WO2013069741A1 - プロフィラグリンc末端ドメイン特異的抗体及びその用途 - Google Patents
プロフィラグリンc末端ドメイン特異的抗体及びその用途 Download PDFInfo
- Publication number
- WO2013069741A1 WO2013069741A1 PCT/JP2012/079015 JP2012079015W WO2013069741A1 WO 2013069741 A1 WO2013069741 A1 WO 2013069741A1 JP 2012079015 W JP2012079015 W JP 2012079015W WO 2013069741 A1 WO2013069741 A1 WO 2013069741A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- terminal domain
- gene
- antibody
- filaggrin
- abnormality
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6881—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids from skin
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/20—Dermatological disorders
- G01N2800/202—Dermatitis
Definitions
- the present invention provides a profilagrin C-terminal domain specific antibody and its use, particularly a method for detecting filaggrin gene abnormality using the antibody and a kit for the detection.
- the keratin fibers in the granule layer of the epidermis bind to and aggregate with a protein called filaggrin when keratinized to form a specific form called “keratin pattern”.
- the precursor of filaggrin is called profilagrin and is stored in keratohyalin granules of the granule layer.
- Profilagrin has a structure in which filaggrin repeats in which 10 to 12 filaggrin molecules are continuously connected are interposed between the N-terminal domain and the C-terminal domain (FIG. 1). With keratinization, these domains are cut out to generate individual filaggrins.
- filaggrin is decomposed to create a natural moisturizing factor (NMF).
- NMF natural moisturizing factor
- the components of NMF are amino acids and their derivatives, which are known to be essential factors for skin moisturization (Horii I, Kawasaki K, Koyama J, et al. J Dermatol. 1983; 10: 25-33).
- filaggrin causes a decrease in NMF, resulting in a decrease in the water retention capacity of the stratum corneum.
- filaggrin genetic anomalies are responsible for fish phosphorosis and some atopic dermatitis (Sandilands A, Terron-Kwiatkowski A, Hull PR, et al. Nat Genet 2007; 39: 650-654), the surprising result that filaggrin abnormality has a direct effect not only on the water retention capacity of the stratum corneum but also on the barrier function due to the decrease in NMF.
- filaggrin genetic abnormalities are estimated to be about half in Europe and about 30% in Japan. From this point, it is well understood that atopic dermatitis is a multi-factorial disease, but it is very important to determine whether there is an abnormality in the filaggrin gene in the future. It is. In addition, it is possible to predict that a subject who has not developed atopic dermatitis is likely to suffer from atopic dermatitis in the future if an abnormality of the filaggrin gene is discovered.
- filaggrin repeat which is the most important region in the profilaggrin gene
- the abnormality of the filaggrin gene varies from one ethnic group to another.
- An object of the present invention is to provide a novel profilagrin C-terminal domain-specific antibody, a method for detecting filaggrin gene abnormality using the antibody, and a kit for the detection.
- the present inventor has found that a filaggrin gene abnormality can be easily and non-invasively detected with a C-terminal domain-specific antibody, and has completed the present invention.
- this application includes the following inventions.
- An antibody specific for the C-terminal domain of the human profilagrin gene wherein the C-terminal domain is a peptide consisting of the amino acid sequence set forth in SEQ ID NO: 1, or one or several amino acids of the amino acid sequence are
- a method for detecting an abnormality of the filaggrin gene the step of detecting the presence or absence of the C-terminal domain in the target skin sample using the antibody of (1), and the C-terminal domain not detected A step of determining that the subject filaggrin gene is abnormal.
- the presence or absence of a filaggrin gene can be easily detected from the presence or absence of a C-terminal domain in a skin sample using the domain-specific antibody. Furthermore, since the profilagrin gene abnormality can cause atopic dermatitis, the use of the antibody of the present invention makes it possible to easily and non-invasively diagnose or predict atopic dermatitis.
- the schematic diagram which shows the structure of the profilagrin gene (Chr1q21.3 / Human) which has 12 filaggrin repeats, and the variation
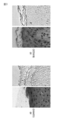
- Immunostaining of normal skin normal wrinkles (+ / +)
- atopic dermatitis skin AD wrinkles (+ / +)
- the left side is the result of staining with hematoxylin and eosin (HE staining)
- the right side is the result of staining with profilagulin C-terminal domain specific antibody and chromogenic substrate DAB (3,3'-diaminobenzidine) (FLG -C).
