WO2014115457A1 - 摺動部材及びその製造方法 - Google Patents
摺動部材及びその製造方法 Download PDFInfo
- Publication number
- WO2014115457A1 WO2014115457A1 PCT/JP2013/084212 JP2013084212W WO2014115457A1 WO 2014115457 A1 WO2014115457 A1 WO 2014115457A1 JP 2013084212 W JP2013084212 W JP 2013084212W WO 2014115457 A1 WO2014115457 A1 WO 2014115457A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- acid
- hard
- branched
- plating layer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16J—PISTONS; CYLINDERS; SEALINGS
- F16J9/00—Piston-rings, e.g. non-metallic piston-rings, seats therefor; Ring sealings of similar construction
- F16J9/26—Piston-rings, e.g. non-metallic piston-rings, seats therefor; Ring sealings of similar construction characterised by the use of particular materials
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/22—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the deposition of inorganic material, other than metallic material
- C23C16/26—Deposition of carbon only
- C23C16/27—Diamond only
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C28/00—Coating for obtaining at least two superposed coatings either by methods not provided for in a single one of groups C23C2/00 - C23C26/00 or by combinations of methods provided for in subclasses C23C and C25C or C25D
- C23C28/30—Coatings combining at least one metallic layer and at least one inorganic non-metallic layer
- C23C28/32—Coatings combining at least one metallic layer and at least one inorganic non-metallic layer including at least one pure metallic layer
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C28/00—Coating for obtaining at least two superposed coatings either by methods not provided for in a single one of groups C23C2/00 - C23C26/00 or by combinations of methods provided for in subclasses C23C and C25C or C25D
- C23C28/30—Coatings combining at least one metallic layer and at least one inorganic non-metallic layer
- C23C28/32—Coatings combining at least one metallic layer and at least one inorganic non-metallic layer including at least one pure metallic layer
- C23C28/322—Coatings combining at least one metallic layer and at least one inorganic non-metallic layer including at least one pure metallic layer only coatings of metal elements only
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C28/00—Coating for obtaining at least two superposed coatings either by methods not provided for in a single one of groups C23C2/00 - C23C26/00 or by combinations of methods provided for in subclasses C23C and C25C or C25D
- C23C28/30—Coatings combining at least one metallic layer and at least one inorganic non-metallic layer
- C23C28/34—Coatings combining at least one metallic layer and at least one inorganic non-metallic layer including at least one inorganic non-metallic material layer, e.g. metal carbide, nitride, boride, silicide layer and their mixtures, enamels, phosphates and sulphates
- C23C28/343—Coatings combining at least one metallic layer and at least one inorganic non-metallic layer including at least one inorganic non-metallic material layer, e.g. metal carbide, nitride, boride, silicide layer and their mixtures, enamels, phosphates and sulphates with at least one DLC or an amorphous carbon based layer, the layer being doped or not
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C28/00—Coating for obtaining at least two superposed coatings either by methods not provided for in a single one of groups C23C2/00 - C23C26/00 or by combinations of methods provided for in subclasses C23C and C25C or C25D
- C23C28/30—Coatings combining at least one metallic layer and at least one inorganic non-metallic layer
- C23C28/34—Coatings combining at least one metallic layer and at least one inorganic non-metallic layer including at least one inorganic non-metallic material layer, e.g. metal carbide, nitride, boride, silicide layer and their mixtures, enamels, phosphates and sulphates
- C23C28/347—Coatings combining at least one metallic layer and at least one inorganic non-metallic layer including at least one inorganic non-metallic material layer, e.g. metal carbide, nitride, boride, silicide layer and their mixtures, enamels, phosphates and sulphates with layers adapted for cutting tools or wear applications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D3/00—Electroplating: Baths therefor
- C25D3/02—Electroplating: Baths therefor from solutions
- C25D3/04—Electroplating: Baths therefor from solutions of chromium
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D5/00—Electroplating characterised by the process; Pretreatment or after-treatment of workpieces
- C25D5/48—After-treatment of electroplated surfaces
- C25D5/50—After-treatment of electroplated surfaces by heat-treatment
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D7/00—Electroplating characterised by the article coated
- C25D7/10—Bearings
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16J—PISTONS; CYLINDERS; SEALINGS
- F16J9/00—Piston-rings, e.g. non-metallic piston-rings, seats therefor; Ring sealings of similar construction
- F16J9/28—Piston-rings, e.g. non-metallic piston-rings, seats therefor; Ring sealings of similar construction of non-metals
Definitions
- the present invention relates to a sliding member and a manufacturing method thereof. More specifically, the present invention relates to a sliding member in which a predetermined hard chromium plating layer and a hard carbon layer are laminated in this order on the surface of a substrate, and a method for manufacturing the same.
- This piston ring for an automobile engine is a piston ring for an automobile engine having a sliding portion that slides in the presence of lubricating oil, and a hard carbon thin film containing 25 atomic% or less of hydrogen atoms on the sliding surface of the sliding portion. Is covered.
- the adhesion of the hard carbon thin film is improved and the durability is improved by subjecting the sliding surface of the sliding part before coating with the hard carbon thin film to chrome plating. Improved.
- the present invention has been made in view of such problems of the conventional technology. And an object of this invention is to provide the sliding member which can implement
- the inventors of the present invention made extensive studies to achieve the above object. As a result, the inventors have found that the above object can be achieved by laminating a predetermined hard chromium plating layer and a hard carbon layer in this order on the surface of the base material, and have completed the present invention.
- the sliding member of the present invention includes a base material, a hard chromium plating layer mainly composed of chromium formed on the surface of the base material, and a carbon element formed on the hard chromium plating layer. And a hard carbon layer mainly composed of And in the sliding member of this invention, the hydrogen concentration of a hard chromium plating layer is 150 mass ppm or less.
- the manufacturing method of the sliding member of this invention is one form of the method of producing the said sliding member of this invention.
- the surface of the base material in which the hard chromium plating layer which has chromium as a main component was formed on the surface is heat-processed at the temperature of 250 degreeC or more, and the hydrogen concentration of a hard chromium plating layer is 150.
- a hard carbon layer mainly composed of carbon element is formed on the hard chromium plating layer.
- a base material, a hard chromium plating layer mainly composed of chromium formed on the surface of the base material, and a carbon element formed mainly on the hard chromium plating layer are mainly configured.
- the hydrogen concentration of the hard chromium plating layer is 150 mass ppm or less. Therefore, the sliding member which can implement
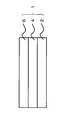
- FIG. 1 is an explanatory diagram showing an outline of a sliding member according to an embodiment of the present invention.
- the sliding member 1 of this embodiment includes a base material 2, a hard chromium plating layer 4 mainly composed of chromium, and a hard chromium plating layer formed on the surface of the base material 2. 4 and a hard carbon layer 6 mainly composed of a carbon element.
- the hard chromium plating layer 4 has a hydrogen concentration of 150 ppm by mass or less.
- the surface of the base material on which the hard chromium plating layer mainly composed of chromium is formed is heated at a temperature of 250 ° C. or higher. It was found that the adhesion between the hard chromium plating layer and the hard carbon layer is remarkably improved by setting the hydrogen concentration of the hard chromium plating layer to 150 mass ppm or less.
- the hard chrome plating layer contains a large amount of hydrogen in the formation stage.
- the higher the temperature of heat treatment the more hydrogen can be released.
- the heat treatment when the heat treatment is performed at 400 ° C. or higher, the hardness of the chromium plating layer decreases, and the chromium plating layer Therefore, it is preferable to perform the heat treatment at a temperature of less than 400 ° C.
- the sliding member of the present invention can be used either in the presence of a lubricant or in the absence of a lubricant (so-called dry conditions) from the viewpoint of exhibiting excellent peeling resistance.
- a lubricant base for example, base oil
- the presence of the lubricant It is preferably used in a lubricant.
- examples of the sliding member include a valve-type sliding member, an intake / exhaust-type sliding member, and a driving-system sliding member such as various internal combustion engines (particularly automobile engines) such as a 4-cycle and 2-cycle engines. be able to.
- a driving-system sliding member such as various internal combustion engines (particularly automobile engines) such as a 4-cycle and 2-cycle engines.
- the present invention is not limited to these, and examples include a variable swash plate and a rotary vane of a refrigerant compressor.
- sliding member for the valve train and intake / exhaust system examples include, for example, a piston ring, a piston pin, a piston (or a piston skirt (a piston skirt means a skirt portion of a piston)), a cylinder (or a cylinder liner), Plunger, check valve, valve guide, connecting rod, bush, crankshaft, cam lobe, cam journal, rocker arm, valve spring, shim, lifter, vane pump rotating vane, vane pump housing, timing chain, sprocket, chain guide (or chain guide) Shoe), chain tensioner (or chain tensioner shoe), etc.
- Examples of the drive system sliding member include gears, gears, chains, belts, rolling bearings, sliding bearings, oil pumps, etc., such as automatic transmissions, continuously variable transmissions, manual transmissions, and final reduction gears. it can.
- a base material in this invention not only a high purity iron member, an aluminum member, and a titanium member but iron alloy members, such as stainless steel (steel material), a copper alloy member, an aluminum alloy member, a magnesium alloy member, titanium What comprised the metal members, such as an alloy member, can also be applied.
- iron alloy members such as stainless steel (steel material)
- a copper alloy member, an aluminum alloy member, a magnesium alloy member, titanium What comprised the metal members, such as an alloy member can also be applied.
- a base material composed of non-metallic members such as resin members such as various rubbers and plastics, ceramic members, and carbon members.
- an iron alloy member, an aluminum alloy member, and a magnesium alloy member are preferable in that they can be easily applied to sliding parts of existing machines and devices, and can contribute widely to energy saving measures in various fields.
- an iron alloy member, an aluminum alloy member, a magnesium alloy member, a titanium alloy member, or the like may be provided with a thin film coating such as titanium nitride (TiN) or chromium nitride (CrN).
- iron alloy member it is preferable to use, for example, an iron-based alloy containing nickel, copper, zinc, chromium, cobalt, molybdenum, lead, silicon, titanium, or any combination thereof as an alloy element.
- an iron-based alloy containing nickel, copper, zinc, chromium, cobalt, molybdenum, lead, silicon, titanium, or any combination thereof.
- high carbon chromium bearing steel defined as SUJ2 in JIS G4805
- alloy tool steel carburized steel
- low alloy chilled cast iron tempered carbon steel
- hardened steel can be used.
