtain 0.3481 g of acid-washed carbon material (23C). The obtained acid-washed carbon material (23C) was used as the nitrogen-containing carbon alloy of Example 23. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 24 ⁇ FeAA2, iron chloride (II) tetrahydrate added (3-Py) 3 -TAz mixture carbon material re-firing and acid treatment (24C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace) 0.3881 g of the acid-cleaned carbon material (12C) of Example 12 was weighed into a quartz boat and installed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement rotary furnace. Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (24B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. The obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.3104 g of acid-cleaned carbon material (24C). The obtained acid-washed carbon material (24C) was used as the nitrogen-containing carbon alloy of Example 24. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 25 ⁇ FeAA2, iron (II) chloride tetrahydrate added (3-Py) 3 -TAz mixture carbon material re-firing and acid treatment (25C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace) 0.4201 g of the acid-cleaned carbon material of Example 13 (13C) was weighed into a quartz boat and installed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement rotary furnace. Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (25B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then allowed to stand overnight to obtain 0.3019 g of an acid cleaned carbon material (25C). The obtained acid cleaned carbon material (25C) was used as the nitrogen-containing carbon alloy of Example 25. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 26 ⁇ FeAA2, iron (II) chloride tetrahydrate added (4-Py) 3 -Py mixture carbon material refiring and acid treatment (26C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace) Weighing 0.2360 g of the acid-cleaned carbon material of Example 14 (14C) into a quartz boat and installing it in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas displacement rotary furnace, Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (26B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. The obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.1370 g of acid-washed carbon material (26C). The obtained acid-washed carbon material (26C) was used as the nitrogen-containing carbon alloy of Example 26. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 27 ⁇ FeAA2, Fe (II) chloride tetrahydrate added (4-Py) 3 -TAz mixture carbon material activation and acid treatment (27C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace) 0.460 g of acid-cleaned carbon material (8C) of Example 8 was weighed into a quartz boat and installed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement rotary furnace. Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the nitrogen was reduced to 20 mL / min, the temperature was raised from 30 ° C. to 1000 ° C. at 5 ° C./min, and when the temperature reached 1000 ° C., the quartz tube was rotated at 1.3 rpm. After holding at 1000 ° C. for 40 minutes, the nitrogen was switched to nitrogen mixed with 10% CO 2 (20 mL per minute) and held for 20 minutes. Then, it cooled to room temperature over 3 hours, and obtained 0.3053g of carbon materials (27B).
- the carbon material (27B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then allowed to stand overnight to obtain 0.2793 g of acid-cleaned carbon material (27C). The obtained acid cleaned carbon material (27C) was used as the nitrogen-containing carbon alloy of Example 27. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- Example 28 ⁇ Ammonia activation and acid treatment of carbon material in FeAA2, iron chloride (II) tetrahydrate added (4-Py) 3 -TAz mixture (28C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace) Weigh out 0.2093 g of the acid-cleaned carbon material (8C) of Example 8 in a quartz boat and install it in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement rotary furnace. Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the nitrogen was reduced to 20 mL / min, the temperature was raised from 30 ° C. to 1000 ° C. at 5 ° C./min, and when the temperature reached 1000 ° C., the quartz tube was rotated at 1.3 rpm. After holding at 1000 ° C. for 40 minutes, the nitrogen was changed to nitrogen mixed with 10% NH 3 (20 mL / min) and held for 20 minutes. Then, it cooled to room temperature over 3 hours, and obtained carbon-material (28B) 0.1176g.
- the carbon material (28B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.1053 g of acid-washed carbon material (28C). The obtained acid-washed carbon material (28C) was used as the nitrogen-containing carbon alloy of Example 28. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- (4-Py) 2 -TAz was synthesized with reference to FIG. 2.60 g of 4-cyanopyridine and 59 mg of NaOMe were dissolved in 7.5 mL of MeOH and stirred at room temperature for 4 hours. Then, 2.03 g of formamidine hydrochloride in DMF was added dropwise and stirred for 16 hours. After the solvent was distilled off, the mixture was stirred at 125 ° C. for 16 hours, 25 mL of water was added, and the precipitated solid was washed with water and toluene to obtain 2.5 g of (4-Py) 2 -TAz.
