WO2013038604A1 - 核酸精製方法、核酸抽出方法、及び核酸精製用キット - Google Patents
核酸精製方法、核酸抽出方法、及び核酸精製用キット Download PDFInfo
- Publication number
- WO2013038604A1 WO2013038604A1 PCT/JP2012/005384 JP2012005384W WO2013038604A1 WO 2013038604 A1 WO2013038604 A1 WO 2013038604A1 JP 2012005384 W JP2012005384 W JP 2012005384W WO 2013038604 A1 WO2013038604 A1 WO 2013038604A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- exchange resin
- nucleic acid
- cation exchange
- sample
- acid purification
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/10—Processes for the isolation, preparation or purification of DNA or RNA
- C12N15/1003—Extracting or separating nucleic acids from biological samples, e.g. pure separation or isolation methods; Conditions, buffers or apparatuses therefor
- C12N15/1006—Extracting or separating nucleic acids from biological samples, e.g. pure separation or isolation methods; Conditions, buffers or apparatuses therefor by means of a solid support carrier, e.g. particles, polymers
- C12N15/101—Extracting or separating nucleic acids from biological samples, e.g. pure separation or isolation methods; Conditions, buffers or apparatuses therefor by means of a solid support carrier, e.g. particles, polymers by chromatography, e.g. electrophoresis, ion-exchange, reverse phase
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D15/00—Separating processes involving the treatment of liquids with solid sorbents; Apparatus therefor
- B01D15/08—Selective adsorption, e.g. chromatography
- B01D15/26—Selective adsorption, e.g. chromatography characterised by the separation mechanism
- B01D15/34—Size-selective separation, e.g. size-exclusion chromatography; Gel filtration; Permeation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D15/00—Separating processes involving the treatment of liquids with solid sorbents; Apparatus therefor
- B01D15/08—Selective adsorption, e.g. chromatography
- B01D15/26—Selective adsorption, e.g. chromatography characterised by the separation mechanism
- B01D15/36—Selective adsorption, e.g. chromatography characterised by the separation mechanism involving ionic interaction, e.g. ion-exchange, ion-pair, ion-suppression or ion-exclusion
- B01D15/361—Ion-exchange
- B01D15/362—Cation-exchange
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D15/00—Separating processes involving the treatment of liquids with solid sorbents; Apparatus therefor
- B01D15/08—Selective adsorption, e.g. chromatography
- B01D15/26—Selective adsorption, e.g. chromatography characterised by the separation mechanism
- B01D15/36—Selective adsorption, e.g. chromatography characterised by the separation mechanism involving ionic interaction, e.g. ion-exchange, ion-pair, ion-suppression or ion-exclusion
- B01D15/361—Ion-exchange
- B01D15/363—Anion-exchange
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J39/00—Cation exchange; Use of material as cation exchangers; Treatment of material for improving the cation exchange properties
- B01J39/04—Processes using organic exchangers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J41/00—Anion exchange; Use of material as anion exchangers; Treatment of material for improving the anion exchange properties
- B01J41/04—Processes using organic exchangers
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6806—Preparing nucleic acids for analysis, e.g. for polymerase chain reaction [PCR] assay
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/88—Integrated analysis systems specially adapted therefor, not covered by a single one of the groups G01N30/04 - G01N30/86
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/96—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation using ion-exchange
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/88—Integrated analysis systems specially adapted therefor, not covered by a single one of the groups G01N30/04 - G01N30/86
- G01N2030/8809—Integrated analysis systems specially adapted therefor, not covered by a single one of the groups G01N30/04 - G01N30/86 analysis specially adapted for the sample
- G01N2030/8813—Integrated analysis systems specially adapted therefor, not covered by a single one of the groups G01N30/04 - G01N30/86 analysis specially adapted for the sample biological materials
- G01N2030/8827—Integrated analysis systems specially adapted therefor, not covered by a single one of the groups G01N30/04 - G01N30/86 analysis specially adapted for the sample biological materials involving nucleic acids
Definitions
- the present technology relates to a nucleic acid purification method, a nucleic acid extraction method, and a nucleic acid purification kit. More specifically, the present invention relates to a method for purifying a nucleic acid by adsorbing impurities with an ion exchange resin.
- Nucleic acid amplification reactions such as PCR (Polymerase Chain Reaction) and LAMP (Loop-Mediated Isothermal Amplification) have been applied in various fields in biotechnology. For example, in the medical field, diagnosis based on the base sequence of DNA or RNA is performed, and in the agricultural field, DNA identification is used for determination of genetically modified crops.
- PCR Polymerase Chain Reaction
- LAMP Loop-Mediated Isothermal Amplification
- nucleic acid in a small amount of sample can be amplified and detected with high efficiency.
- the amount of nucleic acid contained in the sample is extremely small, it may be less than the lower limit of detection.
- detection may not be possible because the nucleic acid to be amplified is not contained in a volume of the sample that can be introduced into the reaction field. In these cases, it is effective to introduce a nucleic acid extracted by purification or concentration in advance into the reaction field.
- the main object of the present technology is to provide a nucleic acid purification method that is simple in operation and capable of highly efficiently purifying nucleic acids in a short time.
- the present technology includes a procedure for adsorbing a substance contained in a sample containing nucleic acid with an ion exchange resin, and the ion exchange resin includes a cation exchange resin and an anion exchange resin.
- the cation exchange resin can adsorb cations such as proteins and metal salts having positive charges contained in the sample.
- the anion exchange resin can adsorb mainly anions having negative charges other than nucleic acids contained in the sample.
- the exclusion molecular weight is a compound that is difficult to enter into the pores of the ion exchange resin among various compounds to be adsorbed on the ion exchange resin, that is, it is difficult to be adsorbed into the pores of the ion exchange resin.
- the molecular weight of the compound with the lowest molecular weight In other words, a compound having a molecular weight higher than the exclusion limit molecular weight is difficult to be adsorbed on the ion exchange resin.
- the first cation exchange resin having a higher exclusion limit molecular weight is likely to selectively adsorb the protein.
- the second cation exchange resin having a lower exclusion limit molecular weight mainly tends to selectively adsorb cations such as metal salts.
- the substance is adsorbed by the anion exchange resin and the second cation exchange resin after the substance is adsorbed by the first cation exchange resin.
- the sample is introduced from the upper layer side of a column having the first cation exchange resin in the upper layer and the anion exchange resin and the second cation exchange resin in the lower layer. Also good.
- the protein in the sample is mainly selectively adsorbed by the first cation exchange resin, and then the metal in the sample by the second cation exchange resin and the anion exchange resin.
- the procedure may be a procedure in which a substance contained in the sample diluted with a buffer solution is adsorbed by the ion exchange resin, and the pH of the buffer solution is 4.0 to 8.0.
- the cation exchange resin is preferably a strongly acidic cation exchange resin.
- the counter ion of the first cation exchange resin is preferably Na + (sodium ion).
- the counter ion of the second cation exchange resin is preferably H + (proton).
- the anion exchange resin is preferably a strongly basic anion exchange resin.
- the counter ion of the anion exchange resin is preferably OH ⁇ (hydroxide ion).
- the ratio of the ion exchange capacity of the anion exchange resin to the second cation exchange resin is preferably 50% to 150%.
- the ion exchange capacity refers to the total number of exchange groups per unit amount of the ion exchange resin.
- the counter ion of the second cation exchange resin is H +
- the ion exchange capacity refers to the total number of H + contained in 1 ml of the second cation exchange resin.
- the ratio of the ion exchange capacity of the anion exchange resin to the second cation exchange resin is 50% to 150%, the pH before and after adsorption of the substance contained in the sample by the ion exchange resin It is possible to more stably suppress fluctuations in In the nucleic acid purification method, it is more preferable to adsorb the substance by adding a nonionic surfactant and / or a nonionic hydrophilic polymer compound to the sample. By adding a nonionic surfactant and / or a nonionic hydrophilic polymer compound to the sample, it is possible to suppress physical adsorption of nucleic acids to the resin.