- nickel chrome steel SNC415, SNC815)
- nickel chrome molybdenum steel SNCM220, SNCM415, SNCM420, SNCM616, SNCM815)
- chrome steel SCr415, SCr420
- chrome molybdenum steel SCM415, SCM4108 prescribed by JIS.
- SCM420, SCM421, SCM822 manganese steel
- SnC420 manganese chrome steel
- the surface hardness of the iron member or iron alloy member is preferably Rockwell hardness (C scale) and HRC 45-60.
- C scale Rockwell hardness
- HRC 45-60 the durability of the hard carbon layer can be maintained even under sliding conditions under a high surface pressure of about 700 MPa like a cam follower member, which is effective. If the surface hardness is less than HRC45, it may be buckled and peeled easily under high surface pressure.
- the surface roughness of the iron member or iron alloy member is preferably an arithmetic average roughness Ra of 0.1 ⁇ m or less from the viewpoint of sliding stability. When it exceeds 0.1 ⁇ m, scuffing is locally formed, and the friction coefficient may be greatly improved.
- the aluminum alloy member for example, a hypoeutectic aluminum alloy or a hypereutectic aluminum alloy containing 4 to 20% by mass of silicon (Si) and 1.0 to 5.0% by mass of copper (Cu) is used.
- Si silicon
- Cu copper
- AC2A, AC8A, ADC12, ADC14 etc. which are prescribed
- the surface hardness of the aluminum member or aluminum alloy member is preferably Brinell hardness and is HB 80 to 130. If the surface hardness of the aluminum member or aluminum alloy member is out of the above range, the aluminum member or aluminum alloy member may be easily worn when the surface hardness is less than HB80.
- the surface roughness of the aluminum member or the aluminum alloy member is preferably an arithmetic average roughness Ra of 0.1 ⁇ m or less from the viewpoint of sliding stability. When it exceeds 0.1 ⁇ m, scuffing is locally formed, and the friction coefficient may be greatly improved.
- magnesium alloy members include magnesium-aluminum-zinc (Mg-Al-Zn), magnesium-aluminum-rare earth metal (Mg-Al-REM), magnesium-aluminum-calcium (Mg-Al-Ca). ), Magnesium-zinc-aluminum-calcium (Mg-Zn-Al-Ca), magnesium-aluminum-calcium-rare earth metal (Mg-Al-Ca-REM), magnesium-aluminum-strontium (Mg-Al- Sr), magnesium-aluminum-silicon (Mg-Al-Si), magnesium-rare earth metal-zinc (Mg-REM-Zn), magnesium-silver-rare earth metal (Mg-Ag-REM), or magnesium- Yttrium-rare earth metal ( g-Y-REM) system, or it is preferable to use pertaining to any combination thereof. Specific examples include AZ91, AE42, AX51, AXJ, ZAX85, AX
- the surface hardness of the magnesium member or the magnesium alloy member is preferably Brinell hardness and is HB45 to 95. If the surface hardness of the magnesium member or magnesium alloy member deviates from the above range, the magnesium member or magnesium alloy member may be easily worn when the surface hardness is less than HB45.
- the surface roughness of the magnesium member or the magnesium alloy member is preferably an arithmetic average roughness Ra of 0.1 ⁇ m or less from the viewpoint of sliding stability. When it exceeds 0.1 ⁇ m, scuffing is locally formed, and the friction coefficient may be greatly improved.
- examples of the hard chromium plating layer in the present invention include those containing chromium as a main component.
- “having chromium as a main component” means that the chromium content in the plating layer is 50 mass% or more.
- the composition of the hard chromium plating layer may vary slightly depending on the type of plating bath and the electrodeposition conditions, but usually 0.03 to 0.1% by mass of hydrogen, 0.2 to 0.5% by mass of oxygen, and the balance Chrome.
- the hydrogen concentration of the hard chromium plating layer in the sliding member of the present invention needs to be 150 mass ppm or less, preferably 10 mass ppm or more and 140 mass ppm or less, and 25 mass ppm or more and 110 mass ppm or less.
- the heat treatment temperature is preferably 260 ° C. or more and less than 400 ° C., and when the hydrogen concentration is 25 mass ppm or more and 110 mass ppm or less. Is preferably 290 ° C. or higher and 360 ° C. or lower.
- a hard carbon layer formed by a chemical vapor deposition (CVD) method or a physical vapor synthesis (PVD) method which will be described later, has a high internal stress in the hard carbon layer itself compared to a surface treatment such as plating, and is hard.
- the hardness of the carbon layer is remarkably high. Therefore, when applied to a sliding member of a machine part, there is a possibility that the hard carbon layer may be peeled off from the base material or the hard carbon layer may be cracked.
- the internal stress can be relaxed and improved while maintaining the adhesion between the hard carbon layer and the substrate.
- the surface hardness of the hard chrome plating layer is micro Vickers hardness at a load of 10 g, usually about 800 to 1000 HV, preferably 700 HV or more, and more preferably 750 HV or more. 800 HV or more is more preferable.
- the thickness of the hard chrome plating layer is preferably 0.05 to 200 ⁇ m, more preferably 0.3 to 100 ⁇ m. If the thickness is less than 0.05 ⁇ m, it is easy to wear out, and the friction reducing effect may be lost immediately due to initial wear. On the other hand, if the thickness exceeds 200 ⁇ m, the residual stress in the layer increases, and there is a possibility of peeling from the substrate.
- the crystalline or amorphous thing mainly comprised by the carbon element can be mentioned.
- the crystalline material include those made of diamond.
- the bonding form between carbons consists of both a diamond structure (SP 3 bond) and a graphite structure (SP 2 bond), or a structure mainly composed of a graphite structure (SP 2 bond).
- DLC diamond-like carbon
- an amorphous material in which the bonding form between carbons consists of both a diamond structure (SP 3 bond) and a graphite structure (SP 2 bond) it consists only of carbon element (not including hydrogen).
- Amorphous carbon amorphous carbon (aC)
- hydrogen amorphous carbon containing hydrogen aC: H
- metal amorphous carbon MeC partially containing metal elements such as titanium (Ti) and molybdenum (Mo)
- ta-C tetrahedral amorphous carbon which is amorphous and has a carbon-carbon bond form mainly composed of a diamond structure (SP 3 bond).
- the hard carbon layer is formed by a method such as a CVD method or a PVD method.
- a CVD method such as a thermal CVD method or a plasma CVD method
- the hard carbon layer contains hydrogen derived from a raw material organic compound (for example, hydrocarbon gas), and the hydrogen concentration of the hard carbon layer is typical. Is 15 to 40 atomic%.
- the PVD method such as ion plating method using carbon beam, arc type ion plating method, laser ablation method, sputtering method, magnetron sputtering method can be controlled to contain or not contain hydrogen. it can. As the hydrogen concentration of the hard carbon layer is smaller, the friction reduction effect is obtained.
- the hydrogen concentration of the hard carbon layer is preferably 40 atomic percent or less, more preferably 25 atomic percent or less, and more preferably 10 atomic percent or less. More preferably, it is more preferably 5 atomic% or less, more preferably 2 atomic% or less, more preferably 0.3 atomic% or less, and 0.1 atomic% or less. Is preferred.
- hydrogen amorphous carbon (aC: H) containing hydrogen that is 1 atomic% or less and over 0 atomic%, amorphous carbon not containing hydrogen (aC), tetrahedral carbon containing no hydrogen (ta-C) Is preferable.
- the hard carbon layer has a multilayer structure of two or more layers, and the outermost layer has hydrogen concentration of 1 atomic% or less and more than 0 atomic%. It is also possible to use hydrogen amorphous carbon (aC: H) containing hydrogen or amorphous carbon containing no hydrogen.
- aC hydrogen amorphous carbon
- the “outermost layer” refers to a range from the outermost surface to 5% based on the thickness of the hard carbon layer, and typically refers to a range from the outermost surface to a depth of 1.0 ⁇ m.
- the layer be the outermost layer.
- the surface roughness of the hard carbon layer is an arithmetic average roughness Ra, preferably 0.1 ⁇ m or less, more preferably 0.08 ⁇ m or less, still more preferably 0.05 ⁇ m or less, It is especially preferable that it is 0.03 micrometer or less.
- the surface roughness is an arithmetic average roughness Ra exceeding 0.1 ⁇ m, scuffing is locally formed, and the friction coefficient may be increased. Since the smoother surface is better, the lower limit of the roughness is not particularly defined. However, in actuality, the surface of the appropriate roughness as described above is finished in consideration of the cost of the manufacturing process. In addition, it is suitable from the surface of sliding stability that surface roughness is 0.08 micrometer or less by arithmetic mean roughness Ra.
- the lubricant applied to the sliding member of the present invention examples include oxygen-containing organic compounds.
- the oxygen-containing organic compound is not particularly limited as long as it is an organic compound containing oxygen in the molecule.
- it may be an oxygen-containing organic compound composed of carbon, hydrogen and oxygen, and contains other elements such as nitrogen, sulfur, halogen (fluorine, chlorine, etc.), phosphorus, boron, metal, etc. in the molecule.
- It may be an oxygen-containing organic compound.
- Oxygen organic compounds and their derivatives are preferred.
- an oxygen-containing organic compound having a low sulfur content or no sulfur is more preferable.
- “derivatives” typically include, for example, nitrogen-containing compounds, phosphorus-containing compounds, sulfur and sulfur-containing compounds, boron-containing compounds, halogens, and the like. Although it refers to a compound obtained by reacting an element, a halogen element-containing compound, a metal element, a metal-containing compound or the like (regardless of organic or inorganic), it is not particularly limited.
- the oxygen-containing organic compound examples include a compound having a hydroxyl group, a carboxyl group, a carbonyl group, etc., a compound having an ester bond, an ether bond, etc. (these have two or more groups or bonds). May be used). It is preferable to have one or more groups or bonds selected from hydroxyl group, carboxyl group, carbonyl group and ester bond, and one or more groups or bonds selected from hydroxyl group, carboxyl group and ester bond.
- the oxygen-containing organic compound is more preferably an oxygen-containing organic compound, more preferably an oxygen-containing organic compound having one or more groups selected from hydroxyl groups or carboxyl groups, and one or more hydroxyl groups. Particularly preferred are oxygen-containing organic compounds.
- Alcohols are represented by the following general formula (I)
- the alcohols (1) include the following. ⁇ Monohydric alcohols (1-1) ⁇ Divalent alcohols (1-2) ⁇ Trivalent or higher alcohols (1-3) ⁇ Alkylene oxide adducts of the above three alcohols (1-4) ⁇ One or a mixture of two or more selected from the above four alcohols (1-5)
- the monohydric alcohol (1-1) has one hydroxyl group in the molecule.
