WO2012081276A1 - 亜鉛基合金ショット - Google Patents
亜鉛基合金ショット Download PDFInfo
- Publication number
- WO2012081276A1 WO2012081276A1 PCT/JP2011/067102 JP2011067102W WO2012081276A1 WO 2012081276 A1 WO2012081276 A1 WO 2012081276A1 JP 2011067102 W JP2011067102 W JP 2011067102W WO 2012081276 A1 WO2012081276 A1 WO 2012081276A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- zinc
- shot
- based alloy
- vickers hardness
- alloy shot
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B24—GRINDING; POLISHING
- B24C—ABRASIVE OR RELATED BLASTING WITH PARTICULATE MATERIAL
- B24C11/00—Selection of abrasive materials or additives for abrasive blasts
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/05—Metallic powder characterised by the size or surface area of the particles
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/02—Making metallic powder or suspensions thereof using physical processes
- B22F9/06—Making metallic powder or suspensions thereof using physical processes starting from liquid material
- B22F9/08—Making metallic powder or suspensions thereof using physical processes starting from liquid material by casting, e.g. through sieves or in water, by atomising or spraying
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C1/00—Making non-ferrous alloys
- C22C1/02—Making non-ferrous alloys by melting
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C18/00—Alloys based on zinc
- C22C18/02—Alloys based on zinc with copper as the next major constituent
Definitions
- Vickers hardness means that measured under the conditions of a test force of 0.4093 N and a retention time of the test force of 10 to 15 s in "JIS Z 2244". Although “ ⁇ HV 0.05” is displayed, it is abbreviated as “ ⁇ HV” in the text.
- alloy composition means "mass%", unless it refuses.
- the “average particle size” of a shot means “median diameter: 50% value of cumulative distribution” unless otherwise specified.
- Zinc shot consisting only of zinc has lower explosion sensitivity of dust cloud resulting from shot crushing and higher explosion lower limit concentration compared to aluminum base alloy shot and stainless steel shot.
- Patent Document 1 paragraph 0004 zinc shot is likely to cause darkening in the product to be treated, and is 40 to 50 HV in Vickers hardness display, so it is soft and the surface treatment effect is insufficient and blasting time is required (Patent Document 1 paragraph 0004) ).
- Patent Documents 1 to 6 zinc-based alloys to which various alloying elements are added have been proposed for the purpose of suppressing the occurrence of darkening and improving the hardness of zinc shots.
- Patent Documents 1 and 2 Ni in Patent Document 3, Mn in Patent Document 4, Cu and Mn in Patent Document 5, and Mg in Patent Document 6 to solve these problems. It has been proposed to do.
- the present invention is a Cu-added zinc-based alloy shot which can be easily prepared to have a relatively high hardness, and the shot itself is less corroded (rust generation), and further, the shot is refined by use. It is an object of the present invention to provide a zinc-based alloy shot of a novel constitution in which the wear and tear do not increase so much and a method of manufacturing the same.
- the inventors of the present invention have the following constitution, and it is easy to prepare a conventional Cu-containing zinc-based alloy having relatively high hardness, and The inventors of the present invention have found that the shot itself can be inhibited from corrosion (rust generation), and the present invention has the following constitution.
- the present invention is a zinc-based alloy shot, which contains a main additive element Cu for the purpose of increasing the Vickers hardness etc. and a sub-additive element Fe for the purpose of the increase of the Vickers hardness and corrosion inhibition.
- the zinc-based alloy shot according to the present invention is characterized in that it has a hardness of 40 to 150 HV.
- the zinc-based alloy shot of the present invention further increases Vickers hardness by adding a small amount of Fe as a sub-additive element together with Cu which is a main additive element to the zinc-based alloy shot, and corrosion of the shot itself (discoloration over time) Is suppressed (see the corrosion test results described later). As a result, the product value (mainly appearance) of the shot is increased.
- Patent Document 3 See Comparative Examples 2 and 3 in Tables 1 and 2).
- the zinc-based alloy shot according to the present invention does not contain Ni or Mn, which is a target of the PRTR system, as in Patent Documents 3 to 5, and is desirable also from the viewpoint of environmental protection and work safety.