- the said substance is a foreign material, for example.
- the contaminant includes, for example, a protein and a metal salt.
- Contaminants include various peptides, sugars, salts, low-molecular anions (eg, fumaric acid, phthalic acid, humic acid, fulvic acid) that are not necessary for analysis of nucleic acids in samples in addition to proteins and metal salts. These substances may be contained.
- the present technology includes a procedure for sonicating a sample containing nucleic acid, a procedure for adsorbing a substance contained in the sample with a cation exchange resin and an anion exchange resin, and electrophoresis.
- a nucleic acid extraction method for performing each of the steps of concentrating the nucleic acid by blocking the moving nucleic acid.
- the electrophoresis may be performed on the nucleic acid into which an intercalator having an anionic functional group is inserted.
- the electrophoresis is performed by mixing the sample, a compound having a functional group that undergoes a dehydration condensation reaction with a carboxyl group contained in the substance contained in the sample, and a condensing agent for the dehydration condensation reaction. It may be performed on the nucleic acid.
- the present technology also includes a cation exchange resin, an anion exchange resin, a nucleic acid purification instrument that holds the cation exchange resin and the anion exchange resin inside and can distribute a sample containing nucleic acid.
- a kit for purifying nucleic acid is provided.
- the present technology provides a nucleic acid purification method that is simple to operate and can extract nucleic acids with high efficiency in a short time.
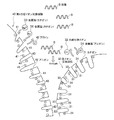
- FIG. 3 is a schematic diagram conceptually illustrating a state in which impurities are adsorbed by the first cation exchange resin according to the first embodiment of the present technology.
- FIG. 3 is a schematic diagram conceptually illustrating a state in which impurities are adsorbed by a second cation exchange resin and an anion exchange resin according to the first embodiment of the present technology. It is a schematic diagram for demonstrating the procedure of the nucleic acid purification method which concerns on 2nd Embodiment of this technique.
- FIG. 6 is a graph showing the results (Tt values) of LAMP reaction and RT-LAMP reaction after adsorption treatment with an ion exchange resin (Example 6).
- FIG. 9 is a graph showing the results (Tt values) of LAMP reaction and RT-LAMP reaction after adsorption treatment with an ion exchange resin (Example 7). It is a graph which shows the result of the LAMP reaction of a sample (Example 12). 10 is a graph showing the results of RT-LAMP reaction of samples (Example 12).
- Nucleic acid purification kit and nucleic acid purification method according to the first embodiment of the present technology (1) Nucleic acid purification kit (2) Nucleic acid purification method 2. Nucleic acid purification kit and nucleic acid purification method according to the second embodiment of the present technology; Nucleic acid extraction method
- Nucleic acid purification kit and nucleic acid purification method according to the first embodiment of the present technology (1) Nucleic acid purification kit First, the nucleic acid purification kit used in the nucleic acid purification method according to the first embodiment of the present technology is illustrated in FIG. ) And will be described. 1A to 1C are schematic diagrams for explaining the procedure of the nucleic acid purification method according to the first embodiment of the present technology.
- a nucleic acid purification kit denoted by reference numeral 1 adsorbs a substance other than nucleic acid (hereinafter referred to as a contaminant) contained in a sample and purifies the nucleic acid.
- the nucleic acid purification kit 1 mainly includes an ion exchange resin 10 and a nucleic acid purification instrument 3 that holds the ion exchange resin 10 inside and can distribute a sample containing nucleic acid.
- the sample used in the present technology is not particularly limited, for example, a swab, oral swab, saliva, serum, plasma, peripheral blood monocytic cells, cerebrospinal fluid, feces, urine, cultured cells, Biological samples such as biopsy tissues can be mentioned. Examples of the sample include river water, seawater, soil and the like in addition to the biological sample.
- the nucleic acid purification instrument 3 holds the ion exchange resin 10 inside and distributes the sample.
- the shape and material of the nucleic acid purification instrument 3 are not particularly limited as long as the ion exchange resin 10 is held inside and the sample can be distributed.
- the nucleic acid purification instrument 3 as shown in FIG. 1A, it is possible to use a commercially available sample tube or chip having a shape with an open top.
- the nucleic acid purification instrument 3 may be formed, for example, in a cylindrical shape with an upper part and a lower part opened. In this case, a chip or the like can be separately used to collect the sample flowing out from the lower part after the sample is distributed to the nucleic acid purification instrument 3 and adsorbed by the ion exchange resin 10.
- the ion exchange resin 10 adsorbs impurities contained in the sample.
- the ion exchange resin 10 includes a cation exchange resin and an anion exchange resin.
- the said foreign material contains protein and a metal salt, for example.
- Contaminants include various peptides, sugars, salts, low-molecular anions (eg, fumaric acid, phthalic acid, humic acid, fulvic acid) that are not necessary for analysis of nucleic acids in samples in addition to proteins and metal salts. These substances may be contained.
- the cation exchange resin mainly adsorbs cationic impurities.
- the cation exchange resin preferably includes a first cation exchange resin and a second cation exchange resin having a lower exclusion limit molecular weight than the first cation exchange resin.
- the first cation exchange resin is used to mainly adsorb proteins contained in the sample.
- the second cation exchange resin is mainly used for adsorbing cations (cations) such as metal ions contained in the sample.
- the first cation exchange resin is not particularly limited as long as it can adsorb the protein contained in the sample, but is preferably a strong acid cation exchange resin. At this time, the first cation exchange resin preferably uses SO 3 ⁇ as a fixed ion.
- the counter ion is not particularly limited, and calcium ion (Ca 2+ ), copper ion (Cu 2+ ), zinc ion (Zn 2+ ), magnesium ion (Mg 2+ ), potassium ion (K + ), Examples include ammonium ion (NH 4 + ), sodium ion (Na + ), proton (H + ), and the like.
- the order of ion exchange strength (selectivity of ions) when these ions are used is as follows: Ca 2+ > Cu 2+ > Zn 2+ > Mg 2+ > K + > NH 4 + > Na + > H + . Therefore, considering the ion exchange strength, the counter ion is preferably H + . On the other hand, when the counter ion is H + , the pH of the sample tends to shift to the acidic side. On the other hand, when the counter ion is Na + , the function of adsorbing the metal salt is weaker than when the counter ion is H + , but it is possible to suppress fluctuations in the pH of the sample.
- the first cation exchange resin is preferably a strong acid cation exchange resin (Na + type strong acid cation exchange resin) having Na + as a counter ion.
- the second cation exchange resin is not particularly limited as long as it can adsorb cations such as metal salts contained in the sample, but is preferably a strongly acidic cation exchange resin.
- the second cation exchange resin preferably uses SO 3 ⁇ as a fixed ion.
- the counter ion is not particularly limited. However, in consideration of the order of the ion exchange strength (selectivity of ions) described above, the counter ion of the second cation exchange resin is proton (H + ) Is more preferable.
- the anion exchange resin adsorbs anions (anions) contained in the sample.
- the anion exchange resin is not particularly limited as long as it can adsorb an anion such as a metal salt contained in the sample, but is preferably a strongly basic anion exchange resin.
- the anion exchange resin can be any of, for example, a type I strong base anion exchange resin having a trimethylammonium group and a type II having a dimethylethanolammonium group.
- the order of the strength (ion selectivity) for the exchange adsorption of anions is HSO 4 ⁇ > NO 3 ⁇ > Br ⁇ > Cl ⁇ > HCO 3 ⁇ > HCOO ⁇ > CH 3 COO. -> F -> OH - become.
- the order of the strength for exchanging anions is HSO 4 ⁇ > NO 3 ⁇ > Br ⁇ > Cl ⁇ > HCO 3 ⁇ > OH ⁇ > HCOO ⁇ > CH. 3 COO ⁇ > F ⁇ .