- Decanol (1-decanol, 2-decanol, 4-decanol, 3,7-dimethyl-1-octanol, 2,4,6-trimethylheptanol, etc.), undecanol, dodecanol, tridecanol, tetradecanol, pentadecane
- Monovalent alkyl alcohols having 1 to 40 carbon atoms (these alkyl groups, such as diol, hexadecanol, heptadecanol, octadecanol (stearyl alcohol, etc.), nonadecanol, eicosanol, heneicosanol, tricosanol, tetracosanol, etc.
- alkenyl alcohols having 2 to 40 carbon atoms such as ethenol, propenol, butenol, hexenol, octenol, decenol, dodecenol, octadecenol (eg oleyl alcohol)
- alkenyl groups may be linear or branched, and the position of the double bond is arbitrary
- Alkyl aryl alcohols (these alkyl groups may be linear or branched, and the substitution position of the alkyl group or hydroxyl group is arbitrary), etc .; 6- (4-oxy -3,5-di-tert-butyl-anilino) -2,4-bis- (n-octyl-thio) -1,3,5- Triazine or the like, and mixtures thereof.
- the friction of the sliding surface formed by the hard carbon layer of the sliding member and other sliding members made of any material type can be further reduced, and the volatility is low and the high temperature condition (
- straight chain or branched alkyl alcohols or alkenyl alcohols having 12 to 18 carbon atoms such as oleyl alcohol and stearyl alcohol are used because they can exhibit a friction reducing effect even under sliding conditions of internal combustion engines or the like. Is more preferable.
- the dihydric alcohol (1-2) specifically has two hydroxyl groups in the molecule.
- the substitution position of the hydroxyl group is also arbitrary); cyclohexanediol, methylcyclohexanediol, etc.
- Alkyl cycloalkanediols (the alkyl group may be linear or branched, and the substitution position of the alkyl group and hydroxyl group is arbitrary), benzenediol (catechol, etc.), methylbenzenediol, ethylbenzenediol, butyl Benzenediol (such as p-tert-butylcatechol), dibutylbenzenediol (such as 4,6-di-tert-butyl-resorcin), 4,4′-thiobis- (3-methyl-6-tert-butyl-phenol) 4,4′-butylidenebis- (3-methyl-6-tert-butyl-phenol), 2,2′-methylenebis- (4-methyl-6-tert-butyl-phenol), 2,2′-thiobis-
- the friction of the sliding surface formed by the hard carbon layer of the sliding member and other sliding members made of any material type can be further reduced, so that ethylene glycol, propylene glycol, neo Pentyl glycol, 1,4-butanediol, 1,5-pentanediol, neopentyl glycol, 1,6-hexanediol, 2-methyl-2,4-pentanediol, 2-ethyl-2-methyl-1,3 Use propanediol, 1,7-heptanediol, 1,8-octanediol, 1,9-nonanediol, 1,10-decanediol, 1,11-undecanediol, 1,12-dodecanediol, etc.
- hindered alcohols having a molecular weight of 300 or more, preferably 400 such as 2,6-di-tert-butyl-4- (3,5-di-tert-butyl-4-hydroxy-benzyl) phenyl alcohol Is preferable in that it does not volatilize even under high temperature conditions (for example, sliding conditions for internal combustion engines, etc.), is excellent in heat resistance, exhibits a friction reducing effect, and can impart excellent oxidation stability.
- the trihydric or higher alcohols (1-3) are specifically those having three or more hydroxyl groups, and usually 3 to 10 valent, preferably 3 to 6 valent polyhydric alcohols are used. It is done. Specific examples include trimethylol alkanes such as glycerin, trimethylolethane, trimethylolpropane, trimethylolbutane, erythritol, pentaerythritol, 1,2,4-butanetriol, 1,3,5-pentanetriol, 1,2 , 6-hexanetriol, 1,2,3,4-butanetetrol, sorbitol, adonitol, arabitol, xylitol, mannitol, etc., and polymers or condensates thereof (for example, diglycerin, triglycerin, tetraglycerin, etc.
- Condensation compounds such as glycerin di- and 8-mer, di-trimethylol propane and other tri-methylol propane di- to octamer, di-pentaerythritol di- and tetramer, etc., sorbitan, sorbitol glycerin condensate, etc. (Intramolecular contraction Compound, the intermolecular condensation compounds or self-condensation compound), and the like.
- saccharides such as xylose, arabitol, ribose, rhamnose, glucose, fructose, galactose, mannose, sorbose, cellobiose, mannose, isomaltose, trehalose, sucrose can be used.
- glycerin trimethylolalkane (eg, trimethylolethane, trimethylolpropane, trimethylolbutane), pentaerythritol, 1,2,4-butanetriol, 1,3,5-pentane Triol, 1,2,6-hexanetriol, 1,2,3,4-butanetetrol, sorbitol, sorbitan, sorbitol glycerin condensate, tri- to hexavalent polyhydric alcohols such as adonitol, arabitol, xylitol, mannitol and the like A mixture of these is more preferable, glycerin, trimethylolethane, trimethylolpropane, pentaerythritol, sorbitan and a mixture thereof are more preferable, and the oxygen content is 20% or more, preferably 30% or more, particularly preferably 4 It is particularly preferably a polyhydric alcohol%
- the alkylene oxide adduct (1-4) is an alkylene oxide adduct of the above alcohols (1-1 to 3).
- the alcohols have 2 to 6 carbon atoms, preferably carbon atoms. Examples include those obtained by adding an alkylene oxide of 2 to 4 or a polymer or copolymer thereof to hydrocarbyl etherification or hydrocarbyl esterification of a hydroxyl group of an alcohol.
- alkylene oxide having 2 to 6 carbon atoms examples include ethylene oxide, propylene oxide, 1,2-epoxybutane ( ⁇ -butylene oxide), 2,3-epoxybutane ( ⁇ -butylene oxide), and 1,2-epoxy-1 -Methylpropane, 1,2-epoxyheptane, 1,2-epoxyhexane and the like.
- ethylene oxide, propylene oxide, and butylene oxide are preferable, and ethylene oxide and propylene oxide are more preferable from the viewpoint of excellent low friction.
- the polymerization mode of the oxyalkylene group is not particularly limited, and may be random copolymerized or block copolymerized.
- alkylene oxide when added to a polyhydric alcohol having 2 to 6 hydroxyl groups, it may be added to all hydroxyl groups or only to some hydroxyl groups.
- Carboxylic acids (2) are represented by the following general formula (II)
- carboxylic acids (2) include the following. Aliphatic monocarboxylic acids (fatty acids) (2-1) . Aliphatic polycarboxylic acids (2-2) ⁇ Carbocyclic carboxylic acids (2-3) ⁇ Heterocyclic carboxylic acids (2-4) ⁇ A mixture of two or more selected from the above four carboxylic acids (2-5)
- the aliphatic monocarboxylic acids (fatty acids) (2-1) are specifically aliphatic monocarboxylic acids having one carboxyl group in the molecule, such as methanoic acid, ethanoic acid (acetic acid), propane. Acids (propionic acid), butanoic acid (butyric acid, isobutyric acid, etc.), pentanoic acid (valeric acid, isovaleric acid, pivalic acid, etc.), hexanoic acid (caproic acid, etc.), heptanoic acid, octanoic acid (caprylic acid, etc.), Nonanoic acid (such as pelargonic acid), decanoic acid, undecanoic acid, dodecanoic acid (such as lauric acid), tridecanoic acid, tetradecanoic acid (such as myristic acid), pentadecanoic acid, hexadecanoic acid (such as palmitic acid), heptadecano
- Examples of the aliphatic polycarboxylic acids (2-2) include ethanedioic acid (oxalic acid), propanedioic acid (malonic acid, etc.), butanedioic acid (succinic acid, methylmalonic acid, etc.), pentanedioic acid.
- carbocyclic carboxylic acids (2-3) are specifically carboxylic acids having one or more carboxyl groups in the molecule in the carbocycle, such as cyclohexane monocarboxylic acid, methylcyclohexane.
- heterocyclic carboxylic acids (2-4) are specifically heterocyclic carboxylic acids having one or more carboxyl groups in the molecule, such as furan carboxylic acid and thiophene. Examples thereof include heterocyclic carboxylic acids having 5 to 40 carbon atoms such as carboxylic acid and pyridinecarboxylic acid (nicotinic acid, isonicotinic acid and the like).
- Esters (3) are represented by the following general formula (III)
- esters (3) include the following. ⁇ Esters of fatty acid monocarboxylic acids (fatty acids) (3-1) . Esters of aliphatic polycarboxylic acids (3-2) ⁇ Esters of carbocyclic carboxylic acids (3-3) ⁇ Esters of heterocyclic carboxylic acids (3-4) ⁇ Alkylene oxide adducts of alcohols or esters (3-5) ⁇ Any mixture selected from the above five types of esters (3-6)
- the esters listed in 3-1 to 5 above may be complete esters in which all hydroxyl groups or carboxyl groups are esterified, or partial esters in which some hydroxyl groups or carboxyl groups remain.
- the fatty acid monocarboxylic acid (fatty acid) ester (3-1) is one or more selected from the above-mentioned fatty acid monocarboxylic acids (2-1), and the above monovalent, divalent, or trivalent ester.
- Specific examples of the aliphatic monocarboxylic acid include glycerol monooleate, glycerol diolate, sorbitan monooleate, and sorbitan diolate.
- a fatty acid ester having a linear or branched hydrocarbon group having 6 to 30 carbon atoms specifically, a fatty acid having the hydrocarbon group and an aliphatic monohydric alcohol or aliphatic polyhydric alcohol. Particularly preferred are esters. Details will be described later.
- esters (3-1) other than fatty acid ester-based ashless friction modifiers include fatty acid esters having a linear or branched hydrocarbon group having 1 to 5 carbon atoms or 31 to 40 carbon atoms. Examples thereof include esters composed of a fatty acid having such a hydrocarbon group and an aliphatic monohydric alcohol or aliphatic polyhydric alcohol.
- those having a kinematic viscosity at 100 ° C. of 1 to 100 mm 2 / s can be used as a lubricating base oil, and can usually be distinguished from the particularly preferred fatty acid ester-based ashless friction modifier.