- the carbon material (30B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then allowed to stand overnight to obtain 0.4573 g of acid-cleaned carbon material (30C). The obtained acid-washed carbon material (30C) was used as the nitrogen-containing carbon alloy of Example 30. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Example 40 ⁇ FeAA2, iron chloride (II) tetrahydrate added (4-Py) 2 -TAz mixture carbon material refiring and acid treatment (40C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace) Weighing 0.2536 g of the acid-cleaned carbon material (30C) of Example 30 into a quartz boat and installing it in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas-substitution rotary furnace, Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (40B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and left overnight to obtain 0.1837 g of acid-cleaned carbon material (40C). The obtained acid-washed carbon material (40C) was used as the nitrogen-containing carbon alloy of Example 40. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- the carbon material (31B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.4526 g of acid-washed carbon material (31C). The obtained acid-washed carbon material (31C) was used as the nitrogen-containing carbon alloy of Example 31. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Example 41 ⁇ FeAA2, iron chloride (II) tetrahydrate added (4-Py) 2 Ph-TAz mixture carbon material refiring and acid treatment (41C)> (Infusibilization and carbonization treatment Baking method B Vacuum gas replacement) 0.1872 g of the acid-cleaned carbon material (31C) of Example 31 was weighed into a quartz boat and installed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement rotary furnace. Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (41B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. The obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.1496 g of acid-cleaned carbon material (41C). The obtained acid cleaned carbon material (41C) was used as the nitrogen-containing carbon alloy of Example 41. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- the carbon material (32B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant liquid were repeated until there was no coloration. After washing with water, it was filtered and air dried. Furthermore, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.7823 g of acid-washed carbon material (32C). The obtained acid cleaned carbon material (32C) was used as the nitrogen-containing carbon alloy of Example 32. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Example 42 ⁇ FeAA2, iron chloride (II) tetrahydrate added (4-Py- ⁇ -) 3 -TAz mixture carbon material refiring and acid treatment (42C)> (Infusibilization and carbonization treatment Baking method B Vacuum gas replacement)
- the acid-cleaned carbon material (32C) 0.3465 g of Example 32 was weighed into a quartz boat and placed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement rotary furnace. Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (42B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.3109 g of acid-washed carbon material (42C). The obtained acid cleaned carbon material (42C) was used as the nitrogen-containing carbon alloy of Example 42. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- the carbon material (33B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant liquid were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then allowed to stand overnight to obtain 0.4257 g of acid-washed carbon material (33C). The obtained acid cleaned carbon material (33C) was used as the nitrogen-containing carbon alloy of Example 33. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Example 43 ⁇ Refiring and acid treatment of carbon material of FeAA2, iron chloride (II) tetrahydrate added 4,4'-Py-Py mixture (43C)> (Infusibilization and carbonization treatment, firing method B, vacuum gas replacement) Weighing 0.3116 g of the acid-cleaned carbon material (33C) of Example 33 into a quartz boat and installing it in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas displacement rotary furnace, Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (43B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then allowed to stand overnight to obtain 0.2123 g of acid-cleaned carbon material (43C). The obtained acid cleaned carbon material (43C) was used as the nitrogen-containing carbon alloy of Example 43. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- the carbon material (34B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant liquid were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.1562 g of acid-washed carbon material (34C). The obtained acid-washed carbon material (34C) was used as the nitrogen-containing carbon alloy of Example 34. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Example 44 ⁇ Refiring and acid treatment of carbon material of FeAA2, iron chloride (II) tetrahydrate added 3,3'-Py-Py mixture (44C)> (Infusibilization and carbonization treatment, firing method B, vacuum gas replacement) 0.0886 g of the acid-cleaned carbon material (34C) of Example 34 was weighed into a quartz boat and placed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas displacement rotary furnace, Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (44B) was pulverized with an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until no coloration occurred. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then allowed to stand overnight to obtain 0.0554 g of an acid cleaned carbon material (44C). The obtained acid cleaned carbon material (44C) was used as the nitrogen-containing carbon alloy of Example 44. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- the carbon material (35B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant liquid were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.4752 g of acid-washed carbon material (35C). The obtained acid-washed carbon material (35C) was used as the nitrogen-containing carbon alloy of Example 35. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Example 45 ⁇ Refiring and acid treatment (45C) of carbon material of 1,2- (4-Py) 2-Ethylene mixture containing FeAA2 and iron (II) chloride tetrahydrate> (Infusibilization and carbonization treatment, firing method B, vacuum gas replacement furnace) Weighing 0.2146 g of the acid-cleaned carbon material of Example 35 (35C) into a quartz boat and installing it in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas displacement rotary furnace, Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (45B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.1604 g of an acid cleaned carbon material (45C). The obtained acid-washed carbon material (45C) was used as the nitrogen-containing carbon alloy of Example 45. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Example 36 ⁇ FeAA2, iron chloride (II) tetrahydrate added (4-Py) (2-Py) 2 -TAz mixture carbon material synthesis (36C)> (Adjustment of (4-Py) (2-Py) 2 -TAz) Chem. Comm, 2002, 1356-1357. (4-Py) (2-Py) 2 -TAz was adjusted with reference to FIG. 200 mg of NaH was added to 5 g of 4-cyanopyridine and 5 g of 2-cyanopyridine, heated at 180 ° C. for 30 minutes, cooled to room temperature, dissolved in 500 mL of toluene with heating, and filtered.
- 4-Py) (2-Py) 2 -TAz was adjusted with reference to FIG. 200 mg of NaH was added to 5 g of 4-cyanopyridine and 5 g of 2-cyanopyridine, heated at 180 ° C. for 30 minutes, cooled to room temperature, dissolved in 500 mL of toluene with heating, and
- the obtained toluene solution was extracted with an aqueous solution obtained by dissolving 3 g of NiCl 2 .6H 2 O in 200 mL of deionized water, 5 g of KCN was added thereto, the resulting precipitate was filtered and dried, recrystallized with ethanol, 2 g of (4-Py) (2-Py) 2 TAz was obtained.
- FeAA2 iron (II) chloride tetrahydrate added (4-Py) (2-Py) 2 -TAz mixture preparation
- the carbon material (36B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and then left overnight to obtain 0.6788 g of acid-washed carbon material (36C). The obtained acid cleaned carbon material (36C) was used as the nitrogen-containing carbon alloy of Example 36. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Example 46 ⁇ FeAA2, iron chloride (II) tetrahydrate added (4-Py) (2-Py) 2 -TAz mixture carbon material refiring and acid treatment (46C)> (Infusibilization and carbonization treatment, firing method B, vacuum gas replacement furnace) Weighing 0.5099 g of the acid-cleaned carbon material (36C) of Example 36 into a quartz boat and installing it in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas displacement rotary furnace, Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (46B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. The obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.3840 g of acid-washed carbon material (46C). The obtained acid cleaned carbon material (46C) was used as the nitrogen-containing carbon alloy of Example 46. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- (4-Py) 3 -Ph was synthesized with reference to FIG. 157 mg 1,3,5-tribromobenzene, 250 mg 4-pyridineboronic acid, 80 mg Pd (dppf) Cl 2 .CH 2 Cl 2 , 1.60 g of sodium carbonate was dissolved in 20 mL of 1,4-dioxane and 20 mL of pure water, degassed by nitrogen bubbling, and then heated to reflux for 30 hours. After extraction with dichloromethane and evaporation of the solvent, the residue was purified by alumina column chromatography (chloroform). Finally, 4.9 g of (4-Py) 3 -Ph was obtained by MeOH cooking.