- type I can adsorb chloride ions (Cl ⁇ ) and the like more accurately. Therefore, it is preferable that the anion exchange resin is type I and has a hydroxide ion (OH ⁇ ) as a counter ion.
- the nucleic acid purification kit 1 includes additives such as nonionic surfactants and nonionic compounds such as nonionic hydrophilic polymer compounds so as not to adsorb nucleic acids along with the adsorption of impurities. It may be.
- nonionic compound nonionic surfactants, such as Brij35, Tween20, or TritonX100, are mentioned, for example.
- nonionic hydrophilic high molecular compounds such as polyethyleneglycol and polyhydroxyethylcellulose, are also mentioned. These exemplified nonionic compounds may be used alone or in combination of two or more.
- the nucleic acid purification kit 1 may contain a chelating additive such as EDTA.
- the nucleic acid purification kit 1 may contain a buffer solution.
- the buffer for the buffer include HomoPIPES (pH: 3.9 to 5.1, pKa: 4.55), MES (pH: 5.5 to 7.0, pKa: 6.15), Bis-Tris (pH: 5.7-7.3, pKa: 6.46), ADA (pH: 5.8-7.4, pKa: 6.60), PIPES (pH: 6.1-7.
- each of the buffering agents exemplified above refers to an appropriate range as the pH of the sample to which the buffer solution has been added.
- pKa of each buffer illustrated above refers to pKa at 20 ° C. excluding HomoPIPES (for HomoPIPES, pKa at 37 ° C. is indicated).
- the pH of the buffer in which the sample is diluted is preferably 4-8.
- HomoPIPES, MES, Bis-Tris, and ADA are preferably used among the buffers exemplified above in the present embodiment.
- nucleic acid purification method according to the related technology of the present technology will be described.
- the nucleic acid purification method according to the related art include a method of adsorbing sample impurities using zeolite (see, for example, Patent Document 2).
- nucleic acid purification method it is possible to remove only a cation among anions and cations contained in a sample such as a biological sample. Therefore, in the nucleic acid purification method according to the related art, protons are released by purification of the sample, and the pH of the liquid containing the sample is lowered (shifted to the acidic side).
- the nucleic acid amplification reaction may be performed at a pH of 7 to 9 after the sample is purified, it is necessary to set the pH of the liquid containing the sample to be contacted with the zeolite to a high value (set to the alkali side) in advance. is there. For this reason, in the nucleic acid purification method according to the related art, the operation may be complicated.
- RNA is contained in a sample, there is a possibility of causing degradation of the RNA.
- the nucleic acid purification method according to the related technique includes a step of diluting the sample with a mixed reagent of an alkaline compound and a surfactant (for example, SDS) and then heating at a high temperature. Therefore, also from this point, in the nucleic acid purification method according to the related technique, the operation may be complicated. In addition, when RNA is contained in a sample, there is a possibility of causing degradation of the RNA.
- a surfactant for example, SDS
- anion may remain in the sample after the sample is purified.
- the sample may contain an anionic surfactant. Therefore, when the nucleic acid is electrophoresed after the sample is purified, the electrolyte concentration in the sample is increased, and the sample is likely to generate heat. Therefore, also from this point, if RNA is contained in the sample, there is a possibility of causing degradation of the RNA. Furthermore, the efficiency of electrophoretic concentration of nucleic acids may decrease due to the occurrence of sample convection due to heat generation.
- nucleic acid purification method which will be described in detail below, has been found as a result of intensive studies by the inventors of the present application, does not require complicated operations, and makes the operations very simple and short.
- nucleic acid can be purified with high efficiency in time.
- the sample A containing the nucleic acid accommodated in the container 5 and the buffer solution obtained by diluting the sample A are filled into the pipette tip 51 attached to the pipette 50. (See FIG. 1A).
- the sample A filled in the pipette tip 51 and the buffer solution obtained by diluting the sample A are injected into the nucleic acid purification instrument 3 (see FIG. 1B).
- the nucleic acid purification instrument 3 holds the ion exchange resin 10 inside. Therefore, impurities contained in the sample A are adsorbed by the ion exchange resin 10.
- sample A purified by adsorbing impurities by the ion exchange resin 10 passes through the ion exchange resin 10 and is stored in the lower part of the nucleic acid purification instrument 3 (see FIG. 1C).
- the sample A can be subjected to a nucleic acid amplification reaction.
- the sample A can be added to a solid phase nucleic acid amplification reagent to perform a nucleic acid amplification reaction.
- the sample can be desalted with the ion exchange resin 10 to convert the double helix DNA into a single-stranded DNA, and the DNA can be appropriately denatured before the nucleic acid amplification reaction.
- FIG. 2 is a schematic diagram conceptually illustrating a state in which the first cation exchange resin adsorbs impurities in FIG. 1B.
- FIG. 3 is a schematic diagram conceptually illustrating a state in which the second cation exchange resin and the anion exchange resin adsorb impurities in FIG. 1B.
- the first cation exchange resin 20 included in the ion exchange resin 10 adsorbs impurities included in the sample A
- a symbol B is a nucleic acid contained in the sample A
- a symbol C is a protein that is an example of a contaminant contained in the sample A.
- the first cation exchange resin 20 is a strongly acidic cation exchange resin having sodium ions (Na + ) as counter ions.
- the first cation exchange resin 20 has an anion 21 such as SO 3 ⁇ and Na + 22 which is a counter ion. Further, the first cation exchange resin 20 is formed with a region 23 for adsorbing protein C.
- the protein C contained in the sample A passed through the nucleic acid purification instrument 3 is positively charged, it is adsorbed to the first cation exchange resin 20 having the anion 21 on the surface. Is done. For example, protein C is adsorbed on the region 23.
- the nucleic acid B is negatively charged, the nucleic acid B is not adsorbed by the first cation exchange resin 20 but is induced to flow by a flow of a buffer solution or the like contained in the sample A.
- the counter ion of the first cation exchange resin 20 is Na + 22
- the metal salt is harder to adsorb (is hard to desalt) than the case where the counter ion is H + , and protein C is selected. Adsorption is possible.
- the pH of the buffer for diluting sample A is preferably set to 4-8.
- the nucleic acid B is negatively charged more stably, and only the contaminants (mainly protein C) are positive. It can be charged. Therefore, only the protein C can be stably adsorbed on the first cation exchange resin 20, and the nucleic acid B can be purified with higher efficiency.
- the first cation exchange resin 20 is a strongly acidic cation exchange resin, the first cation exchange resin 20 has a negative charge under a wide range of pH conditions (for example, pH: 3 to 13).
- the protein C can be stably adsorbed to the first cation exchange resin 20 even if the sample is acidified. Furthermore, by setting the pH of sample A to 4 to 8 (acidifying), the activity of the RNase can be suppressed, so that RNA can be suitably purified.
- the exclusion limit molecular weight of the first cation exchange resin 20 is defined such that the protein C contained in the sample A is taken into the region 23. More specifically, the exclusion limit molecular weight of the first cation exchange resin 20 is preferably 5000 or more. When the exclusion limit molecular weight of the first cation exchange resin 20 is 5000 or more, the protein C is particularly selectively adsorbed and easily removed from the contaminants.
- the average volume particle diameter of the first cation exchange resin 20 is preferably 1 ⁇ m or more and 3000 ⁇ m or less. Even when the average volume particle diameter of the first cation exchange resin 20 is not less than 1 ⁇ m and not more than 3000 ⁇ m, the protein C is particularly selectively adsorbed and easily removed from impurities.
- the specific gravity of the particles of the first cation exchange resin 20 is preferably 0.5 or more and 2.5 or less. Even when the specific gravity of the first cation exchange resin 20 is not less than 0.5 and not more than 2.5, the protein C is adsorbed selectively and easily removed in the contaminants. Further, the specific gravity of the particles themselves is more preferably 1.0 or more and 2.5 or less. When the specific gravity is 1.0 or more and 2.5 or less, the particles are easily settled in the solution, whereby the particles can be easily removed from the solution.