- Examples of these include, for example, trimethylolpropane caprylate, trimethylolpropane pelargonate, pentaerythritol 2-ethylhexanoate, pentaerythritol pelargonate, etc., having 3 to 40 carbon atoms, preferably 4 to 18 carbon atoms, Particularly preferably, trivalent or higher polyols having 4 to 12 carbon atoms, particularly trivalent or higher polyols having a neopentyl structure, and monocarboxylic acids having 1 to 40 carbon atoms, preferably 4 to 18 carbon atoms, particularly preferably 6 to 12 carbon atoms.
- polyol esters such as single esters or complex esters with one or more selected from acids and mixtures thereof, or those further added with alkylene oxide. These may be complete esters in which all hydroxyl groups or carboxyl groups are esterified, and may be partial esters in which some hydroxyl groups or carboxyl groups remain, but are preferably complete esters, and the hydroxyl group value is usually 100 mgKOH / g or less, more preferably 50 mgKOH / g or less, particularly preferably 10 mgKOH / g or less.
- the kinematic viscosity of these lubricating base oils at 100 ° C. is preferably 2 to 60 mm 2 / s, particularly preferably 3 to 50 mm 2 / s.
- ester (3-2) of the aliphatic polyvalent carboxylic acid is one or more selected from the above-mentioned aliphatic polyvalent carboxylic acids (2-2), and the above-mentioned monovalent, divalent, or 3 And esters with one or more alcohols selected from alcohols (1-1 to 3) having higher valences.
- dibutyl maleate, ditridecyl glutarate, di-2-ethylhexyl adipate, diisodecyl adipate, ditridecyl adipate, di-2-ethylhexyl sebacate, etc. preferably 4 to 4 carbon atoms.
- 1 to 2 or more polycarboxylic acids selected from 1 to 18, particularly preferably 6 to 12 dicarboxylic acids, and 1 to 4 carbon atoms, preferably 4 to 18 carbon atoms, particularly preferably 6 to 14 carbon atoms.
- Diesters selected from monohydric alcohols or a mixture of two or more diesters, copolymers of these diesters (for example, dibutyl maleate) and polyalphaolefins having 4 to 16 carbon atoms, alpha olefins for acetic anhydride, etc. And an ester of a compound to which an alcohol is added and an alcohol having 1 to 40 carbon atoms.
- those having a kinematic viscosity at 100 ° C. of 1 to 100 mm 2 / s can be used as the lubricating base oil.
- ester (3-3) of the carbocyclic carboxylic acids one or more selected from the above-mentioned carbocyclic carboxylic acids (2-3) and the above-mentioned monovalent, divalent, or trivalent or higher
- esters with one or more selected from alcohols (1-1 to 3) Specific examples include aromatic carboxylic acid esters such as phthalic acid esters, trimellitic acid esters, pyromellitic acid esters, and salicylic acid esters. Of these, those having a kinematic viscosity at 100 ° C. of 1 to 100 mm 2 / s can be used as the lubricating base oil.
- ester (3-4) of the heterocyclic carboxylic acid may be one or more selected from the above-mentioned heterocyclic carboxylic acids (2-4), and the above-mentioned monovalent, divalent, or 3 Examples thereof include esters with one or more alcohols selected from monohydric or higher alcohols (1-1 to 3). Of these, those having a kinematic viscosity at 100 ° C. of 1 to 100 mm 2 / s can be used as the lubricating base oil.
- the alkylene oxide adduct (3-5) of alcohols or esters is one or more selected from the above-mentioned monovalent, divalent, or trivalent or higher alcohols (1-1 to 3). And those obtained by adding an alkylene oxide to the ester, and those obtained by adding an alkylene oxide to the above (3-1-4) ester. Of these, those having a kinematic viscosity at 100 ° C. of 1 to 100 mm 2 / s can be used as the lubricating base oil.
- Ethers (4) are represented by the following general formula (IV)
- ethers (4) include the following. .Saturated or unsaturated aliphatic ethers (4-1) ⁇ Aromatic ethers (4-2) ⁇ Cyclic ethers (4-3) ⁇ A mixture of two or more selected from the above three ethers (4-4)
- saturated or unsaturated aliphatic ethers include dimethyl ether, diethyl ether, di-n-propyl ether, diisopropyl ether, dibutyl ether, diisobutyl.
- aromatic ethers (4-2) include anisole, phenetole, phenyl ether, benzyl ether, phenylbenzyl ether, ⁇ -naphthyl ether, ⁇ -naphthyl ether, polyphenyl ether, perphenyl ether, and the like.
- Fluoro ethers and the like which may have a saturated or unsaturated aliphatic group (the saturated or unsaturated aliphatic group may be linear or branched, and the position of the unsaturated bond is arbitrary). And the substitution position and number are arbitrary). These are preferably in a liquid state under the use conditions, particularly at room temperature.
- cyclic ethers (4-3) include cyclic compounds having 2 to 40 carbon atoms such as ethylene oxide, propylene oxide, trimethylene oxide, tetrahydrofuran, tetrahydropyran, dioxane, glycidyl ethers and the like.
- Ethers which may have saturated or unsaturated aliphatic groups, carbocycles, carbocycles having saturated or unsaturated aliphatic groups (these saturated or unsaturated aliphatics may be linear It may be branched, the position of the unsaturated bond is arbitrary, and the substitution position and number are also arbitrary).
- Ketones (5) have the following general formula (V)
- ketones (5) include the following. .Saturated or unsaturated aliphatic ketones (5-1) ⁇ Carbocyclic ketones (5-2) ⁇ Heterocyclic ketones (5-3) ⁇ Ketone alcohols (5-4) Ketone acids (5-5) ⁇ A mixture of two or more selected from the above five types of ketones (5-6)
- saturated or unsaturated aliphatic ketones (5-1) include acetone, methyl ethyl ketone, methyl propyl ketone, methyl isopropyl ketone, methyl butyl ketone, methyl isobutyl ketone, pinacolone, diethyl ketone, butyrone, and diisopropyl.
- Saturated or unsaturated aliphatic ketones having 1 to 40 carbon atoms such as ketone, methyl vinyl ketone, mesityl oxide, and methylfeptenone (the saturated or unsaturated aliphatic may be linear or branched, unsaturated The position of the saturated bond is arbitrary).
- carbocyclic ketones (5-2) include cyclobutanone, cyclopentanone, cyclohexanone, acetophenone, propiophenone, butyrophenone, valerophenone, benzophenone, dibenzyl ketone, 2-acetonaphthone and the like.
- carbocyclic ketones having 1 to 40 carbon atoms which may have a saturated or unsaturated aliphatic group (the saturated or unsaturated aliphatic group may be linear or branched, unsaturated The position of the saturated bond is arbitrary, and the substitution position and number are also arbitrary.)
- heterocyclic ketones (5-3) include carbocyclic ketones having 1 to 40 carbon atoms such as acetothienone and 2-acetofurone, which are saturated or unsaturated aliphatic. It may have a group (the saturated or unsaturated aliphatic group may be linear or branched, the position of the unsaturated bond is arbitrary, and the substitution position and number are also arbitrary).
- ketone alcohol (ketol) (5-4) specifically, for example, a ketone having 1 to 40 carbon atoms such as acetol, acetoin, acetoethyl alcohol, diacetone alcohol, phenacyl alcohol, benzoin, etc.
- examples thereof include alcohols, which may have a carbocyclic or heterocyclic ring, and may have a carbocyclic or heterocyclic ring having a saturated or unsaturated aliphatic group (these saturated or unsaturated).
- the aliphatic group may be linear or branched, the position of the unsaturated bond is arbitrary, and the substitution position and number are also arbitrary).
- ketone acids (5-5) include ⁇ -ketone acids such as pyruvic acid, benzoylformic acid and phenylpyruvic acid, ⁇ -ketone acids such as acetoacetic acid, propionylacetic acid and benzoylacetic acid, Examples thereof include ketonic acids having 1 to 40 carbon atoms such as levulinic acid and ⁇ -ketonic acids such as ⁇ -benzoylpropionic acid.
- Aldehydes (6) have the following general formula (VI)
- aldehydes (6) include the following. ⁇ Saturated or unsaturated aliphatic aldehydes (6-1) ⁇ Carbocyclic aldehydes (6-2) ⁇ Heterocyclic aldehydes (6-3) ⁇ A mixture of two or more selected from the above three aldehydes (6-4)
- saturated or unsaturated aliphatic aldehydes include formaldehyde, acetaldehyde, propionaldehyde, butyraldehyde, isobutyraldehyde, valeraldehyde, isovaleraldehyde, pivalin aldehyde, capronaldehyde, Ptoaldehyde, caprylaldehyde, pelargonaldehyde, chemical purine aldehyde, undecyl aldehyde, laurin aldehyde, tridecyl aldehyde, myristic aldehyde, pentadecyl aldehyde, palmitic aldehyde, margarine aldehyde, stearaldehyde, acrolein, crotonaldehyde, propiol aldehyde, C1-C40 saturated or unsaturated aliphatic aldehydes such as
- carbocyclic aldehydes (6-2) include benzaldehyde, o-tolualdehyde, m-tolualdehyde, p-tolualdehyde, salicylaldehyde, cinnamaldehyde, ⁇ -naphthaldehyde, ⁇ -C1-C40 carbocyclic aldehydes such as naphthaldehyde, etc., which may have a saturated or unsaturated aliphatic group (these may be saturated or unsaturated aliphatic linear It may be branched, the position of the unsaturated bond is arbitrary, and the position and number of substitution are arbitrary).
- heterocyclic aldehydes (6-3) include, for example, heterocyclic aldehydes having 1 to 40 carbon atoms such as furfural, which have a saturated or unsaturated aliphatic group.
- saturated or unsaturated aliphatic groups may be linear or branched, the position of the unsaturated bond is arbitrary, and the position and number of substitution are also arbitrary).
- carbonates (7) include dimethyl carbonate, diethyl carbonate, di-n-propyl carbonate, diisopropyl carbonate, di-n-butyl carbonate, diisobutyl carbonate, ditertbutyl carbonate, dipentyl carbonate, and dihexyl carbonate.
- Examples of the derivatives of the oxygen-containing organic compounds (1) to (7) include, for example, nitrogen-containing compounds, phosphorus-containing compounds, sulfur and sulfur-containing compounds, boron-containing compounds, halogen elements and halogens.
- nitrogen-containing compounds, phosphorus-containing compounds, sulfur and sulfur-containing compounds, boron-containing compounds, halogen elements and halogens Although the compound etc. which are obtained by making an element containing compound, a metal element, a metal containing compound, etc. (regardless of organic and inorganic) react are mentioned, it does not restrict
- guide_body is normally used as an additive, even when used as a base oil, the effect is not specifically limited.