- the immuno-staining figure of the skin of atopic dermatitis which has an abnormality in the profilaggrin gene (S2889X; K4022X).
- the left side is the result of staining with hematoxylin and eosin (HE staining)
- the right side is the result of staining with profilagulin C-terminal domain specific antibody and chromogenic substrate DAB (3,3'-diaminobenzidine) (FLG) -C).
- the first aspect of the present invention is an antibody specific for the C-terminal domain of the human profilagrin gene, wherein the C-terminal domain is a peptide consisting of the amino acid sequence set forth in SEQ ID NO: 1 or a variant thereof, I will provide a.
- filaggrin is roughly classified into type I and type II.
- profilaguline refers to type I.
- Profilagrin has a structure in which filaggrin repeats in which 10 to 12 filaggrin molecules are continuously connected are interposed between the N-terminal domain and the C-terminal domain (FIG. 1). The full-length sequence of the C-terminal domain consisting of 157 amino acids is shown in SEQ ID NO: 2.
- amino acid sequence set forth in SEQ ID NO: 1 shows no homology with any of profilaggrin C-terminal domain, type II profilaggrin, and further hofilin, which is a profilagrin-like molecule, and is specific for type I Is an array. Therefore, an antibody specific for the amino acid sequence does not cross-react with these similar proteins.
- the antibody obtained as an antigen is specific for the C-terminal domain of the human profilagrin gene that does not recognize a peptide having a sequence similar to the amino acid sequence set forth in SEQ ID NO: 1 in the skin. Any one may be used. For example, one or several amino acids of the amino acid sequence shown in SEQ ID NO: 1 are deleted or substituted, or one or several amino acids are added to the amino acid sequence. It may be a peptide consisting of an amino acid sequence.
- the above peptides can be obtained by chemical synthesis according to conventional methods.
- the antibody of the present invention can be obtained by administering the peptide as an antigen, preferably an antigen complex bound to a suitable carrier of the peptide, to mammals such as rats, mice, rabbits and the like.
- the dose of the antigen or antigen complex per animal is 0.1 to 100 mg when an adjuvant is not used, and 10 to 1000 ⁇ g when an adjuvant is used, but is not limited thereto.
- adjuvants include Freund's complete adjuvant (FCA), Freund's incomplete adjuvant (FIA), and aluminum hydroxide adjuvant.
- Immunization is performed mainly by injecting intravenously, subcutaneously or intraperitoneally.
- the immunization interval is not particularly limited, and immunization is carried out 1 to 10 times, preferably 2 to 5 times at intervals of several days to several weeks, preferably 2 to 5 weeks. 6 to 60 days after the last immunization day, the antibody titer was measured by Western blotting, enzyme immunoassay (ELISA or EIA), radioimmunoassay (RIA), etc. It is preferable to collect blood to obtain antiserum.
- Polyclonal antibodies can be purified by affinity chromatography using a column to which an antigen peptide is bound, or other purification methods well known to those skilled in the art, such as ion exchange chromatography, gel filtration chromatography, and high performance liquid chromatography. it can.
- the antibody of the present invention may be a monoclonal antibody.
- a monoclonal antibody can be prepared according to an existing method using a peptide having the amino acid sequence shown in SEQ ID NO: 1 or a variant thereof.
- the present invention provides a method for detecting an abnormality of a filaggrin gene, wherein the presence or absence of the C-terminal domain in a target skin sample is detected using the profilagrin C-terminal domain-specific antibody. And a step of determining that the filaggrin gene of interest is abnormal when the C-terminal domain is not detected.
- abnormality of filaggrin gene means one or more mutations in the filaggrin gene that inhibit the formation of the profilagrin C-terminal domain. Examples of the mutation include a mutation S2889X terminated with serine located at position 2889 of profilagrin, and a mutation K4022X terminated with lysine located at position 4022 (FIG. 1).
- An abnormality in the profilagrin gene leads to atopic dermatitis. Therefore, in the method of the present invention, when the C-terminal domain is not detected, it can be determined that the subject suffers from or is likely to suffer from atopic dermatitis.
- the detection of the presence or absence of the C-terminal domain is performed by an immunological method known to those skilled in the art, for example, an immunoassay (ELISA, RIA, etc.).
- the skin sample can be collected from the subject's arm, foot, face, etc. by any method.
- the subject is a mammal, preferably a human, suspected of having a skin disease caused by filaggrin gene abnormality, particularly atopic dermatitis.
- the method of collecting the skin sample is not particularly limited, but the tape stripping method is preferred because it is simple and noninvasive.