- D1 is an anion such as a chloride ion
- D2 is a cation such as a sodium ion
- D3 is a cation such as a magnesium ion.
- the anion exchange resin 30 is an I-type strongly basic anion exchange resin having hydroxide ions (OH ⁇ ) as counter ions.
- the anion exchange resin 30 has a cation 31 such as CH 2 N (CH 3 ) 3 + and OH ⁇ 32 which is a counter ion.
- the anion exchange resin 30 is formed with a region 33 that adsorbs anions such as chloride ions D1.
- the chloride ion D1 contained in the sample A passed through the nucleic acid purification instrument 3 is negatively charged, it is adsorbed by the anion exchange resin 30 having the cation 31 on the surface. .
- the chloride ion D1 is adsorbed in the region 33.
- the nucleic acid B is also negatively charged, the nucleic acid B has a larger volume than anions such as chloride ions D1, and thus is difficult to be adsorbed on the anion exchange resin 30.
- the exclusion limit molecular weight of the anion exchange resin 30 is preferably 100 or more and 2000 or less.
- the exclusion limit molecular weight of the anion exchange resin 30 is 100 or more and 2000 or less, the adsorption of the nucleic acid B is suppressed with higher accuracy, and the cations such as the chloride ion D1 are selectively adsorbed and easily removed.
- the average volume particle size of the anion exchange resin 30 is preferably 1 ⁇ m or more and 3000 ⁇ m or less. Even when the average volume particle diameter of the first cation exchange resin 20 is not less than 1 ⁇ m and not more than 3000 ⁇ m, it becomes easier to selectively adsorb and remove cations such as chloride ions D1 with higher accuracy.
- the specific gravity of the anion exchange resin 30 is preferably 0.5 or more and 2.5 or less. Even when the specific gravity of the anion exchange resin 30 is not less than 0.5 and not more than 2.5, it becomes easier to selectively adsorb and remove cations such as chloride ions D1 with higher accuracy. Furthermore, the specific gravity of the particles themselves is more preferably 1.0 or more and 2.5 or less. When the specific gravity is 1.0 or more and 2.5 or less, the particles are easily settled in the solution, whereby the particles can be easily removed from the solution.
- the second cation exchange resin 40 is a strongly acidic cation exchange resin having H + ions (protons) as counter ions.
- the second cation exchange resin 40 has an anion 41 such as SO 3 ⁇ and H + 42.
- the second cation exchange resin 40 is formed with a region 43 that adsorbs cations such as sodium ions D2 and magnesium ions D3.
- the cations such as sodium ion D2 and magnesium ion D3 contained in the sample A passed through the nucleic acid purification instrument 3 are positively charged. Adsorbed on the cation exchange resin 40. For example, sodium ions D2 and magnesium ions D3 are adsorbed in the region 43. On the other hand, since the nucleic acid B is negatively charged, it is guided by the flow of the buffer solution or the like contained in the sample A without being adsorbed by the second cation exchange resin 40.
- the second cation exchange resin 40 has an exclusion limit molecular weight lower than that of the first cation exchange resin 20. Therefore, the surface area per unit volume is increased, and desalting is easier than the first cation exchange resin 20. Moreover, when the 2nd cation exchange resin 40 has a proton as a counter ion, it becomes easier to desalinate than the 1st cation exchange resin 20.
- the exclusion limit molecular weight of the second cation exchange resin 40 is 100 or more and 2000 or less. When the exclusion limit molecular weight of the second cation exchange resin 40 is 100 or more and 2000 or less, it becomes possible to more accurately adsorb cations such as sodium ion D2 and magnesium ion D3.
- the average volume particle diameter of the second cation exchange resin 40 is preferably 1 ⁇ m or more and 3000 ⁇ m or less. Even when the average volume particle diameter of the second cation exchange resin 40 is 1 ⁇ m or more and 3000 ⁇ m or less, it becomes possible to adsorb cations such as sodium ions D2 and magnesium ions D3 with higher accuracy. Further, the average volume particle diameter of the second cation exchange resin 40 is preferably 1 ⁇ m or more and 2000 ⁇ m or less. When the average volume particle diameter of the second cation exchange resin 40 is 1 ⁇ m or more and 2000 ⁇ m or less, it is possible to keep the pressure when passing the solution through the ion exchange resin low.
- the specific gravity of the second cation exchange resin 40 is preferably 0.5 or more and 2.5 or less. Even if the specific gravity of the second cation exchange resin 40 is 0.5 or more and 2.5 or less, it becomes possible to adsorb cations such as sodium ions D2 and magnesium ions D3 with higher accuracy. Further, the specific gravity of the particles themselves is more preferably 1.0 or more and 2.5 or less. When the specific gravity is 1.0 or more and 2.5 or less, the particles are easily settled in the solution, whereby the particles can be easily removed from the solution.
- the ratio of the ion exchange capacity of the anion exchange resin 30 to the second cation exchange resin 40 is preferably 50% to 150%.
- the ratio of the ion exchange capacity is 50% to 150%, desalting can be performed while suppressing fluctuation of the pH of the sample more stably.
- the ratio of the dose of the anion exchange resin 30 to the second cation exchange resin 40 is preferably 50% to 150%. Even when the proportion of the above dose is 50% to 150%, desalting can be performed while suppressing fluctuations in pH of the sample more stably.
- a nonionic surfactant such as Brij35, Tween 20, or Triton X100 is contained in the sample A in order to prevent nucleic acid B from being adsorbed by the ion exchange resin. It is preferable to be added as appropriate. In addition, it is preferable that a nonionic compound such as a nonionic hydrophilic polymer compound such as polyethylene glycol or polyhydroxyethylcellulose is also appropriately added to the sample A.
- the impurities contained in the sample can be obtained by using the ion exchange resin 10 made of a cation exchange resin and an anion exchange resin as an adsorption carrier. Can be adsorbed.
- the direct adsorption process can be performed without going through a complicated pretreatment process.
- nucleic acids can be extracted with high efficiency in a short time by a very simple operation.
- all the processes can be performed in the time for several seconds, and a nucleic acid can be purified in a short time.
- the nucleic acid purification method according to the present embodiment for example, since it does not include a washing step like silica solid phase extraction, it can be operated with a small amount of sample, and the apparatus can be downsized. realizable. Further, according to the nucleic acid purification method according to the present embodiment, it is possible to subject the sample to an adsorption treatment of impurities under acidic conditions (preferably, pH: 4 to 8). That is, according to the nucleic acid purification method according to the present embodiment, a step of making the sample alkaline or heating it is not necessary. Therefore, when RNA is contained in the sample, the sample can be purified while suppressing degradation of RNA. Moreover, according to the nucleic acid purification method according to the present embodiment, the action of RNase can be suppressed by performing ultrasonic disruption in an acidic region.
- the sample is not diluted, so that inhibition of the nucleic acid amplification reaction due to impurities appears significantly.
- contaminants present in the sample are removed, and thus the nucleic acid amplification reaction is performed by adding the nucleic acid directly to the solid-phased nucleic acid amplification reagent. be able to.
- the cation exchange resin As the cation exchange resin, the first cation exchange resin and the second cation exchange resin having a lower exclusion limit molecular weight than the first cation exchange resin. It is possible to use a cation exchange resin. Therefore, even when the metal salt and protein are contained in the contaminants in the sample, both can be efficiently removed.
- the first cation exchange resin and the second cation exchange resin are strongly acidic cation exchange resins, they are more efficient. It is. Further, when the counter ion of the first cation exchange resin is Na + or the counter ion of the second cation exchange resin is H + , impurities can be removed more efficiently.
- the anion exchange resin is a strongly basic cation exchange resin
- impurities can be more efficiently removed.