- R and R ′ in the general formulas (I) to (VII) are each independently an alkyl group, alkenyl group, alkylene group, cycloalkyl group, alkylcycloalkyl group, aryl group, alkylaryl group, arylalkyl group.
- These hydrocarbon groups may further have one or more groups or bonds selected from a hydroxyl group, a carboxyl group, a carbonyl group, an ester bond, an ether bond, carbon,
- An element other than hydrogen and oxygen for example, nitrogen or sulfur (for example, a heterocyclic compound), halogen (fluorine, chlorine, etc.), phosphorus, boron, metal, etc. may be contained.
- the hydrocarbon group has no limitation on the number of carbon atoms, but preferably has 1 to 40 carbon atoms, more preferably 2 to 30 carbon atoms, and particularly preferably 3 to 20 carbon atoms.
- alkyl group examples include methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, isobutyl group, sec-butyl group, tert-butyl group, straight chain or branched pentyl group, straight chain or Branched hexyl group, linear or branched heptyl group, linear or branched octyl group, linear or branched nonyl group, linear or branched decyl group, linear or branched undecyl group , Linear or branched dodecyl group, linear or branched tridecyl group, linear or branched tetradecyl group, linear or branched pentadecyl group, linear or branched hexadecyl group, linear or branched A branched heptadecyl group, a linear or branched octadecyl group, a
- alkenyl group examples include a vinyl group, a linear or branched propenyl group, a linear or branched butenyl group, a linear or branched pentenyl group, a linear or branched hexenyl group, and a linear chain.
- branched heptenyl group a linear or branched octenyl group, a linear or branched nonenyl group, a linear or branched decenyl group, a linear or branched undecenyl group, a linear or branched dodecenyl group Group, linear or branched tridecenyl group, linear or branched tetradecenyl group, linear or branched pentadecenyl group, linear or branched hexadecenyl group, linear or branched heptadecenyl group, linear or Branched octadecenyl group, linear or branched nonadecenyl group, linear or branched icocenyl group, linear or branched henicosenyl group, linear or branched dococenyl group, linear or branched tricosenyl group , Linear or Such alkenyl
- examples of the cycloalkyl group include cycloalkyl groups having 3 to 40 carbon atoms such as cyclopentyl group, cyclohexyl group, cycloheptyl group and cyclooctyl group, preferably cycloalkyl groups having 3 to 20 carbon atoms, particularly A cycloalkyl group having 5 to 8 carbon atoms is preferred.
- examples of the alkylcycloalkyl group include a methylcyclopentyl group, a dimethylcyclopentyl group (including all structural isomers), a methylethylcyclopentyl group (including all structural isomers), a diethylcyclopentyl group (all Including structural isomers), methylcyclohexyl group, dimethylcyclohexyl group (including all structural isomers), methylethylcyclohexyl group (including all structural isomers), diethylcyclohexyl group (all structural isomers) ), Methylcycloheptyl group, dimethylcycloheptyl group (including all structural isomers), methylethylcycloheptyl group (including all structural isomers), diethylcycloheptyl group (all structural isomers) Alkyl cycloalkyl groups having 4 to 40 carbon atoms such as Gerare, preferably an alkyl cycloal
- the aryl group is an aryl group having 6 to 20 carbon atoms such as a phenyl group or a naphthyl group, preferably an aryl group having 6 to 10 carbon atoms.
- the alkylaryl group includes a tolyl group (including all structural isomers), an ethylphenyl group (including all structural isomers), a linear or branched propylphenyl group (all structural isomers). ), Linear or branched butylphenyl group (including all structural isomers), linear or branched pentylphenyl group (including all structural isomers), linear or branched Branched hexylphenyl group (including all structural isomers), linear or branched heptylphenyl group (including all structural isomers), straight chain or branched octylphenyl group (all structural isomers) ), Linear or branched nonylphenyl group (including all structural isomers), linear or branched decylphenyl group (including all structural isomers), linear or branched Branched undecylphenyl group (all structural isomers ), Mono-substituted phenyl group
- the alkyl group further includes an aryl group, an alkylaryl group, an arylalkyl group;
- An alkylaryl group having 7 to 40 carbon atoms preferably an alkylary group having 7 to 20 carbon atoms, and the like.
- arylalkyl groups include benzyl, phenylethyl, phenylpropyl (including propyl isomers), phenylbutyl (including butyl isomers), and phenylpentyl (pentyl isomers).
- arylalkyl groups having 7 to 40 carbon atoms such as phenylhexyl groups (including isomers of hexyl groups), preferably arylalkyl groups having 7 to 20 carbon atoms, particularly preferably 7 to 7 carbon atoms. 12 arylalkyl groups.
- the oxygen-containing organic compounds described above can be used in the same manner even if they are derivatives thereof.
- a compound obtained by sulfurizing one kind selected from the above alcohols, carboxylic acids, esters, ethers, ketones, aldehydes, and carbonates, and a halogenated (fluorinated, chlorinated, etc.) compound a compound obtained by sulfurizing one kind selected from the above alcohols, carboxylic acids, esters, ethers, ketones, aldehydes, and carbonates, and a halogenated (fluorinated, chlorinated, etc.) compound.
- reaction products with sulfuric acid, nitric acid, boric acid, phosphoric acid and esters or metal salts of these acids reaction products with metals, metal-containing compounds, or amine compounds.
- reaction products for example, Mannich reaction products, acylation reaction products, amides, etc.
- reaction products for example, Mannich reaction products, acylation reaction products, amides, etc.
- alcohols for example, benzyl alcohols, benzyl alcohols, benzyl alcohols, benzyl alcohols, benzyl alcohols, benzyl alcohols, benzyl alcohols, benzyl alcohols, benzyl alcoholsulfate, amides, etc.
- Examples of the amine compound include ammonia, monoamine, diamine, and polyamine. More specifically, ammonia; methylamine, ethylamine, propylamine, butylamine, pentylamine, hexylamine, heptylamine, octylamine, nonylamine, decylamine, undecylamine, dodecylamine, tridecylamine, tetradecylamine, penta Decylamine, hexadecylamine, heptadecylamine, octadecylamine, stearylamine, dimethylamine, diethylamine, dipropylamine, dibutylamine, dipentylamine, dihexylamine, diheptylamine, dioctylamine, dinonylamine, didecylamine, diundecylamine, Didodecylamine, ditridecylamine, ditetradecy
- Examples include compounds having an alkyl group or alkenyl group having 8 to 20 carbon atoms in the above monoamine, diamine, or polyamine, or heterocyclic compounds such as N-hydroxyethyloleylimidazoline; alkylene oxide adducts of these compounds; and mixtures thereof it can.
- aliphatic amines having an alkyl group or alkenyl group having 10 to 20 carbon atoms such as decylamine, dodecylamine, tridecylamine, heptadecylamine, octadecylamine, oleylamine and stearylamine (these may be linear A branched example) may be mentioned as a preferred example.
- oxygen-containing organic compound derivatives preferred examples include carboxylic acid amides having 8 to 20 carbon atoms such as oleic acid amide.
- oxygen-containing organic compound is used alone (that is, 100% by mass) on the sliding surface formed by the hard carbon layer of the sliding member and another sliding member made of an arbitrary material type, Exhibits excellent low-friction characteristics, but added oxygen-containing organic compounds to lubricant base materials (for example, base oils) and additives, or various media (only lubricant base materials and additives)
- lubricant base materials for example, base oils
- additives for example, base oils
- various media only lubricant base materials and additives
- such lubricants include, for example, mineral oil, synthetic oil, natural fats and oils, diluent oil, grease, wax, hydrocarbons having 3 to 40 carbon atoms, hydrocarbon solvents, organic solvents other than hydrocarbons, water, etc.
- an oxygen-containing organic compound in an arbitrary ratio to various media such as a mixture thereof, in particular, a medium that is liquid, grease-like or wax-like at sliding conditions or at room temperature.
- the content of the oxygen-containing organic compound to be contained in these media is not particularly limited, but usually the lower limit is 0.001% by mass, preferably 0.05% by mass, more preferably 0.1% by mass. % And may be contained in excess of 3.0% by mass.
- the upper limit is 100% by mass as described above, preferably 50% by mass, more preferably 20% by mass, still more preferably 10% by mass, particularly preferably 5% by mass, and 0.1 to Even with the addition of a small amount of about 2% by mass, excellent low friction characteristics can be exhibited.
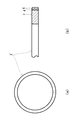
- FIG. 3 is a plan view of a piston ring 1 ′ which is an example of a sliding member 1 in which a predetermined hard chromium plating layer 4 and a predetermined hard carbon layer 6 are formed on the outer peripheral surface (side surface) of the substrate 2. It is a partial sectional view (b) of a) and a circumferential direction.
- the hard chrome plating layer 4 and the hard carbon layer 6 are laminated on the sliding surface of the substrate 2 in this order. Since it has such a structure, even if the outer peripheral surface (side surface) of the piston ring slides on a cylinder bore, which is an unillustrated counterpart material, and friction occurs, it is possible to exhibit peeling resistance and low friction characteristics.
- FIG. 4 shows a piston ring 1 as an example of a sliding member 1 in which a predetermined hard chromium plating layer 4 and a predetermined hard carbon layer 6 are formed on the outer peripheral surface (side surface) and upper and lower surfaces of the substrate 2.
- a predetermined hard chromium plating layer 4 and a predetermined hard carbon layer 6 are formed on the outer peripheral surface (side surface) and upper and lower surfaces of the substrate 2.
- the hard chromium plating layer 4 and the hard carbon layer 6 are arranged in this order on the sliding surface and the contact surface of the substrate 2.
- the lubricant is a lubricating oil composition used in combination with a piston ring for an automobile engine.
- the lubricant base oil contains a fatty acid ester-based ashless friction modifier, an aliphatic amine-based ashless friction modifier, polybutenyl. Succinimide, polybutenyl succinimide derivative or zinc dithiophosphate and any combination thereof are included.
- the lubricating base oil is not particularly limited and may be any kind as long as it is normally used as a base oil of a lubricating oil composition, such as mineral oil, synthetic oil, fats and mixtures thereof. Can be used. Also, it may be a mixture of two or more mineral base oils or two or more synthetic base oils. Furthermore, the mixing ratio of two or more kinds of base oils in the above mixture is not particularly limited and can be arbitrarily selected.