- the zinc-based alloy shot of the present invention can relatively reduce the Cu content. For this reason, generation
- shot blasting the shot with little wear loss is again shot onto the work after being shot onto the work, that is, it is used after being circulated, but the occurrence of the wear is suppressed, so the life of the zinc based alloy shot is long Become.
- FIG. 1 schematically shows the composition range (black portion) of the present invention in the phase diagram of the ternary alloy composition of the zinc-based alloy shot.
- the zinc-based alloy shot of the present invention contains Fe as a sub-additive element together with Cu as a main additive element for the purpose of increasing the hardness.
- the above-described Cu acts to increase the mechanical strength and hardness (Vickers hardness) of the zinc alloy, and if the content of Cu is too low, it is difficult to obtain such an action.
- the Cu content is high, although mechanical strength and Vickers hardness are improved, toughness (impact resistance) tends to decrease.
- the above-mentioned Fe is a small amount added (containing) and cooperates with Cu to act to increase the hardness (Vickers hardness) and also to act to suppress corrosion (discoloration reduction). If the Fe content is too low, it is difficult to obtain those effects. However, when the Fe content is high, as in the case where the Cu content is high, although mechanical strength and Vickers hardness are improved, toughness (impact resistance) tends to decrease.
- the chemical component composition is appropriately selected from the balance of Vickers hardness and toughness.
- the deburring ability and the cleaning ability are not sufficient, and if it exceeds 150 HV, cracking and wear of the zinc-based alloy shot progress in deburring and cleaning. This makes it easy to do so and increases the consumption of shots. This is due to the low toughness of the zinc based alloy shot.
- the surface of the alloy product may be damaged, or it may be processed into a satin-like shape more than necessary to maintain a predetermined surface roughness.
- the Cu content 1.5 to 10.0% and the Cu content of Patent Document 2 Relatively lower than 1.8 to 13.0% is considered to be because the hardness of the shot is significantly increased by the Fe content.
- the Cu content can be greatly reduced to obtain a shot of the same hardness, and the shot It is possible to suppress the reduction of the toughness of (see the section of Vickers hardness of the blast evaluation test of the example).
- the total content of the elements (unavoidable impurities) other than the three components (Zn, Cu, Fe) contained in the zinc-based alloy shot is as small as possible.
- the toughness is likely to be low (cracks are likely to occur), leading to a reduction in life.
- the Fe can be used as all or part of the sub-additive element of the present invention.
- the amount of Fe contained as an impurity in Zn and Cu is approximately the same as the amount of Fe required for a zinc-based alloy shot, it is not necessary to add Fe again, and is required for a zinc-based alloy shot If the amount is smaller than the amount of Fe, a shortfall may be added.
- raw materials (base metals) of Zn which is a base element common zinc base metal (99.97% or more) of JISH 2107, most pure zinc base metal (99.995% or more), special type zinc base metal (99.99%) And the like.
- the Fe content of ordinary zinc metal is 0.005% or less.
- the electric copper metal (99.96% or more) of JISH2121 etc. can be mentioned.
- regulated by JISG 0203 can be used suitably.
- the average particle size (median diameter) of the zinc-based alloy shot in the present invention varies depending on the strength of the article to be treated and the treatment purpose, but is usually in the range of 0.1 to 3.5 mm, from the viewpoint of productivity and demand. , 0.3 to 2.3 mm, and more preferably 0.3 to 1.2 mm. If the average particle size is too small, it is difficult to obtain sufficient deburring ability, cleaning ability and peening effect (for example, application of compressive residual stress). Conversely, if the average particle diameter is too large, the object to be treated may be damaged by surface treatment (deburring, cleaning, shot peening treatment, etc.), or it may be processed into a satin-like shape more than necessary to obtain a predetermined surface roughness. The degree can not be maintained.
- the corrosion of the shot is suppressed by the addition of Fe (sub-additive element) as described above. It is not transferred to the surface of the workpiece (workpiece). Therefore, when applied to the surface treatment of a light alloy product made of an aluminum alloy, a zinc alloy or a magnesium alloy, it can be expected to suppress the generation of darkening of the object to be treated (work).