- the counter ion of the anion exchange resin is OH - if it is, can be removed more efficiently contaminants.
- the ratio of the ion exchange capacity of the anion exchange resin to the second cation exchange resin is set to 50% to 150%, thereby changing the pH of the sample. Can be suppressed. Therefore, as described above, for example, when the sample contains RNA, the sample can be purified while suppressing degradation of RNA.
- nucleic acid purification method it is possible to use a nonionic compound such as a nonionic surfactant or a nonionic hydrophilic polymer compound.
- a nonionic compound such as a nonionic surfactant or a nonionic hydrophilic polymer compound.
- the nucleic acid can be prevented from adsorbing to the matrix of the ion exchange resin, and impurities can be efficiently removed.
- FIGS. 4A to 4C are schematic diagrams for explaining the procedure of the nucleic acid purification method according to the second embodiment of the present technology. is there.
- a nucleic acid purification kit denoted by reference numeral 101 includes an ion exchange resin 10 and a nucleic acid purification instrument 3 that is held inside the ion exchange resin 10 and can distribute a sample containing nucleic acid.
- a cation exchange resin and an anion exchange resin are used for the ion exchange resin 10.
- the first cation exchange resin 20 and the second cation exchange resin 40 are used as the cation exchange resin.
- the nucleic acid purification kit and the nucleic acid purification method according to this embodiment are different from the nucleic acid purification kit and the nucleic acid purification method according to the first embodiment of the present technology in that the first cation exchange resin 20 is the anion exchange resin 30. And the second cation exchange resin 40 is mainly different in that the nucleic acid is purified while being contained in the upper layer (the side into which the sample is injected). Therefore, in this embodiment, the point that the 1st cation exchange resin 20 is provided in the upper layer with respect to the anion exchange resin 30 and the 2nd cation exchange resin 40 is mainly demonstrated.
- the first cation exchange resin 20 is accommodated in the nucleic acid purification instrument 3 on the upper layer side than the anion exchange resin 30 and the second cation exchange resin 40. Therefore, the sample A injected from the upper layer side first comes into contact with the first cation exchange resin 20 (see FIG. 4B).
- any of the anion exchange resin 30 and the 2nd cation exchange resin 40 may be accommodated in the upper layer, and each is mixed. May be accommodated in the lower layer of the first cation exchange resin 20.
- the purified sample A is stored in the lower layer (see FIG. 4C).
- the anion exchange resin 30 and the second cation exchange resin are adsorbed. 40 adsorbs impurities. Therefore, according to the nucleic acid purification method according to the second embodiment of the present technology, it is possible to remove a metal salt having a smaller volume after removing a protein having a larger volume, and more efficiently contaminating substances. Can be removed.
- nucleic acid extraction method including the nucleic acid purification method according to each embodiment of the present technology will be described.
- a procedure for sonicating a sample containing nucleic acid, a substance contained in the sample by a cation exchange resin and an anion exchange resin are used as a pretreatment for performing a nucleic acid amplification reaction.
- a procedure for sonicating a sample containing nucleic acid, a substance contained in the sample by a cation exchange resin and an anion exchange resin are used as a pretreatment for performing a nucleic acid amplification reaction.
- a procedure for sonicating a sample containing nucleic acid, a substance contained in the sample by a cation exchange resin and an anion exchange resin are used as a pretreatment for performing a nucleic acid amplification reaction.
- a procedure for sonicating a sample containing nucleic acid, a substance contained in the sample by a cation exchange resin and an anion exchange resin are used as
- the above procedures are not particularly limited. For example, all procedures can be performed in the same cell. When performing each procedure in the same cell, it is possible to provide the cell with an ultrasonic wave generation unit that performs ultrasonic processing. Moreover, it is possible to accommodate a cation exchange resin and an anion exchange resin in the cell.
- the cation exchange resin and the anion exchange resin the ion exchange resin 10 (the first cation exchange resin 20, the anion exchange resin 30, the second cation exchange resin 10 used in the nucleic acid purification method according to each embodiment of the present technology described above).
- Cation exchange resin 40 can be used.
- a negative electrode and a positive electrode for electrophoresis can be provided in the cell, and a blocking portion (for example, a dialysis membrane, a polymer gel, etc.) for blocking the electrophoretic nucleic acid can be provided.
- a blocking portion for example, a dialysis membrane, a polymer gel, etc.
- examples of the intercalator include compounds having a sulfo group as a functional group.
- examples of the intercalator include 9,10-anthraquinone-2,6-disulfonic acid, sodium anthraquinone-1-sulfonate, disodium anthraquinone-2,7-disulfonate, anthraquinone-1,5. -Sodium disulphonate, sodium anthraquinone-2-sulfonate and the like.
- the isoelectric point of the nucleic acid can be adjusted in the electrophoresis of the nucleic acid. That is, the nucleic acid can be concentrated and purified while controlling the electrophoresis speed of the nucleic acid.
- a carboxyl group such as protein which is one of the impurities contained in the sample, can be subjected to a dehydration condensation reaction with a compound such as N-hydroxysuccinimide (NHS), ethanolamine, or ethylenediamine.
- a carbodiimide compound can be used as a condensing agent.
- carbodiimide compounds include 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), 4- (4,6-dimethoxy-1,3,5-triazin-2-yl)
- EDC 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride
- EDC 4- (4,6-dimethoxy-1,3,5-triazin-2-yl)
- Examples include -4-methylmorpholinium chloride (DMT-MM), dicyclohexylcarbodiimide (DCC), diisopropylcarbodiimide (DIC), and the like. This makes it possible to increase the difference in isoelectric point between nucleic acid and protein that undergo dehydration condensation reaction with carboxyl groups in the protein even when proteins are present in the sample during electrophoresis of nucleic acids. Thus, nucleic acid and protein can be separated with high accuracy.
- a nucleic acid purification method comprising a procedure of adsorbing a substance contained in a sample containing nucleic acid with an ion exchange resin, wherein the ion exchange resin comprises a cation exchange resin and an anion exchange resin.
- the ion exchange resin comprises a cation exchange resin and an anion exchange resin.
- a first cation exchange resin and a second cation exchange resin having a smaller exclusion limit molecular weight than the first cation exchange resin are used.
- the nucleic acid purification method as described.
- the sample flows in from the upper layer side of a column having the first cation exchange resin in the upper layer and the anion exchange resin and the second cation exchange resin in the lower layer.
- the procedure is a procedure in which a substance contained in the sample diluted with a buffer solution is adsorbed by the ion exchange resin, and the pH of the buffer solution is 4.0 to 8.0.
- To (4) The nucleic acid purification method according to any one of (4).
- (6) The nucleic acid purification method according to any one of (1) to (5), wherein the cation exchange resin is a strongly acidic cation exchange resin.
- a procedure for sonicating a sample containing nucleic acid, a procedure for adsorbing a substance contained in the sample with a cation exchange resin and an anion exchange resin, and damaging the nucleic acid moving by electrophoresis A nucleic acid extraction method for performing each of the steps of concentrating the nucleic acid.
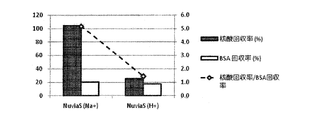
- This protein / nucleic acid mixed solution was sufficiently stirred at room temperature, and then 200 ⁇ L was dropped onto a strongly acidic cation exchange resin-enclosed spin column and sufficiently stirred. Thereafter, the mixture was centrifuged at 12000 G for 2 minutes to spin down the mixture.
- the absorbance of the spin-down solution was measured with NanoDrop D-1000 (manufactured by Thermo Fisher Scientific Inc.), and the nucleic acid purification ability was evaluated from the difference in absorbance before and after treatment with the strongly acidic cation exchange resin.
- the absorbance of BSA was evaluated in the Protein A280 mode, and the absorbance of nucleic acid was evaluated by the absorbance of Cy3 in the microarray mode.