- a lubricating oil fraction obtained by subjecting crude oil to atmospheric distillation and vacuum distillation can be desolvated, solvent extraction, hydrocracking, solvent dewaxing, hydrorefining, sulfuric acid washing, clay Paraffinic or naphthenic oils, normal paraffins, etc., which are purified by appropriately combining purification treatments such as treatment, can be used, and those obtained by solvent purification and hydrorefining treatment are generally used.
- synthetic oils include poly- ⁇ -olefins (eg, 1-octene oligomers, 1-decene oligomers, ethylene-propylene oligomers), poly- ⁇ -olefin hydrides, isobutene oligomers, isobutene oligomers.
- isoparaffin alkylbenzene, alkylnaphthalene, diester (eg, ditridecyl glutarate, dioctyl adipate, diisodecyl adipate, ditridecyl adipate, dioctyl sebacate, etc.), polyol ester (eg, trimethylolpropane caprylate, trimethylolpropane pelargol , Trimethylolpropane esters such as trimethylolpropane isostearinate; pentaerythritol 2-ethylhexanoate, pentaerythritol Pentaerythritol esters such as pelargonate), polyoxyalkylene glycols, dialkyldiphenyl ethers and polyphenyl ethers.
- diester eg, ditridecyl glutarate, dioctyl adipate, diisodecyl adip
- preferred examples include poly- ⁇ -olefins such as 1-octene oligomers and 1-decene oligomers or hydrides thereof.
- esters polyol esters are particularly preferable. Typical examples include gear oil, automotive engine oil, transmission oil, turbine oil, spindle oil, and the like.
- the sulfur content in the lubricating base oil is not particularly limited, but is preferably 0.2% by mass or less, more preferably 0.1% by mass or less, and even more preferably 0.05% by mass based on the total amount of the base oil. % Or less is preferable.
- the sulfur content of hydrorefined mineral oil or synthetic base oil is 0.005 mass% or less, or contains substantially no sulfur content (5 massppm or less), so these should be used as the base oil. Is preferred.
- the aromatic content in the lubricating base oil is not particularly limited, but the total aromatic content is 15% by mass or less in order to maintain a low friction characteristic for a long period of time as a lubricating oil composition. It is preferably 10% by mass or less, more preferably 5% by mass or less. That is, when the total aromatic content of the lubricating base oil exceeds 15% by mass, oxidation stability is inferior, which is not preferable.
- the “total aromatic content” means an aromatic fraction content measured in accordance with ASTM D2549.
- this aromatic fraction contains alkylbenzene, alkylnaphthalene, anthracene, phenanthrene, and alkylated products thereof, compounds in which four or more benzene rings are condensed, or heterogeneous compounds such as pyridines, quinolines, phenols, naphthols, etc. Compounds having aromatics are included.
- the kinematic viscosity of the lubricating base oil is not particularly limited, but when used as a lubricating oil composition, the kinematic viscosity at 100 ° C. is preferably 2 mm 2 / s or more, more preferably 3 mm 2 / s. s or more. On the other hand, the kinematic viscosity at 100 ° C. is preferably 20 mm 2 / s or less, more preferably 10 mm 2 / s or less, and particularly preferably 8 mm 2 / s or less. When the kinematic viscosity at 100 ° C.
- the lubricating base oil is less than 2 mm 2 / s, it is not preferable because sufficient abrasion resistance cannot be obtained and evaporation characteristics may be deteriorated.
- the kinematic viscosity exceeds 20 mm 2 / s, it is difficult to exhibit low friction performance, and low temperature performance may be deteriorated.
- the mixture etc. which arbitrarily mixed 2 or more types of base oils chosen from the said base oil can be used and the kinematic viscosity in 100 degreeC is in said preferable range, kinematic viscosity of base oil alone Even if is other than the above, it can be used.
- the lubricating base oil is 2 mm 2 / s or more is sufficient to form an oil film, has excellent lubricity, and has a smaller base oil evaporation loss under high conditions. Obtainable.
- the kinematic viscosity at 100 ° C. to 20 mm 2 / s or less, the fluid resistance becomes small, so that a composition having a smaller frictional resistance at the lubrication point can be obtained.
- the viscosity index of the lubricating base oil is not particularly limited, but is preferably 80 or more. When used as a lubricating oil composition, it is preferably 100 or more, and 120 or more. Is more preferable, and may be 140 or more and 250 or less. By selecting a lubricating base oil having a high viscosity index, it is possible to obtain a composition that not only consumes less oil and has excellent low-temperature viscosity characteristics, but also has an excellent friction reducing effect.
- One or both of the fatty acid ester-based ashless friction modifier and the aliphatic amine-based ashless friction modifier to be used has 6 to 30 carbon atoms, preferably 8 to 24 carbon atoms, and particularly preferably 10 to 20 carbon atoms. Mention may be made of fatty acid esters having a linear or branched hydrocarbon group, fatty acid amine compounds, and any mixtures thereof. When the carbon number is outside the range of 6 to 30, the friction reducing effect may not be sufficiently obtained.
- straight chain or branched hydrocarbon group having 6 to 30 carbon atoms include hexyl group, heptyl group, octyl group, nonyl group, decyl group, undecyl group, dodecyl group, tridecyl group, tetradecyl group.
- the alkyl group and alkenyl group include all possible linear structures and branched structures, and the position of the double bond in
- fatty acid ester examples include esters of a fatty acid having 6 to 30 carbon atoms and an aliphatic monohydric alcohol or aliphatic polyhydric alcohol.
- glycerol monooleate Particularly preferred examples include glycerine diolate, sorbitan monooleate, and sorbitan diolate.
- aliphatic amine compound examples include aliphatic monoamines or alkylene oxide adducts thereof, aliphatic polyamines, imidazoline compounds, and derivatives thereof.
- fats such as laurylamine, lauryldiethylamine, lauryldiethanolamine, dodecyldipropanolamine, palmitylamine, stearylamine, stearyltetraethylenepentamine, oleylamine, oleylpropylenediamine, oleyldiethanolamine and N-hydroxyethyloleylimidazoline
- Amine amine compounds, amine alkylene oxide adducts such as N, N-dipolyoxyalkylene-N-alkyl (or alkenyl) (carbon number 6 to 28) of these aliphatic amine compounds, carbon numbers in these aliphatic amine compounds 2 to 30 monocarboxylic acids (fatty acids, etc.) and polycarboxylic acids having 2 to 30 carbon atom
- the content of one or both of the fatty acid ester-based ashless friction modifier and the aliphatic amine-based ashless friction modifier contained in the lubricating oil composition is not particularly limited, but is 0.
- the content is preferably from 05 to 3.0% by mass, more preferably from 0.1 to 2.0% by mass, particularly preferably from 0.5 to 1.4% by mass.
- the content is less than 0.05% by mass, the friction reducing effect tends to be small, and when it exceeds 3.0% by mass, the solubility in the lubricating oil and the storage stability are remarkably deteriorated, and precipitates are easily generated. Therefore, it is not preferable.
- PIB in these general formulas represents a polybutenyl group
- the number average molecular weight obtained by polymerizing a high purity isobutene or a mixture of 1-butene and isobutene with a boron fluoride catalyst or an aluminum chloride catalyst is 900 to 3500, preferably Obtained from 1000-2000 polybutene.
- n in the above general formula is preferably an integer of 1 to 5, more preferably an integer of 2 to 4, from the viewpoint of excellent cleanability.
- the polybutene is used to remove a trace amount of fluorine and chlorine remaining due to a catalyst in the production process by an appropriate method such as an adsorption method or sufficient water washing, and more desirably 10 ppm by mass or less. Particularly preferably, it may be used after removing to 1 ppm by mass or less.
- a method for producing such polybutenyl succinimide is not particularly limited.
- the polybutene chlorinated product or polybutene from which chlorine or fluorine has been sufficiently removed and maleic anhydride are reacted at 100 to 200 ° C.
- the polybutenyl succinic acid obtained can be obtained by reacting with polyamines such as diethylenetriamine, triethylenetetramine, tetraethylenepentamine and pentaethylenehexamine.
- a boron compound or an oxygen-containing organic compound is allowed to act on the compound represented by the above general formula (VIII) or (IX), thereby remaining amino groups and imino.
- examples thereof include so-called boron-modified or acid-modified compounds obtained by neutralizing or amidating part or all of one or both of the groups.
- boron-containing polybutenyl succinimide particularly boron-containing bispolybutenyl succinimide, is most preferred at the present time.
- Examples of the boron compound include boric acid, borates, and borate esters.
- examples of the boric acid include orthoboric acid, metaboric acid, and tetraboric acid.
- ammonium borates such as ammonium salt etc., specifically, ammonium metaborate, ammonium tetraborate, ammonium pentaborate, and ammonium octaborate, are mentioned as a suitable example.
- an ester of boric acid and preferably an alkyl alcohol having 1 to 6 carbon atoms more specifically, monomethyl borate, dimethyl borate, trimethyl borate, monoethyl borate, diethyl borate
- Preferable examples include triethyl borate, monopropyl borate, dipropyl borate, triplypropyl borate, monobutyl borate, dibutyl borate, and tributyl borate.
- the mass ratio “B / N” between the boron content B and the nitrogen content N is usually 0.1 to 3, preferably 0.2 to 1. .
- oxygen-containing organic compound examples include formic acid, acetic acid, glycolic acid, propionic acid, lactic acid, butyric acid, valeric acid, caproic acid, enanthic acid, caprylic acid, pelargonic acid, capric acid, and undecyl acid.
- Lauric acid tridecanoic acid, myristic acid, pentadecanoic acid, palmitic acid, margaric acid, stearic acid, oleic acid, nonadecanoic acid and eicosanoic acid, etc., monocarboxylic acids having 1 to 30 carbon atoms, oxalic acid, phthalic acid, trimellit Examples thereof include polycarboxylic acids having 2 to 30 carbon atoms such as acid and pyromellitic acid, anhydrides or ester compounds thereof, alkylene oxides having 2 to 6 carbon atoms, and hydroxy (poly) oxyalkylene carbonate.
- the content of one or both of polybutenyl succinimide and a derivative thereof is not particularly limited, but is preferably 0.1 to 15% by mass, and 1.0 to 12% by mass. Is more preferable. If it is less than 0.1% by mass, the cleaning effect may be poor, and if it exceeds 15% by mass, it is difficult to obtain a cleaning effect corresponding to the content, and the demulsibility tends to deteriorate.