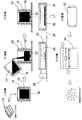
- the zinc-based alloy shot of the present invention includes, for example, dropping molten molten metal into a cooling medium such as water, solidifying and depositing in the cooling medium, and drying the solidified solid. It is desirable to classify and manufacture a granular body. Since the molten metal is rapidly cooled by dropping the molten metal melt into the cooling medium, it has a finer and more uniform structure than a general casting material. When used as shot blasting or shot peening, the zinc-based alloy shot is loaded with a very large external force, so that a fine and uniform structure improves mechanical properties such as impact resistance and tensile strength. Can be suitably used as a zinc-based alloy shot. The case of manufacturing using the aforementioned manufacturing method will be specifically described below (see FIG. 2).
- an ingot (raw material) 12 of a base element (Zn) and an additive element (Cu and Fe) is weighed and introduced into the crucible 14 so as to have a set alloy composition ratio.
- the crucible 14 is heated by a heating means (resistance heating) 15 to melt the ingot (base metal) mixture charged to obtain a molten metal 16.
- the melting and heating temperature at this time varies depending on the alloy composition and production scale, but is usually set appropriately in the range of 550 to 700.degree.
- the melting point of each element is as follows. Zn: 419.6 ° C., Cu: 1083.4 ° C., Fe: 1535 ° C.
- the molten metal 16 is introduced into the molten metal holding vessel 18.
- the molten metal holding vessel 18 is provided with a heating means (resistance heating) 20, and can hold the molten metal 16 so as not to be cooled more than necessary when the zinc-based alloy shot is manufactured.
- the molten metal holding temperature at this time varies depending on the alloy composition and production scale, but is usually set appropriately in the range of 450 to 650.degree.
- a dropping nozzle 22 for dropping the molten metal is provided at the bottom of the molten metal holding vessel 18, and a cooling medium 24 such as water is introduced at the lower part of the nozzle 22, and a cooling means (cooling tower) 26 is attached.
- a tank 28 is arranged.
- the cooling medium 24 may be oil or the like.
- the molten metal 16 in the molten metal holding container 18 is dropped from the dropping nozzle 22 to be in contact with air when passing through the dropping nozzle 22 and reaching the cooling medium 24, and further to be in contact with the cooling medium 24. Spheroidizes under the influence of surface tension as it cools.
- the temperature of the cooling medium 24 is raised by contact with the molten metal, which causes the rapid cooling of the molten metal to be prevented. Therefore, the cooling medium 24 is maintained at the set temperature by the cooling means (cooler) 26.
- the set cooling temperature is usually 60 ° C. or less. When the temperature exceeds 60 ° C., the water in contact with the dropped molten metal (droplet) is boiled to cause the interface to be vaporized, which makes it difficult to exhibit the rapid cooling action.
- particles of zinc alloy 30 are deposited. This is collected, dried by a drier (rotary drier) 32, and then classified by a classifier (vibrating sieve) 34 to obtain a zinc-based alloy shot. Classification is performed so as to obtain a predetermined particle size in accordance with the intended use of the zinc-based alloy shot.
- the manufacturing method of a zinc base alloy shot is not limited to the said drop granulation method.
- known methods such as a gas atomizing method, a centrifugal atomizing method, and a water atomizing method can be appropriately selected according to the shape, particle size, and the like of the target zinc-based alloy shot.
- each zinc-based alloy shot was carried out with the alloy composition shown in Table 1 in the method (droplet granulation method) shown in FIG. 2 described above.
- Each shot thus manufactured was classified to prepare a shot for projection of each sample having an average particle diameter (median diameter) of 1.0 mm. And about the shot of each sample, the test of each following item was performed and evaluation was performed.
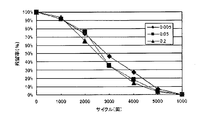
- Shot blast evaluation test 100 kg of the shot (average particle diameter of 1.0 mm) of each sample prepared above was treated with "The Ervin Test Machine (manufactured by Ervin)" at a projection speed of 60 m / s using steel (Rockwell hard With the target of 65 HRC (JIS G0202, defined in JIS Z2245), 5000 shots (shots) were made.