- NaCl manufactured by Wako Pure Chemical Industries, Ltd.
- Wako Pure Chemical Industries, Ltd. NaCl (manufactured by Wako Pure Chemical Industries, Ltd.) is added to the protein-nucleic acid mixed solution so that the final concentration becomes 0.09% by mass, and the treatment with a strongly acidic ion exchange resin is similarly performed. I did it.
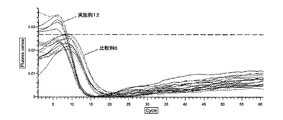
- FIG. 6 shows the results of the LAMP reaction of the sample subjected to the adsorption treatment of the impurities with the ion exchange resin and the sample not subjected to the adsorption treatment.
- LAMP reaction first, a sample, enzyme, fluorescent dye, nucleic acid monomer, buffer, primer set for target nucleic acid chain amplification, and probe for real-time measurement were mixed to prepare a LAMP reaction solution. Each concentration was determined according to Loopamp® DNA® amplification reagent kit (manufactured by Eiken Chemical Co., Ltd.) and Loopamp® RNA® amplification reagent kit (manufactured by Eiken Chemical Co., Ltd.). The reaction temperature was 63 ° C.
- the LAMP reaction was measured using a thermal cycler Chromo4 (Bio-Rad, USA) capable of real-time measurement. 1Cycle was set to 1min.
- the probe is a quenching probe QP probe (J-bio21, Japan: http://www.j-bio21.co.jp/tech/qpmethod.htm ([July 19, 2011], title: QP method) ), A decrease in fluorescence intensity is observed with nucleic acid amplification. As shown in FIG. 6, the nucleic acid amplification reaction could be detected in the sample subjected to the adsorption treatment of the contaminants with the ion exchange resin.
- the LAMP reaction described below was performed under the same conditions as described above.
- the concentration of Na + was measured with a Horiba Cardy Sodium Compact Ion Meter (C-122) (manufactured by Horiba, Ltd.). Further, the pH was measured with a pocket pH meter S2K712 (manufactured by ISFETCOM). The evaluation results are shown in FIG. Table 1 summarizes the evaluation results.
- the pH of the sample after adsorption treatment is lower than that before adsorption treatment (oxidizes) compared to the case of using Na + type cation exchange resin.
- Example 1 Spin filter column (Ultrafree-MC, 0.45 ⁇ m) with strong acid cation exchange resin (Nuvia S (Na + type) (manufactured by Bio Rad)): 100mg, strong acid cation exchange resin (AG1-X8 (H + type) ) (Manufactured by Bio Rad)): 50 mg, and a strongly basic anion exchange resin (AG50W-X8 (OH - type) (manufactured by Bio Rad)): 50 mg were weighed in. The concentration of Na + before the adsorption treatment was 0.45 mg / mL, and the pH was 4.9.
- strong acid cation exchange resin Nuvia S (Na + type) (manufactured by Bio Rad)
- AG1-X8 H + type
- a strongly basic anion exchange resin AG50W-X8 (OH - type) (manufactured by Bio Rad)
- MES buffer pH 5
- bovine whole blood was added in a 1 / 10-fold dilution to prepare a sample.
- the sample was sufficiently stirred at room temperature, and then 200 ⁇ L was dropped onto the ion exchange resin-encapsulated spin column and sufficiently stirred. Thereafter, the mixture was centrifuged at 12000 G for 2 minutes to spin down the mixture. Thereafter, the Na + concentration and pH in the sample were measured.
- Example 2> Compared to Example 1, strongly acidic cation exchange resin (Nuvia S (Na + type) (manufactured by Bio Rad)): 100 mg, strongly acidic cation exchange resin (AG1-X8 (H + type) (Bio Rad) )): 50 mg, and strongly basic anion exchange resin (AG50W-X8 (OH - type) (manufactured by Bio Rad)): instead of 50 mg, strongly acidic cation exchange resin (AG1-X8 (H + type) ) (Bio Rad)): 50 mg and strongly basic anion exchange resin (AG50W-X8 (OH - type) (Bio Rad)): Evaluation was carried out in the same manner as in Example 1 except that 50 mg was used. did.
- Example 3> Compared with Example 2, a strongly acidic cation exchange resin (AG1-X8 (H + type) (manufactured by Bio Rad Co.)): 50 mg and a strongly basic anion exchange resin (AG50W-X8 (OH - form) (Bio Rad)): Instead of using 50 mg, strongly acidic cation exchange resin (AG1-X8 (H + type) (manufactured by Bio Rad)): 70 mg and strongly basic anion exchange resin (AG50W-X8 (OH - type) (manufactured by Bio Rad Co.)): except for using 50mg was evaluated in the same manner as in example 2.
- Table 3 summarizes the evaluation results in Examples 1 to 3 and Comparative Examples 1 and 2.
- Example 1 Example 2, and Example 3 a comparative example was obtained by using an ion exchange resin in which an H + type strongly acidic cation exchange resin and an OH ⁇ type strong basic anion exchange resin were mixed. Compared to 1 and Comparative Example 2, the sample could be desalted with higher accuracy. Moreover, in Example 1, Example 2, and Example 3, compared with the comparative example 2, the pH change of the sample was able to be suppressed more accurately.
- Example 2 and Example 3 the desalting effect and the pH of the strongly acidic cation exchange resin and the strongly basic anion exchange resin can be equalized or as the equivalent ion exchange capacity. It was suggested that the change can be suppressed.
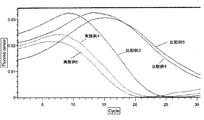
- Example 5> Compared with Example 4, strongly acidic cation exchange resin (Nuvia S (Na + type) (manufactured by Bio Rad)): 100 mg, strongly acidic cation exchange resin (AG1-X8 (H + type) (Bio Rad) )): 50 mg, and strongly basic anion exchange resin (AG50W-X8 (OH - type) (manufactured by Bio Rad)): instead of 50 mg, strongly acidic cation exchange resin (AG1-X8 (H + type) ) (Bio Rad)): 50 mg and strongly basic anion exchange resin (AG50W-X8 (OH - type) (Bio Rad)): evaluated in the same manner as in Example 4 except that 50 mg was used. did.
- Example 3 The sample was evaluated in the same manner as in Example 4 except that a sample to which NaCl was not added was used, and the sample was not dropped onto an ion exchange resin-encapsulated spin column.
- Example 4 As compared with Example 4, it was evaluated in the same manner as in Example 4 except that the sample was not dropped onto the ion exchange resin-encapsulated spin column.
- Example 4 and 5 and Comparative Examples 3 to 5 The summary of the evaluation results in Examples 4 and 5 and Comparative Examples 3 to 5 is shown in FIGS. From Example 4, Example 5, and Comparative Example 4, it was found that the Tt value in the LAMP reaction and the RT-LAMP reaction was reduced by performing desalting of the sample with an ion exchange resin. From Example 4, Example 5, and Comparative Example 4, it was suggested that the progress of the LAMP reaction is improved by desalting the sample with an ion exchange resin. Moreover, from Example 4 and Example 5, it turns out that the influence on LAMP reaction by using strongly acidic cation exchange resin (Nuvia S (Na + type) (made by BioRad)) as an ion exchange resin is small. It was.
- strongly acidic cation exchange resin Nuvia S (Na + type) (made by BioRad)
- Example 6 Spin filter column (Ultrafree-MC, 0.45 ⁇ m) with strong acid cation exchange resin (Nuvia S (Na + type) (manufactured by Bio Rad)): 100mg, strong acid cation exchange resin (AG1-X8 (H + type) ) (Manufactured by Bio Rad)): 50 mg, and a strongly basic anion exchange resin (AG50W-X8 (OH - type) (manufactured by Bio Rad)): 50 mg were weighed in.