- the lubricating oil composition has the following general formula (X)
- R 4 , R 5 , R 6 and R 7 in the above formula (X) each independently represent a hydrocarbon group having 1 to 24 carbon atoms.
- these hydrocarbon groups include linear or branched alkyl groups having 1 to 24 carbon atoms, linear or branched alkenyl groups having 3 to 24 carbon atoms, and cycloalkyl groups having 5 to 13 carbon atoms.
- the alkyl group or alkenyl group may be any of primary, secondary, and tertiary.
- R 4 , R 5 , R 6 and R 7 include a methyl group, an ethyl group, a propyl group, a butyl group, a pentyl group, a hexyl group, a heptyl group, an octyl group, a nonyl group, and a decyl group.
- Alkyl groups such as undecyl group, dodecyl group, tridecyl group, tetradecyl group, pentadecyl group, hexadecyl group, heptadecyl group, octadecyl group, nonadecyl group, icosyl group, heicosyl group, docosyl group, tricosyl group and tetracosyl group, and propenyl group , Isopropenyl group, butenyl group, butadienyl group, pentenyl group, hexenyl group, heptenyl group, octenyl group, nonenyl group, decenyl group, undecenyl group, dodecenyl group, tridecenyl group, tetradecenyl group, pentadecenyl group, hexadecenyl group, and heptadecenyl group Oct
- the hydrocarbon group which R 4 , R 5 , R 6 and R 7 can take include all possible linear structures and branched structures, and the position of the double bond of the alkenyl group.
- the bonding position of the alkyl group to the cycloalkyl group, the bonding position of the alkyl group to the aryl group, and the bonding position of the aryl group to the alkyl group are arbitrary.
- the hydrocarbon group is a linear or branched alkyl group having 1 to 18 carbon atoms, an aryl group having 6 to 18 carbon atoms, or a linear or branched group.
- Particularly preferred is a linear alkylaryl group.
- zinc dithiophosphate examples include zinc diisopropyldithiophosphate, zinc diisobutyldithiophosphate, zinc di-sec-butyldithiophosphate, zinc di-sec-pentyldithiophosphate, zinc di-n-hexyldithiophosphate, di- -Sec-hexyl dithiophosphate zinc, di-octyl dithiophosphate zinc, di-2-ethylhexyl dithiophosphate zinc, di-n-decyl dithiophosphate zinc, di-n-dodecyl dithiophosphate zinc, diisotridecyl dithiophosphate zinc and these The mixture etc. which concern on arbitrary combinations of these are mentioned.
- the content of the zinc dithiophosphate is not particularly limited, but from the viewpoint of exerting a higher friction reduction effect, it is preferably 0.1% by mass or less based on the total amount of the composition and in terms of phosphorus element, Moreover, it is still more preferable that it is 0.06 mass% or less, and it is especially preferable that zinc dithiophosphate does not contain.
- a conventional method can be arbitrarily adopted, and is not particularly limited. Specifically, hydrocarbon groups corresponding to the above R 4 , R 5 , R 6 and R 7 are used. It can be synthesized by reacting the alcohol or phenol possessed with diphosphorus pentoxide (P 2 O 5 ) to give dithiophosphoric acid and neutralizing it with zinc oxide. In addition, it cannot be overemphasized that the structure of the said zinc dithiophosphate changes with raw material alcohol to be used. In addition, two or more kinds of zinc dithiophosphates included in the general formula (X) can be mixed and used at an arbitrary ratio.
- the lubricating oil composition is extremely excellent when used on a sliding surface formed by a sliding member coated with a hard carbon layer and another sliding member made of any material type. Although it exhibits low friction characteristics, metal detergents, antioxidants, viscosity index improvers, other ashless friction modifiers, etc., especially for the purpose of enhancing the performance required as lubricating oil compositions for internal combustion engines Ashless dispersant, antiwear agent or extreme pressure agent, antirust agent, nonionic surfactant, antiemulsifier, metal deactivator, antifoaming agent, etc., alone or in combination Performance can be improved.
- any compound usually used as a metal detergent for lubricating oil can be used.
- alkali metal or alkaline earth metal sulfonates, phenates, salicylates, naphthenates, and the like can be used alone or in combination.
- examples of the alkali metal include sodium (Na) and potassium (K)
- examples of the alkaline earth metal include calcium (Ca) and magnesium (Mg).
- Specific preferred examples include Ca or Mg sulfonates, phenates and salicylates.
- the total base number and addition amount of these metal detergents can be arbitrarily selected according to the required performance of the lubricating oil.
- the total base number is 0 to 500 mgKOH / g, preferably 150 to 400 mgKOH / g by the perchloric acid method, and the amount added is usually 0.1 to 10% by mass based on the total amount of the composition.
- any compound usually used as an antioxidant for lubricating oil can be used.
- the amount of the antioxidant added is usually 0.01 to 5% by mass based on the total amount of the composition.
- a so-called non-dispersion type viscosity index improver such as a copolymer of one or more monomers selected from various methacrylic acid esters and hydrogenated products thereof
- a so-called dispersion type viscosity index improver obtained by copolymerizing various methacrylic acid esters containing a nitrogen compound can be exemplified.
- viscosity index improvers include non-dispersed or dispersed ethylene- ⁇ -olefin copolymers (for example, propylene, 1-butene, 1-pentene, etc.) and hydrogen thereof
- examples thereof include a compound, polyisobutylene and a hydrogenated product thereof, a styrene-diene hydrogenated copolymer, a styrene-maleic anhydride ester copolymer, and a polyalkylstyrene.
- the molecular weight of these viscosity index improvers needs to be selected in consideration of shear stability.
- the number average molecular weight of the viscosity index improver is, for example, 5000 to 1000000, preferably 100000 to 800000 for dispersed and non-dispersed polymethacrylates, 800 to 5000 for polyisobutylene or a hydride thereof, ethylene- In the case of an ⁇ -olefin copolymer or a hydride thereof, 800 to 300,000, preferably 10,000 to 200,000 is preferable.
- Such viscosity index improvers may be contained singly or in any combination of two or more, but the content is usually 0.1 to 40.0% by mass based on the lubricating oil composition. Is desirable.
- ashless friction modifiers include ashless friction modifiers such as boric acid esters, higher alcohols, aliphatic ethers, metal friction modifiers such as molybdenum dithiophosphate, molybdenum dithiocarbamate, and molybdenum disulfide. Is mentioned.
- ashless dispersants include polybutenylbenzylamine and polybutenylamine having a polybutenyl group having a number average molecular weight of 900 to 3500, and polybutenyl succinimide having a polybutenyl group having a number average molecular weight of less than 900 And derivatives thereof.
- the antiwear agent or extreme pressure agent includes disulfide, sulfurized fat and oil, sulfurized olefin, phosphate ester containing 1 to 3 hydrocarbon groups having 2 to 20 carbon atoms, thiophosphate ester, phosphite ester, Examples thereof include thiophosphite esters and amine salts thereof.
- examples of the rust preventive include alkyl benzene sulfonate, dinonyl naphthalene sulfonate, alkenyl succinate and polyhydric alcohol ester.
- nonionic surfactant and the demulsifier examples include polyalkylene glycol nonionic surfactants such as polyoxyethylene alkyl ether, polyoxyethylene alkylphenyl ether, and polyoxyethylene alkyl naphthyl ether.
- metal deactivator examples include imidazoline, pyrimidine derivatives, thiadiazole, benzotriazole, and thiadiazole.
- examples of the antifoaming agent include silicone, fluorosilicone, and fluoroalkyl ether.
- the content is based on the total amount of the composition, other friction modifiers, other ashless dispersants, antiwear agents or extreme pressure agents, rust prevention
- the agent and demulsifier can be appropriately selected from the range of 0.01 to 5% by mass, the metal deactivator 0.005 to 1% by mass, and the antifoaming agent 0.0005 to 1% by mass.
- Example 1 A hard chromium plating layer having a thickness of about 100 ⁇ m was formed by electrolytic plating on the surface of a piston ring base material of an automobile engine. Next, the piston ring base material on which the hard chromium plating layer was formed was heat-treated at 250 ° C. in the atmosphere. Thereafter, a hard carbon layer not containing hydrogen was formed on the hard chrome plating layer of the piston ring base material by the PVD method to obtain the piston ring of this example.
- the hard chrome plating layer which is an intermediate process, using another piston ring in which the hard chrome plating layer is formed in the same batch, heat treatment is performed under the same conditions as the piston ring manufacturing process, and the hard chrome plating layer
- the hydrogen concentration of was investigated.
- a sample cut into an appropriate size was placed in a crucible, heated to about 1000 ° C., and the generated gas was analyzed.
- the carrier gas was argon (Ar), the hydrogen concentration in the discharged gas was measured, and the amount of hydrogen was calculated from the flow rate of the carrier gas.
- the same measurement was performed on the sample on which the hard chrome plating layer was not formed, and only the amount of hydrogen contained in the hard chrome plating layer was calculated by subtracting the background value.
- the hydrogen concentration of the hard chrome plating layer was 148 ppm.
- Example 2 Except that the temperature of the heat treatment was 280 ° C., the same operation as in Example 1 was repeated to obtain the piston ring of this example. At that time, the same operation was repeated to examine the hydrogen concentration of the hard chromium plating layer.
- Example 3 Except that the temperature of the heat treatment was 300 ° C., the same operation as in Example 1 was repeated to obtain the piston ring of this example. At that time, the same operation was repeated to examine the hydrogen concentration of the hard chromium plating layer.
- Example 4 Except that the temperature of the heat treatment was 350 ° C., the same operation as in Example 1 was repeated to obtain the piston ring of this example. At that time, the same operation was repeated to examine the hydrogen concentration of the hard chromium plating layer.
- Example 5 Except that the temperature of the heat treatment was 400 ° C., the same operation as in Example 1 was repeated to obtain the piston ring of this example. At that time, the same operation was repeated to examine the hydrogen concentration of the hard chromium plating layer.
- Example 1 (Comparative Example 1) Except that the heat treatment was not performed, the same operation as in Example 1 was repeated to obtain the piston ring of this example. At that time, the same operation was repeated to examine the hydrogen concentration of the hard chromium plating layer.
- Example 2 (Comparative Example 2) Except that the temperature of the heat treatment was 100 ° C., the same operation as in Example 1 was repeated to obtain the piston ring of this example. At that time, the same operation was repeated to examine the hydrogen concentration of the hard chromium plating layer.