- Tape stripping is a method of collecting a stratum corneum sample by applying a piece of adhesive tape to the skin surface layer, peeling it off, and attaching the skin stratum corneum to the peeled adhesive tape. If the tape stripping method is used, a skin sample containing a profilagrin gene can be easily obtained, and a filaggrin gene abnormality can be detected non-invasively.
- the preferred method of tape stripping is to first clean the surface of the skin with, for example, ethanol to remove sebum, dirt, etc., and lightly place an adhesive tape piece cut to an appropriate size (for example, 5 ⁇ 5 cm) on the skin surface, This is done by applying a uniform force to the entire tape and pressing it flat, and then peeling off the adhesive tape with a uniform force.
- the adhesive tape may be a commercially available cellophane tape or the like, for example, Scotch Superstrength Mailing Tape (3M), cellophane tape (Cellotape (registered trademark); Nichiban Co., Ltd.) or the like.
- soluble components For extraction of soluble components from the tape-stripped stratum corneum, neutral to weakly alkaline buffers containing nonionic surfactants such as the buffers described below can be used, but are not limited thereto. Not: 100 ⁇ ⁇ mM TrisHCl + 0.14 M NaCl + 0.1% Triton X100].
- the tape with the stratum corneum adhered is cut into small pieces with scissors and transferred to a container such as an Eppendorf tube, immersed in a small amount of the above-mentioned buffer, and by stirring by overturning, soluble components can be extracted efficiently.
- the extracted soluble component is subjected to immunoassay or the like using the antibody of the present invention, and the presence or absence of a profilagrin gene abnormality is determined.
- the present invention provides a kit for detecting an abnormality of the filaggrin gene, which contains the antibody.
- the antibody of the present invention may be contained in a container.
- the kit further includes reagents necessary for carrying out the above detection method, such as enzyme complex, substrate solution, stop solution, washing solution, enzyme complex diluent, assay buffer, control, when detecting abnormalities by ELISA. , Sample diluents, conjugates, etc., and instructions for explaining the procedure of the method may be included.
- Immunohistochemical staining Normal skin with no abnormalities in the profilagrin gene and skin sections of atopic dermatitis, and skin sections of atopic dermatitis (S2889X or K4022X) in which the profilagulin gene is mutated were prepared . Immunohistochemical staining of the skin section was performed by the method described in Kamata et al (J. Biol. Chem., Vol. 284, Issue 19, 12829-12836, May 8, 2009).
- Profilagulin C-terminal domain-specific antibody was obtained by immunizing rabbits with a synthetic peptide having the amino acid sequence of CKASAFGKDHPRYYATYINKDP (manufactured by Sigma).
- the profilaggrin gene C-terminal domain-specific antibody of the present invention it is possible to easily detect the presence or absence of many known filaggrin gene abnormalities without sequentially confirming by sequencing, It becomes possible to determine whether the subject's skin is or is likely to suffer from atopic dermatitis.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Urology & Nephrology (AREA)
- Hematology (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- Organic Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Pathology (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- General Physics & Mathematics (AREA)
- Food Science & Technology (AREA)
- Cell Biology (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Peptides Or Proteins (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES12848401.1T ES2650733T3 (es) | 2011-11-09 | 2012-11-08 | Anticuerpo específico para el dominio C-terminal de la profilagrina y su uso |