- Corrosion rate (%) 100 ⁇ total corrosion area (mm 2) / total sample surface area (mm 2) From FIG. 5 showing the result of the corrosion test, the corrosion rate is remarkable only by slightly containing Fe (0.0025 to 0.25%) It can be seen that
- the zinc-based alloy shot of the present invention containing the sub-additive element Fe together with the main additive element Cu easily secures the Vickers hardness and the shot life (toughness) is practically sufficient. Furthermore, it has been proved that the corrosion resistance is also excellent.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
- Powder Metallurgy (AREA)
- Contacts (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020137015097A KR101846413B1 (ko) | 2010-12-16 | 2011-07-27 | 아연기 합금 쇼트 |
| US13/993,780 US9707664B2 (en) | 2010-12-16 | 2011-07-27 | Zinc-based alloy shot |
| BR112013014944A BR112013014944B8 (pt) | 2010-12-16 | 2011-07-27 | grânulo de liga à base de zinco |
| CN201180059609.0A CN103370173B (zh) | 2010-12-16 | 2011-07-27 | 锌基合金丸 |
| MX2013006799A MX356628B (es) | 2010-12-16 | 2011-07-27 | Pieza fundida con aleación a base de zinc. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010280807A JP2012125900A (ja) | 2010-12-16 | 2010-12-16 | 亜鉛基合金ショット |
| JP2010-280807 | 2010-12-16 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012081276A1 true WO2012081276A1 (ja) | 2012-06-21 |
Family
ID=46244392
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/067102 Ceased WO2012081276A1 (ja) | 2010-12-16 | 2011-07-27 | 亜鉛基合金ショット |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US9707664B2 (enExample) |
| JP (1) | JP2012125900A (enExample) |
| KR (1) | KR101846413B1 (enExample) |
| CN (1) | CN103370173B (enExample) |
| BR (1) | BR112013014944B8 (enExample) |
| MX (1) | MX356628B (enExample) |
| WO (1) | WO2012081276A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113737056A (zh) * | 2021-09-09 | 2021-12-03 | 湘潭大学 | 一种Zn-Se基合金材料及其制备方法和应用 |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104294086B (zh) * | 2014-11-10 | 2016-09-14 | 华玉叶 | 一种高铜锌合金及其制备方法 |
| JP5939339B2 (ja) * | 2015-06-04 | 2016-06-22 | 新東工業株式会社 | 亜鉛基合金ショット |
| CN106702212A (zh) * | 2015-11-16 | 2017-05-24 | 上海交通大学 | 医用可降解Zn-Cu-X合金材料及其制备方法 |
| JP6965926B2 (ja) * | 2017-06-21 | 2021-11-10 | 新東工業株式会社 | 亜鉛基合金ショット及びその製造方法 |
| CN113512667B (zh) * | 2021-06-22 | 2022-03-29 | 北京科技大学 | 一种高耐蚀高强韧加工性能优良Zn-Cu-Ti-Mo合金与板材及制备方法 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0970758A (ja) * | 1995-09-05 | 1997-03-18 | Sinto Brator Co Ltd | ショット |
| JP2002224962A (ja) * | 2001-01-30 | 2002-08-13 | Sinto Brator Co Ltd | ショット |
| JP2009226535A (ja) * | 2008-03-21 | 2009-10-08 | Lianyungang Beautech Metal Abrasive Co Ltd | 亜鉛合金ショット |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2046302A (en) * | 1979-03-02 | 1980-11-12 | Mitsui Mining & Smelting Co | Zinc alloy powder |
| JPS60120763A (ja) | 1983-12-06 | 1985-06-28 | Nippon Oil Co Ltd | 粉粒体野外貯蔵物の表面処理剤 |
| JPS60120763U (ja) * | 1984-01-25 | 1985-08-15 | 日本鉱業株式会社 | 亜鉛シヨツトの製造装置 |
| JPS6138870A (ja) * | 1984-07-30 | 1986-02-24 | Dowa Teppun Kogyo Kk | メカニカルプレ−テイング用混合粉体およびこれを使用した連続メカニカルプレ−テイング法 |
| JPH11320416A (ja) * | 1998-05-13 | 1999-11-24 | Toho Zinc Co Ltd | 亜鉛合金ショット |
| JP4309004B2 (ja) | 1999-12-14 | 2009-08-05 | 東邦亜鉛株式会社 | 亜鉛合金ショット |
| JP4774883B2 (ja) | 2005-09-21 | 2011-09-14 | 新東工業株式会社 | 亜鉛基合金ショット |