- Example 3 (Comparative Example 3) Except that the temperature of the heat treatment was 200 ° C., the same operation as in Example 1 was repeated to obtain the piston ring of this example. At that time, the same operation was repeated to examine the hydrogen concentration of the hard chromium plating layer. Table 1 shows a part of the specifications of the above examples.
- the piston ring is exemplified as the sliding member, but the present invention is not limited to this.
- a moving member can be mentioned.
- Specific examples of the sliding members of the valve train and intake / exhaust systems include piston pins, pistons (or piston skirts (piston skirts mean skirt portions of pistons)), cylinders (or cylinder liners), plungers, Check valve, valve guide, connecting rod, bush, crankshaft, cam lobe, cam journal, rocker arm, valve spring, shim, lifter, vane pump rotating vane, vane pump housing, timing chain, sprocket, chain guide (or chain guide shoe) And a chain tensioner (or chain tensioner shoe).
- Specific examples of the sliding members of the drive system include gears, gears, chains, belts, rolling bearings, sliding bearings, oil pumps, etc., such as automatic transmissions, continuously variable transmissions, manual transmissions, and final reduction gears. Can be mentioned.
- sliding member of the present invention is not limited to the above-described one, and slides in the presence of grease, biodiesel fuel, light oil fuel, gas-to-liquid (GTL) fuel, high-octane gasoline fuel, or the like. It can also be applied to cases.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Mechanical Engineering (AREA)
- Electrochemistry (AREA)
- General Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- Lubricants (AREA)
- Pistons, Piston Rings, And Cylinders (AREA)
- Electroplating Methods And Accessories (AREA)
- Other Surface Treatments For Metallic Materials (AREA)
- Sliding-Contact Bearings (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP13872669.0A EP2949783B1 (en) | 2013-01-28 | 2013-12-20 | Sliding member and method for producing same |
| CN201380071358.7A CN104955984B (zh) | 2013-01-28 | 2013-12-20 | 滑动构件和其制造方法 |
| US14/763,011 US9759323B2 (en) | 2013-01-28 | 2013-12-20 | Sliding member and method for producing same |
| US15/672,849 US10451184B2 (en) | 2013-01-28 | 2017-08-09 | Sliding member and method for producing same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013-013290 | 2013-01-28 | ||
| JP2013013290A JP6063758B2 (ja) | 2013-01-28 | 2013-01-28 | 摺動部材及びその製造方法 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/763,011 A-371-Of-International US9759323B2 (en) | 2013-01-28 | 2013-12-20 | Sliding member and method for producing same |
| US15/672,849 Division US10451184B2 (en) | 2013-01-28 | 2017-08-09 | Sliding member and method for producing same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014115457A1 true WO2014115457A1 (ja) | 2014-07-31 |
Family
ID=51227257
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/084212 Ceased WO2014115457A1 (ja) | 2013-01-28 | 2013-12-20 | 摺動部材及びその製造方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US9759323B2 (enExample) |
| EP (1) | EP2949783B1 (enExample) |
| JP (1) | JP6063758B2 (enExample) |
| CN (1) | CN104955984B (enExample) |
| WO (1) | WO2014115457A1 (enExample) |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6432465B2 (ja) * | 2014-08-26 | 2018-12-05 | 三菱マテリアル株式会社 | 接合体、ヒートシンク付パワーモジュール用基板、ヒートシンク、接合体の製造方法、ヒートシンク付パワーモジュール用基板の製造方法、及び、ヒートシンクの製造方法 |
| JP6090303B2 (ja) * | 2014-12-24 | 2017-03-08 | マツダ株式会社 | エンジン |
| JP6643253B2 (ja) * | 2014-12-26 | 2020-02-12 | ソマール株式会社 | シール部材及びシール構造 |
| CN108368448A (zh) * | 2015-12-11 | 2018-08-03 | 道康宁东丽株式会社 | 除了用于图像形成装置的滑动构件以外的滑动构件、部件以及机械装置的噪音降低方法 |
| CN105889502B (zh) * | 2016-04-27 | 2017-12-05 | 重庆驰山机械有限公司 | 一种活塞环 |
| US10563764B2 (en) | 2017-05-26 | 2020-02-18 | Mahle International Gmbh | Coated steel piston ring |
| CN107313079A (zh) * | 2017-07-31 | 2017-11-03 | 安徽沃德气门制造有限公司 | 一种汽车气门快速镀铬工艺 |
| CN107387567A (zh) * | 2017-09-19 | 2017-11-24 | 张家港保税区通勤精密机械有限公司 | 一种高耐磨发热小的轴承衬 |
| DE102017220152B4 (de) | 2017-11-13 | 2021-01-21 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Antriebsanordnung für ein Zweirad |
| JP7061006B2 (ja) | 2018-04-20 | 2022-04-27 | 株式会社豊田中央研究所 | 摺動部材と摺動機械 |
| US10844857B2 (en) | 2018-06-19 | 2020-11-24 | Ingersoll-Rand Industrial U.S., Inc. | Compressor system with purge gas system |
| US11164883B2 (en) * | 2018-06-27 | 2021-11-02 | Sandisk Technologies Llc | Three-dimensional memory device containing aluminum-silicon word lines and methods of manufacturing the same |
| JP6551766B2 (ja) * | 2018-11-19 | 2019-07-31 | 三菱重工業株式会社 | 積層部材、並びに、これを用いた羽根車、圧縮機及びエンジン |
| CN110205659B (zh) * | 2019-07-17 | 2020-06-16 | 广州三孚新材料科技股份有限公司 | 一种电镀锡添加剂及其制备方法 |
| JP2022076932A (ja) * | 2020-11-10 | 2022-05-20 | 日本アイ・ティ・エフ株式会社 | 摺動部材、潤滑油および摺動機構 |
| WO2022101416A1 (en) * | 2020-11-13 | 2022-05-19 | Nanofilm Vacuum Coating (Shanghai) Co., Ltd. | Piston rings and methods of manufacture |
| CN113154036A (zh) * | 2020-11-13 | 2021-07-23 | 纳峰真空镀膜(上海)有限公司 | 活塞环及制造方法 |
| US20250075794A1 (en) * | 2023-08-28 | 2025-03-06 | Hamilton Sundstrand Corporation | Piston rings and barrel sleeves |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004043837A (ja) * | 2002-07-09 | 2004-02-12 | Nissin Electric Co Ltd | 機械部品及びその製造方法並びに電気機械製品 |
| JP2005002888A (ja) | 2003-06-12 | 2005-01-06 | Nissan Motor Co Ltd | 自動車エンジン用ピストンリング及びこれに用いる潤滑油組成物 |
| JP2005003094A (ja) * | 2003-06-12 | 2005-01-06 | Nissan Motor Co Ltd | 自動車用エンジン |
| JP2011157610A (ja) * | 2010-02-03 | 2011-08-18 | Tocalo Co Ltd | Dlc膜被覆部材およびその製造方法 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1479946B1 (en) * | 2003-05-23 | 2012-12-19 | Nissan Motor Co., Ltd. | Piston for internal combustion engine |
| JP4973971B2 (ja) * | 2003-08-08 | 2012-07-11 | 日産自動車株式会社 | 摺動部材 |
| US7771821B2 (en) * | 2003-08-21 | 2010-08-10 | Nissan Motor Co., Ltd. | Low-friction sliding member and low-friction sliding mechanism using same |
| CN100482379C (zh) * | 2005-10-27 | 2009-04-29 | 鸿富锦精密工业(深圳)有限公司 | 一种压铸模仁及其制备方法 |
-
2013
- 2013-01-28 JP JP2013013290A patent/JP6063758B2/ja active Active
- 2013-12-20 WO PCT/JP2013/084212 patent/WO2014115457A1/ja not_active Ceased
- 2013-12-20 US US14/763,011 patent/US9759323B2/en active Active
- 2013-12-20 CN CN201380071358.7A patent/CN104955984B/zh not_active Expired - Fee Related
- 2013-12-20 EP EP13872669.0A patent/EP2949783B1/en active Active
-
2017
- 2017-08-09 US US15/672,849 patent/US10451184B2/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004043837A (ja) * | 2002-07-09 | 2004-02-12 | Nissin Electric Co Ltd | 機械部品及びその製造方法並びに電気機械製品 |
| JP2005002888A (ja) | 2003-06-12 | 2005-01-06 | Nissan Motor Co Ltd | 自動車エンジン用ピストンリング及びこれに用いる潤滑油組成物 |
| JP2005003094A (ja) * | 2003-06-12 | 2005-01-06 | Nissan Motor Co Ltd | 自動車用エンジン |
| JP2011157610A (ja) * | 2010-02-03 | 2011-08-18 | Tocalo Co Ltd | Dlc膜被覆部材およびその製造方法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2949783A4 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2949783A4 (en) | 2016-02-24 |
| US10451184B2 (en) | 2019-10-22 |
| US20150362072A1 (en) | 2015-12-17 |
| JP2014145098A (ja) | 2014-08-14 |
| EP2949783A1 (en) | 2015-12-02 |
| US20170335964A1 (en) | 2017-11-23 |
| JP6063758B2 (ja) | 2017-01-18 |
| US9759323B2 (en) | 2017-09-12 |
| CN104955984B (zh) | 2018-06-12 |
| CN104955984A (zh) | 2015-09-30 |
| EP2949783B1 (en) | 2019-10-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6063758B2 (ja) | 摺動部材及びその製造方法 | |
| US7771821B2 (en) | Low-friction sliding member and low-friction sliding mechanism using same | |
| US8206035B2 (en) | Low-friction sliding mechanism, low-friction agent composition and method of friction reduction | |
| JP4824407B2 (ja) | Dlc接触面を有するシステム、該システムの潤滑方法及び該システム用潤滑油 | |
| JP4614427B2 (ja) | 低摩擦摺動機構、手動変速機及び終減速機 | |
| US7951756B2 (en) | System having DLC contact surfaces, method of lubricating the system, and lubricant for the system | |
| JP2005098289A (ja) | 冷媒圧縮機 | |
| CN100587045C (zh) | 低摩擦滑动机构、低摩擦剂组合物以及减小摩擦的方法 | |
| JP2005097570A (ja) | 低摩擦摺動部材及びこれを用いた低摩擦摺動機構 | |
| JP4840635B2 (ja) | 低摩擦含油摺動機構 | |
| JP4973972B2 (ja) | 無段変速機用低摩擦摺動部材及びこれに用いる無段変速機油組成物 | |
| JP5561546B2 (ja) | 摺動機構 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13872669 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14763011 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2013872669 Country of ref document: EP |