- the carbon material (50B) was pulverized with an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until no coloration occurred. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and then left overnight to obtain 0.5957 g of acid-washed carbon material (50C). The obtained acid cleaned carbon material (50C) was used as the nitrogen-containing carbon alloy of Example 50. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Example 60 ⁇ FeAA2, iron chloride (II) tetrahydrate added (4-Py) 3 -Ph mixture carbon material re-firing and acid treatment (60C)> (Infusibilization and carbonization treatment Baking method B Vacuum gas replacement) Weighing 0.3017 g of the acid-cleaned carbon material (50C) of Example 50 into a quartz boat and installing it in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement rotary furnace, Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (60B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and left overnight as it was to obtain 0.2314 g of acid-cleaned carbon material (60C). The obtained acid cleaned carbon material (60C) was used as the nitrogen-containing carbon alloy of Example 60. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Feal2 and iron chloride (II) tetrahydrate added (HIm) 3 -TAz mixture (51A) 3.1936 g was weighed into a quartz boat and inserted into a tubular furnace with 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz It was installed in the center of the tube, and nitrogen was circulated at 200 mL per minute for 30 minutes at room temperature. The temperature was raised from 30 ° C. to 700 ° C. at a rate of 5 ° C. per minute and held at 700 ° C. for 1 hour. Then, it cooled to room temperature over 3 hours, and obtained carbon-material (51B) 0.8524g.
- the carbon material (51B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and left overnight as it was to obtain 0.5107 g of acid-washed carbon material (51C). The obtained acid cleaned carbon material (51C) was used as the nitrogen-containing carbon alloy of Example 51. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Example 61 ⁇ FeAA2, iron chloride (II) tetrahydrate added (HIm) 3 -TAz mixture carbon material refiring and acid treatment (61C)> (Infusibilization and carbonization treatment, firing method C, vacuum gas displacement rotary furnace) Weighing 0.3101 g of the acid-cleaned carbon material of Example 51 (51C) into a quartz boat and installing it in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas displacement rotary furnace, Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (61B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and then left overnight to obtain 0.1959 g of an acid cleaned carbon material (61C). The obtained acid cleaned carbon material (61C) was used as the nitrogen-containing carbon alloy of Example 61. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- the carbon material (52B) was pulverized in an agate mortar, washed with water, filtered and air-dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then allowed to stand overnight to obtain a non-acid cleaned carbon material (52C). The obtained non-acid cleaned carbon material (52C) was used as the nitrogen-containing carbon alloy of Example 52. The specific surface area was measured by the BET method. The results are shown in the column before acid cleaning in Table 2 below.
- Example 62 ⁇ Refiring and acid treatment (62C) of carbon material of 1,2- (4-Py) 2-Ethane mixture containing FeAA2, iron (II) chloride tetrahydrate> (Infusibilization and carbonization treatment, firing method B, vacuum gas replacement furnace) 0.1546 g of the acid-cleaned carbon material (53C) of Example 53 was weighed into a quartz boat and placed in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas replacement rotary furnace. Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (62B) was pulverized with an agate mortar, washed with water, filtered and air-dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and left as it was overnight to obtain a non-acid cleaned carbon material (62C). The obtained non-acid cleaned carbon material (62C) was used as the nitrogen-containing carbon alloy of Example 62. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Example 54 Carbon Material Synthesis of Poly (4-VinylPyridine) Mixture with Addition of FeAA2 and Iron (II) Chloride Tetrahydrate (54C)>
- the carbon material (54B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and left overnight to obtain 0.6881 g of acid-washed carbon material (54C). The obtained acid cleaned carbon material (54C) was used as the nitrogen-containing carbon alloy of Example 54. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- Example 64 ⁇ Refiring and Acid Treatment of Carbon Material of Mixture of Poly (4-VinylPyridine) Added with FeAA2 and Iron (II) Chloride Tetrahydrate (64C)> (Infusibilization and carbonization treatment, firing method B, vacuum gas replacement furnace) Weighing 0.3244 g of the acid-cleaned carbon material of Example 54 (54C) into a quartz boat and installing it in the center of a 4.0 cm ⁇ (inner diameter 3.6 cm ⁇ ) quartz tube inserted into a vacuum gas displacement rotary furnace, Nitrogen was circulated at 200 mL per minute for 1 minute at room temperature. Next, the inside of the tube was evacuated with a vacuum pump, and nitrogen substitution was repeated three times.