| JP4907985B2 (ja) * | 2005-12-27 | 2012-04-04 | 三井金属鉱業株式会社 | フッ素除去方法 |
| CN1943992B (zh) * | 2006-09-29 | 2010-05-12 | 连云港倍特金属磨料有限公司 | 一种锌合金喷丸 |
| CN100537806C (zh) * | 2007-10-19 | 2009-09-09 | 戴国水 | 一种微合金化高强度锌合金 |
| CN101413075A (zh) * | 2008-12-16 | 2009-04-22 | 连云港倍特金属磨料有限公司 | 一种锌合金喷丸 |
| JP5007776B2 (ja) * | 2009-10-30 | 2012-08-22 | 新東工業株式会社 | 亜鉛基合金ショット |
-
2010
- 2010-12-16 JP JP2010280807A patent/JP2012125900A/ja active Pending
-
2011
- 2011-07-27 BR BR112013014944A patent/BR112013014944B8/pt active IP Right Grant
- 2011-07-27 WO PCT/JP2011/067102 patent/WO2012081276A1/ja not_active Ceased
- 2011-07-27 KR KR1020137015097A patent/KR101846413B1/ko active Active
- 2011-07-27 MX MX2013006799A patent/MX356628B/es active IP Right Grant
- 2011-07-27 US US13/993,780 patent/US9707664B2/en active Active
- 2011-07-27 CN CN201180059609.0A patent/CN103370173B/zh active Active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0970758A (ja) * | 1995-09-05 | 1997-03-18 | Sinto Brator Co Ltd | ショット |
| JP2002224962A (ja) * | 2001-01-30 | 2002-08-13 | Sinto Brator Co Ltd | ショット |
| JP2009226535A (ja) * | 2008-03-21 | 2009-10-08 | Lianyungang Beautech Metal Abrasive Co Ltd | 亜鉛合金ショット |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113737056A (zh) * | 2021-09-09 | 2021-12-03 | 湘潭大学 | 一种Zn-Se基合金材料及其制备方法和应用 |
| CN113737056B (zh) * | 2021-09-09 | 2022-05-27 | 湘潭大学 | 一种Zn-Se基合金材料及其制备方法和应用 |
Also Published As
| Publication number | Publication date |
|---|---|
| BR112013014944A2 (pt) | 2016-09-13 |
| US9707664B2 (en) | 2017-07-18 |
| US20130259737A1 (en) | 2013-10-03 |
| MX2013006799A (es) | 2013-07-29 |
| BR112013014944B8 (pt) | 2020-06-23 |
| CN103370173B (zh) | 2016-04-20 |
| KR101846413B1 (ko) | 2018-04-06 |
| KR20130128414A (ko) | 2013-11-26 |
| BR112013014944B1 (pt) | 2020-06-02 |
| CN103370173A (zh) | 2013-10-23 |
| JP2012125900A (ja) | 2012-07-05 |
| MX356628B (es) | 2018-06-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101237904B1 (ko) | 아연계 합금 쇼트 | |
| WO2016166779A1 (ja) | ダイカスト用アルミニウム合金およびこれを用いたアルミニウム合金ダイカスト | |
| WO2012081276A1 (ja) | 亜鉛基合金ショット | |
| JP5787215B2 (ja) | 亜鉛基合金ショット | |
| JP5939339B2 (ja) | 亜鉛基合金ショット | |
| JP4165794B2 (ja) | ショット | |
| JP2007039748A (ja) | 耐熱性Al基合金 | |
| JPH111706A (ja) | 耐衝撃性鉄系合金球状粒子 | |
| JP2009226535A (ja) | 亜鉛合金ショット | |
| JP4774883B2 (ja) | 亜鉛基合金ショット | |
| WO2018235272A1 (ja) | アルミニウム合金およびアルミニウム合金鋳物品 | |
| TWI771436B (zh) | 鋅基合金珠及其製造方法 | |
| JP6965926B2 (ja) | 亜鉛基合金ショット及びその製造方法 | |
| JP7322611B2 (ja) | 亜鉛合金及びその製造方法 | |
| US10266916B2 (en) | Crystal grain refiner for magnesium alloy, containing aluminum, a method for preparing magnesium alloy, and magnesium alloy manufactured by same method |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201180059609.0 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11848044 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20137015097 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13993780 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: MX/A/2013/006799 Country of ref document: MX |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11848044 Country of ref document: EP Kind code of ref document: A1 |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112013014944 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112013014944 Country of ref document: BR Kind code of ref document: A2 Effective date: 20130614 |