- strong acid cation exchange resin Nuvia S (Na + type) (manufactured by Bio Rad)
- AG1-X8 H + type
- a strongly basic anion exchange resin AG50W-X8 (OH - type) (manufactured by Bio Rad)
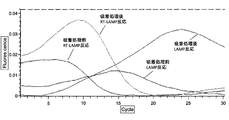
- Example 7 As compared with Example 6, evaluation was performed in the same manner as in Example 6 except that Brij35 and EDTA were not added to the sample.
- Example 6 The evaluation results in Example 6 and Example 7 are shown in FIGS. Table 4 summarizes the evaluation results.
- Example 6 it was found that the Tt value was smaller in both the LAMP reaction and the RT-LAMP reaction than in the adsorption treatment by performing the adsorption treatment of the sample impurities with the ion exchange resin.
- Example 7 it was found that the Tt value was higher in both the LAMP reaction and the RT-LAMP reaction than in the adsorption treatment by performing the adsorption treatment of the sample impurities with the ion exchange resin. This is considered to be due to the fact that in Example 6, the recovery rate of nucleic acid was increased by adding Brij35 and 1 mM EDTA to the sample as compared to Example 7.
- Example 8 Spin filter column (Ultrafree-MC, 0.45 ⁇ m) with strong acid cation exchange resin (Nuvia S (Na + type) (manufactured by Bio Rad)): 100mg, strong acid cation exchange resin (AG1-X8 (H + type) ) (Manufactured by Bio Rad)): 50 mg, and a strongly basic anion exchange resin (AG50W-X8 (OH - type) (manufactured by Bio Rad)): 50 mg were weighed in.
- strong acid cation exchange resin Nuvia S (Na + type) (manufactured by Bio Rad)
- AG1-X8 H + type
- a strongly basic anion exchange resin AG50W-X8 (OH - type) (manufactured by Bio Rad)
- Example 7 prepare 50 mM MES buffer (pH 5), add bovine whole blood to 10% by volume, add bifidobacteria sonicated to 1000 copy / uL, and add 0.5 volume of Brij35. % Was added.
- no nonionic surfactant or nonionic hydrophilic polymer was added to the sample.
- the nucleic acid recovery rate was evaluated in the same manner as in Test Example 1.
- Example 9 Compared to Example 8, evaluation was performed in the same manner as in Example 8 except that Brij35 was added to the sample before the adsorption treatment with the ion exchange resin so as to be 0.5% by volume as a nonionic surfactant. went.
- Example 10 Evaluation was performed in the same manner as in Example 9 except that Tween 20 was used instead of Brij35 as a nonionic surfactant as compared with Example 9.
- Example 11 Evaluation was performed in the same manner as in Example 9 except that PEG 20000, which is a nonionic hydrophilic polymer, was used instead of Brij 35, as compared with Example 9.
- Table 5 shows the evaluation results in Examples 8 to 11.
- Example 7 Compared to Example 7, all of the nucleic acid recovery rates were high in Examples 8 to 10, and a nonionic surfactant (Brij35, Tween20) or a nonionic hydrophilic polymer (PEG20000) was added to the sample. It was proved that the recovery rate of nucleic acid is increased by the adsorption treatment.
- a nonionic surfactant Brij35, Tween20
- a nonionic hydrophilic polymer PEG20000
- Example 12 Comparison between Adsorption Treatment According to the Present Technology and Adsorption Treatment with Zeolite
- Example 12 and Comparative Example 6 a comparison experiment between the adsorption treatment according to the present technology and the adsorption treatment with zeolite was performed.
- Example 12> A sample prepared in the same manner as the sample used in Example 7 was prepared.
- the LAMP reaction and the RT-LAMP reaction were carried out in the same manner as in Example 7 and evaluated.
- Example 12 and Comparative Example 6 The evaluation results in Example 12 and Comparative Example 6 are shown in FIG. 12 (LAMP reaction) and FIG. 13 (RT-LAMP reaction). From Example 12 and Comparative Example 6, the nucleic acid amplification reaction progressed more in the case where the sample impurities were adsorbed with the ion exchange resin than when the sample impurities were adsorbed with the zeolite. It was possible to make it good.
- the nucleic acid purification method according to the present technology is simple in operation and can extract nucleic acids with high efficiency in a short time. Therefore, it can be applied to nucleic acid purification processes for nucleic acid amplification reactions such as PCR (Polymerase Chain Reaction) and LAMP (Loop-Mediated Isothermal Amplification) methods, and nucleic acids contained in samples containing only trace amounts or very low concentrations of nucleic acids. Can be used to purify.
- PCR Polymerase Chain Reaction
- LAMP Loop-Mediated Isothermal Amplification
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Analytical Chemistry (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Physics & Mathematics (AREA)
- Biochemistry (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Immunology (AREA)
- Plant Pathology (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Crystallography & Structural Chemistry (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
- Treatment Of Liquids With Adsorbents In General (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP12832581.8A EP2757156B1 (en) | 2011-09-13 | 2012-08-28 | Nucleic acid purification method, nucleic acid extraction method, and nucleic acid purification kit |
| US14/239,340 US9498737B2 (en) | 2011-09-13 | 2012-08-28 | Method of purifying nucleic acids, method of extracting nucleic acids and kit for purifying nucleic acids |
| CN201280043107.3A CN103781907B (zh) | 2011-09-13 | 2012-08-28 | 纯化核酸的方法、提取核酸的方法和用于纯化核酸的试剂盒 |
| US15/295,720 US10023860B2 (en) | 2011-09-13 | 2016-10-17 | Method of purifying nucleic acids and kit for purifying nucleic acids |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011198949A JP5899731B2 (ja) | 2011-09-13 | 2011-09-13 | 核酸精製方法、核酸抽出方法、及び核酸精製用キット |
| JP2011-198949 | 2011-09-13 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/239,340 A-371-Of-International US9498737B2 (en) | 2011-09-13 | 2012-08-28 | Method of purifying nucleic acids, method of extracting nucleic acids and kit for purifying nucleic acids |
| US15/295,720 Continuation US10023860B2 (en) | 2011-09-13 | 2016-10-17 | Method of purifying nucleic acids and kit for purifying nucleic acids |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013038604A1 true WO2013038604A1 (ja) | 2013-03-21 |
Family
ID=47882857
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/005384 Ceased WO2013038604A1 (ja) | 2011-09-13 | 2012-08-28 | 核酸精製方法、核酸抽出方法、及び核酸精製用キット |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US9498737B2 (enExample) |
| EP (1) | EP2757156B1 (enExample) |
| JP (1) | JP5899731B2 (enExample) |
| CN (1) | CN103781907B (enExample) |
| WO (1) | WO2013038604A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023095704A1 (ja) * | 2021-11-25 | 2023-06-01 | 富士フイルム株式会社 | 核酸検査方法および検査キット |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5899731B2 (ja) * | 2011-09-13 | 2016-04-06 | ソニー株式会社 | 核酸精製方法、核酸抽出方法、及び核酸精製用キット |