- the carbon material (64B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, left to room temperature, and left overnight to obtain 0.2116 g of acid-cleaned carbon material (64C). The obtained acid cleaned carbon material (64C) was used as the nitrogen-containing carbon alloy of Example 64. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 2 below.
- the carbon material (C2B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and allowed to stand overnight to obtain 0.0709 g of acid-cleaned carbon material (C2C). The obtained acid cleaned carbon material (C2C) was used as the nitrogen-containing carbon alloy of Comparative Example 2. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- the carbon material (C3B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and left overnight as it was to obtain 0.0696 g of acid-cleaned carbon material (C3C). The obtained acid cleaned carbon material (C3C) was used as the nitrogen-containing carbon alloy of Comparative Example 3. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- the carbon material (C4B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. The obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and then left overnight to obtain 0.0107 g of acid-washed carbon material (C4C). The obtained acid cleaned carbon material (C4C) was used as the nitrogen-containing carbon alloy of Comparative Example 4. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- the carbon material (C5B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand at room temperature, and left as it was overnight to obtain an acid cleaned carbon material (C5C). The obtained acid-washed carbon material (C5C) was used as the nitrogen-containing carbon alloy of Comparative Example 5. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- FeAA2 iron (II) chloride tetrahydrate added (2-Py) 3 -TAz mixture preparation) (2-Py) 3 -TAz 6.30 g (manufactured by SIGMA ALDRICH), FeAA2 0.403 g and iron (II) chloride tetrahydrate 6.30 g were added, and then mechanically pulverized and mixed to obtain FeAA2, iron chloride (II ) Tetrahydrate added (2-Py) 3 -TAz mixture (C6A) was obtained.
- the carbon material (C6B) was pulverized in an agate mortar, and concentrated hydrochloric acid washing, centrifugal filtration, and removal of the supernatant were repeated until there was no coloration. After washing with water, it was filtered and air dried. Further, the obtained carbon material was vacuum-dried at 110 ° C. for 3 hours, allowed to stand to room temperature, and allowed to stand overnight to obtain 1.1290 g of acid-washed carbon material (C6C). The obtained acid cleaned carbon material (C6C) was used as the nitrogen-containing carbon alloy of Comparative Example 6. The specific surface area was measured by the BET method. The results are shown in the column after acid cleaning in Table 1 below.
- the nitrogen-containing carbon alloys of Examples 1 to 28, Examples 30 to 36, Examples 40 to 46, Examples 50 to 54, and Examples 60 to 64 have a sufficiently large specific surface area and exhibit catalyst performance. It was found that the ORR voltage shown was sufficiently high. Furthermore, it was found that the yield of nitrogen-containing carbon alloy was also good. Further, it was found that a higher ORR voltage was obtained in the nitrogen-containing carbon alloy to which the organometallic complex was added during the production. It can also be seen that a higher ORR voltage is obtained by providing a re-baking step. Furthermore, the yield is improved by providing a re-baking step. On the other hand, it was found that the comparative example had an insufficient increase in specific surface area and had a low voltage indicating catalyst performance, which was insufficient.
- thermocompression-bonded film was taken out from the two polyimide films, and the catalyst layer was transferred to both sides of the proton conducting film by peeling off the Teflon (registered trademark) sheet which is the base of the cathode coating film and the anode coating film.
- Teflon registered trademark
- An electrode composite membrane was obtained. This electrode composite membrane was immersed in a 0.5 mol / L sulfuric acid aqueous solution for 10 hours, washed with ion-exchanged water, and dried to obtain the desired electrode composite membrane.
- the non-platinum catalyst ink for cathode (21E) prepared from the nitrogen-containing carbon alloy material of the present invention in place of the modified product (28E) used in (1) -1 by the same procedure as (1) to (5) above. ), (24E), (27E) and comparative carbon material inks for cathodes (C3E), (C5E), and assembling evaluation batteries (Cell-2) to (Cell-6), and (6) Similarly, current-voltage measurement was performed. The voltage at a current value of 2A is shown in Table 2.
- a nitrogen-containing carbon alloy having a sufficiently high oxygen reduction activity can be obtained.
- the nitrogen-containing carbon alloy obtained by the production method of the present invention can be used as a carbon catalyst.
- the yield of a nitrogen-containing carbon alloy can be raised and productivity can be improved.
- Such a carbon catalyst is preferably used for a fuel cell or an environmental catalyst and has high industrial applicability.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Catalysts (AREA)
- Inert Electrodes (AREA)
- Carbon And Carbon Compounds (AREA)
- Fuel Cell (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013136547 | 2013-06-28 | ||
| JP2013-136547 | 2013-06-28 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014208740A1 true WO2014208740A1 (ja) | 2014-12-31 |
Family
ID=52142063
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/067220 Ceased WO2014208740A1 (ja) | 2013-06-28 | 2014-06-27 | 含窒素カーボンアロイの製造方法、含窒素カーボンアロイ及び燃料電池触媒 |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP2015027934A (enExample) |
| WO (1) | WO2014208740A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112186208A (zh) * | 2020-10-14 | 2021-01-05 | 天津工业大学 | 一种氮、硫共掺杂碳基氧还原催化剂及其制备方法和应用 |
| CN117603191A (zh) * | 2023-10-26 | 2024-02-27 | 华南师范大学 | 一种有机电子传输材料及其制备方法和应用 |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101692852B1 (ko) * | 2015-07-02 | 2017-01-06 | 인하대학교 산학협력단 | 코발트 기반 산소환원반응용 촉매 및 이의 제조방법 |
| JP6560932B2 (ja) * | 2015-08-24 | 2019-08-14 | 旭化成株式会社 | 窒素含有炭素材料及びその製造方法、含窒素炭素材料用前駆体組成物、並びに燃料電池用電極 |
| CN111909132A (zh) * | 2019-05-07 | 2020-11-10 | 北京鼎材科技有限公司 | 一种有机材料及采用该材料的有机电致发光器件 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010064555A1 (ja) * | 2008-12-02 | 2010-06-10 | 日清紡ホールディングス株式会社 | 炭素触媒及びその製造方法、これを用いた電極及び電池 |
| WO2012096023A1 (ja) * | 2011-01-14 | 2012-07-19 | 昭和電工株式会社 | 燃料電池用電極触媒の製造方法、燃料電池用電極触媒およびその用途 |
| WO2013125503A1 (ja) * | 2012-02-20 | 2013-08-29 | 富士フイルム株式会社 | 含窒素カーボンアロイとその製造方法、カーボンアロイ触媒および燃料電池 |
| JP2013212970A (ja) * | 2012-04-03 | 2013-10-17 | M & S Kenkyu Kaihatsu Kk | ポリトリアジンテレフタルアミド樹脂炭化物及びその製造法。 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5689379B2 (ja) * | 2011-07-19 | 2015-03-25 | 日清紡ホールディングス株式会社 | 触媒担持用担体、触媒担持体、電極及び電池 |
| CN103747872B (zh) * | 2011-08-08 | 2016-06-08 | 昭和电工株式会社 | 氧还原催化剂的制造方法以及其用途 |
-
2014
- 2014-06-27 WO PCT/JP2014/067220 patent/WO2014208740A1/ja not_active Ceased
- 2014-06-27 JP JP2014133151A patent/JP2015027934A/ja not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010064555A1 (ja) * | 2008-12-02 | 2010-06-10 | 日清紡ホールディングス株式会社 | 炭素触媒及びその製造方法、これを用いた電極及び電池 |
| WO2012096023A1 (ja) * | 2011-01-14 | 2012-07-19 | 昭和電工株式会社 | 燃料電池用電極触媒の製造方法、燃料電池用電極触媒およびその用途 |
| WO2013125503A1 (ja) * | 2012-02-20 | 2013-08-29 | 富士フイルム株式会社 | 含窒素カーボンアロイとその製造方法、カーボンアロイ触媒および燃料電池 |
| JP2013212970A (ja) * | 2012-04-03 | 2013-10-17 | M & S Kenkyu Kaihatsu Kk | ポリトリアジンテレフタルアミド樹脂炭化物及びその製造法。 |
Non-Patent Citations (2)
| Title |
|---|
| M. LEFEVRE ET AL.: "ACTIVITY FOR O2 REDUCTION OF HEAT-TREATED FE/N/C CATALYSTS PREPARED WITH CARBON BLACK MODIFIED BY NITROGEN-BEARING FUNCTIONALITIES", ECS TRANSACTIONS, vol. 3, no. 1, 2006, pages 201 - 210 * |
| R. KOBAYASHI ET AL.: "Novel N-Doped Carbon Cathode Catalyst for Polymer Electrolyte Membrane Fuel Cells Formed on Carbon Black", CHEMISTRY LETTERS, vol. 38, 2009, pages 396 - 397 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112186208A (zh) * | 2020-10-14 | 2021-01-05 | 天津工业大学 | 一种氮、硫共掺杂碳基氧还原催化剂及其制备方法和应用 |
| CN112186208B (zh) * | 2020-10-14 | 2022-05-27 | 天津工业大学 | 一种氮、硫共掺杂碳基氧还原催化剂及其制备方法和应用 |
| CN117603191A (zh) * | 2023-10-26 | 2024-02-27 | 华南师范大学 | 一种有机电子传输材料及其制备方法和应用 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2015027934A (ja) | 2015-02-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5820408B2 (ja) | 含窒素カーボンアロイとその製造方法、カーボンアロイ触媒および燃料電池 | |
| JP6320333B2 (ja) | 複合体、複合体の製造方法及び燃料電池触媒 | |
| JP5608595B2 (ja) | 含窒素カーボンアロイ、その製造方法及びそれを用いた炭素触媒 | |
| JP2016102037A (ja) | 含窒素カーボンアロイの製造方法、含窒素カーボンアロイ及び燃料電池触媒 | |
| CN103299464B (zh) | 燃料电池用电极催化剂的制造方法、燃料电池用电极催化剂和其用途 | |
| KR102014985B1 (ko) | 복합체, 이를 포함하는 전극 촉매, 그 제조방법 및 이를 이용한 연료전지 | |
| JP5325355B2 (ja) | 燃料電池用電極触媒の製造方法、燃料電池用電極触媒およびその用途 | |
| JP6454350B2 (ja) | 含窒素カーボンアロイの製造方法、含窒素カーボンアロイ及び燃料電池触媒 | |
| WO2013008501A1 (ja) | 酸素還元触媒およびその製造方法、並びに固体高分子形燃料電池 | |
| WO2014208740A1 (ja) | 含窒素カーボンアロイの製造方法、含窒素カーボンアロイ及び燃料電池触媒 | |
| JP5918156B2 (ja) | カーボンアロイ材料、カーボンアロイ触媒および燃料電池の製造方法 | |
| US20150376218A1 (en) | Method for manufacturing nitrogen-containing carbon alloy, nitrogen-containing carbon alloy, and fuel cell catalyst | |
| JP2009231049A (ja) | 白金担持カーボン、燃料電池用触媒、電極膜接合体、および燃料電池 | |
| WO2015199125A1 (ja) | 含窒素カーボンアロイの製造方法、含窒素カーボンアロイ及び燃料電池触媒 | |
| JP2021063246A (ja) | 還元反応用電極、還元反応用電極の製造方法、および還元反応用電極を用いた反応デバイス | |
| KR101768121B1 (ko) | 리튬 공기 전지 | |
| JP5837355B2 (ja) | 燃料電池用電極触媒の製造方法、燃料電池用電極触媒およびその用途 | |
| JP5837356B2 (ja) | 燃料電池用電極触媒の製造方法、燃料電池用電極触媒およびその用途 | |
| Chakraborty et al. | Triazine‐Based Porous Organic Polymer Electrocatalysts: Utility and Design Strategy |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14817590 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14817590 Country of ref document: EP Kind code of ref document: A1 |