| KR1020147011999A KR102025446B1 (ko) | 2011-11-09 | 2012-11-08 | 프로필라그린 c 말단 도메인 특이적 항체 및 그의 용도 |
| US14/356,985 US20140287431A1 (en) | 2011-11-09 | 2012-11-08 | Antibody specific to profilaggrin c-terminal domain, and use thereof |
| CN201280066201.0A CN104039827B (zh) | 2011-11-09 | 2012-11-08 | 丝聚合蛋白原c末端结构域特异性抗体及其用途 |
| EP12848401.1A EP2778174B1 (en) | 2011-11-09 | 2012-11-08 | Antibody specific to profilaggrin c-terminal domain, and use thereof |
| HK15102350.3A HK1201856B (en) | 2011-11-09 | 2012-11-08 | Antibody specific to profilaggrin c-terminal domain, and use thereof |
| US16/136,929 US20190079080A1 (en) | 2011-11-09 | 2018-09-20 | Antibody specific to profilaggrin c-terminal domain, and use thereof |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011245483A JP5771123B2 (ja) | 2011-11-09 | 2011-11-09 | プロフィラグリンc末端ドメイン特異的抗体及びその用途 |
| JP2011-245483 | 2011-11-09 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/356,985 A-371-Of-International US20140287431A1 (en) | 2011-11-09 | 2012-11-08 | Antibody specific to profilaggrin c-terminal domain, and use thereof |
| US16/136,929 Division US20190079080A1 (en) | 2011-11-09 | 2018-09-20 | Antibody specific to profilaggrin c-terminal domain, and use thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013069741A1 true WO2013069741A1 (ja) | 2013-05-16 |
Family
ID=48290111
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/079015 Ceased WO2013069741A1 (ja) | 2011-11-09 | 2012-11-08 | プロフィラグリンc末端ドメイン特異的抗体及びその用途 |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US20140287431A1 (enExample) |
| EP (1) | EP2778174B1 (enExample) |
| JP (1) | JP5771123B2 (enExample) |
| KR (1) | KR102025446B1 (enExample) |
| CN (1) | CN104039827B (enExample) |
| ES (1) | ES2650733T3 (enExample) |
| WO (1) | WO2013069741A1 (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8956859B1 (en) | 2010-08-13 | 2015-02-17 | Aviex Technologies Llc | Compositions and methods for determining successful immunization by one or more vaccines |
| WO2020076770A1 (en) * | 2018-10-08 | 2020-04-16 | Yale University | Selective binding and aggregation of human keratin in the skin barrier using human filaggrin subdomains sd67 and sd150 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4162259B2 (ja) * | 1991-04-26 | 2008-10-08 | ビオメリュー・ソシエテ・アノニム | リウマチ性関節炎抗体により認識される抗原、抗原製剤及びその適用方法 |
| CA2570887C (en) * | 2004-06-18 | 2014-09-16 | Genentech, Inc. | Tumor treatment |
| GB0525492D0 (en) * | 2005-12-15 | 2006-01-25 | Univ Dundee | Filaggrin |
| JP2007217325A (ja) * | 2006-02-16 | 2007-08-30 | Kose Corp | プロフィラグリン及び/又はフィラグリン産生促進剤 |
| WO2011068166A1 (ja) * | 2009-12-03 | 2011-06-09 | 株式会社資生堂 | ブレオマオシン水解酵素の活性を指標としたアトピー性皮膚炎による乾燥肌改善剤のスクリーニング方法 |
-
2011
- 2011-11-09 JP JP2011245483A patent/JP5771123B2/ja active Active
-
2012
- 2012-11-08 EP EP12848401.1A patent/EP2778174B1/en active Active
- 2012-11-08 KR KR1020147011999A patent/KR102025446B1/ko not_active Expired - Fee Related
- 2012-11-08 US US14/356,985 patent/US20140287431A1/en not_active Abandoned
- 2012-11-08 CN CN201280066201.0A patent/CN104039827B/zh active Active
- 2012-11-08 ES ES12848401.1T patent/ES2650733T3/es active Active
- 2012-11-08 WO PCT/JP2012/079015 patent/WO2013069741A1/ja not_active Ceased
-
2018
- 2018-09-20 US US16/136,929 patent/US20190079080A1/en not_active Abandoned
Non-Patent Citations (10)
| Title |
|---|
| CHERVET L. ET AL.: "Missing C-terminal filaggrin expression, NFkappaB activation and hyperproliferation identify the dog as a putative model to study epidermal dysfunction in atopic dermatitis", EXP. DERMATOL., vol. 19, no. 8, 2010, pages E343 - E346, XP055067522 * |
| DATABASE GENBANK [online] 6 November 2011 (2011-11-06), IRVINE AD. ET AL.: "Definition: Homo sapiens filaggrin (FLG)", XP003033827, Database accession no. NM_002016 * |
| FALLON PG. ET AL.: "A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming", NAT. GENET., vol. 41, no. 5, 2009, pages 602 - 608, XP055067518 * |
| HORII I; KAWASAKI K; KOYAMA J ET AL., J DERMATOL., vol. 10, 1983, pages 25 - 33 |
| JUN'ICHI SAKABE: "Filaggrin Idenshi Hen'i ni yotte Naze Filaggrin ga Teika suru noka : C Mattan Ryoiki no Nazo", SKIN RESEARCH, 2011 NEN 10 GATSU, vol. 10, no. 16, pages 25 - 28, XP008174081 * |
| KAMATA ET AL., J. BIOL. CHEM., vol. 284, no. 19, 8 May 2009 (2009-05-08), pages 12829 - 12836 |
| KATO, NORITO: "Treatment Guidelines and New Treatments for Atopic Dermatitis", JOURNAL OF THE JAPANESE DERMATOLOGICAL ASSOCIATION, vol. 120, no. 13, 2010, pages 2564 - 2565 |
| NORITO KATO: "Treatment Guidelines and New Treatments for Atopic Dermatitis", JOURNAL OF THE JAPANESE DERMATOLOGICAL ASSOCIATION, vol. 120, no. 13, 2010, pages 2564 - 2565 |
| SANDILANDS A; TERRON-KWIATKOWSKI A; HULL PR ET AL., NAT GENET, vol. 39, 2007, pages 650 - 654 |
| See also references of EP2778174A4 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN104039827A (zh) | 2014-09-10 |
| EP2778174B1 (en) | 2017-11-01 |
| EP2778174A4 (en) | 2015-06-24 |
| US20190079080A1 (en) | 2019-03-14 |
| HK1201856A1 (en) | 2015-09-11 |
| CN104039827B (zh) | 2016-12-14 |
| JP5771123B2 (ja) | 2015-08-26 |
| KR20140089366A (ko) | 2014-07-14 |
| KR102025446B1 (ko) | 2019-09-25 |
| JP2013100249A (ja) | 2013-05-23 |
| ES2650733T3 (es) | 2018-01-22 |
| EP2778174A1 (en) | 2014-09-17 |
| US20140287431A1 (en) | 2014-09-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Di Zenzo et al. | Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid | |
| Nguyen et al. | Antibodies against keratinocyte antigens other than desmogleins 1 and 3 can induce pemphigus vulgaris–like lesions | |
| US8198412B2 (en) | Antibodies that recognize cutting edge within the TGF-β activation controlling region | |
| JP2014521098A (ja) | 病理学バイオマーカーアッセイ | |
| JP5580347B2 (ja) | ヒトbplpタンパク質由来のペプチド、該ペプチドをコードするポリヌクレオチド及び該ペプチドに対する抗体 | |
| CN102388308B (zh) | 单体和二聚形式的脂连蛋白受体片段和使用方法 | |
| Reese-Petersen et al. | Evaluation of a novel biomarker of type XXVIII collagen formation, PRO-C28, in samples from cancer and heart failure with preserved ejection fraction patients | |
| JP5771123B2 (ja) | プロフィラグリンc末端ドメイン特異的抗体及びその用途 | |
| RU2662973C2 (ru) | Пептидомиметики | |
| JP5943945B2 (ja) | プロフィラグリンc末端ドメイン特異的抗体及びその用途 | |
| JP2011226882A (ja) | 泌尿器系疾患マーカー及びその抗体、並びに泌尿器系疾患診断用キット | |
| Chen et al. | High expression level of homocitrulline is correlated with seborrheic keratosis and skin aging | |
| HK1201856B (en) | Antibody specific to profilaggrin c-terminal domain, and use thereof | |
| EP2918600B1 (en) | Novel peptide and use thereof | |
| JP2648952B2 (ja) | 種々の病理的状態に於いてps2遺伝子から特異的に発現される蛋白質及びその断片、該蛋白質及び/又はその断片から得られる抗体、並びに病理的状態に対する検出、診断及び治療への該蛋白質、その断片及び抗体の適用 | |
| JP2915530B2 (ja) | ラミニン フラグメント | |
| Staab-Weijnitz et al. | Assessment of Collagen in Translational Models of Lung Research | |
| EP1423708B1 (fr) | Compositions et methodes pour le dosage de l'aa4rp | |
| CN108484762A (zh) | 一种甲胎蛋白检测用抗体IgM及应用 | |
| JPH11511973A (ja) | 大腸細胞および大腸癌細胞関連核酸分子、タンパク質およびペプチド | |
| JP2007332122A (ja) | 抗nc1モノクローナル抗体反応性ペプチド | |
| JPWO2001062915A1 (ja) | 新規蛋白質及びそれをコードする遺伝子 | |
| WO2001090196A1 (en) | Immunoassay method for x-type phospholipase a¿2? | |
| Khovidhunkit | DYSBETALIPOPROTEINEMIA (TYPE III HYPERLIPOPROTEINEMIA) | |
| CN1916635A (zh) | 结缔组织生长因子测定方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12848401 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20147011999 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14356985 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REEP | Request for entry into the european phase |
Ref document number: 2012848401 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012848401 Country of ref document: EP |