| CN107980065B (zh) * | 2015-05-11 | 2021-09-07 | 3M创新有限公司 | 用于减少核酸扩增抑制的组合物 |
| FR3038616B1 (fr) | 2015-07-06 | 2020-11-06 | Gl Biocontrol | Procede de purification et de concentration d'acides nucleiques. |
| WO2020047095A1 (en) * | 2018-08-28 | 2020-03-05 | The Scripps Research Institute | Use of non-covalent immobilization in dna encoded libraries |
| KR102654881B1 (ko) * | 2023-06-23 | 2024-04-17 | 주식회사 진시스템 | 핵산 분리 장치 및 방법 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005080555A (ja) | 2003-09-08 | 2005-03-31 | Arkray Inc | 核酸の濃縮方法 |
| JP2007516425A (ja) * | 2003-05-13 | 2007-06-21 | アイシス ファーマシューティカルズ インコーポレイティッド | 後の質量分析法を用いた分析のために、溶液捕獲によって核酸を迅速に精製する方法 |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6914137B2 (en) * | 1997-12-06 | 2005-07-05 | Dna Research Innovations Limited | Isolation of nucleic acids |
| US6310199B1 (en) | 1999-05-14 | 2001-10-30 | Promega Corporation | pH dependent ion exchange matrix and method of use in the isolation of nucleic acids |
| US6270970B1 (en) * | 1999-05-14 | 2001-08-07 | Promega Corporation | Mixed-bed solid phase and its use in the isolation of nucleic acids |
| US6504021B2 (en) * | 2000-07-05 | 2003-01-07 | Edge Biosystems, Inc. | Ion exchange method for DNA purification |
| US20030065285A1 (en) * | 2001-07-23 | 2003-04-03 | Higuchi William I. | Method and apparatus for increasing flux during reverse iontophoresis |
| US20050032105A1 (en) * | 2001-10-12 | 2005-02-10 | Bair Robert Jackson | Compositions and methods for using a solid support to purify DNA |
| GB0212826D0 (en) * | 2002-05-31 | 2002-07-10 | Dna Res Innovations Ltd | Materials and methods relating to polyions and substance delivery |
| US20040016702A1 (en) * | 2002-07-26 | 2004-01-29 | Applera Corporation | Device and method for purification of nucleic acids |
| GB0229287D0 (en) * | 2002-12-16 | 2003-01-22 | Dna Res Innovations Ltd | Polyfunctional reagents |
| US8158354B2 (en) | 2003-05-13 | 2012-04-17 | Ibis Biosciences, Inc. | Methods for rapid purification of nucleic acids for subsequent analysis by mass spectrometry by solution capture |
| KR100647306B1 (ko) * | 2004-12-23 | 2006-11-23 | 삼성전자주식회사 | 아미노기와 카르복실기를 포함하고 제1 pH에서 양전하를띠는 물질을 이용하여 핵산을 분리하는 방법 |
| KR100657957B1 (ko) * | 2005-04-12 | 2006-12-14 | 삼성전자주식회사 | 아미노기와 카르복실기를 포함하고 제1 pH에서 양전하를띠는 물질을 이용하여 핵산을 분리하는 방법 및 상기방법에 사용될 수 있는 핵산 분리용 고상 물질 |
| WO2008035991A2 (en) * | 2006-09-19 | 2008-03-27 | Michael Ronald Cook | A nucleic acid extraction method |
| GB2445441B (en) * | 2006-09-26 | 2010-06-30 | Ge Healthcare Bio Sciences | Nucleic acid purification method |
| US8287848B2 (en) * | 2006-10-03 | 2012-10-16 | Tris Pharma Inc | Formulations containing an ionic mineral-ion exchange resin complex and uses thereof |
| CA2703884C (en) | 2007-11-05 | 2015-03-17 | Eiken Kagaku Kabushiki Kaisha | Method and kit for preparation of sample for use in nucleic acid amplification |
| CN103255130B (zh) * | 2008-04-30 | 2015-12-23 | 斯特莱科生物有限公司 | 高纯度质粒dna制备物及其制备方法 |
| FR2933989B1 (fr) * | 2008-07-16 | 2013-03-08 | Commissariat Energie Atomique | Procede de purification de microorganismes presents dans des echantillons liquides |
| DE102008063001A1 (de) * | 2008-12-23 | 2010-06-24 | Qiagen Gmbh | Nukleinsäureaufreinigungsverfahren |
| US8415467B2 (en) * | 2009-12-14 | 2013-04-09 | Betty Wu | Method and materials for separating nucleic acid materials |
| JP5899731B2 (ja) * | 2011-09-13 | 2016-04-06 | ソニー株式会社 | 核酸精製方法、核酸抽出方法、及び核酸精製用キット |
-
2011
- 2011-09-13 JP JP2011198949A patent/JP5899731B2/ja not_active Expired - Fee Related
-
2012
- 2012-08-28 WO PCT/JP2012/005384 patent/WO2013038604A1/ja not_active Ceased
- 2012-08-28 US US14/239,340 patent/US9498737B2/en not_active Expired - Fee Related
- 2012-08-28 EP EP12832581.8A patent/EP2757156B1/en not_active Not-in-force
- 2012-08-28 CN CN201280043107.3A patent/CN103781907B/zh not_active Expired - Fee Related
-
2016
- 2016-10-17 US US15/295,720 patent/US10023860B2/en active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007516425A (ja) * | 2003-05-13 | 2007-06-21 | アイシス ファーマシューティカルズ インコーポレイティッド | 後の質量分析法を用いた分析のために、溶液捕獲によって核酸を迅速に精製する方法 |
| JP2005080555A (ja) | 2003-09-08 | 2005-03-31 | Arkray Inc | 核酸の濃縮方法 |
Non-Patent Citations (3)
| Title |
|---|
| HUBER, C.G. ET AL.: "On-line cation exchange for suppression of adduct formation in negative-ion electrospray mass spectrometry of nucleic acids", ANAL.CHEM., vol. 70, no. 24, 1998, pages 5288 - 5295, XP002953264 * |
| IKEGAMI, T. ET AL.: "Anion- and cation-exchange MicroHPLC utilizing poly(methacrylates)-coated monolithic silica capillary columns", ANAL.SCI., vol. 23, no. 1, 2007, pages 109 - 113, XP055143215 * |
| See also references of EP2757156A4 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023095704A1 (ja) * | 2021-11-25 | 2023-06-01 | 富士フイルム株式会社 | 核酸検査方法および検査キット |
Also Published As
| Publication number | Publication date |
|---|---|
| US20170029804A1 (en) | 2017-02-02 |
| US9498737B2 (en) | 2016-11-22 |
| EP2757156B1 (en) | 2017-05-24 |
| CN103781907B (zh) | 2016-08-17 |
| JP5899731B2 (ja) | 2016-04-06 |
| EP2757156A4 (en) | 2015-08-26 |
| US10023860B2 (en) | 2018-07-17 |
| CN103781907A (zh) | 2014-05-07 |
| JP2013059275A (ja) | 2013-04-04 |
| US20140197107A1 (en) | 2014-07-17 |
| EP2757156A1 (en) | 2014-07-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10023860B2 (en) | Method of purifying nucleic acids and kit for purifying nucleic acids | |
| JP5821358B2 (ja) | 核酸抽出方法及び核酸抽出用カートリッジ | |
| CA2693654C (en) | Polynucleotide capture materials, and methods of using same | |
| EP2912174B1 (en) | Method and materials for isolation of nucleic acid materials | |
| EP4079853A1 (en) | Automated and manual methods for isolation of extracellular vesicles and co-isolation of cell-free dna from biofluids | |
| JP2007516722A (ja) | マイクロ流体装置および沈殿試薬を使用した核酸を単離する方法およびキット | |
| US20150299769A1 (en) | Method for lysing a fixed biological sample | |
| CN114196669B (zh) | 一种用于核酸提取的漂洗液 | |
| KR20060064619A (ko) | 샘플 제조 방법 및 장치 | |
| WO2017041013A1 (en) | Small-molecule mediated size selection of nucleic acids | |
| US11492655B2 (en) | Method of detecting a nucleic acid | |
| JP2009065849A (ja) | 核酸の抽出方法 | |
| JP6121780B2 (ja) | 生物学的試料の選択的調製のためのアミン化合物 | |
| JP7068183B2 (ja) | 単一洗浄溶出バッファー溶液を使用する核酸精製システム | |
| US20250019757A1 (en) | Purification chemistries and formats for sanger dna sequencing reactions on a micro-fluidics device | |
| WO2025003501A1 (en) | Nucleic acid preparation | |
| AU2016203610B2 (en) | Polynucleotide capture materials, and methods of using same | |
| JP2005304354A (ja) | 核酸増幅法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12832581 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14239340 Country of ref document: US |
|
| REEP | Request for entry into the european phase |
Ref document number: 2012832581 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012832581 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |